Summary
Tuberous sclerosis (TS) is a rare disorder exhibiting multi-systemic benign neoplasms. We hypothesized the origin of TS neoplastic cells derived from the neural crest given the heterogeneous ecto-mesenchymal phenotype of the most common TS neoplasms. To test this hypothesis, we employed Cre-loxP lineage tracing of myelin protein zero (Mpz)-expressing neural crest cells (NCCs) in spontaneously developing renal tumors of Tsc2+/−/Mpz(Cre)/TdTfl/fl reporter mice. In these mice, ectopic renal tumor onset was detected at 4 months of age increasing in volume by 16 months of age with concomitant increase in the subpopulation of tdTomato+ NCCs from 0% to 6.45% of the total number of renal tumor cells. Our results suggest that Tsc2+/− mouse renal tumors arise from domiciled proliferative progenitor cell populations of neural crest origin that co-opt tumorigenesis due to mutations in Tsc2 loci. Targeting neural crest antigenic determinants will provide a potential alternative therapeutic approach for TS pathogenesis.
Subject area: molecular physiology, stem cells research, cancer;
Graphical abstract
Highlights
-
•
Renal and hepatic tumors in Tsc2+/− mice comprise of neural crest cells (NCCs)
-
•
NCC proliferation increased with Tsc2+/− mice tumor volume and multiplicity
-
•
Multipotent Tsc2+/− NCCs induce tumors in ectodermally derived and non-neural tissues
-
•
Pharmacologically targeting NCCs offers an alternate treatment for tuberous sclerosis
Molecular physiology; Stem cells research; Cancer;
Introduction
Tuberous sclerosis (TS) is characterized by highly variable benign neoplasms and hamartomatous lesions in the brain, skin, heart, lungs, and kidneys (Dabora et al., 2001), seizures, and TS-associated neuropsychiatric disorders (de Vries et al., 2015). Heterozygous mutations of the tuberous sclerosis genes TSC1 or more frequently TSC2 have been causatively linked to the abnormal cytological proliferation characterizing tumors in patients with TS (Carsillo et al., 2000; Giannikou et al., 2016; Jones et al., 1999; Sato et al., 2002; Smolarek et al., 1998; Yu and Henske, 2010); however, not all tumors exhibit mutations in these genes (Sancak et al., 2005; Tyburczy et al., 2015). These TSC1/2 mutations lead to hyperactivation of the mechanistic target of rapamycin (mTOR) pathway in a subset of the tumors (Clements et al., 2015; Goncharova et al., 2002), which have since informed the Food and Drug Administration-approved use of mTOR inhibitors for treatment of TS (Barrera et al., 2013; Bissler et al., 2008; Franz et al., 2013; McCormack et al., 2011). Although inhibition of the mTOR complexes 1/2 (mTORC1/2) exerts cytostatic effects on tumor cells, the treatment is transient and not curative (Barrera et al., 2013; Davies et al., 2011; Yao et al., 2014), causing recurrence of tumors upon cessation of therapy (Bee et al., 2018; McCormack et al., 2011). This limitation in the therapeutic effects of mTOR pathway inhibitors could well be due to the fact that many patient tumors do not exhibit TSC mutations (Beauchamp et al., 1998; Niida et al., 1999, 2001) and express the tuberin protein (Badri et al., 2013; Johnson et al., 2002). Given the debilitating phenotypes in multiple organs resulting in poor clinical outcomes, it is imperative we urgently resolve the source of pathogenic cells in TS. Not only will such knowledge foster the development of more effective treatment and prophylaxis for TS, providing relief to over 2 million sufferers worldwide (Kingswood et al., 2016), understanding the origin of TS lesions and tumors will clarify the sporadic and unpredictable nature of disease pathogenesis. Currently, the precise identification of the source of pathogenic cells in TS remains unclear (Henske et al., 2016; Henske and McCormack, 2012; Lam et al., 2018).
Histologically, TS neoplasms commonly comprise immature cells exhibiting either neuronal characteristic, as found in cortical tubers, subependymal giant giant-cell astroocytomas, and retinal astrocytic hamartomas, and non-neuronal phenotypes found mostly outside of central nervous system (CNS) organs (Brigo et al., 2018; Delaney et al., 2014; Feliciano, 2020). While evidence supporting the postulate that CNS lesions originate due to mTOR-induced neural dysplasia, hyperexcitability, abnormal differentiation, and defective maturation of neural progenitor cells during embryonic development is quite compelling (Feliciano et al., 2012; Lin et al., 2016; Park et al., 2018), the ontogenetic mechanisms of non-CNS lesions of TS is not well understood. For instance, some research groups have recently made strides to identify the potential origin of the TS neoplastic cells outside the CNS albeit non-concordant findings, including the hypotheses that lymphangioleiomyomatosis (LAM), the main pulmonary manifestation of TS, is caused by cells of a pleural mesothelial origin (Clements et al., 2020) or uterine origin (Guo et al., 2020). Similarly, postulates of a neural crest origin for renal angiomyolipomas (AMLs), the most common cause of mortality in adult TS (Lam et al., 2018), and patients with LAM have been proposed by others. These investigators have observed that lesions in LAM and renal AMLs comprise heterogeneous cells including those that consistently express markers of smooth muscle cells (α-SMA, vimentin, and desmin), melanocytes (HMB45, MART-1), and adipocytes (AdPLA2) (Taveira-DaSilva and Moss, 2015), cell types that are phenotypically mesenchymal and in most cases foreign to their host tissue interstitium (Henske and McCormack, 2012). Coupled with the multi-organ occurrence of TS lesions (Delaney et al., 2014; Henske and McCormack, 2012), the pathogenesis of LAM and non-CNS lesions in TS seems reminiscent of developmental paradigms of neural crest lineages that give rise to portions of the embryonic mesenchyme either as trunk neural crest cells (TNCCs), cranial neural crest cells (CNCC), and cardiac or vagal neural crest cells (NCCs) (Baggiolini et al., 2015). These neural crest subtypes are postulated to determine TS disease heterogeneity (Delaney et al., 2014; Henske and McCormack, 2012), and the timing of Tsc1/2 mutations in these cells during embryogenesis is predicted to govern the severity of the TS and LAM phenotype (Delaney et al., 2014). However, no experimental data exist to support the hypothesis which remains to be directly and comprehensively investigated (Delaney et al., 2014). Cumulatively, these studies do suggest that TS neoplasms have an embryogenic origin.
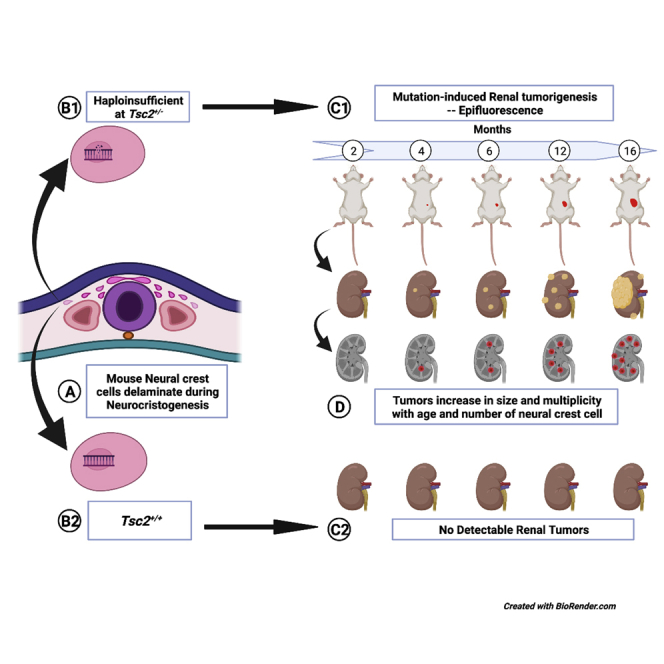
Given the relatively high frequency of renal lesions in TS, TS-associated lymphangioleiomyomatosis (TS-LAM), and sporadic LAM (S-LAM) (Dixon et al., 2011; Rakowski et al., 2006; Siroky et al., 2011), we employed a tuberous sclerosis Tsc2+/− reporter mouse model that spontaneously develops renal cystadenomas (Onda et al., 1999) to test the hypothesis that mouse renal neoplasms are neurocristopathies, pathologies borne of a neural crest lineage. The expression of the tdTomato (TdT) reporter gene in these mice is governed by a Cre-recombinase-driven event under the control of the myelin protein zero (Mpz) promoter, a neural crest lineage marker (Yamauchi et al., 1999). This enables tissues derived from the neural crest to be detected in these mice and pathogenic cells of neural crest lineage to be determined and tracked in growing tumors of the tuberous sclerosis mice and in postmortem biochemical analyses. Mpz expression has previously been associated with multipotent post-migratory differentiating neural crest progenitors that exhibit fate restrictions including smooth muscle-like cells, glia, and neurons dependent on the cellular context and instructive environmental cues (Dupin and Coelho-Aguiar, 2013; Hagedorn et al., 1999). HMB45-positive angiomyolipoma and cystic single cells have also been identified in otherwise normal renal parenchyma (Siroky et al., 2011) reminiscent of migratory pathogenic neural crest-derived cells that defines our hypothesis (Pacheco-Rodriguez and Moss, 2010). The slow development of these tumors in the heterozygote Tsc2 mice (Kwiatkowski, 2010; Onda et al., 1999) mimics the gradual benign growth pattern of TS hamartomas in most patients making this mouse model a suitable translational animal model for this study.
Using a non-invasive imaging strategy comprising epifluorescent IVIS spectral imaging and small animal ultrasound, we report the earliest detection of ectopic renal neoplasms in these tuberous sclerosis mice at 4 months of age. We resolved that a small population of primitive neural crest precursors, whose number increases with increasing tumor volume and maturity, populates renal tumors in the tuberous sclerosis mice and could source renal tumor ontogenesis in TS.
Results
Generation of the tuberous sclerosis reporter mouse model
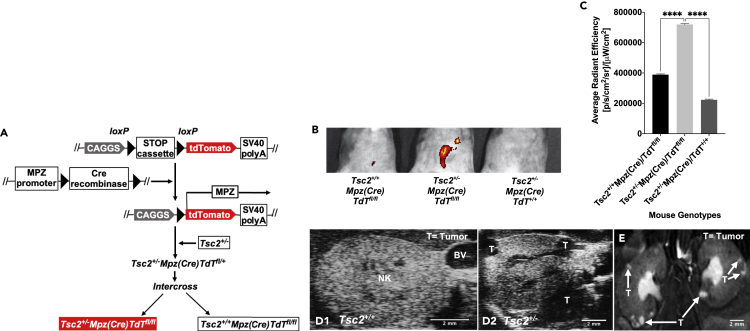
To determine whether TS tumor cells originate from a neural crest lineage, we generated a recapitulative mouse model by crossing floxed B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J reporter mice with transgenic B6N.FVB-Tg(Mpz-Cre)26Mes/J mice. Using genotyping studies, we next selected Mpz(Cre)/TdTfl/fl reporter mice progeny expressing TdT driven by the myelin protein zero promoter (Mpz) (Yamauchi et al., 1999). Upon breeding these mice with the Tsc2+/− tuberous sclerosis mice (Onda et al., 1999) and F1 hybrid crosses of resulting Tsc2+/−/Mpz(Cre)/TdTfl/+ progeny, experimental mice denoting genotypes Tsc2+/−/Mpz(Cre)/TdTfl/fl, Tsc2+/−/Mpz(Cre)/TdT+/+, Tsc2+/+/Mpz(Cre)/TdTfl/fl, and wild-type control Tsc2+/+/Mpz(Cre)/TdT+/+ mice were aged for 16 months to allow for tumor development. A schematic of genetic crosses involved in the development of this mouse model is displayed in Figure 1A. These crossings generate an animal model that allows for systemic epifluorescent detection of neural crest lineage cells in developing TS tumors by Cre-recombinase-mediated insertion of the Mpz promoter to drive tdTomato expression (Liu et al., 2015; Yamauchi et al., 1999). Tumor tracking in this Tsc2+/− reporter mice was performed beginning at 2 months of age at biweekly intervals using sequential In Vivo Imaging System (IVIS) spectral imaging detecting tdTomato excitation/emission spectra at 570 nm/620 nm and small animal ultrasound. Small animal magnetic resonance imaging was used to confirm sites of tumor development prior to excision for subsequent analysis. Figure 1B displays results of IVIS spectral imaging of an array of 16-month-old littermate progeny of the selected genotypes used in this study showing tdTomato epifluorescence to be perceptibly brightest in the abdominal region of only the Tsc2+/−/Mpz(Cre)/TdTfl/fl mice. Spectral quantification of tdTomato epifluorescence revealed significantly higher average radiant efficiency measurements (p < 0.0001∗∗∗) in Tsc2+/−/Mpz(Cre)/TdTfl/fl mice compared to other mouse genotypes in two different sets of mice imaging studies (Figure 1C). TS tumorigenesis in this select mouse group was further verified by ultrasound imaging resolving regions representing multiple peripheral tumors in the kidneys of Tsc2+/−/Mpz(Cre)/TdTfl/fl mice (Figure D2) compared to tumor-less renal tissue in the Tsc2+/+/Mpz(Cre)/TdTfl/fl mice (Figure D1). High-resolution magnetic resonance imaging confirmed the presence of bilateral cortical tumor growth in both kidneys of the Tsc2+/−/Mpz(Cre)/TdTfl/fl mice (Figure 1E). Tsc2+/−/Mpz(Cre)/TdT+l+ mice spontaneously developed renal tumors which could not be detected by tdTomato epifluorescence. Hepatic lesions were also detected in the 16-month-old Tsc2+/−/Mpz(Cre)/TdTfl/fl mice by ultrasound imaging (Figure S1B) compared to cross-sectional images of non-tumorigenic hepatic tissues recovered from Tsc2+/+/Mpz(Cre)/TdTfl/fl mice (Figure S1A). The presence of hepatic lesions in the Tsc2+/−/Mpz(Cre)/TdTfl/fl mice was further confirmed by high-resolution MRI (Figure S1C).
