Abstract
COVID‐19 is a complex disease, and many difficulties are faced today especially in the proper choice of pharmacological treatments. The role of antiviral agents for COVID‐19 is still being investigated and evidence for immunomodulatory and anti‐inflammatory drugs is quite conflicting, whereas the use of corticosteroids is supported by robust evidence. The use of heparins in hospitalized critically ill patients is preferred over other anticoagulants. There are conflicting data on the use of convalescent plasma and vitamin D. According to the World Health Organization (WHO), many vaccines are in Phase III clinical trials, and some of them have already received marketing approval in European countries and in the United States. In conclusion, drug repurposing has represented the main approach recently used in the treatment of patients with COVID‐19. At this moment, analysis of efficacy and safety data of drugs and vaccines used in real‐life context is strongly needed.
LINKED ARTICLES
This article is part of a themed issue on The second wave: are we any closer to efficacious pharmacotherapy for COVID 19? (BJP 75th Anniversary). To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.10/issuetoc
Keywords: clinical research, covid‐19, repurposing drugs, review, vaccines
Abbreviations
- ARDS
acute respiratory distress syndrome
- CHMP
Committee for Medicinal Products for Human Use
- DIC
disseminated intravascular coagulation
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- ICU
intensive care unit
- LMWH
low MW heparin
- MAD
Monoclonal Antibody Discovery
- NSAIDs
non‐steroidal anti‐inflammatory drugs
- PRAC
Pharmacovigilance Risk Assessment Committee
- RdRp
RNA‐dependent RNA polymerase
- WHO
World Health Organization
1. INTRODUCTION
The vast majority of countries around the world are going through the second wave of COVID‐19 pandemic, which constitutes an imminent threat to society, in terms of both loss of human lives and devastating economic consequences. COVID‐19 is a complex disease with several clinical phases of progression, affecting many organs apart from the respiratory tract. Its progression seems to follow four main stages (Cordon‐Cardo et al., 2020). During the first stage, the SARS‐CoV‐2 binds to epithelial cells and starts its replication. The main proteins involved in the cell entry of SARS‐CoV‐2 are ACE2 receptors and TMPRSS2 (Fehr & Perlman, 2015; Hoffmann et al., 2020). Many patients can be asymptomatic at this stage, and the innate immune response is commonly limited. In the second stage, the virus migrates down the respiratory tract, leading to the occurrence of many symptoms, such as fever, cough, shortness of breath, fatigue, muscle pain, headache, loss of taste or smell, sore throat, nausea or vomiting, and diarrhoea (Liu et al., 2020). In this stage, the innate immune response is triggered and an increase in the level of CXCL10 or other innate response cytokine (the so‐called cytokine storm) is observed as well (Qian et al., 2013; Wang et al., 2011). The third stage is represented by a multisystem inflammation. Given the seriousness of symptoms (dyspnoea and hypoxia, ground glass infiltrate and progression to acute respiratory distress syndrome [ARDS], and possible cardiac, kidney and liver damage), patients frequently require hospitalization (Guo et al., 2020). In this phase, abnormal coagulation biomarkers can be detected and a generalized hyperinflammatory state is common (Del Valle et al., 2020; Leppkes et al., 2020). Lastly, a number of patients may reach the most critical and lethal stage (the last one), which is characterized by endothelial damage, thrombosis and multiorgan dysfunction (Nadkarni et al., 2020). The combinations of severe respiratory failure and multiorgan failure, acute neurological disease, and venous and arterial thromboembolic events contribute to the increase in mortality at this stage. In Figure 1, the main COVID‐19 symptoms, in terms of organs and tissues affected, are shown.
FIGURE 1.
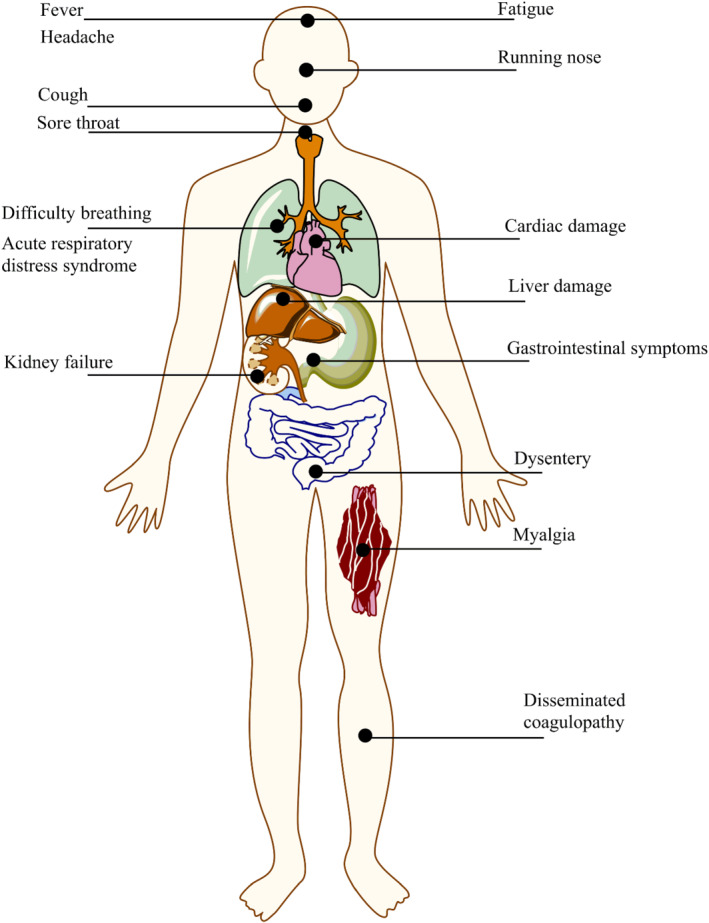
The main symptoms related to COVID‐19, as related to the organs and tissues affected
Recent studies have discussed the role of genetic errors and gene loci, mainly in IFN‐mediated antiviral signalling and chemokine‐mediated/inflammatory signalling, in life‐threatening COVID‐19 cases (McCoy et al., 2020) as well as the association between autoimmune diseases and COVID‐19‐related complications (Karaderi et al., 2020). In addition, multiple variants of SARS‐CoV‐2 are circulating globally: the variant B.1.1.7 from the United Kingdom; the variant B.1.351 from South Africa that shares some mutations with B.1.1.7; and the variant P.1 from Brazil. All these variants could spread more easily than other variants, which may lead to more cases of COVID‐19 (Centers for Disease Control and Prevention [CDC], 2021).
The clinical features of COVID‐19 and the interpatient variability in its progression demonstrate the complexity of this disease, but they also explain difficulties faced in the proper choice of pharmacological treatments. Last May, we reported an overview of the benefit/risk profile of pharmacological treatments used in patients suffering from COVID‐19 (Scavone et al., 2020). Considering new evidence recently acquired on the effect of different pharmacological treatments in patients with COVID‐19, in this paper, we aim to provide an up‐to‐date overview of medicines, including antivirals, anti‐inflammatory and immune‐modulating drugs, anticoagulants and other therapies that have been used around the world to treat COVID‐19 and related evidence in terms of efficacy profile from interventional and observational clinical studies. Readers should be aware that data from observational studies do not give highly valid data due to their intrinsic limitations, design and chosen pharmacological combinations. Safety warnings recently analysed by regulatory agencies were reported as well. Lastly, an update of vaccines under advanced clinical development is provided.
2. CLINICAL EVIDENCE OF ANTIVIRAL AGENTS
A large number of antiviral agents are currently being evaluated for COVID‐19. Figure 2 shows the main drugs evaluated for COVID‐19 and their mechanisms of action.
FIGURE 2.
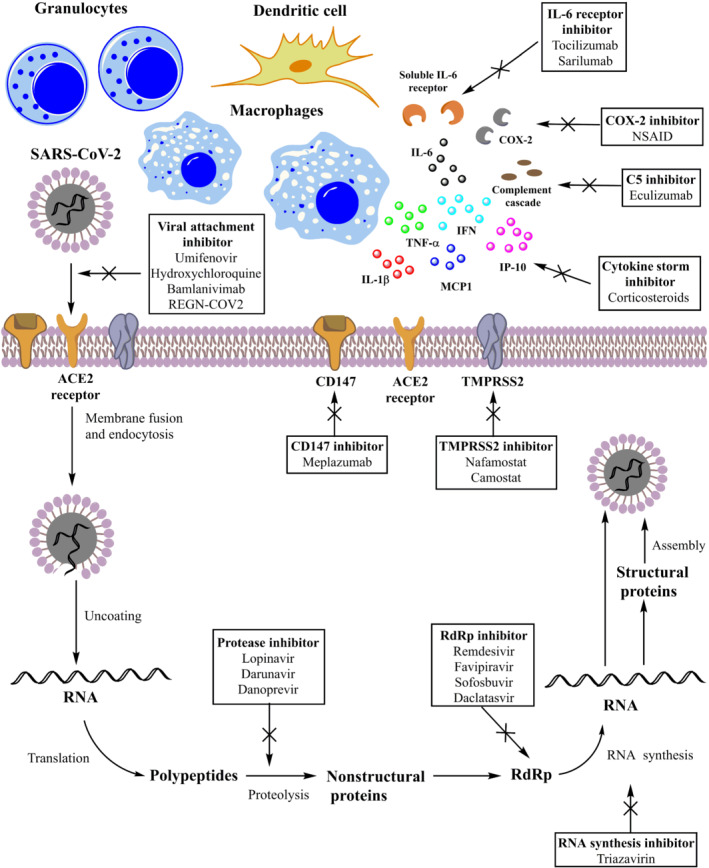
Main drugs evaluated for treatment of COVID‐19 and their probable mechanisms of action
Remdesivir is a nucleotide analogue that inhibits the RNA‐dependent RNA polymerase (RdRp), essential for viral RNA synthesis of MERS‐CoV, SARS‐CoV and SARS‐CoV‐2. A randomized, double‐blind, placebo‐controlled, multicentre trial, conducted on hospitalized adults with laboratory‐confirmed SARS‐CoV‐2 infection, showed no difference for the time to clinical improvement between the remdesivir and placebo groups (Wang, Zhang, et al., 2020). A randomized, open‐label trial, evaluating the efficacy of 5 or 10 days of remdesivir treatment compared with the standard care in hospitalized patients with confirmed severe acute respiratory syndrome and moderate COVID‐19 pneumonia, showed that patients treated with 10‐day course of remdesivir had no statistically significant difference in clinical status compared with standard care. On the contrary, those treated with 5‐day course of remdesivir had a statistically significant difference in clinical status, but the difference was defined as uncertain for clinical importance (Spinner et al., 2020). A double‐blind, randomized, placebo‐controlled trial (NIAID‐ACTT‐1) in adults hospitalized for COVID‐19 showed that remdesivir was superior to placebo in shortening the time to recovery. Specifically, patients receiving remdesivir had a median recovery time of 10 days compared with 15 days of patients receiving placebo (Beigel et al., 2020). Finally, a randomized, open‐label, Phase III trial on hospitalized patients with severe COVID‐19 not requiring mechanical ventilation, which were randomized to receive intravenous remdesivir for 5 or 10 days, showed no difference in terms of clinical status between the two groups (Goldman et al., 2020). A meta‐analysis of these clinical trials showed that the treatment with remdesivir for 10 days increased the recovery rate at Day 14 in severe COVID‐19 patients ([RR] = 1.5, 95% ; [CI] 1.33–1.7) and at Day 28 in moderate and severe COVID‐19 patients (RR = 1.14, 95% CI 1.06–1.22). Additionally, in all patients, remdesivir decreased the mortality rate at Day 14, but not at Day 28 (Elsawah et al., 2020). Based on the results of the NIAID‐ACTT‐1 trial, the European Medicines Agency (EMA) has granted a conditional marketing authorization to remdesivir for the treatment of adults and adolescents from 12 years of age with COVID‐19 pneumonia who require supplemental oxygen. Remdesivir is the first medicine that was recommended for COVID‐19 in Europe through a rolling review procedure. However, on 20 November 2020, the World Health Organization (WHO) has issued a conditional recommendation against the use of remdesivir in hospitalized patients with COVID‐19, regardless of disease severity, as there is no evidence demonstrating an improvement of survival or other outcomes during its use in these patients (WHO, n.d.). Currently, the Committee for Medicinal Products for Human Use (CHMP) of EMA is evaluating data on mortality at Day 28 derived from the NIAID‐ACTT‐1 trial. Moreover, the EMA has requested the full Solidarity data in order to assess if any changes are needed to the marketing authorization of remdesivir. In terms of safety, the EMA is also evaluating a signal for kidney toxicity (EMA, n.d.).
Lopinavir/ritonavir is a combination of lopinavir, a protease inhibitor with high specificity for HIV‐1 and HIV‐2, and ritonavir, an inhibitor of cytochrome P450, in order to increase lopinavir plasma concentration (Scavone et al., 2020). Proteases play a key role in the viral life cycle because they are responsible for the release of functional viral proteins. Therefore, their inhibition results in the production of immature virus particles (Uzunova et al., 2020). First results from randomized trials in patients with COVID‐19 did not show any benefit for this combination compared with the standard care alone (Cao, Wang, et al., 2020; Li, Xie, et al., 2020), In addition, the RECOVERY trial showed no reductions in 28‐day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death for the lopinavir/ritonavir group (Horby, Mafham, et al., 2020). A recent meta‐analysis showed no difference between the lopinavir/ritonavir combination and the standard of care in terms of progression to more severe state, mortality and virological cure on Days 7–10. Moreover, no difference in efficacy was observed with lopinavir/ritonavir compared with umifenovir or hydroxychloroquine (Bhattacharyya et al., 2020). Another meta‐analysis demonstrated no significant difference in terms of negative PCR results between patients treated with lopinavir/ritonavir and those treated with the standard care (Wang, Wu, et al., 2020). A retrospective study, carried out in non‐severe patients with COVID‐19, showed no improvement in the prognosis or shortening of clinical course with lopinavir/ritonavir treatment (Gao et al., 2020). On the contrary, a retrospective cohort study showed that the combined antiviral therapy (lopinavir/ritonavir plus umifenovir) is more effective than lopinavir/ritonavir monotherapy (Deng et al., 2020). Based on the available evidence, the regular use of lopinavir/ritonavir in the treatment of COVID‐19 cannot be supported and further clinical trials are needed.
Favipiravir is a drug authorized for the treatment of influenza virus infections in Japan. It is a prodrug converted into the active form able to inhibit the RdRp, essential for viral replication. A randomized clinical trial, comparing the efficacy and safety of favipiravir with that of umifenovir in hospitalized patients with COVID‐19, demonstrated a higher efficacy for favipiravir than umifenovir (P = .01). The most commonly reported adverse events were liver enzyme abnormalities, psychiatric disorders, gastrointestinal symptoms and serum uric acid elevations. (Chen, Zhang, et al., 2020). An open‐label study, evaluating the effects of favipiravir or lopinavir/ritonavir in patients with COVID‐19 who were also treated with aerosol inhalation of IFN‐α, showed a faster viral clearance and a better chest computed tomography (CT) change for the favipiravir group (Cai et al., 2020). On the contrary, the combination of favipiravir and inhaled IFN‐β‐1b showed no difference for inflammatory biomarkers at hospital discharge and for the overall length of hospital stay when compared with hydroxychloroquine (Khamis et al., 2020). Another study failed in demonstrating a virological effect and clinical benefits of baloxavir marboxil and favipiravir. Authors concluded that this result could be determined by the insufficient concentrations of these drugs relative to their antiviral activities (Lou et al., 2020). An adaptive, multicentre, Phase II/III clinical trial of favipiravir compared with standard of care in hospitalized patients with moderate COVID‐19 pneumonia demonstrated a rapid antiviral response with favipiravir (Ivashchenko et al., 2020). Based on these preliminary results, the Russian Ministry of Health granted, in May 2020, a fast‐track marketing authorization to favipiravir for the treatment of COVID‐19 patients. Currently, two clinical trials are ongoing to evaluate the efficacy and safety of favipiravir alone (NCT04336904) or in combination with tocilizumab (NCT04310228) for the treatment of COVID‐19. Based on the available evidence, favipiravir is effective in alleviating symptoms and in the clinical improvement of COVID‐19 patients, but further studies are needed to prove its benefit in terms of viral clearance, oxygen support requirement and mortality.
