Abstract
Viral infection and chronic maternal inflammation during pregnancy are correlated with a higher prevalence of autism spectrum disorder (ASD). However, the pathoetiology of ASD is not fully understood; moreover, the key molecules that can cross the placenta following maternal inflammation and contribute to the development of ASD have not been identified. Recently, the pro-inflammatory cytokine, interleukin-17A (IL-17A) was identified as a potential mediator of these effects. To investigate the impact of maternal IL-17A on offspring, C57BL/6J dams were injected with IL-17A-expressing plasmids via the tail vein on embryonic day 12.5 (E12.5), and maternal IL-17A was expressed continuously throughout pregnancy. By adulthood, IL-17A-injected offspring exhibited behavioral abnormalities, including social and cognitive defects. Additionally, maternal IL-17A promoted metabolism of the essential amino acid tryptophan, which produces several neuroactive compounds and may affect fetal neurodevelopment. We observed significantly increased levels of kynurenine in maternal serum and fetal plasma. Thus, we investigated the effects of high maternal concentration of kynurenine on offspring by continuously administering mouse dams with kynurenine from E12.5 during gestation. Obviously, maternal kynurenine administration rapidly increased kynurenine levels in the fetal plasma and brain, pointing to the ability of kynurenine to cross the placenta and change the KP metabolites which are affected as neuroactive compounds in the fetal brain. Notably, the offspring of kynurenine-injected mice exhibited behavioral abnormalities similar to those observed in offspring of IL-17A-conditioned mice. Several tryptophan metabolites were significantly altered in the prefrontal cortex of the IL-17A-conditioned and kynurenine-injected adult mice, but not in the hippocampus. Even though we cannot exclude the possibility or other molecules being related to ASD pathogenesis and the presence of a much lower degree of pathway activation, our results suggest that increased kynurenine following maternal inflammation may be a key factor in changing the balance of KP metabolites in fetal brain during neuronal development and represents a therapeutic target for inflammation-induced ASD-like phenotypes.
Keywords: Maternal immune activation, chronic inflammation, interleukin-17A, ASD-like phenotypes, kynurenine
Introduction
Viral infection in women during pregnancy is associated with an increased prevalence of neurodevelopmental disorders such as autism spectrum disorder (ASD) and psychiatric disorders such as schizophrenia (SCZ) in offspring.1,2 In the widely used maternal immune activation (MIA) rodent model, offspring from pregnant mice infected with influenza virus or injected intraperitoneally (i.p.) with lipopolysaccharide (LPS) or synthetic double strand RNA [polyinosinic-polycytidylic acid; poly(I:C)], a viral infection mimetic that strongly induces inflammation, exhibit behavioral abnormalities similar to those observed in ASD and/or SCZ.3,4 Administration of poly(I:C) results in a prominent increase in several serum cytokines, such as tumor necrosis factor-α (TNF-α), interferon-β (IFN-β), interleukin-6 (IL-6), IL-1β, and IL-17A. Recent evidence suggests that IL-17A is a possible mediator underpinning MIA-induced ASD pathogenesis.5 -10 MIA-induced abnormalities associated with ASD were reported to be completely rescued in offspring of poly(I:C)-injected mothers pretreated with IL-17A blocking antibody. Additionally, direct injection of recombinant IL-17A into the fetal brain resulted in similar abnormalities in cortical development and ASD-like behavioral phenotypes. 5 However, the ability of IL-17A to pass through the placental barrier and act directly on fetal brain cells remains controversial.
The kynurenine pathway (KP) is the major route of the essential amino acid L-tryptophan (TRP) catabolism in mammalian cells. The first-rate limiting enzyme of the KP is indoleamine-2,3-dioxygenase1 (IDO1), which has been widely studied due to its important role in mediating immunotolerance, especially materno-embryonic-tolerance. 11 IDO1 is activated by several pro-inflammatory cytokines, such as IL-6, TNF-α, and IFNs. Activation of the KP following neuroinflammation can generate various endogenous neuroactive KP metabolites. The pivotal metabolite of the pathway, L-kynurenine (KYN), is the precursor of several neuroactive metabolites. Previous studies have shown that kynurenic acid (KYNA), a KP metabolite, might be involved in various psychiatric disorders, such as SCZ and depression. In SCZ, elevated levels of KYNA are observed in postmortem brains and cerebrospinal fluid,12,13 and are most likely associated with the cognitive deficits observed in SCZ. Experimentally, increased levels of KYNA in the rat embryonic brain are associated with cognitive impairments in adulthood. 14 Furthermore, we and others have demonstrated that endogenous KYN, KYNA, and anthranilic acid (AA) levels are markedly increased in mice with deletion of kynurenine 3-monooxygenase (KMO), which is the second rate-limiting enzyme in KP and catalyzes KYN into 3-heydroxykynurenine (3-HK). These studies demonstrated that the offspring of KMO knockout dams exhibit both ASD-like and depression-like behaviors.15,16 Goeden et al 17 reported that acute increases in KYN levels in maternal blood rapidly increase KP metabolite levels in the fetal brain, indicating the capability of KYN to cross the placenta and increase the levels of neuroactive compounds in the fetal brain. Although KP metabolites are neuroactive and possibly affect embryonic neurodevelopment, 18 the relationship between MIA and changes in KP metabolites during pregnancy is unclear.
Given that the possible association of the KP and its neuroactive metabolites with inflammation, cognitive impairment, and fetal neuro development, we assumed that maternal inflammation (MIA) induced by IL-17A is associated with the pathology of ASD, mediated via activation of the KP. Therefore, we investigated the relationship between MIA and KP metabolites in offspring in this study. In the first set of experiments, we induced chronic MIA by continuous expression of IL-17A in C57BL/6J mice from embryonic day 12.5 (E12.5) and investigated behavioral changes related to ASD, cognitive function, and depression. Since we hypothesized that KYN was a key metabolite that crossed the placenta and affected fetal brain development, we administered high concentrations of KYN to pregnant C57BL/6J mice on E12.5 and performed the same behavioral tests in offspring in complementary experiments.
Materials and Methods
Animals
This study was carried out in C57BL/6J mice obtained from Japan SLC, Inc. (Hamamatsu, Japan). Animals were maintained on a 12:12 hours light/dark cycle (lights on at 7:00 a.m.) in a temperature- and humidity-controlled specific pathogen-free animal facility at Kansai Medical University. The mice had ad libitum access to food and water. The protocols for all animal experiments were approved by the Committee of Animal Care at Kansai Medical University and Tohoku University. All experiments were conducted in accordance with approved guidelines and regulations (approved number: #20-044 and #2019MdA-310).
Plasmid DNA
pCpG-mcs was purchased from InvivoGen (San Diego, CA, USA). pCpG-Muil17a was constructed by inserting the BglII/NheI fragment containing mouse Il17a coding region into the BglII/NheI site of pCpG-msc, as described previously. 19
Experimental design of maternal immune activation (MIA) model and L-kynurenine (KYN) administration
Mice were mated overnight, and females were checked for the vaginal plugs in the next morning, designated as embryonic day 0.5 (E0.5).
Experiment 1: MIA model
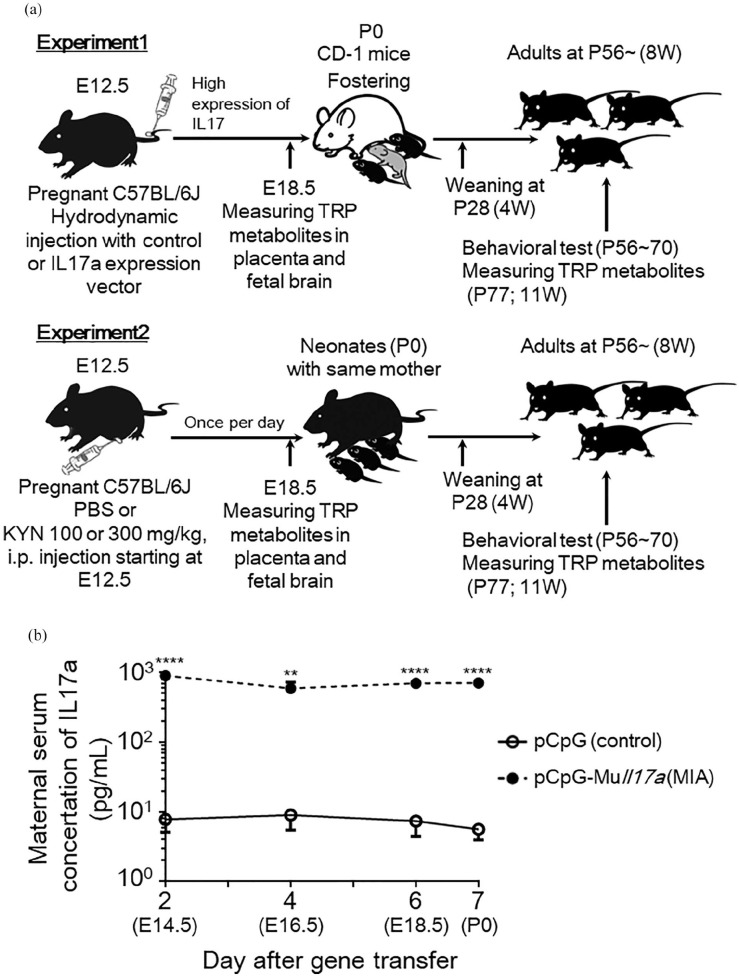
The prepared plasmid pCpG-Muil17a was dissolved in normal saline and injected into the tail veins of pregnant female mice over 5 seconds on E12.5 (MIA mice), as described previously. 19 The injection volume was approximately 9% (v/w) of body weight. The doses of pCpG-Muil17a used were 0.1 pmol/mouse. To eliminate the possibility of tissue damage and/or inflammation from the hydrodynamic injection, a control plasmid, which was an empty vector without the Il17a coding sequence (pCpG-mcs), was injected (0.1 pmol/mouse, control mice). To confirm mIl17a expression, maternal serum was collected on 2, 4, 6, and 7 days after gene transfer, and the concentration of IL-17A was measured using ELISA, according to the manufacturer’s protocol (R&D Systems, Inc., Minneapolis, MN, USA). Gestation and parturition were allowed to proceed normally. However, when maternal stress following hydrodynamic injections impacted the nursing of pups by dams, CD-1 mice were used as fostering mothers from postnatal day 0 (P0) until the pups were weaned on P28 for both control and MIA mice (Figure 1(a)).
Figure 1.
Experimental design of maternal immune activation (MIA) and kynurenine administration. (a) Experiment 1: dams were injected with pGpG-mcs (control vector) or pCpG-Muil17a (IL-17A-expressing vector) on embryonic day 12.5 (E12.5) at a dose of 0.1 pmol/mouse. CD-1 mice were used as fostering mothers on postnatal day 0 (P0) until weaning of pups on P28 for both control and MIA mice. Experiment 2: dams were injected intraperitoneally with vehicle (5%NaOH + phosphate-buffered saline; PBS) or kynurenine (KYN) at a dose of 100 or 300 mg/kg body weight/day from E12.5 until E19.0. All pups remained with the same dams until weaning on P28. For both experiments, maternal serum, placenta, fetal-pooled plasma and brain regions were acquired at E18.5 for the measurement of tryptophan (TRP) metabolites. Behavioral tests were performed in adult animals between P56 and P70. All animals were euthanized on P77 for measurement of TRP metabolites in adult brain region. (b) Serum concentrations of maternal IL-17A (N = 3-4 mice per group, 3 independent experiments) at E14.5 until birth (E19.5) in pCpG-mcs- or pCpG-Muil17a-injected mothers. Data are represented as means ± standard error of the mean (SEM). Student’s t-test, **P < .01, ****P < .0001 versus control (pCpG-mcs-injected mice).
