Abstract
The medial amygdala (MeA) is critical for the expression of a broad range of social behaviors, and is also connected to many other brain regions that mediate those same behaviors. Here, we summarize recent advances towards elucidating mechanisms that enable the MeA to regulate a diversity of social behaviors, and also consider what role the MeA plays within the broader network of regions that orchestrate social sensorimotor transformations. We outline the molecular, anatomical, and electrophysiological features of the MeA that segregate distinct social behaviors, propose experimental strategies to disambiguate sensory representations from behavioral function in the context of a social interaction, and consider to what extent MeA function may overlap with other regions mediating similar behaviors.
The neural circuits that regulate social behaviors are assembled according to at least two organizational principles (Fig 1): single brain regions can coordinate several different social behaviors such as aggression, reproduction, parenting, and sociability, as well as non-social behavior such as feeding and self-grooming [1,2]. At the same time, behaviors as complex as social interactions are orchestrated by a broad network of regions throughout the brain. How single brain regions regulate several different social behaviors, and how single social behaviors are coordinated by broad networks throughout the brain remain significant conceptual questions within social neuroscience. One unique node within the social behavior network is the medial amygdala (MeA). The MeA is critical for a wide range of social behaviors, contains both sensory and behavioral properties, and is functionally connected to a broad network of limbic regions throughout the brain. Here, we consider the organization and function of the MeA as an avenue towards addressing conceptual questions that arise in mapping the social behavior network, and propose experimental approaches towards furthering this line of understanding.
Fig. 1. Organizational principles of neural circuits regulating social behavior.
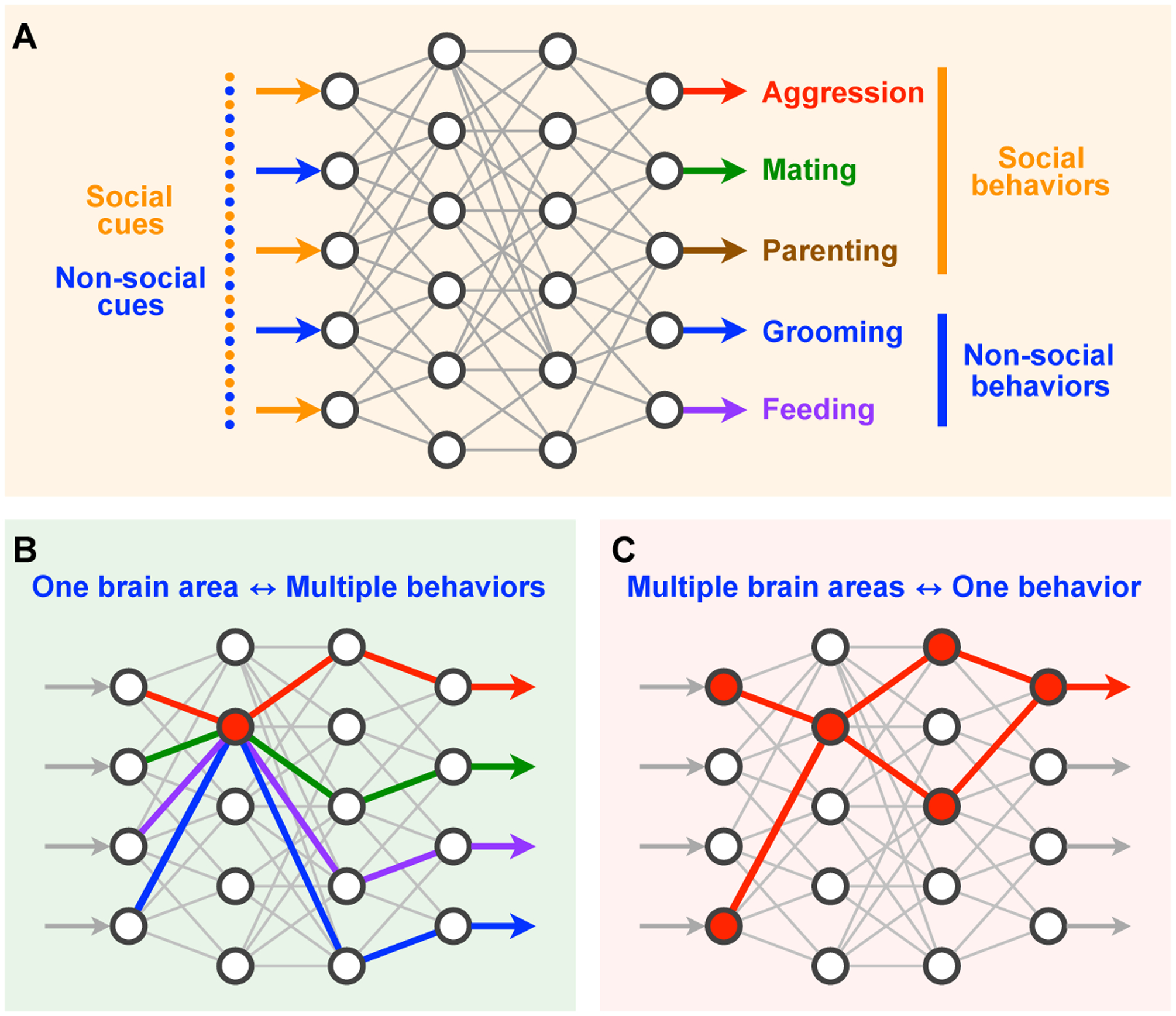
(A) Schematic showing broad networks of interacting brain regions coordinate transformation of sensory inputs into social and non-social behavioral outputs. (B) A single brain region can regulate multiple different behaviors. (C) Multiple brain regions can interact to coordinate a single behavior.
The medial amygdala
The MeA is a division of the amygdalar complex which plays a critical role in social behaviors (Fig 2) [3,4]. It is distinct from the more commonly studied amygdalar divisions such as the basolateral amygdala (BLA) and the central amygdala (CeA), which are primarily involved in regulating positive and negative valence, and responses to fear and stress [5–8]. Compared to other amygdalar divisions, the MeA has unique anatomical, cytoarchitectural, and functional properties [3,9]. It receives strong afferent vomeronasal input from the accessory olfactory system, which carry essential social cues, and is the primary brain region that relays pheromonal signals to the rest of the brain [10–13]. The MeA also receives indirect inputs from the main olfactory system through the cortical amygdala (CoA) [10].
Fig. 2. The MeA controls multiple types of social behaviors.
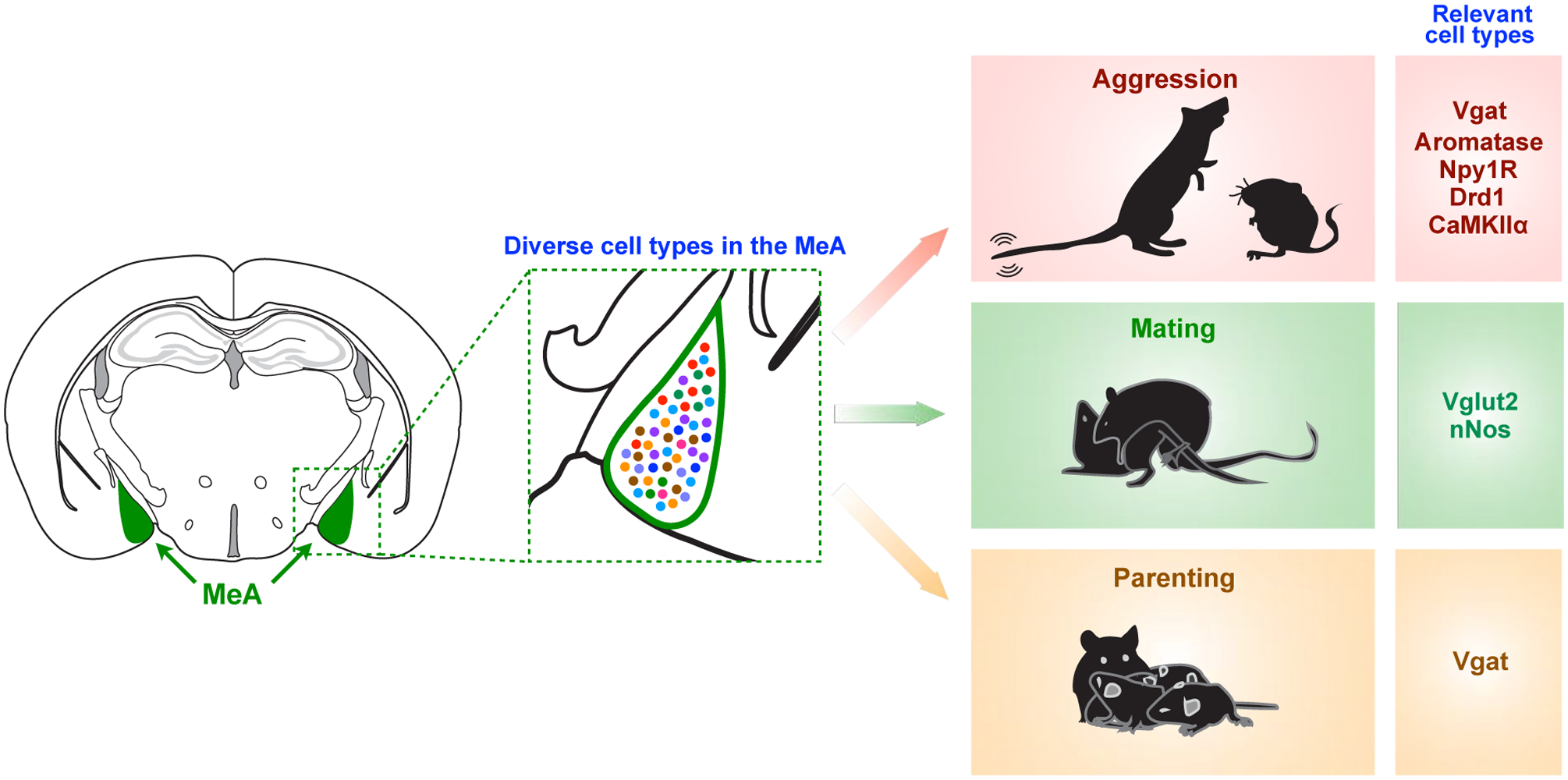
The MeA, which consists of diverse cell types, plays a critical role in a broad range of social behaviors including aggression, mating, and parenting. Each behavioral function is regulated by specific cell types in the MeA, summarized in Table 1.
