Abstract
Study Objectives
We assessed whether the synchrony between brain regions, analyzed using electroencephalography (EEG) signals recorded during sleep, is altered in subjects with post-traumatic stress disorder (PTSD) and whether the results are reproducible across consecutive nights and subpopulations of the study.
Methods
A total of 78 combat-exposed veteran men with (n = 31) and without (n = 47) PTSD completed two consecutive laboratory nights of high-density EEG recordings. We computed a measure of synchrony for each EEG channel-pair across three sleep stages (rapid eye movement [REM] and non-REM stages 2 and 3) and six frequency bands. We examined the median synchrony in 9 region-of-interest (ROI) pairs consisting of 6 bilateral brain regions (left and right frontal, central, and parietal regions) for 10 frequency-band and sleep-stage combinations. To assess reproducibility, we used the first 47 consecutive subjects (18 with PTSD) for initial discovery and the remaining 31 subjects (13 with PTSD) for replication.
Results
In the discovery analysis, five alpha-band synchrony pairs during non-REM sleep were consistently larger in PTSD subjects compared with controls (effect sizes ranging from 0.52 to 1.44) across consecutive nights: two between the left-frontal and left-parietal ROIs, one between the left-central and left-parietal ROIs, and two across central and parietal bilateral ROIs. These trends were preserved in the replication set.
Conclusion
PTSD subjects showed increased alpha-band synchrony during non-REM sleep in the left frontoparietal, left centro-parietal, and inter-parietal brain regions. Importantly, these trends were reproducible across consecutive nights and subpopulations. Thus, these alterations in alpha synchrony may be discriminatory of PTSD.
Keywords: post-traumatic stress disorder, sleep electroencephalography, phase synchronization, reproducibility of results
Statement of Significance.
Sleep disturbances are a hallmark of post-traumatic stress disorder (PTSD). However, previous research to identify objective markers of sleep disturbances, which could potentially help guide sleep-focused clinical interventions, have yielded inconsistent results. To address this issue, the authors compared the synchrony of activity between different brain regions in subjects with and without PTSD to identify reliable and reproducible differences. They found increased synchrony in PTSD subjects during dreamless sleep between the frontal and posterior brain regions. Importantly, these trends were reproducible across consecutive nights and subpopulations of the study.
Post-traumatic stress disorder (PTSD) is a debilitating psychiatric disorder triggered by one or more traumatic events. Sleep disturbances, which may be manifested as nightmares, flashbacks, and heightened arousal, are a hallmark symptom of PTSD [1]. Hence, identifying objective markers specific to sleep disturbances in PTSD subjects could have important clinical implications, including the possibility of providing a prognostic indicator of resilience to trauma and of developing interventions that allow vulnerable individuals to become resilient [2]. Motivated by such potential implications, a handful of single-night polysomnography (PSG) studies have analyzed the spectral power of electroencephalography (EEG) signals in individuals with and without PTSD during sleep, in an attempt to identify discriminatory brain activities [2–4]. Although these studies suggest the presence of detectable differences in certain EEG features between PTSD and non-PTSD subjects, the nature and magnitude of the differences reported to date are inconsistent across studies. For example, an early study showed that high-frequency EEG power, previously implicated in hyperarousal in insomnia [5], is elevated in PTSD subjects relative to controls and positively correlated with subjective hyperarousal [3]. However, a subsequent study controlled for PTSD co-morbidities did not reveal any elevation of high-frequency EEG power in PTSD subjects [4], illustrating the challenge of identifying brain-activity changes that are specific to individuals with PTSD. Motivated similarly, but with the intent to find reproducible differences, we recently identified reduced low-frequency EEG activity and increased high-frequency EEG activity in subjects with PTSD compared with those without PTSD, as potential indicators of heightened central arousal during sleep [6].
Another prominent aspect of brain function that may be altered in PTSD subjects during sleep is the spatiotemporal connectivity in electrical brain signals (i.e. the synchrony in brain activity between different brain regions), as has been suggested in other neurological disorders [7, 8]. Consistent with this conjecture, a recent PSG study [9]−the only one to date focused on EEG synchrony in PTSD subjects during sleep−reported alterations during two sleep stages: wake after sleep onset and non-rapid eye movement (NREM) sleep. In this study, the authors computed the synchrony values for pairwise connections between six EEG channels that covered the frontal, central, and occipital brain regions in subjects with (n = 38) and without PTSD (n = 38; age and sex matched) for a range of frequency bands and then assessed the ratios between these synchrony values. They identified 13 synchrony ratios that are significantly altered in subjects with PTSD compared with controls and showed that these are strongly correlated with diagnostic and symptom-severity measures of PTSD. Three other studies assessed EEG synchrony between brain regions during resting wakefulness in subjects with and without PTSD and reported significant alterations in the network obtained by EEG channel pairs spanning the frontal, central, and parietal regions [10–12].
None of these studies, however, have examined whether the identified changes in synchrony between EEG signals in different brain regions are consistent across consecutive nights of recordings or are reproducible in an independent subpopulation of the study data. This may partly explain why previous findings have been inconsistent across studies. Here we tested the hypothesis that EEG synchrony is altered in PTSD subjects during sleep with a focus on addressing three research questions: whether (1) synchrony is altered within and between the two brain hemispheres, (2) alterations occur during NREM and REM sleep, and (3) the findings are reproducible across consecutive nights and different subpopulations of a study. To this end, we collected and analyzed 64-channel high-density EEG (hd-EEG) recordings from 78 combat-exposed veteran men (31 with PTSD) during two consecutive nights. Using the first 47 consecutive subjects (18 with PTSD), we initially evaluated a measure of synchrony [13] between six brain regions (within and between hemispheres for frontal, central, and parietal regions covered by 38 EEG channels) for two consecutive nights, during three sleep stages (NREM stages 2 [N2] and 3 [N3], and REM), at a range of frequency bands (0.5–32 Hz, spanning slow oscillations under 1 Hz involved in memory processing [14] to beta-band, high-frequency oscillations implicated in central arousal [15]). Finally, we assessed the reproducibility of the findings in the remaining 31 subjects (13 with PTSD).
Materials and Methods
Study data
We recruited subjects from a population of combat-exposed veterans. Subjects were excluded if they were under any medications known to affect sleep or wakefulness within 2 weeks of study enrollment, or had a current diagnosis of severe depression, psychotic or bipolar disorder, substance or alcohol abuse within the past 3 months, a significant or unstable acute or chronic medical condition, current post-concussive symptoms or rehabilitation treatment for traumatic brain injury, or a current sleep disorder other than insomnia or nightmares. We assessed sleep quality via the Pittsburgh Sleep Quality Index [16] and the Insomnia Severity Index (ISI) [17]; mood, anxiety, psychosis, and substance use disorders via the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders IV Axis I Disorders [18]; and depression via the self-reported Patient Health Questionnaire-9 [19]. We assessed the presence and severity of PTSD via the Clinician Administered PTSD Scale [20] (CAPS) and the presence of sleep disorders via a structured clinical interview developed at the University of Pittsburgh [21]. All subjects provided written informed consent in accordance with the protocol approved by the University of Pittsburgh Institutional Review Board (Pittsburgh, PA) and the U.S. Army Medical Research and Development Command Human Research Protection Office (Ft. Detrick, MD).