Experiment 2: KYN administration
The mice were divided into 2 groups: KYN-treated animals were intraperitoneally administered with KYN [100 or 300 mg/kg bodyweight, dissolved in 5% NaOH and 0.2 M phosphate-buffered saline (PBS), pH 7.4] from E12.5 to E19.0 once per day (+KYN mice), whereas the control dams were injected intraperitoneally with the vehicle (5% NaOH and 0.2 M PBS) from E12.5 to E19.0 once per day (+PBS mice) (Figure 1(a)). L-kynurenine hydrate was purchased from Tokyo Chemical Industry CO., LTD (Tokyo, Japan). The dose of KYN was selected based on previous studies.17,18,20 Gestation, parturition, and weaning of pups proceeded normal. All pups remained with the same dams until weaning on P28, following which the mice were group-housed at a maximum of 5 per cage with same-sex littermates.
For both experiments, offspring were subsequently used for behavioral tests from P56 to P70. Mice were euthanized under anesthesia for collection of blood and brains, 1 week following the conclusion of all behavioral testing to minimize the influence of behavioral tests. Each measurement was obtained from an analysis of offspring from at least 3 pregnant females used for each treatment, yielding at least 7 to 10 offspring in total for analysis.
Sample collection for maternal serum, placenta, fetal plasma and brain
Mice were deeply anesthetized with 2% isoflurane (FUJIFILM Wako Pure Chemical Co., Osaka, Japan), and blood was collected from the abdominal vena cava from each dams at E18.5. Offspring were sacrificed and trunk blood was collected as pooled plasma from the same dam. At the same time, placenta was collected from each offspring. The entire brain was quickly removed, and the fetal brain was manually dissected into the pallium (cortex) and subpallium (basal ganglia) on ice-cold saline and immediately frozen because these regions have a distinct molecular patterning and strikingly different developmental potentials.
RNA extraction and reverse transcription quantitative real-time PCR (RT-qPCR)
Total RNA was extracted from the brains of E14.5 fetuses using the Invitrogen Trizol protocol (Thermo Fisher Scientific Inc, MA, USA), and reverse transcribed using PrimeScript II 1st strand cDNA synthesis kit (TaKaRa Bio Inc, Shiga, Japan) according to the manufactures’ protocols. RT-qPCR with SYBR green detection was performed using a LightCycler® Nano (Roche Diagnostics, Mannheim, Germany). Original primers for qPCR were designed using Universal ProbeLibrary Assay Design Center (Roche Diagnostics, Mannheim, Germany). The forward and reverse primer sequences used in this study are given in Table 1. The primers were assessed based on the specificity of the PCR product and efficiency. Specificity was verified by single peak in melting curve analysis. The qPCR assay for unknown samples was performed simultaneously with positive control samples in the same run. The cycling parameters for all genes were as followings: initial denaturation at 95°C for 10 minutes, then 45 cycles at 95°C for 10 seconds, 60°C for 10 seconds, and 72°C for 10 seconds, sequentially. All transcripts in each known sample were measured in duplicates. Expression values were normalized to that of 18S rRNA and reported in units of 2-ΔCq, where ΔCq is the difference of Cq values between the target and 18S rRNA transcripts in the same sample.
Table 1.
Sequence of primers and product size.
| Real-time PCR | Accession no. | Forward primer (5′-3′) | Reverse primer (5′-3′) | Amplicon (bp) |
|---|---|---|---|---|
| IL-17A receptor subunit | ||||
| mIl-17ra | NM008359.2 | TGTGTTGCATGTTGAGTGGA | GGACGGACAGCTCTGCAC | 74 |
| mIl-17rc | NM178942.1 | TGAAGTCCGGGACAGCAT | CACCATCTGTAGACACATTGAGC | 65 |
| Reference gene | ||||
| 18S rRNA | NM003278.3 | GCCGCTAGAGGTGAAATTCTT | CGTCTTCGAACCTCCGACT | 106 |
Behavioral tests
All behavioral tests were performed between 9:00 a.m. and 6:00 p.m. Male mouse offspring at ages of P56-P70 were used. The open-field test (OFT), Y-maze, novel object recognition test (NORT), 3-chamber social approach test, 5-trial social recognition memory test, and forced swim test (FST) were performed sequentially, as described previously. 21 To reduce the influence of prior tests, the sequence of behavioral tests was ordered from a low to high degree of stress. Behavioral tests were performed in an experimental room which was sound-attenuated and air-regulated, and mice were habituated in this room for 1 hour prior.
Open-field test (OFT)
The OFT was performed according to the method outlined in previous studies, with minor modifications.16,21 To measure locomotor activity and anxiety in a novel environment, each mouse was placed in a transparent acrylic cage with a gray frosted Plexiglas floor (40 cm × 40 cm × 30 cm). The locomotion behaviors and time spent in each area were measured every 1 minute for 10 minutes using the Any-maze video-tracking system (Stoelting Co., Wood Dale, IL). A light (200 lux) was positioned 100 cm above the center of the floor. The arena was cleaned between testing session with 70% ethanol. To assess the process of habituation to the novelty of the arena, mice were exposed to the apparatus for 10 minutes per day over 2 consecutive days. Habituation behavior was evaluated as the percentage of total distance traveled on the second day to that traveled on the first day. Although habituation in the OFT upon repeated exposures depends on the inbred mouse strain, inbred C57BL/6J male mice normally show habituation and decreased activity levels. 22
Y-maze test
Spontaneous alternation behavior of mice in a Y-maze, an index of short-term memory, was assessed according to the methods outlined in previous reports.16,21 The Y-maze apparatus consisted of gray frosted Plexiglas, with each arm measuring 40 cm × 10 cm × 12 cm (L × W × H), tapering to 3-cm wide at the bottom. The arms converged to a triangular center, 4 cm per side. Each mouse was placed at the end of 1 arm and allowed to move freely throughout the maze during an 8-minutes session. The series of arm entries was observed visually and recorded. Spontaneous alternation behavior was defined as the consecutive entry into all 3 arms (ie, arm A, arm B, and arm C) in triplet sets (ie, ABC, ACB, BAC, BCA, CAB, and CBA). Alternation behavior was calculated as the ratio of actual alternations to possible alternations (defined as the total number of arm entries—2) ×100, and was presented as a percentage, as described previously. 21
Novel-object recognition test (NORT)
The NORT was performed in accordance with the method outlined in previous reports.21,23 The test procedure consisted of 3 sessions: habituation, training, and retention. The habituation session consisted of a 10-minutes exploration time in an acrylic cage with a gray frosted Plexiglas floor (30 cm × 30 cm × 35 cm) without any objects for 2 days. During the training session, 2 objects were placed in a back corner of the box. The objects included a wooden square pyramid, a golf ball, and a cylindrical metal dry cell, which were different in shape, color, and material, but were of similar size. Each mouse was individually placed midway toward the front of the box, and the total time spent exploring the object was recorded for 10 minutes. A mouse was considered to be exploring the object when their heads was facing the object or when they were touching or sniffing the object. During the retention session, the mouse was placed back into the same cage 24 hours after the training session, but one familiar object used during training was replaced with a novel object. The mouse was then allowed to explore freely for 5 minutes, and the time spent exploring each object was recorded. Throughout the experiments, the objects were used in a counterbalanced manner in terms of their physical complexity and emotional neutrality. The discrimination index, calculated as the ratio of the time spent exploring the novel object (retention session) over the total time spent exploring both objects, was used to measure cognitive function.
Three-chamber social approach test
The three-chamber social approach test is known as Crawley’s sociability and preference for social novelty protocol and has been successfully employed to study social affiliation and memory. The test was performed according to the methods outlined in previous studies with minor modifications.5,24 Experimental mice were habituated in a three-chamber arena for 10 minutes/day for 2 days before testing. Age- and size-matched C57BL/6J male target subjects (“stranger1” and “stranger2”) were habituated by placing them inside wire cages for 30 minutes before the test. The social test apparatus consisted of an acrylic cage (57 cm × 45 cm × 30 cm) with partitions dividing the cage into 3 chambers. The wire cages used to contain the stranger male mice were cylindrical with 11-cm high, 10.5-cm bottom diameter, and 1-cm bars spaced (Galaxy Cup, Spectrum Diversified Designs). For the sociability test, the test animal was introduced to the middle and left chamber to habituate for 10 minutes. Following this period, the middle chamber doors were opened, and the test mouse was allowed to freely explore all 3 chambers for additional 10 minutes. The test mouse was then returned to the middle chamber; subsequently, an unfamiliar mouse (stranger 1) was introduced into the wire cage in one of the side-chambers, while the other wire cage was empty (E) on the other side-chamber. The doors were then opened, and the test animal was allowed to freely explore all 3 chambers over a 10-minutes session. Following this session (session 1), the test mouse was returned to the middle chamber. A novel stranger mouse (stranger 2) was put in the previously empty wire cage; the test mouse was allowed to explore for a 10-minutes session again (session 2). The time spent by the mouse (nose-point) in close proximity (~2 cm) to the wire cages was measured as interaction time (ie, sniffing time) to evaluate the approach behavior. Each session was recorded, and the object exploration time was analyzed using the Any-maze tracking system. Arenas and contents were thoroughly cleaned with 70% ethanol between sessions. Multiple social targets from different home cages were used for testing to prevent potential odorant confounds from target home cages.
Five-trial social recognition memory test
This test assesses cognition and sociability, namely the ability to recognize novel versus familiar animals. The test was performed according to the methods outlined in previous reports.21,25 Experiment mice were habituated in the test arena for 10 minutes/day for 2 days before testing. Age- and size-matched C57BL/6J male stimulus mice were used and habituated to a cylinder holder made of transparent acryl (30-cm high, 10.5 cm-diameter with 16 holes of 0.5-cm diameter) for 30 minutes before testing. In this test, the same stimulus animal was used for both acquisition and recognition phases (intruder 1). Over the course of multiple exposures, experiment mice became habituated to intruders and no longer regarded as interesting as they did for completely novel intruders. During testing, each experiment mouse was given four 5-minutes exposures, with 15 to 20 minutes interval period, in a transparent acrylic box (60 cm × 60 cm × 30 cm). In the fifth trial, the experiment mice encountered an entirely novel intruder (intruder 2). All test trials were recorded with Any-maze tracking system, and total investigating time was subsequently analyzed. The time spent by each mouse (nose-point) in close proximity (~2 cm) to the cylinder holder was measured as interaction time (ie, sniffing time, approach) to evaluate the approach behavior.
Forced swim test (FST)
The FST is a standardized test of depressive-like behaviors, whereby depression is inferred from increased durations of immobility. This test was conducted as described previously,16,19 with minor modifications. Mice were individually placed in a transparent glass cylinder (20-cm high, 8-cm diameter) that contained water at 22 ± 1°C to a depth of 13.5 cm and were forced to swim. All mice were subjected to 15-minutes training under same conditions 24 hours before testing. Each test was video-recorded and the duration of immobility was analyzed using TopScan behavior analysis system (CleverSys, Inc., VA, USA) during the last 5 minutes of the 6-minutes test.
