Abstract
Social mammals with more numerous and stronger social relationships live longer, healthier lives. Despite the established importance of social relationships, our understanding of the neurobiological mechanisms by which they are pursued, formed, and maintained in primates remains largely confined to highly controlled laboratory settings which do not allow natural, dynamic social interactions to unfold. In this review, we argue that the neurobiological study of primate social behavior would benefit from adopting a neuroethological approach, that is, a perspective grounded in natural, species-typical behavior, with careful selection of animal models according to the scientific question at hand. We highlight macaques and marmosets as key animal models for human social behavior and summarize recent findings in the social domain for both species. We then review pioneering studies of dynamic social behaviors in small animals, which can inspire studies in larger primates where the technological landscape is now ripe for an ethological overhaul.
Introduction:
Social distancing measures implemented to slow the spread of COVID-19 have triggered a worldwide craving for social contact, leading to surges in anxiety, depression, and loneliness [1], as well as rebellion against these measures [2]. This social desire is deeply rooted in our evolutionary history [3]: most of our closest nonhuman primate relatives live in groups in which they form differentiated relationships with conspecifics [4]. Moreover, social mammals (including humans) with more numerous and stronger social relationships live longer, healthier lives and have more offspring [5].
Despite the established importance of social connection, our understanding of the neurobiological mechanisms by which social relationships are pursued, formed, and maintained in primates (including humans) remains largely confined to laboratory settings. These settings have limited the range of social behaviors studied to highly controlled, non-species-typical behaviors. Recent technological advances have removed the physical constraints once required, greatly increasing the range of behaviors that can now be studied. Here, we argue that the neurobiological study of primate social behavior would benefit from adopting a neuroethological approach, that is, a perspective grounded in natural, species-typical behavior [6–9], with careful selection of the animal model used [10].
The neuroethological approach:
Pioneering ethologists Konrad Lorenz, Niko Tinbergen, and Karl Von Frisch argued that behavior must be understood in terms of its evolutionary origins, adaptive significance (‘ultimate’ mechanisms), ontogeny, and physiological mechanisms (‘proximate’ mechanisms) [6]. The bidirectional interactions between ultimate and proximate mechanisms of behavior form the basis of neuroethology. This approach focuses on studying neural activity during the expression of species-typical behaviors.
The neuroethological approach has provided rich insights into the brains of many animals. For example, research into the natural behavior of bats led scientists to discover their use of timing differences between the sound of an emitted vocalization and its subsequent echo to identify and localize a target prey [11]. With this behavioral foundation, neuroscientists used the acoustic attributes of echolocation signals to reveal the specialized functional organization of the bat’s auditory cortex [12]. Today, bats continue to be a popular model system in neuroethology for a diverse range of species-typical behaviors from spatial navigation [13] to social interaction [14], as both their behavior in their natural habitat and the underlying neurobiology are studied hand-in-hand. Importantly, our understanding of the specialized organization of the bat’s auditory cortex may have been limited without an ethological understanding of behavior.
The neuroethological approach relies on the premise that individual species’ neural circuits are refined by evolution to support a specific function with adaptive significance. Despite strong evidence for such adaptive specializations [15], some functions are shared more widely either due to inheritance through common descent (i.e. homologies) [16] or through convergent evolution --that is the independent evolution of similar features in species of different descent (i.e. analogies). Therefore, animal models should be carefully chosen according to the function investigated (Krogh’s principle, [10,17]) as well as shared ancestry with what they are intended to model [10].
Non-human primates: key model systems for human social behavior
Primates, including humans, are distinguished from many other mammals by their complex societies and their sophisticated social cognition. Most primates live in multi-layered societies and form differentiated social relationships based on status, friendships, alliances and kinship [8]. Navigating these societies depends not only on recognizing others and responding to them appropriately, but also understanding the relationships between others and using that knowledge to strategically plan behavior [18,19]. These distinctive skills have shaped the evolution of primate brains and may depend on specialized neural mechanisms unique to primates [8,20]. Despite these generalizations, it is important to emphasize that there is a wide range of social adaptations amongst the living 250 primate species [21]. A comparative approach, focused on a much broader array of species, will be necessary to discover which social computations are shared widely and which are species-specific.
Two nonhuman primates, rhesus macaques (Macaca mulatta) and common marmosets (Callithrix jacchus), are most commonly used in neurobiology today. These animals have distinct social, cognitive and physiological characteristics, which make them well-suited to the neurobiological investigation of different aspects of human social behavior. Due to genetic, physiological, behavioral, and cognitive similarities to humans, rhesus macaques, Old World primates that last shared a common ancestor with humans about 22 million years ago, are the most commonly used nonhuman primate in biomedical research. Their sophisticated social behaviors and well-defined neurobiological similarities to humans make them best suited for investigating the mechanisms that mediate complex social skills (e.g., strategic reasoning, perspective-taking, theory of mind). Marmosets, New World primates that last shared a common ancestor with humans about 38 million years ago, possess brains with clear homologies to humans [22] and share several behavioral traits with humans, such as pair-bonding, male parental investment [23] and a rich vocal repertoire [24], which make them well-suited for the study of vocal communication (see section below) and other social functions like cooperation [25]. Moreover, marmosets offer other advantages for husbandry and technological innovation, including small body size, rapid reproduction through twinning, and a relatively small lissencephalic brain [25]. In the next two sections we highlight early efforts to study the neurophysiology of social behavior in macaques and marmosets.