To assess habitual sleep, we asked the subjects to complete a sleep diary for 10 consecutive days, immediately before the commencement of the study. To minimize the effects of caffeine on sleep, we instructed the subjects to limit their caffeine intake to no more than 2 cups of coffee per day (or the equivalent) in a 2-week period prior to the laboratory phase of the study. In addition, because our study involved combat veterans, a majority of whom consume alcohol, we did not exclude subjects who may have had a past history (prior to at least 3 months before the study) of alcohol use disorder (AUD). However, we instructed the subjects not to ingest more than 2 alcoholic drinks per day (28 drinks) in a 2-week period prior to the study. To ensure that the subjects strictly followed our guidelines for caffeine and alcohol consumption, we asked them to complete a 2-week medication history questionnaire at the commencement of the laboratory study.
All subjects spent two consecutive nights and days in the University of Pittsburgh Medical Center’s sleep laboratory. On night 1, they arrived at 08:00 pm and were fitted with a PSG system, which consisted of a 64-channel hd-EEG montage (HydroCel Geodesic Sensor Net [without sponge inserts] Electrical Geodesics Inc., Eugene, OR) and bipolar electrodes for submentalis electromyogram signals. The montage included two reference channels for electrooculogram signals and one unused common reference channel. Thus, the montage consisted of 61 EEG-specific channels. To improve comfort and alleviate pressure from the sensor net cap, we provided the subjects with gauze-like padding (Spandage Tubular Elastic Retainer Net, Medi-Tech International Corp., Brooklyn, NY). The subjects slept undisturbed from 11:00 pm until 07:00 am, while the PSG system collected EEG recordings throughout the entire night of sleep (night 1). On the next day morning (day 1), we removed the PSG system and asked the subjects to perform multiple tests to assess daytime alertness and cognitive functions. At 09.00 pm, we refitted the subjects with the PSG system and repeated the same procedures on night 2 and day 2 until their discharge at 08:00 pm on the second day.
We evaluated daytime cognitive performance on days 1 and 2 by measuring reaction time (RT) on the psychomotor vigilance task (PVT) [22], which assesses alertness, and the RT of correct trials on the N-back task (0-back, 1-back, and 2-back) [23], which assesses working memory. Subjects performed six PVT sessions at 2-h intervals and three N-back memory task sessions at 4-h intervals on a dedicated personal computer. For the N-back task, subjects were instructed to monitor a series of squares presented at different locations on a computer screen and press the number “2” key on the keyboard with their right index finger on trials in which the square was displayed in the position presented “N” trials previously (N = 0, 1, or 2). For all other trials, they were instructed to press the number “3” key. Following these instructions, subjects were presented with 30-s blocks of 0-back, 1-back, and 2-back stimuli in a random order. Between blocks, a fixation cross was displayed on the screen during 15-s rest periods.
The study population consisted of 85 combat-exposed veterans (78 men and 7 women) ranging in age from 18 to 50 years. Of these subjects, 37 (31 men and 6 women) met the diagnostic criteria for PTSD. Because women respond differently to PTSD stressors [24] and there was only one woman among the 48 non-PTSD subjects, we restricted our analyses to the 78 men. We split this sample into a “discovery” set comprising the first 47 consecutive subjects of the study (18 with PTSD), from whom we obtained our initial findings, and a “replication” set comprising the remaining 31 subjects (13 with PTSD) for assessing the reproducibility of the initial findings. Table 1 shows the demographics of the 78 subjects in the study. For the sleep architecture parameters of this population, we refer the reader to the recent study by Wang et al. [6] (see Table 2 in that paper), which used the same dataset as the one used in this study.
Table 1.
Clinical characteristics and sleep-diary variables for the 78 combat-exposed veteran men
| Variable | PTSD (n = 31) | Non-PTSD (n = 47) | Group comparison |
|---|---|---|---|
| Mean (SD) | Mean (SD) | P-value* | |
| Age (y) | 31.3 (4.7) | 32.8 (6.2) | 0.358 |
| PSQI | 8.9 (2.8) | 4.1 (2.4) | <0.001 |
| ISI | 14.2 (4.8) | 3.8 (4.2) | <0.001 |
| PHQ-9 | 5.8 (2.6) | 1.4 (2.5) | <0.001 |
| CAPS | 51.4 (16.8) | 8.6 (7.9) | <0.001 |
| Hyperarousal | 19.0 (7.1) | 3.3 (4.0) | <0.001 |
| Intrusion | 10.7 (5.8) | 0.6 (1.8) | <0.001 |
| Avoidance | 16.9 (8.8) | 1.7 (3.5) | <0.001 |
| Sleep diary† | |||
| Time in bed (min) | 453.0 (100.6) | 465.0 (55.3) | 0.580 |
| Total sleep time (min) | 414.3 (77.0) | 444.1 (52.5) | 0.035 |
| Sleep efficiency (%) | 92.8 (9.5) | 95.6 (3.4) | 0.004 |
| Sleep latency (min) | 27.8 (17.1) | 10.0 (5.9) | <0.001 |
| WASO (min) | 10.9 (18.2) | 4.6 (5.5) | 0.051 |
Bold values represent p < 0.05. CAPS = Clinician Administered PTSD Scale; ISI = Insomnia Severity Index; PHQ-9 = Patient Health Questionnaire-9; PSQI = Pittsburgh Sleep Quality Index; WASO = wakefulness after sleep onset.
*Wilcoxon rank-sum test.
†PTSD, n = 30.
PSG recordings and preprocessing
We recorded hd-EEG data referenced to the linked mastoids at a sampling rate of 250 Hz. We visually scored sleep stages in 30-s epochs according to the criteria of the American Academy of Sleep Medicine [25]. We filtered the EEG data to eliminate noise while preserving the signals within the bandwidth of 0.5–50.0 Hz. Subsequently, we segmented the EEG data into 5-s epochs, and used previously validated algorithms to reject muscle-movement and ocular artifacts [26–28]. We performed these preprocessing steps via custom scripts written in MATLAB (The MathWorks Inc., Natick, MA).
