Abstract
Background
Parquetina nigrescens is among the evergreen plants native to West Africa. It is used in the management of various ailments including anemia, fever, asthma and diabetes. This study evaluated the antidiabetic and antihyperlipidemic effect of Parquetina nigrescens in streptozotocin–nicotinamide-induced type 2 diabetic rats.
Methods
Type 2 diabetes mellitus was induced in overnight fasted rats with a single intraperitoneal injection of streptozotocin (60 mg/kg), followed by the administration of nicotinamide (120 mg/kg) after an interval of 15 min. Diabetic rats were orally administered with; 200, 400 and 800 mg/kg of aqueous extract of Parquetina nigrescens (AEPN), metformin (180 mg/kg) and glibenclamide (1 mg/kg) for two weeks. The effect of treatments on fasting blood glucose, serum insulin, leptin, adiponectin, homa-ir, lipid profile, body weight, pancreatic antioxidants parameters, hepatic glycogen content, glucose-6-phosphate activity, α-amylase inhibition, α-glucosidase inhibition, lipase inhibition and histology of the organs were evaluated.
Results
Data from this study showed that treatment with AEPN produced a significant reduction (p < 0.05) in fasting blood glucose, glucose-6-phosphatase activity, serum lipase, total triglyceride, total cholesterol, low-density lipoproteins, very low-density lipoprotein, atherogenic index, coronary risk index, pancreatic α-amylase, α-glucosidase and lipase activities. Treatment with AEPN also produced a significant (p < 0.05) increase in; glucose tolerance, glycogen content, leptin, adiponectin and pancreatic antioxidants (glutathione, superoxide dismutase, catalase and high-density lipoproteins). The histology of the organ showed regeneration of the pancreatic tissue after treatment with AEPN.
Conclusions
This study showed that AEPN exhibited antidiabetic and antihyperlipidemic activity in streptozotocin–nicotinamide-induced type 2 diabetic rats.
Keywords: Antioxidant, Diabetes, Parquetina nigrescens, Streptozotocin, Histology
Antioxidant; Diabetes; Parquetina nigrescens; Streptozotocin; Histology.
1. Introduction
Diabetes mellitus (DM) has been regarded as a fast-growing epidemic, with over 425 million people estimated to be affected in 2017 and this has been proposed to increase by 35.35% before 2045 [1]. Diabetes is a metabolic disorder resulting from dysfunction in carbohydrate, protein and fatty acid metabolism, leading to persistent hyperglycemia [2]. Type 2 diabetes mellitus (T2DM) is the most prevalent type of diabetes mellitus, accounting for more than 90% of all the morbidity and mortality associated with DM [1]. This is most times characterized by pancreatic β-cell dysfunction and resistance to insulin action, with resultant elevation of blood glucose [3]. The availability of antidiabetic agents such as insulin, biguanides, sulphonylureas, α-glucosidase inhibitors and other groups of antidiabetic drugs has brought some relief to diabetic patients. However, some of these drugs are expensive and not totally accessible especially in the developing countries and are accompanied by side effects including hypoglycemia, dizziness, lactic acidosis and among others [4, 5]. These concerns raised a strong drive for the development of effective ethnomedicines because they are believed to be cheap, more accessible to the diabetic patients in developing countries and are considered to be safe [6].
Parquetina nigrescens belongs to a family called Periplocaceae, it is a perennial plant with twining stems and a base tapering 10–15cm long and 6–8cm broad. Common names for this plant include; Ogbo in Yoruba, kwankwanin in Hausa and Mgbidimgbe in Igbo, Nigeria [7]. The plant is used locally to treat fever and pains, menstrual disorders, helminthiasis, diabetes, rickets, diarrhea, wounds and sexual dysfunction [8, 9, 10]. Various parts of the plant have been documented to be safe [7, 11, 12] and to have antioxidant, erythrocyte membrane stabilizing, antilipogenic, antiulcer, analgesic, anti-inflammatory and antipyretic activities [9, 13, 14, 15]. The plant has also been reported to ameliorate alloxan-induced type 1 diabetes mellitus (T1DM) in rats [16].
Only 10% of people affected with diabetes mellitus have type 1 with 90% of individual with type-2 diabetes mellitus (T2DM). Natural products have over the years been a dependable and inexhaustible source of natural substances for the treatment of various diseases. For cultural and economic reasons, a number of African people use traditional medicine in conjunction with orthodox medicine for the treatment of various diseased conditions, including diabetes mellitus [17]. The World Health Organization has recommended the evaluation of the potential beneficial effects of ethnomedicines [18]. This study, therefore evaluated the antidiabetic and antihyperlipidemic effect of the aqueous extract of parquetina nigrescens (AEPN) in streptozotocin–nicotinamide (STZ-NIC) induced type 2 diabetic rats.
2. Materials and methods
2.1. Drugs and assay kits
Sodium citrate (Guangzou jhd Chemical Reagents Co., Ltd, China), citric acid, (Guangzou jhd Chemical Reagents Co., Ltd, China), streptozotocin (Sigma-Aldrich, Germany), nicotinamide (Qualigens Fine Chemicals, GSK India), D-glucose (Loba Chemie Pvt Ltd, Mumbai), sucrose (Loba Chemie Pvt Ltd, Mumbai), metformin (Bristol-Myers Squibb, New York, United State), glibenclamide (Sanofi Aventis, Paris, France), orlistat (GlaxoSmithKline), glucometer and strip (Roche Diagnostics, Rotkreuz, Switzerland). Triglyceride, cholesterol, high-density lipoprotein, glutathione, catalase, superoxide dismutase and 2-ThioBarbituric Acid Reactive Substances (TBARS) kits were products of Fortress Diagnostic, United Kingdom. Insulin and leptin kits were products of Crystal Chrom, Drowner, IL, United State, adiponectin kit (enzyme immunoassay (EIA)) is a product of Ray Biotech, Norcross, GA, USA, and serum lipase colorimetric kit is a product of BioAssay Systems, Hayward, CA, USA.
2.2. Plant collection and extraction
Parquetina nigrescens was collected at Aderoju Area, Ilorin, Kwara State, Nigeria. The plant was identified at the Department of Plant Biology, University of Ilorin, a sample was deposited and a voucher number (UILH/01/019/876) was given. The fresh whole plant of Parquetina nigrescens was washed, cleaned and dried in the shade at room temperature for two weeks. The dried plant was reduced to the powdered form by a milling machine. Two hundred grams (200 g) of powdered plant was soaked in two litres (2L) of solvent (distilled water) for 72 h with intermittent agitation. After 72 h, the supernatant was decanted, allowed to settle and filtered with a Whatman paper (No 1). The filtrate was evaporated to dryness on a water bath at a temperature of 40 °C [19]. The concentrated extract was named aqueous extract of Parquetina nigrescens (AEPN). The percentage yield of the extract was calculated using Eq. (1).
| (1) |
2.3. Experimental animals
Male Wistar rats (150–160 g) were obtained from the Department of Biochemistry, University of Ilorin, the animals were housed in standard plastic cages in the Animal House of the Department of Biochemistry, University of Ilorin. They were maintained under standard conditions of light and darkness (12/12 h) with free access to food and water ad libitum.
