Abstract
The response to the ongoing COVID‐19 pandemic has been characterized by draconian measures and far too many important unknowns, such as the true mortality risk, the role of children as transmitters and the development and duration of immunity in the population. More than a year into the pandemic much has been learned and insights into this novel type of pandemic and options for control are shaping up. Using a historical lens, we review what we know and still do not know about the ongoing COVID‐19 pandemic. A pandemic caused by a member of the coronavirus family is a new situation following more than a century of influenza A pandemics. However, recent pandemic threats such as outbreaks of the related and novel deadly coronavirus SARS in 2003 and of MERS since 2012 had put coronaviruses on WHOs blueprint list of priority diseases. Like pandemic influenza, SARS‐CoV‐2 is highly transmissible (R 0 ~ 2.5). Furthermore, it can fly under the radar due to a broad clinical spectrum where asymptomatic and pre‐symptomatic infected persons also transmit the virus—including children. COVID‐19 is far more deadly than seasonal influenza; initial data from China suggested a case fatality rate of 2.3%—which would have been on par with the deadly 1918 Spanish influenza. But, while the Spanish influenza killed young, otherwise healthy adults, it is the elderly who are at extreme risk of dying of COVID‐19. We review available seroepidemiological evidence of infection rates and compute infection fatality rates (IFR) for Denmark (0.5%), Spain (0.85%), and Iceland (0.3%). We also deduce that population age structure is key. SARS‐CoV‐2 is characterized by superspreading, so that ~10% of infected individuals yield 80% of new infections. This phenomenon turns out to be an Achilles heel of the virus that may explain our ability to effectively mitigate outbreaks so far. How will this pandemic come to an end? Herd immunity has not been achieved in Europe due to intense mitigation by non‐pharmaceutical interventions; for example, only ~8% of Danes were infected across the 1st and 2nd wave. Luckily, we now have several safe and effective vaccines. Global vaccine control of the pandemic depends in great measure on our ability to keep up with current and future immune escape variants of the virus. We should thus be prepared for a race between vaccine updates and mutations of the virus. A permanent reopening of society highly depends on winning that race.
Keywords: Coronavirus, COVID‐19, mortality, pandemic, superspreading
Coronaviruses: An Era of New Pandemic Threats
SARS‐CoV‐2, the virus that causes COVID‐19, has led to the first confirmed coronavirus pandemic, and to many it has come as a surprise. We have experienced regular influenza pandemics for centuries [1], but there have been signs for some time that something new was on the horizon. A first warning came with the deadly outbreak of SARS‐CoV in Asia in 2003, in which 10% of known cases died; the outbreak was controlled, and the virus eliminated rapidly despite the high transmissibility. Then MERS‐CoV emerged in the Middle East in 2012, a virus with a far higher mortality rate but a poorer ability to spread among humans; it remains a pandemic threat to this day [2]. Previously, coronaviruses were thought to cause only mild illness in humans as the four existing human coronaviruses merely cause a common cold, but after these outbreaks, coronaviruses were put on WHOs blueprint list of priority diseases [3]. Predicting the severity and virus family of the next pandemic is difficult, but one thing is certain: Pandemics will occur intermittently in the future, as they have done historically [1].
Pandemic influenza has been characterized by an emerging novel virus that has adapted to spread effectively among humans. It has historically been accompanied by a shift in mortality to younger ages [4, 5]. But the COVID‐19 deaths are largely affecting the elderly, with a mean of ~80 years in Denmark. Likewise, only 2.7% of Danish COVID‐19 deaths have occurred in people younger than 60 years of age as of February 15, 2021 [6]. This is quite different from historic influenza pandemics [7]: the Spanish Flu (1918), the Asian Flu (1957), the Hong Kong Flu (1968), and the Swine Flu (2009). In both the 1918 and 2009 pandemics, the mean age at death was 25–30 years, and 95% of deaths occurred in people younger than 65 years of age because of a greater degree of immunity in the older generations. The pandemics of 1957 and 1968 were somewhere in between these extremes in terms of age distribution [8]. A historical timeline of pandemics is seen in Fig. 1.
Fig. 1.
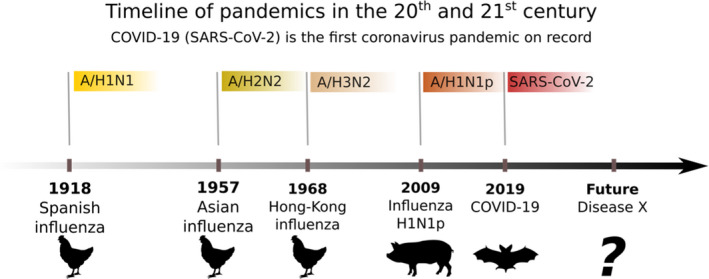
Timeline of respiratory viral pandemics in the 20th and 21st century. After a century of influenza A pandemics, a pandemic coronavirus emerged. In 1918, 1957, and 1968 pandemics are thought to have arisen from birds in Asia, whereas in 2009 originated in Mexican pigs. The origin of SARS‐CoV‐2 is thought to be Chinese bats. The colored labels indicate the pathogen responsible for the disease in question.
Apart from this striking difference in the age distribution of mortality, SARS‐CoV‐2 seems to spread in clusters—temporally as well as geographically. This might in part be due to the concept of superspreading which was also a characteristic of SARS‐CoV and MERS‐CoV.
In the following, we will focus on the lessons from the COVID‐19 pandemic and extract key insights that may point a way forward to end this world crisis.
What Makes SARS‐CoV‐2 So Dangerous?
SARS‐CoV‐2 is a highly contagious respiratory pathogen that can spread directly through droplets and indirectly through fomite. In addition, there are several reports of superspreader events where aerosolized spread is the most likely explanation. However, the relative importance of fomite and aerosols remains unknown [9]. The basic reproduction number, R 0 (the average number of contacts infected by each infected person), is around 2.5 [10] in the absence of control. This is on par with the Spanish Flu [11, 12], meaning that a large fraction of the population needs to be immune in order to stop the epidemic from growing. This fraction (F) is classically estimated using the following formula [13]:
In the case of SARS‐CoV‐2, this means that around 60% of the population must be immune in order to reach herd immunity according to this formula. This in turn means that a very large number of people would be infected if we let the epidemic run its natural course.
As SARS‐CoV‐2 is a newly emerged pathogen meaning there is no specific pre‐existing immunity, it is assumed that almost everyone is susceptible to infection.