The MeA consists of four anatomical subdivisions—anterodorsal (MeAad), anteroventral (MeAav), posterodorsal (MeApd), and posteroventral (MeApv). These subregions have markedly different anatomical features from one another, such as circuit connectivity and cytoarchitecture. While MeAad and MeApd contain a larger fraction of inhibitory neurons, MeAav and MeApv contain more excitatory neurons [14]. MeA subregions also project to a diverse set of brain areas mostly distributed in the amygdala and hypothalamus (Fig 3) [9,15]. While these subregions project densely to areas such as the bed nucleus of stria terminalis (BNST) and the medial preoptic area (MPOA), different MeA subregions project to different subregions of the BNST and ventral medial hypothalamus (VMH). The MeAad also projects back to the accessory olfactory bulb (AOB) (Fig 3) [9]. Interestingly, many of the regions which are targets of MeA outputs also send reciprocal projections back to the same MeA subregions which they received input from. For example, the BNST, MPOA, VMH, posterior amygdala (PA) and ventral premammillary nucleus (PMV) all send reciprocal projections back to the MeA [4,9,16–21]. In addition to these reciprocal projections, the MeA also receives unidirectional input from thalamic nuclei and entorhinal and perirhinal cortices [20,22]. Although anatomical mapping of inputs and outputs of human MeA has not been well established, many of these projections observed rodents are conserved in non-human primates. For example, inputs from olfactory bulbs, hypothalamic nuclei, amygdalar nuclei, BNST, entorhinal cortex have all been observed in primate MeA [23–26].
Fig. 3. Projection patterns of different MeA subregions.
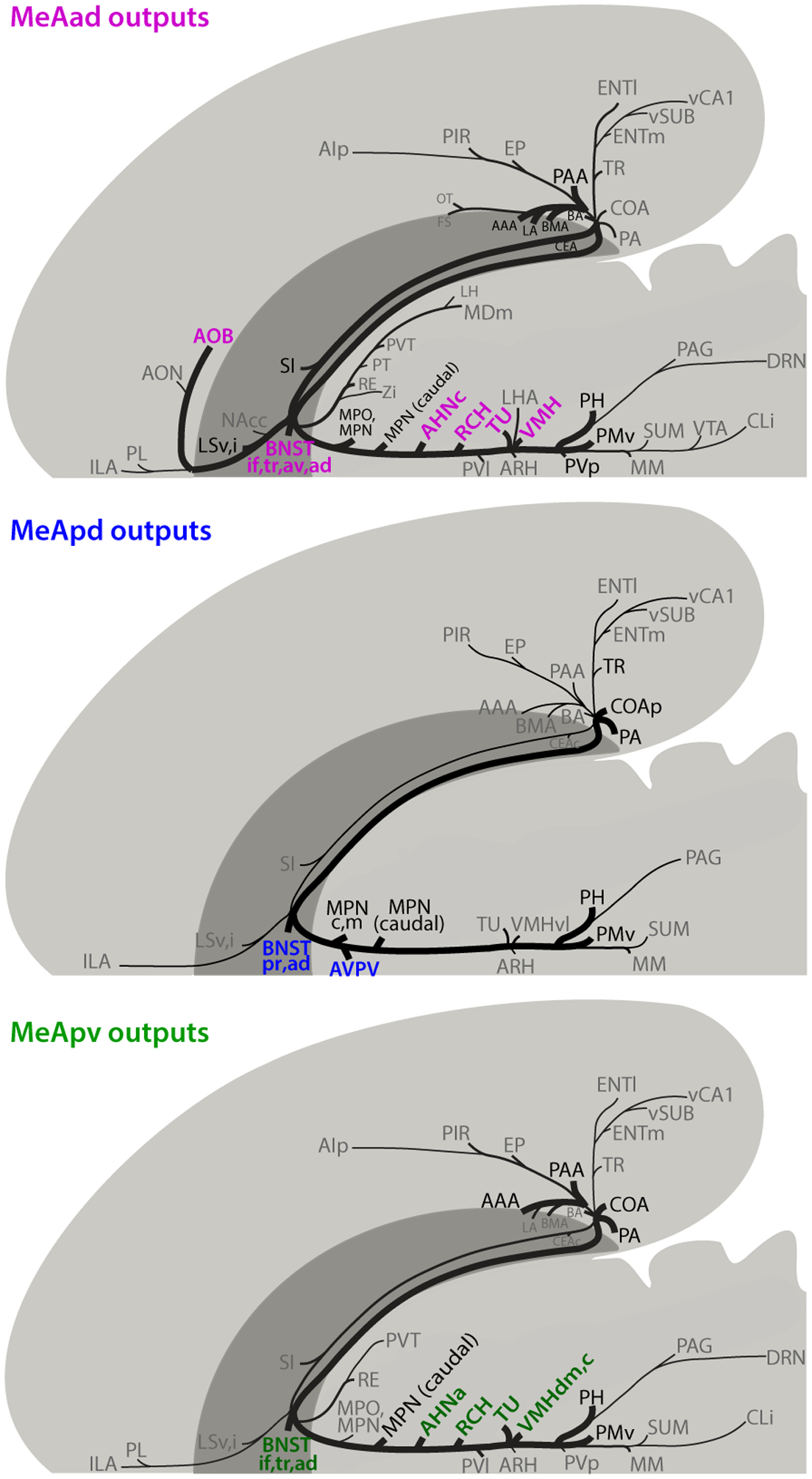
Schematics showing axonal projections from the MeAad (A), MeApd (B), and MeApv (C) in rats. Major brain targets that are unique to a particular MeA subregion are highlighted in color. Figures adapted with permission from Canteras et al. [9].
Distinct sub-classes of inhibitory output neurons in the MeA arise from separate embryonic lineages (Dbx1 and Foxp2) that originate from a unique migratory stream in the preoptic area (POA) of the telencephalon [27]. These two lineages display unique anatomical, electrophysiological, and behavioral properties, in a sexually dimorphic manner [28,29]. In addition to the telencephalon, the diencephalon also appears to be a major source of GABAergic and glutamatergic output neurons in the MeA [30–33]. Furthermore, recent single-cell RNA sequencing studies have provided a high-resolution picture of the molecular architecture of MeA neuronal subpopulations in mice (Fig 4) [34,35]. These studies have identified a number of transcriptionally distinguishable subgroups within both the GABAergic and glutamatergic neurons. These subpopulations are demarcated by the expression of a combination of different genes, notably neuropeptide genes. Moreover, a comparison between male and female MeA identified a number of sexually differentially expressed genes in both GABAergic and glutamatergic subpopulations [35], some of which (e.g. Greb1 and Brs3) were also identified in previous microarray studies using bulk MeA tissues [36]. Interestingly, GABAergic neurons appear to display greater sexually dimorphic gene expression than glutamatergic neurons, consistent with the sexually dimorphic behavioral functions of GABAergic neurons (discussed below) [35]. One open question is how these molecularly distinct subpopulations within the MeA relate to varying input-output circuitry and behavioral function.
