Abstract
Background
Hyperkalemia is common among hemodialysis (HD) patients and has been associated with adverse clinical outcomes. Previous studies considered a single serum potassium (K) measurement or time-averaged values, but serum K excursions out of the target range may be more reflective of true hyperkalemia events. We assessed whether hyperkalemia excursions lead to an elevated risk of adverse clinical outcomes.
Methods
Using data from 21 countries in Phases 4–6 (2009–18) of the Dialysis Outcomes and Practice Patterns Study (DOPPS), we investigated the associations between peak serum K level, measured monthly predialysis, over a 4-month period (‘peak K’) and clinical outcomes over the subsequent 4 months using Cox regression, adjusted for potential confounders.
Results
The analysis included 62 070 patients contributing a median of 3 (interquartile range 2–6) 4-month periods. The prevalence of hyperkalemia based on peak K was 58% for >5.0, 30% for >5.5 and 12% for >6.0 mEq/L. The all-cause mortality hazard ratio for peak K (reference ≤5.0 mEq/L) was 1.15 [95% confidence interval (CI) 1.09, 1.21] for 5.1–5.5 mEq/L, 1.19 (1.12, 1.26) for 5.6–6.0 mEq/L and 1.33 (1.23, 1.43) for >6.0 mEq/L. Results were qualitatively consistent when analyzing hospitalizations and a cardiovascular composite outcome.
Conclusions
Among HD patients, we identified a lower K threshold (peak K 5.1–5.5 mEq/L) than previously reported for increased risk of hospitalization and mortality, with the implication that a greater proportion (>50%) of the HD population may be at risk. A reassessment of hyperkalemia severity ranges is needed, as well as an exploration of new strategies for effective management of chronic hyperkalemia.
Keywords: hemodialysis, hospitalization, hyperkalemia, mortality, potassium
INTRODUCTION
Hyperkalemia (HK) is common among patients with end-stage kidney disease (ESKD) undergoing maintenance hemodialysis (HD) [1–3]. Marked or complete impairment of renal potassium (K) excretion can lead to HK [4] and subsequent increased mortality risk [5, 6], most likely due to cardiac arrhythmias [7]. The burden of HK to patients [8–10] and healthcare systems [11] is well known. Common strategies to avoid and/or treat HK include lowering dietary potassium intake, lowering dialysate K and initiating K-binding resins (K binders); other options include initiating loop diuretics or sodium bicarbonate [9, 10, 12–14]. Because renin–angiotensin–aldosterone system inhibitor (RAASi) agents can increase serum K levels [15, 16], discontinuing RAASi use can also be used as an antihyperkalemic measure [17]; however, this strategy may lead to suboptimal management of cardiovascular (CV) complications of ESKD, particularly associated with heart failure [18]. The extent to which these strategies are initiated in HD patients in response to HK episodes is unknown.
Large cohort studies have demonstrated an elevated risk of adverse events in HD patients with serum K levels >5.5 or >6.0 mEq/L [19–22]. These studies utilized a variety of methodological strategies, including capturing short-term risk within 96 h of K measurement [19], assessing long-term risk following a single K measurement over multiple years [20] and investigating 3-month time-averaged serum K [21, 22]. Serial K measurements may be more informative than a single K measure by being more sensitive to day-to-day fluctuations in serum K [23, 24] and may be aligned with the concept that K excursions may lead to events such as arrhythmias and sudden cardiac death. However, simply averaging recent serum K levels may overlook some at-risk patients because this measure does not reflect variability or potential excursions out of the target range (e.g. monthly serum K levels of 4.0, 6.0 and 5.0 would result in a 3-month average value of 5.0).
As a potentially improved measure of risk due to HK, we investigated any HK excursions out of the target range that patients experienced over the past 4 months. Retrospectively assessing ‘target achievement’ as a mortality risk factor has been studied among HD patients in the mineral and bone disorder (MBD) area [25, 26], but to our knowledge, not for K. We hypothesize that HD patients with more severe HK excursions over 4 months have a higher risk of mortality and other adverse clinical outcomes over the subsequent 4 months.
MATERIALS AND METHODS
Data source
The Dialysis Outcomes and Practice Patterns Study (DOPPS) is an international prospective cohort study of patients ≥18 years of age treated with in-center HD in 21 countries. Maintenance HD patients were randomly selected from national samples of HD facilities in each country for inclusion in the DOPPS database; detailed information is included in prior publications [27, 28] and at http://www.dopps.org. Study approval and patient consent were obtained as required by national and local ethics committee regulations. Information on patient demographics and comorbidity history was abstracted from medical records at DOPPS enrollment in each study phase. Data on predialysis laboratory values (monthly) and medication prescriptions (every 4 months) were abstracted from medical records at baseline and during follow-up. This analysis used data from DOPPS Phase 4 (2009–11), Phase 5 (2012–15) and Phase 6 (2015-2018) and included all 21 countries: the USA, Canada, Japan, China, Russia, Turkey, seven European countries (Belgium, France, Germany, Italy, Spain, Sweden and the UK), six Gulf Cooperation Council (GCC) countries (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia and United Arab Emirates), Australia and New Zealand.
Variables and study design
The primary exposure variable was ‘peak K’, defined as the highest value of a monthly predialysis serum K observed during the 4-month exposure interval (Figure 1) and categorized as >6.0, 5.6–6.0, 5.1–5.5 and ≤5.0 mEq/L (i.e. never exceeding 5.0 over the interval). Because a 4-month interval to define the exposure period was required, we excluded patients who were enrolled in DOPPS for <4 months. We also required that patients had serum K measured in at least 3 of the 4 months. Figure 2 details the number of patients meeting the eligibility criteria for each analysis.
