Abstract
In the absence of effective countermeasures, human convalescent plasma has been widely used to treat severe acute respiratory syndrome coronavirus 2, the causative agent of novel coronavirus disease 19 (COVID‐19), including among patients with innate or acquired immunosuppression. However, the association between COVID‐19‐associated mortality in patients with immunosuppression and therapeutic use of convalescent plasma is unknown. We review 75 reports, including one large matched‐control registry study of 143 COVID‐19 patients with hematological malignancies, and 51 case reports and 23 case series representing 238 COVID‐19 patients with immunosuppression. We review clinical features and treatment protocols of COVID‐19 patients with immunosuppression after treatment with human convalescent plasma. We also discuss the time course and clinical features of recovery. The available data from case reports and case series provide evidence suggesting a mortality benefit and rapid clinical improvement in patients with several forms of immunosuppression following COVID‐19 convalescent plasma transfusion. The utility of convalescent plasma or other forms of antibody therapy in immune‐deficient and immune‐suppressed patients with COVID‐19 warrants further investigation.
Keywords: FFP transfusion, transplantation—solid organ, transfusion practices (oncology–hematology)
1. INTRODUCTION
Convalescent plasma is a passive antibody therapy that has been used to prevent or treat infectious diseases for more than a century. 1 , 2 In the absence of effective countermeasures, human convalescent plasma has been widely used to treat severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causative agent of novel coronavirus disease 19 (COVID‐19). Convalescent plasma has received full or conditional regulatory authorization in the United States and many other countries for therapeutic use in adults and children hospitalized with suspected or laboratory‐confirmed SARS‐CoV‐2 positive COVID‐19. 3 , 4 The evidence supporting the use of convalescent plasma to treat patients with COVID‐19 is not definitive. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 There is evidence that supports the therapeutic use of convalescent plasma among patients with COVID‐19 who are treated earlier in the disease course using plasma with sufficient antibody levels. 18 , 19 In contrast, however, several large clinical trials that transfused severely ill patients later in the disease course have suggested there is no mortality benefit conferred from COVID‐19 convalescent plasma. 16 , 17
Although there are many studies evaluating the clinical efficacy of convalescent plasma in otherwise immunocompetent patients, there is a paucity of studies evaluating the use of convalescent plasma in COVID‐19 patients with immunosuppression or immunodeficiency. Patients with immunosuppression or immunodeficiency have been disproportionately affected by the COVID‐19 pandemic, 20 and often present with persistent SARS‐CoV‐2 infection and may shed viable SARS‐CoV‐2 for months. 21 In this context, we summarize the growing number of contemporary case reports and case series of the clinical experiences of COVID‐19 patients with primary and secondary immunosuppression who were treated with specific neutralizing antibodies via COVID‐19 convalescent plasma transfusion. Importantly, evaluation of the clinical responses to convalescent plasma transfusion in immunosuppressed patients who cannot generate innate immune responses may provide an optimal opportunity to assess the effect of convalescent plasma per se.
2. METHODS
We included investigations of the impact of human convalescent plasma therapy on COVID‐19 patient mortality in patients with primary or secondary immunosuppression, which included case series, case reports, and media reports in lay press. References for this review were identified through searches of the online PubMed and MEDLINE databases for articles published from January 1, 2020 to April 10, 2021. Keywords used in the search included: (convalescent plasma or convalescent serum) AND COVID‐19 (and Medical Subject Headings terms; MeSH terms). During review of abstracts, additional keywords used to search for relevant articles included: immunosuppressed, immunocompromised, immunodeficient, cancer, transplant, malignancy, and agammaglobulinemia. Relevant articles and data were also identified through non‐systematic searches in Google Scholar and medRΧiv. Articles resulting from these searches and relevant references cited in those articles were examined.
To be considered eligible for inclusion, studies must have: (1) included hospitalized patients with primary or secondary immunosuppression and a confirmed diagnosis of COVID‐19, (2) used convalescent plasma as a COVID‐19 treatment, and (3) reported patient mortality. Using an a posteriori approach, studies were further restricted to include only the following: (1) hypogammaglobulinemia or x‐linked agammaglobulinemia, (2) common variable immune deficiency, (3) hematological malignancy, or (4) solid organ transplants. Although our search yielded articles describing use of COVID‐19 convalescent plasma in the context of other potentially relevant conditions (myasthenia gravis, 22 trisomy 21, 23 Sjögrens syndrome, 24 hemodialysis, 25 and rheumatoid arthritis 26 , 27 ), there were very few represented patient cases, thus, our a posteriori restriction provided more robust evidence on fewer cohorts of patients. Two reviewers (J.W.S and S.A.K.) independently screened the titles and abstracts of all studies identified by the search to determine eligibility. Studies that were deemed potentially eligible had their full text reviewed (J.W.S and S.A.K.) to determine if they met the criteria for inclusion in the review. Disagreement was resolved by consensus. Three reviewers (J.W.S, S.A.K., and S.K.F.) extracted study and patient characteristics as well as clinical information, and extracted data were independently reviewed (K.A.S.). All procedures accessed public information and did not require ethical review as determined by the Mayo Clinic Institutional Review Board in accordance with the Code of Federal Regulations, 45 CFR 46.102, and the Declaration of Helsinki.
