Abstract
The Great Oxygenation Event (GOE), ca. 2.4 billion years ago, transformed life and environments on Earth. Its causes, however, are debated. We mathematically analyze the GOE in terms of ecological dynamics coupled with a changing Earth. Anoxygenic photosynthetic bacteria initially dominate over cyanobacteria, but their success depends on the availability of suitable electron donors that are vulnerable to oxidation. The GOE is triggered when the difference between the influxes of relevant reductants and phosphate falls below a critical value that is an increasing function of the reproductive rate of cyanobacteria. The transition can be either gradual and reversible or sudden and irreversible, depending on sources and sinks of oxygen. Increasing sources and decreasing sinks of oxygen can also trigger the GOE, but this possibility depends strongly on migration of cyanobacteria from privileged sites. Our model links ecological dynamics to planetary change, with geophysical evolution determining the relevant time scales.
Subject terms: Microbial ecology, Palaeoecology, Biogeochemistry, Atmospheric chemistry
The Great Oxygenation Event (GOE) 2.4 billion years ago is believed to have been critical for the evolution of complex life. Here, Olejarz et al. propose a model suggesting that competition between major bacterial groups could have triggered the GOE in a feedback loop with geophysical processes.
Introduction
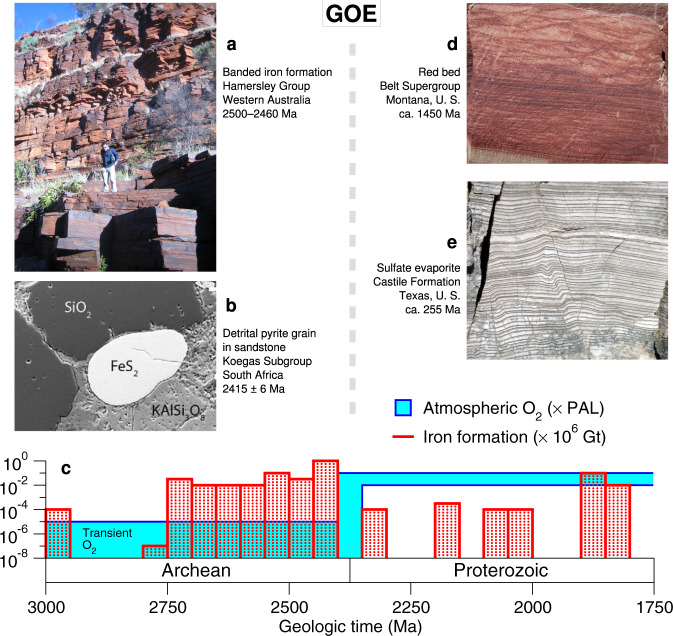
The Great Oxygenation Event (GOE), ~2.4 billion years ago, records a major turning point in the history of our planet. While pO2 may have fluctuated during the GOE1,2, its results are clear: multiple lines of geological and geochemical evidence document the initial rise of O2 to permanent prominence in the atmosphere and surface ocean3. These include (1) a sharp drop in iron formation deposition4, (2) the appearance of red beds in continental sedimentary successions5 and calcium sulfates in marine environments6, (3) the retention of iron in ancient weathering horizons7, (4) the loss of detrital uraninite and other redox-sensitive minerals from fluvial and deltaic sandstones8, and (5) the loss of a mass-independent sulfur-isotopic signature best explained in terms of photochemical reactions in an essentially oxygen-free atmosphere9,10. Detrital pyrite and uraninite suggest that, prior to the GOE, pO2 was <1.6 × 10−4 of present atmospheric levels (PAL)11, whereas modeling of mass-independent S-isotopic fractionation limits Archean pO2 to ~10−5 of PAL or lower9,12,13. Figure 1 summarizes the main geological and geochemical lines of evidence for the GOE.
Fig. 1. Evidence for the GOE.
Multiple lines of geologic and geochemical evidence support the view that oxygen gas first became a permanent component of Earth’s atmosphere and surface ocean ca. 2.4 billion years ago. Sedimentary iron formation (a), which requires transport of ferrous iron through the ocean, is abundant in successions that predate the GOE but uncommon afterward (c, with resurgences around 1900–1850 and 715–660 Ma). Similarly, redox-sensitive minerals such as pyrite (FeS2) occur in detrital facies before the GOE (b) but not afterward. In contrast, red beds (d) and sulfate salts (e), which bespeak O2 in surface environments, have the opposite time distribution, gaining prominence only after the GOE. It is estimated that atmospheric pO2 increased from <10−5 to 1–10% of PAL at this time (c). (Data on iron formations in (c) are taken from Bekker et al.4. The blue shaded region denoting atmospheric O2 levels is only notional, as it is possible that atmospheric pO2 dropped below 1% of PAL during the Proterozoic1,2.).
While there is consensus on when the biosphere began to accumulate oxygen, debate continues about the physical and/or biological drivers of this transition. As emphasized by Kasting14, the GOE required an increase in the rate of oxygen production and/or a decrease in rates of oxygen consumption. Cyanobacterial photosynthesis is generally accepted as the key source of oxygen, but many models to explain the GOE tacitly assume that cyanobacteria were abundant prior to the GOE and so rely on physical events as the proximal drivers of environmental state change14–16. Proposed physical drivers include hydrogen escape to space associated with the photodissociation of H2O and CH4 in the upper atmosphere17,18, a temporal shift to more oxidized volcanic gases as subaerial volcanism increased along with craton expansion19,20, or a decrease in hydrothermal iron fluxes into the ocean as vent temperatures declined19,21,22. Limited phosphorus availability in Archean oceans has also been suggested as limiting rates of photosynthesis and carbon burial23–25.
Goldblatt et at.26 considered that oxygenic photosynthesis was well established prior to the GOE but that atmospheric methane oxidation suppressed oxygen levels. They modeled a low-level steady state of oxygen and a high-level steady state—the latter being characterized by an ozone layer that shields the troposphere from ultraviolet radiation, limiting the rate of methane oxidation. These steady states of oxygen can overlap, resulting in bistability and hysteresis. Also related to methane oxidation, Konhauser et al.27 proposed a coupled biological-geological driver for the GOE, concluding that a reduced flux of nickel to the oceans in the late Archean limited methanogen activity, thereby capping the supply of biogenic methane.
