Abstract
We propose a novel conceptual framework to describe the relationship between placental malaria and adverse infant neurodevelopmental outcomes. This conceptual framework includes three distinct stages: 1) maternal and environmental risk factors for the development of placental malaria; 2) placental pathology and inflammation associated with placental malarial infection; and 3) postnatal impacts of placental malaria. The direct, indirect, and bidirectional effects of these risk factors on infant neurodevelopment across the three stages have been critically examined. These factors ultimately culminate in an infant phenotype that not only leads to adverse birth outcomes, but also to increased risks of neurological, cognitive, and behavioural deficits that may impact the quality of life in this high-risk population. Multiple risk factors have been identified in this conceptual framework, nonetheless, based on the current evidence, a key knowledge gap is the uncertainty as to which are the most important and how exactly they interact.
Keywords: Malaria, Malaria in Pregnancy, Placental Malaria, Neurodevelopment
Malaria remains a global public health concern. Despite continuing efforts and substantial funding, cases of malaria are rising. Between 2015 and 2017, the global number of malaria cases increased from 211 million to 219 million, with a concomitant increase in deaths (WHO, 2018). Approximately 97% of malaria cases occur in sub-Saharan Africa (SSA) and South-East Asia (WHO, 2018) – regions that are burdened by poor sustainable child development indices (Black et al., 2017).
Nearly 85.3 million pregnancies occurred in areas of Plasmodium falciparum transmission in 2007 (Dellicour et al., 2010). A unique risk when infected with P. falciparum during pregnancy is placental malaria (PM), which is prevalent in ~32.3% of pregnant women in SSA (Guyatt and Snow, 2004). Parasitised erythrocytes sequester in the placenta via expression of variant surface antigen 2-chondroitin sulfate A, allowing binding to placental chondroitin sulphate A in the placental intervillous space. This causes a maternal immune-inflammatory response, placental pathologic changes and poor birth outcomes. PM is a significant risk factor for gestational hypertension and preeclampsia (Adam et al., 2011, Sartelet et al., 1996), both of which are well-known predeterminants of adverse birth outcomes. It is likely that the combination of these factors may negatively impact infant neurodevelopment and their long-term outcomes. Neurodevelopment, a complex interplay of genes, the intra- and extra-uterine environment, family socioeconomic status and the developing brain, is essential for optimal motor, socioemotional, neurosensory, and cognitive functioning. Low socioeconomic status, infection, inflammation, and aberrant placental function are well-established independent predictors of adverse short- and long-term neurodevelopmental outcomes. These risk factors are prevalent in malaria-endemic low- and middle-income countries (LMICs), creating circumstances where they may combine to increase risk of neurodevelopmental adversities. Approximately 250 million children worldwide, the majority residing in SSA and South-East Asia, are not reaching their developmental potential (Black et al., 2017). PM may be an important contributor to pathways of causation of impaired neurodevelopment and future developmental disorders.
Converging evidence from animal and human studies demonstrate that PM can adversely impact fetal and infant neurodevelopment. In animal models, offspring born to malaria-infected pregnant mice displayed impaired non-spatial learning, memory and depressive-like behaviour in infancy that persisted through to adulthood (McDonald Chloë R et al., 2015). In human studies, the cingulate sulcus developed faster in fetuses of malaria-infected women compared to uninfected controls, though no statistically significant difference between head circumference and brain volumes or overall rate of sulci development was seen (Rijken et al., 2012). While this evidence is contradictory, it does indicate that exposure to PM has some degree of impact on brain development. Finally, in a recent case report of placental malaria-discordant dizygotic twins, lower motor, cognitive, and language scores were seen in the PM-exposed twin, relative to the PM-unexposed twin, at both 1- and 2-years chronologic age (Conroy et al., 2019) indicating poorer neurodevelopmental outcomes as a result of exposure to PM. Given this evidence, more research is required to define the incidence of various types and degrees of neurodevelopmental impairment in infants affected by PM.
A comprehensive synthesis of the existing literature was conducted, and a conceptual framework developed to propose potential pathways by which PM leads to adverse neurodevelopment. As shown in Figure 1 (and supported by evidence presented in Table 1), this conceptual framework is presented in three stages: 1) maternal and environmental risk factors for the development of PM; 2) placental pathology and dysfunction associated with PM infection; and 3) postnatal impacts of PM. These have direct, indirect, and bidirectional effects on infant neurodevelopment and ultimately, culminate in an infant phenotype where not only are there adverse birth outcomes commonly associated with PM, but also neurological, cognitive, and behavioural deficits that impact the quality of life in this high-risk population from infancy and beyond. Despite a comprehensive review of the literature, as summarised in Table 1, a key knowledge gap based on the current level of evidence is the uncertainty as to which the most important risk factors in this framework are and how exactly they interact. Hence, this novel conceptual framework has been proposed to guide this critical line of research along with potentially informing healthcare policy and clinical practice.
Figure 1: Conceptual framework to describe the progression from placental malaria to adverse infant neurodevelopment.
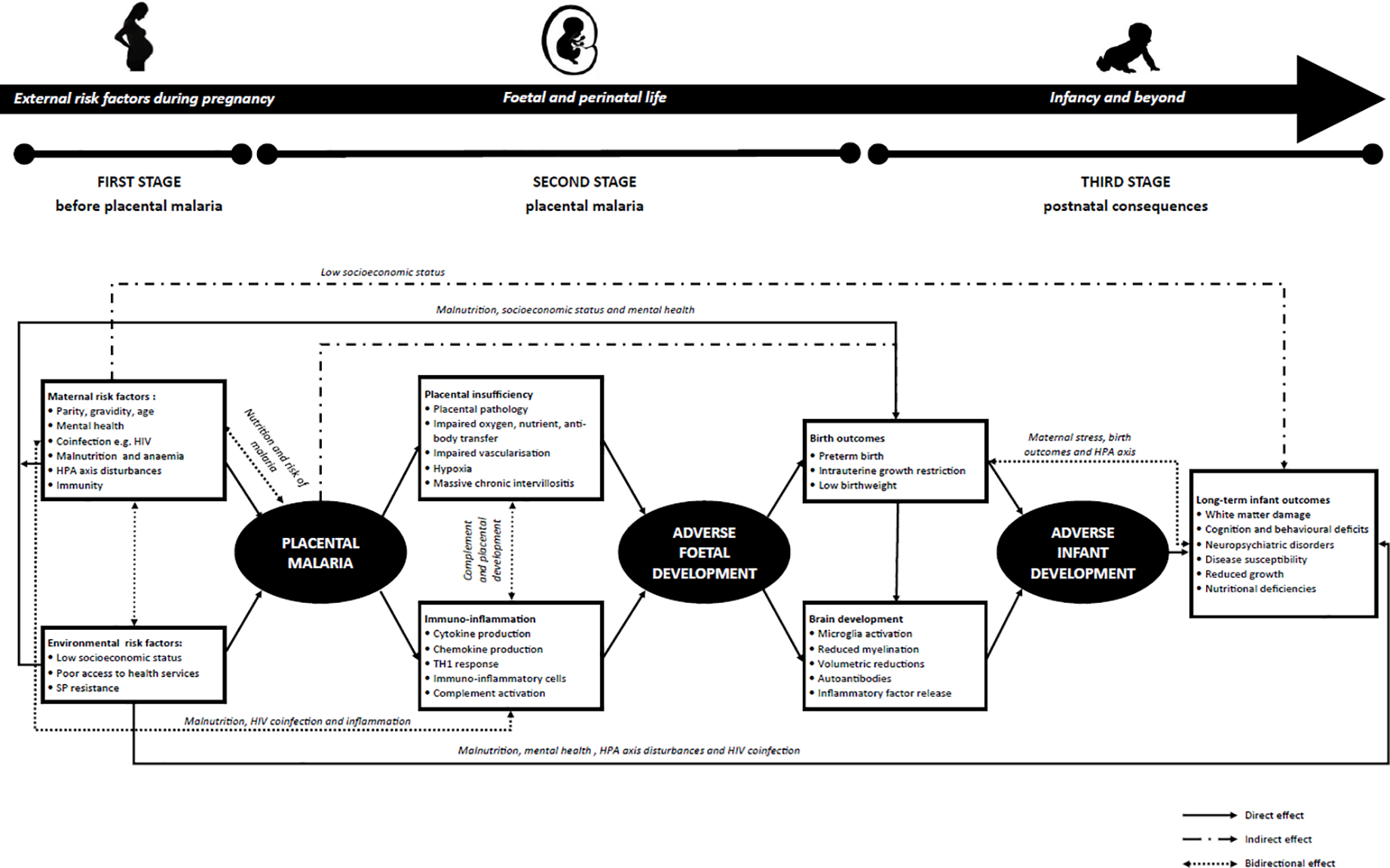
Risk factors are present at three distinct stages: 1) prior to the development of placental malaria; 2) during placental malaria infection; and 3) postnatally. These risk factors act directly, indirectly, and bidirectionally to culminate in an infant phenotype where we see evidence of neurological, cognitive, and behavioural deficits that impact this high-risk population from infancy, through childhood, adolescence, and into adulthood.
