Abstract
As a respiratory viral infection caused by a novel coronavirus, COVID‐19 became rapidly pandemic within a few months. Despite the wide range of manifestations and organ involvement in COVID‐19 patients, the exact pathogenesis of severe and fatal types of COVID‐19 and causes involved with the individual base of the disease is not yet understood. Several studies have reported clinical, laboratory, and histopathological data in favor of vascular injury in multiple organs of critically ill patients with COVID‐19 as a result of hyperactive immune response, inflammation, and cytokine storm. Also, both clinical and histopathological evidence points to such vascular involvements in the skin. Given the ease of clinical examinations and skin biopsy and the lower risks of transmission of COVID‐19 to healthcare workers, the present review article was conducted to investigate the vascular skin manifestations of COVID‐19 patients clinically and/or histopathologically as helpful clues for better understanding the pathogenesis and predicting the prognosis of the disease, especially in severe cases.
Keywords: coagulopathy, coronavirus, COVID‐19, cytokine, histopathology, inflammation, ischemia, necrosis, severe acute respiratory syndrome coronavirus 2 (SARS‐COV‐2), skin, skin biopsy cutaneous, skin manifestation, vascular injury, vasculitis, vasculopathy
1. INTRODUCTION
COVID‐19 is a newly discovered contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐COV‐2). In the late 2019, the disease was first reported in China as a new viral pneumonia with acute respiratory distress syndrome (ARDS)‐like changes in the lungs. Information about this disease has been gradually increasing. Recently, the studies show that other organs and systems are involved with it, including the kidney, heart, gastrointestinal tract, blood vessels, skin, and nervous system. 1
COVID‐19 disease has wide range of presentations, from the common cold to severe respiratory involvement. Given the involvement of many organs in this disease, and the effect of underlying diseases such as hypertension and diabetes as risk factors for the severe type of the disease, other mechanisms, such as different host immune responses and genetic factors, may be involved in the pathogenesis and severity of the disease in addition to the direct effect of the virus. 1 , 2 , 3
Histopathological studies of involved organs are one of the useful ways for understanding the underlying mechanisms and therapeutic approaches to a new unknown disease.
The skin can provide a window into internal changes of the body and mechanisms that are not readily visible. In many diseases, skin involvements are mutually related to systemic diseases; for instance, chronic inflammatory skin diseases such as psoriasis and lichen planus can increase the risk of metabolic syndrome and atherosclerosis. 4 , 5 Certain skin manifestations can suggest internal organ involvement and underlying systemic diseases such as neutrophilic dermatoses and autoimmune bullous diseases as markers for underlying malignancies, drugs, and collagen vascular diseases. 6 , 7 Moreover, cutaneous vascular lesions such as vasculitis or vasculopathic reaction may be seen because of the infections, drugs, rheumatologic disorders, genetic predisposition, coagulopathies, or internal malignancies. 8 , 9 The majority of these conditions can be diagnosed by examining or the skin sampling of cutaneous lesions.
Given the high transmission rate of COVID‐19 as a global pandemic and its high prevalence among healthcare workers, heavy burden in most of the affected communities 10 and unclear mechanism of the death, biopsy, autopsy, and examination of the internal involved organs, such as the lungs, kidneys, and heart, are both difficult and helpful in identifying the pathogenesis and mechanism of mortality of the disease. Given the accessibility of skin lesions and the simplicity of their examination, collecting biopsy specimens from these lesions and carefully examining them can provide valuable information about the pathogenesis, severity mechanisms, prognosis, and even therapeutic protocols of this new disease with lower risks.
However, in the COVID‐19 era, it is valuable to know the possible cutaneous changes caused by SARS‐COV‐2, either specific or non‐specific, because of the particular position of the skin as the most visible organ. It can be crucial from several standpoints.
Some non‐specific skin lesions, such as maculopapular exanthema or urticaria in asymptomatic patients or in patients with mild flu‐like symptoms in COVID‐19 time, may occur because of a mild SARS‐COV‐2 infection and, if left unknown, may cause further transmission.
On another note, although the exact association of severe cutaneous vascular lesions and disease severity is not clear in COVID‐19 patients, the onset of cutaneous vascular lesions in COVID‐19 patients, especially older patients or those with underlying diseases and potential risk factors, may portend poor prognosis requiring further attention and more aggressive treatment approaches with a focus on vascular complications.
In this review, we examine the clinical and histopathological changes associated with cutaneous manifestations of COVID‐19 with vascular components reported in the literature so far. Also, we discuss the possible mechanisms of these findings by evaluating evidence of vascular involvements in organs other than the skin in COVID‐19 and infection‐associated cutaneous vascular involvement.
2. MATERIALS AND METHODS
The present review study was carried out by searching databases, including PubMed, Scopus, Google Scholar, Web of Science, and Medline, for relevant evidence using keywords such as SARS‐COV‐2, COVID‐19, coronavirus‐related vascular injury, vasculitis, vasculopathy, coagulopathy, ischemia, necrosis, cutaneous manifestation, inflammation, and histopathological changes. Case reports, case series, and original and review articles about the histopathological or clinical assessment of the cutaneous manifestations of COVID‐19 with vascular components were included.
3. DISCUSSION
3.1. Cutaneous manifestations of COVID‐19 reflecting vascular injury
Different types of cutaneous manifestations of COVID‐19 have been reported so far. These manifestations can be classified into pseudo‐chilblain lesions, vesicular lesions, urticarial lesions, maculopapules, and livedo/necrosis. The association between different types of cutaneous involvement and the severity of COVID‐19 and the importance of them has not been clearly defined in studies.
Iran was one of the first countries affected by the COVID‐19 pandemic. Mucocutaneous manifestations observed in definite and suspected cases of COVID‐19 included papular or generalized urticaria, morbilliform rashes, petechiae, lesions resembling cherry angiomas, periorbital edema, conjunctivitis, strawberry tongue, painful swelling with erythema inthedistal upper extremities, such as Kawasaki disease, painful tongue ulcers and abrupt onset of small pruritic papulovesicular lesions progressing to purpura and healing within 4 to 5 days.
Here, we discuss some studies that have carried out clinical and pathological evaluations of cutaneous vascular manifestations in COVID‐19 patients. More studies have been listed in Table 1.
TABLE 1.