Other investigations of the carbon isotope record have argued that the δ13C value of marine carbonate increased from the Archean into the Proterozoic28,29, raising the possibility that biological innovations acted as triggers for the GOE. In contrast to geophysical models, Ward et al.30 modeled the GOE as a direct response to the origin of oxygenic photosynthesis. This implicitly assumes that cyanobacteria hold an inherent selective advantage over anoxygenic photoautotrophs, but the dominance of anoxygenic photosynthetic bacteria in modern environments where light is present but oxygen is not (e.g., ref. 31) casts substantial doubt on this assumption. The competitive advantage of anoxygenic photosynthetic bacteria over cyanobacteria may relate to the lower metabolic cost of deriving electrons from donors other than water, or it may reflect the direct inhibition of oxygenic photosynthesis by sulfide32 or ferrous iron33. In the presence of sulfide, some cyanobacteria can shut down Photosystem II and use H2S as an electron donor for anoxygenic photosynthesis34,35. The inhibitory effect of Fe2+ is less clear, as Ward et al.36 show that cyanobacteria live in hot spring waters that contain both ferrous iron and modest amounts of oxygen. Where Fe2+ was present and O2 was absent, however, cyanobacteria were not ecologically important36. Moreover, a growing array of geochemical data is interpreted as evidence for “whiffs of oxygen”—spatially and temporally limited oxygen oases in the Archean biosphere—requiring oxygenic photosynthesis hundreds of millions of years before the GOE37–39.
A natural scenario, then, is that cyanobacteria were present but relatively scarce well before the GOE, rising to global ecological prominence coincident with the environmental transformation. Jones et al.40 argued that as the ratio of alternative electron donors (they focused on Fe2+, but it is the sum of non-H2O electron donors that is key) to phosphorus declined through time, environmental opportunities for oxygenic photoautotrophs increased. Knoll and Nowak41 developed a simple mathematical model to describe the competition between anoxygenic photosynthetic bacteria and cyanobacteria on a changing Earth. Their model keeps track of the abundances of anoxygenic photosynthetic bacteria, cyanobacteria, ferrous iron, and oxygen. Simulations revealed that the GOE can be triggered as the planetary removal rate of oxygen from the atmosphere declines, although that primitive model was not analytically solved. Ozaki et al.42 used both modeling and experimental evidence, arguing once again that oxygenic photoautotrophs evolved long before the GOE, but that geochemical conditions throughout the Archean favored primary production by anoxygenic photosynthetic bacteria. They further argued that anoxygenic photoferrotrophs, being well adapted to low-light conditions, inhabited the bottom of the photic zone, thereby diminishing the supply of upwelling nutrients to cyanobacteria (see also ref. 43). This control on the proliferation of oxygenic bacteria would have remained in place as long as there was a sufficiently large supply of reducing agents from the deep ocean.
Here, we advance the model of Knoll and Nowak41 by additionally keeping track of the abundance of phosphate, because the amount of ferrous iron or other alternative electron donors relative to phosphate influences the competition between anoxygenic photosynthetic bacteria and cyanobacteria. We also properly account for the loss of ferrous iron due to the proliferation of anoxygenic photoautotrophs, and we correctly incorporate loss of dioxygen due to iron oxidation. We can now investigate the effects of a declining influx of iron and an increasing influx of phosphate as consequences of planetary change, while concurrently probing the influences of biological innovations and of changes in sources and sinks of oxygen. Our model thus focuses consideration of the GOE on interactions between the physical and biological Earth.
Results
Based on the present-day distribution of photosynthetic bacteria31, we assume a competitive advantage for anoxygenic photosynthetic bacteria in early environments where electron donors such as Fe2+, H2S, or H2 were present. We also assume the contemporaneous existence of environments where cyanobacterial populations could thrive, providing a seedbed for migration. Non-marine waters provide an example of the latter, supported by the branching of non-marine taxa from basal nodes in cyanobacterial phylogenies44,45 and also by the presence of stromatolites in Archean lacustrine successions46, despite the likelihood that many Archean lakes and rivers had low levels of potential electron donors such as Fe2+ and H2S47.
Following Jones et al.40 and Ozaki et al.42, we use Fe (iron) and P (phosphorus) to represent the environment, which is similar to the H2 and P employed in other studies48,49. The logic of this choice is that in Archean oceans, Fe2+ is thought to have been the principal electron donor for anoxygenic photosynthesis50,51, whereas P governed total rates of photosynthesis. (Kasting14 argued that H2 was key to photosynthesis on the early Earth, a view supported by low iron concentrations in some early Archean stromatolites52.). In any event, under the conditions of low P availability thought to have characterized early oceans25,40,49,53–55, anoxygenic photosynthesis would have depleted limiting nutrients before alternative electron donors were exhausted. In consequence, rates of photosynthetic oxygen production would be low. As iron availability declined and/or P availability increased, the biosphere would inevitably reach a point where P would remain after Fe2+ had been depleted, expanding the range of environments where cyanobacteria are favored by natural selection42.
Our model keeps track of the abundances of anoxygenic photosynthetic bacteria (APB), x1, cyanobacteria, x2, and three crucial chemicals: iron(II) (Fe2+), y1, phosphate (PO43−), y2, and dioxygen (O2), z. Both types of bacteria require phosphate for reproduction. APB needs iron(II) (or some other suitable reductant) as an electron donor in photosynthesis. The following five equations describe the reproduction and death of APB and of cyanobacteria as well as the dynamics of iron(II), phosphate, and dioxygen:
| 1 |
Here, we have omitted to write symbols for those rate constants that, for understanding the GOE, can be set to one without loss of generality (Supplementary Note 1). Each remaining rate constant is a free parameter. Equations (1) thus satisfy redox balance by construction. We are left with a system that has five main parameters: c specifies the rate of reproduction of cyanobacteria; f1 and f2 denote the rates of supply of iron(II) and phosphate, respectively; a denotes biogenic production of oxygen; b denotes geochemical consumption of oxygen. Note that iron(II) and phosphate are also removed by geochemical processes at a rate proportional to their abundance. In addition, iron(II) is used up during anoxygenic photosynthesis, and iron(II) reacts with oxygen and is thereby removed from the system. Phosphate is used up during the growth of APB and cyanobacteria. (We investigate extensions of the model that incorporate bounded bacterial growth rates and organic carbon in Supplementary Note 2 and Supplementary Note 3, respectively.)