In the beginning of the pandemic, there were reports of a high case fatality rate of around 2.3% and 19% getting severe disease requiring oxygen therapy and/or ICU admission. Some speculated correctly that we were just seeing the tip of the iceberg and thus overestimating these figures while others disagreed [14, 15, 16, 17]. We are now certain that these proportions are way too high. Estimating the true proportions of infected people that are hospitalized, admitted to the ICU, or die is best done with serology data. We have shown examples of such calculations based on serology, hospital, and mortality data from Denmark, Spain, and Iceland in Table 1 [18, 19, 20, 21, 22, 23, 24]. See our Danish report with SSI for details on assumptions and calculations [25].
Table 1.
shows estimates of the proportion of all infected individuals who are hospitalized, admitted to the ICU and die. We base our estimates of the number of infected individuals by inferring from seroprevalence studies [19, 21, 22, 23, 24]
| Testing period | Seroprevalence estimate | Hospitalization rate | ICU rate | Infection fatality rate (IFR) | |
|---|---|---|---|---|---|
| Danish seroprevalence study, round 2 | 17/8‐4/9 | 2.2% (1.8–2.6%) | 2.2% (1.9–2.7%) | – | 0.49% (0.41–0.60%) |
| Danish seroprevalence study, round 3 | 14/12–8/1 | 3.9% (3.3–4.6%) | 3.0% (2.6–3.6%) | – | 0.55% (0.46–0.65%) |
| Danish blood donors, week 4 of 2021 | 25/1–29/1 | 8.1% (6.9–8.9%) | 2.4% (2.2–2.8%) | – | 0.46% (0.42–0.54%) |
| Spanish data, ENECOVID | 27/4‐11/5 | 6.1% 1 | 2.59% | 0.24% | 0.85% |
| Data from Iceland | Post first wave seroprevalence | 0.9% (0.8–0.9%) | 3.6% | 0.9% | 0.3% |
We have adjusted the crude Spanish estimate of 5.0% for estimated sensitivity (82.1%) and specificity (100%) of the used IgG POCT.
It is interesting that the ICU rate is higher and the IFR lower in Iceland than in Denmark and Spain. Perhaps, the Icelandic IFR is simply lower because of the younger age pattern of cases, suggesting elderly were better shielded from infection [18, 26]. ICU and hospitalization rates are difficult to compare across countries as those depend on local admission criteria and ICU definitions.
It is expected that the measured IFR would vary greatly depending on the demography of each country and other factors. For example, when we applied the Spanish age‐specific IFRs to the far younger demography of Ethiopia, we found an all‐age IFR of only 0.10%, compared to 0.85% measured in Spain. To truly know the IFR in low‐income settings, we would need national serology studies and complete COVID‐19 mortality statistics. But our Ethiopia example gives a sense that the measured IFR may vary 10‐fold between countries with an aging and a young population. Large meta‐analyses have found similar effects [27, 28].
The WHO published a meta‐analysis estimating the global IFR to be ~0.23% [29]. Another meta‐analysis based on all available serology studies estimated a mean IFR of 0.68% (0.53–0.82%) [30]. These large differences show the importance of referring to a specific population or age stratum when stating an IFR.
Although the COVID‐19 IFR is many times lower than for SARS and MERS, the quick and efficient spread of the SARS‐CoV‐2 virus has already given rise to many more infections and deaths. An alternative measure to the official death count is excess in all‐cause mortality—above what is expected for a specific time of the year. The European EUROMOMO surveillance system allows for timely tracking of excess mortality in European countries and offers both historical and contemporary incidence in mortality (https://www.euromomo.eu/). Excess mortality follows the pandemic wave patterns in Europe over the last year. Excess mortality is clearly highest in the older age groups, but a slightly significant excess mortality is also seen in the age group of 15–44 years. No excess mortality is seen in the group of 0–14 years.
Finally, it is becoming increasingly clear that the disease burden is not adequately described by acute illness and mortality alone. An unknown proportion of recovered patients experience longer lasting and, in some instances, debilitating symptoms such as fatigue, dyspnea, chest pain, joint pain, anosmia, and dysgeusia [31]. Only with time, and from ongoing study of large, representative populations of seropositive individuals, we will understand the duration of these sequelae and get a better idea of the true proportion of all infected individuals that experiences them.
Does SARS‐CoV‐2 Have a Weak Spot?
Throughout the COVID‐19 pandemic, news stories about superspreading events—in which a single person infected a large number of people within a short timeframe—have been cropping up regularly. By now, there is significant evidence from outbreaks and RNA sequence analyses that these are not just isolated events. Rather, a marked heterogeneity in transmission is part of the signature of SARS‐CoV‐2 [32, 33, 34]. Many outbreaks involving such superspreading have already been documented, and a database of more than 2000 cases has been compiled [35]. We have included a few clear examples [36, 37, 38, 39, 40, 41] where one individual infects several others (Table 2).
Table 2.
shows examples [36, 37, 38, 39, 40, 41] of evident COVID‐19 superspreader events, meaning that they occurred in a limited time period so that it most likely represents multiple secondary infection from a single superspreader
| Location | Event type and comments | Date (duration) | Estimated number of secondary infections from one superspreader | Participants | Attack rate |
|---|---|---|---|---|---|
| Skagit County, USA | Choir practice with social distancing transmission 1 | March 10 (2.5 h) | 52 | 61 | 87% |
| Calgary, Australia | Service and party in a church with social distancing 1 | Mid‐March (a few hours) | 23 | 41 | 59% |
| Guangzhou, China | Restaurant, asymptomatic superspreader 2 | January 24 (one lunch period) | 9 | 91 | 11% |
| Edmonton, Canada | Bonspiel (curling event) | March 11–14 (4 days) | 23 2 | 72 3 | 33% |
| Chicago, USA | A dinner, a funeral, and a birthday party | February‐March (three distinct events) | 10 | – | – |
| Zhejiang province, China | Bus ride and worship event (WE) 1 |
Bus ride: 100 mins WE: 150 mins |
Bus 1: 0 Bus 2: 23 WE, others: 7 WE, total: 30 |
Bus 1: 60 Bus 2: 68 WE, others: 172 WE, total: 300 |
Bus 1: 0 % Bus 2: 35% WE, others: 4% WE, total: 10% |
A long list of 1400 outbreaks is available in the following database: https://docs.google.com/spreadsheets/d/1c9jwMyT1lw2P0d6SDTno6nHLGMtpheO9xJyGHgdBoco/edit
Highly probable case of aerosolized transmission.
High probability of at least some tertiary infections.
“Roughly 72 attendees”.
Superspreading is known to have played a significant role in some previous coronavirus outbreaks, such as SARS and MERS [35, 42, 43], and is one of the epidemiological footprints that differentiate them from pandemic influenza [44].