EEG synchrony
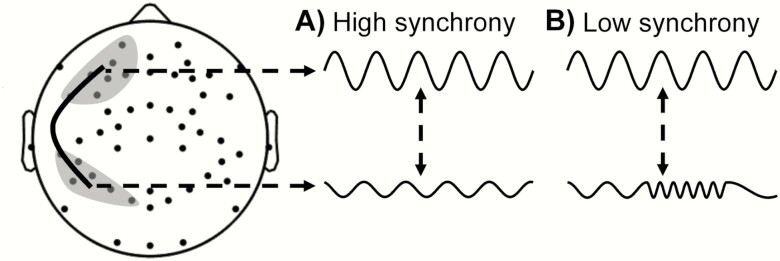
To assess whether synchrony between electrical signals recorded from different pairs of EEG channels during sleep differs between subjects with and without PTSD, we used the phase synchrony metric, which is defined as the absolute value of the average phase difference between two time-series signals over a given time interval. This measure is typically normalized to be between 0 (no synchrony) and 1 (complete synchrony). Figure 1 shows a schematic to illustrate the concept of phase synchrony between two signals. In A, the signals, each from a different region on the scalp, are otherwise identical except that one is a scaled and phase-shifted version of the other. In this case, because the phase difference is constant over time, the two signals are deemed to be completely synchronized (attaining the maximum value of 1). In B, the upper signal is the same as in A, but the lower signal shows a time-dependent variation in frequency, which alters the phase difference between the two signals over time. In this case, the absolute value of the average phase difference (computed by averaging the phase leads and lags over a given time interval) is smaller in B than in A (attaining a value between 0 and 1). Phase synchrony attains a minimum value of 0 (no synchrony) when the phase difference varies randomly over time.
Figure 1.
Two scenarios depicting phase synchrony between a pair of EEG signals from two brain regions (shaded gray regions connected via a solid black curve). (A) High-synchrony scenario and (B) low-synchrony scenario.
To compute the phase synchrony between a pair of EEG channels for each sleep stage, we used a multitaper approach [29] to estimate the cross-spectra and computed a measure of synchrony, the weighted phase lag index (WPLI) [13], by taking a weighted average of the signs of the imaginary components of the cross-spectra across artifact-free 5-s epochs spanning that sleep stage. The WPLI metric is robust to noise artifact often observed in real-world signals, which can lead to errors in the estimation of phase synchrony [13]. Using this method, we estimated the phase synchrony for all 1,830 (= 61 × 60/2) pairs of signals among the 61 EEG channels for each of the 5 sleep stages (Wake, NREM stage 1 [N1], N2, N3, and REM). Then, we computed the mean WPLI value in six frequency bands (slow oscillations [0.5–1.0 Hz], delta [1–4 Hz], theta [4–8 Hz], alpha [8–12 Hz], sigma [12–16 Hz], and beta [16–32 Hz]) for each EEG channel pair and sleep stage. This resulted in a total of 54,900 (= 1,830 × 6 × 5) combinations of WPLI values for the 1,830 EEG channel pairs, six frequency bands, and five sleep stages for each subject.
WPLI analysis
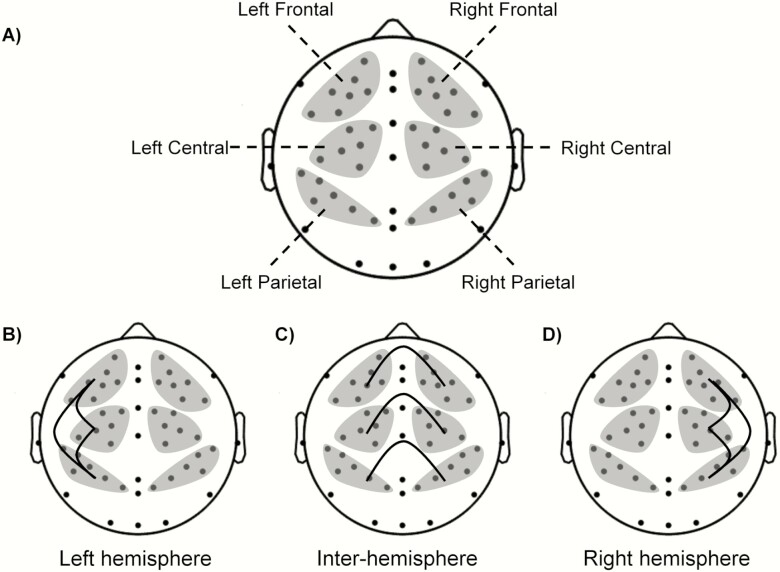
Based on the findings from previous studies that found alterations in synchrony between the frontal, central, and parietal brain regions and to increase the likelihood of identifying true associations between phase synchrony and the PTSD class, we limited the number of comparisons by combining the EEG channels into six regions of interest (ROIs; Figure 2A) and restricting our analysis to three sleep stages (N2, N3, and REM).
Figure 2.
(A) Topographical map showing EEG electrodes covering the frontal, central, and parietal regions of interest (ROIs; shaded gray regions). Overall, we investigated pairwise phase synchrony between six ROIs. (B) The three synchrony pairs among the three left-hemisphere ROIs, (C) six inter-hemispheric ROIs, and (D) three right-hemisphere ROIs.
Given the sparse literature on synchrony in subjects with PTSD during sleep, we selected frequency bands in which EEG activity during sleep is related to known neurophysiological mechanisms. Specifically, alpha and beta activity are related to arousals [3], sigma activity during N2 sleep is related to sleep spindles [30], slow oscillations and delta activity during N3 sleep are related to sleep depth [31], and theta activity during REM sleep is related to emotional memory processing [32]. Hence, we examined the WPLI for the following 10 frequency-band and sleep-stage combinations: the alpha, sigma, and beta bands (3 frequency bands) during N2 sleep; slow oscillations, delta, alpha, and beta bands (4 frequency bands) during N3 sleep; and the theta, alpha, and beta bands (3 frequency bands) during REM sleep. For each of the 10 combinations, we evaluated the synchrony obtained from 9 groups of EEG channel pairs between the 6 ROIs (within the 3 ROIs in each hemisphere and between the 6 ROIs across the two hemispheres; Figure 2, B–D). For each group, we computed the median WPLI over the EEG channel pairs connecting two given ROIs. For example, to compute the alpha synchrony during N2 sleep between the left-frontal and left-central ROIs, which contain 7 and 6 EEG channels, respectively (Figure 2A), we computed the median of the 42 (= 7 × 6) alpha WPLI values during N2 sleep over the EEG channel pairs between these two ROIs. Overall, for the 10 sleep-stage and frequency-band combinations, we analyzed 90 synchrony pairs (see Supplementary Tables S7 and S8 for the results of 180 ROI pairs that were not included in this analysis).
Statistical analysis
To assess group differences for each of the 90 synchrony pairs between subjects with and without PTSD, we computed two statistical measures: the Mann–Whitney statistic (U) of the Wilcoxon rank-sum test, which assesses the difference in group medians, and a robust version of Cohen’s d, a measure of effect size computed by replacing the population mean with the 20% trimmed mean and the population standard deviation with the square root of the 20% winsorized variance [33]. To correct for multiple comparisons, we created 5,000 permuted datasets by randomly shuffling the label of each subject in the two groups and, for each such dataset, recorded the maximum absolute value of U across the 90 pair-wise comparisons to generate a probability distribution for the maximum U obtained by random chance. We then computed the corrected p-value for each synchrony pair as the fraction of the 5,000 permutations that exceeded the true absolute value of U. If this p-value was below 0.05, we deemed that particular synchrony pair to have passed the multiple-comparison test [34].
