Abstract
Background and Purpose
Detecting antibodies against muscle-specific tyrosine kinase (MuSK Abs) is essential for diagnosing myasthenia gravis (MG). We applied an in-house cell-based assay (CBA) to detect MuSK Abs.
Methods
A stable cell line was generated using a lentiviral vector, which allowed the expression of MuSK tagged with green fluorescent protein in human embryonic kidney 293 (HEK293) cells. Serum and anti-human IgG antibody conjugated with red fluorescence were added. The presence of MuSK Abs was determined based on the fluorescence intensity and their colocalization in fluorescence microscopy. Totals of 218 serum samples collected from 177 patients with MG, 31 with other neuromuscular diseases, and 10 healthy controls were analyzed. The CBA results were compared with those of a radioimmunoprecipitation assay (RIPA) and an enzyme-linked immunosorbent assay (ELISA).
Results
The MuSK-HEK293 cell line stably expressed MuSK protein. The CBA detected MuSK Abs in 34 (19.2%) of 177 samples obtained from patients with MG and in none of the participants having other neuromuscular diseases or in the healthy controls. The clinical characteristics of the patients with MuSK MG determined based on the CBA were strongly correlated with known clinical features of MuSK MG. There was an almost perfect agreement between the results of the CBA and those of the RIPA (Cohen's kappa=0.880, p<0.001) and ELISA (Cohen's kappa=0.982, p<0.001).
Conclusions
The results of the in-house CBA showed excellent agreement with both the RIPA and ELISA. Our in-house CBA can be considered a reliable method for detecting MuSK Abs.
Keywords: myasthenia gravis, muscle-specific tyrosine kinase, cell-based assay, diagnosis
INTRODUCTION
Muscle-specific tyrosine kinase (MuSK) is a transmembrane protein required for the differentiation and maintenance of the neuromuscular junction that plays an important role in the clustering of acetylcholine receptors (AChRs). Reportedly 40–70% of generalized myasthenia gravis (MG) patients without AChR antibodies (AChR Abs) have antibodies against MuSK (MuSK Abs). Diagnosing MuSK-Ab-positive MG (MuSK MG) is important since patients with MuSK Abs often exhibit adverse responses to pyridostigmine, are likely to benefit from plasma exchange, and are considered to be less responsive to thymectomy.1 Although patients with MuSK MG display some characteristic clinical features, including predominant bulbar involvement and muscle atrophy,2,3 a substantial proportion of patients with AChR-Ab-positive MG and double-seronegative MG (AChR-Ab negative and MuSK-Ab negative) also demonstrate bulbar weakness.4,5 In addition, it can be difficult to discriminate MuSK MG from AChR-Ab-positive MG based solely on clinical information.6,7 Therefore, detecting MuSK Abs is crucial for the proper diagnosis and management of MuSK MG.
The radioimmunoprecipitation assay (RIPA) is a widely used method for detecting autoantibodies in patients with MG. However, the radiation hazard associated with this assay means that it can only be performed in a small number of laboratories with trained personnel and an appropriate license. Antibodies can also be detected using cell-based assays (CBAs) that utilize living cells, thus mimicking the physiological microenvironment. CBAs have recently been shown to be more sensitive in detecting autoantibodies in MG than is the conventional RIPA. Up to 66% of patients with MG who were previously believed to be negative for AChR Abs were found to have antibodies against clustered AChR through the application of a CBA.8,9 Other studies have similarly demonstrated the presence of MuSK Abs through CBAs that were previously not detected in an RIPA.10,11 In short, a CBA provides a higher sensitivity for the diagnosis of MG without the radiation hazard associated with an RIPA. Therefore, an increasing number of patients who would previously have been regarded as seronegative are now being correctly diagnosed by using CBAs.
In the present study, we developed an in-house CBA and used it to evaluate the serostatus of MuSK Abs in patients with MG. We also tested MuSK Abs in patients with other neuromuscular diseases and in healthy volunteers as negative controls. We evaluated the reliability of the in-house CBA by comparing the results with those from a commercially available RIPA and enzyme-linked immunosorbent assay (ELISA).
METHODS
Patients
We tested 218 serum samples collected from October 2016 to September 2019: 177 were obtained from patients with MG, 8 from patients with Lambert-Eaton myasthenic syndrome (LEMS), 17 from patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), 3 from patients with amyotrophic lateral sclerosis, 3 from patients with idiopathic inflammatory myopathy, and 10 from healthy controls. MG was diagnosed based on clinical features, decrement responses in low-frequency repetitive nerve stimulation, improvement of symptoms after the intramuscular injection of neostigmine, and serum concentrations of AChR Abs and/or MuSK Abs. LEMS, CIDP, amyotrophic lateral sclerosis, and idiopathic inflammatory myopathy were diagnosed based on commonly used diagnostic criteria for each disease.12,13,14,15
The antibody profiles of the 177 patients with MG were categorized into 5 groups based on the results of commercially available AChR Ab and MuSK Ab assays, which were previously applied while diagnosing MG: 1) AChR-Ab positive and MuSK Abs not tested, 2) AChR-Ab negative and MuSK-Ab positive, 3) AChR-Ab negative and MuSK-Ab borderline, 4) double seronegative (AChR-Ab negative and MuSK-Ab negative), and 5) AChR-Ab negative and MuSK Abs not tested. MuSK Abs were not tested in those patients who refused the test while diagnosing MG. All patients provided written informed consent, and the Yonsei University Severance Hospital Institutional Review Board approved this study (No. 4-2016-0601).
