Abstract
Rotation with different active ingredients is among the most effective and recommended strategies to preserve the efficacy of anticoccidial drugs and reduce the emergence of resistance. Tools such as anticoccidial sensitivity tests (ASTs) are ideally used to make rational rotation programs and bring benefits to production. The objective of this study was to evaluate the sensitivity of E. acervulina (EA) and E. maxima (EM) from 3 different regions in Brazil, by using four ASTs. Feces samples weighing 6 to 7 kg were collected in the regions of São Paulo, Paraná, and Minas Gerais. Prevalent oocysts from feces were filtered, identified, and quantified to conduct 2 ASTs with EA and 2 with EM. The same experimental design was used in every AST (4 replicates per treatment, with 6 birds each, for a total of 240 birds). Treatment groups were a nonchallenged and nonmedicated control group (T1), a challenged and nonmedicated control group (T2), and the other groups challenged and treated with the following compounds: lasalocid (90 ppm – T3), maduramycin (6 ppm – T4), decoquinate (30 ppm – T5), nicarbazin+semduramicin (66 ppm – T6), monensin (110 ppm – T7), salinomycin (66 ppm – T8), narasin+nicarbazin (100 ppm – T9), and nicarbazin (125 ppm – T10). At the end of each AST (20 d), the percent change (delta value) between the treated group (T3 to T10) and the control group (T2) was calculated for the following variables: body weight gain, feed conversion ratio, lesion score, and an indicator of percentage of optimal anticoccidial activity (POAA) that included T2. Different sensitivity levels of EA and EM isolates could be identified. As a whole, drugs from T5 and T3 groups showed higher delta values when compared to other compounds, whereas the lowest sensitivity levels of these isolates were observed in groups T4 and T7. Despite some limiting factors, ASTs can be a good tool for strategic selection of anticoccidial drugs in order to maintain efficacy and extend the lifespan of these molecules.
Key words: coccidiosis, anticoccidial sensitivity test, broiler chicken, anticoccidial resistance, anticoccidial drug
INTRODUCTION
Coccidiosis control should be among the pillars of poultry health to achieve better production results. Recent financial estimates suggest that the costs involved with prophylaxis, treatment, and production losses due to coccidiosis can amount to around U$14 billion worldwide a year (Blake et al., 2020a). More than 80% of the drugs for coccidiosis control were introduced between 1948 and 1980. The first cases of resistance were described from one to 11 yr later, for a mean of 4.2 yr (Chapman, 1997; Noack et al., 2019). Most of these drugs have been in use since then, thus leading to many assessments of resistance or loss of sensitivity (Yadav and Gupta, 2001; Conway et al., 2001; Peek and Landman, 2003; Bafundo et al., 2008; Arabkhazaeli et al., 2013; Zhang et al., 2013; Lan et al., 2017).
Nonstop use of anticoccidials can potentially lead to gene modification in Eimeria spp. Nevertheless, practical molecular methods are still unavailable to evaluate these changes (Blake et al., 2020b). Therefore, anticoccidial sensitivity tests (ASTs) need to be performed in vivo, consequently narrowing the choice of different drugs and Eimeria strains for testing (Peek and Landman, 2011). The parameters evaluated in ASTs may vary among lesion score (Johnson and Reid, 1970; Mcdougald et al., 1987), oocyst multiplication by OPG (Lan et al., 2017; Chasser et al., 2020), performance parameters such as food conversion ratio (FCR), body weight gain (BWG), and mortality (Stephan et al., 1997; Chapman, 1998), or the combination of several parameters in formulas generating indexes (Stephan et al., 1997; Arabkhazaeli et al., 2013; Lan et al., 2017).
In Brazil, there has been very little use of ASTs. In contrast, in the United States and Europe, due to a greater availability of resources and the close relationship between universities and the pharmaceutical industry, these ASTs are performed quite often to guide the selection of anticoccidial drugs, however, they are rarely published in literature. ASTs also represent the most feasible tool to assess these drugs’ efficacy in product registration or extension trials required by regulatory authorities (Chapman, 1997; Naciri et al., 2003; Peek and Landman, 2003).
Regardless of the method chosen to assess sensitivity to anticoccidials, it is important to bear in mind what most impacts each broiler chicken company's environment and to strive for a cost-benefit balance between what will bring higher returns, welfare, and sustainability to that business. In modern poultry production, effective coccidiosis control is related to the correct use of available tools. In this sense, good rearing practices and prophylactic drugs are the first line of action, followed by vaccines and alternative products (Blake et al., 2017; Fatoba and Adeleke, 2018).
Considering human population growth and the increasing need for poultry meat to provide dietary protein, anticoccidials have been playing, and will continue to play, a fundamental role in coccidiosis control, the enhancement of poultry health and welfare, the effectiveness of production, and economic and environmental sustainability (Kadykalo et al., 2018).
It is important to know the sensitivity level of the different field Eimeria spp. in each broiler chicken company in order to make more assertive decisions regarding rotational management of anticoccidials. This is done to maintain the available compounds’ efficacy and achieve optimal rates of coccidiosis prevention and productivity gain. The objective of this study was to evaluate the sensitivity of E. acervulina and E. maxima from three different regions in Brazil to the compounds chiefly used locally by means of 4 ASTs.
MATERIALS AND METHODS
Ethics Statement
All procedures involving animals were conducted according to the standards of the Committee on Ethics for the Use of Animals (CEUA, acronym in Portuguese), at the Centro de Amparo à Pesquisa Veterinária (Veterinary Research Support Center). AST 1 Protocol: 0003/2018; AST 2 Protocol: 006/2019; AST 3 Protocol: 0009/2020; AST 4 Protocol: 0010/ 2020.
Sampling and Origin of Samples
Samples were collected at 3 broiler chicken companies in 3 Brazilian states on different dates: São Paulo (2018), Paraná (2019), and Minas Gerais (2020). The 2020 samples were submitted to 2 ASTs: one with E. acervulina isolates and the other with E. maxima isolates. In this study, the term AST refers to the complete set of parameters evaluated, not only to lesion scores.
