Abstract
Introduction.
Obesity is a prevalent condition that accounts for significant morbidity and mortality across the globe. Despite substantial effort, most obesity pharmacotherapies have proven unsafe or ineffective. The use of obese mouse models provides unique insight into the hormones and mechanisms that regulate appetite and metabolism. Paramount among these models are the “obese” and “diabetic” mice that revealed the powerful satiety hormone leptin, revolutionizing obesity research.
Areas Covered.
In this article, we discuss work on leptin therapy, and the clinical response to leptin in humans. The authors describe the use of modern mouse genetics to study targetable mechanisms for genetic forms of human obesity. Additionally, they describe mouse models of neuromodulation and their utility in unraveling neural circuits that govern appetite and metabolism.
Expert opinion.
Combining past and present models of obesity is required for the development of safe, effective, and impactful obesity therapy. Current research in obesity can benefit from repositories of genetically engineered mouse models to discover interactions between appetitive systems and circuits. Combining leptin therapy with other satiety signals comprising the gut-brain axis is a promising approach to induce significant enduring weight loss.
Keywords: animal models, anti-obesity drugs, appetite, gut-brain axis, leptin, leptin resistance, mouse models, neuromodulation, obesity, syndromic obesity
1. Introduction
Obesity, defined as a Body Mass Index (BMI) of 30 or above, is a condition that effects 12% of adults worldwide, and is projected to effect 49% of adults in the United States by 2030 [1,2]. Obesity-related complications, most commonly coronary heart disease and end-stage renal disease, contributed to 7.1% of deaths worldwide in 2015 [1,3]. The effects of obesity on health have been recognized for centuries, if not millennia [4]. Therapies for obesity date back just as far, beginning with the fundamental concepts of eating less and exercising more. Hippocrates, the “father of medicine”, extolled the virtues of healthy diet and prescribed diet and exercise to his patients with obesity [5].
By the mid 18th century, obesity (or “Corpulency”) was being discussed at the Royal Society of London, as were its therapies [6]. A case study from this time gives an enthusiastic appeal for soap as a weight-loss supplement due to its “singular power of rendering oil or fat mixable with water” [7]. The study claims that a man who drank a pint of soapy water for two years every night before bed lost “two stone” (12.7 kg), without the assistance of any other medication. Remarkably, the use of soap in weight loss persisted into the 20th century with pseudoscience obesity “cures” like Fatoff: a heavily advertised “corpulency reducer” that consisted of 90 percent water and 10 percent soap [8].
Pharmacological cures for obesity in the early-to-mid 20th century were underscored by a fundamental lack of understanding about the regulation of weight gain. Moreover, the weight loss market had been flooded with “curealls” and hoax remedies (like Fatoff) for decades, minimizing public and academic perceptions of anti-obesity drugs [9]. At the time, animal models to study obesity in the laboratory setting were not widely used. While the agouti mouse was known to have increased weight, the dermatological features interested researchers more than the overweight phenotype [10]. Without preclinical models, weight loss drugs were stumbled upon incidentally in the clinic, with unfortunate results. Amphetamines are a prime example. In 1937, a study using the amphetamine beta-aminopropylbenzene to treat “nervous exhaustion” noted that 25% of patients showed rapid, sustained weight loss [11]. Shortly thereafter, amphetamines became widely developed and prescribed as weight loss drugs with limited understanding of their physiological effects. After years of rampant prescription, amphetamines were removed from the market due to multiple side effects, including addiction and numerous fatalities [12].
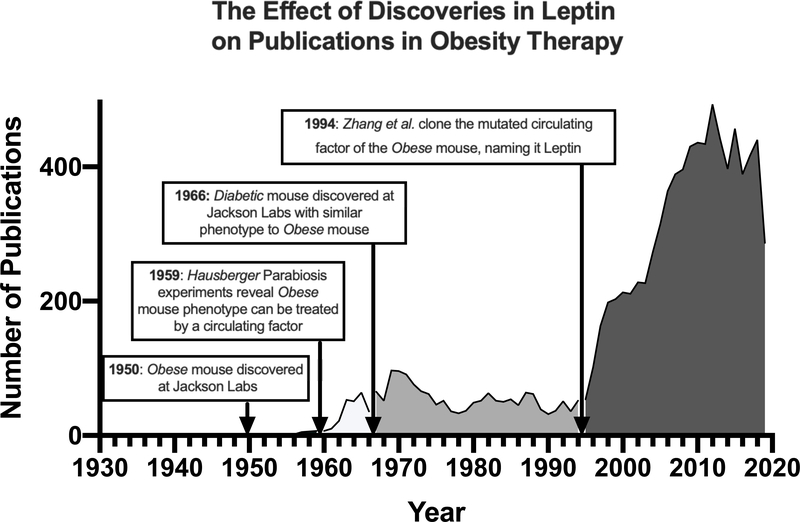
The scientific rigor of obesity research arrived with the advent of animal models for obesity. Inbred mouse strains, first developed in the early 20th century, were gaining acceptance as models of human physiology.[13] These inbred strains were bred specifically to reduce genetic variability of previous outbred strains and provide reproducible, translatable data. Two specific mutations in inbred mouse strains: the “obese” (ob/ob) and “diabetic” (db/db) mouse mutations, were instrumental in legitimizing obesity research and the molecular basis for metabolic regulation. After decades of research, we now know that ob/ob mice model deficiency in the hormone leptin, while db/db mice model deficiency in the leptin receptor. The discovery of the leptin system changed the zeitgeist for obesity therapy from quackery and happenstance to testable hypotheses, as evidenced by increases in anti-obesity publications (Fig. 1). This article will review some landmark discoveries in the leptin system that form the basis for obesity research, as well as the modern mouse models of obesity research influenced by leptin.
Figure 1: The association of landmark papers using leptin mouse models with the volume of publications in obesity drug discovery.
Pubmed search using mesh search terms: drugs, anti-obesity (search performed May, 2020).
2. Obese Mice, Diabetic Mice, and the Rise and Fall of Leptin
In the summer of 1949, “some very plump young mice” were discovered in C57BL/6J mice at the Jackson Labaratory in Bar Harbor, Maine [14]. These “plump” mice weighed in excess of 90 grams, triple the weight of their littermates, earning them the phenotype designation of “ob” for “obese”. Breeding studies revealed that the ob phenotype was a recessive mutation, and that homozygotes were not only obese, but also infertile and mildly diabetic.
In 1965 a second metabolic mutation was characterized at Jackson Labs, this time in the mouse strain C57Bl/KsJ [15]. This mutation, in contrast to the ob mutation, resulted in comparatively modest weight gain, with females averaging weights of 55 grams. This new mutation caused mild obesity and profound diabetes (blood sugars exceeding 300 mg/100 mL by 10 weeks of age). Aptly named “db” for “diabetic”, these mice, like ob/ob mice, were infertile and exhibited small ovaries with immature follicles. Interestingly, transplanting ovaries from ob/ob mice and db/db mice into wild type mice fully rescued ovarian function [16]. Because the mutated ovaries could still respond to endocrine signals from non-mutant animals, the ovarian transplantation studies implied that the ob and db mutations did not directly produce dysfunction of these peripheral tissues. Instead, the signals sent to the ovaries in ob/ob and db/db mice appeared to be dysregulated. To researchers at Jackson Labs, this implied that ob and db were responsible for a central signaling defect, possibly shared by both mice [17].
Douglas Coleman and the researchers at Jackson Labs speculated that loss of a “circulating factor” could be the cause of the obesity and diabetes in these mouse models [18]. The strongest evidence for ob and db mutations causing obesity through a circulating factor came from pioneering parabiosis experiments. Parabiosis is the surgical joining of two animal’s circulatory systems, and began to be used experimentally at the turn of the 20th century [19]. By the late 1960s parabiotic studies were in their heyday, employed by almost every field of biomedical research [20]. Parabiosis between healthy and diseased rodents was used to determine if humoral factors from a healthy mouse could rescue the diseased mouse (for example returning to healthy blood pressure, or recovering from radiation) [20]. Parabiosis was then the natural step to identify the “circulating factor” that linked ob and db mutations to obesity.
2.1. Parabiosis studies using Ob/Ob and Db/Db
The first parabiosis experiments on ob/ob mice were performed by Franz Hausberger in 1959 at Jefferson Medical College [21]. These experiments, in contrast to conventional parabiosis studies, were used primarily to graft adipose tissue between ob/ob mice and wild type mice. Hausberger surgically joined an ob/ob mouse and a wild type littermate for 2–5 months, and then surgically separated the mice, leaving adipose tissue from the ob/ob mouse in the wild type mouse and adipose tissue from the wild type mouse in the ob/ob mouse. He observed that after separation, the ob/ob adipose tissue in the wild type mouse regressed, while the wild type adipose tissue in the ob/ob mouse took on an ob/ob adipose phenotype. This indicated to Hausberger that the ob/ob mouse lacked a “secreted factor”, which he believed acted directly on the adipose tissue. Unfortunately, Hausberger did not report the effects that parabiosis itself had on the ob/ob mice.
Ten years later, Coleman’s first parabiosis experiments coupled a db/db mouse to a wild type C57Bl/KsJ mouse in search of a factor that might sensitize the db/db animals to insulin and normalize their blood sugar [22]. These conventional parabiosis studies measured the effect of shared circulation on the mice during parabiosis. Instead of rescuing the diabetic phenotype, parabiosis between db/db and wild type mice lead to the starvation and swift death (median survival time 23 days) of the wild type animals. Similar results had been found in a parabiosis study 10 years earlier, where rats with hypothalamic lesions had been surgically fused with uninjured rats [23]. Rats with bilateral ventromedial hypothalamic lesions overeat, gain weight, and become obese. Healthy, lesion-less rats coupled to obese, lesioned rats suffered the same fate as the wild type mice coupled to db/db mice: anorexia and eventual death. These studies led to the hypothesis that db/db and lesioned mice overproduced a satiety factor to which they were somehow unable to respond. The hypothalamic lesioning experiments implied that receptors for the satiety response were located in the ventromedial hypothalamus.