The mechanism behind superspreading is not yet fully determined. It is not clear whether superspreading events can primarily be ascribed to large inter‐individual variability in viral shedding over the duration of an infectious period, or if it is perhaps a highly temporal phenomenon, with short‐lived spikes in shedding. It is clear that certain behaviors and procedures which facilitate aerosolization can at least contribute. These can range from everyday occurrences such as singing, to medical procedures such as intubation and tracheoscopy. Some studies found large variations in viral shedding and viral load between infected individuals [45, 46], but it is not clear that these were not just representing various stages of infectivity even though some cases point to specific persons being biological superspreaders. Most compelling, in one study from China, a single person caused a superspreading event, then went on to also infect everyone at home, suggesting that it was a particular superspreading person, rather than a singular event [41]. However, in several superspreading events, behavior seems to play a role—examples of high‐risk activities are whistling and singing. This suggests that superspreading is a property of action also. Needless to say, the presence of a large (typically indoor) crowd is also a risk factor.
With a basic reproductive number of around 2.5 [10], such a marked heterogeneity in transmission entails that the majority of infected individuals hardly transmit the disease at all. In Fig. 2a, a simulated infection chain is shown, clearly showing how the spread of the disease is entirely dependent on superspreaders [47].
Fig. 2.
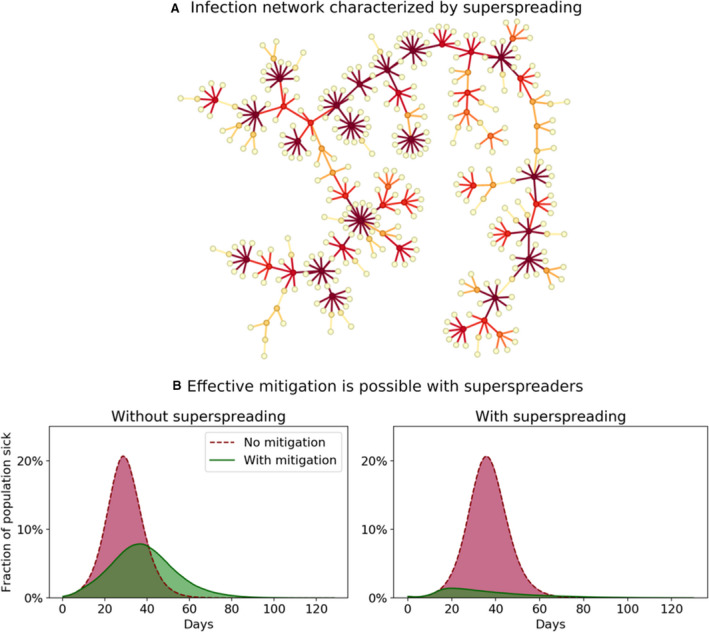
Simulations of an agent‐based model with network and superspreaders (see full model and assumptions in [47]). (A) A single infection tree—the result of a model simulation of superspreading. The epidemic spreads due to a small proportion of individuals who are highly infectious, while the majority do not transmit the disease. (B) Effect of mitigating in the public domain to reduce opportunities for superspreading. If a sizable proportion of infections are caused by superspreaders, the simulations show that just reducing contacts in the public space (that is, outside households and workplaces/schools), has a large mitigation effect (right subpanel); but without superspreaders in the model, not much is gained (left subpanel). Data for panel B from [47]. In these simulations, superspreaders are individuals with a higher personal reproductive number, thus having the potential to transmit the disease to many in an unmitigated scenario. Drastically reducing the number of different persons that one meets (by e.g., banning large gatherings) thus has an outsized effect in a disease characterized by superspreading, providing an opportunity for improved mitigation. The theoretical background for this effect is explored in ref. [49].
This transmission heterogeneity can be summarized by the overdispersion parameter k (a number that—for small values of k—approximates the fraction of people that are responsible for 80% of the transmissions) [42], with lower k denoting a more heterogeneous transmission—that is, one prone to superspreading events. For SARS‐CoV, this was estimated to be 0.16, corresponding to a high level of transmission heterogeneity, while estimates for SARS‐CoV‐2 have been even more dramatic—around 0.10 [32, 33, 48]. This indicates that for SARS‐CoV‐2, the 10% most infectious individuals are responsible for approximately 80% of the transmission. Pandemic influenza, on the other hand, is much more homogenously spread, with an estimated k value close to 1[44]. As we discuss below, this has significant consequences, and so we argue that the k value deserves widespread recognition, similar to the reproduction number R 0. From a mathematical standpoint, this amounts to saying that it is not just the mean of the infectiousness distribution which matters, but also its variance.
Mathematical models have been used to study the impact that superspreading has on the effectiveness of mitigation strategies, demonstrating that efficiency of such strategies primarily relies on reducing social mixing in society [47], including for example a ban of large gatherings. Capturing these phenomena requires modeling on the level of individuals, and this is not possible within traditional compartmental epidemiological models which assume completely random mixing. In popular terms, these models assume that each individual goes to a new job each minute and returns to a new home every evening.
The main finding is that the ability of superspreaders to transmit the disease to anywhere near their full potential can be effectively curbed by even a moderate reduction in the number of contacts that any given person has during an infectious period. For a more homogeneously transmitted disease, this would not be the case. In that case, it would be necessary to reduce the number of distinct social contacts very close to the reproductive number R0 to achieve significant mitigation.
As illustrated in Fig. 2a, superspreading has a tendency to lead to bursty infection chains which then have an increased chance of dying out, as the chain effectively terminates if none of the recently infected persons are themselves superspreaders [49].
Thus, superspreading is in full agreement with the bursty, geographically clustered outbreaks seen during this pandemic [50].
Figure 2b shows the result of reducing contacts outside households and work in an agent‐based model. For a virus prone to superspreading the impact is substantial, while it has little impact for a more homogeneously transmitted virus. Thus, superspreading represents an Achilles heel of SARS‐CoV‐2, making the epidemic vulnerable to even moderate reductions in contacts. This, in turn, explains the high effectiveness of lockdown strategies.
It may be tempting to think that superspreading is merely a product of some people having many contacts—that is, social heterogeneity. This social aspect of superspreading is probably partly true as socially active people are more likely to infect more. Interestingly, socially hyperactive people also have higher risk of becoming infected, meaning that highly active people are also super‐receivers. A modeling study found that social heterogeneity lowers the herd immunity threshold, even in the absence of mitigation [51]. Purely biological superspreading that does not correlate with the superspreaders’ own probability of becoming infected does not change the herd immunity threshold.
We saw earlier how superspreading drastically improves the effect of mitigation strategies which rely on reducing contacts. It is known that social heterogeneity leads to clustering of cases and so increases the effectiveness of another form of mitigation, namely test‐trace‐isolate strategies [51]. Since cases also have a tendency to occur in clusters in this case, superspreading too should make contact tracing easier and more effective. This is especially true if backward contact tracing is performed, since any given infection is quite likely to stem from a superspreader [52].
