From the start of the global coronavirus disease 2019 (COVID‐19) pandemic, much attention has been focused on how severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) affects pregnancy. The current evidence suggests that pregnant women may be at increased risk for more severe COVID‐19 disease, and an increase in maternal death rate has been observed worldwide. 1 , 2 However, mothers are concerned not only for their own wellbeing, but also for that of their unborn child. This concern might well be justified, as a global increase in stillbirths (up to 28%) has been observed during this pandemic. 2 Several studies have also reported increases in adverse pregnancy outcomes in SARS‐CoV‐2‐infected mothers, such as preterm delivery and low birthweight. 1
Many placental findings have been associated with both symptomatic and asymptomatic COVID‐19. These mainly included non‐specific signs of maternal or fetal vascular malperfusion, villitis, and intervillositis. Although all of these have been connected to fetal morbidity in the past, none seemed to be specific for a placental SARS‐CoV‐2 infection. 3
As the pandemic progressed, rare reports were published on placental SARS‐CoV‐2 infection with diffuse viral localisation in syncytiotrophoblast. Histologically, these cases showed variable syncytiotrophoblast necrosis and histiocytic intervillositis. Neonatal outcomes in these cases were highly variable, ranging from asymptomatic babies to stillbirths in up to 45% of cases. 4
Our first experience with severe SARS‐CoV‐2 placentitis was in early 2021, when a 22‐year‐old primipara carrying twins presented at 36 weeks with severe pre‐eclampsia and rupture of membranes (case 1). A week earlier, she had tested positively for SARS‐CoV‐2, and had mild symptoms. Examination on admission revealed intrauterine fetal death (IUFD) of one twin and severe fetal distress in the other, for whom therapy was stopped because of diffuse (hypoxic) cerebral damage. This dramatic turn of events raised one question: ‘could this be COVID?’
On sectioning, the dichorionic, diamniotic placenta was diffusely affected by large, patchy, irregular, solid areas with whitish discolouration (Figure 1A). Microscopically, unusually prominent syncytiotrophoblast necrosis, involving 70% of the placenta, and mild to moderate histiocytic intervillositis were seen (Figure 1B,C). The infiltrate was composed of histiocytes, intermixed with smaller numbers of CD8‐negative or CD4‐positive T cells, and neutrophils. There was no villitis. An additional finding was strong, diffuse, linear C4d deposition at the surface of syncytiotrophoblast, strongest in vital areas (Figure 2B). SARS‐CoV‐2 nucleocapsid protein immunohistochemistry showed diffuse, strong staining in villous trophoblasts (Figure 2A). The presence of SARS‐CoV‐2 was confirmed by reverse transcription quantitative real time polymerase chain reaction (RT‐qPCR) on RNA extracted from formalin‐fixed paraffin‐embedded (FFPE) material. Sequencing of the virus failed, but mutation‐specific polymerase chain reactions did not show variants of concern [tested for the 20I/501Y.V1 (UK), 20H/501Y.V2 (South African) and 20J/501Y.V3 (Brazilian) variants].
Figure 1.
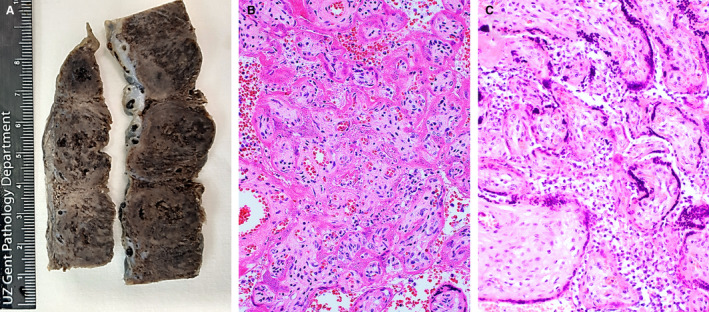
A, Macroscopic view of the placenta of case 1 showing large, irregular, solid areas with whitish discolouration. B, Haematoxylin and eosin (H&E)‐stained section of the placenta of case 1, showing necrotic syncytiotrophoblasts, collapse of the intervillous space, and some histiocytes in the remaining intervillous spaces. The villous stroma is well preserved. C, H&E‐stained section of the placenta of case 2, showing prominent histiocytic intervillositis. Syncytiotrophoblasts show pyknotic nuclei and focal loss of nuclear staining, indicating necrosis.