Table 1:
Summary table of studies included in the literature review and conceptual framework
| Reference | Stage in conceptual framework | Risk factor | Findings |
|---|---|---|---|
| Achan J et al. (2012) | First stage | Maternal coinfection | In this study, Lopinavir-ritonavir-based antiretroviral therapy, as compared with nonnucleoside reverse-transcriptase inhibitor-based antiretroviral therapy, was seen to reduce the incidence and recurrence of malaria. |
| Adam I et al. (2011) | First stage Second stage |
Maternal gravidity Placental pathology |
In this study, placental malaria was significantly associated with preeclampsia. At univariate analysis, primigravidae were associated with preeclampsia. |
| Adamo AM & Oteiza (2010) | First stage | Maternal nutrition | This study found that zinc deficiency during gestation may increase the risk of behavioural and neurological disorders in infancy, adolescence and adulthood. |
| Agostoni et al. (2010) | First stage Third stage |
Maternal malnutrition Nutritional deficiencies |
This expert panel presented guidelines on the nutrition of preterm infants. This commentary discusses the conflicting data surrounding iron supplementation, and highlights studies that have found adversities associated with excessive iron supplementation. |
| Agudelo OM et al. (2014) | Second stage | Hypoxia | The authors found that the hypoxia markers, COX-1 and COX-2, were significantly higher in women with PM, when compared to uninfected controls. |
| Altshuler G (1993) | Second stage | Hypoxia Impaired vascularisation |
A thorough review of studies associating neurodevelopmental disorders with placental pathology. |
| Aucott et al. (2008) | First stage Third stage |
HPA axis disturbances Low birthweight |
This study aimed to evaluate the relationship between cortisol concentrations and short-term outcomes in low birthweight infants. The authors found that infants with high cortisol concentrations had significantly higher odds of severe intraventricular haemorrhage. |
| Basiloius et al. (2015) | Second stage Third stage |
Placental insufficiency White matter damage |
A systematic review summarising the neurological outcomes of placental insufficiency in animal models, including reduced hippocampal and corpus callosum size, hypomyelination and neuronal apoptosis. |
| Basu S et al. (2017) | First stage | Maternal nutrition | This study found that maternal iron deficiency anaemia was associated with lower fetal hippocampal volumes and brain-derived neurotrophic factor, and that the degree of affection was proportional to the severity of maternal anaemia. |
| Bergman K et al. (2007) | First stage | Maternal mental health | In this study, maternal stress during pregnancy was negatively correlated with mental development and positively associated with fearfulness in infants. |
| Black MM et al. (2017) | Third stage | Cognition and behavioural deficits | This review used proxy measures of stunting and poverty and found that ∼250 million children in LMICs are at risk of not reaching their developmental potential. The majority of these infants reside in SSA (94.8 million) and South Asia (88.8 million). |
| Black RE et al. (2008) | Third stage | Nutritional deficiencies | A comprehensive review of the health consequences of maternal and child undernutrition. In particular, a 1.73-point decrease in IQ was seen for every 10g/L decrease in haemoglobin in children. |
| Blencowe H et al. (2013) | Third stage | Birth outcomes Cognition and behavioural deficits |
This study found that approximately 2.7% and 4.4% of preterm births who survived beyond the first month had moderate/severe and mild neurodevelopmental impairment, respectively. |
| Boeuf P et al. (2013) | Third stage | Birth outcomes | This study found alterations of amino acid transport in PM cases, particularly in cases with local inflammation. The authors conclude that, as opposed to PM, inflammation is more likely to blame for compromised amino acid transport and subsequent IUGR. |
| Boeuf P et al. (2008) | Second stage | Hypoxia | This study found significantly higher levels of the hypoxia marker, hypoxia inducible factor-1α, in PM cases. However, the authors conclude that there was little evidence to suggest PM itself causes hypoxia. |
| Boivin MJ et al. (2007) | Third stage | Disease susceptibility Cognition and behavioural deficits |
In a Ugandan cohort, 21.4% of children who had cerebral malaria had cognitive deficits after discharge. |
| Bouyou-Akotet MK et al. (2005) | First stage | Maternal mental health | This study found significantly higher cortisol concentrations among P. falciparum-infected women, relative to uninfected women. Furthermore, cortisol levels were significantly higher among P. falciparum-infected primigravidae relative to uninfected primigravidae. |
| Brabin BJ (1983) | Second stage | Impaired oxygen, nutrient, antibody transfer | It was seen that the maximum parasite rate of pregnant women recruited at ANC visits in Western Kenya occurred at 13–16 weeks’ gestation. It is likely that the presence of parasites at this time can disrupt vasculogenesis and impair placental transfer. |
| Brabin BJ (1991) | First stage Third stage |
Maternal gravidity Birth outcomes |
This study found that the risk of LBW is increased among primigravidae in malaria-endemic areas, significantly correlating with parasite rate at delivery. |
| Buehler MR (2011) | Second stage Third stage |
Cytokine production Neuropsychiatric disorders |
This paper details a model describing the impact of pro-inflammatory cytokines on fetal brain development, and the relation of cytokines to neuropsychiatric disorders, including autism and schizophrenia. |
| Chawanpaiboon S et al. (2018) | Third stage | Birth outcomes | A systematic review of global, regional and national levels of preterm birth in in 2014. There was an estimated 14.84 million live preterm births. |
| Conroy AL et al. (2019) | Third stage | Cognition and behavioural deficits | A case report of the neurodevelopmental outcomes in placental malaria-discordant dizygotic twins. The PM-exposed twin showed consistent delays across cognitive, motor and language domains, relative to the unexposed twin. |
| Conroy AL et al. (2013) | Second stage | Complement activation | This study investigated the role of complement. Complement is seen to be elevated in PM cases and, interestingly, blocking C5a in murine model improved feto-placental vessel development and fetal growth and survival and reduced placental vascular resistance. |
| Cusick SE et al. (2014) | First stage | Maternal nutrition | Vitamin D insufficiency is associated with severe malaria in children; the authors suggest a potential role for vitamin D deficiency in the aetiology of severe malaria. |
| Darling AL et al. (2017) | First stage Third stage |
Maternal nutrition Cognition and behavioural difficulties |
In this study, the offspring of women who were Vitamin D insufficient during gestation had adversities in motor and social development, relative to offspring of women who were Vitamin D sufficient. Maternal 25(OH)D deficiency was associated with mental and motor deficits in infants 16–18 months, poorer gross and fine motor development at 30 months, and lower social development at 42 months. However, associations were not seen at older ages. |
| Dellicour et al. (2010) | First stage | Maternal parity, gravidity, age | Using global estimates, it was concluded that 85.3 million pregnancies occurred in areas with P. falciparum transmission in 2007. |
| Djontu JC et al. (2016) | Second stage | Cytokine production | This study investigated the expression of cytokines in mothers and infants exposed to PM. Placental and peripheral malaria infection correlated positively with peripheral plasma levels of IL-6. |
| Dombrowski JG et al. (2017) | Third stage | Reduced growth | This study from Brazil found that P. falciparum infection during pregnancy significantly increased the odds of the occurrence of small head and microcephaly in exposed infants, relative to unexposed infants. |
| Duthie & Reynolds (2013) | First stage | HPA axis disturbances | A comprehensive review of changes to the HPA axis during pregnancy, and the impact on fetal outcomes. With relation to this framework, the HPA axis is dysregulated by maternal stress, increasing transfer of glucocorticoids from mother to fetus, which are associated with LBW and adverse infant neurodevelopmental outcomes |
| Fitri LE et al. (2015) | Second stage Third stage |
Cytokine production Low birthweight |
This study investigated the influence of concentrations of IL-17 and IL-10 in malaria-infected placentas on fetal weight. The authors concluded that sequestration of parasites directly causes LBW in PM cases, while cytokines act indirectly. |
| Fox et al. (2012) | Third stage | Autoantibodies Cognition and behavioural deficits |
This review discusses the possible association between maternal autoantibody production during gestation, and infant neurodevelopmental consequences. |
| Fried M et al. (1998) | Second stage | TH1 response | In this study, cytokine concentrations were measured in PM positive and negative placentas. Significantly higher TNF-α and TGF-β concentrations and significantly lower IL-10 concentrations were seen in PM positive placentas, leading authors to conclude that maternal malaria increases the concentration of type-1 cytokines. |
| Gelaye B et al. (2016) | First stage | Maternal mental health | This systematic review summarised the prevalence of perinatal depression and its association with infant/child outcomes in LMICs. |
| Gibson RS et al. (1991) | Third stage | Disease susceptibility Nutritional deficiencies |
This cross-sectional study investigated zinc status in children in a subdistrict of Papua New Guinea. Authors believe that suboptimal zinc status seen may be due to the high prevalence of malaria. |
| Gibson RS et al. (1998) | First stage | Maternal nutrition | This study assessed zinc status and infection among rural Malawian women. Hair zinc concentrations was significantly associated with malaria infection (p=0.01). |
| Girardi G et al. (2006) | Second stage | Immuno-inflammatory cells Complement activation |
Using a mouse model of spontaneous miscarriage and IUGR, the authors demonstrate that complement activation is a necessary intermediary in placental and fetal injury. |
| Giurgescu C (2009) | First stage | Maternal mental health HPA axis disturbances |
This review highlights the association between maternal cortisol levels and preterm birth. |
| Guyatt HL et al. (2004) | First stage | Maternal parity, gravidity, age | A comprehensive review on the pathological effects of malaria in pregnancy; data from 15 studies indicate an overall prevalence of malaria of 32.3% among pregnant women. |
| Harrington W et al. (2009) | First stage | SP resistance | A prospective delivery cohort examining the IPTp on level of parasitaemia and inflammation in the placenta. The use of IPTp was associated with an increase in resistant parasites, increased parasitaemia and more intense placental inflammation. |