Fig. 4. Major cell types and neuronal subtypes of the MeA defined by single-cell RNA-seq.
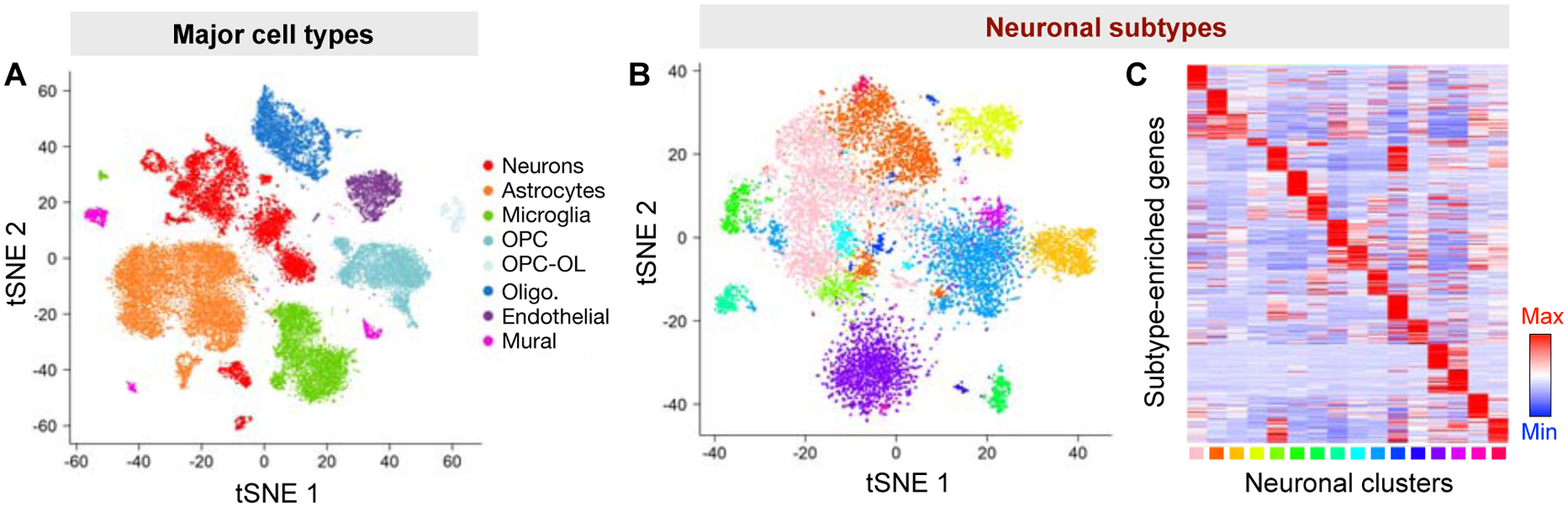
The MeA consists of diverse cell types (A) as well as a number of transcriptionally distinguishable neuronal subtypes (B, C). (A) and (B), tSNE visualization of MeA major cell types and neuronal subtypes. (C) Heatmap showing the expression pattern of MeA neuronal subtype markers across different neuronal subtypes. Figures adapted, with permission, from [35].
Functions of the MeA in various social behaviors
An important question is what specific role the MeA plays in social sensorimotor transformation. As the MeA receives strong afferents from the AOB and is only two synapses away from the VNO, it plays an important role in processing pheromonal signals and differentiating social sensory cues [12,13]. In vivo electrophysiological and optical imaging studies have found that the MeA is critical for differentiating between various social cues, including males, females, pups, and predator cues [37,38], and its responses to male and female conspecific odors are more specific than are AOB responses [38]. Population-based coding of social cues in the MeA is dependent on prior sexual experience, and relies on the action of oxytocin [37]. Importantly, disruptions in these coding schemes impair the animal’s ability to differentiate between different social targets and select the appropriate behavioral output [39]. Additionally, cFos immunoreactivity studies have found that MeApv activity is modulated by the social rank of social stimuli [40].
Beyond representation of social sensory cues, the MeA also regulates the generation of social behavioral outputs (Fig 2, Table 1). In particular, the MeApd subregion is important for the expression of aggression. c-Fos labeling studies have found elevated activity in MeA during male aggressive behaviors such as attack and mounting [4,41,42], and lesion studies implicated the MeA in aggressive behavior [43,44]. Recent studies using cell-type specific manipulations have shown that optogenetic stimulation of GABAergic, but not glutamatergic, neurons of the MeApd in males promotes aggression in a time-locked manner [45]. Conversely, optogenetic silencing of MeApd GABAergic neurons acutely suppresses ongoing aggression [45]. These findings support the notion that the MeA contains GABAergic neurons that positively promote aggression (however it does not imply that all MeA GABAergic neurons play a unitary role in promoting aggression). Similarly, ablating or chemogenetic silencing aromatase+ GABAergic neurons in the MeApd decreases inter-male aggression in males as well as maternal aggression against intruder males in females [46], and chemogenetic activation of Npy1R+ neurons in the MeA promotes aggression [47], suggesting that aggression can be controlled by specific neuronal subtypes within GABAergic population. Given that the MeA consists of heterogeneous subtypes of GABAergic neurons [34,35], it remains an open possibility that a subset of GABAergic neurons (such as certain local interneurons) may play a distinct (or even opposite) role in aggression. Interestingly, optogenetic stimulation of CaMKIIα+ neurons in the MeApv promotes aggression priming, the tendency to more readily express aggression after a recent aggressive encounter in males [48]. Although CaMKIIα is more specifically expressed in excitatory neurons in cortical regions as well as glutamatergic neurons within the MeApv, it labels both GABAergic and glutamatergic neurons throughout the MeA [35].
Table 1.
Behavioral functions of cell type-specific MeA neurons
| MeA subregion |
Cell type | Type of Manipulation | Construct | Change in Behavior | References |
|---|---|---|---|---|---|
| MeApd | Vgat | Optogenetic | ChR2 | ↑ aggression in males | Hong et al. 2014 [45] |
| eNpHR3 | ↓ aggression in males | ||||
| MeApd | Aromatase | Genetic ablation | Casp3 | ↓ intermale aggression in males | Unger et al. 2014 [46] |
| ↓ maternal aggression in females | |||||
| Chemogenetic | hM4Di | ↓ intermale aggression in males | |||
| MeA | Npy1R | Chemogenetic | hM3Dq | ↑ aggression in males | Padilla et al. 2016 [47] |
| Npy1 R (BNST projection) | Optogenetic | ChR2 | ↑ aggression in males | ||
| MeApv | CaMKIIα | Optogenetic | ChR2 (low freq) | ↓ aggression priming in males | Nordman et al. 2020 [48] |
| ChR2 (high freq) | ↑ aggression priming in males | ||||
| CaMKIIα (VMH and BNST projection) | Optogenetic | ChR2 (high freq) | ↑ aggression priming in males | ||
| ArchT | ↓ aggression priming in males | ||||
| MeApd/pv | nNOS | Optogenetic | eNpHR3 | ↓ female attractive response to male pheromone Darcin | Demir et al. 2020 [54] |
| MeApv | Vglut2 | Chemogenetic | hM4Di | ↓ sexual receptivity (lordosis) in females | Ishii et al. 2017 [53] |
| MeApd | Vgat | Optogenetic | ChR2 (low int) | ↑ pup grooming in females and males | Chen et al. 2019 [35] |
| ChR2 (high int) | ↑ pup attack in males | ||||
| eNpHR3 | ↓ pup grooming in females | ||||
| MeA | Ucn3 | Optogenetic | ChR2 | ↑ social novelty preference in males | Shemesh et al. 2016 [64] |
| Chemogenetic | hM4Di | ↓ social novelty preference in males | |||
| MeApv | Drd1 (VMH projection) | Optogenetic | ChR2 | ↑ avoidance of threat in males | Miller et al. 2019 [67] |
| Jaws | ↓ avoidance of threat in males | ||||
| Drd1 (BNST projection) | Optogenetic | ChR2 | ↑ approach to threat in males (including conspecific aggression) | ||
| Jaws | ↓ approach to threat in males |
Male aggression is historically thought to be related to reproductive drive, as males often compete for mating opportunities [49]. Indeed, Fos activity shows intermingled cells activated during fighting and mating in the MeApd [42], suggesting that the mechanism of reproductive behaviors may be partially linked to that of aggressive behavior. While lesion studies in males have implicated the MeA in mating behavior [50], its precise role remains to be further investigated. In contrast to males, female sexual behavior involves a different set of behavioral repertoires such as lordosis and seems to rely more heavily on the MeApv [51–53]. Although the MeApv is thought to be mainly involved in predator defensive behavior, Fos labeling shows that the male lacrimal pheromone ESP1 selectively activates neurons in the MeApv [53]. Moreover, the neurons that respond to sex pheromones in females are largely different from those responding to predator odors. Additionally, exposure to the male urinary protein Darcin induces vocalization and scent marking in females, and stimulation of Darcin-responsive neurons in the MeApv are sufficient to recapitulate these behaviors [54].
