Abstract
Background:
The brain’s endocannabinoid system, the primary target of cannabis, has been implicated in psychosis. The endocannabinoid, anandamide, is elevated in cerebrospinal fluid of patients with schizophrenia. Fatty acid amide hydrolase (FAAH) controls brain anandamide levels, however, it is unknown if FAAH is altered in vivo in psychosis or related to positive psychotic symptoms.
Methods:
Twenty-seven patients with schizophrenia-spectrum disorders and 36 healthy control participants completed high-resolution positron emission tomography (PET) scans with the novel FAAH radioligand, [11C]CURB, and structural MRI. Data were analyzed using the validated irreversible two-tissue compartment model with a metabolite-corrected arterial input function.
Results:
FAAH did not differ significantly between patients with psychotic disorders and healthy controls (F1,62.85=0.48, p=.49). In contrast, lower FAAH predicted greater positive psychotic symptom severity, with the strongest effect observed for the positive symptom dimension, which includes suspiciousness, delusions, unusual thought content, and hallucinations (F1,26.69=12.42, p=.002; Cohen’s f=0.42, large effect). Shorter duration of illness (F1,26.95=13.78, p=.001; Cohen’s f=0.39; medium-to-large effect) and duration of untreated psychosis predicted lower FAAH (F1,26.95=6.03, p=.021, Cohen’s f=0.27, medium effect). These results were not explained by past cannabis exposure or current intake of antipsychotic medications. FAAH exhibited marked differences across brain regions (F7,112.62=175.85, p<1×10−56; Cohen’s f>1). Overall, FAAH was higher in females than in males (F1,62.84=10.05, p=.002; Cohen’s f=0.37).
Conclusions:
This first study of brain FAAH in psychosis indicates that FAAH may represent a biomarker of disease state of potential utility for clinical studies targeting psychotic symptoms or as a novel target for interventions to treat psychotic symptoms.
Keywords: imaging, schizophrenia, endocannabinoid, positron emission tomography, psychosis, fatty acid amide hydrolase
Introduction
Evidence from in vivo brain imaging, post-mortem studies, and physiological measures support a role of the endocannabinoid system in schizophrenia and other psychotic disorders (1).
The endocannabinoid system plays important roles in motivation, learning and stress responses, primarily by modulation of neurotransmitters including glutamate, GABA, and dopamine (2). The endocannabinoid system consists of the CB1 and CB2 receptors, endogenous ligands anandamide and 2-arachidonoyl glycerol (2-AG) and their respective biosynthetic and catabolic enzymes. Brain levels of anandamide are governed primarily by fatty acid amide hydrolase (FAAH; 3), which has a distinct distribution including dense expression in striatum (4) - the major site of dopaminergic abnormalities in schizophrenia (5). Circuit-level alterations present in schizophrenia, including at cortico-striatal and corticolimbic circuits, suggest a pivotal role of the endocannabinoid system given its primary role in regulating neurotransmitter function across the brain (6–9).
In vivo imaging of brain cannabinoid CB1 receptors and elevations of anandamide, an endogenous cannabinoid CB1 receptor agonist, in cerebrospinal fluid and peripheral blood in patients with psychosis provide evidence of endocannabinoid system disturbances that appear to be independent of exposure to cannabis (10). In vivo imaging of brain cannabinoid CB1 receptors has revealed alterations in schizophrenia, with the largest effects observed in first-episode or unmedicated individuals (table S1; 11–14). Beyond the CB1 receptor, no other major endocannabinoid system target has ever been quantified in vivo in the brains of patients with psychotic disorders.
Dramatic elevations of anandamide levels were observed in multiple cohorts of patients with psychosis (10, 15–18; table S2). Cerebrospinal fluid anandamide levels were associated with psychotic symptoms in patients with schizophrenia or schizophreniform disorder (10). The same association between cerebrospinal fluid anandamide and persistent psychosis-like symptoms was observed in otherwise healthy cannabis users (18), suggesting the endocannabinoid system may be more broadly linked with psychosis-spectrum experiences. Lower baseline anandamide was associated with lower rates of conversion to psychosis in psychosis-risk in non-affected twins who later converted to psychosis, suggesting that such changes in anandamide may reflect an adaptive response during disease progression (15, 17, 19, 20).
Despite two decades of attention to anandamide in psychosis (10, 15) to-date there is no report of brain FAAH, its primary regulatory enzyme, in vivo in psychotic disorders.
[11C]CURB is the carbon-11 labeled form of a FAAH inhibitor with high selectivity and specificity for FAAH (21). [11C]CURB has good brain penetration in humans (21), high test-retest reproducibility (<10% variability between scans) and high within-subject reliability (22). High specificity for FAAH was demonstrated by a ≥95% reduction in [11C]CURB binding ([11C]CURB λk3) following pre-treatment with the selective FAAH inhibitor PF-04457845 (1 mg, p.o.; 22). [11C]CURB is sensitive to physiologically relevant changes in levels of FAAH protein, as observed in individuals heterozygous or homozygous for the FAAH rs324420 (C385A) variant A-allele that is associated with reduced levels of FAAH protein (23).
We hypothesized that lower FAAH, as measured by [11C]CURB λk3, will be related to a diagnosis of a psychotic disorder and positive psychotic symptom severity. Brain regions were selected based on their relevance for schizophrenia and the endocannabinoid system. We also explored whether duration of untreated psychosis, duration of illness, sex or brain region predict FAAH levels.
