Abstract
Background:
There is significant interest in understanding the role of modifiable vascular risk factors contributing to dementia risk across age groups.
Objective:
Risk of dementia onset was assessed in relation to vascular risk factors of hypertension and hypercholesterolemia among cognitively normal APOE ε4 carriers and non-carriers.
Methods:
In a sample of prospectively characterized longitudinal cohort from the National Alzheimer’s Coordinating Center database, 9,349 participants met criteria for normal cognition at baseline, had a CDR-Global (CDR-G) score of zero, and had concomitant data on APOE ε4 status and medical co-morbidities including histories of hypertension and hypercholesterolemia. Multivariable Cox proportional hazards models adjusted for well-known potential confounders were used to compare dementia onset among APOE ε4 carriers and non-carriers by young (≤ 65 years) and old (> 65 year) age groups.
Results:
519 participants converted to dementia within an average follow up of 5.97 years. Among older APOE ε4 carriers, hypercholesterolemia was related to lower risk of dementia (HR (95% CI), 0.68 (0.49–0.94), p = 0.02). Among older APOE ε4 non-carriers, hypertension was related to higher risk of dementia (HR (95% CI), 1.44 (1.13–1.82), p = 0.003). These results were corroborated among a subset with autopsy data characterizing underlying neuropathology. Among younger participants, vascular risk factors did not impact dementia risk, likely from a lower frequency of vascular and Alzheimer’s as etiologies of dementia among this cohort.
Conclusion:
A history of hypercholesterolemia related to a lower risk of dementia among older APOE ε4 carriers, while hypertension related to a higher risk of dementia among older APOE ε4 non-carriers.
Keywords: APOE ε4, dementia, dementia risk, depression, hypercholesterolemia, hypertension, longitudinal cohort study, mixed dementia, neuropathology, normal cognition cohort
INTRODUCTION
Given the lack of therapies to cure dementing illnesses, it is crucial to identify and treat modifiable risk factors when present to prevent or decrease the odds of dementia onset. Many studies have evaluated environmental, lifestyle, and clinical factors on dementia risk [1–3]. With prevention of dementia being a major focus, clinical trials targeting cognitively unimpaired individuals at risk for Alzheimer’s disease (AD) using Apolipoprotein ε4 (APOE ε4) as a surrogate are ongoing [4, 5].
APOE ε4 carrier status is associated outright with worse cognitive abilities [6] in addition to its reported impact on cardiovascular disease, stroke, and late life depression [7–10]. Among APOE ε4 carriers, physical inactivity, smoking and alcohol use, depression, and sleep disturbances increase dementia risk by age group [11, 12]. There is also significant interest in understanding the role of vascular disease risk factors contributing to dementia risk given the prevalence of mixed dementia among the older population [13].
Hypertension in midlife and the development of a cognitive decline years later in life is well supported, while late life randomized clinical trials targeting it have shown inconsistent results [14]. The combination of hypertension and hypercholesterolemia has also been reported to increase risk of AD [15] and vascular dementia in later life [16], but other studies report no association between plasma cholesterol and AD dementia [17, 18]. Hypercholesterolemia has been reported as a high-risk factor in midlife measurements [15, 19–23] and strikingly as low risk in late life measurements impacting dementia onset [23–25], but the contribution from APOE ε4 genotype in these findings is still debated. Hypertension and type 2 diabetes taken together are associated with an increased risk of AD and cognitive decline among APOE ε4 carriers [26–29].
In evaluating this slew of results, it is often difficult to parse which of these reported effects of vascular risk factors with regards to APOE ε4 carrier status are specific to a) all-cause dementia versus specific dementia etiologies like AD or mixed pathology; and b) how these results are impacted by variation in the frequency of dementia etiologies among young versus older age individuals [30].
We therefore undertook the current study in the National Alzheimer’s Coordinating Center (NACC) dataset that includes a highly ascertained cohort across multiple sites across the United States with consistent cognitive characterization, including those with normal cognition at baseline, some of whom developed dementia during their longitudinal follow. We had two initial hypotheses: 1) Among older (> 65 years), who often have mixed dementia pathologies, a history of hypertension or hypercholesterolemia would be associated with an increased risk of all-cause dementia among APOE ε4 carriers compared to APOE ε4 non-carriers; 2) The key results of these risk factors on all-cause dementia onset would be corroborated among the subset of participants with a clinical diagnosis of AD dementia and underlying mixed dementia pathology.
MATERIALS AND METHODS
Participants and study design
This is a retrospective cohort study using the NACC dataset. The complete dataset includes participant information collected from 39 past and present Alzheimer’s Disease Centers (ADC) funded by the National Institute on Aging. Data from the uniform data set (UDS) maintained by NACC between September 2005 and September 2018 was used for the present analysis. All contributing ADCs are required to obtain informed consent from their participants and maintain their own separate IRB review and approval from their institution prior to submitting data to NACC. Details on approximately annual data collection and data curating are well documented [31]. In brief, NACC data are collected by trained clinicians and clinic personnel from participants and their co-participants (usually a close friend or family member). The UDS is collected using a standardized evaluation of participants. All of the ADC personnel use the same standard forms and coding guidebooks that provide guidance on filling out the forms. The UDS is longitudinal, and its protocol requires approximately annual follow-up for as long as the participant is able to be involved. Late-stage participants forced to drop out due to health may continue to be followed strictly for autopsy purposes. Determinations of cognitive status in NACC are based on a clinical consensus after review of all available information at each center. In addition, there is evidence supporting good agreement on measures from the NACC neuropathology form across centers used in this study [32].
Clinical and pathological assessment
All participants included had completed CDR® Dementia Staging Instrument score and had a Clinical Dementia Rating-Global (CDR-G) scale of zero at initial visit. The CDR-G scale assesses the participant’s current cognitive and functional status. The CDR-G ratings are calculated using a complex algorithm and range from 0 (no dementia) to 3 (severe dementia) [33]. To enable consistent evaluations for the purpose of this analysis, normal functional status was defined as CDR-G = 0 and dementia was defined as CDR-G ≥ 1.