In addition to regulating interactions with adult conspecifics, the MeA also controls parenting behaviors. Early studies found that lesioning the MeA promotes parenting behavior such as pup retrieval, which seems to suggest that the MeA suppresses parenting behavior [55–57]. However, recent studies with greater cell-type and temporal specificity identify a more nuanced role for the MeA in parenting. Optogenetic stimulation of GABAergic neurons in the MeA promotes pup-directed parenting behaviors in both male and female mice, whereas high intensity stimulation in males induces infanticidal behavior [35]. Infanticidal behavior may depend on pheromonal input from the VNO-AOB system [58,59]. In virgin male mice (which more readily demonstrate infanticidal behaviors), the AOB and MeA both display high levels of Fos activity in response to pups [60], and impairments of VNO signaling induce pup retrieval in virgin males [61]. Thus, elevated VNO activity in response to pup pheromones in naïve males may hyperactivate the MeA to promote pup-directed aggression.
Finally, the MeA is also important for social recognition memory, which gives temporal permanence to an individual’s social interactions. Local infusion of oxytocin receptor antagonist into the MeA impairs social memory [62], and social memory requires oxytocin-dependent LTD at incoming synapses from the AOB to the MeA [63]. In addition, manipulations of the CRFR2 receptor and its associated ligand (Ucn3) in the MeA disrupt social novelty seeking [64]. In addition to the well-defined social functions of the MeA described above, MeA is also engaged in other behavioral contexts, such as social learning [65], group social dynamics [64], self-grooming behaviors [45], and inter-species interaction such as defensive behavior towards and avoidance of predators [37,53,66,67].
Importantly, although the function of the MeA has been difficult to elucidate in human and non-human primates, its involvement in social processes appears to be conserved. Recording and imaging studies in primates have found that MeA neurons respond to complex features of the social environment, such as facial expression, facial identity, pair bonding, and jealousy [68–71]. Additionally, lesion studies in primates suggest a role for the MeA in social functioning. While no studies have systematically lesioned only the MeA, large lesions that include the MeA along with other amygdalar nuclei result in decreased fear and aggression [72,73]. These studies in primates are complemented by studies in humans as well. Human MeA is thought to be important in social affiliation and maternal bonding [74,75]. Moreover, the MeA is often grouped as part of the CMA (centromedial amygdala, which includes the central nucleus of the amygdala), and the CMA is active during empathy and social reward [76,77]. Although no human lesion studies have specifically affected the MeA, large lesions of the amygdala that include MeA result in deficits in emotion recognition, while lesions that spare MeA do not affect social recognition [78,79].
Disambiguating social sensory processing vs. behavioral control
Given that the MeA sits at the crossroads of both sensory representations and behavioral outputs, an important question is whether the MeA is primarily involved in processing sensory stimuli, orchestrating behavioral outputs, or both. The question of disentangling sensory vs behavioral function can be difficult to address in any brain region, as behavioral outputs are strongly dependent on and linked to sensory states. Mimicking a pattern of activity that precisely recapitulates a sensory representation could theoretically produce the appropriate behavioral response to that stimulus. Similarly, silencing or impairing sensory regions can prevent expression of a behavior (as is the case in the VNO) [80,81], not necessarily because sensory regions are the main locus where social behavior outputs are determined, but because the requisite sensory representation is not relayed to downstream nuclei which control the appropriate social behavioral response. However, this seems unlikely to be the case in the MeA—while the MeA is only two synapses away from the vomeronasal sensory neurons, synchronous stimulation of broad classes of MeA populations induces robust behavioral repertoires in a time-locked manner across a variety of social contexts, described in Table 1. These stimulations are unlikely to precisely mimic endogenous sensory representations; if anything, they might disrupt them. That these manipulations produce specific social behavioral actions such as aggression, parenting, and sociosexual approach suggests that the MeA not only plays a role in relaying sensory cues to downstream targets, but itself controls behavioral outputs. Therefore, the MeA sits at the nexus of sensorimotor transformations and may be the earliest stage in the vomeronasal pathway in which specific social behavioral outputs are determined.
To determine how the MeA contributes to sensorimotor transformations, one important question is whether individual neurons in the MeA are selectively tuned to sensory cues vs. behavioral outputs. If individual MeA neurons represent both sensory cues and behavioral outputs, what activity patterns in these cells may differentiate the representations of each? If, however, sensory vs behavioral information is represented in different subsets of cells, what molecular, anatomical, or electrophysiological properties may differentiate these populations? Future studies that use in vivo electrophysiology or calcium imaging in specific cell-types or projection-specific populations may elucidate these mechanisms.
The question of whether sensory stimuli or behavioral decisions are represented in a population of neurons is an ongoing question in many areas of neuroscience, yet particularly challenging to tackle in the field of social neuroscience. Dissociating the neural correlates of sensory signals from behavioral output requires task parameters with enough temporal resolution to separate sensory cues from behavior. Although this resolution is frequently achieved in fields of neuroscience that study decision-making using tasks that have precise trial structures, often with a delay imposed [82–84], such resolution is very difficult to achieve in naturalistic settings such as social interactions, as any given social behavior may have tremendous variability from one event to another, even in the same animal. Different bouts of aggression, for example, may be associated with different contexts and internal states and can have varying latencies, durations, and combinations of behavioral syllables (chasing, mounting, biting). Moreover, the rapid timescale in which sensory cues are transformed to social behavioral decisions makes it difficult to dissociate the two, compounded by the fact that social cues (odor, tactile, auditory) are usually experienced in close physical proximity to the other social agent, rendering it difficult to disentangle sensory experience from behavior actions, such as approach, sniffing, and mounting. This is further complicated by the continually evolving nature of the stimulus itself—rather than responding to a single tone or odor, the animal is responding to another animal, which itself has dynamic behaviors and internal states that form a feedback loop between the two animals [1,85,86], so the sensory space and corresponding internal state of each animal is continually evolving during the course of an interaction. In spite of these hurdles, social neuroscientists are already finding creative ways to circumvent these challenges by developing tasks with clear trial structures where social sensory cues and behavior decisions can be more easily isolated [87–91]. We anticipate that the development of more such tasks will lead to a clearer understanding of sensorimotor transformation in the context of social behaviors.
How does one brain area mediate several different social behaviors?
An important question that is relevant across brain regions which mediate diverse, sometimes conflicting, functions is what mechanisms might be engaged to segregate different behavioral states from each other (Fig 1). The MeA serves as a useful example to consider how molecular, anatomical, and activity-dependent features are engaged to promote distinct behaviors (Fig 2, Table 1).
The MeA is a cytoarchitecturally diverse nucleus containing many different subtypes of neurons [34,35]. Mounting evidence suggests that different subpopulations may play different roles in behavior (Table 1). For example, optogenetic manipulation of GABAergic and glutamatergic neurons in the MeA has revealed that GABAergic neurons promote aggression and pup-directed parenting while glutamatergic neurons promote self-grooming, respectively [35,45]. Ablating or silencing aromatase+ GABAergic neurons in MeApd decreases aggression in both male and female mice [46]. Activation of Npy1R-expressing neurons in the MeA promotes aggression [47]. Silencing of Nos1+ neurons in MeA in females interrupts the expression of behavioral repertoires induced by the male urinary protein Darcin—ultrasonic vocalization and urinary scent marking [54]. Additionally, Vglut2+ cells in MeApv are important for the expression of sexually receptive lordosis behavior in females [53], and deletion of Oxtr in aromatase-expressing neurons impairs male preference for female interactions [39]. It is important to note that when manipulation of a molecularly defined cell type can modulate a behavior, it does not mean that all neurons within that cell type play a unitary role in that behavior. Given the heterogeneity of neuronal subtypes, it is always a possibility that a behavior is controlled by a subset of neurons within that cell type and/or that different subsets of that cell type may exert distinct or even opposite roles. Finer dissection of the aforementioned MeA cell types will provide new insights into the functional organization of heterogeneous MeA neurons in diverse social behaviors. Moreover, while many studies use neuromodulators or their receptors as genetic markers to manipulate molecularly defined cell types in the MeA, the functional roles of these genes have been much less understood. The remarkably diverse convergence of neuromodulator systems in the MeA suggests that neuromodulation may pay a key role in shaping behaviors depending on the motivational state of the animal.
