ABSTRACT
Background
Associations between sugar consumption and cardiometabolic health, taking into account the physical form of sugar-containing foods (liquid vs. solid) and the type of sugars consumed [free sugars (FSs) vs. naturally occurring sugars (NOSs)], remain to be thoroughly documented.
Objective
The objective was to examine whether FS and NOS intakes from drinks and solid foods are associated with cardiometabolic risk factors in a sample of French-speaking adults from the province of Quebec, Canada.
Methods
Data were collected as part of the cross-sectional PREDISE (PRÉDicteurs Individuels, Sociaux et Environnementaux) study (n = 1019, 18–65 y old; 50% women). FS and NOS intakes were assessed by three 24-h dietary recalls using a self-administered, web-based application. Diet quality was assessed using the Alternative Healthy Eating Index–2010. Participants underwent on-site clinical assessment of cardiometabolic risk factors, including blood pressure, waist circumference, BMI, and fasting blood sampling (glucose, insulin, C-reactive protein, blood lipids). Multivariable linear regression models were performed to examine the associations between sugar intake and cardiometabolic risk factors with sociodemographic characteristics, lifestyle variables, and diet quality entered as covariates.
Results
In fully adjusted models, FS intake from drinks was associated with fasting insulin (1.06%; 95% CI: 0.30%, 1.84%; P = 0.006) and with insulin resistance as estimated using the HOMA model (1.01%; 95% CI: 0.19%, 1.84%; P = 0.02). All metabolic variables that were significantly associated with NOS intake from solid foods in minimally adjusted models were no longer significant after entering sociodemographic and lifestyle variables (e.g., educational and income levels, smoking, physical activity, daily energy intake) and diet quality in the models.
Conclusions
Our data from an adult sample showed that unfavorable and favorable associations with cardiometabolic risk factors observed, respectively, for FS intake from drinks and NOS intake from foods are mostly explained by sociodemographic and lifestyle variables, as well as by diet quality.
Keywords: sugar intakes, free sugars, naturally occurring sugars, drinks, solid foods, diet quality, sociodemographic characteristics, lifestyle variables, cardiometabolic health, adults
Introduction
Recently, sugars have attracted growing scientific and media attention (1). Countries and health organizations have set recommendations for added, free, or total sugar intake (2). Chemically, the sugar molecules may be the same but the state they are in foods (added, free, or naturally occurring) changes the definition we give them. As defined by the USDA, added sugars are “caloric sweeteners that are added to foods as ingredients during food processing, during food preparation, or at the table” (3). According to the WHO, the definition of free sugars (FSs) includes all added sugars plus sugars in fruit juices (4). Total sugars, in turn, correspond to the sum of FSs and sugars that are naturally present in foods [naturally occurring sugars (NOSs)] such as fruit or milk.
The WHO recommends limiting FS intake to a maximum of 10% of daily energy (%E) and even proposes to aim at a 5%E limit as a conditional recommendation, particularly to prevent dental caries (4). The Scientific Advisory Committee on Nutrition advising the UK government organizations also recommends a maximum of 5%E as FSs in order to limit total energy intake and the risk of dental caries (5). Health Canada, through the Canada's dietary guidelines for health professionals and policy makers issued along with the 2019 version of the Canada's food guide, endorses the WHO recommendations (6). In order to limit FS intake, Health Canada recommends reducing consumption of highly processed food and sugary drinks and consuming nutritious food containing little to no FSs (6).
For over a decade, an increasing number of studies on sugars and their effects on cardiometabolic outcomes and risk factors have been published in the literature (1). However, most studies were on sugar-sweetened beverages (SSBs) (7), whose consumption is clearly linked with adverse health outcomes (8, 9) and which is sometimes used as a proxy for total sugar consumption (10). The impact of some aspects of sugar consumption, such as the type of sugars (total, added, free, or naturally occurring) consumed (8) and the physical form of foods in which they are consumed (liquid vs. solid; i.e., drinks vs. solid foods) (11), has not been as thoroughly studied (7). This literature gap impedes the comprehensive understanding of the complex associations between sugar intake and health outcomes. Furthermore, associations observed between diet and health may be far more complex than strictly based on the effect of single nutrients (12). The fact that sugar intake can be a marker of diet quality suggests that the associations between sugars and health may be mediated, at least in part, by diet quality (13). In fact, a high intake of added or free sugars may be the reflection of a poorer diet quality—namely, through nutrient dilution if nutrient-dense foods are replaced with foods rich in added or free sugars (14, 15).
