Abstract
The Inonotus obliquus (I. obliquus) mushroom was traditionally used to treat various gastrointestinal diseases. For many years, mounting evidence has indicated the potential of I. obliquus extracts for treatment of viral and parasitic infections. Furthermore, substances from I. obiquus have been shown to stimulate the immune system. The most promising finding was the demonstration that I. obliquus has hypoglycemic and insulin sensitivity potential. This review summarizes the therapeutic potential of I. obliquus extracts in counteracting the progression of cancers and diabetes mellitus as well as their antiviral and antiparasitic activities and antioxidant role. As shown by literature data, various authors have tried to determine the molecular mechanism of action of I. obliquus extracts. Two mechanisms of action of I. obliquus extracts are currently emerging. The first is associated with the broad-sense impact on antioxidant enzymes and the level of reactive oxygen species (ROS). The other is related to peroxisome proliferator-activated receptor gamma (PPARγ) effects. This receptor may be a key factor in the anti-inflammatory, antioxidant, and anti-cancer activity of I. obliquus extracts. It can be concluded that I. obliquus fits the definition of functional food and has a potentially positive effect on health beyond basic nutrition; however, studies that meet the evidence-based medicine (EBM) criteria are needed.
Keywords: Inonotus obliquus, Anti-Cancer, Anti-inflammation, Mushroom, Anti-virus
Graphical abstract
Highlights
-
•
Extracts or polysaccharides from I. obliquus exhibit an anti-cancer potential in vitro.
-
•
Extracts or polysaccharides from I. obliquus exhibit anti-inflammation potential.
-
•
Extracts or polysaccharides from I. obliquus exhibit hypoglycemic and insulin sensitivity potential.
Abbreviations
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- Bax
Bcl-2-associated X protein B-cell
- Bcl-2
B- cell lymphoma 2
- CAT
catalase
- COX-2
cyclooxygenase-2
- DPPH•
2,2-diphenyl-1-picrylhydrazyl
- GPx
glutathione peroxidase
- H2DCFDA
2′,7′-dichlorodihydrofluorescein diacetate
- HCV
Hepatitis C Virus
- HO-1
heme oxygenase-1
- IFNγ
interferon gamma
- IL-12
interleukin 12
- IL-1β
interleukin 1 beta
- IL-2
interleukin 2
- IL-4
interleukin 4
- IL-6
interleukin 6
- IL-10
interleukin 10
- iNOS
inducible nitric oxide synthase
- IκBα
inhibitor kappa B alpha
- LPS
lipopolysaccharides
- MDA
malondialdehyde
- MMPs
matrix metalloproteinases
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor 2
- p-AKT
phospho-protein kinase B
- PGE2
prostaglandin E2
- PI3k
phosphatidylinositol 3-kinase
- p-mTOR
phospho-mammalian target of rapamycin
- PPARγ
peroxisome proliferator-activated receptor gamma
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- STZ
streptozotocin
- T2DM
type 2 diabetes mellitus
- TGF-β
transforming growth factor beta
- TIMPs
tissue metallopeptidase inhibitors
- TLR2
toll-like receptor 2
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor alpha
- XOD
xanthine oxidase
1. Introduction
Inonotus obliquus (I. obliquus) (Ach. ex Pers.) Pilát, belonging to the family Hymenochaetaceae, is a black-brown plant parasitic fungus.1,2 This mushroom has also at least a few common and regional names such as [Pol.] czerniak brzozy, czyreń, czernidło, czarcie oko, or czanga, which most likely has been coined from the Russian word "chaga".3 The genus Inonotus is widespread in North America, Asia, and Europe and includes approximately 100 species.3,4 In Europe, this genus is represented only by 4 species, with I. obliquus as one of them.4 Although it is widely distributed in North America, Asia, and Europe, I. obliquus is on the list of partially protected species of mushrooms in Poland.5
I. obliquus is a primary tree parasite causing decomposition of live trunks.6 It has been seen on many trees species such as alder, beech, maple, rowan, hornbeam, poplar, oak, ash, willow, plane-tree, chestnut, and walnut, but the main hosts of I. obliquus are various species of birch.3,7 It should be noted that the reports on the occurrence of I. obliquus on different species of deciduous trees are not reliable, because other fungi of the genus Inonotus are very often confused with the analyzed species.3,7
I. obliquus infects approximately 30–50-year-old trees through wounds in the bark and can grow on the trunk for another 30–80 years.3,5 A few years after penetrating the trunk of live trees, it produces sclerotia (vegetative or asexual fruiting bodies) with a lumpy irregular shape, cracked surface, and black-brown color. The interior of the sclerotium itself is made of rust-brown, yellow-veined, very dense mycelium.3 The sclerotia grow very slowly, reaching a diameter of more than 10 cm after 10–15 years.5 On old trees, the growths can exceed 50 cm in diameter.3 After many years, the host tree dies and annual fruiting bodies of the sexual stage appear. Fruiting bodies of this stage develop in the warm season of the year in places with the most advanced rot. They very rarely grow on live trees.3,8 This type of fruiting bodies may have considerable sizes (up to 3–4 m in length and up to 50 cm in width) and are eaten very quickly by insects, which are assumed to be the main (besides the wind) spore-spreading vector of I. obliquus.3
Numerous scientific studies have shown that the some assumptions of folk medicine beliefs are reasonable. Mexican and European folk medicine plants have been tested on in vivo or in vitro models, e.g. Jasminum fruticans, Mentha longifolia, and Artemisia absinthium extracts, which show anthelmintic or antibacterial effects.9,10 Given these examples, the search for new potentially active, natural compounds in folk medicine extends the present knowledge and help to find a promising cure to such prevalent diseases as cancers, parasitic infestations, or bacterial infections. Therefore, the usefulness of I. obliquus extract in medicine, e.g. in the treatment of diabetes and parasitic and viral infections, was evaluated. However, there has been no work so far, presenting the current scientific findings on I. obliquus in a concise and substantive form. Therefore, the aim of this work is to present the current state of knowledge of the biological properties of the I. obliquus mushroom and the potential possibilities of its medical use.
2. Application of I. obliquus in folk medicine
In folk medicine, rational premises are intertwined with elements of magic. The rational premises are based on observation of the natural environment and finding substances that alleviate disease symptoms. In many countries, this is the only way in which people can improve their health due to poverty in society or difficult access to scientific medicine. I. obliquus has been used in folk medicine since ancient times. One of the oldest documents confirming the use of its conks for medicinal purposes is the work by Hippocrates "Corpus Hippocraticum".3 The father of medicine used infusions of this mushroom externally to wash wounds.
In Eastern Europe, the Chaga mushroom has been used since the 12th century. Historical sources describe healing of a lip tumor in a Kiev Kniaz.11 I. obliquus was used in traditional medicine for many indications by the people of Siberia.12 The fungus was applied due to its antiparasitic, anti-tuberculosis, anti-inflammatory, and gastrointestinal properties.13 It was also recommended for heart and liver diseases. Most often, it was used in the form of infusions, inhalations, or aqueous macerates.13 Also antiseptic soaps containing I. obliquus were prepared for external use. In the middle of the 20th century, Chaga infusions were used as a substitute for tea in Siberia.14 Relatively early attention was paid to the potential I. obliquus antitumor or supportive effect in cancer treatment, which was particularly important before the era of scientific oncology. Such descriptions can be found in popular literature, e.g. in Aleksander Solzhenitsyn’s Cancer Ward.14
In Asia (China, Japan, Korea), Russia, and the Baltic countries, extracts of Chaga mushroom were used due to their beneficial effects on the plasma lipid system and heart function as well as antibacterial, anti-inflammatory, and anti-cancer activity.15 Furthermore, the antioxidant activity of I. obliquus may be important for prevention of free radical-related civilization diseases (atherosclerosis, cancer, diabetes, accelerated aging, and degenerative diseases of the central nervous system).16 Chaga extracts have been shown to inhibit the reproduction of hepatitis C virus (HCV) and human immunodeficiency viruses (HIV).17
The only objective method to assess the effectiveness of any therapy in scientific medicine is to perform research that meets the criteria of evidence-based medicine (EBM). Unfortunately, no observations from folk medicine or folk tales meet these criteria. Therefore, while drawing information from these sources, it is necessary to conduct scientific research that will either refute or confirm the folk premises. Importantly, the highest EBM standards should be maintained during the research, giving grounds for verifying folk knowledge.
