Abstract
The human immune system is not adequately equipped to eliminate new microbes and could result in serious damage on first exposure. This is primarily attributed to the exaggerated immune response (inflammatory disease), which may prove detrimental to the host, as evidenced by SARS‐CoV‐2 infection. From the experiences of Novel Coronavirus Disease‐19 to date, male patients are likely to suffer from high‐intensity inflammation and disease severity than the female population. Hormones are considered the significant pillars of sex differences responsible for the discrepancy in immune response exhibited by males and females. Females appear to be better equipped to counter invading respiratory viral pathogens, including the novel SARS‐CoV‐2, than males. It can be hypothesized that females are more shielded from disease severity, probably owing to the diverse action/influence of estrogen and other sex hormones on both cellular (thymus‐derived T lymphocytes) and humoral immunity (antibodies).
Keywords: COVID‐19, estrogen, gender biasness, immune system, inflammation, SARS‐CoV‐2, sex hormones
Highlights
Hormones are considered as significant pillars of sex differences and influence both the innate as well as adaptive immune responses.
Sex hormones and their potential role in the immune responses has not been completely understood.
Females are more shielded from disease severity probably owing to their unique hormonal constitution.
In females, the immunological cells have been noted to restrict the spread of infections as compared to males.
Males suffer from increased severity of respiratory infections and are less prone to autoimmune disorders as compared to the female counterparts.
Estrogen and other sex hormones play a key role both in restricting the inflammatory responses and in effective clearance of pathogens including the novel Coronairus.
Abbreviations
- ACE2
angiotensin‐converting enzyme‐2
- Bcl‐2
B cell lymphoma‐2
- CD22
cluster of differentiation 22
- CD4
cluster of differentiation 4
- CD8
cluster of differentiation 8
- CLR
C‐type lectin receptors
- COVID‐19
novel coronavirus disease‐19
- DAMP
damage‐associated molecular patterns
- DC
dendritic cells
- IL
interleukin
- ISG
IFN‐stimulated genes
- NK
natural killer cells
- NLR
nucleotide‐binding oligomerization domain (NOD)‐like receptors
- PRR
pattern recognition receptors
- RAS
rennin–angiotensin system
- RBD
receptor‐binding domain
- RLR
retinoic acid‐inducible gene‐like receptor
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- Shp‐1
SC homology 2 (SH2) domain‐containing protein‐tyrosine phosphatase
- TGF‐β
tissue growth factor‐β
- TH1
T helper 1 cells
- TLR
toll‐like receptors
- TMPRS
type II transmembrane serine protease 2
- TNF‐α
tumor necrosis factor‐alpha
- Vcam‐1
vascular cell adhesion 1
1. INTRODUCTION
Respiratory viral diseases are commonly caused by adenovirus, influenza virus, rhinovirus, parainfluenza virus, respiratory syncytial virus (RSV), coronavirus, which replicate and grow in the respiratory tract. 1 These viruses enter via the mucosal lining of respiratory, alimentary, and genitourinary tracts. They may also enter the human body through the outer surface of the eye and exposed skin, where they replicate by interfering with the synthesis of interferons (IFNs) and macrophages in the host system. After the dissemination in the body, the virus develops mechanisms, such as constant variations of surface glycoproteins, to escape from the host's defense mechanism, that is, circulating antibodies and various immune cells. Most respiratory viruses, including influenza and coronaviruses, have several hosts and are typically transmitted from animals to humans, and subsequently human to humans.2, 3, 4 During repeated jumps/spill‐overs from one host to the other (animal–human, animal–animal, human–animal, and human–human), these viruses undergo significant antigenic variations, which invariably help them to counter the immune responses of the host and establish the infection. The available data on the SARS‐CoV‐2 pandemic indicates higher incidence, morbidity, and mortality among the male population than women suggesting a peculiar predisposition.5, 6, 7, 8 The differential mortality rate between women and men associated with COVID‐19 might suggest a protective role for the sex steroids. 9 SARS‐CoV‐2 utilizes transmembrane protease serine 2 (TMPRSS2) to facilitate its entry into the host cell. TMPRSS2 contributes to the process of viral entry by facilitating the Spike glycoprotein into the S1 (receptor‐binding) and S2 (membrane fusion) domains. 10 Preliminary investigation using single‐cell RNA sequences analysis identified that the expression of TMPRSS2 was significantly higher in males as compared to females. 11 Although the findings were significant, our understanding of the relationship between TMPRSS2 expression and lung immunity remains poorly understood. However, it has opened up new opportunities to utilize sex steroid‐based therapies 11 for improving the disease outcome in patients with COVID‐19.
Androgen receptors can simplify the process of viral entry as they are transcription promoters for TMPRSS2. Therefore, the androgen receptor gene variants can affect androgen sensitivity, and diseases such as prostate cancer and androgenetic alopecia can contribute to COVID‐19 outcomes and hospitalization. 6 In addition to the significant differences between women and men in immune response, nonimmune components, like autophagy, mitochondrial functionality, and cholesterol biosynthesis have marked changes that contribute to the unique response trajectories 12 against the COVID‐19. Gene expression studies 13 conducted using individual expression profiles obtained from upper airway and blood have already identified profound discrepancies in the responses to SARS‐CoV‐2 infection. The COVID‐19‐infected male patients have an increased risk of cardiovascular complications, leading to increased mortality. High cardiovascular vulnerability exhibited by male patients can be attributed to the male‐biased regulation of multiple pathomechanisms 14 observed in COVID‐19. The expression of angiotensin‐converting enzyme‐2 (ACE2) in various tissues, including the heart is male‐biased as it is an X‐linked gene. 15 Therefore, the circulating and tissue levels of ACE2 might impact the virulence of SARS‐CoV‐2 in the heart. The plasma concentrations of ACE2 were observed to be high in male patients with heart disease, 16 contributing to a higher fatality rate in men due to COVID‐19. This review investigates the gender influence and underlying immunological mechanisms in the outcome of respiratory viral infections, including SARS‐CoV‐2.
1.1. Methodology of literature selection
A systematic literature review was performed to collect data by exploring authentic academic databases, including ScienceDirect, Scopus, PubMed, and Google Scholar, as well as, public and government health organization websites, such as the World Health Organization (WHO), European Centre for Disease Prevention and Control of the European Union (ECDC), National Institutes of Health of United States (NIH), Guidelines International Network, and Chinese guidelines on Novel Coronavirus” resources. For critical covering and compilation of most recent and relevant literature contents, the key terms searched include respiratory viral infections, coronavirus, immunological aspects, factors affecting COVID‐19 mortality, androgen and progesterone, ageing, inflammation, innate and adaptive immune responses, gender disparity, and its influence on the immune response, and estrogen action on T and B cells. After literature assortment, the obtained data were carefully examined, and only closely matched studies were considered for critical discussion while excluding the irrelevant or generalized studies.
