Abstract
Background: Several underlying diseases have been associated with unfavorable COVID-19 related outcomes including asthma and Chronic Obstructive Pulmonary Disease (COPD), however few studies have reported risks that are adjusted for confounding variables. This study aimed to examine the adjusted risk of COVID-19 related hospitalsation, intensive care unit (ICU) admission, and mortality in patients with vs. without asthma or COPD.
Methods: A systematic review of major databases was undertaken for studies published between 1/12/2019 and 19/4/2021. Studies reporting the adjusted (for one or more confounder) risks of either hospitalsation, ICU admission, or mortality in asthmatics or COPD patients (control group = no asthma or no COPD) were identified. Risk of bias was determined via the QUIPS tool. A random effect meta-analysis was undertaken.
Findings: 37 studies were eligible for analysis, with a total of 1,678,992 participants. The pooled ORs of COVID-19 hospitalsation in subjects with asthma and COPD was 0.91 (95% CI 0.76–1.09) and 1.37 (95% CI 1.29–1.46), respectively. For ICU admission, OR in subjects with asthma and COPD was 0.89 (95% CI 0.74–1.07) and 1.22 (95% CI 1.04–1.42), respectively. For mortality, ORs were 0.88 (95% CI 0.77–1.01) and 1.25 (95% CI 1.08–1.34) for asthma and COPD, respectively. Further, the pooled risk of mortality as measured via Cox regression was 0.93 (95% CI 0.87–1.00) for asthma and 1.30 (95% CI 1.17–1.44) for COPD. All of these findings were of a moderate level of certainty.
Interpretation: COPD was significantly associated with COVID-19 related hospital admission, ICU admission, and mortality. Asthma was not associated with negative COVID-19 related health outcomes. Individuals with COPD should take precautions to limit the risk of COVID-19 exposure to negate these potential outcomes. Limitations include differing population types and adjustment for differing cofounding variables. Practitioners should note these findings when dealing with patients with these comorbidities.
Review Protocol Registration: https://www.crd.york.ac.uk/prospero/.
Keywords: COVID-19, COPD, asthma, mortality, hospitalsation, meta-analysis, ICU, intensive care
Introduction
In March 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a global pandemic, and as of 3rd February 2021, over 103,000,000 confirmed cases have been diagnosed in more than 130 countries and areas, resulting in ~2,238,000 deaths to date (1). Several risk factors associated with increasing severity of the disease have been reported, including age (2), obesity (3), and underlying conditions such as hypertension (4), and diabetes (5).
An important risk factor for unfavorable COVID-19 outcomes is Chronic Obstructive Pulmonary Disease (COPD); a group of lung conditions including emphysema and chronic bronchitis (6), primarily caused by tobacco smoking, with air pollution, genetic factors, diet and tuberculosis also contributing to the disease (7).
COPD has been associated with increased risks of unfavorable outcomes in non-COVID-19 related pneumonia (8). For COVID-19, some primary studies have questioned whether COPD is associated with worse outcomes (9), whilst the majority of reviews conclude that COPD patients yield significantly worse outcomes than those without (10–13) and others report no effects (14).
An additional risk factor for COVID-19 related complications is the presence of asthma, a common allergy that can cause breathing difficulties including coughing, wheezing, breathlessness and a tight chest (15). Asthma exacerbations have been shown to be strongly associated with other respiratory viral infections, including previous coronaviruses (16, 17). Although some primary studies have reported associations between asthma and negative COVID-19 outcomes, the majority of reviews that have examined associations of COVID-19 outcomes and asthma have concluded a lack of association between asthma and negative COVID-19 outcomes (18, 19).
One limitation of all of the systematic reviews, to date, on COVID-19 outcomes and asthma or COPD is that they report on risk that has not been adjusted for any potential confounding factors, making the true risks of these comorbidities, and subsequent clinical implications, difficult (20)—indeed, of the 16 similar meta-analyses that were published in 2021 (as of April 2021), none of them reported exclusively on adjusted risks; they either report unadjusted risks or the inclusion of adjusted or unadjusted risks is unclear. Several primary studies report on adjusted risks that are lower than the unadjusted risks in several COVID-19 related outcomes, including in asthma (21) and COPD (22). Furthermore, several studies advocate the use of pooling adjusted effect sizes (23, 24), especially in the case of determining COVID-19 related risks (20, 25).
The aim of this review was to examine the risks of negative COVID-19 outcomes in subjects with asthma or COPD, that have been adjusted for one or more COVID-19 related risk factor, including age, sex, smoking status (20, 25), or comorbid disease. Specifically our aims were to assess:
Adjusted risk of COVID-19 related hospitalsation in subjects with vs. without asthma or COPD.
Adjusted risk of COVID-19 related intensive care unit (ICU) admission in subjects with vs. without asthma or COPD.
Adjusted risk of COVID-19 related overall mortality in subjects with vs. without asthma or COPD.
This review has the potential to inform clinicians regarding the true risks of unfavorable COVID-19 outcomes in patients with asthma and COPD, increase awareness in people of the potential risks should they contract COVID-19 and to inform healthcare and public health policies.
Methods
Study Registration
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (26), and was registered on 29th June 2020 with the international prospective register of systematic reviews (PROSPERO: protocol ID CRD42020194155)—note that the full PRIMSA checklist can be found in Supplementary Table 1 and justifications of any deviations from the registered protocol can be found in Supplementary Table 2.
Search Strategy
Databases were searched from 1/12/2019 to 19/4/2021 including Embase, MEDLINE, Pubmed, Scopus, Web of Science, CINAHL, The Cochrane library UK clinical Research Network: Portfolio database, and the International Standard Registered Clinical/soCial sTudy Number (ISRCTN) registry, using the following search terms:
(SARSCoV-2 OR 2019-nCoV OR COVID-19 OR coronavirus OR “Wuhan Coronavirus”)
AND
(2019 or 2020)
AND
(asthma* OR COPD OR “chronic obstructive pulmonary disease”)
No other limiters were applied.
Study Selection
Two researchers (MV,SW) independently screened titles and abstracts of all identified studies after duplicates were removed. Discrepancies between reviewers were resolved by discussion before screening full texts independently against the inclusion criteria. If it was not possible to determine whether a study met the inclusion criteria from the title and/or abstract, it was marked for a full paper review. Where necessary, the reviewers contacted corresponding authors to request missing information or clarification. All references were imported to Mendeley.
Study Inclusion and Exclusion
Two reviewers (MV & SW) independently screened all titles and abstracts. The relevance of each study was assessed according to the inclusion and exclusion criteria. Studies were included if they met the following criteria.
Population
Studies including humans with COPD and/or asthma and a confirmed case (via polymerase chain reaction or antibody test) of COVID-19 were included in this review. Children <18 yrs and animal studies were excluded from this review. We also excluded studies on previous human coronaviruses: 229E, NL63, OC43, HKU1, MERS-CoV, and SARS-CoV.
Intervention
Observational studies, including case-control and cohort studies were included. Randomized studies that reported the prognostic role of asthma/COPD in post-hoc analyses (e.g., Cox regression models) were also included.