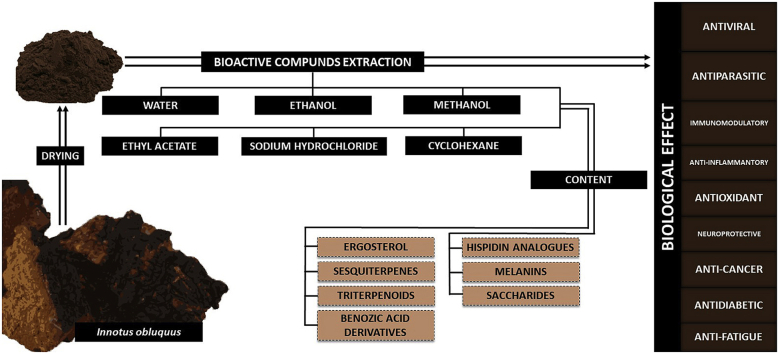
3. Chemical composition and approaches to extraction of substances from I. obliquus
Only young and fresh sclerotia growing on birches, harvested throughout the year, are used in medicine.3 Sclerotia should be harvested in uncontaminated areas distant from sources of pollution, which may accumulate in the fungus.18 In the natural environment, I. obliquus grows in a cool climate with high seasonal temperature fluctuations, freezing, UV radiation, as well as occurrence of bacterial and viral infections.19,20 In response to numerous stressors, I. obliquus has developed complex defense mechanisms. These mechanisms include the production of various bioactive substances: antioxidants, triterpenoids, ergosterol and its peroxide, sesquiterpenes, benzoic acid derivatives, hispidin analogues, and melanins. In addition, high expression of antioxidant enzymes has been detected in I. obliquus.21, 22, 23, 24, 25, 26 Moreover, many reports have highlighted that the polysaccharide fraction present in I. obliquus extracts is the largest group of active compounds, besides phenols. Therefore, many investigations have been performed to evaluate the polysaccharide content in this mushroom extracts, also in various conditions of extraction such as freeze, hot air, or vacuum drying methods.27 As reported by Ma et al. I. obliquus extracts contain many low molecular polysaccharides, which are considered antioxidant agents responsible for this activity of these mentioned extracts.27 This antiradical property has been repeatedly proved in the literature inter alia by Ma et al. or Cui et al. who tested the antioxidant properties of I. obliquus saccharides in vitro or in vivo on rat livers, respectively.1,27 The water extract contained 19.76–26.51% of neutral sugars, in which the sugar composition varied depending on the drying method, but rhamnose, galactose, and glucose were the dominant sugars, whereas arabinose or mannose were the least abundant components.27 The chemical structure of I. obliquus polysaccharides was also strictly related to the method applied, indicating that the saccharides were characterized by a dense spherical, branching, and elongated rod structure.27
Currently, many approaches for isolation of substances from I. obliquus have been described; they are summarized in Table 1. They can be divided into techniques yielding the whole of isolated substances, individual fractions, or even single active substances from I. obliquus.1,28,29 The use of total aqueous or ethanol extracts is the most similar approach to the methods used in folk medicine.28,30 These types of extracts are suitable for direct consumption. Unfortunately, the application of other methods of total extraction using organic solvents such as methanol, ethyl acetate, or cyclohexane prevents the use of such a product.30, 31, 32 From a practical point of view, the use of a whole extract in scientific research is very important for practical natural or herbal medicine. In addition, many substances present in the whole extract can abolish or enhance each other’s activity. On the other hand, it is not possible to investigate the exact mechanism of specific substances in a non-fractionated extract. Another approach is to use individual fractions obtained from I. obliquus. In the literature, there are descriptions of research on various substances isolated from I. obliquus, including melanin, inodothiol, flavans, triterpenoids, steroids, polyphenols, or polysaccharides.1,24,33, 34, 35, 36 This type of approach makes it possible to determine precisely which substance has therapeutic effects. Currently, the most frequently performed research is focused on the polysaccharide fraction (summarized in Table 1). As shown in the literature, this is a very promising group of substances associated with anti-cancer activity and insulin-improving sensitivity.37,38 It should be noted that proteins contained in extracts are not studied. The extraction methods denature the protein at an early stage of isolation. In the case of aqueous extracts, the isolation of the substance is carried out at a temperature between 80 and 100 °C.37,39,40 Similarly, various organic solvents (ethanol, methanol, cyclohexane, etc.) as well as multi-stage extraction methods can destroy the bioactive active ingredients contained in I. obliquus.
Table 1.
Approaches to isolation and administration of extracts or various substances isolated from Inonotus obliquus.
| TYPES OF EXTRACTS FROM Inonotus obliquus TESTED | REFERENCES |
|---|---|
| ENTIRE WATER EXTRACTS | 28,30,31,39,41, 42, 43, 44, 45 |
| ENTIRE ETHANOL EXTRACTS | 1,30,36,41,42,46, 47, 48 |
| ENTIRE METHANOL EXTRACTS | 30,32,41,49 |
| ENTIRE SODIUM HYDROXIDE EXTRACTS | 41 |
| ENTIRE CYCLOHEXANE EXTRACTS | 31 |
| ENTIRE ETHYL ACETATE EXTRACTS | 31 |
| MELANIN FRACTION | 24,29,50 |
| INODOTIOL FRACTION | 33,51 |
| FLAVAN FRACTION | 34 |
| TRITERPENOIDS AND STEROIDS FRACTIONS | 1,35,52, 53, 54 |
| POLYSACCHARIDE FRACTIONS | 1,37,62, 63, 64, 65, 66, 67, 68, 69,38,55, 56, 57, 58, 59, 60, 61 |
| POLYPHENOLIC FRACTION | 1,32,36,70 |
4. Biological properties of Inonotus obliquus extracts
Many different properties of extracts or substances derived from I. obliquus have been described to date. These include antiviral, antidiabetic, antioxidant, antiparasitic, immunomodulatory, anti-inflammatory, neuroprotective, anticancer properties in vitro, and recently antifatigue effects.1,30,36,37,39,65,71 Relevant literature data are summarized in Table 2.
Table 2.
Properties of different extracts from Inonotus obliquus. In vitro means a cell culture model or chemical analysis (cell-free model) in vivo means experiments performed on animals. To date, no human experiments have been performed.
| PROPERTIES OF DIFFERENT EXTRACTS FROM Inonotus obliquus | TYPE OF BIOLOGICAL MODEL USED | REFERENCES |
|---|---|---|
| ANTIVIRAL | in vivo | 17,39,72, 73, 74 |
| ANTIDIABETIC | in vivo/in vitro | 29,37,55,56,75,76 |
| ANTIOXIDANT/ROS SCAVENGER | in vivo/in vitro | 1,30,59,60,70,75,77, 78, 79, 80, 81, 82,36,83, 84, 85, 86, 87,37,42,50,55, 56, 57, 58 |
| ANTIFATIGUE | in vivo | 71 |
| ANTIPARASITIC | in vivo | 63, 64, 65 |
| IMMUNOMODULATORY | in vivo/in vitro | 33,48,61,62,64,88, 89, 90 |
| ANTI-INFLAMMATORY | in vivo/in vitro | 33,36,41,57,86,91 |
| NEUROPROTECTIVE | in vivo/in vitro | 34,66,82,92 |
| ANTI-CANCER POTENTIAL | in vitro | 28,30,49,51,63,67,75,93, 94, 95,31,32,38,43, 44, 45, 46, 47 |
4.1. Antioxidant properties
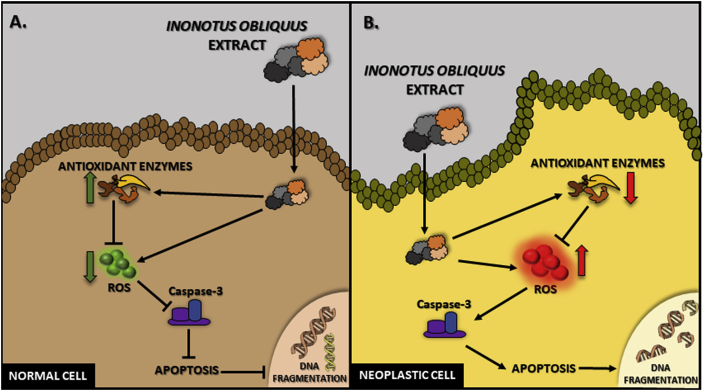
The antioxidant properties of extracts and individual fractions of substances obtained from I. obliquus are the most frequently studied parameter in scientific publications. To determine the antioxidant potential, methods based on the stable 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) are used.1,57,61,77,85,96, 97, 98 Less often, another stable 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical is used.70,79 The DPPH• and ABTS methods are based only on the antioxidant properties of chemical substances and do not fully reflect the antioxidative potential of a given extract or substance.99,100 Methods based on the impact on the activity or expression of antioxidant enzymes as well as the formation of reactive oxygen species (or scavenging) in living cells reflect better the actual state in the organism. To date, the effects of I. obliquus extracts on the activity and/or expression of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), xanthine oxidase (XOD) in cell cultures and mouse tissues have been described.30,37,55,57,59,66,78 In addition, extracts from I. obliquus affected reactive oxygen species (ROS) production measured with the malonic dialdehyde (MDA) and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) methods.58,63,92 Interestingly, the effects were different depending on the type of cell. Szychowski et al. showed that extracts from I. obliquus reduced the amount of ROS in normal cells30,58; in turn, numerous research teams described an increase in ROS in cancer cell lines.30,67
Flavan derivatives, polysaccharides, and 3,4-dihydroxybenzalacetone isolated from I. obliquus have also been shown to have neuroprotective effects in neurodegenerative disease models such as Alzheimer and Parkinson diseases and the SH-SY5Y cell line.34,66,92 These substances were found to protect against oxidative stress or increase SOD expression. Similarly, in other cells considered normal, such as pancreatic (RINm5F cell line) and hepatic (L02 cell line) cells, I. obliquus polysaccharides protected against H2O2-induced damage.42,58,77
4.2. Anti-inflammatory and immunomodulatory effects