Further experimentation to test this hypothesis came with parabiosis experiments that included the ob/ob mouse [24]. Previous experiments that crossed ob/ob and db/db mice served a dual purpose: revealing the exact phenotypic overlap of the two mutations on the same strain, and protecting against rejection (or “disharmony”) after parabiosis surgery [25]. These experiments revealed that different mechanisms drove the obese and diabetic mutations, one seemingly ligand-based, and the other seemingly receptor-based. When ob/ob mice were paired with wild type mice, the obese phenotype was partially rescued. This showed, in classic parabiosis fashion, that the ob phenotype resulted from the deficiency in a blood-borne factor. When db/db and ob/ob mice were paired together, the ob/ob mouse would starve and die (median survival time of 26 days), very closely matching the parabiosis experiments of db/db and wild type mice. These experiments provided further evidence that the ob/ob and db/db mutations resulted in the insufficiency of a humoral satiety factor in the former and an inability to respond to the factor in the latter.
2.2. Leptin: a new age of molecular obesity
Search for the humoral satiety factor lasted until the early nineties, when advancements in molecular genetics and painstaking work by the Friedman Laboratory finally elucidated the mutated gene in ob/ob mice. Forty-four years after their discovery, positional cloning revealed a nonsense mutation in amino acid 105 of an unnamed gene in ob/ob mice [26]. The Friedman lab named the product of this gene “leptin”: “derived from the Greek root “leptos” for “thin,” the notion being that leptin kept a mouse (and humans) from becoming obese” [27].
The obesity field erupted after the seminal discovery of the “obese gene product”. One year later, the causative mutation in db/db mice was cloned from a protein with similarities to cytokine receptors (what we now know as leptin receptor; LepR) [28]. The mutation was subsequently found to be a splicing defect that eliminated key signaling domains of the long-form of LepR, rendering it ineffective at regulating satiety and metabolism [29]. Recombinant leptin was shown to induce weight loss by central and peripheral administration in ob/ob mice, but not db/db mice [30]. Further, even wild type mice were shown to have decreased appetite and weight loss after chronic intraperitoneal (IP) administration of leptin [31]. These experiments demonstrated that leptin is a powerful satiety hormone released by the adipose mass that acts in an endocrine fashion on LepR in the ventromedial hypothalamus, inducing satiety and increasing metabolism. The buzz around leptin had reached a fever-pitch after this string of high-impact publications revealed the molecular mechanisms for obesity.
Since the discovery of leptin and leptin receptor, many of the downstream signaling mechanisms have been revealed. Leptin binding to LepR activates two downstream pathways necessary for satiety: JAK2 phosphorylation of STAT3 and SH2B1 recruitment of IRS proteins to activate PI3K.[32–35] Leptin binding to LepR causes phosphorylation of the transcription factor STAT3, which in the arcuate nucleus increases expression of anorexigenic hormones (CART and POMC) and decreases expression of feeding hormones (AGRP and NPY).[36–38] Activation of PI3K confers the more immediate signaling of LepR, especially by mediating the effect of leptin signaling on both depolarizing and hyperpolarizing neuronal subpopulations.[39–41] Additionally, pSTAT3 forms a negative feedback loop by increasing the transcription of Suppressor of Cytokine Signaling 3 (SOCS3), which inhibits the JAK-STAT activation by LepR.[42,43] SOCS3 and the tyrosine phosphatase PTP1B are negative regulators of LepR, and loss of either of these proteins results in leptin hypersensitivity and resistance to diet-induced obesity in mice.[44–46] Pharmacological inhibitors of SOCS3 and PTP1B are promising approaches to enhance leptin signaling in mice and patients with obesity.
Direct evidence for leptin signaling through the hypothalamus emerged from intracerebroventricular (ICV) cannulation and leptin injection. Initial studies in rats and mice revealed that central administration of leptin produced rapid (<1hr) decreases in food intake and transcription of Neuropeptide Y (NPY).[47,48] Additional studies revealed that peripheral and central administration of leptin also reduced food intake, and that mice deficient in the cytokine receptor IL-1RI were not responsive to central leptin administration.[49] Further, ICV injection of leptin and subsequent staining of Fos (a canonical readout of neuron activation), identified the specific hypothalamic nuclei activated by leptin administration.[50,51] Microinjection of leptin into the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), and lateral hypothalamus (LH), and lesioning studies of the ARC using monosodium glutamate, revealed that the ARC was the primary site of leptin’s satiety effect.[52,53] The central effects of leptin in humans has been studied through functional Magnetic Resonance Imaging. When shown pictures of food, subjects had increased activation in the ventromedial striatum in a leptin-deficient state.[54] This implies that leptin signaling decreases “wanting” signals for food through the ventromedial striatum, and provides evidence for a leptin-driven central satiety mechanism in humans.
2.3. Leptin as a therapuetic
Unfortunately, the promising effects of leptin in lean mice did not translate to a universal human obesity therapy. Unlike the ob/ob mouse, most individuals with obesity did not harbor mutations in the leptin gene, so direct replacement of leptin in most of these individuals would not be a simple cureall [55]. Even more damning, human obesity is characterized by a marked increase in leptin levels, not a deficiency that could be treated with exogenous leptin like in the ob/ob mouse [56]. Even in other obese mouse models like Diet Induced Obese (DIO), Agouti (Avy), and New Zealand Obese (NZO) mice, chronic leptin treatment was ineffective at producing the robust weight loss seen in ob/ob and even lean mice [57]. A clinical trial in which 54 lean and 73 patients with obesity administered subcutaneous recombinant leptin daily showed extremely variable effects in both groups [58]. Patients with obesity were concurrently placed on a 500 calorie deficit diet, and only a modest weight loss trend was observed after 24 weeks. Leptin administration in mice and humans with obesity revealed a state of “leptin resistance” where weight-loss signals from leptin are relatively ineffective. The mechanisms behind leptin resistance are contentious and complex. An initial human study found a decreased ratio of leptin in the cerebrospinal fluid compared to plasma leptin in patients with obesity. This implied that leptin resistance in obesity could be caused by impaired transport across the blood brain barrier (BBB).[59] Further investigation of this hypothesis in mice has been technically challenging and resulted in divergent data. [60,61] Subsequent studies have also shown that DIO mice are resistant to both central and peripheral leptin, suggesting that the resistance may be downstream of leptin transport into the brain.[62] Overcoming leptin resistance in obesity is an active area of research, and could allow leptin to re-emerge as a universal obesity therapy [63].
Despite it’s disappointing efficacy in treating obesity, leptin monotherapy has utility in patients with dysregulated adipose signaling. Metreleptin, recombinant human leptin, is clinically effective at treating hypothalamic amenorrhea and lipodystrophy (especially lipodystrophy caused by anti-retroviral therapy for HIV).[64,65] Further, metreleptin ameliorated hyperactivity and improved cognitive, emotional, and behavioral symptoms in three patients with anorexia.[66] Metreleptin also improved insulin sensitivity and decreased de novo lipogenesis (a contributor to hepatic steatosis) in eleven patients with lipodystrophy.[67] Moreover, leptin dysregulation in obesity plays a role in atherosclerosis and cardiovascular disease, possibly through immune-mediated and inflammatory pathways.[68–70]
2.4. Adipokines
The discovery of leptin not only revolutionized obesity research, but also emphasized the role of adipose tissue as and endocrine organ. When leptin was cloned, adipose tissue was beginning to be recognized as more than just a depot of triglycerides. Adipsin (complement factor D), and tumor necrosis factor alpha (TNFa) were first revealed to be secreted by adipose tissue and mediate inflammation and insulin resistance in distal tissues.[71] Since the discovery of these initial bioactive molecules released by adipose (“adipokines”), over 600 adipokines have been identified, revealing the complex role of adipose in maintaining homeostasis.[72] Of these adipokines, many have therapeutic promise, improving insulin resistance in rodent models.[73] Adiponectin is a particularly attractive therapeutic candidate, as its levels decrease in obesity and administration of adiponectin decreases body weight and increases insulin sensitivity and release.[74,75] Dipeptidyl peptidase 4 (DPP-4) is an adipokine that has been targeted therapeutically since 2006.[76] DPP-4 inhibitors such as sitagliptin, saxagliptin, and linagliptin are used in the management of type 2 diabetes by increasing the activity of the incretins that DPP-4 degrades.[77] A multitude of adipokines like resistin, visfatin, chemerin, and omentin reinforce the link between adipokine dysregulation, inflammation, insulin resistance, cardiovascular disease, and metabolic syndrome.[78–81] Investigation of adipokine signaling is revealing novel obesity pathophysiology, and leading towards therapeutics to treat the sequalae of obesity.
3. Modern Mouse Models: Reverse Translation from Human to Mouse
In light of the disappointing outcomes of leptin monotherapy, the utility of mouse models in designing human obesity therapies needed to be rethought. The original mouse models of obesity were spontaneous mutations resulting in remarkable phenotypes, requiring decades of research to pinpoint their genomic origin. Modern mouse models reverse this paradigm by targeting a gene of interest and subsequently observing a phenotype. This, coupled with advances in uncovering human genetic causes of obesity, allows researchers to investigate relevant genes for human obesity in mouse models.
3.1. Mouse models of human syndromic obesity
Mouse models of rare forms of human congenital obesity have provided insights into the mechanisms of human satiety and weight gain. Though less generalizable, mice engineered in this way model actual human mutations, and therefore are directly applicable to treating clinical obesity. “Syndromic obesity” refers to severe congenital obesity that occurs with other stereotypical phenotypes (e.g. dysmorphic features and developmental abnormalities) [82]. The two most common forms of syndromic obesity are Bardet-Beidel syndrome (BBS) and Prader Willi syndrome (PWS) [82]. Mouse models engineered to study these diseases have helped to reveal specific genes implicated in obesity in these syndromes, while answering broader questions about regulation of appetite and satiety.
3.1.1. Bardet Beidel syndrome
Bardet Beidel Syndrome is a heterogenous genetic disorder whose primary features include retinal dystrophy, obesity, polydactyly, and hypogonadism [83]. The incidence of obesity in the BBS population is reported to be 72–86% [84]. Molecularly, BBS mutations lead to ciliary defects, which lead to dysfunction in receptor trafficking and cytoskeletal abnormalities [85]. Mutations in 19 different genes have been associated with BBS (BBS1-BBS19). Of these genes, 8 are associated with the “BBSome”, a complex that effects trafficking to cilia and the cell membrane [86].