Methods and Materials
Participants
In total 63 [11C]CURB PET scans were analyzed, including 27 participants with a psychotic disorder and 36 age-matched healthy controls between 18 and 40 years-old. Participants with psychotic disorders were recruited from a tertiary care psychiatric hospital (Centre for Addiction and Mental Health, CAMH) and healthy control participants were recruited from the Toronto-area community. Recruitment and data collection were conducted between April 2013 and December 2018. Psychosis participants met DSM-IV criteria for schizophrenia, schizophreniform disorder, delusional disorder, schizoaffective disorder, or psychosis not otherwise specified, as determined with the Structured Clinical Interview for DSM-IV Axis I Disorders and confirmed by a psychiatrist (RM). Healthy control participants had no history of DSM-IV axis I disorders or substance use, no family history of psychotic disorders, and were medication-free except for oral contraceptives. All participants screened negative for drugs of abuse and did not meet criteria for a substance use disorder at the time of the study. Participants were excluded if they had unstable medical or neurological illness, history of severe head trauma, metal implants precluding an MRI scan or were pregnant or breastfeeding. Psychotic symptom severity was determined by the Positive and Negative Syndrome Scale (PANSS), and duration of illness was calculated from the onset of frank psychotic symptoms. Duration of untreated psychosis was calculated per participants’ own recollection and clinical notes when available of commencement of symptoms until first antipsychotic exposure. The present analysis includes twenty-seven healthy control participants that have been reported in prior publications (22–25).
This study was approved by the Research Ethics Board at CAMH. All psychosis patients demonstrated capacity to provide informed consent as assessed by the MacArthur competence assessment tool. All participants provided written informed consent, in accordance with the Declaration of Helsinki, after procedures were explained thoroughly.
Positron Emission Tomography
PET scans were performed using a 3D high resolution research tomograph (HRRT) brain tomograph (CPS/Siemens, Knoxville, TN, USA), which measures radioactivity in 207 slices with an interslice distance of 1.22 mm. An intravenous line was inserted into the participant’s antecubital vein for tracer injection and an arterial cannula was inserted into the radial artery of their opposite arm for arterial blood sampling. To reduce head movement, each participant was fitted with a custom thermoplastic mask used with a head-fixation system. A transmission scan using a single photon point source, 137Cs (t1/2=30.2 years, Eγ = 662 keV), was acquired to correct the emission data for the attenuation of the emission photons through the head and support. Following the transmission scan, [11C]CURB (357.27 ± 25.28 MBq) was infused over 1 minute at a constant rate (Harvard Apparatus, Holliston, MA, USA) and data were acquired in list mode for 60 minutes following injection.
Arterial Sampling of [11C]CURB in Plasma
Arterial samples were taken continuously for the first 22.5 minutes after [11C]CURB injection with an automatic blood sampling system (ABSS; Model PBS-101, Veenstra Instruments, Joure, The Netherlands). Manual samples were taken 3, 7, 12, 15, 20, 30, 45, and 60 minutes after injection. Radioactivity in whole blood and plasma (1,500 RCF, 5 minutes) was counted using a Packard Cobra II or Wizard 2480 γ-counter (Packard Instrument Co., Meridian, CT) cross-calibrated with the PET system. Concentration of parent radioligand and its metabolites was determined in each manual sample (except the one at 15 minutes) as previously validated (21). Blood-to-plasma radioactivity ratios were fitted using a biexponential function and parent plasma fraction using a Hill function. A dispersion- and metabolite-corrected arterial plasma input function was generated as previously described (21).
Kinetic analysis
A two-tissue-compartment model with irreversible binding was used to quantify binding of [11C]CURB to FAAH. The validated outcome measure for [11C]CURB, the composite parameter λk3, is independent of blood flow, a common limitation of irreversible radioligands (21).
Region of interest analysis
Proton density (PD)-weighted brain MR images required for the delineation of each region of interest were obtained for each subject using a 3T MR-750 scanner (General Electric Medical Systems, Milwaukee, WI). Time-activity curves were extracted for the regions of interest using a validated and reproducible in-house imaging pipeline (21).
FAAH rs324420 polymorphism genotyping
The FAAH rs324420 polymorphism affects FAAH protein levels (26), therefore rs324420 genotype was determined as described previously (23). Participants were categorized according to their alleles (C/C, A/C, or A/A). Out of 65 participants who completed PET and MRI scans, two exhibited the A/A genotype, both in the healthy control group. Therefore, in order to better match genotypes across groups, these two A/A participants are excluded from the primary analysis. Including the A/A participants does not change the outcome of the analysis, and results including the A/A participants are presented in the supplement.
Statistical Analysis
Statistical analysis was conducted using SPSS 25 (IBM Corp). For the purpose of mixed-model figure generation, mixed model fit, residuals, and 95% confidence intervals were generated with lme4 (27) and visreg (28) for R. All figures were created using GraphPad Prism.
Demographics were examined for any group differences using independent-samples t-tests or chi-square tests. The relationship between [11C]CURB λk3 and schizophrenia diagnosis, psychotic symptoms, sex, duration of illness and duration of untreated psychosis, were tested using a random effects linear mixed model with group and genotype as fixed factors, region of interest as a repeated within-subject fixed factor with a diagonal covariance structure, participant identification number as a random effect (including intercept), and regional [11C]CURB λk3 as the dependent variable. Linear mixed models are robust to missing data and do not assume sphericity or compound symmetry (29). Effect sizes (Cohen’s f) were calculated using estimated marginal means, sum of squares, or R2 from SPSS 25. As described by Cohen (30), effect sizes were interpreted as follows, Cohen’s f = 0.1 a small effect, Cohen’s f=0.25 a medium effect, and Cohen’s f=0.40 a large effect. All statistical tests were two-tailed, with α=0.05.