Data collection
Basic demographic and clinical data of the patients, including sex, age at onset, age at diagnosis, initial symptoms, presence of MG crisis, Myasthenia Gravis Foundation of America (MGFA) clinical classification, and the state of immunosuppressive treatment, were recorded by reviewing medical records. The MGFA clinical classification represents the severity of MG and ranges from class I (ocular muscle weakness only) to class V (intubation required).16 The results for AChR Ab and MuSK Ab titers performed during the course of diagnoses were also recorded. AChR Abs were analyzed using a commercially available RIPA (anti-AChR-binding antibody, Seoul Clinical Laboratories, Seoul, Korea), with a result of AChR Abs >0.5 nmol/L considered positive. An anti-MuSK Ab assay was applied using a commercially available RIPA (anti-MuSK Ab, Athena Diagnostics, Worcester, MA, USA). Before June 2015, the results were reported as negative (<10 titer units), borderline (≥10 titer units and <20 titer units), or positive (≥20 titer units). After June 2015, the results were provided in numerical values, and a result of >0.02 nmol/L was considered positive.
Development of a stable cell line expressing MuSK
MuSK cDNA was amplified and cloned into the Sgf I and Mlu I sites of the pLenti-C-mGFP-P2A-Puro cloning vector (OriGene, Rockville, MD, USA). The cloned MuSK plasmids or empty vector plasmids were transfected with lentiviral packaging plasmid (OriGene) into human embryonic kidney 293 (HEK293) cells (Korean Cell Line Bank, Seoul, Korea) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) on cell culture dishes. The transfected cells were cultured in a cell culture incubator at 37℃ and 5% CO2, with the culture medium changed after 12 h of incubation. After two days of incubation, the first batch of lentiviral supernatant was harvested, and 10 mL of fresh cell culture medium was added to the cell culture. The day after the first harvest, the second batch of viral supernatant was collected. The lentiviral supernatant was filtered through a 0.45 µm filter. To generate a stable HEK293 cell line expressing MuSK, the MuSK cDNA containing lentiviral particles was added to the target HEK293 cells, which were cultured in the medium containing 1 µg/mL puromycin dihydrochloride (Life Technologies, Carlsbad, CA, USA). The puromycin-resistant cells were isolated after 1 week and then cloned by serial dilution. The individual stable transduced clones were screened for green fluorescent protein (GFP) expression using fluorescence microscopy. The expression of MuSK mRNA and protein was confirmed using the reverse-transcription polymerase chain reaction (RT-PCR) with three different primer pairs and Western blotting with an anti-MuSK antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), respectively. The following primer pairs were used for the RT-PCR: MuSK1, forward 5′-CCTTCAGCGGAACTGAGAAA-3′ and reverse 5′-GAGTCCTGGCTTGGTGATAAA-3′; MuSK2, forward 5′-AGTGCCTACTCCTATTCCCATTT-3′ and reverse 5′-CTCTCCGATGTCTCTCACATATTC-3′; MuSK3, forward 5′-GGCAGAATTTGACAACCCTAAC-3′ and reverse 5′-GCCATCTCGCACGTAGTAAA-3′; and GAPDH, forward 5′-ATGGCACCGTCAAGGCTGAGA-3′ and reverse 5′-GGCATGGACTGTGGTCATGAG-3′. Untransduced HEK293 cells and HEK293 cells transduced with empty vectors were used as negative controls. The cells were progressively cryofrozen to −80℃ in solution comprising 90% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) and 10% dimethyl sulfoxide (Sigma Aldrich, St. Louis, MO, USA), and then stored in liquid nitrogen on the following day.
Immunofluorescence staining and analysis
After thawing, the stably transduced HEK293 cells expressing MuSK tagged with GFP were seeded on a coverslip (SPL Life Science, Pocheon, Korea) coated with poly-L-lysine (Sigma Aldrich) and placed in wells of 24-well cell culture plates. The cells were grown in DMEM (GE Healthcare, Chicago, IL, USA) supplemented with 10% FBS (GE Healthcare), 100 units/mL penicillin (Invitrogen, Carlsbad, CA, USA), and 100 µg/mL streptomycin (Invitrogen) at 37℃ and 5% CO2 to 70–80% confluence. The culture medium was gently removed and the cells were rinsed three times with DMEM containing 20 mM HEPES. The cells were incubated with rabbit polyclonal anti-MuSK antibody (Invitrogen; 50 µg/mL final concentration) or sera from patients or controls (diluted 1:20) in Dulbecco's Modified Eagle's medium (DMEM) containing 1% bovine serum albumin and 20 mM HEPES, for 1 h at room temperature. The cells were washed three times with DMEM containing 20 mM HEPES and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature. After washing with PBS, the cells were incubated with goat ant-rabbit IgG conjugated with Alexa Fluor 594 (Abcam, Cambridge, UK; dilution 1:750, 2.67 µg/mL final concentration) to detect rabbit polyclonal MuSK Abs, or with goat anti-human IgG (H+L) conjugated with Alexa Fluor 594 (Invitrogen; dilution 1:750, 2.67 µg/mL final concentration) to detect MuSK Abs in patients' serum for 45 min at room temperature in the dark. The goat anti-human IgG (H+L) used in the present study binds to both heavy and light chains of human immunoglobulin. After washing three times with PBS, the coverslips were gently removed from the 24-well plates and placed on microscope slides with VECTASHIELD antifade mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA). The slides were stored in darkness at 4℃ before being observed using fluorescence microscopy.