Rationale for participant inclusion/exclusion criteria was to determine the likelihood of onset of dementia (transition to CDR-G ≥ 1) across the groups of interest. All participants included in this analysis had known APOE ε4 status and complete data from their medical comorbidities profile of interest. Young and old participants groups were separated for analysis based on 65 years being the common cutoff used for young onset dementia [34].
Data on hypercholesterolemia, hypertension, thyroid disease, and vitamin B12 deficiency were collated from the medical history of the participants at initial visit in the NACC database. There was no secondary validation of the medical diagnosis with a supporting biomarker, including data on LDL or total cholesterol levels and a limited number of subjects in NACC had data on statin use and therefore these data were not used for further analysis given likely biases in the data.
For secondary analyses, primary etiological diagnosis as determined by clinician at last visit was collated for each participant that underwent transition to dementia (CDR-G). Clinicians are asked to mark only one condition as primary to the observed cognitive impairment which is documented in UDS Form D1. A subset of participants included in the analysis had neuropathology of AD (Braak stages III-VI and moderate or frequent neuritic plaques), Lewy body pathology (Brainstem-predominant, Limbic or amygdala-predominant, Neocortical, or have Lewy bodies present but region unspecified), vascular pathology (ischemic, hemorrhagic, or other vascular pathology), had any two of the above three pathologies documented concomitantly (mixed dementia), or frontotemporal lobar degeneration pathologies.
Statistical analysis
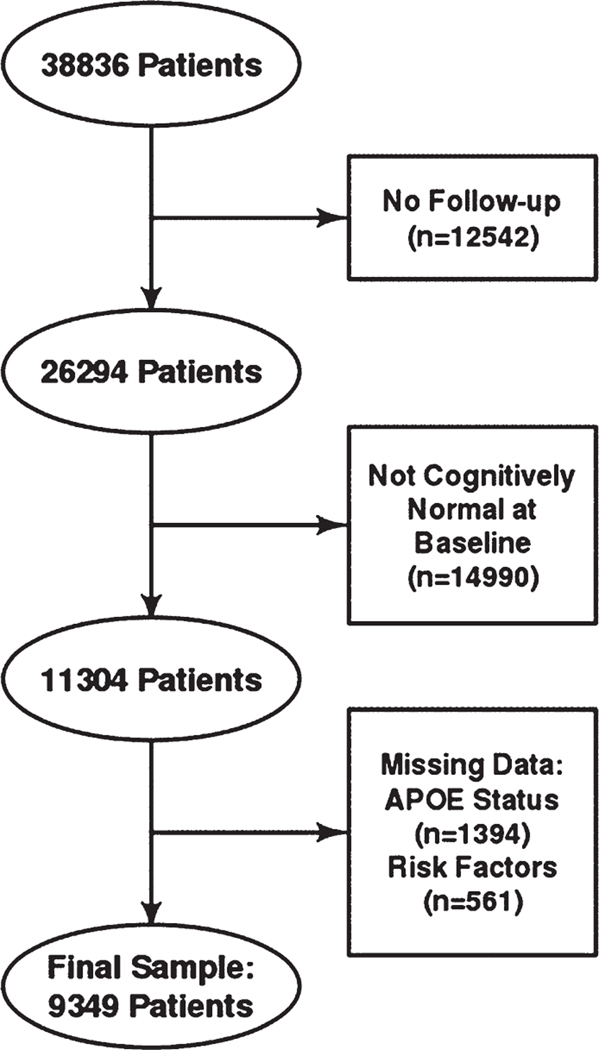
9,349 participants were included in the analysis and of them 519 (5.55%) converted to dementia. Flow chart for participant selection, limiting participants to those without missing data of interest is noted in Fig. 1. Sex (male, female), active depression in the past two years, hypertension, hypercholesterolemia, thyroid disease, and vitamin B12 deficiency (present versus absent) were included in the models as dichotomous variables. Age, education, and smoker years (one point per year) were included in the models as continuous variables. Shapiro-Wilks test was conducted for continuous variables to assess normality. A t-test was applied to compare normally distributed continuous variables, Wilcoxon rank sum test was applied to compare non-normally distributed continuous variables, and Pearson’s Chi-square test was applied to compare categorical variables. Normally distributed measures were summarized as means (standard deviations), non-normally distributed continuous measures were described using median and inter quartile range, and categorical factors were presented as frequencies (percentages).
Fig. 1.
Participant selection flow chart.
Our primary goal was to evaluate how vascular risk factors predicted risk of dementia onset within older APOE ε4 carrier groups compared to older non-carriers as APOE ε4 peak dementia risk is in the over 65 years age group [4]. As we expected to identify different risk factors across these subgroups, a stratified approach was chosen. Compared to the alternative higher order interaction approach, a stratified approach was expected to deliver more parsimonious models for clearer interpretation. Multivariable Cox proportional hazard regression was employed as our study’s goal was to identify risk factors instead of making predictions on clinical outcomes [35]. To assess association between time to develop dementia (with event of interest being onset of dementia defined as Clinical Dementia Rating Global scale CDR-G ≥ 1) and potential risk factors, among APOE ε4 carriers and non-carriers separately for age ≤ 65 and age > 65 separately. There were 310 patients (3.3%) who died the same year or one year after their last follow up. This low death rate suggested death was not a competing risk in the analysis arms. Multicollinearity was assessed for all variables for their impact on final results.
There were over 2500 participants per APOE ε4 carrier group or the age categories defined. Secondary comparisons stratifying by both these factors yielded sample sizes of at least 900. With this smaller sample size and the dementia conversion rate of 5% at 5 years seen in this cohort, there was 80% power to detect hazard ratios of at least 1.6 for risk factors for stratification by age or APOE ε4 carrier status, and hazard ratios of more than 2.0 for risk factors with stratification for both factors. Calculations assume a prevalence of at least 40% among the risk factors, as was observed for both hypertension and hypercholesterolemia.