In conclusion, superspreading seems to represent an Achilles heel of SARS‐CoV‐2, which opens up possibilities for particularly effective mitigation, far more than what could ever be achieved for pandemic influenza. We argue that models used to explore the pandemic trajectory should take heterogeneity into account when evaluating possible mitigation strategies (and not just view it as “statistical noise”).
Unanswered Questions
What role do children play in the COVID‐19 pandemic?
In prior influenza pandemics, children played a major role as transmitters. It was therefore surprising that so few children figured among known cases in the early phase of the COVID‐19 pandemic. This could be explained by children typically having only mild symptoms, but it was suspected early on that children were less susceptible and infectious than adults [53, 54]. Could it be true that children are not important transmitters in this pandemic? The best way to answer this question is by testing for SARS‐CoV‐2‐antibodies in local outbreak settings or in randomized population samples; however, there are ethical and legal concerns when drawing blood from healthy children.
For adolescents (14–20 years old), new evidence has since clarified that this age group indeed plays an important role in the pandemic. High school outbreaks have been reported all over the world. The latest Danish evaluation of population seroprevalence found the second highest seroprevalence in the 12–19 years old age group (6.6% vs. 3.9% in the general population) [22]. A meta‐analysis [55] found similar seroprevalences in adolescents and adults in population‐wide screening studies of several different countries. Secondary household attack rates were as high or higher for adolescents compared to adults.
For younger children, the same analysis found a lower seroprevalence in this age group than in adults [55]. However, to the best of our knowledge, none of the used antibody tests have been validated on pediatric populations. A German study found no difference between viral load in children and adults suggesting that children might be as infectious as adults [56].
A meta‐analysis of contact tracing studies suggests a lower probability of secondary infections in children than adults, but the study was not conclusive [55]. When excluding studies with a high risk of bias (e.g., testing only symptomatic contacts—i.e., fewer children), this lower probability became non‐significant [57]. A meta‐analysis of contact tracing studies suggests a lower probability of secondary infections in children than adults, but the study was not conclusive [55]. When excluding studies with a high risk of bias (e.g., testing only symptomatic contacts—i.e., fewer children), this lower probability became non‐significant [57].
Finally, household contact studies show a lower probability of a child being the index case of a household [58]. However, this could be due to a bias in ascertaining the index person—typically a symptomatic adult—masking the possibility that it was an asymptomatic child who brought the disease into the household in the first place.
Since the reopening of countries following the initial lockdowns, several notable outbreaks have been reported in younger pediatric populations. Examples include a youth overnight camp in Georgia for age 6‐19 years in the United States, where mass PCR testing revealed an attack rate of at least 44% among campers [59]. Additionally, 41 of 825 schools in Berlin had to close two weeks after reopening due to school outbreaks [60]. In the United States, serious concerns were raised over reopening schools after the summer [61].
Studies in which all pupils, teachers, and their home contact are all tested—preferably using antibody tests—regardless of symptoms are the most informative and less biased. A study of this kind was performed at a high school in Oise, France, and underscored the high susceptibility and transmissibility in adolescents [62].
In conclusion, while susceptibility and infectiousness of children were downplayed for a long time, it has become increasingly clear (from the above‐mentioned serology studies) that adolescents play an important role in this pandemic. The question remains open for younger children, an age group rarely tested. We do not, however, have evidence to suggest they can be disregarded. Furthermore, with the rise of the new British B.1.1.7 variant, there is evidence from Israel that this variant leads to high attack rates even among young children [63]. Knowing the infectiousness of young children is naturally of key importance in informing policy decisions about keeping young children in schools and institutions. It is, however, very clear that children and adolescents have very mild infections—the reason for this remains a mystery, but a tempting explanation is a better innate immune response in children [64]. An exception to this is the multisystem inflammatory syndrome in children (MIS‐C) after infection with SARS‐CoV‐2 which in some aspects resembles Kawasaki disease. Patients present with fever and severe illness involving two or more organ systems. The suggested pathogenesis involves post‐infectious immune dysregulation. The syndrome is rare, and when it occurs it has a mortality rate of around 1.5%. The possibility of sequelae in children after SARS‐CoV‐2 is another important point, but the data so far are inconclusive, and more research is needed to truly understand the impact of COVID‐19 in children [65, 66]. This is important in weighing the risks and benefits of vaccinating children against COVID‐19 [67, 68].
SARS‐CoV‐2 immunity
In the early stages of the COVID‐19 pandemic, there was intense debate over the immune response to SARS‐CoV‐2. Some researchers argued for long‐lasting, effective immunity—even suggesting that we create immunity passports. Others, however, doubted that antibody responses would be lasting and remain highly prevalent in recovered individuals. Early on some were even concerned that the antibodies might not even be neutralizing [69, 70].
We now know that most people do in fact develop a lasting antibody response, lasting at least several months—and several studies have found antibodies to be neutralizing [24, 71, 72, 73, 74, 75]. There is evidence to suggest that cellular immunity is robust as well—and it might prove important if antibody titers decline [76]. Interestingly, between 20 and 50% of unexposed individuals (that is, from blood drawn before the pandemic virus existed) display significant SARS‐CoV‐2 specific T‐cell response, possibly originating from immunity to the common and related cold coronaviruses [77]. The implications of this are still uncertain, but it would be interesting to examine the effect of this on SARS‐CoV‐2 susceptibility. More research is needed, and it would be particularly interesting to examine differences in pre‐existing immune responses between different age groups including children and elderly.
Re‐challenge studies in macaques also points toward a protective immune response [57, 78]. There is thus a theoretical basis for immunity.
An interesting case of real‐life immunity was reported in a fishery vessel outbreak with a PCR‐confirmed attack rate of 85.2% (104 of 122 individuals). Three previously recovered individuals with neutralizing antibodies were on board and none of them experienced any symptoms nor tested positive in the PCR test. This real‐life situation thus provides evidence of the protective effect of neutralizing antibodies (p = 0.002, Fisher's exact test) [79]. Another notable real‐life example was seen at an overnight summer school retreat in Wisconsin in the summer of 2020 reported by the CDC [80]. There was a great outbreak with an attack rate of 76% (116 cases) among the 152 attendees. 24 of the participants had positive serologic results before going to the camp—all of these got negative RT‐PCR results. The report provides no statistical test for this apparent immunity, but we have performed a Fisher’s exact test showing p < 0.001. Thus, there is both theoretical and real‐life evidence of immunity.
On the other hand, there have been reports of a few credible reinfection events in Hong Kong, Belgium, and the Netherlands [81, 82]. Highly concerning is the growing evidence from Manaus, Northern Brazil, where herd immunity following the 1st wave was later overcome by a new variant dominating the 2nd wave [83, 84, 85].