We posit iron(II) as the primary electron donor for anoxygenic photosynthesis, and for simplicity of presentation, we refer to y1 and f1 in this context. However, as noted above, y1 and f1 can similarly represent the abundances and influxes of other alternative electron donors, especially dihydrogen (H2)56,57 and hydrogen sulfide (H2S)58. Our model, its analytical solution, and the conclusions that follow hold equally well by considering any of these electron donors or all together.
We also include small migration rates, u1 and u2, which allow for the possibility that APB and cyanobacteria persist in privileged sites from which they can migrate into the main arena of competition. On the Archean Earth, these parameters could have been affected by the flow of water and by surface winds. For the mathematical analysis presented in the main text, we assume that these rates are negligibly small.
The GOE represents the transition from a world dominated by APB (Equilibrium E1) to one that is dominated by cyanobacteria (Equilibrium E2) (Figs. S1, S2). On a slowly changing planet, the abundances of APB and cyanobacteria and of the three chemicals are approximately in steady state. Therefore, we consider the fixed points of Eqs. (1).
Pure equilibria
In the absence of APB and cyanobacteria, the abiotic equilibrium abundances of iron(II) and of phosphate are given by f1 and f2, respectively, and there is no oxygen in the system. If f1f2 > 1, then APB can emerge. Subsequently, the system settles to Equilibrium E1, where only APB are present and there is still no oxygen. E1 is stable against invasion of cyanobacteria if
| 2 |
This condition can be fulfilled if the influx of iron, f1, is large enough, or if the influx of phosphate, f2, is small enough. The term on the right-hand side of the inequality is an increasing function of the reproductive rate, c, of cyanobacteria.
If cf2 > 1, then the system admits another equilibrium, E2, where only cyanobacteria are present and oxygen is abundant. Equilibrium E2 is stable against invasion of APB if
| 3 |
The left-hand side of the inequality is positive. If the right-hand side is negative (that is, if f1 < c), then the condition certainly holds. If the right-hand side is positive, then the condition can be fulfilled if the influx of phosphate, f2, is large enough, or if the production of oxygen, a, is large enough. In other words, the dominance of cyanobacteria after the GOE can be guaranteed by a sufficiently large supply of phosphate or sufficiently large production of oxygen. It may or may not be possible for the proportional removal rate of oxygen, b, to become small enough for the condition to be fulfilled.
Mixed equilibrium
If Conditions (2) and (3) are either both satisfied or both not satisfied, then the system also admits an interior equilibrium, . If Conditions (2) and (3) are both satisfied, then Equilibrium is unstable; if those conditions are both not satisfied, then Equilibrium is a stable mixed equilibrium where both types of bacteria coexist. Equilibrium is characterized by the stable coexistence of APB and cyanobacteria if
| 4 |
Condition (4) is understood as follows. If b is sufficiently large, then there is not enough atmospheric oxygen for rusting to render E2 stable against invasion of APB before E1 loses stability; the result is stable coexistence. But if b is sufficiently small, then rusting causes E2 to become stable before E1 becomes unstable. The critical value of b therefore depends on the input of atmospheric oxygen for Equilibrium E2; it is an increasing function of the reproductive rate of cyanobacteria and of their rate of production of oxygen.
If a < 1, then bistability is not possible. In this case, for Equilibrium E2, dioxygen is depleted by rusting before there is any significant loss of iron(II). As a result, E2 cannot gain stability before E1 loses stability, regardless of the values of b or c.
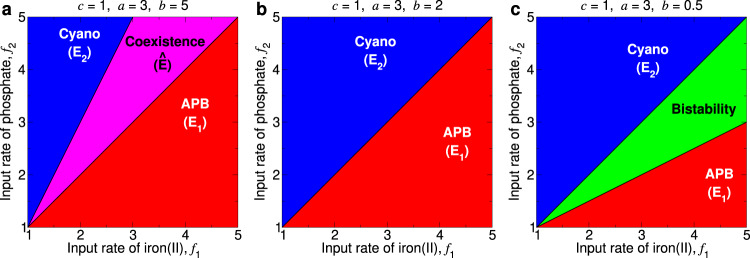
Figure 2 shows, for different values of b, the behavior of the system as a function of f1 and f2.
Fig. 2. The stability properties of Equilibrium E1 (APB dominate) and Equilibrium E2 (cyanobacteria dominate) depend on the input rate of iron(II), f1, and the input rate of phosphate, f2.
High values of f1 and low values of f2 promote stability of E1 and instability of E2. Low values of f1 and high values of f2 promote instability of E1 and stability of E2. a If the proportional consumption rate of oxygen, b, is large, then intermediate values of f1 and f2 lead to both E1 and E2 being unstable, with Equilibrium corresponding to stable coexistence. b For an intermediate value of b, either E1 is stable with E2 unstable, or E1 is unstable with E2 stable. c If b is small, then intermediate values of f1 and f2 lead to both E1 and E2 being stable.
Transition from Equilibrium E1 to Equilibrium E2
The transition between Equilibria E1 and E2 can be achieved by reducing the supply of iron(II), f1, since such a reductant is required for anoxygenic photosynthesis. When this happens, we lose the stability of E1 and gain the stability of E2.