Comparison
Comparator groups include humans with confirmed COVID-19 and no evidence of COPD and/or asthma.
Outcomes
Studies had to report one or more of the following:
Number of COVID-19 cases hospitalised vs. COVID-19 cases non-hospitalised cases.
Number of hospitalised COVID-19 cases treated in intensive care unit (ICU) vs. hospitalsation but not admitted for ICU care.
Number of COVID-19 related deaths vs. survival.
Furthermore, studies were excluded if they were:
Not written in English.
Not peer reviewed (e.g., preprints).
Studies in a non-adult (<18 years) population.
Had insufficient data to calculate an adjusted odds ratio (aOR; adjusted for more than one COVID-19 related covariate) related to the stated outcomes.
Data Extraction
Data was extracted by two reviewers (MT & MV) and included: first author, study title, date of study, dates in which study data were collected, country, aim/objective, study type, number of participants, disease investigated, method of disease diagnoses, method of COVID-19 diagnosis, outcome type, sample size, participant characteristics, adjusted OR and 95% confidence intervals (CIs) (or raw data in which an adjusted odds ratio could be calculated), details of confounding variables the OR was adjusted for. Where data was missing, required clarification or particular variables of interest were not reported in the paper, corresponding authors were contacted to enable inclusion in the meta-analysis, and given 2 weeks to respond. If no response was received within 2 weeks, or the data was unavailable, these studies were excluded.
Quality Assessment
Risk of bias was assessed by two independent researchers (MT & MV) using the Quality In Prognosis Studies (QUIPS) tool (27). The QUIPS is a non-scoring appraisal tool for assessing the scientific validity of articles, which requires the identification of whether or not relevant information is present in each article using a yes, no or not applicable rating, with an overall verdict of “low,” “medium,” or “high” risk of bias. Any discrepancies over the final risk of bias verdict was made by consensus, with involvement of a third review author (SP) where necessary.
Statistical Analysis
Due to anticipated heterogeneity, a random-effects model was conducted using the DerSimonian and Laird method, with studies weighted according the inverse variance, using Comprehensive Meta-Analysis (28). The meta-analysis was conducted using the following steps:
Adjusted odds ratios (aORs), or adjusted Hazard Ratios (aHRs) and 95% CIs were inputted (with significance set as p = 0.05). Note that if the raw data were available, a binary logistic regression was conducted.
Heterogeneity between studies was assessed using the I2 statistic (29). If high (>50%) heterogeneity was found, sub-group analyses were conducted based on total participants (>10 vs. <10k participants).
Publication bias was assessed with a visual inspection of funnel plots and with the Egger bias test (30). As per the recommendations by Fu et al. (31) and Sterne et al. (32), these tests were only conducted if the number of studies in each analysis exceeded ten.
Sensitivity analyses were conducted to assess the robustness of the pooled effect sizes through the one study removed method.
Certainty of Evidence
To ascertain the certainty of the evidence, the Grading of Recommendations, Assessment, Development and Evaluations (33) (GRADE) framework was used.
Results
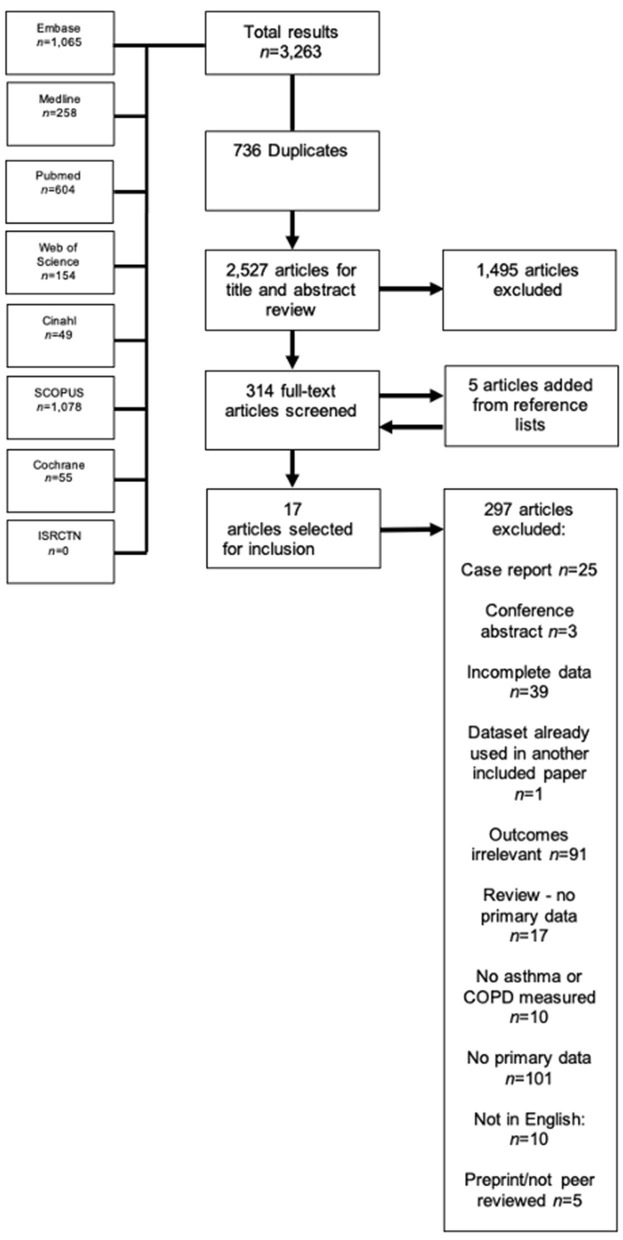
The literature search yielded 3,701 results, of which 780 were duplicates and were automatically removed, leaving 2,921 studies to be screened using the title and abstract. Of these studies, 416 full-texts were screened, where five extra studies were obtained by way of reference lists, resulting in 421 full texts that were finally screened. Thirty-eight studies appeared to be eligible for inclusion, however one (34) was excluded because the reported 95% CIs were not symmetrical, and therefore could not be pooled, leaving 37 finally eligible for inclusion (21, 35–69). The full PRISMA flowchart is shown in Figure 1, and a full list of excluded studies with reasons for exclusion can be found in Supplementary Table 2. There were a total of 1,678,992 participants across the included studies, with a mean age range of 45.7–81.9 years. Of the included studies, 10 (38, 42, 46, 48, 51, 55, 56, 58, 66, 69) examined outcomes in both asthma and COPD, seven (21, 43, 50, 52, 63, 64) examined outcomes exclusively in asthma, and the remaining 20 studies (37, 39–41, 44, 45, 47, 49, 53, 57, 59–62, 65, 67, 68, 70, 71) reported on outcomes exclusively regarding COPD. All but one study was classified as having low risk of bias (see Supplementary Table 4 for full QUIPS scoring). Full descriptive characteristics of included studies are shown in Table 1.
Figure 1.
PRISMA flowchart of included studies.
Table 1.