What is the best way to control the epidemic until vaccine immunity is achieved?
While the world awaits widespread distribution of effective vaccines, it is critical to find a sustainable and acceptable way of living while suppressing the epidemic until we have vaccine‐induced immunity, especially among the elderly and others at high risk. In our opinion, this is best achieved by measures that reduce excessive contacts in the public space, to avoid superspreading events [47].
While most countries adapted draconian measures, Sweden did not use lockdowns during the first wave and remained a semi‐open society with open borders. Early on, Sweden had a high death toll which can be explained by a late implementation of their control measures, a full two weeks after the other Nordic countries went into lockdown. In Sweden, these measures were focused on voluntary changes in mobility and work behavior and, importantly, a further restriction of gatherings from a maximum of 500 to a maximum of 50 persons, as well as intensified efforts to secure elderly in nursing homes, while schools for children under 16 years remained open. With this relatively light control strategy, they achieved epidemic control around May 1st, so that the effective reproductive number was below 1 over the summer; until autumn where partial lifting of this ban, along with seasonal change, resulted in a substantial second wave (Fig. 3). We wonder if the situation in Sweden during May‐September showed us the potency of restrictions on large gatherings, isolated from the effect of other factors imposed in a full lockdown.
Fig. 3.
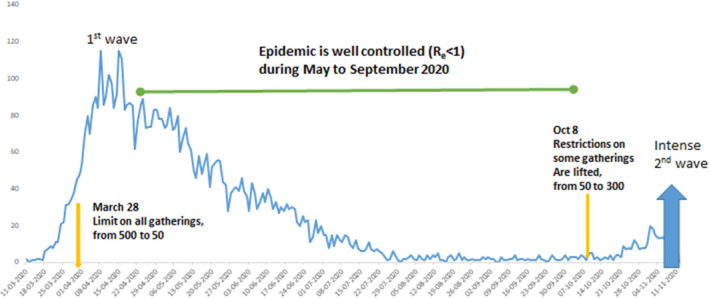
A sustainable control strategy in Sweden? On March 28th, Sweden introduced a ban on events >50 persons and the daily numbers of deaths started to decline a few weeks after [96]. On October 8, some gatherings were again allowed up to 300. Many other factors were in effect in Sweden, including working from home, less traveling, more effective shielding of the elderly, closed universities, and the seasonal changes in temperature and humidity. But borders remained open, as did schools for children up to 16 years of age in this time period.
In our view, the Swedish success is that of getting to R e below 1—albeit too late—while maintaining a fairly free and open society. Furthermore, had this been achieved 2 weeks sooner, then Sweden would not have suffered the large death toll in the spring.
Some have argued that allowing R e to somewhat exceed 1 could be desirable because it allows herd immunity to slowly build up in the population (Great Barrington Declaration) [86]. In our view, however, this comes at an unacceptably high cost in terms of disease burden and deaths. We computed that cost for Denmark, by multiplying the IFR and the hospitalization rate, assuming the final epidemic size would be 60% of the Danish population (Table 3). Using our estimates based on the latest two seroprevalence studies and the latest blood donor data from Denmark (week 4 of 2021) this gives us.
Table 3.
shows the hypothetical cost of controlled, natural herd immunity in Denmark in terms of deaths and hospitalizations. The resulting figures are far greater than the current cumulative burden of ~2300 deaths and ~12,000 hospitalizations in our country (as of Feb 16, 2021).
| COVID‐19 Hospitalizations | % of population hospitalized | COVID‐19 Deaths | % of population dead | |
|---|---|---|---|---|
| Estimates based on seroprevalence study, round 2 | 76,500 (64731–93500) | 1.3% (1.1–1.6%) | 17,100 (14469–20900) | 0.29% (0.25–0.36%) |
| Estimates based on seroprevalence study, round 3 | 105,538 (89478–124727) | 1.8% (1.5–2.1%) | 19,154 (16239–22636) | 0.33% (0.28–0.39%) |
| Estimates based on Danish blood donor serology study; week 4, 2021 | 82,993 (75533–97426) | 1.4% (1.3–1.7%) | 16,059 (14616–18852) | 0.28% (0.25–0.32%) |
We found that natural herd immunity in Denmark would lead to ~20,000 deaths and ~90,000 hospitalizations. In developing countries, the cost of following such a strategy would presumably be far less dramatic, due to having low proportions of people above 60 years of age. In Denmark, this age group accounts for 97.3% of COVID‐19 deaths as of February 15, 2021 [6]. One might suggest isolating the elderly and chronically ill and allowing herd immunity to develop in the rest of the population. In a sense, the numbers above actually already account for that because isolation of elderly and chronically ill has already been a part of the Danish strategy from the start. From the seroprevalence data, it is also clear that this has actually been quite successful. In the third round of the national seroprevalence study, the seroprevalence was estimated at 1.9% (0.9–3.4%) in those above 65 years of age while it was 7.3% (5.3–9.9%) in those between 20 and 29 years of age. In a situation with higher infection rates in society, it seems more difficult to avoid infections in nursing homes, hospitals, and in the elderly part of the general population. In that case, the estimates of mortality and hospitalizations above are too low.
In addition, to avoid hospital overburdening, the reproductive number would have to be kept close to 1 (meaning a similar degree of restrictions to those needed to keep R e < 1) until significant effects of herd immunity kick in—and this is a very slow process that is nowhere near happening in any western countries despite high death tolls.
On the contrary, with strict border control and quarantining of incoming travelers, a strategy of testing, contact tracing, local outbreak control combined with social distancing and hygiene measures has allowed to suppress the epidemic resulting in very low death tolls in islands such as Iceland, Faeroe Islands, South Korea, Taiwan, and New Zealand despite having quite open societies during most of the pandemic [87]. Acting quickly to get R e below 1 while disease prevalence is still low is, in our view, the best way to keep an open society in the long term.
However, the situation has recently been complicated by new, faster spreading variants such as lineage B.1.1.7, commonly known as the UK variant. This variant requires even tougher restrictions than what has been necessary until now, due to increased (around 50%) higher transmissibility [88].
How will this End?
Historically, influenza pandemics ended when sufficient immunity had built up in the population, even in the recent 2009 pandemic when the vaccine only became available after several waves [89]. We see four mutually non‐exclusive ways of ending the crisis:
A highly effective and widely available treatment of COVID‐19
Herd immunity achieved by natural infection of at least 60% of the population
Herd immunity achieved by mass vaccinations
Widespread availability of inexpensive rapid tests for repeated mass testing
Several treatments are in use, but none have been proven to drastically improve the prognosis of the disease. Remdesivir seemed initially to improve mortality in a specific subgroup of hospitalized patients [90], but a later meta‐analysis by the WHO found no reduction in mortality for Remdesivir nor three other studied drugs (hydroxychloroquine, lopinavir, and interferon beta‐1a). Furthermore, a recent Cochrane review concludes that there is currently no evidence‐based treatment for COVID‐19 [91]. Combining this knowledge with the current vaccine advances, a game changer of a treatment does not seem to be the most plausible way out of the crisis in any near future.