Like humans, mice with mutated BBSome proteins become obese. Bbs2−/−, Bbs4−/−, and Bbs6−/− mice are hyperphagic, and gain significantly more weight than WT mice [87]. Interestingly, all 3 knockout mice exhibit leptin resistance, and show no feeding or body weight changes to leptin administration [87]. The BBSome was found to directly interact with LepR in the hypothalamus, and mice with mutant BBSome proteins showed decreased hypothalamic leptin receptor signaling [88]. A knockin mouse model of the most common mutation in BBS (Bbs1M390R/M390R) also exhibits obesity, in addition to sperm flagellar defects (male infertility), and hydrocephaly [89]. The Bbs1fl/fl mouse has been useful in creating conditional BBS knockout models to decipher which tissues require the BBSome to regulate satiety. Crossing the Bbs1fl/fl mouse with a neuron-specific cre (NestinCre/Bbs1fl/fl) and an adipose-specific cre (AdipCre/Bbs1fl/fl) resulted in obesity in only the NestinCre/Bbs1fl/fl mice, indicating that neural Bbs1 is necessary for maintaining normal weight. Furthermore, eliminating Bbs1 in only LepR+ cells (LepRCre/Bbs1fl/fl) also lead to obesity in mice [90]. In vitro experiments revealed that the BBSome is necessary for leptin receptor trafficking to the cell surface [90]. Gene therapy using Adeno-Associated virus (AAV) has been successful in treating the retinal degeneration of Bbs4−/− mice, however similar approaches have not yet been attempted in the hypothalamus to treat obesity [91].
3.1.2. Prader Willi syndrome
Prader Willi Syndrome is caused by deficient expression of the Prader Willi Critical Region (PWCR) of chromosome 15q11.2-q13 [92]. PWS usually is caused by the loss of a paternally imprinted region of chromosome 15 by paternal deletion, maternal uniparental disomy of chromosome 15, or imprinting defect. Angelman Syndrome is caused by the loss of the maternally imprinted region on the q arm of chromosome 15, and is phenotypically similar to PWS until two years of age, when hyperphagia and obesity become apparent in PWS [92]. Feeding behaviors in PWS are classically divided into two phases: poor feeding and hypotonia in infancy, with a reversal to hyperphagia and obesity that occurs between 18 and 36 months [93]. More recently, the nutritional phases of PWS have been divided into 4 main phases, with phase 3 (8 years old – adulthood) characterized by insatiable hunger [93]. During this phase, individuals with PWS seem unable to feel full. The compulsion to eat can be so severe that individuals with PWS have been reported to gain up to 20lbs in one weekend, and can eat to the point of life-threatening gastric rupture [94]. Complications from obesity account for the majority of mortality in individuals with PWS [95].
The Prader Willi Critical Region encodes five protein-coding genes, three of which have been made into knockout mouse models: MKRN3, MAGEL2, and NECDIN [92]. Knockout mouse models of these genes have helped to unravel the specific role of each of these genes in the clinical presentation of PWS. In addition to altered feeding behavior, PWS is also characterized by developmental delay, hypogonadism and infertility, and oppositional behavioral problems [96]. Necdin-deficient mice exhibit cognitive behaviors reminiscent of PWS, including skin-scraping activity that mirrors skin-picking seen in individuals with PWS [97]. Additionally, loss of oxytocin- and luteinizing hormone releasing hormone neurons in Necdin-deficient mice implicates Necdin in the disordered pubertal development characteristic of PWS. MKRN3 also is involved in pubertal development, as evidenced by the Mkrn3 knockout mouse, which exhibits precocious puberty [98]. Mkrn3−/− mice do not model the obesity associated with PWS. However, Magel2−/− mice exhibit decreased weight at birth, and a subsequent increase in weight gain and adiposity at 5–6 weeks of age, matching the nutritional phases of PWS [99]. By 16 weeks, Magel2−/− mice have 18% body fat, double that of wild-type littermates [99]. Surprisingly, at 16 weeks Magel2−/− mice eat 10% less than wild-type littermates, which contrasts with the insatiable appetites of individuals with PWS. Similar to the other mouse models of PWS, Magel2−/− mice also have reproductive defects [100]. Thus, the obesity phenotype seen in PWS appears to be driven by decreased MAGEL2 expression, while the reproductive phenotype appears to involve genes throughout the Prader Willi Critical Region.
The mechanism behind the increased adiposity of the Magel2−/− mouse is similar to that of BBS mouse models: decreased surface-expression of leptin receptor in the hypothalamus [101]. Like BBS mice, Magel2−/− mice are resistant to leptin administration, and show no decrease in food intake after leptin treatment [102]. Moreover, this leptin de-sensitization occurs between week 4 and 6, overlapping with the switch from low weight and adiposity to increased weight and adiposity in these mice [103]. Magel2 is a MAGE protein, a family of proteins that inhibit the activity of E3 protein ligases, thereby protecting proteins from degradation. Lack of Magel2 in Magel2−/− mice leads to decreased expression of LepR in the hypothalamus, likely through increased lysosomal degradation of LepR, and decreased trafficking of LepR to the cell surface [101].
3.1.3. Leptin receptor trafficking, therapeutic implications
Mouse models of BBS and PWS underscore the importance of LepR localization on the cell surface of hypothalamic neurons. The effect of LepR trafficking on appetite and metabolism also can be appreciated in mouse models with silenced Endospanin-1 (Endo1, also known as OB-RGRP or LEPROT). Endo1 is encoded on the same gene as LepR via alternative splicing, and directly interacts with LepR by trafficking the receptor inside the cell and targeting LepR for lysosomal degredation [104]. Thus, Endo1 opposes Magel2 and BBSome proteins, which increase LepR trafficking to the cell surface and decrease LepR degradation. Though an Endo1 knockout mouse has not been reported, stereotactic injection of Endo1-specific shRNA has been used to silence Endo1 in the mouse hypothalamus [105]. Endo1 knockdown in the mouse hypothalamus imparts resistance to diet-induced obesity (DIO) [105]. Mice injected with Endo1 shRNA showed significantly decreased body weight and fat mass after being fed high fat diet when compared to mice injected with sham RNA. In mice that are already obese, Endo1 knockdown increases weight loss when mice are switched to a lean diet, and decreases weight gain when obese mice are maintained on a high fat diet [106]. Whether or not knockdown of Endo1 can overcome decreased LepR surface expression in BBS and PWS mouse models has yet to be investigated.
3.2. TBC1D1, an obesity variant
While syndromic obesities clearly link certain genetic loci to obesity, other loci implicated in human obesity have been discovered through linkage analysis. One such locus was discovered on chromosome 4p (4p15.1) using data from the San Antonio Family Diabetes Study [107]. Subsequent studies revealed that the variant R125W in the gene TBC1D1 within 4p15.1 correlated with obesity in females [108]. TBC1D1 is a gene expressed primarily in skeletal muscle and is important for regulating glucose and lipid uptake by muscle cells. Using these human data, mouse models that manipulate TBC1D1 were engineered to investigate the role of this gene in obesity. Coincidentally, it was revealed that a Tbc1d1 variant in Swiss Jim Lambert (SJL) mice conferred resistance to DIO in this mouse strain [109]. This mouse model implied that variations in the Tbc1d1 gene could alter weight gain in two directions, either protecting against or exacerbating obesity. In order to study the R125W variant that predisposes humans to obesity, Tbc1d1 with the R125W mutation was transfected into mouse skeletal muscle [110]. Introduction of the R125W variant of TBC1D1 into the tibialis anterior muscle in mice impaired insulin-stimulated glucose transport, providing a possible mechanism for obesity in individuals with the variant. Germline knockin models of Tbc1d1 variants also produce obesity in mice on lean diets, lending further evidence that this gene regulates energy uptake and storage [111]. Like mouse models of BBS and PWS, Tbc1d1 mice are examples of “reverse translation”: using clinical phenotypes to derive basic research models that investigate mechanisms underlying human disease.
3.3. Melanocortin receptor 4, the most common cause of human monogenic obesity
Other mouse models of obesity have shown promising correlation to human obesity. Research on the melanocortin 4 receptor (MC4R) is a prime example of the modern interplay between mouse models, the genetic basis for human obesity, and development of effective anti-obesity pharmaceuticals. The viable yellow Agouti mouse (Avy) was one of the original mouse models of obesity, characterized by its late-onset obesity (Agouti obesity syndrome) and unique coloring [10]. The Agouti gene was the first obesity factor cloned (2 years before leptin), and cloning revealed that the mutation in the Avy mouse caused ectopic expression of Agouti peptide [112]. Unlike ob and db mice, the Avy mouse at the time did not have a reciprocal receptor knockout mouse. This changed in 1997, when researchers developed a MC4R knockout mouse, which recapitulated the phenotype of Agouti obesity syndrome and implicated MC4R as the receptor that mutant Agouti antagonized [113]. Like LepR, MC4R is a hypothalamic receptor that induces satiety when stimulated by ligands from neurons in the arcuate nucleus (ARC) [114]. Using these data from mouse models, it was revealed that mutations in MC4R produce the most common monogenic form of human obesity [115]. The strong evidence that MC4R receptor regulates mouse and human body weight led to the development of additional mouse models that modulate MC4R function. One such model disrupts Melanocortin 2 Receptor Accessory Protein 2 (MRAP2), a protein that interacts directly with MC4R, leading to profound obesity in mice [116]. Human studies revealed that loss-of-function mutations in MRAP2 lead to hyperphagic obesity associated with hyperglycemia and hypertension in children and adults [116,117]. Notably, a second generation MC4R agonist, setmelanotide, has shown promising results in reducing hyperphagia and obesity in patients with rare genetic obesities, and is currently in a phase III clinical trial for patients with BBS (Clinical trial # NCT03746522) [118].
4. Mouse Models of Neuromodulation to Treat Obesity
Rodent models are instrumental in revealing not only the genetic basis of obesity, but also the neuroanatomic basis for obesity. Rodent models are useful for manipulating neuronal populations that modulate appetite and satiety. “Neuromodulation” of appetitive circuits in mice through various techniques has revealed complex neuronal circuits that interact to modulate feeding behavior. One of the first neuromodulation experiments in rodent models was electrolytic ablation of the hypothalamus, which lead to marked adiposity in lesioned rats [119]. Ablation studies, along with research on hormone receptors revealed the role of the hypothalamus in integrating appetite and satiety cues.