Non-significant interactions were removed from the model. Sex was not balanced across groups, and in light of evidence that brain levels of endocannabinoid proteins including FAAH differ by sex (31, 32), analyses were conducted with sex included in the model. Analyses including common sources of confounding as covariates (age, antipsychotic medication use, and self-reported use of tobacco or cannabis) are presented after reporting results without these variables.
All analyses included hippocampus, amygdala, limbic striatum (LST), associative striatum (AST), sensorimotor striatum (SMST; 33), anterior cingulate cortex, medial prefrontal cortex, and dorsolateral prefrontal cortex. In the present analyses (mixed effects model) a significant main effect – in the absence of a significant interaction with region of interest – indicates that the main effect is controlled for all regions studied.
Results
Sample characteristics
In total, 63 [11C]CURB PET scans were analyzed, including 27 participants with a psychotic disorder and 36 healthy control participants. Sample characteristics are presented in table 1. One psychosis participant who completed PET and MRI scans had an incidental finding, identified during the MRI scan, that compromised region of interest delineation for the amygdala, hippocampus and limbic striatum in the affected hemisphere, therefore for this participant [11C]CURB λk3 values for these regions were excluded from the statistical analysis.
Table 1.
Demographics and clinical measures (mean ± SD)
| Healthy control | Psychotic disorder | F/chi2 | p | |
|---|---|---|---|---|
| n | 36 | 27 | ||
| Age (years) | 24.32±5.17 | 25.10±5.34 | 0.347 | .56 |
| BMI | 23.73±3.62 | 25.09±4.54 | 1.745 | .19 |
| male/female | 19/17 | 22/5 | 5.59 | .018 |
| Tobacco non-smoker/smokera | 35/1 | 22/5 | 4.44 | .035 |
| Cigarettes per day (in smokers) | 17.5 | 14.81 ± 8.34 | 0.087 | .78 |
| Cannabis exposures | ||||
| >10 lifetime | 1 | 16 | ||
| >100 lifetime | 0 | 12 | ||
| >10 in past year | 0 | 6 | ||
| FAAH genotype CC/AC | 25/11 | 16/11 | 0.704 | .40 |
| Medications | ||||
| Antidepressant | - | 9 | ||
| Benzodiazepine | - | 2 | ||
| Stimulant | - | 3 | ||
| Antipsychotics: AP-free/AP-current | - | 16/11 | ||
| Mean antipsychotic dose (chlorpromazine-equivalent)b Sub-therapeutic dosec (n=7) | 70±42 mg/day | |||
| Therapeutic dose (n=4) | 446±313 mg/day | |||
| PANSS Positive Score (SD) | - | 16.26±3.57 | ||
| PANSS Total Score (SD) | - | 56.81±11.04 | ||
| Diagnosis | ||||
| SZ/SF/SA/DD/NOS | - | 18/6/1/1/1 | ||
| [11C]CURB PET Scan | ||||
| Amount injected (MBq) | 361.10±22.57 | 352.16±28.12 | 1.96 | .17 |
| Mass (μg) | 1.63±0.92 | 1.97±1.37 | 1.39 | .24 |
| Specific activity (GBq/pmol) | 91.13±46.30 | 81.47±53.52 | 0.59 | .45 |
SZ: schizophrenia SF: schizophreniform disorder; SA: schizoaffective disorder; DD: delusional disorder; NOS: Psychotic disorder not otherwise specified;
Tobacco smoking status determined using the Fagerstrom Test for Nicotine Dependence
Mean dose for those taking antipsychotics; chlorpromazine equivalent doses calculated per Andreasen et al. (34). Lurasidone was first converted to an equivalent olanzapine dose (42) before following the method of (40).
Sub-therapeutic defined as doses below the equivalent starting dose of chlorpromazine (~200 mg/day)
Eight of 27 psychosis participants were antipsychotic-naïve at the time of the PET scan, 16 were antipsychotic-free (including antipsychotic-naïve participants), seven were receiving sub-therapeutic doses and only four were taking therapeutic doses (table 1 and table S3; chlorpromazine equivalent doses were calculated per Andreasen et al., 2010, 34). Four patients had a history of cannabis abuse or dependence but did not meet criteria within the six months preceding the PET scan. The patient group had a higher proportion of males, more smokers, and more participants with a history of cannabis use than the healthy control group (table 1).
[11C]CURB PET imaging results
Psychotic symptom severity and diagnosis
FAAH did not differ significantly between healthy control participants and patients with psychosis (F1,62.85=0.48, p=.49; Cohen’s f=0.08; figure 1), or patients with a diagnosis of schizophrenia (F1,53.87=0.37, p=.55; figure 1). This outcome was not altered by controlling for past-year cannabis exposure (F1,62.86=0.85, p=.36; schizophrenia: F1,53.87=0.43, p=.52), cigarettes-per-day (F1,62.82=0.75, p=.39; schizophrenia: F1,53.82=0.75, p=.39) or antipsychotic dose (F1,62.84=0.14, p=.71; schizophrenia: F1,53.87=0.09, p=.76).