Studies on cutaneous vascular manifestations of COVID‐19
| Reference | Skin findings with vascular features | Pathological finding of vascular injury | Lab |
|---|---|---|---|
| Original articles | |||
| 57 | Livedoid lesions, perniosis, chilblain erythema, maculohemorrhagic rash | Vasodilation, endothelial swelling, perivascular infiltration of lymphocytes and eosinophils, microthrombosis, severe vascular damage, RBC extravasation | — |
| 16 | 19% showed pseudo‐chilblain, 6% showed livedo/necrosis | — | — |
| 58 | Chilblain of the toes (more common) and fingers with mild pain and pruritus, all patients had good prognosis without any complications. | Skin biopsy was performed in six patients. The findings include perivascular and perieccrine lymphocytic infiltration with vasculopathy changes, dermal edema, RBC extravasation and thrombosis limited to the papillary dermis. | Chemistry and coagulation tests were normal, only elevated d‐dimer in one patient without any complication. RT‐PCR was positive only in one case. |
| 59 | Swelling and edema of the toes, erythemato‐violaceous macules and purpuric lesions, in video capillaroscopy: pericapillary edema, abnormal shape and dimension, microhemorrhages and capillary dilation | Dermal edema, RBC extravasation, superficial, and deep perivascular and perieccrine lymphocytic infiltration, endothelial swelling, fibrin thrombus, granular deposition of c3 in the vessel wall in DIF study | Negative RT‐PCR test, negative IgG antibody against the nucleocapsid protein of SARS‐CoV‐2 but positive for antibody against S1 spike protein of virus in some patients, other blood test were normal |
| 60 |
Pernio‐like lesions (18%), retiform purpura (6.4%). Patients with pernio had better prognosis than patients with retiform purpura. |
— | — |
| 15 | Acro‐ischemia that presented as acral cyanosis, skin bulla, and progression to dry gangrene | — | High d‐dimer, fibrinogen and FDP in all cases, prolonged PT and DIC in four cases. d‐dimer and FDP levels progressively elevated consistent with COVID‐2019 exacerbation. |
| Review articles | |||
| 61 | Petechial lesions, retiform purpura, livedoid lesions, acro‐ischemia, chilblain‐like lesions, urticarial vasculitis, Kawasaki‐like lesions | Superficial and deep perivascular infiltration of lymphocytes, endothelial swelling, fibrinoid necrosis, vascular thrombosis, leukocytoclastic vasculitis, RBC extravasation | — |
| 62 | The most commonly reported skin finding was chilblain‐like lesions (400, 40.1%), followed by maculopapular lesions (230, 23.1%), vesicular lesions (101, 10.1%), urticarial lesions (87, 21.8%), livedoid/necrotic lesions (23, 2.3%), and other/non‐descript rashes/skin lesions (197, 19.8%). Pain/burning was reported in at least 96 cases, and itch was reported in at least 268 cases. | — | — |
| 63 | Erythematous, urticarial, and vesicular (chicken pox‐like or varicelliform), Pete‐chiae rash, livedo reticularis, reactivation of oral herpes simplex virus type 1 (HSV‐1), vascular lesions and peculiar (perniosis‐like) skin lesions | — | — |
| 64 | Vascular complications including acro‐ischemia, livedo‐like necrosis, chilblain‐like eruptions | Histopathological investigations in three patients who died from COVID‐19 revealed hyaline thrombi in microvessels of skin. | — |
| Case series | |||
| 65 | Chilblain‐like acral lesion | Necrotic keratinocytes, dermal edema, perivascular and perieccrine lymphocytic infiltration, endothelialitis, microthromboses, fibrin deposition, immunoreactant deposits on vessels | — |
| 66 | Chilblain acral lesions | RBC extravasation, dermal edema, lymphocytic vasculitis, fibrinoid necrosis, microthrombosis, superficial and deep perivascular and perieccrine infiltration of lymphocytes with extension to subcutis tissue, SARS‐COV2 spike protein in the endothelial cells of vessels and epithelial cells of eccrine glands | Normal CBC, normal coagulation test, minimally elevated d‐dimer in one patient |
| 67 | Petechial and purpuric lesions (7.7%), necrosis (7.7%), pernio (1.9%). Skin manifestations were most commonly seen in elder patients (>55 y) with comorbidities. | — | — |
| 68 | Pernio‐like lesions in 318 (63%) of patients, 9.2% with associated acrocyanosis | Perivascular lymphocytic infiltration without vasculitis in five patients, small vessel lymphocytic vasculitis without microthrombosis in one patient, lymphocytic vasculitis with thrombosis in one patient | — |
| Case reports | |||
| 69 | Transient livedo reticularis several days after onset of COVID‐19 | Not reported but they proposed microthrombosis as etiology of vascular cutaneous presentation of COVID‐19 | — |
| 70 | Fever, respiratory distress and skin mottling (like sepsis induced cutaneous changes) in a newborn patient from the symptomatic mother | — |
O2 sat: 93% CBC‐NL |
| 17 | Cutaneous features consistent with vaso‐occlusive or vasculopathicethiology, including retiform purpura with obvious inflammation, bulla formation and necrosis, chilblain‐like lesions in the acral sites or livedoid rash on the extremities in three cases | Histopathologic studies of the lung and skin of these patients indicated a microvascular injury with thrombosis and complement deposition in the vessel walls. Co‐localization of SARS‐CoV2‐specific spike glycoproteins with complement components in the lung and skin was also documented. | Mild thrombocytopenia (in two cases), high d‐dimer level (in three of five cases) |
| 14 | Erythematous‐violaceous lesions on the toes of a child with pruritus and burning, progressing to purpuric lesions with necrotic crust within a few days | — | — |
| Letter to Editor | |||
| 71 |
Maculopapular exanthems were the most frequent manifestation observed (10). Pseudo‐chilblain (9), palpable purpura (4), and livedo reticularis (1) |
Endothelial swelling, perivascular and periadnexal lymphocytic sometime neutrophilic infiltration, thrombosis | — |
| 72 | Retiform purpura with hemorrhagic blister and crust on the lower extremities, PTE during hospitalization | Thrombosis, deposition of IgM, C3, C9, and fibrinogen in the vessel wall | Elevated serum level of d‐dimer and acute phase reactant, leukopenia and after PTE, thrombocytopenia |
| 73 | Acro‐ischemia: purpuric lesions, hemorrhagic bullae and necrosis in the acrals | Small vessel vasculitis, RBC extravasation and neutrophil infiltration | Elevated CRP and d‐dimer level, leukocytosis with neutrophilia and lymphocytopenia, Plt and coagulation test were normal. |
| 12 | Petechial skin rash in one COVID‐19 patient mimicking other viral infections like dengue | — | — |
Key findings:
| |||
Abbreviations: CBC, complete blood count; CBC‐NL, complete blood count‐normal level; DIC, disseminated intravascular coagulation; DIF, differential; FDP, fibrinogen degradation product; PT, prothrombin time; PTE, pulmonary thromboembolism; RBC, red blood cells; RT‐PCR, reverse transcription polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