| Imamura T et al. (2002) | Second stage | Placental pathology | This study investigated tissue factor expression and fibrin deposition in P. falciparum infected placentas. The tissue occupancy ratio of fibrin was found to be 5-fold higher in infected relative to uninfected placentas. The authors conclude that perivillous fibrin clot formations narrow and plug the intervillous space, interrupting placental blood supply. |
| Kauye F et al. (2014) | First stage | Maternal mental health | This study found that common mental disorders at out-patients services in Malawi were commonly misdiagnosed as malaria. |
| Keelan JA et al. (1999) | Second stage Third stage |
Cytokine production Preterm birth |
This study investigated inflammation in the gestational tissue of women with preterm labour. Significantly higher cytokine concentrations were seen in amniotic and chorionic-decidual tissues from women with preterm deliveries. |
| Keim SA et al. (2014) | First stage Third stage |
Maternal malnutrition Cognition and behavioural deficits |
This study investigated the impact of maternal and cord blood 25(OH)D concentrations on child development and behaviour. IQ at 7y was associated with both maternal and cord blood 25(OH)D concentrations. |
| Kuban KC et al. (2009) | Third stage | Reduced growth | An investigation into developmental correlates of congenital microcephaly and at 2-years in extremely preterm infants. In this cohort, the authors found that congenital microcephaly is only a risk factor for cognitive impairment if microcephaly persists to 2-years. |
| Lang B et al. (2005) | Second stage | Autoantibodies | In a cohort of Kenyan children, levels of antibodies to the voltage-gated calcium channels increased with the severity of malaria. |
| Lang B et al. (2003) | Second stage | Autoantibodies | A review discussing the associations between autoantibodies and central nervous system disorders. |
| Le Hesran Y et al. (1997) | Third stage | Disease susceptibility | This study conducted in Cameroon found that offspring exposed to PM were more likely to develop a malaria infection between 4–6 months of age and have higher parasite prevalence rates at 5–8 months of age. |
| Lee AC et al. (2017) | Third stage | Birth outcomes | This review found that, in 2012, approximately 23.3 million infants in low- and middle-income countries were born small for gestational age. |
| Liew J et al. (2015) | Second stage | Autoantibodies | This study investigated the autoantibody profile of patients infected with P. knowlesi relative to uninfected patients. |
| Loureiro B et al. (2017) | First stage Third stage |
Maternal nutrition White matter damage Cognition and behavioural deficits |
In this study, infants with severe anaemia at birth displayed white matter injuries, which were related to global developmental delay, behavioural and learning problems. |
| Martinez E et al. (1998) | Second stage Third stage |
Cytokine production White matter damage |
In this study, amniotic fluid IL-6 was an independent risk factor for periventricular leukomalacia and intraventricular haemorrhage. |
| McDonald CR et al. (2015) | Third stage | Cognition and behavioural deficits | In a murine model of malaria, offspring born to malaria-infected pregnant mice displayed impaired non-spatial learning, memory and depressive-like behaviour in infancy that persisted through to adulthood. |
| McDonald CR et al. (2013) | Second stage Third stage |
Complement activation Impaired vascularisation Cognition and behavioural deficits |
A review discussing the role of the complement system in placental malaria, placental insufficiency and adverse neurodevelopmental outcomes. |
| McDonald CR et al. (2015) | Second stage Third stage |
Complement activation Cognition and behavioural deficits |
A review of the role of complement in pregnancy, and the possible consequences on infant neurodevelopmental outcomes. |
| Menendez C et al. (2000) | Third stage | Birth outcomes | This study investigated the impact of malaria-associated placental changes on birth weight. The severe fibrin deposition was significantly associated with increased risk of preterm LBW at univariate and multivariate analysis. |
| Moore JM et al. (1999) | First stage | Maternal coinfection | A study investigating cytokine production in HIV-positive/negative PM-infected/uninfected women. It was concluded that HIV-mediated cytokine dysregulation and impaired interferon-gamma responses increased the susceptibility of HIV-positive pregnant women to malaria. |
| Moormann AM et al. (1999) | Second stage Third stage |
Cytokine production Intrauterine growth restriction |
In this study, cytokine expression was compared in malaria infected and uninfected placentas. Significantly increased concentrations of IL-1β, IL-8, and TNF-α were seen in infected placentas, and increased TNF-α or IL-8 was associated with IUGR. |
| Mount AM et al. (2004) | First stage | Maternal coinfection | A comparison of serum concentrations of antibodies to placental type variant surface antigens among pregnant women. Concentrations of IgG to variant surface antigens were lower in HIV-infected women than in HIV-uninfected women. |
| Muehlenbachs A et al. (2010) | Second stage | Massive chronic intervillositis | This study developed a histological grading scheme for PM, including the presence of massive chronic intervillositis. The literature suggests massive chronic intervillositis is reported in 1.7–6.9% of PM cases. |
| Muehlenbachs A et al. (2006) | First stage Second stage |
Maternal parity, gravidity and age Impaired oxygen, nutrient, antibody transfer | This cross-sectional study assessed levels of soluble vascular endothelial growth factor receptor 1 (also known as soluble Fms-like tyrosine kinase-1), a preeclampsia biomarker, in primigravidae with either PM, hypertension, or both. Levels were found to be significantly increased, suggesting soluble Fms-like tyrosine kinase-1 may be involved in the relationship between chronic PM and hypertension in primigravidae. |
| Mutabingwa TK et al. (2005) | Third stage | Disease susceptibility | This study found that infants who were born to mothers with PM were more likely to have malaria earlier than infants born to unexposed mothers. Offspring of multigravida mothers with PM were more likely to have malaria during infancy, while it appeared that offspring of primigravida had reduced susceptibility to PM. |
| Naing C et al. (2016) | First stage | Maternal coinfection | A meta-analysis on the coinfection of HIV and malaria. It is estimated that there is an estimated pooled prevalence of HIV and malaria coinfection of 12% among pregnant women. |
| Nosarti C et al. (2010) | Third stage | Birth outcomes White matter damage Cognition and behavioural deficits |
This book discussed the evidence associating adverse birth outcomes (preterm birth and low birthweight) with an increase in the prevalence neurodevelopmental adversities. |
| Nzila A et al. (2014) | First stage | Environmental risk factors | A review investigating the impact of folate supplementation during pregnancy on SP efficacy. The authors summarise evidence suggesting the use of folate may negate SP efficacy. |
| Opsjln SL et al. (1993) | Second stage Third stage |
Cytokine production Preterm birth |
This study measured levels of TNF, IL-1 and IL-6 in human pregnancies; these cytokines were seen to increase at the onset of labour, suggesting they may play a role in the onset of normal labour. The authors suggest that higher levels of these cytokines in PM cases may be a risk factor for preterm labour. |
| Ordi J et al. (1998) | Second stage Third stage |
Massive chronic intervillositis Low birthweight |
In this study, massive chronic intervillositis is associated with PM, predominantly among primigravidae. Further, massive chronic intervillositis was associated with low birthweight. |
| Paintlia MK et al. (2008) | Second stage Third stage |
Immuno-inflammatory cells Reduced myelination White matter damage |
This study investigated neuroinflammation and white matter injury in a series of mouse models. The authors found evidence of hypomyelination and astrogliosis in the brain of offspring of murine neuroinflammation models. The authors conclude that cerebral white matter injury may be due to maternal immune activation, such as occurs in PM. |
| Postels DG et al. (2018) | Third stage | Disease susceptibility Cognition and behavioural difficulties |
This study investigated electroencephalogram findings in survivors of cerebral malaria from Uganda and Malawi. It was seen that 44% and 11.3% of infants from Uganda and Malawi, respectively, had neurological sequelae at discharge. |
| Rijken MJ et al. (2012) | Second stage | Brain development | A Thailand-based longitudinal observational investigation into the effect of malaria in pregnancy in pregnancy on foetal cortical brain development. Foetuses of infected and uninfected women showed no difference in foetal cortical development or brain volumes during pregnancy. However, the cingulate sulcus matured significantly faster in foetuses of malaria infected women, suggesting some neurodevelopmental involvement. |
| Rogerson SJ and Boeuf (2007) | Second stage Third stage |
Placental insufficiency Immuno-inflammation Birth outcomes |
A comprehensive review on the relationship between malaria in pregnancy and foetal growth restriction. Of note, this review describes the role of intervillous monocytes, placental hypoxia, and impaired placental transfer that has been reported in the literature. |
| Rogerson SJ et al. (2018) | First stage Third stage |
Maternal malnutrition Nutritional deficiencies |
This review discusses, among other factors, the association between malaria in pregnancy and maternal and infant anaemia. |
| Saraf et al. (2015) | First stage Third stage |
Maternal malnutrition Nutritional deficiencies |
A systematic review on global maternal and newborn Vitamin D status. It was found that Vitamin D deficiency is present in 54% of pregnant women and 75% of newborns globally. |
| Sartelet H et al. (1996) | Second stage | Placental insufficiency | This study from Senegal found that pre-eclampsia was significantly associated with malaria in pregnancy (p<0–03). |
| Scherjon SA et al. (1998) | Third stage | Cognition and behavioural deficits | This study investigated neurodevelopmental outcomes in IUGR, preterm infants. Adverse neurodevelopmental outcomes at 3-years were significantly associated with low head circumference (p=0.01). |
| Schwarz NG et al. (2008) | Third stage | Disease susceptibility | This study from Gabon investigated the effect of PM on the risk of malaria in the first 30 months of their offspring’s life. Offspring of women with active PM at delivery were at significantly higher risk of clinical malaria in the first 30 months of life. |
| Shulman CE et al. (2001) | First stage Third stage |
Maternal malnutrition Low birthweight |