Figure 1.
Generation of tuberous sclerosis reporter mouse model
(A) Schematic of genetic crosses to generate Tsc2+/− reporter mouse models shows floxed R26Sortm9(CAG-tdTomato)Hze mice (female, N = 10 animals) were crossed with Tg(Mpz-Cre)26Mes mice (male, N = 10 animals) expressing Cre recombinase under the control of myelin protein zero (Mpz) promoter. Mice with Mpz(Cre)tm1.1JDar mouse progeny (male/female, N = 8 animals) were then mated with Tsc2+/− mice (male/female, N = 10 animals) to source Tsc2+/−/Mpz(Cre)/TdTfl/fl tuberous sclerosis reporter mouse models (shown in red) (male/female, N = 24 animals) that spontaneously develop renal tumors driven by neural crest cells labeled with tdTomato.
(B) IVIS spectral images of abdominopelvic cavities of 1.3-yr-old Tsc2+/−/Mpz(Cre)/TdTfl/fl tuberous sclerosis mouse model compared to age-matched Tsc2+/-Mpz(Cre)TdT+/+ and Tsc2+/+Mpz(Cre)TdTfl/fl mice distinguishing epifluorescence of tdTomato-expressing regions.
(C) Average radiant efficiency was used to compare tdTomato epifluorescence in these mouse models by one-way analysis of variance (ANOVA) and post-hoc Dunnett's test (p < 0.0001∗∗∗∗). Small animal ultrasound scans of sagittal sections of normal kidney (NK) parenchyma in adult wild-type mice imaged on a viewing stage in supine position (D1) in comparison to renal sagittal sections in Tsc2+/-Mpz(Cre)TdTfl/fl mice (D2) with labeled sites of tumors (T). BV denotes blood vessels. Rapid magnetic resonance images of kidneys (E) in Tsc2+/-Mpz(Cre)TdTfl/fl mice identifying tumors (T) shown with arrows. See also Figures S1A–S1C comparing ultrasound and MRI images of tumorigenic hepatic tissues in Tsc2+/-Mpz(Cre)/TdTfl/fl mice to normal liver tissue of Tsc2+/+Mpz(Cre)/TdTfl/fl mice.
Renal tumorigenesis in Tsc2+/− mice correlates with onset and proliferation of neural crest precursor populations
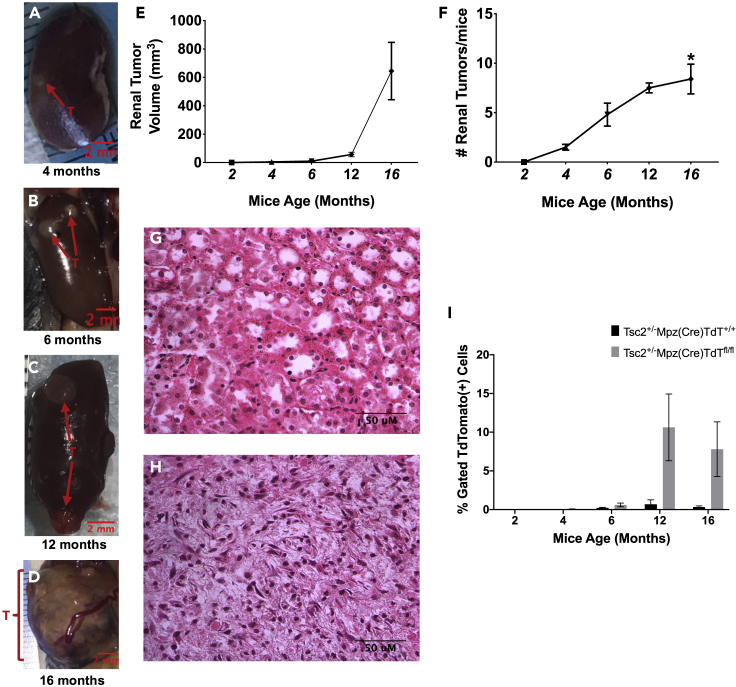
Prior to our study, the earliest reported detection of renal neoplasms in tuberous sclerosis Tsc2+/− mouse models was at 6 months of age (Kobayashi et al., 1999; Kwiatkowski, 2010; Lam et al., 2018; Onda et al., 1999), and earlier polycystic lesions occurring in the Dermo1Cre;Tsc2fl/fl mice led to mouse lethality by 3 weeks of age (Ren et al., 2016). We report the earliest detection of ectopic fluid-filled renal cysts spontaneously developing in Tsc2+/−/Mpz(Cre)/TdTfl/fl by 4 months of age (Figure 2A). These tumors were detectable by both IVIS spectral imaging and ultrasound. By 6 months of age, multiple solid tumors and/or cysts containing denser fluid can be observed (Figure 2B), which increased in volume by 12 months (Figure 2C), and by 16 months, enlarged, dysmorphic, inflamed renal tissue coalescing with multiple cysts could be observed (Figure 2D). Individual renal tumor volumes varied widely within the same kidney and mice at all ages, doubling in the mean volume from 4.68 ± 1.5 mm3 at 4 months of age (N = 4 mice) to 9.66 ± 1.9 mm3 at 6 months (N = 5 mice), to 56.8 ± 16.0 mm3 at 12 months (N = 3 mice), and then increasing by over 1,100% to 645 ± 202 mm3 by 16 months of age (Figure 2E). The average number of renal tumors per mice increased from 1.50 ± 0.3 (N = 4 mice; p = 0.895) in 4-month-old mice, to 4.80 ± 2.6 (N = 5 mice; p = 0.213) in 6-month-old mice, and to 7.50 ± 0.7 (N = 3 mice; p = 0.068) in 12-month-old mice, reaching statistical significance in 16-month-old mice (8.40 ± 3.4 tumors; N = 5 mice; ∗p = 0.025) compared to the 2-month-old mice (Figure 2F).
Figure 2.
Renal tumorigenesis in Tsc2+/− mice correlates with onset and proliferation of neural crest precursor populations
(A–D) Earliest reported detection of ectopic renal tumors in the Tsc2+/-Mpz(Cre)TdTfl/fl mice at 4 months of age (A), then pictured at 6 months (B), 12 months (C), and 16 months (D).
(E) Tumor volume increased from 4.68 ± 1.5 mm3 at 4 months of age (male/female, N = 5 animals) to 9.66 ± 1.9 mm3 at 6 months (male/female, N = 6 animals), to 56.8 ± 16.0 mm3 at 12 months (male/female, N = 5 animals), and then increasing by over 1,100% to 645 ± 202 mm3 by 16 months of age (male/female, N = 6 animals).
(F–H) (F) The average number of tumors per mice increased proportionally during the same period from 1.50 ± 0.3 (N = 5 animals; p = 0.895) in 4-month-old mice, to 4.80 ± 2.6 (N = 6 animals) in 6-month-old mice, and 7.50 ± 0.7 (N = 5 animals; p = 0.068) in 12-month-old mice, reaching statistical significance in 16-month-old mice (8.40 ± 3.4; ∗p = 0.025; N = 6 animals) compared to the 2-month-old mice. Representative images of hematoxylin/eosin (H/E)-stained immunohistochemical slides of renal tissue cross sections (4μM thickness) from 12-month-old Tsc2+/+/Mpz(Cre)/TdTfl/fl mice (G) and Tsc2+/−/Mpz(Cre)/TdTfl/fl mice (H).
(I) Renal tumors from 4- to 16-month-old Tsc2+/−Mpz(Cre)TdTfl/fl mice were dissociated, gated for live/dead staining (Sytox Green(−)/DAPI(−)/DRAQ5(+)), and sorted for tdTomato expression in comparison with renal cortical cells obtained from age-matched Tsc2+/+Mpz(Cre)TdTfl/fl mice.