Measurements of tryptophan and its metabolites
Serum was diluted (4:1, v/v) in 10% perchloric acid (PCA) for measurements of KP metabolites. After thorough mixing, the precipitated proteins were removed by centrifugation (7000 g × 10 minutes, 4°C), as described previously. 26 Brain tissues were weighed and homogenized (1:3, w/v) in 10% PCA. After homogenization, the precipitated proteins were removed by centrifugation (17,000 g × 30 minutes, 4°C), and 40 µL of the supernatant was subjected to high-performance liquid chromatography (HPLC) analysis for measurements of KP metabolites. TRP metabolites were measured as reported previously and according to the manufacturer’s protocol, with slight modifications. 26 TRP, KYN, KYNA, AA, and 3-HAA were gradient-eluted from a reverse-phase chromatography column (CAPCELL PAK ADME S3, 100 mm [L] × 2.1 mm [internal diameter; ID], 3-µm particle size, Osaka Soda Co., LTD, Osaka, Japan) with a mobile phase containing 10 mM ammonium formate buffer (pH 4.0), and 0% to 25% (v/v) gradient of acetonitrile at a flow rate of 0.2 mL/minutes. TRP and KYN were detected using an ultraviolet and visible spectrophotometric detector (UV detector, SPD-20A, Shimadzu Co., Kyoto, Japan; UV wavelengths for TRP and KYN were 280 and 254 nm, respectively). KYNA, AA, and 3-HAA were detected using a fluorescence spectrometric detector (RF-10Axs, Shimadzu Co., Kyoto, Japan) under the following conditions: for KYNA, the excitation (Ex) wavelength was 334 nm, and the emission (Em) wavelength was 380 nm; for AA and 3-HAA, the Ex wavelength was 320 nm, and the Em wavelength was 420 nm. 3-HK was measured using HPLC with an electrochemical detector (Eicom ECD-300; oxidation potential: +0.55 V) and a chromatographic column (EICOMPAK SC-50DS, 150 mm [L] × 3.0 mm [ID], 3-µm particle size, Eicom Co., Kyoto, Japan) with a mobile phase of 0.59% (v/v) phosphoric acid, 0.27 mM EDTA, 8.9 mM sodium heptane sulfonic acid, 0.9% (v/v) trimethylamine, and 1.5% (v/v) acetonitrile at a flow rate of 0.5 mL/minutes.
Statistical analysis
All results are expressed as means ± standard error of mean (SEM) for each group. Student’s t-test was used to compare 2 sets of data. Intergroup comparisons were made using 1-way ANOVA, followed by the Tukey’s multiple comparison test. For comparisons of more than 3 groups, two-way ANOVA was used, followed by the Bonferroni’s multiple comparison test. GraphPad Prism version 8.4.3 (GraphPad Software, San Diego, USA) was used for statistical analysis and generation of graphs. A P-value less than .05 was considered statically significant.
Results
Maternal IL-17A promoted behavioral abnormalities in offspring
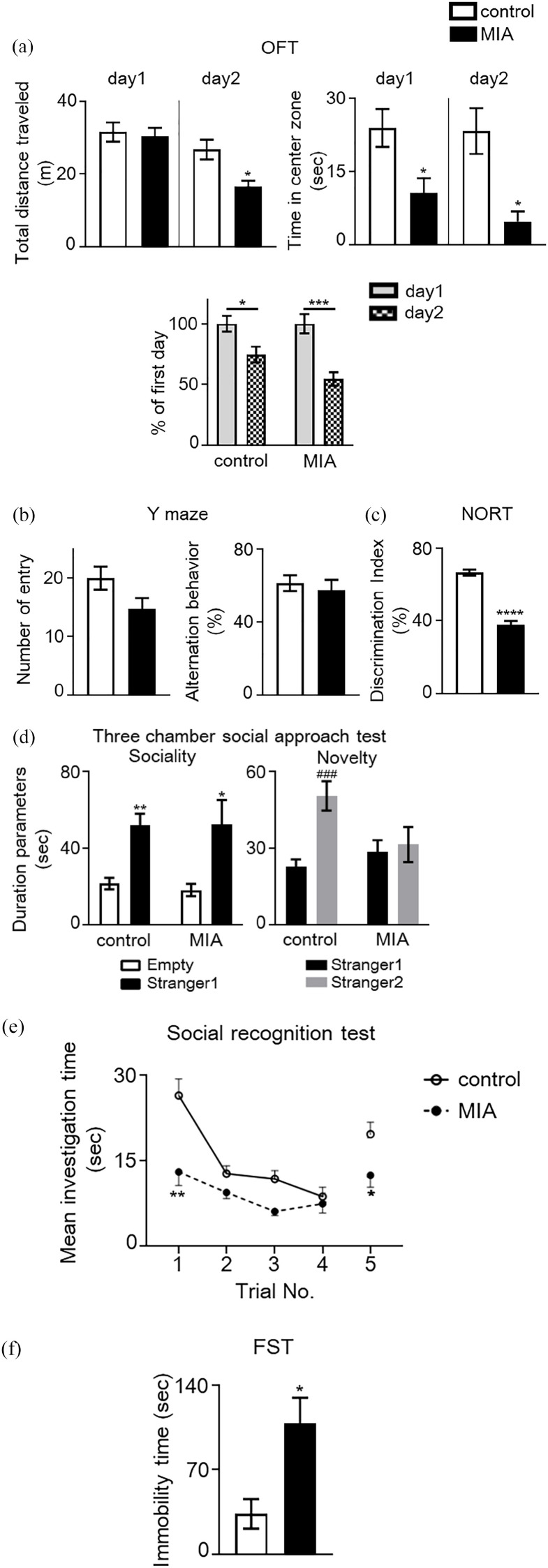
ASD-relevant behavioral abnormalities have been reported in adult poly(I:C)-induced MIA offspring.5,8 To confirm that the MIA mice used in this study exhibited relevant behavioral abnormalities, we conducted several behavioral tests, including the novel OFT, Y-maze, NORT, three-chamber social approach, five-trial social recognition memory tests, and FST. In the OFT, no significant difference was observed in total distance traveled by MIA mice in a novel environment on day 1, and habituation was unchanged on day 2, compared to control mice (Figure 2(a)). A significant decrease was observed in total distance traveled by MIA mice in the habituated environment on day 2, compared to control mice. A significant decrease in time spent in the center of the open field was observed as well, which is an indicator of increased anxiety-like behavior, as observed in MIA mice across both days (Figure 2(a): Student’s t-test, day 1, t = 2.621, P < .05 and day 2, t = 2.800, P < .05, vs control mice). In the Y-maze test, MIA mice demonstrated a tendency to engage in reduced spontaneous activity and unaltered working memory when compared to control mice, as indicated by the percentage of spontaneous alternation behaviors (Figure 2(b)). In the NORT, performance was significantly lower in MIA mice than in control mice (Student’s t-test: t = 10.46, P < .0001; Figure 2(c)). In the social approach test which used a three-chamber box, both MIA and control mice spent more time in the chamber containing a stranger mouse than in the empty chamber [2-way ANOVA: Fstranger1 (1, 30) = 24.79, P < .0001; FMIA (1, 30) = 0.05, P = .8172; Fstranger 1 × MIA (1, 30) = 0.09, P = .7676; Figure 2(d)]. No significant difference was observed between MIA and control mice in the time spent in the chamber containing the stranger mouse. However, when both stranger and familiar mice were placed in different chambers, control mice spent a significantly longer time in the chamber containing the stranger mouse than in that containing the familiar mouse, but the durations were not significantly different to those of MIA mice [2-way ANOVA: Fstranger2 (1, 30) = 8.38, P < .001; FMIA (1, 30) = 1.53, P = .226; Fstranger 2 × MIA (1, 30) = 5.63, P = .0243; Figure 2(d)]. In the five-trial social recognition memory test, MIA mice exhibited significant differences in mean investigation time in the first trial with intruder 1 and in the fifth trial with a novel intruder [2-way repeated measure ANOVA: FMIA (1, 16) = 11.75, P < .01; Ftrials (2.09, 31.35) = 17.19, P < .0001; FMIA × trials (4, 60) = 3.529, P = .0119; Figure 2(e)]. These results indicated that MIA altered social behavior and social recognition ability. In addition, the MIA mice exhibited significantly increased immobility time in the FST, compared to control mice (Student’s t-test, t = 3.248, P < .05, Figure 2(f)).
Figure 2.
Behavioral abnormalities in maternal immune activation (MIA) mice. (a) Locomotor activity and anxiety-like behavior in novel and habituated environments. Total distance traveled for 10 minutes was measured as an index of locomotor activity on 2 consecutive days. Time spent in each area was measured. A significant decrease in time spent in the center of the open field, which is an indicator of increased anxiety-like behavior, was observed in MIA mice, but not in control mice (Student’s t-test, *P < .05 vs control mice). Additionally, habituation to a novel environment was evaluated. The ratio (percentage) of total distance traveled on the second day to that traveled on the first day was significantly decreased in both groups during repeated trials, suggesting no changes in habituation of MIA mice to the novel environment [two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test: *P < .05, ***P < .001 vs percentage of the first day]. (b) Spontaneous activity and working memory in the Y-maze test. Total arm entries and alternation behavior were measured during an 8-minutes session. (c) Object recognition memory was measured in a novel object-based recognition test (NORT). A memory retention session was performed 24 hours after the training session. The discrimination index was calculated as described in the Methods (Student’s t-test: ***P < .001 vs control mice). (d) Three-chamber social approach test. Duration parameters are presented as investigation times (two-way ANOVA with Bonferroni’s multiple comparison test: *P < .05, **P < .01 vs Empty; ###P < .001 vs stranger1). No significant difference was observed between groups. Empty, empty cage. (e) Five-trial social recognition memory test. The investigation time in repeated trials was significantly shorter in MIA mice than in control mice (Trials 1 and 5; two-way repeated measures ANOVA: *P < .05, **P < .01 vs control mice). (f) Immobility of MIA and control mice in a forced swimming test (FST) (Student’s t-test: *P < .05 vs control mice). Data are presented as means ± standard error of the mean (SEM; n = 7-11).