This study investigated the relationship between PM, anaemia and low birthweight. Overall, a significant interaction was seen between chronic or past malaria and severe anaemia, and their effects on birthweight. |
| Skinner-Adams TS et al. (1998) | First stage Second stage Third stage |
Maternal coinfection Placental insufficiency Immuno-inflammation Long-term infant outcomes |
This study investigated the antimalarial activities of six commonly used antiretroviral agents. P. falciparum growth in vitro was inhibited by the HIV–1 protease inhibitors saquinavir, ritonavir, and indinavir. This information suggests a potentially protective interaction between HIV, PM and infant outcomes. |
| Soni PN et al. (1993) | Second stage | Autoantibodies | This South African study investigated the presence of anticardiolipin antibodies and P. falciparum infection. The authors concluded that there is a high prevalence of these antibodies in acute falciparum malaria cases. |
| Steketee RW et al. (1996a) | First stage Third stage |
Maternal coinfection Disease susceptibility |
This Malawian study investigated the association between HIV and P. falciparum infection in pregnant women. Parasitaemia density and placental infection was significantly higher in HIV-infected women (p<0.001) and newborns of HIV-infected women had higher rates of umbilical cord blood parasitaemia. |
| Steketee RW et al. (1996b) | Third stage | Low birthweight | This study prospectively evaluated malaria prevention in pregnant women in rural Malawi between 1987–1990. In this population, it was found that clearing placenta and umbilical cord parasites resulted in a 3–5% reduction in infant mortality rate and reduce 35% of preventable LBW deliveries. |
| Suchdev PS et al. (2017) | First stage Second stage |
Maternal malnutrition Immuno-inflammation |
This review assessed the relationship between nutrition, inflammation and child neurodevelopment in low-resource settings. These authors hypothesise a bidirectional relationship between inflammation and nutrition, wherein nutrition can directly impact immune function and the inflammatory response. |
| Suguitan AL et al. (2003) | Second stage Third stage |
Cytokine production Preterm birth |
This study measured concentrations of IFN-γ, TNF-α, IL-4 and IL-10 in placental plasma of malaria-infected and -uninfected Cameroonian women with premature and full-term deliveries. Elevated TNF-α and IL-10 were considered risk factors for malaria-associated preterm birth. |
| Sylvester B et al. (2016) | Third stage | Disease susceptibility | This Tanzanian study found that, in the first 2 years of life, exposure to P. falciparum in utero both reduced the time from birth to first clinical malaria episode and increased their frequency. |
| ter Kuile et al. (2004) | First stage | Maternal coinfection | This review includes 11 studies indicating higher peripheral and placental malaria in HIV-infected women. Malaria in pregnancy was associated with a higher HIV-1 viral concentrations, though reports of mother-to-child-transmission was conflicting. |
| Umbers AJ et al. (2011) | Second stage | Impaired oxygen, nutrient, antibody transfer | This study investigated concentrations of insulin growth factors-1 and -2, to determine whether PM and inflammation were associated with disturbances in the insulin-like growth factor axis, and thus played a role in fetal growth restriction. insulin growth factor-1 was significantly reduced in PM cases (p=0.007) and in their neonates (p=0.002). |
| Umbers AJ et al. (2013) | Second stage | Impaired vascularisation | Trophoblast invasion, migration and viability were measured in pregnant women with malaria infection. Trophoblast invasion (p=0.06) and migration (p=0.004) was reduced in response to serum of malaria-infected pregnant women, relative to uninfected women pregnant. |
| Veerhuis R et al. (2011) | Second stage | Complement activation | This review discusses the role of complement in central nervous system development, and evidence of the production of neurotoxic substances in response to complement activation. |
| Walther B et al. (2012) | Third stage | Reduced growth | This study investigated immune responses in 12-month-old offspring of PM cases. Lower growth rates were seen in infants born to mothers with PM; infants had significantly lower weight (p=0.032) and head circumference (p=0.041) at 12 months. No differences were seen at birth, implying that reduced growth at 12 months was associated with PM. |
| WBG (2016) | First stage | Low socioeconomic status | This report estimated global poverty. In 2013, ∼389 million people survived on <USD$1.90/day. |
| Weobong B et al. (2014) | First stage | Maternal mental health | This study investigated the adverse maternal and infant consequences of antenatal depression in Ghana. Interestingly, antenatal depression was associated with significant reductions in bed net use (p=0.005). |
| WHO (2017) | First stage | Maternal malnutrition | The WHO estimates that ∼10.9% of women of reproductive age in the African region are underweight (body mass index <18.5 kg/m2) and a median prevalence of 47.3% pregnant women are anaemic in the African region. |
| WHO (2018) | First stage | The latest World Malaria Report at the time of publication. | |
| Yamada M et al. (1989) | Second stage | Complement activation | This study assessed 20 placentas from PM cases in Malawi. It was seen that 15% of placentas stained strongly for the complement factor, C3. |
| Yoon BH et al. (1997) | Second stage Third stage |
Cytokine production White matter damage |
This study investigated cytokine expression in the amniotic fluid of women with complicated preterm gestations. Significant differences were seen in the concentrations of IL-1β and IL-6 between infants with and without white matter lesions, support the authors’ hypothesis that inflammatory cytokines play a role in the genesis of brain white matter lesions. |
| Zimmerman AW et al. (2007) | Second stage | Autoantibodies | In this study, serum reactivity to prenatal, postnatal, and adult rat brain proteins was tested in mothers with autistic children and maternal and child controls. Reactivity to prenatal rat brain proteins was seen in autistic children and their mothers, and children with other neurodevelopmental disorders. These patterns of reactivity differed to controls and suggest a role of maternal antibodies in the development of autism and possibly other |
| neurodevelopmental disorders, as well as evidence of transfer of autoantibodies across the placenta. |
First Stage: Risk factors for Placental Malaria
While numerous factors are involved in the dynamics of malaria transmission, the proposed conceptual framework will focus on the maternal and environmental risk factors that increase the risk of PM, and thereby may increase the risk of neurodevelopmental adversities.
Maternal risk factors for placental malaria
It is well known that, in malaria-endemic areas, infection is more common in younger compared to older pregnant women, and in primigravidae or secundigravidae compared to women who have been pregnant more than twice (Rogerson et al., 2018). Age, rather than gravidity, is considered a stronger predictor of malaria infection in pregnant women (Rogerson and Boeuf, 2007).
Maternal macro- and micro-nutrient malnutrition is closely associated with both risk of malaria and, independently, with adverse neurodevelopmental outcomes. One third of the global population is zinc deficient and, in developing countries, zinc deficiency is a significant risk factor for disease and illness. Zinc deficiencies are associated with malaria prevalence in pregnant women and children (Gibson et al., 1991, Gibson and Huddle, 1998), though this association is not consistent among children. Chronic haemolysis due to malaria increases urinary excretion of zinc, resulting in maternal zinc deficiency which can cause fetal brain malformations during gestation, impacting cell signalling, neurotransmission and neurological disorders in infancy and beyond (Adamo and Oteiza, 2010). Maternal and newborn 25-hydroxyvitamin D (25(OH)D) concentrations are highly correlated, with 54% of pregnant women and 75% of neonates diagnosed with 25(OH)D deficiency (Saraf et al., 2016). Low levels of plasma 25(OH)D are associated with severe malaria in children (Cusick et al., 2014). Some studies, (but not others) have found that maternal 25(OH)D deficiency is associated with mental and motor deficits and lower intelligence quotient (IQ) in infants (Darling et al., 2017) (Keim et al., 2014).
In the African region, the median prevalence of anaemia is 47.3% among pregnant women (WHO, 2017) due to numerous risk factors, including malaria. Adequate maternal nutrition is vital for optimal transplacental exchange and fetal growth and women with severe anaemia due to malaria have an increased risk of low birthweight (LBW) infants (Shulman et al., 2001). Malaria infection is associated with infant and maternal anaemia (Rogerson et al., 2018). Among the other causes of anaemia, maternal and infant iron deficiency anaemia are associated with poor infant neurodevelopmental outcomes – maternal iron deficiency anaemia is associated with lower fetal hippocampal volumes and brain-derived neurotrophic factor (Basu et al., 2017), while severe anaemia in neonates correlates with white matter injury, global developmental delay, learning, and behavioural difficulties (Loureiro et al., 2017). A meta-analysis estimated that every 10g/L decrease in haemoglobin in children is associated with a 1.73 point decrease in IQ (Black et al., 2008), with significant improvements in IQ scores seen in children receiving iron supplementation.
However, iron deficiency is often associated with other nutritional deficiencies and co-morbidities. Furthermore, iron supplementation can have adverse effects leading to iron overload in patients with chronic haemolytic conditions (Agostoni et al., 2010), so while reducing risk of iron deficiency is a well-established public health principle, the role of iron supplementation is uncertain in some populations. Nonetheless, it can be speculated that PM-induced maternal and infant anaemia could exacerbate anaemia due to nutritional deficiencies and contribute to infant neurodevelopmental adversities.
Lastly, Suchdev et al. (2017) suggest a bidirectional interaction between nutrition and inflammation, wherein inflammation negatively impacts nutritional status, and poor nutrition negatively impacts immune function (Suchdev et al., 2017). Potentially, reduced immune function due to malnutrition may increase the risk of PM.
In summary, nutritional deficiencies play a role in both increasing the risk of malaria and poor birth outcomes and, independently, the risk of poor neurodevelopmental outcomes. This relationship appears to be better defined with regards to severe iron deficiency, but less clear for other nutrients including zinc and 25(OH)D. Further research into the role of nutritional deficits as a risk factor for PM and subsequent infant morbidities is recommended.
