Abstract
Mouse models of radiation-induced thymic lymphoma are commonly used to study the biological effects of total-body irradiation (TBI) on the formation of hematologic malignancies. It is well documented that radiation-induced thymic lymphoma can be inhibited by protecting the bone marrow (BM) from irradiation; however, the mechanisms underlying this phenomenon are poorly understood. Here, we aimed to address this question by performing transplantation of BM cells from genetically engineered mice that have defects in tumor immunosurveillance or occupying different thymic niches. We found that BM cells from mice that have impaired tumor immunosurveillance, by deleting tumor necrosis factor alpha (TNF±), interferon gamma (IFNγ) or perforin-1 (PRF1), remained sufficient to suppress the formation of radiation-induced thymic lymphoma. On the other hand, BM cells from Rag2−/−; γc−/− mice and Rag2−/− mice, which have defects in occupying thymic niches beyond double negative (DN2) and DN3, respectively, failed to inhibit radiation-induced lymphomagenesis in the thymus. Taken together, based on our findings, we propose a model where unirradiated BM cells suppress radiation-induced lymphomagenesis in the thymus by competing with tumor-initiating cells for thymic niches beyond the DN3 stage.
Editor’s note.
The online version of this article (DOI: https://doi.org/10.1667/RADE-20-00221.1) contains supplementary information that is available to all authorized users.
INTRODUCTION
Exposure of the bone marrow (BM) to ionizing radiation from cancer therapy or a nuclear disaster is associated with a significant increase in the risk of hematologic malignancies (1–4). Experimentally, total-body irradiation (TBI) frequently induces thymic (T-cell) lymphomas in certain strains of mice such as BALB/c and C57BL/6 (5–7). Although the thymus is the primary site where lymphoma cells develop (8, 9), protecting the bone marrow, and not the thymus, from radiation is necessary and sufficient to suppress the formation of thymic lymphoma (10). For example, Kaplan and Brown demonstrate that radiation-induced thymic lymphoma in mice can be effectively inhibited by shielding one femur from radiation (11, 12) or by receiving a BM transplant from a unirradiated donor (13–15). Moreover, transplantation of BM cells from previously irradiated donors failed to suppress the development of radiation-induced lymphomas (9, 13, 14, 16). Together, these findings reveal that unirradiated BM cells play an essential role in suppressing radiation-induced lymphomagenesis in the thymus. However, mechanisms underlying this phenomenon remain poorly understood. Here, we performed a series of BM transplantation experiments to test the hypothesis that protection of the BM from TBI prevents the formation of radiation-induced thymic lymphoma through two possible mechanisms: 1. tumor immunosurveillance and 2. niche competition.
MATERIALS AND METHODS
Mouse Strains
All animal procedures for this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Duke University (Durham, NC). Mouse strains used in the study include C57BL/6J mice, B6.SJL mice, tumor necrosis factor alpha knockout (TNFα−/−) mice, interferon gamma knockout (IFNγ−/−) mice, Perforin-1 knockout (PRF1−/−) mice and IgM−/− mice (all acquired from Jackson Laboratory, Bar Harbor, ME), as well as Rag2−/−; γc−/− mice, Rag2−/− mice and C57BL/6NTac mice (all acquired from Taconic Biosciences, Rensselaer, NY). Mice that were 8-to-10 weeks old were used as BM donors. For mice that received BM transplantation, both male and female mice were used and were equally distributed to receive BM cells from different donors or PBS vehicle.
Small Animal Irradiation
The irradiation experiments were performed using the X-RAD 320 Biological Irradiator (Precision X-ray Inc., North Branford, CT). Mice were irradiated at 50 cm from the radiation source at a dose rate of 2.2 Gy/min with 320 kVp X rays, using 12.5 mA and a filter consisting of 2.5 mm aluminum and 0.1 mm copper. The dose rate was measured using an ion chamber by members of the Radiation Safety Division at Duke University.
Induction of Radiation-Induced Thymic Lymphoma and Bone Marrow Transplantation
Four-week-old B6.SJL (CD45.1) mice served as recipient mice, and received four weekly fractions of 1.8 Gy TBI to induce thymic lymphoma (6). At 6 h after the final TBI, recipient mice received PBS vehicle or 1 × 107 whole BM cells from donor mice with various genotypes via intravenous injection according to methods described elsewhere (16). Mice were euthanized when they showed signs of sickness or reached experimental end points. The presence of lymphoma in various organs including the thymus, the spleen, the liver and the kidneys was examined during necropsy, as described elsewhere (16).