So far, few research teams have tried to determine the impact of extracts obtained from I. obliquus on the immune system.33,88,89 Fan et al. have shown that water-soluble polysaccharides obtained from I. obliquus increased the proliferation of murine peritoneal lymphocytes.38 Polysaccharides from I. obliquus also stimulated the production of tumor necrosis factor-alpha (TNFα) by mouse macrophages. TNFα production increased in direct proportion to the increasing concentration of the tested polysaccharides.38 Similarly, Chen et al.101 observed that polysaccharides from I. obliquus increased the proliferation of splenocytes and lymphocytes. In addition, I. obliquus increased the secretion of cytokines such as interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-12 (IL-12), and TNFα and intensified the phagocytic processes. I. obliquus also significantly increased Bax expression and inhibition of Bcl-2 101. In turn, the latest research has shown that I. obliquus extracts very strongly reduced TNFα secretion in the RAW 264.7 cell line.41 Similarly, Chen et al. showed that polysaccharides from I. obliquus affected the Th1/Th2 lymphocyte ratio and Th17/Treg in the colon, mesenteric lymph nodes, and spleen of male BALB/c mice.89 Using a mouse model, the authors showed that the studied polysaccharides from I. obliquus could be used to treat inflammatory bowel disease. Other studies performed on BALB/c mice showed that extracts from I. obliquus suppressed Th2 and Th17 immune response.33 It is currently thought that I. obliquus extracts or their polysaccharide fraction inhibit inflammatory reactions.41,57 Most studies show a reduction in the production and/or secretion of proinflammatory cytokines such as interleukin-1 beta (IL-1β), interferon gamma (IFNγ), and TNFα.41,57,89 In vivo studies showed that an I. obliquus extract administered to Sprague-Dawley rats for 7 days protected the animals against the development of induced inflammation. The same studies showed that the I. obliquus extract exhibited analgesic properties as well.16 Park et al. tried to elucidate the mechanism of the anti-inflammatory and analgesic effects of I. obliquus extracts on the RAW 264.7 cell line.16 It was demonstrated that the extract caused a decrease in the production of nitric oxide (NO) and prostaglandin E2 (PGE2).16 In addition, LPS-stimulated macrophages produced nitric oxide synthase (iNOS), which was inhibited by the I. obliquus extract. Furthermore, the tested extract inhibited lipopolysaccharide (LPS)-induced cyclooxygenase-2 (COX-2) production.16 The I. obliquus extract also reduced mRNA production and expression of TNFα and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) induced by LPS.16 Recent studies have shown that individual fractions from I. obliquus can act antagonistically. Wold et al. proved that some polysaccharide fractions increased NO production by murine macrophage and dendritic cell lines J774. A1 and D2SC/1 62. It cannot be excluded that, depending on the type of cells tested, the extracts or individual substances from I. obliquus may increase or inhibit NO production.
4.3. Antiviral, antibacterial, and antiparasitic properties of I. obliquus
Data on the antiviral activity of I. obliquus extracts are very limited. To date, it has been shown that polysaccharides derived from the aqueous fraction of I. obliquus inhibit the protease from HIV type 1 (HIV-1), and thus impede the entry of virions into cells.39 In other studies, Shibnev et al. showed that the I. obliquus water extract was active against the HCV.17 At 48 h after addition of the I. obliquus aqueous extract to the HCV-infected embryonic porcine kidney epithelial inoculated line (SPEV), the amount of the virus in the cells was inversely proportional to the concentration of the added extract. At the highest concentrations, the extract completely stopped or significantly inhibited the reproduction of HCV. Moreover, it was shown that the addition of the extract to the cells 24 h before their exposure to the virus protected the SPEV cells from infection.17 In another experiment, an aqueous extract of I. obliquus showed an effect against the herpes virus - Herpes simplex type 1 (HSV-1) in normal kidney cells (Vero) of infected Cercopithecus aethiops.102 The presented studies confirm the antiviral effect of I. obliquus and indicate its potential use in the treatment of diseases associated with viral infections. In addition, the polysaccharide fraction of I. obliquus exhibited a broad-spectrum antiviral activity against feline herpesvirus 1, feline influenza virus H3N2 and H5N6, feline panleukopenia virus, and feline infectious peritonitis virus, which cause respiratory and gastrointestinal diseases in cats.73 Similarly, Seo and Choi showed that ethanolic extracts from I. obliquus inhibited the entry of murine norovirus (MNV) and feline calicivirus (FCV) into RAW264.7 cells 72.
In the available literature, there are only two reports suggesting the antibacterial or probiotic effect of I. obliquus extracts. Niu et al. showed that various fractions derived from I. obliquus stimulated NO production and additionally increased phagocytosis in RAW 264.7 cells.61 As demonstrated by Hu et al. I. obliquus polysaccharide was found to regulate the gut microbiota in chronic pancreatitis in mice.103 Moreover, the authors suggest that administration of I. obliquus polysaccharides could regulate the gut microbiota composition and diversity to a healthy profile in mice with chronic pancreatitis.
To date, only one team has studied the antiparasitic aspects of I. obliquus extracts and polysaccharides 63–65. Toxoplasma gondii (T. gondii) is an obligate intracellular protozoan that causes toxoplasmosis in humans and many warm-blooded animals. Approximately 30%–50% of the population is infected with T. gondii worldwide.104 Xu et al. described that I. obliquus polysaccharides significantly reduced the abortion rate, inhibited the decreases in progesterone (P) and estriol (E3) levels and the increase in the MDA level, and increased the activities of SOD and GSH.64 Furthermore, I. obliquus polysaccharides inhibited the production of inflammatory cytokines, such as TNFα, IL-6, IFNγ, IL-1β, and IL-17A, and promoted the production of the anti-inflammatory cytokine interleukin-10 (IL-10) and transforming growth factor (TGF)-β in T. gondii-infected pregnant mice.64 Similarly, Xu et al. described that I. obliquus polysaccharides significantly decreased the liver coefficient and the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), MDA, and nitric oxide NO. In turn, the polysaccharides increased the levels of antioxidant enzymes SOD and GSH.63 As mentioned previously, I. obliquus polysaccharides also effectively decreased the expression of serum TNFα, IL-6, IL-1β, IFNγ, and interluekin-4 (IL-4) in T. gondii-infected mice. The authors found that I. obliquus polysaccharides down-regulated the levels of toll-like receptor 2 (TLR2) and toll-like receptor 4 (TLR4) and phosphorylation of NF-κB p65 and inhibitor kappaBα (IκBα), but up-regulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1).63 Lastly, Ding et al. showed that I. obliquus polysaccharides significantly improved the spermatogenic capacity, ameliorated pathological damage to testis, and increased the levels of serum testosterone, luteinizing hormone, and follicular-stimulating hormone in T. gondii-infected male mice. I. obliquus polysaccharides effectively up-regulated the expression of testicular steroidogenic acute regulatory protein (StAR), P450scc, and 17β-HSD. These compounds further enhanced testicular phosphatidylinositol 3-kinase (PI3K), phospho-protein kinase B (p-AKT), and phospho-mammalian target of rapamycin (p-mTOR) expression levels.65
4.4. Anti-cancer potential
In medical databases, there are many publications on the anti-cancer activity of I. obliquus extracts in vitro. The anti-cancer potential of the extracts or substances isolated from I. obliquus and tested on different tumor cell lines are described in Table 3. Unfortunately, studies on animals are very limited and there are no reliable publications on humans.57,105 Most studies have been conducted on cell lines originating from the digestive system, such as AGS, HCT-116, HT-29, SW620, SGC-7901, DLD-1, and CACO-2 30,38,46,105. Extracts from I. obliquus have been shown to inhibit proliferation and/or are cytotoxic to these human gastrointestinal tumor cell lines. Similarly, their cytotoxic, anti-proliferative, or proapoptotic effects have been demonstrated in cell lines from many other tissues and systems, e.g. lung, cervix, mammary gland, ovary, liver, lymphoid cancers (A549, MCF-7, HepG2, HepG3B, HeLa, MCF-7, HL-60, PA-1, H1264, H1299) (described in detail in Table 3).28,32,43,49,94,95,106
Table 3.