Disease severity of COVID‐19 was delineated using a six‐level ordinal scale, with higher scores indicating more progressed clinical course of COVID‐19 at the time of convalescent plasma transfusion. 28 Scores on the ordinal scale were define as follows: a score of 1 indicated not hospitalized; 2, hospitalized and not receiving supplemental oxygen; 3, hospitalized and receiving supplemental oxygen; 4, hospitalized and receiving oxygen supplementation administered by a high‐flow nasal cannula or noninvasive ventilation; 5, hospitalized and receiving extracorporeal membrane oxygenation or invasive mechanical ventilation; and 6, death.
In all studies but one large matched‐control study, 29 there were no appropriate and comparable control groups. Thus, no analytical statistics analyses were performed, and no novel measures of probability are reported. Descriptive statistics are presented as frequencies and percentages based on numerical values reported in primary literature. These descriptive statistics should not be used to infer definitive treatment effects.
2.1. Aggregate results
The literature search yielded 831 reports, of which 75 reports met the eligibility criteria and were included in the systematic review (Figure 1). This review includes one large matched‐control registry study of 143 patients with hematological malignancies, and 51 case reports and 23 case series representing 238 COVID‐19 patients with primary immunosuppression due to Agammaglobulinemia (X‐linked or autosomal) or Common Variable Immunodeficiency, and secondary immunosuppression related to hematological malignancies and solid organ transplants who were transfused with convalescent plasma. A summary overview of patient characteristics, COVID‐19 therapies used (including convalescent plasma), and clinical symptomology of these patients is available as Tables S1–S3. Mortality among these hospitalized COVID‐19 patients with immunosuppression who were treated with convalescent plasma was 16% (60 of 381 patients) with ~60% of patients demonstrating rapid clinical improvement within 5 days of convalescent plasma therapy, see Table 1.
FIGURE 1.
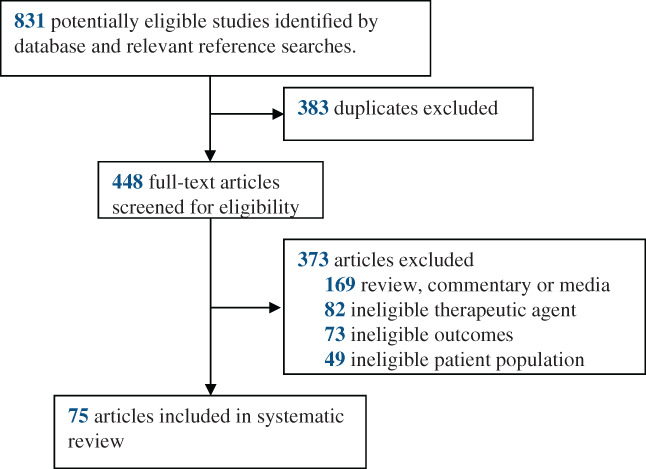
Flow chart of the study selection [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Summary of hospital course and disposition of coronavirus disease 19 (COVID‐19) patients with immunosuppression after transfusion of convalescent plasma
| Condition | No. patients | COVID‐19 disease severity scale a | Illness onset to treatment (days) a | Mortality (n, %) a | Rapid improvement in supplemental oxygen (≤5 days) (n, %) a | Discharge (days) a |
|---|---|---|---|---|---|---|
| Primary immunosuppression | ||||||
| Agammaglobulinemia | 15 | 3 (2–5) | 27 (12–69) | 1, 7% | 3 of 6, 50% | 10 (1–50) |
| Common variable immune deficiency | 7 | 3 (2–5) | 20 (11–28) | 1, 14% | 2 of 2, 100% | 10 (7–13) |
| Secondary immunosuppression | ||||||
| Hematological malignancies | 150 | 3 (2–5) | 26 (2–103) | 30, 20% | 37 of 55, 67% | 27 (1–148) |
| Solid organ transplants | 66 | 3 (2–5) | 9 (2–31) | 9, 14% | 25 of 37, 68% | 18 (2–58) |
Note: Data are presented as mean (range), count (n), or mean (%).
Data were not reported for all patients, please see Tables S1–S3 for additional information. WHO Disease Severity Scale: 1 (not hospitalized), 2 (hospitalized, no supplemental oxygen), 3 (hospitalized, non‐high flow supplemental oxygen), 4 (hospitalized, high flow supplemental oxygen), 5 (hospitalized, intubated or extracorporeal membrane oxygenation), 6 (deceased).