2. RESPIRATORY VIRAL INFECTIONS
Respiratory diseases are the leading causes of morbidity and death in children and older people. 17 Respiratory viral infections affect the airways (breathing passage) and lungs. Most virus‐associated respiratory infections are self‐limited and affect the upper airway, causing milder symptoms such as sore throat and running nose. 18 Nevertheless, these can involve the lower airways in the elderly and children resulting in wheezing, breath shortness, and pneumonia.1, 19 The viruses are inhaled through the droplets and aerosols that float in the air when a person sneezes, coughs, and talks. The virus enters, invades with the help of specific receptors, and multiplies within the respiratory epithelial cells. Although the human body is equipped with innate immunity as the first line of defense in mucus lining and secretory antibodies, the virus may successfully evade inactivation from the nasopharynx to the alveolar membrane by triggering activation of innate immune‐related responses.20, 21, 22 The host‐cell and virus interaction occur in three stages: The phase where immune cells sense the virus, initiate innate antiviral responses, and stimulate the adaptive immune response. Later, T and B immune cells clear the infection by executing infected/damaged cells and enforcing antibody‐assisted immune responses, respectively. In the final phase, innate immune cells fix damaged tissues and produce mediators that implement the immune response's homeostasis. 23 Type I and III INFs are secreted by immune and tissue‐specific cells, which elicit an immune response that is purely antiviral. Lung dendritic cells (DCs) presenting viral antigens drain into lymphatics, where they encounter naïve T cells. These cells activate and re‐enter the lungs to react with resident myeloid cells, causing the release of either pro‐ or anti‐inflammatory cytokines to act against infected cells. 24
Among the most common respiratory infection‐causing viruses, the Influenza virus is responsible for frequent seasonal viral infections. Influenza virus has four main types, wherein the Type A (H1N1, H2N2, H3N2, H5N1, H5N5, H5N8, H7N7, H7N9, H7N3, H9N2, H10N8), and B (Yamagata and Victoria) cause frequent human and animal/bird infections, and Type C, and D are mostly confined to animals (pigs and cattle). Influenza viruses adapt themselves in different hosts, and undergo genetic variations, and are responsible for epidemics and pandemics. Moreover, RSV belonging to the Paramyxovirus group causes annual outbreaks of respiratory infections, especially during the winter season. The onset of pulmonary symptoms peaks during wet months in the geographical regions with high annual precipitation and cooler months in hot and dry areas. The RSV causes both upper and lower respiratory tract infections in adults, with the virus entering through contact with mucous membranes and by droplet aerosols. Increasing severity of RSV infection is noted in persons with comorbid conditions, adults with severe combined immunodeficiency, and lung or bone marrow transplantation patients. 25 Other viruses that are responsible for respiratory infections include the Rhinovirus, Coronavirus, human Adenovirus, Enterovirus, Parainfluenza virus (Types 1–4), and human Metapneumovirus.26, 27
3. VARIABLE CLINICAL OUTCOMES IN RELATION TO GENDER
A previous study had assessed the variations in the plasma viral load, anti‐SARS‐CoV‐2 antibodies, and cytokines among males and females diagnosed with moderate COVID‐19. The results of the study revealed that there was no significant difference in the viral load among both sexes. Nevertheless, it was observed that in males, higher activities of plasma cytokines and weakened T cell responses correlated with the worst disease outcomes. In contrast, female patients revealed increased activities of innate cytokines that positively correlated with unfavorable disease outcomes and efficient T cell activities during favorable outcomes. 28 Increased ACE‐2 expression on the cells of various organs attributed to the sex hormones, and X‐chromosome mediated overexpression of androgens and ACE‐2 receptors could predispose males to severe complications 29 of COVID‐19. Significance of age and sex influences on the COVID‐9 mortality rates, a study from Italy by Gallo et al. 30 proposed data be interpreted based on standardized mortality trend ratio, that considers both age and sex factors and its relation to the disease outcome. Although the X‐chromosome codes for the pattern recognition receptors (PRRs) and toll‐like receptors (TLRs) that effectively function to identify and destroy the pathogens, an exaggerated and uncontrolled inflammatory response that potentially harms host cells may be attributed to the increased severity of COVID‐19 among males. 31 In another study from China by Zheng et al., 32 it was noted that increased age and male sex were risk factors for respiratory oxygen supplementation and other intensive care support among more than 1700 patients observed. 32
Age‐related decreased expression of sex steroid hormones may facilitate increased proinflammatory responses during COVID‐19. Estrogen and testosterone may act as anti‐inflammatory mediators and minimize disease severity. Therefore, the therapeutic efficacy of hormone replacement therapy among COVID‐19 patient management must be explored. 33 A higher risk of death from COVID‐19 was associated with the male sex. Other risk factors of death in both the sexes included were older age, presence of co‐morbidities like cardiovascular disease, cerebrovascular disease, diabetes, chronic kidney disease, hypertension, obesity, smoking, chronic obstructive pulmonary disease, and co‐infection with human immunodeficiency virus (HIV) and tuberculosis.34, 35 An assessment of the effect of age and sex on COVID‐19 mortality in the European region was recently reported. The results of the study revealed that irrespective of age, the male gender was noted to be at increased risk of death due to COVID‐19 than the female counterparts of the same age. 36 COVID‐19 is reported to cause venous thromboembolic events that can result in adverse outcomes. The exaggerated coagulopathic state observed in patients can lead to the formation of fibrin clots. There will be an elevation in the D‐dimer level when these clots start to dissolve. It has been already established that an elevated D‐dimer level is associated with the disease severity 37 in COVID‐19. In a meta‐analysis involving 33 970 patients (60.1% men), men were found to experience venous thromboembolic events more than women (1.5‐times more) in the case of hospitalized patients 38 with COVID‐19. Myocardial injury is another important COVID‐19‐related adverse event and it is sex‐dependent. 39
4. INNATE IMMUNE MECHANISM DURING SARS‐COV‐2 INFECTION
The cells of innate immunity, mainly white blood cells (WBCs), such as basophils, eosinophils, monocytes, mast cells, DCs, natural killer (NK) cells, macrophages, neutrophils, and Langerhans cells, are activated at the site of viral entry. The PRRs present in host cells play a crucial role in initiating an immunological reaction during infection. They are evolutionarily conserved molecules that help in the detection of invading pathogens. They identify molecules, namely damage‐associated molecular patterns (DAMPs), released from pathogen‐infected, damaged, and dead cells alerting the immunity system of imminent danger to host cells. PRRs are categorized into four major classes, including TLRs, C‐type lectin receptors (CLRs), a retinoic acid‐inducible gene I (RIG‐1)‐like receptors (RLRs), and nucleotide‐binding oligomerization domain (NOD)‐like receptors. TLRs are single membrane spanned receptors seen on the surface of macrophages, DCs, NK cells, and T and B lymphocytes. TLRs are subdivided into ten types (TLR1 to TLR10). TLRs identify dangerouss molecules, such as viral nucleic acids, and proteins present in infected cells activating the innate immunity and DCs, which cause the release of proinflammatory cytokines. If the innate immunity cannot eliminate infection, the adaptive immune responses are stimulated via antigen‐presenting cells. The adaptive immune response eliminates infection by activating both cells mediated immunity (CMI) and humoral immunity (HI). 40 RLRs function as intracellular pattern recognition molecules that recognize the virus and facilitate the host immune response. They stimulate the release of inflammatory cytokines, including IFNs, and control the response by phosphorylation or ubiquitination.
NLRs are intracellular sensor molecules that work alongside pathogen‐associa‐inflammatory response. This causes the release of cytokines, chemokines, cell adhesion molecules, and immunoreceptors during the early response to an infection, activates genes, and enables the adaptive immune response.
The infected host cells trigger the secretion of virus‐specific IFNs α and γ (IFN‐α and IFN‐γ), various pro‐inflammatory cytokines that include interleukin‐1 (IL‐1), IL‐6, IL‐18, and tumor necrosis factor‐alpha (TNF‐α). Interferons induce the adaptive immune response against the virus via stimulating many genes in immune cells. The activated immune cells either destroy infected cells or cause the deactivation of virus particles with antibodies. 41 Stimulation of the IFN‐α and IFN‐γ is critical to restrict the virus proliferation within the host cells at the initial phase of the infection. IFN‐γ and IFN‐α are incredibly effective in limiting the SARS‐CoV‐2 spread and can orchestrate innate and adaptive immunity. Viruses, including SARS‐CoV, and MERS‐CoV employ various mechanisms to attack the immune response governed by type I IFNs. Recently, reports have confirmed the expression of that IFNs during SARS‐CoV‐2 infection. 42 Furthermore, Signal transducer and activator of transcription 1 (STAT1) translocation following IFN‐stimulated genes (ISGs) stimulation have also been determined in the SARS‐CoV‐infected lungs. In addition to these observations, plasmacytoid DCs (pDCs) also secrete IFNs during SARS‐CoV infection indicating the indispensable role of IFNs throughout viral infections. Although the genetic basis of limiting the effect of COVID‐19 infection is not entirely understood, ISGs, lymphocyte antigen 6 complex locus E, and IFN‐induced transmembrane family (IFITM) proteins may regulate virus‐host cell interactions both in favor and against infection. 43
5. GENDER VARIATION AND ITS INFLUENCE ON THE IMMUNE RESPONSE
The infectious disease outcome significantly varies between males and females. Females suffer less severe consequences from infection than males due to the strong cellular and humoral immunity. It is also perceived that males are vulnerable to infections and cancers, whereas females are more prone to autoimmune diseases. For example, males exhibit 40% more viral RNA than females in acute HIV infection and show a higher mortality rate in hepatitis infected cases. Moreover, numerous viral, bacterial, and fungal infections lead to gender‐biased diseases. Hepatitis, syphilis, influenza, fungal meningitis, and Lyme diseases are commonly occurring in males, whereas tapeworm, onychomycosis, and bacteremia infections are often diagnosed in females. 44 These differences could be due to several factors including gonadal hormones, expression of receptors of gonadal hormones, microbiome, epigenetics, and the X chromosome associated genes. In comparison to males, females appear to have a more robust immunity system compared to their male, which makes them more efficient in clearing the infection. It also makes them more susceptible to autoimmune diseases by aggressively responding to self‐antigens. 45
Many recent reports have demonstrated a clear sex‐specific bias for COVID‐19 with males more likely to exhibit increased mortality rates compared with female patients with similar ages, ethnic, and social backgrounds.46, 47 The male patients are likely to show more serious manifestations accompanied by a higher fatality rate though it also varies among various countries.48, 49 The highest ratios of COVID‐19‐associated male/female mortalities have been reported in Greece, Romania, Netherlands, and the Dominican Republic.49, 50 This sex‐biased illness severity and mortality might be ascribed to varying immune response, where females mount more robust cell‐mediated, innate, and humoral immunity levels than male counterparts. 51 Though females have a more robust immune response following infection with pathogenic microorganisms, the intensified immune response can also trigger immunopathology. A study of 198 COVID‐19 patients showed women had better T cell activation compared to men. In men, age was negatively correlated with poor T cell response, which was associated with adverse disease outcomes. This was not seen in women who fared worse if levels of innate immune cytokines were higher. 28 It is crucial to examine the reasons for gender variation in disease manifestations and outcomes in SARS‐CoV‐2.