Anti-cancer potential of extracts or substances isolated from Inonotus obliquus tested on different tumor cell lines.
| CANCER CELL LINE | ASSAYS | REFERENCES |
|---|---|---|
| A549 human lung adenocarcinoma | LDH release | 28,95 |
| Caspase-3 activity | 67,95 | |
| DNA fragmentation | ||
| Cell viability | 28,32,49,67,68,95 | |
| Cell migration | 28 | |
| Cell proliferation | ||
| ROS production | 67 | |
| Cytotoxicity | 31 | |
| HeLa human cervical cancer | Cell viability | 49,51 |
| Caspase-3 activity | 51 | |
| Cell migration | ||
| 4T1 mouse mammary carcinoma | Cell viability | 47 |
| MCF-7 human breast cancer | Cell viability | 47,49,68 |
| AGS human gastric cancer | Cell viability | 49 |
| HEK-293 human embryonic kidney | Cell viability | 49 |
| PA-1 human ovarian teratocarcinoma | Cell viability | 32 |
| U937 human myeloid leukemia cell | Cell viability | 32 |
| HL-60 human myeloid leukemia | Cell viability | 32 |
| CACO-2 human colon carcinoma | Cell viability | 30 |
| LDH release | ||
| ROS production | ||
| Cell proliferation | ||
| HT-29 human colorectal adenocarcinoma | Cell viability | 28,46 |
| DNA synthesis | ||
| Cell proliferation | ||
| LDH release | 28 | |
| Cell migration | ||
| H1264 lung adenocarcinoma | LDH release | 95 |
| Caspase-3 activity | ||
| DNA fragmentation | ||
| Cell viability | ||
| HCT-116 human colon carcinoma | Cell viability | 45 |
| LDH release | ||
| Caspase-3 activity | ||
| H1299 human non-small cell lung cancer | LDH release | 95 |
| Caspase-3 activity | ||
| DNA fragmentation | ||
| Cell viability | ||
| B16–F10 murine skin melanoma | Cell viability | 44,68,93 |
| Caspase-3 activity | 44 | |
| Cell cycle inhibition | ||
| Cell migration | 93 | |
| Calu-6 human pulmonary adenocarcinoma | LDH release | 95 |
| Caspase-3 activity | ||
| DNA fragmentation | ||
| Cell viability | ||
| HEC-1B human endometrial adenocarcinoma | Cell viability | 68 |
| P388 mouse leukemia | Caspase-3 activity | 94 |
| Cell viability | ||
| DNA fragmentation | ||
| Fao rat hepatoma | Cell viability | 28 |
| Cell proliferation | ||
| LDH release | ||
| Cell migration | ||
| P19 mouse embryo teratocarcinoma | Cell viability | 28 |
| Cell proliferation | ||
| LDH release | ||
| Cell migration | ||
| C6 rat glioma | Cell viability | 28 |
| Cell proliferation | ||
| LDH release | ||
| Cell migration | ||
| Hep3B human hepatoma | Cell viability | 43,107 |
| Cell cycle inhibition | 43 | |
| HepG2 human hepatoma | Cell viability | 43,107 |
| Cell cycle inhibition | 43 | |
| ROS production | 108 | |
| Hur7 human hepatoma | Cell viability | 68 |
| KATO-III human stomach carcinoma | Cell viability | 68 |
| DLD-1 human colon carcinoma | DNA fragmentation | 40 |
| SGC-7901 human gastric carcinoma | Cell viability | 38 |
|
SK-OV3 human ovary adenocarcinoma |
Cell viability | 68 |
| SW156 kidney adenocarcinoma | Cell viability | 68 |
| SW620 human colorectal adenocarcinoma | Cell proliferation | 105 |
Unfortunately, many of these studies lack in-depth experiments. In some publications, the authors have limited themselves to reporting whether a given extract is toxic to cells without elucidation of the molecular mechanism of action, using only one parameter. These include investigations of 4T1, MCF-7, AGS, HEK-293, PA-1, U937, HL-60, P388, Hur7, HEC-1B, KATO-III, SG-7901, SK-OV3, and SW156 cell lines.32,38,47,49,68 Interestingly, the majority of studies were conducted on the A549 line, which is a human lung adenocarcinoma line with the best documented anti-cancer potential of I. obliquus extracts or fractions of substances derived from the extract.28,31,67,68,95 In publications elucidating the molecular mechanism of extracts, the authors consistently emphasize the role of ROS in the mechanism of action of substances derived from I. obliquus.28,30 The authors also agree that extracts from I. obliquus intensify the process of apoptosis measured by capase-3 activity or the level of DNA fragmentation and stop the cell cycle in the G0 phase.44,95,109 Very important in migration/metastasis of tumors are matrix metalloproteinases (MMPs).110 Interestingly, it has been shown that, although the I. obliquus extract was not toxic to the A549 cell line at low concentrations, it statistically significantly reduced cell migration.111 The authors attributed this effect to the declining MMP-2 and MMP-9 expression and the increasing expression of tissue metallopeptidase inhibitor-2 (TIMP-2) as well as the reduced expression of NF-κB.111 Similarly, a decrease in NF-κB expression in A549 induced by I. obliquus extracts has been described.109 Moreover, it has been reported that I. obliquus polysaccharides inhibit the expression of MMP-2, MMP-7, and MMP-9 in B16–F10 mouse cells.93 Recently, Zhang et al. described that inotodiol from I. obliquus decreases the expression of MMP-2 and MMP-9 in HeLa human cells and reduces cell invasiveness.51 Unfortunately, the tests performed in vivo are very limited. To date, it has been shown that tumors of the 3LL mouse lung cancer line implanted in C57BL/6 mice developed significantly more slowly in animals treated with an I. obliquus aqueous extract. In the experiment, it was shown that the tumor in mice receiving the extract was by 60% smaller and the number of metastases decreased by 25%. Interestingly, ingestion of I. obliquus reduced the body weight of the mice and increased their body temperature, which may have contributed to the protection against cancer, as suggested by the researchers.112 Furthermore, I. obliquus extracts have been shown to inhibit the development of two tumor lines: melanoma B16–F10 and sarcoma-180 after implantation into Balbc/c strain mice.44,49 It has also been shown that ergosterol isolated from I. obliquus inhibited the development of human colorectal cancer in the C57BL/6 mouse strain.105 It is currently believed that the high content of polysaccharides is responsible for the anti-cancer potential of I. obliquus.67,111 However, the anti-cancer potential have been found in all types of extracts used.113
4.5. Hypoglycemic and insulin sensitivity action
ROS play a key role in many signaling pathways. Moreover, the relationship between oxidative stress and metabolic disorders such as type 2 diabetes (T2DM), cancer, obesity, and cardiovascular disease is well described.114 To date, it has been shown that I. obliquus polysaccharides have hypoglycemic activity.115,116 Unfortunately, the exact mechanism of action has not been elucidated. It has been described that I. obliquus polysaccharides in streptozotocin (STZ)-induced diabetic Wistar rats reduced blood glucose levels and restored the structure of β-cells after diabetes-induced cellular damage.117 I. obliquus polysaccharides lowered the level of lipid peroxidation products (such as low-density lipoprotein), whereas the high-density lipoprotein cholesterol level was enhanced.117 Moreover, Wang et al. reported that I. obliquus polysaccharides enhanced the serum levels of insulin and alleviated the metabolic derangement of glucose enzymes in STZ-induced diabetic mice.56 In the same experimental model, I. obliquus polysaccharides with the chromium (III) complex significantly decreased fasting blood glucose levels, plasma insulin levels, and body weight in mice.55 This is consistent with the previously described studies of total extracts. Cha et al. demonstrated that I. obliquus dietary treatment lowered serum glucose and leptin levels and alleviated obesity-related complications in T2DM OLETF rats.118 Similarly, Sun et al. described that feeding the experimental mice with I. obliquus improved serum insulin levels, moderately expanded the pancreatic islets, and reduced pancreatic injuries in alloxan-induced diabetic mice.119 In turn, I. obliquus polysaccharides elevated insulin levels in C57BL/6 mice with diabetic nephropathy; however, the cholesterol and triglyceride levels remained unaffected.120 Recently, I. obliquus polysaccharide has been reported to help to alleviate pancreatic acinar atrophy and weight loss in chronic pancreatitis mice induced by diethyldithiocarbamate. Hu et al. postulated that I. obliquus polysaccharides possessed strong antioxidant activity for scavenging free radicals in vitro and in vivo, which could be beneficial for chronic pancreatitis therapy in mice.57
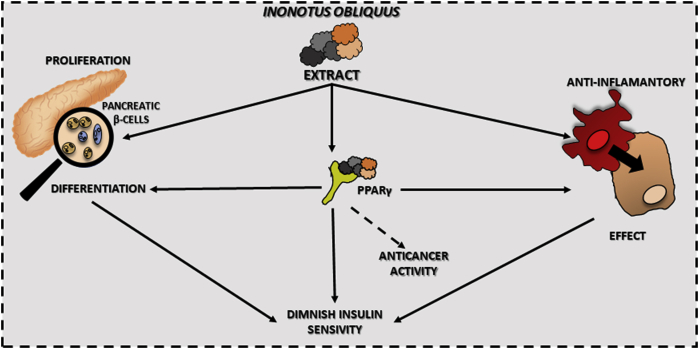
Peroxisome proliferator-activated receptor gamma (PPARγ) is a key receptor involved in insulin resistance and cell sensitivity to this hormone.121 Moreover, PPARγ is also involved in anti-inflammatory action, by controlling activation and expression of NF-κB and pro-inflammatory cytokines.122 However, only one paper showed that I. obliquus extract activated adipogenesis of 3T3-L1 preadipocytes, enhanced the expression PPARγ target genes, and increased triacylglycerol accumulation.123
5. Conclusions and perspectives
For decades, extracts of I. obliquus have been used as a remedy for numerous diseases in folk medicine. Recent discoveries have provided evidence of the appropriateness of using these drugs in vitro. Extracts from I. obliquus prepared in various solvents as well as individual fractions of substances derived from the fungus show antiviral, antibacterial, immunostimulating, and anti-tumor activity in vitro. To date, various studies have suggested significant therapeutic potential of substances derived from I. obliquus. Based on the literature data, it can be concluded that the effectiveness of extracts and/or substances derived from I. obliquus is based on two main mechanisms. The first one is the effect of I. obliquus on the production of ROS and/or on mechanisms of ROS scavenging, which varies depending on the cell type. The proposed scheme of the effect is described in Fig. 1.