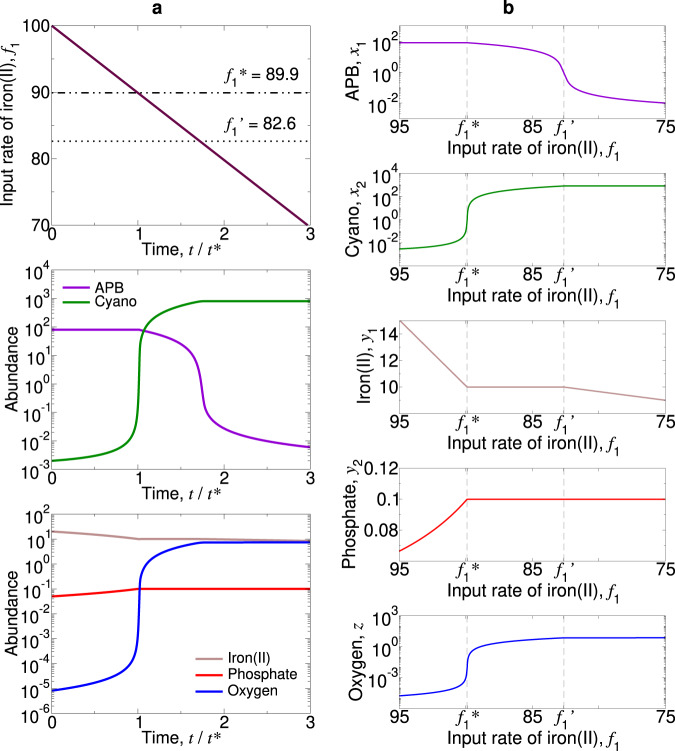
The transition is gradual if b > c(a − 1). Figure 3 shows gradual oxygenation due to decreasing f1. In this case, the transition occurs via the mixed equilibrium, , where both types of bacteria coexist (Fig. 4). A subsequent increase in f1 can cause APB to regain dominance (Fig. S3a).
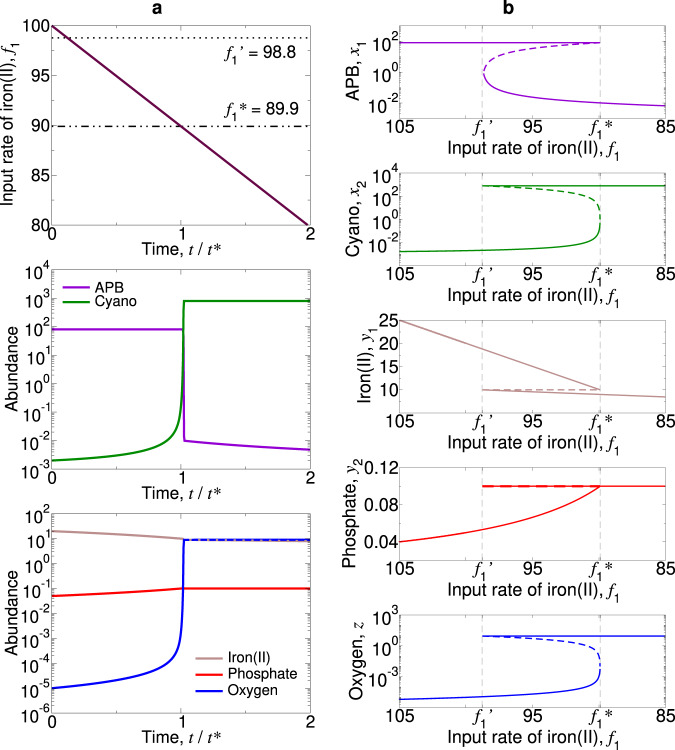
Fig. 3. The GOE can be triggered by a decline in the influx of iron(II) and is gradual if b > c(a − 1).
Equilibrium E1 (APB dominate) loses stability and Equilibrium E2 (cyanobacteria dominate) gains stability when f1 drops below and , respectively. We set f2 = 80, c = 10, a = 10, b = 100, and u1 = u2 = 10−3. a We simulate Eqs. (8) from Supplementary Note 1 with α1 = α2 = β1 = β2 = 1, and we set f1 = 100 − 40(t/105). t* denotes the time at which Equilibrium E1 loses stability. b There is stable coexistence of both types of bacteria for .
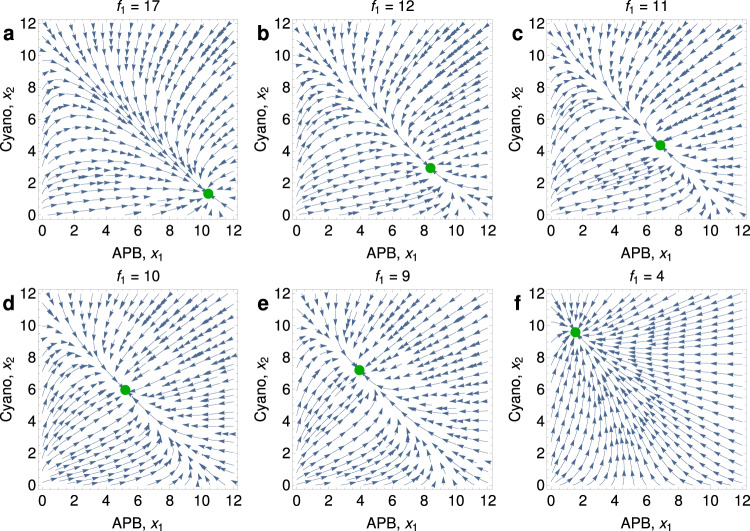
Fig. 4. Phase portraits of Eqs. (14) from Supplementary Note 1 illustrating a gradual transition are shown for six distinct values of f1, the influx rate of iron(II).
For values of f1 = 17 (a), 12 (b), 11 (c), 10 (d), 9 (e), and 4 (f), the stable equilibrium (green dot) moves continuously from a world that is dominated by APB to one that is dominated by cyanobacteria. Parameter values are f2 = 10, c = 1, a = 10, b = 12, u1 = u2 = 1, and α1 = α2 = 1. The GOE is gradual.
Alternatively, if b < c(a − 1), then the transition is sudden (i.e., discontinuous). Figure 5 shows rapid oxygenation due to decreasing f1. In this case, E2 is already stable before E1 loses stability (Fig. 6). This results in bistability and hysteresis: Once the world is dominated by cyanobacteria, moderate fluctuations in the supply rate of iron would no longer change the status quo (Fig. S3b).
Fig. 5. The GOE can be triggered by a decline in the influx of iron(II) and is sudden if b < c(a − 1).