Figure 2.
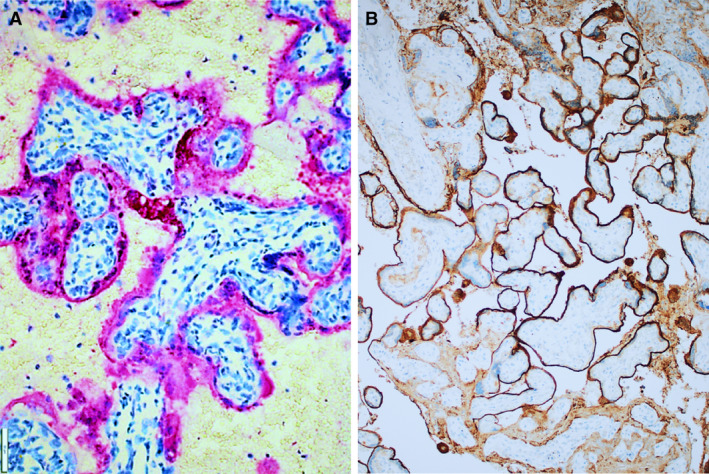
A, Immunohistochemistry for severe acute respiratory syndrome coronavirus 2 on the placenta of case 1, showing diffuse positive staining of trophoblasts (red). B, C4d immunohistochemistry on the placenta of case 1, showing strong and diffuse linear staining at the surfaces of syncytiotrophoblasts.
At autopsy, the IUFD twin showed no signs of inflammation, thrombotic events, or any other specific findings. Nasal swabs in both children tested negative for SARS‐CoV‐2.
One month later, a similar story ensued (case 2), when a SARS‐CoV‐2‐positive 28‐year‐old woman, with a singleton pregnancy of 31 weeks, was admitted with contractions and signs of fetal distress; luckily, this child did well. Placental examination resulted in similar findings, but with less extensive syncytiotrophoblast necrosis (20–30%) and more pronounced intervillositis. Remarkably, similar, strong C4d positivity was also observed. SARS‐CoV‐2 infection was confirmed immunohistochemically. Sequencing of FFPE‐extracted RNA showed infection with the SARS‐CoV‐2 20I/501Y.V1 variant (UK; B.1.1.7). Nasal swabs were negative for the virus.
To test the robustness of our findings, we repeated SARS‐CoV‐2 immunohistochemistry on 14 placentas of SARS‐CoV‐2‐infected mothers, without signs of intervillositis or trophoblast necrosis. On eight of these, RT‐qPCR was performed. No (false)‐positive results were observed. We also immunostained all 14 of the above‐mentioned placentas, as well as infarcted regions in five randomly selected placentas from SARS‐CoV‐2‐negative mothers, for C4d, but none of these showed any positivity.