In a follow up analysis to confirm the main group results and to evaluate whether their significance is consistent among those with specific clinical diagnosis of AD dementia and neuropathological corroboration of mixed dementia (AD, vascular, and/or Lewy body pathology), Kaplan-Meier survival curves were compared for each of these diagnoses on hypertension and hypercholesterolemia by using the log-rank test and a Schoenfeld residual method was used to assess proportionality of hazards and p values were larger than 0.05. Given the reduced sample sizes within these subgroups, multivariable models were not fit. All tests were two-tailed and performed at a significance level of 0.05. Given the a priori hypotheses on hypertension and hypercholesterolemia alone, we considered these results without false discovery rate (FDR) correction [36]. FDR corrections across models for each of the other variables are provided for interpretation. R version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
Data availability
The data used in the study are publicly accessible after a formal request to NACC.
RESULTS
The demographics of participants in the cohort are described in Table 1 and Supplementary Table 1. The average age at initial visit who converted to dementia on follow up was 77.6 years (Std dev 10.1), with a time to dementia conversion from initial evaluation at 71.6 months (Std dev 32.1). In the overall cohort, APOE ε4 carriers had a significant increase in risk (1.56 (95% CI: 1.28–1.89, p < 0.001) for dementia as expected.
Table 1.
Participant demographics. Median, IQR (inter quartile range)
| APOE ε4 carriers | APOE ε4 non-carriers | p | |
|---|---|---|---|
| (n = 2,806) | (n = 6,543) | ||
| Age at initial visit [y] Median [IQR] | 69.00 [64.00;76.00] | 72.00 [66.00;79.00] | < 0.001 |
| ≤ 65 y | 32.61% | 24.58% | |
| > 65 y | 67.39% | 75.42% | |
| Follow up time [y] Median [IQR] | 4.81 [2.70;7.45] | 4.88 [2.40;7.88] | 0.86 |
| Dementia at follow up | 6.34% | 5.21% | 0.032 |
| Education [y] Median [IQR] | 16.00 [14.00;18.00] | 16.00 [14.00;18.00] | 0.49 |
| Smoker y Median [IQR] | 0 [0–15] | 0 [0–18] | 0.071 |
| Female sex | 65.65% | 65.29% | 0.76 |
| Diabetes | 9.62% | 10.13% | 0.473 |
| Hypertension | 43.66% | 46.39% | 0.016 |
| Hypercholesterolemia | 50.68% | 43.31% | < 0.001 |
| Vitamin B12 deficiency | 2.28% | 2.43% | 0.72 |
| Thyroid disease | 15.22% | 16.60% | 0.10 |
| Active depression in past 2 y | 16.71% | 15.97% | 0.39 |
| APOE ε4/ε4 homozygotes | 9.1% | - | - |
> 65 years, older age group
Among all older participants, the median age at initial visit was 75.0 [IQR 70.0; 80.0], 34.5% of participants were APOE ε4 carriers. APOE ε4 allele significantly increased the risk of dementia onset (HR 1.5 (95% CI (1.2–1.8), p < 0.001), along with recent depression history, thyroid disease, and hypertension, while higher education and hypercholesterolemia were related to lower risk of all-cause dementia onset (Table 2A).
Table 2.
Multivariable Cox proportional hazard regression for young and old age groups
| a: > 65 y (Old) Variables | Hazard ratio (95% CI) | p | FDR |
|---|---|---|---|
| Education, y | 0.94 (0.91, 0.97) | < 0.001 | 0.001 |
| Smoker, y | 0.99 (0.99, 1.003) | 0.265 | 0.53 |
| Sex - Male versus Female | 1.12 (0.92, 1.37) | 0.261 | 0.261 |
| Diabetes - present versus absent | 0.91 (0.66, 1.27) | 0.586 | 0.846 |
| Hypertension - present versus absent | 1.35 (1.11, 1.63) | 0.002 | NA |
| Hypercholesterolemia - present versus absent | 0.77 (0.63, 0.93) | 0.007 | NA |
| Vit B12 deficiency - present versus absent | 1.03 (0.56, 1.87) | 0.933 | 0.933 |
| Thyroid disease - present versus absent | 1.29 (1.03, 1.63) | 0.029 | 0.058 |
| Depression - present versus absent | 1.40 (1.1, 1.79) | 0.007 | 0.014 |
| APOE ε4 + ve status | 1.50 (1.24, 1.82) | < 0.001 | 0.001 |
| b. ≤ 65 y (Young) Variables | Hazard ratio (95% CI) | p | FDR |
| Education, y | 0.89 (0.81, 0.98) | 0.015 | 0.015 |
| Smoker, y | 0.996 (0.972, 1.021) | 0.74 | 0.74 |
| Sex - Male versus Female | 1.76(0.98, 3.15) | 0.059 | 0.118 |
| Diabetes - present versus absent | 1.11 (0.37, 3.31) | 0.846 | 0.846 |
| Hypertension - present versus absent | 0.79 (0.4, 1.59) | 0.511 | NA |
| Hypercholesterolemia - present versus absent | 0.92 (0.48, 1.75) | 0.8 | NA |
| Vit B12 deficiency - present versus absent | 1.35 (0.18, 10.05) | 0.767 | 0.933 |
| Thyroid disease - present versus absent | 0.53 (0.16, 1.73) | 0.292 | 0.292 |
| Depression - present versus absent | 1.76 (0.95, 3.26) | 0.07 | 0.07 |
| APOE ε4 + ve status | 0.79 (0.43, 1.45) | 0.452 | 0.452 |
FDR, False discovery rate.
Among older APOE ε4 carriers, hypercholesterolemia decreased risk of dementia onset during follow up (HR 0.68 (95% CI (0.49–0.94), p = 0.02) (Fig. 2A), while active depression in the last two years increased dementia risk (HR 1.56 (95% CI (1.05–2.31), p = 0.028). Among older APOE ε4 non-carriers, higher education years mitigated dementia onset risk (HR 0.92 (95% CI (0.889–0.96), p < 0.001), while hypertension increased all-cause dementia risk (HR 1.435 (95% CI (1.134, 1.816), p = 0.0026) (Fig. 2b, Table 3).
Fig. 2.
A) Kaplan Meier curves for older APOE ε4 carriers with hypercholesterolemia as a significant predictor for all-cause dementia. B) Kaplan Meier curves for older APOE ε4 non-carriers with hypertension as a significant predictor for all-cause dementia.