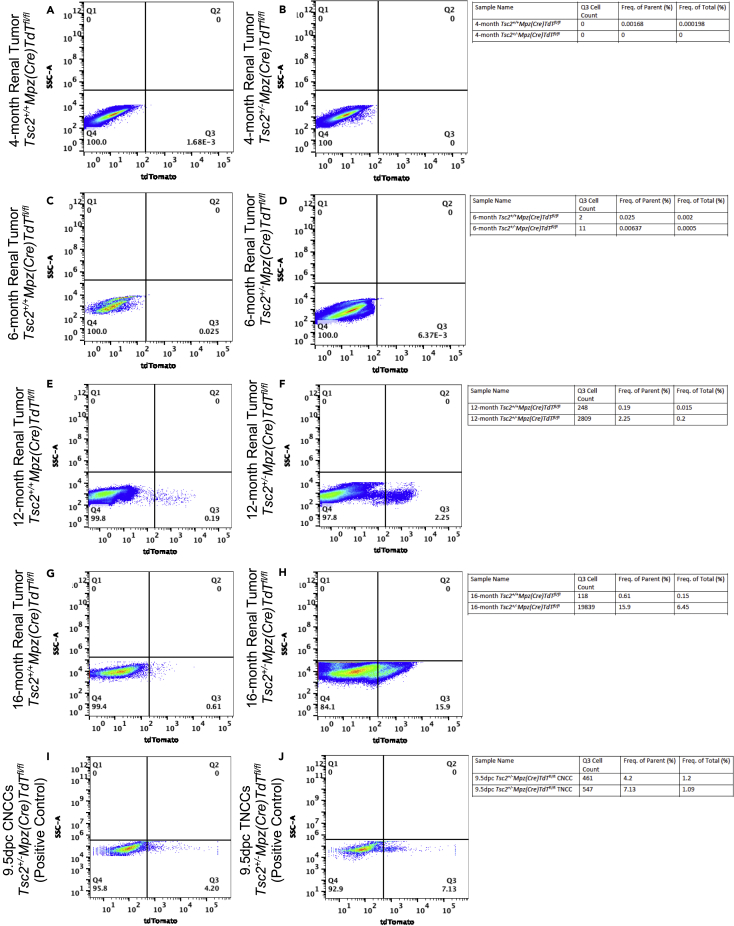
Tsc2+/− mice renal tumors and comparative renal cortical tissues were excised at 4, 6, 12, and 16 months. Representative images of hematoxylin/eosin (H/E)-stained immunohistochemical slides of renal tissue cross sections (4 μM thickness) from 12-month-old Tsc2+/+/Mpz(Cre)/TdTfl/fl mice (Figure 2G) and Tsc2+/−/Mpz(Cre)/TdTfl/fl mice (Figure 2H) are depicted. Tsc2+/− reporter mice renal tissues and tumors were dissociated into single cell populations for flow cytometric sorting of tdTomato+ NCCs. Results indicate that the significant increase in renal tumor volume (Figure 2E) and number of tumors (Figure 2F) in Tsc2+/−/Mpz(Cre)/TdTfl/fl mice over the 16-month-period correlated with a marked increase in the fraction of gated tdTomato+ cells from an average of 0.60% beginning at 6 months, to 10.6% at 12 months, and 7.8% of the total number of sorted cells at 16 months (Figure 2I) compared to sorted cell fractions from Tsc2+/+/Mpz(Cre)/TdTfl/fl mice and Tsc2+/−/Mpz(Cre)/TdT+/+ following gating protocols for live cells (DAPI(−)/Sytox Green(−)/DRAQ5(+)) and tdTomato(+). Representative dot plots comparing gating results of flow cytometric separation of renal tissue and tumor cells between Tsc2+/+/Mpz(Cre)/TdTfl/fl and Tsc2+/−/Mpz(Cre)/TdTfl/fl obtained at 4 months (Figures 3A and 3B), 6 months (Figures 3C and 3D), 12 months (Figures 3E and 3F), and 16 months (Figures 3G and 3H), respectively, are also displayed. Single cell suspensions of CNCCs (Figure 3I) and TNCCs (Figure 3J) excised from 9.5dpc Tsc2+/-Mpz(Cre)TdTfl/fl mice embryos were used as positive controls to comparatively assess the emergence of tdTomato+ NCCs in tuberous sclerosis Tsc2+/− mice. The resolution of tdTomato+ cells in renal tumor cell dissociates indicates active Mpz promoter activity in cells of a neural crest lineage. This unique cell population likely results from migrants from the neural crest that retain their proliferative and stem cell character and differentiate into source pathogenic tumor cells of TS, as phenotypic manifestations of mono-allelic and biallelic mutations at the Tsc2 gene locus. Although the fraction of these tdTomato-expressing cells in both the gated and whole-cell populations analyzed by flow cytometry is quite low, they are proportional in quantity to the NCC precursors recoverable from 9.5dpc embryonic cranial tissue and mesodermal somite used as our cranial and trunk NCC controls, respectively (Figures 3I and 3J).
Figure 3.
Flow cytometric analysis of tdTomato-positive neural crest precursors in Tsc2+/− reporter mice renal tumors
Representative flow cytometric data comparing single-cell events obtained between renal cortical tissue of Tsc2+/+Mpz(Cre)TdTfl/fl mice and Tsc2+/−Mpz(Cre)TdTfl/fl reporter mice at 4 months (A and B), 6 months (C and D), 12 months (E and F), and 16 months (G and H) are displayed, respectively. Single-cell suspensions of cranial neural crest cells (CNCCs) (I) and trunk neural crest cells (TNCCs) (J) excised from the 9.5dpc Tsc2+/-Mpz(Cre)TdTfl/fl mice embryos were used as positive controls to assess the emergence of tdTomato+ neural crest cells in tuberous sclerosis mice. Flow cytometric data shown is one representative experiment of doublet studies performed at different times using N = 2-3 mice/genotype/age group/experiment. Acquisition and analyses of flow cytometric data was performed on a Bio-Rad S3e cell sorter and analyzed using FlowJo 10.7.1/FCS Express 7 Plus software. All statistics were performed using a one-way ANOVA with post-hoc Dunnett test for multiple comparisons.
Characterization of neural crest lineage cells in Tsc2+/− mouse renal tumors
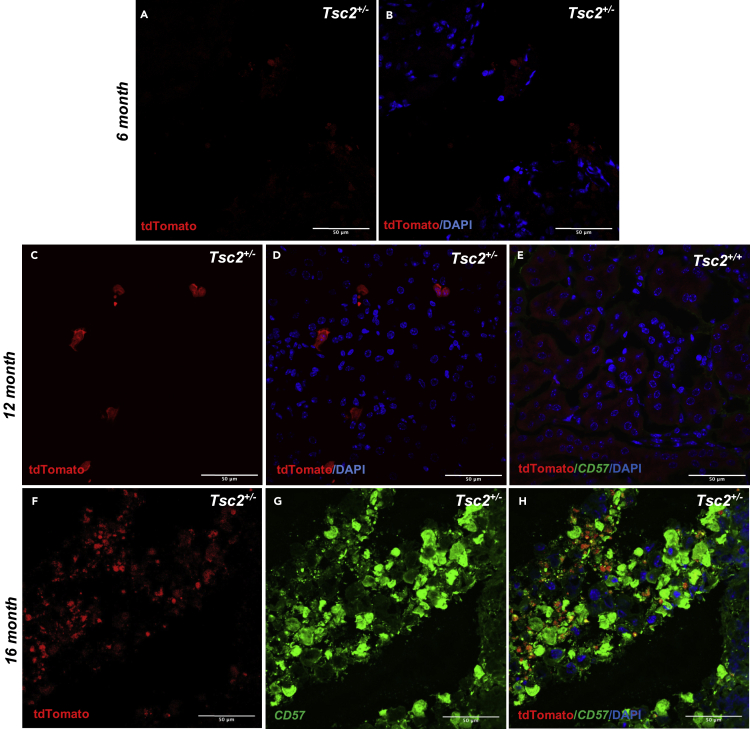
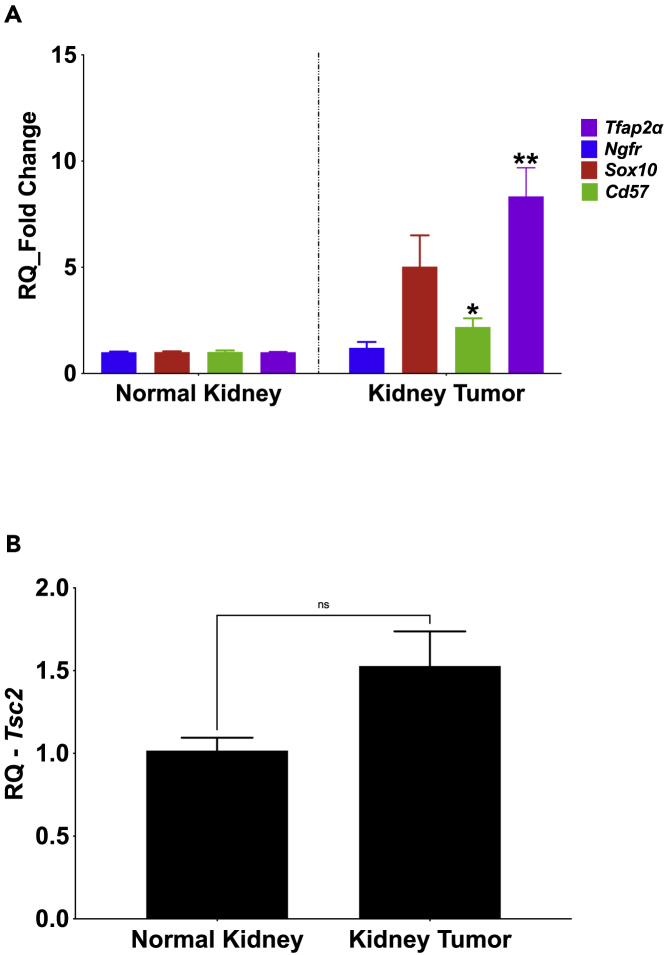
Immunohistochemical preparations of excised renal tumor slices (4 μm thickness) obtained from 6-month-old Tsc2+/−/Mpz(Cre)/TdTfl/fl mice revealed red fluorescent punctate cell masses occurring singly or in small colonies in the renal tumor slices following fluorescence excitation using the Texas Red chromatic filter (Figures 4A and 4B). The proportion of red fluorescent cells in renal tumors increased with age where even more colonies can be seen in the 12-month-old Tsc2+/−/Mpz(Cre)/TdTfl/fl mouse tumor slices (Figures 4C and 4D) and 16-month-old Tsc2+/−/Mpz(Cre)/TdTfl/fl mouse tumor slices (Figures 4F–4H) compared to renal tissue wildtype at the Tsc2 locus (Figure 4E). Double immunolabeling studies using the 16-month-old renal tumor slices reveal significant expression of neural crest marker CD57 (β-1,3-glucuronyltransferase (B3GAT1)) in select renal tumor cells by confocal microscopy, along with 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining and endogenous tdTomato expression reporting the tumor's neural crest phenotype (Figure 3H). These tdTomato+ cell masses were also observed in hepatic tumor slices obtained from Tsc2+/−/Mpz(Cre)/TdTfl/fl mice (Figures S1D and S1E). The myelin protein zero (Mpz)-driven tdTomato expression of cells in renal and hepatic tumors of the Tsc2+/− reporter mice is a direct demonstration of a neural crest lineage for these tumors. Although we have not yet successfully extracted sufficient reliable genomic and proteomic material from tdTomato+ flow-sorted cells for downstream applications in this pilot study, RT-qPCR analysis of excised whole renal tumors from Tsc2+/-Mpz(Cre)TdTfl/fl mice compared to age-matched Tsc2+/+/Mpz(Cre)TdTfl/fl mice renal tissue exhibited significantly higher expression of select neural crest markers Ap2α (p = 4.40 × 10−3∗∗) and CD57 (p = 0.05∗) (Figure 5A) in renal tumors assessed including Tsc2-null and Tsc2-expressing mouse renal tumors. In aggregate, Tsc2+/− mice renal tumors assessed in this study did not exhibit significantly different Tsc2 gene expression compared to renal cortical tissues in normal mice (p = 0.0824) (Figure 5B) in concordance with findings from our previous studies using this mouse model (D'Armiento et al., 2016).
Figure 4.
Visualizing neural crest lineage cells in Tsc2+/− mouse renal tumors
(A–D) Representative tdTomato and merged tdTomato/DAPI images of immunohistochemical slices (4 μM thickness) of renal tumors showing punctate singlets and colonies of tdTomato+ neural crest cell populations obtained in 6-month-old (A and B) and 12-month-old (C and D) Tsc2+/-Mpz(Cre)TdTfl/fl mice viewed using an excitation filter of 540/45 nm in a confocal microscope.
(E–H) (E) Comparative image panel of renal tissue from wild-type 12-month-old mice. Double immunolabeling of renal tumor slices obtained from 16-month-old Tsc2+/-Mpz(Cre)TdTfl/fl mice reveal tandem endogenous expression of Mpz-driven tdTomato red fluorescent protein (F), Alexa Fluor 488-labeled CD57 neural crest marker expression (G), and tdTomato/CD57/DAPI merged images (H) confirming neural crest identity of these cells. Confocal and fluorescence imaging was performed using N = 3 slides; 2 sections/slide to visualize each renal tumor/tissue. Scale bar represents 50μm. See also Figures S1D and S1E for fluorescent imaging of immunohistochemical slices of hepatic lesions of Tsc2+/-Mpz(Cre)/TdTfl/fl mice showing punctate tdTomato+ NCCs.
Figure 5.
Neural crest marker expression in Tsc2+/− mouse renal tumors
(A) RNA extracts of Tsc2+/+Mpz(Cre)TdTfl/fl mice kidney parenchyma and Tsc2+/-Mpz(Cre)TdTfl/fl mice renal tumors from 16-month-old mice analyzed by RT-qPCR for neural crest marker expression depict significantly increased expression of transcription factor activating protein—Tfap2α (p = 4.40 × 10−3∗∗) and CD57 (β-1,3-glucuronyltransferase (B3GAT1)) (p = 0.05∗).
(B) There was no statistically significant difference in Tsc2 gene expression state between wild-type kidney tissues and renal tumors in Tsc2+/− mice (p = 0.0824). RT-qPCR experiments were performed using triplicate biological samples applied in PCR plates in quadruplicates.