2.4. Experimental procedure
The experiment was performed in accordance with the procedures laid down by the University of Ilorin Ethics Committee for care and use of laboratory animals and in accordance with the principles of laboratory animal care by the National Institute of Health (NIH publication No. 85-23, which was revised in 1985). The rats were acclimatized for one week before the start of the experiment and Ethical clearance was gotten from the Ethical Review Committee of the University of Ilorin, Ilorin, Nigeria. The experiment was given an approval no: UERC/ASN/2020/2001.
2.5. Induction of T2DM
T2DM was induced in overnight fasted rats (18 h) by a single intraperitoneal injection of streptozotocin (60 mg/kg) and after 15 min, the animals were given nicotinamide (120 mg/kg) intraperitoneally. Streptozotocin was dissolved in citrate buffer (pH 4.5) while nicotinamide was dissolved in normal saline. Hyperglycemia was confirmed by elevated fasting blood glucose, determined at 72 h and on day 7 after injection. The rats with FBG ≥200 mg/dL were considered diabetic, regrouped and used for this study [20].
2.6. Oral glucose tolerance test (OGTT)
The oral glucose tolerance test was carried out in overnight fasted (18 h) rats and the animals were divided into six groups (n = 5). The animals were administered with normal saline (1 mL/kg), AEPN (200, 400, and 800 mg/kg), metformin (180 mg/kg) and glibenclamide (1 mg/kg) respectively via oral route. The doses selected were based on the result of our pilot study. Thirty minutes after treatment, animals were orally fed with glucose (2 g/kg) and blood was withdrawn from the retro-orbital sinus under ether inhalation at 0, 30, 60, 90,120 and 180 min post treatment [21]. The fasting blood glucose was estimated using glucose oxidase–peroxidase reactive strips (Roche Diagnostics, USA).
2.7. Experimental design for multiple-dose study
Diabetic rats were divided into seven groups of (n = 7) animals; group I consist of normoglycemic animals and received normal saline (1 mL/kg), group II to VII consist of diabetic animals and received normal saline (1 mL/kg), AEPN (200 mg/kg), AEPN (400 mg/kg), AEPN (800 mg/kg), metformin (180 mg/kg), and glibenclamide (1 mg/kg) respectively. Treatments were given orally via oral cannula for 14 days. Fasting blood glucose (FBG) was taken 24 h before starting the treatment and was termed day 0 then on day 1 (24 h after treatment), 5, 10, and 14 using an Accu-check glucometer and glucose oxidase–peroxidase reactive strips [22].
2.7.1. Collection of blood and organs
At the end of the experiment, rats were anesthetized using 25 mL of diethyl ether in an airtight glass chamber; blood was collected via cardiac puncture and was placed into sample bottles. Kidney, pancreas and liver were harvested from the rats and weighed appropriately before being stored in 10% formalin.
2.7.2. Evaluation of serum insulin, leptin, adiponectin and lipase levels
Blood samples were collected and centrifuged at 3000 rpm for 15 min, after which the serum was collected using a Pasteur pipette into clean sample bottles. Serum insulin and leptin levels were assayed using enzyme-linked immunosorbent assay (ELISA). Adiponectin level was quantified using an enzyme immunoassay, serum lipase activity was quantified using a colorimetric kit. Each assay was followed through using the manufacturer's protocol and expressed as standard units.
2.7.3. Homeostasis model assessment of insulin resistance (HOMA-IR)
HOMA-IR was calculated using Eq. (2) [23]:
| (2) |
2.7.4. Evaluation of serum lipid, atherogenic index (AI) and coronary risk index (CRI)
Serum total cholesterol (TC), triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) levels were determined by enzymatic methods using commercial assay kits. The experiment was performed in accordance with the manufacturer's protocol. Serum low-density lipoprotein cholesterol (LDL-C) was calculated using Friedewald's Eq. (3) [24]:
| (3) |
Very low density lipoprotein cholesterol (VLDL-C), AI and CRI were calculated using Eqs. (4), (5), and (6) respectively [25, 26, 27].
| (4) |
| (5) |
| (6) |
2.7.5. Evaluation of hepatic glucose-6-phosphatase activity and glycogen content
Glucose-6-phosphatase (Glc-6-Pase) activity was determined following Bagniski method [27], samples and control were prepared in similar manner except that aliquots of the microsomal suspensions were pipetted after adding ascorbic acid-trichloroacetic acid, and optical density was read at a wavelength of 700 nm using a spectrophotometer (Biotek-Power Wave). The hepatic glycogen content was evaluated using Vander-Vries method [28], optical density was read at a wavelength of 700 nm using a spectrophotometer (Biotek-Power Wave).
2.7.6. Evaluation of pancreatic markers of oxidative stress
Measurements of pancreatic oxidative stress; thiobarbituric acid reactive substances (TBARS), glutathione (GSH), catalase (CAT) and superoxide dismutase (SOD) activities were carried out using commercial kits. The enzymes; MDA, GSH, CAT concentrations were measured following the manufacturer's protocols.
2.7.7. Evaluation of body weight and relative organ weight
The animals were weighed on the first and last day of the experiment and the differences were noted. After the experiment, animals were sacrificed, organs were removed, weighed, and the relative organ weight (ROW) was calculated using Eq. (7);
| (7) |
2.8. In-vitro mechanistic study
Alpha-amylase inhibition (A1), alpha-glucosidase inhibition (GI), and lipase inhibition (LI) assays were carried out following standard methods of Odeyemi [29], Sancheti and Leo [30], Lewis and Liu [31] with slight modifications with regards to the concentrations used. Biotek-Power Wave XS spectrophotometer was used for the analysis. AEPN (1, 2, 3, 4, and 5) represent 50, 100, 150, 200, and 250 μg/mL respectively. The concentrations of the acarbose and orlistat used were 250 and 100 μg/mL respectively. The percentage inhibitions were calculated using Eqs. (8), (9), and (10) respectively [29, 30, 31]:
| (8) |
| (9) |
| (10) |
2.9. Histology
Liver, kidney and pancreas were fixed in 10% neutral buffered formalin and then dehydrated by successively passing through a gradient of mixtures of ethyl alcohol and water. The samples were rinsed with xylene and embedded in paraffin. Organs sections (5 μm thickness) were cut, stained (hematoxylin and eosin dye) and examined under light microscope.