3. PRIMARY IMMUNODEFICIENCY
3.1. Agammaglobulinemia
Patients with Agammaglobulinemia do not produce endogenous antibodies and require regular intravenous infusions or subcutaneous injections of immunoglobulins to avoid serial infections from common pathogens. 30 However, immunoglobulin replacement therapy cannot protect immunosuppressed patients against pathogens for which antibodies are uncommon or absent in the immunoglobulin donor pool, such as the SARS‐CoV‐2 virus. 30 We identified 15 patients from six case reports 31 , 32 , 33 , 34 , 35 , 36 and three case series. 37 , 38 , 39 The COVID‐19 patients with agammaglobulinemia were predominantly male and primarily young to middle‐aged (range, 3–40 years). These patients also had a wide range of disease severity (range, 2–5) assessed using a six‐level ordinal scale to assess the clinical course of COVID‐19. 28 Overall, the observed mortality rate was 7% (1 of 15 patients) and rapid improvement in supplemental oxygen was observed in three of six patients (50%) that reported on these metrics. In a case series, three male patients with X‐linked Agammaglobulinemia (aged 10, 24 and 40 years) with prolonged COVID‐19 who required oxygen support were treated with broad‐spectrum antibiotics with limited or no clinical improvement. 37 Each patient then received convalescent plasma, resulting in rapid clinical improvement in all three patients and hospital discharge within 72 hours for the two adult patients. 37
3.2. Common variable immunodeficiency
Common variable immunodeficiency represents a heterogeneous collection of immunodeficiencies commonly characterized by intrinsic B‐cell defects and suppressed antibody production. 40 Patients with common variable immunodeficiency often present with inflammatory and autoimmune disorders, which are suspected to elevate patient risk for progression to severe COVID‐19. 38 , 40 We identified seven patients with common variable immunodeficiency from three case reports 39 , 41 , 42 and one case series. 38 Among these seven patients, ~50% were female, ~50% were receiving mechanical ventilation or extracorporeal membrane oxygenation, and one mortality was observed. In the case series, four patients with antibody‐deficient common variable immunodeficiency diagnosed with severe or life‐threatening COVID‐19 were transfused with convalescent plasma for COVID‐19 therapy. 38 Three of these patients survived following convalescent plasma transfusion, including two patients whose clinical symptomatology required mechanical ventilation or extracorporeal membrane oxygenation. In two case studies of a young male (37 years) and young female (25 years) who had life‐threatening COVID‐19 which required mechanical ventilation or extracorporeal membrane oxygenation, both patients demonstrated rapid clinical improvement resulting in successful weaning from invasive ventilation within 48 h. 41 , 42
These data from patients with primary immunodeficiencies suggest that COVID‐19 convalescent plasma could be a valuable therapeutic approach, but further studies should be performed to determine the therapeutic outcomes of convalescent plasma transfusion in patients with abnormal capacity to produce antibody responses.
4. SECONDARY IMMUNODEFICIENCY
4.1. Hematological malignancy
Hematological malignancies are associated with deficits in both humoral and cellular immunity which may contribute to increased risk of progression to severe COVID‐19. 29 Many treatments of hematologic malignancies, such as the cornerstone of treatment (anti‐CD‐20 monoclonal antibodies), may lead to prolonged B‐cell depletion and impaired immune responses. 43 , 44 From 32 case reports 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 and 11 case series, 27 , 44 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 we identified 150 COVID‐19 patients with hematological malignancies treated with convalescent plasma. Among these 150 patients, the mortality rate was 20% (30 of 150 patients), 37 patients demonstrated rapid clinical improvement after convalescent plasma transfusion, and the average time between convalescent plasma transfusion and hospital discharge was 27 days. In a case series, 17 patients with B‐cell lymphopenia and protracted COVID‐19 received convalescent plasma with high neutralizing antibody titers. 44 Although patients received plasma late in the disease course (7–83 days after symptom onset), 16 patients demonstrated improvement of clinical symptoms and reduction in SARS‐CoV‐2 RNAemia within 7–14 days. One patient, who required mechanical ventilation, died. 44 Similarly, in a separate cohort of 14 patients with hematological malignancies, 78 most patients exhibited improvement in clinical symptomatology, including reduced oxygen requirements, after treatment with convalescent plasma. Notably, a patient with protracted COVID‐19, evidenced by three separate COVID‐19‐related hospitalizations over a 100+ day period, and with lymphoma‐associated B‐cell immunodeficiency demonstrated rapid reductions in fever, oxygen requirements, and lung infiltrates (via chest computed tomography) forthwith after two separate convalescent plasma transfusions separated by ~90 days. 46
At the time of writing, there is one large matched‐control study using registry data from the COVID‐19 and Cancer Consortium (CCC19) international consortium. 29 The 30‐day mortality was evaluated in hospitalized adults with hematologic malignancy and COVID‐19, comparing 143 patients transfused with convalescent plasma and 823 matched controls who received standard care treatment. Death within 30 days occurred in 13.3% (19 of 143 patients) in the patients transfused with convalescent plasma and 24.8% (204 of 823 patients) in the matched control group. Among these patients with hematologic malignancy, convalescent plasma treatment was associated with a lower risk of death after adjustment for potential confounding factors (hazard ratio, 0.60; 95% confidence interval [CI], 0.37–0.97) or after propensity‐score matching (hazard ratio, 0.52; 95% CI, 0.29–0.92). Although these data are consistent with a mortality benefit associated with administration of COVID‐19 convalescent plasma in the context of hematological malignancy, this observational study should not be inferred for causality.