Evaluation of reproducibility
We assessed the reproducibility of the findings by partitioning the entire sample into two subsamples−one for initial “discovery” and another for “replication”. Because no single indicator sufficiently describes whether a replication is a success [35], we evaluated reproducibility using three different criteria by determining whether (1) the “replication” analysis showed a statistically significant effect (Wilcoxon rank-sum test, p < 0.05) in the same direction as the initial finding, (2) the effect size of the “replication” analysis fell within the 95% confidence interval (CI) of the initial finding, and (3) the analysis combining the “discovery” and “replication” data showed a statistically significant effect, in terms of both the rank-sum test (p < 0.05) and the 95% CIs of the effect size for the “combined” analysis excluding zero [35, 36]. We used a bootstrap approach with 5,000 replicates to determine the 95% CI of the effect sizes [37].
Assessing the effect of confounders on synchrony pairs
The findings of this study may be confounded by variations in the subjects’ age range, history of AUD, or sleep architecture parameters [6] between the two groups. To assess this possibility, we separately analyzed each of these factors. First, to assess the effect of age, for each synchrony pair, we computed Pearson’s correlation coefficient using values from subjects in the “combined” set for each night, one night at a time. We deemed a synchrony pair to be correlated with age if the correlation coefficients for each of the two nights were significant (p < 0.05). For these synchrony pairs, we computed their difference between subjects with and without PTSD (effect size and rank-sum p-value) using age-corrected and uncorrected values to assess whether age confounded our findings.
For assessing the effect of past AUD on the discriminative synchrony pairs, we performed a two-factor analysis of variance, with PTSD status as one factor and past history of AUD as the second factor in the “combined” set. Similarly, we assessed the effect of percent time spent in N3 sleep (which was ~3% lower in subjects with PTSD compared with those without PTSD; Wang et al. [6], Table 2) by using it as a continuous factor in a generalized linear regression framework with the discriminatory synchrony pair during N3 sleep as the other factor. We ruled out the possibility that the difference in N3 sleep duration confounded our findings if the estimated coefficient for a given synchrony pair remained significant (p < 0.05) for each night after accounting for the percentage of N3 sleep.
Association of the discriminative synchrony pairs
To identify associations between our findings and clinical scores, we analyzed the correlation between the synchrony values of electrode pairs that were discriminative of PTSD and subjective measures of PTSD, such as scores on the CAPS and the ISI. Specifically, we computed Spearman’s correlation coefficient (ρ) between the synchrony pair and the CAPS or ISI score using values from the “combined” set for each night of recording.
To assess the association between the discriminatory synchrony pairs and daytime measures of cognitive performance, we first computed the mean RT for each PVT session and then averaged the values across all six sessions to obtain the average mean RT for day 1. Similarly, we computed the mean RT for correct responses on the 0-back, 1-back, and 2-back working memory tasks for each session and averaged the values over all three sessions to obtain the average mean RT for correct responses on day 1. We repeated these steps to obtain the data for day 2 (see Supplementary Table S4 for the mean values of these scores for the two groups on each of the two days). We then computed ρ between night 1 values of each discriminatory synchrony pair and day 1 values of PVT mean RT and the 2-back mean RT. We chose the 2-back task for this purpose because it is the most difficult among the N-back tasks assessed in this study. Similarly, we computed the correlation between night 2 values of the synchrony pairs and day 2 values of the daytime cognitive performance measures.
Results
Discovery analysis
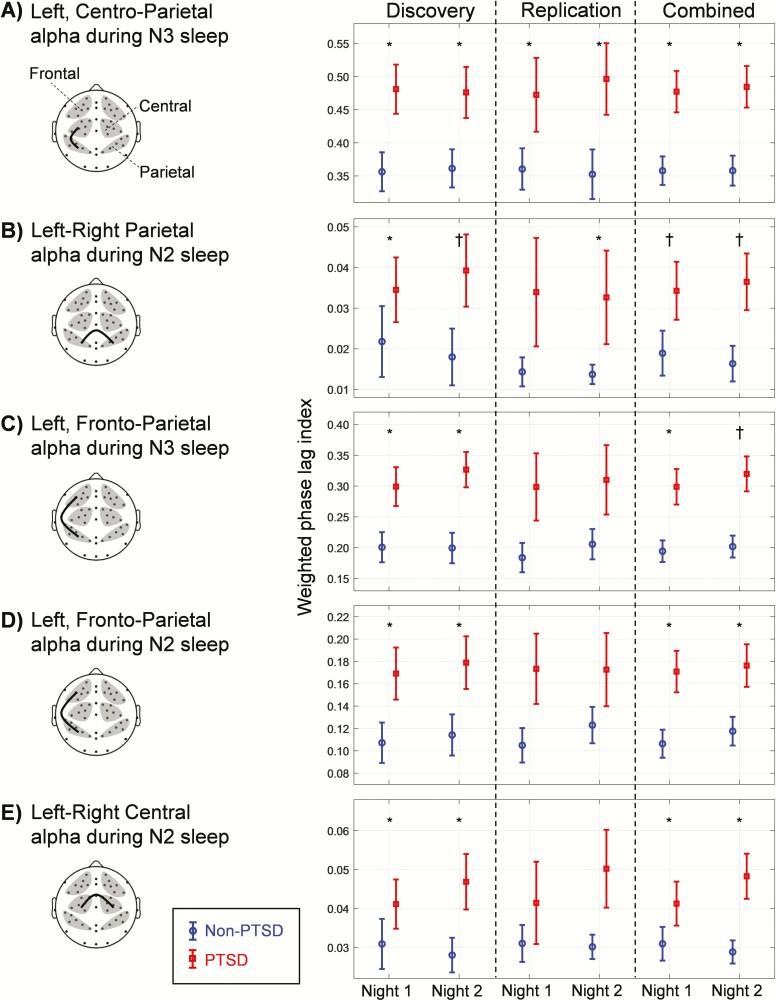
Among the 90 synchrony pairs, we identified 5 pairs where PTSD subjects showed significantly greater synchrony (Wilcoxon rank-sum test, p < 0.05) compared with non-PTSD subjects (Figure 3, Discovery; plots show the mean and standard error of the mean for each group) on both nights (see Supplementary Table S1 for a summary of the 85 non-significant pairs). All five pairs were from the alpha band during NREM sleep. Of these, two were between the left-frontal and left-parietal ROIs, one between the left-central and left-parietal ROIs, and two between corresponding ROIs (central or parietal) across hemispheres. Of these, the left-right parietal alpha synchrony during N2 sleep also passed the multiple-comparison test (Figure 3B, Discovery; p = 0.015) on night 2 but not on night 1 (p = 0.063).