As discussed in Section 4, aiming for natural herd immunity is undesirable due to the substantial cost in terms of disease burden and lives lost. This is further complicated by the fact that new and more contagious variants like B.1.1.7 have emerged and are replacing the original variant in many countries, thus requiring an even higher percentage of the population to recover from infection in order to achieve herd immunity. Furthermore, allowing widespread infections while building up herd immunity increases the risk of escape mutations that can cause reinfections and give rise to future epidemic waves even though herd immunity has been established. This is a quite probable explanation of recent events in Manaus, Brazil, where a second, deadlier wave of COVID‐19 has hit the Amazonas capital after an estimated attack rate of 76% in the first wave had apparently conferred herd immunity [84, 85]. Genetic sequencing points to the immune escape lineage P1 playing a major role in the second wave. In December, 51% of the sequenced SARS‐CoV‐2 genomes in the Amazonas belonged to the P1 strain and in January this figure had risen to 91% [83].
Preliminary data from the Novavax COVID‐19 vaccine trial in South Africa—where an escape variant, B.1.351, is highly prevalent—points toward 60% (19.9‐80.1%) protection against symptomatic, confirmed COVID‐19 in HIV‐negative, vaccinated individuals. Of concern is that the 1/3 of participants who were seropositive at entry (thought to have been due to the original SARS‐CoV‐2 strain in the first wave) had no protection relative to the placebo group. This (along with the Manaus data) points to an unfortunate preliminary conclusion—the naturally acquired immunity does little to nothing to protect against reinfection with escape variants. Luckily, at least the Novavax vaccine seems to offer some protection. It will be interesting to see if this holds true for the other vaccine candidates. Based on in vitro studies on 8 human sera and sera from non‐human primates, Moderna has found preliminary evidence suggesting that their mRNA‐based COVID‐19 vaccine (mRNA‐1273) might not induce as high neutralizing antibody titers against the B.1.351 lineage relative to prior strains. The titers are still expected high enough to confer immunity, but out of caution, Moderna has already sent an emerging variant booster vaccine into trial (mRNA‐1273.351) against the B.1.351 variant [92]. By rapidly updating vaccines, we will have a more sustainable weapon against the new variants than allowing recurrent waves of new escape variants to confer herd immunity. Even though the vaccines do not completely protect against mild infections, all of the approved vaccines in the EU confer very high protection against severe disease [93].
A combination of natural and vaccine‐based immunity is also possible, however, and one could argue that broadly imposed restrictions do no longer have ethical merit once those vulnerable to severe outcomes of infection have been vaccinated. However, hospitalization rates are not as age dependent as the fatality rate, so care must be taken that hospitals are not overwhelmed by quick lifting of measures. Also—immune senescence might leave many elderly vulnerable even after vaccination. Gradual reduction of restrictions while maintaining Re around or below 1 based on hospital admissions might be the best way for a balanced return to a normal society. As more and more risk groups are vaccinated, we should expect a lowering of the risk of hospitalization meaning that an increase in infection rates will not necessarily significantly increase hospitalizations. Mathematical modeling will be crucial in informing the timing of reopening attempts—who and how many must be vaccinated before a COVID‐19 wave in an open society is unable to overburden hospitals?
Regardless of how herd immunity is achieved, SARS‐CoV‐2 is likely to become endemic and may cause occasional large resurgences, either due to waning of antibodies or due to the appearance of immune escape variants [94]. These phenomena mean that we might need to repeatedly vaccinate a large part of the population—for example, each winter as we do for the seasonal flu.
It is thus clear that COVID‐19 should not just be viewed as a temporary pandemic phenomenon, and that sustainable strategies are required. On a positive note, rapid tests have now become widely available, and these can significantly increase the speed of outbreak detection in vulnerable settings and of contact tracing in general. If rapid tests become cheaper and available for home use, they could realistically be used for recurrent mass testing of the entire population in order to curb the spread—such as it was successfully done in Slovakia in October 2020 [95].
Ørskov S, Nielsen BF, Føns S, Sneppen K, Simonsen L. The COVID‐19 pandemic: key considerations for the epidemic and its control. APMIS. 2021; 129: 408–420.
References
- 1. Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics — implications for policy. N Engl J Med. 2009;360:2595–8. [DOI] [PubMed] [Google Scholar]
- 2. Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID‐19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. https://www.who.int/medicines/ebola‐treatment/WHO‐list‐of‐top‐emerging‐diseases/en/
- 4. Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K, et al. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178:53–60. [DOI] [PubMed] [Google Scholar]
- 5. Murray CJL, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. The Lancet. 2006;368:2211–18. [DOI] [PubMed] [Google Scholar]
- 6. https://experience.arcgis.com/experience/aa41b29149f24e20a4007a0c4e13db1d
- 7. Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, et al. Comparing SARS‐CoV‐2 with SARS‐CoV and influenza pandemics. Lancet Infect Dis. 2020;20:e238–e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Viboud C, Miller M, Olson DR, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr. 2010;2:RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. https://www.who.int/news‐room/commentaries/detail/transmission‐of‐sars‐cov‐2‐implications‐for‐infection‐prevention‐precautions
- 10. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200306‐sitrep‐46‐covid‐19.pdf?sfvrsn=96b04adf_4
- 11. Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis. 2008;197:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mills CE, Robins JM, Lipsitch M. Transmissibility of 1918 pandemic influenza. Nature. 2004;432:904–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fine P, Eames K, Heymann DL. “Herd Immunity”: a rough guide. Clin Infect Dis. 2011;52:911–6. [DOI] [PubMed] [Google Scholar]
- 14. Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–42. [DOI] [PubMed] [Google Scholar]