FIGURE 1:
Illustration of data collection and study design. Exposure variable is peak K, i.e. the peak serum K level, measured monthly predialysis over the 4-month exposure. For clinical outcomes, patients were followed for the next 4 months. For medication change outcomes, prescriptions at the end of the 4-month exposure period were compared with the beginning of that period. Multiple exposure/outcome periods per patient may be included.
FIGURE 2:
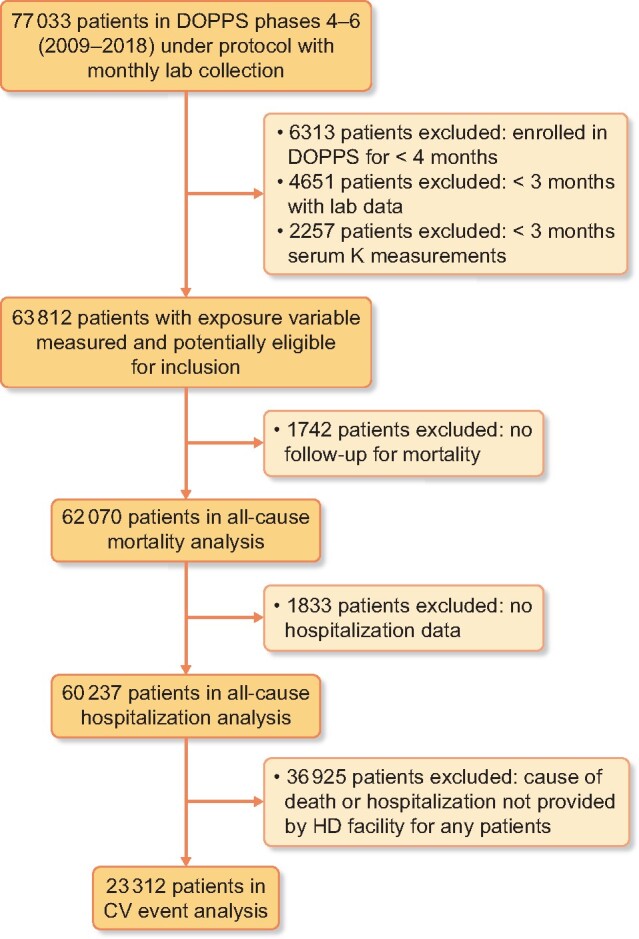
Flow chart illustrating inclusion/exclusion criteria for analyses of clinical outcomes. Primary analysis: all-cause mortality. A large proportion of patients were unavailable for CV event analyses due to electronic health record transfers from US providers that do not include information on the cause of death or hospitalization.
Our study design is illustrated in Figure 1; we divided time into 4-month intervals to assess whether peak K over a 4-month period was associated with an elevated risk of adverse clinical outcomes over the subsequent 4 months. Multiple exposure–outcome periods per patient were included to the extent that data were available, that is, the unit of analysis was the 4-month period. The primary outcome was all-cause mortality. Secondary outcomes included all-cause hospitalization and a composite CV outcome of CV death or CV hospitalization. Time at risk began at the end of the 4-month exposure interval and ended after the event of interest, 7 days after leaving the facility due to transfer or change in modality, loss to follow-up, administrative end of the study phase or end of the 4-month follow-up interval (whichever occurred first).
Statistical analyses
We summarized the distribution of HK excursions for each threshold and reported the distribution of peak K by country. We reported patient characteristics for included versus excluded patients and by peak K among patients included in the primary all-cause mortality analysis.
We assessed the frequency of initiation—by region and peak K—of five serum K management strategies: RAASi discontinuation, K binder initiation, loop diuretic initiation, sodium bicarbonate initiation and lowering dialysate K. The outcome was defined by the prescription at the end of the 4-month exposure period. Evaluation of RAASi discontinuation was restricted to patients prescribed a RAASi at the beginning of the period (i.e. because only users could discontinue). Similarly, evaluations of medication initiation were restricted to patients not prescribed the medication at the beginning of the period. The dialysate K lowering (by ≥0.5 mEq/L) analysis was restricted to patients prescribed dialysate K ≥2.5 mEq/L at the beginning of the period, because dialysate K levels <2.5 mEq/L would rarely be lowered below 2.0 mEq/L. Exclusion criteria for medication analyses are further described in Supplementary data, Figure S1.
We used Cox regression for time-to-event analyses of clinical outcomes. Models were stratified by DOPPS phase and country and within-facility and within-patient clustering was accounted for by using robust sandwich covariance estimators. Potential confounders selected for adjustment included age, sex, Black race, vintage, 13 comorbidities [coronary artery disease, cerebrovascular disease, heart failure, peripheral vascular disease, other CV disease, cancer (nonskin), diabetes, gastrointestinal bleeding, hypertension diagnosis, lung disease, neurologic disease, psychiatric disorder and recurrent cellulitis/gangrene], albumin, phosphorus, hemoglobin, catheter use, body mass index (BMI), lowest serum K value during the 4-month exposure period (‘valley K’; to account for the likely U-shaped association between serum K and outcomes [29]) and frequency of K measurement. To expand on our primary findings, we performed additional analyses for the number of months (over the 4-month interval) during which serum K exceeded HK excursion thresholds of 5.0, 5.5 and 6.0 mEq/L; for more specific CV events; varying the level of covariate adjustment; by international region; for subgroups defined a priori [diabetes, age, time since HD initiation (vintage) and history of heart failure] and substituting mean serum K over 4 months or the single most-recent serum K as the exposure variable.