Descriptive characteristics of included studies.
| Authors | Study design | Country | Total n | Age (mean) | Percentage female | Type of outcome(s) measured | Disease | Method of asthma diagnosis | Method of COPD diagnosis | Confounding/adjusted variables | Conflict of interest | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atkins et al. (38) | Cohort | UK | 268,704 | 73.1 | NR | Hospitalsation risk; mortality risk | Asthma or COPD | “existing diagnoses were available from baseline questionnaires (2006–2010) eliciting participant reports of doctor-diagnosed disease. New disease diagnoses since baseline were from linked electronic medical records to hospital inpatient routine data (to March 2017), coded according to the International Classification of Diseases 10th revision (ICD-10)” | Age group, sex, ethnicity, education, baseline assessment centre, CHD, Atrial fibrillation, stroke, hypertension, T2D, CKD, depression, dementia, asthma, COPD, Osteoporosis, previous delirium, previous pneumonia, previous falls/fragility fractures. | Reported—none declared | Low | |
| Attaway et al. (39) | Cohort | USA | 2527 | NR | NR | Hospitalsation risk; ICU admission risk; mortality risk | COPD | – | “Patients were asked if they had a diagnosis of COPD, and the diagnosis was confirmed if it was also included in the patient's medical chart” | Age, race, sex, BMI, smoking status (current vs. former), hypertension, cancer, diabetes mellitus, coronary artery disease, immunosuppressive therapy. | Reported—none declared | Low |
| Aveyard et al. (55) | Retrospective cohort | UK | 811 | NR | NR | Mortality risk | Asthma and COPD | NR | NR | Age, sex, ethnicity, socioeconomic status, region of England, body-mass index (categorical variable), and smoking status (with current intensity of smoking as categorical variables), on-smoking-related illness (hypertension, type 1 diabetes, chronic liver disease, chronic neurological disease) and smoking-related illness (coronary heart disease, stroke, atrial fibrillation, type 2 diabetes, chronic kidney disease). | Reported—several potential conflicts declared | Low |
| Azoulay et al. (59) | Retrospective cohort | France | 376 | NR | NR | Mortality risk | COPD | – | NR | Age, comorbidities (asthma, diabetes, COPD, hypertension, immunosuppression), time from viral symptom onset to ICU admission, acute kidney injury, and troponin | Reported—none declared | Low |
| Bloom et al. (69) | Retrospective cohort | UK | 47,398 | NR | NR | Mortality risk | Asthma and COPD | NR | NR | Age, sex, ethnicity, smoking, obesity, malignancy, chronic cardiac disease, CKD, and centre | Reported—several potential conflicts declared | Low |
| Cellina et al. (40) | Retrospective observational | Italy | 246 | 63.0 | 31.0% | Mortality risk | COPD | – | NR | Age, diabetes, and radiological outcomes | Reported—none declared | Low |
| Choi et al. (21) | Cohort | Korea | 7,590 | NR | NR | ICU admission risk; mortality risk | Asthma | “An asthma diagnosis was determined when patients visited the hospital (at least once) due to asthma symptoms from January 2019 to December 2019. Furthermore, only patients who met the following criteria during the assessment period were regarded as having asthma: (1) ICD- 10 codes for asthma (J45 and J46) as primary diagnosis or first sub-diagnosis; and (2) prescription of asthma medications on at least 2 occasions during outpatient visits or prescription of asthma medication following an outpatient visit and admission with treatment using systemic corticosteroids during the assessment period.” | – | Age, sex, and underlying conditions | Reported—none declared | Low |
| Choi et al. (54) | Retrospective cohort | South Korea | 4,057 | NR | 60.4% | Mortality risk | Asthma | NR | – | Age, sex, obesity, systolic blood pressure, diastolic blood pressure, heart rate, temperature, diabetes, hypertension, heart failure, chronic heart disease, chronic obstructive pulmonary disease, chronic kidney disease, cancer, chronic liver disease, rheumatic or autoimmune disease, and dementia. | Reported—none declared | Low |
| De Vito et al. (41) | Retrospective observational | Italy | 87 | 72 (median) | 35.6% | Mortality risk | COPD | – | NR | Age >72 years, Hypertension, > 3 comorbidities, >5 comorbidities, non-compliance, moderate ARDS, lymphocyte <900/mm3 | Reported—none declared | Low |
| De Vito et al. (57) | Retrospective cohort | Italy | 264 | 81.9 (10.1) | 62.5% | Mortality risk | COPD | – | NR | Age, sex, hypertension, diabetes, neurological syndrome, hypokinetic disease, autonomy, fever + dyspnoea, LMWH | Reported—none declared | Low |
| Giannouchos et al. (42) | Cross-sectional | Mexico | 89,756 | 46.2 | 43.6% | Hospitalsation risk; ICU admission risk | Asthma and COPD | NR | NR | Age, gender, smoking, CKD, diabetes, immunosuppression, obesity, hypertension, CVD, asthma or COPD | Reported—none declared | Low |
| Girardin et al. (56) | Retrospective cohort | USA | 4,446 | NR | NR | Mortality risk | Asthma and COPD | NR | COPD was defined as presence of chronic bronchitis or emphysema. | Age, sex, PAD, low income, asthma, ethnicity, obesity, CAD, cancer, smoking, diabetes, auto-immune disease, hyperlipidaemia, sleep apnoea, hypertension | Reported—none declared | Low |
| Grandbastien et al. (43) | Cross-sectional | France | 106 | 63.5 (median) | 37.7% | ICU admission ris | Asthma | “clinical diagnosis of asthma based on the clinical history recorded by medical staff” | – | Age, sex, hypertension, diabetes, body mass index <30, and heart failure | Reported—one author reports conflict of interest with pharmaceutical companies | Low |
| Grasselli et al. (60) | Retrospective cohort | Italy | 3,988 | NR | 20.1% | Mortality risk | COPD | - - | NR | Age, sex, respiratory support type, HTN, hypercholesterolemia, heart disease, T2D, malignancy, ACE inhibitor therapy, ARB therapy, statin, diuretic, PEEP at admission, Fio2 at admission, Pao2/Fio2 at admission | Reported—several potential conflicts declared | Low |
| Guan et al. (66) | Retrospective cohort | China | 39,420 | 55.7 (NR) | NR | Mortality risk | Asthma and COPD | NR | NR | Age, sex, other systemic comorbidities | Reported—none declared | Low |
| Gupta et al. (44) | Cohort | USA | 2,215 | 60.5 | 35.2% | Mortality risk | COPD | – | “Per chart review” | Age, sex, race, hypertension, diabetes, body mass index, coronary artery disease, congestive heart failure, current smoking status, active cancer, duration of symptoms before ICU admission, and covariates assessed at ICU admission (lymphocyte count, ratio of the PaO2 to the fraction of inspired oxygen [FIO2], shock, and the kidney, liver, and coagulation components of the sequential organ failure assessment score). | Reported—several authors report conflict of interest | Low |