Maternal IL-17A activated the KP and increased KYN levels in maternal serum, placenta and fetus
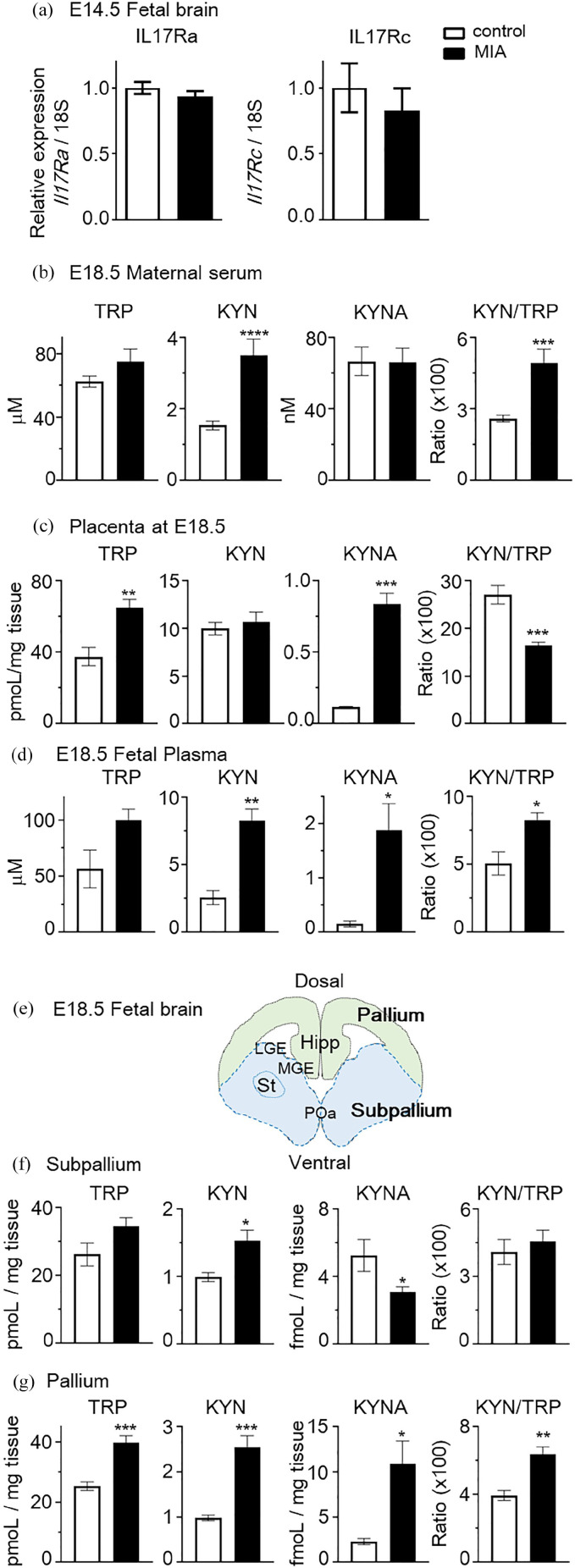
Administration of poly(I:C) to dams increases the serum levels of IL-17A in dams and IL-17A receptor subunit A (IL-17Ra) expression in the cortex of the fetal brain. These results suggest that maternal IL-17A-dependent pathway mediates abnormal phenotypes in offspring following in utero MIA due to exposure of the fetal brain to increased levels of IL-17A. 5 To confirm whether MIA mice used in this study exhibited increased levels of IL-17Ra in the fetal cortex, we examined IL-17Ra and IL17-Rc (receptor subunit for IL-17A) mRNA expression using qPCR. No significant changes were noted in the expression of IL-17Ra and IL-17Rc mRNA in fetal brain on E14.5 following induction of MIA (Student’s t-test; mIl17ra, t = 1.070, P > .05; mIl17rc, t = 0.6846, P > .05; Figure 3(a)). Several pro-inflammatory cytokines, such as IL-6 (upstream of IL-17A), TNF-α and IFNs, are able to induce the KP. We therefore hypothesized that IL-17A may induce the KP. KYN levels in maternal serum were significantly increased in MIA mice relative to those in control pregnant mice (control vs MIA, 1.54 ± 0.12 vs 3.51 ± 0.46 µM; t = 5.28, P < .0001, Student’s t-test; Figure 3(b)). AA levels were significantly decreased in MIA mice compared to those in control mice (t = 2.31, P < .05, Student’s t-test; Table 2). No significant differences were observed in the levels of KYNA (Figure 3(b)), 3-HK and 3-HAA (Table 2) in maternal serum. The KYN/TRP ratio (reflecting IDO1 and TDO activity) exhibited higher values in MIA mice than in control mice (control vs MIA, 2.61 ± 0.14 vs 4.92 ± 0.60; t = 4.85, P < .0001, Student’s t-test; Figure 3(b)).
Figure 3.
Increased IL-17A levels in mothers transfected with pCpG-MuIl17a resulted in elevated levels of kynurenine (KYN) in the dams and fetus. (a) Relative IL-17Ra and IL-17Rc mRNA levels in embryonic day 14.5 (E14.5) fetal brains derived from pCpG-mcs- or pCpG-MuIl17a gene-transfected mothers at E12.5 (N = 4-5 mice per group, 2 independent experiments). Serum (b) and placenta (c) concentrations of tryptophan (TRP) metabolites in E18.5 dams (N = 7-11 mice per group, 3 independent experiments) after pCpG-mcs or pCpG-MuIl17a gene transfection into pregnant dams on E12.5. (d) Concentrations of TRP metabolites in fetal plasma at E18.5 (N = 3-5 pooled plasma per group). (e) Schematic presentation of E18.5 fetal brain structure. Hipp, hippocampus; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; POa, preoptic area; St, striatum. (f and g) Concentrations of TRP metabolites were measured in fetal brain regions of subpallium (f) and pallium (g) (N = 6-9 fetus per group, 2 independent experiments). Data are presented as means ± standard error of the mean (SEM). Student’s t-test, *P < .05, **P < .01, ***P < .001, ****P < .0001, vs control mice.
Table 2.
Tryptophan metabolites in maternal serum, placenta, fetal plasma and brain following pCpG-MuIl17a transfer at E18.5.
| 3-HK | 3-HAA | AA | |
|---|---|---|---|
| Maternal serum (nM) | |||
| Control | 56.7 ± 9.14 | 70.7 ± 20.2 | 251.7 ± 24.7 |
| MIA | 72.1 ± 7.42 | 135.4 ± 23.9 | 165.0 ± 21.4* |
| Placenta (fmoL/mg tissue) | |||
| Control | 47.1 ± 11.0 | 19.2 ± 4.14 | 257.6 ± 29.1 |
| MIA | 36.5 ± 2.57 | 17.0 ± 0.59 | 103.9 ± 10.4*** |
| Fetal plasma (nM) | |||
| Control | 39.1 ± 3.81 | 50.8 ± 7.86 | 309.2 ± 108.5 |
| MIA | 74.2 ± 8.81* | 453.3 ± 32.3**** | 245.5 ± 37.0 |
| Fetal brain (fmoL/mg tissue) | |||
| Subpallium | |||
| Control | 117.5 ± 17.8 | 7.8 ± 0.66 | 24.7 ± 3.87 |
| MIA | 108.4 ± 7.68 | 9.4 ± 1.18 | 29.8 ± 3.21 |
| Pallium | |||
| Control | 81.3 ± 10.9 | 10.3 ± 1.26 | 19.6 ± 1.99 |
| MIA | 80.0 ± 9.51 | 10.1 ± 0.93 | 33.5 ± 5.87 |
Data are presented as means ± standard error of the mean (SEM).
P < .05, ***P < .001, ****P < .0001 vs control.
In the placenta, TRP and KYNA levels were significantly increased in MIA mice relative to those in control pregnant mice (control vs MIA, TRP; 37.6 ± 5.10 vs 65.0 ± 4.71 pmoL/mg tissue; t = 3.58, P < .01, KYNA; 118.4 ± 1.93 vs 836.7 ± 75.9 fmoL/mg tissue; t = 6.46, P < .001, Student’s t-test; Figure 3(c)). 3-HK levels trended to be higher in MIA mice, though without reaching reach statistical significance. In contrast, AA levels were significantly decreased in MIA mice (257.6 ± 29.1 vs 103.9 ± 10.4 fmoL/mg tissue; t = 6.31, P < .001, Student’s t-test; Table 2). No significant changes were observed in KYN and 3-HAA levels (Figure 3(c)) and Table 2). The KYN/TRP ratio was significantly decreased in MIA mice (control vs MIA, 27.1 ± 1.94 vs 16.4 ± 0.78; t = 6.27, P < .001, Student’s t-test; Figure 3(c)).
The levels of KYN in maternal serum were significantly increased in MIA-dams; therefore, we assessed whether maternal IL-17A affected KP metabolites in fetal plasma and brain. KYN levels were significantly increased in MIA fetal plasma compared to control mice (control vs MIA, KYN; 2.59 ± 0.51 vs 8.26 ± 0.89 µM; t = 4.55, P < .01, Student’s t-test; Figure 3(d)). It was surprising that the KYNA levels in MIA offspring were greater than 10-fold higher than those of control fetus animals (156.1 ± 54.4 vs 1886 ± 489.1 nM; t = 2.65, P < .05, Student’s t-test). TRP levels trended to be higher in MIA fetal plasma than in control fetus, though without reaching statistical significance (Student’s t-test: t = 2.45, P = .050). 3-HK and 3-HAA levels were also significantly increased in MIA fetal plasma compared to control mice (Table 2). AA levels varied in control fetus and did not reach statistical significance. The KYN/TRP ratio was significantly higher in MIA fetus than in control fetus (control vs MIA, 5.09 ± 0.86 vs 8.30 ± 0.52; t = 3.43, P < .05, Student’s t-test; Figure 3(d)). The subpallium is the source of various GABAergic interneuron, including striatum, and the pallium consists of the cerebral cortex and hippocampus (Figure 3(e)). KYN levels were significantly increased in both brain regions in MIA mice (Figure 3(f) and (g)). KYNA levels were significantly increased only in the pallium, and significantly deceased in the subpallium of MIA mice, as compared to control mice. AA levels trended to be higher in both regions of MIA mice than in control mice, though without reaching statistical significance (Table 2). No significant differences were observed in the levels of 3-HK and 3-HAA in both regions of fetal brain (Table 2). The KYN/TRP ratio was significantly higher in the pallium of MIA mice compared to control mice, but no changes were observed in the subpallium (Figure 3(f) and (g)). We also investigated whether KYN promotes the production of IL-17A in dams following KYN administration after 90 minutes in E18.5 mice. The concentration of IL-17A was under 10 pg/mL and almost undetectable (supplemental Figure 1). These results suggested that MIA induced by high maternal concentrations of IL-17A promoted KP, and KYN was a potential key metabolite that crossed the placenta and affected the fetal brain development.