Immunofluorescence staining was evaluated using a fluorescence microscope (Zeiss, Oberkochen, Germany). The intensity of fluorescence on the cell membrane and the colocalization of the GFP-labeled MuSK (green fluorescence) with Alexa Fluor 594-labeled antibody (red fluorescence) was assessed using a previously described visual scoring system: 0 for no labeling of the transduced cells, 0.5 for very weak labeling of a few cells with no definite colocalization, 1 for weak labeling of some cells with colocalization, 2 for labeling of 20–50% of cells with accurate colocalization, 3 for labeling of 50–80% of cells with perfect colocalization, and 4 for labeling of all transduced cells showing perfect colocalization.9,17 A score of 1 or more was considered a positive result. The results were interpreted using consensus by two independent investigators (M.K. and S.W.K.) who were blinded to the clinical data.
Enzyme-linked immunosorbent assay
All 218 serum samples were evaluated with MuSK Abs ELISA (IBL International, Männedorf, Germany) according to the manufacturer's instructions. Both positive and negative control serum samples were used to monitor the performance of the ELISA. The concentration of the MuSK Abs was calculated by referring to standard curves. All assays were performed in duplicate, with the final concentration calculated by averaging the results. The result was considered positive if the concentration of the MuSK Abs was >0.4 U/mL.
Statistical analysis
The data are expressed as percentages for categorical variables and mean±standard deviation values for continuous variables, depending on the assumption of normality. Overall comparisons between three groups were conducted by a chi-square test for categorical variables and ANOVA for continuous variables. Cohen's kappa coefficient was used to analyze the agreement in determining a positive result for MuSK Abs between the CBA, RIPA, and ELISA. The CBA and ELISA were applied during this study using the same samples, whereas the RIPA had been applied previously during the diagnostic workup; this meant that CBA and RIPA were performed using different samples. Cohen's kappa coefficients of 0.41–0.60, 0.61–0.80, and 0.81–1.00 were interpreted as indicating moderate, substantial, and almost perfect levels of agreement, respectively. All p values were two-sided, and a p value of <0.05 was considered statistically significant. Statistical analyses were performed using R software (version 3.4.3, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Overall results of the CBA
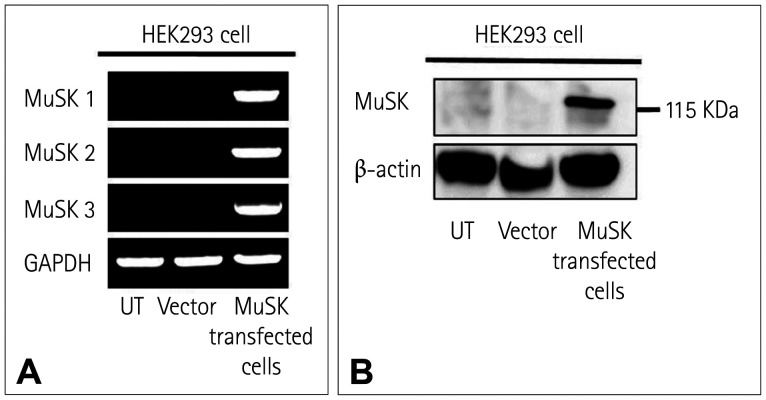
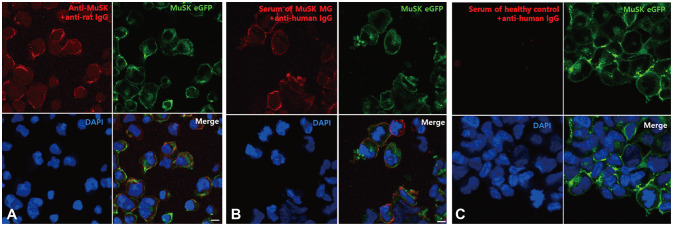
The stable expression of MuSK mRNA and protein in the MuSK-HEK293 cell line was confirmed by RT-PCR and Western blotting, respectively (Fig. 1). In fluorescence microscopy, green fluorescence was observed along the cell surface of the MuSK-HEK293 cells, indicating the expression of MuSK at the cell membranes. After adding the anti-MuSK Abs or sera of patients who had previously been diagnosed with MuSK MG, the cell membranes were stained with red fluorescence that was colocalized with the green fluorescence (Fig. 2). No red fluorescence was detected when the same procedure was conducted with the sera of negative controls. Overall, 34 (15.6%) of the 218 samples tested for the MuSK Abs using the CBA displayed a positive result for MuSK Abs. All samples with positive MuSK Ab results were from patients diagnosed with MG. None of the 41 samples from patients with other neuromuscular diseases or the healthy controls showed positive results for MuSK Abs (Supplementary Table 1 in the online-only Data Supplement). Representative images of the CBA for each level of visual scoring are shown in Supplementary Fig. 1 (in the online-only Data Supplement).
Fig. 1. Confirmation of the expression of MuSK mRNA and protein. A: Reverse-transcription polymerase chain reaction revealed the stable expression of MuSK mRNA in the MuSK-HEK293 cell line, whereas no bands were detected in either untransduced HEK293 cells or HEK293 cells transduced with empty vectors. B: Western blotting also confirmed MuSK expression (band at 122 kDa) in the MuSK-HEK293 cell line. Lanes of untransduced HEK293 cells or HEK293 cells transduced with empty vectors showed no bands. GAPDH: glyceraldehyde 3-phosphate dehydrogenase, HEK293: human embryonic kidney 293, MuSK: muscle-specific tyrosine kinase, UT: untransduced cells, Vector: HEK293 cells transduced with lentiviral vector that does not contain the MuSK gene.