AST 1
Approximately 6 kg of feces were collected at a broiler chicken company in the state of São Paulo in September 2018. The samples were collected at 8 different poultry farms, where E. maxima oocysts were most prevalent. This company did not report the anticoccidial program used at the time of sampling.
AST 2
Approximately 7 kg of feces were collected at a broiler chicken company in the state of Paraná in September 2019. The samples were collected at 12 different poultry farms, where E. acervulina oocysts were prevalent. The shuttle anticoccidial program was nicarbazin + narasin (1–21 d) and monensin (22 d until 5 d prior to slaughter) at the time of sampling, as reported by the broiler chicken company.
AST 3
Approximately 7 kg of feces were collected in February 2020 at a broiler chicken company in the state of Minas Gerais. The samples were from 6 different poultry farms with a high prevalence of E. acervulina and E. maxima. Therefore, it was decided to conduct an AST for each species of Eimeria. In this case, it was for E. acervulina. According to this company, the shuttle anticoccidial program at the time of collection was nicarbazin + semduramicin (1–21 d), and salinomycin (for 22 d up to slaughter).
AST 4
The same isolate as in AST 3. However, E. maxima oocysts were set aside for this experiment.
Feces Collection, Inoculum, and Identification of Eimeria Species
The selection of poultry farms was based on their history of coccidiosis challenge in previous flocks (presence of lesions observed in necropsy indicative of the target Eimeria species) informed by the company's veterinarian. It was also confirmed on the spot by necropsy just before collection. The flock age ranged from 21 to 35 d. Fresh feces samples, free of litter and cecal content, were collected at several places in the poultry houses on each farm. After collection, the feces samples were immediately placed in a potassium dichromate solution, at the rate of 100 mL solution for 900 g feces. The solution consisted of 2.5 g potassium dichromate diluted in 97.5 mL distilled water. Samples were placed in polyethylene terephthalate bottles at room temperature and forwarded to a laboratory within three days of collection.
Oocysts present in the feces were filtered and microscopically identified (by size). This was then confirmed by a pre-test to determine the appearance and location of the lesions (Long and Reid, 1982). The filtration procedure was conducted to separate a single target species for the in vivo test, although other oocysts present in the sample may have passed the filtering process. The large amount of feces collected made it possible to achieve the number of oocysts to each AST with no need to propagate them in live chickens. Obtained oocysts were quantified in the final volume.
To assess virulence and dose of Eimeria before each AST, a pre-test was carried out with different oocyst doses. Thirty-5 birds were included in the pre-test. They were divided into 7 groups of 5 birds each. Each group received a dilution of the original inoculum, according to the total volume of the isolate, at the age of 14 d. The lesion score was evaluated (Johnson and Reid, 1970) in all birds at 20 days of age (6 d postinoculation). The group in which at least 80% of the birds showed lesion score ≥2 and 20% weight gain reduction, with minimal mortality, was elected as the infecting dose for the AST (Mcdougald et al., 1987).
Birds and Experimental Design
One-day-old male Cobb 500 broiler chicks were housed in Eimeria-free suspended cages. They received feed and water ad libitum throughout the experimental period. The same experimental design was used in the four ASTs. A total of 240 broilers were included and divided into 10 treatments of 4 replicates, with 6 birds each (Table 1). The birds received a (nonmedicated) standard starter feed up to 12 d of age and then medicated feed with the respective anticoccidial up to 20 d of age (Lan et al., 2016).
Table 1.
In feed anticoccidial treatments included in the four anticoccidial sensitivity tests (ASTs).
| Treatment | Anticoccidial | Eimeria challenge | Concentration (%) | Dose (ppm) | Inclusion (mg/kg) |
|---|---|---|---|---|---|
| T1 | No | No | |||
| T2 | No | Yes | |||
| T3 | Lasalocid | Yes | 20 | 90 | 450 |
| T4 | Maduramycin | Yes | 1 | 6 | 600 |
| T5 | Decoquinate | Yes | 6 | 30 | 500 |
| T6 | Nicarbazin + Semduramicin | Yes | 11 | 66 | 600 |
| T7 | Monensin | Yes | 20 | 110 | 550 |
| T8 | Salinomycin | Yes | 12 | 66 | 550 |
| T9 | Narasin + Nicarbazin | Yes | 16 | 100 | 625 |
| T10 | Nicarbazin | Yes | 25 | 125 | 500 |
All birds from treatment groups T2 to T10 were inoculated with 1 mL of Eimeria oocyst solution at 14 d of age (concentrations were AST 1: 100,000 E. maxima oocysts/bird; AST 2: 150,000 E. acervulina oocysts/bird; AST 3: 80,000 E. acervulina oocysts/bird; AST 4: 25.000 E. maxima oocysts/bird) orally (gavage). Treatment group T1 (negative control) received 1 mL of distilled water, also by gavage.
Every bird and all leftover feed were weighed at 20 d of age for weight gain and feed conversion ratio calculations. All birds were euthanized on the same day, and coccidiosis lesion scoring was performed by using a 0 to 4 scale (Johnson and Reid, 1970). Percentage of Optimum Anticoccidial Activity (POAA) was calculated based on weight and mortality data. POAA = (SGR in the medicated group [T3 to T10] – SGR in the challenged and nonmedicated group [T2]) / (SGR in the nonchallenged and nonmedicated group [T1] – SGR in the challenged and nonmedicated group [T2]) × 100%, where SGR (Survival and Growth Rate) was defined as the final weight (all birds in the cage + weight of the dead birds) divided by the initial weight (prechallenge) (Rathinam and Chapman, 2009; Lan et al., 2017). The Eimeria isolates were considered resistant (R) if the POAA was ≤50%, partially resistant (PR) if the POAA was between 51%-74%, and sensitive (S) if the POAA was ≥75% (Rathinam and Chapman, 2009).