The first report of skin manifestations associated with COVID‐19 was published by Recalcati et al in Italy, included severe urticaria, chickenpox‐like vesicles, and erythematous rashes, mainly involving the trunk, was documented in 20.4% of the 88 COVID‐19 patients examined. They also found cutaneous lesions not to be correlated with the disease severity. 11
The other skin manifestations reported so far include petechiae, pernio‐like lesions, dengue‐like lesions, that is, petechial lesions with thrombocytopenia as a presenting sign and livedoid vascular eruption with hematuria. 12 , 13
Mazzotta et al reported the case of a 13‐year‐old boy suspected of COVID‐19 with erythematous‐violaceous lesions in the plantar surface of his toes that changed to purpuric and bullous lesions and finally healed within 2 weeks. They described these cutaneous symptoms as acute transient self‐healing acro‐ischemic lesions because of their benign course. 14
Zhang et al reported seven critical COVID‐19 patients with acro‐ischemia that emerged as acrocyanosis and bullae and progressed to dry gangrene. They reported high d‐dimer levels in all the patients and prolonged prothrombin time in four of them. According to the findings of this study, these types of cutaneous manifestations suggest hypercoagulopathy‐related mechanisms at least in severe cases of COVID‐19. 15
In an original study on 375 COVID‐19 cases, Casas et al reported five major patterns for COVID‐19 skin manifestations, two of which had vascular features, including pseudo‐chilblain lesions, with a 19% prevalence and more common in younger patients, and livedoid/ necrotic lesions, with a 6% prevalence and more common in older adults. About the association of these lesions with the severity of the disease, pseudo‐chilblain lesions were associated with a milder course of COVID‐19, and livedo/necrotic lesions with the severe form of the disease (10% mortality rate in the latter group). 16
The histopathologic and laboratory findings obtained by Magro et al in five patients with severe COVID‐19 were characterized by respiratory failure. In three of them, cutaneous features were consistent with vaso‐occlusion or vasculopathy, including retiform purpura with an obvious inflammation, bulla formation and necrosis, chilblain‐like lesions in the acral sites and livedoid rash on the extremities with markedly elevated d‐dimer levels. The histopathology of the lung and skin showed microvascular injuries with thrombosis and complement deposition in the vessel walls in these patients. The co‐localization of SARS‐COV‐2‐specific spike glycoproteins with complement components was also documented in the lung and skin. The authors therefore hypothesized that a generalized complement‐mediated thrombotic microvascular injury syndrome had developed at least in the severe cases of COVID‐19. They supported this hypothesis by reporting an increase in the dead space and the lack of response to the increase in pulmonary compliance in the patients with pulmonary involvement of COVID‐19, which was not justified by ARDS alone. 17
According to the studies mentioned above or shown in Table 1, we can categorize cutaneous vascular lesions into two main groups, including transient mild lesions and severe major lesions. Most of the lesions in the first group, such as pseudo‐chilblain and some types of livedo reticularis, are mild in COVID‐19 patients and not associated with systemic hematological disorders or may be because of transient non‐specific mechanisms, but lesions in the second group, such as necrotic lesions, hemorrhagic bullae, and gangrene, are rare and may be an indicator of systemic coagulopathic complications related to infections or a hyperactive immune system.
3.2. Mechanisms of cutaneous involvement in viral infections
The wide range of causes and underlying mechanisms of the skin involvement in viral infections can be categorized into three groups 18 , 19 :
Virus directly affecting the skin, for example, in papulovesicular or ulcerative lesions in human herpesviruses (HHV), poxvirus and parapoxvirus, measles, rubella, and verrucous lesions in human papillomavirus (HPV).
The host immune responses to the virus causing manifestations: Given the differences in immunological responses between different individuals and the effect of genetics on response type, these manifestations are not necessarily visible in all the individuals involved. The range of potential cutaneous manifestations include urticaria, erythema multiforme, erythema nodosum, Gianotti‐Crosti syndrome, non‐specific morbilliform eruptions, vasculitis, serum sickness‐like reaction, Kawasaki disease, necrolytic acral erythema, non‐specific cutaneous manifestations of viral hemorrhagic fever, pityriasis rosea, erythema annulare centrifugum, erosive lichen planus, and acute generalized exanthematous pustulosis.
Tumor proliferation caused or triggered by viruses such as Kaposi sarcoma because of HHV8, squamous cell carcinoma because of HPV and lympho‐proliferative disorders because of the Epstein‐Barr virus.
SARS‐COV‐2 enters and infects the host cells through the receptors of angiotensin‐converting enzyme 2 (ACE2), which is expressed in different body organs in humans, including the lung, heart, brain, skeletal muscles, kidney, intestine, smooth muscles, and endothelial cells. 20 , 21 , 22 The dysfunction of ACE2 as a protective negative regulator of cardiovascular function in the renin‐angiotensin system caused by intrinsic factors, such as genetic polymorphism, or extrinsic factors, such as infections, can cause vascular complications, including hypertension and thrombotic events. 23 , 24 , 25
Research suggests that the cutaneous involvements discussed in SARS‐COV‐2 infections have been observed in only about 25% of the patients. Some of these involvements are also common in other respiratory infections, such as urticaria, erythema multiforme, pityriasis rosea, and morbilliform eruptions. 11 Non‐invasive immune reactions to infections in vulnerable patients normally cause these non‐specific and non‐alarming lesions.
However, some other skin lesions, especially in people with more severe disease, can indicate a systemic and significant underlying reaction in these patients and may be associated with deterioration of the disease course. Therefore, accurate identification of these manifestations and evaluation of the underlying causes of them may be helpful in the survival of these patients. These manifestations, according to reports so far, include petechia and purpura, chilblain‐like lesions, acro‐ischemia, necrosis, and livedo reticularis all of which are indicative of a kind of vascular involvement such as coagulopathy and thrombotic thrombocytopenia, leukocytoclastic, lymphocytic, ANCA‐associated or septic vasculitis and vasculopathy, complement‐mediated vascular injury, cryoglobulinemia, antiphospholipid antibody syndrome, and vaso‐occlusion. 14 , 15 , 17 , 18 , 26 Some of these reactions are mild and transient while others are indicative of systemic reactions. Table 1 presents a list of further histopathological and clinical studies on the cutaneous manifestations associated with COVID‐19 with an emphasis on vascular involvements.