Low or high dose of KYN injection significantly increased KP metabolite levels in maternal serum, placenta and fetus
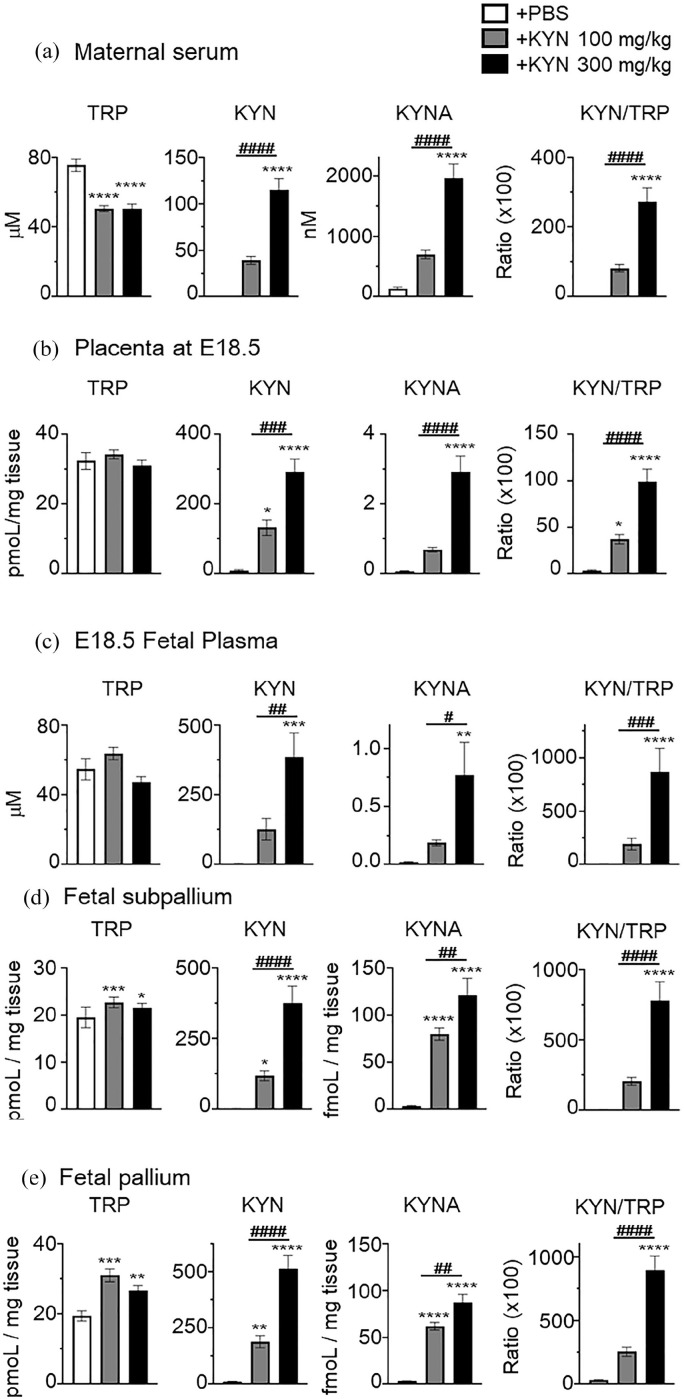
We measured KP metabolites in maternal serum 90 minutes after final administration of KYN at E18.5. As expected, KYN was significantly increased compared to mice treated with vehicle (PBS), as previously reported (Figure 4(a)) 17 (+PBS vs 100 mg/kg vs 300 mg/kg, 0.62 ± 0.09 vs 39.2 ± 4.33 vs 115.2 ± 12.2 µM; ****P < .0001 vs +PBS, ####P < .0001 vs +KYN 100 mg/kg, one-way ANOVA followed by Tukey’s multiple comparisons test; Figure 4(a)). KYNA levels were also increased after KYN administration (+PBS vs 100 mg/kg vs 300 mg/kg, 134.2 ± 23.6 vs 701.4 ± 70.3 vs 1957 ± 237.8 nM; ****P < .0001 vs +PBS, ####P < .0001 vs +KYN 100 mg/kg, one-way ANOVA followed by Tukey’s multiple comparisons test; Figure 4(a)). Both doses of KYN administered increased KYN and KYNA levels in maternal serum, but only a 300 mg/kg KYN administration reached statistical significance as compared to +PBS mice. TRP levels were significantly decreased following injection with both doses of KYN (+PBS vs +KYN 100 mg/kg vs +KYN 300 mg/kg, 75.6 ± 3.57 vs 50.9 ± 1.71 vs 50.7 ± 2.63 µM; ****P < .0001 vs +PBS, one-way ANOVA followed by Tukey’s multiple comparisons test; Figure 4(a)). The KYN/TRP ratio was significantly higher following KYN administration as compared to that in +PBS mice (+PBS vs +KYN 100 mg/kg vs +KYN 300 mg/kg, 1.28 ± 0.22 vs 81.9 ± 10.4 vs 273.7 ± 38.7; ****P < .0001 vs +PBS, ####P < .0001 vs +KYN 100 mg/kg, 1-way ANOVA followed by Tukey’s multiple comparisons test; Figure 4(a)). 3-HK and 3-HAA also increased after KYN administration, but only 3-HK reached statistical significance as compared to that in +PBS mice (Table 3). On the other hand, the AA levels were significantly decreased (Table 3). These levels returned to control levels within 12 hours of the administration (data not shown), suggesting that the KYN effect is very rapid in KP metabolites in dams.
Figure 4.
Low or high dose of kynurenine (KYN) administration significantly increased the levels of tryptophan (TRP) metabolites in maternal serum, placenta, fetal plasma and brain. We measured TRP metabolites 90 minutes after final intraperitoneal injection in pregnant mice on embryonic day 18.5 (E18.5) with either PBS (vehicle), low (100 mg/kg), or high (300 mg/kg) dose of kynurenine (KYN). The concentrations of TRP, KYN, kynurenic acid (KYNA) and the ratio of KYN/TRP in maternal serum (a) (N = 6-8), placenta (b) (N = 11-16, 3 independent experiments), fetal plasma (c) (N = 4-5 pooled plasma per group), and fetal brain region of subpallium (d) and pallium (e) (N = 22-30, 3 independent experiments). Data are presented as means ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, *P < .05, **P < .01, ***P < .001, ****P < .0001, versus + PBS mice, #P < .05, ##P < .01, ###P < .001, ####P < .0001, versus + KYN 100 mg/kg mice.
Table 3.
Tryptophan metabolites in maternal serum, placenta, fetal plasma and brain following low and high dose of kynurenine (KYN) injection at E18.5.
| 3-HK | 3-HAA | AA | |
|---|---|---|---|
| Maternal serum (nM) | |||
| +PBS | 42.6 ± 5.00 | 109.6 ± 28.7 | 210.5 ± 37.5 |
| +KYN 100 mg/kg | 477.3 ± 30.0**** | 243.2 ± 39.0 | 89.8 ± 12.6*** |
| +KYN 300 mg/kg | 1034 ± 99.0****, #### | 393.8 ± 81.3 | 93.3 ± 11.7*** |
| Placenta (fmoL/mg tissue) | |||
| +PBS | 45.3 ± 7.06 | 24.7 ± 5.61 | 167.3 ± 11.5 |
| +KYN 100 mg/kg | 771.4 ± 52.4**** | 367.5 ± 58.9*** | 109.1 ± 6.20**** |
| +KYN 300 mg/kg | 860.3 ± 162.7**** | 363.8 ± 45.5*** | 90.6 ± 5.67**** |
| Fetal plasma (nM) | |||
| +PBS | 40.6 ± 3.97 | 31.6 ± 13.7 | 239.3 ± 49.8 |
| +KYN 100 mg/kg | 545.3 ± 35.9 | 405.8 ± 58.0**** | 313.6 ± 35.1 |
| +KYN 300 mg/kg | 1738 ± 420.3****, ### | 568.0 ± 135.1*** | 1038 ± 229.9***, ### |
| Fetal brain (fmoL/mg tissue) | |||
| Subpallium | |||
| +PBS | 79.0 ± 9.77 | 7.13 ± 0.83 | 21.3 ± 1.72 |
| +KYN 100 mg/kg | 285.4 ± 42.4** | 24.5 ± 2.71 | 24.6 ± 1.47 |
| +KYN 300 mg/kg | 606.8 ± 57.4****, #### | 85.4 ± 9.18****, #### | 36.3 ± 3.61***, ## |
| Pallium | |||
| +PBS | 115.3 ± 26.8 | 10.1 ± 1.13 | 15.5 ± 1.40 |
| +KYN 100 mg/kg | 363.2 ± 35.7** | 28.5 ± 6.33 | 17.5 ± 0.98 |
| +KYN 300 mg/kg | 660.6 ± 75.4****, ### | 80.2 ± 8.88****, #### | 15.5 ± 1.60 |
Data are presented as means ± standard error of the mean (SEM).
P < .001, ****P < .0001 versus + PBS. ##P < .01, ###P < .001, ####P < .0001 versus + KYN 100 mg/kg.
As in maternal serum, maternal administration of KYN also increased the levels of KYN, KYNA, 3-HK, and 3-HAA, but the AA levels were significantly decreased in the placenta (Figure 4(b) and Table 3). The levels of KYN and KYNA were significantly increased compared to mice treated with vehicle (KYN; +PBS vs +KYN 100 mg/kg vs +KYN 300 mg/kg, 9.9 ± 1.61 vs 132.4 ± 22.4 vs 293.1 ± 36.7 pmoL/mg tissue, KYNA; 0.076 ± 0.01 vs 0.69 ± 0.065 vs 2.92 ± 0.47 pmoL/mg tissue, *P < .05, ****P < .0001 vs +PBS, ###P < .001, ####P < .0001 vs +KYN 100 mg/kg, one-way ANOVA followed by Tukey’s multiple comparisons test; Figure 4(b)). The KYN/TRP ratio was significantly higher following KYN administration than that in +PBS mice (+PBS vs +KYN 100 mg/kg vs +KYN 300 mg/kg, 34.0 ± 7.31 vs 372.8 ± 51.2 vs 991.5 ± 137.9; *P < .05, ****P < .0001 vs +PBS, ####P < .0001 vs +KYN 100 mg/kg, 1-way ANOVA followed by Tukey’s multiple comparisons test; Figure 4(b)).
Next, we analyzed KP metabolites in the fetus following the final administration of KYN after 90 minutes. As in maternal serum and placenta, the maternal administration of KYN also increased the levels of all KP metabolites in the fatal plasma (Figure 4(c) and Table 3). The levels of KYN and KYNA were increased compared to those in mice treated with vehicle (KYN; +PBS vs +KYN 100 mg/kg vs +KYN 300 mg/kg, 3.39 ± 0.38 vs 126.6 ± 38.8 vs 386.1 ± 87.3 µM, KYNA; 0.22 ± 0.05 vs 1.87 ± 0.25 vs 7.73 ± 2.83 µM, **P < .01, ***P < .001, ****P < .0001 vs +PBS, #P < .05, ##P < .01, ###P < .001 vs +KYN 100 mg/kg, one-way ANOVA followed by Tukey’s multiple comparisons test; Figure 4(c)), but only 300 mg/kg KYN administration reached statistical significance as compared to that in +PBS mice.
A high dose of KYN administered to the dam significantly increased the levels of all KP metabolites in the fetal brain, regardless of the brain region (Figure 4(d) and (e), Table 3). A low dose of KYN administration also increased the levels of KYN, KYNA, and 3-HK in both regions of the fetal brain (Figure 4(d) and (e) and Table 3). AA levels were only increased by a high dose of KYN administration in the pallium, but no changes were observed in the subpallium (Table 3).