The hypothalamus is a complex brain region composed of at least 11 major nuclei, each regulating multiple homeostatic functions. Adding to this complexity is the heterogeneity of hypothalamic cell types, recently tallied at 34 distinct neuronal cell types in a single cell RNAseq study [120]. In order to study these and other heterogeneous neuronal populations, modern mouse models of obesity have evolved beyond simple ablation studies and conventional knockout models. In the ARC nucleus, for example, two intermingled neuronal populations communicate opposing appetitive cues to MC4R receptors. One population, Proopiomelanocortin positive (POMC+) neurons, are activated by leptin and induce satiety. This was discovered using a transgenic mouse model expressing enhanced Green Fluorescent Protein (eGFP) in POMC neurons, which allowed the excitability of POMC neurons to be specifically probed in brain slices after leptin administration [121]. The other population, Agouti-Related Peptide positive (AGRP+) neurons, send hunger signals, necessary for feeding. Though AGRP injections were known to induce feeding, the conventional AGRP knockout mouse had no dysregulation in feeding behavior [122]. To further investigate this circuit, a Cre-lox system was employed in mice to more specifically ablate AGRP and POMC neurons in the hypothalamus [123]. Diphtheria toxin receptor (DTR) controlled by a loxP-flanked stop cassette was selectively expressed in AGRP+ cells by Cre recombinase under the control of the AGRP promoter (AGRP-cre). IP injection of diphtheria toxin in these mice killed AGRP neurons in the ARC, leading to decreases in food intake and body weight within 48 hrs, and eventual starvation. This implied that AGRP+ ARC neurons are necessary for regulation of appetite. Moreover, when DTR was expressed in POMC neurons and mice were given diphtheria toxin, mice became hyperphagic and gained weight. Thus, the push and pull of appetite by AGRP+ and POMC+ neurons in the ARC was established using Cre-lox mouse models.
Current mouse models can manipulate neural circuits that control appetite within milliseconds. These highly engineered mouse models modulate neural activity with direct electrical currents, light, designer chemicals, and electromagnetic waves.
4.1. Deep brain stimulation
Deep brain stimulation (DBS) is a direct method of electrical neuromodulation used in mice to influence appetite and satiety. In DBS, current is passed between surgically implanted electrodes in the brain to stimulate neural firing. DBS was first shown to decrease feeding when mice were presented with highly palatable food [124]. In these experiments, electrodes were implanted into the nucleus accumbens (NA), the projection target of the brain’s reward pathway. Stimulation of the NA decreased “binge eating” in these animals, and lead to significant weight loss after 4 days of chronic stimulation. Further studies revealed that stimulation of the NA shell inhibited neuronal firing in the Lateral Hypothalamic Area (LHA), providing a possible link between stimulation of the NA and canonical hypothalamic regulation of feeding behavior [125]. DBS as a therapy for human obesity has been attempted with a wide range of results. Numerous case studies have noted success from DBS of the NA, LHA, and Ventromedial Nucleus (VMN) of the hypothalamus, and have been recently reviewed [126]. DBS of the LHA in 4 patients with PWS was ineffective at inducing weight loss or decreasing food cravings, instead causing stimulation-induced manic symptoms in 2 patients [127]. In contrast, DBS of the LHA in 3 patients with refractory obesity was safe, and possibly effective as 2 of the patients had more than a 10% decrease in body weight after 2.5 years of stimulation [128]. More robust studies of DBS in the general population are necessary to fully understand the safety and efficacy of this therapy in treating obesity.
4.2. Optogenetics
DBS, like ablation studies, lacks the specificity to target specific neuronal subtypes. To simulate particular neuronal populations, the Cre-lox system had to be adapted to express modulatory receptors controlled by cell-type-specific Cre drivers. Ideally, these modulatory receptors would be responsive only to external stimuli applied by the experimenter, to eliminate confounding endogenous signaling from the mice. Studies on the phototaxis responses of the green algae Chlamydomanas reinhardtii revealed a unique class of opsin-related proteins called channelrhodopsins that produced light-gated ion conductance in the eye spot of these organisms [129]. When packaged into a lentiviral vector, Channelrhodopsin-2 (ChR2) evoked blue-light-dependent spike chains in rat hippocampal neurons in vitro, establishing channelrhodopsins as a tool for selectively stimulating rodent neurons [130].
Stimulation and inhibition of appetitive circuits by light-sensitive channels (optogenetics), is in the forefront in the study of obesity over the last decade. Many studies use stereotactic injection of Adeno-Associated viruses (AAV) to infect neurons with channelrhodopsin. To further dissect the functionality of AGRP and POMC neurons in the ARC, AGRP-Cre and POMC-Cre mice were again used to discriminate between the two neuronal populations [131]. These mice were stereotactically injected with AAV-FLEX-rev-ChR2:tdtomato, a virus encoding a fluorescently-labelled ChR2 that would only be expressed in neurons that also expressed Cre. Stimulation of AGRP+ ARC neurons resulted in an 8-fold increase in food consumption over 1 hr in mice with high levels of ChR2 expression. POMC stimulation decreased food consumption over a longer time course, resulting in a cumulative decrease in food intake and body weight after 24hrs.
Optogenetic mouse models have also shed light on novel circuits that regulate appetite and body weight. One such circuit projects from the dorsal raphe nucleus (DRN) of the brainstem to canonical feeding centers in the hypothalamus like the LHA [132]. Like the ARC, the DRN is comprised of 2 populations of neurons that have opposite effects on feeding [133]. Unlike AGRP+ and POMC+ neurons in the ARC which are characterized by unique neuropeptides, the DRN subpopulations are characterized by general inhibitory (Vgat+) or excitatory (VGLUT3+) markers. Injection of Cre-dependent AAV5-EF1a-DIO-ChR2-EYFP into the DRN of Vgat-IRES-Cre mice lead to an increase in food consumption that scaled with photostimulation. Conversely, optogenetic stimulation of the ChR2-infected DRN of VGLUT3-IRES-Cre mice induced the opposite effect, a significant decrease in feeding. In addition to ChR2, this study employed Arch3.0, a light-induced proton pump that inhibits neuronal firing by hyperpolarizing the cell (the opposite effect of ChR2 stimulation). Inhibition of the respective DRN circuits with light-activated Arch3.0 produced the converse outcome of stimulation with ChR2, providing evidence that these circuits can independently regulate appetite bi-directionally.
Optogenetic studies in mice have been valuable in studying obesity outside of the brain as well. Through a combination of techniques, including optogenetics, it was discovered that leptin acts through the sympathetic nervous system to induce lipolysis in white adipose tissue (WAT) [134]. This was a remarkable finding, indicating that leptin induces weight loss not only through appetite suppression and a general increase in metabolic rate, but also through a direct neural pathway back to the adipose tissue that initially secretes leptin. This study used a Cre-dependent fluorescent reporter (Rosa26-LSL-Tdtomato) crossed with tyrosine hydroxylase Cre (TH-Ccre) to visualize sympathetic innervation of WAT. Then, using this same TH-Cre mouse, ChR2 was expressed in sympathetic neurons by crossing these animals to a transgenic Cre-dependent ChR2 mouse line (Rosa26-LSL-ChR2-YFP). Unilateral optogenetic stimulation of these sympathetic neurons targeted at the inguinal fat pad decreased fat mass to 23% of the untreated side. More recently, it was revealed that the sympathetic stimulation of lipolysis in WAT is dependent on leptin signaling in the ARC through both AGRP+ and POMC+ neurons [135].
Though conventional optogenetics is a powerful tool for neuromodulation, it is not without drawbacks. Notably, the need for chronic cannulation and attachment of a wired device can be limiting to long-term studies and behavioral assays. Recent innovation in lightweight, wireless optogenetic devices can alleviate some of the experimental strain imposed by hardware issues.[136] Wireless optogenetics has been used in mouse models of DIO, preventing weight gain in mice fed a high fat diet and inducing weight loss in DIO mice by stimulating “beige fat” [137].
4.3. Chemogenetics
Other methods of neuromodulation eliminate the need for light-producing hardware altogether. “Chemogenetics” uses designer compounds to activate engineered G protein-coupled receptors (GPCRs) that modulate neuronal excitability and transmission [138]. These GPCRs, also known as designer receptors exclusively activated by designer drug (DREADDs), can be genetically encoded and expressed in particular tissues and cell types through viral or transgenic methods. Clozapine-N-oxide (CNO) is the designer drug used to activate DREADDs, as it is an inert, bioavailable compound that interacts solely with DREADDs [138]. Expression of an inhibitory DREADD (hM4D), in ARCAGRP+ neurons using the AGRP-Cre mouse, reduces feeding by 40% after injection of CNO [139]. These appetite-inducing ARCAGRP+ neurons were known to act on paraventricular hypothalamus SIM1+ neurons (PVHSIM1+), but the rest of the circuit was not known. Expression of the same inhibitory DREADD in PVHSIM1+ neurons revealed a new appetitive circuit from the PVH to the DRN in the brainstem. Injection of CNO into mice expressing hM4D in PVHSIM1+ neurons decreased synaptic transmission to the DRN and recapitulated the 40% feeding reduction produced by inhibiting ARCAGRP+ neurons [139].
Chemogenetic study of the DRN has extended our knowledge of this circuit. In addition to optogenetic stimulation and inhibition of DRNVgat+ neurons, chemogenetic neuromodulation has revealed the role of these neurons in the regulation of appetite and obesity. Crossing Vgat-IRES-Cre mice to ob/ob mice permitted evaluation of this circuit in an obese mouse model. Expression of the DREADD hM4D in the DVN revealed weight loss and feeding reduction over 24 days of CNO injection that was reversed after CNO was withdrawn [133]. In a follow-up study, the DRN of Vgat-IRES-Cre mice was injected with AAV5-hSyn-DIO-hM3D(Gq)-mCherry, a virus that induced expression of the excitatory hM3D DREADD in DRNVgat+ neurons of these mice [140]. Activation of DRNVgat+ neurons with CNO decreased thermogenesis in mice, indicating that DRNVgat+ neurons both increase appetite and decrease energy expenditure. Further, these neurons were shown to exert their effect on feeding through projections to the Bed Nucleus of the Stria Terminalis (BNST), and the Dorsomedial Hypothalamus (DMH), while regulating temperature chiefly through descending projections to the raphe palladius (RPa).