3.3. Extra cutaneous manifestations indicating vascular injury and thrombosis in COVID‐19
Both arterial and venous thrombotic events were reported in 31% of critically ill ICU patients with COVID‐19 despite receiving thromboprophylaxis. 27 The wide range of central or peripheral nervous system involvements, including agitation, confusion, disorientation, headache, dizziness, seizure, acute cerebrovascular attacks, perfusion disorders, anosmia, dysgeusia, neuralgia, and neuropathy, reported in critically ill COVID‐19 patients with a higher d‐dimer levels than non‐critical patients suggest cerebrovascular accidents in these patients. 28 Five COVID‐19 patients aged below 50 years also presented with a large‐vessel stroke. 29 Neurologic manifestations including this thrombotic event were also reported in association with the SARS and Middle East Respiratory Syndrome (MERS) outbreak with positive polymerase chain reaction (PCR) in cerebrospinal fluid analysis. 30 , 31 , 32 However, it was not positive for COVID in some studies. 33 , 34
Other systemic features of COVID‐19 patients include manifestations of kidney involvement, such as hematuria, proteinuria, and increased blood urea nitrogen and creatinine levels. Mortality is also higher in the patients with kidney involvement compared to the patients without evidence of kidney involvement. Despite the unclear mechanism of kidney injury in these patients, direct damage by the virus and immunological mechanisms have been reported as the potential causes. 2 , 35 , 36 Moreover, the viral inclusion bodies detected in the endothelial cells of the kidney in some COVID‐19 patients suggest vascular mechanisms. 22 Acute kidney injury and tubular necrosis were also reported in some SARS and MERS patients. 37 , 38 , 39
Some studies proposed that the ventilation‐perfusion mismatch in patients with COVID‐19 can be related to microvascular injuries. It can be explained as severe and resistant hypoxia was observed in these patients while it is not usually seen in the case of typical ARDS with the same therapeutic intervention. So, vascular injury can be a proposed etiology in this disorder. 17 , 40
3.4. Vascular mechanisms revealed by histopathology in COVID‐19 patients
In some patients with severe COVID‐19, histopathological examinations suggested vascular involvements such as endothelial injury, fibrinoid necrosis, microvascular thrombosis, red blood cell (RBC) extravasation, leukocytoclasia, infiltration of the inflammatory cells, including the neutrophils, monocytes, and lymphocytes, and complement deposition in the vessel walls of the skin, heart, kidney, and lung biopsies; however, there are conflicting results about the detection of SARS‐COV‐2 particles in these involved tissue, except for lung involvement. 17 , 41
In the autopsy of three patients with COVID, obvious endothelitis has been reported in various organs such as the lung, heart, kidney, liver, and intestines. Viral inclusion bodies were also detected in the endothelial cells of some involved organs, such as the kidneys. It has been proposed that this widespread endothelial involvement is caused by inflammatory cells recruitment because of direct virus stimulation or immune system response and induces generalized endothelial dysfunction, pro‐coagulation state, microcirculation disturbance, and ischemia, respectively. 22
Table 1 lists some histopathological evaluations of cutaneous COVID‐19 lesions indicating vascular injury.
3.5. The possible mechanisms of vascular injury in COVID‐19
-
Vasculitis and thromboembolic events: There appear to be relationships between inflammation, endothelial injury, and thrombosis. 5 , 42 Systemic vasculitis causes endothelial damage in the context of widespread inflammation in the body by activating the endothelial cells, increasing expressions of adhesive molecules and tissue factor and recruitment of the inflammatory cells. This diffuse endothelial damage induces thrombotic complications, caused by increasing thrombin and von Willebrand factor (VWF), decreasing thrombomodulin, protein C, and other anticoagulant factors production, which can finally lead to multiorgan damage. 43 , 44 , 45
Systemic vasculitis has different types such as leukocytoclastic, lymphocytic, ANCA‐associated, complement‐mediated, cryoglobulinemia, septic, and so on, and various causes such as autoimmune connective tissue diseases, malignancies, drugs, and infections. 9 , 18
A study on pathological changes in different organs in SARS infection found the main targets of virus to include the lungs, the immune system, and systemic small vessels. Moreover, histopathological examinations found systemic vasculitis to include edema, localized fibrinoid necrosis and infiltration of monocytes, lymphocytes and plasma cells into vessel walls in the heart, the lung, the liver, the kidney, the adrenal glands, the stroma of striated muscles, and thrombosis in small veins. 46 Maybe, these pathological changes in favor of vasculitis are also present in COVID‐19. 47
Infections and immunization are important factors in the pathogenesis of acute hemorrhagic edema of infancy as an abnormal form of cutaneous small vessel vasculitis, which usually affects children aged 4 to 24 months. Infection with human coronavirus NL63 comorbid with recurrent rash was reported in a child. 48
Vaso‐occlusion: After the stimulation of the immune system and the production of pro‐inflammatory cytokines, some infections increase thrombin production and cause anticoagulant pathway dysfunction. The thrombin released during the process also increases inflammation. This damaged cycle progresses to microthrombosis, diffuse intravascular coagulation, and multiorgan failure. Increased immune responses and cytokine storms were found to cause thrombotic disorders in critically ill COVID‐19 patients. This hypothesis was confirmed with the observation of high d‐dimer levels in most of these patients. 3 , 10 , 49
The expression polymorphisms of the genes involved in complement or coagulation pathway scan increase vulnerability to vascular thrombotic events; for instance, a mutation in the complement regulatory gene causes uncontrolled complement activation and lethal thrombotic events in catastrophic antiphospholipid syndrome. 50 , 51 The vascular deposition of the complement also plays a critical role in many thrombotic syndromes, and infections can activate the complement cascade. 52 , 53 , 54 As discussed earlier, complement deposition in the vessel walls of the lung and skin has been reported in some SARS‐COV‐2 patients. 17
Thrombocytopenia, prolonged prothrombin time and partial thromboplastin time, and elevated serum levels of d‐dimer, and antiphospholipid antibodies (anticardiolipin antibody, anti B2 glycoprotein) were derived from the laboratory data of three critical COVID‐19 patients with clinically significant coagulopathy (bilateral lower limbs and digital ischemia of the hand and multiple cerebral infarcts). It is recommended that further studies be conducted to confirm certain pathological roles of these antibodies in COVID‐19 patients. 26
Cutaneous manifestations are in favor of vascular injuries, vasculitis, and vasculopathies in the majority of critically ill COVID‐19 patients. There were also reports on the role of antithrombotic and anti‐inflammatory agents in treating COVID‐19. 55 , 56 The present review was therefore performed to summarize evidence for these features in the skin and internal organs and discuss its relevance and ultimately decide on the possibility of skin involvements for reflecting events in visceral tissues.