An increase in proinflammatory cytokines level was recorded in the case of MERS and SARS‐CoV. SARS‐CoV positive individuals showed an increased level of cytokines, including IL‐6, IL‐12, IL‐1B, putative internal head protein 10 (IP10), IFNγ, and monocyte chemoattractant protein 1 (MCP1), whereas IL‐17, IL‐15, TNF‐α, and IFNγ levels raised in MERS‐infected persons. The explicit mechanism of COVID‐19 immunopathology remains to be elucidated, and the utmost information is based on MERS and SARS‐CoV. Some studies have revealed an increased concentration of TNF‐α, IL‐2, IL‐6, IL‐7, IL‐10, IFNγ, MCP1, granulocyte stimulating factor, and macrophage inflammatory protein 1‐α in critical ill COVID‐19 patients.52, 53 Among these modulators, IL‐10 and IL‐6 displayed a positive association with milder COVID‐19 cases, whereas a strong correlation was observed between IL‐6 and severely infected groups. 53 Therefore, inhibiting the activity of IL‐6 has been suggested to decrease the severity 52 of COVID‐19, and clinical investigations are underway to scrutinize the therapeutic effectiveness of antibodies against IL‐6 or IL‐6 receptors.
High mobility group box 1 protein (HMGB1) is another important contributing factor and immunomodulator for sex‐biased risk of COVID‐19, severity, and mortality. The release of endogenous DAMPs by stressed or infected cells can trigger the immune response by interaction with PRRs.54, 55 A large number of DAMPs, like IL‐33, decorin, biglycan, defensin, RNA, DNA, versican, F‐actin, fibrinogen, and HMGB1, have been recognized. 54 As the chronic inflammatory disorders led to an increase in HMGB1 level, the severity extent in COVID‐19‐infected persons with associated inflammatory comorbidities might be linked with HMGB1. 55 Sex‐biased differential immune response results in different mechanisms to clear out the injured cells and viral load. Apoptosis is typical in stressed/infected pulmonary endothelial cells in females, whereas males are recognized to undergo necrotic cell death. Physiological conditions such as pregnancy make the women tolerant to the allogenic paternal antigen of the fetus. The fetal cells have been shown to enter the maternal blood and persist for a long time. Thus, women can precisely control the excessive immune activation to foreign antigens, as the virus‐associated organ damages during the COVID‐19 infection is due to excessive immune activation and inflammation of the individuals' own immune system. The cytokine release syndrome in COVID‐19 patients is due to excessive immune activation, leading to tissue and organ damage. It is also known that the bats are able to live with the Coronaviruses by controlling the unwanted inflammatory response in the body and weakened INF activation. 56
5.1. Effect of the X chromosome on immunity
The X chromosome defines a few immunity‐related functions of the cell. It regulates the genes coding for immunity, which are necessary to synthesize microRNAs (miRNAs). 57 As females carry two X chromosomes and forestall excessive X chromosomal responses, it is inactivated by transcriptional silencing during developmental stages, resulting in cellular mosaicism. 57 Two X chromosomes give women an advantage in mounting more efficient and rapid immune response which may also be related to female hormones. Behavioral differences between women and men also play some role in differential susceptibility and outcomes. Women are less likely to smoke or drink and have a lower burden of chronic diseases such as hypertension; chronic lung diseases hence may have lower mortality due to COVID‐19.
6. VITAMIN D AND SEX DIFFERENCES
Among the factors that affect COVID‐19 mortality, the vitamin D status of the patient has received a special position due to the role played in modulating the immune system as well as on the renin–angiotensin system. 58 A sex‐specific association was previously suggested between the vitamin D status and mortality rate of COVID‐19 in the older population. 58 The deficiency of vitamin D is believed to predispose susceptible individuals to infections, including COVID‐19. In a recent study conducted among the patients from Switzerland, older males showed a higher susceptibility to vitamin D deficiency than their female counterparts. 59 Vitamin D deficiency can further increase X‐chromosome‐linked the renin–angiotensin system (RAS) activity, making the male population susceptible to ACE2 receptor dysregulation. This association is believed to improve the COVID‐19‐associated mortality in males mainly because of the increased susceptibility to COVID‐19 induced cytokine storm. Vitamin D has a key role in limiting the rapid increase of circulating pro‐inflammatory cytokines, thereby controlling the hyperinflammatory stage/cytokine storm‐related to COVID‐19. 59 Furthermore, the role played by Vitamin D3 in controlling the differentiation of T regulatory cells is estrogen‐dependent. Estrogen was found to enhance the gene expression of vitamin D receptors in CD4 + T cells and at the same time reduce the expression of the cytochrome P450 component of 25‐hydroxyvitamin D(3)‐24‐hydroxylase enzyme, CYP24A1, that inactivates Vitamin D3. The immunomodulatory potential of Vitamin D3 is sex‐related, therefore leading to a role in the outcome and lethality of COVID‐19. Therefore, vitamin D supplementation can be considered an important strategy to reduce COVID‐19 infection.
6.1. Estrogen
The fourth type of estrogen, estetrol, is found only during pregnancy. The molecular structure of estrogen and its various forms is shown in Figure 1. The estrogen activities influence females' health, as noted by the development of fibroids, predisposed to breast cancer when it increases, and susceptible to osteoporosis in case of decreased activities. 60 The role of estrogen during microbial infections has been recently established. 61 For example, Channappanavar et al. 62 infected both males and females mice with SARS‐CoV to investigate the role of sex hormones on the survival rate of animals. Results showed a more severe form of the disease in ovariectomized female mice than that to control. Furthermore, the death number was not increased in castrated male mice due to SARS‐CoV infection compared to the control group, indicating a pivotal role of estrogen in the disease onset. Moreover, interference of 17β‐estradiol with hepatitis C life cycle suggests a direct inhibitory action of steroid on the virus, rather than only the modulation of host intracellular homeostatic processes. As the sterols can potentially modify virus infection capacity, pretreatment with estrogen is likely to enhance protective actions in acute lung injury due to the anti‐inflammatory effects. 63 A previous study suggested that estrogen could lower the activities of CMI, HI, and innate immune cells, like neutrophils and NK cells.
Figure 1.
