Abstract
The COVID‐19 pandemic has impacted the entire world, causing a great number of mortality of humans and affecting the economy, while conservation efforts are finally recognized to prevent further pandemics. The wildlife rehabilitation centers (WRCs) play a relevant role in animal welfare; nevertheless, they also represent an imminent risk of pathogen transmission between humans‐to‐animals and between animals. Moreover, WRCs could spread pathogens into natural habitats through the reintroduction of infectious individuals. These biosafety concerns at WRCs may increase as the economic and social impact of the COVID‐19 extends. We explored the current situation of Latin American WRCs under the COVID‐19 pandemic to determine the feasibility of SARS‐CoV‐2 introduction, amplification, and spread within these institutions. We surveyed WRCs from eight Latin American countries. We found that pandemic is affecting these institutions in many aspects: workers with symptoms compatible with COVID‐19, reduced economic resources, and lack of information and support from governmental authorities. These have forced WRCs to reduce the workforce, veterinary visits, and animal food rations and to increase the number of animals released. This scenario generates a risky environment for the transmission of SARS‐CoV‐2, especially for felids, mustelids, and non‐human primates. Therefore, it is imperative to respect quarantine periods, monitor incoming patients, increase biosecurity measures, develop and apply guidelines and recommendations for the protection of personnel and biosafety of enclosures and instruments. It is of utmost importance the proper and safer reintroduction of recovered wildlife.
Keywords: conservation, COVID‐19, health risks, Latin America, SARS‐CoV‐2, wildlife rehabilitation centers
Wildlife rehabilitation centers in Latin America are impacted with the current COVID‐19 pandemic. Some centers are experiencing economic and logistical challenges that have vastly impacted budgets, operations, and supply restocking. The wildlife rehabilitation centers could be a potential risky environment for the introduction, amplification, and spread of SARS‐CoV‐2, especially for felids, mustelids, and non‐human primates; species commonly admitted and with scientific evidence known to amplify and transmit this virus. Preventive measures should be established at wildlife rehabilitation centers to reduce the risk of SARS‐CoV‐2 transmission, including respecting quarantine periods, monitoring new patients, isolate symptomatic animals, increasing biosecurity measures, and developing guidelines and recommendations for protection of personnel, enclosures, and instruments.

RESUMEN
La pandemia de COVID‐19 ha impactado mundialmente, provocando una alta mortalidad en humanos y afectando la economía, resaltando la importancia de los esfuerzos de conservación para prevenir nuevas pandemias. Los centros de rehabilitación de vida silvestre juegan un papel relevante en el bienestar animal, sin embargo, también representan un riesgo inminente de transmisión de patógenos entre humanos a animales y entre animales. Además, los centros de rehabilitación de vida silvestre podrían propagar patógenos a hábitats naturales mediante la reintroducción de individuos infecciosos. Estas preocupaciones de bioseguridad en centros de rehabilitación de vida silvestre pueden aumentar a medida que se extiende el impacto económico y social del COVID‐19. Exploramos la situación actual de centros de rehabilitación de vida silvestre latinoamericanos durante la pandemia de COVID‐19 para determinar la viabilidad de la introducción, amplificación y propagación del SARS‐CoV‐2 dentro de estas instituciones. Encuestamos centros de rehabilitación de vida silvestre de ocho países latinoamericanos y encontramos que la pandemia está afectando a estas instituciones en muchos aspectos: trabajadores con síntomas compatibles con COVID‐19, recursos económicos reducidos y falta de información y apoyo de las autoridades gubernamentales. Estos han obligado a centros de rehabilitación de vida silvestre a reducir la mano de obra, las visitas veterinarias y las raciones de alimentos para animales, así como aumentar el número de animales liberados. Este escenario genera un entorno de riesgo para la transmisión del SARS‐CoV‐2, especialmente para félidos, mustélidos y primates no humanos. Por lo tanto, es imperativo respetar los períodos de cuarentena, monitorear a los pacientes que ingresan, incrementar las medidas de bioseguridad, desarrollar y aplicar lineamientos y recomendaciones para la protección del personal y la bioseguridad. Es de suma importancia la reintroducción adecuada y segura de la vida silvestre recuperada.
As a result of anthropogenic pressures that include poisonings, electrocutions, hunting accidents, illegal trade, antagonistic interactions with humans and domestic animals, Wildlife Rehabilitation Centers (WRCs) have been established all over Latin America to rescue, rehabilitate, and release native fauna (Romero et al., 2019; Stidworthy, 2016). Although there are different categories and terms used to describe primary care centers for wildlife species throughout Latin America, we will conceptualize them as WRCs in this commentary. A few Latin American countries (e.g., Costa Rica, Chile, Mexico) have legislation regarding the functions, obligations, and requirements of WRC's; however, the lack of guidance and support from government institutions is common in all Latin American countries. This implies that the systems for handling, recovering, and releasing animals are dependent on variable economic resources, infrastructure, and knowledge of professionals; however, in several occasions due to a limitation of economic resources, many of the technical responsibilities depend on the empirical decisions of the owners, or other staff in charge of the site.