Figure 1:
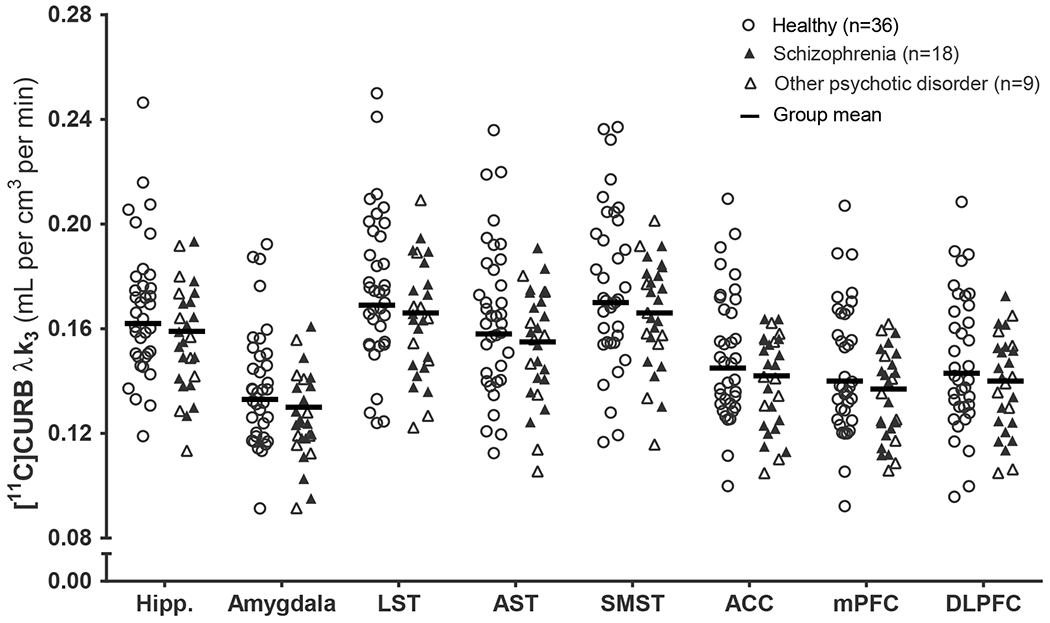
[11C]CURB λk3 in healthy controls and patients with psychotic disorders. Means are adjusted for sex and FAAH rs324420 genotype.
Hipp: hippocampus; LST: limbic striatum; AST: associative striatum; SMST: sensorimotor striatum; ACC: anterior cingulate cortex; mPFC: medial prefrontal cortex; DLPFC: dorsolateral prefrontal cortex.
Sample characteristics
Across the combined patient sample, PANSS positive score was significantly associated with FAAH, such that lower FAAH predicted higher PANSS positive score (F1,26.92=4.46, p=.044; Cohen’s f=0.31; medium-to-large effect; figure S2). This effect was not altered after controlling for antipsychotic dose (F1,26.91=5.73, p=.024), cigarettes-per-day (F1,26.91=5.18, p=.031) or past-year cannabis exposure (F1,26.9=4.33, p=.047).
It is well-recognized that the PANSS positive scale reflects several distinct clinical dimensions, and items on the full PANSS scale are well described by a 5-factor model (35, 36). Therefore, we investigated whether individual clinical dimensions were specifically associated with FAAH. Here, we report that FAAH was significantly associated with the positive symptom dimension (F1,26.69=12.42, p=.002; Cohen’s f=0.42, large effect; figure 2), and this effect was not altered after controlling for antipsychotic dose (F1,26.66=15.19, p=.001), cigarettes-per-day (F1,26.90=13.85, p=.001) or past-year cannabis use (F1,26.71=12.20, p=.002).
Figure 2:
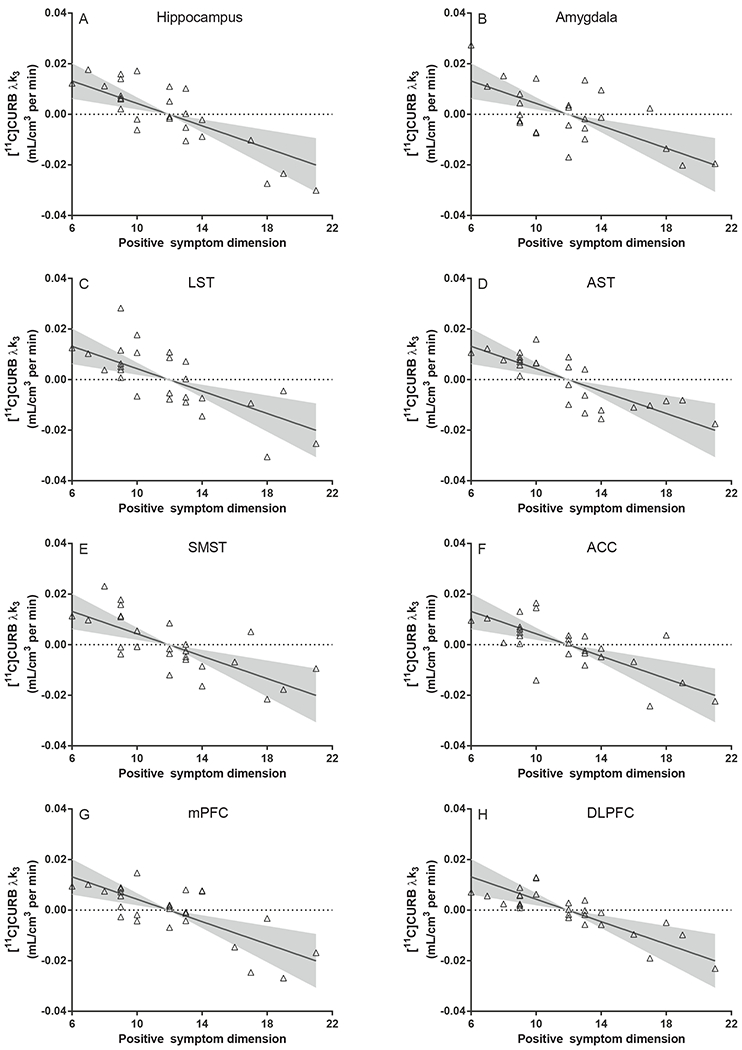
Partial residuals plots of [11C]CURB λk3 and the positive symptom dimension in psychosis patients (n=27). Fit line and shaded areas reflect the model fit and 95% confidence interval of the overall random effects linear mixed model, controlling for FAAH rs324420 genotype, sex and including all brain regions (n=8).