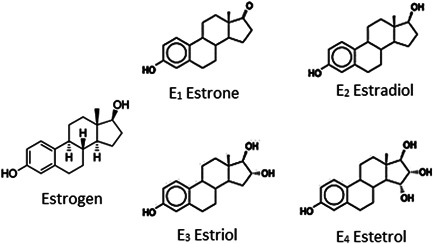
The molecular structure of estrogen and its derivatives
Estrogen was found to inhibit proinflammatory cytokines, like IL‐4, IL‐10, IL‐12, and TGF‐β, regulate immune cell apoptosis, and enhance the activities of anti‐inflammatory cytokines, including NK cells, IL‐1, and IL‐6. IL‐17, IL‐23, and TNF‐α. The effect of sex hormones on the vulnerability to Zika and herpes simplex virus‐2 (HSV‐2) infection has previously been explored. 64 Additionally, the immunomodulatory activities of 17β‐estradiol and its effect on innate immune responses against bacterial infection were reported. A previous study noted that in females, the replication of influenza A virus within nasal epithelial cells was inhibited compared to that in males. This was supported by the fact that respiratory epithelial cells have receptors for binding estrogen, that is, G‐protein‐coupled estrogen receptor. These receptors were noted to regulate cell function, signaling pathways, and the inflammatory response.
6.2. Estrogen receptor (ER)
There are two subcategories of ER, ERα, and ERβ. These are studied for their potential role in immunomodulation. Estrogens receptors present on the immune cells regulate cellular functions of the adaptive and innate immune system and play a noteworthy contribution in immune cell development. 65 ER‐mediated regulation of immune cells and signaling may have a key role in the gender‐based differences in innate and adaptive immune pathways. Most immune cells, including plasma cells, monocytes, macrophages, DCs, T lymphocytes, mast cells, and NK cells, express ERα and ERβ, which are self‐regulated.
6.3. Role of ER receptors
ERs mediate the immunomodulatory effector function through different mechanisms including acting as a ligand‐dependent transcription factor for regulating gene expression and promoting epigenetic changes, participating in membrane‐initiated steroid signaling to generating rapid responses and dose‐dependent effect on innate immune cell function and development. 65 The immune‐related functions of ERs are mediated more by the ER‐α subset than by ER‐β. ER‐α participates in activating, proliferation, and differentiation of T cells. It contributes to T cell‐mediated inflammation and regulates T cell functions. ER enhances the proinflammatory responses of TLR and the cytotoxicity of NK cells and ameliorates the activities of TNF‐α, IL‐1, and ‐6. ERs can modulate invariant natural killer T cells (iNKT), a type of T cell that causes the death of infected and tumor cells. Experimental studies on mice have shown increased cytokine production in females only in estrogen presence but not in ovariectomized mice and mice supplied with artificial iNKT cell ligands. Thus, estrogen induces proinflammatory cytokines and NK cells that clear abnormal cells and pathogens more efficiently. 66
6.4. The action of estrogen on T and B cells
T cell‐mediated immune responses are necessary to prevent autoimmune disorders and cancers. The estrogen hormone modulates the T cells differentiation, including cytotoxic, helper, and memory cells. It regulates the transcription of the IFN‐γ gene, which is essential for the release of cytokines, type II IFNs, and other immune cells participating in adaptive and innate immune responses.63, 67, 68 Estrogen upregulates the Th‐1 transcription factor T‐bet, a key transcriptional factor of type 1 T helper cells (Th1) required to clear pathogens and maintain immunity. It suppresses the microRNA‐dependent expression of IFN‐γ. MicroRNAs are noncoding RNAs that regulate RNA silencing and gene expression. The regulation of microRNA transcription during viral infection correlates with the interferon signaling pathway. Estrogen suppresses host sterol synthesis, especially the mevalonate‐isoprenoid branch of cholesterol biosynthesis, required by the virus to survive in the host. It facilitates the expansion of Treg cells, and self‐tolerance to antigens and prevents the development of the autoimmune disease.69, 70
6.5. Estrogen action on B cells
6.5.1. Cluster of differentiation 22 (CD22) gene
Estrogen affects cell differentiation, function, and activity by regulating the CD22 gene belonging to the sialic acid‐binding immunoglobulin type lectin (SIGLEC) family. This gene is expressed predominantly on mature B cells and helps to regulate the overactivity of immune cells and prevents autoimmunity.
6.5.2. Shp‐1 gene
The gene codes for the Src homology 2 (SH2) domain‐containing protein‐tyrosine phosphatase, which plays an important role in lymphocyte activation, differentiation, and proliferation. It also regulates B cell receptor (BCR)‐mediated signal transduction, which is related to B cell activation. The binding of antigen to the BCR initiates signal transduction, which involves a change in the receptor oligomerization (interaction of more than one polypeptide chain) and other assessment regarding the antigen type and its presentation to the helper T cells.
6.5.3. B cell lymphoma‐2 (Bcl‐2) gene
Bcl‐2 is a gene belonging to the family of Bcl‐2 proteins associated with the regulation of apoptosis either by inducing or inhibiting cell death.
6.5.4. Vascular cell adhesion 1 (Vcam‐1) gene
This codes for Vcam protein 1 and is also called CD‐108. It is an adhesion molecule mediating the adhesion of lymphocytes, eosinophils, monocytes, and basophils to the vascular endothelium. Experimental studies have provided evidence regarding whether estrogen protects isolated primary B cells from B cell receptor‐mediated apoptosis and its ability to activate B cells to autoreactivity, making women more prone to autoimmune diseases.71, 72
6.6. Role of estrogen in ACE2
ACE2 acts as a coreceptor for the SARS‐CoV‐2 entry into the host cell. ACE is an important enzyme of RAS. 73 Estrogen activity on the ACE receptor may profoundly contribute to the gender‐based differences between males and females in the SARS‐COV‐2 infection and outcome of COVID‐19.
6.7. RAS
RAS is a cascade of hormone systems that maintains blood pressure (BP), fluid and electrolyte balance. A drop in renal blood volume or decreased BP stimulates the release of Renin from renal cells (juxtaglomerular cells), which acts on angiotensinogen secreted by hepatic cells. Renin cleaves angiotensinogen into angiotensin I (Ang. I), and further metabolism of these products is executed by ACE. 73
6.8. ACE
It is secreted by the epithelium of the lungs and kidneys. It may be ACE1 mostly or along with ACE2. ACE1 acts on Ang. I and converts it into angiotensin II (Ang. II), which acts as a vasoconstrictor, raising BP. ACE2 antagonizes the activity of Ang. II by converting Ang. II into angiotensin 1‐7, which helps in BP homeostasis and regulates the transport of neutral amino acids (alanine, valine, leucine, etc.) across the membrane by acting as a vasodilator. However, respiratory viruses such as Coronavirus use ACE2 on the membrane as a coreceptor for their entry into the host cell, as they have more affinity to bind to these receptors. 74 Experimental evidence in rats found that ACE2 activity or its levels are lower in females than in male rats. 75 The decreased levels of ACE2 in females are due to estrogen (E2) activity, whose levels frequently change from birth to puberty, menopause, and pregnancy.
6.9. Androgen and progesterone
Progesterone is a hormone produced by the corpus luteum during the menstrual cycle and placenta during pregnancy. It exerts its effects via progesterone receptors, which are seen on NK cells, T cells, macrophages, and DCs. Experimental studies revealed that progesterone relaxes bronchial smooth muscles during lung inflammation, acting as vasodilators, and in allergic inflammation of the lung, progesterone promotes the release of TH1 cells and cytokines, like IL‐1β, ‐4, ‐5, ‐6, ‐10, ‐22, and TNF‐α. 76 Treatment of lung inflammation with progesterone reduced inflammation and restored tissue homeostasis 77 (Figure 2). Progesterone increases Tregs' production that is anti‐inflammatory and increases Th17 and CD8+, which protects from an adverse immune response that is fatal for the host. 46 Vitamin D regulates the immune response and modulates IL‐6 via progesterone‐induced blocking factor. Administration of exogenous progesterone led to rapid recovery by elevating IL‐6 and ‐22, TGF‐β, Th17 expressing CD39, and cell multiplication. It also reduced protein leakage in the airway and upregulated AREG in the lungs which improves pulmonary functions. Progesterone‐based compounds alter cellular activity and signaling to influence infections outcome in the respiratory tract.
Figure 2.