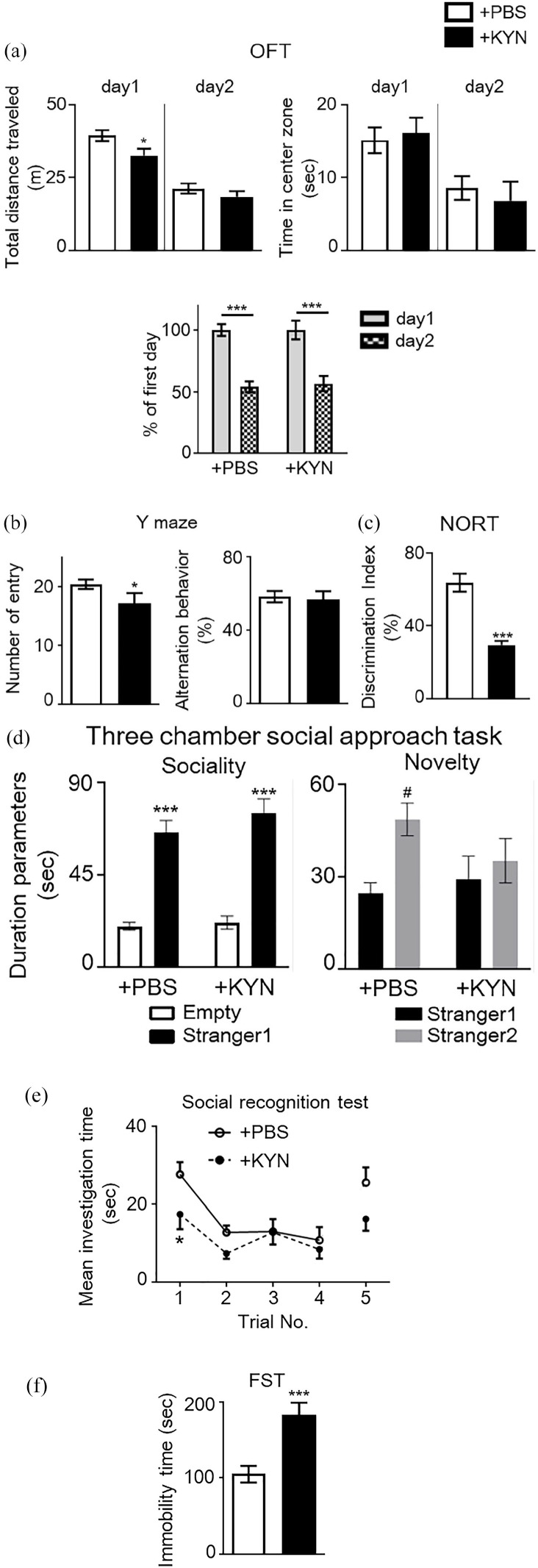
Consecutive maternal administration of high concentrations of KYN promoted behavioral abnormalities in offspring
Pocivavsek et al 18 demonstrated high embryonic KYN treatment (the amount is equivalent to approximately 300 mg/kg per day) from E15 to E22 in rat induced hippocampus-related cognitive dysfunction later in life. Therefore, we consecutively administered high concentrations of KYN to pregnant mice and conducted the same behavioral tests as those carried out in MIA mice. We subsequently investigated behavioral changes in the offspring. In the OFT, KYN-administered mice (+KYN mice) exhibited a significant decrease in total distance traveled in a novel environment on day 1 (Student’s t-test, t = 2.156, P < .05; Figure 5(a)) but not on day 2 (Student’s t-test, t = 1.002, P > .05), and habituation remained unchanged on day 2, when compared with PBS-administered mice (+PBS mice) (Figure 5(a)). No significant differences were observed in the time spent in the center area of the open field in +PBS and +KYN mice across both days (Figure 5(a)). In the Y-maze test, +KYN mice exhibited significantly lower spontaneous activity and unchanged working memory when compared with +PBS mice (Figure 5(b)). In the NORT, +KYN mice displayed significantly poorer performance when compared to +PBS mice (Student’s t-test: t = 4.67, P < .001; Figure 5(c)). We further investigated sociability and social novelty using a three-chamber box. Both +PBS and +KYN mice spent more time in the chamber containing a stranger mouse than in the empty chamber [2-way ANOVA: Fstranger1 (1, 38) = 96.25, P < .0001; FKYN administration (1, 38) = 1.23, P = .2735; Fstanger1 × KYN administration (1, 38) = 0.56, P = .4582; Figure 5(d)]. No significant difference was observed between +PBS and +KYN mice, in the chamber containing the stranger mouse. When both stranger and familiar mice were placed in different chambers, +PBS mice spent a longer time in the chamber containing the stranger mouse than in that containing the familiar mouse, but the durations were not significantly different to those for +KYN mice [2-way ANOVA; Fstranger2 (1, 37) = 6.60, P < .05; FKYN administration (1, 37) = 0.60, P = .4442; Fstanger2 × KYN administration (1, 37) = 2.34, P = .1346; Figure 5(d)]. In the five-trial social recognition memory test, +KYN mice exhibited significant differences in the mean investigation time in the first trial with intruder 1 [two-way repeated measure ANOVA: FKYN administration (1, 30) = 6.984, P < .05; Ftrials (4, 65) = 5.665, P < .001; FKYN administration × trials (4, 30) = 0.879, P = .5003; Figure 5(e)]. These results indicated that maternal administration of high-dose KYN altered social behavior and social recognition ability, similar to that observed in MIA mice. Further, +KYN mice exhibited significantly longer immobility time in the FST when compared to +PBS mice (Student’s t-test, t = 4.019, P < .001; Figure 5(f)).
Figure 5.
Behavioral abnormalities in animals receiving a high dose of kynurenine (KYN) during the prenatal period. (a) Locomotor activity and anxiety-like behavior in a novel and habituated environments. Total distance traveled for 10 minutes was measured as an index of locomotor activity on 2 consecutive days. Time spent in each area was also measured. No significant difference in time spent in the center of the open field, which is an indicator of increased anxiety-like behavior, was observed between groups (Student’s t-test, *P < .05 vs + PBS mice). Additionally, habituation to a novel environment was evaluated. The ratio (percentage) of total distance traveled on the second day to that traveled on the first day was significantly decreased in both mouse groups over repeated trials, suggesting no differences in habituation to a novel environment [two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test: ***P < .001 vs percentage of the first day]. (b) Spontaneous activity and working memory in the Y-maze test. Total arm entries and alternation behavior were measured during an 8-minutes session (Student’s t-test, *P < .05 vs + PBS mice). (c) Object recognition memory was measured in a novel object-based recognition test (NORT). Memory retention session was performed 24 hours after the training session. The discrimination index was calculated as described in the Methods (Student’s t-test: ***P < .001 vs control mice). (d) Three-chamber social approach test. Duration parameters are presented as investigation times (two-way ANOVA with Bonferroni’s multiple comparison test: ***P < .001 vs Empty; #P < .05 vs stranger1). No significant differences were observed between groups. Empty, empty cage. (e) Five-trial social recognition memory test. The investigation time in repeated trials was significantly shorter in MIA mice than in control mice (Trial 1; two-way repeated measures ANOVA: *P < .05 vs control mice). (f) Immobility of + PBS and + KYN mice in a forced swimming test (FST) (Student’s t-test: ***P < .001 vs control mice). Data are presented as means ± standard error of the mean (SEM; n = 7-11).
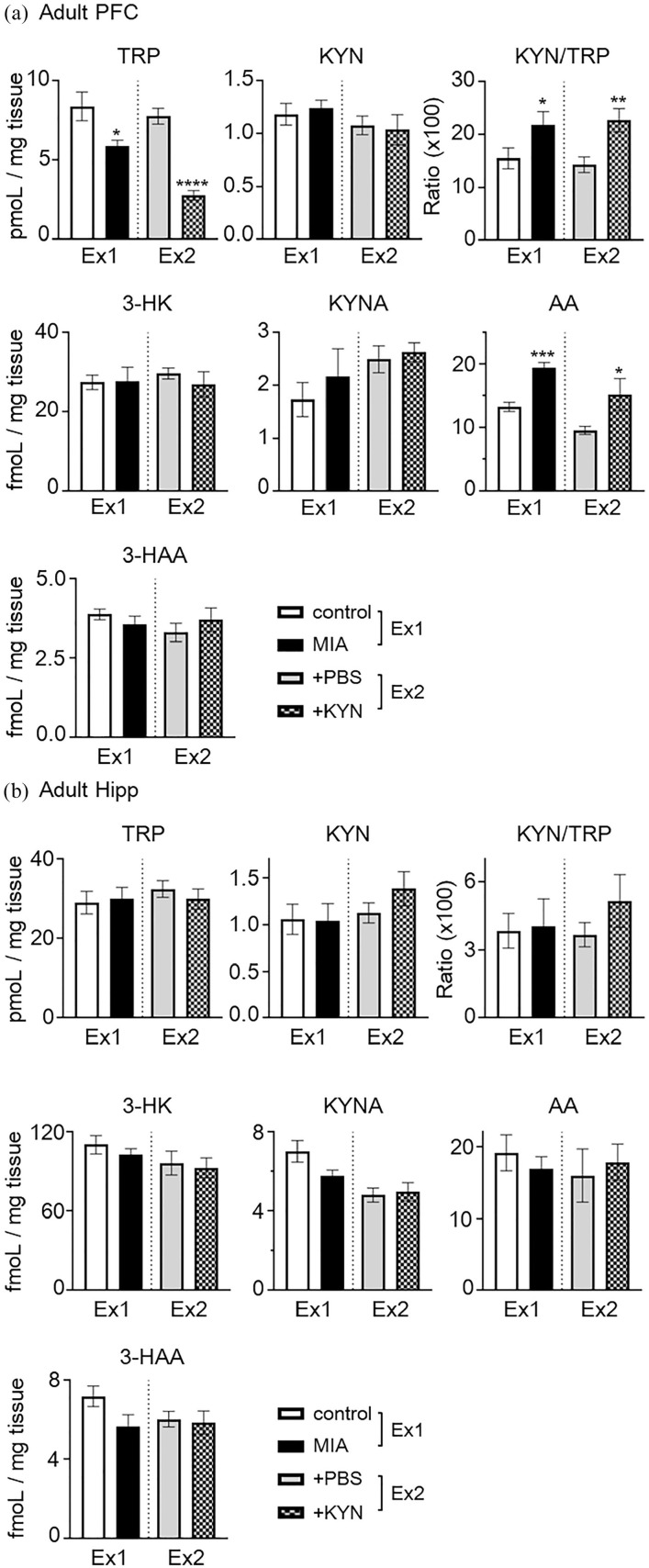
Changes in KP metabolite concentrations in the adult brain following MIA and maternal administration of high concentrations of KYN
As MIA induced by poly(I:C) influences the expression of KP enzymes in adult rats 27 and our model animals showed impaired cognitive function and depressive-like behavior, we assessed concentrations of KP metabolites in the adult prefrontal cortex (PFC) and hippocampus (Hipp) following MIA (experiment 1; Ex1) and maternal KYN administration (experiment 2; Ex2).
In PFC, AA levels were significantly increased in MIA and maternal KYN administrated mice (+KYN), as compared to those in control or +PBS mice (control vs MIA, 13.3 ± 0.74 vs 19.4 ± 0.88 fmol/mg tissue; 45.7% increase; t = 5.28, P < .001; +PBS vs +KYN, 9.6 ± 0.64 vs 15.2 ± 2.50 fmol/mg tissue; 58.3% increase; t = 2.65, P < .05 Student’s t-test; Figure 6(a)). TRP levels were significantly decreased in the MIA and +KYN mice (control vs MIA, 8.4 ± 0.90 vs 5.9 ± 0.35 pmol/mg tissue; 29.8% decrease; t = 2.57, P < .05, +PBS vs +KYN, 7.8 ± 0.50 vs 2.8 ± 0.30 pmol/mg tissue; 64.1% decrease; t = 7.40, P < .001, Student’s t-test). However, in both experiments, no significant changes in levels of KYN, 3-HK, or 3-HAA were observed in the PFC. The KYN/TRP ratio was significantly higher in the PFC of MIA and +KYN mice, as compared to control mice or +PBS mice (control vs MIA, 15.5 ± 1.95 vs 23.0 ± 2.52, t = 2.38, P < .05; +PBS vs +KYN, 14.3 ± 1.46 vs 22.7 ± 2.25, t = 3.02, P < .01, Student’s t-test). On the other hand, no TRP metabolites were significantly altered in the hippocampus of MIA nor +KYN mice, as compared to control/+PBS mice (Figure 6(b)).
Figure 6.