In contagious infections, the simplest way of tissue sampling can be vital in limiting the transmission of the disease to healthcare workers. 55 , 56 , 74 , 75 , 76
The authors of this study have concentrated on various aspects of COVID‐19, especially dermatologic concerns. 77 , 78 , 79 , 80 , 81 , 82 , 83 We believe that vascular injuries may be one of the key pathomechanisms of COVID‐19 in various vital organs; awareness of dermatologic manifestation may offer an approach to earlier diagnosis and more rapid therapeutic approaches toward similar systemic events.
Various types of vascular injuries of skin in our own tertiary center and in patients were admitted by COVID‐19 infection (Figures 1, 2, 3, 4, 5, 6). Because of certain condition of these patients, we were not able to histopalogically evaluate these vascular injuries that were obviously a COVID‐related dermatologic manifestation.
FIGURE 1.
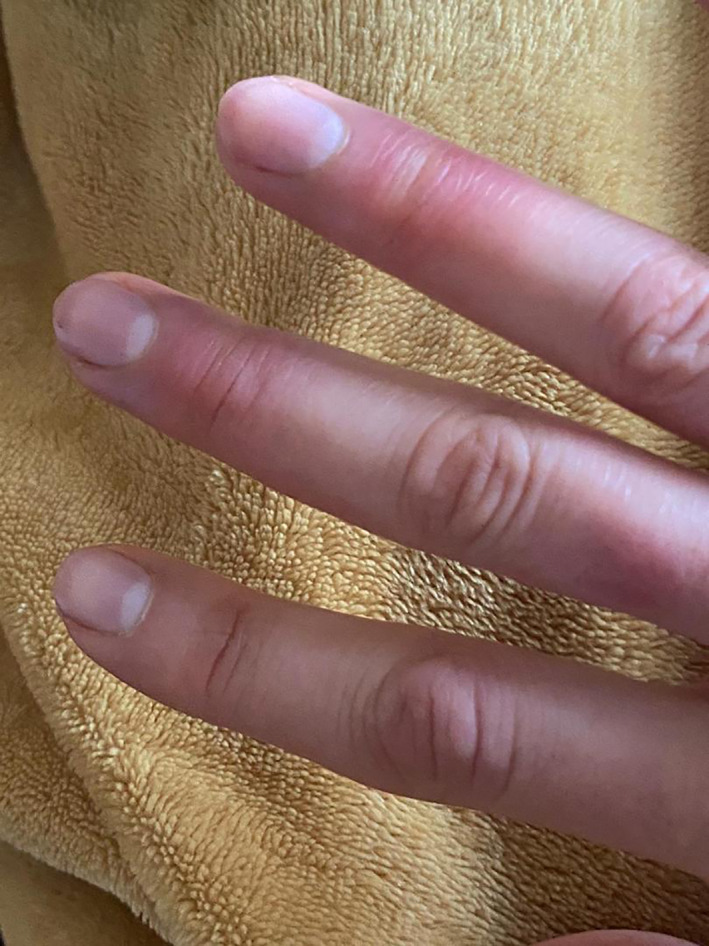
Acral pernio/chilblain‐like lesions
FIGURE 2.
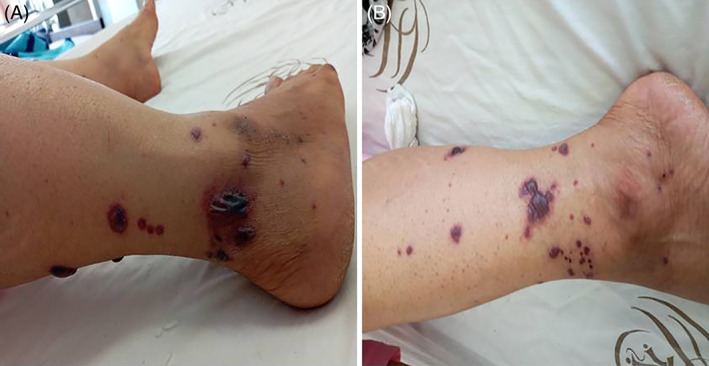
A,B, Diffuse vascular injury
FIGURE 3.
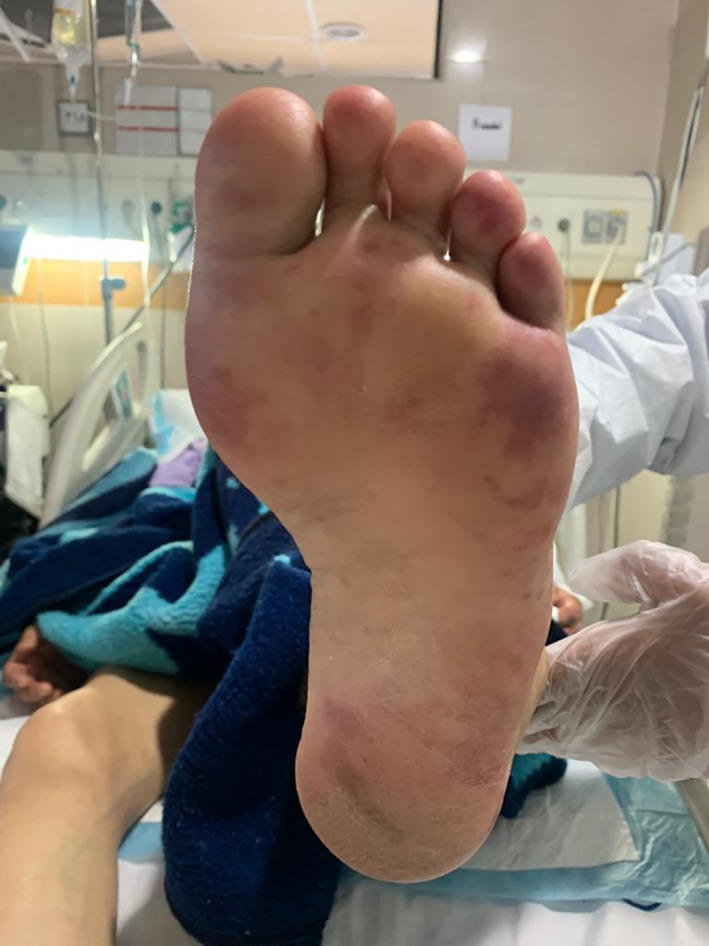
Acral vasculopathic lesions
FIGURE 4.