In addition to molecular features such as cell types, the MeA also uses unique connectivity patterns to compartmentalize different social behaviors. Each MeA subregion send projections to a number of downstream targets (Fig 2), which may each serve unique functions. Activation of projection of MeA Npy1R+ neurons to BNST but not VMHvl promotes territorial aggression [47]. Additionally, MeApv Drd1+ neurons project to both BNST and VMHdm, yet stimulation of the BNST projection induces approach of threatening stimuli, whereas stimulation of the VMHdm pathway induces avoidance of the same stimuli [67]. Moreover, activation of MeApv projections to VMHvl and BNST, but not MPOA or LS, promotes aggression in males [48]. Synaptic inputs from MeApv to VMHvl and BNST are potentiated after foot shock exposure, and depotentiating them with low frequency stimulation prevents stress-induced aggression after foot shock [92]. Additional studies elucidating the role of specific MeA projections in different behavioral contexts will yield further insight into anatomical features used to dissociate different behaviors. Finally, some studies have also examined the functional role of inputs onto MeA. Activating inhibitory inputs from AgRP neurons that suppress MeA GABAergic neurons inhibits aggression [47]. Stimulating inputs from dorsal raphe nucleus onto MeA is also shown to suppress attack, but it is unclear which MeA subdivision or neuronal type that mediates this effect [93]. It also remains to be determined whether individual MeA neurons that project to different downstream areas receive the same or distinct neuronal inputs. Future studies that combine projection-specific input mapping such as TRIO with circuit manipulations may identify similar mechanisms in the MeA [94].
A third mechanism by which the MeA dissociates distinct social behaviors is through coding strategies at the population level. For example, in vivo microendoscopic calcium imaging identifies distinct populations of neurons that encode social cues such as male, female, pup, and predator odors [11,37]. In male mice, more MeA neurons are tuned to female cues, and in female mice, more MeA neurons are tuned to male cues, which may aid in mediating reproductive behaviors in both sexes [38,39]. These population-based coding schemes are highly dependent on prior sexual history in males, as well as the actions of the neuropeptide oxytocin [37,39]. In males that lack Oxtr expression in MeA aromatase neurons, strong population responses to female cues become less tuned and more broadly responsive to predator and male cues [39]. These tuning properties to distinct social cues may overlap with molecular and anatomical features defined above. For example, cells that preferentially respond to a particular social stimulus may belong to a molecularly-defined or projection-specific subpopulation of neurons. Further studies recording from specific populations of neurons will clarify these possibilities.
In addition to representation of social sensory cues, different activity patterns are associated with distinct behavioral outputs. For example, fiber photometry recordings of GCaMP6 activity show moderate activity during pup grooming and high activity during pup attack [35]. This is consistent with scalable optogenetic activation experiments that promote distinct behaviors at different levels of intensity—low level stimulation promotes pup grooming, whereas high-level stimulation promotes infanticidal attack behavior [35]. Moreover, scalable optogenetic activation of MeApd GABAergic neurons can lead to mounting (low-intensity stimulation) vs attack (high-intensity stimulation) in inter-male interactions [45,48]. Similarly, low frequency optogenetic stimulation of MeApv CamKIIα+ neurons suppresses aggression, while high frequency stimulation increases aggression [48]. These studies provide intriguing insights into how different levels of neural activity in MeA may promote different behaviors. Multiple possibilities may explain these observations. Different levels of activity may potentially recruit different subpopulations of neurons that play distinct roles in a particular behavior—for instance, pup retrieval and infanticidal behaviors may be modulated by two distinct ensembles of neurons that are preferentially activated by low or high intensity stimulations. Alternatively, different levels of activity may recruit different sizes of a common population such that the size of the recruited population modulates different behavioral outputs. Finally, higher levels of activity may reflect the same ensemble of neurons firing at a higher frequency. Distinguishing between these various possibilities will require future studies using in vivo electrophysiology or calcium imaging that confer cellular identity resolution.
How is one brain region integrated into the broader circuitry for social behavior?
Although the MeA plays a critical role in a diverse set of social behaviors, there are also several other brain regions and circuits that contribute to the same social processes. For example, neural representation of distinct social cues such as conspecific sex, social identity, dominance status, and offspring has been observed in other brain regions such as the VMHvl [42,95,96], as well as the prefrontal cortex [97–101] and hippocampus [102,103]. Aggressive behaviors are also modulated by a broad network of regions, including most notably VMHvl [42,87,95], as well as the lateral septum [104], nucleus accumbens [91], posterior amygdala [105–107], BNST [108], dorsomedial hypothalamus [109] and periaqueductal gray (PAG) [110]. Parenting and infanticidal functions of the MeA overlap with other regions including MPOA [111–116], and sensory regions such as VNO [58–61] and auditory cortex [117–119]. Finally, social recognition memory is also mediated by a broad network of areas including hippocampal areas CA2 [120,121] and vCA1 [102,122–124], prefrontal cortex [125], and olfactory circuits [126]. Although this is not an exhaustive description of all circuits and regions that share overlapping functions with the MeA, it serves to illustrate heterogenous brain regions that form a larger network for complex social behaviors.
Given that a multitude of regions beyond the MeA are implicated in any given social behavior, we can ask whether these brain regions play similar or different roles and how they contribute to making specific social decisions. One conceptual question is where a particular social decision is made. Are social decisions made in a single locus, or shaped and modulated by multiple brain areas? Manipulations of neural components using optogenetics and chemogenetics suggests that different brain areas may serve overlapping functions in a behavior—for example, manipulations of MeA, VMHvl, and PAG all lead to increases or deficits in aggressive behavior. However, another possibility is that while manipulations of several regions may all lead to an apparently common behavioral output, each may play unique roles in modulating that behavior that are hidden when using binary manipulations to activate or silence a circuit. For example, a recent study used multi-site recording strategies to identify that elements of an aggressive encounter are hierarchically represented in a step-wise, linear fashion along the VMHvl-lPAG-jaw circuit [110]. Within this circuit, male social sensory cues represented in the VMHvl are relayed through a monosynaptic circuit to the lPAG and jaw, which represent finer aspects of the aggressive bout—lPAG neurons are selective for the attack, while the jaw muscle is selective for the bite specifically [110]. The use of a similar experimental framework may prove informative in understanding the coding properties of the MeA in relation to its in inputs and outputs as well. Although it is currently unclear how MeA signals compare with signals in downstream regions such as VMHvl, the MeA may function as an intermediate layer of processing by integrating chemosensory input from the vomeronasal network with behavior outputs and routing these signals to appropriate downstream regions mediating specific social behaviors, such as aggression and parenting. Alternatively, some overlap in function may indeed exist between some nuclei, and this may serve as a redundant or compensatory mechanism to ensure that critical functions are maintained even when circuit dysregulation occurs in a brain region.
Parsing out these possible models may be best achieved using multi-site or brain-wide recording strategies that enable direct comparison of coding strategies across nuclei [127–132]. For example, simultaneous paired recordings from the MeA and VMHvl during bouts of aggression, or MeA and MPOA during parenting, would enable time-locked comparison of neural dynamics in both regions, potentially revealing distinct coding properties during the sensory cues, onset, and completion of behavior bouts. The resolution required for such analysis may not be attainable when comparing coding properties across studies which have performed recordings from single brain regions. It may also provide an additional layer of experimental rigor in social behaviors where imposition of a trial structure is not feasible. In these cases, although behavioral variability across events may confound comparison of crosstrial neural dynamics, simultaneous recordings of two brain regions would still enable time-locked comparison during a single behavior event. Additionally, as the distinct roles of MeA subregions and cell-types in various behaviors were mostly studied in different research groups using different technologies and behavioral paradigms, it would be important to directly compare these cell-types using identical experimental conditions. New developments in machine learning-based animal tracking and behavior analysis also show promise to streamline behavior analysis across laboratories and paradigms [127,133–136].
Concluding remarks
Although this review has focused on the organization and functions of the MeA, many of the conceptual considerations and challenges can be extended and applied to other brain regions which also mediate a variety of social behaviors. The basic principles by which single brain regions coordinate multiple social behaviors, and multiple brain regions interact to orchestrate a single social behavior stand out as critical knowledge gaps facing the field of social neuroscience as we continue to articulate a social behavior network in the brain. Increasingly precise tools for tracing, recording, and manipulating specific circuit components will enable the dissection of molecular, anatomical, and electrophysiological mechanisms that segregate behavioral functions within a single brain region. Employing multi-site recording strategies of multiple regions simultaneously will enable comparison of regional heterogeneity of function on a fine scale during social encounters. Furthermore, the development of social behavior assays that take advantage of a trial structure will enable us to more readily disambiguate the neural representations of sensory and motor components of a social interaction. The expansion of experimental tools and novel behavioral assays will prove useful in addressing the range of conceptual issues under consideration and contribute to a clearer understanding of how social behaviors are coordinated by neural circuits.