Neurophysiology of social behavior in macaques
Neurobiological studies in macaques have traditionally neglected species-typical behaviors, mainly due to the physical constraints imposed both to accommodate neural recording equipment and to control for movements that may complicate inferences about the measured patterns of neural activity. Despite these limitations, studies of the “Social Brain” in macaques have attempted to approximate the fundamental principles of real social interactions within the constraints of a controlled laboratory setting using computer-based social tasks.
One set of studies explored the prioritization of cues to social status and mate quality in rhesus macaques [26,27]. The value of these cues was reflected in monkeys’ choices, as well as how long they looked at them, and this information was encoded by neurons in the reward system, including ventral striatum [28] and orbitofrontal cortex [27], as well as the fronto-parietal attention network [29]. Importantly, these studies utilized species-typical social stimuli with known adaptive value in the wild [30,31] to evoke spontaneous, rather than trained, patterns of orienting behaviors. Recent studies recording neural activity while showing naturalistic and socially-relevant stimuli, such as conspecific faces, uncovered neural correlates of social information including identity, social status, gaze and facial expression in the amygdala [32,33], orbitofrontal cortex (OFC) [34] and posterior superior temporal sulcus (pSTS) [35]. Viewing videos of conspecifics interacting [36,37] or direct encounter with a human face [38], revealed selective encoding of eye contact in the amygdala and PFC. Finally, Hayashi and colleagues showed that monkeys’ spontaneous gaze betrayed an anticipation of human actors’ impending actions driven by false beliefs, and that the medial PFC causally mediated this process [39].
Implementation of dyadic social decision-making tasks was an important next step in studying more naturalistic social interactions in controlled laboratory settings. For example, Chang and colleagues [40] developed a reward allocation task in which a monkey could deliver a reward to himself, his partner, both animals or neither, to probe the neural mechanisms mediating other-regarding decisions. Monkeys were generally prosocial, preferring to reward a recipient monkey rather than let the reward go to waste [40]. Neurons in both ACCg [40] and amygdala [41] responded similarly to rewards delivered to self and other, and these neural signals predicted the frequency of prosocial choices. Using a similar approach, a recent study showed that synchronization between mPFC and the amygdala predicted more prosocial choices [42] and a second study [43] demonstrated that ACC causally contributes to formation of prosocial preferences.
Perhaps the most sophisticated approach has been the application of game theory to probe the neural basis of strategic decision making with a partner. Early game theoretic studies focused solely on interactions with a computer opponent [44,45]. Haroush and Williams (2015) were among the first to apply game theory to monkeys making decisions to cooperate or defect with another monkey in the Prisoner’s Dilemma game [46]. In a recent study, using a variant of the classic “chicken” game with an option to cooperate, Ong and colleagues found that monkeys’ choices could not be explained by reinforcement learning, counterfactual learning, or simple strategies like tit-for-tat or win-stay-lose-shift, but instead required a sophisticated model including the goals and strategies of the other player [47]. Neurons in mid-STS and ACCg signaled this abstract strategic information, and these signals were sensitive to social context and not reducible to physical social cues. Thus, allowing macaques to engage in richer social interactions unveiled a previously undiscovered wealth of cognitive and motivational signals in key nodes within the primate social brain network.
A neuroethological approach to marmoset vocal communication
Most neurophysiological studies of vocal communication in primates have used head-restrained macaques either spontaneously vocalizing or trained to detect or discriminate vocalizations [48] while being physically isolated from conspecifics. In those studies, stimulation of brainstem neurons are sufficient to evoke vocalizations, and frontal cortex lesions only briefly impair call production, suggesting frontal involvement is unnecessary [49]. However, such distinctly unnatural experimental conditions may fail to engage the full neural circuitry that mediates vocal communication during live social interactions. More recent studies in marmosets, leveraging wireless neurotechnology specially adapted to this small primate, have explored the role of the PFC during natural, dynamic and interactive vocal communication [50] and reveal a different picture.
Marmosets are notably volubile primates. Their proclivity to produce vocal signals at high rates is thought to be an adaptation to the dense vegetation of the forest habitats where they lived throughout their evolutionary history [51]. Their rich and context-dependent vocal repertoire has been characterized in detail, both in the laboratory [52] and in the wild [24]. Importantly, marmosets only engage in rich vocal behavior when unrestrained [53], making wireless technologies essential to its neuroscientific study (see Box 2). Miller & Wang 2006 developed an elegant paradigm, “Virtual Marmosets” (VMs), which parametrically manipulated various dimensions of vocalizations and broadcasted them to marmosets to test specific questions, from call recognition to social decision-making. Firing rates of neurons recorded in prefrontal (PFC) and premotor cortical areas (PMC) were modulated throughout vocal interactions with a VM [54] and were correlated with conversation length. Moreover, patterns of activity across the population of recorded neurons predicted whether the monkeys would vocalize in response to hearing a call [55]. These findings indicate that PFC and PMC play a critical role in call production during naturalistic social interactions, which was not apparent from prior studies in restrained macaques. Perturbation or lesion studies will be required to establish a causal role.