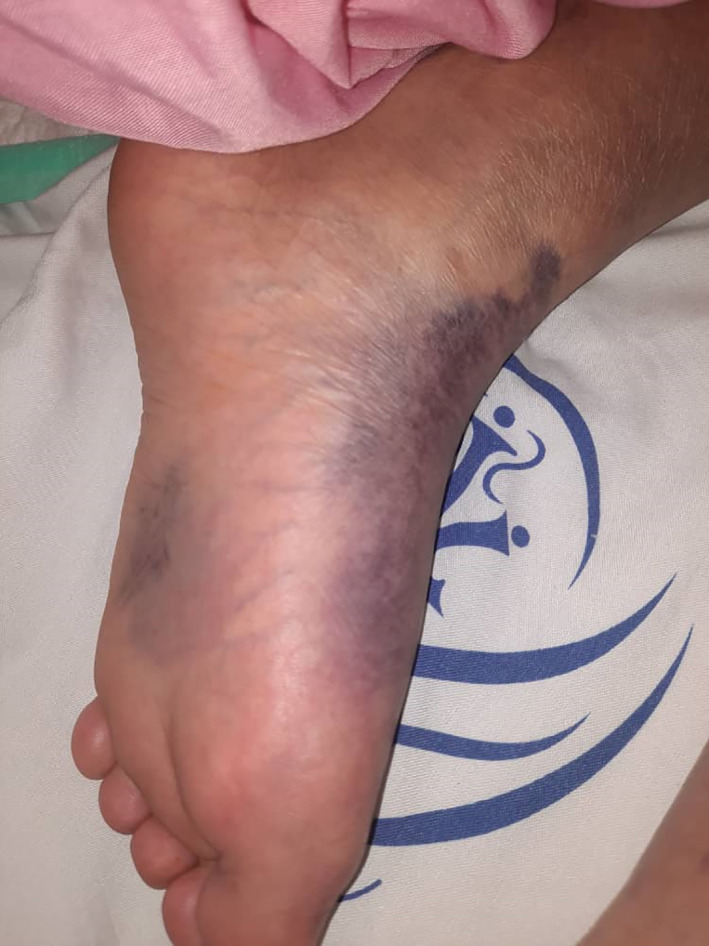
Acral vasulopathic lesions
FIGURE 5.
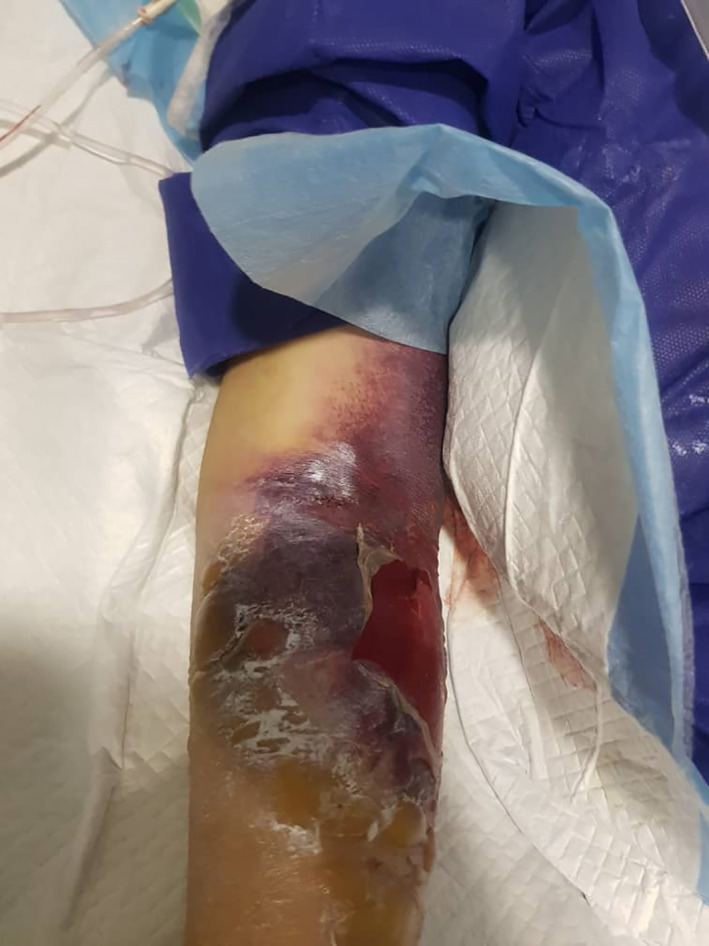
A patient in poor condition intensive care unit (ICU) admitted and features of dermatologic manifestation in disseminated intravascular coagulation (DIC) phase
FIGURE 6.
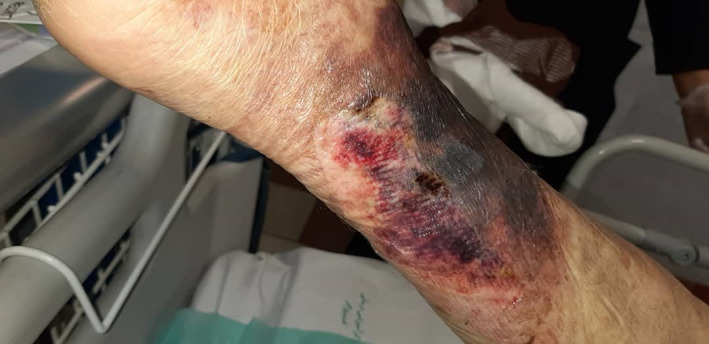
Another intensive care unit (ICU) admitted patient in disseminated intravascular coagulation (DIC) phase and hemorrhagic ulceronecrotic lesions
4. CONCLUSION
COVID‐19 is a new infectious disease with a wide range of signs, symptoms, and severities in different individuals. Contrary to the initial assumptions about this disease as only a severe lung infection, different manifestations emerged over time involving different organs in addition to the lungs, which can be explained by genetic and immunological differences among people in responding to infections coupled with direct viral damage. Given the specific conditions of this infection and its high transmission rate, its pathogenesis cannot be determined by performing simple evaluative procedures such as biopsies of internal organs. As an accessible body organ, the skin can be used as a prognostic and diagnostic guide in many systemic diseases such as coagulation dysfunction, inflammations, immunological disorders, and infections. Dermatologic signs might be the “first presenter” for COVID‐19, as the manifestations can occur before main signs of the disease. Given the reports about certain clinical cutaneous manifestations with a vascular pattern and pathological skin examinations in COVID disease, it may be helpful to pay attention to these symptoms and evaluate parameters such as coagulation test results, peripheral blood smear, complement levels, rheumatologic markers, cryoglobulin levels, antiphospholipid antibodies, and skin biopsy, especially in critically ill patients. Also, in addition to reporting the types of cutaneous manifestations witnessed, these studies should also pay attention to the role of these manifestations in the prediction of prognosis of COVID‐19 patients; for example, patients with pernio‐like lesions may have a better prognosis than patients with livedoid lesions and retiform purpura.