Discussion
The present study demonstrates through lineage tracing that renal angiomyolipomas and hepatic tumors in Tsc2+/− reporter mouse models harbor NCCs. The occurrence of this population of NCCs was confirmed using fluorescent and confocal microscopy, flow cytometric analyses, and immunohistochemistry, substantiating the lineage tracing results. The lineage tracing studies utilizing the myelin protein zero (Mpz) promoter, therefore, are representative of expression of the ectodermal neural crest. The immunologic expression of canonical neural crest marker CD57 in tdTomato-positive renal tumor cells (Figure 3H) further confirms their neural crest cellular identity, as well as the differential expression of multiple neural crest markers in diseased mouse renal tumors compared to healthy kidney tissues. The multipotency demonstrated by the expression pattern suggests that the tumor cell subpopulations are migrant neural crest progenitor relics of neurocristogenesis that have co-opted tumorigenesis in their various domiciled organs. Interestingly, the NCCs increased in number as the mice aged and the tumors increased in size. The described findings potentially allow for the development of efficacious targeted therapy for this untreated rare disease.
It is well established that neural crest progenitor cells are destined to delaminate from the neural tube, undergo epithelial-mesenchymal transitions (EMT), and invade multiple organs to give rise to CNCCs and trunk and vagal NCCs (Minarcik and Golden, 2003; Osumi-Yamashita et al., 1994; Serbedzija et al., 1992). NCCs are variably lineage restrictive in their multipotency, maintaining their ability to differentiate into few or many different cell types (Achilleos and Trainor, 2012; Baroffio et al., 1991; Calloni et al., 2009; Krispin et al., 2010; Motohashi et al., 2011), while retaining their multipotent self-renewal capacity even into adulthood in mammals (Bhatt et al., 2013). Interestingly, these cytogenetic activities mimic routine tumor cell physiology with ample evidence existing to demonstrate that cancer cells co-opt many of the genetic and molecular mechanisms used by developing NCCs including EMT, proliferation, migration, and differentiation (Maguire et al., 2015). Furthermore, the fate of these migratory and post-migratory NCCs is not completely determined, even after domiciliation in target organs, because they continue to generate multiple tissue derivatives (Maguire et al., 2015). The predilection of renal neoplasms in the Tsc2+/− mice and in most patients with TS could well be due to these embryogenetic lineage restrictions in the tumorigenic Tsc2+/− NCC subpopulations. Additionally, renal organs could also possess the most conducive microenvironment to co-opting tumorigenesis in these cells due to the presence of activating factors such as hormone stimulation (Yu et al., 2009).
The findings from the foregoing studies present a number of interesting possibilities. Given the embryonic nature of NCCs, it can be assumed that tumors in the tuberous sclerosis Tsc2+/− mouse model harbor NCCs possessing mono-allelic germline mutations at the Tsc2 gene loci. As haploinsufficient TS mutations have been shown to suffice for TS and LAM pathogenicity similar to Tsc2−/− neoplastic cells (D'Armiento et al., 2016; Julian et al., 2017; Martin et al., 2017; Peri et al., 2017; Tam et al., 2019), multipotent Tsc2+/− NCCs could induce tumor development in both ectodermally-derived neural and non-neural tissues (Feliciano, 2020; Ferrans et al., 2000) located in multiple organs that are ontogenetic destinations for neural crest progenitor cells (Delaney et al., 2014). Supporting this postulate is the recent delineation of catabolic signaling potentially inducing mesenchymal lineage specificity in human pluripotent stem cell-derived NCCs upon complete ablation of Tsc2 expression (Delaney et al., 2020). Similarly, Tsc1−/− NCCs were observed to drive the development of sclerotic craniofacial bone lesions in a floxed Tsc1−/−/Mpz mouse model (Fang et al., 2015). Furthermore, concomitant increase in tumor volume and number with increases in the proportion of tdTomato+ NCCs identified in this study could indicate that more Tsc2+/− NCCs initiate increased interstitial paracrine signaling that thus co-opt a greater number of host cells expanding proliferation and secretion of cystic volume. More tdTomato+ NCCs engaging in self-renewal proliferative processes can account for large numbers of tumors in the same or varied organs which altogether index more aggressive tumorigenic phenotypes.
Mutations in the Tsc1 and predominantly the Tsc2 gene loci give rise to constitutive mTOR kinase activity and unregulated cell growth and metabolism causing TS tumors (El-Hashemite et al., 2003; Giannikou et al., 2016; Inoki et al., 2003; Kenerson et al., 2007). However, in studies conducted by our group and others (Hartman et al., 2009; Higa et al., 2009; Lee et al., 2010; Li et al., 2014), several mTOR-independent pathogenic mechanisms for TS have been demonstrated in diseased tissues haploinsufficient at the Tsc2 locus. Such exemplary mTOR-independent signaling was observed in our studies demonstrating the requirement of the misexpression of DNA transcription factor High-Mobility Group (Hmga2) in mesenchymal and epithelial tumors, and the concomitant expression of upstream and downstream targets of Hmga2 such as insulin-like growth factor 2 mRNA-binding protein 2 (Igf2bp2), let-7, and lin28 in Tsc2+/− mouse tumors (Berner et al., 1997; D'Armiento et al., 2007; D'Armiento et al., 2016; Hunter et al., 2002; Kazmierczak et al., 1996; Tallini et al., 2000). This implicates an active Hmga2 pathway inducing tumorigenesis in these study models. Given our findings and above that TS tumors are phenotypically mesenchymal and originate from the neural crest and the exclusive expression of Hmga2 in embryonic undifferentiated mesenchyme (Chiappetta et al., 1996; Hock et al., 2006), Hmga2 misexpression could potentially define the ability of Tsc2+/− NCCs to form mesenchymal tumors. Furthermore, it is evident that the pathogenic cells of TS possess an enduring stemness characteristic. HMGA2 as a stemness gene has been implicated in NCC fate specification (Macri et al., 2016) and self-renewal of NCC derivative neural stem cells (Nishino et al., 2008), regulating these developmental events in a manner similar to the gene's effect in propagating mammalian tumorigenesis (Macri et al., 2016; Nishino et al., 2008). NCC progenitors differentiate into ecto-mesenchymal tissue derivatives following EMT during embryogenesis (Duband, 2000; Duband et al., 1995). Given our prior determination of the necessity for expression of Hmga2 for mesenchymal tumorigenesis in Tsc2+/− mice, the gene's expression in 100% of human and mice TS tumors, and its influence in increasing the number of renal tumors (D'Armiento et al., 2016), Hmga2 could be acting downstream of NCC delamination and Tsc2 mutational events to determine the tumorigenic potential and destiny of migratory NCCs causative for TS neoplasms.
These studies provide evidence for a neural crest origin for tumorigenic cells in lesions from Tsc2+/− mouse models and suggest that a treatment approach specifically targeting neural crest progenitor cells could potentially provide an alternative to mTOR inhibitors as treatment for TS. While mTOR inhibitors remain cytostatic to TS tumorigenic cells (Henske and McCormack, 2012), inhibitors to neural crest markers such as AP2α expressed in renal tumors examined in this study have the potential to inhibit DNA transcription and stymy tumor cell growth leading to an alternative or adjunctive therapeutic agent for TS.
Limitations of the study
Given the multi-systemic occurrence of neoplasms in patients with TS as well as in the Tsc2+/− reporter mouse model, it would have been prudent to distinguish the genomic characteristic of individual tumors assessed from the same or different diseased mouse organ(s). However, the low proportion and viability of flow-sorted tdTomato+ renal tumor cells resolved in this study limited our ability to perform downstream biochemical analysis of excised tumors, necessitating our pooling of tumors for analysis. This was not an ideal methodology for comprehensive testing of our hypothesis that Tsc2+/− mouse renal tumors were neurocristopathic because the characteristic phenotype of these different tumors is thus not known. Future single-cell RNA sequencing studies of dissociated Tsc2+/− mouse renal tumors will overcome this limitation. Additionally, the inability of the majority of Tsc2+/− mice to spontaneously develop pulmonary tumors, as well as other regularly occurring tumors and phenotypes of patients with TS, limits its appropriateness as a model of TS and specifically LAM, central to our laboratory investigations. The short life span of the Mpz-driven tdTomato expression in renal tumors limits our ability for prolonged microscopic examination of renal tumor slices by confocal or fluorescence microscopy and employing anti-tdTomato antibodies did not diminish the quenching of the fluorescent signal.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-CD57 | Santa Cruz Biotechnology | Cat# sc-6261; RRID: AB_627130 |
| Donkey anti-mouse IgG Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A21202; RRID:AB_141607 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| DMEM/F12 (1:1) | Thermo Fisher Scientific | Cat# 11320033 |
| EmbryoMax DMEM - High Glucose, Low Bicarbonate without sodium pyruvate | Millipore Sigma | Cat# SLM-220-M |
| ESGRO Leukemia Inhibitory Factor (LIF) | Millipore Sigma | Cat# ESG1106 |
| Fetal bovine serum (FBS) | GE Healthcare Biosciences | Cat# SH30071.03 |
| EmbryoMax ES Fetal Bovine Serum | Millipore Sigma | Cat# ES-009-C |
| Penicillin-Streptomycin | Thermo Fisher Scientific | Cat#15140122 |
| Collagenase I | Worthington Biochemicals | Cat# LS004196 |
| DNase I | Millipore Sigma | Cat# 4716728001 |
| Elastase | Worthington Biochemicals | Cat# LS002294 |
| Normal Donkey Serum | Sigma-Aldrich | Cat# D9663 |
| Sodium Azide | Sigma-Aldrich | Cat# S2002 |
| DRAQ5 | Biolegend | Cat# 424101 |
| Isoflurane | Henry Schein | Cat# 1311758 |
| RBC Lysis Buffer | Santa Cruz Biotechnology | Cat# sc-296258 |
| Sytox Green Nucleic Acid Stain | Thermo fisher Scientific | Cat# S7020 |
| DAPI | Thermofisher Scientific | Cat# D1306 |
| β-mercaptoethanol | Thermofisher Scientific | Cat# O3446I |
| Sodium Pyruvate | Thermofisher Scientific | Cat# 11-360-070 |
| L-Glutamine | Millipore Sigma | Cat# G7513 |
| Minimum essential media nonessential amino acids (MEM NEAA) | Thermofisher Scientific | Cat# 11095080 |
| Basic fibroblast growth factor (bFGF) | Millipore Sigma | Cat# GF003 |
| Triton X-100 | Thermofisher Scientific | Cat# BP151-500 |
| Bovine Serum Albumin | Millipore Sigma | Cat# A8412 |
| Normal Donkey Serum | Millipore Sigma | Cat# D9663 |
| Prolong Diamond Anti-fade Mountant with DAPI | Thermofisher Scientific | Cat#P36971 |
| Critical Commercial Assays | ||
| RNeasy Mini Kit | Qiagen Inc. | Cat# 74106 |
| High-Capacity cDNA Reverse Transcription Kit | Thermo Fisher Scientific | Cat# 4368813 |
| QIAamp DNA Mini Kit | Qiagen Inc. | Cat# 51304 |
| Deposited data | ||
| Original data for Figures 2J–2S | Mendeley | https://doi.org/10.17632/tsjk7wm5s8.1#file-7f9e656d-e11b-4ab4-be87-6e99139b3ab9 |
| Experimental Models: Cell Lines | ||
| STO Feeder Cell Line | ATCC | Cat# CRL:1503 RRID:CVCL_3420 |
| Experimental Models: Organisms/Strains | ||
| Mus musculus: B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J | The Jackson Laboratory | Cat# JAX:007909 RRID:IMSR_JAX:007909 |