Fig. 1.
Proapoptotic and antioxidant action of Inonotus obliquus extracts in normal and neoplastic cells described in literature.
The other mechanism is based on the action via the PPARγ receptor. Experimental evidence suggests that extracts and/or substances obtained from I. obliquus may affect the level of expression or activity of the PPARγ receptor.123 In this way, extracts from I. obliquus can reduce insulin resistance. However, it cannot be excluded that the anti-cancer mechanism of action of I. obliquus extracts also involves the PPARγ receptor. The proposed scheme of the effects is described in Fig. 2. Unfortunately, due to the lack of data on the involvement of the PPARγ receptor in the anti-tumor activity of I. obliquus extracts, further research is required. It can be concluded that I. obliquus fits the definition of functional food and has a potentially positive effect on health beyond basic nutrition; however, studies that meet the EBM criteria are needed. Taking the above into account, we suppose that the I. obliquus extracts can be included in cancer therapies in the future; however, more specialistic analysis should be performed, especially on in vivo models.
Fig. 2.
Antidiabetic and anti-inflammatory action of Inonotus obliquus extracts with key involvement of the PPARγ receptor described in literature.
CRediT authorship contribution statement
Konrad A. Szychowski: Data curation, Formal analysis, Writing - original draft. Bartosz Skóra: Writing - original draft. Tadeusz Pomianek: Writing - original draft. Jan Gmiński: Writing - original draft.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgements
This work was supported by statutory funds from the University of Information Technology and Management in Rzeszow, Poland (DS 507-03-01 GW). The authors declare no conflict of interests.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2020.08.003.
Contributor Information
Konrad A. Szychowski, Email: kszychowski@wsiz.rzeszow.pl.
Bartosz Skóra, Email: bskora@wsiz.rzeszow.pl.
Tadeusz Pomianek, Email: tpomianek@wsiz.rzeszow.pl.
Jan Gmiński, Email: jgminski@wsiz.rzeszow.pl.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cui Y., Kim D.-S.S., Park K.-C.C. Antioxidant effect of Inonotus obliquus. J Ethnopharmacol. 2005;96(1-2):79–85. doi: 10.1016/j.jep.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Dai Y.C. Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience. 2012;53(1):49–80. doi: 10.1007/s10267-011-0134-3. [DOI] [Google Scholar]
- 3.Szczepkowski A., Piętka J., Grzywacz A. [Biology and medicinal properties of the chaga mushroom Inonotus obliquus (Fr.) Pilát] Sylwan. 2013;157(3):223–233. [Google Scholar]
- 4.Wagner T., Fischer M. Natural groups and a revised system for the European poroid Hymenochaetales (Basidiomycota) supported by nLSU rDNA sequence data. Mycol Res. 2001;105(7):773–782. doi: 10.1017/S0953756201004257. [DOI] [Google Scholar]
- 5.Zheng W., Miao K., Liu Y. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl Microbiol Biotechnol. 2010;87(4):1237–1254. doi: 10.1007/s00253-010-2682-4. [DOI] [PubMed] [Google Scholar]
- 6.Manka K., Sobiczewski P., Manka M., Fiedorow Z. Państwowe Wydawnictwo Rolnicze i Leśne; Warszawa: 2005. Fitopatologia Lesna. [Google Scholar]
- 7.Ryvarden L., Gilbertson R., Eide A. Vol. 7. Fungiflora; Oslo: 1994. European Polypores: Part 2: Meripilus-Tyromyces. (Synopsis fungorum). [Google Scholar]
- 8.Cha J.Y., Lee S.Y., Lee S.Y., Chun K.W. Basidiocarp formation by Inonotus obliquus on a living paper birch tree. For Pathol. 2011;41(2):163–164. doi: 10.1111/j.1439-0329.2010.00687.x. [DOI] [Google Scholar]
- 9.Kozan E., Küpeli E., Yesilada E. Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. J Ethnopharmacol. 2006;108(2):211–216. doi: 10.1016/j.jep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Aghapoor K., Mohsenzadeh F., Shakeri A., Darabi H.R., Ghassemzadeh M., Neumueller B. Catalytic application of recyclable silica-supported bismuth (III) chloride in the benzo [N, N]-heterocyclic condensation. J Organomet Chem. 2013;743:170–178. [Google Scholar]
- 11.Perevedentseva L. Use of wild-growing mushrooms for therapeutic purposes in the perm territory, Russia. Indian J Environ Health. 2013;2(4):236–242. [Google Scholar]
- 12.Harpe J de La. 1801. Abrégé de l’Histoire Générale Des Voyages Continué Par Comeiras. [Google Scholar]
- 13.Saar M. Fungi in khanty folk medicine. J Ethnopharmacol. 1991;31(2):175–179. doi: 10.1016/0378-8741(91)90003-V. [DOI] [PubMed] [Google Scholar]
- 14.Solženicyn A.I. 2007. Le pavillon des cancéreux roman. [Google Scholar]
- 15.Shashkina M.Y., Shashkin P.N., Sergeev A.V. Chemical and medicobiological properties of chaga. Pharm Chem J. 2006;40(10):560–568. doi: 10.1007/s11094-006-0194-4. (review) [DOI] [Google Scholar]
- 16.Park Y.-M.M., Won J.-H.H., Kim Y.-H.H., Choi J.-W.W., Park H.-J.J., Lee K.-T.T. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J Ethnopharmacol. 2005;101(1-3):120–128. doi: 10.1016/j.jep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Shibnev V.A., Mishin D.V., Garaev T.M., Finogenova N.P., Botikov A.G., Deryabin P.G. Antiviral activity of Inonotus obliquus fungus extract towards infection caused by hepatitis C virus in cell cultures. Bull Exp Biol Med. 2011;151(5):612–614. doi: 10.1007/s10517-011-1395-8. [DOI] [PubMed] [Google Scholar]
- 18.Shashkina M.Y., Shashkin P.N., Sergeev A.V. Chemical and medicobiological properties of chaga. Pharm Chem J. 2006;40(10):560–568. doi: 10.1007/s11094-006-0194-4. (review) [DOI] [Google Scholar]
- 19.Bolwell P.P., Page A., Piślewska M., Wojtaszek P. Pathogenic infection and the oxidative defences in plant apoplast. Protoplasma. 2001;217(1-3):20–32. doi: 10.1007/BF01289409. [DOI] [PubMed] [Google Scholar]
- 20.Zucconi L., Ripa C., Selbmann L., Onofri S. Effects of UV on the spores of the fungal species Arthrobotrys oligospora and A. ferox. Polar Biol. 2002;25(7):500–505. doi: 10.1007/s00300-002-0371-1. [DOI] [Google Scholar]
- 21.Taji S., Yamada T. Wada S ichi, Tokuda H, Sakuma K, Tanaka R. Lanostane-type triterpenoids from the sclerotia of Inonotus obliquus possessing anti-tumor promoting activity. Eur J Med Chem. 2008;43(11):2373–2379. doi: 10.1016/j.ejmech.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima Y., Sato Y., Konishi T. Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga) Chem Pharm Bull (Tokyo) 2007;55(8):1222–1226. doi: 10.1248/cpb.55.1222. [DOI] [PubMed] [Google Scholar]
- 23.Lee I.K., Kim Y.S., Jang Y.W., Jung J.Y., Yun B.S. New antioxidant polyphenols from the medicinal mushroom Inonotus obliquus. Bioorg Med Chem Lett. 2007;17(24):6678–6681. doi: 10.1016/j.bmcl.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 24.Babitskaya V.G., Shcherba V.V., Ikonnikova N.V. Melanin complex of the fungus Inonotus obliquus. Prikl Biokhim Mikrobiol. 2000;36(4):444. [PubMed] [Google Scholar]
- 25.Mizuno T., Zhuang C., Abe K. Antitumor and hypoglycemic activities of polysaccharides from the sclerotia and mycelia of inonotus obliquus (Pers.: Fr.) pil. (Aphyllophoromycetideae) Int J Med Mushrooms. 1999;1(4):301–316. doi: 10.1615/IntJMedMushr. [DOI] [Google Scholar]
- 26.Rhee S.J., Cho S.Y., Kim K.M., Cha D.S., Park H.J. A comparative study of analytical methods for alkali-soluble β-glucan in medicinal mushroom, Chaga (Inonotus obliquus) LWT - Food Sci Technol (Lebensmittel-Wissenschaft -Technol) 2008;41(3):545–549. doi: 10.1016/j.lwt.2007.03.028. [DOI] [Google Scholar]
- 27.Ma L., Chen H., Zhu W., Wang Z. Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Res Int. 2013;50(2):633–640. doi: 10.1016/j.foodres.2011.05.005. [DOI] [Google Scholar]
- 28.Lemieszek M.K., Langner E., Kaczor J. Anticancer effects of fraction isolated from fruiting bodies of chaga medicinal mushroom, inonotus obliquus (Pers.:Fr.) Pilát (aphyllophoromycetideae): in vitro studies. Int J Med Mushrooms. 2011;13(2):131–143. doi: 10.1615/IntJMedMushr. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.H., Hyun C.K. Insulin-sensitizing and beneficial lipid-metabolic effects of the water-soluble melanin complex extracted from Inonotus obliquus. Phyther Res. 2014;28(9):1320–1328. doi: 10.1002/ptr.5131. [DOI] [PubMed] [Google Scholar]