CONFLICT OF INTEREST
The authors declare no conflicts of interest with respect to the publication of this article.
AUTHOR CONTRIBUTIONS
The authors equally contributed to all stages of this study. All members of the research team reviewed the manuscript and the data and completely agreed on the research steps.
ACKNOWLEDGMENTS
We would like to show our gratitude to the Rasool Akram Medical Complex Clinical Research Development Center (RCRDC) for its technical and editorial assists. This study did not receive any funding.
Sadeghzadeh‐Bazargan A, Rezai M, Najar Nobari N, Mozafarpoor S, Goodarzi A. Skin manifestations as potential symptoms of diffuse vascular injury in critical COVID‐19 patients. J Cutan Pathol. 2021;48(10):1266–1276. 10.1111/cup.14059
DATA AVAILABILITY STATEMENT
The data of this study are available by the corresponding author on reasonable request.
REFERENCES
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Raspir Med. 2020;8(6):e46‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol. 2018;36(1):21‐28. [DOI] [PubMed] [Google Scholar]
- 5. Nasiri S, Sadeghzadeh‐Bazargan A, Robati R, et al. Subclinical atherosclerosis and cardiovascular markers in patients with lichen planus: a case–control study. Indian J Dermatol Venereol Leprol. 2019;85(2):138‐144. [DOI] [PubMed] [Google Scholar]
- 6. Nelson CA, Stephen S, Ashchyan HJ, James WD, Micheletti RG, Rosenbach M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dematol. 2018;79(6):987‐1006. [DOI] [PubMed] [Google Scholar]
- 7. Patel F, Wilken R, Patel FB, et al. Pathophysiology of autoimmune bullous diseases: nature versus nurture. Indian J Dermatol. 2017;62(3):262‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brogan P, Eleftheriou D. Vasculitis update: pathogenesis and biomarkers. Pediatr Nephrol. 2018;33(2):187‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharlala H, Adebajo A. Virus‐induced vasculitis. Cur Rheumatol Rep. 2008;10(6):449‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult in patients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 12. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otto MA. Skin manifestations are emerging in the coronavirus pandemic. The Hospitalist . https://www.the‐hospitalist.org/hospitalist/article/220183/coronavirus‐updates/skin‐manifestations‐are‐emerging‐coronavirus‐pandemic. Accessed April 3, 2020.
- 14. Mazzotta F, Troccoli T. Acute Acroischemia in the Child at the Time of COVID‐19. International Federation of Podiatrists. https://www.fipifp.org/wpcontent/uploads/2020/04/acroischemia-ENG.pdf. Accessed April 16, 2020 [Google Scholar]
- 15. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41(10):E006. [DOI] [PubMed] [Google Scholar]
- 16. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. China: Elsevier; 2018. [Google Scholar]
- 19. Drago F, Ciccarese G, Gasparini G, et al. Contemporary infectious exanthems: an update. Future Microbiol. 2017;12:171‐193. [DOI] [PubMed] [Google Scholar]
- 20. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus—a first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111(20):2605‐2610. [DOI] [PubMed] [Google Scholar]
- 22. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID‐19: the case for health‐care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crackower MA, Sarao R, Oudit GY, et al. Angiotensin‐converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822‐828. [DOI] [PubMed] [Google Scholar]
- 24. Clarke NE, Turner AJ. Angiotensin‐converting enzyme 2: the first decade. Int J Hypertens. 2012;2012:307315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monteil V, Kwon H, Prado P, et al. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell. 2020;181(4):905‐913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382(17):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kloka FA, Kruipb MJHA, Meerc NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Int J Stroke. 2018;13(6):612‐632.29786478 [Google Scholar]
- 28. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oxley TJ, Mocco J, Majidi S, et al. Large‐vessel stroke as a presenting feature of Covid‐19 in the young. N Engl J Med. 2020;382(20):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Umapathi T, Kor AC, Venketasubramanian N, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol. 2004;251(10):1227‐31221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marc D, Dominique JF, Élodie B, et al. Human Coronavirus: Respiratory Pathogens Revisited as Infectious Neuroinvasive, Neurotropic, and Neurovirulent Agents. CRC Press; 2013:93‐122. [Google Scholar]
- 32. Arabi YM, Balkhy HH, Hayden FG, et al. Middle East Respiratory Syndrome. N Engl J Med. 2017;376(6):584‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382(23):2268‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID‐19): encephalopathy. Cureus. 2020;12(3):e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lotfi B, Farshid S, Dadashzadeh N, et al. Is coronavirus disease 2019 (COVID‐19) associated with renal involvement? A review of century infection. Jundishapur J Microbiol. 2020;13(4):e102899. [Google Scholar]
- 36. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019‐nCoV patients. medRxiv. 2020. [Google Scholar]
- 37. Peiri JSM, Chu CM, Cheng VCC, et al. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767‐1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chu KH, Tsang WK, Tang CS, et al. Acute renal impairment in coronavirus‐associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid‐19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies. Chinese J Pathol. 2020;49(5):411‐417. [DOI] [PubMed] [Google Scholar]
- 42. Roumen‐Klappe EM, den Heijer M, van Uum SH, et al. Inflammatory response in the acute phase of deep vein thrombosis. J Vasc Surg. 2002;35(4):701‐706. [DOI] [PubMed] [Google Scholar]
- 43. Eleftheriou D, Ganesan V, Hong Y, Klein NJ, Brogan PA. Endothelial injury in childhood stroke with cerebral arteriopathy: a cross‐sectional study. Neurology. 2012;79(21):2089‐2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shah V, Christov G, Mukasa T, et al. Cardiovascular status after Kawasaki disease in the UK. Heart. 2015;101(20):1646‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tomasson G, Monach PA, Merkel PA. Thromboembolic disease in vasculitis. Curr Opin Rheumatol. 2009;21(1):41‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Samuel J, Green AJ, Josephson SA. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection. JAMA Neurol. 2020;77(6):679‐680. [DOI] [PubMed] [Google Scholar]