Evaluation of Drug Sensitivity
To analyze the results of these four experiments, they were combined according to the challenge (E. acervulina or E. maxima). All performance data were described as a delta value of experimental group (T3 to T10) in relation to the positive control group (T2) for the parameters BWG, FCR, and lesion score (LS). The delta was calculated by performance (FCR, BWG, and LS) of experimental group minus positive control group, divided by performance of experimental group, multiplied by 100. Hence, when percentual delta is positive, it means that experimental group was numerically superior and when negative, numerically inferior to the positive control group. Note that a positive delta does not mean necessarily better result, for example, FCR a positive delta means that FCR of experimental group was superior that positive control. This is not a good result, but remains numerically superior. POAA data were described according to the result generated by the formula itself.
Statistical Analysis
To assess the influence of the use or nonuse of the anticoccidial drugs, percentual delta of FCR, BWG, LS, and POAA variables were combined by meta-analysis models. For each experimental group of each AST, a pooled effect was calculated. Inverse variance for the mean of continuous measures was used to obtain the pooled effect. This pooled effect represents a weighted mean of all values and a confidence interval of 95% is presented considering variability intragroup and inter-group. This means that is not a simple weighted mean but can be interpreted as well. Weight of each value was defined by proportion of their inverse variance as described by Schwarzer (2007). These calculations are presented by a specific kind of graphic in meta-analysis denominated Forestplot, can be found each value, pooled effect, weights of each value and confidence interval. In addition, variability between values should be tested to choose fixed or random effects models. Heterogeneity was tested by I-square test. This test evaluates that the differences between measures are random or more than expected. When heterogeneity is significant (I-square > 70–80% or P < 0.05), random effect models should be used to calculate pooled effect. When not significant, fixed effect models should be used. This procedure is necessary not to validate the results obtained, but to validate methods used to combine them. Calculations were generally challenge type and compound type. Results were presented by pooled effect, 95% confidence interval, I-square result, and their P-value. Correlations between lesion score and performance variables were calculated with Pearson´s correlation coefficient (data was tested for normal distribution by Shapiro-Wilk test ad presented symmetric). The coefficient, the confidence interval 95%, and the P-value were presented.
All analyses were processed in R environmental (R Core Team, 2019), “stats” package (basic package), “ggplot2” (Wickham, 2010) and “meta” (Schwarzer, 2007).
RESULTS
The positive control (T2) was the group that showed the worst performance results and the highest lesion score in four ASTs when compared to the groups that received any of the compounds (T3 to T10). Table 2 shows the pooled effects obtained in meta-analysis models for delta (Δ) BWG at 20 d. A positive effect was observed regardless of the anticoccidial drug used (Overall): 6.52% BWG with confidence interval (CI) from 2.21 to 10.80% in challenges with E. acervulina and 5.25% BWG with CI from −1.12 to 11.60% in challenges with E. maxima. In the E. acervulina challenge, the most numerically outstanding compound was decoquinate (DEC) (Δ = 13.95%, CI −0.79 to 28.6%), and the lowest numerical delta value in BWG was maduramycin (MAD) (Δ = 2.08%, CI −10.3 to 14.4%). In the E. maxima challenge lasalocid (LAS) (Δ = 10.35%, CI −10.9 to 31.6%) showed the highest delta in BWG, and monensin (MON) (Δ = 3.10%, CI −12.8 to 19.0%) had the lowest delta value in BWG. In both challenges, there was an overlap in the confidence interval, indicating no statistical difference for BWG.
Table 2.
Pooled effects obtained in meta-analysis models for delta body weight gain at 20 d.
| CI 95% |
|||||
|---|---|---|---|---|---|
| Challenge | Treatment | Pooled effects (%) | Lower | Upper | I2 and P-value heterogeneity |
| E. acervulina | Overall | 6.52 | 2.21 | 10.8 | 0%, P = 1.0 |
| Lasalocid | 9.08 | −2.39 | 20.5 | 0%, P = 0.7 | |
| Maduramycin | 2.08 | −10.3 | 14.4 | 0%, P = 0.9 | |
| Decoquinate | 13.95 | −0.79 | 28.6 | 0%, P = 0.8 | |
| Nicarbazin+semduramicin | 7.89 | −5.20 | 20.9 | 0%, P = 0.7 | |
| Monensin | 3.90 | −5.22 | 13.0 | 0%, P = 0.3 | |
| Salinomycin | 5.72 | −8.06 | 19.5 | 0%, P = 0.7 | |
| Narasin+Nicarbazin | 7.07 | −7.29 | 21.4 | 0%, P = 0.6 | |
| Nicarbazin | 6.57 | −5.18 | 18.3 | 0%, P = 0.8 | |
| E. maxima | Overall | 5.25 | −1.12 | 11.6 | 0%, P = 1.0 |
| Lasalocid | 10.35 | −10.9 | 31.6 | 0%, P = 0.7 | |
| Maduramycin | 5.02 | −20.2 | 30.2 | 0%, P = 0.9 | |
| Decoquinate | 9.84 | −8.22 | 27.9 | 0%, P = 0.5 | |
| Nicarbazin+semduramicin | 3.47 | −15.1 | 22.1 | 0%, P = 0.9 | |
| Monensin | 3.10 | −12.8 | 19.0 | 0%, P = 0.9 | |
| Salinomycin | 3.97 | −12.5 | 20.4 | 0%, P = 0.7 | |
| Narasin+Nicarbazin | 3.73 | −15.6 | 23.1 | 0%, P = 0.8 | |
| Nicarbazin | 4.71 | −9.95 | 19.4 | 0%, P = 0.8 | |
CI: Confidence interval; I2: I-square test percentual and P-value of this. I-square is a heterogeneity test that evaluates if the differences between measures are random or more than expected. When heterogeneity is significant (I-square > 70–0% and/or P < 0.05), random effect models were used to calculate pooled effect. When not significant, fixed effect models were used. This procedure is necessary not to validate the results obtained, but to validate methods used to combine them. Significant or not significant differences between pooled effects of each compound can be evaluated by comparing confidence intervals and their intersections. Significant differences are when there is no intersections between CI.