Highlights.
The medial amygdala (MeA) is critical for processing social sensory signals and regulating a broad range of social behaviors.
The MeA coordinates multiple social behaviors through distinct cell types, projections, or population activity.
The MeA is embedded in a broader neural network that orchestrates social sensorimotor transformation.
Multiple brain regions interact in a distributed brain network to orchestrate a single social behavior.
Acknowledgements
We would like to thank Dayu Lin, Joshua Corbin, Samara Miller, Emily Wu, Lyle Kingsbury, James Dang, and an anonymous reviewer for thoughtful comments on this manuscript. We also thank Samara Miller for providing part of the illustration in Fig 2. This work was supported in part by NIH grants T32-NS048004, R01-NS113124, and RF1-DA048811, a Searle Scholar Award, a Klingenstein-Simons Fellowship, a Packard Foundation Fellowship, a McKnight Scholar Award, a Vallee Scholar Award, a Mallinckrodt Scholar Award, and a Keck Foundation Junior Faculty Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References
- 1.Chen P, Hong W: Neural Circuit Mechanisms of Social Behavior. Neuron 2018, 98:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanson LW: Cerebral hemisphere regulation of motivated behavior. Brain Res 2000, 886:113–164. [DOI] [PubMed] [Google Scholar]
- 3.Petrulis A: Structure and Function of the Medial Amygdala. Handb Behav Neurosci 2020, 26:39–61. [Google Scholar]
- 4.Newman SW: The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci 1999, 877:242–257. [DOI] [PubMed] [Google Scholar]
- 5.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, et al. : Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010, 468:277–282. [DOI] [PubMed] [Google Scholar]
- 6.Janak PH, Tye KM: From circuits to behaviour in the amygdala. Nature 2015, 517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pignatelli M, Beyeler A: Valence coding in amygdala circuits. Curr Opin Behav Sci 2019, 26:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyeler A, Dabrowska J: Neuronal diversity of the amygdala and the bed nucleus of the stria terminalis. Handb Behav Neurosci 2020, 26:63–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canteras NS, Simerly RB, Swanson LW: Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 1995, 360:213–245. [DOI] [PubMed] [Google Scholar]
- 10.Keshavarzi S, Power JM, Albers EH, Sullivan RK, Sah P: Dendritic Organization of Olfactory Inputs to Medial Amygdala Neurons. J Neurosci 2015, 35:13020–13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Dulac C: Neural coding of sex-specific social information in the mouse brain. Curr Opin Neurobiol 2018, 53:120–130. [DOI] [PubMed] [Google Scholar]
- 12.Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del Mar Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A: Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol 2007, 504:346–362. [DOI] [PubMed] [Google Scholar]
- 13.Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Banon I, Crespo C, Insausti R, Martinez-Marcos A: Segregated pathways to the vomeronasal amygdala: differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci 2007, 25:2065–2080. [DOI] [PubMed] [Google Scholar]
- 14.Keshavarzi S, Sullivan RK, Ianno DJ, Sah P: Functional properties and projections of neurons in the medial amygdala. J Neurosci 2014, 34:8699–8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardo-Bellver C, Cadiz-Moretti B, Novejarque A, Martinez-Garcia F, Lanuza E: Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat 2012, 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu G, Cornea A, Simerly RB: Sexual differentiation of projections from the principal nucleus of the bed nuclei of the stria terminalis. J Comp Neurol 2003, 460:542–562. [DOI] [PubMed] [Google Scholar]
- 17.Akesson TR, Simerly RB, Micevych PE: Estrogen-concentrating hypothalamic and limbic neurons project to the medial preoptic nucleus. Brain Res 1988, 451:381–385. [DOI] [PubMed] [Google Scholar]
- 18.Canteras NS, Simerly RB, Swanson LW: Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 1994, 348:41–79. [DOI] [PubMed] [Google Scholar]
- 19.Soden ME, Miller SM, Burgeno LM, Phillips PEM, Hnasko TS, Zweifel LS: Genetic Isolation of Hypothalamic Neurons that Regulate Context-Specific Male Social Behavior. Cell Rep 2016, 16:304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cadiz-Moretti B, Otero-Garcia M, Martinez-Garcia F, Lanuza E: Afferent projections to the different medial amygdala subdivisions: a retrograde tracing study in the mouse. Brain Struct Funct 2016, 221:1033–1065. [DOI] [PubMed] [Google Scholar]
- 21.Canteras NS, Simerly RB, Swanson LW: Connections of the posterior nucleus of the amygdala. J Comp Neurol 1992, 324:143–179. [DOI] [PubMed] [Google Scholar]
- 22.McDonald AJ, Mascagni F: Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 1997, 77:445–459. [DOI] [PubMed] [Google Scholar]
- 23.Turner BH, Gupta KC, Mishkin M: The locus and cytoarchitecture of the projection areas of the olfactory bulb in Macaca mulatta. J Comp Neurol 1978, 177:381–396. [DOI] [PubMed] [Google Scholar]
- 24.Amaral DG, Veazey RB, Cowan WM: Some observations on hypothalamo-amygdaloid connections in the monkey. Brain Res 1982, 252:13–27. [DOI] [PubMed] [Google Scholar]
- 25.McDonald AJ: Cortical pathways to the mammalian amygdala. Prog Neurobiol 1998, 55:257–332. [DOI] [PubMed] [Google Scholar]
- 26.Martin LJ, Powers RE, Dellovade TL, Price DL: The bed nucleus-amygdala continuum in human and monkey. J Comp Neurol 1991, 309:445–485. [DOI] [PubMed] [Google Scholar]
- 27.Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG: Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci 2009, 12:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lischinsky JE, Sokolowski K, Li P, Esumi S, Kamal Y, Goodrich M, Oboti L, Hammond TR, Krishnamoorthy M, Feldman D, et al. : Embryonic transcription factor expression in mice predicts medial amygdala neuronal identity and sex-specific responses to innate behavioral cues. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matos HY, Hernandez-Pineda D, Charpentier CM, Rusk A, Corbin JG, Jones KS: Sex Differences in Biophysical Signatures across Molecularly Defined Medial Amygdala Neuronal Subpopulations. eNeuro 2020, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soma M, Aizawa H, Ito Y, Maekawa M, Osumi N, Nakahira E, Okamoto H, Tanaka K, Yuasa S: Development of the mouse amygdala as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J Comp Neurol 2009, 513:113–128. [DOI] [PubMed] [Google Scholar]
- 31.Zhao T, Szabo N, Ma J, Luo L, Zhou X, Alvarez-Bolado G: Genetic mapping of Foxb1-cell lineage shows migration from caudal diencephalon to telencephalon and lateral hypothalamus. Eur J Neurosci 2008, 28:1941–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bupesh M, Legaz I, Abellan A, Medina L: Multiple telencephalic and extratelencephalic embryonic domains contribute neurons to the medial extended amygdala. J Comp Neurol 2011, 519:1505–1525. [DOI] [PubMed] [Google Scholar]
- 33.Morales L, Castro-Robles B, Abellan A, Desfilis E, Medina L: A novel telencephalon-opto-hypothalamic morphogenetic domain coexpressing Foxg1 and Otp produces most of the glutamatergic neurons of the medial extended amygdala. J Comp Neurol 2021. [DOI] [PubMed] [Google Scholar]
- 34.Wu YE, Pan L, Zuo Y, Li X, Hong W: Detecting Activated Cell Populations Using Single-Cell RNA-Seq. Neuron 2017, 96:313–329 e316. [DOI] [PubMed] [Google Scholar]
- 35.Chen PB, Hu RK, Wu YE, Pan L, Huang S, Micevych PE, Hong W: Sexually Dimorphic Control of Parenting Behavior by the Medial Amygdala. Cell 2019, 176:1206–1221 e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM: Modular genetic control of sexually dimorphic behaviors. Cell 2012, 148:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, Murthy VN, Dulac C: Neuronal Representation of Social Information in the Medial Amygdala of Awake Behaving Mice. Cell 2017, 171:1176–1190 e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergan JF, Ben-Shaul Y, Dulac C: Sex-specific processing of social cues in the medial amygdala. Elife 2014, 3:e02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao S, Bergan J, Lanjuin A, Dulac C: Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee W, Dowd HN, Nikain C, Dwortz MF, Yang ED, Curley JP: Effect of relative social rank within a social hierarchy on neural activation in response to familiar or unfamiliar social signals. Sci Rep 2021, 11:2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veening JG, Coolen LM, de Jong TR, Joosten HW, de Boer SF, Koolhaas JM, Olivier B: Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur J Pharmacol 2005, 526:226–239. [DOI] [PubMed] [Google Scholar]