Equilibrium E2 (cyanobacteria dominate) gains stability and Equilibrium E1 (APB dominate) loses stability when f1 drops below and , respectively. We set f2 = 80, c = 10, a = 10, b = 80, and u1 = u2 = 10−3. a We simulate Eqs. (8) from Supplementary Note 1 with α1 = α2 = β1 = β2 = 1, and we set f1 = 100 − 40(t/105). t* denotes the time at which Equilibrium E1 loses stability. b Bifurcation plots reveal bistability for .
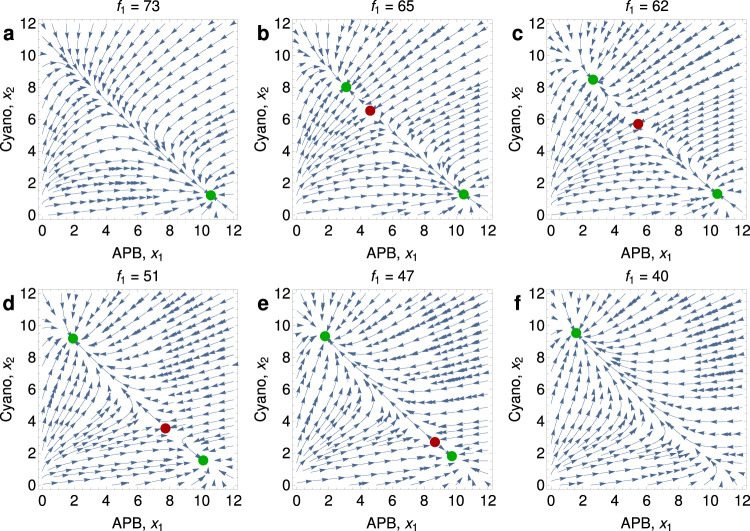
Fig. 6. Phase portraits of Eqs. (14) from Supplementary Note 1 illustrating a sudden transition are shown for six distinct values of f1, the influx rate of iron(II).
For f1 = 73 (a), there is a single stable equilibrium (green dot) describing a world dominated by APB. For values of f1 = 65 (b), 62 (c), 51 (d), and 47 (e), there is a second stable equilibrium (green dot) describing the dominance of cyanobacteria, and in addition, there is an unstable equilibrium (red dot). The unstable equilibrium moves as the value of f1 changes. For f1 = 40 (f), the only stable equilibrium is the one where cyanobacteria dominate. Parameter values are f2 = 10, c = 1, a = 10, b = 1, u1 = u2 = 1, and α1 = α2 = 1. The GOE is triggered by a saddle-node bifurcation and is sudden.
The effects of increasing f2 are nearly identical to those of decreasing f1. Increasing the supply of phosphate results in loss of stability of E1 and gain of stability of E2. This is because as f2 rises, APB proliferate, inducing a concomitant depletion of iron reserves. The transition is gradual if b > c(a − 1) (Fig. S4) or sudden if b < c(a − 1) (Fig. S5). The critical values of f1 and f2 are robust to changes in u2 (Figs. S6a, S6b).
Another possibility is that the GOE resulted from an increase in the parameter c, which denotes the reproductive rate of cyanobacteria, as affected by biological mutations. We cannot exclude the possibility that cyanobacterial performance and, therefore, primary production increased as a function of genetic innovations; however, the observation that even today oxygenic photosynthesis by cyanobacteria is limited when alternative electron donors are present places limits on such speculation. The parameter c could also be affected by geophysical or geochemical properties unrelated to oxygen consumption and independent of iron(II) or phosphate flux, such as temperature, pH, salinity, or availability of trace nutrients or other resources. The transition can be gradual (Fig. S7) or sudden (Fig. S8), depending on whether b > c(a − 1) or b < c(a − 1) when c is such that Equilibrium E1 becomes unstable. Similar to the critical values of f1 and f2, the critical value of c for triggering a GOE is robust to changes in u2 (Fig. S6c).
Yet another possibility is that the GOE was triggered by an increase in parameter a, which measures the production rate of oxygen (Fig. S9), or by a reduction in parameter b, which denotes the proportional consumption rate of oxygen (Fig. S10). For this transition to occur, however, it is essential that u2 is sufficiently large. Moreover, the critical values of a and b are strongly dependent on the magnitude of u2. If a is not large enough, then it is not possible for a reduction in b to trigger a GOE, regardless of how small b becomes (Fig. S11).
A GOE resulting from an increase in a or a decrease in b is necessarily sudden. This is because as a rises or b declines, Equilibrium E2, which is characterized by abundance of oxygen, may eventually gain stability, while Equilibrium E1 remains stable. If a becomes sufficiently large or b becomes sufficiently small, then E1 may cease to exist, and a saddle-node bifurcation results in rapid oxygenation.
Effects of migration rate
The migration rates, u1 and u2, have negligible effects on the abundance of APB for Equilibrium E1 and on the abundances of cyanobacteria and oxygen for Equilibrium E2. The principal effects of the migration rates are to determine the abundance of APB for Equilibrium E2 and the abundances of cyanobacteria and oxygen for Equilibrium E1. As such, u1 and u2 control the magnitude of the decline in APB across the GOE and the magnitude of the rise in cyanobacteria and oxygen across the GOE (Figs. S12, S13).
Discussion
Our analytical investigation of ecological dynamics indicates that a switch in ecological dominance from APB to cyanobacteria would have been sufficient to spawn the GOE. Accordingly, the competitive advantage of cyanobacteria, c, the influx of suitable reductants, f1, and the influx of phosphate, f2, that appear as parameters in Condition (2) are key considerations for determining when the GOE began. As extant cyanobacteria display low fitness in sunlit but anoxic environments, this observation must condition any proposed mechanisms by which c could have substantially increased around the time of the GOE. In contrast, a decrease in f1 and/or an increase in f2 comprise robust mechanisms for initiating the GOE. Decreasing Fe and increasing P fluxes to the oceans are both predicted by secular cooling of the mantle (and hydrothermal systems), continental emergence, and increasing oxidant supply as the GOE began22,24,53–55. Our analysis reveals the time at which the GOE began to be determined by the difference, f1 − f2, in these influxes. It does not depend on these influxes individually. This functional dependence is preserved under the assumption that bacterial growth rates are limited (Supplementary Note 2, Fig. S14), as are the possibilities of both gradual and sudden transitions (Figs. S15, S16). These results also hold when explicitly accounting for organic carbon (Supplementary Note 3, Fig. S17).