Analysis of Thymocytes by Flow Cytometry
Total thymocytes were isolated from the thymus according to methods described elsewhere (16). A total of 1 × 106 live thymocytes were blocked with a rat anti-mouse CD16/32 antibody (BD Pharmingene, San Diego, CA) and stained with a combination of the antibodies including PE-Cy5 conjugated anti-mouse CD4 (clone: GK1.5), PE conjugated anti-mouse CD8 (clone: 53–6.7), APC conjugated anti-mouse CD44 (clone: IM7), FITC conjugated anti-mouse CD45.2 (clone: 104), and APC-eFluor780 conjugated anti-mouse CD45.1 (clone: A20), all acquired from eBioscience™ Inc. (San Diego, CA), as well as BV421 conjugated anti-mouse CD25 antibodies (clone: PC61; BioLegend® Inc., San Diego, CA). All antibodies were diluted 1:400. Data were collected from at least 100,000 single cells using FACSCanto™ (BD Pharmingen) and analyzed using FlowJo (Tree Star Inc., Ashland, OR) without knowledge of the genotype or treatment by a single observer (C-LL).
Analysis of Hematopoietic Stem/Progenitor Cells (HSPCs) in the Bone Marrow by Flow Cytometry
Whole BM cells were isolated from femurs and tibias according to methods described elsewhere (16). A total of 3 × 106 live BM cells were blocked using a rat anti-mouse CD16/32 antibody (BD Pharmingen) and stained with PE-Cy5 conjugated anti-mouse CD3e (clone: 145–2C11), PE-Cy5 conjugated anti-mouse CD4 (clone: GK1.5), PE-Cy5 conjugated anti-mouse CD8 (clone: 53–6.7) PE-Cy5 conjugated anti-mouse B220 (clone: RA3–6B2), PE-Cy5 conjugated PE-Cy5 conjugated anti-mouse CD11b (clone: M1/70), PE-Cy5 conjugated anti-mouse Gr-1 (clone: RB6–8C5), PE-Cy5 conjugated anti-mouse Ter119 (Ly-76), PE conjugated anti-mouse Sca1 (clone: D7), APC conjugated anti-mouse cKit (clone: 2B8), FITC conjugated anti-mouse CD45.2 (clone: 104; eBioscience) and APC-eFluor780 conjugated anti-mouse CD45.1 (clone: A20; e eBioscience). All antibodies were diluted 1:400. Data were collected from at least 500,000 single cells using FACSCanto (BD Pharmingen) and analyzed using FlowJo (Tree Star Inc.) without knowledge of the genotype or treatment by a single observer (C-LL).
Analysis of Peripheral Blood Mononuclear Cells (PBMCs) by Flow Cytometry
PBMCs were isolated according to methods described elsewhere (16). PBMCs were blocked using a rat anti-mouse CD16/32 antibody (BD Pharmingen) and stained with FITC conjugated anti-mouse CD45.2 (clone: 104), APC-eFluor780 conjugated anti-mouse CD45.1 (clone: A20), PE conjugated anti-mouse B220 (clone: RA3–6B2), PE-Cy5 conjugated anti-mouse CD11b (clone: M1/70) (all acquired from eBioscience) and Brilliant Violet 421 conjugated anti-mouse CD3e antibodies (clone: 145–2C11; BioLegend). All antibodies were diluted 1:400. Data were collected from at least 20,000 single cells by FACSCanto (BD Pharmingen) and analyzed using FlowJo (Tree Star, Inc) without knowledge of the genotype or treatment by a single observer (C-LL).
Statistical Analysis
For all experiments, data are presented as mean ± standard error (SE) and each data point represents one mouse. Student’s t test (two-tailed) was performed to compare the means of two groups. One-way analysis of variance (ANOVA) was performed to compare the means of more than two independent groups. For rad0069ation-induced thymic lymphoma experiments, Kaplan-Meier analysis was performed followed by the log-rank test. Significance was assumed at P < 0.05. GraphPad Prism 8 (GraphPad Software Inc., LaJolla, CA) was used for the calculation of the statistics.