The emergence of COVID‐19 in late 2019 and its subsequent global spread has impacted the entire world, and Latin America is no exception. WRCs are likely to be impacted in this scenario, as they may be economically dependent on volunteers, educational programs, tourism, or a mix of those activities, all of which have been suspended or heavily reduced. Beyond potentially reduced budgets, logistic challenges may also emerge, such as restocking food and medical supplies.
Despite the potential role of WRCs in biodiversity conservation, it is of utmost importance to consider their inherent and previously recognized health risks (Karesh, 1995). The WRCs represent an interface in which multiple species interactions occur boosting infection risks as shown in Figure 1. These emerging challenges may increase the risk of pathogen introduction, amplification, and spread of infectious diseases at, within, and from WRCs. These health risks are a growing conservation concern due to the risk of SARS‐CoV‐2 introduction into native environments of Latin America and the potential susceptibility of endemic wildlife species.
FIGURE 1.
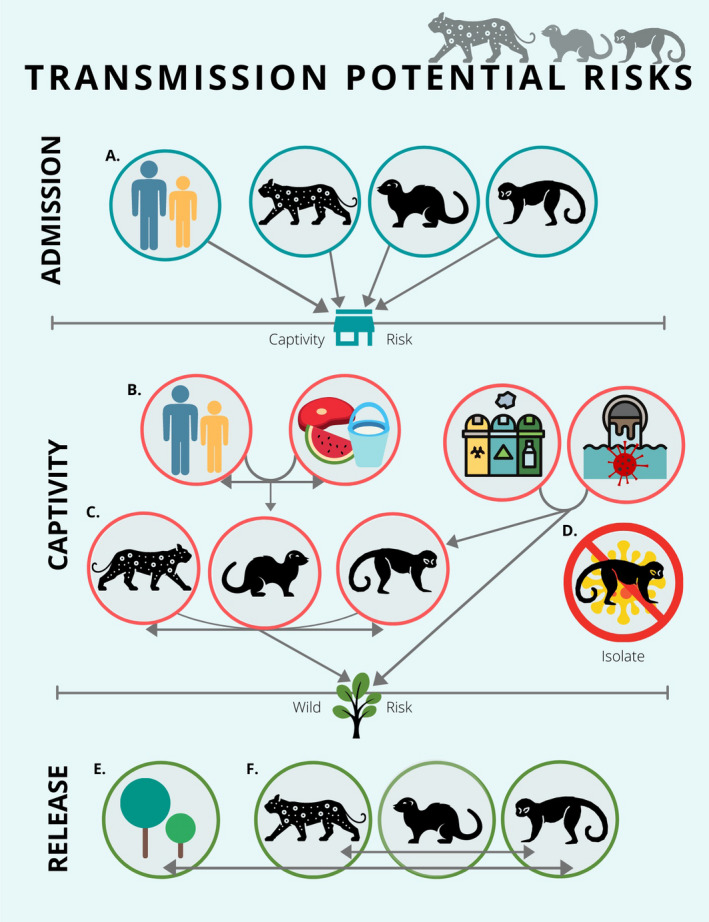
Transmission Potential Risks: The entry of wild animals and humans (workers and visitors) (a) to rehabilitation centers favor the risk of introduction of pathogens into the premises. The direct (b) and indirect (c) contact between captive animals with humans, other captive animals and fomites within wildlife rehabilitation centers may favor pathogen amplification. The isolation (d) of symptomatic animals compatible with SARS‐CoV‐2 clinical signs should be mandatory to prevent a potential transmission of the infectious agent. The release of animals from the wildlife rehabilitation centers and disposal of wastewater and other fomites (e.g., garbage) favor the risk of spread of pathogens into wild environments (e) and the potential pathogen transmission to wild individuals of the same or different species (f)
1. THE CURRENT SCENARIO IN LATIN AMERICAN WRCS
Here, we explored the current situation of WRCs in Latin America under the COVID‐19 pandemic to assess the feasibility of SARS‐CoV‐2 introduction, amplification, and spread in these institutions, and assessed the possibility for governmental and technical support. We contacted 35 WRCs operating in eight Latin American countries (Argentina, Bolivia, Brazil, Costa Rica, Chile, Peru, Ecuador, and Mexico) and surveyed them anonymously during June 2020.