4.2. Solid organ transplant
Recipients of solid organ transplants are known to be vulnerable to viral infections secondary to a weakened T‐cell mediated immune response. 86 From 10 case reports 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 and 10 case series, 25 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 we identified 66 COVID‐19 patients who received a solid organ transplant and who were treated with convalescent plasma. Of these 66 patients, mortality was observed in nine patients (14%), 4 patients remain hospitalized at the end of the observation period, and 25 patients demonstrated rapid improvement in clinical symptomatology. In a case series, 13 hospitalized patients with COVID‐19 who were solid organ transplant recipients were transfused with two units of convalescent plasma and received concomitant therapies, primarily broad spectrum antibiotics and hydroxychloroquine. 97 Nine patients were discharged home without readmission, of which eight had de‐escalating oxygen support within 7 days of plasma transfusion. One patient had a prolonged hospital admission and three patients died. Of the three patients who expired, two patients received plasma more than 2 weeks after symptom onset—a timeframe which may be associated with a reduced mortality benefit of COVID‐19 convalescent plasma. 106 These data are consistent with a recent systematic review of clinical outcomes of COVID‐19 among solid organ transplant recipients. 86
5. CONCLUSIONS
The available data from case reports and case series provide evidence suggesting a mortality benefit and rapid clinical improvement in patients with several forms of immunosuppression following COVID‐19 convalescent plasma transfusion. In contrast to ongoing studies of convalescent plasma efficacy in clinical trials where the majority of patients are otherwise immunocompetent and thus mount their own protective antibody responses, 5 convalescent plasma use in immunosuppressed patients represents a situation where exogenous antibody is given in the setting of an immune deficit. However, there is some hesitancy about using convalescent plasma due to concern that it could select for the emergence of SARS‐CoV‐2 variants, given reports that new SARS‐CoV‐2 variants were found in a COVID‐19 patient with immunosuppression treated with plasma. 67 As described elsewhere, 107 it is highly unlikely that convalescent plasma is the source of SARS‐CoV‐2 variants currently circulating, rather these variants were more likely selected by natural immune responses in infected patients and convalescent plasma may be one of few potential therapeutics for emerging SARS‐CoV‐2 variants.
The case series and case reports summarized here provide evidence suggesting a mortality benefit and rapid clinical improvement in patients with several forms of immunosuppression following COVID‐19 convalescent plasma transfusion. These findings were an important component of the scientific evidence considered by the US Food and Drug Administration suggesting a longer potential therapeutic window for COVID‐19 convalescent plasma among immunosuppressed or immunodeficient patients than is evident in immunocompetent patients. Although these summary findings are encouraging for the use of therapeutic convalescent plasma in COVID‐19 patients with immunosuppression, well‐controlled, published data in these populations remain lacking, including only one large matched treatment‐control study 29 and no randomized controlled trials. As such, the data should not be used to infer definitive treatment effects. The utility of convalescent plasma or other forms of antibody therapy in immunosuppressed or immunodeficient patients with COVID‐19 warrants further investigation.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Table S1. Descriptive summary of COVID‐19 patients with immunosuppression who received convalescent plasma
Table S2. Clinical therapeutic summary of COVID‐19 patients with immunosuppression who received convalescent plasma
Table S3. Clinical Summary of COVID‐19 patients with immunosuppression who received convalescent plasma.
ACKNOWLEDGMENTS
This research was supported, in part, by National Heart, Lung, and Blood Institute (F32HL154320 to JWS; 5R35HL139854 to MJJ; RO1 HL059842 to AC) and Natural Sciences and Engineering Research Council of Canada (PDF‐532926‐2019 to SAK).
Senefeld JW, Klassen SA, Ford SK, et al. Use of convalescent plasma in COVID‐19 patients with immunosuppression. Transfusion. 2021;61:2503–2511. 10.1111/trf.16525
Jonathon W. Senefeld, Stephen A. Klassen, Shane K. Ford, Nigel S. Paneth, Arturo Casadevall, and Michael J. Joyner contributed equally to this study.
Funding information Natural Sciences and Engineering Research Council of Canada, Grant/Award Number: PDF‐532926‐2019; National Heart, Lung, and Blood Institute, Grant/Award Numbers: 5R35HL139854, F32HL154320, R01HL059842
REFERENCES
- 1. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130:1545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ripoll JG, van Helmond N, Senefeld JW, Wiggins CC, Klassen SA, Baker SE, et al. Convalescent plasma for infectious diseases: historical framework and use in COVID‐19. Clin Microbiol Newsl. 2021;43:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID‐19. J Clin Invest. 2020;130:2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration . Clinical memorandum for the emergency use authorization of COVID‐19 convalescent plasma, 2020.
- 5. Klassen SA, Senefeld JW, Johnson PW, Carter RE, Wiggins CC, Shoham S, et al. The effect of convalescent plasma therapy on mortality among patients with COVID‐19: Systematic review and meta‐analysis. Mayo Clin Proc. 2021;96(5):1262–1275. 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID‐19) patients transfused early with convalescent plasma containing high‐titer anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike protein IgG. Am J Pathol. 2021;191:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, Christensen PA, et al. Treatment of coronavirus disease 2019 (COVID‐19) patients with convalescent plasma. Am J Pathol. 2020;190:1680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rasheed AM, Fatak DF, Hashim HA, Maulood MF, Kabah KK, Almusawi YA, et al. The therapeutic potential of convalescent plasma therapy on treating critically‐ill COVID‐19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 2020;28:357–66. [PubMed] [Google Scholar]