- 42.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ: Functional identification of an aggression locus in the mouse hypothalamus. Nature 2011, 470:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemble ED, Blanchard DC, Blanchard RJ, Takushi R: Taming in wild rats following medial amygdaloid lesions. Physiol Behav 1984, 32:131–134. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi LK, Gladstone CD: Medial amygdaloid lesions and the regulation of sociosexual behavioral patterns across the estrous cycle in female golden hamsters. Behav Neurosci 1988, 102:268–275. [DOI] [PubMed] [Google Scholar]
- 45.Hong W, Kim DW, Anderson DJ: Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 2014, 158:1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unger EK, Burke KJ Jr., Yang CF, Bender KJ, Fuller PM, Shah NM: Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep 2015, 10:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, Quintana A, Zweifel LS, Ronnekleiv OK, Kelly MJ, et al. : Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci 2016, 19:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordman JC, Ma X, Gu Q, Potegal M, Li H, Kravitz AV, Li Z: Potentiation of Divergent Medial Amygdala Pathways Drives Experience-Dependent Aggression Escalation. J Neurosci 2020, 40:4858–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hashikawa K, Hashikawa Y, Falkner A, Lin D: The neural circuits of mating and fighting in male mice. Curr Opin Neurobiol 2016, 38:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kondo Y: Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiol Behav 1992, 51:939–943. [DOI] [PubMed] [Google Scholar]
- 51.Rajendren G, Moss RL: The role of the medial nucleus of amygdala in the mating-induced enhancement of lordosis in female rats: the interaction with luteinizing hormone-releasing hormone neuronal system. Brain Res 1993, 617:81–86. [DOI] [PubMed] [Google Scholar]
- 52.DiBenedictis BT, Ingraham KL, Baum MJ, Cherry JA: Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol Behav 2012, 105:554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishii KK, Osakada T, Mori H, Miyasaka N, Yoshihara Y, Miyamichi K, Touhara K: A Labeled-Line Neural Circuit for Pheromone-Mediated Sexual Behaviors in Mice. Neuron 2017, 95:123–137 e128. [DOI] [PubMed] [Google Scholar]
- 54.Demir E, Li K, Bobrowski-Khoury N, Sanders JI, Beynon RJ, Hurst JL, Kepecs A, Axel R: The pheromone darcin drives a circuit for innate and reinforced behaviours. Nature 2020, 578:137–141. [DOI] [PubMed] [Google Scholar]
- 55.Fleming AS, Vaccarino F, Luebke C: Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav 1980, 25:731–743. [DOI] [PubMed] [Google Scholar]
- 56.Numan M, Numan MJ, English JB: Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Horm Behav 1993, 27:56–81. [DOI] [PubMed] [Google Scholar]
- 57.Sheehan T, Paul M, Amaral E, Numan MJ, Numan M: Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience 2001, 106:341–356. [DOI] [PubMed] [Google Scholar]
- 58.Isogai Y, Wu Z, Love MI, Ahn MH, Bambah-Mukku D, Hua V, Farrell K, Dulac C: Multisensory Logic of Infant-Directed Aggression by Males. Cell 2018, 175:1827–1841 e1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trouillet AC, Keller M, Weiss J, Leinders-Zufall T, Birnbaumer L, Zufall F, Chamero P: Central role of G protein Galphai2 and Galphai2(+) vomeronasal neurons in balancing territorial and infant-directed aggression of male mice. Proc Natl Acad Sci U S A 2019, 116:5135–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tachikawa KS, Yoshihara Y, Kuroda KO: Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J Neurosci 2013, 33:5120–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG: Galanin neurons in the medial preoptic area govern parental behaviour. Nature 2014, 509:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferguson JN, Aldag JM, Insel TR, Young LJ: Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 2001, 21:8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gur R, Tendler A, Wagner S: Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol Psychiatry 2014, 76:377–386. [DOI] [PubMed] [Google Scholar]
- 64.Shemesh Y, Forkosh O, Mahn M, Anpilov S, Sztainberg Y, Manashirov S, Shlapobersky T, Elliott E, Tabouy L, Ezra G, et al. : Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics. Nat Neurosci 2016, 19:1489–1496. [DOI] [PubMed] [Google Scholar]
- 65.Twining RC, Vantrease JE, Love S, Padival M, Rosenkranz JA: An intra-amygdala circuit specifically regulates social fear learning. Nat Neurosci 2017, 20:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ: Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 2005, 46:647–660. [DOI] [PubMed] [Google Scholar]
- 67.Miller SM, Marcotulli D, Shen A, Zweifel LS: Divergent medial amygdala projections regulate approach-avoidance conflict behavior. Nat Neurosci 2019, 22:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brothers L, Ring B, Kling A: Response of neurons in the macaque amygdala to complex social stimuli. Behav Brain Res 1990, 41:199–213. [DOI] [PubMed] [Google Scholar]
- 69.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG: Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol 2007, 97:1671–1683. [DOI] [PubMed] [Google Scholar]
- 70.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK: Facial-expression and gaze-selective responses in the monkey amygdala. Curr Biol 2007, 17:766–772. [DOI] [PubMed] [Google Scholar]
- 71.Leonard CM, Rolls ET, Wilson FA, Baylis GC: Neurons in the amygdala of the monkey with responses selective for faces. Behav Brain Res 1985, 15:159–176. [DOI] [PubMed] [Google Scholar]
- 72.Meunier M, Bachevalier J, Murray EA, Malkova L, Mishkin M: Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci 1999, 11:4403–4418. [DOI] [PubMed] [Google Scholar]
- 73.Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG: The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behav Neurosci 2001, 115:515–544. [PubMed] [Google Scholar]
- 74.Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC: Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci 2012, 32:14729–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Atzil S, Touroutoglou A, Rudy T, Salcedo S, Feldman R, Hooker JM, Dickerson BC, Catana C, Barrett LF: Dopamine in the medial amygdala network mediates human bonding. Proc Natl Acad Sci U S A 2017, 114:2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB: Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct 2012, 217:783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN: Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 2010, 49:3276–3285. [DOI] [PubMed] [Google Scholar]
- 78.Adolphs R, Baron-Cohen S, Tranel D: Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci 2002, 14:1264–1274. [DOI] [PubMed] [Google Scholar]
- 79.Becker B, Mihov Y, Scheele D, Kendrick KM, Feinstein JS, Matusch A, Aydin M, Reich H, Urbach H, Oros-Peusquens AM, et al. : Fear processing and social networking in the absence of a functional amygdala. Biol Psychiatry 2012, 72:70–77. [DOI] [PubMed] [Google Scholar]
- 80.Stowers L, Holy TE, Meister M, Dulac C, Koentges G: Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 2002, 295:1493–1500. [DOI] [PubMed] [Google Scholar]
- 81.Kimchi T, Xu J, Dulac C: A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 2007, 448:1009–1014. [DOI] [PubMed] [Google Scholar]
- 82.Harvey CD, Coen P, Tank DW: Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 2012, 484:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Driscoll LN, Pettit NL, Minderer M, Chettih SN, Harvey CD: Dynamic Reorganization of Neuronal Activity Patterns in Parietal Cortex. Cell 2017, 170:986–999 e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alabi OO, Fortunato MP, Fuccillo MV: Behavioral Paradigms to Probe Individual Mouse Differences in Value-Based Decision Making. Front Neurosci 2019, 13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kingsbury L, Huang S, Wang J, Gu K, Golshani P, Wu YE, Hong W: Correlated Neural Activity and Encoding of Behavior across Brains of Socially Interacting Animals. Cell 2019, 178:429–446 e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kingsbury L, Hong W: A Multi-Brain Framework for Social Interaction. Trends Neurosci 2020, 43:651–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D: Hypothalamic control of male aggression-seeking behavior. Nat Neurosci 2016, 19:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Golden SA, Heins C, Venniro M, Caprioli D, Zhang M, Epstein DH, Shaham Y: Compulsive Addiction-like Aggressive Behavior in Mice. Biol Psychiatry 2017, 82:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Covington HE 3rd, Newman EL, Tran S, Walton L, Hayek W, Leonard MZ, DeBold JF, Miczek KA: The Urge to Fight: Persistent Escalation by Alcohol and Role of NMDA Receptors in Mice. Front Behav Neurosci 2018, 12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Golden SA, Jin M, Shaham Y: Animal Models of (or for) Aggression Reward, Addiction, and Relapse: Behavior and Circuits. J Neurosci 2019, 39:3996–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Golden SA, Jin M, Heins C, Venniro M, Michaelides M, Shaham Y: Nucleus Accumbens Drd1-Expressing Neurons Control Aggression Self-Administration