- 30.Szychowski K.A., Rybczyńska-Tkaczyk K., Tobiasz J., Yelnytska-Stawasz V., Pomianek T., Gmiński J. Biological and anticancer properties of Inonotus obliquus extracts. Process Biochem. 2018;73(June):180–187. doi: 10.1016/j.procbio.2018.07.015. [DOI] [Google Scholar]
- 31.Géry A., Dubreule C., André V. Chaga (inonotus obliquus), a future potential medicinal fungus in oncology? A chemical study and a comparison of the cytotoxicity against human lung adenocarcinoma cells (A549) and human bronchial epithelial cells (BEAS-2B) Integr Canc Ther. 2018;17(3):832–843. doi: 10.1177/1534735418757912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima Y., Nishida H., Matsugo S., Konishi T. Cancer cell cytotoxicity of extracts and small phenolic compounds from Chaga [Inonotus obliquus (persoon) Pilat] J Med Food. 2009;12(3):501–507. doi: 10.1089/jmf.2008.1149. [DOI] [PubMed] [Google Scholar]
- 33.Nguyet T.M.N., Lomunova M., Le B.V. The mast cell stabilizing activity of Chaga mushroom critical for its therapeutic effect on food allergy is derived from inotodiol. Int Immunopharm. November 2017;2018(54):286–295. doi: 10.1016/j.intimp.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 34.Zou C.-X., Wang X.-B., Lv T.-M. Flavan derivative enantiomers and drimane sesquiterpene lactones from the Inonotus obliquus with neuroprotective effects. Bioorg Chem. December 2019;2020:103588. doi: 10.1016/j.bioorg.2020.103588. 96. [DOI] [PubMed] [Google Scholar]
- 35.Sagayama K., Tanaka N., Fukumoto T., Kashiwada Y. Lanostane-type triterpenes from the sclerotium of Inonotus obliquus (Chaga mushrooms) as proproliferative agents on human follicle dermal papilla cells. J Nat Med. 2019;73(3):597–601. doi: 10.1007/s11418-019-01280-0. [DOI] [PubMed] [Google Scholar]
- 36.Van Q., Nayak B.N., Reimer M., Jones P.J.H., Fulcher R.G., Rempel C.B. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell screening assay. J Ethnopharmacol. 2009;125(3):487–493. doi: 10.1016/j.jep.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Wang J., Hu W., Li L. Antidiabetic activities of polysaccharides separated from Inonotus obliquus via the modulation of oxidative stress in mice with streptozotocin-induced diabetes. PLoS One. 2017;12(6):1–19. doi: 10.1371/journal.pone.0180476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan L., Ding S., Ai L., Deng K. Antitumor and immunomodulatory activity of water-soluble polysaccharide from Inonotus obliquus. Carbohydr Polym. 2012;90(2):870–874. doi: 10.1016/j.carbpol.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Ichimura T., Watanabe O., Maruyama S. Inhibition of HIV-1 protease by water-soluble lignin-like substance from an edible mushroom, fuscoporia obliqua. Biosci Biotechnol Biochem. 1998;62(3):575–577. doi: 10.1271/bbb.62.575. [DOI] [PubMed] [Google Scholar]
- 40.Hu H., Zhang Z., Lei Z., Yang Y., Sugiura N. Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. J Biosci Bioeng. 2009;107(1):42–48. doi: 10.1016/j.jbiosc.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Javed S., Mitchell K., Sidsworth D. Inonotus obliquus attenuates histamine-induced microvascular inflammation. PLoS One. 2019;14(8):1–16. doi: 10.1371/journal.pone.0220776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao X., Santhanam R.K., Xue Z. Antioxidant, α-amylase and α-glucosidase activity of various solvent fractions of I. obliquus and the preventive role of active fraction against H 2 O 2 induced damage in hepatic L02 cells as fungisome. J Food Sci. 2020;85(4):1060–1069. doi: 10.1111/1750-3841.15084. [DOI] [PubMed] [Google Scholar]
- 43.Youn M.-J., Kim J.-K., Park S.-Y. Chaga mushroom (Inonotus obliquus) induces G0/G1 arrest and apoptosis in human hepatoma HepG2 cells. World J Gastroenterol. 2008;14(4):511–517. doi: 10.3748/wjg.14.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youn M.J., Kim J.K., yeol Park S. Potential anticancer properties of the water extract of Inontus obliquus by induction of apoptosis in melanoma B16-F10 cells. J Ethnopharmacol. 2009;121(2):221–228. doi: 10.1016/j.jep.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Tsai C.C., Li Y.S., Lin P.P. Inonotus obliquus extract induces apoptosis in the human colorectal carcinoma’s HCT-116 cell line. Biomed Pharmacother. 2017;96(1018):1119–1126. doi: 10.1016/j.biopha.2017.11.111. [DOI] [PubMed] [Google Scholar]
- 46.Lee H.S., Kim E.J., Kim S.H. Ethanol extract of Innotus obliquus (Chaga mushroom) induces G 1 cell cycle arrest in HT-29 human colon cancer cells. Nutr Res Pract. 2015;9(2):111. doi: 10.4162/nrp.2015.9.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao F., Xia G., Chen L. Chemical constituents from Inonotus obliquus and their antitumor activities. J Nat Med. 2016;70(4):721–730. doi: 10.1007/s11418-016-1002-4. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z., Yu D., Li L. Three-phase partitioning for the extraction and purification of polysaccharides from the immunomodulatory medicinal mushroom inonotus obliquus. Molecules. 2019;24(3):1–14. doi: 10.3390/molecules24030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung M.J., Chung C.-K., Jeong Y., Ham S.-S. Anticancer activity of subfractions containing pure compounds of Chaga mushroom ( Inonotus obliquus ) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells. Nutr Res Pract. 2010;4(3):177–182. doi: 10.4162/nrp.2010.4.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burmasova M.A., Utebaeva A.A., Sysoeva E.V., Sysoeva M.A. Melanins of inonotus obliquus: bifidogenic and antioxidant properties. Biomolecules. 2019;9(6):1–9. doi: 10.3390/biom9060248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang S.-D., Yu L., Wang P. Inotodiol inhibits cells migration and invasion and induces apoptosis via p53-dependent pathway in HeLa cells. Phytomedicine. 2019;60(May):152957. doi: 10.1016/j.phymed.2019.152957. [DOI] [PubMed] [Google Scholar]
- 52.Frye L.L., Leonard D.A. Lanosterol analogs: dual-action inhibitors of cholesterol biosynthesis. Crit Rev Biochem Mol Biol. 1999;34(2):123–140. doi: 10.1080/10409239991209246. [DOI] [PubMed] [Google Scholar]
- 53.Wold C.W., Gerwick W.H., Wangensteen H., Inngjerdingen K.T. Bioactive triterpenoids and water-soluble melanin from Inonotus obliquus (Chaga) with immunomodulatory activity. J Funct Foods. 2020;71(March):104025. doi: 10.1016/j.jff.2020.104025. [DOI] [Google Scholar]
- 54.Zou C.X., Hou Z.L., Bai M. Highly modified steroids fromInonotus obliquus. Org Biomol Chem. 2020;18(20):3908–3916. doi: 10.1039/d0ob00474j. [DOI] [PubMed] [Google Scholar]
- 55.Wang C., Chen Z., Pan Y., Gao X., Chen H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem Toxicol. 2017;108:498–509. doi: 10.1016/j.fct.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Wang J., Wang C., Li S. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed Pharmacother. 2017;95:1669–1677. doi: 10.1016/j.biopha.2017.09.104. [DOI] [PubMed] [Google Scholar]
- 57.Hu Y., Sheng Y., Yu M. Antioxidant activity of Inonotus obliquus polysaccharide and its amelioration for chronic pancreatitis in mice. Int J Biol Macromol. 2016;87:348–356. doi: 10.1016/j.ijbiomac.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Sim Y.C., Lee J.S., Lee S. Effects of polysaccharides isolated from Inonotus obliquus against hydrogen peroxide-induced oxidative damage in RINm5F pancreatic cells. Mol Med Rep. 2016;14(5):4263–4270. doi: 10.3892/mmr.2016.5763. [DOI] [PubMed] [Google Scholar]
- 59.Hu Y., Shi S., Lu L. Effects of selenizing modification on characteristics and antioxidant activities of Inonotus obliquus polysaccharide. Macromol Res. 2017;25(3):222–230. doi: 10.1007/s13233-017-5030-z. [DOI] [Google Scholar]