BOX2: Video-based automatic behavioral tracking.
Video-based behavioral tracking has delivered impressive improvements in efficiency, generalizability across taxa and contexts, as well as availability to the larger scientific community. In recent years a new deep-learning-based software package, DeepLabCut [69], enabled video-based motion tracking in any species and any context. This software radically democratized automatic behavioral tracking for the wider scientific community and was cited more than 450 times in the last two years [70]. Labuguen and colleagues (2020) labeled more than 13,000 images of macaques in the wild and showed robust pose tracking using DeepLabCut [71]. Building on this machine learning approach, Bala et al. 2020 tracked up to two monkeys simultaneously, in three-dimensional space, with impressive accuracy [72]. Finally, Nourizonoz et al. 2020 developed an ultra-fast, multi-camera closed-loop tracking system capable of providing simultaneous close-up views of mouse-lemurs in large naturalistic environments (up to 100m3) [64]. They combined this hardware with DeepLabCut-based pose estimation to identify specific poses on-the-fly (Figure 2). In parallel to advances in pose tracking, automatic classification of poses into meaningful behaviors, such as grooming, are being developed [73].
Dynamic natural social interaction in small animal models: inspiration for studies in macaques
The neuroethological approach adopted in marmosets has yet to reach macaques. This is in part due to the unique challenges imposed by macaques whose size and dexterity has made the development of robust neurotechnology compatible with free behavior very difficult. Marmoset studies, as well as recent work in other small animals, leveraging specialized technologies, can inspire future neuroethological investigations involving larger primate species. Here, we highlight a few remarkable example studies in non-primate species before discussing the technological landscape applicable to macaques.
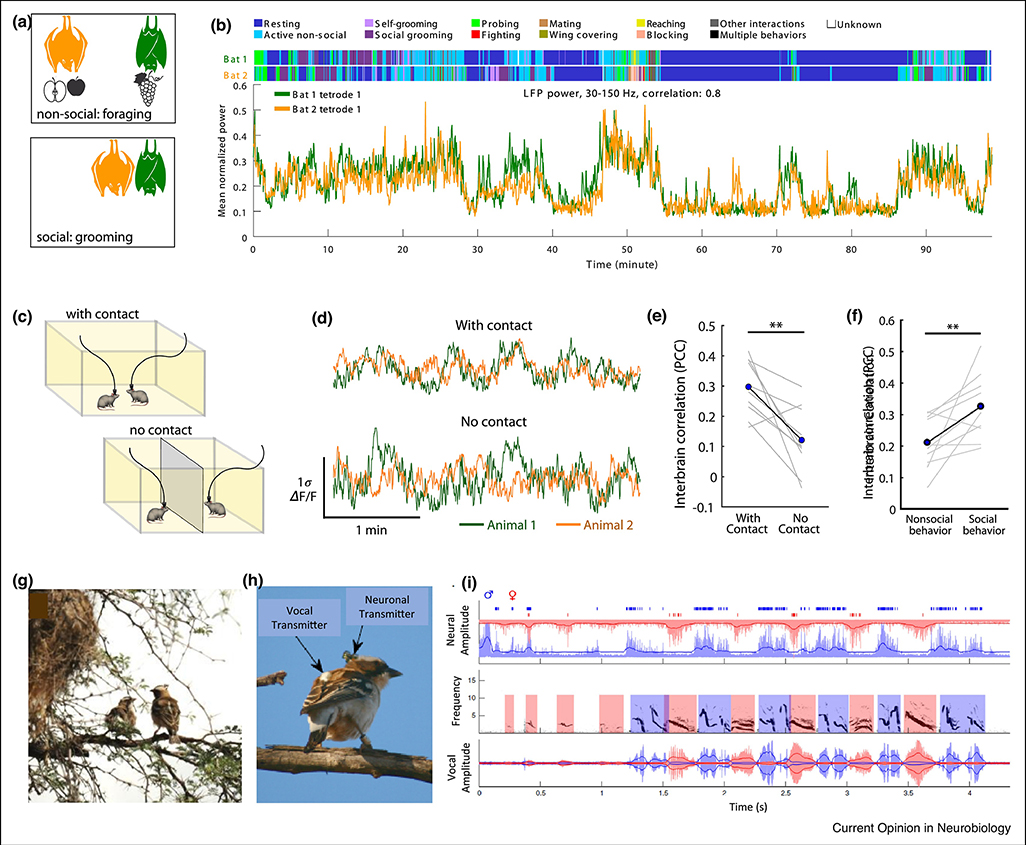
In a landmark study, Zhang and Yartsev (2019) recorded wirelessly from the frontal cortex of pairs of bats engaged in a wide range of natural social interactions, including vocalizing, grooming, fighting and mating (Figure 1A–B) [56]. Behaviors were quantified through a combination of computer vision technology and frame-by-frame video scoring by human observers. Remarkably, neurophysiological signals in interacting bats were synchronized over timescales from seconds to hours (Figure 1B). Synchrony was only observed when bats were together in the same physical space, could not be explained by common sensory inputs (e.g. computer playbacks of conspecific calls), or coordinated behavior. The higher the neural synchrony between bats, the more frequently they interacted with one another. Finally, increases in correlated brain activity preceded initiation of contact, suggesting neural synchrony drives rather than merely reflects social interactions.