RESULTS
To determine the role of tumor immunosurveillance in regulating radiation-induced thymic lymphomagenesis, we conducted transplantation experiments using BM cells from genetically engineered mice that have defects in cytokine production or the function of cytotoxic lymphocytes. It has been reported that treatment with TNFα or IFNγ after TBI significantly decreases the incidence of radiation-induced thymic lymphoma (17–19). To examine the role of TNFα and IFNγ, B6.SJL (CD45.1) mice received four weekly doses of 1.8 Gy TBI and transplanted these mice with BM cells from adult wild-type (C57BL/6J), TNFα−/− and IFNγ−/− mice (all of them are CD45.2). A cohort of irradiated mice that did not receive BM cells (PBS vehicle) served as a positive control for the development of radiation-induced thymic lymphomas. Examination of the engraftment of wild-type, TNFα−/− and IFNγ−/− BM cells in PBMCs showed similar levels of donor-derived repopulation in CD3+ T cells, B220+ B cells and CD11b+ myeloid cells (Supplementary Fig. S1; https://doi.org/10.1667/RADE-20-00221.1.S1). Unexpectedly, we observed that transplantation of BM cells from either wild-type, TNFα−/− or IFNγ−/− mice significantly prevented the formation of radiation-induced thymic lymphomas compared to irradiated mice that did not receive BM cells (P < 0.0001) (Fig. 1A).
FIG. 1.
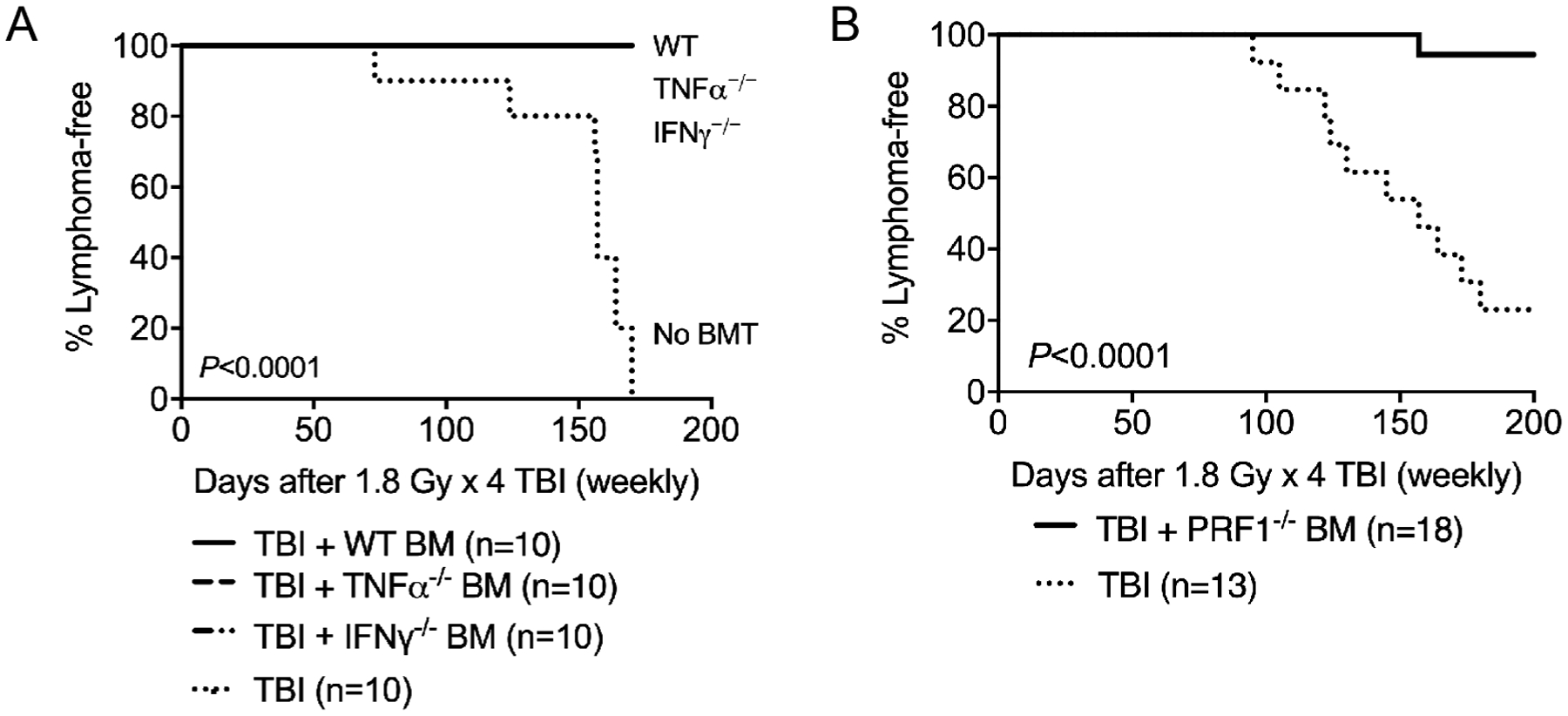
Bone marrow cells from mice that have defects in immunosurveillance remain sufficient to suppress radiation-induced lymphomagenesis in the thymus. Four-week-old B6.SJL (CD45.1) mice received one dose of 1.8 Gy TBI for four consecutive weeks. Bone marrow transplantation was performed 6 h after final TBI. Each recipient was injected intravenously with 1 × 107 bone marrow cells from mice on a CD45.2 background. Both male and female mice were used. Panel A: Lymphoma-free survival of irradiated mice that received bone marrow cells from wild-type (WT), TNFα−/− or IFNγ−/− mice. Irradiated mice that did not receive bone marrow cells (No BMT) were used as a positive control for the development of radiation-induced thymic lymphoma. The lines of irradiated mice that received bone marrow cells from WT, TNFα−/− or IFNγ−/− mice are superimposed. P Value was calculated using log-rank test. Panel B: Lymphoma-free survival of irradiated mice that received bone marrow cells from either PRF1−/− mice or did not receive bone marrow cells. P value was calculated using log-rank test.