| Mus musculus: B6N.FVB-Tg(Mpz-Cre)26Mes/J | The Jackson Laboratory | Cat# JAX:017927 RRID:IMSR_JAX:017927 |
| Mus musculus: B6. Cg-Mpz(Cre)tm1.1JDar | This paper | N/A |
| Mus musculus: B6.129S4-Tsc2tm1Djk/J Mus musculus | The Jackson Laboratory | Cat# JAX:004686 RRID:IMSR_JAX:004686 |
| Mus musculus: C57Bl/6J | The Jackson Laboratory | Cat# JAX:000664 RRID:IMSR_JAX:000664 |
| Oligonucleotides | ||
| Primer: R26R - Forward | AAAGTCGCTCTGAGTTGTTAT | N/A |
| Primer: R26R - Reverse | GGAGCGGGAGAAATGGATATG | N/A |
| Primer: Mpz - Forward | CCACCACCTCTCCATTGCAC | N/A |
| Primer: Mpz – Reverse | ATGTTTAGCTGGCCCAAATG | N/A |
| Primer: Tsc2 - Forward | CAAACCCACCTCCTCAAGCTTC | N/A |
| Primer: Tsc2 – Reverse | AGACTGCCTTGGGAAAAGCG | N/A |
| Tsc2 (Mm00442004_m1) | Thermofisher Scientific | Cat# 4331182 |
| CD57 (B3gat1) (Mm00661498_m1) | Thermofisher Scientific | Cat# 4331182 |
| Tfap2aα (Mm00495574_m1) | Thermofisher Scientific | Cat# 4331182 |
| Ngfr (Mm00446296_m1) | Thermofisher Scientific | Cat# 4331182 |
| Sox10 (Mm00569909) | Thermofisher Scientific | Cat# 4331182 |
| Software and Algorithms | ||
| Prism 9 (version 9.11) | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| VEVOLab 4.1 | Visualsonics | https://www.visualsonics.com/product/software/vevo-lab |
| Paravision 6.0 | Bruker | https://www.bruker.com/service/support-upgrades/software-downloads/mri.html |
| Living Image version 4.5 | Perkin Elmer | https://www.perkinelmer.com/product/li-software-for-spectrum-1-seat-add-on-128113 |
| GeneAmp 7900 SDS software | Thermofisher Scientific | https://www.thermofisher.com/order/catalog/product/4350490#/4350490 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jeanine D’Armiento (jmd12@cumc.columbia.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Original data for Figures 3A–3J in the paper is available (Mendeley Data DOI: https://doi.org/10.17632/tsjk7wm5s8.1#file-7f9e656d-e11b-4ab4-be87-6e99139b3ab9)
Experimental model and subject details
Mouse genetic studies
Animal experimentation was performed according to the Declaration of Helsinki convention for the use and care of animals and approved by the Institutional Animal Care and Use Committee (IACUC) at Columbia University Medical Center under protocol #AC-AAAR3401. The tuberous sclerosis Tsc2+/− transgenic reporter mice was generated by crossing floxed B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J reporter mice (JAX #007909, RRID:IMSR_JAX:007909) (female, N = 10 animals, 10 weeks old) with transgenic B6N.FVB-Tg(Mpz-Cre)26Mes/J mice (JAX #017927, RRID:IMSR_JAX:017927) (male, N = 10 animals; 10 weeks old) encoding a cell adhesion molecule myelin protein zero (P0) promoter driving expression of cre recombinase specifically in neural crest and Schwann cells (Kaku et al., 2012; Sowa et al., 2013; Yamauchi et al., 1999). These mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained on a C57BL/6J background. B6. Cg-Mpz(Cre)tm1.1JDar progeny (male/female, N = 8 animals), obtained after backcrosses (N > 5) to R26 mice, were then bred with tuberous sclerosis Tsc2+/− mice (RRID:IMSR_JAX:004686) (male/female, 10 weeks old, N = 10 animals) containing a neo-cassette targeted disruption of the second coding exon in the Tsc2 gene (Onda et al., 1999). Following F1 hybrid crosses of Tsc2+/−/Mpz(Cre)/TdTfl/+ mice and genotyping analysis, Tsc2+/−/Mpz(Cre)/TdTfl/fl (male/female, N = 22 animals), Tsc2+/−/Mpz(Cre)/TdT+/+ (male/female, N = 13 animals), Tsc2+/+/Mpz(Cre)/TdTfl/fl (male/female, N = 15 animals), and control Tsc2+/+/Mpz(Cre)/TdT+l+ (male/female, N = 12 animals) mice development was monitored for 16 months and sacrificed for experiments. A schematic of genetic crosses involved in the development of this mouse model is displayed in Figure 1A. Tracking of spontaneous renal tumor development in all mouse genotypes commenced at 2 months of age, using tumor tracking protocols described below.
All mouse genotypes were confirmed by Southern hybridization of genomic DNA prepared from tail biopsies. Mouse sample sizes for each genotype utilized in this study are adequate to measure Tsc2+/--induced tumorigenesis detectable by tdTomato expression of neural crest cells expressing the Cre recombinase under the control of Mpz promoter, in comparison to mouse progeny wildtype for Tsc2 and non-transgenic R26R mice. Given the time required for tumors to develop in the Tsc2+/− mice, the 80-90% rate of spontaneous tumorigenesis in the kidneys and liver, the number of pups per litter (∼7 pups), the number of pups per litter yield for each genotype, the isogenicity of strains specified for genetic crosses, and comparative studies between mouse genotypes, such an assumption can be made for the four genotypes being compared, using mouse attrition rate of 8%, statistical power of 0.8 with double-sided α error of 0.05 (Festing and Altman, 2002). Blinding was assured in this study because mouse genetic crosses to obtain desired genotypes, assessments of resulting phenotypes, and selection of mice for comparative imaging studies, were all performed by an independent laboratory technician without knowledge of the hypothesis being tested. Genotyping results determined assignments of mouse pups to the different genetic backgrounds. Inbred littermates were used in each mouse genotype category and mice were age- and sex-matched for each group. Mouse littermates were also selected for genetic crosses at random. All attempts at replication in this study yielded successful reproducibility 90% of the time. All tumor tracking, measurement and excision experiments were repeated 2-3 times on different days per age group.
Method details
Tumor tracking protocols
Non-invasive monitoring of renal tumor development in the TS reporter mouse was performed by sequential IVIS spectral imaging, small animal ultrasound and confirmatory magnetic resonance imaging (MRI) as described below. Tumor monitoring began at 2 months of age with biweekly intervals up to 16 months of age. All animal imaging, data acquisition and analysis was performed at the Oncology and Precision Therapeutics and Imaging Core (OPTIC) of the Columbia University Medical Center in accordance with the guidance of the Institutional Animal Care and Use Committee.
Biofluorescence imaging
Mice were anesthetized with inhaled 2.5% isoflurane (Cat# 1311758, IsoThesia™, Henry Schein, Melville, NY), and the abdomen of the animal was fully shaved using electric shears and depilatory cream (NairTM Church & Dwight Co. Trenton, NJ). Mice were then positioned supine on the warming stage of the In Vivo Imaging System (IVIS) Spectrum (Perkin Elmer, Santa Clara, CA) directly facing the camera sensor to monitor heart rate and body temperature, and capture fluorescence images. In vivo imaging system parameters were determined using standard optimization protocols with spectral unmixing. Each IVIS imaging procedure was completed within 5-10 minutes; animals were monitored until fully recovered from anesthesia. Fluorescence images were analyzed and total radiant efficiency ([photons/s]/[μW/cm2]) calculated using the Living Image version 4.5 software (Perkin Elmer, Santa Clara, CA).
High frequency ultrasound imaging
Animals were anesthetized with 2.5% isoflurane (IsoThesia™, Henry Schein, Melville, NY) and placed on an electric heating pad to prevent hypothermia; abdominal hair was removed as described. Animals were positioned supine to monitor heart rate and body temperature. Images of renal sagittal sections were obtained using the VisualSonics Vevo® 2100 Imaging System with 550D scan head (FUJIFILM VisualSonics Inc., Toronto, ON) at 55 megahertz. The 3-D Mode was used for advanced data acquisition and analysis, with virtual sections obtained in all directions (x-, y-, z- and other plane variations). Each ultrasound was completed within 10–15 minutes; animals were monitored until fully recovered from anesthesia. Ultrasound images were analyzed using the VEVOLab 4.1 software provided by Visualsonics (Toronto, ON).
Magnetic Resonance Imaging (MRI)
The MRI examination of the abdomen of the mice was performed to confirm findings of ultrasound scans in some experimental mice. MRI was performed with a 9.4 Tesla animal scanner (Bruker BioSpin, Germany). Animals were anesthetized by inhalation of isoflurane (IsoThesia™, Henry Schein, Melville, NY) (3% induction and 1-2% maintenance) and were kept homeothermic by a water-circulating system to keep the MRI bed at 37°C. A Rapid Imaging with Refocused Echoes sequence was used to acquire multi-slice images in the long axis of both kidneys by using the following parameters: TR/TE = 2600/30 ms, averages = 4, RARE factor = 8, matrix = 256x256, field of view = 40x40 mm2, slice thickness = 0.5 mm. MRI images were analyzed utilizing the Paravision 6.0 software from Bruker BioSpin.
Mouse renal tumor processing and flow cytometry
Individual renal tumor length and width per kidney pair per mice were measured using digital calipers following mice sacrifice. Tumor volumes were estimated using the formular: (Width2 X Length)/2 (Faustino-Rocha et al., 2013; Sápi et al., 2015). Renal tumor and tissues biopsied from Tsc2+/-/Mpz(Cre)/TdTfl/fl, Tsc2+/-/Mpz(Cre)/ TdT+/+, Tsc2+/+/Mpz(Cre) /TdTfl/fl, and control Tsc2+/+/Mpz(Cre)/TdT+/+ mice at 4, 6, 12, and 16 months were pooled by age group and digested into single cell suspension (Neelisetty et al., 2015). Samples were minced in DMEM-F12 media supplemented with 2% fetal bovine serum (FBS) (#SH30071.03, Hyclone, GE Healthcare Biosciences, Piscataway, NJ) using fine sterile razors and digested with a cocktail of collagenase I (170mg/L, Cat# LS004196, Worthington Biochemical, Lakewood, NJ), DNase I (0.33 U/ml; Cat# 4716728001, Millipore Sigma, St. Louis, MO) and elastase (0.075 U/ml, Cat# LS002294, Worthington Biochemical, Lakewood, NJ) in a shaker at a speed of 85rpm at 37°C for 45 mins. Tissue particles were vigorously triturated and re-incubated in the same conditions for another 30 mins then passed through 100μm, 70μm, and 40μm cell strainers sequentially. Cell suspensions were centrifuged for 5 min at 300 x g at room temperature, supernatant aspirated, and the erythrocytes lysed using 1× RBC lysis buffer (Cat# sc-296258, Santa Cruz Biotechnology, Dallas, TX) for 10 mins. Following subsequent centrifugation for 5 min at 300 x g, cell pellets were resuspended in PBS containing 2% FBS and DAPI (Cat# D1306, Thermofisher Scientific, Grand Island, NY) or Sytox green viability dye (Cat# S7020, Thermofisher Scientific, Grand Island, NY) in the dark for 10 mins. Cells were washed in cold PBS, filtered with 40μm cell strainer, and resuspended in PBS containing 2% FBS, DNase I for cell sorting, and DRAQ5 (1:1000, Cat# 424101, BioLegend, San Diego, CA) in some experiments to distinguish viable cells. Flow cytometry was performed using a BD FACS Aria II (Becton Dickinson, Franklin Lakes, NJ, USA) at the Columbia Stem Cell Initiative (CSCI) Flow Cytometry Core Facility and cells were sorted into tubes pre-incubated with DMEM-F12 containing 2% FBS. Single cells were sequentially selected on FSC-A/SSC-A and FSC-A/FSC-H dot plots and bandpass filters 586/15, 530/30, 450/50, or 715/50 were used to acquire tdTomato and viability dye signals. Tubes with unstained cells were used to set compensation and at least 50,000 events were collected for each tumor or tissue sample sorted into tdTomato(+) vs tdTomato(-) fractions per experiment. Data analysis was performed using FlowJo 10.7.1 (BD Biosciences, Franklin Lakes, NJ) or FCS Express 7 Plus (De Novo Software, Glendale, CA).