- 60.Wang C., Li W., Chen Z. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, α-amylase and α-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Res Int. July 2017;2018(103):280–288. doi: 10.1016/j.foodres.2017.10.058. [DOI] [PubMed] [Google Scholar]
- 61.Niu H., Song D., Mu H., Zhang W., Sun F., Duan J. Investigation of three lignin complexes with antioxidant and immunological capacities from Inonotus obliquus. Int J Biol Macromol. 2016;86:587–593. doi: 10.1016/j.ijbiomac.2016.01.111. [DOI] [PubMed] [Google Scholar]
- 62.Wold C.W., Kjeldsen C., Corthay A. Structural characterization of bioactive heteropolysaccharides from the medicinal fungus Inonotus obliquus (Chaga) Carbohydr Polym. December 2017;2018(185):27–40. doi: 10.1016/j.carbpol.2017.12.041. [DOI] [PubMed] [Google Scholar]
- 63.Xu L., Sang R., Yu Y., Li J., Ge B., Zhang X. The polysaccharide from Inonotus obliquus protects mice from Toxoplasma gondii-induced liver injury. Int J Biol Macromol. 2019;125:1–8. doi: 10.1016/j.ijbiomac.2018.11.114. [DOI] [PubMed] [Google Scholar]
- 64.Xu L., Yu Y., Sang R. Inonotus obliquus polysaccharide protects against adverse pregnancy caused by Toxoplasma gondii infection through regulating Th17/Treg balance via TLR4/NF-κB pathway. Int J Biol Macromol. 2020;146:832–840. doi: 10.1016/j.ijbiomac.2019.10.051. [DOI] [PubMed] [Google Scholar]
- 65.Ding X., Ge B., Wang M. Inonotus obliquus polysaccharide ameliorates impaired reproductive function caused by Toxoplasma gondii infection in male mice via regulating Nrf2-PI3K/AKT pathway. Int J Biol Macromol. 2020;151:449–458. doi: 10.1016/j.ijbiomac.2020.02.178. [DOI] [PubMed] [Google Scholar]
- 66.Han Y., Nan S., Fan J., Chen Q., Zhang Y. Inonotus obliquus polysaccharides protect against Alzheimer’s disease by regulating Nrf2 signaling and exerting antioxidative and antiapoptotic effects. Int J Biol Macromol. 2019;131:769–778. doi: 10.1016/j.ijbiomac.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 67.Jiang S., Shi F., Lin H. Inonotus obliquus polysaccharides induces apoptosis of lung cancer cells and alters energy metabolism via the LKB1/AMPK axis. Int J Biol Macromol. 2020;151:1277–1286. doi: 10.1016/j.ijbiomac.2019.10.174. [DOI] [PubMed] [Google Scholar]
- 68.Kim Y.O., Park H.W., Kim J.H., Lee J.Y., Moon S.H., Shin C.S. Anti-cancer effect and structural characterization of endo-polysaccharide from cultivated mycelia of Inonotus obliquus. Life Sci. 2006;79(1):72–80. doi: 10.1016/j.lfs.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 69.Eid J.I., Das B. Molecular insights and cell cycle assessment upon exposure to Chaga (Inonotus obliquus) mushroom polysaccharides in zebrafish (Danio rerio) Sci Rep. 2020;10(1):7406. doi: 10.1038/s41598-020-64157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang B.S., Lee I.-K., Yun B.-S. Phenolic compounds from the fungus Inonotus obliquus and their antioxidant properties. J Antibiot (Tokyo) 2016;69(2):108–110. doi: 10.1038/ja.2015.83. [DOI] [PubMed] [Google Scholar]
- 71.Zhang C.J., Guo J.Y., Cheng H. Spatial structure and anti-fatigue of polysaccharide from Inonotus obliquus. Int J Biol Macromol. 2020;151:855–860. doi: 10.1016/j.ijbiomac.2020.02.147. [DOI] [PubMed] [Google Scholar]
- 72.Seo D.J., Choi C. Inhibition of murine norovirus and feline calicivirus by edible herbal extracts. Food Environ Virol. 2017;9(1):35–44. doi: 10.1007/s12560-016-9269-x. [DOI] [PubMed] [Google Scholar]
- 73.Tian J., Hu X., Liu D., Wu H., Qu L. Identification of Inonotus obliquus polysaccharide with broad-spectrum antiviral activity against multi-feline viruses. Int J Biol Macromol. 2017;95:160–167. doi: 10.1016/j.ijbiomac.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng W., Miao K., Liu Y. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl Microbiol Biotechnol. 2010;87(4):1237–1254. doi: 10.1007/s00253-010-2682-4. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X., Bao C., Zhang J. Inotodiol suppresses proliferation of breast cancer in rat model of type 2 diabetes mellitus via downregulation of β-catenin signaling. Biomed Pharmacother. 2018;99:142–150. doi: 10.1016/j.biopha.2017.12.084. [DOI] [PubMed] [Google Scholar]
- 76.Xu H.-Y., Sun J.-E., Lu Z.-M., Zhang X.-M., Dou W.-F., Xu Z.-H. Beneficial effects of the ethanol extract from the dry matter of a culture broth of Inonotus obliquus in submerged culture on the antioxidant defence system and regeneration of pancreatic β-cells in experimental diabetes in mice. Intergovernmental Panel on Climate Change. Nat Prod Res. 2010;24(6):542–553. doi: 10.1080/14786410902751009. [DOI] [PubMed] [Google Scholar]
- 77.Wang C., Gao X., Santhanam R.K. Effects of polysaccharides from Inonotus obliquus and its chromium (III) complex on advanced glycation end-products formation, α-amylase, α-glucosidase activity and H2O2-induced oxidative damage in hepatic L02 cells. Food Chem Toxicol. 2018;116(February):335–345. doi: 10.1016/j.fct.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 78.Yong T., Chen S., Liang D. Actions of inonotus obliquus against hyperuricemia through XOD and bioactives screened by molecular modeling. Int J Mol Sci. 2018;19(10):1–11. doi: 10.3390/ijms19103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu X., Wu P., Wang T., Yan L., Lin M., Chen C. Synergistic effects of surfactant-assisted biodegradation of wheat straw and production of polysaccharides by Inonotus obliquus under submerged fermentation. Bioresour Technol. November 2018;2019(278):43–50. doi: 10.1016/j.biortech.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 80.Niu H., Song D., Mu H., Zhang W., Sun F., Duan J. Investigation of three lignin complexes with antioxidant and immunological capacities from Inonotus obliquus. Int J Biol Macromol. 2016;86:587–593. doi: 10.1016/j.ijbiomac.2016.01.111. [DOI] [PubMed] [Google Scholar]
- 81.Liang L., Zhang Z., Wang H. Antioxidant activities of extracts and subfractions from Inonotus Obliquus. Int J Food Sci Nutr. 2009;60(sup2):175–184. doi: 10.1080/09637480903042279. [DOI] [PubMed] [Google Scholar]
- 82.Nakajima Y., Nishida H., Nakamura Y., Konishi T. Prevention of hydrogen peroxide-induced oxidative stress in PC12 cells by 3,4-dihydroxybenzalacetone isolated from Chaga (Inonotus obliquus (persoon) Pilat) Free Radic Biol Med. 2009;47(8):1154–1161. doi: 10.1016/j.freeradbiomed.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 83.Yun J.S., Pahk J.W., Lee J.S., Shin W.C., Lee S.Y., Hon E.K. Inonotus obliquus protects against oxidative stress-induced apoptosis and premature senescence. Mol Cell. 2011;31(5):423–429. doi: 10.1007/s10059-011-0256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang S quan, Ding S., Fan L. Antioxidant activities of five polysaccharides from Inonotus obliquus. Int J Biol Macromol. 2012;50(5):1183–1187. doi: 10.1016/j.ijbiomac.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 85.Mu H., Zhang A., Zhang W., Cui G., Wang S., Duan J. Antioxidative properties of crude polysaccharides from Inonotus obliquus. Int J Mol Sci. 2012;13(7):9194–9206. doi: 10.3390/ijms13079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Debnath T., Park S.R., Kim D.H., Jo J.E., Lim B.O. Anti-oxidant and anti-inflammatory activities of Inonotus obliquus and germinated brown rice extracts. Molecules. 2013;18(8):9293–9304. doi: 10.3390/molecules18089293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Du X.J., Mu H.M., Zhou S., Zhang Y., Zhu X.L. Chemical analysis and antioxidant activity of polysaccharides extracted from Inonotus obliquus sclerotia. Int J Biol Macromol. 2013;62:691–696. doi: 10.1016/j.ijbiomac.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L., Lin D., Li H. Immunopotentiating effect of Inonotus obliquus fermentation products administered at vaccination in chickens. Mol Cell Probes. 2018;41(July):43–51. doi: 10.1016/j.mcp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y.F., Zheng J.J., Qu C. Inonotus obliquus polysaccharide ameliorates dextran sulphate sodium induced colitis involving modulation of Th1/Th2 and Th17/Treg balance. Artif Cells Nanomed Biotechnol. 2019;47(1):757–766. doi: 10.1080/21691401.2019.1577877. [DOI] [PubMed] [Google Scholar]