Human Immunodeficiency Virus (HIV) and malaria coinfection have an estimated pooled prevalence of 12% among pregnant women (Naing et al., 2016). There are several important interactions between maternal HIV infection and PM. Maternal HIV infection increases severity of malaria by impairing antibody development to variant surface antigens expressed by malaria-infected erythrocytes, dysregulating cytokine production and reducing protective interferon-gamma responses (Moore et al., 2000, Mount et al., 2004). Moreover, malaria increases HIV viral load in pregnant women, mother-to-child transmission, and it hastens progression from HIV to acquired-immunodeficiency-syndrome (ter Kuile et al., 2004). HIV-infected women have higher rates of PM compared to uninfected women and higher rates of umbilical cord parasitaemia are reported in HIV-infected, compared to uninfected, newborns (Steketee et al., 1996a). Concurrently, there is an increased risk of antimalarial treatment failure in HIV-infected patients. However, antiretroviral treatment may mitigate malaria by inhibiting P. falciparum growth in vitro (Skinner-Adams et al., 2004). Malaria incidence was significantly reduced in Ugandan infants taking antiretroviral treatments (Achan et al., 2012). Therefore, while HIV-infection may increase susceptibility to and severity of PM, HIV treatment can play a protective role against malaria infection.
Poor maternal mental health may increase the likelihood of PM and directly contribute to poor birth and neurodevelopmental outcomes. Firstly, antenatal stress/mental health can increase vulnerability to malaria; indeed, antenatal depression is significantly associated with bed net non-use during pregnancy (Weobong et al., 2014). Secondly, prenatal depression is widespread in LMICs, with a recent meta-analysis finding a pooled prevalence estimate of antepartum depression of 25.3% (Gelaye et al., 2016). Thirdly, prenatal depression and stress are themselves well-known independent risk factors for poor infant cognitive and socioemotional development (Bergman et al., 2007). This relationship between maternal mental health and PM requires further investigation; of note, common mental disorders have been misdiagnosed as malaria due to commonality of symptoms, including headache, fatigue and joint pain (Kauye et al., 2014). Mental health is commonly under recognised and undertreated in LMICs (Gelaye et al., 2016), and continued vigilance on the burden of both malaria and maternal mental health is required.
Environmental risk factors for placental malaria
Access to antimalarials and malaria prevention tools are essential for malaria elimination, and lack of access to these services is a key risk factor for PM. In malaria-endemic areas, intermittent preventative treatment of malaria in pregnancy (IPTp) with the antifolate sulfadoxine-pyrimethamine (SP) is distributed at antenatal care visits alongside free long-lasting insecticidal-treated bed nets (LLINs). IPTp-SP is only administered in the second trimester due to concerns over its teratogenic effects, hence pregnant women rely mostly on LLINs for malaria prevention in their first trimester. Access to these interventions remains limited; in 2017, only 61% of pregnant women slept under an insecticide-treated bed net and 22% received the recommended three or more doses of IPTp in the African region (WHO, 2018). Clinical resistance to SP has been increasingly reported, with concerns regarding treatment efficacy in pregnancy (Harrington et al., 2009). The use of IPTp-SP in resistant areas can increase the proportion of resistant parasites, the level of placental parasitisation and placental inflammation (Harrington et al., 2009). Folic acid supplementation is recommended in pregnancy to prevent fetal neural tube defects; however, the concomitant use of antifolate and folate may negate SP efficacy (reviewed by (Nzila et al., 2014)). Therefore, while benefitting the majority, IPTp-SP could paradoxically worsen PM and potentially worsen downstream infant neurodevelopment.
Globally, SSA accounts for 50% of the extreme poor, with ~389 million people surviving on <USD$1.90/day in 2013 (WBG, 2016). Poor-quality housing that allows infected mosquitoes to enter and the inability to afford malaria prevention tools exacerbates infection risk. One of the most frequently cited reasons for not owning a LLIN is lack of money. Further to increasing the risk of malaria, children born to socioeconomically disadvantaged parents have higher odds of neurological abnormalities, suggesting there may be additive effects of low socioeconomic status and malaria infection in impairing neurodevelopment.
Second Stage: Placental pathology and inflammation associated with Placental Malaria infection
Placental development
PM-induced placental pathologic changes include infarction, decreased villous surface area, reduced capillaries per villus and fibrin deposition. These can cause placental insufficiency (or placental dysfunction), a serious condition characterised by impaired placental transfer, metabolic, endocrine and immune function.
Parasite sequestration causes infiltration of maternal macrophages and monocytes and secretion of chemotactic β-chemokines by fetal villous and maternal mononuclear cells; β-chemokines attract more monocytes and inflammatory factors and initiate an inflammatory cascade (McDonald C. R. et al., 2015). Macrophage migration inhibitory factor, a cytokine believed to retain and activate macrophages, is significantly higher in the placental intervillous space of PM cases (Rogerson and Boeuf, 2007). Macrophages are associated with fibrin deposition, and the tissue occupancy ratio of fibrin can be fivefold higher in PM (Imamura et al., 2002). Fibrin deposition can disrupt placental blood flow and impair gaseous exchange (Imamura et al., 2002), and perivillous fibrin deposition has been independently associated with increased risk of preterm birth (Menendez et al., 2000).
Monocytes and fibrin in the intervillous space impair placental and fetal growth, likely due to the development of ‘fibrin nets’ around parasitised erythrocytes and macrophages that narrow and/or plug intervillous spaces, mechanically impairing blood flow and transplacental exchange (Imamura et al., 2002). Heightened inflammatory cells and mediators can dysregulate trophoblast invasion into the maternal uterine wall, which is essential for transformation of the spiral arteries and increased placental blood flow (Umbers et al., 2013). Inadequate trophoblast invasion (and consequent incomplete remodelling of the spiral arteries) is considered the main cause of placental ischaemia and hypoxic conditions, and may explain PM-associated fetal growth restriction and preeclampsia. In malaria-endemic areas, peak prevalence of infection occurs at 13–20 weeks gestation, coinciding with the period where increased uterine blood flow is required to meet fetal metabolic demands (Brabin, 1983). The presence of parasitaemia during this period is believed to further disrupt vasculogenesis and impair placental blood circulation.
Massive chronic intervillositis (MCI), an idiopathic inflammatory lesion of the placenta, can show widespread fibrin deposition. MCI is associated with PM, particularly amongst primigravidae and in areas of ineffective malaria control and antimalarial resistance (Ordi et al., 1998). MCI is associated with adverse pregnancy outcomes, including intrauterine growth restriction (IUGR) (Ordi et al., 1998).
With reference to the placental effects of PM on neurodevelopment, many associations are seen. Firstly, reduced hippocampal and corpus callosum size, hypomyelination and neuronal apoptosis are seen in the offspring of animal models of placental insufficiency (Basilious et al., 2015). Secondly, placentas of infants with neurodevelopmental disorders demonstrate features of placental insufficiency and ischemia (Altshuler, 1993). Lastly, both MCI and placental insufficiency are associated with poor birth outcomes, which are themselves related to adverse neurodevelopment. Indeed, placental insufficiency is one of the most common causes of fetal growth restriction, and given its association with PM, it is logical that this pathway contributes significantly to reduced fetal growth.
However, cases of MCI and PM are rare (reported in 1.7–6.9% of PM cases (Muehlenbachs et al., 2010)) and many environmental and maternal factors can cause placental insufficiency, irrespective of PM. Nonetheless, PM may be an important mediator for placental insufficiency and/or MCI in malarious areas. Indeed, it is recommended that malaria be considered in the differential diagnosis of MCI (Ordi et al., 1998).The neurodevelopmental outcome of infants exposed to MCI has not been studied, but may be an important gap in knowledge for which research is needed.
Inflammatory factors
PM-associated inflammation is known to impair glucose and amino-acid transport (Boeuf et al., 2013, Umbers et al., 2011), suggesting a direct role of PM-induced placental inflammation on reduced fetal growth. There are varying reports as to what cytokines are implicated in PM, though reports of inflammation are consistent. Markers of oxidative stress and hypoxia are seen in PM, and conditions suggestive of hypoxia are known to occur in PM cases (Agudelo et al., 2014, Boeuf et al., 2008); however, the role of placental hypoxia in the pathogenesis of PM is not always supported in the literature (Boeuf et al., 2008).
Placental hypoxia enhances apoptosis in term human trophoblasts and increases production of pro-inflammatory cytokines interleukin (IL)-1α, IL-1β, and Tumour Necrosis Factor (TNF)-α (Rogerson and Boeuf, 2007). In PM, there is evidence of a Type-1 helper T-cell (TH1)-cytokine/TH2 immune response disequilibrium, characterised by increased TH1-cytokines in placental blood and tissue and lower TH2-cytokines (Fried et al., 1998).
Cytokines have been associated with perinatal brain injury and the detrimental impact of maternal immune stimulation and cytokines on short- and long-term neurodevelopment has been widely discussed. Cytokines are known to stimulate astrocytes and microglia in the fetal brain to produce more pro-inflammatory cytokines, disrupting neuronal plasticity and brain growth (Buehler, 2011). Microglia activation in the fetal brain causes astrogliosis, an abnormal increase in astrocytes due to neuronal death, reducing oligodendrocytes and myelination in the postnatal brain and causing cerebral white matter injury (Paintlia et al., 2008). Animal models of prenatal immune activation show fewer oligodendrocytes, apoptosis in white matter and deficits in neurogenesis, and elevated pro-inflammatory cytokines are seen in the plasma and cerebrospinal fluid of patients with neuropsychiatric disease, including schizophrenia and autism (Buehler, 2011).
There are inconsistencies regarding whether levels of IL-6, a TH2-cytokine with both pro- and anti-inflammatory properties, increase or decrease in response to malaria. When compared to uninfected placentas, infected placentas have significantly decreased IL-6 (Moormann et al., 1999), though associations between increased levels of IL-6 and placental and peripheral P. falciparum parasitaemia have been reported (Djontu et al., 2016). Importantly, elevated IL-6 levels in neonatal cord blood and amniotic fluid is an independent risk factor for intraventricular haemorrhage and periventricular leukomalacia in preterm infants; white matter lesions are seen in neonates born to mothers with elevated concentrations of TNF-α, IL-1β and IL-6 in their amniotic fluid, while periventricular leukomalacia is seen in neonates with increased levels of IL-6 in cord blood plasma (Martinez et al., 1998, Yoon et al., 1997). Severe neurodevelopmental delays/deficits are common in children with intraventricular haemorrhage and periventricular leukomalacia.