Tybor et al. (1) systematically reviewed all published studies on dietary sugars and identified knowledge gaps in the complex relation between sugar intake and health outcomes. In designing the present study, we considered some of the research questions related to sugar and health that were identified by Tybor et al. as not yet having clear answers, such as “What factors modify the effects of sugars intake on cardiovascular disease outcomes?” and “Does type, form, source, or timing of dietary sugars have different effects on cardiovascular disease outcomes?” (1). More precisely, the objective of the present study was to cross-sectionally examine the associations of the intake of FSs and NOSs from drinks and solid foods with cardiometabolic risk factors in an age- and sex-representative sample of French-speaking adults from the province of Quebec, Canada. We hypothesized that the type of sugars (free vs. naturally occurring) and the physical form of foods in which they are consumed (solid foods vs. drinks) are differently associated with cardiometabolic risk factors and that these associations are largely explained by sociodemographic characteristics, lifestyle variables, and diet quality.
Methods
Participants and procedures
The PREDISE (PRÉDicteurs Individuels, Sociaux et Environnementaux) study is a cross-sectional, web-based, multicentered study that was designed to assess how individual, social, and perceived environmental factors are associated with adherence to dietary guidelines (16). Participants were recruited between August 2015 and April 2017 using random-digit dialing in 5 different administrative regions of the province of Quebec, Canada: Capitale-Nationale/Chaudière-Appalaches, Estrie, Mauricie, Montréal, and Saguenay-Lac-St-Jean. A total of 76 728 individuals were contacted and 1849 eligible individuals accepted to participate. Participants were asked to fill out online questionnaires and to complete three 24-h dietary recalls (1149 completed at least one 24-h dietary recall). Pregnant women (n = 2) were excluded. The final sample is representative based on sex and predetermined age groups (18–34, 35–49, and 50–65 y) of the 5 administrative regions and includes 1147 participants (50.2% women). Participants were mostly White (94.3%) and 44.5% had a university degree. Following completion of the questionnaires, participants were subsequently invited to a research center partner of the project for the measurement of cardiometabolic risk factors: blood pressure [systolic blood pressure (SBP) and diastolic blood pressure (DBP)] assessment (sitting, after 10-min rest, mean of 3 measures taken 3 min apart; Digital BPM HEM-907XL model; Omron), anthropometric measurements [height, weight, waist circumference (WC) (standardized protocol)], fasting blood sampling 12-h fast; serum; [blood glucose (Hexokinase method; Roche Modular P System), insulin (Cobas 6000; Roche Diagnostics), C-reactive protein (CRP; serum, ELISA; High Sensitivity C-Reactive Protein Enzyme Immunoassay Test Kit, model BC-1119; Biocheck, Inc.), triglycerides (TGs), total cholesterol (TC), and HDL cholesterol (Roche Modular P System; Roche Diagnostics) and LDL cholesterol (calculated with the use of the Friedewald equation)]. The detailed methodology is described elsewhere (17). For the present study, participants who did not come to their research center visit (n = 123) and who were not fasted before blood sampling (n = 5) were excluded. Therefore, this study sample includes 1019 participants (50.9% women, 92.2% White, 45.1% with a university degree). Written informed consent was obtained from all participants. The project was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committees of Université Laval (ethics number: 2014–271), Centre Hospitalier Universitaire de Sherbrooke (ethics number: MP-31–2015-997), Montreal Clinical Research Institute (ethics number: 2015–02), and Université du Québec à Trois-Rivières (ethics number: 15–2009-07.13).