It has been shown that cytotoxic lymphocytes play a critical role in suppressing the development of spontaneous lymphomas in mice. For example, IFNγ−/− mice, PRF1−/− mice, and IFNγ/PRF1 double-knockout mice all have a significantly higher incidence of developing lymphomas due to impaired function of cytotoxic lymphocytes (20, 21). To investigate the role of cytotoxic lymphocytes, B6.SJL mice (CD45.1) received fractionated TBI followed by transplantation with BM cells from PRF1−/− mice (CD45.2) and PBS vehicle. Multi-lineage engraftment of PRF1−/− BM was observed in PBMCs, although the repopulation in CD11b+ cells by PRF1−/− BM cells was significantly lower than that by wild-type, TNFα−/− and IFNγ−/− BM cells (Supplementary Fig. S1). However, transplantation of BM cells from PRF1−/− mice also significantly suppressed the formation of radiation-induced thymic lymphoma (P < 0.0001) (Fig. 1B). Taken together, our results demonstrate that transplantation with unirradiated BM cells from TNFα−/−, IFNγ−/− or PRF1−/− mice is sufficient to prevent the formation of radiation-induced thymic lymphoma.
In mouse models of spontaneously developed T-cell acute lymphoblastic leukemia (T-ALL), it has been shown that BM-derived hematopoietic progenitors inhibit malignant transformation of resident progenitors in the thymus by competing for the double negative 3 (DN3) niche (22). To test the hypothesis that unirradiated BM cells suppress radiation-induced lymphomagenesis in the thymus through niche competition, we conducted transplantation experiments using BM cells from Rag2−/−; γc−/− mice, which cannot occupy thymic niches beyond double negative 2 (DN2) (23) or Rag2−/− mice, which cannot occupy thymic niches beyond DN3 (24). B6.SJL mice (CD45.1) received fractionated TBI followed by transplantation with PBS vehicle as well as BM cells from Rag2−/−; γc−/− mice and wild-type controls on the same genetic background (C57BL/6NTac) (23). Both donor strains are CD45.2. The engraftment of wild-type and Rag2−/−; γc−/− BM cells was examined in PBMCs. While wild-type and Rag2−/−; γc−/− BM had similar engraftment in CD11b+ myeloid cells, Rag2−/−; γc−/− BM showed significantly lower engraftment in CD3+ T cells and B220+ B cells compared to wild-type BM (Supplementary Fig. S2; https://doi.org/10.1667/RADE-20-00221.1.S1). Remarkably, our data showed that transplantation with wild-type BM cells significantly protected mice from radiation-induced thymic lymphoma compared to mice that received PBS vehicle (P = 0.0004) or Rag2−/−; γc−/− BM cells (P = 0.0071). However, Rag2−/−; γc−/− BM cells did not significantly suppress radiation-induced thymic lymphomagenesis (P = 0.2553 compared to PBS vehicle) (Fig. 2A).