Darunavir is an inhibitor of dimerization and of the HIV‐1 protease, whereas cobicistat is an inhibitor of cytochromes P450 that increases plasma concentrations of darunavir (Deeks, 2018). At present, there are conflicting in vitro data on the effect of darunavir in inhibiting SARS‐CoV‐2 viral replication (Alshaeri & Natto, 2020; De Meyer et al., 2020). Results from a single‐centre, randomized and open‐label trial of darunavir/cobicistat plus IFN‐α‐2b, compared with IFN alpha‐2b alone, in COVID‐19 patients showed no difference in the proportion of negative PCR results at Day 7 between the two groups (Chen, Liu, et al., 2020). Nicolini et al. (2020) reported the results on the real‐life use of darunavir/cobicistat in severe COVID‐19 patients. Their findings showed that, although well tolerated, this treatment did not reduce mortality in COVID‐19. On the contrary, a case–control study showed a lower mortality for darunavir/cobicistat group than the control group (odds ratio [OR] 0.07, 95% CI 0.01–0.52, P = .009) in critically ill patients with SARS‐CoV‐2 infection (Kim et al., 2020).
Sofosbuvir and daclatasvir are direct‐acting antivirals that represent potential candidates for the treatment of COVID‐19. A trial, evaluating the effectiveness of sofosbuvir and daclatasvir compared with ribavirin in patients with severe COVID‐19, showed an RR of death of 0.17 (95% CI 0.04–0.73, P = .02) for the sofosbuvir/daclatasvir group (Eslami et al., 2020). Similarly, a randomized controlled clinical trial conducted in adults with moderate or severe COVID‐19 showed that the group treated with sofosbuvir/daclatasvir plus standard care had a significant reduction of the duration of hospital stay compared with standard care alone (Sadeghi et al., 2020). A single‐centre, randomized, controlled trial of adults with moderate COVID‐19, comparing the treatment with sofosbuvir, daclatasvir and ribavirin to the standard care, demonstrated instead no difference in terms of median duration of hospital stay, number of intensive care unit (ICU) admissions and the number of deaths between groups, but the cumulative incidence of recovery was higher in the sofosbuvir/daclatasvir/ribavirin group (Abbaspour Kasgari et al., 2020). Further investigations in larger clinical trials are needed.
Nafamostat and camostat mesilate are inhibitors of TMPRSS211, a protease essential for the penetration of coronaviruses into the cell (Mascolo et al., 2020). First evidence showed a clinical improvement with the use of nafamostat and hydroxychloroquine, or nafamostat and favipiravir in severe COVID‐19 patients (Doi et al., 2020; Iwasaka et al., 2020). Moreover, nafamostat was also effective in three cases of elderly patients with COVID‐19 pneumonia. Both drugs are currently being evaluated in different clinical trials (www.clinicaltrials.gov).
Ivermectin, a drug used for parasite infestations, has the ability to reduce, in vitro, the viral RNA of SARS‐CoV‐2. The ICON study, a chart review of hospitalized patients with confirmed COVID‐19 treated with or without ivermectin, showed a lower mortality in the ivermectin group, but no difference was found for the extubation rates or length of stay (Rajter et al., 2020). Another retrospective study conducted in hospitalized patients showed that a single dose of ivermectin (200 μg·kg−1) did not improve clinical and microbiological outcomes of patients with severe COVID‐19 (Camprubí et al., 2020). Preliminary results from a randomized, controlled, Phase III, clinical trials, showed the efficacy of the combination of ivermectin and doxycycline, compared with placebo (NCT04523831).
Finally, monoclonal antibodies (Figure 2), such as meplazumab, REGN‐COV2 and bamlanivimab, have been developed to prevent the viral attachment of SARS‐CoV‐2 to host cells.
Meplazumab is an inhibitor of CD147, a glycoprotein involved in the viral entry of SARS‐CoV‐2 by interacting with the coronavirus S protein. CD147 has also a pro‐inflammatory activity and regulates cytokine secretion and leukocyte chemotaxis during viral infections. Preliminary results showed that meplazumab compared with the control group was associated with a faster improvement of pneumonia (Bian et al., 2020). Two clinical trials are ongoing to evaluate the safety and efficacy of meplazumab in patients with COVID‐19 (NCT04275245 and NCT04586153).
REGN‐COV2 is a cocktail of two monoclonal antibodies (casirivimab and imdevimab) targeting the spike protein of SARS‐CoV‐2. First descriptive data showed that REGN‐COV2 is able to reduce the viral load and the time to alleviate symptoms in non‐hospitalized patients with COVID‐19. REGN‐COV2 also showed positive trends in reducing medical visits (Regeneron Pharmaceuticals, Inc., n.d.). Clinical trials are currently ongoing to evaluate its efficacy and safety for the treatment (NCT04425629, NCT04426695 and NCT04381936) or prevention (NCT04452318) of COVID‐19. On 21 November 2020, the US Food and Drug Administration (FDA) granted an emergency authorization for REGN‐COV2 for the treatment of mild to moderate COVID‐19 in patients aged ≥12 years and weighing at least 40 kg and at high risk for progressing to severe COVID‐19. This approval is based on data from a clinical trial demonstrating a reduction of COVID‐19‐related hospitalization or emergency room visits within 28 days after treatment in patients at high risk for disease progression (FDA, n.d.‐b).
Bamlanivimab is a recombinant, human IgG1 monoclonal antibody directed against the spike protein of SARS‐CoV‐2. A Phase I study of bamlanivimab in hospitalized patients with COVID‐19 was successfully completed (Eli Lilly and Company, n.d.), and an interim analysis of an ongoing Phase II clinical trial in outpatients with mild or moderate COVID‐19 (BLAZE‐1) showed that one of three doses of bamlanivimab accelerates the natural decline in viral load over time (Chen, Nirula, et al., 2020). Based on these results, on 9 November 2020, bamlanivimab was authorized by the FDA for the treatment of mild to moderate COVID‐19 in adults and paediatric patients (≥12 years), who are at high risk for progressing to severe COVID‐19 or hospitalization. Bamlanivimab is recommended to be administered as soon as possible after a positive COVID‐19 test and within 10 days of symptom onset. Currently, bamlanivimab is being evaluated in a Phase III clinical trial assessing the prevention of COVID‐19 in residents and staff at long‐term care facilities (BLAZE‐2 and NCT04497987) and in the National Institutes of Health (NIH)‐led ACTIV‐2 study in ambulatory COVID‐19 patients. In a press release, Lilly (2021) recently announced that bamlanivimab prevented COVID‐19 at nursing homes in the BLAZE‐2 trial, reducing risk by up to 80% for residents. On 9 February 2021, the FDA issued an emergency use authorization for bamlanivimab and etesevimab, another monoclonal antibody that is specifically directed against the spike protein of SARS‐CoV‐2, administered together for the treatment of mild to moderate COVID‐19 in adults and paediatric patients who are at high risk for progressing to severe COVID‐19 (FDA, n.d.‐a). Lastly, a new monoclonal antibody is currently under early stage of development in Italy at the Monoclonal Antibody Discovery (MAD) Lab of the Fondazione Toscana Life Sciences. This investigational therapy was obtained starting from convalescent plasma. At this moment, researchers found that the antibody is able to bind the spike protein and disable the virus. Thus, this therapy could serve to both prevent and treat COVID‐19 (The Florentine, 2020).
Regarding monoclonal antibodies' safety profile, allergic and infusion‐related reactions—resulting from the activation of the immune system in response to the antibody— were frequently observed. Their symptoms might include flushing, itching, shortness of breath or low BP (Lloyd et al., 2021).
In conclusion, the role of antiviral agents for COVID‐19 is still being investigated. Remdesivir is the only one recommended in Europe for COVID‐19, but it was also recently questioned for efficacy and safety and is evaluated by the EMA. Regarding monoclonal antibodies, recent evidence suggests that these drugs are effective in patients at early stage of the disease, preventing the progression of COVID‐19 and reducing the morbidity and mortality of infection and the frequency of hospitalizations (Cohen, 2021). The cost of these drugs might represent a challenge for healthcare systems. In the United States, for instance, the doses will be free of charge, even though some patients might have to pay for them. In November 2020, the federal government waived co‐payments for the cost of administering the treatment for people covered by Medicare (The New York Times, 2021).
3. CLINICAL EVIDENCE OF IMMUNOMODULATORY AND ANTI‐INFLAMMATORY AGENTS
Since the beginning of the COVID‐19 pandemic, the determining role of inflammatory cytokines in the worsening of clinical conditions has been identified. Indeed, one of the reasons underlying the occurrence of serious symptoms (Figure 1) is represented by the increase in levels of pro‐inflammatory cytokines. These mediators are responsible for the so‐called cytokine storm that, in turn, induces ARDS, organ failure and sepsis (Guo et al., 2020). Many drugs, acting through different mechanisms, are able to block or reduce the effects mediated by the cytokine storm (Figure 2).
Tocilizumab is a monoclonal antibody (Figure 2), authorized for the treatment of many diseases, including rheumatoid arthritis, which is active against IL‐6 receptors, one of the key mediators of the inflammatory process (Scott, 2017). At the beginning of the pandemic, the drug was tested in China to reduce lung complications in 20 patients with severe SARS‐CoV‐2 infection, being associated with a reduction of oxygen requirement, resolution of CT lesions, normalization of lymphocyte count, reduction of C‐reactive protein levels and hospital discharge (Xu et al., 2020). A few months ago, many trials evaluating the effects of tocilizumab started in Italy. However, the availability of new evidence from ongoing clinical trials has led to a change of course. Indeed, the study carried out by the Local Health Unit‐IRCCS of Reggio Emilia was stopped early, after the enrolment of 126 patients with COVID‐19 pneumonia who did not require invasive or semi‐invasive mechanical ventilation procedures. The reason for the early conclusion lies in the absence of benefit in treated patients in terms of either worsening (access to the ICU) or survival (Italian Medicines Agency, 2020a). Similarly, the results of the COVACTA trial did not reveal a benefit of tocilizumab over placebo in patients with severe COVID‐19 pneumonia. Indeed, no differences in clinical status between patients treated with tocilizumab and placebo were found. In addition, no differences between groups were found regarding the percentage of patients who died by Week 4 and in ventilator‐free days (Roche, 2020). Despite the negative results of these trials, tocilizumab was associated with positive outcome in other clinical studies. For instance, the single‐arm trial TOVICID‐19 evaluated the effects of tocilizumab in 180 hospitalized patients with SARS‐CoV‐2 infection, oxygen saturation at rest ≤ 93% or requiring oxygen support or mechanical ventilation. The results revealed that the treatment with tocilizumab reduced lethality at 30 days, although its effects at 14 days was less relevant (Perrone et al., 2020). Lewis et al. reported the results of an observational cohort study of hospitalized patients who received tocilizumab 400 mg intravenously once in addition to standard of care or standard of care alone. Out of 3580 patients, 497 were treated with tocilizumab. The propensity score analysis showed that a lower number of deaths occurred in tocilizumab‐treated patients and that tocilizumab was associated with improved survival compared with controls (P > .001). No differences were found between groups in term of adjusted time to hospital discharge, whereas patients treated with tocilizumab had longer adjusted ICU length of stay and a higher adjusted infection rate (both p > .001) than controls. It was unclear whether the infections could have contributed to prolonged stay in the ICU in patients receiving tocilizumab or not (Lewis et al., 2020). Tleyjeh et al. carried out a systematic review and meta‐analysis of 24 studies, including five randomized controlled trials and 19 observational studies. The results demonstrated that tocilizumab is able to reduce the risk of mechanical ventilation in hospitalized COVID‐19 patients but not the short‐term mortality, at least according to the results of randomized controlled trials. On the contrary, data from observational studies suggested an association between tocilizumab and lower mortality. In terms of safety profile, no higher risk of infections or any other adverse events was found with tocilizumab use (Tleyjeh et al., 2020). Given the conflicting evidence currently available on the effects of tocilizumab in COVID‐19 patients, no firm conclusion can be drawn. We should wait for the results of other studies, such those from the RECOVERY trial (University of Oxford, 2020b). Furthermore, biochemical parameters like serum IL‐6 levels and TH17 cells may contribute to select subgroups of COVID‐19 patients that would specifically benefit from the treatment with tocilizumab (Cacciapuoti et al., 2020).
Conflicting results were also obtained for sarilumab (Figure 2), another antibody belonging to the same class as tocilizumab. Benucci et al. evaluated the efficacy of sarilumab in eight hospitalized patients (mean age: 62 years). The drug was administered in a dose of 400 mg in combination with hydroxychloroquine, azithromycin, darunavir, cobicistat and enoxaparin. Seven patients had an improvement of the Horowitz index (oxygenation expressed by an increased SpO2/FiO2 ratio). A progressive reduction in the serum amyloid A and CRP inflammation parameters was observed, and patients were discharged within 14 days of hospitalization (Benucci et al., 2020). Another study evaluated the effects of sarilumab in 53 patients with severe COVID‐19. Almost 70% of patients were also treated with darunavir/ritonavir, whereas 94% were received also hydroxychloroquine. Thirty‐nine patients were treated in medical wards, whereas 14 in ICU. Among patients who received the drug in the medical wards, at 19‐day median follow‐up, almost 90% significantly improved. Among patients who received sarilumab in ICU, almost 36% were still alive at the last follow‐up. The overall mortality rate was 5.7% (Gremese et al., 2020). Data from a further retrospective study, which was carried in 15 hospitalized patients, demonstrated that improvements in respiratory parameters were observed in 10 patients after sarilumab administration. Five patients who received sarilumab died (Montesarchio et al., 2020). Overall, the majority of clinical studies carried out on sarilumab were observational and limited by a low number of patients. In addition, many other drugs were coadministered. Thus, also for this drug, further clinical data are strongly needed.