We used multiple imputations, assuming data were missing at random, to impute missing variables using the sequential regression multiple imputation method by IVEware [30]. Results from 20 such imputed data sets were combined for the final analysis using Rubin’s formula [31]. The proportion of missing data was 23% for medication prescriptions, 13% for comorbidity history and ≤5% for all other variables. All analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Study sample
Our primary analysis included 244 177 4-month periods across 62 070 patients. Individual patients contributed a median of 3 [interquartile range (IQR) 2–6, range 1–9] of these 4-month periods. We excluded 14 963 patients (19%) due to missing data on the outcome (i.e. no follow-up for mortality) or exposure (i.e. no 4-month period with at least three serum K measurements) as illustrated in Figure 2. Excluded patients had a shorter time since HD therapy initiation (median 0.9 versus 2.2 years) and other differences specific to incident HD patients (e.g. lower serum albumin, lower hemoglobin, greater catheter use) but minimal differences in age or comorbidity history (Supplementary data, Table S1).
Descriptive data
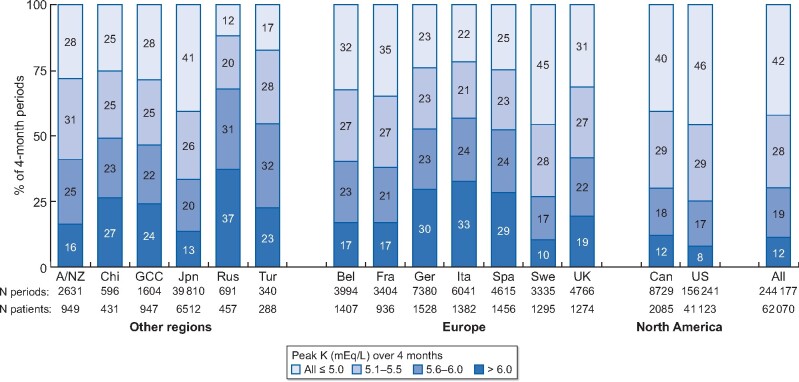
The distribution of the number of HK excursions over the 4-month periods is shown in Figure 3 using different serum K thresholds: >5.0, 5.5 and 6.0 mEq/L. Peak K over the 4-month periods reached >6.0 mEq/L in 12% of periods, 5.6–6.0 mEq/L in 19% of periods, 5.1–5.5 mEq/L in 28% of periods and never exceeded 5.0 mEq/L in 42% of 4-month periods. Patients in Russia, Italy, Germany and Spain were most likely to have a severe (>6.0 mEq/L) HK excursion, while patients in the USA, Sweden, Canada and Japan were least likely (Figure 4).
FIGURE 3:

Number of months with an HK excursion over a 4-month period.
FIGURE 4:
Prevalence and severity of HK excursions, by country. A/NZ, Australia/New Zealand; Chi, China; Jpn, Japan; Rus, Russia; Tur, Turkey; Bel, Belgium; Fra, France; Ger, Germany; Ita, Italy; Spa, Spain; Swe, Sweden; UK, United Kingdom; Can, Canada; US, United States. Columns may not sum to 100% due to rounding.
Patient characteristics by peak K are summarized in Tables 1 and 2. Baseline patient characteristics for measures that are largely time-fixed are shown in Table 1 (unit of analysis: patient). Patients with higher peak K levels tended to be younger, were less likely to be black, had a longer time since HD initiation and were less likely to have diabetes; minimal differences were observed for other comorbidities. Dynamic patient characteristics that may vary longitudinally within patients are shown in Table 2 (unit of analysis: patient period); during periods with higher peak K levels, mean phosphorus levels were higher and mean albumin levels were slightly higher. The prevalence of RAASi and K binder therapy was higher during periods with higher peak K levels; loop diuretic use ranged from 19 to 25% and sodium bicarbonate use was rare (≤5%).
Table 1.
Fixed baseline patient characteristics by peak serum K in the past 4 months
| Characterisitcs | Peak serum K (mEq/L) during the 4-month exposure period |
|||
|---|---|---|---|---|
| ≤5.0 | 5.1–5.5 | 5.6–6.0 | >6.0 | |
| Patients, n (%) | 27 631 (45) | 16 653 (27) | 10 857 (17) | 6929 (11) |
| Characteristics | ||||
| Age (years), mean ± SD | 65.2 ± 14.4 | 63.6 ± 14.6 | 62.1 ± 14.7 | 60.9 ± 14.9 |
| Sex (men), % | 58 | 58 | 57 | 57 |
| Race (Black), % | 31 | 26 | 21 | 17 |
| Vintage (years), median (IQR) | 1.6 (0.5–4.3) | 2.5 (0.7–5.6) | 3.2 (1.0–6.5) | 3.3 (1.1–6.7) |
| Body mass index (kg/m2), mean ± SD | 27.7 ± 7.0 | 27.4 ± 7.0 | 26.9 ± 6.8 | 26.3 ± 6.4 |
| Comorbid conditions, % | ||||
| Coronary artery disease | 26 | 27 | 27 | 29 |
| Cerebrovascular disease | 9 | 10 | 9 | 10 |
| Heart failure | 26 | 25 | 24 | 24 |
| Peripheral vascular disease | 18 | 20 | 20 | 23 |
| Other CV disease | 20 | 21 | 22 | 22 |
| Cancer (non-skin) | 9 | 9 | 9 | 10 |
| Diabetes | 60 | 58 | 54 | 49 |
| Gastrointestinal bleeding | 4 | 4 | 4 | 5 |
| Hypertension diagnosis | 80 | 81 | 82 | 83 |
| Lung disease | 9 | 9 | 10 | 10 |
| Neurologic disease | 7 | 7 | 7 | 9 |
| Psychiatric disorder | 19 | 19 | 20 | 19 |
| Recurrent cellulitis, gangrene | 5 | 7 | 7 | 8 |
Table 2.