Overall, the use of anticoccidials resulted in a reduction of −2.15% (CI −3.37 to −0.93%) in FCR in challenges with E. acervulina and a reduction of −2.13% (CI −3.45 to −0.81%) in challenges with E. maxima. The compounds DEC (Δ = −4.38%, CI –6.15 to −2.61%) and nicarbazin+semduramicin (NIC+SEM) (Δ = −3.37%, CI −4.47 to −2.27%) presented lower delta in FCR compared to MON (Δ = −0.02%, CI –1.38 to 1.34%) and narasin+nicarbazin (NAR+NIC) (Δ = −0.10%, CI −1.86 to 1.66%) in challenges with E. acervulina (Table 3). In challenge with E. maxima, LAS (Δ = -5.07%, CI −5.29 to −4.85%) showed lower delta in FCR compared to MON (Δ = 1.10%, CI −2.40 to 4.60%), NAR+NIC (Δ = 0.67%, CI −4.11 to 5.45%) and nicarbazin (NIC) (Δ = −3.53%, CI −4.37 to −2.69%) (Table 3).
Table 3.
Pooled effects obtained in meta-analysis models for delta of feed conversion ratio.
| CI 95% |
|||||
|---|---|---|---|---|---|
| Challenge | Treatment | Pooled effects (%) | Lower | Upper | I2 and P-value heterogeneity |
| E. acervulina | Overall | −2.15 | −3.37 | −0.93 | 100%, P = 0 |
| Lasalocid | −3.01 | −5.55 | −0.48 | 99%, P < 0.01 | |
| Maduramycin | 0.77 | −3.10 | 4.64 | 100%, P < 0.01 | |
| Decoquinate | −4.38 | −6.15 | −2.61 | 99%, P < 0.01 | |
| Nicarbazin+semduramicin | −3.37 | -−4.47 | −2.27 | 98%, P < 0.01 | |
| Monensin | −0.02 | −1.38 | 1.34 | 97%, P < 0.01 | |
| Salinomycin | −2.68 | −7.45 | 2.09 | 100%, P < 0.01 | |
| Narasin+Nicarbazin | −0.10 | −1.86 | 1.66 | 99%, P < 0.01 | |
| Nicarbazin | −4.41 | −7.38 | −1.43 | 100%, P< 0.01 | |
| E. maxima | Overall | −2.13 | −3.45 | −0.81 | 100%, P = 0 |
| Lasalocid | −5.07 | −5.29 | −4.85 | 76%, P = 0.04 | |
| Maduramycin | −2.95 | −7.18 | 1.27 | 100%, P = 0 | |
| Decoquinate | −2.19 | −8.73 | 4.36 | 100%, P = 0 | |
| Nicarbazin+semduramicin | −2.63 | −4.92 | −0.35 | 100%, P < 0.01 | |
| Monensin | 1.10 | -2.40 | 4.60 | 100%, P < 0.01 | |
| Salinomycin | −2.42 | −7.36 | 2.52 | 100%, P < 0.01 | |
| Narasin+Nicarbazin | 0.67 | −4.11 | 5.45 | 100%, P = 0 | |
| Nicarbazin | −3.53 | −4.37 | −2.69 | 97%, P < 0.01 | |
CI: Confidence interval; I2: I-square test percentual and P-value of this. I-square is a heterogeneity test that evaluates if the differences between measures are random or more than expected. When heterogeneity is significant (I-square > 70–80% and/or P< 0.05), random effect models were used to calculate pooled effect. When not significant, fixed effect models were used. This procedure is necessary not to validate the results obtained, but to validate methods used to combine them. Significant or not significant differences between pooled effects of each compound can be evaluated by comparing confidence intervals and their intersections. Significant differences are when there is no intersections between CI.
Birds treated with LAS (Δ = −55.89%, CI −85.44 to −26.34%) or NIC (Δ = −33.63%, CI −42.19 to −25.08%) presented more reduction in E. acervulina lesion scores compared to birds treated with NAR+NIC (Δ = −18.70%, CI −22.13 to −15.26%) (Table 4). LAS (Δ = −71.34%, CI −78.39 to −64.29%) showed higher reduction in lesion score in challenges with E. maxima compared to NIC+SEM (Δ = −15.01%, CI −32.26 to 2.24%), MAD (Δ = −15.20%, CI −21.04 to –9.36%), salinomycin (Δ = −17.28%, CI −30.29 to −4.27%), and NIC (Δ = −17.96%, CI −24.98 to −10.94%) (Table 4).
Table 4.
Pooled effects obtained in meta-analysis models for delta lesion score.
| CI 95% |
|||||
|---|---|---|---|---|---|
| Challenge | Treatment | Pooled effects (%) | Lower | Upper | I2 and P-value heterogeneity |
| E. acervulina | Overall | −117.83 | −117.89 | −177.77 | 100%, P < 0.001 |
| Lasalocid | −55.89 | −85.44 | −26.34 | 100%, P < 0.001 | |
| Maduramycin | −58.48 | −147.40 | 30.43 | 100%, P < 0.001 | |
| Decoquinate | −503.27 | −1252.84 | 246.30 | 100%, P < 0.001 | |
| Nicarbazin+semduramicin | −46.34 | −105.78 | 13.11 | 100%, P < 0.001 | |
| Monensin | −20.59 | −31.56 | −9.63 | 100%, P < 0.001 | |
| Salinomycin | −29.23 | −46.42 | −12.03 | 100%, P < 0.001 | |
| Narasin+Nicarbazin | −18.70 | −22.13 | −15.26 | 100%, P < 0.001 | |
| Nicarbazin | −33.63 | −42.19 | −25.08 | 100%, P < 0.001 | |
| E. maxima | Overall | −62.91 | −144.41 | 18.59 | 100%, P < 0.001 |
| Lasalocid | −71.34 | −78.39 | −64.29 | 100%, P < 0.001 | |
| Maduramycin | −15.20 | −21.04 | −9.36 | 100%, P < 0.001 | |
| Decoquinate | −294.16 | −795.60 | 207.27 | 100%, P < 0.001 | |
| Nicarbazin+semduramicin | −15.01 | −32.26 | 2.24 | 100%, P < 0.001 | |
| Monensin | −38.48 | −106.79 | 29.82 | 100%, P < 0.001 | |
| Salinomycin | −17.28 | −30.29 | −4.27 | 100%, P < 0.001 | |
| Narasin+Nicarbazin | −33.86 | −80.34 | 12.62 | 100%, P < 0.001 | |
| Nicarbazin | −17.96 | −24.98 | −10.94 | 100%, P < 0.001 | |
CI: Confidence interval; I2: I-square test percentual and P-value of this. I-square is a heterogeneity test that evaluates if the differences between measures are random or more than expected. When heterogeneity is significant (I-square > 70–80% and/or P < 0.05), random effect models were used to calculate pooled effect. When not significant, fixed effect models were used. This procedure is necessary not to validate the results obtained, but to validate methods used to combine them. Significant or not significant differences between pooled effects of each compound can be evaluated by comparing confidence intervals and their intersections. Significant differences are when there is no intersections between CI.