LST: limbic striatum; AST: associative striatum; SMST: sensorimotor striatum; ACC: anterior cingulate cortex; mPFC: medial prefrontal cortex; DLPFC: dorsolateral prefrontal cortex.
While our study was powered to only test FAAH with positive psychotic symptoms, the other four psychotic symptom dimensions were not associated with FAAH (negative: F1,26.91=0.19, p=.66; disorganization: F1,26.90=1.16, p=.29; excitability: F1,26.94=0.53, p=.47; depression/anxiety: F1,26.91=0.86, p=.36). Similarly, FAAH was not associated with PANSS total score (F1,29.04=0.96, p=.34) or PANSS negative score (F1,26.92=0.22, p=.64).
Duration of illness and duration of untreated psychosis
Duration of illness was significantly associated with FAAH (F1,26.95=13.78, p=.001; Cohen’s f=0.39; medium-to-large effect; figure S3) such that FAAH was higher with longer duration of illness, and this result was not affected by controlling for age (F1,26.94=13.50, p=.001). The association of duration of illness with FAAH remained significant even after removing the two patients with the longest duration of illness (F1,24.95=12.97, p=.001). Further, longer duration of untreated psychosis was associated with higher FAAH (F1,26.95=6.03, p=.021; Cohen’s f=0.27; medium effect; figure S4), and the result held after controlling for age (F1,26.96=5.96, p=.022). Controlling for antipsychotic dose did not meaningfully alter the associations of FAAH with duration of illness (F1,26.95=13.30, p=.001) or duration of untreated psychosis (F1,26.95= 5.63, p=.025).
Impact of sex on FAAH
Given the imbalance of sex across groups, and in light of evidence that brain levels of endocannabinoid proteins including FAAH differ by sex (31, 32), we investigated the impact of sex on FAAH across the combined study sample. Females had higher FAAH across the combined study sample such that FAAH was 9-12% higher in females than in males (F1,62.84=10.05, p=.002; Cohen’s f=0.37, medium-to-large effect; figure 3).
Figure 3:

[11C]CURB λk3 in males and females in the entire sample (n=63). Group means are adjusted for diagnostic group and FAAH rs324420 genotype.
Hipp: hippocampus; LST: limbic striatum; AST: associative striatum; SMST: sensorimotor striatum; ACC: anterior cingulate cortex; mPFC: medial prefrontal cortex; DLPFC: dorsolateral prefrontal cortex.
Regional organization of FAAH in brain
Brain region significantly predicted FAAH (F7,112.62=175.85, p<1x10−56; Cohen’s f>1, large effect), such that the highest FAAH was observed in the LST and SMST across the whole sample. Compared to the LST, FAAH was 4-6% lower in AST (p<1x10−8) and hippocampus (p=.006), 14-17% lower in ACC (p<1x10−27), mPFC (p<1x10−34) and DLPFC (p<1x10−30) and 21% lower in the amygdala (p<1x10−33; p-values Bonferroni corrected).
Discussion
To our knowledge, this is the first in vivo quantification of brain FAAH in patients with psychotic disorders. Lower FAAH was associated with greater positive psychotic symptoms, independent of diagnosis of a psychotic disorder, including schizophrenia. Shorter duration of illness and duration of untreated psychosis was associated with lower brain FAAH. We demonstrated a robust effect of brain region on FAAH and observed higher FAAH in females relative to males in the combined sample.
Across diagnoses, patients with greater symptom severity had lower FAAH, in agreement with a previous observations linking anandamide levels to disease state (37). The strongest effect was seen with the positive symptom dimension, which includes suspiciousness, delusions, unusual thought content, and hallucinations (36). The 20-25% variation in FAAH, as observed across patients from low to high symptom severity (figure 2), is of plausible functional significance. This change in FAAH is of comparable magnitude to the FAAH rs324420 (C385A) genotype variant (23), which exhibits a documented behavioural phenotype accompanied by changes to fronto-limbic functional connectivity (38). Greater positive psychotic symptom severity was associated with lower FAAH (figures 2 and supplementary figure S2), replicating the same relationship observed with symptoms and FAAH measured in peripheral blood mononuclear cells in a mixed sample of first-episode patients with affective or non-affective psychosis (39). Biological markers linked to behavioural presentations are complementary to, and may in some cases be more informative than a categorical diagnosis for elucidating the neurobiology underlying clinical state (40–43). Only one CB1 PET study explored associations with PANSS 5-factor dimensions, and observed associations with negative factor items in a subgroup of untreated and first-episode patients (12).