Fig. 2. CBA to detect anti-MuSK Abs. When HEK293 cells expressing MuSK were incubated with (A) commercially available polyclonal anti-MuSK Abs or (B) sera from patients with MuSK-Ab-positive MG, anti-IgG antibody binding (red fluorescence) was observed along the cell surface, indicating the binding of MuSK Abs to MuSK. This colocalized with GFP-labeled MuSK expressed on the surface of HEK293 cells (green fluorescence). In contrast, (C) anti-human IgG antibody binding (red fluorescence) was not observed after adding the sera from the healthy control. Scale bar: 10 µm. CBA: cell-based assay, DAPI: 4′,6-diamidino-2-phenylindole, eGFP: enhanced green fluorescent protein, HEK293: human embryonic kidney 293, MG: myasthenia gravis, MuSK Abs: antibodies against muscle-specific tyrosine kinase.
Demographics and clinical features of the patients with MG
The present analysis was applied to 177 samples from the patients with MG (66 males and 111 females). The age of the patients at the time of sampling was 47.0±16.0 years. Ocular MG and generalized MG were present in 45 (25.4%) patients and 132 (74.6%) patients, respectively. Twenty-five (14.1%) patients had experienced a myasthenic crisis and 77 (43.5%) had undergone a thymectomy.
Autoantibody profiles
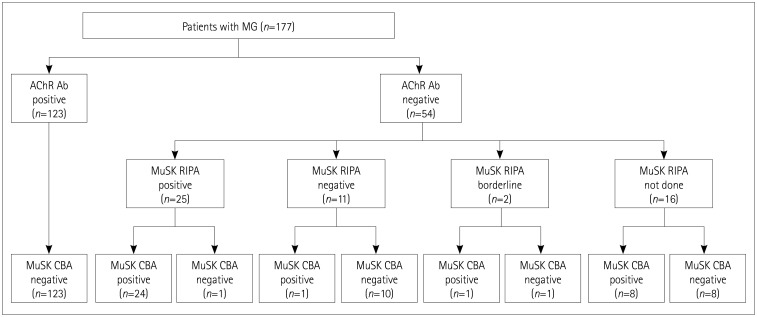
The serostatus of the patients with MG based on the RIPA and CBA is presented in detail in Fig. 3. The serostatus of the samples according to the RIPA was classified as follows: 123 AChR-Ab-positive MG, 25 AChR-Ab-negative and MuSK-Ab-positive MG, 11 double-seronegative MG, 16 AChR-Ab-negative MG without testing for MuSK Abs, and 2 AChR-Ab-negative MG patients with borderline results for MuSK Abs. The CBA for the MuSK Abs showed a positive result in 24 (96.0%) of 25 MuSK-Ab-positive MG patients, 8 (50.0%) of 16 AChR-Ab-negative MG patients with unknown MuSK Ab status, 1 (50%) of 2 AChR-Ab-negative MG patients with borderline MuSK Abs, and 1 (9.1%) of 11 double-seronegative patients. The results of the CBA for the MuSK Abs were negative in all of the remaining patients.
Fig. 3. Serostatus of 177 patients with MG determined by MuSK RIPA and MuSK CBA. AChR Ab: acetylcholine receptor antibody, CBA: cell-based assay, MG: myasthenia gravis, MuSK: muscle-specific tyrosine kinase, RIPA: radioimmunoprecipitation assay.
Comparison of clinical features between the three groups
We classified the patients with MG into three groups based on the presence of MuSK Abs as evaluated by CBA and of AChR Abs as tested using a commercial RIPA: AChR-Ab-positive and MuSK-Ab-negative MG (AChR MG, n=123), AChR-Ab-negative and MuSK-Ab-positive MG (MuSK MG, n=34), and AChR-Ab-negative and MuSK-Ab-negative MG (double-seronegative MG, n=20). Detailed clinical features of these groups are provided in Table 1. Patients with MuSK MG demonstrated a marked female predominance (p<0.001), were more likely to show bulbofacial presentation (p=0.041), more frequently had MGFA Class ≥III (p<0.001), and were frequently treated with corticosteroids (p=0.006), cyclosporine (p=0.005), or mycophenolate mofetil (p=0.005). Patients with AChR MG were more likely to have undergone a thymectomy (p<0.001).
Table 1. Clinical characteristics of the patients with MG classified based on the results of the in-house CBA for MuSK Abs and commercially available test for AChR Abs.