2.10. Data collection and analysis
Data were collected from the different methods used above and analyzed using GraphPad Prism version 8.03 for Windows (GraphPad Software, San Diego, CA, USA). The results were expressed as mean ± SEM and a comparison of mean values between different groups was performed by one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison tests. Value of p < 0.05 was considered statistically significant.
3. Results
3.1. Percentage yield of the extract
The percentage yield of the extract was 6.12%
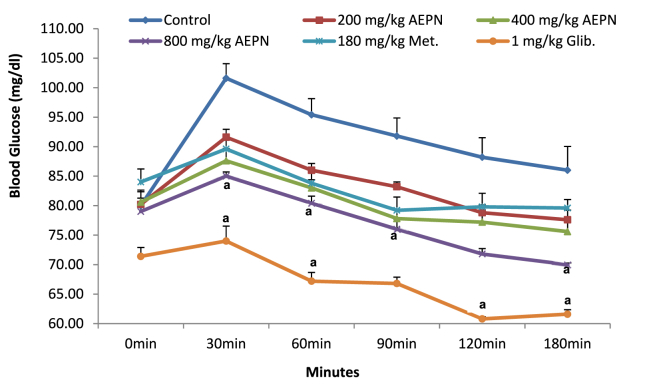
3.2. Effect of AEPN on oral glucose tolerance test
Thirty minutes after oral glucose load, there was a significant increase (p < 0.05) in glucose level for the control group (21.26 %) as compared to 12.44, 7.99, 7.06, 7.25, and 3.51 observed in AEPN (200, 400, and 800 mg/kg), metformin and glibenclamide respectively when compared to their basal values. Administration of AEPN (800 mg/kg) and glibenclamide significantly increased (p < 0.05) glucose tolerance ability of the rats and prevent glucose surge within the period of 30–180 min as compared to control (Figure 1).
Figure 1.
Effect of AEPN on oral glucose tolerance test. n = 5, Data are expressed as mean ± S.E.M. ap < 0.05 is significant difference in comparison with control, Met. is metformin and Glib. is glibenclamide.
3.3. Multiple-dose study
Administration of the extract (800 mg/kg) produced a significant reduction (p < 0.05) in glucose level with a percentage reduction of 27.01 after 24 h of treatment. On day 5, AEPN (400 and 800 mg/kg) and glibenclamide-treated groups had a significant reduction (p < 0.05) in fasting blood glucose and the percentage reduction were 37.37, 45.97 and 36.62 respectively. On the 10th day of treatment, AEPN (400 and 800 mg/kg), metformin and glibenclamide groups produced a significant reduction (p < 0.05) in fasting blood glucose with percentage reduction of 43.20, 54.50, 42.58 and 46.47 respectively. On the last day of experiment (14th day), AEPN (200, 400 and 800 mg/kg), metformin and glibenclamide-treated groups had a significant reduction (p < 0.05) in FBG with percentage reduction of 37.05, 47.09, 60.18, 49.28 and 52.58 respectively (Table 1).
Table 1.
Effect of AEPN on fasting blood glucose of diabetic animals.
| Groups | Fasting blood glucose (mg/dL) |
||||
|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 5 | Day 10 | Day 14 | |
| I | 81.34 ± 4.23 | 82.40 ± 2.34 | 81.20 ± 2.50 | 80.40 ± 1.50 | 82.60 ± 1.43 |
| II | 256.6 ± 9.21 | 259.40 ± 8.12b | 253.20 ± 7.90b | 251.00 ± 4.91b | 257.00 ± 4.90b |
| III | 224.5 ± 7.39 | 232.80 ± 9.34 | 188.80 ± 4.01 | 171.00 ± 4.47 | 141.40 ± 2.04a |
| IV | 206.3 ± 8.45 | 167.0 ± 11.38 | 129.00 ± 2.55a | 117.60 ± 1.63a | 109.60 ± 3.09a |
| V | 211.1 ± 6.78 | 154.00 ± 4.15a | 114.40 ± 2.99a | 96.00 ± 1.55a | 84.20 ± 1.88a |
| VI | 209.0 ± 9.54 | 174.20 ± 6.78 | 151.80 ± 3.12 | 120.80 ± 3.26a | 116.80 ± 2.31a |
| VII | 213.5 ± 11.45 | 182.40 ± 10.1 | 135.20 ± 6.24a | 114.60 ± 1.86a | 101.20 ± 1.39a |
n = 7, Data are expressed as mean ± S.E.M, ap < 0.05 and bp ≤ 0.05 are statistical significant differences in comparison with diabetic control and normal control group respectively. Group I-VII are normal control, diabetic control, AEPN (200 mg/kg), AEPN (400 mg/kg), AEPN (800 mg/kg), metformin (180 mg/kg), and glibenclamide (1 mg/kg) group respectively.
3.4. Effect of AEPN on serum insulin, leptin, adiponectin, and homa-ir, and lipase
Induction of T2DM produced a significant increase (p < 0.05) in serum insulin, homa-ir, lipase and a decrease in leptin and adiponectin levels when compared to normal control. Administration of the extract (800 mg/kg) and glibenclamide significantly reduced (p < 0.05) serum insulin by 44.60 and 41.70 % as compared to diabetic control (Table 2). Treatment with AEPN (800 mg/kg) produced a significant increase in serum leptin and the percentage increase was found to be 45.92. Adiponectin concentration was significantly increased (p < 0.05) in groups treated with AEPN (400 and 800 mg/kg), metformin and glibenclamide and the percentage increase were found to be 5.51, 5.50, 5.04 and 5.40 respectively. Administration of AEPN (400 and 800 mg/kg), metformin and glibenclamide significantly decreased (p < 0.05) the homa-ir (76.07, 84.79, 78.79 and 77.76) and lipase levels (43.64, 56.55, 43.32 and 44.27) when compared with diabetic untreated group (Table 2).
Table 2.
Effect of AEPN on serum insulin, leptin, adiponectin, and homa-ir, and lipase.
| Groups | Insulin (μiu/mL) | Leptin (ng/mL) | Adiponectin (ng/mL) | Homa-ir | Lipase (u/L) |
|---|---|---|---|---|---|
| I | 30.00 ± 1.61 | 1.30 ± 0.05 | 5987.38 ± 11.38 | 6.04 ± 0.26 | 503.95 ± 2.63 |
| II | 68.60 ± 1.44b | 0.53 ± 0.02b | 5665.82 ± 20.05b | 44.93 ± 1.50b | 1588.53 ± 9.76b |
| III | 52.00 ± 1.58b | 0.72 ± 0.02b | 5827.36 ± 18.26 | 23.12 ± 0.74b | 1110.02 ± 2.36b |
| IV | 46.80 ± 1.50 | 0.83 ± 0.03 | 5995.28 ± 18.28a | 10.75 ± 0.52a | 895.88 ± 31.57a |
| V | 38.00 ± 1.14a | 0.98 ± 0.01a | 5996.13 ± 12.67a | 6.83 ± 0.31a | 690.32 ± 5.51a |
| VI | 42.40 ± 1.21 | 0.78 ± 0.03b | 5967.76 ± 24.54a | 9.53 ± 0.66a | 900.60 ± 2.88a |
| VII | 40.00 ± 0.89a | 0.81 ± 0.05 | 5991.84 ± 36.32`a | 9.99 ± 0.23a | 885.36 ± 9.82a |
n = 7, Data are expressed as mean ± S.E.M, ap < 0.05 and bp ≤ 0.05 are statistical significant differences in comparison with diabetic control and normal control group respectively. Group I-VII are normal control, diabetic control, AEPN (200 mg/kg), AEPN (400 mg/kg), AEPN (800 mg/kg), metformin (180 mg/kg) and glibenclamide (1 mg/kg) group respectively.