Associations between psychotic symptoms and endocannabinoid system disturbances vary across studies (supplementary tables S1 and S2), however most studies in brain reported greater symptom severity with larger deviations of cannabinoid CB1 receptors from the levels of healthy controls (11, 12, 14; but see 13). Although some studies reported that higher anandamide levels were related to lower symptoms, treatment and/or symptom remission was nevertheless accompanied by normalization of anandamide toward the levels of healthy controls (16, 19). Taken together, the present results along with studies of brain CB1 receptors, cerebrospinal fluid and peripheral blood all support an association of endocannabinoid state with psychosis state as measured by symptom severity (37).
Furthermore, the present results provide preliminary evidence that FAAH may change with disease progression (supplementary figures S3 and S4), potentially providing a biomarker of disease stage. To our knowledge, PET studies of the cannabinoid CB1 receptor have not reported whether brain CB1 receptors are associated with illness course, although the largest effects in CB1 receptor PET studies were observed in untreated and antipsychotic-naive patients (12, 13). As most patients in the present study have a duration of illness below 18 months (n=20; supplementary figure S3), future studies should examine FAAH before illness onset (e.g., clinical high risk) and in chronic patients, to better understand the role of FAAH in illness stage.
Across all regions examined and controlling for diagnostic group, FAAH was higher in females than in males in agreement with wider reports of sex differences in the endocannabinoid system of humans and animals (31, 44–47). Similarly, women had higher platelet FAAH activity than men (44), although in peripheral blood mononuclear cells FAAH levels were lower in women than in men (39). Sex hormones and their targets affect FAAH activity in human leukocytes (48), and thus may likewise contribute to sex differences in brain. The functional relevance of higher FAAH in females is not known, but in line with differences in CB1 receptors (31), sex is an important variable to be considered in clinical trials and future investigations of the endocannabinoid system, and is particularly relevant in studies with psychosis patients.
The present results should be interpreted in light of the following considerations. In the present study, patients had low total antipsychotic exposure. Although results did not change after controlling for antipsychotic dose and groups did not differ in FAAH according to past or current antipsychotic exposure (Supplemental Results), it is unknown whether the same results would be observed following chronic antipsychotic use or higher doses. Patient medication use did not meaningfully affect results as psychotic symptoms were related to FAAH even when controlling for the use of anti-depressants, stimulants, or benzodiazepines (Supplemental Results). The present study did not measure anandamide levels, however the only study published to-date reporting brain FAAH and peripheral blood N-acylethanolamines observed higher anandamide with lower [11C]CURB λk3 in abstinent patients with alcohol use disorder (49). Although not all participants were subjected to urine toxicology on the day of the PET scan, whenever urine toxicology was repeated on the scan day the results matched the negative drug screen results observed at study baseline.
Patients in the present study varied in symptom severity from mild to moderately severe. Considering the results of the present study, it might be expected that a sample of patients with high positive symptom severity – such as chronic or treatment-resistant patients – would exhibit reduced FAAH relative to healthy controls or patients in remission. Estimates of duration of untreated psychosis relied on retrospective patient self-report and clinical notes when available, however, the short period of illness in this primarily first-episode sample (48% within 1 year, 78% with 2 years of symptom onset) minimizes the likelihood of unreliability of this information.
As in previous clinical studies with [11C]CURB, we did not measure the plasma free fraction; therefore a possible contribution of the radioligand protein binding to the results of the present study cannot be ruled out.
Finally, as with other endocannabinoid PET studies in this population (11, 13, 14) the patient sample in the present study consisted primarily of males. We observed lower FAAH in males relative to females and although the sex-by-group interaction was not significant, the primarily male composition of the patient group limits our ability to make group-specific conclusions. Restricting the analysis to a male-only sample did not reveal significant differences in FAAH between patients and healthy control participants (Supplemental Results). The contribution of sex to FAAH must therefore be explored in large samples of both patients and healthy controls with an equal number of females and males within each clinical group.
This research is particularly timely in light of global trends toward cannabis legalization. Epidemiological studies link cannabis use with increased risk of psychosis (50, 51), and early initiation with a younger age of psychosis onset (52, 53). No biological mechanism for these associations has been identified. Prior reports showing significantly lower brain FAAH in cannabis use disorders (24, 25) position FAAH as a potential molecular link between cannabis use and psychosis.
Conclusions
To our knowledge, this is the first in vivo quantification of brain FAAH in patients with psychotic disorders, showing no significant difference between patients and healthy controls. Lower FAAH activity predicted greater positive psychotic symptom severity, with the strongest effect observed for the positive symptom dimension. Lower FAAH was also associated with shorter duration of illness and duration of untreated psychosis. We also observed higher FAAH in females relative to males in the combined sample.
Future studies should investigate whether FAAH changes with symptom remission, and from the prodromal stages to psychosis onset and chronic psychosis. This study opens up opportunities to better understand the molecular underpinnings of cannabis-induced psychosis and perhaps offers a novel target for interventions to treat psychotic symptoms.