Changes in tryptophan (TRP) metabolite concentrations in the prefrontal cortex (PFC) and hippocampus (Hipp) of MIA (experiment 1; Ex1) or maternal KYN administration (experiment 2; Ex2). TRP metabolite concentrations were determined in the PFC (a) and Hipp (b) of mice at 11 weeks of age (postnatal day 77). Each column represents means ± standard error of the mean (SEM; n = 7-11). Data were analyzed using a Student’s t-test, *P < .05, **P < .01, ***P < .001, ****P < .0001 versus control mice or + PBS mice in each experiment.
Discussion
Herein, we show that chronic maternal IL-17A expression during the pregnancy resulted in ASD-relevant behavioral phenotypes in offspring. High levels of maternal IL-17A also resulted in cognitive dysfunction and depression-like behavior. Notably, our results suggested that MIA induced by high concentration of IL-17A promoted the KP in mother and fetus and its pivotal metabolite, KYN, may be a key molecule that crossed the placenta and affected fetal brain development, resulting in behavioral abnormalities.
Our behavioral observations following chronic IL-17A expression during gestation are largely consistent with previous work using the poly(I:C)-induced MIA model.5,8 Offspring following MIA induced by IL-17A exhibited altered sociability, increased anxiety-like and depression-like behavior, and impaired cognitive function (Figure 2). Shin et al 6 showed that poly(I:C)-induced MIA promoted abnormal cortical formation in offspring, and that cortical abnormalities were preferentially localized in the primary somatosensory cortex dysgranular zone (SIDZ), resulting in the dysregulation of neuronal activity and ASD-like behaviors. Previous studies have also demonstrated that pretreatment with IL-17A blocking antibody rescued abnormal communication, social interaction deficits, and anxiety-behaviors in adult offspring from poly(I:C)-injected mothers. 5 Taken together, these results indicate that the IL-17A in pregnant mice is critical for mediating the MIA-induced behavioral phenotype in offspring. In contrast with these studies, Reed et al 7 demonstrated that IL-17A can temporarily rescue social deficits in adult offspring from poly(I:C)-injected mothers. They also showed that the direct injection of IL-17A in the SIDZ of adult offspring exposed to MIA suppressed abnormal neuronal activity and improved sociability phenotypes. These data suggested that IL-17A exerts bifacial roles and can induce 2 opposing behavioral outcomes, depending on when its upregulation occurs (during embryonic brain development or in the adult brain).
High IL-17 concentration during gestation may play important roles at various levels, such as dysregulation of blood pressure, redox balance, and maternal factors that may independently regulate nutrient transfer, growth, and development in fetus. 28 Several studies have suggested that IL-17 crosses the placenta and reaches developing brain tissue prior to BBB formation (nearly E15.0 in mice), thereby, initiating downstream intracellular signal pathways, such as nuclear factor-kappa B (NF-κB) and extracellular signal-regulated kinase (ERK).5,9 These signaling factors may directly alter synaptic gene expression and/or broader signaling cascades. However, trans-placental passage of IL-17 has not been demonstrated directly, such as using radiotracer studies. Despite the high levels of maternal IL-17A, no significant changes in IL-17Ra mRNA expression in the cortex of the fetal brain were observed (Figure 3(a)). Further, we were unable to detect mRNA of Il-17a in the fetal brain, and protein expression of IL-17A in fetal plasma (data not shown). Therefore, we speculate that another molecule may be responsible for MIA-induced phenotypes. The KP of TRP degradation comprises several neuroactive metabolites that influence brain function in health and disease. The KP is induced by several cytokines, and changes in KP metabolism during development are involved in the pathophysiology of neurodevelopmental and psychiatric disorders, such as ASD and SCZ.15,29 In addition, acute increases in KYN in maternal blood lead rapidly to substantial elevations in KYN levels in the placenta as well as fetal plasma and brain. 17 This study indicated that KYN crossed from the mother to the placenta and reached the fetus in vivo. Therefore, we investigated whether chronic IL-17A expression during gestation influences the levels of KP metabolites in dams, placenta and offspring. First, we observed significantly higher levels of KYN and increased the ratio of KYN/TRP in MIA-maternal serum than those in control (Figure 3(b)). KYNA levels were significantly elevated in the placenta, however, KYN levels remained unchanged and the KYN/TRP ratio was significantly decreased in the MIA-placenta compared to controls (Figure 3(c)). Next, we observed significantly higher levels of several KP metabolites and an increased the ratio of KYN/TRP in the plasma of MIA-offspring than those in control (Figure 3(d)). Specifically, the KYNA levels in fetal plasma were greater than 10-fold higher than those in the control fetus. In normal pregnancy, the KYN/TRP ratio is significantly increased in maternal serum compared to non-pregnant women and there are high levels of IDO1expression and KP activity in the placenta to prevent rejection of the allogenic fetus by maternal T cells.11,30 During pregnancy, the maternal immune system also changes and maintains a certain tolerance to the fetal allograft while preserving innate and adaptive immune mechanisms to protect against microbial challenges. 31 Under physiological conditions, the IL-17A pathway down-regulated TRP catabolism and completely antagonized the induction of IDO1 by IFN-γ in neutrophils during fungal infection. 32 However, during Candidemia, an one of opportunistic infection, significantly higher IL-17A concentrations in patients serum have been shown alongside high KYN levels, compared with those in non-candidemic patients. 33 These results suggested that the role of IL-17A on KP activation may depend on host immune conditions. Our data cannot exclude the possibility that IL-17A indirectly induced KP activation in maternal circulation; however, KYN levels in serum were significantly increased and placental KP activation seems to be blocked by high IL-17A. Under physiological conditions, very little KYNA crosses through the placenta. 17 On the other hand, previous studies have reported that the increased production of IL-17A rapidly increased not only the BBB, but also intestinal epithelial barrier permeability. Both barriers are held together by tight junctions, similar to the placental barrier. 34 These results suggested that high concentrations of IL-17A changed the permeability of the placenta, resulting not only in maternal KYN, but also in the high concentrations of KYNA in the placenta, being transferred and affecting the fetus. In additional, we demonstrated that in the pallium of fetal brain, which includes the brain hippocampus and neocortex, the KYN and KYNA levels and the ratio of KYN/TRP were significantly higher in MIA-offspring than in controls (Figure 3(g)). Forrest et al 35 demonstrated that the prenatal inhibition of KMO, which diverts KYN metabolism to KYNA, significantly increased the levels of KYN and KYNA in fetal brain. They also showed that increased KYNA in fetal brain leads to structural and functional changes in the Hipp of adult offspring.36 -39 In particular, a high concentration of KYNA during early neuronal development changed protein expression patterns associated with neuronal development and excitatory- inhibitory balance, 38 which was also related to the pathophysiological mechanisms of ASD. These results suggested that maternal inflammation affected KP metabolites and that increased levels of KYN and KYNA may be linked to neurodevelopment.
To test our hypothesis, we investigated whether a high dose of KYN during gestation affected fetal development in the absence of inflammation. The administration of low (100 mg/kg) or high (300 mg/kg) concentration of KYN in pregnant dams significantly increased the levels of KP metabolites rapidly as shown in previous studies (Figure 4 and Table 3)17,18,20,40 and these significant increases in maternal serum returned to basal levels within 12 hours (data not shown). We also found that the maternal administration of KYN significantly increased the levels of KYN and KYNA in fetal plasma and brain rapidly. We observed that animals with highly elevated KYN during neurodevelopment exhibited impairments in cognitive and social behavior in adulthood (Figure 5). Further, these animals exhibited depressive-like behavior, which is consistent with KMO deletion phenotypes. 16 Pocivavsek et al 18 demonstrated that KYN-treatment in rat dams with 100 mg/kg in diet from E15 (equal to E13 in mice) to 22 elevated the levels of KYNA in fetal forebrain. The amount of KYN they used was equivalent to approximately 300 mg/kg body weight/day, which is the same dose that for the high KYN treatment in our experiment. Their results provide evidence that a continuous increase in brain KYNA levels during the late prenatal period induces Hipp-related cognitive and learning dysfunctions. Pershing et al 41 who belong to the same laboratory as Pocivavsek, also showed that an exposure to high concentrations of KYNA during the last week of gestation resulted in age-dependent changes in N-methyl-D-aspartate (NMDA) receptor expression and cognitive performance. Liu et al 20 showed that KYN-treatment in neonatal mice at a dose of 2 × 200 mg/kg/day i.p. at P7-16 enhanced sensitivity to d-amphetamine-induced increase in locomotor activity and mild impairments in prepulse inhibition and memory. Iaccarino et al 40 demonstrated that adult rats injected with 100 mg/kg KYN, i.p., during the neonatal period at P7-10 exhibited decreased social behavior and locomotor activity, but underwent no changes in attentional function or fear conditioning behaviors. Varge et al 42 showed that the systemic administration of 300 mg/kg, i.p., in adult mice increased anxiety-like behavior and abolished the formation of object recognition memory. Collectively, previous studies and our results suggest that KYN was a key metabolite that crossed the placenta, and high concentrations of KYN and/or KYNA affected fetal brain development. However, we did not observe anxiety-related behaviors in KYN-treated mice, and social impairments were less evident in KYN-treated mice than in MIA-mice. Therefore, the possibility of direct effects of IL-17A in the fetal brain cannot be ruled out, and different factors may be implicated in the pathology underpinning MIA-induced ASD.
Measurement of KP metabolites in the PFC of adult MIA- and KYN-treated mice (P77) revealed significantly lower levels of TRP and higher levels of AA, and no significant changes in other metabolites in both mice compared to control/PBS-treated animals (Figure 6(a)). However, we observed no significant changes in KP metabolites in Hipp (Figure 6(b)). Guillemin et al43,44 reported that KP activation in ASD children resulted in an altered balance between KP metabolites and increased production of quinolinic acid (QUIN), which is an agonist of NMDA receptors and an endogenous neurotoxin. Liang et al 45 reported the levels of AA were higher in ASD children than in the healthy subjects. AA acts as an intermediate factor in the KP, serving as a potential biomarker for other neurodevelopmental disorders 21 and a target for the treatment of SCZ. 46 Bryn et al 47 reported that the mean serum levels of KYNA were significantly lower in individuals with ASD than in healthy controls, but no significant differences were observed in QUIN levels. Further, reduction of quinolinate phosphoribosyltransferase (QPRT), which catabolizes 3-HAA to QUIN, was reported to alter neuronal morphology of differentiation in an in vitro cell line. Chemical inhibition or complete gene deletion of QPRT was lethal upon induction of neuronal differentiation, but no effects on cell proliferation and levels of KP metabolites in vivo were observed. 48 Clark et al 27 reported that MIA rats exhibited an augmented inflammatory status in adolescence (P35), but no changes in the brain levels of several KP metabolites, in both P35 and P60 offspring were noted. They also demonstrated that MIA alone did not exert long-lasting effects on the expression of inflammatory cytokines on P60, whereas MIA offspring exhibited blunted responses of several cytokines and KP metabolites to a secondary immune challenge with LPS in young adulthood (P60). These results suggest progressive impairments of the inflammatory response, induction of KP enzymes, and imbalance in KP metabolites as the brain matures. Our data are partially consistent with previous work. The changes in KP metabolites in ASD still remain controversial. Possible explanations for these inconsistencies include age differences,49,50 subtype of ASD, comorbid conditions in clinical samples, 47 or species differences. 51
In conclusion, our results indicate that chronic gestational IL-17A is relevant to ASD-like behavioral changes, cognitive dysfunction, and depressive-like behavior in mice. Maternal IL-17A promotes the KP to generate the crucial metabolite, KYN, which may change the balance of KP metabolites in fetal brain during neurodevelopment. In addition, maternal high-dose KYN in the absence of inflammation causes similar behavioral changes in offspring, but the responses are blunted. These results collectively suggest that KYN or KP metabolites are key molecules involved in the pathogenesis of ASD following maternal inflammation. Translational approaches, which integrate metabolomics will help shed light on the molecular and biological underpinnings of ASD.