3.5. Effect of extract on lipid profile
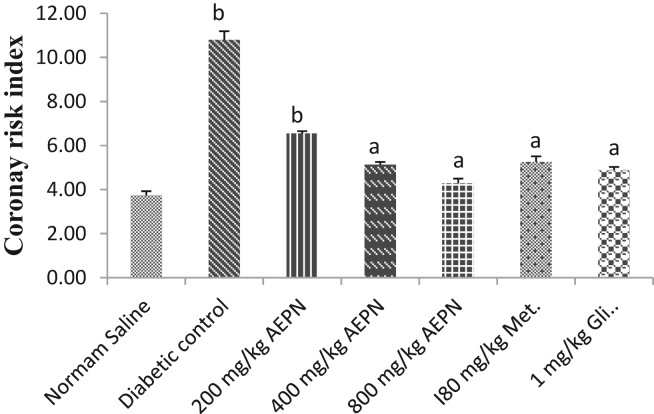
The diabetic untreated group had a significant increase (p < 0.05) in TC, LDL-C and a decrease in HDL-C in comparison with the normal control group. Administration of AEPN (800 mg/kg), metformin and glibenclamide significantly decreased (p < 0.05) the TG (37.67, 41.94 and 39.45) and VLDC-C (37.66, 41.94 and 39.47) as presented in Table 3. Treatment with AEPN (800 mg/kg) and glibenclamide significantly decreased (p < 0.05) TC values by 37.17 and 40.31 %. The HDL-C value was also significantly increased (p < 0.05) by 41.37 and 39.28 in groups treated with AEPN (400 and 800 mg/kg). Administration of AEPN (800 mg/kg), metformin and glibenclamide significantly reduced (p < 0.05) LDL-C value by 49.6, 39.56 and 49.06 % respectively (Table 3). The atherogenic (AI) and coronary risk indexes (CRI) were significantly increased (p < 0.05) following the induction of T2DM. Treatment with AEPN (400 and 800 mg/kg) and metformin lowered atherogenic index dose-dependently and the percentage reduction were found to be 39.79, 41.84 and 38.77 % respectively (Supplementary Figure 1). Treatment with AEPN (200, 400 and 800 mg/kg), metformin and glibenclamide significantly lowered CRI value by 57.14, 60.24, 51.34, 71.4 and 54.77 % respectively (Figure 2).
Table 3.
Effect of AEPN on lipid profile.
| Groups | TG (mg/dL) | TC (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | VLDL-C (mg/dL) |
|---|---|---|---|---|---|
| I | 112.66 ± 1.66 | 127.98 ± 1.68 | 34.564 ± 1.46 | 70.884 ± 2.54 | 22.53 ± 0.33 |
| II | 170.34 ± 1.65 | 191.72 ± 2.65b | 17.86 ± 0.61b | 139.79 ± 2.62b | 34.07 ± 0.33 |
| III | 132.60 ± 1.79 | 169.10 ± 1.15 | 25.88 ± 0.45 | 116.70 ± 1.13 | 26.52 ± 0.36 |
| IV | 113.70 ± 3.02 | 149.68 ± 1.24 | 29.24 ± 0.83a | 97.70 ± 0.94 | 22.74 ± 0.60 |
| V | 106.18 ± 2.20a | 120.52 ± 0.70a | 28.38 ± 1.32a | 70.904 ± 1.50a | 21.24 ± 0.44a |
| VI | 98.90 ± 1.43a | 128.88 ± 1.75 | 24.76 ± 0.97 | 84.34 ± 2.76a | 19.78 ± 0.29a |
| VII | 103.08 ± 3.72a | 114.84 ± 1.86a | 23.62 ± 0.96 | 70.60 ± 0.78a | 20.62 ± 0.74a |
n = 7, Data are expressed as mean ± S.E.M, ap < 0.05 and bp ≤ 0.05 are statistical significant differences in comparison with diabetic control and normal control group respectively. Group I-VII are normal control, diabetic control, AEPN (200 mg/kg), AEPN (400 mg/kg), AEPN (800 mg/kg), metformin (180 mg/kg), and glibenclamide (1 mg/kg) group respectively.
Figure 2.
Effect of AEPN on serum coronary risk index. n = 7, Data are expressed as mean ± S.E.M, ap < 0.05 and bp ≤ 0.05 are statistical significant differences in comparison with diabetic control and normal control group respectively.
3.6. Effect of AEPN on hepatic glycogen content and Glc- 6-pase activity
The results obtained showed a significant reduction (p < 0.05) in the liver glycogen and an increase in hepatic Glc-6-Pase activity in the diabetic untreated group as compared to the normal control. Administration of AEPN (800 mg/kg), metformin (180 mg/kg) and glibenclamide (1 mg/kg) significantly increased (p < 0.05) the glycogen level by 43.11, 40.32 and 40.57 % (Supplementary Figure 2). Treatment with AEPN (800 mg/kg), metformin and glibenclamide for 14 days significantly lowered (p < 0.05) Glc- 6-Pase activity by 47.99, 48.66 and 44.63 % in comparison with diabetic control group (Supplementary Figure 3).
3.7. Effect of AEPN on pancreatic markers of oxidative stress
Induction of T2DM produced a significant increase in TBARS and a significant decrease (p < 0.05) in GSH, SOD and CAT levels when compared to the normal control group (Table 4). Administration of AEPN (400 and 800 mg/kg) and metformin significant decreased (p < 0.05) the elevated TBARS by 44.73, 48.86 and 43.66 % respectively (Table 4). Groups treated with AEPN (400 and 800 mg/kg), metformin and glibenclamide produced a significant (p < 0.05) increase in GSH value by 59.90, 62.04, 56.99 and 56.58 % respectively. Treatment with AEPN (400 and 800 mg/kg) and glibenclamide increased the SOD value by 48.71, 58.89 and 45.78% in comparison with diabetic control (Table 4). There was a significant increase in CAT level for the group treated with AEPN (800 mg/kg) and the percentage increase was 43.10 as compared to diabetic control (Table 4).