Table 3.
Multivariable Cox proportional hazard regression for older (>65 y) APOE ε4 carriers and non-carriers
|
APOE ε4 non-carriers | |||
|---|---|---|---|
| Variables | Hazard ratio (95% CI) | p | FDR |
| Education, y | 0.92 (0.9, 0.96) | < 0.001 | 0.002 |
| Smoker, y | 0.99 (0.99, 1.006) | 0.77 | 0.828 |
| Sex - Male versus Female | 1.21 (0.95, 1.54) | 0.13 | 0.262 |
| Diabetes - present versus absent | 1.02 (0.70, 1.50) | 0.91 | 0.909 |
| Hypertension - present versus absent | 1.44 (1.13, 1.82) | 0.003 | NA |
| Hypercholesterolemia - present versus absent | 0.82 (0.65, 1.04) | 0.11 | NA |
| Vit B12 deficiency - present versus absent | 0.91 (0.426, 1.93) | 0.80 | 0.799 |
| Thyroid disease- present versus absent | 1.31 (0.98, 1.74) | 0.06 | 0.256 |
| Depression- present versus absent | 1.30 (0.96, 1.78) | 0.09 | 0.125 |
|
APOE ε4 carriers | |||
| Education, y | 0.98 (0.93, 1.04) | 0.50 | 0.495 |
| Smoker, y | 0.99 (0.98, 1.002) | 0.10 | 0.404 |
| Sex - Male versus Female | 1.001 (0.71, 1.42) | 0.99 | 0.994 |
| Diabetes - present versus absent | 0.72 (0.38, 1.36) | 0.31 | 0.718 |
| Hypertension - present versus absent | 1.22 (0.89, 1.69) | 0.22 | NA |
| Hypercholesterolemia - present versus absent | 0.68 (0.49, 0.94) | 0.02 | NA |
| Vit B12 deficiency - present versus absent | 1.45 (0.53, 3.97) | 0.47 | 0.699 |
| Thyroid disease - present versus absent | 1.32 (0.89, 1.95) | 0.16 | 0.322 |
| Depression - present versus absent | 1.56 (1.05, 2.31) | 0.03 | 0.112 |
FDR, False discovery rate.
≤ 65 years, younger age group
Within the younger age group, the median age at initial visit at 61.0 years [IQR 56.0; 64.0], 32.7% of participants were APOE ε4 carriers among them. APOE ε4 did not significantly increase risk of dementia onset (HR 0.8 (95% CI (0.4–1.5), p = 0.452). Additionally, none of the clinical factors evaluated impacted all-cause dementia onset except higher educational attainment decreasing its risk (Table 2B).
Within both young APOE ε4 carriers and non-carrier subgroups, vascular risk factors did not differentially impact the risk in progressing from normal cognition to all-cause dementia. There was a significant effect of higher education years in mitigating dementia risk among young APOE ε4 carriers (HR 0.84 (95% CI (0.72–0.97), p = 0.015) and male sex in increasing all-cause dementia risk among young APOE ε4 non-carriers (HR 2.28 (95% CI (1.13–4.6), p = 0.022) (Table 4).
Table 4.
Multivariable Cox proportional hazard regression for younger (≤65 y) APOE ε4 carriers and non-carriers
|
APOE ε4 non-carriers | |||
|---|---|---|---|
| Variables | Hazard ratio (95% CI) | p | FDR |
| Education, y | 0.91 (0.81, 1.03) | 0.14 | 0.191 |
| Smoker, y | 1.003 (0.98, 1.03) | 0.83 | 0.828 |
| Sex - Male versus Female | 2.28 (1.13, 4.59) | 0.02 | 0.088 |
| Diabetes - present versus absent | 0.74 (0.17, 3.32) | 0.70 | 0.909 |
| Hypertension - present versus absent | 0.45 (0.18, 1.17) | 0.10 | NA |
| Hypercholesterolemia - present versus absent | 1.24 (0.57, 2.69) | 0.59 | NA |
| Vit B12 deficiency - present versus absent | 2.19 (0.29, 16.37) | 0.45 | 0.699 |
| Thyroid disease - present versus absent | 0.57 (0.13, 2.49) | 0.46 | 0.458 |
| Depression - present versus absent | 1.90 (0.91, 3.95) | 0.09 | 0.125 |
|
APOE ε4 carriers | |||
| Education, y | 0.84 (0.72, 0.97) | 0.02 | 0.03 |
| Smoker, y | 0.98 (0.93, 1.04) | 0.51 | 0.828 |
| Sex - Male versus Female | 0.99 (0.33, 2.95) | 0.99 | 0.994 |
| Diabetes - present versus absent | 2.11 (0.43, 10.40) | 0.36 | 0.718 |
| Hypertension - present versus absent | 2.22 (0.73, 6.71) | 0.16 | NA |
| Hypercholesterolemia - present versus absent | 0.48 (0.16, 1.48) | 0.20 | NA |
| Thyroid disease - present versus absent | 0.31 (0.04, 2.44) | 0.26 | 0.351 |
| Depression - present versus absent | 1.63 (0.50, 5.27) | 0.41 | 0.414 |
Vitamin B12 deficiency not included in the model as no subject was recorded to be deficient. FDR, False discovery rate.
Analysis of specific underlying etiologies of dementia
Clinical etiology for the participants who converted to dementia during follow up is provided by age group in Supplementary Table 2. The younger age group compared to the older age group had a lower frequency of a primary clinical diagnosis of AD dementia (27.3% versus 70.6%) and vascular dementia (3% versus 13.1%) and a higher frequency of frontotemporal dementia (54.5% versus 5.6%) (χ2 = 41.3, p < 0.00001). Among the subgroup in whom neuropathology data was available, the results are presented in Supplementary Table 3 noting the high prevalence of vascular and AD pathology among the older age group.
In the supplementary analyses, when participants were categorized according to subgroups with clinical and neuropathology data of specific dementia etiologies, similar patterns for the effect of hypercholesterolemia and hypertension were noted between the ‘all-cause dementia’ analysis and in the supplementary analyses for the older age group. Dementia-free survival time was higher in older APOE ε4 carrier participants with hypercholesterolemia with a clinical AD diagnosis (log-rank test: p = 0.02) and lower among those with hypertension with a clinical AD diagnosis (log-rank test: p = 0.02) (Supplementary Figure 1A, B).