The survey was informal in nature, as the intention was not to carry out any statistical analysis. The survey was sent to recognized WRCs from Latin American countries and to professional groups with representatives from these types of institutions. Closed‐ended questions were the primary format, with the objective of finding any variation of pre‐pandemic procedures with those at the time they were surveyed related to the quarantine/isolation/biosecurity/release procedures used by the WRCs. We focused on groups of animals (felids, mustelids and non‐human primates) that are recognized as at risk for SARS‐CoV‐2. Other wild animals at risk (e.g., bats) were not considered because these animal species are rarely kept in captivity by a WRC in Latin America.
As expected, some WRCs are experiencing economic and logistical challenges that have vastly impacted budgets, operations, and supply restocking. We obtained reports of on‐duty WRCs’ workers with COVID‐19 compatible symptoms, very few WRCs that monitor the health of animals before admission, and several WRCs are admitting high‐risk species, such as felids, mustelids, and non‐human primates. These processes may contribute to the SARS‐CoV‐2 introduction into WRCs. Human‐to‐animal transmission, specifically to captive felids (cougar [Puma concolor], tigers [Panthera tigris], and lions [Panthera leo]), captive mustelids (American minks [Neovison vison]), and cats (Felis catus; felidae), has already occurred (Enserink, 2020; Oreshkova et al., 2020; Ruiz‐Arrondo et al., 2020). Moreover, cats, ferrets (Mustela putorius furo; mustelidae), and rhesus and crab‐eating macaques (Macaca mulatta and Macaca fascicularis; cercopithecidae) are susceptible to this virus as demonstrated in experimental infections (Bosco‐Lauth et al., 2020; Chan et al., 2020; Deng et al., 2020). Based on this evidence, the amplification of pathogens within WRCs maintaining potentially susceptible felids, mustelids, and non‐human primates may also occur. Moreover, species of these taxa could also infect individuals of the same species according to experimental infections in cats and ferrets (Deng et al., 2020; Shi et al., 2020; Sia et al., 2020).
Because of the way the majority of Latin American WRCs are managed, their main expenses are primarily covered by donations, visitations, and eventually from funds obtained for conservation or research, with limited capacity to save; thus, COVID‐19 restrictions impacted on their main income sources. For these reasons, some of the contacted WRCs have implemented restricted food rations aiming to secure the nutrition of the animals in the mid‐term. Other WRCs have reduced personnel (more than 85% in specific cases) or have interrupted regular veterinary visits. Very few of the contacted centers monitor the health of animals during captivity. These difficulties and restrictions in WRCs operations may further increase the risk of pathogen amplification within their facilities. Lastly, although indirect transmission of SARS‐CoV‐2 remains unclear, infection through fomites has been proposed and surface swab samples have been found positive for SARS‐CoV‐2 RNA (Ong et al., 2020). Consequently, fomites (i.e., food and water utensils) could also represent a biohazard for the amplification of this virus within WRCs.
Finally, the spread of SARS‐CoV‐2 from WRCs to the Neotropic wild populations is a potential risk, as those WRCs contacted typically release felids, mustelids, and non‐human primates without testing prior to liberation. Beyond the lack of testing, some WRCs have considered or have activated emergency measures, such as increasing the number of released animals. Some of these released individuals may not be in an adequate health condition. In one WRC, more than 60% of captive animals were released due to budget constraints caused by the pandemic. Further, experimental evidence has demonstrated a significant transmission of SARS‐CoV‐2 via air transmission between ferrets (Richard et al., 2020). Additionally, SARS‐CoV‐2 RNA has been detected in wastewaters, where viable infectious viral particles could remain in this media (Ahmed et al., 2020; La Rosa et al., 2020; Wu et al., 2020). Indeed, wastewater has been proposed as an interface for human–wildlife SARS‐CoV‐2 transmission (Franklin & Bevins, 2020; Nabi & Khan, 2020) and should be regarded as a biohazard for the spread of pathogens from WRCs into natural habitats.
2. CONSERVATION CONCERNS AND THE SARS‐COV‐2 INFECTION
Latin America holds 12 felidae (wild felids), 14 mustelidae (ferrets, badgers, weasels, otters), and 149 platyrrhine (new world monkeys) native species, which include 25 species currently classified as “endangered” (one felidae, four mustelidae, and 20 plathyrrhine; International Union for the Conservation of Nature—[IUCN], 2020). The former two families include species involved in natural and successful experimental infections (with post‐transmission to conspecifics), underlining the serious possibility that these Latin American species are susceptible to SARS‐CoV‐2 and may be infectious afterward. Although only minks have shown mortality due to SARS‐CoV‐2 (Enserink, 2020), and the findings are reported in farms with distinct conditions and species, we currently do not know the clinical outcomes, virulence, case fatality rate, and eventual chronic effects of this virus on Latin American felids and mustelids. In the case of platyrrhines, experimental infections with SARS‐CoV‐1 in the common marmoset (Callithrix jacchus) showed that these primates develop symptoms similar to the human disease (Carrion & Patterson, 2012); however, this species appears to be resistant to SARS‐CoV‐2 infection based on experimental conditions (Lu et al., 2020). The Platyrrhini parvorder is a very diverse taxonomic group, and further research is needed to disregard the possibility of SARS‐CoV‐2 infection of these Latin American primates and subsequent transmission if introduced into these populations.