Table 4.
Effect of AEPN on pancreatic markers of oxidative stress.
| Groups | TBARS |
GSH |
SOD |
CAT |
|---|---|---|---|---|
| (nmole MDA/mg protein) | (nmole/mg protein) | (units/mg protein) | (nmole H2O2/min /mg protein) |
|
| I | 15.15 ± 0.14 | 12.06 ± 0.62 | 18.15 ± 0.88 | 50.33 ± 1.14 |
| II | 95.75 ± 1.91b | 3.20 ± 0.22b | 6.17 ± 0.55b | 18.12 ± 1.34b |
| III | 63.46 ± 1.68b | 5.17 ± 0.19b | 10.57 ± 0.54b | 23.74 ± 1.41b |
| IV | 52.92 ± 1.74a | 7.98 ± 0.19a | 12.03 ± 0.96a | 28.36 ± 1.11b |
| V | 48.97 ± 2.05a | 8.43 ± 0.66a | 15.01 ± 0.48a | 31.85 ± 1.49a |
| VI | 53.95 ± 2.77a | 7.44 ± 0.38a | 10.17 ± 0.79b | 27.74 ± 1.04 |
| VII | 58.42 ± 1.28b | 7.37 ± 0.49a | 11.38 ± 1.35a | 26.09 ± 1.24 |
n = 7, Data are expressed as mean ± S.E.M, ap < 0.05 and bp ≤ 0.05 are statistical significant differences in comparison with diabetic control and normal control group respectively. Group I-VII are normal control, diabetic control, AEPN (200 mg/kg), AEPN (400 mg/kg), AEPN (800 mg/kg), metformin (180 mg/kg) and glibenclamide (1 mg/kg) group respectively.
3.8. Effect of AEPN on α-amylase, α-glucosidase, and lipase enzyme
The highest test dose of the extract (250 μg/mL) produced a significant inhibition (p < 0.05) of α-amylase enzyme (52.83 %) in a manner similar to acarbose (100 μg/mL) with 54.67 % inhibition. The same concentration of AEPN and acarbose inhibited α-glucosidase enzyme by 48.56 and 87.51 respectively. Both the extract (250 μg/mL) and orlistat (50 μg/mL) produced a significant (p < 0.05) inhibition of lipase enzyme with percentage inhibition of 54.47 and 83.60 respectively (Table 5).
Table 5.
Effect of AEPN on α-amylase, α-glucosidase, and lipase enzyme.
| Plant extract | Percentage inhibition (%) |
||
|---|---|---|---|
| Pancreatic α-amylase | Pancreatic α-glucosidase | Pancreatic lipase | |
| AEPN1 | 29.09 ± 0.21b | 10.79 ± 2.61b | 10.04 ± 0.27b |
| AEPN2 | 34.96 ± 0.35 | 14.03 ± 0.94b | 14.13 ± 0.42b |
| AEPN3 | 37.17 ± 0.82 | 27.89 ± 0.35b | 26.11 ± 2.85b |
| AEPN4 | 40.04 ± 0.27 | 37.92 ± 0.88b | 33.89 ± 1.26b |
| AEPN5 | 42.83 ± 0.51a | 48.56 ± 0.64a | 54.47 ± 1.91a |
| Acarbose | 54.67 ± 0.45a | 87.51 ± 0.27a | Not used |
| Orlistat | Not used | Not used | 83.60 ± 0.20a |
| Enzyme | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b |
n = 3, Data are expressed as mean ± S.E.M. ap≤0.05 is statistical significant difference in comparison with enzyme, bp≤0.05 is statistical significant difference in comparison with acarbose or orlistat. AEPN (1, 2, 3, 4, and 5), Acarbose and orlistat are at concentration of 50, 100, 150, 200, 250, 250 μg/mL and 100 μg/mL respectively.
3.9. Effect of AEPN on body weight, organ weight and relative organ weight
The result of this study showed a significant (p < 0.05) weight reduction in diabetic control group (15.55%) on the last day of the experiment, no significant weight variations were observed for all the treatment groups (Table 6). Diabetic control group had a significant increase in KW, RKW, LW, RLW, and a significant decrease in PW and RPW. The percentage variations in comparison with control group were found to be 28.62, 41.90, 41.34, 52.16, 52.66 and 43.92 respectively (Table 6 and Supplementary Table 1).
Table 6.
Effect of AEPN on body weight and relative organ weight.
| Groups | Body weight |
Relative organ weight (%) |
|||
|---|---|---|---|---|---|
| Initial (g) | Final (g) | RKW (%) | RLW (%) | RPW (%) | |
| I | 148.40 ± 1.29 | 157.40 ± 2.56 | 1.22 ± 0.06 | 3.88 ± 0.17 | 1.07 ± 0.04 |
| II | 151.80 ± 3.04 | 128.20 ± 1.66a | 2.10 ± 0.07b | 8.11 ± 0.50b | 0.60 ± 0.06b |
| III | 148.20 ± 2.67 | 143.40 ± 3.57 | 1.41 ± 0.06 | 4.53 ± 0.27 | 1.05 ± 0.04 |
| IV | 157.00 ± 4.70 | 151.60 ± 4.76 | 1.20 ± 0.05 | 4.90 ± 0.35 | 1.01 ± 0.06 |
| V | 159.32 ± 3.09 | 149.70 ± 5.98 | 1.15 ± 0.09 | 3.80 ± 0.38 | 1.11 ± 0.03 |
| VI | 149.40 ± 1.17 | 146.60 ± 2.69 | 1.31 ± 0.03 | 4.10 ± 0.26 | 1.09 ± 0.06 |
| VII | 152.00 ± 3.15 | 145.00 ± 3.82 | 1.31 ± 0.06 | 4.00 ± 0.20 | 1.00 ± 0.05 |
n = 7, Data are expressed as mean ± S.E.M. ap≤0.05 and bp≤0.05 are statistical significant differences in comparison with initial weight and normal control group respectively. RKW, RLW and RPW are relative kidney, liver and pancreas weight.