Supplementary Material
Acknowledgements
This work was supported by a NARSAD Independent Investigator’s Grant (Grant No. 21977 [to RM]) and grants from the National Institute of Mental Health (Grant Nos. R21MH103717 and R01MH113564 [to RM]). This research was supported, in part, by funding from the Canada Research Chairs program (Canada Research Chair in Pharmacogenomics [to RFT]), Canadian Institutes of Health Research (Foundation Grant No. FDN-154294 [to RFT]), and the Centre for Addiction and Mental Health (to RFT). This study was supported, in part, by the National Institute of Health and National Institute of Drug Abuse (Grant No. R21AA022246 [to IB]), the Ontario Mental Health Foundation (to IB), and a Canadian Institutes of Health Research Project Grant (Grant No. PG-38934 [to IB]).
We thank the staff of the Centre for Addiction and Mental Health Research Imaging Centre, Marcos Sanchez for biostatistical support and advice and Dr. Sina Hafizi for his senior postdoctoral support during the study execution.
Presented in part or in full at meetings of the Society of Nuclear Medicine and Molecular Imaging (June 22–25, 2019), American College for Neuropsychopharmacology (Dec 9–13, 2018 and Dec 8–11, 2019), and the International Cannabinoid Research Society (June 23–26, 2017).
RM has received travel support and speaker fees from Janssen Inc and consulting fees from Otsuka-Lundbeck Canada. RFT has consulted for Apotex and Quinn Emanuel, and Ethismos on unrelated topics. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fernandez-Espejo E, Viveros M-P, Núñez L, Ellenbroek BA, Rodriguez de Fonseca F (2009): Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology. 206:531–549. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R, Parker LA (2013): The endocannabinoid system and the brain. Annual review of psychology. 64:21–47. [DOI] [PubMed] [Google Scholar]
- 3.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. (2001): Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 98:9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero J, Hillard CJ, Calero M, Rabano A (2002): Fatty acid amide hydrolase localization in the human central nervous system: an immunohistochemical study. Brain Res Mol Brain Res. 100:85–93. [DOI] [PubMed] [Google Scholar]
- 5.Laruelle M, Abi-Dargham A, Van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. (1996): Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences. 93:9235–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katona I, Freund TF (2008): Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nature Medicine. 14:923–930. [DOI] [PubMed] [Google Scholar]
- 7.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. (2008): Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in neurosciences. 31:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hariri AR, Gorka A, Hyde LW, Kimak M, Haider I, Ducci F, et al. (2009): Divergent Effects of Genetic Variation in Endocannabinoid Signaling on Human Threat- and Reward-Related Brain Function. Biological Psychiatry. 66:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossong MG, Jansma JM, Bhattacharyya S, Ramsey NF (2014): Role of the endocannabinoid system in brain functions relevant for schizophrenia: an overview of human challenge studies with cannabis or 9-tetrahydrocannabinol (THC). Prog Neuropsychopharmacol Biol Psychiatry. 52:53–69. [DOI] [PubMed] [Google Scholar]
- 10.Minichino A, Senior M, Brondino N, Zhang SH, Godwlewska BR, Burnet PWJ, et al. (2019): Measuring Disturbance of the Endocannabinoid System in Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, et al. (2010): Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. NeuroImage. 52:1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceccarini J, De Hert M, Van Winkel R, Peuskens J, Bormans G, Kranaster L, et al. (2013): Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. NeuroImage. 79:304–312. [DOI] [PubMed] [Google Scholar]
- 13.Ranganathan M, Cortes-Briones J, Radhakrishnan R, Thurnauer H, Planeta B, Skosnik P, et al. (2016): Reduced Brain Cannabinoid Receptor Availability in Schizophrenia. Biol Psychiatry. 79:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgan F, Laurikainen H, Veronese M, Marques TR, Haaparanta-Solin M, Solin O, et al. (2019): In Vivo Availability of Cannabinoid 1 Receptor Levels in Patients With First-Episode Psychosis. JAMA Psychiatry. 76:1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D (1999): Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 10:1665–1669. [DOI] [PubMed] [Google Scholar]
- 16.De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V (2003): Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids in health and disease. 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leweke FM, Giuffrida A, Koethe D, Schreiber D, Nolden BM, Kranaster L, et al. (2007): Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophrenia research. 94:29–36. [DOI] [PubMed] [Google Scholar]
- 18.Morgan CJ, Page E, Schaefer C, Chatten K, Manocha A, Gulati S, et al. (2013): Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. The British Journal of Psychiatry. 202:381–382. [DOI] [PubMed] [Google Scholar]
- 19.Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, et al. (2004): Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 29:2108–2114. [DOI] [PubMed] [Google Scholar]
- 20.Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, et al. (2009): Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry. 194:371–372. [DOI] [PubMed] [Google Scholar]