Dietary assessment
On 3 unannounced randomly allocated days during a period of 21 d, participants had to complete a 24-h dietary recall, in which they were asked to indicate what they had eaten the day before. The number of completed recalls was evenly distributed across the 7 d of the week (17). The 24-h dietary recalls were completed using an automated, self-administered, web-based 24-h dietary recall instrument (R24W). In our sample, 98.9% of participants completed 3 web-based 24-h dietary recalls and all participants completed at least 2 web-based 24-h recalls.
The R24W has been previously shown to be a valid tool for measuring the intakes of energy and macro- and micronutrients as well as for assessing diet quality in our population (18–20). The nutritional value of foods declared by participants is automatically calculated by the R24W using data from the Canadian Nutrient File (21). For each individual, dietary intake data used in this study are based on the average of all available recalls. Total sugar content of each food item was differentiated into FS and NOS in the R24W database using a step-by-step systematic methodology (22) inspired by an algorithm developed by Louie et al. (23) and thereafter modified by Bernstein et al. (24), and using the WHO's FS definition: “Free sugars include monosaccharides and disaccharides added to foods and beverages by the manufacturer, cook or consumer, and sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates” (4). The complete sugar differentiation methodology used is presented elsewhere (22). Main food sources of sugars were based on food categories determined by the Canadian Bureau of Nutritional Sciences and used to assess main sources of total sugars in the 2015 Canadian Community Health Surveys (25). The drinks category included alcoholic beverages, hot drinks (coffee, hot chocolate, tea), energy drinks, soft drinks, sports drinks, plant-based beverages, water, fruit juices, fruit drinks, vegetable juices, milk, milkshakes, smoothies, and powders and concentrates used to reconstitute those drinks. Solid foods were all foods not included in the drinks category.
Diet quality
A higher Alternative Healthy Eating Index–2010 (AHEI) score (maximum: 110) indicates a diet that is associated with a significant reduction in chronic disease risk (cardiovascular disease, type 2 diabetes, cancer, and neurodegenerative disease) and all-cause mortality (26, 27). For the purpose of this study, a partial AHEI score was used to assess diet quality and was entered as a covariate in linear regression models. To avoid overadjustment for %E as FS from liquid sources and %E as NOS from solid sources, “sugar-sweetened beverages and fruit juice” and “whole fruits” subscores were removed from the original score. Thus, a partial score (maximum: 90) was calculated for each participant based on their intakes of various food groups (vegetables, whole grains, nuts and legumes, red/processed meat, and alcohol) and specific nutrients [trans fat, long-chain (n–3) fats (EPA + DHA), (n–3) PUFAs, and sodium] (27).
Plausibility of self-reported energy intakes
The plausibility of self-reported energy intakes was determined using a method described by Huang et al. (28). Reported energy intake was compared with predicted energy requirements calculated with the equations from the Institute of Medicine (29). A ratio of self-reported energy intake over predicted energy requirements <0.78 implied underreporting of energy intake, whereas a ratio >1.22 suggested overreporting of energy intake.
HOMA-IR
The HOMA-IR was calculated by multiplying fasting glucose (millimoles/liter) by fasting insulin (picomoles/liter) and dividing the product by 135, as proposed by Matthews et al. (30).
Continuous metabolic syndrome risk score (z score)
To assess a continuous metabolic syndrome risk score (MS z score), we used formulas that were previously documented (31) in which each component was normalized at the reference threshold of the National Cholesterol Education Program–Adult Treatment Panel III (NCEP-ATP III) for men and women separately (32). More precisely, normalized, continuous MS z scores were calculated as follows:
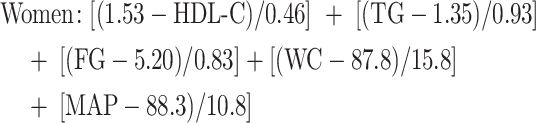 |
(1) |
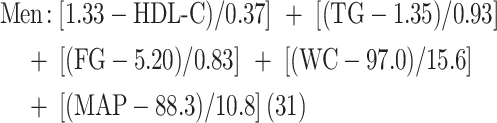 |
(2) |
where HDL-C = HDL cholesterol (millimoles/liter); FG = fasting glucose (millimoles/liter); MAP = mean arterial pressure [DBP (mm Hg) multiplied by 2 + SBP (mm Hg)/3]; denominators are finite population SDs of the corresponding variables specific to the PREDISE sample and were calculated while taking into consideration the stratified sampling methods of the PREDISE study.