- 90.Kim Y.-R. Immunomodulatory activity of the water extract from medicinal mushroom inonotus obliquus. Mycobiology. 2005. 2005;33(3):158–162. doi: 10.4489/MYCO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi S.Y., Hur S.J., An C.S. Anti-inflammatory effects of inonotus obliquus in colitis induced by dextran sodium sulfate. J Biomed Biotechnol. 2010;2010:943516. doi: 10.1155/2010/943516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gunjima K., Tomiyama R., Takakura K. 3,4-dihydroxybenzalacetone protects against Parkinson’s disease-related neurotoxin 6-OHDA through Akt/Nrf2/glutathione pathway. J Cell Biochem. 2014;115(1):151–160. doi: 10.1002/jcb.24643. [DOI] [PubMed] [Google Scholar]
- 93.Lee K.R., Lee J.S., Lee S. Polysaccharide isolated from the liquid culture broth of Inonotus obliquus suppresses invasion of B16-F10 melanoma cells via AKT/NFB signaling pathway. Mol Med Rep. 2016;14(5):4429–4435. doi: 10.3892/mmr.2016.5771. [DOI] [PubMed] [Google Scholar]
- 94.Nomura M., Takahashi T., Uesugi A., Tanaka R., Kobayashi S. Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspase-3-dependent apoptosis. Anticancer Res. 2008;28(5 A):2691–2696. [PubMed] [Google Scholar]
- 95.Baek J., Roh H.-S., Baek K.-H. Bioactivity-based analysis and chemical characterization of cytotoxic constituents from Chaga mushroom (Inonotus obliquus) that induce apoptosis in human lung adenocarcinoma cells. J Ethnopharmacol. 2018;224:63–75. doi: 10.1016/j.jep.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 96.Fu L., Chen H., Dong P., Zhang X., Zhang M. Effects of ultrasonic treatment on the physicochemical properties and DPPH radical scavenging activity of polysaccharides from mushroom Inonotus obliquus. J Food Sci. 2010;75(4):C322–C327. doi: 10.1111/j.1750-3841.2010.01590.x. [DOI] [PubMed] [Google Scholar]
- 97.Xiang Y., Xu X., Li J. Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn stover medium. Food Chem. 2012;134(4):1899–1905. doi: 10.1016/j.foodchem.2012.03.121. [DOI] [PubMed] [Google Scholar]
- 98.Xu X., Wu Y., Chen H. Comparative antioxidative characteristics of polysaccharide-enriched extracts from natural sclerotia and cultured mycelia in submerged fermentation of Inonotus obliquus. Food Chem. 2011;127(1):74–79. doi: 10.1016/j.foodchem.2010.12.090. [DOI] [Google Scholar]
- 99.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 100.Floegel A., Kim D.O., Chung S.J., Koo S.I., Chun O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal. 2011;24(7):1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- 101.Chen Y., Huang Y., Cui Z., Liu J. Purification, characterization and biological activity of a novel polysaccharide from Inonotus obliquus. Int J Biol Macromol. 2015;79:587–594. doi: 10.1016/j.ijbiomac.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 102.Kapp K., Püssa T., Vuorela H., Välimaa H. 2017. Antiviral effect of Inonotus obliquus (Pers.:Fr.) Pilat extract against herpes simplex virus type 1 in vitro. [DOI] [Google Scholar]
- 103.Hu Y., Teng C., Yu S. Inonotus obliquus polysaccharide regulates gut microbiota of chronic pancreatitis in mice. AMB Express. 2017;7(1):39. doi: 10.1186/s13568-017-0341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Montazeri M., Sharif M., Sarvi S., Mehrzadi S., Ahmadpour E., Daryani A. A systematic review of in vitro and in vivo activities of anti-toxoplasma drugs and compounds (2006–2016) Front Microbiol. 2017;8(JAN):25. doi: 10.3389/fmicb.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kang J.-H., Jang J.-E., Mishra S.K. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J Ethnopharmacol. 2015;173:303–312. doi: 10.1016/j.jep.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 106.Lee S.H., Hwang H.S., Yun J.W. Antitumor activity of water extract of a mushroom, Inonotus obliquus, against HT-29 human colon cancer cells. Phyther Res. 2009;23(12):1784–1789. doi: 10.1002/ptr.2836. [DOI] [PubMed] [Google Scholar]
- 107.Zou C., Zhang Y.-Y., Bai M., Huang X.-X., Wang X.-B., Song S.-J. Aromatic compounds from the sclerotia of Inonotus obliquus. Nat Prod Res. October 2019:1–4. doi: 10.1080/14786419.2019.1677656. [DOI] [PubMed] [Google Scholar]
- 108.Li Z., Mei J., Jiang L. Chaga medicinal mushroom, inonotus obliquus (agaricomycetes) polysaccharides suppress tacrine-induced apoptosis by ROS-scavenging and mitochondrial pathway in HepG2 cells. Int J Med Mushrooms. 2019;21(6):583–593. doi: 10.1615/IntJMedMushrooms. 2019030857. [DOI] [PubMed] [Google Scholar]
- 109.Wang Q., Mu H., Zhang L., Dong D., Zhang W., Duan J. Characterization of two water-soluble lignin metabolites with antiproliferative activities from Inonotus obliquus. Int J Biol Macromol. 2015;74(20120731):507–514. doi: 10.1016/j.ijbiomac.2014.12.044. [DOI] [PubMed] [Google Scholar]
- 110.Quintero-Fabián S., Arreola R., Becerril-Villanueva E. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9(December):1–21. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee K.R., Lee J.S., Song J.E., Ha S.J., Hong E.K. Inonotus obliquus-derived polysaccharide inhibits the migration and invasion of human non-small cell lung carcinoma cells via suppression of MMP-2 and MMP-9. Int J Oncol. 2014;45(6):2533–2540. doi: 10.3892/ijo.2014.2685. [DOI] [PubMed] [Google Scholar]
- 112.Arata S., Watanabe J., Maeda M. Continuous intake of the Chaga mushroom (Inonotus obliquus) aqueous extract suppresses cancer progression and maintains body temperature in mice. Heliyon. 2016;2(5) doi: 10.1016/j.heliyon.2016.e00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duru K.C., Kovaleva E.G., Danilova I.G., Bijl P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phyther Res. 2019;33(8):1966–1980. doi: 10.1002/ptr.6384. [DOI] [PubMed] [Google Scholar]
- 114.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu P., Xue J., Tong S., Dong W., Wu P. Structure Characterization and Hypoglycaemic Activities of two polysaccharides from inonotus obliquus. Molecules. 2018;23(8):1948. doi: 10.3390/molecules23081948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang M., Zhao Z., Zhou X. Simultaneous use of stimulatory agents to enhance the production and hypoglycaemic activity of polysaccharides from inonotus obliquus by submerged fermentation. Molecules. 2019;24(23):4400. doi: 10.3390/molecules24234400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Diao B., Jin W., Yu X. Protective effect of polysaccharides from inonotus obliquus on streptozotocin-induced diabetic symptoms and their potential mechanisms in rats. Evid Base Complement Alternat Med. 2014;2014:841496. doi: 10.1155/2014/841496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cha J.Y., Jun B.S., Yoo K.S., Hahm J.R., Cho Y.S. Fermented chaga mushroom (Inonotus obliquus) effects on hypolipidemia and hepatoprotection in Otsuka Long-Evans Tokushima fatty (OLETF) rats. Food Sci Biotechnol. 2006;15:122–127. [Google Scholar]
- 119.Sun J.-E., Ao Z.-H., Lu Z.-M. Antihyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. J Ethnopharmacol. 2008;118(1):7–13. doi: 10.1016/j.jep.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 120.Chou Y.-J., Kan W.-C., Chang C.-M. Renal protective effects of low molecular weight of inonotus obliquus polysaccharide (LIOP) on HFD/STZ-Induced nephropathy in mice. Int J Mol Sci. 2016;17(9):1535. doi: 10.3390/ijms17091535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olefsky J.M. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. J Clin Invest. 2000;106(4):467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clark R.B. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71(3):388–400. doi: 10.1189/jlb.71.3.388. [DOI] [PubMed] [Google Scholar]
- 123.Joo J.I., Kim D.H., Yun J.W. Extract of Chaga mushroom (Inonotus obliquus) stimulates 3t3-l1 adipocyte differentiation. Phyther Res. 2010;24(11):1592–1599. doi: 10.1002/ptr.3180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.