The persistence of SARS‐CoV‐2 in ecological communities after its hypothetical introduction may be supported by the potential wide host spectrum of this virus. Experimental infections have reported that tree shrews (Tupaia belangeri; Soricidae) show viral replication and shedding capability without showing any clinical signs (Zhao et al., 2020). Syrian hamsters (Mesocricetus auratus; Critecidae) were also susceptible and infectious to conspecifics after experimentally exposed to SARS‐CoV‐2 (Chan et al., 2020; Sia et al., 2020). Latin America harbors 62 soricids (including shrews) and 508 cricetids (including new world mice, rats) (IUCN, 2020). The Sigmodon hispidus (forest species reported as a pest on palm plantations) could adapt to urban scenarios within the proximity of captive wildlife (González Campos, 2017) and supports SARS‐CoV‐2 infection within WRCs. The mustelid American mink, an original North American species, has successfully invaded the Patagonia region (Jaksic et al., 2002), while domestic cats are abundant across Latin America and have been detected as a potential source of pathogens for native felid species (e.g., Mora et al., 2015). The large populations of these invasive carnivores may act as an efficient long‐term reservoir of SARS‐CoV‐2 for endemic wildlife populations. Finally, susceptibility studies based on statistical modeling, artificial intelligence, in vitro cell culture infections, and in silico 3D structure modeling (ACE2‐SARS‐CoV‐2 spike protein interactions) have consistently included non‐human primates (especially of the Catarrhini parvorder), Chiropterans (particularly Rhinolophids), rodents, viverrids, and pholidots as potential SARS‐CoV‐2 hosts (e.g., Melin et al., 2020). If these findings are correct, several Neotropical species would be susceptible to the virus, which includes threatened and endangered species (e.g., jaguar, howler and spider monkey) and suggests that as the COVID‐19 pandemic escalates in humans, it may instigate a new threat to these vulnerable species (Damas et al., 2020).
The wide variability of potential host species for SARS‐CoV‐2 could favor the maintenance of the virus in host species of conservation interest (Haydon et al., 2002; Viana et al., 2014). The persistence of pathogens in reservoirs has been associated with their capacity to substantially reduce populations or even cause extinctions of susceptible species (De Castro & Bolker, 2005). A potential effect on wild carnivore populations (e.g., predators such as jaguars, pumas, and meso‐carnivores) can generate a negative effect on the trophic cascade with disruption of Latin American ecosystems, as a worst‐case scenario in the medium to long‐term. Moreover, we should be cautious under the potential evolutionary paths of this novel CoV, which, as its predecessor SARS‐CoV‐1, could develop adaptations or mechanisms that favor cross‐species transmission and spillover to naïve wild animal species (Decaro & Lorusso, 2020; Li, 2013; Olival et al., 2020).
3. RECOMMENDATIONS
As a result of the current knowledge on SARS‐CoV‐2 and past experience concerning emerging multi‐host pathogens, we suggest a review of admissions procedures for new animals to WRCs which include: application of a quarantine period in isolated premises prior to admission; testing of newly admitted individuals belonging to species potentially susceptible to SARS‐CoV‐2; implementing guidelines for testing wildlife for the virus, including assessment of species risk, case definition, biosafety; and that sample handling and storage have been provided as specified by the Office International des Epizooties (OIE) and the Center for Disease Control and Prevention (CDC) (CDC, 2020a, 2020b, 2021; OIE, 2020a).
To avoid the potential amplification of SARS‐CoV‐2 within the WRC premises, biosecurity is critical. Poor biosecurity in wildlife handling has led to outbreaks in these populations (Gray et al., 2018). Guidelines and recommendations to build biosecurity protocols have been developed by the International Wildlife Rehabilitation Council, Wildlife Health Australia, and the Australian Department of Agriculture, Fishing and Forestry, among others (Miller, 2012; Reiss & Woods, 2011; Wildlife Health Australia, 2018). These documents include information on adequate quarantine, use of personal protective equipment, disinfection, and disposal of biohazardous materials. Biosecurity considerations for the management of non‐domestic species under the current pandemic have been formulated by joint initiatives, such as the Zoo and Aquarium All Hazards Preparedness, Response, and Recovery Fusion Center (https://zahp.aza.org), OIE and others. To block zoonotic spread, the following should be applied: limit the number of personnel to the minimum necessary, maintain physical distance between personnel, identify and isolate symptomatic animals with clinical signs compatible to SARS‐CoV‐2 and report them to the local authorities, minimize the amount of time people are in contact with animals, wear clean dedicated clothing, gloves, masks, face covering, and footwear (especially with species considered particularly susceptible), and clean and disinfect all reusable equipment and utensils (CDC, 2021; OIE, 2020a, 2020b).