4.4. Magnetogenetics
An emerging method of neuromodulation used in obesity research is termed “magnetogenetics”. As the name implies, magnetogenetics involves genetically-encoded channels that can be controlled by magnetic fields [141]. These genetically encoded channels fuse the cation channel TRPV4 (or TRPV1) to ferritin, an iron-storing protein. The opening of the TRP channel can be controlled by applying a magnetic field to the fusion protein and changing its conformation, allowing ions to flow through the channel and depolarize or hyperpolarize the cell (depending on the channel). This concept was first shown to be effective in vivo by controlling tactile responses in zebrafish, and reward preferences in mice [142]. Magnetogenetics subsequently was used to regulate food intake in mice. Ad-FLEX-anti-GFP-TRPV1/GFP-ferritin was injected into the VMH of glucokinase-Cre (GK-Cre) mice to target the glucose-sensing neurons in the brain [143]. Stimulation of these neurons with a magnetic field increased feeding to levels comparable to optogenetic stimulation of the same neurons with ChR2. This method boasts advantages over optogenetics (notably the lack of hardware on the mice). However, the original TRPV1-based magnetogenetics model (Magneto2.0) recently has come under scrutiny [144]. Though the technique is still in its infancy, magnetogenetics provides a hardware-free approach to neuromodulation that maintains the temporal control of optogenetics.
5. Conclusions
Mouse models of obesity are central to our current understanding of appetite and weight regulation. The discovery of ob/ob and db/db mice at Jackson Labs paved the way for mice to become an essential model organism for obesity research. Since the cloning of leptin using the ob/ob mouse mutation in 1994, enthusiasm for discovering successful obesity therapies has boomed. Current mouse models have moved away from spontaneous mutations like ob/ob and db/db towards more targeted approaches. Insights into the genetic basis for human obesity coupled with modern methods for genetically engineering mice facilitates the development of models that more accurately reflect human disease. Using these new mouse models, promising therapies are being developed with efficacy in treating human obesity. Further, modern methods of neuromodulation have provided insights into the signals and circuits modulating feeding in real time. Indeed, transgenic mice have powered the ability to dissect individual neural circuits that regulate feeding, metabolism, energy expenditure, and food-based reward (Fig 2). Modulating these circuits with electrodes, light, and designer drugs produces profound and highly specific changes in feeding and body weight. Though clinical therapies that can target appetitive circuits with the precision of transgenic mouse models are still a long way off, they could be the solution for safe, effective, and personalized obesity therapy.
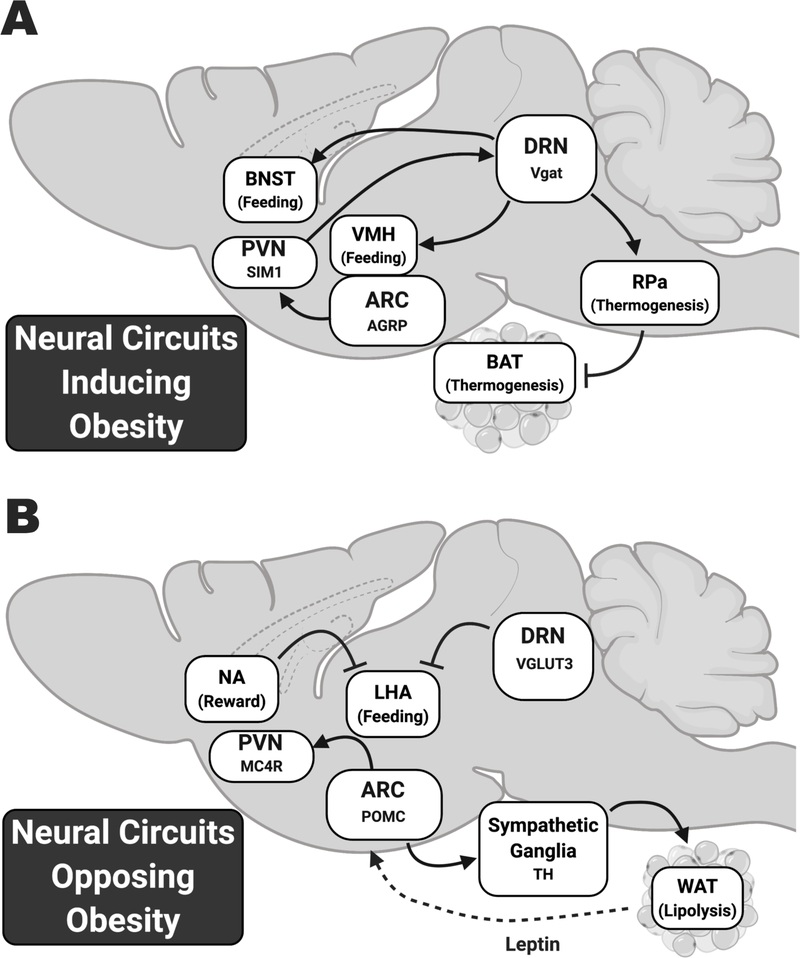
Figure 2: Neural circuits that induce or oppose obesity revealed by mouse models of neuromodulation.
A) Circuits that induce feeding or inhibit thermogenesis. In the Arcuate Nucleus (ARC), neurons that send positive feeding cues are characterized by Agouti Related Peptide (AGRP) and their downstream targets in the Dorsal Raphe Nucleus (DRN) are characterized by Vesicular GABA Transporter (Vgat). B) Circuits that induce satiety or lipolysis. ARC neurons that send these signals are characterized by Proopiomelanocortin (POMC), while DRN neurons that oppose feeding are characterized by Vesicular Glutamate Transporter 3 (VGLUT3). While most of these circuits have been investigated with optogenetics or chemogenetics, Deep Brain Stimulation (DBS) of the Nucleus Accumbens (NA) shows that these neurons reduce feeding by inhibiting the Lateral Hypothalamic Area (LHA). AGRP, Agouti Related Peptide; ARC, Arcuate nucleus; BNST, Bed Nucleus of the Stria Terminalis; BAT, Brown Adipose Tissue; DRN, Dorsal Raphe Nucleus; LHA, Lateral Hypothalamic Area; MC4R, Melanocortin 4 Receptor; NA, Nucleus Accumbens; PVN, Paraventricular Nucleus; RPa, Raphe Palladius; POMC, Proopiomelanocortin; TH, Tyrosine Hydroxylase; VMH, Ventromedial Hypothalamus; Vgat, Vesicular GABA Transporter; VGLUT3, Vesicular Glutamate Transporter 3; WAT, White Adipose Tissue. Created with BioRender.com
6. Expert Opinion
Monogenic mouse models have been invaluable for elucidating the molecular mechanisms underlying obesity and metabolic disease. By 2015, mouse models of 221 distinct genes had been studied for obesity-related phenotypes [145]. This list is continually expanding, as are the tools that allow researchers to interrogate the mechanisms behind obesity. Large-scale projects like The GENSAT Project at Rockefeller University have developed hundreds of Bacterial Artificial Chromosome (BAC)-driven GFP and Cre mouse lines that have been used in over 1000 publications [146]. Multi-institutional, international projects have developed suites of neuron specific Cre-drivers and toolboxes of transgenic mice with Cre-dependent optogenetic channels for dissecting the neural circuits that underlie mammalian behavior [147,148]. These herculean efforts provide obesity researchers with thousands of combinations of transgenic mice that can study the interactions between appetitive pathways. While individual circuits are being rigorously mapped and stimulated, discovery of how these pathways work in concert to evoke feeding responses is a crucial next step to precisely modulating satiety in human obesity.
Combining pharmaceuticals that regulate appetite and metabolism can synergistically enhance the effect either signal alone exerts on body weight. This concept has produced a resurgent focus on leptin therapy for obesity. In particular, coupling leptin with gastrointestinal hormones that regulate satiety has proven to increase weight loss over either therapy alone (Table 1).[149] These gastrointestinal hormones are thought to act distally on receptors in the brain and vagus nerve, thus forming a gut-brain axis of satiety. Leptin’s interaction with gut hormones in the nervous system could overcome leptin resistance in obesity, similar to the possible effects of endospanin-1 inhibition [104]. Combining these approaches with calorie restriction could lead to increased efficacy in treating weight loss, as well as benefiting longevity and aging [69].
Table 1: Leptin-sensitizing gut hormones.
Satiety-signaling gut hormones combined with leptin therapy can induce greater effects on weight loss than either hormone alone in rodent models. Exogenous administration of these hormones with leptin can re-sensitize leptin resistant animals to leptin signaling. The therapeutic potential of these hormones in combination with leptin is highlighted by the current production and use of analogues to many of these hormones in clinical practice. GLP-1, Glugagon-Like Peptide 1; IL-6, Interleukin 6; LepR, Leptin Receptor; ICV, Intracerebroventricular.
| Hormone | Hormone Analogue | Efficacy with Leptin | References |
|---|---|---|---|
| Amylin (pancreatic B cells) | Pramlintide (Symlin, Astrazeneca) | Synergistic weight loss effect with leptin, mediated by IL-6 in the Ventromedial Hypothalamus, also enhances GLP-1 weight loss | [150–152] |
| GLP-1 (intestinal L-cells) | Exendin-4/Exenatide (Byetta, Astrazeneca) | Restores leptin sensitivity, along with glucagon receptor agonist greatly enhances weight loss over leptin alone, also enhances Amylin weight loss | [157,158] |
| Uroguanylin (intestinal epithelium) | Plecanatide (Trulance, Salix), Linaclotide (Linzess, Allergan & Ironwood) | Re-sensitizes obese mice to leptin, synergistic effect with central co-administration with leptin, induces thermogenesis in brown adipose tissue | [160,161,163] |
| Cholecystokinin (Intestinal I-cells) | Sincalide (Kinevac, Bracco Diagnostics) | Synergistic effect with low-dose ICV leptin, only peripheral Cholecystokinin coupled with ICV leptin was effective | [167,168] |
| Peptide YY (Intestinal L-cells) | N/A | Leptin therapy coupled with Peptide YY increased weight loss over either alone, and extended anorectic effect of Peptide YY | [169] |
One such gastrointestinal, leptin-sensitizing hormone is amylin, a hormone secreted along with insulin from pancreatic β-cells. Amylin and its analogue, pramlintide, increase leptin responsiveness in rats with diet-induced obesity and overweight humans [150]. Importantly, weight loss was synergistic, and administration of both amylin and leptin elicited greater weight loss than either alone [151]. Amylin’s effect on leptin is mediated by IL-6 in the VMH, which enhances LepR signalling [152]. Amylin Pharmaceuticals Inc and Takeda Pharmaceutical Co. Ltd. were developing pramlintide/metreleptin combination treatment for obesity, but discontinued this project in 2011 citing safety concerns [153].