Chloroquine and hydroxychloroquine (Figure 2) are noteworthy too. These are antimalarial drugs that have also been used for the treatment of different rheumatic diseases (Mascolo et al., 2018). Both drugs show many pharmacological effects, including the stabilization of the lysosomal membranes, the inhibition of PG synthesis, polymorphonuclear chemotaxis and phagocytosis, and a possible interference with the production of IL‐1 by monocytes and inhibition of the release of superoxide by neutrophils (Italian Medicines Agency, n.d.). These drugs inhibit the replication of SARS‐CoV‐2 in experimental models (Figure 2) (Italian Medicines Agency, n.d.). The efficacy of hydroxychloroquine, as a prophylactic agent, was evaluated in 821 subjects who had family or occupational exposure with a subject with COVID‐19. The study results revealed no difference in the incidence of new onset of COVID‐19 between participants treated with hydroxychloroquine (11.8%) and those who received placebo (14.3%). Adverse events were more commonly observed with hydroxychloroquine than with placebo, although no serious adverse reactions were reported (Boulware et al., 2020). Many clinical trials evaluated the efficacy and safety of hydroxychloroquine as therapeutic agent. Gautret et al. carried out a single‐arm interventional study, which evaluated the effects of hydroxychloroquine (plus azithromycin) in 36 hospitalized patients with COVID‐19. Authors reported a significant reduction in viral load on Day 6 in patients treated with hydroxychloroquine compared with controls. The addition of azithromycin to hydroxychloroquine therapy significantly contributed to the reduction of viral load (Gautret et al., 2020). Another study enrolled 11 hospitalized patients treated with hydroxychloroquine and azithromycin. After 5–6 days of starting treatment, the nasopharyngeal swab gave a positive result in 8/10 patients. These virological results are in contrast with those reported by Gautret et al. and raise doubts about the effectiveness of the drugs combination (Molina et al., 2020). A further study found no difference in viral load reduction and clinical outcomes between hydroxychloroquine and standard of care (Chen, Xia, et al., 2020). In addition, the results of a randomized, double‐blind, placebo‐controlled clinical trial (carried out in 491 outpatients at an early stage of COVID‐19) found no differences in symptom severity over 14 days between hydroxychloroquine and placebo (relative difference 12%; p = .117) (Skipper et al., 2020). The results of another randomized study showed that on Day 14, 100% of patients treated with chloroquine were discharged from the hospital, compared with 50% in the lopinavir/ritonavir group. However, it should be underlined that patients in the chloroquine group were younger and they had started the treatment earlier (Huang et al., 2020). Lastly, the preliminary results of the SOLIDARITY study seem to suggest that hydroxychloroquine and the combination of lopinavir/ritonavir and IFN‐based regimens have little or no effect on mortality at 28 days or on hospital course (WHO, 2020a). Alongside with data on the efficacy profile of chloroquine and hydroxychloroquine, many data on the safety profile of both drugs were collected as well. The EMA drew the attention on risks of serious adverse reactions (including heart rhythm disturbances), highlighting the need for prescribers to closely monitor patients treated with both drugs (EMA, 2020c). In addition, the EMA recommended the use of these medicines only in clinical trials or in national emergency management programmes in hospitalized patients under close monitoring (EMA, 2020b). The FDA also highlighted the need for patients' monitoring when treated with hydroxychloroquine, especially regarding the risk of severe changes in heart rhythm (tachycardia, atrial fibrillation and torsades de pointes) and recommended the use of these drugs only in the context of clinical trials (US FDA, 2020). In June 2020, the WHO suspended the hydroxychloroquine treatment arm in the multicentre clinical trial SOLIDARITY (Italian Medicines Agency, 2020b). Lastly, on November 2020, the Pharmacovigilance Risk Assessment Committee (PRAC) of the EMA recommended updating the product information for chloroquine or hydroxychloroquine due to the risk of psychiatric disorders and suicidal behaviour (EMA, 2020e). In conclusion, data related to the efficacy and safety profile of antimalarial drugs are quite conflicting. We should wait for the results of clinical trials currently ongoing (www.clinicaltrials.gov).
Corticosteroids, especially dexamethasone and methylprednisolone, could be considered one of the mainstays in the treatment of COVID‐19, especially for patients with severe disease, to prevent its worsening and complications and to resolve its serious symptoms. As reported in Figure 2, corticosteroids are associated with multiple mechanisms, including the increase in the gene transcription of anti‐inflammatory cytokines; the decrease in gene transcription of pro‐inflammatory cytokines, chemokines and adhesion molecules; and a reduction in inflammatory response. All these effects would contribute to suppress the cytokine storm. The preliminary results of the RECOVERY trial provided the most significant evidence supporting the use of dexamethasone. In this controlled, open‐label trial, patients were randomized to receive oral or intravenous dexamethasone 6 mg·day−1 for up to 10 days (n = 2104) or the usual care alone (n = 4321). According to the level of respiratory support, the incidence of death was lower in dexamethasone group among patients receiving invasive mechanical ventilation or oxygen without invasive mechanical ventilation (Horby, Lim, et al., 2020). These results, together with those from other randomized clinical studies (Jeronimo et al., 2020; Tomazini et al., 2020), showed that dexamethasone could be considered a treatment option in hospitalized subjects with severe COVID‐19 infection who require oxygen therapy, whether or not they have mechanical ventilation (invasive or not invasive). Based on the preliminary results of the RECOVERY trial, the CHMP of the EMA concluded that dexamethasone can be considered for patients who require oxygen therapy, and the EMA is endorsing its use in adults and adolescents at a dose of 6 mg once a day for up to 10 days (EMA, 2020d). Recently, the WHO REACT Working Group carried out a meta‐analysis of seven randomized clinical trials that evaluated the efficacy of corticosteroids (dexamethasone, hydrocortisone or methylprednisolone) in 1703 critically ill patients with COVID‐19. The results showed that, compared with usual care or placebo, the OR for the association with mortality was 0.64 P < .001) for dexamethasone, 0.69 (P = .13) for hydrocortisone and 0.91 (P = .87) for methylprednisolone. Sixty‐four serious adverse events were observed among patients randomized to corticosteroids and 80 serious adverse events among patients randomized to usual care or placebo. Authors concluded that the administration of systemic corticosteroids, compared with usual care or placebo, was associated with lower 28‐day all‐cause mortality (Sterne et al., 2020). Lastly, several clinical trials are currently underway or in development to evaluate corticosteroids for the treatment of COVID‐19 (www.clinicaltrials.gov).
Similarly to corticosteroids, non‐steroidal anti‐inflammatory drugs (NSAIDs) (Figure 2) and paracetamol represent valuable therapeutic options in patients with COVID‐19, especially for outpatients at early stage (not severe form), to treat fever and muscle pain (EMA, 2020a). At the beginning of the outbreak, the role of NSAIDs was misjudged due to some concerns that arose from few studies (Capuano, Scavone, et al., 2020). Specifically, in March 2020, the French Minister of Health recommended the use of paracetamol instead of ibuprofen or oral cortisone for the treatment of fever in patients with COVID‐19. This statement was supported also by the French authorities, which announced that NSAIDs might worsen clinical conditions of patients with COVID‐19 (Ministère de la Santé, 2020). Recently, new clinical data have become available regarding the use of NSAIDs in patients with COVID‐19. For instance, Rinott et al. carried out a retrospective cohort study on 403 patients with COVID‐19. Of the entire cohort, 87 patients received ibuprofen and 316 did not. No significant difference was found between groups in terms of mortality and respiratory support. The comparison of ibuprofen users with paracetamol users did not reveal any difference in mortality rates or the need for respiratory support (Rinott et al., 2020). A prospective cohort study enrolled patients with COVID‐19, who were interviewed about NSAID use and infection outcomes (such as death, admission, severity, time to clinical improvement, oxygen requirement and length of stay). According to study's results, neither the acute use of ibuprofen nor chronic NSAID use was associated with a greater risk of mortality compared with non‐use (adjusted hazard ratio [HR]: 0.632 [95% CI 0.073–5.441; p = .6758] and adjusted HR 0.492 [95% CI 0.178–1.362; p = .1721], respectively) (Abu Esba et al., 2020). Lastly, a further multicentre, observational study, which collected data related to 1222 hospitalized patients with COVID‐19 (of whom 54 were routinely prescribed NSAIDs prior to admission), found no evidence that routine NSAID use was associated with higher COVID‐19 mortality (Bruce et al., 2020). Based on these results and according to the recommendation from the EMA, paracetamol remains the first‐line option for the treatment of fever, and there is no reason for patients who are taking NSAIDs for chronic diseases to stop taking them (EMA, 2020a).
Other drugs able to reduce the hyperinflammation are currently under evaluation in patients with COVID‐19, including baricitinib, ruxolitinib and eculizumab. For instance, the effects of baricitinib, a JAK inhibitor that is able to suppress the cytokine storm and to interrupt the passage and intracellular assembly of SARS‐CoV‐2 into the target cells (Zhang et al., 2020), were evaluated in 12 patients with mild–moderate COVID‐19 pneumonia. At Week 1 and Week 2, the drug improved the clinical and laboratory parameters, and none of the patients required ICU support (Cantini, Niccoli, Matarrese, et al., 2020). The same research group carried out an observational, retrospective, multicentre study in hospitalized patients with moderate COVID‐19 pneumonia to compare the 2‐week effectiveness and safety of baricitinib and lopinavir/ritonavir (n = 113) compared with hydroxychloroquine and lopinavir/ritonavir (n = 78). The results showed that the fatality rate and the ICU admission were significantly lower among patients treated with baricitinib (Cantini, Niccoli, Nannini, et al., 2020). Based on data currently available, no firm conclusion can be drawn on the effects of baricitinib in COVID‐19 patients. In addition, due to a possible increased risk of herpes zoster and simplex infections, a group of Italian researchers suggested that the use of baricitinib should be considered with extreme caution (Favalli et al., 2020). Ruxolitinib is a JAK1 and JAK2 inhibitor (El Bairi et al., 2020) whose effects are currently evaluated in the RUXCOVID‐DEVENT trial (NCT04377620) in COVID‐19 patients with ARDS requiring mechanical ventilation. Lastly, two recent studies highlighted the benefits deriving from the treatment with eculizumab (Annane et al., 2020; Laurence et al., 2020). This is a monoclonal antibody that inhibits the terminal portion of the complement cascade involved in the inflammatory response. The study carried out by Laurence et al. (2020) reported the experience of three critical COVID‐19 patients who experienced a marked decline in d‐dimers and neutrophil counts after eculizumab treatment. The results of the proof‐of‐concept study carried out by Annane et al. showed that, in a population of 80 patients with severe COVID‐19 admitted to ICU, the estimated survival was 82.9% with eculizumab and 62.2% without eculizumab (log‐rank test, P = .04). Based also on the positive results on rapid decrease in lactate, blood urea nitrogen, bilirubin levels and a rapid increase in platelet count and prothrombin time observed with eculizumab, authors suggested that the drug could improve survival and reduce hypoxia in patients with severe COVID‐19 (Annane et al., 2020).
In conclusion, many immunomodulatory and anti‐inflammatory drugs have been tested in patients with COVID‐19, but today, the evidence is quite conflicting for most of them. In addition, many of the published studies are observational or suffer from many limitations, including the lack of a sample size calculation or control groups and the use of surrogate endpoints (viral load instead of mortality rate). At present, the highest number of concluded clinical studies was found for tocilizumab, hydroxychloroquine and corticosteroids. Among these drugs, only the use of corticosteroids seems to be supported by robust evidence, whereas data related to the efficacy and safety of tocilizumab and hydroxychloroquine are quite conflicting. Therefore, further data from randomized controlled trial or well‐designed observational studies are strongly needed.
4. ANTICOAGULANT AND ANTIPLATELET DRUGS
As shown in Figure 1, among the most serious clinical complications of COVID‐19, there is the onset of a coagulopathy that is a cause of death in COVID‐19 patients along with respiratory failure (Asakura & Ogawa, 2020). At the beginning, the state of hyperinflammation and hypercoagulability was identified as disseminated intravascular coagulation (DIC) (Marietta et al., 2020). However, the pathophysiology of COVID‐19‐associated DIC is different from the classic one (septic or traumatic DIC) (Asakura & Ogawa, 2020). In fact, in COVID‐19 patients, the most common pattern of coagulopathy is characterized by increased levels of fibrinogen and d‐dimer, a mild prolongation of PT/aPTT and a mild thrombocytopenia, which can also be absent in some patient (Atallah et al., 2020; American Society of Hematology, 2020b).
The exact mechanisms contributing to coagulopathy in COVID‐19 patients are not completely understood. In general, inflammation and coagulation are known to be linked by different molecular signals. Pro‐inflammatory mediators can stimulate the expression of intravascular tissue factor, leukocyte adhesion molecules and plasminogen activator inhibitor‐1 (PAI‐1) (Gozzo et al., 2020). Moreover, inflammation can activate the coagulation cascade by overexpressing thrombin both systemically and locally in the lungs, leading to fibrin deposition and subsequent tissue damage. SARS‐CoV‐2 could also directly damage vascular endothelial cells through its bond to ACE2, which could represent the first injury triggering the abnormal coagulation (Li, Liu, et al., 2020). In this context, the generalized hypercoagulable state of COVID‐19 patients could be due to the involvement of Type II pneumocytes, the extensive pulmonary microvascular network and the extensive hyperinflammatory state that is similar to the macrophage activation syndrome. Finally, the development of hypoxemia, secondary to ARDS, might also activate the coagulation cascade and could contribute to endothelial dysfunction (Gozzo et al., 2020; McGonagle et al., 2020).
A better understanding of the thromboembolic risk in patients suffering from COVID‐19 could help to optimize both diagnostic strategies and pharmacological management (Capuano, Rossi, & Paolisso, 2020; Lodigiani et al., 2020). In this regard, an observational study found that the in‐hospital mortality in patients who required mechanical ventilation was lower for those treated with anticoagulants than those not receiving the anticoagulant treatment (Paranjpe et al., 2020). Undoubtedly, heparins, either unfractionated or in low MW forms (LMWH), used for blocking or limiting the state of hypercoagulability, represent a good therapeutic option in patients with COVID‐19. Apart from anticoagulant properties, heparins mitigate the inflammatory state exercising non‐anticoagulant mechanisms such as inhibition of heparanase activity, chemokine and cytokine neutralization, interference with leukocyte trafficking, neutralization of extracellular cytotoxic histones and reduction of viral cellular entry (Buijsers et al., 2020). Therefore, the benefit of using heparins could be related to the ability of blocking both coagulation and inflammation. Accordingly, a retrospective observational study demonstrated that heparins improved the coagulation dysfunction of COVID‐19 patients and exerted anti‐inflammatory effects by reducing IL‐6 and increasing lymphocyte percentage (SHI et al., 2020). Another observational study found that the treatment with heparin was associated with a lower mortality in hospitalized patients with COVID‐19 (Ayerbe et al., 2020).
The WHO has recommended the use of LMWH, such as enoxaparin, in patients hospitalized with COVID‐19 to prevent venous thromboembolism (WHO, 2020b). The optimal anticoagulant dosage to be used in patients with COVID‐19 is still being debated. Guidelines recommend the use of prophylactic doses of LMWH in hospitalized COVID‐19 patients unless contraindicated, but not in non‐hospitalized patients (Gozzo et al., 2020; NIH, 2020; WHO, 2020b). As reported by the American Society of Hematology (2020a), many protocols have adopted an intermediate‐intensity dose (administering the usual daily LMWH dose twice daily) or even a therapeutic‐intensity dose strategy for thromboprophylaxis based on local experience. Another guideline suggests the use, in acutely/critically ill patients with COVID‐19, of a standard dose of anticoagulant over intermediate (LMWH twice daily or increased weight‐based dosing) or full treatment dosing and to prefer unfractionated heparins over LMWH in patients at high risk of bleeding (including those with severe renal failure) (Moores et al., 2020). A recent retrospective study, evaluating the effects of different doses of LMWH on the incidence of bleeding in COVID‐19 patients admitted to ICUs, showed that the use of therapeutic doses did not increase the risk of bleeding. Moreover, the study suggested the importance of applying a risk stratification based on d‐dimer values for critically ill patients with COVID‐19 (Pavoni et al., 2020). Based on these considerations, a close clinical monitoring and an individual patient evaluation for the risk of thrombosis and bleedings should be applied (Gozzo et al., 2020). Currently, several different clinical trials are ongoing to evaluate the treatment with heparin in hospitalized patients with COVID‐19 (www.clinicaltrials.gov).