Cytokines have been associated with poor birth outcomes. TNF-α, IL-1, and IL-6 can increase prostaglandin synthesis, hormones involved in cervical ripening and labour induction, causing preterm labour and preterm rupture of membranes (Opsjln et al., 1993). High TNF-α is associated with IUGR (Moormann et al., 1999) and increased IL-6 is seen in preterm deliveries (Keelan et al., 1999). Specifically concerning malaria, elevated TNF-α and IL-10 are risk factors for malaria-associated preterm birth (Suguitan et al., 2003) and levels of interferon-gamma are significantly higher in primigravidae with malaria and are associated with LBW (Fried et al., 1998). It has been hypothesised that cytokines act indirectly on birth outcomes in PM, by increasing immune responses that mechanically block nutrients and oxygen (Fitri et al., 2015) impairing fetal growth.
Overall, the evidence from the literature suggests a mediating and indirect role of cytokines. Pro-inflammatory cytokines that cross the placenta are capable of damaging fetal white matter, while increased immune responses due to the presence of cytokines can mechanically impair transplacental exchange and fetal growth. Thus, in PM, TH1/ TH2 imbalances and elevated cytokine levels may mediate fetal white matter damage and poor birth outcomes (and subsequently impact neurodevelopment), though the exact pathways remain unclear.
The complement system may also play a role. It comprises a series of plasma proteins, which are crucial for innate immune responses to infection. Complement components are markedly increased in PM (Yamada et al., 1989). In the presence of malaria-infected erythrocytes, C5a causes monocyte release of the anti-angiogenic factor soluble Fms-like tyrosine kinase-1, which binds to vascular endothelial growth factor and placental growth factor preventing signalling and preventing promotion of angiogenesis (McDonald et al., 2013) thus impacting placental growth and function. High levels of soluble Fms-like tyrosine kinase-1 are seen in primigravidae with hypertension, PM, or both (Muehlenbachs et al., 2006). C5a in maternal and umbilical cord plasma is associated with spontaneous miscarriage and IUGR (Girardi et al., 2006); blocking C5a and its receptor in a mouse model of PM increased placental vascularisation and offspring growth and survival (Conroy et al., 2013).
In the central nervous system, complement plays a critical role in synapse formation and development (Veerhuis et al., 2011). However, activation of the complement system in response to disease in the brain promotes inflammation and production of pro-inflammatory cytokines and neurotoxic substances by glial cells (Veerhuis et al., 2011). Thus, maternal and fetal complement activation in response to PM may directly impair placental development, fetal growth and lead to brain injury. Targeting complement as a therapeutic strategy to prevent poor birth outcomes in PM cases has been suggested (McDonald C. R. et al., 2015) and continued research into this area should be encouraged.
Autoantibodies
Autoantibodies have the potential to cross the placenta and are increased in pregnant women (Fox et al., 2012) and parasitic infections, including malaria (Liew et al., 2015). Severe malaria patients have shown elevated levels of anticardiolipin antibodies (Soni et al., 1993), and autoantibodies against voltage-gated ion channels are seen in children with malaria (Lang et al., 2005). Autoantibodies are hypothesised to be involved in central nervous system disorders, though more research is required to define their role (Lang et al., 2003).
Maternal autoantibodies that recognise fetal brain antigens, and subsequent maternal autoantibody-induced brain injury, may underlie neurodevelopmental disorders, including behavioural and cognitive deficits (Fox et al., 2012). Indeed, serum from mothers of children with autism, developmental delay and attention-deficit/hyperactivity disorder is seen to react with prenatal rat brain proteins, but not with serum from control mothers (Zimmerman et al., 2007). Investigation into brain-reactive autoantibodies in women with PM has not yet been conducted but should be considered in future research.
Third Stage: Birth and early life outcomes as a consequence of Placental Malaria that affect infant neurodevelopment
Malaria infection, preterm birth, fetal growth restriction and low birthweight
PM is a risk factor for both preterm birth, fetal growth restriction and LBW. Annually, ~15 million infants are born preterm; the highest rates of preterm birth are in SSA and Asia, contributing to >60% of annual global preterm births (Chawanpaiboon et al., 2019). Similarly, in LMICs an estimated 23.3 million are born annually with fetal growth restriction, or small for gestational age (Lee et al., 2017). The risk of LBW is increased among primigravidae in malaria-endemic areas, significantly correlating with parasite rate at delivery (Brabin, 1991), and clearing placenta and umbilical cord parasites resulted in a 3–5% reduction in infant mortality rate and reduced 35% of preventable LBW deliveries (Steketee et al., 1996b). It is well-recognised that preterm birth, fetal growth restriction and LBW are associated with altered structural and functional brain maturation, adverse cognitive and behavioural deficits (Nosarti et al., 2010) and mild/moderate neurodevelopmental impairment (Blencowe et al., 2013).
Disease susceptibility
In addition to its effects on central nervous system development via effects on maternal immuno-inflammatory responses and placental function, PM increases malaria susceptibility among exposed infants. Offspring born to mothers with PM have a significantly increased risk of clinical malaria (Schwarz et al., 2008), higher parasite prevalence rates (Le Hesran et al., 1997) and a shorter time from birth to first clinical malaria episode (Sylvester et al., 2016) when compared to unexposed infants. Interestingly, interactions have been seen between gravidity and risk of malaria; two previous studies have found that infants born to multigravidae with PM have a higher risk of malaria compared to infants born to primigravidae, and in fact, infants born to primigravidae had a reduced risk of malaria compared to unexposed infants or infants born to PM-positive multigravidae (Mutabingwa et al., 2005, Schwarz et al., 2008). However, this interaction with gravidity is not consistent in the literature (Sylvester et al., 2016).
In malaria-endemic areas, infants <5 are at increased risk of malaria and the development of severe disease. Increased disease susceptibility is particularly concerning as cerebral malaria is a critical risk factor for adverse neurodevelopment. In 2000, neurological deficits were seen in 4.4% of infants <5 who had suffered from cerebral malaria in SSA, with recent reports finding neurological sequelae in 21.4% to 44.0% of cerebral malaria survivors in Uganda (Boivin et al., 2007, Postels et al., 2018). These findings highlight that firstly, mechanisms of immune responses in PM-exposed infants need to be further researched, especially given their potential impact on long-term neurodevelopmental outcomes, and secondly, women of all gravidities must be protected malaria.
Reduced growth
Infants exposed to PM have alterations in growth patterns including significantly lower body mass index-for-age, weight-for-age, and weight-for-length at 3, 6, and 12 months, and lower weight and head circumference at 12 months compared to unexposed infants (Walther et al., 2012). Infants exposed to malaria in pregnancy have increased odds of a small head (HC <1 SD below the median) and microcephaly (HC <2 SD below the median), relative to controls (Dombrowski et al., 2017). Lower head circumference is associated with adverse neurodevelopmental outcome at 3 years and major neurologic abnormalities and lower IQ scores at 8 years (Scherjon et al., 1998). Interestingly, microcephaly that persists until 2 years of age only is associated with abnormal cognitive and motor outcomes, suggesting microcephaly is a risk factor for cognitive impairment if there is no catch-up growth of head circumference (Kuban et al., 2009). This implies an indirect and long-term effect of PM on neurodevelopment via offspring growth; if PM impairs growth, particularly beyond infancy and into childhood, it is possible this will lead to persistent neurodevelopmental adversities.
Infant Hypothalamic-Pituitary-Adrenocortical Axis Disturbances
The Hypothalamic-Pituitary-Adrenocortical (HPA) axis is compromised in P. falciparum malaria; cortisol is significantly higher in women with malaria, particularly primigravidae and a positive correlation between cortisol concentration and parasite load at delivery is evident (Bouyou-Akotet et al., 2005). The HPA axis is also dysregulated by maternal stress, increasing transfer of glucocorticoids from mother to fetus (Duthie and Reynolds, 2013). Increased fetal exposure to glucocorticoids is associated with LBW and is believed to lead to adverse infant neurodevelopmental outcomes (Duthie and Reynolds, 2013). High cortisol concentrations are positively associated with preterm birth (Giurgescu, 2009) and severe intraventricular haemorrhage in extremely LBW infants (Aucott et al., 2008). Speculatively, disruption of the maternal HPA axis due to PM and maternal stress can lead to HPA axis dysfunction in infants (primarily with regards to cortisol concentrations), which may contribute to poor infant birth and neurodevelopmental outcomes. Future research is required to clarify this relationship.
Conclusion
We have provided a comprehensive synthesis of the literature and a corresponding novel conceptual framework to identify the potential pathways that may explain adverse neurodevelopmental outcomes as a consequence of exposure to PM. The rationale for this proposal is based on three well-established findings. PM and associated maternal and environmental risk factors are common in LMICs, and can adversely impact neurodevelopment; PM leads to poor birth outcomes, including preterm birth, LBW, and IUGR, which are associated with short- and long-term adverse neurological outcomes; and finally, PM is associated with poor infant neurodevelopmental outcomes in animal and human studies.