A safer release of wildlife back into their habitats must fulfill the recommendations for animal translocations suggested by the IUCN Conservation Translocation Specialist Group (IUCN, 2013), the implementation of a regional criteria for animal release developed with local wildlife authorities including a disease risk analysis following IUCN recommendations (Jakob‐Hoff, 2014), the guidelines for wildlife rehabilitations (Miller, 2012), and “the hierarchy of controls to reduce the risk of SARS‐CoV‐2” spreading between humans and wildlife recommended by CDC (2021). Guidelines for quarantine and health screening protocols prior to the release of wildlife have been developed by specialist groups (Woodford, 2000). The topics in this document also include treatments, vaccinations, and ethical considerations.
We believe that drafting similar documents in Spanish and Portuguese, or the translation of the previously mentioned sources into these languages is an urgent task for the Latin American community working in wildlife health, in addition to a multilevel communication plan led by expert associations. This plan should target the communication of information related to the pandemic progress and the status of investigations related to new reservoirs and biosafety recommendations. The audience should include all Latin American countries and local WRCs for adaptation to their operations (Figure 2).
FIGURE 2.
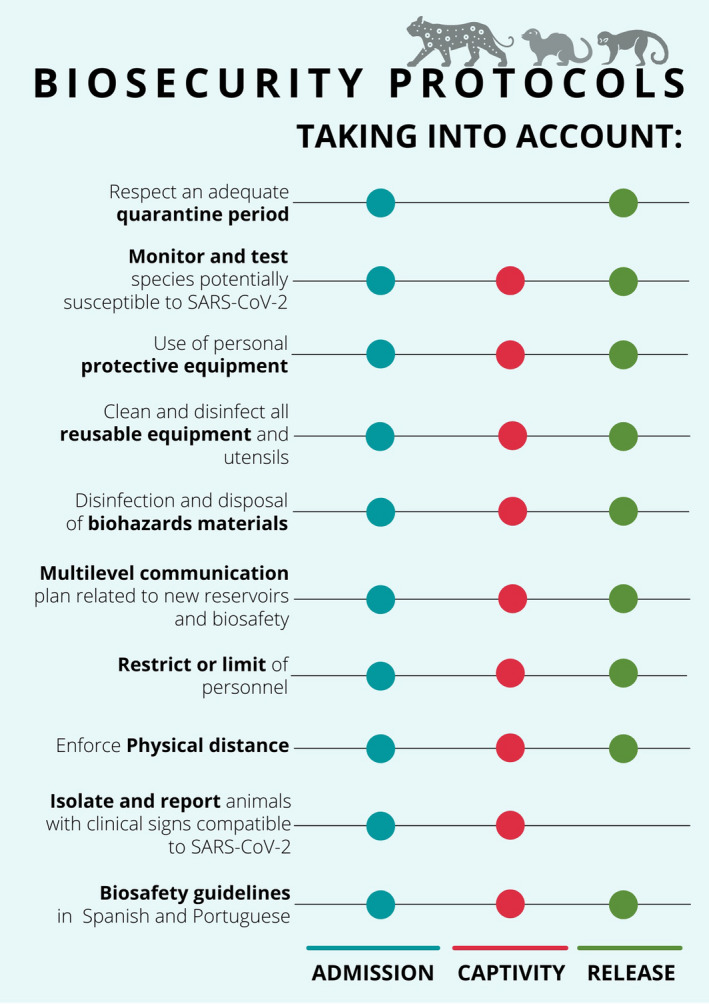
Biosecurity processes recommended for wildlife rehabilitation centers in Latin America to prevent entry, amplification, and dissemination of infectious agents such as SARS‐CoV‐2
Despite working directly with the authorities in charge of protecting wildlife and being a part of the solution for the protection and rescue of individuals who require ex‐situ recovery, the vast majority of WRCs in Latin America appear to not receive financial support or guidance from public institutions. Undoubtedly, WRCs have had unattended limitations much before the COVID‐19 pandemic, and this may be an opportunity to address this situation.