Figure 3.
Group differences for the five WPLI synchrony features across the three analysis sets (discovery, replication, and combined). All five WPLI features were from the alpha band (8–12 Hz) during non-rapid-eye-movement (NREM) sleep stages 2 (N2) and 3 (N3) in the (A) left, centro-parietal, (B) left-right parietal, (C and D) left, frontoparietal, and (E) left-right central regions of interest (ROI) pairs. The discovery, replication, and combined sets consisted of 18, 13, and 31 PTSD subjects and 29, 18, and 47 non-PTSD subjects, respectively. The plotted values are the group means of the WPLIs based on ROI pairs. Error bars indicate standard errors of the mean. Asterisks indicate significant group differences at p < 0.05. Daggers indicate significant group differences at p < 0.05 after correcting for multiple comparisons.
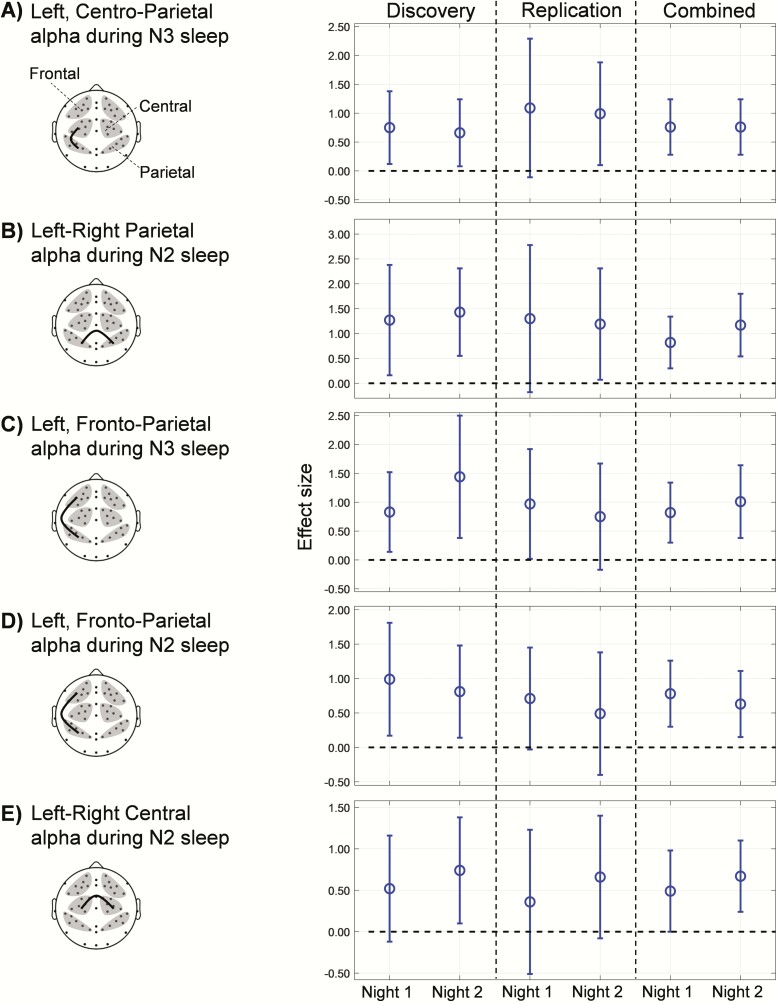
The five synchrony pairs also showed moderately large effect sizes (0.52–1.44) on each of the two nights [Figure 4, Discovery; plots show robust Cohen’s d and the 95% CIs]. Except for the CI for the left-right central alpha effect size during N2 sleep (Figure 4E, Discovery) on night 1, none of the CIs included zero in the “discovery” analysis, indicating that, for four out of the five pairs, synchrony was significantly greater in PTSD subjects compared with non-PTSD subjects on both nights.
Figure 4.
Comparisons of effect sizes for the five WPLI synchrony features across the three analysis sets (discovery, replication, and combined). Open circles denote effect sizes (Cohen’s robust d; positive values indicate that the WPLI is larger in PTSD subjects than in non-PTSD subjects). Error bars indicate the 95% CIs of the effect sizes; the dashed lines indicate an effect size of zero. The effect sizes of the replication set fell within the CIs of the discovery set for all five features. The lower bound of the 95% CIs of the effect sizes for the combined set is at or above the zero dashed line, indicating that the observed effects are significant.
Replication analysis
In the “replication” set, the trends across the five synchrony pairs remained similar to those of the corresponding “discovery” set (Figure 3, Replication). The difference in synchrony for the left, centro-parietal ROI pair during N3 sleep was statistically significant and in the same direction as in the “discovery” set (Criterion 1, Evaluation of Reproducibility, Methods Section) on both nights (Figure 3A, Replication: Wilcoxon rank-sum test, p = 0.039 for both nights). The difference for the left-right parietal ROI pair during N2 sleep satisfied Criterion 1 on night 2 (Figure 3B, Replication: p = 0.043), but not on night 1 (p = 0.075). The differences between the other three pairs did not meet Criterion 1 on either night (Figure 3, C–E, Replication).
The effect size values of the “replication” set (Figure 4, Replication, open circles) fell well within the 95% CIs of the “discovery” set (Criterion 2) for each of the five pairs on both nights.
For the “combined” set (n = 78 subjects [31 with PTSD]), the differences in synchrony for the five pairs were statistically significant (Criterion 3) for each of the two nights (Figure 3, Combined: Wilcoxon rank-sum test, p < 0.05 for all pairs), where the difference for the left-right parietal ROI pair during N2 sleep also passed the multiple-comparison test (Figure 3B, Combined: night 1, p = 0.016 and night 2, p = 0.004). The difference for the left, frontoparietal ROI pair during N3 sleep also passed the multiple-comparison test on night 2 (Figure 3C, Combined: p = 0.045) but not on night 1 (p = 0.190). For four of the five pairs in the “combined” set, the lower bound of the CI of the effect size exceeded zero (Figure 4, A–D, Combined). Similar to the “discovery” set, the lower bound of the CI of the effect size for the left-right central ROI pair during N2 sleep crossed the zero value on night 1 (Figure 4E; Combined).
Overall, we found that the results satisfied two of the three criteria for four of the five synchrony pairs and, hence, showed good reproducibility. Supplementary Table S2 shows the numerical values for the results shown in Figures 3 and 4.
Assessing the effect of confounders on the identified synchrony pairs
None of the five discriminative pairs were correlated with age. In fact, only 2 of the 90 synchrony pairs were correlated with age, although neither pair was discriminative of PTSD, either before or after age correction.