Multiple risk factors are involved in this conceptual framework and a key knowledge gap is the uncertainty as to which are the most important and most prevalent and how exactly they interact. While it is hypothesised that there are direct, indirect and bidirectional roles at play, further research should investigate whether additive, augmentative, synergistic or even protective relationships exist. Field studies are required to corroborate the true impact of PM on infants exposed in utero, and long-term follow-up to identify the contribution of PM to infant neurodevelopmental and health outcomes. Validating this relationship will increase awareness and advocate for interventions to prevent malaria in pregnancy that may ultimately help to improve infant neurological outcomes and prevent neurodevelopmental impairments later in life. Clinical practices and targeted early interventions need to be set in place to treat this high-risk population.
Finally, it is noteworthy that pregnant women remain mostly unprotected in their first trimester, coinciding with peak parasitaemia prevalence, and are often excluded from population-targeted drug administration programs. Development of methods of prophylaxis that are safe during the first trimester is urgently needed, as well as continued vigilance in increasing access and use of LLINs throughout pregnancy.
Funding:
This work was supported by a Mater Foundation Principal Research Fellowship to Associate Professor Samudragupta Bora and The University of Queensland Research Training Program and Frank Clair Scholarships to Ms. Harriet L.S. Lawford. The funding sources had no role in the writing of the manuscript or the decision to submit it for publication.
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- HIV
Human Immunodeficiency Virus
- HPA
Hypothalamic-Pituitary-Adrenocortical
- IL
Interleukin
- IQ
Intelligence Quotient
- IPTp
Intermittent Preventative Treatment of Malaria in Pregnancy
- IUGR
Intrauterine Growth Restriction
- LBW
Low Birthweight
- LLIN
Long-Lasting Insecticidal-Treated Bed Nets
- LMIC
Low- and Middle-Income Country
- MCI
Massive Chronic Intervillositis
- PM
Placental Malaria
- SP
Sulfadoxine-pyrimethamine
- TH1/2
Type-1/2 Helper T-cell
Footnotes
Conflict of Interest: The authors have no potential or perceived conflicts of interest relevant to this article to disclose.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Ethical Approval: Not required.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achan J, Kakuru A, Ikilezi G, Ruel T, Clark TD, Nsanzabana C, et al. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med 2012;367(22):2110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam I, Elhassan EM, Mohmmed AA, Salih MM, Elbashir MI. Malaria and pre-eclampsia in an area with unstable malaria transmission in Central Sudan. Malar J 2011;10:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo AM, Oteiza PI. Zinc deficiency and neurodevelopment: the case of neurons. Biofactors 2010;36(2):117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 2010;50(1):85–91. [DOI] [PubMed] [Google Scholar]
- Agudelo OM, Aristizabal BH, Yanow SK, Arango E, Carmona-Fonseca J, Maestre A. Submicroscopic infection of placenta by Plasmodium produces Th1/Th2 cytokine imbalance, inflammation and hypoxia in women from northwest Colombia. Malaria Journal 2014;13(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler G Some placental considerations related to neurodevelopmental and other disorders. J Child Neurol 1993;8(1):78–94. [DOI] [PubMed] [Google Scholar]
- Aucott SW, Watterberg KL, Shaffer ML, Donohue PK, Group PS. Do cortisol concentrations predict short-term outcomes in extremely low birth weight infants? Pediatrics 2008;122(4):775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilious A, Yager J, Fehlings MG. Neurological outcomes of animal models of uterine artery ligation and relevance to human intrauterine growth restriction: a systematic review. Dev Med Child Neurol 2015;57(5):420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Kumar D, Anupurba S, Verma A, Kumar A. Effect of maternal iron deficiency anemia on fetal neural development. Journal of Perinatology 2017:1. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child & Adolescent Psychiatry 2007;46(11):1454–63. [DOI] [PubMed] [Google Scholar]
- Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, et al. Early childhood development coming of age: science through the life course. Lancet 2017;389(10064):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243–60. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res 2013;74 Suppl 1:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeuf P, Aitken EH, Chandrasiri U, Chua CLL, McInerney B, McQuade L, et al. Plasmodium falciparum malaria elicits inflammatory responses that dysregulate placental amino acid transport. PLoS pathogens 2013;9(2):e1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeuf P, Tan A, Romagosa C, Radford J, Mwapasa V, Molyneux ME, et al. Placental hypoxia during placental malaria. J Infect Dis 2008;197(5):757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics 2007;119(2):e360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyou-Akotet MK, Adegnika AA, Agnandji ST, Ngou-Milama E, Kombila M, Kremsner PG, et al. Cortisol and susceptibility to malaria during pregnancy. Microbes and Infection 2005;7(11):1217–23. [DOI] [PubMed] [Google Scholar]
- Brabin B An assessment of low birthweight risk in primiparae as an indicator of malaria control in pregnancy. Int J Epidemiol 1991;20(1):276–83. [DOI] [PubMed] [Google Scholar]
- Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ 1983;61(6):1005–16. [PMC free article] [PubMed] [Google Scholar]
- Buehler MR. A proposed mechanism for autism: an aberrant neuroimmune response manifested as a psychiatric disorder. Med Hypotheses 2011;76(6):863–70. [DOI] [PubMed] [Google Scholar]
- Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7(1):e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy AL, Bangirana P, Muhindo MK, Kakuru A, Jagannathan P, Opoka RO, et al. Case Report: Birth Outcome and Neurodevelopment in Placental Malaria Discordant Twins. Am J Trop Med Hyg 2019;100(3):552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy AL, Silver KL, Zhong K, Rennie M, Ward P, Sarma JV, et al. Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell Host Microbe 2013;13(2):215–26. [DOI] [PubMed] [Google Scholar]
- Cusick SE, Opoka RO, Lund TC, John CC, Polgreen LE. Vitamin D insufficiency is common in Ugandan children and is associated with severe malaria. PloS one 2014;9(12):e113185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling AL, Rayman MP, Steer CD, Golding J, Lanham-New SA, Bath SC. Association between maternal vitamin D status in pregnancy and neurodevelopmental outcomes in childhood; results from the Avon Longitudinal Study of Parents and Children (ALSPAC). The British journal of nutrition 2017;117(12):1682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 2010;7(1):e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djontu JC, Siewe Siewe S, Mpeke Edene YD, Nana BC, Chomga Foko EV, Bigoga JD, et al. Impact of placental Plasmodium falciparum malaria infection on the Cameroonian maternal and neonate’s plasma levels of some cytokines known to regulate T cells differentiation and function. Malaria Journal 2016;15:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski JG, Souza RM, Lima FA, Bandeira CL, Murillo O, Costa DS, et al. Plasmodium falciparum infection during pregnancy impairs fetal head growth: prospective and populational-based retrospective studies. bioRxiv 2017:203059. [Google Scholar]
- Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology 2013;98(2):106–15. [DOI] [PubMed] [Google Scholar]
- Fitri LE, Sardjono TW, Rahmah Z, Siswanto B, Handono K, Dachlan YP. Low Fetal Weight is Directly Caused by Sequestration of Parasites and Indirectly by IL-17 and IL-10 Imbalance in the Placenta of Pregnant Mice with Malaria. Korean J Parasitol 2015;53(2):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Amaral D, Van de Water J. Maternal and fetal antibrain antibodies in development and disease. Developmental neurobiology 2012;72(10):1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol 1998;160(5):2523–30. [PubMed] [Google Scholar]
- Gelaye B, Rondon MB, Araya R, Williams MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry 2016;3(10):973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson RS, Heywood A, Yaman C, Sohlstrom A, Thompson LU, Heywood P. Growth in children from the Wosera subdistrict, Papua New Guinea, in relation to energy and protein intakes and zinc status. Am J Clin Nutr 1991;53(3):782–9. [DOI] [PubMed] [Google Scholar]
- Gibson RS, Huddle J-M. Suboptimal zinc status in pregnant Malawian women: its association with low intakes of poorly available zinc, frequent reproductive cycling, and malaria. Am J Clin Nutr 1998;67(4):702–9. [DOI] [PubMed] [Google Scholar]
- Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006;203(9):2165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurgescu C Are maternal cortisol levels related to preterm birth? J Obstet Gynecol Neonatal Nurs 2009;38(4):377–90. [DOI] [PubMed] [Google Scholar]
- Guyatt HL, Snow RW. Impact of Malaria during Pregnancy on Low Birth Weight in Sub-Saharan Africa. Clinical Microbiology Reviews 2004;17(4):760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W, Mutabingwa T, Muehlenbachs A, Sorensen B, Bolla M, Fried M, et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proceedings of the National Academy of Sciences 2009;106(22):9027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Sugiyama T, Cuevas LE, Makunde R, Nakamura S. Expression of tissue factor, the clotting initiator, on macrophages in Plasmodium falciparum-infected placentas. J Infect Dis 2002;186(3):436–40. [DOI] [PubMed] [Google Scholar]
- Kauye F, Jenkins R, Rahman A. Training primary health care workers in mental health and its impact on diagnoses of common mental disorders in primary care of a developing country, Malawi: a cluster-randomized controlled trial. Psychol Med 2014;44(3):657–66. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999;181(6):1530–6. [DOI] [PubMed] [Google Scholar]