- 15. https://www.bmj.com/content/368/bmj.m606/rr‐5
- 16. https://www.nytimes.com/2020/03/04/health/china‐lessons‐aylward.html
- 17. https://www.who.int/docs/default‐source/coronaviruse/clinical‐management‐of‐novel‐cov.pdf
- 18. https://www.covid.is/data‐old
- 19. https://www.isciii.es/Noticias/Noticias/Paginas/Noticias/PrimerosDatosEstudioENECOVID19.aspx?fbclid=IwAR3LuOScys3SZc37J5IdXB95k2LU5SRZ6Ujji583S1NQQPWUNHToNOu8EIA
- 20. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/situacionActual.htm
- 21. https://www.ssi.dk/‐/media/arkiv/dk/aktuelt/nyheder/2020/notat‐‐‐covid‐19‐prvalensundersgelsen.pdf?la=da
- 22. https://files.ssi.dk/praevalensundersoegelse_runde3
- 23. https://bloddonor.dk/coronavirus/
- 24. Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E. Humoral immune response to SARS‐CoV‐2 in Iceland. N. Engl. J. Med. 2020;383:1724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ørskov S, Føns S, Haase N, Simonsen L. Fokusrapport: Mørketal; 2020. SSI: https://www.ssi.dk/‐/media/arkiv/dk/aktuelt/sygdomsudbrud/covid19/fokusrapport‐‐‐uge‐35‐‐‐mrketallet‐‐‐final.pdf?la=da
- 26. Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS‐CoV‐2 in the Icelandic population. N Engl J Med. 2020;382:2302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghisolfi S, et al. Predicted COVID‐19 fatality rates based on age, sex, comorbidities, and health system capacity. medRxiv. 2020; 2020.06.05.20123489 [DOI] [PMC free article] [PubMed]
- 28. Levin AT, Hanage WP, Owusu‐Boaitey N, Cochran KB, Walsh SP, Meyerowitz‐Katz G. Assessing the age specificity of infection fatality rates for COVID‐19: systematic review, meta‐analysis, and public policy implications. Eur. J. Epidemiol. 2020;35:1123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ioannidis J. The infection fatality rate of COVID‐19 inferred from seroprevalence data. Bull World Health Organ 2020;99:19‐33F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyerowitz‐Katz G, Merone L. A systematic review and meta‐analysis of published research data on COVID‐19 infection‐fatality rates. medRxiv. 2020; 2020.05.03.20089854 [DOI] [PMC free article] [PubMed]
- 31. Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, et al. Epidemiology and transmission of COVID‐19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Endo A, Abbott S, Kucharski AJ, Funk S. Estimating the overdispersion in COVID‐19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller D, Martin MA, Harel N, Tirosh O, Kustin T, Meir M, et al. Full genome viral sequences inform patterns of SARS‐CoV‐2 spread into and within Israel. Nat Commun. 2020;11. 10.1038/s41467-020-19248-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. https://medium.com/@codecodekoen/covid‐19‐superspreading‐events‐database‐4c0a7aa2342b
- 36. Hamner L, Dubbel P, Capron I, Ross A, Jordan A, Lee J, et al. High SARS‐CoV‐2 Attack rate following exposure at a choir practice — Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606–10. [DOI] [PubMed] [Google Scholar]
- 37. https://calgary.ctvnews.ca/i‐would‐do‐anything‐for‐a‐do‐over‐calgary‐church‐hopes‐others‐learn‐from‐their‐tragic‐covid‐19‐experience‐1.4933461
- 38. https://nationalpost.com/news/how‐an‐edmonton‐curling‐tournament‐became‐a‐hotspot‐for‐the‐covid‐19‐outbreak‐in‐canada
- 39. Ghinai I, Woods S, Ritger KA, McPherson TD, Black SR, Sparrow L, et al. Community transmission of SARS‐CoV‐2 at Two Family Gatherings — Chicago, Illinois, February–March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu J, Gu J, Li K, Xu C, Su W, Lai Z, et al. COVID‐19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerging Infect Dis J. 2020;26:1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shen YE, Li C, Dong H, Wang Z, Martinez L, Sun Z, et al. Community outbreak investigation of SARS‐CoV‐2 transmission among bus riders in Eastern China. JAMA Internal Med 2020;180(12):1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lloyd‐Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong G, Liu W, Liu Y, Zhou B, Bi Y, Gao GF. MERS, SARS, and Ebola: the role of super‐spreaders in infectious disease. Cell Host Microbe. 2015;18:398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fraser C, Cummings DAT, Klinkenberg D, Burke DS, Ferguson NM. Influenza transmission in households during the 1918 pandemic. Am. J. Epidemiol. 2011;174:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kleiboeker S, Cowden S, Grantham J, Nutt J, Tyler A, Berg A, et al. SARS‐CoV‐2 viral load assessment in respiratory samples. J. Clin. Virol. 2020;129:104439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pujadas E, Chaudhry F, McBride R, Richter F, Zhao S, Wajnberg A, et al. SARS‐CoV‐2 viral load predicts COVID‐19 mortality. Lancet Respiratory Med. 2020;8:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sneppen K, Taylor RJ, Simonsen L. Impact of Superspreaders on dissemination and mitigation of COVID‐19. medRxiv, 2020: 2020.05.17.20104745
- 48. Kirkegaard JB, Sneppen K. Variability of individual infectiousness derived from aggregate statistics of COVID‐19. medRxiv, 2021: 2021.01.15.21249870
- 49. Nielsen BF, Simonsen L, Sneppen K. COVID‐19 Superspreading Suggests Mitigation by Social Network Modulation. Physical Review Letters. 2021;126. [DOI] [PubMed] [Google Scholar]
- 50. Eilersen A, Sneppen K. COVID‐19 superspreading in cities versus the countryside. medRxiv, 2020: 2020.09.04.20188359
- 51. Nielsen BF, Sneppen K, Simonsen L, Mathiesen J. Social network heterogeneity is essential for contact tracing. medRxiv. 2020: 2020.06.05.20123141
- 52. Endo A, Leclerc QJ, Knight GM, Medley GF, Atkins KE, Funk S, et al. Implication of backward contact tracing in the presence of overdispersed transmission in COVID‐19 outbreaks. Wellcome Open Res. 2021;5:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li X, Xu W, Dozier M, He Y, Kirolos A, Theodoratou E. The role of children in transmission of SARS‐CoV‐2: A rapid review. Journal of Global Health. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Munro APS, Faust SN. Children are not COVID‐19 super spreaders: time to go back to school. Arch Dis Child. 2020;105:618–9. [DOI] [PubMed] [Google Scholar]
- 55. Viner RM, Mytton OT, Bonell C, Melendez‐Torres GJ, Ward J, Hudson L, et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared With Adults. JAMA Pediatr. 2021;175:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jones TC, Mühlemann B, Veith T, Biele G, Zuchowski M, Hofmann J, et al. An analysis of SARS‐CoV‐2 viral load by patient age. medRxiv. 2020; 2020.06.08.20125484