| Harrison et al. (45) | Retrospective cohort | USA | 31,461 | 50 (median) | 54.5% | Mortality risk | COPD | – | NR | Age, sex, ethnicity, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, rheumatic disease, peptic ulcer disease, mild liver disease, moderate/severe liver disease, diabetes, hemiplegia or paraplegia, renal disease, any malignancy, metastatic solid tumor, AIDS/HIV | Reported—several authors report conflict of interest | Low |
| Hernandez-Galdamez et al. (46) | Cross-sectional | Mexico | 211,003 | 45.7 | 45.3% | Hospitalsation risk; ICU admission risk; mortality risk | Asthma and COPD | “The information is obtained through a dichotomous questionnaire that the physician fills with the information provided by the patient.” | Age, sex, CKD, immunosuppression, diabetes, hypertension, cardiovascular disease, COPD or asthma, obesity and smoking. | Reported—none declared | Low | |
| Ho et al. (64) | Retrospective cohort | USA | 10,523 | 58.35 (18.81) | 45.8% | Hospitalsation risk; ICU admission risk; mortality risk | Asthma | NR | Age, sex, BMI, race, COVID-19 disease severity, Charleston Comorbidity Index, COPD, C-reactive protein (>150 mg/L), interleukin-6 (>80 mg/L), ferritin (>2,000 ng/L), D-dimer (>2.0 mg/L), use of anticoagulation, use of corticosteroids, and smoking (current and former). | Reported—none declared | Low | |
| Hu et al. (47) | Cohort | China | 821 | NR | NR | Mortality risk | COPD | – | “COPD patients diagnosed by lung function” | Age, sex, hypertension, diabetes, CAD, CVD, Malignancy, CKD, chronic liver disease | Reported—none declared | Low |
| Hu et al. (72) | Retrospective cohort | China | 213 | 44 (median) | NR | ICU admission risk | COPD | – | NR | Age, Dyspnoea, Poor appetite, WBC>10 ×10-9/l, D-dimer>0.5 mg/l, Albumin <35 g/L, ALT, AST, LDH. | Reported—none declared | Low |
| Jiang et al. (68) | Retrospective cohort | China | 281 | NR | NR | Mortality risk | COPD | – | NR | Age, sex, anorexia, comorbidities, CD8+ count, lymphocyte count, CRP, D-dimer, LDH, high sensitivity troponin I, osmotic pressure, PCT, and SOFA score on ICU admission | Reported—none declared | Low |
| Kammar-Garcia et al. (51) | Cohort | Mexico | 13,842 | NR | NR | Hospitalsation risk; ICU admission risk; mortality risk | Asthma and COPD | “Self-report and defined as present or absent” | Age, sex, pneumonia, diabetes, asthma or COPD, immunosuppression, hypertension, CVD, obesity, CKD, other comorbidities | Not reported | Medium | Low |
| Lee et al. (67) | Retrospective cohort | South Korea | 4,610 | NR | NR | Mortality risk | COPD | – | Medical records—Identification of COPD patients with ICD-10 codes (J43 and J44 except J43.0) | Age, sex, and Charleston Comorbidity Index score | Reported—none declared | Low |
| Li et al. (53) | Case-series | China | 204 | 68 (median) | 51% | Mortality risk | COPD | – | NR | None | Reported—none declared | Low |
| Mahdavinia et al. (52) | Case-series | USA | 1,003 | NR | NR | Hospitalsation risk; mortality risk | Asthma | “asthma diagnosis based on Global Initiative for Asthma (GINA) guidelines” | - | None | Reported—none declared | Low |
| Martos-Benitez et al. (37) | Retrospective cohort | Mexico | 38,324 | 46.9 (15.7) | 41.7% | ICU admission risk; mortality risk | COPD | – | NR | Age, sex, smoking habit, time from symptoms onset to medical contact, and all the comorbidities | Reported—none declared | Low |
| Murillo-Zamora et al. (58) | Retrospective cohort | Mexico | 66,123 | NR | NR | Mortality risk | Asthma and COPD | NR | NR | Age, sex, diagnosed pneumonia at admission, tobacco use, obesity, COPD, diabetes, arterial hypertension, immunosuppression, CKD | Reported—none declared | Low |
| Parra-Bracamonte et al. (48) | Cohort | Mexico | 331,298 | 44 (median) | 46.2% | Mortality risk | Asthma and COPD | As confirmed by dataset used—no specific method reported | Age, sex, smoking status, hospitalsation, pneumonia, hypertension, obesity, diabetes, cardiopathy, COPD or asthma, immunosuppressed, CKD, other complications. | Not reported | Low | |
| Rosenthal et al. (63) | Retrospective cohort | USA | 727 | 49.46 (17.93) | NR | Hospitalsation risk | Asthma | NR | – | Age, BMI, race, and a number of comorbidities (chronic kidney disease, coronary artery disease or congestive heart failure, diabetes, and hypertension) | Reported—none declared | Low |
| Timerlake et al. (65) | Retrospective cohort | USA | 274 | NR | NR | ICU admission risk; mortality risk | COPD | – | NR | Age, sex, race, admission diagnosis (COVID-19 vs. other), CAD, and obesity | Reported—several potential conflicts declared | Low |
| Wang et al. (61) | Case-series | China | 339 | 69 (median) | 51.0% | Mortality risk | COPD | – | NR | Age, CVD, cerebrovascular disease | Reported—none declared | Low |
| Wang et al. (62) | Retrospective cohort | China | 141 | 64 (median) | 30.0% | Mortality risk | COPD | – | NR | Ventilation status, creatinine ?104 umol/; vs. <104 umol/l and chronic renal diseases | Reported—none declared | Low |
| Wang et al. (70) | Case-series | USA | 1,827 | 54 (median) | 67.4% | Hospitalsation risk; ICU admission risk; mortality risk | COPD | - | NR | Age, sex, race, marital status, educational level, insurance type, smoking history, BMI, diabetes, CKD, CLD, CVD, HTN, allergic rhinitis | Reported—several potential conflicts declared | Low |
| Wu et al. (49) | Retrospective observational | China | 443 | NR | NR | ICU admission risk | COPD | – | NR | Age, sex, smoking status, diabetes, hypertension, coronary heart disease, cerebrovascular diseases, hepatitis B infection, cancer, chronic renal diseases, immunodeficiency. | Reported—none declared | Low |
| Yoshida et al. (71) | Case-series | USA | 776 | 60.5 (16.1) | NR | ICU admission risk; mortality risk | COPD | – | NR | Age, sex, hospital site, and the Charleston Comorbidity Index | Reported—none declared | Low |
| Zhu et al. (50) | Cohort | UK | 492,768 | NR | NR | Hospitalsation risk | Asthma | Measurement of genetic asthma phenotypes | - | Age, sex, race/ethnicity, and BMI | Reported—none declared | Low |
Meta-Analysis
Risk of COVID-19 Related Hospitalsation
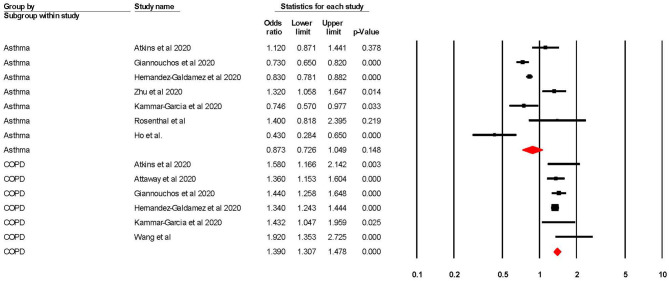
When adjusted for one or more comorbidity, the pooled aOR was 0.87 (95% CI 0.73–1.05; p = 0.15; I2 = 85.36) for asthma and 1.39 (95% CI 1.31–1.48; p = < 0.001; I2 = 4.24) for COPD (see Table 2 and Figure 2). The sensitivity analysis found that the removal of any one study did not significantly change the direction of results for either asthma or COPD (see Supplementary Figures 1, 2 for full details).