Dynamic patient characteristics during the 4-month exposure period by peak serum K
| Characterisitcs | Peak serum K (mEq/L) during the 4-month exposure period |
|||
|---|---|---|---|---|
| ≤5.0 | 5.1–5.5 | 5.6–6.0 | >6.0 | |
| 4-month periods, n (%) | 102 516 (42) | 67 949 (28) | 45 385 (19) | 28 327 (12) |
| Valley Ka (mEq/L), % | ||||
| <3.5 | 9 | 2 | 1 | 1 |
| 3.5–3.9 | 34 | 9 | 5 | 4 |
| ≥4.0 | 57 | 90 | 94 | 95 |
| Lab values (mean of 4 months) | ||||
| Potassium (mEq/L), mean ± SD | 4.3 ± 0.3 | 4.9 ± 0.2 | 5.2 ± 0.3 | 5.7 ± 0.4 |
| Albumin (g/dL), mean ± SD | 3.70 ± 0.41 | 3.76 ± 0.39 | 3.78 ± 0.39 | 3.79 ± 0.40 |
| Hemoglobin (g/dL), mean ± SD | 10.9 ± 1.0 | 10.9 ± 1.1 | 11.0 ± 1.1 | 11.0 ± 1.1 |
| Phosphorus (mg/dL), mean ± SD | 4.9 ± 1.1 | 5.3 ± 1.2 | 5.5 ± 1.3 | 5.7 ± 1.5 |
| PTH (pg/mL), median (IQR) | 280 (163 452) | 300 (174 491) | 298 (169 506) | 295 (161 512) |
| Calcium (mg/dL), mean ± SD | 9.0 ± 0.6 | 9.0 ± 0.6 | 9.0 ± 0.6 | 8.9 ± 0.7 |
| Catheter use (%) | 17 | 16 | 15 | 18 |
| Dialysate potassiumb (mEq/L), % | ||||
| 1.0–1.5 | 2 | 5 | 8 | 14 |
| 2.0–2.5 | 68 | 77 | 80 | 75 |
| 3.0–4.0 | 30 | 18 | 12 | 12 |
| Other HK-related treatments, % | ||||
| RAASi use | 34 | 38 | 40 | 42 |
| K binder | 5 | 7 | 11 | 19 |
| Loop diuretic | 25 | 21 | 19 | 20 |
| Sodium bicarbonate | 4 | 4 | 4 | 5 |
Lab values represent the mean of up to 4 monthly measurements during the exposure period.
PTH, parathyroid hormone.
aValley K is the lowest serum K measured over the 4-month interval.
bDialysate potassium: 99% of dialysate K values in the 2.0–2.5 group were exactly 2 mEq/L and 94% of values in the 3.0–4.0 group were exactly 3 mEq/L.
HK excursions and initiation of K-lowering strategies
While the prescription prevalences are shown in Table 2, the frequency of initiating HK management strategies is illustrated in Table 3 by region and peak K. RAASi discontinuation was observed in 7–10% of users over the 4-month period and was not more likely to occur among patients who had an HK excursion >6.0 mEq/L. Initiation of loop diuretics and sodium bicarbonate was rare (<3%) and also did not vary by peak K in the past 4 months. Among patients with a peak K >6.0 mEq/L in the past 4 months, K binder initiation was more common in Europe (7%) and Japan (6%) than in North America (2%). Among patients with a dialysate K ≥2.5 mEq/L, 22% of North American patients were prescribed a lower dialysate K 4 months later, and this proportion was much greater if the patients had a peak K >6.0 (64%) versus ≤5.0 mEq/L (12%) in the past 4 months. In Europe, lowering the dialysate K was twice as common when peak K was >6.0 (15%) versus ≤5.0 mEq/L (8%).
Table 3.
HK excursions over a 4-month period and initiation of K-lowering strategies by region
| Regions | RAASi discontinuation | K binder initiation | Loop diuretic initiation | Sodium bicarbonate initiation | Dialysate K lowered |
|---|---|---|---|---|---|
| North America | |||||
| No. of eligible periodsa | 32 242 | 90 335 | 74 924 | 88 963 | 22 646 |
| Percentage of strategy initiatorsb | 8.4 | 0.5 | 1.8 | 0.6 | 22.2 |
| Percentage of strategy initiators by peak K (mEq/L) in past 4 months | |||||
| ≤5.0 | 8.8 | 0.3 | 2.0 | 0.6 | 11.6 |
| 5.1–5.5 | 8.1 | 0.4 | 1.6 | 0.5 | 29.5 |
| 5.6–6.0 | 7.7 | 0.6 | 1.7 | 0.7 | 48.2 |
| >6.0 | 9.0 | 1.7 | 1.8 | 0.6 | 63.5 |
| Japan | |||||
| No. of eligible periodsa | 16 533 | 29 332 | 25 376 | 33 574 | 155 |
| Percentage of strategy initiatorsb | 9.0 | 1.9 | 0.7 | 0.1 | — |
| Percentage of strategy initiators by peak K (mEq/L) in past 4 months | |||||
| ≤5.0 | 10.1 | 0.5 | 0.9 | 0.1 | — |
| 5.1–5.5 | 8.9 | 1.3 | 0.6 | 0.0 | — |
| 5.6–6.0 | 8.7 | 2.9 | 0.6 | 0.1 | — |
| >6.0 | 7.1 | 6.2 | 0.5 | 0.1 | — |
| Europe | |||||
| No. of eligible periodsa | 9,700 | 20 896 | 17 119 | 24 375 | 9576 |
| Percentage of strategy initiatorsb | 9.6 | 2.9 | 2.0 | 0.9 | 11.4 |
| Percentage of strategy initiators by peak K (mEq/L) in past 4 months | |||||
| ≤ 5.0 | 10.3 | 0.9 | 2.9 | 0.7 | 7.8 |
| 5.1–5.5 | 9.5 | 1.6 | 1.9 | 1.1 | 10.8 |
| 5.6–6.0 | 8.9 | 3.5 | 1.6 | 0.8 | 14.4 |
| >6.0 | 9.8 | 6.7 | 1.5 | 1.0 | 14.7 |
Peak serum potassium: highest value of serum K over the 4-month exposure period.