Some of the proposals to explore these issues may include (1) the promotion of a communication platform between WRCs, government, and academic institutions, (2) the integration and development of surveillance programs with Animal Health and Environmental Agencies, (3) the generation of training programs for local professionals related to WRCs (i.e., managers, veterinarians, biologists), (4) the access to public funds for research promotion through the integration of WRC research projects, (5) the negotiation of public fund initiatives to allocate resources proceeding from activities related to the use of nature, such as ecotourism, hunting, and extractive activities (i.e., mining, logging) to implement the rescue, release and monitoring programs, (6) it is worth considering the recommendation on animal testing from the CDC (2021) on integrating a collaborative effort with public and animal health officials using a One Health approach. This represents an example of the many initiatives to explore in order to tackle the hurdles faced by the WRCs in Latin America and minimize the risks associated to the transmission and dissemination of viruses like SARS‐CoV‐2 from anthropogenic to natural environments.
Chaves and Montecino‐Latorre contributed equally to this study.
Associate Editor: Jennifer Powers
Handling Editor: Jennifer Powers
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
REFERENCES
- Ahmed, W. , Angel, N. , Edson, J. , Bibby, K. , Bivins, A. , O'Brien, J. W. , Choi, P. M. , Kitajima, M. , Simpson, S. L. , Li, J. , Tscharke, B. , Verhagen, R. , Smith, W. J. M. , Zaugg, J. , Dierens, L. , Hugenholtz, P. , Thomas, K. V. , & Mueller, J. F. (2020). First confirmed detection of SARS‐CoV‐2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID‐19 in the community. Science of the Total Environment, 728, 138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco‐Lauth, A. M. , Hartwig, A. E. , Porter, S. M. , Gordy, P. W. , Nehring, M. , Byas, A. D. , VandeWoude, S. , Ragan, I. K. , Maison, R. M. , & Bowen, R. A. (2020). Experimental infection of domestic dogs and cats with SARS‐CoV‐2: Pathogenesis, transmission and response in cats. PNSA, 117(42), 26382–26388. 10.1073/pnas.2013102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion, R. Jr , & Patterson, J. L. (2012). An animal model that reflects human disease: The common marmoset (Callithrix jacchus). Current Opinion in Virology, 2, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2020a). Interim guidance for SARS‐CoV‐2 testing in North American wildlife. Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention . (2020b). Evaluation for SARS‐CoV‐2 testing in animals. Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention . (2021). Reducing the risk of SARS‐CoV‐2 spreading between people and wildlife. Centers for Disease Control and Prevention. [Google Scholar]
- Chan, J.‐F.‐W. , Zhang, A. , Yuan, S. , Poon, V.‐K.‐M. , Chan, C.‐C.‐S. , Lee, A.‐C.‐Y. , Chan, W.‐M. , Fan, Z. , Tsoi, H.‐W. , Wen, L. , Liang, R. (2020). Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID‐19) in golden Syrian hamster model: Implications for disease pathogenesis and transmissibility. Clinical Infectious Diseases, 71, 2428–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas, J. , Hughes, G. M. , Keough, K. C. , Painter, C. A. , Persky, N. S. , Corbo, M. , Hiller, M. , Koepfli, K.‐P. , Pfenning, A. R. , Zhao, H. , Genereux, D. P. , Swofford, R. , Pollard, K. S. , Ryder, O. A. , Nweeia, M. T. , Lindblad‐Toh, K. , Teeling, E. C. , Karlsson, E. K. , & Lewin, H. A. (2020). Broad host range of SARS‐CoV‐2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proceedings of the National Academy of Sciences 117(36), 22311–22322. 10.1073/pnas.2010146117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro, F. , & Bolker, B. (2005). Mechanisms of disease‐induced extinction. Ecology Letters, 8, 117–126. [Google Scholar]
- Decaro, N. , & Lorusso, A. (2020). Novel human coronavirus (SARS‐CoV‐2): A lesson from animal coronaviruses. Veterinary Microbiology, 244, 108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W. , Bao, L. , Liu, J. , Xiao, C. , Liu, J. , Xue, J. , Lv, Q. I. , Qi, F. , Gao, H. , Yu, P. , Xu, Y. , Qu, Y. , Li, F. , Xiang, Z. , Yu, H. , Gong, S. , Liu, M. , Wang, G. , Wang, S. , … Qin, C. (2020). Primary exposure to SARS‐CoV‐2 protects against reinfection in rhesus macaques. Science, 369(6505), 818–823. 10.1126/science.abc5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink, M. (2020). Coronavirus rips through Dutch mink farms, triggering culls. Science, 368, 1169. [DOI] [PubMed] [Google Scholar]
- Franklin, A. B. , & Bevins, S. N. (2020). Spillover of SARS‐CoV‐2 into novel wild hosts in North America: A conceptual model for perpetuation of the pathogen. Science of the Total Environment, 733, 139358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Campos, J. (2017). Caracterización y determinación de densidad poblacional de roedores plaga en el Parque Zoológico Minerva, Quetzaltenango, Guatemala, 2016. Universidad De San Carlos De Guatemala. [Google Scholar]
- Gray, M. J. , Spatz, J. A. , Carter, E. D. , Yarber, C. M. , Wilkes, R. P. , & Miller, D. L. (2018). Poor biosecurity could lead to disease outbreaks in animal populations. PLoS One, 13, e0193243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon, D. T. , Cleaveland, S. , Taylor, L. H. , & Laurenson, M. K. (2002). Identifying reservoirs of infection: A conceptual and practical challenge. Emerging Infectious Diseases, 8, 1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Union for the Conservation of Nature (2020). The IUCN Red List of Threatened Species.