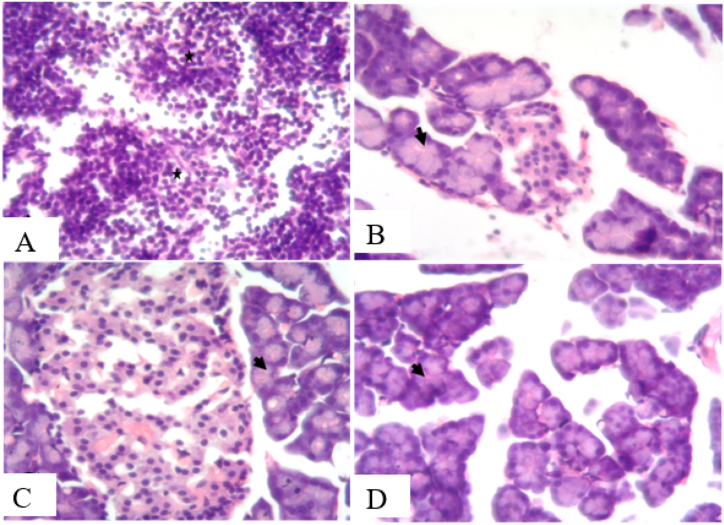
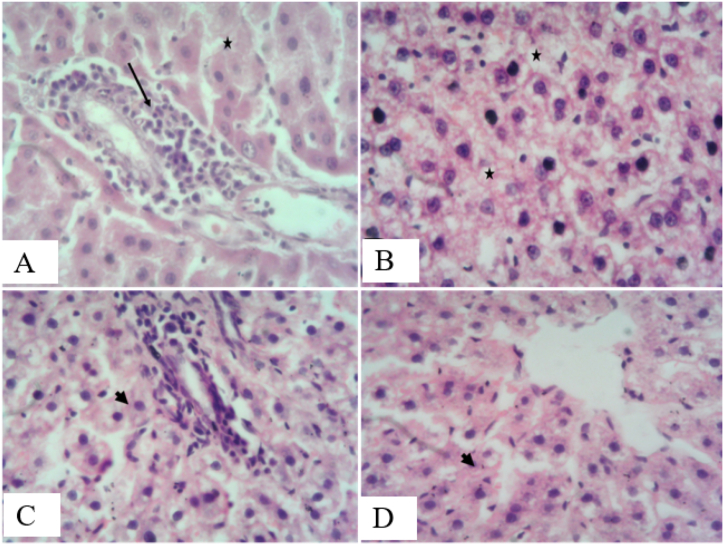
3.10. Protective effect of AEPN on organ histology
The pancreas section of the normal control had normal pancreatic islet while section of the diabetic untreated group showed destruction of the acinar architecture and islet structure (Figure 3A). The pancreas sections of the extract treated groups showed varying degrees of regeneration (Figure 3B-D), evident by the increased hyperchromasia of the acinar and islet cells nuclei and abundant cytoplasm. The pancreas sections of metformin, and glibenclamide treated groups showed little or no regeneration. Liver section of normal control group showed normal architecture whereas the diabetic untreated group showed moderate hepatocellular degeneration with focal bile duct inflammation and cellular infiltration (Figure 4A). An increased dose-dependent improvement in hepatocytes architecture were seen in the AEPN treated groups (Figure 4B-D). Liver sections of metformin, and glibenclamide were unremarkable when compared with the extract treated groups, especially with glibenclamide treated group, fatty degeneration was seen, and hemorrhage in metformin treated group. Kidney sections of normal control group showed kidney with preserved architecture, diabetic untreated group showed kidney with renal tubular necrosis and cellular infiltration (Supplementary Figure 4A). Sections of other treatment groups showed varying levels of tubular degeneration but groups treated with 200 and 400 mg/kg of AEPN showed improved renal glomerular and tubular structures (Supplementary Figure 4B–C).
Figure 3.
Effect of AEPN on histology of the pancreas. (H and E X400)
Figure 4.
Effect of AEPN on histology of the liver. (H&E X400)
A (streptozotocin induced Diabetes): section shows complete destruction of acinar architecture and islet structures (black asterisk) with presence of acinar cells, B (streptozotocin induced Diabetes and treatment with 200 mg/kg AEPN): section shows focal acinar cell necrosis with increased hyperchromasia of some acinar cell nuclei and abundant cytoplasm (black arrowhead, evidence of regeneration). C (streptozotocin induced Diabetes and treatment with 400 mg/kg AEPN): section shows normal islet of Langerhans with increase vascularization and most acinar cell nuclei and cytoplasm shows increased hyperchromasia, D (streptozotocin induced Diabetes and treatment with 800 mg/kg AEPN): section shows focal necrosis with increased hyperchromasia of many acinar cell nuclei and abundant cytoplasm.
A (streptozotocin induced Diabetes): section shows hepatocellular degeneration, necrosis (black asterisk) and focal bile duct inflammation with cellular infiltration (black arrow), B (streptozotocin induced Diabetes and treatment with 200 mg/kg AEPN): section shows diffuse hepatocytes cytoplasmic degeneration and multifocal single hepatocellular nuclear pyknosis (necrosis, black asterisk), C (streptozotocin induced Diabetes and treatment with 400 mg/kg AEPN): section shows moderate hepatocellular degeneration with regenerative hepatocytes and focal periportal cellular infiltration, D (streptozotocin induced Diabetes and treatment with 800 mg/kg AEPN): section shows presence of increased regenerative hepatocytes.
4. Discussion
Orthodox medicines are commercially available for the management of T2DM but all present with their various side effects ranging from hypoglycemia, weight gain, headache, dizziness, lactic acidosis, liver injury and cardiopathy [5]. Plants with reported antidiabetic activity include Ocimum gratissimum, Zingiber officinale and Ficus exasperata to mention a few [32, 33]. Screening of more plants with antidiabetic potentials is vital to providing accessible and affordable treatment for this scourge, especially in low income countries where the incidence of T2DM is currently on the rise. In this study, the antidiabetic and antihyperlipidemic effect of parquetina nigrescens was evaluated in STZ-NIC induced T2DM rats.
As shown by the oral glucose tolerance test, the ability of the AEPN (800 mg/kg) and glibenclamide (1 mg/kg) to prevent glucose surge within 30 min to 3 h post-oral glucose load could be attributed to a delay in carbohydrate digestion in the digestive tract caused by inhibition of α-glucosidase and α-amylase and hence prevention of postprandial hyperglycemia. Previous studies have suggested that the glucose-lowering effect of AEPN might be due to its ability to improve glucose utilization and or attenuate insulin resistance as well [34, 35]. The effect of the extract in the multiple-dose study showed its ability to attenuate persistent hyperglycemia and this is in tandem with the work of Saba et al. (2010) where an aqueous extract of P. nigrescens was found to ameliorate T1DM.
Two major adipokines; leptin and adiponectin have been reported to play important roles in the regulation of cardiovascular and metabolic homeostasis. Leptin is an adipose tissue-derived hormone which acts directly on the hypothalamus, thereby regulating food intake and energy expenditure and it has been reported to be involved in pathways that influence the risk of cardiovascular diseases from diabetes [36]. Administration of AEPN increased both leptin and adiponectin concentration, the synergistic effects of both might have caused a reduction in glucose levels as both have been implicated in glucose homeostasis [37]. Although few studies have examined the putative association between leptin and adiponectin in diabetes and their results have not been consistent. A negative association was observed from this study which corroborated the work of Matsuzawa et al. (2004) where decrease leptin and adiponectin was reported for T2DM [38].