In our study population, 37% of the 78 subjects (61% of the 31 with PTSD) had a past history of AUD. Nonetheless, a two-factor analysis of variance carried out to account for this potential confounding factor revealed that AUD history did not significantly contribute to, or interact with, the PTSD status for any of the five synchrony pairs for either of the two nights (p-values for the contribution of AUD ranged from 0.37 to 0.82 and those for the interaction between PTSD and AUD ranged from 0.14 to 0.97 across the five pairs in both nights).
Increased alpha synchrony in the left, frontoparietal and left, centro-parietal ROI pairs during N3 sleep were unaffected by the reduced percentage of N3 sleep (~3%; Wang et al. [6], Table 2) observed in subjects with PTSD across both nights (p-values of the regression coefficients for each of the two synchrony pairs were less than 0.01 after accounting for the percentage of N3 sleep across both nights).
Association between synchrony and clinical scores
Only the synchrony between the left-right parietal ROI pair was significantly correlated with the CAPS score (ρ = 0.36 for both night 1 and night 2) and the ISI score (ρ = 0.36 for night 1, ρ = 0.42 for night 2; Supplementary Table S3) across both nights. For the remaining synchrony pairs, the correlation with each score was positive but not significant.
Association between synchrony and daytime cognitive performance
Three of the five identified synchrony pairs showed significant positive correlations with the 2-back mean RT on correct responses (Supplementary Table S3): the alpha synchrony between the left, frontoparietal ROI pair during both N2 and N3 sleep (ρ: 0.25–0.30), and between the left-right central ROI pair during N2 sleep (ρ = 0.30 for night 1, ρ = 0.40 for night 2). Only the synchrony between the left, centro-parietal ROI pair during N3 sleep showed a significant positive correlation with the PVT mean RT (Supplementary Table S3; ρ = 0.30 for night 1, ρ = 0.31 for night 2). For the remaining synchrony pairs, the correlations with each score were positive but not significant.
Discussion
In this study, our primary objective was to assess whether synchrony in EEG signals during sleep differs between PTSD and non-PTSD subjects across regions within and between the two brain hemispheres. The results revealed that PTSD subjects had larger alpha synchrony during NREM sleep between the left, centro-parietal (Figure 4A), left-right parietal (Figure 4B), and the left, frontoparietal (Figure 4, C and D) ROI pairs compared with non-PTSD subjects. These findings were consistent across nights and the trends were reproduced in the “replication” set.
Increased alpha synchrony in PTSD subjects during NREM sleep
Several EEG and neuroimaging studies have revealed the importance of frontoparietal networks (which span the frontal, central, and parietal brain regions in both hemispheres) in memory consolidation processes during NREM sleep [38–40]. These processes are impaired in PTSD and other psychiatric disorders [14], suggesting that functional differences in frontoparietal networks between healthy subjects and subjects with these disorders may be related to impairments in memory processes in the latter. Furthermore, studies of alpha synchrony in frontoparietal networks indicate that this EEG feature is an indicator of vigilance. Whereas some sleep studies have shown that in healthy individuals, alpha synchrony decreases with increasing sleep depth (decreasing vigilance) [41, 42], studies on daytime cognitive performance have found that alpha synchrony increases in task-relevant regions of the brain only while they perform the task [43–47]. Interestingly, the cognitive functions examined in the latter studies are impaired in PTSD and other psychiatric disorders, such as mild cognitive impairment [48] and depression [49]. Together, these findings suggest that the increased alpha synchrony we observed in PTSD subjects during NREM sleep is associated with disturbed sleep (due to hypervigilance) and may influence cognitive memory processes.
If this conjecture is correct, we would expect frontoparietal alpha synchrony during NREM sleep to be correlated with PTSD severity and daytime performance on cognitive tasks. As clinical scores and data on daytime cognitive performance were available for our study population, we analyzed correlations between the five identified alpha-synchrony pairs and PTSD clinical scores as well as daytime performance measures of alertness and working memory. Consistent with our expectations, we found that all five alpha synchrony values were positively correlated with the CAPS and ISI scores, with alpha synchrony in the left-right parietal ROI pair achieving significance across both nights (Supplementary Table S3). Additionally, we found positive correlations between alpha synchrony in the left, frontoparietal ROI pair during NREM sleep and the mean RT of correct responses in the 2-back working memory task and between alpha synchrony in the left, centro-parietal ROI pair and the PVT mean RT (Supplementary Table S3). These results indicate that, in our study population, increased alpha synchrony during NREM sleep is associated with PTSD severity and cognitive performance deficits. Prospective investigations of EEG synchrony comparing subjects with and without PTSD during sleep, followed by an assessment of daytime cognitive performance, should enable us to further corroborate these findings.
Comparisons with other studies on EEG synchrony in subjects with PTSD
A handful of studies have reported alterations in EEG synchrony between different brain regions in PTSD subjects compared with controls [9–12]. Of these, three found such alterations during resting wakefulness. Lee et al. [10] computed synchrony between 62 EEG channels pairwise in the delta through gamma bands (32–50 Hz) for each subject in a non-PTSD group and a PTSD group that included subjects on medication. Then, for each group, they averaged the pairwise synchrony values across subjects and, setting the overall mean synchrony of the group plus one standard deviation as the threshold, obtained binary synchrony matrices for each frequency band. From this analysis, the authors found that beta and gamma synchrony in the frontal and central regions across both hemispheres are reduced in the PTSD group relative to the non-PTSD group. In contrast, we did not find any differences in beta synchrony in any sleep stage between the two groups in our subject population, where none of the subjects were being medicated. In a similar study, Kim et al. [12] compared PTSD subjects on medication against controls to identify differences in patterns of pairwise directional synchrony (a frequency-independent measure) among 16 EEG channels during resting wakefulness. They found that compared with controls, PTSD subjects showed increased synchrony between pairs of electrodes spanning the frontal, parietal, and temporal regions of the left hemisphere, and decreased synchrony between those spanning the frontal, parietal, and occipital regions of the right hemisphere. We found similar increases in synchrony in the alpha band among regions in the left hemisphere, but no reductions in synchrony among regions in the right hemisphere. The differences between our findings and those of Lee et al. [10] on beta-band synchrony or those of Kim et al. [12] on the pattern of within-hemispheric changes in synchrony could be attributed to any of a number of methodological differences, including the subject population (PTSD subjects were on medication in both studies, but not in our study), the synchrony metrics used, and the state of the subject (sleep vs. wakefulness).
Imperatori et al. [11], in another study carried out during resting wakefulness, examined synchrony in the frequency bands between 0.5 and 60 Hz using a metric similar to the WPLI (lagged coherence) in PTSD subjects who were free of co-morbidities and medication. They found that compared with healthy controls, subjects with PTSD showed increased alpha synchrony between two electrodes, one placed at the parietal-midline between the two hemispheres and the other placed in the right-parietal region. However, they did not find evidence for increased synchrony between the electrodes placed on the left and right parietal regions. In our study, which involved a similar population of subjects, PTSD subjects showed increased alpha synchrony between corresponding parietal ROIs in the left and right hemispheres during N2 sleep compared with controls (Figure 3B). The difference between these two findings may be attributed to the fact that they examined the subjects during resting wakefulness.