- 9. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hegerova L, Gooley TA, Sweerus KA, Maree C, Bailey N, Bailey M, et al. Use of convalescent plasma in hospitalized patients with COVID‐19: case series. Blood. 2020;136:759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng QL, Yu ZJ, Gou JJ, Li GM, Ma SH, Zhang GF, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xia X, Li K, Wu L, Wang Z, Zhu M, Huang B, et al. Improved clinical symptoms and mortality among patients with severe or critical COVID‐19 after convalescent plasma transfusion. Blood. 2020;136:755–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abolghasemi H, Eshghi P, Cheraghali AM, Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S, et al. Clinical efficacy of convalescent plasma for treatment of COVID‐19 infections: Results of a multicenter clinical study. Transfus Apher Sci. 2020;59:102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rogers R, Shehadeh F, Mylona EK, Rich J, Neill M, Touzard‐Romo F, et al. Convalescent plasma for patients with severe COVID‐19: a matched cohort study. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altuntas F, Ata N, Yigenoglu TN, Basci S, Dal MS, Korkmaz S, et al. Convalescent plasma therapy in patients with COVID‐19. Transfus Apher Sci. 2020;60:102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horby PW, Estcourt L, Peto L, Emberson JR, Staplin N, Spata E, et al. Convalescent plasma in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. medRxiv. 2021. 10.1101/2021.03.09.21252736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vazquez C, et al. A randomized trial of convalescent plasma in Covid‐19 severe pneumonia. N Engl J Med. 2021;384:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. Convalescent plasma antibody levels and the risk of death from Covid‐19. N Engl J Med. 2021;384(11):1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Libster R, Perez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384(7):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenbaum L. The untold toll—the Pandemic's effects on patients without Covid‐19. N Engl J Med. 2020;382:2368–71. [DOI] [PubMed] [Google Scholar]
- 21. Aydillo T, Gonzalez‐Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of viable SARS‐CoV‐2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aksoy E, Oztutgan T. COVID‐19 presentation in association with myasthenia gravis: a case report and review of the literature. Case Rep Infect Dis. 2020;2020:8845844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez Z, Shane AL, Verkerke H, Lough C, Zimmerman MG, Suthar M, et al. COVID‐19 convalescent plasma clears SARS‐CoV‐2 refractory to remdesivir in an infant with congenital heart disease. Blood Adv. 2020;4:4278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol. 2020;92:1890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindemann M, Krawczyk A, Dolff S, Konik M, Rohn H, Platte M, et al. SARS‐CoV‐2‐specific humoral and cellular immunity in two renal transplants and two hemodialysis patients treated with convalescent plasma. J Med Virol. 2021;93:3047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerber V, Velay A, Boehn L, Solis M, Kaeuffer C, Rougier E, et al. Protracted SARS‐CoV‐2 pneumonia with rituximab treatment: about two cases. J Med Virol. 2021;93(7):4141–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kenig A, Ishay Y, Kharouf F, Rubin L. Treatment of B‐cell depleted COVID‐19 patients with convalescent plasma and plasma‐based products. Clin Immunol. 2021;227:108723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without azithromycin in mild‐to‐moderate Covid‐19. N Engl J Med. 2020;383:2041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson MA, Henderson JP, Shah PK, Rubinstein SM, Joyner MJ, Choueiri TK, et al. Convalescent plasma and improved survival in patients with hematologic malignancies and COVID‐19. medRxiv. 2021. 10.1101/2021.02.05.21250953. [DOI] [Google Scholar]
- 30. El‐Bohy M, Poowuttikul P, Secord E. Humoral immune deficiencies of childhood. Pediatr Clin North Am. 2019;66:897–903. [DOI] [PubMed] [Google Scholar]
- 31. Mira E, Yarce OA, Ortega C, Fernandez S, Pascual NM, Gomez C, et al. Rapid recovery of a SARS‐CoV‐2‐infected X‐linked agammaglobulinemia patient after infusion of COVID‐19 convalescent plasma. J Allergy Clin Immunol Pract. 2020;8:2793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Milosevic I, Jovanovic J, Stevanovic O. Atypical course of COVID‐19 in patient with Bruton agammaglobulinemia. J Infect Dev Ctries. 2020;14:1248–51. [DOI] [PubMed] [Google Scholar]
- 33. Flaherty C. Man leaves hospital after three month battle with Covid‐19. Impartial Reporter 2020.
- 34. Hovey JG, Tolbert D, Howell D. Burton's agammaglobulinemia and COVID‐19. Cureus. 2020;12:e11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iaboni A, Wong N, Betschel SD. A patient with X‐linked Agammaglobulinemia and COVID‐19 infection treated with Remdesivir and convalescent plasma. J Clin Immunol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buckland MS, Galloway JB, Fhogartaigh CN, Meredith L, Provine NM, Bloor S, et al. Treatment of COVID‐19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun. 2020;11:6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jin H, Reed JC, Liu STH, Ho HE, Lopes JP, Ramsey NB, et al. Three patients with X‐linked agammaglobulinemia hospitalized for COVID‐19 improved with convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(10):3594–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meyts I, Bucciol G, Quinti I, Neven B, Fischer A, Seoane E, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147(2):520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ho HE, Mathew S, Peluso MJ, Cunningham‐Rundles C. Clinical outcomes and features of COVID‐19 in patients with primary immunodeficiencies in new York City. J Allergy Clin Immunol Pract. 2021;9:490–3.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abbott JK, Gelfand EW. Common variable immunodeficiency: diagnosis, management, and treatment. Immunol Allergy Clin North Am. 2015;35:637–58. [DOI] [PubMed] [Google Scholar]