Table 2.
Meta-analysis showing the pooled adjusted risk of unfavorable COVID-19 outcomes in subjects with asthma or COPD.
| Study details | Meta-analysis | Heterogeneity | Publication bias | GRADE rating | |||
|---|---|---|---|---|---|---|---|
| Lung disease | Number of studies | Number of participants | Odds ratio (95% CI) | p-value | I2 | Egger bias and p-value | |
| Hospitalisation | |||||||
| Asthma | 7 | 1,087,689 | 0.873 (0.726–1.049) | 0.148 | 85.355 | 0.747p = 0.678 | Moderate (downgraded due to high heterogeneity) |
| COPD | 6 | 588,025 | 1.390 (1.307–1.478) | <0.001 | 4.236 | 1.453p = 0.050 | Moderate (downgraded due to possible publication bias) |
| ICU admission | |||||||
| Asthma | 4 | 167,849 | 0.746 (0.545–1.020) | 0.067 | 87.198 | −1.979p = 0.653 | Moderate (downgraded due to high heterogeneity) |
| COPD | 9 | 197,108 | 1.336 (1.139–1.566) | <0.001 | 66.643 | 1.537p = 0.075 | Moderate (downgraded due to high heterogeneity) |
| Mortality (aORs) | |||||||
| Asthma | 7 | 876,759 | 0.827 (0.711–0.961) | 0.013 | 61.481 | 0.007p = 0.996 | Moderate (downgraded due to high heterogeneity) |
| COPD | 17 | 950,502 | 1.276 (1.176–1.385) | <0.001 | 34.508 | 0.881p = 0.038 | Moderate (downgraded due to possible publication bias) |
| Mortality (aHRs from Cox regression models) | |||||||
| Asthma | 4 (5 outcomes) | 122,786 | 0.930 (0.865–1.000) | 0.049 | 64.176 | 1.400p = 0.414 | Moderate (downgraded due to high heterogeneity) |
| COPD | 8 (9 outcomes) | 123,886 | 1.296 (1.170–1.436) | <0.001 | 88.386 | 2.179p = 0.093 | Moderate (downgraded due to high heterogeneity) |
GRADE, Grading of Recommendations, Assessment, Development, and Evaluations; COPD, Chronic Obstructive Pulmonary Disease; aOR, adjusted odds ratio; aHR, adjusted hazard ratio.
Figure 2.
Forest plot showing odds ratios (adjusted for at least one confounder) for COVID-19 related hospitalisation in subjects with asthma or Chronic Obstructive Pulmonary Disease (COPD).
Risk of COVID-19 Related ICU Admission
When adjusted for one or more comorbidity, the pooled aOR was 0.75 (95% CI 0.55–1.02; p = 0.07; I2 = 87.20) for asthma and 1.34 (95% CI 1.14–1.57; p = <0.001; I2 = 66.64) for COPD (see Table 2 and Figure 3). The sensitivity analysis found that for asthma the aOR became significant with the removal of one study (46) (OR = 0.65 95% CI 0.44–0.97 p = 0.04). The removal of any one study did not significantly change the direction of results for COPD (see Supplementary Figures 3, 4 for full details).
Figure 3.
Forest plot showing odds ratios (adjusted for at least one comorbidity) for COVID-19 related intensive care admission in subjects with asthma or Chronic Obstructive Pulmonary Disease (COPD).
Risk of COVID-19 Related Mortality
When adjusted for one or more comorbidity, the pooled aOR was 0.83 (95% CI 0.71–0.96; p = 0.01; I2 = 61.48) for asthma and 1.28 (95% CI 1.18–1.39; p = < 0.001; I2 = 34.51) for COPD (see Table 2 and Figure 4). The sensitivity analysis found that for asthma the aOR became non-significant with the removal of one study (46) (OR = 0.83 95% CI 0.66–1.05 p = 0.118), and the results did not significantly change for COPD when any one study was removed (see Supplementary Figures 5, 6 for full details).
Figure 4.
Forest plot showing odds ratios (adjusted for at least one comorbidity) for COVID-19 related overall mortality in subjects with asthma or Chronic Obstructive Pulmonary Disease (COPD).
Regarding studies that reported aHRs in the form of Cox regression models, the pooled risk of mortality was 0.93 (95% CI 0.87–1.00; p = 0.049; I2 = 64.18) for asthma and 1.30 (95% CI 1.17–1.44; p = <0.001; I2 = 88.39) for COPD (see Table 2 and Figure 5). The sensitivity analysis found that the removal of any one study did not significantly change the direction of results for COPD, and the removal of any one of three studies (56, 58, 69) changed the significance of results in asthma (see Supplementary Figures 7, 8 for full details).
Figure 5.
Forest plot showing Cox regression hazard ratios (adjusted for at least one comorbidity) for COVID-19 related overall mortality in subjects with asthma or Chronic Obstructive Pulmonary Disease (COPD).
Certainty of Evidence Using the GRADE Approach
Using the GRADE (33) approach, all of the results were rated as being a “moderate” level of certainty. The two reasons why the level of evidence was not rated as “high” was because of either (1) high heterogeneity, or (2) the presence of publication bias.
Sub-Group Analyses
When sub-grouped between studies with >10 vs. <10k participants, no significant changes were found, except for in risk of mortality (as measured by Cox regression) in participants with COPD. It was found that studies with >10k participants yielded significantly lower (p = 0.001) risk of mortality (aHR = 1.13 95% CI 1.10–1.17) when compared to studies that had <10k participants (aHR = 1.59 95% CI 1.31–1.94), and also yielded lower heterogeneity in this subgroup (>10k = 36.19%; <10k = 58.32%). Although the differences between sub-groups were significant, both pooled aHRs were still, respectively, statistically significant. Full information can be found in Table 3 and in Supplementary Figures 9–16.
Table 3.