- International Union for the Conservation of Nature. Species Survival Commission . (2013). Guidelines for reintroductions and other conservation translocations. International Union for the Conservation of Nature Species Survival Commission. [Google Scholar]
- Jakob‐Hoff, R. M. , MacDiarmid, S. C. , Lees, C. , Miller, P. S. , Travis, D. , & Kock, R. (2014). Manual of procedures for wildlife disease risk analysis. World Organisation for Animal Health. , 160 pp. Published in association with the International Union for Conservation of Nature and the Species Survival Commission. [Google Scholar]
- Jaksic, F. M. , Agustín Iriarte, J. , Jiménez, J. E. , & Martínez, D. (2002). Invaders without frontiers: Cross‐border Invasions of exotic mammals. Biological Invasions, 4, 157–173. [Google Scholar]
- Karesh, W. B. (1995). Wildlife rehabilitation: Additional considerations for developing countries. Journal of Zoo and Wildlife Medicine, 26, 2–9. [Google Scholar]
- La Rosa, G. , Iaconelli, M. , Mancini, P. , Bonanno Ferraro, G. , Veneri, C. , Bonadonna, L. , Lucentini, L. , & Suffredini, E. (2020). First detection of SARS‐CoV‐2 in untreated wastewaters in Italy. Science of the Total Environment, 736, 139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. (2013). Receptor recognition and cross‐species infections of SARS coronavirus. Antiviral Research, 100, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S. , Zhao, Y. , Yu, W. , Yang, Y. , Gao, J. , Wang, J. , Kuang, D. , Yang, M. , Yang, J. , Ma, C. , Xu, J. (2020). Comparison of non‐human primates indentified the suitable model for COVID‐19. Signal Transduction and Targeted Therapy, 5, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin, A. D. , Janiak, M. C. , Marrone, F. , Arora, P. S. , & Higham, J. P. (2020). Comparative ACE2 variation and primate COVID‐19 risk. Commun Biol, 3, 641. 10.1038/s42003-020-01370-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. (2012). Minimum standards for wildlife rehabilitation, 4e. National Wildlife Rehabilitators Association and International Wildlife Rehabilitation Council. [Google Scholar]
- Mora, M. , Napolitano, C. , Ortega, R. , Poulin, E. , & Pizarro‐Lucero, J. (2015). Feline immunodeficiency virus and feline leukemia virus infection in free‐ranging guignas (Leopardus guigna) and sympatric domestic cats in human perturbed landscapes on Chiloé Island, Chile. Journal of Wildlife Diseases, 51, 199–208. [DOI] [PubMed] [Google Scholar]
- Nabi, G. , & Khan, S. (2020). Risk of COVID‐19 pneumonia in aquatic mammals. Environmental Research, 188, 109732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office International des Epizooties ‐ OIE . (2020b). Guidelines for working with free‐ranging wild mammals in the era of the COVID‐19 pandemic.