FIG. 2.
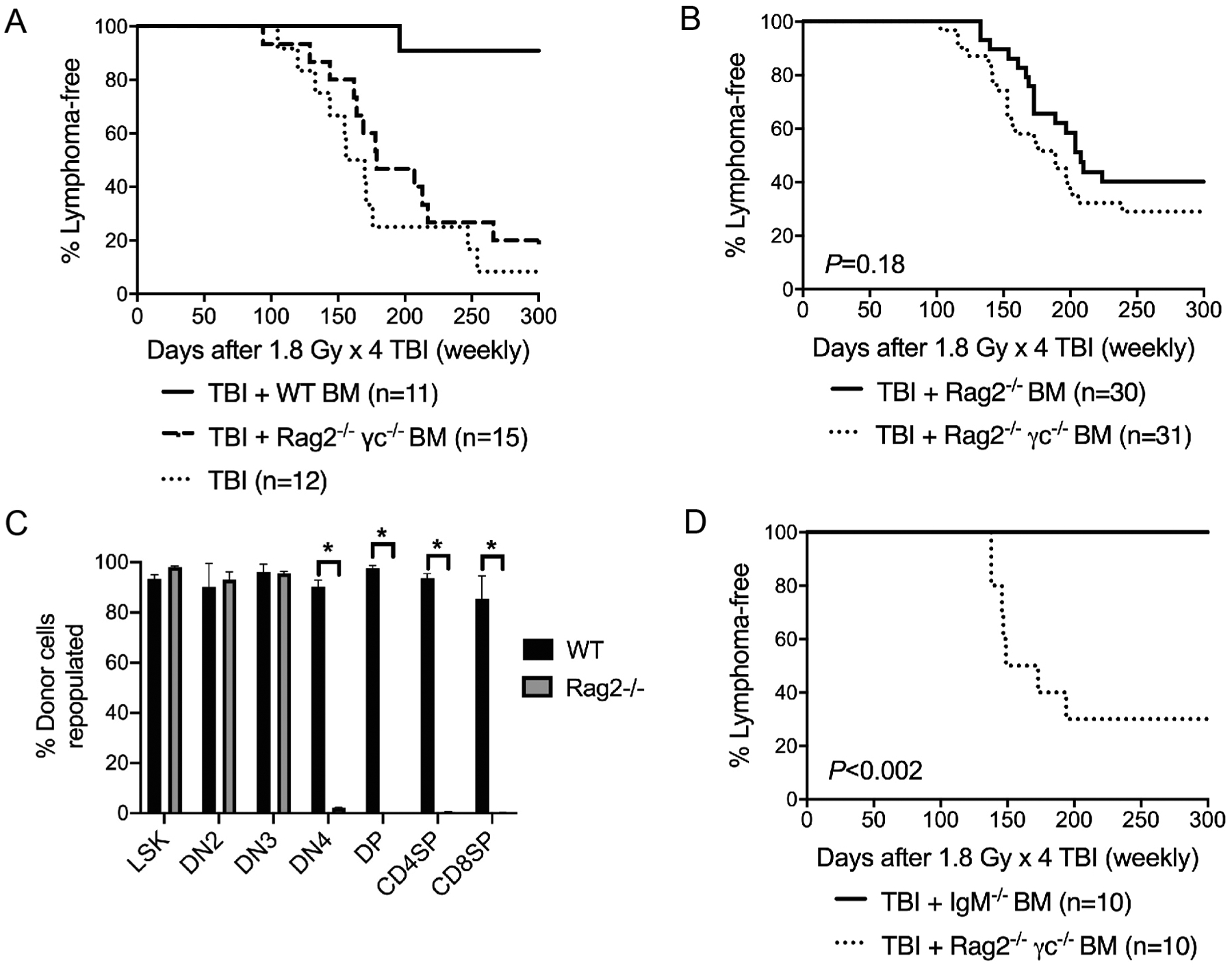
Unirradiated bone marrow cells suppress the formation of radiation-induced thymic lymphoma through niche competition. Four-week-old B6.SJL (CD45.1) mice received one dose of 1.8 Gy TBI for four consecutive weeks. Bone marrow transplantation (BMT) was performed 6 h after final TBI. Each recipient was intravenously injected with 1 × 107 bone marrow cells from mice on a CD45.2 background. Both male and female mice were used. Panel A: Lymphoma-free survival of irradiated mice that received bone marrow cells from either wild-type (WT) or Rag2−/−; γc−/− mice or did not receive bone marrow cells (No BMT). P = 0.0004 for WT BM vs. no BMT; P = 0.0071 for WT BM vs. Rag2−/−; γc−/− BM; P = 0.2553 for Rag2−/−; γc−/− BM vs. no BMT. P value was calculated using log-rank test. Panel B: Lymphoma-free survival of irradiated mice that received bone marrow cells from Rag2−/−; γc−/− or Rag2−/− mice. These data are the summary of results from three independent experiments. P value was calculated using log-rank test. Panel C: Bone marrow cells and thymocytes were harvested to examine the repopulation of donor-derived cells by flow cytometry. Donor-derived repopulation was examined in LSK (Lineage− Sca1+ cKit+) cells in the BM as well as DN2 (CD4− CD8− CD25+ CD44+), DN3 (CD4− CD8− CD25+ CD44−), DN4 (CD4− CD8− CD25− CD44−), DP (CD4+ CD8+), CD8SP (CD8+) and CD4SP (CD4+) thymocytes. n = 5 mice for wild-type and n = 7 mice for Rag2−/−. Data are presented as mean 6 SE. *P < 0.05 (two-tailed t test). Panel D. Lymphoma-free survival of irradiated mice that received bone marrow cells from Rag2−/−; γc−/− or IgM−/− mice. P value was calculated by log-rank test.