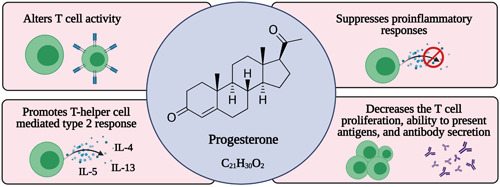
Actions of progesterone related to immunity
6.10. Action of testosterone associated with immunity
Testosterone is the principal androgen present in males for male traits and reproductive activity. It exerts effects on binding with receptors, forming a complex that then moves into the nucleus, binds with transcriptional regulators on DNA, and controls its activity. Testosterone modulates the functions of lung immune cells, such as decreasing the expression of innate lymphoid cells 2 in males involved in allergic reactions attenuating airway inflammation. 78 Experimental evidence suggests that testosterone binds to T cell receptors and inhibits their differentiation and proliferation. It was found to have no role in B cell differentiation or proliferation. Testosterone inhibits T cell differentiation by activating the protein tyrosine phosphatase nonreceptor type 1 gene. This gene reduces tyrosine kinase 2 phosphorylation that stimulates lymphocytes and T cell differentiation. Thus, testosterone will increase Th1 responses and activate CD8 cells simultaneously. Additionally, it decreases NK cell and TNF‐α activities and increases anti‐inflammatory cytokines such as IL‐10. Th1 mediates cell‐mediated immune reactions towards intracellular viruses and bacteria via the secretion of IFNs and IL‐2 and IL‐10. IL‐2 enables cell‐mediated immunity through T cells and prevents autoimmune diseases. IL‐10 facilitates host defense against pathogens invasion by shielding host tissue damage and maintaining tissue homeostasis.79, 80
6.11. Role of estrogen in DNA methylation, epigenetic changes, and histone modifications
DNA methylation implicates adding a methyl group at the 5‐position of cytosine nucleotides adjacent to guanine, forming CpG islands. It is catalyzed by methyltransferase enzymes, and this methylation process of DNA causes a change in histone modification. Histone modification includes acetylation and methylation of histone protein of chromatin catalyzed by histone acetyltransferase and histone methyltransferase. These processes add acetyl or methyl groups to lysine residues (positive charge) of histone tails, changing their charge and subsequent gene activation. It can be demethylated or deacetylated by histone deacetylase or demethylase mask positive charge on histone inactivates gene function.81, 82 Estrogen receptors, especially ERα, directly interact with transcriptional factors involved in histone modification, such as acetylation, phosphorylation, and methylation. Thus, it has a role in these modifications in eliciting an immune response against viral infections. Estrogen receptors cause epigenetic changes in the genome. Epigenetic modifications include DNA methylation and histone modifications. These epigenetic changes are heritable wherein phenotypic changes are inherited by progeny. During such changes, the DNA sequence does not alter, but a difference in the reading frame of the DNA alters its function. Human breast epithelial cells undergo cancerous changes because of estrogen, mediated by ERs, their direct genotoxic effects, and the ability to cause DNA methylation. 83
7. IMMUNOLOGICAL RESPONSES IN GENDER FEMALE AND MALE
Sex hormones regulate the immune system via receptors present on immune cells, including proliferation, differentiation, and maturation. A review reports that 17β‐estradiol (E2) and progesterone are strong immune‐modulators, which may reverse the immune dysregulation, leading to the adverse clinical manifestation of cytokine storm 84 in COVID‐19. Women exhibit a different immune response to vaccinations and gender‐based biological factors are key determinants of susceptibility to infections and clinical outcomes.
7.1. Innate immune responses
The immune response is the body's natural way of eliminating a pathogen and ensures cells' normal function. There are sex differences seen in all classes of vertebrates from insects to lizards and birds to mammals. In all these, there are lowered adaptive and innate immune responses in males compared to females. 85 The difference in the immunity related to innate immune cells among males and females is germline‐encoded (a genetic constituent). There is a difference in identifying viruses by pattern recognition molecules by the immunity system of female and male genders. TLR activation, retinoic acid‐inducible gene (RIG), and so forth, and release of type I IFNs and related responses were observed more often in females than in males. 86 There is increased cytokine stimulation and production by females than males, governed by gender‐specific chromosomal expression. This differential expression of PRRs is important for gender‐based responses by innate immune cells. The number and activities of innate immune cells vary between females and males. In males, the frequency of NK cells is higher, whereas the phagocytic activity of macrophages and antigen‐presenting cell activity is higher among females. 87 The response of innate lymphoid cells and secretions of cytokines is also superior in females. Dysregulated activity of innate lymphoid cells leads to autoimmune diseases. Notably, females are more vulnerable to autoimmune diseases due to the lower number of these innate lymphoid cells.
7.2. Adaptive immune responses
Adaptive immunity protects against invading pathogens and toxic metabolic byproducts while ensuring the safety of host cells (self). The thymus gland plays a pivotal role in adaptive immunity by producing mature T cells. These T cells constitute helper (cluster of differentiation 4 [CD4]) T cells, cytotoxic (cluster of differentiation 8 [CD8]) T cells, and memory cells. There is a high activity of helper T cells in females, whereas the increased activity of cytotoxic T cells was detected in males. The better fighting capacity against infection in females is due to increased CD4 T cells. In contrast, the ability to kill cancer cells and other invaders is greater among males due to the high activities of CD8 T cells. The greater antibody responses among females are attributed to increased B lymphocyte counts 88 (Figure 3).
Figure 3.
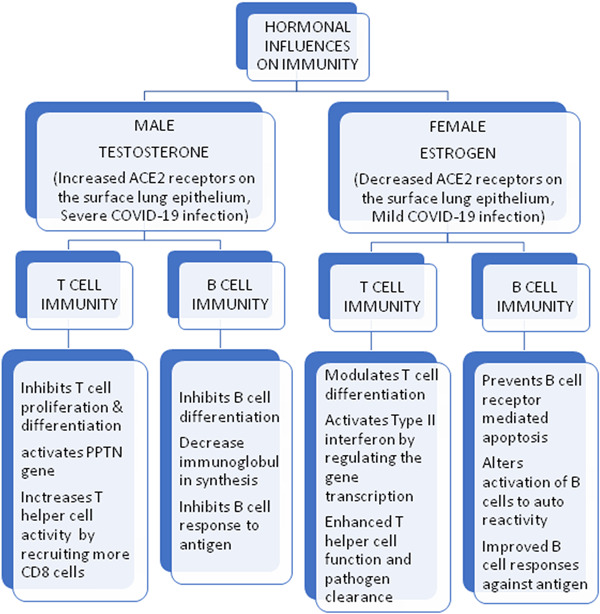
The hormonal differences between males and females on T and B cells
8. INFLUENCES OF SEX HORMONES ON LUNG FUNCTION
Gender‐based differences in the prevalence of lung disorders, like asthma, cancer, pulmonary arterial hypertension, and obstructive pulmonary diseases have been observed. Several human and animal‐based studies have detected substantial differences between males and females in respiratory physiology. 89 The sex hormone such as estrogen and its receptor can influence cellular processes such as proliferation and migration in the lung, which might affect the activity of vascular cells, pulmonary tissue, and immune cells. There is a varied functional difference shown by lung cells related to immunity, like macrophages, DCs, eosinophils, NK cells, and neutrophils by the actions of sex hormones and it is discussed in detail below.
8.1. Macrophages
Macrophages of the lung, also called alveolar macrophages, are the first defense system seen in the lower airway tract. They are the mediators of inflammation and tissue remodeling. The female sex hormone estrogen shortens the proinflammatory phase and prolongs the inflammatory phase required for immunomodulation and remodeling. 90 Progesterone inhibits the release of NO from activated macrophages and inhibits tissue injury. The male sex hormone testosterone reduces the formation of the TNF‐α in AM and decreases NO. 91
8.2. Dendritic cells
These are the cells that present antigen material to MHC (major histocompatibility cells) molecules and activate T cells. They also act as a connecting link between adaptive and innate immunity by giving instructions either to Th1 (antiviral) or Th2 (antiparasitic or allergic reaction) or Th17 (autoimmunity or antibacterial) type of response. 92 It elicits a Th1 immune response during viral infection and tries to clear the invading virus, but the respiratory virus can block the Th1 immune response. Mouse studies on DCs revealed that DCs are regulated by epigenetic modification, especially during the inflammatory response against a respiratory virus. The epigenetic modification of the transcriptional regulator H3K4me3 (activator of the release of type 1 IFNs) blocks the Th1 response and activates the Th2 response. 93
8.3. Eosinophils
They are immune system components involved in allergic airway disease and asthma. The development of asthma has environmental factors and most viral infections. Respiratory viruses trigger asthma during the infection process because these asthmatic individuals have low innate immunity (impaired type I & III INFs). 94 Eosinophilic cells detect viral particles with the help of PAMPs and become activated. These activated eosinophils present these viral antigens to lymph nodes and activate T cells secreting cytokines, eliciting an inflammatory response. 95 The female hormone estrogen increases the activated eosinophils and inflammatory process compared to the male hormone testosterone. 96 Thus, females are more prone to eosinophilia than males. 97 It also decreases viral multiplication by the secretions of granular components that contain the enzyme NO synthase. NO (nitric oxide) acts against viral particles, inhibiting its replication process in the host.