Glucagon-Like Peptide 1 (GLP-1) is an anorexigenic hormone secreted by intestinal enteroendocrine cells that induces satiety by binding its receptor (GLP-1R) in neurons of the hypothalamus and vagus nerve. Specifically, GLP-1, and its analogue, exendin-4, stimulate GLP-1R in POMC+ ARC neurons, which also express LepR [154]. However, GLP-1 also modulates leptin’s action through receptors on the vagus nerve [155]. Treating DIO mice with exendin-4 restores leptin sensitivity, and accentuates leptin-induced weight loss and satiety [156]. A glucagon receptor/GLP-1R co-agonist showed even greater efficacy when coupled with leptin therapy in DIO mice [157]. Treating DIO mice with leptin alone did not decrease weight. However, GLP-1/glucagon produced 26% weight loss after 33 days, while both GLP-1/glucagon and leptin produced 44% weight loss after 33 days. Combination therapy using GLP-1 and amylin analogues together also induces synergistic, long-lasting effects on weight loss in rats [158].
Uroguanylin (UGN) is yet another gut hormone that has shown efficacy in dual therapy with leptin. Uroguanylin is a hormone released by intestinal cells postprandially to induce satiety through its receptor, guanylyl cyclase C (GUCY2C) in the hypothalamus [159]. Central uroguanylin administration induces weight loss by stimulating thermogenesis in brown adipose tissue and increasing peripheral metabolism [160]. GUCY2C protein and mRNA is co-expressed in hypothalamic LepR+ neurons, providing a possible mechanism for UGN’s effect on appetite and metabolism [161,162]. Central administration of UGN re-sensitizes DIO mice to leptin, and co-administration of leptin and UGN in the brain induced greater weight loss than administration of either hormone alone [163].
DIO rodent models provided the first insights into these leptin-sensitizing agents and their possible efficacy in human weight loss. FDA approved analogues for each of these hormones are currently used in the clinic. The amylin analog pramlintide (Symlin, Astrazeneca) is currently approved for use to boost the effectiveness of mealtime insulin in diabetes [164]. Various GLP-1R agonists are currently used for inducing weight loss and boosting glucose-dependent insulin secretion in diabetes [165]. The uroguanylin analogue plecanatide (Trulance, Salix) and GUCY2C agonist linaclotide (Linzess, Allergan and Ironwood) are approved for to treat Chronic Idiopathic Constipation (CIC), and Irritable Bowel Syndrome with Constipation (IBS-C) [166]. Combining these therapies with leptin has yet to be proven safe and effective in humans. The promising animal data, coupled with approved therapies that simulate these appetitive gut hormones, presents a tractable approach for combination leptin therapy in the treatment of obesity.
Article highlights.
Obesity is a prevalent, high-risk condition that has benefitted from mice as pre-clinical models.
Mice with mutations in leptin and the leptin receptor laid the groundwork for our current understanding of the regulation of appetite and metabolism.
Genetic loci that are implicated in human obesity have been targeted in mouse models to provide attractive pre-clinical models for drug discovery.
Many current mouse models of obesity focus on dissecting neurological circuits implied in appetite and the regulation of metabolism.
Modern obesity studies in mouse models take advantage of large repositories of transgenic mice to modulate specific cell types in the brain using optogenetics, chemogenetics, and magnetogenetics.
Combining gut hormones that signal satiety with leptin therapy reverses “leptin resistance” in rodent models of obesity and could prove to be a safe and effective obesity therapy in humans.
Acknowledgments
Funding:
The authors are supported by the National Institutes of Health, via grants P30 CA56036 R01 CA204881 and R01 CA206026 from the National Cancer Institute to S Waldman and from National Institute of Diabetes and Digestive and Kidney Diseases via grant 1F30DK127639 to J Barton, the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation, the Courtney Ann Diacont Memorial Foundation, the Defense Congressionally Directed Medical Research Program via grants W81XWH-17-1-0299, W81XWH-17-PRCRP-TTSA, W81XWH-19-1-0067 and W81XWH-19-1-0263 and Targeted Diagnostics and Therapeutics Inc.
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of interest (*)
Papers of considerable interest (**)
- 1.Collaborators GBDO. Health effects of overweight and obesity in 195 countries over 25 years. New England Journal of Medicine. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward ZJ, Bleich SN, Cradock AL, et al. Projected US state-level prevalence of adult obesity and severe obesity. New England Journal of Medicine. 2019;381(25):2440–2450. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel M, Jensen MD, Ryan DH, et al. Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity. 2014;22(S2):S5–S39. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocrine reviews. 2018;39(2):79–132.* A thorough review on the evolving science of obesity therapy
- 5.Precope J Hippocrates on diet and hygiene. Zeno; 1952. [Google Scholar]
- 6.Short T A Discourse Concerning the Causes and Effects of Corpulency: Together with the Method for Its Prevention and Cure. By Thomas Short MD. Roberts J, near the Oxford Arms in Warwick Lane; 1727. [Google Scholar]
- 7.Flemyng M A Discourse on the Nature, Causes, and Cure of Corpulency. Illustrated by a Remarkable Case, Read Before the Royal Society, November 1757. And Now First Published, by Malcolm Flemyng, M.D. L. Davis and C. Reymers; 1760. (A Discourse on the Nature, Causes, and Cure of Corpulency. Illustrated by a Remarkable Case, Read Before the Royal Society, November 1757. And Now First Published, by Malcolm Flemyng, M.D). [Google Scholar]
- 8.American Medical A, Cramp AJ. Nostrums and Quackery: Articles on the Nostrum Evil and Quackery Reprinted from the Journal of the American Medical Association. Press of American medical association; 1921. (Nostrums and Quackery). [Google Scholar]
- 9.Haslam D Weight management in obesity–past and present. International journal of clinical practice. 2016;70(3):206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacArthur JW. Genetics of Body Size and Related Characters. I. Selecting Small and Large Races of the Laboratory Mouse. The American Naturalist. 1944. 1944/03/01;78(775):142–157. [Google Scholar]
- 11.Nathanson MH. The central action of betaaminopropylbenzene (benzedrine): clinical observations. Journal of the American Medical Association. 1937;108(7):528–531. [Google Scholar]
- 12.Cohen PA, Goday A, Swann JP. The return of rainbow diet pills. American journal of public health. 2012;102(9):1676–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ericsson AC, Crim MJ, Franklin CL. A brief history of animal modeling. Missouri medicine. 2013;110(3):201. [PMC free article] [PubMed] [Google Scholar]
- 14.Ingalls AM, Dickie MM, Shell GD. Obese, a new mutation in the house mouse. Journal of Heredity. 1950;41:317–318. [DOI] [PubMed] [Google Scholar]
- 15.Hummel KP, Dickie MM, Coleman DL. Diabetes, a New Mutafton in the Mouse. Science. 1966;153(3740):1127. [DOI] [PubMed] [Google Scholar]
- 16.Hummel KP. Transplantation of ovaries of the obese mouse. The Anatomical Record. 1957;128:569. [Google Scholar]
- 17.Johnson LM, Sidman RL. A Reproductive Endocrine Profile in the Diabetes (db) Mutant Mouse1. Biology of Reproduction. 1979;20(3):552–559. [DOI] [PubMed] [Google Scholar]
- 18.Coleman DL. A historical perspective on leptin. Nature Medicine. 2010. 2010/10/01;16(10):1097–1099.** A unique first-hand retrospective on the reception of the original research on ob/ob and db/db mouse mutations
- 19.Sauerbruch F HM. Über Parabiose künstlich vereinigter Warmblüter. Munch Med Wchnschr. 1908. (55):153–156. [Google Scholar]
- 20.Eggel A, Wyss-Coray T. A revival of parabiosis in biomedical research. Swiss medical weekly. 2014;144(0506). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hausberger FX. Behavior of transplanted adipose tissue of hereditarily obese mice. The Anatomical record. 1959;135:109–113.* The first parabiosis studies in ob/ob mice.