Case 1 illustrates the possible dramatic consequences of placental SARS‐CoV‐2 infection. In this case, it is highly likely that the massive trophoblast necrosis was responsible for the deleterious effect on the twins. It is also tempting to speculate that massive release of syncytiotrophoblast fragments in the maternal circulation could have played a role in the pre‐eclampsia. However, how SARS‐CoV‐2 provokes trophoblast necrosis and intervillositis is currently unknown. An interesting finding is that prominent C4d deposition was noted in both infected placentas, similar to, but more extensive than, what has been observed in idiopathic chronic histiocytic intervillositis. 5 This suggests that complement activation may play a role in SARS‐CoV‐2 placentitis, analogously to what has been reported in SARS‐CoV‐2 lung infection. 6 The mechanism of infection is still unclear, as the main binding receptor of SARS‐CoV‐2, angiotensin‐converting enzyme 2, is expressed in a polarised pattern, with the highest expression level on the stromal side of the syncytiotrophoblast. 3 A possible way for the virus to bypass the tight syncytiotrophoblast barrier could be via neonatal Fc receptors that transfer virus–IgG complexes through the fetomaternal barrier, similarly to what has been implicated in placental cytomegalovirus infections. 7 Unfortunately, we were unable to obtain fresh placental tissue or appropriate maternal blood samples for further investigation of complement activation.
In conclusion, we report two cases of severe SARS‐CoV‐2 placentitis, which is rare, but can have a dramatic effect on pregnancy outcome. The main histopathological features of SARS‐CoV‐2 placentitis are syncytiotrophoblast necrosis, histiocytic intervillositis, and strong immunohistochemical positivity for SARS‐CoV‐2 nucleocapsid protein. Deposition of C4d may be an additional hallmark, but deserves further study.
Conflicts of interest
The authors declare no conflicts of interest.
Ethics approval
The use of medical information and tissue for this study was approved by the ethics committee of Ghent University Hospital (EC/045‐2021/mf, 20/03/2021).
Author contributions
S.L. and J.V.D. performed paper concept and design; S.L., J.V.D and I.D. performed development of methodology, and writing, review and revision of the paper; I.D. provided acquisition and clinical information; J.V.C, L.V and B.V. provided analysis and interpretation of results; K.V.D.V, A.D., E.P and S.C provided technical and material support. All authors read, reviewed and approved the final paper.
Acknowledgements
We would like to thank J. H. Von der Thüsen and T. P. P. van den Bosch from MC Erasmus, Rotterdam, for performing the SARS‐CoV‐2 nucleocapsid immunohistochemistry, and W. van Snippenberg (Ghent University) for optimising the RT‐qPCRs.
Libbrecht S, Van Cleemput J, Vandekerckhove L, Colman S, Padalko E, Verhasselt B, Van de Vijver K, Dendooven A, Dehaene I & Van Dorpe J. (2021) Histopathology 79, 674–676. 10.1111/his.14402 A rare but devastating cause of twin loss in a near‐term pregnancy highlighting the features of severe SARS‐CoV‐2 placentitis
References
- 1. Dubey P, Thakur B, Reddy S et al. Current trends and geographical differences in therapeutic profile and outcomes of COVID‐19 among pregnant women—a systematic review and meta‐analysis. BMC Pregnancy Childbirth 2021; 21; 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chmielewska B, Barratt I, Townsend R et al. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob. Health 2021; 9; E759–E772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hecht JL, Quade B, Deshpande V et al. SARS‐CoV‐2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID‐19‐positive mothers. Mod. Pathol. 2020; 33; 2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwartz DA, Baldewijns M, Benachi A et al. Chronic histiocytic intervillositis with trophoblast necrosis are risk factors associated with placental infection from coronavirus disease 2019 (COVID‐19) and intrauterine maternal‐fetal severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) transmission in liveborn and stillborn infants. Arch. Pathol. Lab. Med. 2020; 145; 517–528. [DOI] [PubMed] [Google Scholar]
- 5. Bendon RW, Coventry S, Thompson M et al. The significance of C4d immunostaining in placental chronic intervillositis. Pediatr. Dev. Pathol. 2015; 18; 362–368. [DOI] [PubMed] [Google Scholar]
- 6. Gao T, Hu M, Zhang X et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP‐2‐mediated complement over‐activation. medRxiv 2020. E‐pub, 30 March.
- 7. Maidji E, McDonagh S, Genbacev O et al. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor‐mediated transcytosis. Am. J. Pathol. 2006; 168; 1210–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]