Next, to determine the role of the DN3 niche, B6.SJL (CD45.1) mice received fractionated TBI followed by transplantation with BM cells from either Rag2−/−; γc−/− mice or Rag2−/− mice. Unexpectedly, while mice that received Rag2−/− BM showed a slight delay in tumor onset compared to mice that received Rag2−/−; γc−/− BM (median tumor-free survival is 208 days post-TBI with Rag2−/− BM and 189 days post-TBI with Rag2−/−; γc−/− BM), transplantation with Rag2−/− BM did not significantly inhibit radiation-induced thymic lymphomagenesis (P = 0.18 compared to Rag2−/−; γc−/− BM) (Fig. 2B). To determine the engraftment of wild-type and Rag2−/− donor BM cells, we harvested BM cells and thymocytes 30 days after BM transplantation and examined the repopulation of donor-derived cells (CD45.2). Our data showed that BM cells from wild-type and Rag2−/− mice were equally efficient to repopulate HSPCs (Lineage− Sca1+ cKit+) in the BM as well as DN2 (CD4− CD8− CD25+ CD44+) and DN3 (CD4− CD8− CD25+ CD44−) thymocytes. However, Rag2−/− BM cells had significant defects in repopulating thymocytes beyond DN3, including DN4 (CD4− CD8− CD25− CD44−), double positive (DP) (CD4+ CD8+), CD8 single positive (SP) (CD8+) and CD4SP (CD4+) cells (Fig. 2C).
To exclude the possibility that Rag2−/− BM cells failed to prevent the development of radiation-induced thymic lymphoma due to the defect in producing mature B lymphocytes (24), we repeated the BM transplantation experiments using BM cells from Rag2−/−; γc−/− mice or IgM−/− mice, which cannot produce mature B cells (25). Examination of the engraftment of IgM−/− BM cells in PBMCs showed a marked defect in repopulating B220+ B cells (Supplementary Fig. S3; https://doi.org/10.1667/RADE-20-00221.1.S1). However, unlike Rag2−/− BM, IgM−/− BM significantly suppressed the formation of radiation-induced thymic lymphoma (P < 0.002 compared to Rag2−/−; γc−/− BM) (Fig. 2D). Together, our results demonstrate that unirradiated Rag2−/− BM cells do not fully suppress the development of radiation-induced thymic lymphoma even though they occupy the DN3 niche in the thymus.