Neural crest cell culture
Cranial (CNCC) and trunk (TNCC) neural crest cells were used as positive comparative controls for flow cytometry and RT-qPCR experiments. These tissues were obtained from 9.5dpc Tsc2+/-/Mpz(Cre)/TdTfl/fl fetal mice excavated from pregnant dam. Isolation of CNCCs was performed by cutting a region between head fold and the anterior tip of the otic placode of mouse embryos (Au - Gonzalez Malagon et al., 2019) under a dissecting microscope, while TNCCs were obtained by excising embryonic neural tube between somites 16-22 (Pfaltzgraff et al., 2012). Tissue dissects were placed in the center of sterile matrigel-coated petri dishes containing neural crest media and incubated at 37°C in 5% CO2 to allow for migration of NCCs away from the neural tube. NCC media was conditioned using STO feeder cells (mouse embryonic fibroblasts) (ATCC Cat# CRL-1503, RRID:CVCL_3420) grown overnight in Dulbecco’s modified Eagle’s medium (DMEM, 4500mg/L glucose) (Cat# SLM-220-M, Millipore Sigma, St. Louis, MO) containing 15% fetal bovine serum (FBS) (Cat# ES-009-C, Millipore Sigma, St. Louis, MO), 0.1mM minimum essential media nonessential amino acids (MEM NEAA 100X) (Cat# 11095080), 1 mM sodium pyruvate (Cat# 11-360-070, Thermofisher Scientific, Grand Island, NY), 55 μM β-mercaptoethanol (Cat# O3446I-100, Thermofisher Scientific, Grand Island, NY), 100 units/mL penicillin-streptomycin (Cat#15140122, Thermofisher Scientific, Grand Island, NY) and 2 mM L-glutamine (Cat# G7513, Millipore Sigma, St. Louis, MO). Conditioned media was thereafter supplemented with basic fibroblast growth factor (bFGF, 25ng/ml) (Cat# GF003, Millipore Sigma, St. Louis, MO) and leukemia inhibition factor (LIF, 1000U) (Cat# ESG1106, Millipore Sigma, St. Louis, MO). 48hrs after NCC migration from neural tube, explant tissue is removed and NCCs routinely passaged. NCCs were incubated in DAPI viability dyes for 10 mins prior to flow cytometry experiments.
Immunohistochemistry
Renal tumor and tissues of differentially aged mice were excised, briefly washed in PBS and immediately fixed in 4% paraformaldehyde (PFA) for 2 hours at room temperature. Renal tumors and tissues are then rinsed in PBS and incubated overnight at 4°C in 30% sucrose to preserve endogenous protein expression (Wuidart et al., 2016). Thereafter, fixed tissues/tumors were immediately embedded in OCT compound, snap-frozen in isopentane mixed with dry ice and kept at -80°C. Embedded tissues were used to obtain 4 μM thick sections at the Molecular Pathology Core Facility at Columbia University Medical Center.
For CD57 (β-1,3-glucuronyltransferase (B3GAT1)) immunolabeling (sc-6261; RRID: AB_627130; Santa Cruz Biotechnologies, Dallas, TX), tissue/tumor slices were blocked and permeabilized for 1 hour at room temperature using a mixture of 0.3% Triton X-100 (Cat# BP151-500, Thermofisher Scientific, Grand Island, NY), 1% bovine serum albumin (BSA) (Cat# 8412, Millipore Sigma, St. Louis, MO) and 10% normal donkey serum (NDS) (Cat# D9663, Millipore Sigma, St. Louis, MO), and incubated overnight at 4°C in anti-CD57 diluted in antibody dilution buffer (1% BSA, 1% NDS, 0.3% Triton X-100, 0.01% sodium azide). Following 1-hr secondary antibody incubation using donkey anti-mouse Alexa Fluor 488 (Cat# A21202, RRID:AB_141607, Thermofisher Scientific, Grand Island, NY), slides were cover-slipped using Prolong Diamond Anti-fade Mountant with DAPI (Cat# P36971, Thermofisher Scientific, Grand Island, NY ) and visualized using Ti-E Eclipse inverted fluorescent or confocal microscope (Nikon Instruments Inc., Melville, NY). Immunohistochemistry experiments were repeated twice for each aged mice timepoint using N=3 slides per tissue/tumor, 2-3 sections per slide. The use of mouse tissue samples was approved by the IACUC protocol #AC-AAAR3401.
RT-qPCR and southern blot
Total RNA was isolated from mouse renal tumors and tissue biopsies excised from all mouse genotypes. RNA isolation was performed using RNeasy Mini Kit (#74106, Qiagen Inc., Valencia, CA). High-quality RNA was ensured by UV spectroscopy and incorporating DNase treatment in the protocol. Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription Kit (#4368813; Applied Biosystems, Thermofisher Scientific, Grand Island, NY, USA) (Qin et al., 2011). Quantification of gene expression was assessed in quadruplicates per biological replicate by reverse transcription qPCR using TaqMan probes with FAM dye labels (Thermofisher Scientific, Grand Island, NY), mouse primers for Tsc2 (Mm00442004_m1), B3gat1 (Mm00661498_m1), Tfap2aα (Mm00495574_m1), Ngfr (Mm00446296_m1) and Sox10 (Mm00569909) (Cat# 4331182, Thermofisher Scientific, Grand Island, NY). The ABI 7300 real-time sequence detection system (Applied Biosystems, Thermofisher Scientific, Grand Island, NY) was used along with the GeneAmp 7900 SDS software (Cat# 4350490, Applied Biosystems, Thermofisher Scientific, Grand Island, NY) to convert data into threshold cycle (ΔΔCt) values.
For genotyping studies, DNA was isolated from mice tail snips collected in sterile 1.5ml microcentrifuge tubes by digestion in 50mM Tris, pH 8.0, 100mM ethylenediaminetetraacetic acid (EDTA) (Cat# 17892, Thermofisher Scientific, Grand Island, NY), 0.5% sodium dodecyl sulfate (SDS) (Cat# L3771, Millipore Sigma, St. Louis, MO) and 20mg/ml proteinase K (Cat# AM2548, Thermofisher Scientific, Grand Island, NY). After overnight incubation, a phenol:chloroform (1:1) phase separation was performed, and DNA precipitated from the aqueous layer using a sodium acetate and ethanol mixture. DNA solubilized in TE buffer (10mM Tris, pH 8.0, 1mM EDTA) was further resolved on 1.2-2.7% agarose gel and probe hybridization performed using the following cDNA primer sequences: R26R 5’-AAAGTCGCTCTGAGTTGTTAT-3’ (forward) and 5’-GGAGCGGGAGAAATGGATATG-3’ (reverse); Mpz transgene 5’-CCACCACCTCTCCATTGCAC-3’ (forward) and 5’-ATGTTTAGCTGGCCCAAATG-3’ (reverse); Tsc2 5’-CAAACCCACCTCCTCAAGCTTC-3’ (forward) and 5’-AGACTGCCTTGGGAAAAGCG-3’ (reverse) (Integrated DNA Technologies, Research Triangle Park, NC).
Quantification and statistical analysis
This investigation was utilized as a pilot study to trace the ontogeny of tumorigenic cells in the Tsc2+/- mice; therefore, the less rigorous resource equation method and upper confidence limit (UCL) approach was employed to estimate optimal sample size for mouse groups in this pilot study (Festing, 2006). The time required for tumors to develop in Tsc2+/- mice was considered, as well as the 80-90% rate of spontaneous tumorigenesis in the kidneys, the number of pups per litter yield (∼7 pups), the total number of litters per genotype used, the isogeneity of strains specified for genetic crosses, mouse attrition rate of 8%, and statistical power of 0.8 with double-sided α error of 0.05 (Festing and Altman, 2002; Van Sluyters et al., 2003). Sample sizes in experiments are specified in each figure legend, and all data in the text and figures are expressed as mean ± SEM. Statistical tests are appropriately justified and performed using a one-way ANOVA with post-hoc Dunnett tests comparing treatment conditions to controls where applicable.
Acknowledgments
We thank Tina Zelonina for assistance in mouse breeding and genotyping, tumor excision, and volume measurements. We also thank Christopher Damoci for his technical expertise during small animal imaging and data analysis. This study was supported by grants from the Center for LAM and Rare Lung Diseases at Columbia University. J.D is supported by NIH RO1-HL086936 and Congressionally Directed Medical Research Programs (CDMRP) grant (TS170057). U.U is supported by Congressionally Directed Medical Research Programs (CDMRP) grant (TS170057). M.G is supported by NIH KO8 (HL126071).
Author contributions
Conceptualization, U.U., T.S., K.C., and J.D.; methodology, U.U., T.S., M.G., and J.D.; investigation, U.U.; formal analysis, U.U.; resources, M.G. and J.D.; writing – original draft, U.U., T.S., and J.D.; writing – review & editing, U.U., M.G., K.C., and J.D.; visualization, U.U. and J.D.; supervision, K.C. and J.D.; funding acquisition, M.G. and J.D.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We worked to ensure sex balance in the selection of non-human subjects. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list. The author list of this paper includes contributors from the location where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Published: July 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102684.