Since homa-ir reveals the dynamics between the baseline fasting blood glucose and the responsive insulin, higher homa-ir values therefore, correlate with insulin resistance observed in diabetic animals and this was markedly reduced following the administration of AEPN to values similar to what was obtained in the normal control rats. Hence, our results suggest that administration of AEPN reversed the insulin resistance caused by STZ-NIC treatment. The elevated serum lipid profile observed in diabetic rats in this study has also been previously reported in the study published by Karigidi et al. (2019). The reversal of hypertriglyceridemia and reduction in AI and CRI in AEPN and glibenclamide treated rats may be directly attributed to improvement in insulin resistance level upon therapy. Insulin resistance in T2DM has been reported to be a risk factor for elevated lipid profile and increased risk for coronary diseases [6]. This suggests the possible role of AEPN in ameliorating T2DM and for preventing coronary events.
The effect of liver microsomal Glc-6-Pase has been reported to be increased in diabetic mellitus [39]. The reduced hepatic glycogen content and elevated glucose-6-phosphatase activity in diabetic rats were greatly reversed by AEPN. This suggests the antihyperglycemic effect of AEPN and this effect may be due to reduced hepatic glucose production and or increased sensitivity to insulin [40]. Streptozotocin induces oxidative stress in the body resulting in pancreatic injury, and by extension an increase in fasting blood glucose seen in diabetic animals [41, 42]. Antioxidants have been suggested to have a role in the alleviation of diabetes [6]. Pancreatic antioxidant markers (GSH, SOD and CAT) except TBARS were found to be reduced after 14 days in diabetic control rats. The enhanced activity of antioxidant markers in groups treated with AEPN (400 and 800 mg/kg) may be due to the presence of phytochemicals like alkaloids, phenolics and flavonoids in the plant as this plant has been documented to have antioxidant properties [43].
The highest concentration of the AEPN used for the in-vitro study significantly inhibited the pancreatic α-amylase, α-glucosidase and lipase enzyme. The mechanism of the glucose lowering effect of AEPN may be due in part to the inhibition of these enzymes by AEPN. The weight reduction recorded in the diabetic untreated groups could be correlated with toxic metabolites of streptozotocin and the protective effect of AEPN may be attributed to the presence of rich phytochemicals present in AEPN [43]. This is supported by the previous study of Kayode et al. who documented the presence of all the 20 essential amino acids in the plant [43].
The liver as the major organ saddled with metabolism is prone to toxicity either from the drugs, chemicals, extracts and or their metabolites [44]. The alterations observed in the organ weight and relative organ weight of the diabetic untreated group may be due to the abnormalities found in the histology of the liver (hepatocellular necrosis and cellular infiltration), the kidney (renal tubular necrosis and marked interstitial inflammatory cellular infiltration) and the pancreas (destruction of the pancreatic acinar architecture, focal acinar cell necrosis and increased hyperchromasia). Administration of the different doses of the extract conferred some protections to the organs through the varying degrees of regeneration of the damaged organs. Aqueous extract of Parquetina nigrescens showed a dose-dependent antidiabetic and antihyperlipidemic activity and hence can ameliorates T2DM and some of the coronary events associated with T2DM.
5. Conclusion
Data from this study justified the effectiveness of parquetina nigrescens in ameliorating persistent hyperglycemia, high lipid profile and some markers of organs dysfunction in T2DM. Hence, the folkloric use of parquetina nigrescens in the management of diabetes is valid and should be encouraged.
Declarations
Author contribution statement
Fatimoh Idowu Ojuade: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Olufunke Esan Olorundare: Conceived and designed the experiments.
Olatunde Babatunde Akanbi: Performed the experiments.
Saheed Olanrewaju Afolabi; Anoka Ayembe Njan: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the technical staff of the Department of Pharmacology and Toxicology and the Department of Biochemistry, University of Ilorin, Ilorin, Nigeria.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cho N., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138(1):271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erukainure O.L., Hafizur R.M., Kabir N., Choudhary M.I., Atolani O., Banerjee P., Preissner R., Chukwuma C.I., Muhammad A., Amonsou E.O., Islam M. Suppressive effects of clerodendrum volubile P beauv.[labiatae] methanolic extract and its fractions on type 2 diabetes and its complications. Front. Pharmacol. 2018;9:8. doi: 10.3389/fphar.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantino M.I., Molyneaux L., Limacher-Gisler F., Al-Saeed A., Luo C., Wu T., Twigg S.M., Yue D.K., Wong J. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36(12):3863–3869. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surendran S., Eswaran M.B., Vijayakumar M., Rao C.V. In vitro and in vivo hepatoprotective activity of Cissampelos pareira against carbon-tetrachloride induced hepatic damage. Indian J. Exp. Biol. 2011;49:939–945. [PubMed] [Google Scholar]
- 6.Karigidi K.O., Olaiya C.O. Antidiabetic activity of corn steep liquor extract of Curculigo pilosa and its solvent fractions in streptozotocin-induced diabetic rats. J. Tradit. Complement. Med. 2019 doi: 10.1016/j.jtcme.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imaga N.O., Gbenle G.O., Okochi V.I., Adenekan S.O., Edeoghon S.O., Kehinde M.O., Bamiro S.B., Ajiboye A., Obinna A. Antisickling and toxicological profiles of leaf and stem of Parquetina nigrescens L. J. Med. Plants Res. 2010;4(8):639–643. [Google Scholar]
- 8.Sopeyin A.O., Ajayi G.O. Pharmacognostic study of parquetina nigrescens (Afzel.) Bullock (Periplocaceae) Int. J. Pharmacogn. Phytochem. Res. 2016;8(2):321–326. [Google Scholar]
- 9.Aderibigbe O.R., Odetola A.A., Oluwole F.S., Farombi E.O., Onabanjo O.O., Jiboku O.A. Antioxidant properties of methanol extract of Parquetina nigrescens in ulcerated rats. Int. J. Trop. Med. 2011;6(2):25–29. [Google Scholar]
- 10.Kokwaro J.O. University of Nairobi press; 2009. Medicinal Plants of East Africa. [Google Scholar]
- 11.Femi-olabisi F.J., Faokunla O., Agboola A.O., Olorunyolemi I.M. Biochemical and toxicological evaluations of aqueous extract of parquetina nigrescens (Afzel.) leaves on mifepristone-induced polycystic ovarian syndrome in rats. JDDT. 2020 Apr 15;10(2-s):94–101. [Google Scholar]