In the neuropathology defined sub analyses, dementia-free survival time was higher in older APOE ε4 carrier participants with hypercholesterolemia and mixed dementia (log-rank test: p = 0.03) and lower among those with hypertension and mixed dementia (log-rank test: p = 0.02) (Supplementary Figure 2A, B).
DISCUSSION
Our results from this large well characterized longitudinal cohort clearly shows among older APOE ε4 carriers, a medical history of hypercholesterolemia was related to a lower risk of all-cause dementia. Among older APOE ε4 non-carriers, a medical history of hypertension increased risk of converting to dementia when compared to APOE ε4 carriers. The results were statistically significant even after adjustment for common clinical comorbidities. These results were also consistent within the older age mixed dementia subgroup defined by neuropathology burden of AD, vascular, and Lewy body pathologies. Among younger APOE ε4 carriers compared to non-carriers, hypertension and hypercholesterolemia did not significantly impact the risk of progressing from normal cognition to all-cause dementia.
This study noted that APOE ε4 increases risk of dementia in the older age group but not in the younger age group. A closer age stratification by each decade in the NACC data previously noted that APOE ε4 carrier status and APOE ε4 dose, both significantly predicted risk of progression to AD in all age groups of cognitively normal individuals greater than 60 years but not in those less than or equal to 60 years group [37]. Given the interquartile age range of the young onset dementias in the current study (56–64 years), the findings likely reflect the non-linear effects of APOE ε4 carrier status on dementia risk at younger ages.
Even as the role of hypercholesterolemia on atherogenesis is well appreciated, recent evidence suggests that rather than high-density lipoproteins (HDL) alone, sub fractions of HDL are differentially associated with coronary heart disease [38] and dementia [39]. The role for hypercholesterolemia is therefore not surprisingly more nuanced as a dementia risk with the presence of APOE ε4 genotype. Similar results to ours on the risk lowering effect of hypercholesterolemia on risk of all-cause dementia has been previously reported [23–25] and in relation to APOE ε4 status in population studies [40, 41]. In a clinical trial over 30 weeks, high-cholesterol-APOE ε4 group was found to have slower decline than a high cholesterol non-APOE ε4 allele group [42]. The biological reasons for these findings are still a subject of active investigation.
Hypertension during midlife has been reported to be a risk factor for cognitive decline and dementia in many reports, while optimal blood pressure ranges for older adults may depend on earlier life hypertension history [4, 43–45]. There have been limited number of studies that have evaluated the direct effect of hypertension between APOE ε4 carriers and non-carriers when controlling for other confounders [46]. In the current study, with a larger number of subjects in a longitudinal aging cohort, the risk of dementia onset from hypertension beyond other co-morbidities on dementia onset appears to be significant among APOE ε4 non-carriers.
The differential effects of midlife and late life effects of cardiovascular risk factors on all-cause dementia risk though well-known [10–25], the current results evaluate more closely the role for these factors taking into account age, APOE ε4 carrier status, and common clinical comorbidities likely explaining variations in its relative effect of these comorbidities. In addition, all participants in this analysis started from a normal functional baseline that was formally evaluated unlike some population reports. Our findings on the impact of vascular risk in relation to APOE ε4 status among older subjects has important implications. Identifying individuals at greatest genetic risk for AD and understanding the differential impact of modifiable risk factors may help with providing appropriate individual clinical decision making and risk stratifying patients in a clinical trial of APOE ε4 carriers. In addition, these results prompt us to determine the underlying biology behind the role of hypercholesterolemia among older APOE ε4 carriers in decreasing their dementia risk.
In prior studies, APOE ε4 status has been reported to modify the association between depressive symptoms and dementia [47, 48]. The current report also notes older APOE ε4 carriers with depression have heighted risk of dementia onset. Education has been reported to be a protective influence on the risk of developing dementia even when adjusting for the number of APOE ε4 alleles [49]. In this study, the mitigating effect of education on dementia onset was seen among younger APOE ε4 non-carriers and older APOE ε4 carriers, suggesting its effect is likely modulated by the relative strength of other comorbidities and factors including age and APOE ε4 status.
A recent meta-analysis did not report a difference between sexes on dementia risk between 55 and 85 years and noted women have an increased risk at younger ages [50]. It is likely that multiple environmental and clinical factors contribute to these differences noted across studies [51]. Female APOE ε4 carriers have shown more pronounced AD-like changes in neuroimaging and neuropathological measures when compared to their APOE ε3 homozygous peers [52–54]. The higher proportion of non-AD dementias in the young age group in this study could potentially account for male sex to be associated with higher all-cause dementia risk among young APOE ε4 non-carriers. In addition, the current analysis takes into account other confounders that also have sex differences with age (cardiovascular risk, coronary artery disease, type II diabetes, depression) [55–57] that were often not considered in some of the biomarker focused studies.
The impact of vascular risk factors (hypertension, hypercholesterolemia, diabetes) to moderate APOE-related differences in cognitive performance with age has been noted to differ based on the nature of characterization of the risk factors (self-report versus biomarkers) and between longitudinal versus cross-sectional studies [58–61]. These differences make generalizations across studies challenging but likely provide complementary information on the relative strength of the associations. Moreover, the actual differences in risk even though significant for hypertension and hypercholesterolemia between APOE ε4 carriers and non-carriers is relatively small; these could therefore have been easily missed in earlier studies with smaller subject numbers.
Vascular risk factors hypertension and diabetes have been related to lower performance in visuospatial skills/speed and verbal fluency domains [62]. Vascular co-morbidities, including hypertension and hypercholesterolemia, have been associated with greater impairments in immediate verbal memory and delayed visual memory, verbal reasoning, set shifting, and visuospatial skills [63]. There have been limited studies examining the effect of hypercholesterolemia alone on specific cognitive domains with age and APOE ε4 status.