| Clinical feature | MuSK-Ab positive (n=34) | AChR-Ab positive (n=123) | Double seronegative (n=20) | p | |
|---|---|---|---|---|---|
| Age at onset, years | 35.8±11.9 | 39.5±19.2 | 40.0±17.8 | 0.534 | |
| Sex, male | 2 (3.0) | 58 (47.2) | 6 (30.0) | <0.001*‡ | |
| Symptoms at onset | |||||
| Ocular | 32 (94.1) | 100 (81.3) | 18 (90.0) | 0.171 | |
| Bulbofacial | 15 (44.1) | 29 (23.6) | 4 (20.0) | 0.041* | |
| Limb | 10 (29.4) | 25 (20.3) | 3 (15.0) | 0.427 | |
| Neck | 2 (5.9) | 6 (4.9) | 0 (0.0) | 0.620 | |
| Respiratory | 4 (11.8) | 3 (2.4) | 0 (0.0) | 0.068 | |
| Severity | <0.001*†‡ | ||||
| MGFA class I/II | 10 (29.4) | 65 (52.8) | 16 (80.0) | ||
| MGFA class III/IV/V | 24 (70.6) | 58 (47.2) | 4 (20.0) | ||
| MGFA classification “b” | 32 (94.1) | 45 (51.7) | 7 (63.6) | <0.001*‡ | |
| AChR-Ab positive, initial | 0 (0.0) | 121 (98.4) | 0 (0.0) | <0.001* | |
| AChR-Ab titer, nmol/L | - | 7.7±5.6 | - | - | |
| Thymectomy | 3 (8.8) | 71 (57.7) | 3 (15.0) | <0.001*† | |
| Abnormal RNS response | 28 (82.4) | 92 (74.8) | 10 (50.0) | 0.027†‡ | |
| Treatment | |||||
| Corticosteroid | 31 (91.2) | 78 (63.4) | 12 (60.0) | 0.006*‡ | |
| Azathioprine | 10 (29.4) | 29 (23.6) | 5 (25.0) | 0.765 | |
| Cyclosporine | 5 (14.7) | 2 (1.6) | 1 (5.0) | 0.005* | |
| Mycophenolate mofetil | 5 (14.7) | 2 (1.6) | 1 (5.0) | 0.005* | |
| Tacrolimus | 5 (14.7) | 34 (27.6) | 3 (15.0) | 0.186 | |
Data are mean±standard deviation or n (%) values.
*Statistically significant for AChR-Ab positive vs. MuSK-Ab positive, †Statistically significant for AChR-Ab positive vs. double seronegative, ‡Statistically significant for MuSK-Ab positive vs. double seronegative.
AChR Ab: acetylcholine receptor antibody, CBA: cell-based assay, MG: myasthenia gravis, MGFA: Myasthenia Gravis Foundation of America, MuSK Ab: muscle-specific tyrosine kinase antibody, RNS: repetitive nerve stimulation.
Correlation between CBA and RIPA results
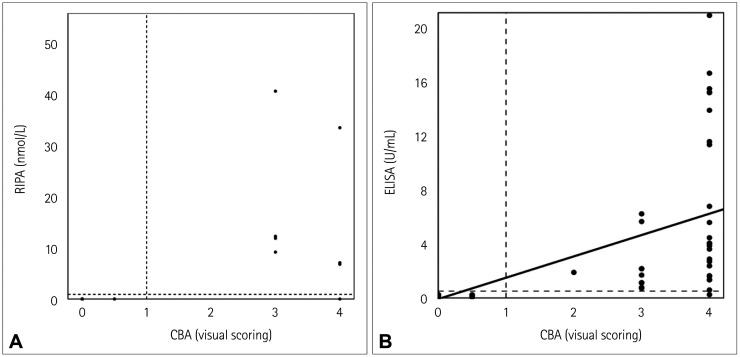
We evaluated the correlation between the results of the RIPA and CBA. The RIPA had previously been applied at the time of the initial diagnosis, whereas the CBA was applied during the present study. Therefore, the RIPA and CBA were applied to different samples obtained in different disease states. There were 38 patients who underwent both the RIPA and CBA. The RIPA was performed during the active disease course in every case. In contrast, 4 of the 38 patients underwent the CBA during clinical remission, whereas the CBA was performed in the remaining 34 patients while they had MG-related symptoms. The median duration between the RIPA and CBA was 8.0 months (interquartile range=2.0–29.3 months). The RIPA results were reported as quantitative values in 20 of these 38 patients. The distribution of the MuSK Ab concentration as evaluated by the RIPA and the results of the CBA measured using a visual grading score in these patients are presented in Fig. 4A. The correlation between the RIPA and CBA results was evaluated in 36 patients after excluding 2 patients whose results in the RIPA were borderline. There was an almost perfect agreement between the results of the CBA measured using visual grading and the results of the RIPA (Cohen's kappa=0.880, p<0.001). Only two samples did not show an agreement between the RIPA and CBA: the result for MuSK Abs was positive only in the CBA in one patient and only in the RIPA in the other patient.
Fig. 4. Correlations between the tests to detect MuSK Abs. A: The correlation between the CBA and the previously performed RIPA was assessed in 20 samples from the patients with MG whose samples had previously been assessed using an RIPA, the results of which were reported as quantitative values. There was an almost perfect agreement between the results of the CBA measured by visual grading and the results of the previously conducted RIPA (Cohen's kappa=0.880, p<0.001). B: Correlation between the results obtained in the CBA and ELISA applied to the same samples was assessed in 177 samples from the patients with MG. There was also an almost perfect agreement between the results of the CBA and ELISA (Cohen's kappa=0.982, p<0.001). CBA: cell-based assay, ELISA: enzyme-linked immunosorbent assay, MG: myasthenia gravis, MuSK Abs: antibodies against muscle-specific tyrosine kinase, RIPA: radioimmunoprecipitation assay.
Correlation between CBA and ELISA results
We further evaluated the correlation between the results for MuSK Abs obtained using the CBA and the commercially available ELISA. The CBA and ELISA of each patient were applied to the same sample. The distribution of the MuSK Ab concentration as evaluated by the ELISA and the results of CBA as measured using visual grading in 177 samples from the patients with MG are presented in Fig. 4B. There was an almost perfect agreement between the results of the CBA and ELISA (Cohen's kappa=0.982, p<0.001). There was one sample that did not demonstrate an agreement between the CBA and ELISA, with the result being positive in the MuSK CBA but negative in the ELISA.