Aspirin (acetylsalicylic acid), an irreversible platelet inhibitor used for conditions such as myocardial infarction, strokes and pre‐eclampsia in pregnant women, has been investigated in COVID‐19 patients. In addition to its anti‐inflammatory and anti‐thrombotic effects, aspirin has shown a significant antiviral activity against DNA and RNA viruses, including different human coronaviruses (Bianconi et al., 2020). Moreover, the use of aspirin has been associated with reduced thrombo‐inflammation and lower rates of clinical complications and in‐hospital mortality in different types of infections (Bianconi et al., 2020). Observational studies demonstrated some benefit in reducing the risk of ICU mortality, acute lung injury and ARDS (W. Chen et al., 2015; Erlich et al., 2011), whereas a larger observational study did not demonstrate any difference between aspirin use and ALI (Kor et al., 2011). Moreover, a randomized, double‐blind, placebo‐controlled, randomized clinical trial, conducted on 390 patients, showed that the use of aspirin compared with placebo did not reduce the risk of ARDS (Kor et al., 2016). First data from a retrospective cohort study of adult patients hospitalized with COVID‐19 showed that aspirin was associated with a decreased risk of mechanical ventilation, ICU admission and in‐hospital mortality, with no difference for major bleedings between aspirin and non‐aspirin users (Chow et al., 2020). The drug will be tested for its effect of reducing blood clots in the world's largest clinical trial of treatments for patients hospitalized with COVID‐19 (RECOVERY trial) (University of Oxford, 2020a). If effective, aspirin may be beneficial because it is safe, accessible and inexpensive.
Oral anticoagulants or other antiplatelet agents (P2Y12 receptor antagonists) are available for clinical use. Their use in SARS‐CoV‐2 infection derives from the effects of the cytokine storm on enhanced platelet activation, thrombotic microangiopathy and clotting, as well as the effects of activated platelets on neutrophil activation (Banik et al., 2021). However, concerns were raised about their interactions with multiple medications that are being used and tested for the treatment of COVID‐19 (Gozzo et al., 2020). For instance, sarilumab and tocilizumab can reduce plasma concentrations of apixaban, rivaroxaban and warfarin, whereas atazanavir and lopinavir/ritonavir can increase drug concentrations of apixaban and rivaroxaban and reduce the active metabolite of clopidogrel and prasugrel (American Society of Hematology, 2020a).
In conclusion, the use of heparins in hospitalized critically ill patients is preferred over other anticoagulants because of the shorter half‐life and fewer drug–drug interactions, being also the first choice for pregnant women.
5. OTHER THERAPIES
Convalescent plasma is a mixture of inorganic and organic compounds, water and proteins (including albumin, immunoglobulins, complement, coagulation and antithrombotic factors). Stringent criteria need to be satisfied in order to be a convalescent donor. Specifically, donors should have the following characteristics: aged between 18 and 65 years, absence of infectious symptoms and a negative test for COVID‐19 14 days after the recovery (Tiberghien et al., 2020). The main procedure to obtain plasma is apheresis, which is based on a continuous centrifugation of blood from the donor (Bloch et al., 2020). Few clinical studies were concluded, and their results could provide new insights regarding the efficacy and safety profile of plasma therapy in COVID‐19 patients. Li, Zhang, et al. reported the results of an open‐label, multicentre, randomized clinical trial carried out in 103 participants with a severe form of COVID‐19, who were randomized to receive convalescent plasma in addition to standard treatment (n = 52) or the standard treatment alone (n = 51). According to the study's results, the convalescent plasma therapy was not associated with a statistically significant improvement in time to clinical improvement within 28 days compared with standard treatment alone (Li, Zhang, et al., 2020). Simonovich et al. carried out a randomized controlled trial in 334 hospitalized adult patients with severe COVID‐19 pneumonia, who were randomized to receive convalescent plasma (n = 228) or placebo (n = 105) in addition to standard treatment. Authors evaluated the effects of the therapy in terms of changes in patient's clinical status 30 days after the intervention. As in the previous study, no difference in terms of efficacy was found between treatments groups at Day 30. Regarding the safety profile, infusion‐related adverse events were detected in 4.8% of patients in the convalescent plasma group and in 1.9% of patients in the placebo group, even though no significant differences were found in the overall incidence of adverse events or serious adverse events (Simonovich et al., 2020). On the other hand, few small trials or case series reported that the convalescent plasma therapy might be beneficial in COVID‐19 patients. For instance, Duan et al. (2020) reported that the administration of 200 ml of convalescent plasma in 10 severe patients led to a significant improvement in clinical symptoms and no severe adverse effects were observed. Lastly, a further case series described the effects of the convalescent plasma therapy in five critically ill patients with COVID‐19 and ARDS, who improved after receiving the therapy (Shen et al., 2020). Despite their positive results, these studies' findings should be interpreted with caution considering the limited number of patients who were enrolled and the study designs that did not allow making any comparison. In conclusion, based on the conflicting evidence currently available, the use of convalescent plasma needs to be considered as investigational. Intravenous immunoglobulins, which are isolated from the pooled plasma of healthy donors, are used for the treatment of many autoimmune and inflammatory diseases and could represent a good option to improve the prognosis of critical‐type patients with COVID‐19 (Cao, Liu, et al., 2020; Galeotti et al., 2020).
Many studies are currently investigating the effects of vitamin D in COVID‐19 patients. This interest derives from the evidence suggesting the role of vitamin D in reducing the risk of cold and acute respiratory infections. Many mechanisms seem to underlie this effect, including the effects of vitamin D on cellular natural immunity and adaptive immunity through the decrease in cytokine storm (this effect was observed on IFN‐γ, TNF‐α and CD4+ T‐cell count (Ali, 2020). In addition, vitamin D improves the production of antimicrobial peptides in the respiratory epithelium, potentially reducing the risk of local infection, and it seems to interact with ACE2 (Mitchell, 2020). Pizzini et al. investigated the association between of vitamin D status and COVID‐19 presentation among 109 patients, using data from the CovILD registry. They found that low vitamin D levels were not associated with poor clinical and radiological outcomes of COVID‐19 (Pizzini et al., 2020). A further study, which was based on data from the UK Biobank, aimed to establish whether blood 25‐hydroxyvitamin D concentration was associated with COVID‐19 risk or not. Data were available for 348,598 UK Biobank participants, of which 449 had COVID‐19 infection. Authors concluded that no association was found in terms of potential association between vitamin D concentrations and the risk of COVID‐19 infection (Hastie et al., 2020). Ultimately, current evidence on a potential correlation between vitamin D and COVID‐19 is contradictory. Therefore, we should wait for results from clinical trials, which are currently underway to evaluate the effects of vitamin D supplementation on mortality, morbidity, prevention and treatment of COVID‐19 (www.clinicaltrials.gov).
6. VACCINES ALREADY AUTHORIZED OR UNDER ADVANCED STAGE OF CLINICAL DEVELOPMENT
According to the WHO, up to 2 February 2021, 63 candidate vaccines are under clinical evaluation and 175 under preclinical evaluation (WHO, 2020c). Specifically, 21 vaccines are already in advanced stage of clinical development, being evaluated in Phase III clinical trials.
In Table 1, an overview of these vaccines is reported. For some of these products, many clinical studies are currently undergoing, but others have already provided preliminary results. Among these 21 vaccines, four were already approved by the EMA and FDA or are under advanced evaluation for the marketing approval: ChAdOx1‐S (AZD1222, AstraZeneca), LNP‐encapsulated mRNA (mRNA‐1273, Moderna), 3 LNP‐mRNAs (BNT162b2, Pfizer) and adenovirus type 26 vector (Ad26.COV2.S, Janssen).
TABLE 1.
Vaccines in Phase III clinical trials
| Manufacturer of COVID‐19 vaccine | Vaccine platform | Type of candidate vaccine | Number of doses and timing of doses | Clinical trial identifier | Estimated study completion date |
|---|---|---|---|---|---|
| University of Oxford/AstraZeneca | Non‐replicating viral vector | ChAdOx1‐S (AZD1222) | 2 doses at Days 0 and 28 | NCT04516746 | February 2023 |
| ISRCTN89951424 | December 2021 | ||||
| NCT04540393 | March 2021 | ||||
| CTRI/2020/08/027170 | March 2021 a | ||||
| NCT04536051 | September 2021 | ||||
| EUCTR2020‐005226‐28‐DE | Not found | ||||
| NCT04400838 | September 2021 | ||||
| Moderna/NIAID | RNA | LNP‐encapsulated mRNA (mRNA‐1273) | 2 doses at Days 0 and 28 | NCT04470427 | October 2022 |
| NCT04649151 | June 2022 | ||||
| BioNTech/Fosun Pharma/Pfizer | RNA | 3 LNP‐mRNAs (BNT162b2) | 2 doses at Days 0 and 21 | NCT04368728 | January 2023 |
| NCT04713553 | April 2021 | ||||
| Janssen Pharmaceutical Companies (Johnson & Johnson) | Non‐replicating viral vector | Adenovirus type 26 vector (Ad26.COV2.S) | 1 dose at Day 0 | NCT04505722 | March 2023 |
| 2 doses at Days 0 and 56 | NCT04614948 | May 2023 | |||
| Novavax | Protein subunit | Full‐length recombinant SARS‐CoV‐2 glycoprotein nanoparticle vaccine adjuvanted with matrix M (SARS‐CoV‐2 rS/matrix‐M1 adjuvant) | 2 doses at Days 0 and 21 | 2020‐004123‐16 | September 2021 a |
| NCT04611802 | December 2022 | ||||
| NCT04583995 | January 2022 | ||||
| Sinovac | Inactivated | Inactivated (CoronaVac) | 2 doses at Days 0 and 14 | NCT04456595 | October 2021 |
| NCT04582344 | April 2021 | ||||
| NCT04617483 | May 2021 | ||||
| NCT04508075 | September 2021 | ||||
| NCT04651790 | March 2022 | ||||
| Wuhan Institute of Biological Products/Sinopharm | Inactivated | Inactivated | 2 doses at Days 0 and 21 | ChiCTR2000034780 | July 2021 |
| ChiCTR2000039000 | December 2020 | ||||
| NCT04612972 | September 2021 | ||||
| NCT04510207 b | September 2021 | ||||
| Beijing Institute of Biological Products/Sinopharm | Inactivated | Inactivated | 2 doses at Days 0 and 21 | NCT04560881 | December 2021 |
| NCT04510207 b | September 2021 | ||||
| Bharat Biotech | Inactivated | Whole‐Virion inactivated | 2 doses at Days 0 and 14 | CTRI/2020/11/028976 | November 2021 a |
| NCT04641481 | March 2022 | ||||
| CanSino Biological Inc./Beijing Institute of Biotechnology | Nonreplicating viral vector | Adenovirus type 5 vector | 1 dose | NCT04526990 | January 2022 |
| NCT04540419 | July 2021 | ||||
| Gamaleya Research Institute | Nonreplicating viral vector | Adeno‐based (rAd26‐S + rAd5‐S, sputnik V) | 2 doses at Days 0 and 21 | NCT04530396 | May 2021 |
| NCT04564716 | April 2021 | ||||
| NCT04642339 | December 2021 | ||||
| NCT04656613 | December 2021 | ||||
| NCT04640233 | September 2021 | ||||
| Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences | Protein subunit | Adjuvanted recombinant protein (RBD‐dimer) expressed in CHO cells | 3 doses at Days 0, 28 and 56 |
ChiCTR2000040153 |
April 2022 |
| Medicago Inc. | VLP | Coronavirus‐like particle COVID‐19 (CoVLP) | 2 doses at Days 0 and 21 | NCT04636697 | April 2022 |
| CureVac AG | RNA | RNA based vaccine (CVnCoV vaccine) | 2 doses at Days 0 and 28 | NCT04674189 | April 2022 |
| NCT04652102 | March 2023 | ||||
| Institute of Medical Biology and Chinese Academy of Medical Sciences | Inactivated | Inactivated | 2 doses at Days 0 and 28 | NCT04659239 | July 2022 |
| Research Institute for Biological Safety Problems, Republic of Kazakhstan | Inactivated | Inactivated | 2 doses at Days 0 and 21 | NCT04691908 | July 2021 |
| Zydus Cadila | DNA | DNA‐based vaccine c | 3 doses at Days 0, 28 and 56 | CTRI/2020/07/026352 | July 2021 a |
| Inovio Pharmaceuticals + International Vaccine Institute and Advaccine (Suzhou) Biopharmaceutical Co., Ltd | DNA | DNA‐based vaccine c | 2 doses at Days 0 and 28 | NCT04642638 | September 2022 |
| AnGes, Takara Bio and Osaka University | DNA | DNA‐based vaccine (AG0301‐COVID19) | 2 doses at Days 0 and 14 | NCT04655625 | March 2022 |
| Clover Biopharmaceuticals Inc., GSK and Dynavax | Protein subunit | SCB‐2019 + AS03 or CpG 1018 adjuvant plus alum adjuvant (native‐like trimeric subunit spike protein vaccine) | 2 doses at Days 0 and 21 | NCT04672395 | July 2022 |
| COVAXX and United Biomedical Inc. | Protein subunit | UB‐612 (multitope peptide‐based S1‐RBD protein‐based vaccine) | 2 doses at Days 0 and 28 | NCT04683224 | March 2023 |
Abbreviations: LNP, lipid nanoparticle; VLP, virus‐like particle.
Dates computed based on the estimated trial duration.
This trial assesses both the Wuhan and Beijing vaccines in the same study.
Administered through intradermal injections.
On 8 December 2020, the preliminary results of Phase III trials of the COVID‐19 vaccine developed by AstraZeneca and Oxford University were published in the Lancet. This vaccine was developed using a chimpanzee adenovirus viral vector that triggers the expression of the spike protein of SARS‐CoV‐2. Once injected, the human cells produce the protein and train the immune system to antibodies and T‐cells against it. The published preliminary results refer to data from four ongoing randomized, controlled trials, which are currently underway across the United Kingdom, Brazil and South Africa (studies ISRCTN89951424, NCT04324606, NCT04400838 and NCT04444674). Adult participants were randomized to receive two doses of the vaccine (both containing 5 × 1010 viral particles [standard dose cohort, SD] or a low dose as first dose and a standard dose as second dose [LD/SD cohort]) or control (meningococcal group A, C, W and Y conjugate vaccine or saline). From April until November 2020, 23,848 participants were enrolled. Of these, 11,636 participants were included in the interim primary efficacy analysis. In SD and LD/SD cohort, the vaccine efficacy was 62.1% and 90%, respectively. Starting from 21 days after the first dose, 10 cases hospitalized for COVID‐19 all in the control arm occurred. One hundred and seventy‐five severe adverse events occurred, of which 84 in the ChAdOx1 nCoV‐19 group and 91 in the control group. Among serious adverse events, there were cases of haemolytic anaemia and transverse myelitis. Authors concluded that the new vaccine is efficacious and it could contribute to control of the disease in this pandemic (Voysey et al., 2020). The British and Indian regulatory agencies have granted marketing approval for the Oxford University/AstraZeneca vaccine. On 29 January 2021, the EMA recommended a conditional marketing authorization for this vaccine too.
Pfizer developed a lipid nanoparticle‐formulated, nucleoside‐modified RNA vaccine that encodes a prefusion stabilized, membrane‐anchored SARS‐CoV‐2 full‐length spike protein, BNT162b2, whose efficacy and safety were recently published in the NEJM (Polack et al., 2020). Specifically, a multinational, placebo‐controlled, pivotal efficacy trial, which is still ongoing, enrolled 43,548 patients aged >16 years of age and who were randomized to receive two doses, 21 days apart, of placebo or the BNT162b2 vaccine (30 μg per dose). A total of 43,448 received injections: 21,720 with the vaccine and 21,728 with placebo. Study's results revealed for the vaccine an efficacy in preventing COVID‐19 equal to 95% (95% CI 90.3–97.6); indeed, after the second dose, eight cases of COVID‐19 were detected among subjects who received BNT162b2 versus 162 among those assigned to placebo. Similar results were found across subjects' subgroups by age, sex, race, ethnicity, baseline body mass index and the co‐morbidities. After the first dose, nine cases of severe COVID‐19 were detected among subjects receiving placebo versus one case in those receiving BNT162b2. BNT162b2 demonstrated a good safety profile, being associated with short‐term, mild‐to‐moderate injection site reactions, fatigue and headache, and no differences were detected between the groups in term of serious adverse events (Polack et al., 2020). This vaccine was first approved in the United Kingdom with its administration started in December 2020, and then it was authorized, during the same month, by the FDA for emergency use in subjects 16 years of age and older. Finally, on 21 December 2020, the EMA has granted a conditional marketing authorization for this vaccine to prevent COVID‐19 in people from 16 years of age.