- Office International des Epizooties ‐ OIE Preparedness and Resilience Department and the OIE ad hoc Group on COVID‐19 and the Human Animal Interface . (2020a). Considerations for sampling, testing, and reporting of SARS‐CoV‐2 in animals. World Organisation for Animal Health. [Google Scholar]
- Olival, K. J. , Cryan, P. M. , Amman, B. R. , Baric, R. S. , Blehert, D. S. , Brook, C. E. , Calisher, C. H. , Castle, K. T. , Coleman, J. T. H. , Daszak, P. , Epstein, J. H. , Field, H. , Frick, W. F. , Gilbert, A. T. , Hayman, D. T. S. , Ip, H. S. , Karesh, W. B. , Johnson, C. K. , Kading, R. C. , … Wang, L.‐F. (2020). Possibility for reverse zoonotic transmission of SARS‐CoV‐2 to free‐ranging wildlife: A case study of bats. PLoS Path, 16(9), e1008758. 10.1371/journal.ppat.1008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, S. W. X. , Tan, Y. K. , Chia, P. Y. , Lee, T. H. , Ng, O. T. , Wong, M. S. Y. , & Marimuthu, K. (2020). Air, surface environmental, and personal protective equipment contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) from a symptomatic patient. JAMA, 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova, N. , Molenaar, R. J. , Vreman, S. , Harders, F. , Oude Munnink, B. B. , Hakze‐van der Honing, R. W. , Gerhards, N. , Tolsma, P. , Bouwstra, R. , Sikkema, R. S. , Tacken, M. G. J. , de Rooij, M. M. T. , Weesendorp, E. , Engelsma, M. Y. , Bruschke, C. J. M. , Smit, L. A. M. , Koopmans, M. , van der Poel, W. H. M. , & Stegeman, A. (2020). SARS‐CoV‐2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance Weekly, 25, 2001005. 10.2807/1560-7917.ES.2020.25.23.2001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss, A. E. , & Woods, R. W. (2011). National zoo biosecurity manual. Department of Agriculture, Fishing and Forestry, Australia. [Google Scholar]
- Richard, M. , Kok, A. , de Meulder, D. , Bestebroer, T. M. , Lamers, M. M. , Okba, N. M. A. , Fentener van Vlissingen, M. , Rockx, B. , Haagmans, B. L. , Koopmans, M. P. G. , Fouchier, R. A. M. , & Herfst, S. (2020). SARS‐CoV‐2 is transmitted via contact and via the air between ferrets. Nature. Communications., 11(1), 1–6. 10.1038/s41467-020-17367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, F. , Espinoza, A. , Sallaberry‐Pincheira, N. , & Napolitano, C. (2019). A five‐year retrospective study on patterns of casuistry and insights on the current status of wildlife rescue and rehabilitation centers in Chile. Revista Chilena De Historia Natural, 92(1), 10.1186/s40693-019-0086-0 [DOI] [Google Scholar]
- Ruiz‐Arrondo, I. , Portillo, A. , Palomar, A. M. , Santibanez, S. , Santibanez, P. , Cervera, C. , & Oteo, J. A. (2020). Detection of SARS‐CoV‐2 in pets living with COVID‐19 owners diagnosed during the COVID‐19 lockdown in Spain: A case of an asymptomatic cat with SARS‐CoV‐2 in Europe. Transboundary and Emerging Diseases, 10.1111/tbed.13803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , Liu, R. , He, X. , Shuai, L. , Sun, Z. , Zhao, Y. , Liu, P. , Liang, L. , Cui, P. , Wang, J. , Zhang, X. , Guan, Y. , Tan, W. , Wu, G. , … Bu, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science, 368, 1016–1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia, S. F. , Yan, L.‐M. , Chin, A. W. H. , Fung, K. , Choy, K.‐T. , Wong, A. Y. L. , Kaewpreedee, P. , Perera, R. A. P. M. , Poon, L. L. M. , Nicholls, J. M. , Peiris, M. , & Yen, H.‐L. (2020). Pathogenesis and transmission of SARS‐CoV‐2 in golden hamsters. Nature, 583, 834–838. 10.1038/s41586-020-2342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stidworthy, M. (2016). Wildlife rescue and rehabilitation guidelines. The Veterinary Record, 179, 364. [DOI] [PubMed] [Google Scholar]
- Viana, M. , Mancy, R. , Biek, R. , Cleaveland, S. , Cross, P. C. , Lloyd‐Smith, J. O. , & Haydon, D. T. (2014). Assembling evidence for identifying reservoirs of infection. Trends in Ecology & Evolution, 29, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildlife Health Australia (2018) National wildlife biosecurity guidelines. Wildlife Health Australia. [Google Scholar]
- Woodford, M. H. (2000). Quarantine and health screening protocols for wildlife prior to translocation and release into the wild. IUCN Species Survival Commission’s Veterinary Specialist Group, Gland, Switzerland, the Office International des Epizooties (OIE), Paris, France, Care for the Wild. U.K., and the European Association of Zoo and Wildlife Veterinarians, Switzerland.
- Wu, F. , Zhang, J. , Xiao, A. , Gu, X. , Lee, W. L. , Armas, F. , Kauffman, K. , Hanage, W. , Matus, M. , Ghaeli, N. , Endo, N. , Duvallet, C. , Poyet, M. , Moniz, K. , Washburne, A. D. , Erickson, T. B. , Chai, P. R. , Thompson, J. , & Alm, E. J. (2020). SARS‐CoV‐2 titers in wastewater are higher than expected from clinically confirmed cases. American Society for Microbiology. 10.1128/mSystems.00614-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Wang, J. , Kuang, D. , Xu, J. , Yang, M. , Ma, C. , Zhao, S. , Li, J. , Long, H. , Ding, K. , Gao, J. , Liu, J. , Wang, H. , Li, H. , Yang, Y. , Yu, W. , Yang, J. , Zheng, Y. , Wu, D. , … Peng, X. (2020). Susceptibility of tree shrew to SARS‐CoV‐2 infection. Scientific Reports, 10, 16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