- 22.Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. American Journal of Physiology-Legacy Content. 1969. 1969/11/01;217(5):1298–1304. [DOI] [PubMed] [Google Scholar]
- 23.Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. The Journal of physiology. 1959;145(2):336–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973. 1973/08/01;9(4):294–298. [DOI] [PubMed] [Google Scholar]
- 25.Coleman DL, Hummel KP. The influence of genetic background on the expression of the obese (ob) gene in the mouse. Diabetologia. 1973. 1973/08/01;9(4):287–293. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432.** The paradigm-shifting publication in obesity therapy revealing the genetic basis for the ob/ob mutation
- 27.Friedman J The long road to leptin. The Journal of clinical investigation. 2016;126(12):4727–4734.* A unique first-hand retrospective on the molecular discovery of leptin
- 28.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–1271. [DOI] [PubMed] [Google Scholar]
- 29.Lee G-H, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. [DOI] [PubMed] [Google Scholar]
- 30.Campfield LA, Smith FJ, Guisez Y, et al. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. [DOI] [PubMed] [Google Scholar]
- 31.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. [DOI] [PubMed] [Google Scholar]
- 32.Vaisse C, Halaas JL, Horvath CM, et al. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nature genetics. 1996;14(1):95–7. [DOI] [PubMed] [Google Scholar]
- 33.Niswender KD, Morton GJ, Stearns WH, et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794–5. [DOI] [PubMed] [Google Scholar]
- 34.Duan C, Li M, Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. The Journal of biological chemistry. 2004;279(42):43684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Zhou Y, Carter-Su C, et al. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Molecular endocrinology (Baltimore, Md). 2007;21(9):2270–81. [DOI] [PubMed] [Google Scholar]
- 36.Fruhbeck G Intracellular signalling pathways activated by leptin. Biochem J. 2006. January 1;393(Pt 1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui Y, Huang L, Elefteriou F, et al. Essential role of STAT3 in body weight and glucose homeostasis. Molecular and cellular biology. 2004;24(1):258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinology and metabolism clinics of North America. 2008;37(4):811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams KW, Sohn J-W, Donato J, et al. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(37):13147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spanswick D, Smith MA, Groppi VE, et al. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390(6659):521–5. [DOI] [PubMed] [Google Scholar]
- 41.Jr J, Elias CF. Donato Frazão R, & The PI3K signaling pathway mediates the biological effects of leptin. Arquivos Brasileiros de Endocrinologia Metabologia. 2010;54(7 SRC - BaiduScholar):591–602. [DOI] [PubMed] [Google Scholar]
- 42.Mori H, Hanada R, Hanada T, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nature medicine. 2004;10(7):739–43. [DOI] [PubMed] [Google Scholar]
- 43.Waelput W, Verhee A, Broekaert D, et al. Identification and expression analysis of leptin-regulated immediate early response and late target genes. The Biochemical journal. 2000;348 Pt 1:55–61. [PMC free article] [PubMed] [Google Scholar]
- 44.Bence KK, Delibegovic M, Xue B, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nature medicine. 2006;12(8):917–924. [DOI] [PubMed] [Google Scholar]
- 45.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, et al. PTP1B regulates leptin signal transduction in vivo. Developmental cell. 2002;2(4):489–495. [DOI] [PubMed] [Google Scholar]
- 46.Mori H, Hanada R, Hanada T, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nature medicine. 2004;10(7):739–743. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz MW, Seeley RJ, Campfield LA, et al. Identification of targets of leptin action in rat hypothalamus. The Journal of clinical investigation. 1996;98(5):1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mistry AM, Swick AG, Romsos DR. Leptin rapidly lowers food intake and elevates metabolic rates in lean and ob/ob mice. The Journal of nutrition. 1997;127(10):2065–2072. [DOI] [PubMed] [Google Scholar]
- 49.Luheshi GN, Gardner JD, Rushforth DA, et al. Leptin actions on food intake and body temperature are mediated by IL-1. Proceedings of the National Academy of Sciences. 1999;96(12):7047–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Dijk G, Thiele TE, Donahey JC, et al. Central infusions of leptin and GLP-1-(7–36) amide differentially stimulate c-FLI in the rat brain. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1996;271(4):R1096–R1100. [DOI] [PubMed] [Google Scholar]
- 51.Elias CF, Kelly JF, Lee CE, et al. Chemical characterization of leptin-activated neurons in the rat brain. Journal of Comparative Neurology. 2000;423(2):261–281. [PubMed] [Google Scholar]
- 52.Satoh N, Ogawa Y, Katsuura G, et al. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neuroscience letters. 1997;224(3):149–152. [DOI] [PubMed] [Google Scholar]
- 53.Tang-Christensen M, Holst JJ, Hartmann B, et al. The arcuate nucleus is pivotal in mediating the anorectic effects of centrally administered leptin. Neuroreport. 1999;10(6):1183–1187. [DOI] [PubMed] [Google Scholar]
- 54.Farooqi IS, Bullmore E, Keogh J, et al. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Considine RV, Considine EL, Williams CJ, et al. Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. The Journal of clinical investigation. 1995;95(6):2986–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New England Journal of Medicine. 1996;334(5):292–295. [DOI] [PubMed] [Google Scholar]
- 57.Halaas JL, Boozer C, Blair-West J, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proceedings of the National Academy of Sciences. 1997;94(16):8878–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. Jama. 1999;282(16):1568–1575.* A clinical trial of leptin therapy revealing dissappointing efficacy in treating obesity
- 59.Schwartz MW, Peskind E, Raskind M, et al. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nature medicine. 1996;2(5):589–593. [DOI] [PubMed] [Google Scholar]
- 60.Harrison L, Schriever SC, Feuchtinger A, et al. Fluorescent blood–brain barrier tracing shows intact leptin transport in obese mice. International Journal of Obesity. 2019;43(6):1305–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. American Journal of Physiology-Endocrinology and Metabolism. 2003. [DOI] [PubMed] [Google Scholar]
- 62.Enriori PJ, Evans AE, Sinnayah P, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5(3):181–194. [DOI] [PubMed] [Google Scholar]
- 63.Myers MG Jr, Leibel RL, Seeley RJ, et al. Obesity and leptin resistance: distinguishing cause from effect. Trends in Endocrinology & Metabolism. 2010;21(11):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. New England Journal of Medicine. 2004;351(10):987–997. [DOI] [PubMed] [Google Scholar]
- 65.Brennan AM, Lee JH, Tsiodras S, et al. r-metHuLeptin improves highly active antiretroviral therapy-induced lipoatrophy and the metabolic syndrome, but not through altering circulating IGF and IGF-binding protein levels: observational and interventional studies in humans. European journal of endocrinology/European Federation of Endocrine Societies. 2009;160(2):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milos G, Antel J, Kaufmann L-K, et al. Short-term metreleptin treatment of patients with anorexia nervosa: rapid on-set of beneficial cognitive, emotional, and behavioral effects. Translational psychiatry. 2020;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baykal AP, Parks EJ, Shamburek R, et al. Leptin decreases de novo lipogenesis in patients with lipodystrophy. JCI insight. 2020;5(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruscica M, Baragetti A, Catapano AL, et al. Translating the biology of adipokines in atherosclerosis and cardiovascular diseases: gaps and open questions. Nutrition, Metabolism and Cardiovascular Diseases. 2017;27(5):379–395. [DOI] [PubMed] [Google Scholar]
- 69.Wilhelmi de Toledo F, Grundler F, Sirtori CR, et al. Unravelling the health effects of fasting: A long road from obesity treatment to healthy life span increase and improved cognition. Annals of Medicine. 2020:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lau DCW, Dhillon B, Yan H, et al. Adipokines: molecular links between obesity and atheroslcerosis. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288(5):H2031–H2041. [DOI] [PubMed] [Google Scholar]
- 71.Cook KS, Min HY, Johnson D, et al. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237(4813):402–405. [DOI] [PubMed] [Google Scholar]
- 72.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. [DOI] [PubMed] [Google Scholar]
- 73.Blüher M Adipokines–removing road blocks to obesity and diabetes therapy. Molecular metabolism. 2014;3(3):230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okamoto M, Ohara-Imaizumi M, Kubota N, et al. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51(5):827–835. [DOI] [PubMed] [Google Scholar]
- 75.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. [DOI] [PubMed] [Google Scholar]
- 76.Lamers D, Famulla S, Wronkowitz N, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60(7):1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ceriello A, Sportiello L, Rafaniello C, et al. DPP-4 inhibitors: pharmacological differences and their clinical implications. Expert opinion on drug safety. 2014;13(sup1):57–68. [DOI] [PubMed] [Google Scholar]
- 78.Tan BK, Adya R, Randeva HS. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends in cardiovascular medicine. 2010;20(5):143–148. [DOI] [PubMed] [Google Scholar]
- 79.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends in Endocrinology & Metabolism. 2010;21(11):660–667. [DOI] [PubMed] [Google Scholar]
- 80.Saddi-Rosa P, Oliveira CSV, Giuffrida FMA, et al. Visfatin, glucose metabolism and vascular disease: a review of evidence. Diabetology & metabolic syndrome. 2010;2(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jamaluddin MS, Weakley SM, Yao Q, et al. Resistin: functional roles and therapeutic considerations for cardiovascular disease. British journal of pharmacology. 2012;165(3):622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huvenne H, Dubern B, Clément K, et al. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obesity facts. 2016;9(3):158–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forsythe E, Beales PL. Bardet-Biedl Syndrome. 2015. [DOI] [PMC free article] [PubMed]
- 84.Forsythe E, Beales PL. Bardet–Biedl syndrome. European journal of human genetics. 2013;21(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ansley SJ, Badano JL, Blacque OE, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. Nature. 2003;425(6958):628–633. [DOI] [PubMed] [Google Scholar]
- 86.Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. [DOI] [PubMed] [Google Scholar]
- 87.Rahmouni K, Fath MA, Seo S, et al. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. The Journal of clinical investigation. 2008;118(4):1458–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seo S, Guo D-F, Bugge K, et al. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Human molecular genetics. 2009;18(7):1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis RE, Swiderski RE, Rahmouni K, et al. A knockin mouse model of the Bardet–Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proceedings of the National Academy of Sciences. 2007;104(49):19422–19427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo D-F, Cui H, Zhang Q, et al. The BBSome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLoS genetics. 2016;12(2):e1005890.** Description of the molecular mechanism for BBS obesity, evidence for reduced trafficking of leptin receptor to the cell membrane
- 91.Simons DL, Boye SL, Hauswirth WW, et al. Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proceedings of the National Academy of Sciences. 2011;108(15):6276–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cassidy SB, Schwartz S, Miller JL, et al. Prader-willi syndrome. Genetics in Medicine. 2012;14(1):10–26. [DOI] [PubMed] [Google Scholar]
- 93.Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader–Willi syndrome. American journal of medical genetics Part A. 2011;155(5):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stevenson DA, Heinemann J, Angulo M, et al. Gastric rupture and necrosis in Prader-Willi syndrome. Journal of pediatric gastroenterology and nutrition. 2007;45(2):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Einfeld SL, Kavanagh SJ, Smith A, et al. Mortality in Prader-Willi syndrome. American journal on mental retardation. 2006;111(3):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elena G, Bruna C, Benedetta M, et al. Prader-Willi syndrome: clinical aspects. Journal of obesity. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muscatelli Fo, Abrous DN, Massacrier A, et al. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader–Willi syndrome. Human molecular genetics. 2000;9(20):3101–3110. [DOI] [PubMed] [Google Scholar]
- 98.Li C, Lu W, Yang L, et al. MKRN3 regulates the epigenetic switch of mammalian puberty via ubiquitination of MBD3. National Science Review. 2020;7(3):671–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bischof JM, Stewart CL, Wevrick R. Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Human molecular genetics. 2007;16(22):2713–2719. [DOI] [PubMed] [Google Scholar]