- 21.Rusjan PM, Wilson AA, Mizrahi R, Boileau I, Chavez SE, Lobaugh NJ, et al. (2013): Mapping human brain fatty acid amide hydrolase activity with PET. J Cereb Blood Flow Metab. 33:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boileau I, Rusjan PM, Williams B, Mansouri E, Mizrahi R, De Luca V, et al. (2015): Blocking of fatty acid amide hydrolase activity with PF-04457845 in human brain: a positron emission tomography study with the novel radioligand [C]CURB. J Cereb Blood Flow Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boileau I, Tyndale RF, Williams B, Mansouri E, Westwood DJ, Le Foll B, et al. (2015): The fatty acid amide hydrolase C385A variant affects brain binding of the positron emission tomography tracer [(11)C]CURB. J Cereb Blood Flow Metab. 35:1237–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boileau I, Mansouri E, Williams B, Le Foll B, Rusjan P, Mizrahi R, et al. (2016): Fatty Acid Amide Hydrolase Binding in Brain of Cannabis Users: Imaging With the Novel Radiotracer [(11)C]CURB. Biol Psychiatry. 80:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson M, Watts J, Da Silva T, Boileau I, Tyndale R, Rusjan P, et al. (2020): Fatty Acid Amide Hydrolase is Reduced Lower in Young Adult Cannabis Users. Addiction Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiang KP, Gerber AL, Sipe JC, Cravatt BF (2004): Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human molecular genetics. 13:2113–2119. [DOI] [PubMed] [Google Scholar]
- 27.Bates D, Mächler M, Bolker B, Walker S (2014): Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823. [Google Scholar]
- 28.Breheny P, Burchett W (2013): Visualization of regression models using visreg. R package. 1–15. [Google Scholar]
- 29.Maxwell SE, Delaney HD (2004): Designing experiments and analyzing data: A model comparison perspective. [Google Scholar]
- 30.Cohen J (2013): Statistical power analysis for the behavioral sciences. Routledge. [Google Scholar]
- 31.Laurikainen H, Tuominen L, Tikka M, Merisaari H, Armio R-L, Sormunen E, et al. (2019): Sex difference in brain CB1 receptor availability in man. NeuroImage. 184:834–842. [DOI] [PubMed] [Google Scholar]
- 32.Craft RM, Marusich JA, Wiley JL (2013): Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life sciences. 92:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang D-R, Huang Y, et al. (2003): Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. Journal of Cerebral Blood Flow & Metabolism. 23:285–300. [DOI] [PubMed] [Google Scholar]
- 34.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C (2010): Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological psychiatry. 67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Oord EJ, Rujescu D, Robles JR, Giegling I, Birrell C, Bukszár J, et al. (2006): Factor structure and external validity of the PANSS revisited. Schizophrenia research. 82:213–223. [DOI] [PubMed] [Google Scholar]
- 36.Lehoux C, Gobeil M-H, Lefèbvre A-A, Maziade M, Roy M-A (2009): The five-factor structure of the PANSS: a critical review of its consistency across studies. Clinical Schizophrenia & Related Psychoses. 3:103–110. [Google Scholar]
- 37.Volk DW, Lewis DA (2019): Insights Into the Pathophysiology of Endocannabinoid Signaling in Schizophrenia. JAMA Psychiatry. [DOI] [PubMed] [Google Scholar]
- 38.Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, et al. (2015): FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature communications. 6:6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bioque M, García-Bueno B, MacDowell KS, Meseguer A, Saiz PA, Parellada M, et al. (2013): Peripheral Endocannabinoid System Dysregulation in First-Episode Psychosis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 38:2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allardyce J, Suppes T, Van Os J (2007): Dimensions and the psychosis phenotype. International journal of methods in psychiatric research. 16:S34–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapur S, Phillips AG, Insel TR (2012): Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Molecular Psychiatry. 17:1174–1179. [DOI] [PubMed] [Google Scholar]
- 42.Arndt S, Andreasen NC, Flaum M, Miller D, Nopoulos P (1995): A Longitudinal Study of Symptom Dimensions in Schizophrenia: Prediction and Patterns of Change. JAMA Psychiatry. 52:352–360. [DOI] [PubMed] [Google Scholar]
- 43.Flaum M, O’Leary DS, Swayze VW, Miller DD, Arndt S, Andreasen NC (1995): Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. Journal of Psychiatric Research. 29:261–276. [DOI] [PubMed] [Google Scholar]
- 44.Cupini L, Bari M, Battista N, Argiro G, Finazzi-Agro A, Calabresi P, et al. (2006): Biochemical changes in endocannabinoid system are expressed in platelets of female but not male migraineurs. Cephalalgia. 26:277–281. [DOI] [PubMed] [Google Scholar]
- 45.Hillard CJ (2018): Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 43:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM (2010): Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proceedings of the National Academy of Sciences. 107:20535–20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabatadze N, Huang G, May RM, Jain A, Woolley CS (2015): Sex Differences in Molecular Signaling at Inhibitory Synapses in the Hippocampus. The Journal of Neuroscience. 35:11252–11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agrò A, Rossi A (2003): Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros Evidence for a synergistic effect of leptin. Journal of Biological Chemistry. 278:32726–32732. [DOI] [PubMed] [Google Scholar]
- 49.Best LM, Williams B, Le Foll B, Mansouri E, Bazinet RP, Lin L, et al. (2020): Lower brain fatty acid amide hydrolase in treatment-seeking patients with alcohol use disorder: a positron emission tomography study with [C-11]CURB. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andreasson S, Allebeck P, Engstrom A, Rydberg U (1987): Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 2:1483–1486. [DOI] [PubMed] [Google Scholar]
- 51.Callaghan RC, Cunningham JK, Allebeck P, Arenovich T, Sajeev G, Remington G, et al. (2012): Methamphetamine Use and Schizophrenia: A Population-Based Cohort Study in California. Am JPsychiat. 169:389–396. [DOI] [PubMed] [Google Scholar]
- 52.Compton MT, Kelley ME, Ramsay CE, Pringle M, Goulding SM, Esterberg ML, et al. (2009): Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 166:1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefanis NC, Dragovic M, Power BD, Jablensky A, Castle D, Morgan VA (2013): Age at initiation of cannabis use predicts age at onset of psychosis: the 7- to 8-year trend. Schizophrenia bulletin. 39:251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