While prior investigations have focused heavily on sources and sinks of oxygen as potential drivers of the GOE, our analysis emphasizes that this possibility hinges critically on competition from cyanobacteria. If u2 was small, then sources and sinks of oxygen, a and b, were mostly irrelevant for initiating the GOE. There is a simple intuition behind this observation. If cyanobacteria were ecologically subordinate to APB and scarce in the Archean, then atmospheric levels of O2 would have remained low. a might have increased and b might have decreased—and fractional changes in these parameters could have been substantial—but when multiplied by low abundance of cyanobacteria, the absolute change in atmospheric O2 levels would also be small.
Our model of ecological dynamics is robust to a broad range of influences on primary production, oxygen generation, and oxygen consumption. Our study also emphasizes that it was not strictly geophysical processes or biological innovations that ushered in the GOE, but rather the interplay between Earth and life as populations adapted to a changing planet.
Methods
We elaborate our mathematical model of ecological dynamics, its analytical solutions, the stability properties of its fixed points, and important considerations behind the GOE in Supplementary Note 1. Here, we provide an abbreviated account of our analysis and key findings.
Ecological dynamics and fixed points
Equations (1) specify the ecological dynamics of APB and cyanobacteria. They set the foundation for our understanding of the GOE. To make progress analytically, we make two simplifying assumptions. First, we assume that, at any given time, Equations (1) are approximately in steady state. This is because f1, f2, c, a, and b change very slowly relative to the typical reproductive lifetimes of the bacteria. Second, we assume that u1 and u2 are both small. Equations (1) become
| 5 |
Equations (5) admit a solution for which the equilibrium abundance of cyanobacteria is zero:
| 6 |
Equations (5) admit another solution for which the equilibrium abundance of APB is zero:
| 7 |
Equations (5) also admit a solution for which the equilibrium abundances of cyanobacteria and APB are both nonzero:
| 8 |
Here, we have set
| 9 |
| 10 |
| 11 |
Dynamical stability
Equilibrium E1, given by Eqs. (6), is stable if p < 0 and unstable if p > 0. From Eq. (9), setting p < 0 and rearranging, we obtain Condition (2). Equilibrium E2, given by Eqs. (7), is stable if q < 0 and unstable if q > 0. From Eq. (10), setting q < 0 and rearranging, we obtain Condition (3). Equilibrium , given by Eqs. (8), is stable if r > 0 and unstable if r < 0. From Eq. (11), setting r > 0 and rearranging, we obtain Condition (4).
Timing and nature of the GOE
The GOE corresponds to a transition between Equilibrium E1 and Equilibrium E2.
One possibility is that the GOE is gradual. Initially, E1 is stable, while E2 is unstable. When E1 loses dynamical stability, a stable interior fixed point, given by Eqs. (8), (9), (10), and (11), appears near E1 in phase space. As parameter values become more favorable to cyanobacteria, the interior fixed point moves toward E2 in phase space, and oxygenation is progressive. When E2 gains dynamical stability, the GOE is complete.
Another possibility is that the GOE is sudden. In this case, E2 gains dynamical stability first. An unstable interior fixed point, given by Eqs. (8), (9), (10), and (11), appears near E2 in phase space, and the interior fixed point moves toward E1 in phase space. When E1 loses dynamical stability, sudden oxygenation results, and the GOE is complete.
Numerical integration
We used the fourth-order Runge-Kutta method to numerically integrate our differential equations.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank W. W. Fischer for providing the image of detrital pyrite. This research was supported by the John Templeton Foundation (grant number 61443).
Author contributions
J.O., Y.I., A.H.K. and M.A.N. performed research and wrote the manuscript.
Data availability
All equations and parameter values are included in this article and in its SI file.
Code availability
Code is available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks James Kasting, Kurt Konhauser, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-23286-7.
References
- 1.Planavsky NJ, Bekker A, Hofmann A, Owens JD, Lyons TW. Sulfur record of rising and falling marine oxygen and sulfate levels during the Lomagundi event. Proc. Natl Acad. Sci. USA. 2012;109:18300–18305. doi: 10.1073/pnas.1120387109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canfield DE, et al. Oxygen dynamics in the aftermath of the Great Oxidation of Earth’s atmosphere. Proc. Natl Acad. Sci. USA. 2013;110:16736–16741. doi: 10.1073/pnas.1315570110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth’s early ocean and atmosphere. Nature. 2014;506:307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 4.Bekker A, et al. Iron formation: the sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes. Econ. Geol. 2010;105:467–508. doi: 10.2113/gsecongeo.105.3.467. [DOI] [Google Scholar]
- 5.Roscoe, S. M. The Huronian Supergroup, a Paleoaphebian succession showing evidence of atmospheric evolution. In Huronian Stratigraphy and Sedimentation (ed. Young, G. M.) Geological Association of Canada special paper no. 12, 31–48 (Pierre Des Marais, 1973).