- 57. Chandrashekar A, Liu J, Martinot AJ, McMahan K, Mercado NB, Peter L, et al. SARS‐CoV‐2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ludvigsson JF. Children are unlikely to be the main drivers of the COVID‐19 pandemic – a systematic review. Acta Paediatr. 2020;109:1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Szablewski CM, Chang KT, Brown MM, Chu VT, Yousaf AR, Anyalechi N, et al. SARS‐CoV‐2 Transmission and Infection Among Attendees of an Overnight Camp — Georgia, June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1023–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. https://www.theguardian.com/world/2020/aug/21/coronavirus‐iurope‐dozens‐schools‐report‐infections‐berlin‐germany‐spain
- 61. https://www.theguardian.com/world/2020/aug/14/school‐reopenings‐covid‐19‐coronavirus‐us
- 62. Fontanet A, Tondeur L, Madec Y, Grant R, Besombes C, Jolly N, et al. Cluster of COVID‐19 in northern France: A retrospective closed cohort study. medRxiv 2020; 2020.04.18.20071134
- 63. Day M. Covid‐19: More young children are being infected in Israel and Italy, emerging data suggest. BMJ. 2021;372:n383. [DOI] [PubMed] [Google Scholar]
- 64. Carsetti R, Quintarelli C, Quinti I, Piano Mortari E, Zumla A, Ippolito G, et al. The immune system of children: the key to understanding SARS‐CoV‐2 susceptibility?. Lancet Child Adolesc Health. 2020;4:414–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. medRxiv. 2021; 10.1101/2021.01.23.21250375 [DOI] [PMC free article] [PubMed]
- 66. Denina M, Pruccoli G, Scolfaro C, Mignone F, Zoppo M, Giraudo I, et al. Sequelae of COVID‐19 in hospitalized children: a 4‐months follow‐up. Pediatr Infect Dis J 2020;39:e458–e459. [DOI] [PubMed] [Google Scholar]
- 67. Nakra N, Blumberg D, Herrera‐Guerra A, Lakshminrusimha S. Multi‐System Inflammatory Syndrome in Children (MIS‐C) following SARS‐CoV‐2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. 2020;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Radia T, Williams N, Agrawal P, Harman K, Weale J, Cook J, et al. Multi‐system inflammatory syndrome in children & adolescents (MIS‐C): A systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Phelan AL. COVID‐19 immunity passports and vaccination certificates: scientific, equitable, and legal challenges. Lancet. 2020;395:1595–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. https://www.the‐scientist.com/news‐opinion/what‐do‐antibody‐tests‐for‐sars‐cov‐2‐tell‐us‐about‐immunity‐‐67425
- 71. Kreer C, Zehner M, Weber T, Ercanoglu MS, Gieselmann L, Rohde C, et al. Longitudinal isolation of potent near‐germline SARS‐CoV‐2‐neutralizing antibodies from COVID‐19 patients. Cell. 2020;182:843–854.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pollán M, Pérez‐Gómez B, Pastor‐Barriuso R, Oteo J, Hernán MA, Pérez‐Olmeda M, et al. Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study. Lancet. 2020;396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ripperger TJ, Uhrlaub JL, Watanabe M, Wong R, Castaneda Y, Pizzato HA, et al. Orthogonal SARS‐CoV‐2 Serological Assays Enable Surveillance of Low‐Prevalence Communities and Reveal Durable Humoral Immunity. Immunity. 2020;53:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang X, Guo X, Xin Q, Pan Y, Hu Y, Li J, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020;71:2688–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, Strålin K, Gorin J‐B, Olsson A, et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID‐19. Cell. 2020;183:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sette A, Crotty S. Pre‐existing immunity to SARS‐CoV‐2: the knowns and unknowns. Nat Rev Immunol. 2020;20:457–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bao L, Deng W, Gao H, Xiao C, Jiayi L, Jing X, et al. Reinfection could not occur in SARS‐CoV‐2 infected rhesus macaques. bioRxiv. 2020. 10.1101/2020.03.13.990226 [DOI] [Google Scholar]
- 79. Addetia A, Crawford KHD, Dingens A, Zhu H, Roychoudhury P, Huang M‐L, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020;58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pray IW, Gibbons‐Burgener SN, Rosenberg AZ, Cole D, Borenstein S, Bateman A, et al. COVID‐19 outbreak at an overnight summer school retreat – Wisconsin, July–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. https://www.businessinsider.com/2‐new‐coronavirus‐reinfection‐cases‐belgium‐netherlands‐hong‐kong‐2020‐8?r=US&IR=T
- 82. To KK‐W, Hung IF‐N, Ip JD, Chu AW‐H, Chan W‐M, Tam AR, et al. Coronavirus Disease 2019 (COVID‐19) Re‐infection by a Phylogenetically Distinct Severe Acute Respiratory Syndrome Coronavirus 2 Strain Confirmed by Whole Genome Sequencing. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Naveca F, Nascimento V, Souza V, Corado A, Nascimento F, Silva G, et al. Phylogenetic relationship of SARS‐CoV‐2 sequences from Amazonas with emerging Brazilian variants harboring mutations E484K and N501Y in the Spike protein. Virological.org. 2021. [Google Scholar]
- 84. Buss LF, Prete CA, Abrahim CMM, Mendrone A, Salomon T, de Almeida‐Neto C, et al. Three‐quarters attack rate of SARS‐CoV‐2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sabino EC, Buss LF, Carvalho MPS, Prete CA, Crispim MAE, Fraiji NA, et al. Resurgence of COVID‐19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397:452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. https://gbdeclaration.org/
- 87. https://ourworldindata.org/
- 88. Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday J, et al. Estimated transmissibility and severity of novel SARS‐CoV‐2 Variant of Concern 202012/01 in England. medRxiv. 2020; 10.1101/2020.12.24.20248822 [DOI]
- 89. Van Kerkhove MD, Hirve S, Koukounari A, Mounts A. Estimating age‐specific cumulative incidence for the 2009 influenza pandemic: a meta‐analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza Other Respir Viruses. 2013;7:872–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid‐19 — preliminary report. N Engl J Med. 2020;383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al. Interventions for treatment of COVID‐19: Second edition of a living systematic review with meta‐analyses and trial sequential analyses (The LIVING Project). PLoS One. 2021;16:e0248132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. https://investors.modernatx.com/news‐releases/news‐release‐details/moderna‐covid‐19‐vaccine‐retains‐neutralizing‐activity‐against/
- 93. Creech CB, Walker SC, Samuels RJ. SARS‐CoV‐2 Vaccines. JAMA. 2021;325:1318. [DOI] [PubMed] [Google Scholar]
- 94. Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID‐19 to endemicity. Science. 2021;371:741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pavelka M, Van‐Zandvoort K, Abbott S, Sherratt K, Majdan M, CMMID COVID‐19 working group et al. The effectiveness of population‐wide, rapid antigen test based screening in reducing SARS‐CoV‐2 infection prevalence in Slovakia. medRxiv. 2020; 2020.12.02.20240648
- 96. https://experience.arcgis.com/experience/09f821667ce64bf7be6f9f87457ed9aa/page/page_0/