aRAASi discontinuation analysis restricted to patients prescribed RAASi during the previous 4-month period, initiation analyses restricted to patients not prescribed the medication during the previous 4-month period and dialysate K lowering (by ≥0.5 mEq/L) analysis restricted to patients prescribed dialysate K ≥2.5 mEq/L during the previous 4-month period.
bStrategy initiators reflect the proportion of eligible patients who initiated the strategy (e.g. discontinued RAASi or new user of other medication). DOPPS countries from other regions (China, Russia, Turkey, GCC, Australia/New Zealand) had too few patients to present stratified results.
—, data were suppressed due to a small sample size in Japan caused by near-uniform dialysate K of 2 mEq/L.
HK excursions and clinical outcomes
For the primary outcome of all-cause mortality, 9448 patients died and the death rate was 0.123 per patient-year; 11% of 4-month follow-up periods were truncated due to censoring. The association between HK excursions and all-cause mortality over the subsequent 4-month period is summarized in Table 4. The all-cause mortality hazard ratio (HR) compared with peak K ≤5.0 mEq/L was 1.33 [95% confidence interval (CI) 1.23, 1.43] for peak K >6.0 mEq/L, 1.19 (1.12, 1.26) for peak K 5.6–6.0 mEq/L and 1.15 (1.09, 1.21) for peak K 5.1–5.5 mEq/L. When dichotomizing at HK thresholds of >5.0, >5.5 and >6.0 mEq/L, the adjusted HR for all-cause mortality ranged from 1.11 to 1.17 for one HK excursion and from 1.20 to 1.29 for two or more HK excursions compared with the reference of no HK excursions over the 4-month period (Supplementary data, Table S2).
Table 4.
HK excursions over a 4-month period and HRs (95% CIs) of clinical outcomes over the subsequent 4 months
| Characteristics | Percentage of 4-month periods | All-cause death | All-cause hospitalization | Composite: CV death or CV hospitalization |
|---|---|---|---|---|
| No. of patients | 62 070 | 60 237 | 23 312 | |
| No. of events | 9448 | 54 804 | 5570 | |
| Event rate (per patient-year) | 0.123 | 0.854 | 0.186 | |
| Peak serum potassium (mEq/L) | ||||
| ≤5.0 | 42 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 5.1–5.5 | 28 | 1.15 (1.09, 1.21) | 1.13 (1.11, 1.16) | 1.13 (1.05, 1.22) |
| 5.6–6.0 | 19 | 1.19 (1.12, 1.26) | 1.16 (1.13, 1.19) | 1.20 (1.10, 1.30) |
| >6.0 | 12 | 1.33 (1.23, 1.43) | 1.28 (1.24, 1.32) | 1.33 (1.21, 1.45) |
Cox regression models, stratified by DOPPS phase and country, simultaneously adjusted for age, sex, Black race, vintage, 13 comorbidities, albumin, phosphorus, hemoglobin, catheter use, BMI, valley K (lowest value of serum K over the 4-month period) and frequency of K measurement. Robust variance estimator used for hospitalizations and CV composite outcome, modeled as a time-to-first event within each 4-month follow-up period and may include multiple events per patient. Peak serum potassium: highest value of serum K over the 4-month exposure period.
The associations of peak K with all-cause hospitalization and a CV composite outcome (Table 4) were also strong and positive. Associations with more specific outcomes (CV death, sudden cardiac death, CV hospitalization, composite of all-cause mortality + CV hospitalization, composite of CV death + hospitalization for myocardial infarction, stroke or heart failure) were directionally consistent with the primary results (Supplementary data, Table S3).
In unadjusted models, the association between HK excursions and all-cause mortality was largely null; only after accounting for differences in patient characteristics (e.g. patients with HK excursions were on average younger and healthier) did we observe a strong association (Supplementary data, Table S4). The primary findings were generally consistent across regions; the association between HK excursions and all-cause mortality was strong in North America and Europe but weaker in Japan, where the lower number of events resulted in a loss of precision (Supplementary data, Table S5). Results were also robust to subgroup analyses by diabetes, age, vintage and history of heart failure (Supplementary data, Table S6). When analyzing mean serum K or the single most recent serum K—rather than peak K—over the 4-month exposure period, we observed a J-shaped association with all-cause mortality (Supplementary data, Table S7). In contrast to the peak K analysis, no elevated mortality rate was observed in the 5.1–5.5 (versus 4.0–5.0) mEq/L group: the HR was 1.01 (95% CI 0.95, 1.06) for mean serum K and 1.00 (0.94, 1.05) for the single most recent serum K level.
DISCUSSION
In this large international cohort study of HD patients we observed consistent associations between one or more HK excursions and mortality, hospitalizations and CV events. Even patients with a peak serum K at levels currently considered mildly hyperkalemic (5.1–5.5 mEq/L) [18] over a 4-month period had a 13–15% greater rate (versus K ≤5.0 mEq/L) of mortality, hospitalization and CV events over the subsequent 4 months. This new finding implies that a much greater proportion (>50%) of the HD population may be at risk for adverse clinical outcomes than previously reported.