- 12.Adu-Amoah L., Agyare C., Kisseih E., Ayande P.G., Mensah K.B. Vol. 1. 2014 Jan1. Toxicity assessment of Erythrophleum ivorense and Parquetina nigrescens. Toxicology report; pp. 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owoyele B.V., Oyelowo O.T., Biliaminu S.A., Alaran O.N., Alimi S.A., Saliu R.S. Hematological and biochemical studies on Parquetina nigrescens root extract in albino rats. J. Appl. Pharmaceut. Sci. 2011;1(10):176–182. [Google Scholar]
- 14.Odetola A.A., Oluwole F.S., Adeniyi B.A., Olatiregun A.M., Ikupolowo O.R., Labode O., Busari K.O., Shorinola J.A. Antimicrobial and gastrointestinal protective properties of Parquetina nigrescens (Afzel.) Bullock. J. Biol. Sci. 2006;6(4):701–705. [Google Scholar]
- 15.Owoyele B.V., Nafiu A.B., Oyewole I.A., Oyewole L.A., Soladoye A.O. Studies on the analgesic, anti-inflammatory and antipyretic effects of Parquetina nigrescens leaf extract. J. Ethnopharmacol. 2009;122(1):86–90. doi: 10.1016/j.jep.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Saba A.B., Oyagbemi A.A., Azeez O.I. Antidiabetic and haematinic effects of Parquetina nigrescens on alloxan induced type-1 diabetes and normocytic normochromic anaemia in Wistar rats. Afr. Health Sci. 2010;10(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 17.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . 2013. Traditional Medicine Strategy: 2014-2023.http://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/ Retrieved September, 2014. [Google Scholar]
- 19.Sofowora A. Spectrum Books Ltd; Ibadan, Nigeria: 1993. Medicinal Plants and Traditional Medicine in Africa; pp. 191–289. [Google Scholar]
- 20.Masiello P., Broca C., Gross R., Roye M., Manteghetti M., Hillaire-Buys D., Novelli M., Ribes G. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47(2):224–229. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- 21.Bonner-Weir S. Morphological evidence for pancreatic polarity of β-cell within islets of Langerhans. Diabetes. 1988;37(5):616–621. doi: 10.2337/diab.37.5.616. [DOI] [PubMed] [Google Scholar]
- 22.Chattopadhyay R.R., Bandyopadhyay M. Effect of Azadirachta indica leaf extract on serum lipid profile changes in normal and streptozotocin induced diabetic rats. Afr. J. Biomed. Res. 2005;8(2):101–104. [Google Scholar]
- 23.Alladi S., Radha Shanmugasundaram K. Induction of hypercholesterolemia by supplementing soy protein with acetate generating amino acids. Nutr. Rep. Int. 1989;40(5):893–900. [Google Scholar]
- 24.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(2):499–502. [PubMed] [Google Scholar]
- 25.Morita T., Oh-hashi A., Takei K., Ikai M., Kasaoka S., Kiriyama S. Cholesterol-lowering effects of soybean, potato and rice proteins depend on their low methionine contents in rats fed a cholesterol-free purified diet. J. Nutr. 1997;127(3):470–477. doi: 10.1093/jn/127.3.470. [DOI] [PubMed] [Google Scholar]
- 26.Dobiás̆ová M., Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL) Clin. Biochem. 2001;34(7):583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 27.Baginski E.S., Foà P.P., Zak B. Determination of rat liver microsomal glucose-6-phosphatase activity: study of citrate and G-6-P inhibition. Anal. Biochem. 1967;21(2):201–207. doi: 10.1016/0003-2697(67)90181-9. [DOI] [PubMed] [Google Scholar]
- 28.Vander-Vries J. Two methods for the determination of glycogen in liver. Biochem. J. 1954;57:410–416. doi: 10.1042/bj0570410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odeyemi S.W. University of Fort Hare; 2015. A Comparative Study of the in Vitro Antidiabetic Properties, Cytotoxicity and Mechanism of Action of Albuca Bracteata and Albuca Setosa Bulb Extracts. Doctoral dissertation. [Google Scholar]
- 30.Sancheti S., Sancheti S., Seo S.Y. Evaluation of antiglycosidase and anticholinesterase activities of Boehmeria nivea. Pak. J. Pharm. Sci. 2010;23(2):236–240. [PubMed] [Google Scholar]
- 31.Lewis D.R., Liu D.J. Direct measurement of lipase inhibition by orlistat using a dissolution linked in vitro assay. Clin. Pharmacol. Biopharm. 2012;16(3):387–393. doi: 10.4172/2167-065X.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguiyi J.C., Obi C.I., Gang S.S., Igweh A.C. Hypoglycaemic activity of Ocimum gratissimum in rats. Fitoterapia. 2000;71(4):444–446. doi: 10.1016/s0367-326x(00)00143-x. [DOI] [PubMed] [Google Scholar]
- 33.Ojewole J.A. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res. 2006;20(9):764–772. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- 34.Donath M.Y., Halban P.A. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47(3):581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- 35.Monnier L., Mas E., Ginet C., Michel F., Villon L., Cristol J.P., Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. J. Am. Med. Assoc. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 36.Satoh N., Naruse M., Usui T., Tagami T., Suganami T., Yamada K., Kuzuya H., Shimatsu A., Ogawa Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27(10):2488–2490. doi: 10.2337/diacare.27.10.2488. [DOI] [PubMed] [Google Scholar]
- 37.Kotani K., Sakane N., Saiga K., Kurozawa Y. Leptin: adiponectin ratio as an atherosclerotic index in patients with type 2 diabetes: relationship of the index to carotid intima–media thickness. Diabetologia. 2005;48(12):2684–2686. doi: 10.1007/s00125-005-0015-4. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzawa Y., Funahashi T., Kihara S., Shimomura I. Adiponectin and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2004;24(2):29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 39.Pushparaj P.N., Low H.K., Manikandan J., Tan B.K., Tan C.H. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2007;111(2):430–434. doi: 10.1016/j.jep.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Bollen M., Keppens S., Stalmans W. Specific features of glycogen metabolism in the liver. Biochem. J. 1998;336(1):19–31. doi: 10.1042/bj3360019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 42.Valentovic M.A., Alejandro N., Carpenter A.B., Brown P.I., Ramos K. Streptozotocin (STZ) diabetes enhances benzo (α) pyrene induced renal injury in Sprague Dawley rats. Toxicol. Lett. 2006;164(3):214–220. doi: 10.1016/j.toxlet.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Kayode O.T., Yakubu M.T. Parquetina nigrescens leaves: chemical profile and influence on the physical and biochemical indices of sexual activity of male Wistar rats. J. Integr. Med. 2017;15(1):64–76. doi: 10.1016/S2095-4964(17)60318-2. [DOI] [PubMed] [Google Scholar]
- 44.Traesel G.K., de Souza J.C., de Barros A.L., Souza M.A., Schmitz W.O., Muzzi R.M., Oesterreich S.A., Arena A.C. Acute and subacute (28 days) oral toxicity assessment of the oil extracted from Acrocomia aculeata pulp in rats. Food Chem. Toxicol. 2014;74(2):320–325. doi: 10.1016/j.fct.2014.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.