Furthermore, the term ‘obesity paradox’ has been used to describe the effect of excess weight improving predicted survival in elderly [64] and risk of dementia [65], with increasing weight loss per decade from midlife to late life a marker for mild cognitive impairment [66]. In this light, it is possible that the presence of hypercholesterolemia among elderly is a proxy for lack of significant weight loss. It is also possible that there are biological explanations for this phenomenon; since brain ApoE4 is less lipidated and stable than are ApoE3 and ApoE2 [67, 68], increasing brain ApoE levels and lipidation has therefore been proposed as a therapeutic approach [69]. Hypercholesterolemia among APOE ε4 carriers could then be a natural counterweight on the poorly lipidated state of ApoE4 decreasing the risk of dementia onset as noted. These intriguing possibilities need biomarker confirmation.
Limitations and strengths
Some key variables including hypertension, hypercholesterolemia, diabetes, thyroid disease, and vitamin B12 deficiency were defined by clinical diagnoses and not based on biomarkers as they were not available to corroborate the diagnoses. Use of CDR-G score of zero to define the functionally unimpaired at baseline also has its limitations as subtle cognitive changes related to early disease progression could be misattributed as normal. Despite the large size of the initial NACC cohort, sub-stratification by age can make the analysis of some subgroups small. Given the smaller number of subjects, possible recruitment biases and lower frequency of vascular and AD etiology as the primary cause of dementia among the younger age group in this cohort, the results should be interpreted with caution.
Small numbers of subjects in some subgroups also limits our analysis on follow up questions of interest to this study, especially when trying to understand the role of concomitant medication use (statins, hypertension medications) on the results given the low frequency of dementia onset within each of the subgroups. The potential limitations from missing data were addressed by limiting analyses to participants with completed data fields in the variables of interest, as no clear rationale for a rigorous imputation methods (e.g., multiple imputation) could be met. Study results would have to be therefore interpreted with caution regarding unforeseen biases from this analysis method. Participant dropout could result in survivorship bias, although it is less likely for three reasons: 1) number of participants followed remain fairly consistent over time across comparison groups (with and without hypercholesterolemia/hypertension), 2) low death rate in the analysis arms as noted earlier, and 3) additionally, survivorship bias alone is not likely to account for protective benefit seen only with hypercholesterolemia and not with hypertension or diabetes which also have a higher risk of early mortality. The study also evaluated the underlying dementia etiology to corroborate the results of all-cause dementia risks to clarify if it applies to AD and mixed dementia pathologies as well. Given the strengths and biases of the NACC cohort, it is likely the current results are generalizable to other prospective research cohorts tracking normal cognition APOE ε4 carriers and non-carriers including clinical trials.
CONCLUSIONS
APOE ε4 status differently impacts the relevance of clinical factors including hypercholesterolemia, hypertension, and depression on the risk of dementia onset. These results are worthy of further pursuit to clarify the nature of the protective effect of hypercholesterolemia among older APOE ε4 carriers.
Supplementary Material
ACKNOWLEDGMENTS
Jagan A. Pillai is supported by the Alzheimer Association, Keep Memory Alive Foundation, NIA K23 AG055685-01 and P30 AG062428-01, and the Jane and Lee Seidman fund.
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG062428-01 (PI James Leverenz, MD) P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P30 AG062421-01 (PI Bradley Hyman, MD, PhD), P30 AG062422-01 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P30 AG062429-01(PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P30 AG062715-01 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20–1609r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-201609.
REFERENCES
- [1].Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11, 718–726. [DOI] [PubMed] [Google Scholar]
- [2].Scott KR, Barrett AM (2007) Dementia syndromes: Evaluation and treatment. Expert Rev Neurother 7, 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Hazlett KE, Figueroa CM, Kandah CC, Kay CD, Matthews MA, Rao SM (2014) Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer’s disease. Front Aging Neurosci 6, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qian J, Wolters FJ, Beiser A, Haan M, Ikram MA, Karlawish J, Langbaum JB, Neuhaus JM, Reiman EM, Roberts JS, Seshadri S, Tariot PN, Woods BM, Betensky RA, Blacker D (2017) APOE-related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts. PLoS Med 14, e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reiman EM, Langbaum JB, Tariot PN (2010) Alzheimer’s prevention initiative: A proposal to evaluate presymptomatic treatments as quickly as possible. Biomark Med 4, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lyall DM, Celis-Morales C, Lyall LM, Graham C, Graham N, Mackay DF, Strawbridge RJ, Ward J, Gill JMR, Sattar N, Cavanagh J, Smith DJ, Pell JP (2019) Assessing for interaction between APOE epsilon4, sex, and lifestyle on cognitive abilities. Neurology 92, e2691–e2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wilson PW, Schaefer EJ, Larson MG, Ordovas JM (1996) Apolipoprotein E alleles and risk of coronary disease. A meta-analysis. Arterioscler Thromb Vasc Biol 16, 1250–1255. [DOI] [PubMed] [Google Scholar]
- [8].Ilveskoski E, Perola M, Lehtimaki T, Laippala P, Savolainen V, Pajarinen J, Penttila A, Lalu KH, Mannikko A, Liesto KK, Koivula T, Karhunen PJ (1999) Age-dependent association of apolipoprotein E genotype with coronary and aortic atherosclerosis in middle-aged men: An autopsy study. Circulation 100, 608–613. [DOI] [PubMed] [Google Scholar]