DISCUSSION
The goal of the current study was to elucidate the mechanisms by which unirradiated BM cells suppress the formation of radiation-induced thymic lymphoma in mice. Previously published studies show that treatment with recombinant TNFα or IFNγ significantly decreases the incidence of radiation-induced thymic lymphoma (17–19). However, our results demonstrate that BM cells lacking either TNFα or IFNγ remain sufficient to inhibit radiation-induced lymphomagenesis in the thymus (Fig. 1 and Table 1). These findings indicate that unirradiated BM cells prevent the development of radiation-induced thymic lymphoma through mechanisms independent of secreting TNFα or IFNγ. In addition, while mice with germline deletion of IFNγ−, PRF1, or both IFNγ/PRF1 are susceptible for developing spontaneous lymphomas due to impaired function of cytotoxic lymphocytes (20, 21), unirradiated PRF1−/− BM cells remain effective for suppressing the formation of radiation-induced thymic lymphoma (Fig. 1 and Table 1). Taken together, our results from transplantation experiments using BM cells from TNFα−/−, IFNγ−/− and PRF1−/− mice indicate that unirradiated BM cells prevent the formation of radiation-induced thymic lymphoma through mechanisms independent of tumor immunosurveillance.
TABLE 1.
Summary of Results of the Bone Marrow Transplantation Experiments
| Bone marrow | Inhibit thymic | |
|---|---|---|
| donors | Biological defects | lymphoma? |
| Wild-type (C57BL6) |
|
Yes |
| TNFα−/− |
|
Yes |
| IFNγ−/− |
|
Yes |
| PRF1−/− |
|
Yes |
| Rag2−/−; γc−/− |
|
No |
| Rag2−/− |
|
No |
| IgM−/− |
|
Yes |
Alternatively, our results reveal that transplantation of wild-type BM cells inhibits the development of radiation-induced thymic lymphoma through niche competition. Martins et al. found that the formation of spontaneously developed T-ALL was suppressed by BM cells from Rag2−/− mice, which occupy the DN3 niche (22). However, our findings demonstrate that although BM cells from Rag2−/− mice are sufficient to occupy the DN3 niche, they do not significantly inhibit radiation-induced lymphomagenesis in the thymus (Fig. 2 and Table 1). These results indicate that critical niches that support the development of radiation-induced thymic lymphoma in C57BL/6 mice are beyond DN3.
Our findings have a significant impact on delineating mechanisms underlying discoveries made using mouse models of radiation-induced thymic lymphoma. For example, a growing body of literature shows that inhibition of radiation-induced apoptosis by either temporarily knocking down p53 during TBI (16), by deleting transcriptional targets of p53 including PUMA (26, 27) and Irf5 (28) or by mutating Mdm2 (29) all significantly suppress the development of radiation-induced thymic lymphoma. One common observation across all of these mouse models is that inhibition of p53-mediated apoptosis protects BM-derived HSPCs from radiation injury. Our results suggest that niche competition is a mechanism by which blocking p53-mediated cell death of bone marrow-derived HSPCs prevents radiation-induced lymphomagenesis in the thymus.
In summary, our findings provide an answer to a long-standing question in the field of radiation biology: Why does dose protection of the bone marrow, but not the thymus, from radiation prevent the formation of thymic lymphoma? Based on the results from our BM transplantation experiments, we propose a model where BM cells suppress radiation-induced lymphomagenesis in the thymus by competing with tumor-initiating cells for thymic niches beyond the DN3 stage. Further investigations are warranted to dissect mechanisms underlying niche competition between unirradiated BM-derived thymic progenitors and lymphoma-initiating cells in the thymus.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Cancer Institute [grant nos. K99CA212198 and R00CA212198 (C-LL)] as well as by the Whitehead Scholar Award from Duke University School of Medicine (C-LL).
C-LL reports research support from Janssen R&D. However, this interest does not present a conflict with the content in this manuscript. The remaining authors have no conflicting financial interests.