The associations we observed between HK and clinical outcomes were directionally consistent with prior large cohort studies; however, we identified an increased risk at serum K levels (5.1–5.5 mEq/L) not recognized by most clinicians as harmful, particularly for predialysis measurements in HD patients. Indeed, the threshold identified in the present study is lower than previously reported [19–22]. A prior DOPPS analysis reported an increased risk of all-cause mortality at serum K levels >5.5 mEq/L, but not as low as 5.1–5.5 mEq/L [20]; discrepancies with our results appeared to be driven by parameterization of the exposure as a single K value versus 4-month peak K, which may have weakened the association between HK and clinical outcomes (Supplementary data, Table S7). Brunelli et al. [19] focused on short-term risk, linking clinical outcomes within 4 days following serum K measurement and found a greater risk of hospitalization at K >5.5 mEq/L and a greater risk of death and emergency department visits at K >6.0 mEq/L. This study, designed to estimate the risk of events shortly following an HK excursion, is more aligned with the potential mechanistic link between individual episodes of HK and specific causes of death and hospitalization, namely arrhythmias and sudden cardiac death induced by HK. Other studies [21, 22] used a 3-month averaged serum K and found an elevated risk of mortality at a mean serum K >5.5 mEq/L. Our approach demonstrates an increased risk even for seemingly mild HK excursions (5.1–5.5 mEq/L) that was not observable when simply averaging the serum K values (Supplementary data, Table S7). It is possible that patients may not be at immediate risk for acute events due to serum K levels of 5.1–5.5 mEq/L, but when considering day-to-day fluctuations in K levels, particularly the increase in the postprandial phase, these slightly elevated levels may indicate a risk of more severe and dangerous (yet unrecognized) HK excursions in the subsequent weeks or months.
Regarding strategies to lower serum K, we focus on the initiation of HK management strategies in secondary analyses. We observed that patients with one or more HK excursions over a 4-month period were not more likely to initiate sodium bicarbonate or loop diuretics. These findings were not necessarily surprising, given prior questions about their effectiveness in HD patients [32, 33]. Although RAASi therapies have been shown to increase serum K levels in HD patients [15, 16], we found that RAASi discontinuation was unrelated to peak K levels over the past 4 months in this HD population. Ideally patients would remain on RAASi therapy, given the probable CV benefits [34], particularly in heart failure and hypertension control, and achieve better K control through other means as first-line therapy. Initiation of two other K management strategies—lowering of dialysate K and initiation of K binders—was more common in patients with higher peak K levels over the past 4 months. Changes in dialysate K were very common in North America, while K binder initiation was more common in Europe and Japan; however, both strategies are short-term treatments to address acutely high serum K levels and are generally ineffective for long-term HK control. Altering the dialysate K is recommended to manage changes in intradialytic serum K levels [13] and has a strong impact on K levels measured immediately postdialysis [35]. However, extracellular K is removed more efficiently than intracellular K (where 98% of the body’s K is stored) during the intradialytic period, so after the HD session is over, there is a K rebound when the intracellular K is redistributed [4]. It is thus not surprising that intradialytic K removal is minimally correlated with predialysis serum K [36] and that HD facilities using uniformly lower dialysate K prescriptions do not have lower predialysis serum K levels [20]. K binders are often used outside of North America [37] to manage high serum K, but efficacy results have been mixed [14, 38] and long-term effectiveness is limited by poor tolerability and potentially serious gastrointestinal side effects [39–41].
Other options for long-term K management include dietary restrictions and new pharmacological therapies. We found that serum K was highest in some European countries, including Spain and Italy, where Mediterranean diets are rich in K [42]. Greater dietary K intake has been associated with increased mortality in HD patients [43]; however, dietary K restrictions are burdensome to patients, can have poor adherence, can negatively impact nutritional status and lead to CV-unhealthy diet patterns [44]. Reducing serum K levels through means that allow for a liberal diet is considered important to patients [44]. Emerging therapies for the long-term treatment of chronic HK, such as the novel potassium binders patiromer and sodium zirconium cyclosilicate (SZC), appear to effectively, sustainably and safely reduce serum K levels [22, 45–47]. SZC has been shown to be safe and effective to reduce predialysis K levels in the HD setting [48]. Patiromer has been shown to reduce serum K levels [49] and allow more RAASi use [50] in the non-dialysis CKD setting, and real-world evidence suggests effectiveness in the HD setting [51]. The impact of these therapies on HK in everyday dialysis practice, as well as their effects on clinical outcomes, requires further investigation.
This study had some limitations. First, while we cannot rule out residual confounding in this observational study, as patients with more severe HK excursions tended to be younger and healthier (Tables 1 and 2), and so adjustment for measured confounders tended to increase the estimated effect (Supplementary data, Table S4). We thus speculate that unmeasured confounding due to patient health status would more likely result in an underestimate than an overestimate of the observed effect. However, we must also consider the possibility that the observed association was driven in part by high K levels being a marker for other adherence risk factors such as skipping medications or missed/shortened HD treatments. Second, we studied clinical and medication outcomes but lacked data on dietary K intake and dietary interventions; we can infer the importance of nutrition based on the strong correlation between peak K and serum phosphorus levels in Table 2. Third, we did not consider the day of the week or the time of day of the serum K measurements. A prior DOPPS analysis [20] showed the difference between serum K levels collected at the first HD session of the week versus midweek was <0.2 mEq/L within each country. While the time of day of the lab draw was not available, it is likely that any misclassification would be nondifferential and thus bias results toward the null (i.e. would result in an underestimate of the HK effect). Fourth, DOPPS collects cross-sectional data every 4 months on most oral medications and thus changes in prescription within each 4-month period are unknown. For example, if K binders were started and stopped during the same 4-month window in response to an HK excursion, then we may be underestimating the reported association. Finally, we have no information on medication history prior to DOPPS enrollment, so we cannot reliably report on the duration of RAASi use prior to discontinuation.