Sub-group analyses showing the pooled adjusted risk of unfavorable COVID-19 outcomes in participants with asthma or COPD stratified >10 vs. <10k participants.
| Study details | Meta-analysis | Heterogeneity | ||||
|---|---|---|---|---|---|---|
| Lung disease | Sub-group | Number of studies | Odds ratio (95% CI) | p-value | Differences between groups | I2 |
| Hospitalisation | ||||||
| Asthma | >10k | 1 | 1.400 (0.818–2.395) | 0.219 | p = 0.079 | 0.000 |
| <10k | 6 | 0.841 (0.697–1.014) | 0.070 | 86.609 | ||
| COPD | >10k | 4 | 1.374 (1.291–1.463) | <0.001 | p = 0.463 | 0.000 |
| <10k | 2 | 1.559 (1.120–2.169) | 0.008 | 67.174 | ||
| ICU admission | ||||||
| Asthma | >10k | 3 | 0.757 (0.537–1.065) | 0.110 | p = 0.748 | 91.376 |
| <10k | 1 | 0.656 (0.295–1.459) | 0.301 | 0.000 | ||
| COPD | >10k | 3 | 1.191 (0.994–1.426) | 0.058 | p = 0.077 | 69.159 |
| <10k | 6 | 1.708 (1.196–2.441) | 0.003 | 65.159 | ||
| Mortality (aORs) | ||||||
| Asthma | >10k | 6 | 0.808 (0.695–0.938) | 0.013 | p = 0.133 | 62.813 |
| <10k | 1 | 1.317 (0.708–2.450) | 0.005 | 0.000 | ||
| COPD | >10k | 7 | 1.251 (1.160–1.349) | <0.001 | p = 0.320 | 37.046 |
| <10k | 10 | 1.425 (1.115–1.821) | 0.005 | 36.935 | ||
| Mortality (aHRs from Cox regression models) | ||||||
| Asthma | >10k | 2 (3 outcomes) | 0.913 (0.852–0.978) | 0.009 | p = 0.529 | 59.036 |
| <10k | 3 | 0.993 (0.772–1.275) | 0.954 | 69.146 | ||
| COPD | >10k | 2 (3 outcomes) | 1.132 (1.097–1.168) | <0.001 | p = 0.001 | 36.191 |
| <10k | 7 | 1.590 (1.305–1.937) | <0.001 | 58.320 | ||
COPD, Chronic Obstructive Pulmonary Disease; aOR, adjusted odds ratio; aHR, adjusted hazard ratio.
Discussion
This meta-analysis included 37 studies examined the adjusted risks of COVID-19 related hospitalsation, ICU-admission, and mortality in populations with and without either asthma or COPD. The analysis suggests with a moderate level of certainty that COPD is a significant risk factor for COVID-19 related hospitalsation, ICU admission, or mortality when the risks were adjusted for at least one comorbidity. Furthermore, with a moderate level of certainty, asthma was not shown to be a significant risk factor for COVID-19 related hospitalsation, ICU admission, or mortality when adjusted for at least one comorbidity.
COPD was shown to be a significant risk factor in all three outcomes, with the sensitivity analysis reporting robustness in all outcomes. These results broadly agree with previous meta-analyses exploring similar outcomes in this population (10–14). When directly comparing reported risks, this study shows a marked decrease in mortality risk (5.69 vs. 1.25) when compared to Lippi and Henry (10), which would be expected. Although the mechanisms that underpin this risk are not clear, several hypotheses, including the increased expression of the angiotensin-converting enzyme 2 (ACE-2) in COPD patients, have been reported as COVID-19's route of entry into susceptible cells (73). Furthermore, it has been reported that morbidity and mortality in COPD patients are frequently related to acute exacerbation (12), and severe respiratory failure (67) which may add to already compromised respiratory capacity among COVID-19 patients (12, 74, 75). Moreover, the effect of smoking could be a reason why people with COPD appear to have increased COVID-19 risks; indeed, a recent systematic review and meta-analysis (76) reported that both current and former smokers have increased risks of COVID-19 related deaths, although these risks do not appear to have been adjusted for any co-variates. Further exploration into adjusted smoking risk, in particular adjusted for COPD and/or asthma presence, would be beneficial.
Other comorbidities have also been shown to be significant risk factors for unfavorable COVID-19 related outcomes including (but not limited to), hypertension (4), diabetes (5), and obesity (3). It is difficult to directly compare our results with previous data as these previous estimates report unadjusted data making true risks of each comorbidity hard to compare. We agree with Jordan et al. (20) and recommend that future studies aim to report risks based on adjustments for, at the very least, age, sex, and smoking status so that true risks can be determined. It is recommended that clinicians continue to consider COPD patients to be at greater risk of COVID-19 related morbid outcomes. Individuals with COPD should take extra precautions to ensure that exposure to COVID-19 is minimal.
Although asthma has been related to worse outcomes in other viral infections, including other forms of coronavirus (16, 17), our analysis did not suggest asthma as a significant risk factor for any of the outcomes measured in this review, apart from mortality (measured as a non-time dependent OR), however sensitivity analysis suggested that the significance of this outcome was subject to the influence of one large study. These results broadly agree with previous meta-analyses that concluded that asthma was not a significant risk factor for either mortality or “severe” health outcomes (14, 18, 35, 77). When directly comparing reported risks across these meta-analyses, this study's mortality risk is lower (0.83 and 0.93 vs. 0.96 and 1.03) (35, 77), which is an expected result given we pooled adjusted ORs and the other meta-analyses were not adjusted for any other covariates. These results, however, need to be interpreted with caution as the included studies have used asthma as an umbrella term and did not differentiate between different types or severities of the disease. The National Health Service (NHS) in the UK has severe asthma listed “high risk of severe outcomes,” and other severities at “moderate risk” of COVID-19 (78), and although this study does not support this, more data is required to differentiate between different severity of asthma, and, as such, individuals with asthma should still aim to minimize their risk of COVID-19 exposure.
Although this is the first review to systematically examine risks of unfavorable COVID-19 outcomes in populations with asthma or COPD with effect sizes adjusted for at least one covariate, our results should be considered within its limitations. Firstly, although the majority were deemed as low risk of bias, the effect of methodological bias cannot be ruled out. Secondly, the pooling of adjusted ORs (with different studies adjusting for different covariates) inherently creates a degree of inconsistency, meaning that the results should be treated only as indicative. Thirdly, there was considerable heterogeneity in some of the reported analyses, especially in the asthmatic populations, which could not be explained by the presence of large studies vs. smaller ones. One probable reason for this is the different asthma diagnosis methods, in particular regarding the type and severity of asthma. Furthermore, there was some evidence of publication bias, which could not be explained. Lastly, meta-analyses have inherent limitations: their findings are dependent on estimates selected from each primary study and thus are dependent on the accuracy of primary studies (79).
Conclusions
COPD is significantly associated with worse COVID-19 related, hospital admission, ICU admission and mortality, even when adjusted for at least one comorbidity. Asthma, when pooling risks were adjusted for other comorbidities, was not associated with a higher risk of COVID-19 related hospitalsation, ICU admission and mortality. Clinicians should note these findings when dealing with patients with these comorbidities. Furthermore, individuals with COPD should take special precautions to limit the risk of COVID-19 exposure to negate these potential outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MT and MV acquisition and analysis. MT, MV, and SP drafted the work. MT and MV verified the underlying data. All authors made substantial contributions to the conception, design of the work, interpretation of data for the work, revising it critically for important intellectual content and final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.668808/full#supplementary-material
References
- 1.World Health Organization . WHO Coronavirus Disease (COVID-19) Dashboard. (2021). Available online at: https://covid19.who.int (accessed February 3, 2021).