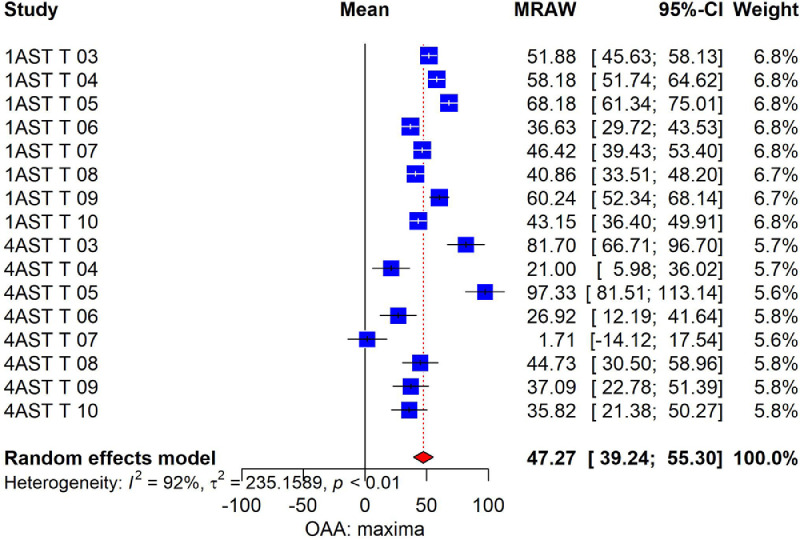
Regarding POAA, which is based only in BWG in the 2 control groups (positive and negative) and the test compound, it is possible to observe that DEC (Δ = 47.07%, CI 25.6 to 68.5%) presented higher percentage of OAA compared to MAD (Δ = 6.60%, CI −6.55 to 19.7%) and MON (Δ = 7.06%, CI −11.4 to 25.5%) in E. acervulina challenged birds. In E. maxima challenged birds, DEC showed higher POAA compared to NIC+SEM, salinomycin and NIC (Table 5). The analysis of POAA classification in R/PR/S in the challenge with E. maxima revealed sensitivity to decoquinate, partial resistance to lasalocid, and resistance to the remaining test compounds. All compounds were classified as resistant in the challenge with E. acervulina. Figure 1 shows the splitting of POAA results in AST 1 and 4, when the challenge was with E. maxima. Details of all parameters can be found in the supplementary material as Forestplot graphics.
Table 5.
Pooled effects obtained in meta-analysis models for percentage of optimal anticoccidial activity (POAA).
| Classification | CI 95% |
|||||
|---|---|---|---|---|---|---|
| Challenge | Treatment | Pooled effects (%) | R/PR/S | Lower | Upper | I2 and P-value heterogeneity |
| E. acervulina | Overall | 22.45 | R | 13.8 | 31.1 | 74%, P < 0.01 |
| Lasalocid | 33.09 | R | −1.27 | 67.4 | 86%, P < 0.01 | |
| Maduramycin | 6.60 | R | −6.55 | 19.7 | 0%, P = 0 | |
| Decoquinate | 47.07 | R | 25.6 | 68.5 | 68%, P = 0 | |
| Nicarbazin+semduramicin | 18.62 | R | 6.3 | 30.9 | 0%, P = 0 | |
| Monensin | 7.06 | R | −11.4 | 25.5 | 48%, P =0 | |
| Salinomycin | 21.63 | R | −4.04 | 47.3 | 74%, P = 0 | |
| Narasin+Nicarbazin | 18.80 | R | 6.30 | 31.3 | 0%, P = 0 | |
| Nicarbazin | 23.72 | R | 0.21 | 47.2 | 70%, P = 0 | |
| E. maxima | Overall | 47.27 | R | 39.2 | 55.3 | 92%, P < 0.01 |
| Lasalocid | 65.9 | PR | 36.7 | 95.1 | 92%, P = 0 | |
| Maduramycin | 40.2 | R | 3.83 | 76.6 | 95%, P = 0 | |
| Decoquinate | 81.8 | S | 53.3 | 110.3 | 91%, P = 0 | |
| Nicarbazin+semduramicin | 34.0 | R | 25.6 | 42.4 | 27%, P = 0 | |
| Monensin | 24.6 | R | -−19.1 | 68.4 | 96%, P = 0 | |
| Salinomycin | 41.6 | R | 35.1 | 48.2 | 0%, P = 0 | |
| Narasin+Nicarbazin | 49.4 | R | 26.8 | 72.1 | 87%, P = 0 | |
| Nicarbazin | 41.8 | R | 35.7 | 47.9 | 0%, P =0 | |
CI: Confidence interval; I2: I-square test percentual and P-value of this. I-square is a heterogeneity test that evaluates if the differences between measures are random or more than expected. When heterogeneity is significant (I-square > 70–80% and/or P < 0.05), random effect models were used to calculate pooled effect. When not significant, fixed effect models were used. This procedure is necessary not to validate the results obtained, but to validate methods used to combine them. Significant or not significant differences between pooled effects of each compound can be evaluated by comparing confidence intervals and their intersections. Significant differences are when there is no intersections between CI.