Supplemental information
References
- Achilleos A., Trainor P.A. Neural crest stem cells: discovery, properties and potential for therapy. Cell Res. 2012;22:288–304. doi: 10.1038/cr.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au - Gonzalez Malagon S.G., Au - Dobson L., Au - Muñoz A.M.L., Au - Dawson M., Au - Barrell W., Au - Marangos P., Au - Krause M., Au - Liu K.J. Dissection, culture and analysis of primary cranial neural crest cells from mouse for the study of neural crest cell delamination and migration. JoVE. 2019:e60051. doi: 10.3791/60051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri K.R., Gao L., Hyjek E., Schuger N., Schuger L., Qin W., Chekaluk Y., Kwiatkowski D.J., Zhe X. Exonic mutations of TSC2/TSC1 are common but not seen in all sporadic pulmonary lymphangioleiomyomatosis. Am. J. Respir. Crit. Care Med. 2013;187:663–665. doi: 10.1164/ajrccm.187.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini A., Varum S., Mateos J.M., Bettosini D., John N., Bonalli M., Ziegler U., Dimou L., Clevers H., Furrer R. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell. 2015;16:314–322. doi: 10.1016/j.stem.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Baroffio A., Dupin E., Le Douarin N.M. Common precursors for neural and mesectodermal derivatives in the cephalic neural crest. Development. 1991;112:301. doi: 10.1242/dev.112.1.301. [DOI] [PubMed] [Google Scholar]
- Barrera P., Simons S.O., Luijk B., Wessels M.J., Heijdra Y.F. Efficacy of sirolimus therapy for chylous effusions in lymphangioleiomyomatosis. Ann. Am. Thorac. Soc. 2013;10:408–409. doi: 10.1513/AnnalsATS.201212-125OC. [DOI] [PubMed] [Google Scholar]
- Beauchamp R.L., Banwell A., McNamara P., Jacobsen M., Higgins E., Northrup H., Short P., Sims K., Ozelius L., Ramesh V. Exon scanning of the entire TSC2 gene for germline mutations in 40 unrelated patients with tuberous sclerosis. Hum. Mutat. 1998;12:408–416. doi: 10.1002/(SICI)1098-1004(1998)12:6<408::AID-HUMU7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bee J., Fuller S., Miller S., Johnson S.R. Lung function response and side effects to rapamycin for lymphangioleiomyomatosis: a prospective national cohort study. Thorax. 2018;73:369–375. doi: 10.1136/thoraxjnl-2017-210872. [DOI] [PubMed] [Google Scholar]
- Berner J.M., Meza-Zepeda L.A., Kools P.F., Forus A., Schoenmakers E.F., Van de Ven W.J., Fodstad O., Myklebost O. HMGIC, the gene for an architectural transcription factor, is amplified and rearranged in a subset of human sarcomas. Oncogene. 1997;14:2935–2941. doi: 10.1038/sj.onc.1201135. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Diaz R., Trainor P.A. Signals and switches in Mammalian neural crest cell differentiation. Cold Spring Harb. Perspect. Biol. 2013;5:a008326. doi: 10.1101/cshperspect.a008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissler J.J., McCormack F.X., Young L.R., Elwing J.M., Chuck G., Leonard J.M., Schmithorst V.J., Laor T., Brody A.S., Bean J. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigo F., Lattanzi S., Trinka E., Nardone R., Bragazzi N.L., Ruggieri M., Martini M., Walusinski O. First descriptions of tuberous sclerosis by Désiré-Magloire Bourneville (1840–1909) Neuropathology. 2018;38:577–582. doi: 10.1111/neup.12515. [DOI] [PubMed] [Google Scholar]
- Calloni G.W., Le Douarin N.M., Dupin E. High frequency of cephalic neural crest cells shows coexistence of neurogenic, melanogenic, and osteogenic differentiation capacities. Proc. Natl. Acad. Sci. U S A. 2009;106:8947. doi: 10.1073/pnas.0903780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsillo T., Astrinidis A., Henske E.P. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. U S A. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappetta G., Avantaggiato V., Visconti R., Fedele M., Battista S., Trapasso F. High level expression of the HMGI (Y) gene during embryonic development. Oncogene. 1996;13:2439–2446. [PubMed] [Google Scholar]
- Clements D., Dongre A., Krymskaya V.P., Johnson S.R. Wild type mesenchymal cells contribute to the lung pathology of lymphangioleiomyomatosis. PLoS One. 2015;10:e0126025. doi: 10.1371/journal.pone.0126025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements D., Miller S., Johnson S.R. Pulmonary Lymphangioleiomyomatosis originates in the pleural mesothelial cell population. Med. Hypotheses. 2020;141:109703. doi: 10.1016/j.mehy.2020.109703. [DOI] [PubMed] [Google Scholar]
- D'Armiento J., Imai K., Schiltz J., Kolesnekova N., Sternberg D., Benson K., Pardo A., Selman M., Smolarek T., Vundavalli M. Identification of the benign mesenchymal tumor gene HMGA2 in lymphangiomyomatosis. Cancer Res. 2007;67:1902–1909. doi: 10.1158/0008-5472.CAN-06-1122. [DOI] [PubMed] [Google Scholar]
- D'Armiento J., Shiomi T., Marks S., Geraghty P., Sankarasharma D., Chada K. Mesenchymal tumorigenesis driven by TSC2 haploinsufficiency requires HMGA2 and is independent of mTOR pathway activation. Cancer Res. 2016;76:844–854. doi: 10.1158/0008-5472.CAN-15-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabora S.L., Jozwiak S., Franz D.N., Roberts P.S., Nieto A., Chung J., Choy Y.S., Reeve M.P., Thiele E., Egelhoff J.C. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D.M., de Vries P.J., Johnson S.R., McCartney D.L., Cox J.A., Serra A.L., Watson P.C., Howe C.J., Doyle T., Pointon K. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin. Cancer Res. 2011;17:4071–4081. doi: 10.1158/1078-0432.CCR-11-0445. [DOI] [PubMed] [Google Scholar]
- de Vries P.J., Whittemore V.H., Leclezio L., Byars A.W., Dunn D., Ess K.C., Hook D., King B.H., Sahin M., Jansen A. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr. Neurol. 2015;52:25–35. doi: 10.1016/j.pediatrneurol.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney S.P., Julian L.M., Pietrobon A., Yockell-Lelièvre J., Doré C., Wang T.T., Doyon V.C., Raymond A., Patten D.A., Kristof A.S. Human pluripotent stem cell modeling of tuberous sclerosis complex reveals lineage-specific therapeutic vulnerabilities. bioRxiv. 2020:683359. [Google Scholar]
- Delaney S.P., Julian L.M., Stanford W.L. The neural crest lineage as a driver of disease heterogeneity in Tuberous Sclerosis Complex and Lymphangioleiomyomatosis. Front. Cell Dev. Biol. 2014;2:69. doi: 10.3389/fcell.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon B.P., Hulbert J.C., Bissler J.J. Tuberous sclerosis complex renal disease. Nephron Exp. Nephrol. 2011;118:e15–e20. doi: 10.1159/000320891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband J.L. Madame Curie Bioscience Database. Landes Bioscience; 2000-2013. Neural crest delamination and migration: integrating regulations of cell interactions, locomotion, survival and fate; pp. 1–42. [DOI] [PubMed] [Google Scholar]
- Duband J.L., Monier F., Delannet M., Newgreen D. Epithelium-mesenchyme transition during neural crest development. Acta Anat. (Basel) 1995;154:63–78. doi: 10.1159/000147752. [DOI] [PubMed] [Google Scholar]
- Dupin E., Coelho-Aguiar J.M. Isolation and differentiation properties of neural crest stem cells. Cytometry. A. 2013;83:38–47. doi: 10.1002/cyto.a.22098. [DOI] [PubMed] [Google Scholar]
- El-Hashemite N., Zhang H., Henske E.P., Kwiatkowski D.J. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet. 2003;361:1348–1349. doi: 10.1016/S0140-6736(03)13044-9. [DOI] [PubMed] [Google Scholar]
- Fang F., Sun S., Wang L., Guan J.L., Giovannini M., Zhu Y., Liu F. Neural crest-specific TSC1 deletion in mice leads to sclerotic craniofacial bone lesion. J. Bone Miner Res. 2015;30:1195–1205. doi: 10.1002/jbmr.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino-Rocha A., Oliveira P.A., Pinho-Oliveira J., Teixeira-Guedes C., Soares-Maia R., da Costa R.G., Colaço B., Pires M.J., Colaço J., Ferreira R. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab. Anim. 2013;42:217–224. doi: 10.1038/laban.254. [DOI] [PubMed] [Google Scholar]
- Feliciano D.M. The neurodevelopmental pathogenesis of tuberous sclerosis complex (TSC) Front. Neuroanat. 2020;14:39. doi: 10.3389/fnana.2020.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano D.M., Quon J.L., Su T., Taylor M.M., Bordey A. Postnatal neurogenesis generates heterotopias, olfactory micronodules and cortical infiltration following single-cell Tsc1 deletion. Hum. Mol. Genet. 2012;21:799–810. doi: 10.1093/hmg/ddr511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrans V.J., Yu Z.X., Nelson W.K., Valencia J.C., Tatsuguchi A., Avila N.A., Riemenschn W., Matsui K., Travis W.D., Moss J. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J. Nippon Med. Sch. 2000;67:311–329. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]
- Festing M.F. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47:5–14. doi: 10.1093/ilar.47.1.5. [DOI] [PubMed] [Google Scholar]
- Festing M.F., Altman D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002;43:244–258. doi: 10.1093/ilar.43.4.244. [DOI] [PubMed] [Google Scholar]
- Franz D.N., Belousova E., Sparagana S., Bebin E.M., Frost M., Kuperman R., Witt O., Kohrman M.H., Flamini J.R., Wu J.Y. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- Giannikou K., Malinowska I.A., Pugh T.J., Yan R., Tseng Y.Y., Oh C., Kim J., Tyburczy M.E., Chekaluk Y., Liu Y. Whole exome sequencing identifies TSC1/TSC2 biallelic loss as the primary and sufficient driver event for renal angiomyolipoma development. PLoS Genet. 2016;12:e1006242. doi: 10.1371/journal.pgen.1006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova E.A., Goncharov D.A., Eszterhas A., Hunter D.S., Glassberg M.K., Yeung R.S., Walker C.L., Noonan D., Kwiatkowski D.J., Chou M.M. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J. Biol. Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- Guo M., Yu J.J., Perl A.K., Wikenheiser-Brokamp K.A., Riccetti M., Zhang E.Y., Sudha P., Adam M., Potter A., Kopras E.J. Single-cell transcriptomic analysis identifies a unique pulmonary lymphangioleiomyomatosis cell. Am. J. Respir. Crit. Care Med. 2020;202:1373–1387. doi: 10.1164/rccm.201912-2445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn L., Suter U., Sommer L. P0 and PMP22 mark a multipotent neural crest-derived cell type that displays community effects in response to TGF-beta family factors. Development. 1999;126:3781–3794. doi: 10.1242/dev.126.17.3781. [DOI] [PubMed] [Google Scholar]
- Hartman T.R., Liu D., Zilfou J.T., Robb V., Morrison T., Watnick T., Henske E.P. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum. Mol. Genet. 2009;18:151–163. doi: 10.1093/hmg/ddn325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henske E.P., Jozwiak S., Kingswood J.C., Sampson J.R., Thiele E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Primers. 2016;2:16035. doi: 10.1038/nrdp.2016.35. [DOI] [PubMed] [Google Scholar]
- Henske E.P., McCormack F.X. Lymphangioleiomyomatosis - a wolf in sheep's clothing. J. Clin. Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa F., Uchihara T., Haranaga S., Yara S., Tateyama M., Oshiro Y., Shiraishi M., Kumasaka T., Seyama K., Fujita J. Malignant epithelioid angiomyolipoma in the kidney and liver of a patient with pulmonary lymphangioleiomyomatosis: lack of response to sirolimus. Intern. Med. 2009;48:1821–1825. doi: 10.2169/internalmedicine.48.2411. [DOI] [PubMed] [Google Scholar]
- Hock R., Witte F., Brocher J., Schutz M., Scheer U. Expression of HMGA2 variants during oogenesis and early embryogenesis of Xenopus laevis. Eur. J. Cell Biol. 2006;85:519–528. doi: 10.1016/j.ejcb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Hunter D.S., Klotzbucher M., Kugoh H., Cai S.L., Mullen J.P., Manfioletti G., Fuhrman U., Walker C.L. Aberrant expression of HMGA2 in uterine leiomyoma associated with loss of TSC2 tumor suppressor gene function. Cancer Res. 2002;62:3766–3772. [PubMed] [Google Scholar]
- Inoki K., Li Y., Xu T., Guan K.L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.R., Clelland C.A., Ronan J., Tattersfield A.E., Knox A.J. The TSC-2 product tuberin is expressed in lymphangioleiomyomatosis and angiomyolipoma. Histopathology. 2002;40:458–463. doi: 10.1046/j.1365-2559.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- Jones A.C., Shyamsundar M.M., Thomas M.W., Maynard J., Idziaszczyk S., Tomkins S., Sampson J.R., Cheadle J.P. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am. J. Hum. Genet. 1999;64:1305–1315. doi: 10.1086/302381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian L.M., Delaney S.P., Wang Y., Goldberg A.A., Doré C., Yockell-Lelièvre J., Tam R.Y., Giannikou K., McMurray F., Shoichet M.S. Human pluripotent stem cell-derived TSC2-haploinsufficient smooth muscle cells recapitulate features of lymphangioleiomyomatosis. Cancer Res. 2017;77:5491–5502. doi: 10.1158/0008-5472.CAN-17-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku M., Komatsu Y., Mochida Y., Yamauchi M., Mishina Y., Ko C.-C. Identification and characterization of neural crest-derived cells in adult periodontal ligament of mice. Arch. Oral Biol. 2012;57:1668–1675. doi: 10.1016/j.archoralbio.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak B., Rosigkeit J., Wanschura S., Meyer-Bolte K., Van de Ven W.J., Kayser K., Krieghoff B., Kastendiek H., Bartnitzke S., Bullerdiek J. HMGI-C rearrangements as the molecular basis for the majority of pulmonary chondroid hamartomas: a survey of 30 tumors. Oncogene. 1996;12:515–521. [PubMed] [Google Scholar]