Finally, in a recent sleep-focused study [9] of subjects with (n = 38) and without PTSD (n = 38; matched for age and sex), Modarres et al. reported a novel set of synchrony-derived markers diagnostic of, and correlated with the severity of, PTSD. Specifically, they first calculated the synchrony between each of 15 electrode pairs among six EEG channels covering the frontal, central, and occipital brain regions. They then computed the ratio of synchrony between one pair of electrodes to that between another pair. However, the physical meaning of a ratio of pairwise synchrony values is unclear. For example, the authors found that the ratio in the alpha band during N2 sleep, of the synchrony between the right-occipital and left-central electrodes (10.8–11.8 Hz) to the synchrony between the right-occipital and left-frontal electrodes (10.2–11.2 Hz), was significantly larger in PTSD subjects compared with controls. This information, however, does not allow one to determine whether the difference arose from an increase in the pairwise synchrony of the numerator, or a reduction in the pairwise synchrony of the denominator in PTSD subjects compared with the corresponding pairwise synchrony values in controls. This ambiguity prevents a direct comparison with our findings.
It is worth noting that none of the studies discussed above assessed whether their results were reproducible across multiple recordings from the same subjects or across an independent subject population. While the realities of time and cost impose constraints in study design, it would be important to ascertain the reproducibility of their results under these conditions.
Synchrony provides information in addition to electrical brain activity
In the present study, alpha-band synchrony between the frontal, central, and parietal brain regions in PTSD subjects increased relative to that in non-PTSD subjects. In contrast, brain activity, as measured by the alpha-band spectral power in these regions, did not differ between the two groups (Supplementary Table S5). Thus, phase synchrony potentially provides information about the interactions between two ROIs that cannot be identified by studying brain-activity patterns in each of the ROIs independently. To the extent that neurological disorders generally involve altered interactions between multiple brain regions and that some disorders share similar alterations in brain activity, our findings deserve further investigation in EEG studies of such disorders.
Comparisons with other measures of synchrony
Various measures have been employed in previous studies to assess synchrony, including coherence (which Modaress et al. [9] used to derive the synchrony ratio) and lagged coherence [11]. Coherence consistently overestimates the synchrony between two EEG signals due to the volume-conduction effect, which results from deep-brain transmissions from a common source that instantaneously impact the electrical activity measured by all EEG electrodes [13]. The WPLI and lagged coherence metrics were developed to address this issue. Both metrics explicitly reject instantaneous phase components from a pair of EEG signals while estimating phase synchrony, thus reducing the volume-conduction effect. To assess how well coherence and lagged coherence would perform relative to the WPLI, we compared the three metrics based on the effect sizes of the five alpha-band synchrony pairs for the “combined” set (Supplementary Table S6). The results obtained using lagged coherence were similar to those obtained using WPLI, as might be expected given that both measures correct for the effects of volume conduction. In contrast, coherence, which overestimated the synchrony for both PTSD and non-PTSD subjects, consistently yielded low effect sizes for all pairs, except for the synchrony between the left and right parietal ROIs.
Limitations of our study
By design, our study used PTSD subjects who were free of co-morbid disorders and sleep-related medications. By comparing these subjects to those without PTSD, we were able to identify discriminatory synchrony markers. However, the same markers might be observed for disorders with symptoms that overlap with PTSD, such as insomnia, depression, and mild traumatic brain injury. Therefore, to test whether alpha-band synchrony during NREM sleep is a PTSD-specific marker, future studies could be conducted on diverse datasets of populations with disorders related to but absent of PTSD.
Another potential limitation of our study is that 37% of the 78 subjects (61% of the 31 with PTSD) in the “combined” set had a past history of AUD, which is known to cause alterations in EEG spectral power in the delta and beta bands that may persist even after several months of abstinence [50]. Whether EEG synchrony is altered in subjects with a past history of AUD compared with healthy controls remains to be investigated. Controlling for AUD in a population of Service members is difficult because a majority of them consume alcohol. Using a prior history of alcohol consumption as an exclusion criterion here would have greatly reduced our sample size and, in turn, our ability to draw any conclusions that could apply to Service members in general. Therefore, we decided not to exclude subjects with prior history of alcohol abuse, but instead advised them to limit their intake to 2 drinks per day for two weeks prior to the study. Two-factor analysis of variance on the identified markers, with PTSD status as one factor and past history of AUD as the second factor revealed that past history of AUD did not significantly contribute to, or interact with, the PTSD status for any of the five synchrony pairs in our study population for either of the two nights. In fact, the identified markers showed the same trends on subjects without previous AUD (n = 49 [12 with PTSD]), suggesting that it is unlikely that past AUD history affected the markers identified in our study.
Conclusion
In conclusion, we found an increase in alpha-band synchrony during NREM sleep in PTSD subjects in the left, frontoparietal, left, centro-parietal, and left-right parietal ROI pairs, in general agreement with a handful of existing reports of altered synchrony between the frontal and posterior brain regions in PTSD subjects. Importantly, these differences were preserved across two consecutive nights and mostly reproduced in a “replication” dataset, demonstrating the potential feasibility of identifying reliable markers of PTSD. Independent validation of our findings will be required to further demonstrate reproducibility and to verify whether these markers are specific to sleep disturbances in PTSD subjects. If successful, such markers could help guide the development of sleep-focused interventions to improve the condition of PTSD subjects.
Supplementary Material
Funding
This work was sponsored by U.S. Defense Health Program (grant No. W81XWH-14-2-0145) and managed by the U.S. Army Military Operational Medicine Program Area Directorate, Ft. Detrick, MD. The University of Pittsburgh Medical Center was partially supported by the Clinical and Translational Science Institute at the University of Pittsburgh (UL1 TR001857).
Conflict of interest statement. This was not an industry-supported study. The authors have indicated no financial conflicts of interest. The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army, the U.S. Department of Defense, or The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. This article has been approved for public release with unlimited distribution.