Abbreviations: PR, Partially Resistant; R, Resistant; S, Sensitive.
Figure 1.
Forestplot of meta-analysis model of optimum anticoccidial activity (POAA) with different treatments in ASTs 1 and 4 (challenge with E. maxima). T03: lasalocid, T04: maduramycin T05: decoquinate, T06: nicarbazin+semduramicin, T07: monensin, T08: salinomycin, T09: narasin+nicarbazin, T 10: nicarbazin. Blue square are the mean of POAA in each experimental group and each AST; red diamond represents pooled effect of all experimental groups considered.
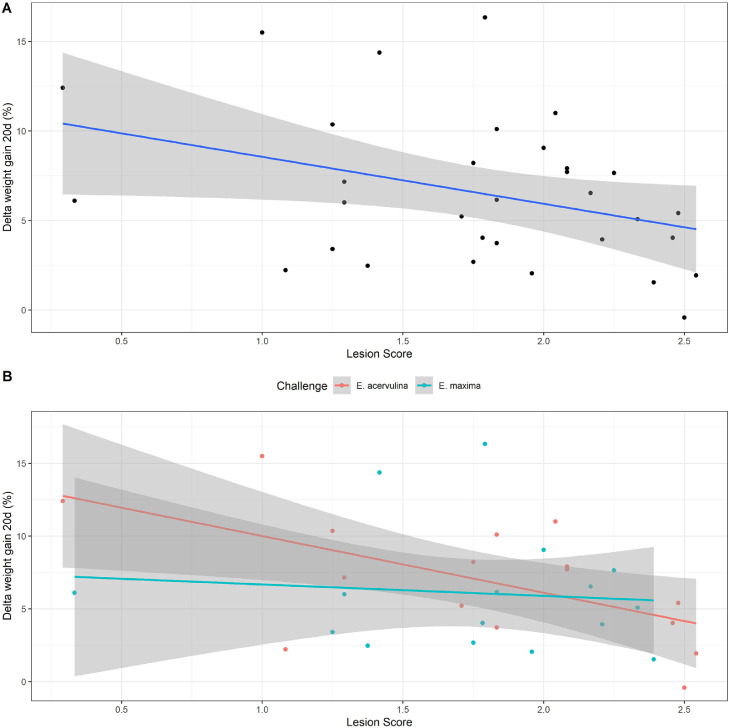
The correlation analysis between lesion score and the variables FCR, BWG, and POAA included treatments T3 to T10. It combined the results of the 4 ASTs and separated by type of challenge (Table 6). No significant correlation between ΔFCR and lesion score was observed. A negative correlation between ΔBWG and LS was observed (r = −36) overall and in the challenge with E. acervulina (r = −0.59), that is, the lower the BWG, the higher the LS (Figure 2). The POAA showed a negative correlation with LS in the overall analysis (r = −0.42), and in the challenges with E. maxima (r = −60) and with E. acervulina (r = −0.43).
Table 6.
Correlation coefficient between lesion scores and other variables.
| Confidence interval for coefficient |
|||||
|---|---|---|---|---|---|
| Variable | Challenge | Correlation coefficient | Lower | Upper | P-value |
| Delta feed conversion 1–20 d | Overall | −0.07 | −0.41 | 0.28 | 0.698 |
| E. acervulina | 0.11 | −0.40 | 0.58 | 0.674 | |
| E. maxima | −0.27 | −0.67 | 0.27 | 0.321 | |
| Delta body weight gain 1–20 d | Overall | −0.36 | −0.63 | −0.02 | 0.041 |
| E. acervulina | −0.59 | −0.84 | −0.13 | 0.017 | |
| E. maxima | −0.10 | −0.57 | 0.42 | 0.715 | |
| POAA | Overall | −0.42 | −0.67 | −0.09 | 0.016 |
| E. acervulina | −0.43 | −0.76 | 0.09 | 0.098 | |
| E. maxima | −0.60 | −0.84 | −0.14 | 0.015 | |
Figure 2.
Scatterplot of correlation between lesion score and delta weight gain, regardless of challenge (A) and separating challenges with E. acervulina and E. maxima (B).
DISCUSSION
The last study of this category carried out in Brazil was conducted by Mcdougald et al., 1987. Sixty samples of Eimeria (a mix of several species – not specified the dose of the inoculum) were isolated and submitted to an extensive test with the 7 compounds most commonly used at that time (monensin, narasin, salinomycin, maduramycin, clopidol, amprolium, and nicarbazin). Although a direct comparison between studies does not seem adequate, the difference in lesion score and BWG between the treated group and the nonmedicated and infected control group reveals a significant change in the last 30 yr. The assessed compounds reduced lesion scores by 52% on average and improved weight gain by 77.7% compared to the control group (Mcdougald et al., 1987). In the present study, the overall average of the used compounds resulted in an improvement of 6.52% in weight gain in challenge with E. acervulina and of 5.52% in challenge with E. maxima. Similar results were found by Conway et al., 2001 in 2 studies. The investigators used salinomycin, monensin, and lasalocid in one of these experiments, and nicarbazin, narasin+nicarbazin, and zoalene in the other study. Arabkhazaeli et al., 2013 also evaluated salinomycin, amprolium+ethopabate, and diclazuril and reported similar results.
Considering FCR as a strong indicator of drug sensitivity, MON and NAR+NIC presented lower delta compared to DEC in challenge with E. acervulina and compared to LAS in challenge with E. maxima. This does not mean that these compounds have completely lost their efficacy, since analysis of the confidence interval reveals different effect possibilities, which may be influenced by different field conditions. An interesting point is that even after 50 yr of use, monensin remains an important tool in coccidiosis control programs (Chapman et al., 2010). In general, a partial loss of efficacy combined with immunity acquisition explains the continuity of ionophore efficacy in the field (Chapman et al., 2010).