and Aggression Seeking in Mice. J Neurosci 2019, 39:2482–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nordman J, Ma X, Li Z: Traumatic Stress Induces Prolonged Aggression Increase through Synaptic Potentiation in the Medial Amygdala Circuits. eNeuro 2020, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nordman J, Li Z: The Dorsal Raphe Regulates the Duration of Attack through the Medial Orbitofrontal Cortex and Medial Amygdala. eNeuro 2020, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, et al. : Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 2015, 524:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Falkner AL, Dollar P, Perona P, Anderson DJ, Lin D: Decoding ventromedial hypothalamic neural activity during male mouse aggression. J Neurosci 2014, 34:5971–5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Remedios R, Kennedy A, Zelikowsky M, Grewe BF, Schnitzer MJ, Anderson DJ: Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 2017, 550:388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kingsbury L, Huang S, Raam T, Ye LS, Wei D, Hu RK, Ye L, Hong W: Cortical Representations of Conspecific Sex Shape Social Behavior. Neuron 2020, 107:941–953 e947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levy DR, Tamir T, Kaufman M, Parabucki A, Weissbrod A, Schneidman E, Yizhar O: Dynamics of social representation in the mouse prefrontal cortex. Nat Neurosci 2019, 22:2013–2022. [DOI] [PubMed] [Google Scholar]
- 99.Zhou T, Zhu H, Fan Z, Wang F, Chen Y, Liang H, Yang Z, Zhang L, Lin L, Zhan Y, et al. : History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 2017, 357:162–168. [DOI] [PubMed] [Google Scholar]
- 100.Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H: Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 2011, 334:693–697. [DOI] [PubMed] [Google Scholar]
- 101.Raam T: Oxytocin-Sensitive Neurons in Prefrontal Cortex Gate Social Recognition Memory. J Neurosci 2020, 40:1194–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A: Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun 2017, 8:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rao RP, von Heimendahl M, Bahr V, Brecht M: Neuronal Responses to Conspecifics in the Ventral CA1. Cell Rep 2019, 27:3460–3472 e3463. [DOI] [PubMed] [Google Scholar]
- 104.Leroy F, Park J, Asok A, Brann DH, Meira T, Boyle LM, Buss EW, Kandel ER, Siegelbaum SA: A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 2018, 564:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamaguchi T, Wei D, Song SC, Lim B, Tritsch NX, Lin D: Posterior amygdala regulates sexual and aggressive behaviors in male mice. Nat Neurosci 2020, 23:1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stagkourakis S, Spigolon G, Liu G, Anderson DJ: Experience-dependent plasticity in an innate social behavior is mediated by hypothalamic LTP. Proc Natl Acad Sci U S A 2020, 117:25789–25799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zha X, Wang L, Jiao ZL, Yang RR, Xu C, Xu XH: VMHvl-Projecting Vglut1+ Neurons in the Posterior Amygdala Gate Territorial Aggression. Cell Rep 2020, 31:107517. [DOI] [PubMed] [Google Scholar]
- 108.Bayless DW, Yang T, Mason MM, Susanto AAT, Lobdell A, Shah NM: Limbic Neurons Shape Sex Recognition and Social Behavior in Sexually Naive Males. Cell 2019, 176:1190–1205 e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zelikowsky M, Hui M, Karigo T, Choe A, Yang B, Blanco MR, Beadle K, Gradinaru V, Deverman BE, Anderson DJ: The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress. Cell 2018, 173:1265–1279 e1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Falkner AL, Wei D, Song A, Watsek LW, Chen I, Chen P, Feng JE, Lin D: Hierarchical Representations of Aggression in a Hypothalamic-Midbrain Circuit. Neuron 2020, 106:637–648 e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fang YY, Yamaguchi T, Song SC, Tritsch NX, Lin D: A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron 2018, 98:192–207 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, Miyamishi K, Zweifel LS, Luo L, Uchida N, et al. : Functional circuit architecture underlying parental behaviour. Nature 2018, 556:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li XY, Han Y, Zhang W, Wang SR, Wei YC, Li SS, Lin JK, Yan JJ, Chen AX, Zhang X, et al. : AGRP Neurons Project to the Medial Preoptic Area and Modulate Maternal Nest-Building. J Neurosci 2019, 39:456–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stagkourakis S, Smiley KO, Williams P, Kakadellis S, Ziegler K, Bakker J, Brown RSE, Harkany T, Grattan DR, Broberger C: A Neuro-hormonal Circuit for Paternal Behavior Controlled by a Hypothalamic Network Oscillation. Cell 2020, 182:960–975 e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kohl J: Parenting - a paradigm for investigating the neural circuit basis of behavior. Curr Opin Neurobiol 2020, 60:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei YC, Wang SR, Jiao ZL, Zhang W, Lin JK, Li XY, Li SS, Zhang X, Xu XH: Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat Commun 2018, 9:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC: Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 2015, 520:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schiavo JK, Valtcheva S, Bair-Marshall CJ, Song SC, Martin KA, Froemke RC: Innate and plastic mechanisms for maternal behaviour in auditory cortex. Nature 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tasaka GI, Feigin L, Maor I, Groysman M, DeNardo LA, Schiavo JK, Froemke RC, Luo L, Mizrahi A: The Temporal Association Cortex Plays a Key Role in Auditory-Driven Maternal Plasticity. Neuron 2020, 107:566–579 e567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hitti FL, Siegelbaum SA: The hippocampal CA2 region is essential for social memory. Nature 2014, 508:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oliva A, Fernandez-Ruiz A, Leroy F, Siegelbaum SA: Hippocampal CA2 sharp-wave ripples reactivate and promote social memory. Nature 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S: Ventral CA1 neurons store social memory. Science 2016, 353:1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meira T, Leroy F, Buss EW, Oliva A, Park J, Siegelbaum SA: A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat Commun 2018, 9:4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deng X, Gu L, Sui N, Guo J, Liang J: Parvalbumin interneuron in the ventral hippocampus functions as a discriminator in social memory. Proc Natl Acad Sci U S A 2019, 116:16583–16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan Y, Singhal SM, Harden SW, Cahill KM, Nguyen DM, Colon-Perez LM, Sahagian TJ, Thinschmidt JS, de Kloet AD, Febo M, et al. : Oxytocin Receptors Are Expressed by Glutamatergic Prefrontal Cortical Neurons That Selectively Modulate Social Recognition. J Neurosci 2019, 39:3249–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, et al. : Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron 2016, 90:609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pereira TD, Tabris N, Li J, Ravindranath S, Papadoyannis ES, Wang ZY, Turner DM, McKenzie-Smith G, Kocher SD, Falkner AL, et al. : SLEAP: Multi-animal pose tracking. bioRxiv 2020:2020.2008.2031.276246.
- 128.Steinmetz NA, Zatka-Haas P, Carandini M, Harris KD: Distributed coding of choice, action and engagement across the mouse brain. Nature 2019, 576:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, Harris KD: Spontaneous behaviors drive multidimensional, brainwide activity. Science 2019, 364:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim CK, Yang SJ, Pichamoorthy N, Young NP, Kauvar I, Jennings JH, Lerner TN,Berndt A, Lee SY, Ramakrishnan C, et al. : Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat Methods 2016, 13:325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sych Y, Chernysheva M, Sumanovski LT, Helmchen F: High-density multi-fiber photometry for studying large-scale brain circuit dynamics. Nat Methods 2019, 16:553–560. [DOI] [PubMed] [Google Scholar]
- 132.Luo TZ, Bondy AG, Gupta D, Elliott VA, Kopec CD, Brody CD: An approach for long-term, multi-probe Neuropixels recordings in unrestrained rats. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M: DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 2018, 21:1281–1289. [DOI] [PubMed] [Google Scholar]
- 134.Nilsson SR, Goodwin NL, Choong JJ, Hwang S, Wright HR, Norville ZC, Tong X, Lin D, Bentzley BS, Eshel N, et al. : Simple Behavioral Analysis (SimBA) – an open source toolkit for computer classification of complex social behaviors in experimental animals. bioRxiv 2020:2020.2004.2019.049452.
- 135.Hong W, Kennedy A, Burgos-Artizzu XP, Zelikowsky M, Navonne SG, Perona P, Anderson DJ: Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc Natl Acad Sci U S A 2015, 112:E5351–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, Datta SR: Mapping Sub-Second Structure in Mouse Behavior. Neuron 2015, 88:1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]