Figure 1. Neural synchrony in socially interacting bats (A-B), mice (C-F) and birds (G-I).
(A-B) Wireless recordings from the frontal cortex of freely behaving pairs of bats, which exhibited a range of both social and non-social behaviors, such as grooming or foraging (A), and showed high inter-brain synchrony. (B) Mean normalized LFP power in the 30–150 Hz band during an example session, simultaneously recorded from two bats. Annotated behaviors are shown above. (C-F) Calcium imaging of neural activity from dmPFC neurons in freely behaving mice revealed inter-brain synchrony. Mice were studied in contact or separated (C). (D) Calcium traces of overall dmPFC activity (mean activity) revealed higher inter-brain correlation in mice that were in contact (E) and that were socially interacting (F). (G-I) Electrophysiological recordings from song birds’ HVC during duetting in their natural habitat (G), using wireless vocal and neuronal transmitters (H). (I) Vocalizations were locked to bursts of premotor HVC activity in the singing bird. Male, blue; female, red. Top panel: neural traces. Middle panel: spectrogram. Lower panel: vocal amplitude. Both male and female are shown for exemplary duet bouts initiated by the female. Solid dark blue and dark red lines outline the root-mean-square envelope of neural and vocal signals. Times of spike occurrence are indicated by blue rasters. Note the precise alternation of neural bursts between interacting males and females. Panels A-B adapted from [56], panels C-F adapted from [57] and panels G-I adapted from [59], with permission.
Strikingly, a second study published simultaneously [57] replicated these findings in dorsomedial (dm)PFC in mice (Figure 1C–F). Correlated neural activity across mice emerged from a subset of dmPFC neurons encoding social information; discarding those cells from analysis reduced neural synchrony across animals. Moreover, synchrony was driven more strongly by dominant animals than subordinates, echoing prior findings in leader-follower pairs of humans [58]. Lastly, synchronized brain activity during social interactions was also found in birds. Hoffmann et al. (2019) recorded extracellular activity in the high vocal center (HVC) of three wild white-browed sparrow-weaver (P. mahali) pairs during both individual vocalizations and duetting in their natural habitat on the Kalahari savanna (Figure 1G–I) [59]. HVC is necessary for both learning and production of song in songbirds [60]. Neural activity in HVC was synchronized in duet partners as birds took turns singing, and neural synchronization varied with degree of vocal synchrony across birds.
Together, these studies potentially reveal a widely shared principle of brain function during natural dynamic social interactions, namely cross-brain synchrony. Importantly, these studies validate--at the single neuron level--observations of neurophysiological synchrony in humans using less direct recording methods such as EEG and fMRI [61]. These studies also highlight the importance of a comparative approach to identify potential universal principles of neural function [10], and inspire studies in macaques, where the technological landscape is now ripe for an ethological overhaul.
Technological advances for macaque social neuroethology
Recent advances in neurotechnology (Box 1) and automated behavioral tracking (Box 2) have extended their applicability to macaques and make it possible to study neural activity in freely-behaving macaques. This paves the way for a true neuroethology of complex social interactions in Old World primates as well. In this section we highlight pioneering studies that make use of such technology.
BOX 1: Wireless neural recording technologies.
Wireless recording of single neuron activity in primates has been available for several years [65], although recent technological advances have made it more powerful and more accessible. Blackrock Microsystems commercialized the Cereplex W headstage in 2015, which permitted simultaneous recording from 96 channels with chronically implanted Utah array(s), opening the door to unrestrained experiments in the lab with minimal technical complexity [62,63,66,67]. The neural data transmission from the headstage to the recording computer is performed through radio-frequency signals captured by antennas, which limits the effective range to about 2–3 meters but permits closed-loop experiments critical to the field of brain-machine interface research [68]. For those whose research requires longer ranges and does not require online access to neural data, dataloggers are a promising option. Companies such as Deuteron Technologies and NeuraLynx offer dataloggers capable of recording up to 128 and 256 channels, respectively, directly onto a micro-SD card embedded in the headstage worn by the animal [56]. Micro-SD cards with capacities up to 1 Tb are now available on the market, and in combination with advanced batteries, can deliver upward of 10 hours of multi-electrode recording with unlimited range. Together, these technologies are expanding the landscape of neuroscience experiments that can be conducted in naturalistic environments while maximizing the safety and welfare of laboratory primates. The slow speed at which technologies widely available in smaller animals have translated to a larger primate is a testament to the significant technological challenges imposed by this specific animal model. The technology used must be carefully tailored to the animal’s ecological niche and biology. It is imperative to extend development and translation of a wide range of neurotechnologies across species.