Compounds showing considerable effects on BWG, FCR, POAA, and lesion scores independent of the main challenge were decoquinate and lasalocid. One hypothesis for this finding would be that these drugs would have “rested” due to the low frequency of use in anticoccidial programs and that their sensitivity was restored. Chapman and Jeffers, 2015 observed this effect of restoration of sensitivity with salinomycin after 5 flocks were reared under different drug programs and vaccine use. It is important to point out that, in our experiments, oocysts were not propagated in live chickens but were filtered and used directly as inoculum, which minimizes oocyst selection. However, the doses used are considered high, and this does not reflect the magnitude of field challenges (Chapman, 1999).
Another relevant fact of this study is that treatment with nicarbazin (T10) apparently was not the most effective in ASTs. This caught our attention, because it is very common for treatment with nicarbazin to stand out in relation to other anticoccidials in similar assessments conducted in the United States and Europe (Mathis et al., 1984; Mcdougald et al., 1986; Bafundo et al., 2008). Although nicarbazin is known to be the most effective compound in coccidiosis control, this might be changing. The reason may be that Brazilian anticoccidial programs have continuously used pure nicarbazin or associated with another ionophore without any rotation. The mechanisms why Eimeria spp. develop resistance against nicarbazin are still poorly understood (Chapman, 1997). However, this issue deserves a deeper understanding, by increasing the number of ASTs in other Brazilian regions to determine how widely this occurs.
Many factors may interfere in the pathogenicity and sensitivity profile of drugs against Eimeria spp., such as region, previous exposure to other drugs, and use of the same drug for a long period (Tan et al., 2017). However, approaches used to detect resistance or virulence factors in several bacteria (Fluit et al., 2001) are not yet available for Eimeria (Blake et al., 2020b). Therefore, even with limiting factors, such as cost and slow procedure, in vivo experiments are the only way to estimate the sensitivity profile of Eimeria spp. to anticoccidials (Peek and Landman, 2011). This study's differential is that multiplication in live birds was not necessary because of the large volume of feces collected (5–7 kg). Literature suggests oocysts multiplication in live birds could result in selection of non-relevant coccidia (Peek and Landman, 2011). Thus, we can state that the results obtained with this methodology are closer to the reality in the field.
Although fighting resistance against anticoccidials is a difficult task, shuttle programs and rotation of compounds are approaches that help prevent or postpone its emergence (Quiroz-Castañeda and Dantán-González, 2015). In shuttle programs, different drugs are alternated in a same flock, whereas in rotation programs, different drugs are alternated after one or several seasonal or rearing cycles (Gussem, 2007). Strictly speaking, rotating between a monovalent ionophore and another may be considered rotation. However, considering that cross-resistance may happen within the same class of ionophores (Weppelman et al., 1977), the relevance of this type of rotation could be questioned (Gussem, 2007). The rotation of compounds – when rationally used – helps restore available drug efficacy since it promotes a rest period between compound usage (Chapman and Jeffers, 2015). Another means of restoring drug efficacy is the use of live vaccines, because they change the oocyst population on the litter with sensitive vaccine strains (Snyder et al., 2021).
Of all the parameters used in this study, the POAA seems to be the least accurate for sensitivity assessment to anticoccidials. Categorization into R, PR, and S does not appear to be a good indicator for understanding the differences in sensitivity level between different inocula. Perhaps for this reason, few scientific studies have adopted this form of description by category of sensitivity to Eimeria spp., differently from what is usually done for bacteria in antibiograms.
This study confirmed and identified different sensitivity levels of E. acervulina and E. maxima to these eight compounds in different Brazilian regions, based mainly in FCR. Sensitivity tests to anticoccidials in wire cages permit a good diagnosis of Eimeria sensitivity. They can substantiate making the decision to change or continue the use of more effective compounds. Even if some compounds do not further lose their effectiveness completely, frequent change – based on sensitivity studies – can identify opportunities for performance and economic gains.
DISCLOSURES
The authors declare that there is no conflict of interests regarding the publication of this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi: https://doi.org/10.1016/j.psj.2021.101233.
Appendix. Supplementary materials
REFERENCES
- Arabkhazaeli F., Modrisanei M., Nabian S., Mansoori B., Madani A. Evaluating the resistance of eimeria spp. field isolates to anticoccidial drugs using three different indices. Iran. J. Parasitol. 2013;8:234–241. [PMC free article] [PubMed] [Google Scholar]
- Bafundo K.W., Cervantes H.M., Mathis G.F. Sensitivity of Eimeria field isolates in the United Sstates: responses of nicarbazin-containing anticoccidials. Poult. Sci. 2008;87:1760–1767. doi: 10.3382/ps.2008-00129. [DOI] [PubMed] [Google Scholar]
- Blake D.P., Knox J., Dehaeck B., Huntington B., Rathinam T., Ravipati V., Ayoade S., Gilbert W., Adebambo A.O., Jatau I.D., Raman M., Parker D., Rushton J., Tomley F.M. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:1–14. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.P., Pastor-Fernández I., Nolan M.J., Tomley F.M. Recombinant anticoccidial vaccines - a cup half full? Infect. Genet. Evol. 2017;55:358–365. doi: 10.1016/j.meegid.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Blake D.P., Worthing K., Jenkins M.C. Exploring Eimeria genomes to understand population biology: Recent progress and future opportunities. Genes (Basel) 2020;11:1–14. doi: 10.3390/genes11091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.D. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl. Int. J. Parasitol. 1998;28:1141–1144. doi: 10.1016/s0020-7519(98)00024-1. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. The development of immunity to Eimeria species in broilers given anticoccidial drugs. Avian Pathol. 1999;28:155–162. doi: 10.1080/03079459994885. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K. Restoration of sensitivity to salinomycin in Eimeria following 5 flocks of broiler chickens reared in floor-pens using drug programs and vaccination to control coccidiosis. Poult. Sci. 2015;94:943–946. doi: 10.3382/ps/pev077. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K., Williams R.B. Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci. 2010;89:1788–1801. doi: 10.3382/ps.2010-00931. [DOI] [PubMed] [Google Scholar]