DISCUSSION
We established an in-house CBA method by generating a stably transfected MuSK-HEK293 cell line. The present findings demonstrate excellent agreement between the results of our in-house CBA and two other methods: RIPA and ELISA. In addition, there were two cases where the serostatus was revealed to be positive for MuSK Abs by the CBA, whereas MuSK Abs were not detected when using an RIPA or ELISA. Our in-house CBA can therefore be considered a reliable method for identifying the presence of MuSK Abs.
The CBA demonstrated high sensitivity and specificity in detecting autoantibodies in patients with MuSK MG. Huda et al.18 demonstrated that the MuSK CBA is highly reliable for detecting MuSK Abs in patients who were previously known to have MuSK MG as determined by an RIPA. Furthermore, the CBA detected MuSK Abs in 8% of the patients who were determined to be double seronegative in a RIPA. A previous multinational study that also used CBA detected MuSK Abs in 13% of the patients with triple-seronegative MG, in whom AChR Abs and MuSK Abs were not detected by an RIPA and antibodies against LRP4 (low-density lipoprotein receptor-related protein 4) were not detected by CBA.11 In particular, the CBA in that study revealed that MuSK-Ab-positive MG is more common among patients with ocular MG than previously thought. In a recent study from South Korea, the CBA demonstrated a higher sensitivity in detecting MuSK Abs than did the conventional RIPA.10 Other studies have similarly demonstrated that the seropositive rate in patients with MG is higher when a CBA is performed in addition to an RIPA.19,20 In the present study, none of the patients with other neuromuscular disorders or the healthy controls showed positive results for MuSK Abs in the CBA. In addition, the CBA was able to detect additional MuSK-Ab-positive cases that were previously thought to be MuSK-Ab negative or borderline based on the RIPA. These results indicate that the present in-house CBA is highly sensitive and specific for detecting MuSK Abs.
In addition to its reliability and concordance with other assays in detecting MuSK Abs, the current CBA has strength in constructing a cell line stably expressing MuSK using a lentivirus. Although the detailed method of transfecting MuSK DNA was not reported for some of the previous studies, most of them used transiently transfected HEK293 cells in CBAs for detecting MuSK Abs.11,21,22 Transient transfection can be performed both easily and rapidly, but it has limitations in the duration of protein expression being short and the transfection needing to be conducted repeatedly. In contrast, a gene of interest could be integrated into the host genome by using a lentivirus, and this method has previously been shown to be efficient and reliable for generating stable cell lines,23 as demonstrated by the use of a lentivirus to generate a cell line that stably expresses MuSK protein in the present study.
Clinical characteristics of patients with MG who were MuSK-Ab positive in the CBA but not in the RIPA have been described previously. Huda et al.18 reported that these patients had a lower age at onset, a lower disease severity, and showed more favorable responses to acetylcholinesterase inhibitor than did MuSK-RIPA-positive patients. Tsonis et al.11 similarly suggested that patients positive for MuSK Abs in a CBA but negative in an RIPA more frequently had ocular MG and less frequently experienced myasthenic crisis than previously thought. These studies suggest that the CBA may be useful for detecting MuSK Abs in milder forms of MG. However, in the present study, the patient who was MuSK-CBA positive but MuSK-RIPA negative was 72 years old and had a maximum severity of MGFA class V, which contrasts with previous studies. This apparent discrepancy may be due to the relatively small number of patients in the current study. In addition, due to the high cost of the MuSK RIPA, most of the tests were only applied to patients with a high probability of having MuSK MG; that is, those with severe bulbar symptoms and who did not respond to acetylcholinesterase inhibitor. This resulted in an RIPA only being applied to three patients with ocular MG. Further assessments of patients with milder disease may reveal additional MuSK-CBA-positive and MuSK-RIPA-negative patients.
One patient in the present study was positive for MuSK Abs in a commercially available RIPA but negative in the CBA. However, the CBA was performed 8 years after the RIPA, during which time the patient remained in pharmacological remission under treatment with azathioprine. Although little is known regarding serial changes in MuSK Ab titers, previous studies have demonstrated the possibility of the negative seroconversion of MuSK Abs. Triplett et al.24 reported a patient with MuSK MG whose serum MuSK Ab concentration indicated negative seroconversion approximately 6 years after treatment with rituximab and azathioprine. Similarly, Hain et al.25 reported a MuSK MG patient whose serum MuSK Ab concentration changed from highly positive to negative after treatment with rituximab. In both of these previous cases, the reduction of the circulating MuSK Ab concentration was accompanied by the improvement of clinical symptoms. This is consistent with another previous observation of clinical improvement being accompanied by a significant reduction or negative seroconversion of MuSK Abs in patients with MuSK MG treated with rituximab.26 Therefore, the different results between the RIPA and CBA in the present study may have been due to the seroconversion of MuSK Abs rather than differences in the sensitivities of the tests. The result of the present ELISA that used the same sample as in the CBA was also negative for MuSK Abs, which may support the hypothesis that the negative result in the CBA resulted from the negative seroconversion of MuSK Abs.