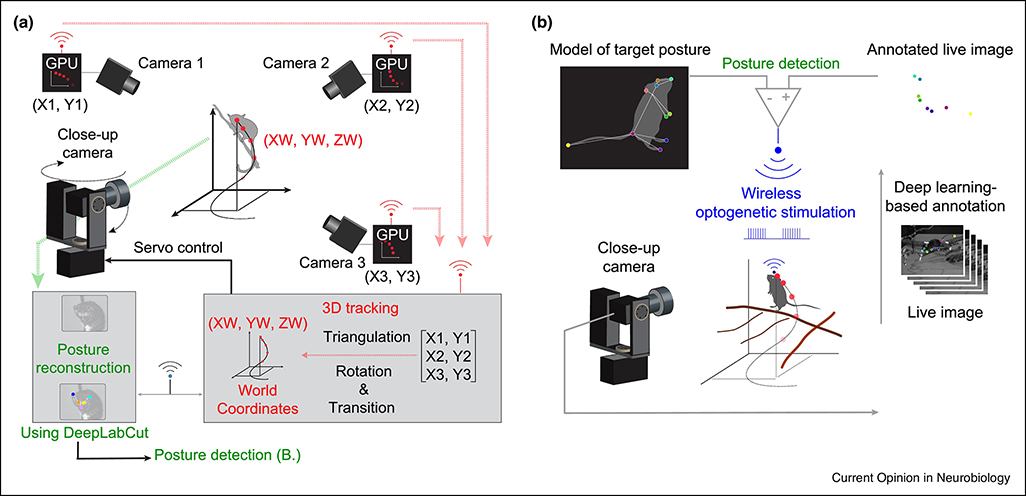
Berger et al. 2020 combined wireless electrophysiology with automated quantification of behavior using video-based computer vision (see Box 2) to investigate how monkeys plan limb movements to reach a distant goal outside haptic space—which cannot be studied in restrained animals [62]. Shahidi et al. 2019 (preprint) recorded activity of dlPFC neurons in macaques foraging in a large open space and showed that reward probabilities and memory for recent outcomes could be decoded from the distribution of activity across the population [63]. Moreover, decoded information predicted monkeys’ subsequent actions better than the actual experimental contingencies did, indicating access to the internal representations used to make their foraging decisions. Finally, Nourizonoz et al. 2020 developed an automatic behavioral tracking system for mouse-lemurs in large naturalistic environments (up to 100m3; Figure 2A; Box 2) [64], which could translate to a larger primate. They combined this hardware with deep learning-based pose estimation (DeepLabCut) to identify specific behaviors online and reinforce them in a closed-loop, either through classical conditioning (both mouse-lemurs and mice) or optogenetic stimulation of the reward system (mice only, Figure 2B).
Figure 2. Pose tracking in naturalistic 3D environments and real-time reinforcement of automatically detected behaviors.
(A) Schematic depiction and spatial arrangement of various EthoLoop components. Multiple infrared cameras (cameras 1–3) with dedicated GPUs process images from different viewing angles. The identity and positions of the detected markers are wirelessly transmitted to a central host computer for 3D reconstruction (triangulation, followed by rotation and transition into real-world 3D coordinates). 3D coordinates are forwarded to control the position and focus of a mounted close-up camera. (B) The images from the close-up system are either saved for offline analysis or processed on-the-fly to trigger optogenetic activation of the animal’s reward system. The close-up camera provides high-resolution live images of the tracked animal, the body parts are then classified in real time using a pre-trained deep-learning network (DeepLabCut). If there is a match between the annotated live image and a geometric model of the posture, a behavioral event is detected. This detection wirelessly triggered the optogenetic stimulation through a portable, battery-powered stimulator. Content adapted from [64], with permission.
These studies demonstrate the feasibility of studying neural activity in unrestrained macaques while precisely quantifying behavior using computer vision technology (Figure 2). Though challenges remain, translating these technologies to the study of complex social interactions in pairs, triads, or even groups of macaques is within reach with improvements in the ability to track and record from multiple animals simultaneously. These advances will allow us to understand how the social brain generates adaptive behavior in the real world, and open new avenues for treatment of social dysfunctions that attend disorders like autism and schizophrenia.
Highlights:
An ethological approach to behavior can reveal both specialized and common neurobiological mechanisms.
Macaques and marmosets are complementary animal models of human social behavior.
We review recent neurophysiology studies of social behavior in macaques and marmosets.
Recent advances in neurotechnologies and automated behavioral tracking now allow neuroethological study of complex social interactions in freely-moving macaques.
ACKNOWLEDGEMENTS:
This work was supported by the National Institutes of Health (R37MH109728, R01MH108627, U01MH121260, R01MH118203), the Human Frontier Science Program, the Canada Banting Fellowship and the Kauffman Foundation.