- 41. Van Damme KFA, Tavernier S, Van Roy N, De Leeuw E, Declercq J, Bosteels C, et al. Case report: convalescent plasma, a targeted therapy for patients with CVID and severe COVID‐19. Front Immunol. 2020;11:596761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ribeiro LC, Benites BD, Ulaf RG, Nunes TA, Costa‐Lima C, Addas‐Carvalho M, et al. Rapid clinical recovery of a SARS‐CoV‐2 infected common variable immunodeficiency patient following the infusion of COVID‐19 convalescent plasma. Allergy Asthma Clin Immunol. 2021;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sacco KA, Abraham RS. Consequences of B‐cell‐depleting therapy: hypogammaglobulinemia and impaired B‐cell reconstitution. Immunotherapy. 2018;10:713–28. [DOI] [PubMed] [Google Scholar]
- 44. Hueso T, Pouderoux C, Pere H, Beaumont AL, Raillon LA, Ader F, et al. Convalescent plasma therapy for B‐cell‐depleted patients with protracted COVID‐19. Blood. 2020;136:2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, et al. Case study: prolonged infectious SARS‐CoV‐2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–12.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baang JH, Smith C, Mirabelli C, Valesano AL, Manthei DM, Bachman M, et al. Prolonged SARS‐CoV‐2 replication in an immunocompromised patient. J Infect Dis. 2021;223(1):23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Balashov D, Trakhtman P, Livshits A, Kovalenko I, Tereshenko G, Solopova G, et al. SARS‐CoV‐2 convalescent plasma therapy in pediatric patient after hematopoietic stem cell transplantation. Transfus Apher Sci. 2021;60(1):102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cinar OE, Sayinalp B, Aladag Karakulak E, Avsar Karatas A, Velet M, Inkaya AC, et al. Convalescent (immune) plasma treatment in a myelodysplastic COVID‐19 patient with disseminated tuberculosis. Transfus Apher Sci. 2020;59:102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clark E, Guilpain P, Filip IL, Pansu N, Le Bihan C, Cartron G, et al. Convalescent plasma for persisting COVID‐19 following therapeutic lymphocyte depletion: a report of rapid recovery. Br J Haematol. 2020;190:e154–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hartman W, Hess A, Connor J. Use of COVID‐19 convalescent plasma as prophylaxis in a patient with new onset ALL. Clin Oncol Case Rep. 2021;4(1). [Google Scholar]
- 51. Honjo K, Russell RM, Li R, Liu W, Stoltz R, Tabengwa EM, et al. Convalescent plasma‐mediated resolution of COVID‐19 in a patient with humoral immunodeficiency. Cell Rep Med. 2021;2:100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karatas A, Inkaya AC, Demiroglu H, Aksu S, Haziyev T, Cinar OE, et al. Prolonged viral shedding in a lymphoma patient with COVID‐19 infection receiving convalescent plasma. Transfus Apher Sci. 2020;59(5):102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lancman G, Mascarenhas J, Bar‐Natan M. Severe COVID‐19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. London J, Boutboul D, Lacombe K, Pirenne F, Heym B, Zeller V, et al. Severe COVID‐19 in patients with B cell Alymphocytosis and response to convalescent plasma therapy. J Clin Immunol. 2021;41:356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lubnow M, Schmidt B, Fleck M, Salzberger B, Muller T, Peschel G, et al. Secondary hemophagocytic lymphohistiocytosis and severe liver injury induced by hepatic SARS‐CoV‐2 infection unmasking Wilson's disease: balancing immunosuppression. Int J Infect Dis. 2021;103:624–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Luetkens T, Metcalf R, Planelles V, Zheng Y, Larragoite ET, Spivak ES, et al. Successful transfer of anti‐SARS‐CoV‐2 immunity using convalescent plasma in an MM patient with hypogammaglobulinemia and COVID‐19. Blood Adv. 2020;4:4864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Malsy J, Veletzky L, Heide J, Hennigs A, Gil‐Ibanez I, Stein A, et al. Sustained response after remdesivir and convalescent plasma therapy in a B‐cell depleted patient with protracted COVID‐19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moore JL, Ganapathiraju PV, Kurtz CP, Wainscoat B. A 63‐year‐old woman with a history of non‐Hodgkin lymphoma with persistent SARS‐CoV‐2 infection who was seronegative and treated with convalescent plasma. Am J Case Rep. 2020;21:e927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Niu A, McDougal A, Ning B, Safa F, Luk A, Mushatt DM, et al. COVID‐19 in allogeneic stem cell transplant: high false‐negative probability and role of CRISPR and convalescent plasma. Bone Marrow Transplant. 2020;55:2354–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rodriguez JA, Bonnano C, Khatiwada P, Roa AA, Mayer D, Eckardt PA. COVID‐19 coinfection with mycobacterium abscessus in a patient with multiple myeloma. Case Rep Infect Dis. 2021;2021:8840536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shankar R, Radhakrishnan N, Dua S, Arora S, Rana M, Sahu DK, et al. Convalescent plasma to aid in recovery of COVID‐19 pneumonia in a child with acute lymphoblastic leukemia. Transfus Apher Sci. 2021;60(1):102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang B, Van Oekelen O, Mouhieddine TH, Del Valle DM, Richter J, Cho HJ, et al. A tertiary center experience of multiple myeloma patients with COVID‐19: lessons learned and the path forward. J Hematol Oncol. 2020;13:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wright Z, Bersabe A, Eden R, Cap A. Successful use of COVID‐19 convalescent plasma in a patient recently treated for follicular lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21(1):66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang LL, Liu Y, Guo YG, Chang J, Gao B, Li ZZ, et al. Convalescent plasma rescued a severe COVID‐19 patient with chronic myeloid leukemia blast crisis and myelofibrosis. Turk J Haematol. 2021;18(1):74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kasik V. Cancer patient who caught COVID‐19 recovered with immune plasma treatment News Splinter, 2020.