8.4. Neutrophils
They act as the front line of the immune system against any infection. During a respiratory viral infection, neutrophils identify viral particles via pattern‐associated molecular recognition molecules. Neutrophils reach virus‐infected sites and produce proinflammatory mediators (cytokines, chemokines, and IFNs) and toxic substances (peroxides, hydrolytic enzymes, and reactive oxygen species [ROS]) that induce pathological changes in the invaded virus. 98 These inflammatory substances that are released damage the virus's DNA, inhibit virus replication, al propagation or induce trigger apoptosis of virally infected cells, thus halting viral spread. 99 Neutrophils also induce innate immunity of the host, increasing antiviral host defense. Neutrophils in the process of host defense against a virus can also cause collateral damage to the host. 100 However, experimental evidence suggests that the invaded virus can block the generation of ROS by neutrophils by impeding the chemokines released from virus‐infected cells. 101 Females are prone to neutrophilic lung inflammation, as the estrogen hormone recruits more neutrophils at the infection site than males. 102
8.5. Natural killer cells
They are components of the immune system that eliminate virus‐ or virus‐infected cells or tumor cells. They are cytotoxic in nature and release interferons at a preliminary stage, sensing the presence of infection. 103 NK cells kill virally infected cells or viruses either by producing cytokines or by secreting cytolytic granules or death receptor‐mediated cytolysis. 104 Activated NK cells secrete interferon‐gamma, making the virus less sustainable in the host and protecting the cells from viral infection acting at distant sites. 105 However, viruses have multiple mechanisms to inhibit the NK cells functioning by downregulating cytotoxic function or by triggering apoptosis, thereby decreasing the number of NK cell activities. 106 Recent reports have shown NK cell‐based therapies to protect against SARS‐CoV‐2 infection. 107 Due to the increased activities of NK cells in males compared to females, there is an elevated cytotoxic response in males 108 (Figure 4).
Figure 4.
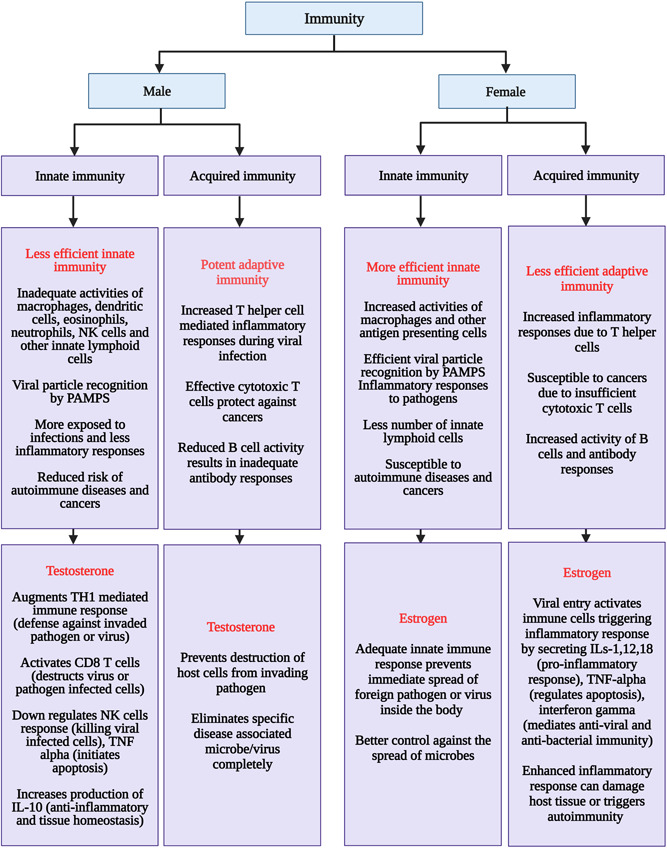
Flow chart of the differential activity of immunity, and hormonal influences in males and females
9. CONCLUSION AND FUTURE PROSPECTS
Several reports have shown high mortality among men than women due to the ongoing pandemic COVID‐19, and men are more likely to be affected by the COVID‐19. Despite the men engaging in high‐risk behaviors, such as smoking and ignoring social distancing, it appears that there is a significant gender‐based difference between women and men in the immune system, susceptibility to COVID‐19 infection, pathobiology of the disease, and disease outcome. Factors, like sex hormones, sex hormone receptor expression, ACE‐2 receptor expression also contribute to the gender‐based differences at the cellular and molecular level. Sex hormones such as estrogen, testosterone, progesterone, and so forth, play a key role in immune responses during infections. On the basis of the available literature, females appear to be better equipped to counter invading respiratory viral pathogens, including the novel SARS‐CoV‐2, than males.
Estrogen seems to regulate innate and adaptive immunity mechanisms, thereby influencing the activities of both cellular and humoral immune responses. ER expressed on immune cells also affects the effector functionalities of immune cells and their development. ACE2 receptors and their expression on various human cells are regulated by estrogen, which might contribute to the gender‐based differences between males and females on vulnerability to COVID‐19 infection and the disease outcome. Other sex hormones, like testosterone and progesterone, also significantly influence immune systems during infections. The differential mortality and morbidity rates due to COVID‐19 in females and males may be because the sex hormones affect the immune responses during SARS‐CoV‐2 infection. Further studies in this regard are warranted to improve understanding regarding the influences of sex hormones and gender bias on the immunological responses during microbial infections and their impact on vaccine development.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Sabitha Vadakedath proposed the idea and provided an initial draft. Venkataramana Kandi provided the title, performed review of the literature, and revised the manuscript. Ranjan K. Mohapatra participated in preparing the draft. Sabitha Vadakedath, Venkataramana Kandi, Ranjan K. Mohapatra, Venkata B. K. Pinnelli, Richa R. Yegurla, Praveen R. Shahapur, and Vikram Godishala performed the revision of the manuscript. Senthilkumar Natesan, Kranti S. Vora, Khan Sharun, Ruchi Tiwari, Muhammad Bilal, and Kuldeep Dhama updated and edited the review. All the authors were involved in the writing and proofreading of the manuscript. All authors have read and approved the final manuscript.
Vadakedath S, Kandi V, Mohapatra RK, et al. Immunological aspects and gender bias during respiratory viral infections including novel Coronavirus disease‐19 (COVID‐19): a scoping review. J Med Virol. 2021;93:5295‐5309. 10.1002/jmv.27081
Contributor Information
Venkataramana Kandi, Email: ramana20021@gmail.com.
Ranjan K. Mohapatra, Email: ranjank_mohapatra@yahoo.com.
Kuldeep Dhama, Email: kdhama@rediffmail.com.