The clinical characteristics of patients with MuSK MG determined based on our in-house CBA correspond well to known clinical features of MuSK MG. The patients with MuSK MG showed a marked female predominance with a mean age of 35.8 years at onset. This is in line with previous studies finding that females constituted 71.9–100% of cases,1,2,10,18,27 and the mean age at onset ranging from 28.9 to 43.5 years.2,10,18 A high proportion of patients demonstrating bulbar symptoms has also been reported previously. The proportion of patients demonstrating an MGFA classification of “b” was 94.1% in the present study and 80–100% in previous studies.4,5,27,28 The proportion of patients with MuSK MG showing abnormal RNS responses was 82.4% in the present study, which was significantly higher than the proportion of patients with double-seronegative MG. This was similarly found in previous studies that analyzed the clinical characteristics of MuSK MG.5,29
Several limitations of the present study need to be considered when interpreting its results. First, the study was conducted at a single center and was based on a relatively small number of patients. Further assessments based on larger numbers of patients are required to strengthen the results. Second, the MuSK RIPA and CBA were applied to different serum samples obtained at different time points, and so both agreements and disagreements between the two assays should be interpreted with caution. Third, a recent study recommended using an IgG Fc gamma-specific secondary antibody in the MuSK CBA to enhance its specificity.18 In that study, the MuSK CBA using anti-human IgG (H+L) detected IgM that bound nonspecifically to MuSK. These nonspecific IgM antibodies failed to inhibit AChR clusters, and are therefore regarded as lacking pathogenic potential. In another study that also used secondary antibodies that react with both heavy and light chains, MuSK Abs were present in 12.5% of AChR-Ab-positive MG patients and in 1.9% of healthy subjects.11 These results may reflect the nonspecific binding of the secondary antibody. Since anti-human IgG (H+L) was also used in the present study, caution is needed in interpreting these results. However, the results of the current CBA demonstrated near perfect agreement with the RIPA, and no samples from patients with other neurological disorders or healthy controls showed positive results in MuSK CBAs.
In conclusion, we generated stably transfected HEK293 cell lines expressing MuSK protein, and the results of a CBA utilizing this cell line demonstrated excellent agreement with those from both the RIPA and ELISA. Moreover, the MuSK CBA detected two additional cases that were MuSK-Ab negative in the RIPA and ELISA. The clinical features of the patients with MuSK MG determined based on the CBA were consistent with the known clinical characteristics of MuSK MG. The present in-house CBA can be used to reliably determine the presence of MuSK Abs.
Acknowledgements
This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2016-0110).
Footnotes
- Conceptualization: Ha Young Shin, Min Ju Kim, Seung Woo Kim.
- Data curation: Ha Young Shin, Min Ju Kim, MinGi Kim, Seung Woo Kim.
- Formal analysis: Ha Young Shin, Seung Woo Kim.
- Funding acquisition: Ha Young Shin.
- Investigation: all authors.
- Methodology: all authors.
- Project administration: Ha Young Shin, Seung Woo Kim.
- Resources: Ha Young Shin, Seung Woo Kim.
- Supervision: Seung Woo Kim, Young-Chul Choi, Seung Min Kim, Ha Young Shin.
- Validation: all authors.
- Visualization: Ha Young Shin, Min Ju Kim, MinGi Kim, Seung Woo Kim.
- Writing—original draft: Ha Young Shin, Min Ju Kim, MinGi Kim, Seung Woo Kim.
- Writing—review & editing: all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2021.17.3.400.
Overall results of MuSK CBAs in patients with MG, other neuromuscular disorders, and healthy controls
The representative images of the CBA in each level of visual scores. Anti-human IgG antibody binding (red fluorescence) is observed along the cell surface which co-localizes with MuSK-GFP expressed on the surface of HEK293 cells (green fluorescence). CBA: cell-based assay, HEK293: human embryonic kidney 293, MuSK-GFP: muscle-specific tyrosine kinase fused to green fluorescent protein
References
- 1.Evoli A, Bianchi MR, Riso R, Minicuci GM, Batocchi AP, Servidei S, et al. Response to therapy in myasthenia gravis with anti-MuSK antibodies. Ann N Y Acad Sci. 2008;1132:76–83. doi: 10.1196/annals.1405.012. [DOI] [PubMed] [Google Scholar]
- 2.Deymeer F, Gungor-Tuncer O, Yilmaz V, Parman Y, Serdaroglu P, Ozdemir C, et al. Clinical comparison of anti-MuSK- vs anti-AChR-positive and seronegative myasthenia gravis. Neurology. 2007;68:609–611. doi: 10.1212/01.wnl.0000254620.45529.97. [DOI] [PubMed] [Google Scholar]
- 3.Farrugia ME, Robson MD, Clover L, Anslow P, Newsom-Davis J, Kennett R, et al. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain. 2006;129(Pt 6):1481–1492. doi: 10.1093/brain/awl095. [DOI] [PubMed] [Google Scholar]
- 4.Kim SW, Sunwoo MK, Kim SM, Shin HY, Sunwoo IN. Repetitive nerve stimulation in MuSK-antibody-positive myasthenia gravis. J Clin Neurol. 2017;13:287–292. doi: 10.3988/jcn.2017.13.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh SJ, Morgan MB, Lu L, Hatanaka Y, Hemmi S, Young A, et al. Different characteristic phenotypes according to antibody in myasthenia gravis. J Clin Neuromuscul Dis. 2012;14:57–65. doi: 10.1097/CND.0b013e318275197c. [DOI] [PubMed] [Google Scholar]
- 6.Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. 2011;44:36–40. doi: 10.1002/mus.22006. [DOI] [PubMed] [Google Scholar]