- 100.Mercer RE, Wevrick R. Loss of magel2, a candidate gene for features of Prader-Willi syndrome, impairs reproductive function in mice. PLoS One. 2009;4(1):e4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wijesuriya TM, De Ceuninck L, Masschaele D, et al. The Prader-Willi syndrome proteins MAGEL2 and necdin regulate leptin receptor cell surface abundance through ubiquitination pathways. Human molecular genetics. 2017;26(21):4215–4230.** Description of the molecular mechanism for obesity in PWS, evidence for decreased leptin receptor trafficking to the cell membrane
- 102.Mercer RE, Michaelson SD, Chee MJS, et al. Magel2 is required for leptin-mediated depolarization of POMC neurons in the hypothalamic arcuate nucleus in mice. PLoS Genet. 2013;9(1):e1003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pravdivyi I, Ballanyi K, Colmers WF, et al. Progressive postnatal decline in leptin sensitivity of arcuate hypothalamic neurons in the Magel2-null mouse model of Prader–Willi syndrome. Human molecular genetics. 2015;24(15):4276–4283. [DOI] [PubMed] [Google Scholar]
- 104.Roujeau C, Jockers R, Dam J. Endospanin 1 Determines the Balance of Leptin-Regulated Hypothalamic Functions. Neuroendocrinology. 2019;108(2):132–141. [DOI] [PubMed] [Google Scholar]
- 105.Couturier C, Sarkis C, Séron K, et al. Silencing of OB-RGRP in mouse hypothalamic arcuate nucleus increases leptin receptor signaling and prevents diet-induced obesity. Proceedings of the National Academy of Sciences. 2007;104(49):19476–19481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vauthier V, Roujeau C, Chen P, et al. Endospanin1 affects oppositely body weight regulation and glucose homeostasis by differentially regulating central leptin signaling. Molecular metabolism. 2017;6(1):159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arya R, Duggirala R, Jenkinson CP, et al. Evidence of a novel quantitative-trait locus for obesity on chromosome 4p in Mexican Americans. The American Journal of Human Genetics. 2004;74(2):272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stone S, Abkevich V, Russell DL, et al. TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Human molecular genetics. 2006;15(18):2709–2720. [DOI] [PubMed] [Google Scholar]
- 109.Chadt A, Leicht K, Deshmukh A, et al. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nature genetics. 2008;40(11):1354. [DOI] [PubMed] [Google Scholar]
- 110.An D, Toyoda T, Taylor EB, et al. TBC1D1 regulates insulin-and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010;59(6):1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen L, Chen Q, Xie B, et al. Disruption of the AMPK–TBC1D1 nexus increases lipogenic gene expression and causes obesity in mice via promoting IGF1 secretion. Proceedings of the National Academy of Sciences. 2016;113(26):7219–7224.* Evidence that TBC1D1 mutations confer obesity phenotypes in mice, reflecting similar phenotypes in humans
- 112.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71(7):1195–1204. [DOI] [PubMed] [Google Scholar]
- 113.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. [DOI] [PubMed] [Google Scholar]
- 114.Tao Y-X. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocrine reviews. 2010;31(4):506–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Farooqi IS, Keogh JM, Yeo GSH, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. New England Journal of Medicine. 2003;348(12):1085–1095. [DOI] [PubMed] [Google Scholar]
- 116.Asai M, Ramachandrappa S, Joachim M, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341(6143):275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baron M, Maillet J, Huyvaert M, et al. Loss-of-function mutations in MRAP2 are pathogenic in hyperphagic obesity with hyperglycemia and hypertension. Nature medicine. 2019;25(11):1733–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kühnen P, Clément K, Wiegand S, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375:240–246. [DOI] [PubMed] [Google Scholar]
- 119.Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. The Anatomical Record. 1940;78(2):149–172. [Google Scholar]
- 120.Chen R, Wu X, Jiang L, et al. Single-cell RNA-seq reveals hypothalamic cell diversity. Cell reports. 2017;18(13):3227–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. [DOI] [PubMed] [Google Scholar]
- 122.Qian S, Chen H, Weingarth D, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Molecular and cellular biology. 2002;22(14):5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gropp E, Shanabrough M, Borok E, et al. Agouti-related peptide–expressing neurons are mandatory for feeding. Nature neuroscience. 2005;8(10):1289–1291. [DOI] [PubMed] [Google Scholar]
- 124.Halpern CH, Tekriwal A, Santollo J, et al. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. Journal of Neuroscience. 2013;33(17):7122–7129.* Evidence that DBS in mouse models can influence feeding behavior
- 125.Wei N, Wang Y, Wang X, et al. The different effects of high-frequency stimulation of the nucleus accumbens shell and core on food consumption are possibly associated with different neural responses in the lateral hypothalamic area. Neuroscience. 2015;301:312–322. [DOI] [PubMed] [Google Scholar]
- 126.Formolo DA, Gaspar JM, Melo HM, et al. Deep brain stimulation for obesity: a review and future directions. Frontiers in neuroscience. 2019;13:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Franco RR, Fonoff ET, Alvarenga PG, et al. Assessment of safety and outcome of lateral hypothalamic deep brain stimulation for obesity in a small series of patients with prader-willi syndrome. JAMA network open. 2018;1(7):e185275–e185275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whiting DM, Tomycz ND, Bailes J, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. Journal of neurosurgery. 2013;119(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nagel G, Ollig D, Fuhrmann M, et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296(5577):2395–2398.* Discovery of Channelrhodopsin-1, laying the groundwork for optogenetic manipulation of weight-regulating neurons
- 130.Boyden ES, Zhang F, Bamberg E, et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8(9):1263–1268. [DOI] [PubMed] [Google Scholar]
- 131.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature neuroscience. 2011;14(3):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Petrovický P, Kadlecová O, Mašek K. Mutual connections of the raphe system and hypothalamus in relation to fever. Brain Research Bulletin. 1981;7(2):131–149. [DOI] [PubMed] [Google Scholar]
- 133.Nectow AR, Schneeberger M, Zhang H, et al. Identification of a brainstem circuit controlling feeding. Cell. 2017;170(3):429–442.** Evidence that neuromodulation of Dorsal Raphe Nucleus neurons regulates feeding bidirectionally in two neuronal populations
- 134.Zeng W, Pirzgalska RM, Pereira MMA, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163(1):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang P, Loh KH, Wu M, et al. A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature. 2020:1–6. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Y, Castro DC, Han Y, et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proceedings of the National Academy of Sciences. 2019;116(43):21427–21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tajima K, Ikeda K, Tanabe Y, et al. Wireless optogenetics protects against obesity via stimulation of non-canonical fat thermogenesis. Nature communications. 2020;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Armbruster BN, Li X, Pausch MH, et al. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences. 2007;104(12):5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→ midbrain pathway for feeding behavior. Neuron. 2014;82(4):797–808.* Elegant use of chemogenetics to describe two nodes of a neural circuit that regulates feeding
- 140.Schneeberger M, Parolari L, Banerjee TD, et al. Regulation of energy expenditure by brainstem GABA neurons. Cell. 2019;178(3):672–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Christiansen MG, Senko AW, Anikeeva P. Magnetic Strategies for Nervous System Control. Annual review of neuroscience. 2019;42:271–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wheeler MA, Smith CJ, Ottolini M, et al. Genetically targeted magnetic control of the nervous system. Nature neuroscience. 2016;19(5):756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stanley SA, Kelly L, Latcha KN, et al. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016. 2016/03/01;531(7596):647–650.* Evidence that magnetogenetics can be used to modulate feeding circuits in mouse models
- 144.Wang G, Zhang P, Mendu SK, et al. Revaluation of magnetic properties of Magneto. Nature Neuroscience. 2019:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yazdi FT, Clee SM, Meyre D. Obesity genetics in mouse and human: back and forth, and back again. PeerJ. 2015;3:e856.* A detailed review of over 200 genes used in mouse models of obesity research
- 146.Gong S, Zheng C, Doughty ML, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. [DOI] [PubMed] [Google Scholar]
- 147.Daigle TL, Madisen L, Hage TA, et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell. 2018;174(2):465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Madisen L, Mao T, Koch H, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature neuroscience. 2012;15(5):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Andreoli MF, Donato J, Cakir I, et al. Leptin resensitisation: a reversion of leptin-resistant states. Journal of Endocrinology. 2019;241(3):R81–R96. [DOI] [PubMed] [Google Scholar]
- 150.Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proceedings of the National Academy of Sciences. 2008;105(20):7257–7262.** Evidence for leptin re-sensitization through amylin agonism
- 151.Turek VF, Trevaskis JL, Levin BE, et al. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology. 2010;151(1):143–152. [DOI] [PubMed] [Google Scholar]
- 152.Le Foll C, Johnson MD, Dunn-Meynell AA, et al. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes. 2015;64(5):1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roujeau C, Jockers R, Dam J. New pharmacological perspectives for the leptin receptor in the treatment of obesity. Frontiers in endocrinology. 2014;5:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dalvi PS, Nazarians-Armavil A, Purser MJ, et al. Glucagon-like peptide-1 receptor agonist, exendin-4, regulates feeding-associated neuropeptides in hypothalamic neurons in vivo and in vitro. Endocrinology. 2012;153(5):2208–2222. [DOI] [PubMed] [Google Scholar]
- 155.Ronveaux CC, Tomé D, Raybould HE. Glucagon-like peptide 1 interacts with ghrelin and leptin to regulate glucose metabolism and food intake through vagal afferent neuron signaling. The Journal of nutrition. 2015;145(4):672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Müller TD, Sullivan LM, Habegger K, et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. Journal of Peptide Science. 2012;18(6):383–393. [DOI] [PubMed] [Google Scholar]
- 157.Clemmensen C, Chabenne J, Finan B, et al. GLP-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes. 2014;63(4):1422–1427.** Evidence for leptin re-sensitization through GLP-1R agonism
- 158.Liberini CG, Koch-Laskowski K, Shaulson E, et al. Combined Amylin/GLP-1 pharmacotherapy to promote and sustain long-lasting weight loss. Scientific reports. 2019;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Valentino MA, Lin JE, Snook AE, et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. The Journal of clinical investigation. 2011;121(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Folgueira C, Beiroa D, Callon A, et al. Uroguanylin action in the brain reduces weight gain in obese mice via different efferent autonomic pathways. Diabetes. 2016;65(2):421–432. [DOI] [PubMed] [Google Scholar]
- 161.Merlino DJ, Barton JR, Charsar BA, et al. Two distinct GUCY2C circuits with PMV (hypothalamic) and SN/VTA (midbrain) origin. Brain Structure and Function. 2019;224(8):2983–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Inoue F, Eckalbar WL, Wang Y, et al. Genomic and epigenomic mapping of leptin-responsive neuronal populations involved in body weight regulation. Nature metabolism. 2019;1(4):475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Folgueira C, Beiroa D, González-Rellán MJ, et al. Uroguanylin improves leptin responsiveness in diet-induced obese mice. Nutrients. 2019;11(4):752.** Evidence for leptin re-sensitization through GUCY2C agonism
- 164.Pullman J, Darsow T, Frias JP. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vascular health and risk management. 2006;2(3):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs in context. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-C agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. The American journal of gastroenterology. 2018;113(3):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Matson CA, Reid DF, Cannon TA, et al. Cholecystokinin and leptin act synergistically to reduce body weight. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2000;278(4):R882–R890. [DOI] [PubMed] [Google Scholar]
- 168.Matson CA, Wiater MF, Kuijper JL, et al. Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides. 1997;18(8):1275–1278. [DOI] [PubMed] [Google Scholar]
- 169.Unniappan S, Kieffer TJ. Leptin extends the anorectic effects of chronic PYY (3–36) administration in ad libitum-fed rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008;295(1):R51–R58. [DOI] [PMC free article] [PubMed] [Google Scholar]