- Keim SA, Bodnar LM, Klebanoff MA. Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behavior. Paediatric and perinatal epidemiology 2014;28(5):434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuban KC, Allred EN, O’Shea TM, Paneth N, Westra S, Miller C, et al. Developmental correlates of head circumference at birth and two years in a cohort of extremely low gestational age newborns. J Pediatr 2009;155(3):344–9 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B, Dale RC, Vincent A. New autoantibody mediated disorders of the central nervous system. Curr Opin Neurol 2003;16(3):351–7. [DOI] [PubMed] [Google Scholar]
- Lang B, Newbold CI, Williams G, Peshu N, Marsh K, Newton CR. Antibodies to voltage-gated calcium channels in children with falciparum malaria. J Infect Dis 2005;191(1):117–21. [DOI] [PubMed] [Google Scholar]
- Le Hesran JY, Cot M, Personne P, Fievet N, Dubois B, Beyeme M, et al. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am J Epidemiol 1997;146(10):826–31. [DOI] [PubMed] [Google Scholar]
- Lee AC, Kozuki N, Cousens S, Stevens GA, Blencowe H, Silveira MF, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: analysis of CHERG datasets. BMJ 2017;358:j3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew J, Amir A, Chen Y, Fong MY, Razali R, Lau YL. Autoantibody profile of patients infected with knowlesi malaria. Clin Chim Acta 2015;448:33–8. [DOI] [PubMed] [Google Scholar]
- Loureiro B, Martinez-Biarge M, Foti F, Papadaki M, Cowan FM, Wusthoff CJ. MRI Patterns of brain injury and neurodevelopmental outcomes in neonates with severe anaemia at birth. Early Hum Dev 2017;105:17–22. [DOI] [PubMed] [Google Scholar]
- Martinez E, Figueroa R, Garry D, Visintainer P, Patel K, Verma U, et al. Elevated Amniotic Fluid Interleukin-6 as a Predictor of Neonatal Periventricular Leukomalacia and Intraventricular Hemorrhage. J Matern Fetal Investig 1998;8(3):101–7. [PubMed] [Google Scholar]
- McDonald CR, Cahill LS, Ho KT, Yang J, Kim H, Silver KL, et al. Experimental malaria in pregnancy induces neurocognitive injury in uninfected offspring via a C5a-C5a receptor dependent pathway. PLoS pathogens 2015;11(9):e1005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Elphinstone RE, Kain KC. The impact of placental malaria on neurodevelopment of exposed infants: a role for the complement system? Trends in parasitology 2013;29(5):213–9. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Tran V, Kain KC. Complement Activation in Placental Malaria. Front Microbiol 2015;6:1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis 2000;181(5):1740–5. [DOI] [PubMed] [Google Scholar]
- Moore JM, Ayisi J, Nahlen BL, Misore A, Lal AA, Udhayakumar V. Immunity to placental malaria. II. Placental antigen-specific cytokine responses are impaired in human immunodeficiency virus-infected women. J Infect Dis 2000;182(3):960–4. [DOI] [PubMed] [Google Scholar]
- Moormann AM, Sullivan AD, Rochford RA, Chensue SW, Bock PJ, Nyirenda T, et al. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. Journal of Infectious Diseases 1999;180(6):1987–93. [DOI] [PubMed] [Google Scholar]
- Mount AM, Mwapasa V, Elliott SR, Beeson JG, Tadesse E, Lema VM, et al. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet 2004;363(9424):1860–7. [DOI] [PubMed] [Google Scholar]
- Muehlenbachs A, Fried M, McGready R, Harrington WE, Mutabingwa TK, Nosten F, et al. A novel histological grading scheme for placental malaria applied in areas of high and low malaria transmission. J Infect Dis 2010;202(10):1608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and maternal-fetal conflict during placental malaria. PLoS Med 2006;3(11):e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutabingwa TK, Bolla MC, Li JL, Domingo GJ, Li X, Fried M, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med 2005;2(12):e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naing C, Sandhu NK, Wai VN. The Effect of Malaria and HIV Co-Infection on Anemia: A Meta-Analysis. Medicine (Baltimore) 2016;95(14):e3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Murray RM, Hack M. Neurodevelopmental outcomes of preterm birth: from childhood to adult life: Cambridge University Press, 2010. [Google Scholar]
- Nzila A, Okombo J, Molloy AM. Impact of folate supplementation on the efficacy of sulfadoxine/pyrimethamine in preventing malaria in pregnancy: the potential of 5-methyl-tetrahydrofolate. J Antimicrob Chemother 2014;69(2):323–30. [DOI] [PubMed] [Google Scholar]
- Opsjln SL, Wathen NC, Tingulstad S, Wiedswang G, Sundan A, Waage A, et al. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol 1993;169(2 Pt 1):397–404. [DOI] [PubMed] [Google Scholar]
- Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol 1998;22(8):1006–11. [DOI] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetyl cysteine. Exp Neurol 2008;210(2):560–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postels DG, Wu X, Li C, Kaplan PW, Seydel KB, Taylor TE, et al. Admission EEG findings in diverse paediatric cerebral malaria populations predict outcomes. Malar J 2018;17(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijken MJ, de Wit MC, Mulder EJ, Kiricharoen S, Karunkonkowit N, Paw T, et al. Effect of malaria in pregnancy on foetal cortical brain development: a longitudinal observational study. Malar J 2012;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson SJ, Boeuf P. New approaches to pathogenesis of malaria in pregnancy. Parasitology 2007;134(Pt 13):1883–93. [DOI] [PubMed] [Google Scholar]
- Rogerson SJ, Desai M, Mayor A, Sicuri E, Taylor SM, van Eijk AM. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 2018;18(4):e107–e18. [DOI] [PubMed] [Google Scholar]
- Saraf R, Morton SM, Camargo CA Jr., Grant CC . Global summary of maternal and newborn vitamin D status - a systematic review. Matern Child Nutr 2016;12(4):647–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartelet H, Rogier C, Milko-Sartelet I, Angel G, Michel G. Malaria associated pre-eclampsia in Senegal. Lancet 1996;347(9008):1121. [DOI] [PubMed] [Google Scholar]
- Scherjon SA, Oosting H, Smolders-DeHaas H, Zondervan HA, Kok JH. Neurodevelopmental outcome at three years of age after fetal ‘brain-sparing’. Early Hum Dev 1998;52(1):67–79. [DOI] [PubMed] [Google Scholar]
- Schwarz NG, Adegnika AA, Breitling LP, Gabor J, Agnandji ST, Newman RD, et al. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis 2008;47(8):1017–25. [DOI] [PubMed] [Google Scholar]
- Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, Peshu N, et al. Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Int Health 2001;6(10):770–8. [DOI] [PubMed] [Google Scholar]
- Skinner-Adams TS, McCarthy JS, Gardiner DL, Hilton PM, Andrews KT. Antiretrovirals as antimalarial agents. The Journal of infectious diseases 2004;190(11):1998–2000. [DOI] [PubMed] [Google Scholar]
- Soni PN, De Bruyn CC, Duursma J, Sharp BL, Pudifin DJ. Are anticardiolipin antibodies responsible for some of the complications of severe acute Plasmodium falciparum malaria? S Afr Med J 1993;83(9):660–2. [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Bloland PB, Chilima B, Mermin JH, Chitsulo L, et al. Impairment of a pregnant woman’s acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg 1996a;55(1 Suppl):42–9. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Campbell CC. Developing effective strategies for malaria prevention programs for pregnant African women. Am J Trop Med Hyg 1996b;55(1 Suppl):95–100. [DOI] [PubMed] [Google Scholar]
- Suchdev PS, Boivin MJ, Forsyth BW, Georgieff MK, Guerrant RL, Nelson CA, 3rd. Assessment of Neurodevelopment, Nutrition, and Inflammation From Fetal Life to Adolescence in Low-Resource Settings. Pediatrics 2017;139(Suppl 1):S23–S37. [DOI] [PubMed] [Google Scholar]
- Suguitan AL Jr., Cadigan TJ, Nguyen TA, Zhou A, Leke RJ, Metenou S, et al. Malaria-associated cytokine changes in the placenta of women with pre-term deliveries in Yaounde, Cameroon. Am J Trop Med Hyg 2003;69(6):574–81. [PubMed] [Google Scholar]
- Sylvester B, Gasarasi DB, Aboud S, Tarimo D, Massawe S, Mpembeni R, et al. Prenatal exposure to Plasmodium falciparum increases frequency and shortens time from birth to first clinical malaria episodes during the first two years of life: prospective birth cohort study. Malar J 2016;15(1):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, van Eijk AM, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg 2004;71(2 Suppl):41–54. [PubMed] [Google Scholar]
- Umbers AJ, Boeuf P, Clapham C, Stanisic DI, Baiwog F, Mueller I, et al. Placental malaria-associated inflammation disturbs the insulin-like growth factor axis of fetal growth regulation. J Infect Dis 2011;203(4):561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbers AJ, Stanisic DI, Ome M, Wangnapi R, Hanieh S, Unger HW, et al. Does malaria affect placental development? Evidence from in vitro models. PLoS One 2013;8(1):e55269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol 2011;48(14):1592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther B, Miles DJ, Waight P, Palmero MS, Ojuola O, Touray ES, et al. Placental malaria is associated with attenuated CD4 T-cell responses to tuberculin PPD 12 months after BCG vaccination. BMC Infect Dis 2012;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WBG. World Bank Group: Poverty and shared prosperity 2016: taking on inequality: World Bank Publications, 2016. [Google Scholar]
- Weobong B, ten Asbroek AH, Soremekun S, Manu AA, Owusu-Agyei S, Prince M, et al. Association of antenatal depression with adverse consequences for the mother and newborn in rural Ghana: findings from the DON population-based cohort study. PLoS One 2014;9(12):e116333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Nutrition in the WHO African Region. Brazzaville: Licence: CC BY-NCSA 3.0 IGO; 2017. [Google Scholar]
- WHO. World malaria report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- Yamada M, Steketee R, Abramowsky C, Kida M, Wirima J, Heymann D, et al. Plasmodium falciparum associated placental pathology: a light and electron microscopic and immunohistologic study. Am J Trop Med Hyg 1989;41(2):161–8. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177(1):19–26. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, et al. Maternal antibrain antibodies in autism. Brain Behav Immun 2007;21(3):351–7. [DOI] [PubMed] [Google Scholar]