- [9].Lagging C, Lorentzen E, Stanne TM, Pedersen A, Soderholm M, Cole JW, Jood K, Lemmens R, Phuah CL, Rost NS, Thijs V, Woo D, Maguire JM, Lindgren A, Jern C, Genetics of Ischaemic Stroke Functional Outcome (GISCOME) network and the International Stroke Genetics Consortium (2019) APOE epsilon4 is associated with younger age at ischemic stroke onset but not with stroke outcome. Neurology 93, 849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Feng F, Lu SS, Hu CY, Gong FF, Qian ZZ, Yang HY, Wu YL, Zhao YY, Bi P, Sun YH (2015) Association between apolipoprotein E gene polymorphism and depression. J Clin Neurosci 22, 1232–1238. [DOI] [PubMed] [Google Scholar]
- [11].Kivipelto M, Rovio S, Ngandu T, Kareholt I, Eskelinen M, Winblad B, Hachinski V, Cedazo-Minguez A, Soininen H, Tuomilehto J, Nissinen A (2008) Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: A population-based study. J Cell Mol Med 12, 2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burke SL, Maramaldi P, Cadet T, Kukull W (2016) Associations between depression, sleep disturbance, and apolipoprotein E in the development of Alzheimer’s disease: Dementia. Int Psychogeriatr 28, 1409–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204. [DOI] [PubMed] [Google Scholar]
- [14].Sierra C (2020) Hypertension and the risk of dementia. Front Cardiovasc Med 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A (2001) Midlife vascular risk factors and Alzheimer’s dis-ease in later life: Longitudinal, population based study. BMJ 322, 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, Ross GW, Havlik RJ, Launer LJ (2000) Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol 20, 2255–2260. [DOI] [PubMed] [Google Scholar]
- [17].Tan ZS, Seshadri S, Beiser A, Wilson PW, Kiel DP, Tocco M, D’Agostino RB, Wolf PA (2003) Plasma total cholesterol level as a risk factor for Alzheimer disease: The Framingham Study. Arch Intern Med 163, 1053–1057. [DOI] [PubMed] [Google Scholar]
- [18].Romas SN, Tang MX, Berglund L, Mayeux R (1999) APOE genotype, plasma lipids, lipoproteins, and AD in community elderly. Neurology 53, 517–521. [DOI] [PubMed] [Google Scholar]
- [19].Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA (2009) Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord 28, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281. [DOI] [PubMed] [Google Scholar]
- [21].Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H (2002) Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 137, 149–155. [DOI] [PubMed] [Google Scholar]
- [22].Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A (1998) Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology 17, 14–20. [DOI] [PubMed] [Google Scholar]
- [23].Anstey KJ, Ashby-Mitchell K, Peters R (2017) Updating the evidence on the association between serum cholesterol and risk of late-life dementia: Review and meta-analysis. J Alzheimers Dis 56, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reitz C, Tang MX, Luchsinger J, Mayeux R (2004) Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol 61, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mielke MM, Zandi PP, Sjogren M, Gustafson D, Ostling S, Steen B, Skoog I (2005) High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 64, 1689–1695. [DOI] [PubMed] [Google Scholar]
- [26].Peila R, White LR, Petrovich H, Masaki K, Ross GW, Havlik RJ, Launer LJ (2001) Joint effect of the APOE gene and midlife systolic blood pressure on late-life cognitive impairment: The Honolulu-Asia aging study. Stroke 32, 2882–2889. [DOI] [PubMed] [Google Scholar]
- [27].Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr Diaz-Arrastia R, Park DC (2013) Risk factors for beta-amyloid deposition in healthy aging: Vascular and genetic effects. JAMA Neurol 70, 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Irie F, Fitzpatrick AL, Lopez OL, Kuller LH, Peila R, Newman AB, Launer LJ (2008) Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: The Cardiovascular Health Study Cognition Study. Arch Neurol 65, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging S (2002) Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 51, 1256–1262. [DOI] [PubMed] [Google Scholar]
- [30].Giannakopoulos P, Hof PR, Savioz A, Guimon J, Antonarakis SE, Bouras C (1996) Early-onset dementias: Clinical, neuropathological and genetic characteristics. Acta Neuropathol 91, 451–465. [DOI] [PubMed] [Google Scholar]
- [31].Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA, NIA Alzheimer’s Disease Centers (2007) The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord 21, 249–258. [DOI] [PubMed] [Google Scholar]
- [32].Montine TJ, Monsell SE, Beach TG, Bigio EH, Bu Y, Cairns NJ, Frosch M, Henriksen J, Kofler J, Kukull WA, Lee EB, Nelson PT, Schantz AM, Schneider JA, Sonnen JA, Trojanowski JQ, Vinters HV, Zhou XH, Hyman BT (2016) Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer’s disease. Alzheimers Dement 12, 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [34].Dufouil C, Richard F, Fievet N, Dartigues JF, Ritchie K, Tzourio C, Amouyel P, Alperovitch A (2005) APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: The Three-City Study. Neurology 64, 1531–1538. [DOI] [PubMed] [Google Scholar]
- [35].Lau B, Cole SR, Gange SJ (2009) Competing risk regression models for epidemiologic data. Am J Epidemiol 170, 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, Devereaux PJ, Thabane L (2017) An introduction to multiplicity issues in clinical trials: The what, why, when and how. Int J Epidemiol 46, 746–755. [DOI] [PubMed] [Google Scholar]
- [37].Bonham LW, Geier EG, Fan CC, Leong JK, Besser L, Kukull WA, Kornak J, Andreassen OA, Schellenberg GD, Rosen HJ, Dillon WP, Hess CP, Miller BL, Dale AM, Desikan RS, Yokoyama JS (2016) Age-dependent effects of APOE epsilon4 in preclinical Alzheimer’s disease. Ann Clin Transl Neurol 3, 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jensen MK, Aroner SA, Mukamal KJ, Furtado JD, Post WS, Tsai MY, Tjonneland A, Polak JF, Rimm EB, Overvad K, McClelland RL, Sacks FM (2018) High-density lipoprotein subspecies defined by presence of apolipoprotein C-III and incident coronary heart disease in four cohorts. Circulation 137, 1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Koch M, DeKosky ST, Goodman M, Sun J, Furtado JD, Fitzpatrick AL, Mackey RH, Cai T, Lopez OL, Kuller LH, Mukamal KJ, Jensen MK (2020) Association of apolipoprotein E in lipoprotein subspecies with risk of dementia. JAMA Netw Open 3, e209250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Evans RM, Emsley CL, Gao S, Sahota A, Hall KS, Farlow MR, Hendrie H (2000) Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: A population-based study of African Americans. Neurology 54, 240–242. [DOI] [PubMed] [Google Scholar]