Footnotes
DECLARATION OF INTEREST: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
* Special Interest
** Outstanding interest
- 1.Hwang T-J, Rabheru K, Peisah C, Reichman W, Ikeda M: Loneliness and social isolation during the COVID-19 pandemic. Int Psychogeriatr 2020, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedo S, Brown N: ‘We don’t consent’: Dramatic scenes at anti-lockdown protest’. Daily Mercury 2020, 10. [Google Scholar]
- 3.Dunbar R, Dunbar R: Grooming, gossip, and the evolution of language. Harvard University Press; 1998, [Google Scholar]
- 4.Cheney DL, Silk JB, Seyfarth RM: Network connections, dyadic bonds and fitness in wild female baboons. R Soc Open Sci 2016, 3:160255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder-Mackler N, Burger JR, Gaydosh L, Belsky DW, Noppert GA, Campos FA, Bartolomucci A, Yang YC, Aiello AE, O’Rand A, et al. : Social determinants of health and survival in humans and other animals. Science 2020, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinde RA: Ethology, its nature and relations with other sciences. Oxford University Press, USA; 1982. [Google Scholar]
- 7.Tinbergen N: The study of instinct. 1951, 237. [Google Scholar]
- 8.Platt ML, Seyfarth RM, Cheney DL: Adaptations for social cognition in the primate brain. Philos Trans R Soc Lond B Biol Sci 2016, 371:20150096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson JM, Watson KK, Platt ML: Decision making: the neuroethological turn. Neuron 2014, 82:950–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yartsev MM: The emperor’s new wardrobe: Rebalancing diversity of animal models in neuroscience research. Science 2017, 358:466–469. [DOI] [PubMed] [Google Scholar]
- 11.Simmons JA, Freedman EG, Stevenson SB, Chen L, Wohlgenant TJ: Clutter interference and the integration time of echoes in the echolocating bat, Eptesicus fuscus. J Acoust Soc Am 1989, 86:1318–1332. [DOI] [PubMed] [Google Scholar]
- 12.Suga N: Biosonar and Neural Computation in Bats. Scientific American 1990, 262:60–68. [DOI] [PubMed] [Google Scholar]
- 13.Genzel D, Yovel Y, Yartsev MM: Neuroethology of bat navigation. Curr Biol 2018, 28:R997–R1004. [DOI] [PubMed] [Google Scholar]
- 14.Omer DB, Maimon SR, Las L, Ulanovsky N: Social place-cells in the bat hippocampus. Science 2018, 359:218–224. [DOI] [PubMed] [Google Scholar]
- 15.Garcia J, Koelling RA: Relation of cue to consequence in avoidance learning. Psychon Sci 1966, 4:123–124. [Google Scholar]
- 16.Darwin C: The origin of species. 6th. John Murray, London; 1859. [Google Scholar]
- 17.Krogh A: THE PROGRESS OF PHYSIOLOGY. Science 1929, 70:200–204. [DOI] [PubMed] [Google Scholar]
- 18.Cheney D, Seyfarth R, Smuts B: Social relationships and social cognition in nonhuman primates. Science 1986, 234:1361–1366. [DOI] [PubMed] [Google Scholar]
- 19.Cheney DL, Seyfarth RM: How monkeys see the world: Inside the mind of another species. University of Chicago Press; 2018, [Google Scholar]
- 20.Rushworth MFS, Mars RB, Sallet J: Are there specialized circuits for social cognition and are they unique to humans? Curr Opin Neurobiol 2013, 23:436–442. [DOI] [PubMed] [Google Scholar]
- 21.Strier KB: Primate Behavioral Ecology. Routledge; 2016. [Google Scholar]
- 22.Fukushima M, Ichinohe N, Okano H: Neuroanatomy of the marmoset. In The Common Marmoset in Captivity and Biomedical Research. Elsevier; 2019:43–62. [Google Scholar]
- 23.Digby LJ, Barreto CE: Social Organization in a Wild Population of Callithrix jacchus. Folia Primatologica 1993, 61:123–134. [DOI] [PubMed] [Google Scholar]
- 24.Bezerra BM, Souto A: Structure and usage of the vocal repertoire of Callithrix jacchus. Int J Primatol 2008, 29:671. [Google Scholar]
- 25.Miller CT, Freiwald WA, Leopold DA, Mitchell JF, Silva AC, Wang X: Marmosets: A Neuroscientific Model of Human Social Behavior. Neuron 2016, 90:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deaner RO, Khera AV, Platt ML: Monkeys Pay Per View: Adaptive Valuation of Social Images by Rhesus Macaques. Current Biology 2005, 15:543–548. [DOI] [PubMed] [Google Scholar]
- 27.Watson KK, Platt ML: Social signals in primate orbitofrontal cortex. Curr Biol 2012, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein JT, Platt ML: Social information signaling by neurons in primate striatum. Curr Biol 2013, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein JT, Deaner RO, Platt ML: Neural correlates of social target value in macaque parietal cortex. Curr Biol 2008, 18:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chance MRA: Attention Structure as the Basis of Primate Rank Orders. Man 1967, 2:503–518. [Google Scholar]
- 31.Higham JP, Brent LJN, Dubuc C, Accamando AK, Engelhardt A, Gerald MS, Heistermann M, Stevens M: Color signal information content and the eye of the beholder: a case study in the rhesus macaque. Behav Ecol 2010, 21:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munuera J, Rigotti M, Salzman CD: Shared neural coding for social hierarchy and reward value in primate amygdala. Nat Neurosci 2018, 21:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taubert J, Flessert M, Wardle SG, Basile BM, Murphy AP, Murray EA, Ungerleider LG: Amygdala lesions eliminate viewing preferences for faces in rhesus monkeys. Proc Natl Acad Sci U S A 2018, 115:8043–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barat E, Wirth S, Duhamel J-R: Face cells in orbitofrontal cortex represent social categories. Proc Natl Acad Sci U S A 2018, 115:E11158–E11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramezanpour H, Thier P: Decoding of the other’s focus of attention by a temporal cortex module. Proc Natl Acad Sci U S A 2020, 117:2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosher CP, Zimmerman PE, Gothard KM: Neurons in the monkey amygdala detect eye contact during naturalistic social interactions. Curr Biol 2014, 24:2459–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demolliens M, Isbaine F, Takerkart S, Huguet P, Boussaoud D: Social and asocial prefrontal cortex neurons: a new look at social facilitation and the social brain. Soc Cogn Affect Neurosci 2017, 12:1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pryluk R, Shohat Y, Morozov A, Friedman D, Taub AH, Paz R: Shared yet dissociable neural codes across eye gaze, valence and expectation. Nature 2020, 586:95–100. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi T, Akikawa R, Kawasaki K, Egawa J, Minamimoto T, Kobayashi K, Kato S, Hori Y, Nagai Y, Iijima A, et al. : Macaques Exhibit Implicit Gaze Bias Anticipating Others’ False-Belief-Driven Actions via Medial Prefrontal Cortex. Cell Rep 2020, 30:4433–4444.e5.* Study shows that where monkeys look when watching a video betrays an understanding of human false-beliefs-driven actions and that the medial prefrontal cortex (mPFC) causally mediates this process. An understanding of others’ false beliefs had not been shown in macaques previously. Replication in wild freely behaving macaques remains critical to confirm the existence of such advanced social cognition in this primate species.