- 6.Blättler CL, et al. Two-billion-year-old evaporites capture Earth’s great oxidation. Science. 2018;360:320–323. doi: 10.1126/science.aar2687. [DOI] [PubMed] [Google Scholar]
- 7.Rye R, Holland HD. Paleosols and the evolution of atmospheric oxygen: a critical review. Am. J. Sci. 1998;298:621–672. doi: 10.2475/ajs.298.8.621. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen B, Buick R. Redox state of the Archean atmosphere: evidence from detrital heavy minerals in ca. 3250–2750 Ma sandstones from the Pilbara Craton, Australia. Geology. 1999;27:115–118. doi: 10.1130/0091-7613(1999)027<0115:RSOTAA>2.3.CO;2. [DOI] [Google Scholar]
- 9.Farquhar J, Bao H, Thiemens M. Atmospheric influence of Earth’s earliest sulfur cycle. Science. 2000;289:756–758. doi: 10.1126/science.289.5480.756. [DOI] [PubMed] [Google Scholar]
- 10.Warke MR, et al. The great oxidation event preceded a paleoproterozoic “snowball Earth”. Proc. Natl Acad. Sci. USA. 2020;117:13314–13320. doi: 10.1073/pnas.2003090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JE, Gerpheide A, Lamb MP, Fischer WW. O2 constraints from Paleoproterozoic detrital pyrite and uraninite. Geol. Soc. Am. Bull. 2014;126:813–830. doi: 10.1130/B30949.1. [DOI] [Google Scholar]
- 12.Pavlov AA, Kasting JF. Mass-independent fractionation of sulfur isotopes in Archean sediments: strong evidence for an anoxic Archean atmosphere. Astrobiology. 2002;2:27–41. doi: 10.1089/153110702753621321. [DOI] [PubMed] [Google Scholar]
- 13.Catling DC, Zahnle KJ. The Archean atmosphere. Sci. Adv. 2020;6:eaax1420. doi: 10.1126/sciadv.aax1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasting JF. What caused the rise of atmospheric O2? Chem. Geol. 2013;362:13–25. doi: 10.1016/j.chemgeo.2013.05.039. [DOI] [Google Scholar]
- 15.Bachan A, Kump LR. The rise of oxygen and siderite oxidation during the Lomagundi Event. Proc. Natl Acad. Sci. USA. 2015;112:6562–6567. doi: 10.1073/pnas.1422319112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catling, D. C. & Kasting, J. F. Atmospheric Evolution on Inhabited and Lifeless Worlds (Cambridge University Press, 2017).
- 17.Catling DC, Zahnle KJ, McKay CP. Biogenic methane, hydrogen escape, and the irreversible oxidation of early earth. Science. 2001;293:839–843. doi: 10.1126/science.1061976. [DOI] [PubMed] [Google Scholar]
- 18.Claire MW, Catling DC, Zahnle KJ. Biogeochemical modelling of the rise in atmospheric oxygen. Geobiology. 2006;4:239–269. doi: 10.1111/j.1472-4669.2006.00084.x. [DOI] [Google Scholar]
- 19.Holland HD. Volcanic gases, black smokers, and the Great Oxidation Event. Geochim. Cosmochim. Acta. 2002;66:3811–3826. doi: 10.1016/S0016-7037(02)00950-X. [DOI] [Google Scholar]
- 20.Gaillard F, Scaillet B, Arndt NT. Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature. 2011;478:229–233. doi: 10.1038/nature10460. [DOI] [PubMed] [Google Scholar]
- 21.Seyfried Jr. WE, Ding K, Berndt ME. Phase equilibria constraints on the chemistry of hot spring fluids at mid-ocean ridges. Geochim. Cosmochim. Acta. 1991;55:3559–3580. doi: 10.1016/0016-7037(91)90056-B. [DOI] [Google Scholar]
- 22.Kump LR, Seyfried Jr. WE. Hydrothermal Fe fluxes during the Precambrian: Effect of low oceanic sulfate concentrations and low hydrostatic pressure on the composition of black smokers. Earth Planetary Sci. Lett. 2005;235:654–662. doi: 10.1016/j.epsl.2005.04.040. [DOI] [Google Scholar]
- 23.Bjerrum CJ, Canfield DE. Ocean productivity before about 1.9 Ga ago limited by phosphorus adsorption onto iron oxides. Nature. 2002;417:159–162. doi: 10.1038/417159a. [DOI] [PubMed] [Google Scholar]
- 24.Hao J, et al. Cycling phosphorus on the Archean Earth: Part II. Phosphorus limitation on primary production in Archean ecosystems. Geochim. Cosmochim. Acta. 2020;280:360–377. doi: 10.1016/j.gca.2020.04.005. [DOI] [Google Scholar]
- 25.Derry LA. Causes and consequences of mid-Proterozoic anoxia. Geophys. Res. Lett. 2015;42:8538–8546. doi: 10.1002/2015GL065333. [DOI] [Google Scholar]
- 26.Goldblatt C, Lenton TM, Watson AJ. Bistability of atmospheric oxygen and the Great Oxidation. Nature. 2006;443:683–686. doi: 10.1038/nature05169. [DOI] [PubMed] [Google Scholar]
- 27.Konhauser KO, et al. Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature. 2009;458:750–754. doi: 10.1038/nature07858. [DOI] [PubMed] [Google Scholar]
- 28.Bjerrum CJ, Canfield DE. New insights into the burial history of organic carbon on the early Earth. Geochem., Geophys., Geosyst. 2004;5:Q08001. doi: 10.1029/2004GC000713. [DOI] [Google Scholar]
- 29.Krissansen-Totton J, Buick R, Catling DC. A statistical analysis of the carbon isotope record from the Archean to Phanerozoic and implications for the rise of oxygen. Am. J. Sci. 2015;315:275–316. doi: 10.2475/04.2015.01. [DOI] [Google Scholar]
- 30.Ward LM, Kirschvink JL, Fischer WW. Timescales of oxygenation following the evolution of oxygenic photosynthesis. Orig. Life Evol. Biosph. 2016;46:51–65. doi: 10.1007/s11084-015-9460-3. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton TL, Bryant DA, Macalady JL. The role of biology in planetary evolution: cyanobacterial primary production in low-oxygen Proterozoic oceans. Environ. Microbiol. 2016;18:325–340. doi: 10.1111/1462-2920.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Beer D, et al. Oxygenic and anoxygenic photosynthesis in a microbial mat from an anoxic and sulfidic spring. Environ. Microbiol. 2017;19:1251–1265. doi: 10.1111/1462-2920.13654. [DOI] [PubMed] [Google Scholar]