- 2.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. (2020) 80:e14–8. 10.1016/j.jinf.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr Clin Res Rev. (2020) 14:655–9. 10.1016/j.dsx.2020.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst JRAAS. (2020) 21:1470320320926899. 10.1177/1470320320926899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. (2020) 14:395–403. 10.1016/j.dsx.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Health Service . Overview: Chronic Obstructive Pulmonary Disease (COPD). (2019). Available online at: https://www.nhs.uk/conditions/chronic-obstructive-pulmonary-disease-copd/ (accessed January 26, 2021).
- 7.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. An official American thoracic society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2010) 182:693–718. 10.1164/rccm.200811-1757ST [DOI] [PubMed] [Google Scholar]
- 8.Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. (2006) 28:346–51. 10.1183/09031936.06.00131905 [DOI] [PubMed] [Google Scholar]
- 9.Schultze A, Walker AJ, MacKenna B, Morton CE, Bhaskaran K, Brown JP, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. (2020) 8:1106–20. 10.1016/S2213-2600(20)30415-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. (2020) 167:105941. 10.1016/j.rmed.2020.105941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung JM, Niikura M, Yang CWT, Sin DD. COVID-19 and COPD. Eur Respir J. (2020) 56:2002108. 10.1183/13993003.02108-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pranata R, Soeroto A, Huang I, Lim M, Santoso P, Permana H, et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. (2020) 24:838–43. 10.5588/ijtld.20.0278 [DOI] [PubMed] [Google Scholar]
- 13.Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS ONE. (2020) 15:e0233147. 10.1371/journal.pone.0233147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS ONE. (2020) 15:e0238215. 10.1371/journal.pone.0238215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health Service . Overview: Asthma. (2018). Available online at: https://www.nhs.uk/conditions/asthma/ (accessed January 26, 2021).
- 16.Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, et al. Viruses and bacteria in acute asthma exacerbations - A GA 2LEN-DARE* systematic review. Allergy Eur J Allergy Clin Immunol. (2011) 66:458–68. 10.1111/j.1398-9995.2010.02505.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busse WW, Lemanske RF, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. (2010) 376:826–34. 10.1016/S0140-6736(10)61380-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soeroto AY, Purwiga A, Roesli R. Asthma does not increase COVID-19 mortality and poor outcomes: a systematic review and meta-analysis. Asian Pac J Allergy Immunol. (2021). 10.12932/AP-110920-0955. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Sunjaya AP, Allida SM, Di Tanna GL, Jenkins C. Asthma and risk of infection, hospitalisation, ICU admission and mortality from COVID-19: Systematic review and meta-analysis. J Asthma. (2021) 1:1–22. 10.1080/02770903.2021.1888116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. (2020) 368:m1198. 10.1136/bmj.m1198 [DOI] [PubMed] [Google Scholar]
- 21.Choi YJ, Park J-Y, Lee HS, Suh J, Song JY, Byun MK, et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J. (2020) 57:2002226. 10.1183/13993003.02226-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cen Y, Chen X, Shen Y, Zhang X-H, Lei Y, Xu C, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—a multi-centre observational study. Clin Microbiol Infect. (2020) 26:1242–7. 10.1016/j.cmi.2020.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. (2005) 143:199–211. 10.7326/0003-4819-143-3-200508020-00006 [DOI] [PubMed] [Google Scholar]
- 24.Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. (2018) 175:631–48. 10.1176/appi.ajp.2018.17111194 [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Jung S, Linton NM, Kinoshita R, Hayashi K, Miyama T, et al. Communicating the risk of death from novel coronavirus disease (COVID-19). J Clin Med. (2020) 9:580. 10.3390/jcm9020580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 28.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta Analysis. Englewood, NJ: Biostat; (2013). [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Smith GD, Schneider M, Minder C. Bias in meta - analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. (2011) 64:1187–97. 10.1016/j.jclinepi.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 32.Sterne JA, Egger M, Moher D. Addressing Reporting Biases. Cochrane Handb Syst Rev Interv Cochrane Book Ser. Chichester: John Wiley & Sons, Ltd; (2008). 10.1002/9780470712184.ch10 [DOI] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. (2008) 336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Islam M, Riaz B, Islam A, Khanam F, Akhter J, Choudhury R, et al. Risk factors associated with morbidity and mortality outcomes of COVID-19 patients on the 28th day of the disease course: a retrospective cohort study in Bangladesh. Epidemiol Infect. (2020) 148:e263. 10.1017/S0950268820002630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Chen J, Chen W, Liu L, Dong M, Ji J, et al. Does asthma increase the mortality of patients with COVID-19?: A systematic review and meta-analysis. Int Arch Allergy Immunol. (2020) 22:1–7. 10.1159/000510953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nogueira PJ, de Araújo Nobre M, Costa A, Ribeiro RM, Furtado C, Bacelar Nicolau L, et al. The role of health preconditions on COVID-19 deaths in portugal: evidence from surveillance data of the first 20293 infection cases. J Clin Med. (2020) 9:2368. 10.3390/jcm9082368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martos-Benítez FD, Soler-Morejón CD, García-del Barco D. Chronic comorbidities and clinical outcomes in patients with and without COVID-19: a large population-based study using national administrative healthcare open data of Mexico. Intern Emerg Med. (2021) 7:1–11. 10.1007/s11739-020-02597-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, et al. Pre-existing comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci. (2020) 75:2224–30. 10.1093/gerona/glaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attaway AA, Zein J, Hatipoglu US. SARS-CoV-2 infection in the COPD population is associated with increased healthcare utilization: an analysis of Cleveland clinic's COVID-19 registry. EClinicalMedicine. (2020) 26:100515. 10.1016/j.eclinm.2020.100515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cellina M, Gibelli D, Valenti Pittino C, Toluian T, Marino P, Oliva G. Risk factors of fatal outcome in patients with COVID-19 pneumonia. Disaster Med Public Health Prep. (2020). 10.1017/dmp.2020.346. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Vito A, Geremia N, Fiore V, Princic E, Babudieri S, Madeddu G, et al. Clinical features, laboratory findings and predictors of death in hospitalized patients with COVID-19 in Sardinia, Italy. Eur Rev Med Pharmacol Sci. (2020) 24:7861–8. 10.26355/eurrev_202007_22291 [DOI] [PubMed] [Google Scholar]