Moderna also developed an RNA vaccine and recently provided data on the achievement of its study's primary efficacy endpoints. The results of the COVE study (NCT04470427), a Phase III, randomized, placebo‐controlled study, which is investigating the efficacy, safety and immunogenicity of mRNA‐1273 SARS‐CoV‐2 vaccine in 30,000 adult subjects in the United States, were recently published in the NEJM. The mRNA‐1273 vaccine has 94.1% efficacy at preventing COVID‐19. Thirty subjects, all receiving placebo, developed severe COVID‐19. Local and systemic reactions occurred in subjects who received the vaccine, but no safety concerns were identified. Serious adverse events were rare, and no differences were found between the placebo and vaccine groups (Baden et al., 2020). On 18 December 2020, the US FDA issued an emergency use authorization for the second vaccine to prevent COVID‐19 in individuals 18 years of age and older. The EMA authorized this vaccine on 6 January 2021.
Recently, some concerns related to clinical studies on Pfizer's and Moderna's vaccines were highlighted, mainly related to suspected COVID‐19 cases not confirmed by PCR, the exclusion of subjects from the efficacy analysis for protocol deviations, and the potential confounding role of medications to treat pain and fever symptoms (Doshi, 2021). Even though the request of raw data related to clinical trials' results is shareable, we should consider that regulatory agencies have been reviewing safety and efficacy data on authorized vaccines. Indeed, on 28 January, the EMA published the latest safety data for Pfizer vaccine. No new safety concerns were identified, including in frail individuals. Therefore, the EMA stated that the benefits of this vaccine continue to outweigh any risks, and there are no recommended changes regarding the use of the vaccine (EMA, 2021).
Lastly, the efficacy and safety of adenovirus type 26 vector (Johnson & Johnson) are currently evaluated in Phase III ENSEMBLE and ENSEMBLE 2, randomized, double‐blind, placebo‐controlled clinical trials (both studies will enrol approximately 90,000 subjects). On 29 January 2021, Johnson & Johnson announced that the vaccine candidate met primary endpoints in interim analysis of Phase 3 ENSEMBLE Trial (Johnson & Johnson, 2021). The FDA scheduled a meeting on 26 February 2021, to discuss the request for emergency use authorization for this vaccine. Finally, on 3 February 2021, the EMA started the rolling review for NVX‐CoV2373, developed by Novavax CZ AS.
Many other vaccines are under evaluation in Phase I–II clinical trials. Some of them are facing delays in achieving the study outcomes. For instance, Sanofi and GSK announced a delay in their adjuvanted recombinant protein‐based COVID‐19 vaccine programme due to a low immune response in adults aged >49 years, for an insufficient antigen concentration. The pharmaceutical companies state that they will carry out a Phase IIb study with an improved antigen formulation (Sanofi, 2020).
In conclusion, immunization programmes with COVID‐19 vaccines have already started around the world. Therefore, preliminary efficacy and safety data from real life will be soon available. In addition, at this moment, evidence suggests that antibodies generated through vaccination with the currently authorized vaccines also recognize SARS‐CoV‐2 variants (CDC, 2021).
7. CONCLUSION
The spread of the new COVID‐19 was inevitably followed by an intense search for therapies able to counteract severe signs and symptoms of this disease. Today, pharmacological researches are focusing on different drug classes, including antivirals, immunomodulatory and anti‐inflammatory agents, anticoagulants and antiplatelet drugs, convalescent plasma and vitamins. Other drugs are currently administered among inpatients and outpatients with COVID‐19, such as antibiotics. The use of these drugs is, on many occasions, necessary, given that patients with COVID‐19 may also develop bacterial infections, such as pneumonia.
Given the absence of a specific drug able to block the replication of SARS‐Cov‐2, drug repurposing has represented the main approach recently used. This is the case, for example, of antivirals, whose role however is still debated. Also for remdesivir, which is the only drug recommended for COVID‐19, some concerns related to its efficacy profile were raised by the WHO based on the results of the open‐label SOLIDARITY trial. Given these concerns, the EMA is currently re‐evaluating the drug.
Many immunomodulatory and anti‐inflammatory drugs have been tested in patients with COVID‐19 as well. Based on current evidence and considering the limitations of published clinical studies on these drugs, no firm conclusions can be drawn. Among these drug classes, tocilizumab, hydroxychloroquine and corticosteroids have been extensively studied, even though only the use of corticosteroids seems to be supported by robust evidence, for both outpatients and inpatients requiring supplemental oxygen. In addition, anti‐inflammatory actions explain the significant role of NSAIDs, mainly ibuprofen and paracetamol, especially in patients suffering from a mild form of COVID‐19 (early stage—manageable at home) to solve symptoms like fever and joint and muscle pain.
The role of heparins is noteworthy too. Indeed, the administration of these drugs in critically ill patients is crucial in order to reduce the thromboembolic risk, which is one of the most serious consequences of COVID‐19.
In addition, based on the results of published studies, the role of convalescent plasma and vitamins is still not clear. Therefore, we should wait for results from clinical trials, which are currently ongoing to evaluate the effects of these therapies on mortality, morbidity, prevention and treatment of COVID‐19.
Other drugs, such as the combination of monoclonal antibodies REGN‐COV2, might represent a powerful strategy to avoid patients' hospitalization and alleviate the burden on the healthcare system. At this moment, REGN‐COV2 and bamlanivimab have received approval from the FDA, whereas the EMA has started the rolling review for REGN‐COV2. The Italian Medicines Agency recently decided to make these treatments available for specific patient populations, while continuing the evaluation of evidence on their efficacy and safety profile.
In conclusion, the results of the studies summarized in this review article were quite conflicting. Apart from methodological limitations of clinical studies, there was a large difference in how studies defined clinical endpoints whether that be mortality, negative PCR testing, hospital discharge or safety profile of drugs. In addition, we should consider that few SARS‐CoV‐2 variants were detected and, at this moment, it is not possible to exclude the possibility that these variants might evade some pharmacological therapies presently effective.
Lastly, out of 21 vaccines in advanced stage of clinical development, three have already obtained marketing approval and two are under evaluation by the EMA. Preliminary efficacy data for these vaccines revealed a high efficacy rate and a good safety profile. However, according to the EMA, further post‐marketing studies are needed in order to better evaluate their effectiveness and immediate and longer term protection in a greater number of subjects.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Fabbro et al., 2019; Alexander, Kelly et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Scavone, C. , Mascolo, A. , Rafaniello, C. , Sportiello, L. , Trama, U. , Zoccoli, A. , Bernardi, F. F. , Racagni, G. , Berrino, L. , Castaldo, G. , Coscioni, E. , Rossi, F. , & Capuano, A. (2022). Therapeutic strategies to fight COVID‐19: Which is the status artis? British Journal of Pharmacology, 179 (10), 2128–2148. 10.1111/bph.15452
Cristina Scavone and Annamaria Mascolo share first authorship.
Francesco Rossi and Annalisa Capuano share lead authorship.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article because no new data were created or analysed in this study.
REFERENCES
- Abbaspour Kasgari, H. , Moradi, S. , Shabani, A. M. , Babamahmoodi, F. , Davoudi Badabi, A. R. , Davoudi, L. , Alikhani, A. , Hedayatizadeh Omran, A. , Saeedi, M. , Merat, S. , Wentzel, H. , Garratt, A. , Levi, J. , Simmons, B. , Hill, A. , & Tirgar Fakheri, H. (2020). Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID‐19 patients with moderate disease compared with standard care: A single‐centre, randomized controlled trial. The Journal of Antimicrobial Chemotherapy, 75(11), 3373–3378. 10.1093/jac/dkaa332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Esba, L. C. , Alqahtani, R. A. , Thomas, A. , Shamas, N. , Alswaidan, L. , & Mardawi, G. (2020). Ibuprofen and NSAID use in COVID‐19 infected patients is not associated with worse outcomes: A prospective cohort study. Infectious Diseases and Therapy, 10, 1–16. 10.1007/s40121-020-00363-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019). The concise guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Buneman, O. P. , Cidlowski, J. A. , Christopoulos, A. , Davenport, A. P. , Fabbro, D. , Spedding, M. , Striessnig, J. , Davies, J. A. , … Wong, S. S. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Other protein targets. British Journal of Pharmacology, 176(Suppl 1), S1–s20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, N. (2020). Role of vitamin D in preventing of COVID‐19 infection, progression and severity. Journal of Infection and Public Health, 13(10), 1373–1380. 10.1016/j.jiph.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaeri, H. K. , & Natto, Z. S. (2020). A contemporary look at COVID‐19 medications: Available and potentially effective drugs. European Review for Medical and Pharmacological Sciences, 24(17), 9188–9195. 10.26355/eurrev_202009_22870 [DOI] [PubMed] [Google Scholar]
- American Society of Hematology . (2020a). COVID‐19 and VTE‐anticoagulation—Hematology.org. https://www.hematology.org/COVID-19/COVID-19-and-vte-anticoagulation
- American Society of Hematology . (2020b). COVID‐19 and coagulopathy—Hematology.org. https://www.hematology.org/COVID‐19/COVID‐19‐and‐coagulopathy
- Annane, D. , Heming, N. , Grimaldi‐Bensouda, L. , Frémeaux‐Bacchi, V. , Vigan, M. , Roux, A. L. , Marchal, A. , Michelon, H. , Rottman, M. , & Moine, P. (2020). Eculizumab as an emergency treatment for adult patients with severe COVID‐19 in the intensive care unit: A proof‐of‐concept study. EClinicalMedicine, 28, 100590. 10.1016/j.eclinm.2020.100590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura, H. , & Ogawa, H. (2020). COVID‐19‐associated coagulopathy and disseminated intravascular coagulation. International Journal of Hematology, 1, 3–57. 10.1007/s12185-020-03029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah, B. , Mallah, S. I. , & AlMahmeed, W. (2020). Anticoagulation in COVID‐19. European Heart Journal—Cardiovascular Pharmacotherapy, 6(4), 260–261. 10.1093/ehjcvp/pvaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayerbe, L. , Risco, C. , & Ayis, S. (2020). The association between treatment with heparin and survival in patients with COVID‐19. Journal of Thrombosis and Thrombolysis, 50(2), 298–301. 10.1007/s11239-020-02162-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden, L. R. , El Sahly, H. M. , Essink, B. , Kotloff, K. , Frey, S. , Novak, R. , Diemert, D. , Spector, S. A. , Rouphael, N. , Creech, C. B. , McGettigan, J. , Khetan, S. , Segall, N. , Solis, J. , Brosz, A. , Fierro, C. , Schwartz, H. , Neuzil, K. , Corey, L. , … Zaks, T. (2020). Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. New England Journal of Medicine NEJMoa2035389, 384, 403–416. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik, J. , Mezera, V. , Köhler, C. , & Schmidtmann, M. (2021). Antiplatelet therapy in patients with COVID‐19: A retrospective observational study. Thrombosis Update, 2, 100026. 10.1016/j.tru.2020.100026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel, J. H. , Tomashek, K. M. , Dodd, L. E. , Mehta, A. K. , Zingman, B. S. , Kalil, A. C. , Hohmann, E. , Chu, H. Y. , Luetkemeyer, A. , Kline, S. , Lopez de Castilla, D. , Finberg, R. W. , Dierberg, K. , Tapson, V. , Hsieh, L. , Patterson, T. F. , Paredes, R. , Sweeney, D. A. , Short, W. R. , … Lane, H. C. (2020). Remdesivir for the treatment of COVID‐19—Final report. The New England Journal of Medicine, 383, 1813–1826. 10.1056/nejmoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benucci, M. , Giannasi, G. , Cecchini, P. , Gobbi, F. L. , Damiani, A. , Grossi, V. , Infantino, M. , & Manfredi, M. (2020). COVID‐19 pneumonia treated with Sarilumab: A clinical series of eight patients. Journal of Medical Virology, 92(11), 2368–2370. 10.1002/jmv.26062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, A. , Kumar, S. , Sarma, P. , Kaur, H. , Prajapat, M. , Shekhar, N. , Bansal, S. , Avti, P. , Hazarika, M. , Sharma, S. , Mahendru, D. , Prakash, A. , & Medhi, B. (2020). Safety and efficacy of lopinavir/ritonavir combination in COVID‐19: A systematic review, meta‐analysis, and meta‐regression analysis. Indian Journal of Pharmacology, 52(4), 313–323. 10.4103/ijp.IJP_627_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, H. , Zheng, Z.‐H. , Wei, D. , Zhang, Z. , Kang, W.‐Z. , Hao, C.‐Q. , Dong, K. , Kang, W. , Xia, J.‐L. , Miao, J.‐L. , Xie, R.‐H. , Wang, B. , Sun, X.‐X. , Yang, X.‐M. , Lin, P. , Geng, J.‐J. , Wang, K. , Cui, H.‐Y. , Zhang, K. , … Zhu, P. (2020). Meplazumab treats COVID‐19 pneumonia: An open‐labelled, concurrent controlled add‐on clinical trial. MedRxiv, 2020(03), 21.20040691. 10.1101/2020.03.21.20040691 [DOI] [Google Scholar]