- 33.Swanner ED, et al. Modulation of oxygen production in Archaean oceans by episodes of Fe(II) toxicity. Nat. Geosci. 2015;8:126–130. doi: 10.1038/ngeo2327. [DOI] [Google Scholar]
- 34.Oren A, Padan E, Avron M. Quantum yields for oxygenic and anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. Proc. Natl Acad. Sci. USA. 1977;74:2152–2156. doi: 10.1073/pnas.74.5.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton TL, Klatt JM, de Beer D, Macalady JL. Cyanobacterial photosynthesis under sulfidic conditions: insights from the isolate Leptolyngbya sp. strain hensonii. ISME J. 2018;12:568–584. doi: 10.1038/ismej.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward LM, et al. Geochemical and metagenomic characterization of Jinata Onsen, a Proterozoic-analog hot spring, reveals novel microbial diversity including iron-tolerant phototrophs and thermophilic lithotrophs. Microbes Environ. 2019;34:278–292. doi: 10.1264/jsme2.ME19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anbar AD, et al. A Whiff of oxygen before the great oxidation event? Science. 2007;317:1903–1906. doi: 10.1126/science.1140325. [DOI] [PubMed] [Google Scholar]
- 38.Satkoski AM, Beukes NJ, Li W, Beard BL, Johnson CM. A redox-stratified ocean 3.2 billion years ago. Earth Planetary Sci. Lett. 2015;430:43–53. doi: 10.1016/j.epsl.2015.08.007. [DOI] [Google Scholar]
- 39.Albut G, et al. Modern weathering in outcrop samples versus ancient paleoredox information in drill core samples from a Mesoarchaean marine oxygen oasis in Pongola Supergroup, South Africa. Geochim. Cosmochim. Acta. 2019;265:330–353. doi: 10.1016/j.gca.2019.09.001. [DOI] [Google Scholar]
- 40.Jones C, Nomosatryo S, Crowe SA, Bjerrum CJ, Canfield DE. Iron oxides, divalent cations, silica, and the early earth phosphorus crisis. Geology. 2015;43:135–138. doi: 10.1130/G36044.1. [DOI] [Google Scholar]
- 41.Knoll AH, Nowak MA. The timetable of evolution. Sci. Adv. 2017;3:e1603076. doi: 10.1126/sciadv.1603076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozaki K, Thompson KJ, Simister RL, Crowe SA, Reinhard CT. Anoxygenic photosynthesis and the delayed oxygenation of Earth’s atmosphere. Nat. Commun. 2019;10:3026. doi: 10.1038/s41467-019-10872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston DT, Wolfe-Simon F, Pearson A, Knoll AH. Anoxygenic photosynthesis modulated Proterozoic oxygen and sustained Earth’s middle age. Proc. Natl Acad. Sci. USA. 2009;106:16925–16929. doi: 10.1073/pnas.0909248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schirrmeister BE, Sanchez-Baracaldo P, Wacey D. Cyanobacterial evolution during the Precambrian. Int. J. Astrobiol. 2016;15:187–204. doi: 10.1017/S1473550415000579. [DOI] [Google Scholar]
- 45.Sanchez-Baracaldo P, Raven JA, Pisani D, Knoll AH. Early photosynthetic eukaryotes inhabited low-salinity habitats. Proc. Natl Acad. Sci. USA. 2017;114:E7737–E7745. doi: 10.1073/pnas.1620089114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buick R. The antiquity of oxygenic photosynthesis: evidence from stromatolites in sulphate-deficient Archaean lakes. Science. 1992;255:74–77. doi: 10.1126/science.11536492. [DOI] [PubMed] [Google Scholar]
- 47.Sleep NH, Bird DK. Evolutionary ecology during the rise of dioxygen in the Earth’s atmosphere. Philos. Trans. R. Soc. Lond. B. 2008;363:2651–2664. doi: 10.1098/rstb.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laakso TA, Schrag DP. Regulation of atmospheric oxygen during the Proterozoic. Earth Planet. Sci. Lett. 2014;388:81–91. doi: 10.1016/j.epsl.2013.11.049. [DOI] [Google Scholar]
- 49.Laakso TA, Schrag DP. A theory of atmospheric oxygen. Geobiology. 2017;15:366–384. doi: 10.1111/gbi.12230. [DOI] [PubMed] [Google Scholar]
- 50.Canfield DE. The early history of atmospheric oxygen: homage to Robert M. Garrels. Annu. Rev. Earth Planet. Sci. 2005;33:1–36. doi: 10.1146/annurev.earth.33.092203.122711. [DOI] [Google Scholar]
- 51.Tutolo BM, Seyfried Jr. WE, Tosca NJ. A seawater throttle on H2 production in Precambrian serpentinizing systems. Proc. Natl Acad. Sci. USA. 2020;117:14756–14763. doi: 10.1073/pnas.1921042117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tice MM, Lowe DR. Hydrogen-based carbon fixation in the earliest known photosynthetic organisms. Geology. 2006;34:37–40. doi: 10.1130/G22012.1. [DOI] [Google Scholar]
- 53.Kipp MA, Stueken EE. Biomass recycling and Earth’s early phosphorus cycle. Sci. Adv. 2017;3:eaao4795. doi: 10.1126/sciadv.aao4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao J, Knoll AH, Huang F, Hazen RM, Daniel I. Cycling phosphorus on the Archean Earth: Part I. Continental weathering and riverine transport of phosphorus. Geochim. Cosmochim. Acta. 2020;273:70–84. doi: 10.1016/j.gca.2020.01.027. [DOI] [Google Scholar]
- 55.Reinhard CT, et al. Evolution of the global phosphorus cycle. Nature. 2017;541:386–389. doi: 10.1038/nature20772. [DOI] [PubMed] [Google Scholar]
- 56.Kharecha P, Kasting J, Siefert J. A coupled atmosphere-ecosystem model of the early Archean Earth. Geobiology. 2005;3:53–76. doi: 10.1111/j.1472-4669.2005.00049.x. [DOI] [Google Scholar]
- 57.Olson JM. Photosynthesis in the Archean Era. Photosynth. Res. 2006;88:109–117. doi: 10.1007/s11120-006-9040-5. [DOI] [PubMed] [Google Scholar]
- 58.Olson KR, Straub KD. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology. 2016;31:60–72. doi: 10.1152/physiol.00024.2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All equations and parameter values are included in this article and in its SI file.
Code is available from the corresponding author upon reasonable request.