REFERENCES
- 1. Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680‐686. [DOI] [PubMed] [Google Scholar]
- 2. Mahal A, Duan M, Zinad DS, et al. Recent progress in chemical approaches for the development of novel neuraminidase inhibitors. RSC Adv. 2021;11:1804‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohapatra RK, Perekhoda L, Azam M, et al. Computational investigations of three main drugs and their comparison with synthesized compounds as potent inhibitors of SARS‐CoV‐2 main protease (Mpro): DFT, QSAR, molecular docking, and in silico toxicity analysis. J. King Saud Univ.–Sci. 2021;33:101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohapatra RK, Das PK, Pintilie L, Dhama K. Infection capability of SARS‐CoV‐2 on different surfaces. Egypt J Basic Appl Sci. 2021;8(1):75‐80. [Google Scholar]
- 5. Arumugam VA, Thangavelu S, Fathah Z, et al. COVID‐19 and the world with co‐morbidities of heart disease, hypertension and diabetes. J Pure Appl Microbiol. 2020;14(3):1623‐1638. [Google Scholar]
- 6. Mohamed MS, Moulin TC, Schiöth HB. Sex differences in COVID‐19: the role of androgens in disease severity and progression. Endocrine. 2020;71:1‐6. 10.1007/s12020-020-02536-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhama K, Khan S, Tiwari R, et al. Coronavirus Disease 2019‐COVID‐19. Clin Microbiol Rev. 2020;33(4):e00028‐20. 10.1128/CMR.00028-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhama K, Patel SK, Pathak M, et al. An update on SARS‐CoV‐2/COVID‐19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med Infect Dis. 2020;37:101755. 10.1016/j.tmaid.2020.101755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinna G. Sex and COVID‐19: a protective role for reproductive steroids. Trends Endocrinol Metab. 2020;32:3‐6. 10.1016/j.tem.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okwan‐Duodu D, Lim EC, You S, Engman DM. TMPRSS2 activity may mediate sex differences in COVID‐19 severity. Signal Transduct Target Ther. 2021;6(1):100. 10.1038/s41392-021-00513-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García‐Pérez BE, González‐Rojas JA, Salazar MI, Torres‐Torres C, Castrejón‐Jiménez NS. Taming the autophagy as a strategy for treating COVID‐19. Cells. 2020;9(12):2679. 10.3390/cells9122679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun M, Shankar R, Ko M, et al. Sex differences in viral entry protein expression, host responses to SARS‐CoV‐2, and in vitro responses to sex steroid hormone treatment in COVID‐19. Res Sq [Preprint]. 2020. 10.21203/rs.3.rs-100914/v1 [DOI] [Google Scholar]
- 14. Ritter O, Kararigas G. Sex‐biased vulnerability of the heart to COVID‐19. Mayo Clin Proc. 2020;95(11):2332‐2335. 10.1016/j.mayocp.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tukiainen T, Villani AC, Yen A, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin‐angiotensin‐aldosterone inhibitors. Eur Heart J. 2020;41(19):1810‐1817. 10.1093/eurheartj/ehaa373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizgerd JP. Lung infection–a public health priority. PLOS Med. 2006;3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaas AK, Chen M, Varkey J, et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell Host Microbe. 2009;6:207‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olenec JP, Kim WK, Lee WM, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy ClinImmunol. 2010;125:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2(5):403‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435‐436. [DOI] [PubMed] [Google Scholar]
- 22. Murawski MR, Bowen GN, Cerny AM, et al. Respiratory syncytial virus activates innate immunity through Toll‐like receptor 2. J Virol. 2009;83:1492‐1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoo JK, Kim TS, Hufford MM, Braciale TJ. Viral infection of the lung: host response and sequelae. J Allergy ClinImmunol. 2013;132(6):1263‐1276. 10.1016/j.jaci.2013.06.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. 2016;38(4):471‐482. 10.1007/s00281-016-0558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shandera WX, Clark E. Common viral respiratory infections. In: Papadakis MA, McPhee SJ, Rabow MW eds., Current Medical Diagnosis and Treatment. McGraw‐Hill; 2020. https://accessmedicine.mhmedical.com/content.aspx?bookid=2683%26sectionid=225054979 [Google Scholar]
- 26. Zhang N, Wang L, Deng X, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92(4):408‐417. 10.1002/jmv.25674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al‐Romaihi HE, Smatti MK, Ganesan N, et al. Epidemiology of respiratory infections among adults in Qatar (2012‐2017). PLOS One. 2019;14(6):e0218097. 10.1371/journal.pone.0218097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID‐19 disease outcomes. Nature. 2020;588(7837):315‐320. 10.1038/s41586-020-2700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Białas AJ, Kumor‐Kisielewska A, Górski P. Ageing, sex, obesity, smoking and COVID‐19—truths, myths and speculations. Adv Respir Med. 2020;88(4):335‐342. 10.5603/ARM.2020.0133 [DOI] [PubMed] [Google Scholar]
- 30. Gallo V, Chiodini P, Bruzzese D, Bhopal R. Age‐and sex‐adjustment and the COVID‐19 pandemic—transformative example from Italy. Int J Epidemiol. 2020;49(5):1730‐1732. 10.1093/ije/dyaa139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salvati L, Biagioni B, Vivarelli E, Parronchi P. A gendered magnifying glass on COVID‐19. Clin Mol Allergy. 2020;18:14. 10.1186/s12948-020-00129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng H, Tan J, Zhang X, et al. Impact of sex and age on respiratory support and length of hospital stay among 1792 patients with COVID‐19 in Wuhan, China. Br J Anaesth. 2020;125(4):e378‐e380. 10.1016/j.bja.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al‐Lami RA, Urban RJ, Volpi E, Algburi A, Baillargeon J. Sex hormones and novel corona virus infectious disease (COVID‐19). Mayo Clin Proc. 2020;95(8):1710‐1714. 10.1016/j.mayocp.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moula AI, Micali LR, Matteucci F, et al. Quantification of death risk in relation to sex, pre‐existing cardiovascular diseases and risk factors in COVID‐19 patients: let's take stock and see where we are. J Clin Med. 2020;9(9):2685. 10.3390/jcm9092685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boulle A, Davies MA, Hussey H, et al. Risk factors for COVID‐19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020:ciaa1198. 10.1093/cid/ciaa1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahrenfeldt LJ, Otavova M, Christensen K, Lindahl‐Jacobsen R. Sex and age differences in COVID‐19 mortality in Europe. Wien Klin Wochenschr. 2020;22;1‐6. 10.1007/s00508-020-01793-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4(7):1178‐1191. 10.1002/rth2.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng R, Liu C, Yang J, et al. Sex differences in the incidence and risk factors of myocardial injury in COVID‐19 patients: a retrospective cohort study. Front Physiol. 2021;12:632123. 10.3389/fphys.2021.632123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manicassamy S, Pulendran B. Modulation of adaptive immunity with toll‐like receptors. Sem Immunol. 2009;21(4):185‐193. 10.1016/j.smim.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haji Abdolvahab M, Moradi‐Kalbolandi S, Zarei M, Bose D, Majidzadeh‐A K, Farahmand L. Potential role of interferons in treating COVID‐19 patients. Int Immunopharmacol. 2021;90:107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li H, Xiong N, Li C, et al. Efficacy of ribavirin and interferon‐α therapy for hospitalized patients with COVID‐19: a multicenter, retrospective cohort study. Int J Infect Dis. 2021;104:641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pfaender S, Mar KB, Michailidis E, et al. LY6E impairs coronavirus fusion and confers immune control of viral disease. bioRxiv. 2020. 10.1101/2020.03.05.979260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. The X chromosome and sex‐specific effects in infectious disease susceptibility. Hum Genomics. 2019;13(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. 2016;6:635. 10.3389/fimmu.2015.00635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gadi N, Wu SC, Spihlman AP, Moulton VR. What's sex got to do with COVID‐19? Gender‐based differences in the host immune response to coronaviruses. Front Immunol. 2020;11:2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sharma G, Volgman AS, Michos ED. Sex differences in mortality from COVID‐ 19 pandemic. JACC: Case Rep. 2020;2:1407‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID‐19 outcomes. Nat Rev Immunol. 2020;20:442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li AJ, Li X. Sex‐dependent immune response and lethality of COVID‐19. Stem Cell Res. 2021;50:102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T‐cell response in women identified by microarray analysis. Genes Immun. 2009;10(5):509‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin‐6 (IL‐6) blockade for coronavirus disease 2019 (COVID‐19)‐induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wan S, Yi Q, Fan S, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID‐19) infected patients. Br J Haematol. 2020;189(3):428‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roh JS, Sohn DH. Damage‐associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18(4):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Andersson U, Ottestad W, Tracey KJ. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID‐19? Mol Med. 2020;26:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Banerjee A, Kulcsar K, Misra V, Frieman M, Mossman K. Bats and Coronaviruses. Viruses. 2019;11(1):41. 10.3390/v11010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pinheiro I, Dejager L, Libert C. X‐chromosome‐located microRNAs in immunity: might they explain male/female differences? The X chromosome‐genomic context may affect X‐located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays. 2011;33:791‐802. [DOI] [PubMed] [Google Scholar]
- 58. Hars M, Mendes A, Serratrice C, et al. Sex‐specific association between vitamin D deficiency and COVID‐19 mortality in older patients. Osteoporos Int. 2020;31(12):2495‐2496. 10.1007/s00198-020-05677-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pagano MT, Peruzzu D, Ruggieri A, Ortona E, Gagliardi MC. Vitamin D and sex differences in COVID‐19. Front Endocrinol (Lausanne). 2020;11:567824. 10.3389/fendo.2020.567824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oestrogen. https://www.healthdirect.gov.au/oestrogen#:~:text=Oestrogen%20is%20one%20of%20the,puberty. Accessed July 20, 2020.