- Chasser K.M., Duff A.F., Wilson K.M., Briggs W.N., Latorre J.D., Barta J.R., Bielke L.R. Research Note: Evaluating fecal shedding of oocysts in relation to body weight gain and lesion scores during Eimeria infection. Poult. Sci. 2020;99:886–892. doi: 10.1016/j.psj.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway D.P., Mathis G.F., Johnson J., Schwartz M., Baldwin C. Efficacy of diclazuril in comparison with chemical and ionophorous anticoccidials against Eimeria spp. in broiler chickens in floor pens. Poult. Sci. 2001;80:426–430. doi: 10.1093/ps/80.4.426. [DOI] [PubMed] [Google Scholar]
- Fatoba A.J., Adeleke M.A. Diagnosis and control of chicken coccidiosis: a recent update. J. Parasit. Dis. 2018;42:483–493. doi: 10.1007/s12639-018-1048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluit A.C., Visser M.R., Schmitz F.J. Molecular detection of antimicrobial resistance. Clin. Microbiol. Rev. 2001;14:836–871. doi: 10.1128/CMR.14.4.836-871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussem M.D. Coccidiosis in poultry : review on diagnosis, control, prevention and interaction with overall gut health. 16th European Symposium on Poultry Nutrition; Strasbourg, France; 2007. pp. 253–261. [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kadykalo S., Roberts T., Thompson M., Wilson J., Lang M., Espeisse O. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents. 2018;51:304–310. doi: 10.1016/j.ijantimicag.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Lan L.H., Sun B.B., Zuo B.X.Z., Chen X.Q., Du A.F. Prevalence and drug resistance of avian Eimeria species in broiler chicken farms of Zhejiang province. China. Poult. Sci. 2017;96:2104–2109. doi: 10.3382/ps/pew499. [DOI] [PubMed] [Google Scholar]
- Lan L., Zuo B., Ding H., Huang Y., Chen X., Du A. Anticoccidial evaluation of a traditional Chinese medicine-Brucea javanica-in broilers. Poult. Sci. 2016;95:811–818. doi: 10.3382/ps/pev441. [DOI] [PubMed] [Google Scholar]
- Long P.L., Reid W.M. University of Georgia, College of Agriculture, Experiment Stations; Athens, GA: 1982. A Guide For The Diagnosis of Coccidiosis in Chickens. [Google Scholar]
- Mathis G.F., McDougald L.R., McMurray B. Drug sensitivity of coccidia from broiler breeder pullets and from broilers in the same integrated company. Avian Dis. 1984;28:453–459. [PubMed] [Google Scholar]
- Mcdougald L.R., Fuller L., Solisa J. Drug-sensitivity of 99 isolates of coccidia from broiler farms. Avian Dis. 1986;30:690–694. [PubMed] [Google Scholar]
- Mcdougald L.R., Silva J.M.L., Solis J., Braga M. A survey of sensitivity to anticoccidial drugs in 60 isolates of coccidia from broiler chickens in Brazil and Argentina Mauricio Braga Published by : American Association of Av. Avian Dis. 1987;31:287–292. [PubMed] [Google Scholar]
- Naciri M., De Gussem K., Fort G., Bernardet N., Nérat F., Chaussé A.M. Pages 826–827 in Spring Meeting of the WPSA French Branch. 2003. Interest of anticoccidial sensitivity tests (ASTs) in the prevention of chicken coccidiosis. [DOI] [PubMed] [Google Scholar]
- Noack S., Chapman H.D., Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019;118:2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001. Avian Pathol. 2003;32:391–401. doi: 10.1080/0307945031000121149. [DOI] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Quiroz-Castañeda R.E., Dantán-González E. Control of avian coccidiosis: Future and present natural alternatives. Biomed. Res. Int. 2015;2015:430610. doi: 10.1155/2015/430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2019. R: A language and environment for statistical computing.https://www.r-project.org/ Acessed Nov. 2020. [Google Scholar]
- Rathinam T., Chapman H.D. Sensitivity of isolates of Eimeria from Turkey flocks to the anticoccidial drugs amprolium, clopidol, diclazuril, and monensin. Avian Dis. 2009;53:405–408. doi: 10.1637/8679-030509-Reg.1. [DOI] [PubMed] [Google Scholar]
- Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7:40–45. http://cran.r-project.org/doc/Rnews/ [Google Scholar]
- Snyder R.P., Guerin M.T., Hargis B.M., Kruth P.S., Page G., Rejman E., Rotolo J., Sears W., Zeldenrust E.G., Whale J., Barta J.R. Restoration of anticoccidial sensitivity to a commercial broiler chicken facility in Canada. Poult. Sci. 2021;100:663–674. doi: 10.1016/j.psj.2020.10.042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan B., Rommel M., Daugschies A., Haberkorn A. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet. Parasitol. 1997;69:19–29. doi: 10.1016/s0304-4017(96)01096-5. [DOI] [PubMed] [Google Scholar]
- Tan L., Li Y., Yang X., Ke Q., Lei W., Mughal M.N., Fang R., Zhou Y., Shen B., Zhao J. Genetic diversity and drug sensitivity studies on Eimeria tenella field isolates from Hubei Province of China. Parasi.t Vectors. 2017;10:1–10. doi: 10.1186/s13071-017-2067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weppelman R.M., Olson G., Smith D.A., Tamas T., Van Iderstine A. Comparison of anticoccidial efficacy, resistance and tolerance of narasin, monensin and lasalocid in chicken battery trials. Poult. Sci. 1977;56:1550–1559. doi: 10.3382/ps.0561550. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: Elegant Graphics for Data Analysis - book review. J. Stat. Softw. 2010;35:1–3. [Google Scholar]
- Yadav A., Gupta S.K. Study of resistance against some ionophores in Eimeria tenella field isolates. Vet. Parasitol. 2001;102:69–75. doi: 10.1016/s0304-4017(01)00512-x. [DOI] [PubMed] [Google Scholar]
- Zhang J.J., Wang L.X., Ruan W.K., An J. Investigation into the prevalence of coccidiosis and maduramycin drug resistance in chickens in China. Vet. Parasitol. 2013;191:29–34. doi: 10.1016/j.vetpar.2012.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.