- Bianconi, V. , Violi, F. , Fallarino, F. , Pignatelli, P. , Sahebkar, A. , & Pirro, M. (2020). Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID‐19? Drugs, 80(14), 1383–1396. 10.1007/s40265-020-01365-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch, E. M. , Shoham, S. , Casadevall, A. , Sachais, B. S. , Shaz, B. , Winters, J. L. , Van Buskirk, C. , Grossman, B. J. , Joyner, M. , Henderson, J. P. , Pekosz, A. , Lau, B. , Wesolowski, A. , Katz, L. , Shan, H. , Auwaerter, P. G. , Thomas, D. , Sullivan, D. J. , Paneth, N. , … Tobian, A. A. R. (2020). Deployment of convalescent plasma for the prevention and treatment of COVID‐19. Journal of Clinical Investigation, 130(6), 2757–2765. 10.1172/JCI138745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware, D. R. , Pullen, M. F. , Bangdiwala, A. S. , Pastick, K. A. , Lofgren, S. M. , Okafor, E. C. , Skipper, C. P. , Nascene, A. A. , Nicol, M. R. , Abassi, M. , Engen, N. W. , Cheng, M. P. , LaBar, D. , Lother, S. A. , MacKenzie, L. J. , Drobot, G. , Marten, N. , Zarychanski, R. , Kelly, L. E. , … Hullsiek, K. H. (2020). A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID‐19. New England Journal of Medicine, 383(6), 517–525. 10.1056/nejmoa2016638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, E. , Barlow‐Pay, F. , Short, R. , Vilches‐Moraga, A. , Price, A. , McGovern, A. , Braude, P. , Stechman, M. J. , Moug, S. , McCarthy, K. , Hewitt, J. , Carter, B. , & Myint, P. K. (2020). Prior routine use of non‐steroidal anti‐inflammatory drugs (NSAIDs) and important outcomes in hospitalised patients with COVID‐19. Journal of Clinical Medicine, 9(8), 2586. 10.3390/jcm9082586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijsers, B. , Yanginlar, C. , Maciej‐Hulme, M. L. , de Mast, Q. , & van der Vlag, J. (2020). Beneficial non‐anticoagulant mechanisms underlying heparin treatment of COVID‐19 patients. eBioMedicine, 59, 102969. 10.1016/j.ebiom.2020.102969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapuoti, S. , De Rosa, A. , Gelzo, M. , Megna, M. , Raia, M. , Pinchera, B. , Pontarelli, A. , Scotto, R. , Scala, E. , Scarano, F. , Scalia, G. , Castaldo, G. , Fabbrocini, G. , Gentile, I. , & Parrella, R. (2020). Immunocytometric analysis of COVID patients: A contribution to personalized therapy? Life Sciences, 261, 118355. 10.1016/j.lfs.2020.118355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Q. , Yang, M. , Liu, D. , Chen, J. , Shu, D. , Xia, J. , Liao, X. , Gu, Y. , Cai, Q. , Yang, Y. , Shen, C. , Li, X. , Peng, L. , Huang, D. , Zhang, J. , Zhang, S. , Wang, F. , Liu, J. , Chen, L. , … Liu, L. (2020). Experimental treatment with favipiravir for COVID‐19: An open‐label control study. Engineering, 6, 1192–1198. 10.1016/j.eng.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camprubí, D. , Almuedo‐Riera, A. , Martí‐Soler, H. , Soriano, A. , Hurtado, J. C. , Subirà, C. , Grau‐Pujol, B. , Krolewiecki, A. , & Muñoz, J. (2020). Lack of efficacy of standard doses of ivermectin in severe COVID‐19 patients. PLoS ONE, 15(11), e0242184. 10.1371/journal.pone.0242184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini, F. , Niccoli, L. , Matarrese, D. , Nicastri, E. , Stobbione, P. , & Goletti, D. (2020). Baricitinib therapy in COVID‐19: A pilot study on safety and clinical impact. Journal of Infection, 81(2), 318–356W.B. Saunders Ltd. 10.1016/j.jinf.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini, F. , Niccoli, L. , Nannini, C. , Matarrese, D. , Di Natale, M. E. , Lotti, P. , Aquilini, D. , Landini, G. , Cimolato, B. , Di Pietro, M. A. , Trezzi, M. , Stobbione, P. , Frausini, G. , Navarra, A. , Nicastri, E. , Sotgiu, G. , & Goletti, D. (2020). Beneficial impact of Baricitinib in COVID‐19 moderate pneumonia; multicentre study. Journal of Infection, 81(4), 647–679W.B. Saunders Ltd. 10.1016/j.jinf.2020.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B. , Wang, Y. , Wen, D. , Liu, W. , Wang, J. , Fan, G. , Ruan, L. , Song, B. , Cai, Y. , Wei, M. , Li, X. , Xia, J. , Chen, N. , Xiang, J. , Yu, T. , Bai, T. , Xie, X. , Zhang, L. , Li, C. , … Wang, C. (2020). A trial of lopinavir–ritonavir in adults hospitalized with severe COVID‐19. New England Journal of Medicine, 382(19), 1787–1799. 10.1056/nejmoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, W. , Liu, X. , Bai, T. , Fan, H. , Hong, K. , Song, H. , Han, Y. , Lin, L. , Ruan, L. , & Li, T. (2020). High‐dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infectious Diseases, 7(3), 1–6. 10.1093/ofid/ofaa102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano, A. , Rossi, F. , & Paolisso, G. (2020). COVID‐19 kills more men than women: An overview of possible reasons. Frontiers in Cardiovascular Medicine, 7, 131. 10.3389/fcvm.2020.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano, A. , Scavone, C. , Racagni, G. , & Scaglione, F. (2020). NSAIDs in patients with viral infections, including COVID‐19: Victims or perpetrators? Pharmacological Research, 157, 104849. 10.1016/j.phrs.2020.104849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2021). New variants of the virus that causes COVID‐19. https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html
- Chen, C. , Zhang, Y. , Huang, J. , Yin, P. , Cheng, Z. , Wu, J. , Chen, S. , Zhang, Y. , Chen, B. , Lu, M. , Luo, Y. , Ju, L. , Zhang, J. , & Wang, X. (2020). Favipiravir versus arbidol for COVID‐19: A randomized clinical trial. 10.1101/2020.03.17.20037432 [DOI] [PMC free article] [PubMed]
- Chen, J. , Liu, D. , Liu, L. , Liu, P. , Xu, Q. , Xia, L. , Ling, Y. , Huang, D. , Song, S. , Zhang, D. , Qian, Z. , Li, T. , Shen, Y. , & Lu, H. (2020). A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease‐19 (COVID‐19). Journal of Zhejiang University (Medical Science), 49(1), 1–10. 10.3785/j.issn.1008-9292.2020.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Xia, L. , Liu, L. , Xu, Q. , Ling, Y. , Huang, D. , Huang, W. , Song, S. , Xu, S. , Shen, Y. , & Lu, H. (2020). Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID‐19. Open Forum Infectious Diseases, 7(7). 10.1093/ofid/ofaa241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P. , Nirula, A. , Heller, B. , Gottlieb, R. L. , Boscia, J. , Morris, J. , Huhn, G. , Cardona, J. , Mocherla, B. , Stosor, V. , Shawa, I. , Adams, A. C. , Van Naarden, J. , Custer, K. L. , Shen, L. , Durante, M. , Oakley, G. , Schade, A. E. , Sabo, J. , … Skovronsky, D. M. (2020). SARS‐CoV‐2 neutralizing antibody LY‐CoV555 in outpatients with COVID‐19. The New England Journal of Medicine, 384, 229–237. 10.1056/nejmoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Janz, D. R. , Bastarache, J. A. , May, A. K. , O'Neal, H. R. , Bernard, G. R. , & Ware, L. B. (2015). Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: A propensity‐adjusted analysis. Critical Care Medicine, 43(4), 801–807. 10.1097/CCM.0000000000000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, J. H. , Khanna, A. K. , Kethireddy, S. , Yamane, D. , Levine, A. , Jackson, A. M. , McCurdy, M. T. , Tabatabai, A. , Kumar, G. , Park, P. , Benjenk, I. , Menaker, J. , Ahmed, N. , Glidewell, E. , Presutto, E. , Cain, S. , Haridasa, N. , Field, W. , Fowler, J. G. , … Mazzeffi, M. A. (2020). Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in‐hospital mortality in hospitalized patients with COVID‐19. Anesthesia & Analgesia, 132, 930–941. 10.1213/ANE.0000000000005292 [DOI] [PubMed] [Google Scholar]
- Cohen, M. S. (2021). Monoclonal antibodies to disrupt progression of early COVID‐19 infection. The New England Journal of Medicine, 384(3), 289–291. 10.1056/NEJMe2034495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon‐Cardo, C. , Pujadas, E. , Wajnberg, A. , Sebra, R. , Patel, G. , Firpo‐Betancourt, A. , Fowkes, M. , Sordillo, E. , Paniz‐Mondolfi, A. , Gregory, J. , Krammer, F. , Simon, V. , Isola, L. , Soon‐Shiong, P. , Aberg, J. A. , Fuster, V. , & Reich, D. L. (2020). COVID‐19: Staging of a new disease. Cancer Cell, 38(5), 594–597. 10.1016/j.ccell.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer, S. , Bojkova, D. , Cinatl, J. , Van Damme, E. , Buyck, C. , Van Loock, M. , Woodfall, B. , & Ciesek, S. (2020). Lack of antiviral activity of darunavir against SARS‐CoV‐2. International Journal of Infectious Diseases, 97, 7–10. 10.1016/j.ijid.2020.05.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks, E. D. (2018). Darunavir/cobicistat/emtricitabine/tenofovir alafenamide: A review in HIV‐1 infection. Drugs, 78(10), 1013–1024. 10.1007/s40265-018-0934-2 [DOI] [PubMed] [Google Scholar]
- Del Valle, D. M. , Kim‐Schulze, S. , Huang, H. H. , Beckmann, N. D. , Nirenberg, S. , Wang, B. , Lavin, Y. , Swartz, T. H. , Madduri, D. , Stock, A. , Marron, T. U. , Xie, H. , Patel, M. , Tuballes, K. , Van Oekelen, O. , Rahman, A. , Kovatch, P. , Aberg, J. A. , Schadt, E. , … Gnjatic, S. (2020). An inflammatory cytokine signature predicts COVID‐19 severity and survival. Nature Medicine, 26(10), 1636–1643. 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, L. , Li, C. , Zeng, Q. , Liu, X. , Li, X. , Zhang, H. , Hong, Z. , & Xia, J. (2020). Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. Journal of Infection, 81(1), e1–e5. 10.1016/j.jinf.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, K. , Ikeda, M. , Hayase, N. , Moriya, K. , Morimura, N. , Maehara, H. , Tagami, S. , Fukushima, K. , Misawa, N. , Inoue, Y. , Nakamura, H. , Takai, D. , Kurimoto, M. , Tokunaga, K. , Yamamoto, M. , Hirayama, I. , Horie, R. , Endo, Y. , Hiwatashi, K. , … Inoue, J. I. (2020). Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with COVID‐19: A case series. Critical Care, 24(1), 392. 10.1186/s13054-020-03078-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi, P. (2021). Peter Doshi: Pfizer and Moderna's “95% effective” vaccines—We need more details and the raw data. The BMJ. https://blogs.bmj.com/bmj/2021/01/04/peter-doshi-pfizer-and-modernas-95-effective-vaccines-we-need-more-details-and-the-raw-data/ [Google Scholar]
- Duan, K. , Liu, B. , Li, C. , Zhang, H. , Yu, T. , Qu, J. , Zhou, M. , Chen, L. , Meng, S. , Hu, Y. , Peng, C. , Yuan, M. , Huang, J. , Wang, Z. , Yu, J. , Gao, X. , Wang, D. , Yu, X. , Li, L. , … Yang, X. (2020). Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proceedings of the National Academy of Sciences of the United States of America, 117(17), 9490–9496. 10.1073/pnas.2004168117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bairi, K. , Trapani, D. , Petrillo, A. , Le Page, C. , Zbakh, H. , Daniele, B. , Belbaraka, R. , Curigliano, G. , & Afqir, S. (2020). Repurposing anticancer drugs for the management of COVID‐19. European Journal of Cancer, 141, 40–61. 10.1016/j.ejca.2020.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eli Lilly and Company . (n.d.). Lilly's neutralizing antibody bamlanivimab (LY‐CoV555) receives interim authorization from Health Canada as a treatment for COVID‐19. Retrieved December 2, 2020, from https://investor.lilly.com/news-releases/news-release-details/lillys-neutralizing-antibody-bamlanivimab-ly-cov555-receives
- Elsawah, H. K. , Elsokary, M. A. , Abdallah, M. S. , & ElShafie, A. H. (2020). Efficacy and safety of remdesivir in hospitalized COVID‐19 patients: Systematic review and meta‐analysis including network meta‐analysis. Reviews in Medical Virology, e2187. 10.1002/rmv.2187 [DOI] [PubMed] [Google Scholar]
- Erlich, J. M. , Talmor, D. S. , Cartin‐Ceba, R. , Gajic, O. , & Kor, D. J. (2011). Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: A population‐based cohort study. Chest, 139(2), 289–295. 10.1378/chest.10-0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami, G. , Mousaviasl, S. , Radmanesh, E. , Jelvay, S. , Bitaraf, S. , Simmons, B. , Wentzel, H. , Hill, A. , Sadeghi, A. , Freeman, J. , Salmanzadeh, S. , Esmaeilian, H. , Mobarak, M. , Tabibi, R. , Jafari Kashi, A. H. , Lotfi, Z. , Talebzadeh, S. M. , Wickramatillake, A. , Momtazan, M. , … Mobarak, S. (2020). The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID‐19. The Journal of Antimicrobial Chemotherapy, 75(11), 3366–3372. 10.1093/jac/dkaa331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency . (2020a). EMA advice on the use of NSAIDs for COVID‐19. 10.1016/s2213 [DOI]
- European Medicines Agency . (2020b). COVID‐19: Chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes. https://www.ema.europa.eu/en/news/COVID-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes
- European Medicines Agency . (2020c). COVID‐19: Reminder of the risks of chloroquine and hydroxychloroquine. https://www.ema.europa.eu/en/news/COVID-19-reminder-risks-chloroquine-hydroxychloroquine
- European Medicines Agency . (2020d). EMA endorses use of dexamethasone in COVID‐19 patients on oxygen or mechanical ventilation. https://www.ema.europa.eu/en/news/ema-endorses-use-dexamethasone-COVID-19-patients-oxygen-mechanical-ventilation
- European Medicines Agency . (2020e). Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 23–26 November 2020. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-23-26-november-2020
- European Medicines Agency . (2021). COVID‐19 vaccine safety update. https://www.ema.europa.eu/en/documents/COVID-19-vaccine-safety-update/COVID-19-vaccine-safety-update-comirnaty-january-2021_en.pdf
- European Medicines Agency . (n.d.). Update on remdesivir—EMA will evaluate new data from Solidarity trial. Retrieved December 1, 2020, from https://www.ema.europa.eu/en/news/update-remdesivir-ema-will-evaluate-new-data-solidarity-trial
- Favalli, E. G. , Biggioggero, M. , Maioli, G. , & Caporali, R. (2020). Baricitinib for COVID‐19: A suitable treatment? The Lancet Infectious Diseases, 20(9), 1012–1013. 10.1016/S1473-3099(20)30262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr, A. R. , & Perlman, S. (2015). Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses: Methods and Protocols, 1282, 1–23. 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration . (n.d.‐a). Coronavirus (COVID‐19) update: FDA authorizes monoclonal antibodies for treatment of COVID‐19. Retrieved February 12, 2021, from https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-authorizes-monoclonal-antibodies-treatment-COVID-19-0
- Food and Drug Administration . (n.d.‐b). Coronavirus (COVID‐19) update: FDA authorizes monoclonal antibodies for treatment of COVID‐19. Retrieved December 1, 2020, from https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-authorizes-monoclonal-antibodies-treatment-COVID-19