- 40.Chang SWC, Gariépy J-F, Platt ML: Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci 2013, 16:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang SWC, Fagan NA, Toda K, Utevsky AV, Pearson JM, Platt ML: Neural mechanisms of social decision-making in the primate amygdala. Proc Natl Acad Sci U S A 2015, 112:16012–16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dal Monte O, Chu CCJ, Fagan NA, Chang SWC: Specialized medial prefrontal-amygdala coordination in other-regarding decision preference. Nat Neurosci 2020, 23:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basile BM, Schafroth JL, Karaskiewicz CL, Chang SWC, Murray EA: The anterior cingulate cortex is necessary for forming prosocial preferences from vicarious reinforcement in monkeys. PLoS Biol 2020, 18:e3000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo H, Cai X, Donahue CH, Lee D: Neural correlates of strategic reasoning during competitive games. Science 2014, 346:340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorris MC, Glimcher PW: Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron 2004, 44:365–378. [DOI] [PubMed] [Google Scholar]
- 46.Haroush K, Williams ZM: Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell 2015, 160:1233–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ong WS, Madlon-Kay S, Platt ML: Neuronal Mechanisms of Strategic Cooperation. 2018, doi: 10.1101/500850.** Using a variant of the classic “chicken” game with an option to cooperate, Ong and colleagues found that macaques’ choices could not be explained by reinforcement learning, counterfactual learning, or simple strategies like tit-for-tat or win-stay-lose-shift, but instead required a sophisticated model including the goals and strategies of the other player. Neurons in mid-STS and ACCg signaled this abstract strategic information, and these signals were sensitive to social context and not reducible to physical social cues.
- 48.Habbershon HM, Ahmed SZ, Cohen YE: Rhesus macaques recognize unique multimodal face-voice relations of familiar individuals and not of unfamiliar ones. Brain Behav Evol 2013, 81:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jürgens U: Neural pathways underlying vocal control. Neurosci Biobehav Rev 2002, 26:235–258. [DOI] [PubMed] [Google Scholar]
- 50.Eliades SJ, Miller CT: Marmoset vocal communication: Behavior and neurobiology. Dev Neurobiol 2017, 77:286–299. [DOI] [PubMed] [Google Scholar]
- 51.Morrill RJ, Thomas AW, Schiel N, Souto A, Miller CT: The effect of habitat acoustics on common marmoset vocal signal transmission. Am J Primatol 2013, 75:904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller CT, Wang X: Sensory-motor interactions modulate a primate vocal behavior: antiphonal calling in common marmosets. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2006, 192:27–38. [DOI] [PubMed] [Google Scholar]
- 53.Roy S, Wang X: Wireless multi-channel single unit recording in freely moving and vocalizing primates. J Neurosci Methods 2012, 203:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller CT, Thomas AW, Nummela SU, de la Mothe LA: Responses of primate frontal cortex neurons during natural vocal communication. J Neurophysiol 2015, 114:1158–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nummela SU, Jovanovic V, de la Mothe L, Miller CT: Social Context-Dependent Activity in Marmoset Frontal Cortex Populations during Natural Conversations. J Neurosci 2017, 37:7036–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang W, Yartsev MM: Correlated Neural Activity across the Brains of Socially Interacting Bats. Cell 2019, 178:413–428.e22.** Study recorded wirelessly from the frontal cortex of bat pairs engaged in a wide range of natural social interactions. Behaviors were quantified through a combination of computer vision technology and frame-by-frame scoring by human observers. Neurophysiological signals in interacting bats were synchronized over timescales from seconds to hours. Synchrony was only observed when bats were together in the same physical space and could not be explained by common sensory inputs or coordinated behavior.