- 66. Hughes CM, Gregory GP, Pierce AB, Druce JD, Catton M, Chong B, et al. Clinical illness with viable SARS‐CoV‐2 virus presenting 72 days after infection in an immunocompromised patient. Infect Control Hosp Epidemiol. 2021;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kemp SA, Collier DA, Datir RP, Ferreira I, Gayed S, Jahun A, et al. SARS‐CoV‐2 evolution during treatment of chronic infection. Nature. 2021;592:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khan AM, Ajmal Z, Raval M, Tobin E. Concurrent diagnosis of acute myeloid leukemia and COVID‐19: a management challenge. Cureus. 2020;12:e9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Khatamzas E, Rehn A, Muenchhoff M, Hellmuth J, Gaitzsch E, Weiglein T, et al. Emergence of multiple SARS‐CoV‐2 mutations in an immunocompromised host. medRxiv. 2021. 10.1101/2021.01.10.20248871. [DOI] [Google Scholar]
- 70. Martinot M, Jary A, Fafi‐Kremer S, Leducq V, Delagreverie H, Garnier M, et al. Remdesivir failure with SARS‐CoV‐2 RNA‐dependent RNA‐polymerase mutation in a B‐cell immunodeficient patient with protracted Covid‐19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McKemey E, Shields AM, Faustini SE, Hill HJ, Barnskaya A, Stamataki Z, et al. Resolution of persistent COVID‐19 after convalescent plasma in a patient with B cell aplasia. J Clin Immunol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Reuken PA, Stallmach A, Pletz MW, Brandt C, Andreas N, Hahnfeld S, et al. Severe clinical relapse in an immunocompromised host with persistent SARS‐CoV‐2 infection. Leukemia. 2021;35:920–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sepulcri C, Dentone C, Mikulska M, Bruzzone B, Lai A, Fenoglio D, et al. The longest persistence of viable SARS‐CoV‐2 with recurrence of viremia and relapsing symptomatic COVID‐19 in an immunocompromised patient—a case study. medRxiv. 2021. 10.1101/2021.01.23.21249554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Szwebel TA, Veyer D, Robillard N, Eshagh D, Canoui E, Bruneau T, et al. Usefulness of plasma SARS‐CoV‐2 RNA quantification by droplet‐based digital PCR to monitor treatment against COVID‐19 in a B‐cell lymphoma patient. Stem Cell Rev Rep. 2021;17:296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Truong TT, Ryutov A, Pandey U, Yee R, Goldberg L, Bhojwani D, et al. Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS‐CoV‐2 infection: A consecutive case series. EBioMedicine. 2021;67 103355. 10.1016/j.ebiom.2021.103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ormazabal Velez I, Indurain Bermejo J, Espinoza Perez J, Imaz Aguayo L, Delgado Ruiz M, Garcia‐Erce JA. Two patients with rituximab associated low gammaglobulin levels and relapsed covid‐19 infections treated with convalescent plasma. Transfus Apher Sci. 2021;103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jeyaraman P, Agrawal N, Bhargava R, Bansal D, Ahmed R, Bhurani D, et al. Convalescent plasma therapy for severe Covid‐19 in patients with hematological malignancies. Transfus Apher Sci. 2021;103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tremblay D, Seah C, Schneider T, Bhalla S, Feld J, Naymagon L, et al. Convalescent plasma for the treatment of severe COVID‐19 infection in cancer patients. Cancer Med. 2020;9(22):8571–8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ferrari S, Caprioli C, Weber A, Rambaldi A, Lussana F. Convalescent hyperimmune plasma for chemo‐immunotherapy induced immunodeficiency in COVID‐19 patients with hematological malignancies. Leuk Lymphoma. 2021;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pal P, Ibrahim M, Niu A, Zwezdaryk KJ, Tatje E, Robinson WR, et al. Safety and efficacy of COVID‐19 convalescent plasma in severe pulmonary disease: a report of 17 patients. Transfus Med. 2020. [DOI] [PubMed] [Google Scholar]
- 81. Betrains A, Godinas L, Woei AJF, Rosseels W, Van Herck Y, Lorent N, et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol. 2021;192(6):1100–1105. [DOI] [PubMed] [Google Scholar]
- 82. Abid MB, Chhabra S, Buchan B, Graham MB, Abedin S, Thapa B, et al. Bronchoalveolar lavage‐based COVID‐19 testing in patients with cancer. Hematol Oncol Stem Cell Ther. 2021;14(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hatzl S, Eisner F, Schilcher G, Kreuzer P, Gornicec M, Eller P, et al. Response to "COVID‐19 in persons with haematological cancers". Leukemia. 2020;34:2265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Biernat MM, Kolasinska A, Kwiatkowski J, Urbaniak‐Kujda D, Biernat P, Janocha‐Litwin J, et al. Early Administration of Convalescent Plasma Improves Survival in patients with hematological malignancies and COVID‐19. Viruses. 2021;13:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Delgado‐Fernandez M, Garcia‐Gemar GM, Fuentes‐Lopez A, Munoz‐Perez MI, Oyonarte‐Gomez S, Ruiz‐Garcia I, et al. Treatment of COVID‐19 with convalescent plasma in patients with humoral immunodeficiency—three consecutive cases and review of the literature. Enferm Infecc Microbiol Clin. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Raja MA, Mendoza MA, Villavicencio A, Anjan S, Reynolds JM, Kittipibul V, et al. COVID‐19 in solid organ transplant recipients: a systematic review and meta‐analysis of current literature. Transplant Rev. 2020;35:100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Antony SJ, Singh J, de Jesus M, Lance J. Early use of tocilizumab in respiratory failure associated with acute COVID ‐19 pneumonia in recipients with solid organ transplantation. IDCases. 2020;21:e00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Choudhury A, Reddy GS, Venishetty S, Pamecha V, Shasthry SM, Tomar A, et al. COVID‐19 in liver transplant recipients—a series with successful recovery. J Clin Transl Hepatol. 2020;8:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Christensen J, Kumar D, Moinuddin I, Bryson A, Kashi Z, Kimball P, et al. Coronavirus disease 2019 viremia, Serologies, and clinical course in a case series of transplant recipients. Transplant Proc. 2020;52:2637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jamir I, Lohia P, Pande RK, Setia R, Singhal AK, Chaudhary A. Convalescent plasma therapy and remdesivir duo successfully salvaged an early liver transplant recipient with severe COVID‐19 pneumonia. Ann Hepatobiliary Pancreat Surg. 2020;24:526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jiang J, Miao Y, Zhao Y, Lu X, Zhou P, Zhou X, et al. Convalescent plasma therapy: helpful treatment of COVID‐19 in a kidney transplant recipient presenting with serve clinical manifestation and complex complications. Clin Transplant. 2020;34:e14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Trimarchi H, Gianserra R, Lampo M, Monkowski M, Lodolo J. Eculizumab, SARS‐CoV‐2 and atypical hemolytic uremic syndrome. Clin Kidney J. 2020;13:739–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yi SG, Rogers AW, Saharia A, Aoun M, Faour R, Abdelrahim M, et al. Early experience with COVID‐19 and solid organ transplantation at a US high‐volume transplant center. Transplantation. 2020;104:2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bartosch J. Lung transplant patient with COVID‐19 recovers following plasma clinical trial. Chicago, IL: The University of Chicago Medicine; 2020. [Google Scholar]
- 95. 59 WMaF . Plasma donation helps save Indiana man diagnosed with COVID‐19, 2020.
- 96. Transplant NBa . One of the first COVID‐19 convalescent plasma recipients supports donor appeal. Give Blood. 2020. [Google Scholar]
- 97. Rahman F, Liu STH, Taimur S, Jacobs S, Sullivan T, Dunn D, et al. Treatment with convalescent plasma in solid organ transplant recipients with COVID‐19: experience at large transplant center in New York City. Clin Transplant. 2020;34:e14089. [DOI] [PubMed] [Google Scholar]
- 98. Fung M, Nambiar A, Pandey S, Aldrich JM, Teraoka J, Freise C, et al. Treatment of immunocompromised COVID‐19 patients with convalescent plasma. Transpl Infect Dis. 2020;23:e13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Katz‐Greenberg G, Yadav A, Gupta M, Martinez‐Cantarin MP, Gulati R, Ackerman L, et al. Outcomes of COVID‐19‐positive kidney transplant recipients: a single‐center experience. Clin Nephrol. 2020;94:318–21. [DOI] [PubMed] [Google Scholar]
- 100. Naeem S, Gohh R, Bayliss G, Cosgrove C, Farmakiotis D, Merhi B, et al. Successful recovery from COVID‐19 in three kidney transplant recipients who received convalescent plasma therapy. Transpl Infect Dis. 2020;23:e13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lima B, Gibson GT, Vullaganti S, Malhame K, Maybaum S, Hussain ST, et al. COVID‐19 in recent heart transplant recipients: clinicopathologic features and early outcomes. Transpl Infect Dis. 2020;22:e13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mehta SA, Rana MM, Motter JD, Small CB, Pereira MR, Stosor V, et al. Incidence and outcomes of COVID‐19 in kidney and liver transplant recipients with HIV: report from the national HOPE in action consortium. Transplantation. 2021;105:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kutzler HL, Poulos CM, Cheema F, O'Sullivan DM, Ali A, Ebcioglu Z, et al. COVID‐19 in solid organ transplant recipients: observations from Connecticut. Transplantation. 2021;105:e6–8. [DOI] [PubMed] [Google Scholar]
- 104. Gupta A, Kute VB, Patel HV, Engineer DP, Banerjee S, Modi PR, et al. Feasibility of convalescent plasma therapy in kidney transplant recipients with severe COVID‐19: a single‐center prospective cohort study. Exp Clin Transplant. 2021;19:304–9. [DOI] [PubMed] [Google Scholar]
- 105. Rodionov RN, Biener A, Spieth P, Achleitner M, Holig K, Aringer M, et al. Potential benefit of convalescent plasma transfusions in immunocompromised patients with COVID‐19. Lancet Microbe. 2021;2:e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID‐19: initial three‐month experience. medRxiv. 2020. 10.1101/2020.08.12.20169359. [DOI] [Google Scholar]
- 107. Casadevall A, Henderson JP, Joyner MJ, Pirofski LA. SARS‐CoV‐2 variants and convalescent plasma: reality, fallacies, and opportunities. J Clin Invest. 2021;131(7):e148832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Descriptive summary of COVID‐19 patients with immunosuppression who received convalescent plasma
Table S2. Clinical therapeutic summary of COVID‐19 patients with immunosuppression who received convalescent plasma
Table S3. Clinical Summary of COVID‐19 patients with immunosuppression who received convalescent plasma.