- 42.Giannouchos TV, Sussman RA, Mier JM, Poulas K, Farsalinos K. Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory–confirmed COVID-19 cases. Eur Respir J. (2020). 10.1101/2020.06.04.20122481. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grandbastien M, Piotin A, Godet J, Abessolo-Amougou I, Ederlé C, Enache I, et al. SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract. (2020) 8:2600–7. 10.1016/j.jaip.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors associated with death in critically Ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. (2020) 180:1436–47. 10.1001/jamainternmed.2020.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. (2020) 17:e3321. 10.1371/journal.pmed.1003321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernández-Galdamez DR, González-Block MÁ, Romo-Dueñas DK, Lima-Morales R, Hernández-Vicente IA, Lumbreras-Guzmán M, et al. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing non-communicable diseases and modifiable risk factors in Mexico. Arch Med Res. (2020) 51:683–9. 10.1016/j.arcmed.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu W, Dong M, Xiong M, Zhao D, Zhao Y, Wang M, et al. Clinical courses and outcomes of patients with chronic obstructive pulmonary disease during the covid-19 epidemic in hubei, china. Int J COPD. (2020) 15:2237–48. 10.2147/COPD.S265004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. (2020) 52:93–8.e2. 10.1016/j.annepidem.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu F, Zhou Y, Wang Z, Xie M, Shi Z, Tang Z, et al. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: a multicenter, retrospective, observational study. J Thorac Dis. (2020) 12:1811–23. 10.21037/jtd-20-1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. (2020) 146:327–9. 10.1016/j.jaci.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.García AK, Mayo J de JV, Zertuche JMV, Hernández ML, López OV, Badilla OS, et al. Impact of comorbidities in Mexican SARS-CoV-2-positive patients: a retrospective analysis in a national cohort. Rev Invest Clin. (2020) 72:151–8. 10.24875/RIC.20000207 [DOI] [PubMed] [Google Scholar]
- 52.Mahdavinia M, Foster KJ, Jauregui E, Moore D, Adnan D, Andy-Nweye AB, et al. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. (2020) 8:2388–91. 10.1016/j.jaip.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li P, Chen L, Liu Z, Pan J, Zhou D, Wang H, et al. Clinical features and short-term outcomes of elderly patients with COVID-19. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. (2020) 97:245–50. 10.1016/j.ijid.2020.05.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi H, Wee JH, Kim SY, Kim J, Kim HI, Park J, et al. Association between asthma and clinical mortality/morbidity in COVID-19 patients using clinical epidemiologic data from Korean disease control and prevention. Allergy. (2020) 76:921–4. 10.22541/au.160279752.20065578/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aveyard P, Gao M, Lindson N, Hartmann-Boyce J, Watkinson P, Young D, et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med. (2021) 76:921–24. 10.1016/S2213-2600(21)00095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girardin J-L, Seixas A, Ramos Cejudo J, Osorio RS, Avirappattu G, Reid M, et al. Contribution of pulmonary diseases to COVID-19 mortality in a diverse urban community of New York. Chron Respir Dis. (2021) 18:1479973120986806. 10.1177/1479973120986806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Vito A, Fiore V, Princic E, Geremia N, Panu Napodano CM, Muredda AA, et al. Predictors of infection, symptoms development, and mortality in people with SARS-CoV-2 living in retirement nursing homes. PLoS ONE. (2021) 16:e0248009. 10.1371/journal.pone.0248009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murillo-Zamora E, Hernandez-Suarez CM. Survival in adult inpatients with COVID-19. Public Health. (2021) 190:1–3. 10.1016/j.puhe.2020.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azoulay E, Fartoukh M, Darmon M, Géri G, Voiriot G, Dupont T, et al. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. (2020) 46:1714–22. 10.1007/s00134-020-06202-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. (2020) 180:1345–55. 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. (2020) 80:639–45. 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang T, Tang C, Chen R, Ruan H, Liang W, Guan W, et al. Clinical features of coronavirus disease 2019 patients with mechanical ventilation: a nationwide study in China. Crit Care Med. (2020) 48:e809–12. 10.1097/CCM.0000000000004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenthal JA, Awan SF, Fintzi J, Keswani A, Ein D. Asthma is associated with increased risk of intubation but not hospitalization or death in coronavirus disease 2019. Ann Allergy Asthma Immunol. (2021) 126:93–5. 10.1016/j.anai.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho KS, Howell D, Rogers L, Narasimhan B, Verma H, Steiger D. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Ann Allergy Asthma Immunol. (2021) 1–7. 10.1016/j.anai.2021.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Timberlake DT, Narayanan D, Ogbogu PU, Raveendran R, Porter K, Scherzer R, et al. Severity of COVID-19 in hospitalized patients with and without atopic disease. World Allergy Organ J. (2021) 14:100508. 10.1016/j.waojou.2021.100508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan W, Liang W, Shi Y, Gan L, Wang H, He J, et al. Chronic respiratory diseases and the outcomes of COVID-19: A nationwide retrospective cohort study of 39,420 cases. J Allergy Clin Immunol Pract. (2021). 10.1016/j.jaip.2021.02.041. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SC, Son KJ, Han CH, Park SC, Jung JY. Impact of COPD on COVID-19 prognosis: a nationwide population-based study in South Korea. Sci Rep. (2021) 11:1–8. 10.1038/s41598-021-83226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang Y, Abudurexiti S, An M-M, Cao D, Wei J, Gong P. Risk factors associated with 28-day all-cause mortality in older severe COVID-19 patients in Wuhan, China: a retrospective observational study. Sci Rep. (2020) 10:1–13. 10.1038/s41598-020-79508-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bloom CI, Drake TM, Docherty AB, Lipworth BJ, Johnston SL, Nguyen-Van-Tam JS, et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. (2021). 10.1016/S2213-2600(21)00013-8. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L, Foer D, Bates DW, Boyce JA, Zhou L. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. (2020) 146:808–12. 10.1016/j.jaci.2020.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshida Y, Gillet SA, Brown MI, Zu Y, Wilson SM, Ahmed SJ, et al. Clinical characteristics and outcomes in women and men hospitalized for coronavirus disease 2019 in New Orleans. Biol Sex Differ. (2021) 12:1–11. 10.1186/s13293-021-00359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu X, Hu C, Yang Y, Chen J, Zhong P, Wen Y, et al. Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China. Ther Adv Respir Dis. (2020) 14:1753466620963035. 10.1177/1753466620963035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leung JM, Yang CX, Tam A, Shaipanich T, Hackett T-L, Singhera GK, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. (2020) 55:2000688. 10.1183/13993003.00688-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. (2006) 173:1114–21. 10.1164/rccm.200506-859OC [DOI] [PubMed] [Google Scholar]
- 75.Rohde G, Wiethege A, Borg I, Kauth M, Bauer T, Gillissen A, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. (2003) 58:37–42. 10.1136/thorax.58.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Umnuaypornlert A, Kanchanasurakit S, Lucero-Prisno DEI, Saokaew S. Smoking and risk of negative outcomes among COVID-19 patients: a systematic review and meta-analysis. Tob Induc Dis. (2021) 19:9. 10.18332/tid/132411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Ao G, Qi X, Xie B. The association between COVID-19 and asthma: a systematic review and meta-analysis. Clin Exp Allergy J Br Soc Allergy Clin Immunol. (2020) 50:1274–7. 10.1111/cea.13733 [DOI] [PubMed] [Google Scholar]
- 78.National Health Service . Who's at Higher Risk From Coronavirus. (2021). Available online at: https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/whos-at-higher-risk-from-coronavirus/ (accessed January 28, 2021).
- 79.IOANNIDIS JPA. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. (2016) 94:485–514. 10.1111/1468-0009.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.