- 7.Stickler DE, Massey JM, Sanders DB. MuSK-antibody positive myasthenia gravis: clinical and electrodiagnostic patterns. Clin Neurophysiol. 2005;116:2065–2068. doi: 10.1016/j.clinph.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Devic P, Petiot P, Simonet T, Stojkovic T, Delmont E, Franques J, et al. Antibodies to clustered acetylcholine receptor: expanding the phenotype. Eur J Neurol. 2014;21:130–134. doi: 10.1111/ene.12270. [DOI] [PubMed] [Google Scholar]
- 9.Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, et al. IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis. Brain. 2008;131(Pt 7):1940–1952. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park KH, Waters P, Woodhall M, Lang B, Smith T, Sung JJ, et al. Myasthenia gravis seronegative for acetylcholine receptor antibodies in South Korea: autoantibody profiles and clinical features. PLoS One. 2018;13:e0193723. doi: 10.1371/journal.pone.0193723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsonis AI, Zisimopoulou P, Lazaridis K, Tzartos J, Matsigkou E, Zouvelou V, et al. MuSK autoantibodies in myasthenia gravis detected by cell based assay--a multinational study. J Neuroimmunol. 2015;284:10–17. doi: 10.1016/j.jneuroim.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 13.Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society-first revision. Eur J Neurol. 2010;17:356–363. doi: 10.1111/j.1468-1331.2009.02930.x. [DOI] [PubMed] [Google Scholar]
- 14.Titulaer MJ, Lang B, Verschuuren JJGM. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10:1098–1107. doi: 10.1016/S1474-4422(11)70245-9. [DOI] [PubMed] [Google Scholar]
- 15.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 16.Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, et al. Myasthenia gravis: recommendations for clinical research standards. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 17.Kang ES, Min JH, Lee KH, Kim BJ. Clinical usefulness of cell-based indirect immunofluorescence assay for the detection of aquaporin-4 antibodies in neuromyelitis optica spectrum disorder. Ann Lab Med. 2012;32:331–338. doi: 10.3343/alm.2012.32.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huda S, Waters P, Woodhall M, Leite MI, Jacobson L, De Rosa A, et al. IgG-specific cell-based assay detects potentially pathogenic MuSK-Abs in seronegative MG. Neurol Neuroimmunol Neuroinflamm. 2017;4:e357. doi: 10.1212/NXI.0000000000000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang T, Leite MI, Senanayake S, Gunaratne PS, Gamage R, Riffsy MT, et al. Clinical and serological study of myasthenia gravis using both radioimmunoprecipitation and cell-based assays in a South Asian population. J Neurol Sci. 2014;343:82–87. doi: 10.1016/j.jns.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Maxwell S, Leite MI, Waters P, Clover L, Fan X, et al. Non-radioactive serological diagnosis of myasthenia gravis and clinical features of patients from Tianjin, China. J Neurol Sci. 2011;301:71–76. doi: 10.1016/j.jns.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Takata K, Stathopoulos P, Cao M, Mané-Damas M, Fichtner ML, Benotti ES, et al. Characterization of pathogenic monoclonal autoantibodies derived from muscle-specific kinase myasthenia gravis patients. JCI Insight. 2019;4:e127167. doi: 10.1172/jci.insight.127167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McConville J, Farrugia ME, Beeson D, Kishore U, Metcalfe R, Newsom-Davis J, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55:580–584. doi: 10.1002/ana.20061. [DOI] [PubMed] [Google Scholar]
- 23.Tandon N, Thakkar KN, LaGory EL, Liu Y, Giaccia AJ. Generation of stable expression mammalian cell lines using lentivirus. Bio Protoc. 2018;8:e3073. doi: 10.21769/BioProtoc.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triplett JD, Hardy TA, Riminton DS, Chu SYK, Reddel SW. Association between musk antibody concentrations and the myasthenia gravis composite score in 3 patients: a marker of relapse? Muscle Nerve. 2019;60:307–311. doi: 10.1002/mus.26609. [DOI] [PubMed] [Google Scholar]
- 25.Hain B, Jordan K, Deschauer M, Zierz S. Successful treatment of MuSK antibody-positive myasthenia gravis with rituximab. Muscle Nerve. 2006;33:575–580. doi: 10.1002/mus.20479. [DOI] [PubMed] [Google Scholar]
- 26.Díaz-Manera J, Martínez-Hernández E, Querol L, Klooster R, Rojas-García R, Suárez-Calvet X, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78:189–193. doi: 10.1212/WNL.0b013e3182407982. [DOI] [PubMed] [Google Scholar]
- 27.Sanders DB, El-Salem K, Massey JM, McConville J, Vincent A. Clinical aspects of MuSK antibody positive seronegative MG. Neurology. 2003;60:1978–1980. doi: 10.1212/01.wnl.0000065882.63904.53. [DOI] [PubMed] [Google Scholar]
- 28.Farrugia ME, Kennett RP, Hilton-Jones D, Newsom-Davis J, Vincent A. Quantitative EMG of facial muscles in myasthenia patients with MuSK antibodies. Clin Neurophysiol. 2007;118:269–277. doi: 10.1016/j.clinph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe GI, Oh SJ. Clinical phenotype of muscle-specific tyrosine kinase-antibody-positive myasthenia gravis. Ann N Y Acad Sci. 2008;1132:71–75. doi: 10.1196/annals.1405.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overall results of MuSK CBAs in patients with MG, other neuromuscular disorders, and healthy controls
The representative images of the CBA in each level of visual scores. Anti-human IgG antibody binding (red fluorescence) is observed along the cell surface which co-localizes with MuSK-GFP expressed on the surface of HEK293 cells (green fluorescence). CBA: cell-based assay, HEK293: human embryonic kidney 293, MuSK-GFP: muscle-specific tyrosine kinase fused to green fluorescent protein