- [41].Hall K, Murrell J, Ogunniyi A, Deeg M, Baiyewu O, Gao S, Gureje O, Dickens J, Evans R, Smith-Gamble V, Unverzagt FW, Shen J, Hendrie H (2006) Cholesterol, APOE genotype, and Alzheimer disease: An epidemiologic study of Nigerian Yoruba. Neurology 66, 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Evans RM, Hui S, Perkins A, Lahiri DK, Poirier J, Farlow MR (2004) Cholesterol and APOE genotype interact to influence Alzheimer disease progression. Neurology 62, 1869–1871. [DOI] [PubMed] [Google Scholar]
- [43].Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH (2014) Midlife hypertension and 20-year cognitive change: The atherosclerosis risk in communities neurocognitive study. JAMA Neurol 71, 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Muller M, Sigurdsson S, Kjartansson O, Aspelund T, Lopez OL, Jonnson PV, Harris TB, van Buchem M, Gudnason V, Launer LJ, Age GE S-RSI (2014) Joint effect of mid- and late-life blood pressure on the brain: The AGES-Reykjavik study. Neurology 82, 2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, Bandeen-Roche K, Coresh J, Gross AL, Windham BG, Knopman DS, Power MC, Rawlings AM, Mosley TH, Gottesman RF (2019) Association of midlife to late-life blood pressure patterns with incident dementia. JAMA 322, 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yasuno F, Tanimukai S, Sasaki M, Ikejima C, Yamashita F, Kodama C, Hidaka S, Mizukami K, Asada T (2012) Effect of plasma lipids, hypertension and APOE genotype on cognitive decline. Neurobiol Aging 33, 2633–2640. [DOI] [PubMed] [Google Scholar]
- [47].Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Pankratz VS, Rocca WA (2006) Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: A prospective cohort study. Arch Neurol 63, 435–440. [DOI] [PubMed] [Google Scholar]
- [48].Irie F, Masaki KH, Petrovitch H, Abbott RD, Ross GW, Taaffe DR, Launer LJ, White LR (2008) Apolipoprotein E epsilon4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: The Honolulu-Asia Aging Study. Arch Gen Psychiatry 65, 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sando SB, Melquist S, Cannon A, Hutton M, Sletvold O, Saltvedt I, White LR, Lydersen S, Aasly J (2008) Risk-reducing effect of education in Alzheimer’s disease. Int J Geriatr Psychiatry 23, 1156–1162. [DOI] [PubMed] [Google Scholar]
- [50].Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang LS, Romero K, Arneric SP, Redolfi A, Orlandi D, Frisoni GB, Au R, Devine S, Auerbach S, Espinosa A, Boada M, Ruiz A, Johnson SC, Koscik R, Wang JJ, Hsu WC, Chen YL, Toga AW (2017) Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol 74, 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mortensen EL, Hogh P (2001) A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology 57, 89–95. [DOI] [PubMed] [Google Scholar]
- [52].Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Gold-stein JM, Kantarci K, Mallampalli MP, Mormino EC, Scott L, Yu WH, Maki PM, Mielke MM (2018) Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement 14, 1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ungar L, Altmann A, Greicius MD (2014) Apolipoprotein E, gender, and Alzheimer’s disease: An overlooked, but potent and promising interaction. Brain Imaging Behav 8, 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative (2014) Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 75, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mosca L, Barrett-Connor E, Wenger NK (2011) Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 124, 2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gale EA, Gillespie KM (2001) Diabetes and gender. Diabetologia 44, 3–15. [DOI] [PubMed] [Google Scholar]
- [57].Salk RH, Hyde JS, Abramson LY (2017) Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull 143, 783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L (1999) The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA 282, 40–46. [DOI] [PubMed] [Google Scholar]
- [59].Small BJ, Graves AB, McEvoy CL, Crawford FC, Mullan M, Mortimer JA (2000) Is APOE–epsilon4 a risk factor for cognitive impairment in normal aging? Neurology 54, 2082–2088. [DOI] [PubMed] [Google Scholar]
- [60].van Vliet P (2012) Cholesterol and late-life cognitive decline. J Alzheimers Dis 30(Suppl 2), S147–162. [DOI] [PubMed] [Google Scholar]
- [61].Appleton JP, Scutt P, Sprigg N, Bath PM (2017) Hypercholesterolaemia and vascular dementia. Clin Sci (Lond) 131, 1561–1578. [DOI] [PubMed] [Google Scholar]
- [62].Miralbell J, Lopez-Cancio E, Lopez-Oloriz J, Arenillas JF, Barrios M, Soriano-Raya JJ, Galan A, Caceres C, Alzamora M, Pera G, Toran P, Davalos A, Mataro M (2013) Cognitive patterns in relation to biomarkers of cerebrovascular disease and vascular risk factors. Cerebrovasc Dis 36, 98–105. [DOI] [PubMed] [Google Scholar]
- [63].Goldstein FC, Ashley AV, Endeshaw YW, Hanfelt J, Lah JJ, Levey AI (2008) Effects of hypertension and hypercholesterolemia on cognitive functioning in patients with Alzheimer disease. Alzheimer Dis Assoc Disord 22, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Freedman DM, Ron E, Ballard-Barbash R, Doody MM, Linet MS (2006) Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 30, 822–829. [DOI] [PubMed] [Google Scholar]
- [65].Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr., Luchsinger JA (2009) Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Arch Neurol 66, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Alhurani RE, Vassilaki M, Aakre JA, Mielke MM, Kremers WK, Machulda MM, Geda YE, Knopman DS, Petersen RC, Roberts RO (2016) Decline in weight and incident mild cognitive impairment: Mayo Clinic Study of Aging. JAMA Neurol 73, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jones PB, Adams KW, Rozkalne A, Spires-Jones TL, Hshieh TT, Hashimoto T, von Armin CA, Mielke M, Bacskai BJ, Hyman BT (2011) Apolipoprotein E: Isoform specific differences in tertiary structure and interaction with amyloid-beta in human Alzheimer brain. PLoS One 6, e14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hu J, Liu CC, Chen XF, Zhang YW, Xu H, Bu G (2015) Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Abeta metabolism in apoE4-targeted replacement mice. Mol Neurodegener 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Serrano-Pozo A, Das S, Hyman BT (2021) APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol 20, 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in the study are publicly accessible after a formal request to NACC.