- 61. Sun Z, Qu J, Xia X, et al. 17β‐Estradiol promotes LC3B‐associated phagocytosis in trained immunity of female mice against sepsis. Int J Biol Sci. 2021;17(2):460‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex‐based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046‐4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lemes RMR, Costa AJ, Bartolomeo CS, et al. 17β‐estradiol reduces SARS‐CoV‐2 infection in vitro. Physiol Rep. 2021;9(2):e14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caine EA, Scheaffer SM, Arora N, et al. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat Commun. 2019;10(1):280. 10.1038/s41467-018-07993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63‐69. 10.1016/j.cellimm.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718‐724. [DOI] [PubMed] [Google Scholar]
- 67. Merrheim J, Villegas J, van Wassenhove J, et al. Estrogen, estrogen‐like molecules and autoimmune diseases. Autoimmun Rev. 2020;19(3):102468. [DOI] [PubMed] [Google Scholar]
- 68. Uehara IA, Soldi LR, Silva MJB. Current perspectives of osteoclastogenesis through estrogen modulated immune cell cytokines. Life Sci. 2020;256:117921. [DOI] [PubMed] [Google Scholar]
- 69. Khan D, Dai R, Ansar Ahmed S. Sex differences and estrogen regulation of miRNAs in lupus, a prototypical autoimmune disease. Cell Immunol. 2015;294(2):70‐79. [DOI] [PubMed] [Google Scholar]
- 70. Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen‐treated mice. J Neuroimmunol. 2005;170(1–2):85‐92. [DOI] [PubMed] [Google Scholar]
- 71. Owen J, Punt J, Stranford S, et al. Kuby Immunology. 7th ed. New York, NY: W.H. Freeman and Company; 2013:102‐104. [Google Scholar]
- 72. Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clinc Invest. 2002;109(12):1625‐1633. 10.1172/JCI14873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fountain JH, Lappin SL. Physiology, Renin‐Angiotensin System. 2020 https://www.ncbi.nlm.nih.gov/books/NBK470410/. Accessed May 9, 2019. [PubMed]
- 74. Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin‐converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013;77(2):301‐308. 10.1253/circj.cj-12-1544 [DOI] [PubMed] [Google Scholar]
- 75. Bhatia K, Zimmerman MA, Sullivan JC. Sex difference in angiotensin converting enzyme modulation of Ang (1‐7) levels in normotensive WKY rats. Am J Hypertens. 2013;26(5):591‐598. 10.1093/ajh/hps088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Oliveira AP, Peron JP, Damazo AS, et al. Female sex hormones mediate the allergic lung reaction by regulating the release of inflammatory mediators and the expression of lung E‐selectin in rats. Respir Res. 2010;11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hall OJ, Limjunyawong N, Vermillion MS, et al. Progesterone‐based therapy protects against influenza by promoting lung repair and recovery in females. PLOS Pathog. 2016;12:e1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cephus JY, Stier MT, Fuseini H, et al. Testosterone attenuates group 2 innate lymphoid cell‐mediated airway inflammation. Cell Rep. 2017;21:2487‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kissick HT, Sanda MG, Dunn LK, et al. Androgens alter T‐cell immunity by inhibiting T‐helper 1 differentiation. Proc Natl Acad Sci USA. 2014;111(27):9887‐9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32(1):23‐63. 10.1615/critrevimmunol.v32.i1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hayakawa T, Nakayama J‐I. Physiological roles of class I HDAC complex and histone demethylase. J Biomed Biotechnol. 2011;2011:129383. 10.1155/2011/129383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mann M, Cortez V, Vadlamudi RK. Epigenetics of estrogen receptor signaling: role in hormonal cancer progression and therapy. Cancers (Basel). 2011;3(3):1691‐1707. 10.3390/cancers3021691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fernandez SV, Wu Y‐Z, Russo IH, et al. The role of DNA methylation in estrogen‐induced transformation of human breast epithelial cells. Cancer Res. 2006;66(8 suppl):375 https://cancerres.aacrjournals.org/content/66/8_Supplement/375.4.article-info [Google Scholar]
- 84. Mauvais‐Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation and COVID‐19 outcomes. Endocrinology. 2020;161:bqaa127. 10.1210/endocr/bqaa127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hill‐Burns EM, Clark AG. X‐Linked variation in immune response in Drosophila melanogaster. Genetics. 2009;183:1477‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN‐α production in females. J Immunol. 2006;177:2088‐2096. [DOI] [PubMed] [Google Scholar]
- 87. Spitzer JA. Gender differences in some host defense mechanisms. Lupus. 1999;8:380‐383. [DOI] [PubMed] [Google Scholar]
- 88. Teixeira D, Longo‐Maugeri IM, Santos JLF, Duarte YAO, Lebrão ML, Bueno V. Evaluation of lymphocyte levels in a random sample of 218 elderly individuals from Sao Paulo city. Rev Bras Hematol Hemoter. 2011;33:367‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Card JW, Zeldin DC. Hormonal influences on lung function and response to environmental agents: lessons from animal models of respiratory disease. Proc Am Thorac Soc. 2009;6(7):588‐595. 10.1513/pats.200904-020RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015;5:15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rettew JA, Huet‐Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll‐like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78:432‐437. [DOI] [PubMed] [Google Scholar]
- 92. Tognarelli EI, Bueno SM, González PA. Immune‐modulation by the human respiratory syncytial virus: focus on dendritic cells. Front Immunol. 2019;10:810. 10.3389/fimmu.2019.00810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Malinczak CA, Rasky AJ, Fonseca W, et al. Upregulation of H3K27 demethylase KDM6 during respiratory syncytial virus infection enhances proinflammatory responses and immunopathology. J Immunol. 2020;204(1):159‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Phipps S, Lam CE, Mahalingam S, et al. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578‐158. [DOI] [PubMed] [Google Scholar]
- 96. Tamaki M, Konno Y, Kobayashi Y, et al. Expression and functional roles of G‐protein‐coupled estrogen receptor (GPER) in human eosinophils. Immunol Lett. 2014;160(1):72‐78. 10.1016/j.imlet.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 97. Riffo‐Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares‐de‐Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37:459‐470. [DOI] [PubMed] [Google Scholar]
- 98. Bai F, Kong KF, Dai J, et al. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis. 2010;202:1804‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Akaike T, Noguchi Y, Ijiri S, et al. Pathogenesis of influenza virusinduced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448‐2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bradley LM, Douglass MF, Chatterjee D, Akira S, Baaten BJ. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus‐induced toll‐like receptor signaling. PLOS Pathog. 2012;8:e1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tripathi S, Verma A, Kim EJ, White MR, Hartshorn KL. LL‐37 modulates human neutrophil responses to influenza A virus. J Leukoc Biol. 2014;96:931‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cabello N, Mishra V, Sinha U, et al. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1150‐L1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lam VC, Lanier LL. NK cells in host responses to viral infections. Curr Opin Immunol. 2017;44:43‐51. 10.1016/j.coi.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007; 28(6):252‐259. [DOI] [PubMed] [Google Scholar]
- 105. Lee SH, Biron CA. Here today‐‐not gone tomorrow: roles for activating receptors in sustaining NK cells during viral infections. Eur J Immunol. 2010;40(4):923‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. van Erp EA, van Kampen MR, van Kasteren PB, de Wit J. Viral infection of human natural killer cells. Viruses. 2019; 11(3):243. 10.3390/v11030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Market M, Angka L, Martel AB, et al. Flattening the COVID‐19 curve with natural killer cell based immunotherapies. Front Immunol. 2020;11:1512. 10.3389/fimmu.2020.01512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lee BW, Yap HK, Chew FT, et al. Age and sex‐related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry. 1996;26(1):8‐15. 10.1002/(SICI)1097-0320(19960315)26:13.0.CO;2-E [DOI] [PubMed] [Google Scholar]