- 57.Kingsbury L, Huang S, Wang J, Gu K, Golshani P, Wu YE, Hong W: Correlated Neural Activity and Encoding of Behavior across Brains of Socially Interacting Animals. Cell 2019, 178:429–446.e16.* Study shows that inter-brain neural synchrony is much higher when mice were socially interacting in the same physical space, which could not be explained by common sensory inputs or coordinated behavior. They further show that synchrony emerges from a subset of dmPFC neurons encoding information about the partner’s behavior and is driven by the dominant animal.
- 58.Jiang J, Chen C, Dai B, Shi G, Ding G, Liu L, Lu C: Leader emergence through interpersonal neural synchronization. Proc Natl Acad Sci U S A 2015, 112:4274–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffmann S, Trost L, Voigt C, Leitner S, Lemazina A, Sagunsky H, Abels M, Kollmansperger S, Maat AT, Gahr M: Duets recorded in the wild reveal that interindividually coordinated motor control enables cooperative behavior. Nat Commun 2019, 10:2577.** This study brought the lab to the field and investigated the neural correlates of vocal duetting behavior in songbird pairs ranging freely in their natural habitat – the South-African Kalahari. Their pioneering approach combines wireless recordings of individual vocalizations and multi-unit vocal premotor neural activity. They find that in the duet-initiating bird, the onset of partner’s contribution to the duet triggers a change in the neural discharges which are time-locked to the bird’s own vocalizations. This change results in inter-individually synchronized neural activity which may, authors suggest, elicit perfectly alternating vocalizations between partners.
- 60.Burke JE, Schmidt MF: Neural Control of Birdsong. eLS 2020, doi: 10.1002/9780470015902.a0029190. [DOI] [Google Scholar]
- 61.Kingsbury L, Hong W: A Multi-Brain Framework for Social Interaction. Trends Neurosci 2020, doi: 10.1016/j.tins.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berger M, Agha NS, Gail A: Wireless recording from unrestrained monkeys reveals motor goal encoding beyond immediate reach in frontoparietal cortex. Elife 2020, 9.** To our knowledge, this is the first study to successfully combine wireless electrophysiology with automated quantification of behavior in rhesus macaques during task performance. They investigate how monkeys plan limb movements to reach a distant goal outside haptic space—which could not be studied in restrained animals.
- 63.Shahidi N, Schrater P, Wright T, Pitkow X, Dragoi V: Population coding of strategic variables during foraging in freely-moving macaques. 2019, doi: 10.1101/811992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nourizonoz A, Zimmermann R, Ho CLA, Pellat S, Ormen Y, Prévost-Solié C, Reymond G, Pifferi F, Aujard F, Herrel A, et al. : EthoLoop: automated closed-loop neuroethology in naturalistic environments. Nat Methods 2020, 17:1052–1059.** Study presents an ultra-fast, multi-camera closed-loop tracking system capable of providing simultaneous close-up views of mouse-lemurs in large naturalistic environments (up to 100mx100m). They combined this sophisticated hardware with DeepLabCut-based pose estimation to identify specific poses online and reinforce those behaviors either through automatic classical conditioning (“RECO” reward-delivery boxes) or optogenetic stimulation of the reward system. To our knowledge, this landmark study is the first to automatically manipulate brain activity and ensuing behavior in a naturalistic environment using minimally invasive technology in a primate. They provide all of the information required to replicate their system in an online platform, etholoop.org.
- 65.Schwarz DA, Lebedev MA, Hanson TL, Dimitrov DF, Lehew G, Meloy J, Rajangam S, Subramanian V, Ifft PJ, Li Z, et al. : Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat Methods 2014, 11:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin M, Borton DA, Komar J, Agha N, Lu Y, Li H, Laurens J, Lang Y, Li Q, Bull C, et al. : Wireless neurosensor for full-spectrum electrophysiology recordings during free behavior. Neuron 2014, 84:1170–1182. [DOI] [PubMed] [Google Scholar]
- 67.Milton R, Shahidi N, Dragoi V: Dynamic states of population activity in prefrontal cortical networks of freely-moving macaque. Nat Commun 2020, 11:1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot J-B, Buse N, Gandar J, Barraud Q, Xing D, et al. : A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016, 539:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nath T, Mathis A, Chen AC, Patel A, Bethge M, Mathis MW: Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat Protoc 2019, 14:2152–2176. [DOI] [PubMed] [Google Scholar]
- 70.Mathis MW, Mathis A: Deep learning tools for the measurement of animal behavior in neuroscience. Curr Opin Neurobiol 2020, 60:1–11. [DOI] [PubMed] [Google Scholar]
- 71.Labuguen R, Matsumoto J, Negrete S, Nishimaru H, Takada M, Go Y, Inoue K-I, Shibata T: MacaquePose: A novel “in the wild” macaque monkey pose dataset for markerless motion capture. bioRxiv, 2020. doi: 10.1101/2020.07.30.229989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bala PC, Eisenreich BR, Yoo SBM, Hayden BY, Park HS, Zimmermann J: OpenMonkeyStudio: Automated Markerless Pose Estimation in Freely Moving Macaques. 2020, doi: 10.1101/2020.01.31.928861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsu AI, Yttri EA: B-SOiD: An Open Source Unsupervised Algorithm for Discovery of Spontaneous Behaviors. bioRxiv, 2020. doi: 10.1101/770271. [DOI] [PMC free article] [PubMed] [Google Scholar]