- 48. Chesser H, Chambliss JM, Zwemer E. Acute hemorrhagic edema of infancy after coronavirus infection with recurrent rash. Case Rep Pediatr. 2017;2017:5637503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chaturvedi S, Braunstein EM, Yuan X, Yu J, Alexander A, Chen H, Gavriilaki E, Alluri R, Streiff MB, Petri M, Crowther MA, McCrae KR, Brodsky RA Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood. 2020; 135(4): 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fremeaux‐Bacchi V, Fakhouri F, Garnier A, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8(4):554‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ruffatti A, Calligaro A, Lacognata CS, et al. Insights into the pathogenesis of catastrophic antiphospholipid syndrome. A case report of relapsing catastrophic antiphospholipid syndrome and review of the literature on ischemic colitis. Clin Rheumatol. 2020;39(4):1347‐1355. [DOI] [PubMed] [Google Scholar]
- 53. Perera TB, Murphy‐Lavoie HM. Purpura fulminans. Stat Pearls Publishing; 2020. http://www.ncbi.nlm.nih.gov/pubmed/30422460. Accessed March 28, 2020. [PubMed] [Google Scholar]
- 54. Uthman IW, Gharavi AE. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256‐263. [DOI] [PubMed] [Google Scholar]
- 55. Seirafianpour F, Sodagar S, Mohammad AP, et al. Cutaneous manifestations and considerations in COVID‐19 pandemic: a systematic review. Dermatol Ther. 2020;33(6):e13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seirafianpour F, Mozafarpoor S, Fattahi N, et al. Treatment of COVID‐19 with pentoxifylline: could it be a potential adjuvant therapy? Dermatol Ther. 2020;33(4):e13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gianotti R, Recalcati S, Fantini F, et al. Histopathological study of a broad spectrum of skin dermatoses in patients affected or highly suspected of infection by COVID‐19 in the northern part of Italy: analysis of the many faces of the viral‐induced skin diseases in previous and new reported cases. Am J Dermatopathol. 2020;42(8):564‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Andina D, Noguera‐Morel L, Bascuas‐Arribas M, et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatric Dermatol. 2020;37(3):406‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34(11):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Freeman E, McMahon D, Lipoff J, et al. The spectrum of COVID‐19‐associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kaya G, Kaya A, Saurat JH. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID‐19: review of the literature. Dermatopathology (Basel). 2020;7(1):3‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jia JL, Kamceva M, Rao S, et al. Cutaneous manifestations of COVID‐19: a preliminary review. J Am Acad Dermatol. 2020;83(2):687‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tang K, Wang Y, Zhang H, Zheng Q, Fang R, Sun Q. Cutaneous manifestations of the coronavirus disease 2019 (COVID‐19): a brief review. Dermatol Ther. 2020;33(4):e13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wollina U, Karadağ AS, Rowland‐Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID‐19 patients: a review. Dermatol Ther. 2020;33(5):13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain‐like acral lesions during the COVID‐19 pandemic (“COVID toes”): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020;83(3):870‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Colmenero I, Santonja C, Alonso‐Riaño M, et al. SARS‐CoV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Askin O, Altunkalem RN, Altinisik DD, et al. Cutaneous manifestations in hospitalized patients diagnosed as COVID‐19. Dermatol Ther. 2020;33(6):e13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Freeman E, McMahon D, Lipoff J, et al. Pernio‐like skin lesions associated with COVID‐19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: transient livedo reticularis. J Am Acad Dermatol. 2020;83(2):e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond). 2020;52(6):427‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rubio‐Muniz C, Puerta‐Peña M, Falkenhain‐López D, et al. The broad spectrum of dermatological manifestations in COVID‐19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol. 2020;34(10):e574‐e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bosch‐Amate X, Giavedoni P, Podlipnik S, et al. Retiform purpura as a dermatological sign of coronavirus disease 2019 (COVID‐19) coagulopathy. J Eur Acad Dermatol Venereol. 2020;34(10):e548‐e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Calvão J, Relvas M, Pinho A, Brinca A, Cardoso JC. Acro‐ischaemia and COVID‐19 infection: clinical and histopathological features. J Eur Acad Dermatol Venereol. 2020;34(11):e653‐e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Najar Nobari N, Montazer F, Seirafianpour F, Nikkhah F, Aryanian Z, Goodarzi A. Histopathological concordance between samples of lung, skin, and other internal organs in COVID‐19. J Cell Mol Anesth. 2021;6(1):81‐88. [Google Scholar]
- 75. Atefi NS, Behrangi E, Mozafarpoor S, Seirafianpour F, Peighambari S, Goodarzi A. N‐acetylcysteine and coronavirus disease 2019: may it work as a beneficial preventive and adjuvant therapy? A comprehensive review study. J Res Med Sci. 2020;25(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kooranifar S, Sadeghipour A, Riahi T, Goodarzi A, Tabrizi S, Davoody N. Histopathologic survey on lung necropsy specimens of 15 patients who died from COVID‐19: a large study from Iran with a high rate of anthracosis. Med J Islam Repub. 2021;35(1):481‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mohamadi MM, Goodarzi A, Aryannejad A, et al. Geriatric challenges in the new coronavirus disease‐19 (COVID‐19) pandemic: a systematic review. Med J Islam Repub Iran. 2020;34(1):841‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nobari NN, Goodarzi A. Patients with specific skin disorders who are affected by COVID‐19: what do experiences say about management strategies? A systematic review. Dermatol Ther. 2020;33(6):e13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sadeghzadeh‐Bazargan A, Behrangi E, Goodarzi A. Systemic retinoids in the COVID‐19 era – are they helpful, safe, or harmful? A comprehensive systematized review study. Iran J Dermatol. 2020;23(suppl 1):S9‐S12. [Google Scholar]
- 80. Sadeghzadeh‐Bazargan A, Behrangi E, Goodarzi A. Cytokine storm and probable role of immunoregulatory drugs in COVID‐19: a comprehensive review study. Iran J Dermatol. 2020;23(suppl 1):S13‐S18. [Google Scholar]
- 81. Najar Nobari N, Seirafianpour F, Mashayekhi F, Goodarzi A. A systematic review on treatment‐related mucocutaneous reactions in COVID‐19 patients. Dermatol Ther. 2020;34(1):e14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mohamadi M, Fattahi N, Goodarzi A, et al. A comprehensive review on COVID‐19 infection and comorbidities of various organs. Acta Med Iranic. 2021;59(1):4‐14. [Google Scholar]
- 83. Najar Nobari N, Seirafianpour F, Dodangeh M, Sadeghzadeh‐Bazargan A, Behrangi E, Mozafarpoor S, Goodarzi A. A systematic review of the histopathologic survey on skin biopsies in patients with Corona Virus Disease 2019 (COVID‐19) who developed virus or drug‐related mucocutaneous manifestations. Exp Dermatol. 2021. 10.1111/exd.14384. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available by the corresponding author on reasonable request.