Supplemental Material
Supplemental material, sj-TIF-1-try-10.1177_11786469211026639 for The Effects of Maternal Interleukin-17A on Social Behavior, Cognitive Function, and Depression-Like Behavior in Mice with Altered Kynurenine Metabolites by Yuki Murakami, Yukio Imamura, Yoshiyuki Kasahara, Chihiro Yoshida, Yuta Momono, Ke Fang, Toshimasa Nishiyama, Daisuke Sakai and Yukuo Konishi in International Journal of Tryptophan Research
Acknowledgments
We are grateful to Drs. Keiko Maekawa and Chisato Takahashi at Doshisha Women’s College of Liberal Arts for HPLC measurements. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Grants-in-Aids for Scientific Research (16K08948 and 17H04364) from the Japan Society for Promotion of Science (JSPS). Additionally, this work reported in this paper has been supported in parts by RIKEN Healthcare and Medical Data Platform Project and Developmental Disorder Data Multi-level Integration Unit of Medical Sciences Innovation Hub Program.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Y.Mu. designed the study, performed the experiments with the help of D.S., analyzed the data, and wrote the manuscript. K.F., Y.Ka., C.Y., and Y.Mo. assisted with experiments. Y.Mu., Y.I., and D.S. interpreted and discussed the results. T.N. and Y.Ko. supervised the project.
ORCID iD: Yuki Murakami  https://orcid.org/0000-0002-7085-4832
https://orcid.org/0000-0002-7085-4832
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Lee BK, Magnusson C, Gardner RM, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015;44:100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aguilar-Valles A, Rodrigue B, Matta-Camacho E. Maternal immune activation and the development of dopaminergic neurotransmission of the offspring: relevance for schizophrenia and other psychoses. Front Psychiatry. 2020;11:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McAlonan GM, Li Q, Cheung C. The timing and specificity of prenatal immune risk factors for autism modeled in the mouse and relevance to schizophrenia. Neurosignals. 2010;18:129-139. [DOI] [PubMed] [Google Scholar]
- 4. Solek CM, Farooqi N, Verly M, Lim TK, Ruthazer ES. Maternal immune activation in neurodevelopmental disorders. Dev Dyn. 2018;247:588-619. [DOI] [PubMed] [Google Scholar]
- 5. Choi GB, Yim YS, Wong H, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shin Yim Y, Park A, Berrios J, et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature. 2017;549:482-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reed MD, Yim YS, Wimmer RD, et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature. 2020;577:249-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gumusoglu SB, Hing BWQ, Chilukuri ASS, Dewitt JJ, Scroggins SM, Stevens HE. Chronic maternal interleukin-17 and autism-related cortical gene expression, neurobiology, and behavior. Neuropsychopharmacology. 2020;45:1008-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong H, Hoeffer C. Maternal IL-17A in autism. Exp Neurol. 2018;299:228-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sasaki T, Tome S, Takei Y. Intraventricular IL-17A administration activates microglia and alters their localization in the mouse embryo cerebral cortex. Mol Brain. 2020;13:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191-1193. [DOI] [PubMed] [Google Scholar]
- 12. Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521-530. [DOI] [PubMed] [Google Scholar]
- 13. Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96-98. [DOI] [PubMed] [Google Scholar]
- 14. Pershing ML, Bortz DM, Pocivavsek A, et al. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology. 2015;90:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erhardt S, Pocivavsek A, Repici M, et al. Adaptive and behavioral changes in kynurenine 3-monooxygenase knockout mice: relevance to psychotic disorders. Biol Psychiatry. 2017;82:756-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tashiro T, Murakami Y, Mouri A, et al. Kynurenine 3-monooxygenase is implicated in antidepressants-responsive depressive-like behaviors and monoaminergic dysfunctions. Behav Brain Res. 2017;317:279-285. [DOI] [PubMed] [Google Scholar]
- 17. Goeden N, Notarangelo FM, Pocivavsek A, Beggiato S, Bonnin A, Schwarcz R. Prenatal dynamics of kynurenine pathway metabolism in mice: focus on kynurenic acid. Dev Neurosci. 2017;39:519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology. 2014;231:2799-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murakami Y, Ishibashi T, Tomita E, et al. Depressive symptoms as a side effect of Interferon-alpha therapy induced by induction of indoleamine 2,3-dioxygenase 1. Sci Rep. 2016;6:29920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu XC, Holtze M, Powell SB, et al. Behavioral disturbances in adult mice following neonatal virus infection or kynurenine treatment – role of brain kynurenic acid. Brain Behav Immun. 2014;36:80-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murakami Y, Imamura Y, Saito K, Sakai D, Motoyama J. Altered kynurenine pathway metabolites in a mouse model of human attention-deficit hyperactivity/autism spectrum disorders: a potential new biological diagnostic marker. Sci Rep. 2019;9:13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: a survey of inbred strains and F1 hybrids. Behav Genet. 2000;30:285-293. [DOI] [PubMed] [Google Scholar]
- 23. Mouri A, Koseki T, Narusawa S, et al. Mouse strain differences in phencyclidine-induced behavioural changes. Int J Neuropsychopharmacol. 2012;15:767-779. [DOI] [PubMed] [Google Scholar]
- 24. Halassa MM, Acsady L. Thalamic inhibition: diverse sources, diverse scales. Trends Neurosci. 2016;39:680-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia KO, Ornellas FL, Martin PK, et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front Aging Neurosci. 2014;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kubo H, Hoshi M, Mouri A, et al. Absence of kynurenine 3-monooxygenase reduces mortality of acute viral myocarditis in mice. Immunol Lett. 2017;181:94-100. [DOI] [PubMed] [Google Scholar]
- 27. Clark SM, Notarangelo FM, Li X, Chen S, Schwarcz R, Tonelli LH. Maternal immune activation in rats blunts brain cytokine and kynurenine pathway responses to a second immune challenge in early adulthood. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:286-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dhillion P, Wallace K, Herse F, et al. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2012;303:R353-R358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Notarangelo FM, Pocivavsek A. Elevated kynurenine pathway metabolism during neurodevelopment: implications for brain and behavior. Neuropharmacology. 2017;112:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sedlmayr P, Blaschitz A, Stocker R. The role of placental tryptophan catabolism. Front Immunol. 2014;5:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aghaeepour N, Ganio EA, McIlwain D, et al. An immune clock of human pregnancy. Sci Immunol. 2017;2:eaan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romani L, Zelante T, De Luca A, Fallarino F, Puccetti P. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J Immunol. 2008;180:5157-5162. [DOI] [PubMed] [Google Scholar]
- 33. Krause R, Zollner-Schwetz I, Salzer HJ, et al. Elevated levels of interleukin 17A and kynurenine in candidemic patients, compared with levels in noncandidemic patients in the intensive care unit and those in healthy controls. J Infect Dis. 2015;211:445-451. [DOI] [PubMed] [Google Scholar]
- 34. Rahman MT, Ghosh C, Hossain M, et al. IFN-gamma, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun. 2018;507:274-279. [DOI] [PubMed] [Google Scholar]
- 35. Forrest CM, Khalil OS, Pisar M, Darlington LG, Stone TW. Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 2013;1504:1-15. [DOI] [PubMed] [Google Scholar]
- 36. Forrest CM, Khalil OS, Pisar M, et al. Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience. 2013;254:241-259. [DOI] [PubMed] [Google Scholar]
- 37. Pisar M, Forrest CM, Khalil OS, et al. Modified neocortical and cerebellar protein expression and morphology in adult rats following prenatal inhibition of the kynurenine pathway. Brain Res. 2014;1576:1-17. [DOI] [PubMed] [Google Scholar]
- 38. Khalil OS, Pisar M, Forrest CM, Vincenten MC, Darlington LG, Stone TW. Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring. Eur J Neurosci. 2014;39:1558-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forrest CM, McNair K, Pisar M, Khalil OS, Darlington LG, Stone TW. Altered hippocampal plasticity by prenatal kynurenine administration, kynurenine-3-monoxygenase (KMO) deletion or galantamine. Neuroscience. 2015;310:91-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iaccarino HF, Suckow RF, Xie S, Bucci DJ. The effect of transient increases in kynurenic acid and quinolinic acid levels early in life on behavior in adulthood: implications for schizophrenia. Schizophr Res. 2013;150:392-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pershing ML, Phenis D, Valentini V, et al. Prenatal kynurenine exposure in rats: age-dependent changes in NMDA receptor expression and conditioned fear responding. Psychopharmacology. 2016;233:3725-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Varga D, Heredi J, Kanvasi Z, et al. Systemic L-Kynurenine sulfate administration disrupts object recognition memory, alters open field behavior and decreases c-Fos immunopositivity in C57Bl/6 mice. Front Behav Neurosci. 2015;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim CK, Essa MM, de Paula Martins R, et al. Altered kynurenine pathway metabolism in autism: implication for immune-induced glutamatergic activity. Autism Res. 2016;9:621-631. [DOI] [PubMed] [Google Scholar]
- 44. Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279:1356-1365. [DOI] [PubMed] [Google Scholar]
- 45. Liang Y, Ke X, Xiao Z, et al. Untargeted metabolomic profiling using UHPLC-QTOF/MS reveals metabolic alterations associated with Autism. Biomed Res Int. 2020;2020:6105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oxenkrug G, van der Hart M, Roeser J, Summergrad P. Anthranilic acid: a potential biomarker and treatment target for schizophrenia. Ann Psychiatry Ment Health. 2016;4:1059. [PMC free article] [PubMed] [Google Scholar]
- 47. Bryn V, Verkerk R, Skjeldal OH, Saugstad OD, Ormstad H. Kynurenine pathway in autism spectrum disorders in children. Neuropsychobiology. 2017;76:82-88. [DOI] [PubMed] [Google Scholar]
- 48. Haslinger D, Waltes R, Yousaf A, et al. Loss of the Chr16p11.2 ASD candidate gene QPRT leads to aberrant neuronal differentiation in the SH-SY5Y neuronal cell model. Mol Autism. 2018;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Comai S, Costa CV, Ragazzi E, Bertazzo A, Allegri G. The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats. Clin Chim Acta. 2005;360:67-80. [DOI] [PubMed] [Google Scholar]
- 50. Kaluzna-Czaplinska J, Gatarek P, Chirumbolo S, Chartrand MS, Bjorklund G. How important is tryptophan in human health? Crit Rev Food Sci Nutr. 2019;59:72-88. [DOI] [PubMed] [Google Scholar]
- 51. Murakami Y, Saito K. Species and cell types difference in tryptophan metabolism. Int J Tryptophan Res. 2013;6:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-TIF-1-try-10.1177_11786469211026639 for The Effects of Maternal Interleukin-17A on Social Behavior, Cognitive Function, and Depression-Like Behavior in Mice with Altered Kynurenine Metabolites by Yuki Murakami, Yukio Imamura, Yoshiyuki Kasahara, Chihiro Yoshida, Yuta Momono, Ke Fang, Toshimasa Nishiyama, Daisuke Sakai and Yukuo Konishi in International Journal of Tryptophan Research