- Galeotti, C. , Kaveri, S. V. , & Bayry, J. (2020). Intravenous immunoglobulin immunotherapy for coronavirus disease‐19 (COVID‐19). Clinical & Translational Immunology, 9(10), e1198. 10.1002/cti2.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, G. , Wang, A. , Wang, S. , Qian, F. , Chen, M. , Yu, F. , Zhang, J. , Wang, X. , Ma, X. , Zhao, T. , Zhang, F. , & Chen, Z. (2020). Brief report: Retrospective evaluation on the efficacy of lopinavir/ritonavir and chloroquine to treat nonsevere COVID‐19 patients. Journal of Acquired Immune Deficiency Syndromes (1999), 85(2), 239–243. 10.1097/QAI.0000000000002452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret, P. , Lagier, J. C. , Parola, P. , Hoang, V. T. , Meddeb, L. , Mailhe, M. , Doudier, B. , Courjon, J. , Giordanengo, V. , Vieira, V. E. , Tissot Dupont, H. , Honoré, S. , Colson, P. , Chabrière, E. , La Scola, B. , Rolain, J. M. , Brouqui, P. , & Raoult, D. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID‐19: Results of an open‐label non‐randomized clinical trial. International Journal of Antimicrobial Agents, 56(1), 105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Goldman, J. D. , Lye, D. C. B. , Hui, D. S. , Marks, K. M. , Bruno, R. , Montejano, R. , Spinner, C. D. , Galli, M. , Ahn, M.‐Y. , Nahass, R. G. , Chen, Y.‐S. , SenGupta, D. , Hyland, R. H. , Osinusi, A. O. , Cao, H. , Blair, C. , Wei, X. , Gaggar, A. , Brainard, D. M. , … Subramanian, A. (2020). Remdesivir for 5 or 10 days in patients with severe COVID‐19. The New England Journal of Medicine, 383, 1827–1837. 10.1056/nejmoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzo, L. , Viale, P. , Longo, L. , Vitale, D. C. , & Drago, F. (2020). The potential role of heparin in patients with COVID‐19: Beyond the anticoagulant effect. A review. Frontiers in Pharmacology, 11. 10.3389/fphar.2020.01307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremese, E. , Cingolani, A. , Bosello, S. L. , Alivernini, S. , Tolusso, B. , Perniola, S. , Landi, F. , Pompili, M. , Murri, R. , Santoliquido, A. , Garcovich, M. , Sali, M. , De Pascale, G. , Gabrielli, M. , Biscetti, F. , Montalto, M. , Tosoni, A. , Gambassi, G. , Rapaccini, G. L. , … Gasbarrini, A. (2020). Sarilumab use in severe SARS‐CoV‐2 pneumonia. EClinicalMedicine, 27, 100553. 10.1016/j.eclinm.2020.100553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y. R. , Cao, Q. D. , Hong, Z. S. , Tan, Y. Y. , Chen, S. D. , Jin, H. J. , Tan, K. S. , Wang, D. Y. , & Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—An update on the status. Military Medical Research, 7(1), 11. 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie, C. E. , Mackay, D. F. , Ho, F. , Celis‐Morales, C. A. , Katikireddi, S. V. , Niedzwiedz, C. L. , Jani, B. D. , Welsh, P. , Mair, F. S. , Gray, S. R. , O'Donnell, C. A. , Gill, J. M. , Sattar, N. , & Pell, J. P. (2020). Vitamin D concentrations and COVID‐19 infection in UK Biobank. Diabetes and Metabolic Syndrome: Clinical Research and Reviews, 14(4), 561–565. 10.1016/j.dsx.2020.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N. H. , Nitsche, A. , Müller, M. A. , Drosten, C. , & Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby, P. , Lim, W. S. , Emberson, J. R. , Mafham, M. , Bell, J. L. , Linsell, L. , Staplin, N. , Brightling, C. , Ustianowski, A. , Elmahi, E. , Prudon, B. , Green, C. , Felton, T. , Chadwick, D. , Rege, K. , Fegan, C. , Chappel, L. C. , Faust, S. N. , Jaki, T. , … Landray, M. J. (2020). Dexamethasone in hospitalized patients with COVID‐19—Preliminary report. The New England Journal of Medicine, 384, 693–704. 10.1056/nejmoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby, P. W. , Mafham, M. , Bell, J. L. , Linsell, L. , Staplin, N. , Emberson, J. , Palfreeman, A. , Raw, J. , Elmahi, E. , Prudon, B. , Green, C. , Carley, S. , Chadwick, D. , Davies, M. , Wise, M. P. , Baillie, J. K. , Chappell, L. C. , Faust, S. N. , Jaki, T. , … Landray, M. J. (2020). Lopinavir–ritonavir in patients admitted to hospital with COVID‐19 (RECOVERY): A randomised, controlled, open‐label, platform trial. The Lancet, 396(10259), 1345–1352. 10.1016/S0140-6736(20)32013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Tang, T. , Pang, P. , Li, M. , Ma, R. , Lu, J. , Shu, J. , You, Y. , Chen, B. , Liang, J. , Hong, Z. , Chen, H. , Kong, L. , Qin, D. , Pei, D. , Xia, J. , Jiang, S. , & Shan, H. (2020). Treating COVID‐19 with chloroquine. Journal of Molecular Cell Biology, 12(4), 322–325. 10.1093/jmcb/mjaa014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italian Medicines Agency . (2020a). COVID‐19: STUDIO RANDOMIZZATO ITALIANO, NESSUN BENEFICIO DAL TOCILIZUMAB. https://www.aifa.gov.it/documents/20142/823882/Comunicato_AIFA_n.600.pdf/158129e8-6cf2-1c5a-1fca-5f6072536f44
- Italian Medicines Agency . (2020b). Idrossiclorochina nella terapia dei pazienti adulti con COVID‐19. https://www.aifa.gov.it/documents/20142/1123276/idrossiclorochina_22.07.2020.pdf/764add8f-f08f-0e26-df75-952986e54b8b
- Italian Medicines Agency . (n.d.). RIASSUNTO DELLE CARATTERISTICHE DEL PRODOTTO 2010. Retrieved December 3, 2020, from https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFieName=footer_008055_013967_RCP.pdf%26retry=0%26sys=m0b1l3
- Ivashchenko, A. A. , Dmitriev, K. A. , Vostokova, N. V. , Azarova, V. N. , Blinow, A. A. , Egorova, A. N. , Gordeev, I. G. , Ilin, A. P. , Karapetian, R. N. , Kravchenko, D. V. , Lomakin, N. V. , Merkulova, E. A. , Papazova, N. A. , Pavlikova, E. P. , Savchuk, N. P. , Simakina, E. N. , Sitdekov, T. A. , Smolyarchuk, E. A. , Tikhomolova, E. G. , … Ivachtchenko, A. V. (2020). AVIFAVIR for treatment of patients with moderate COVID‐19: Interim results of a Phase II/III multicenter randomized clinical trial. Clinical Infectious Diseases, ciaa1176. 10.1093/cid/ciaa1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaka, S. , Shono, Y. , Tokuda, K. , Nakashima, K. , Yamamoto, Y. , Maki, J. , Nagasaki, Y. , Shimono, N. , Akahoshi, T. , & Taguchi, T. (2020). Clinical improvement in a patient with severe coronavirus disease 2019 after administration of hydroxychloroquine and continuous hemodiafiltlation with nafamostat mesylate. Journal of Infection and Chemotherapy, 26(12), 1319–1323. 10.1016/j.jiac.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo, C. M. P. , Farias, M. E. L. , Val, F. F. A. , Sampaio, V. S. , Alexandre, M. A. A. , Melo, G. C. , Safe, I. P. , Borba, M. G. S. , Netto, R. L. A. , Maciel, A. B. S. , Neto, J. R. S. , Oliveira, L. B. , Figueiredo, E. F. G. , Oliveira Dinelly, K. M. , de Almeida Rodrigues, M. G. , Brito, M. , Mourão, M. P. G. , Pivoto João, G. A. , Hajjar, L. A. , … Metcovid Team . (2020). Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID‐19; Metcovid): A randomized, double‐blind, Phase IIb, placebo‐controlled trial. Clinical Infectious Diseases, ciaa1177. 10.1093/cid/ciaa1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson & Johnson . (2021). Johnson & Johnson announces single‐shot Janssen COVID‐19 vaccine candidate met primary endpoints in interim analysis of its Phase 3 ENSEMBLE trial. https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-COVID-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial
- Karaderi, T. , Bareke, H. , Kunter, I. , Seytanoglu, A. , Cagnan, I. , Balci, D. , Barin, B. , Hocaoglu, M. B. , Rahmioglu, N. , Asilmaz, E. , & Taneri, B. (2020). Host genetics at the intersection of autoimmunity and COVID‐19: A potential key for heterogeneous COVID‐19 severity. Frontiers in Immunology, 11(586111), 1. 10.3389/fimmu.2020.586111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamis, F. , Al Naabi, H. , Al Lawati, A. , Ambusaidi, Z. , Al Sharji, M. , Al Barwani, U. , Pandak, N. , Al Balushi, Z. , Al Bahrani, M. , & Al‐Zakwani, I. (2020). Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta‐1b in hospitalized patients with moderate to severe COVID‐19 pneumonia. International Journal of Infectious Diseases, 102, 538–543. 10.1016/j.ijid.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. J. , Choi, S. H. , Park, J. S. , Kwon, Y. S. , Lee, J. , Kim, Y. , Lee, S. Y. , & Choi, E. Y. (2020). Use of darunavir‐cobicistat as a treatment option for critically ill patients with SARS‐CoV‐2 infection. Yonsei Medical Journal, 61(9), 826–830. 10.3349/ymj.2020.61.9.826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kor, D. J. , Carter, R. E. , Park, P. K. , Festic, E. , Banner‐Goodspeed, V. M. , Hinds, R. , Talmor, D. , Gajic, O. , Ware, L. B. , & Gong, M. N. (2016). Effect of aspirin on development of ARDS in at‐risk patients presenting to the emergency department. The LIPS—A randomized clinical trial. JAMA: The Journal of the American Medical Association, 315(22), 2406–2414. 10.1001/jama.2016.6330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kor, D. J. , Erlich, J. , Gong, M. N. , Malinchoc, M. , Carter, R. E. , Gajic, O. , & Talmor, D. S. (2011). Association of prehospitalization aspirin therapy and acute lung injury: Results of a multicenter international observational study of at‐risk patients. Critical Care Medicine, 39(11), 2393–2400. 10.1097/CCM.0b013e318225757f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence, J. , Mulvey, J. J. , Seshadri, M. , Racanelli, A. , Harp, J. , Schenck, E. J. , Zappetti, D. , Horn, E. M. , & Magro, C. M. (2020). Anti‐complement C5 therapy with eculizumab in three cases of critical COVID‐19. Clinical Immunology, 219, 108555. 10.1016/j.clim.2020.108555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppkes, M. , Knopf, J. , Naschberger, E. , Lindemann, A. , Singh, J. , Herrmann, I. , Stürzl, M. , Staats, L. , Mahajan, A. , Schauer, C. , Kremer, A. N. , Völkl, S. , Amann, K. , Evert, K. , Falkeis, C. , Wehrfritz, A. , Rieker, R. J. , Hartmann, A. , Kremer, A. E. , … Herrmann, M. (2020). Vascular occlusion by neutrophil extracellular traps in COVID‐19. eBioMedicine, 58, 102925. 10.1016/j.ebiom.2020.102925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, T. C. , Adhikari, S. , Tatapudi, V. , Holub, M. , Kunichoff, D. , Troxel, A. B. , Montgomery, R. A. , & Sterman, D. H. (2020). A propensity‐matched cohort study of tocilizumab in patients with coronavirus disease 2019. Critical Care Explorations, 2(11), e0283. 10.1097/CCE.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Liu, L. , Zhang, D. , Xu, J. , Dai, H. , Tang, N. , Su, X. , & Cao, B. (2020). SARS‐CoV‐2 and viral sepsis: Observations and hypotheses. The Lancet, 395(10235), 1517–1520. 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Zhang, W. , Hu, Y. , Tong, X. , Zheng, S. , Yang, J. , Kong, Y. , Ren, L. , Wei, Q. , Mei, H. , Hu, C. , Tao, C. , Yang, R. , Wang, J. , Yu, Y. , Guo, Y. , Wu, X. , Xu, Z. , Zeng, L. , … Liu, Z. (2020). Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: A randomized clinical trial. JAMA: The Journal of the American Medical Association, 324(5), 460–470. 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Xie, Z. , Lin, W. , Cai, W. , Wen, C. , Guan, Y. , Mo, X. , Wang, J. , Wang, Y. , Peng, P. , Chen, X. , Hong, W. , Xiao, G. , Liu, J. , Zhang, L. , Hu, F. , Li, F. , Zhang, F. , Deng, X. , & Li, L. (2020). Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID‐19: An exploratory randomized controlled trial. Med, 1, 105–113.e4. 10.1016/j.medj.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly . (2021). Lilly's neutralizing antibody bamlanivimab (LY‐CoV555) prevented COVID‐19 at nursing homes in the BLAZE‐2 trial, reducing risk by up to 80 percent for residents. Eli Lilly and Company. https://investor.lilly.com/news-releases/news-release-details/lillys-neutralizing-antibody-bamlanivimab-ly-cov555-prevented
- Liu, S. T. H. , Lin, H. M. , Baine, I. , Wajnberg, A. , Gumprecht, J. P. , Rahman, F. , Rodriguez, D. , Tandon, P. , Bassily‐Marcus, A. , Bander, J. , Sanky, C. , Dupper, A. , Zheng, A. , Nguyen, F. T. , Amanat, F. , Stadlbauer, D. , Altman, D. R. , Chen, B. K. , Krammer, F. , … Bouvier, N. M. (2020). Convalescent plasma treatment of severe COVID‐19: A propensity score‐matched control study. Nature Medicine, 26(11), 1708–1713. 10.1038/s41591-020-1088-9 [DOI] [PubMed] [Google Scholar]
- Lloyd, E. C. , Gandhi, T. N. , & Petty, L. A. (2021). Monoclonal antibodies for COVID‐19. Journal of the American Medical Association, 325(10), 1015. 10.1001/jama.2021.1225 [DOI] [PubMed] [Google Scholar]
- Lodigiani, C. , Iapichino, G. , Carenzo, L. , Cecconi, M. , Ferrazzi, P. , Sebastian, T. , Kucher, N. , Studt, J. D. , Sacco, C. , Alexia, B. , Sandri, M. T. , & Barco, S. (2020). Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research, 191, 9–14. 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, Y. , Liu, L. , Yao, H. , Hu, X. , Su, J. , Xu, K. , Luo, R. , Yang, X. , He, L. , Lu, X. , Zhao, Q. , Liang, T. , & Qiu, Y. (2020). Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID‐19 patients: An exploratory randomized, controlled trial. European Journal of Pharmaceutical Sciences, 157, 105631. 10.1016/j.ejps.2020.105631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marietta, M. , Ageno, W. , Artoni, A. , De Candia, E. , Gresele, P. , Marchetti, M. , Marcucci, R. , & Tripodi, A. (2020). COVID‐19 and haemostasis: A position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfusion, 18(3), 167–169. 10.2450/2020.0083-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascolo, A. , Berrino, P. M. , Gareri, P. , Castagna, A. , Capuano, A. , Manzo, C. , & Berrino, L. (2018). Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: A review article. Inflammopharmacology, 26(5), 1141–1149. 10.1007/s10787-018-0498-5 [DOI] [PubMed] [Google Scholar]
- Mascolo, A. , Scavone, C. , Rafaniello, C. , Ferrajolo, C. , Racagni, G. , Berrino, L. , Paolisso, G. , Rossi, F. , & Capuano, A. (2020). Renin‐angiotensin system and coronavirus disease 2019: A narrative review. Frontiers in Cardiovascular Medicine, 7, 143. 10.3389/fcvm.2020.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy, K. , Peterson, A. , Tian, Y. , & Sang, Y. (2020). Immunogenetic association underlying severe COVID‐19. Vaccine, 8(4), 1–13. 10.3390/vaccines8040700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle, D. , O'Donnell, J. S. , Sharif, K. , Emery, P. , & Bridgewood, C. (2020). Immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia. The Lancet Rheumatology, 2(7), e437–e445. 10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministère de la Santé . (2020). Liste des messages d'alertes. https://dgs‐urgent.sante.gouv.fr/dgsurgent/inter/detailsMessageBuilder.do;jsessionid=21ECACBF8B1ECE6C542B9126E7A8215F.du‐dgsurgentc2?id=30500%26cmd=visualiserMessage