REFERENCES
- 1.Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood 2003; 102:43–52. [DOI] [PubMed] [Google Scholar]
- 2.Shuryak I, Sachs RK, Hlatky L, Little MP, Hahnfeldt P, Brenner DJ. Radiation-induced leukemia at doses relevant to radiation therapy: modeling mechanisms and estimating risks. J Natl Cancer Inst 2006; 98:1794–806. [DOI] [PubMed] [Google Scholar]
- 3.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res 2012; 177:229–43. [DOI] [PubMed] [Google Scholar]
- 4.Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat Res 2013; 179:361–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan HS. Observations on radiation-induced lymphoid tumors of mice. Cancer Res 1947; 7:141–7. [PubMed] [Google Scholar]
- 6.Kaplan HS, Brown MB. A quantitative dose-response study of lymphoid-tumor development in irradiated C 57 black mice. J Natl Cancer Inst 1952; 13:185–208. [PubMed] [Google Scholar]
- 7.Rivina L, Schiestl R. Mouse models of radiation-induced cancers. Adv Genet 2013; 84:83–122. [DOI] [PubMed] [Google Scholar]
- 8.Furth J Prolongation of life with prevention of leukemia by thymectomy in mice. J Gerontol 1946; 1:46–54. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman M, Hansteen GA, McCune JM, Scott ML, White JH, Weissman IL. Indirect induction of radiation lymphomas in mice. Evidence for a novel, transmissible leukemogen. J Exp Med 1987; 166:1883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kominami R, Niwa O. Radiation carcinogenesis in mouse thymic lymphomas. Cancer Sci 2006; 97:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan HS, Brown MB. Further observations on inhibition of lymphoid tumor development by shielding and partial-body irradiation of mice. J Natl Cancer Inst 1951; 12:427–36. [PubMed] [Google Scholar]
- 12.Kaplan HS, Brown MB. Protection against radiation-induced lymphoma development by shielding and partial-body irradiation of mice. Cancer Res 1952; 12:441–4. [PubMed] [Google Scholar]
- 13.Kaplan HS, Brown MB, Paull J. Influence of bone-marrow injections on involution and neoplasia of mouse thymus after systemic irradiation. J Natl Cancer Inst 1953; 14:303–16. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan HS, Moses LE, Brown MB, Nagareda CS, Hirsch BB. The time factor in inhibition of lymphoid-tumor development by injection of marrow cell suspensions into irradiated C57BL mice. J Natl Cancer Inst 1955; 15:975–9. [PubMed] [Google Scholar]
- 15.Boniver J, Decleve A, Lieberman M, Honsik C, Travis M, Kaplan HS. Marrow-thymus interactions during radiation leukemogenesis in C57BL/Ka mice. Cancer Res 1981; 41:390–2. [PubMed] [Google Scholar]
- 16.Lee CL, Castle KD, Moding EJ, Blum JM, Williams N, Luo L, et al. Acute DNA damage activates the tumour suppressor p53 to promote radiation-induced lymphoma. Nature Commun 2015; 6:8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boniver J, Humblet C, Defresne MP. Tumor necrosis factor and interferon gamma inhibit the development of radiation-induced thymic lymphomas in C57BL/Ka mice. Leukemia 1989; 3:611–3. [PubMed] [Google Scholar]
- 18.Humblet C, Deman J, Rongy AM, Greimers R, Boniver J, Defresne MP. TNF-alpha is involved in the mechanism of murine thymic lymphoma prevention by bone marrow grafting. Adv Exp Med Biol 1994; 355:195–9. [DOI] [PubMed] [Google Scholar]
- 19.Humblet C, Greimers R, Delvenne P, Deman J, Boniver J, Defresne MP. Prevention of murine radiogenic thymic lymphomas by tumor necrosis factor or by marrow grafting. J Natl Cancer Inst 1996; 88:824–31. [DOI] [PubMed] [Google Scholar]
- 20.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 2000; 192:755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 2001; 97:192–7. [DOI] [PubMed] [Google Scholar]
- 22.Martins VC, Busch K, Juraeva D, Blum C, Ludwig C, Rasche V, et al. Cell competition is a tumour suppressor mechanism in the thymus. Nature 2014; 509:465–70. [DOI] [PubMed] [Google Scholar]
- 23.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 1995; 2:223–38. [DOI] [PubMed] [Google Scholar]
- 24.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992; 68:855–67. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 1991; 350:423–6. [DOI] [PubMed] [Google Scholar]
- 26.Labi V, Erlacher M, Krumschnabel G, Manzl C, Tzankov A, Pinon J, et al. Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev 2010; 24:1602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalak EM, Vandenberg CJ, Delbridge AR, Wu L, Scott CL, Adams JM, et al. Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev 2010; 24:1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bi X, Feng D, Korczeniewska J, Alper N, Hu G, Barnes BJ. Deletion of Irf5 protects hematopoietic stem cells from DNA damage-induced apoptosis and suppresses gamma-irradiation-induced thymic lymphomagenesis. Oncogene 2014; 33:3288–97. [DOI] [PubMed] [Google Scholar]
- 29.Carr MI, Roderick JE, Gannon HS, Kelliher MA, Jones SN. Mdm2 phosphorylation regulates its stability and has contrasting effects on oncogene and radiation-induced tumorigenesis. Cell Rep 2016; 16:2618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