- Kenerson H., Folpe A.L., Takayama T.K., Yeung R.S. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum. Pathol. 2007;38:1361–1371. doi: 10.1016/j.humpath.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingswood C., Bolton P., Crawford P., Harland C., Johnson S.R., Sampson J.R., Shepherd C., Spink J., Demuth D., Lucchese L. The clinical profile of tuberous sclerosis complex (TSC) in the United Kingdom: a retrospective cohort study in the Clinical Practice Research Datalink (CPRD) Eur. J. Paediatr. Neurol. 2016;20:296–308. doi: 10.1016/j.ejpn.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Minowa O., Kuno J., Mitani H., Hino O., Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 1999;59:1206–1211. [PubMed] [Google Scholar]
- Krispin S., Nitzan E., Kassem Y., Kalcheim C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development. 2010;137:585. doi: 10.1242/dev.041509. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D.J. Animal models of lymphangioleiomyomatosis (LAM) and tuberous sclerosis complex (TSC) Lymphat Res. Biol. 2010;8:51–57. doi: 10.1089/lrb.2009.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H.C., Siroky B.J., Henske E.P. Renal disease in tuberous sclerosis complex: pathogenesis and therapy. Nat. Rev. Nephrol. 2018;14:704–716. doi: 10.1038/s41581-018-0059-6. [DOI] [PubMed] [Google Scholar]
- Lee P.S., Tsang S.W., Moses M.A., Trayes-Gibson Z., Hsiao L.L., Jensen R., Squillace R., Kwiatkowski D.J. Rapamycin-insensitive up-regulation of MMP2 and other genes in tuberous sclerosis complex 2-deficient lymphangioleiomyomatosis-like cells. Am. J. Respir. Cell Mol. Biol. 2010;42:227–234. doi: 10.1165/rcmb.2009-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhang E., Sun Y., Lee P.S., Zhan Y., Guo Y., Osorio J.C., Rosas I.O., Xu K.F., Kwiatkowski D.J. Rapamycin-insensitive up-regulation of adipocyte phospholipase A2 in tuberous sclerosis and lymphangioleiomyomatosis. PLoS One. 2014;9:e104809. doi: 10.1371/journal.pone.0104809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.V., Hsieh L., Kimura T., Malone T.J., Bordey A. Normalizing translation through 4E-BP prevents mTOR-driven cortical mislamination and ameliorates aberrant neuron integration. Proc. Natl. Acad. Sci. U S A. 2016;113:11330. doi: 10.1073/pnas.1605740113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Jin Y.Q., Chen L., Wang Y., Yang X., Cheng J., Wu W., Qi Z., Shen Z. Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS One. 2015;10:e0123278. doi: 10.1371/journal.pone.0123278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S., Simula L., Pellarin I., Pegoraro S., Onorati M., Sgarra R., Manfioletti G., Vignali R. Hmga2 is required for neural crest cell specification in Xenopus laevis. Dev. Biol. 2016;411:25–37. doi: 10.1016/j.ydbio.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Maguire L.H., Thomas A.R., Goldstein A.M. Tumors of the neural crest: common themes in development and cancer. Dev. Dyn. 2015;244:311–322. doi: 10.1002/dvdy.24226. [DOI] [PubMed] [Google Scholar]
- Martin K.R., Zhou W., Bowman M.J., Shih J., Au K.S., Dittenhafer-Reed K.E., Sisson K.A., Koeman J., Weisenberger D.J., Cottingham S.L. The genomic landscape of tuberous sclerosis complex. Nat. Commun. 2017;8:15816. doi: 10.1038/ncomms15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack F.X., Inoue Y., Moss J., Singer L.G., Strange C., Nakata K., Barker A.F., Chapman J.T., Brantly M.L., Stocks J.M. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. New Engl. J. Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minarcik J.C., Golden J.A. AP-2 and HNK-1 define distinct populations of cranial neural crest cells. Orthod. Craniofac. Res. 2003;6:210–219. doi: 10.1046/j.1601-6335.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- Motohashi T., Yamanaka K., Chiba K., Miyajima K., Aoki H., Hirobe T., Kunisada T. Neural crest cells retain their capability for multipotential differentiation even after lineage-restricted stages. Dev. Dyn. 2011;240:1681–1693. doi: 10.1002/dvdy.22658. [DOI] [PubMed] [Google Scholar]
- Neelisetty S., Alford C., Reynolds K., Woodbury L., Nlandu-Khodo S., Yang H., Fogo A.B., Hao C.M., Harris R.C., Zent R. Renal fibrosis is not reduced by blocking transforming growth factor-β signaling in matrix-producing interstitial cells. Kidney Int. 2015;88:503–514. doi: 10.1038/ki.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niida Y., Lawrence-Smith N., Banwell A., Hammer E., Lewis J., Beauchamp R.L., Sims K., Ramesh V., Ozelius L. Analysis of both TSC1 and TSC2 for germline mutations in 126 unrelated patients with tuberous sclerosis. Hum. Mutat. 1999;14:412–422. doi: 10.1002/(SICI)1098-1004(199911)14:5<412::AID-HUMU7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Niida Y., Stemmer-Rachamimov A.O., Logrip M., Tapon D., Perez R., Kwiatkowski D.J., Sims K., MacCollin M., Louis D.N., Ramesh V. Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am. J. Hum. Genet. 2001;69:493–503. doi: 10.1086/321972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J., Kim I., Chada K., Morrison S.J. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell. 2008;135:227–239. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda H., Lueck A., Marks P.W., Warren H.B., Kwiatkowski D.J. Tsc2(+/–) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J. Clin. Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi-Yamashita N., Ninomiya Y., Doi H., Eto K. The contribution of both forebrain and midbrain crest cells to the mesenchyme in the frontonasal mass of mouse embryos. Dev. Biol. 1994;164:409–419. doi: 10.1006/dbio.1994.1211. [DOI] [PubMed] [Google Scholar]
- Pacheco-Rodriguez G., Moss J. The role of chemokines in migration of metastatic-like lymphangioleiomyomatosis cells. Crit. Rev. Immunol. 2010;30:387–394. doi: 10.1615/critrevimmunol.v30.i4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.M., Lim J.S., Ramakrishina S., Kim S.H., Kim W.K., Lee J., Kang H.C., Reiter J.F., Kim D.S., Kim H.H. Brain somatic mutations in MTOR disrupt neuronal ciliogenesis, leading to focal cortical dyslamination. Neuron. 2018;99:83–97.e7. doi: 10.1016/j.neuron.2018.05.039. [DOI] [PubMed] [Google Scholar]
- Peri S., Caretti E., Tricarico R., Devarajan K., Cheung M., Sementino E., Menges C.W., Nicolas E., Vanderveer L.A., Howard S. Haploinsufficiency in tumor predisposition syndromes: altered genomic transcription in morphologically normal cells heterozygous for VHL or TSC mutation. Oncotarget. 2017;8:17628–17642. doi: 10.18632/oncotarget.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaltzgraff E.R., Mundell N.A., Labosky P.A. Isolation and culture of neural crest cells from embryonic murine neural tube. J. Vis. Exp. 2012:e4134. doi: 10.3791/4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W., Bajaj V., Malinowska I., Lu X., MacConaill L., Wu C.L., Kwiatkowski D.J. Angiomyolipoma have common mutations in TSC2 but no other common genetic events. PLoS One. 2011;6:e24919. doi: 10.1371/journal.pone.0024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski S.K., Winterkorn E.B., Paul E., Steele D.J., Halpern E.F., Thiele E.A. Renal manifestations of tuberous sclerosis complex: incidence, prognosis, and predictive factors. Kidney Int. 2006;70:1777–1782. doi: 10.1038/sj.ki.5001853. [DOI] [PubMed] [Google Scholar]
- Ren S., Luo Y., Chen H., Warburton D., Lam H.C., Wang L.L., Chen P., Henske E.P., Shi W. Inactivation of Tsc2 in mesoderm-derived cells causes polycystic kidney lesions and impairs lung alveolarization. Am. J. Pathol. 2016;186:3261–3272. doi: 10.1016/j.ajpath.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak O., Nellist M., Goedbloed M., Elfferich P., Wouters C., Maat-Kievit A., Zonnenberg B., Verhoef S., Halley D., van den Ouweland A. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype--phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur. J. Hum. Genet. 2005;13:731–741. doi: 10.1038/sj.ejhg.5201402. [DOI] [PubMed] [Google Scholar]
- Sápi J., Kovács L., Drexler D.A., Kocsis P., Gajári D., Sápi Z. Tumor volume estimation and quasi-continuous administration for most effective bevacizumab therapy. PLoS One. 2015;10:e0142190. doi: 10.1371/journal.pone.0142190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Seyama K., Fujii H., Maruyama H., Setoguchi Y., Iwakami S., Fukuchi Y., Hino O. Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis. J. Hum. Genet. 2002;47:20–28. doi: 10.1007/s10038-002-8651-8. [DOI] [PubMed] [Google Scholar]
- Serbedzija G.N., Bronner-Fraser M., Fraser S.E. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Siroky B.J., Yin H., Bissler J.J. Clinical and molecular insights into tuberous sclerosis complex renal disease. Pediatr. Nephrol. 2011;26:839–852. doi: 10.1007/s00467-010-1689-5. [DOI] [PubMed] [Google Scholar]
- Smolarek T.A., Wessner L.L., McCormack F.X., Mylet J.C., Menon A.G., Henske E.P. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am. J. Hum. Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa Y., Imura T., Numajiri T., Takeda K., Mabuchi Y., Matsuzaki Y., Nishino K. Adipose stromal cells contain phenotypically distinct adipogenic progenitors derived from neural crest. PLoS One. 2013;8:e84206. doi: 10.1371/journal.pone.0084206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallini G., Vanni R., Manfioletti G., Kazmierczak B., Faa G., Pauwels P., Bullerdiek J., Giancotti V., Van Den Berghe H., Dal Cin P. HMGI-C and HMGI(Y) immunoreactivity correlates with cytogenetic abnormalities in lipomas, pulmonary chondroid hamartomas, endometrial polyps, and uterine leiomyomas and is compatible with rearrangement of the HMGI-C and HMGI(Y) genes. Lab. Invest. 2000;80:359–369. doi: 10.1038/labinvest.3780040. [DOI] [PubMed] [Google Scholar]
- Tam R.Y., Yockell-Lelièvre J., Smith L.J., Julian L.M., Baker A.E.G., Choey C., Hasim M.S., Dimitroulakos J., Stanford W.L., Shoichet M.S. Rationally designed 3D hydrogels model invasive lung diseases enabling high-content drug screening. Adv. Mater. 2019;31:e1806214. doi: 10.1002/adma.201806214. [DOI] [PubMed] [Google Scholar]
- Taveira-DaSilva A.M., Moss J. Clinical features, epidemiology, and therapy of lymphangioleiomyomatosis. Clin. Epidemiol. 2015;7:249–257. doi: 10.2147/CLEP.S50780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburczy M.E., Dies K.A., Glass J., Camposano S., Chekaluk Y., Thorner A.R., Lin L., Krueger D., Franz D.N., Thiele E.A. Mosaic and intronic mutations in TSC1/TSC2 explain the majority of TSC patients with No mutation identified by conventional testing. PLoS Genet. 2015;11:e1005637. doi: 10.1371/journal.pgen.1005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sluyters R.C., Ballinger B., Bayne K., Cunningham C., Degryse A.-D., Dubner R., Evans H., Gdowski M.J., Knight R., Mench J. National Academies Press (US); 2003. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. [PubMed] [Google Scholar]
- Wuidart A., Ousset M., Rulands S., Simons B.D., Van Keymeulen A., Blanpain C. Quantitative lineage tracing strategies to resolve multipotency in tissue-specific stem cells. Genes Dev. 2016;30:1261–1277. doi: 10.1101/gad.280057.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y., Abe K., Mantani A., Hitoshi Y., Suzuki M., Osuzu F., Kuratani S., Yamamura K. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev. Biol. 1999;212:191–203. doi: 10.1006/dbio.1999.9323. [DOI] [PubMed] [Google Scholar]
- Yao J., Taveira-DaSilva A.M., Jones A.M., Julien-Williams P., Stylianou M., Moss J. Sustained effects of sirolimus on lung function and cystic lung lesions in lymphangioleiomyomatosis. Am. J. Respir. Crit. Care Med. 2014;190:1273–1282. doi: 10.1164/rccm.201405-0918OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Henske E.P. mTOR activation, lymphangiogenesis, and estrogen-mediated cell survival: the "perfect storm" of pro-metastatic factors in LAM pathogenesis. Lymphat Res. Biol. 2010;8:43–49. doi: 10.1089/lrb.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.J., Robb V.A., Morrison T.A., Ariazi E.A., Karbowniczek M., Astrinidis A., Wang C., Hernandez-Cuebas L., Seeholzer L.F., Nicolas E. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc. Natl. Acad. Sci. U S A. 2009;106:2635–2640. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data for Figures 3A–3J in the paper is available (Mendeley Data DOI: https://doi.org/10.17632/tsjk7wm5s8.1#file-7f9e656d-e11b-4ab4-be87-6e99139b3ab9)