This study also had several strengths. First, we utilized a very large international sample of HD patients. Second, we developed an approach to assess HK excursions using peak serum K over 4 months rather than only assessing a single value or deemphasizing HK excursions by averaging high and low K values over time. Third, we chose a ‘mid-term’ 4-month (versus 3-day or 3-year) follow-up period, but acknowledge that different approaches (i.e. short versus long follow-up period) may result in different findings because they functionally address different research questions [52]. Finally, we adjusted for numerous potential confounders, including hypokalemia, to minimize bias; patients with no HK excursions >5.0 mEq/L during the exposure period should not be considered ‘well-controlled’ if serum K levels are too low due to malnutrition, so we adjusted for ‘valley K’ to account for the inclusion of hypokalemic patients in the reference group. While it was important to adjust models for valley K, we chose to focus the article on HK and not hypokalemia.
In summary, we observed clear associations between one or more HK excursions and mortality, hospitalizations and CV events using a novel peak K approach. An increased rate of adverse events was observed for patients with a peak serum K at a lower threshold (5.1–5.5 mEq/L) than previously reported [19–22] and considered mild according to current recommendations [18], with the implication that a greater proportion of the HD population (>50%) may be at risk. A reassessment of existing HK severity ranges is needed, as well as an exploration of new strategies for effective monitoring and management of chronic HK.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial assistance for this article.
FUNDING
This article was funded in part by AstraZeneca. Global support for the ongoing DOPPS Program is provided without restriction on publications. See https://www.dopps.org/AboutUs/Support.aspx for more information.
AUTHORS’ CONTRIBUTIONS
A.K., B.M.R., G.J., K.H. and R.P.-F. designed the study. A.K. analyzed the data and drafted the article. B.M.R., G.J., K.H., C.P.M.Q., P.D.S., K.N. and R.P.-F. revised the article. All authors approved the final version of the manuscript. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions and agrees to ensure that questions pertaining to the accuracy and integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
A.K., B.M.R and R.P.-F. are employees of Arbor Research Collaborative for Health, which administers the DOPPS. G.J., K.H. and C.P.M.Q. are employees of AstraZeneca who receive a salary and own shares. P.D.S. and K.N. have no conflicts of interest to declare. The results presented in this article have not been published previously in whole or part, except in abstract format.
DATA AVAILABILITY
The data that support the findings of this study are available from Arbor Research Collaborative for Health, but restrictions apply to the availability of these data which were used for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Arbor Research Collaborative for Health.
REFERENCES
- 1. Rossignol P, Lamiral Z, Frimat L. et al. Hyperkalaemia prevalence, recurrence and management in chronic haemodialysis: a prospective multicentre French regional registry 2-year survey. Nephrol Dial Transplant 2017; 32: 2112–2118 [DOI] [PubMed] [Google Scholar]
- 2.United States DOPPS Practice Monitor. 2019. http://www.dopps.org/dpm (8 October 2019, date last accessed)
- 3. Xu H, Ashfaq A, Karaboyas A. et al. Prevalence of hyperkalemia in DOPPS: a real-world, international cohort of hemodialysis patients. Poster MP-371. Presented at the ERA-EDTA Congress, Madrid, Spain, 3–6 June 2017. Nephrol Dial Transplant 2017; 32(Suppl 3): iii563
- 4. Hunter RW, Bailey MA.. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant 2019; 34: iii2–iii11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gasparini A, Evans M, Barany P. et al. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant 2019; 34: 1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kovesdy CP, Matsushita K, Sang Y. et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J 2018; 39: 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi HY, Ha SK.. Potassium balances in maintenance hemodialysis. Electrolyte Blood Press 2013; 11: 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palaka E, Grandy S, Darlington O. et al. Associations between serum potassium and adverse clinical outcomes: a systematic literature review. Int J Clin Pract 2020; 74: e1342. [DOI] [PubMed] [Google Scholar]
- 9. Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol 2014; 10: 653–662 [DOI] [PubMed] [Google Scholar]
- 10. Bianchi S, Aucella F, De Nicola L. et al. Management of hyperkalemia in patients with kidney disease: a position paper endorsed by the Italian Society of Nephrology. J Nephrol 2019; 32: 499–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Betts KA, Woolley JM, Mu F. et al. The cost of hyperkalemia in the United States. Kidney Int Rep 2018; 3: 385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noori N, Kalantar-Zadeh K, Kovesdy CP. et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis 2010; 56: 338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pun PH, Middleton JP.. Dialysate potassium, dialysate magnesium, and hemodialysis risk. J Am Soc Nephrol 2017; 28: 3441–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long B, Warix JR, Koyfman A.. Controversies in management of hyperkalemia. J Emerg Med 2018; 55: 192–205 [DOI] [PubMed] [Google Scholar]
- 15. Hammer F, Malzahn U, Donhauser J. et al. A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int 2019; 95: 983–991 [DOI] [PubMed] [Google Scholar]
- 16. Movilli E, Camerini C, Gaggia P. et al. Use of renin-angiotensin system blockers increases serum potassium in anuric hemodialysis patients. Am J Nephrol 2018; 48: 79–86 [DOI] [PubMed] [Google Scholar]
- 17. Bandak G, Sang Y, Gasparini A. et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) project. J Am Heart Assoc 2017; 6: e005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clase CM, Carrero J-J, Ellison DH. et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2020; 97: 42–61 [DOI] [PubMed] [Google Scholar]