References
- 1. Harvey AG, et al. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23(3):377–407. [DOI] [PubMed] [Google Scholar]
- 2. Cowdin N, et al. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res. 2014;232(5):1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woodward SH, et al. PTSD-related hyperarousal assessed during sleep. Physiol Behav. 2000;70(1–2):197–203. [DOI] [PubMed] [Google Scholar]
- 4. Cohen DJ, et al. Quantitative electroencephalography during rapid eye movement (REM) and non-REM sleep in combat-exposed veterans with and without post-traumatic stress disorder. J Sleep Res. 2013;22(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Regestein QR, et al. Daytime alertness in patients with primary insomnia. Am J Psychiatry. 1993;150(10):1529–1534. [DOI] [PubMed] [Google Scholar]
- 6. Wang C, et al. An attempt to identify reproducible high-density EEG markers of PTSD during sleep. Sleep. 2020;43(1). doi: 10.1093/sleep/zsz207 [DOI] [PubMed] [Google Scholar]
- 7. Leistedt SJ, et al. Altered sleep brain functional connectivity in acutely depressed patients. Hum Brain Mapp. 2009;30(7):2207–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corsi-Cabrera M, et al. Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. Sleep. 2012;35(4):501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Modarres MH, et al. Strong correlation of novel sleep electroencephalography coherence markers with diagnosis and severity of posttraumatic stress disorder. Sci Rep. 2019;9(1):4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee SH, et al. Functional connectivity of resting state EEG and symptom severity in patients with post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:51–57. [DOI] [PubMed] [Google Scholar]
- 11. Imperatori C, et al. Aberrant EEG functional connectivity and EEG power spectra in resting state post-traumatic stress disorder: a sLORETA study. Biol Psychol. 2014;102:10–17. [DOI] [PubMed] [Google Scholar]
- 12. Kim J, et al. Hemispheric asymmetry in non-linear interdependence of EEG in post-traumatic stress disorder. Psychiatry Clin Neurosci. 2012;66(2):87–96. [DOI] [PubMed] [Google Scholar]
- 13. Vinck M, et al. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. 2011;55(4):1548–1565. [DOI] [PubMed] [Google Scholar]
- 14. Goerke M, et al. Sleep-dependent memory consolidation and its implications for psychiatry. J Neural Transm (Vienna). 2017;124(Suppl 1):163–178. [DOI] [PubMed] [Google Scholar]
- 15. Perlis ML, et al. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–117. [DOI] [PubMed] [Google Scholar]
- 16. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 17. Bastien CH, et al. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 18. First MB, Spitzer RL.. Structured Clinical Interview for DSM-IV AXIS I Disorders: SCID-I. New York, NY: Biometrics Research Department; 1997. [Google Scholar]
- 19. Löwe B, et al. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9). J Affect Disord. 2004;81(1):61–66. [DOI] [PubMed] [Google Scholar]
- 20. Blake DD, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. [DOI] [PubMed] [Google Scholar]
- 21. Buysse DJ, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khitrov MY, et al. PC-PVT: a platform for psychomotor vigilance task testing, analysis, and prediction. Behav Res Methods. 2014;46(1):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaeggi SM, et al. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18(4):394–412. [DOI] [PubMed] [Google Scholar]
- 24. Richards A, et al. Sex differences in objective measures of sleep in post-traumatic stress disorder and healthy control subjects. J Sleep Res. 2013;22(6):679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silber MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121–131. [PubMed] [Google Scholar]
- 26. Brunner DP, et al. Muscle artifacts in the sleep EEG: automated detection and effect on all-night EEG power spectra. J Sleep Res. 1996;5(3):155–164. [DOI] [PubMed] [Google Scholar]
- 27. Doman J, et al. Automating the sleep laboratory: implementation and validation of digital recording and analysis. Int J Biomed Comput. 1995;38(3):277–290. [DOI] [PubMed] [Google Scholar]
- 28. Liu J, et al. Effects of signal artefacts on electroencephalography spectral power during sleep: quantifying the effectiveness of automated artefact-rejection algorithms. J Sleep Res. 2018;27(1):98–102. [DOI] [PubMed] [Google Scholar]
- 29. Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70(9):1055–1096. [Google Scholar]
- 30. Fogel SM, et al. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35(5):1154–1165. [DOI] [PubMed] [Google Scholar]
- 31. Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36(1):677–695. [DOI] [PubMed] [Google Scholar]
- 32. Nishida M, et al. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19(5):1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Algina J, et al. An alternative to Cohen’s standardized mean difference effect size: a robust parameter and confidence interval in the two independent groups case. Psychol Methods. 2005;10(3):317–328. [DOI] [PubMed] [Google Scholar]
- 34. Maris E, et al. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. [DOI] [PubMed] [Google Scholar]
- 35. Stodden V, Leisch F, Peng RD.. Implementing Reproducible Research. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 36. Open Science Collaboration. Estimating the reproducibility of psychological science. Science. 2015;349(6251):aac4716. [DOI] [PubMed] [Google Scholar]
- 37. Wang C, et al. Identifying Electrophysiological Prodromes of Post-traumatic Stress Disorder: results from a pilot study. Front Psychiatry. 2017;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stickgold R, et al. Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 2005;28(8):408–415. [DOI] [PubMed] [Google Scholar]
- 39. Vahdat S, Fogel S, Benali H, Doyon J. Network-wide reorganization of procedural memory during NREM sleep revealed by fMRI. eLife. 2017;6:e24987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spoormaker VI, et al. Frontoparietal connectivity and hierarchical structure of the brain’s functional network during sleep. Front Neurol. 2012;3:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cantero JL, et al. Alpha EEG coherence in different brain states: an electrophysiological index of the arousal level in human subjects. Neurosci Lett. 1999;271(3):167–170. [DOI] [PubMed] [Google Scholar]
- 42. Nayak CS, et al. Phase synchronization analysis of natural wake and sleep states in healthy individuals using a novel ensemble phase synchronization measure. J Clin Neurophysiol. 2017;34(1):77–83. [DOI] [PubMed] [Google Scholar]
- 43. Palva JM, et al. Phase synchrony among neuronal oscillations in the human cortex. J Neurosci. 2005;25(15):3962–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hummel F, et al. Larger interregional synchrony is associated with greater behavioral success in a complex sensory integration task in humans. Cereb Cortex. 2005;15(5):670–678. [DOI] [PubMed] [Google Scholar]
- 45. Doesburg SM, et al. From local inhibition to long-range integration: a functional dissociation of alpha-band synchronization across cortical scales in visuospatial attention. Brain Res. 2009;1303:97–110. [DOI] [PubMed] [Google Scholar]
- 46. Freunberger R, et al. Dissociation between phase-locked and nonphase-locked alpha oscillations in a working memory task. Hum Brain Mapp. 2009;30(10):3417–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Freunberger R, et al. Alpha phase coupling reflects object recognition. Neuroimage. 2008;42(2):928–935. [DOI] [PubMed] [Google Scholar]
- 48. Babiloni C, et al. Fronto-parietal coupling of brain rhythms in mild cognitive impairment: a multicentric EEG study. Brain Res Bull. 2006;69(1):63–73. [DOI] [PubMed] [Google Scholar]
- 49. Yamada M, Kimura M, Mori T, Endo S. EEG power and coherence in presenile and senile depression. Characteristic findings related to differences between anxiety type and retardation type. Nihon Ika Daigaku Zasshi. 1995;62(2): 176–185. [DOI] [PubMed] [Google Scholar]
- 50. Colrain IM, et al. Alcohol and the sleeping brain. Handb Clin Neurol. 2014;125:415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.