- Mitchell, F. (2020). Vitamin‐D and COVID‐19: Do deficient risk a poorer outcome? The Lancet Diabetes and Endocrinology, 8(7), 570. 10.1016/S2213-8587(20)30183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina, J. M. , Delaugerre, C. , Le Goff, J. , Mela‐Lima, B. , Ponscarme, D. , Goldwirt, L. , & de Castro, N. (2020). No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID‐19 infection. Médecine et Maladies Infectieuses, 50(4), 384. 10.1016/j.medmal.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesarchio, V. , Parella, R. , Iommelli, C. , Bianco, A. , Manzillo, E. , Fraganza, F. , Palumbo, C. , Rea, G. , Murino, P. , De Rosa, R. , Atripaldi, L. , D'Abbraccio, M. , Curvietto, M. , Mallardo, D. , Celentano, E. , Grimaldi, A. M. , Palla, M. , Trojaniello, C. , Vitale, M. G. , … Ascierto, P. A. (2020). Outcomes and biomarker analyses among patients with COVID‐19 treated with interleukin 6 (IL‐6) receptor antagonist sarilumab at a single institution in Italy. Journal for Immunotherapy of Cancer, 8(2), e001089. 10.1136/jitc-2020-001089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores, L. K. , Tritschler, T. , Brosnahan, S. , Carrier, M. , Collen, J. F. , Doerschug, K. , Holley, A. B. , Jimenez, D. , Le Gal, G. , Rali, P. , & Wells, P. (2020). Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest, 158(3), 1143–1163. 10.1016/j.chest.2020.05.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni, G. N. , Lala, A. , Bagiella, E. , Chang, H. L. , Moreno, P. R. , Pujadas, E. , Arvind, V. , Bose, S. , Charney, A. W. , Chen, M. D. , Cordon‐Cardo, C. , Dunn, A. S. , Farkouh, M. E. , Glicksberg, B. S. , Kia, A. , Kohli‐Seth, R. , Levin, M. A. , Timsina, P. , Zhao, S. , … Fuster, V. (2020). Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID‐19. Journal of the American College of Cardiology, 76(16), 1815–1826. 10.1016/j.jacc.2020.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health . (2020). Antithrombotic therapy. COVID‐19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/antithrombotic-therapy/ [PubMed]
- Nicolini, L. A. , Mikulska, M. , Signori, A. , Di Biagio, A. , Portunato, F. , Vena, A. , Giacomini, M. , & Bassetti, M. (2020). Reply to: “Antiviral activity and safety of darunavir/cobicistat for treatment of COVID‐19”. Open Forum Infectious Diseases, 7(8), ofaa321. 10.1093/ofid/ofaa321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe, I. , Fuster, V. , Lala, A. , Russak, A. J. , Glicksberg, B. S. , Levin, M. A. , Charney, A. W. , Narula, J. , Fayad, Z. A. , Bagiella, E. , Zhao, S. , & Nadkarni, G. N. (2020). Association of treatment dose anticoagulation with in‐hospital survival among hospitalized patients with COVID‐19. Journal of the American College of Cardiology, 76(1), 122–124. 10.1016/j.jacc.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavoni, V. , Gianesello, L. , Pazzi, M. , Stera, C. , Meconi, T. , & Frigieri, F. C. (2020). Venous thromboembolism and bleeding in critically ill COVID‐19 patients treated with higher than standard low molecular weight heparin doses and aspirin: A call to action. Thrombosis Research, 196, 313–317. 10.1016/j.thromres.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone, F. , Piccirillo, M. C. , Ascierto, P. A. , Salvarani, C. , Parrella, R. , Marata, A. M. , Popoli, P. , Ferraris, L. , Marrocco‐Trischitta, M. M. , Ripamonti, D. , Binda, F. , Bonfanti, P. , Squillace, N. , Castelli, F. , Muiesan, M. L. , Lichtner, M. , Calzetti, C. , Salerno, N. D. , Atripaldi, L. , … Gallo, C. (2020). Tocilizumab for patients with COVID‐19 pneumonia. The single‐arm TOCIVID‐19 prospective trial. Journal of Translational Medicine, 18(1), 405. 10.1186/s12967-020-02573-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzini, A. , Aichner, M. , Sahanic, S. , Böhm, A. , Egger, A. , Hoermann, G. , Kurz, K. , Widmann, G. , Bellmann‐Weiler, R. , Weiss, G. , Tancevski, I. , Sonnweber, T. , & Löffler‐Ragg, J. (2020). Impact of vitamin D deficiency on COVID‐19—A prospective analysis from the CovILD registry. Nutrients, 12(9), 1–9. 10.3390/nu12092775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack, F. P. , Thomas, S. J. , Kitchin, N. , Absalon, J. , Gurtman, A. , Lockhart, S. , Perez, J. L. , Pérez Marc, G. , Moreira, E. D. , Zerbini, C. , Bailey, R. , Swanson, K. A. , Roychoudhury, S. , Koury, K. , Li, P. , Kalina, W. V. , Cooper, D. , Frenck, R. W. , Hammitt, L. L. , … C4591001 Clinical Trial Group . (2020). Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. The New England Journal of Medicine, 383, 2603–2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Z. , Travanty, E. A. , Oko, L. , Edeen, K. , Berglund, A. , Wang, J. , Ito, Y. , Holmes, K. V. , & Mason, R. J. (2013). Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome–coronavirus. American Journal of Respiratory Cell and Molecular Biology, 48(6), 742–748. 10.1165/rcmb.2012-0339OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajter, J. C. , Sherman, M. S. , Fatteh, N. , Vogel, F. , Sacks, J. , & Rajter, J.‐J. (2020). Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019. Chest, 159, 85–92. 10.1016/j.chest.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regeneron Pharmaceuticals, Inc . (n.d.). Regeneron's REGN‐COV2 antibody cocktail reduced viral levels and improved symptoms in non‐hospitalized COVID‐19 patients. Retrieved December 1, 2020, from https://investor.regeneron.com/news-releases/news-release-details/regenerons-regn-cov2-antibody-cocktail-reduced-viral-levels-and/
- Rinott, E. , Kozer, E. , Shapira, Y. , Bar‐Haim, A. , & Youngster, I. (2020). Ibuprofen use and clinical outcomes in COVID‐19 patients. Clinical Microbiology and Infection, 26(9), 1259.e5–1259.e7. 10.1016/j.cmi.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche . (2020). Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID‐19 associated pneumonia. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm
- Sadeghi, A. , Ali Asgari, A. , Norouzi, A. , Kheiri, Z. , Anushirvani, A. , Montazeri, M. , Hosamirudsai, H. , Afhami, S. , Akbarpour, E. , Aliannejad, R. , Radmard, A. R. , Davarpanah, A. H. , Levi, J. , Wentzel, H. , Qavi, A. , Garratt, A. , Simmons, B. , Hill, A. , & Merat, S. (2020). Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID‐19): A randomized controlled trial. The Journal of Antimicrobial Chemotherapy, 75(11), 3379–3385. 10.1093/jac/dkaa334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanofi . (2020). Sanofi and GSK announce a delay in their adjuvanted recombinant protein‐based COVID‐19 vaccine program to improve immune response in the elderly. https://www.sanofi.com/en/media-room/press-releases/2020/2020-12-11-07-00-00
- Scavone, C. , Brusco, S. , Bertini, M. , Sportiello, L. , Rafaniello, C. , Zoccoli, A. , Berrino, L. , Racagni, G. , Rossi, F. , & Capuano, A. (2020). Current pharmacological treatments for COVID‐19: What's next? British Journal of Pharmacology, 177(21), 4813–4824. 10.1111/bph.15072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, L. J. (2017). Tocilizumab: A review in rheumatoid arthritis. Drugs, 77(17), 1865–1879. 10.1007/s40265-017-0829-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, C. , Wang, Z. , Zhao, F. , Yang, Y. , Li, J. , Yuan, J. , Wang, F. , Li, D. , Yang, M. , Xing, L. , Wei, J. , Xiao, H. , Yang, Y. , Qu, J. , Qing, L. , Chen, L. , Xu, Z. , Peng, L. , Li, Y. , … Liu, L. (2020). Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA: The Journal of the American Medical Association, 323(16), 1582–1589. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, C. , Wang, C. , Wang, H. , Yang, C. , Cai, F. , Zeng, F. , Cheng, F. , Liu, Y. , Zhou, T. , Deng, B. , Vlodavsky, I. , Li, J. , & Zhang, Y. (2020). The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID‐19 patients: A retrospective clinical study. Rappaport Faculty of Medicine, 13(6), 1087–1095. 10.1101/2020.03.28.20046144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovich, V. A. , Burgos Pratx, L. D. , Scibona, P. , Beruto, M. V. , Vallone, M. G. , Vázquez, C. , Savoy, N. , Giunta, D. H. , Pérez, L. G. , del Sánchez, M. L. , Gamarnik, A. V. , Ojeda, D. S. , Santoro, D. M. , Camino, P. J. , Antelo, S. , Rainero, K. , Vidiella, G. P. , Miyazaki, E. A. , Cornistein, W. , … Belloso, W. H. (2020). A randomized trial of convalescent plasma in COVID‐19 severe pneumonia. The New England Journal of Medicine, 384, 619–629. 10.1056/nejmoa2031304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper, C. P. , Pastick, K. A. , Engen, N. W. , Bangdiwala, A. S. , Abassi, M. , Lofgren, S. M. , Williams, D. A. , Okafor, E. C. , Pullen, M. F. , Nicol, M. R. , Nascene, A. A. , Hullsiek, K. H. , Cheng, M. P. , Luke, D. , Lother, S. A. , MacKenzie, L. J. , Drobot, G. , Kelly, L. E. , Schwartz, I. S. , … Boulware, D. R. (2020). Hydroxychloroquine in nonhospitalized adults with early COVID‐19. Annals of Internal Medicine, 173(8), 623–631. 10.7326/M20-4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner, C. D. , Gottlieb, R. L. , Criner, G. J. , Arribas López, J. R. , Cattelan, A. M. , Soriano Viladomiu, A. , Ogbuagu, O. , Malhotra, P. , Mullane, K. M. , Castagna, A. , Chai, L. Y. A. , Roestenberg, M. , Tsang, O. T. Y. , Bernasconi, E. , Le Turnier, P. , Chang, S. C. , Sengupta, D. , Hyland, R. H. , Osinusi, A. O. , … Marty, F. M. (2020). Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: A randomized clinical trial. JAMA: The Journal of the American Medical Association, 324(11), 1048–1057. 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne, J. A. C. , Murthy, S. , Diaz, J. V. , Slutsky, A. S. , Villar, J. , Angus, D. C. , Annane, D. , Azevedo, L. C. P. , Berwanger, O. , Cavalcanti, A. B. , Dequin, P. F. , Du, B. , Emberson, J. , Fisher, D. , Giraudeau, B. , Gordon, A. C. , Granholm, A. , Green, C. , Haynes, R. , … Marshall, J. C. (2020). Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: A meta‐analysis. JAMA: The Journal of the American Medical Association, 324(13), 1330–1341. 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Florentine . (2020). COVID‐19: Monoclonal antibody treatment developed in Siena. https://www.theflorentine.net/2020/11/03/COVID-19-monoclonal-antibody-treatment-developed-in-siena/
- The New York Times . (2021). What to know of COVID‐19 antibody drugs: Cost, availability and more. https://www.nytimes.com/2020/12/23/health/coronavirus-antibody-drugs.html
- Tiberghien, P. , de Lamballerie, X. , Morel, P. , Gallian, P. , Lacombe, K. , & Yazdanpanah, Y. (2020). Collecting and evaluating convalescent plasma for COVID‐19 treatment: Why and how? Vox Sanguinis, 115(6), 488–494. 10.1111/vox.12926 [DOI] [PubMed] [Google Scholar]
- Tleyjeh, I. M. , Kashour, Z. , Damlaj, M. , Riaz, M. , Tlayjeh, H. , Altannir, M. , Altannir, Y. , Al‐Tannir, M. , Tleyjeh, R. , Hassett, L. , & Kashour, T. (2020). Efficacy and safety of tocilizumab in COVID‐19 patients: A living systematic review and meta‐analysis. Clinical Microbiology and Infection, 27, 215–227. 10.1016/j.cmi.2020.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomazini, B. M. , Maia, I. S. , Cavalcanti, A. B. , Berwanger, O. , Rosa, R. G. , Veiga, V. C. , Avezum, A. , Lopes, R. D. , Bueno, F. R. , Silva, M. V. A. O. , Baldassare, F. P. , Costa, E. L. V. , Moura, R. A. B. , Honorato, M. O. , Costa, A. N. , Damiani, L. P. , Lisboa, T. , Kawano‐Dourado, L. , Zampieri, F. G. , … Azevedo, L. C. P. (2020). Effect of dexamethasone on days alive and ventilator‐free in patients with moderate or severe acute respiratory distress syndrome and COVID‐19: The CoDEX randomized clinical trial. JAMA: The Journal of the American Medical Association, 324(13), 1307–1316. 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Oxford . (2020a). Aspirin to be investigated as a possible treatment for COVID‐19 in the RECOVERY trial. https://www.recoverytrial.net/news/aspirin-to-be-investigated-as-a-possible-treatment-for-COVID-19-in-the-recovery-trial
- University of Oxford . (2020b). Welcome—RECOVERY trial. https://www.recoverytrial.net/
- US Food and Drug Administration . (2020). FDA cautions against use of hydroxychloroquine or chloroquine for COVID‐19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-COVID-19-outside-hospital-setting-or
- Uzunova, K. , Filipova, E. , Pavlova, V. , & Vekov, T. (2020). Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS‐CoV‐2. Biomedicine and Pharmacotherapy, 131, 110668. 10.1016/j.biopha.2020.110668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey, M. , Ann, S. , Clemens, C. , Madhi, S. A. , Weckx, L. Y. , Folegatti, P. M. , Aley, P. K. , Angus, B. , Baillie, V. L. , Barnabas, S. L. , Bhorat, Q. E. , Bibi, S. , Briner, C. , Cicconi, P. , Collins, A. M. , Colin‐Jones, R. , Cutland, C. L. , Darton, T. C. , Dheda, K. , … Pollard, A. J. (2020). Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet, 397, 99–111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Nikrad, M. P. , Phang, T. , Gao, B. , Alford, T. , Ito, Y. , Edeen, K. , Travanty, E. A. , Kosmider, B. , Hartshorn, K. , & Mason, R. J. (2011). Innate immune response to influenza A virus in differentiated human alveolar type II cells. American Journal of Respiratory Cell and Molecular Biology, 45(3), 582–591. 10.1165/rcmb.2010-0108OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Wu, T. , Zuo, Z. , You, Y. , Yang, X. , Pan, L. , Hu, Y. , Luo, X. , Jiang, L. , Xia, Z. , & Deng, M. (2020). Evaluation of current medical approaches for COVID‐19: A systematic review and meta‐analysis. BMJ Supportive & Palliative Care, 11, 45–52. 10.1136/bmjspcare-2020-002554 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Zhang, D. , Du, G. , Du, R. , Zhao, J. , Jin, Y. , Fu, S. , Gao, L. , Cheng, Z. , Lu, Q. , Hu, Y. , Luo, G. , Wang, K. , Lu, Y. , Li, H. , Wang, S. , Ruan, S. , Yang, C. , Mei, C. , … Wang, C. (2020). Remdesivir in adults with severe COVID‐19: A randomised, double‐blind, placebo‐controlled, multicentre trial. The Lancet, 395(10236), 1569–1578. 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2020a). “Solidarity” clinical trial for COVID‐19 treatments. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-COVID-19-treatments
- World Health Organization . (2020b). Clinical management of COVID‐19. https://www.who.int/publications/i/item/clinical-management-of-COVID-19 [PubMed]
- World Health Organization . (2020c). DRAFT landscape of COVID‐19 candidate vaccines. https://www.who.int/docs/default-source/a-future-for-children/novel-coronavirus_landscape_COVID-19.pdf?sfvrsn=4d8bd201_1
- World Health Organization . (n.d.). WHO recommends against the use of remdesivir in COVID‐19 patients. Retrieved December 1, 2020, from https://www.who.int/news-room/feature-stories/detail/who-recommends-against-the-use-of-remdesivir-in-COVID-19-patients
- Xu, X. , Han, M. , Li, T. , Sun, W. , Wang, D. , Fu, B. , Zhou, Y. , Zheng, X. , Yang, Y. , Li, X. , Zhang, X. , Pan, A. , & Wei, H. (2020). Effective treatment of severe COVID‐19 patients with tocilizumab. Proceedings of the National Academy of Sciences of the United States of America, 117(20), 10970–10975. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhang, Y. , Qiao, W. , Zhang, J. , & Qi, Z. (2020). Baricitinib, a drug with potential effect to prevent SARS‐COV‐2 from entering target cells and control cytokine storm induced by COVID‐19. International Immunopharmacology, 86, 106749. 10.1016/j.intimp.2020.106749 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no new data were created or analysed in this study.