- 19. Brunelli SM, Du Mond C, Oestreicher N. et al. Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis 2017; 70: 21–29 [DOI] [PubMed] [Google Scholar]
- 20. Karaboyas A, Zee J, Brunelli SM. et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2017; 69: 266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kovesdy CP, Regidor DL, Mehrotra R. et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2007; 2: 999–1007 [DOI] [PubMed] [Google Scholar]
- 22. Kim GH. Pharmacologic treatment of chronic hyperkalemia in patients with chronic kidney disease. Electrolyte Blood Press 2019; 17: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friedman PA, Scott CG, Bailey K. et al. Errors of classification with potassium blood testing: the variability and repeatability of critical clinical tests. Mayo Clin Proc 2018; 93: 566–572 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt ST, Ditting T, Deutsch B. et al. Circadian rhythm and day to day variability of serum potassium concentration: a pilot study. J Nephrol 2015; 28: 165–172 [DOI] [PubMed] [Google Scholar]
- 25. Danese MD, Belozeroff V, Smirnakis K. et al. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 1423–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tangri N, Wagner M, Griffith JL. et al. Effect of bone mineral guideline target achievement on mortality in incident dialysis patients: an analysis of the United Kingdom Renal Registry. Am J Kidney Dis 2011; 57: 415–421 [DOI] [PubMed] [Google Scholar]
- 27. Young EW, Goodkin DA, Mapes DL. et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int 2000; 57(Suppl 74): S74–S81 [DOI] [PubMed] [Google Scholar]
- 28. Pisoni RL, Gillespie BW, Dickinson DM. et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 2004; 44: 7–15 [DOI] [PubMed] [Google Scholar]
- 29. Linde C, Qin L, Bakhai A. et al. Serum potassium and clinical outcomes in heart failure patients: results of risk calculations in 21 334 patients in the UK. ESC Heart Fail 2019; 6: 280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raghunathan TE, Solenberger PW, Van Hoewyk J.. IVEware: Imputation and Variance Estimation Software: User Guide. Ann Arbor, MI: Institute for Social Research, University of Michigan, 2002. [Google Scholar]
- 31. Little RJA, Rubin DB.. Statistical Analysis with Missing Data. New York: John Wiley & Sons, 1987. [Google Scholar]
- 32. Sterns RH, Grieff M, Bernstein PL.. Treatment of hyperkalemia: something old, something new. Kidney Int 2016; 89: 546–554 [DOI] [PubMed] [Google Scholar]
- 33. Watson M, Abbott KC, Yuan CM.. Damned if you do, damned if you don't: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol 2010; 5: 1723–1726 [DOI] [PubMed] [Google Scholar]
- 34. Balamuthusamy S, Srinivasan L, Verma M. et al. Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: a meta-analysis. Am Heart J 2008; 155: 791–805 [DOI] [PubMed] [Google Scholar]
- 35. Dolson GM, Ellis KJ, Bernardo MV. et al. Acute decreases in serum potassium augment blood pressure. Am J Kidney Dis 1995; 26: 321–326 [DOI] [PubMed] [Google Scholar]
- 36. Blumberg A, Roser H, Zehnder C. et al. Plasma potassium in patients with terminal renal failure during and after haemodialysis: relationship with dialytic potassium removal and total body potassium. Nephrol Dial Transplant 1997; 12: 1629–1634 [DOI] [PubMed] [Google Scholar]
- 37. Jadoul M, Karaboyas A, Goodkin DA. et al. Potassium-binding resins: associations with serum chemistries and interdialytic weight gain in hemodialysis patients. Am J Nephrol 2014; 39: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J, Lv MM, Zach O. et al. Calcium-polystyrene sulfonate decreases inter-dialytic hyperkalemia in patients undergoing maintenance hemodialysis: a prospective, randomized, crossover study. Ther Apher Dial 2018; 22: 609–616 [DOI] [PubMed] [Google Scholar]
- 39. Harel Z, Harel S, Shah PS. et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med 2013; 126: 264.e9–264.e24. [DOI] [PubMed] [Google Scholar]
- 40. Laureati P, Xu Y, Trevisan M.. et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant 2019; 35: 1518–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noel JA, Bota SE, Petrcich W. et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med 2019; 179: 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chauveau P, Aparicio M, Bellizzi V. et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant 2018; 33: 725–735 [DOI] [PubMed] [Google Scholar]
- 43. Noori N, Kalantar-Zadeh K, Kovesdy CP. et al. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palmer BF, Colbert G, Clegg DJ.. Potassium homeostasis, chronic kidney disease, and the plant-enriched diets. Kidney360 2020; 1: 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fried L, Kovesdy CP, Palmer BF.. New options for the management of chronic hyperkalemia. Kidney Int Suppl (2011) 2017; 7: 164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosano GMC, Spoletini I, Agewall S.. Pharmacology of new treatments for hyperkalaemia: patiromer and sodium zirconium cyclosilicate. Eur Heart J Suppl 2019; 21: A28–A33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nassif ME, Kosiborod M.. New frontiers for management of hyperkalaemia: the emergence of novel agents. Eur Heart J Suppl 2019; 21: A34–A40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fishbane S, Ford M, Fukagawa M. et al. A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J Am Soc Nephrol 2019; 30: 1723–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bakris GL, Pitt B, Weir MR. et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 2015; 314: 151–161 [DOI] [PubMed] [Google Scholar]
- 50. Agarwal R, Rossignol P, Romero A. et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019; 394: 1540–1550 [DOI] [PubMed] [Google Scholar]
- 51. Kovesdy CP, Rowan CG, Conrad A. et al. Real-world evaluation of patiromer for the treatment of hyperkalemia in hemodialysis patients. Kidney Int Rep 2019; 4: 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dekker FW, de Mutsert R, van Dijk PC. et al. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int 2008; 74: 994–997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Arbor Research Collaborative for Health, but restrictions apply to the availability of these data which were used for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Arbor Research Collaborative for Health.