Statistical analyses
To account for the stratified design of the study, analyses were performed using SURVEY procedures in SAS Studio (version 3.6; SAS Institute). Because the sample size was slightly larger than anticipated, sampling weights were used in order to ensure that our sample remains age- and sex-representative of each region of the province of Quebec included in the study. Missing sociodemographic characteristics, used as covariates, were imputed using the fully efficient fractional imputation method [household income level (n = 94), educational level (n = 6), and physical activity level (either low, moderate, or high; n = 119)], as presented elsewhere (17). Descriptive analyses were used to present population characteristics and to determine food sources of all types of sugars. Sugar intakes were expressed as percentages of daily energy intake (%E). Associations of %E as FSs and NOSs for the overall diet, solid foods, and drinks with AHEI score (total and partial) and AHEI individual components were assessed by univariable correlation analyses. Participants with reported type 1 diabetes (n = 5) were excluded from analyses involving fasting glucose and insulin, HOMA-IR, and MS z score. Participants with CRP concentrations of ≥10 mg/L (n = 60), which indicates acute inflammation process (33), were excluded from analyses involving CRP. Participants taking medication that could affect the studied outcome were removed from specific analyses [WC and BMI: no exclusion; glucose, insulin, and HOMA-IR: exclusion of participants taking antidiabetic medication (n = 41); CRP: exclusion of women taking systemic hormonal contraceptives (n = 79); TGs, LDL cholesterol, HDL cholesterol, and TC:HDL cholesterol: exclusion of participants taking lipid-lowering medication (n = 98); SBP and DBP: exclusion of participants taking antihypertensive medication (n = 116); MS z score: exclusion of participants taking antidiabetic, antihypertensive, lipid-lowering, or heart disease medication (n = 185)].
Multivariable linear regressions were performed to assess the associations between %E as FSs and NOSs and cardiometabolic risk factors (WC, BMI, fasting glucose and insulin, HOMA-IR, CRP, TGs, LDL cholesterol and HDL cholesterol, ratio of TC to HDL cholesterol, SBP and DBP, and MS z score). Fasting glucose, fasting insulin, HOMA-IR, CRP, and TG variables were log-transformed due to the linear regression model residuals not being normally distributed. Different regression models were tested. Model 1 was adjusted for sex, continuous age, and administrative region (Capitale-Nationale/Chaudière-Appalaches, Estrie, Mauricie, Montreal, and Saguenay-Lac-St-Jean). In addition to the variables included in model 1, the following variables were also included in model 2: continuous WC (except when WC or BMI is the studied outcome), educational level [high school or no diploma, Collège d'Enseignement Général et Professionnel (pre-university and technical college institution particular to the province of Quebec educational system, which is considered higher than high school and lower than university), university], household income level in Canadian dollars (<30,000, ≥30,000 to <60,000, ≥60,000 to <90,000, ≥90,000), smoking (yes, no, former smoker), physical activity [low, moderate, high, based on the 2005 International Physical Activity Questionnaire (IPAQ) categorical scoring rules (34)], mean daily energy intake, plausibility of self-reported energy intake (underreporters, plausible reporters, overreporters of energy intake), and the number of 24-h dietary recalls completed on weekend days (0, 1, 2, 3). Model 3 included all covariates from models 1 and 2 and the AHEI partial score. Estimates were also rescaled to represent a change equivalent to the interquartile range of %E from each type and physical form of sugar (Supplemental Tables 1 and 2). Finally, multivariable linear regressions (model 3) were also performed to assess the associations between each of the top 3 sources of FSs (sugars, syrups and confectionary, fruit juices, and baked products) and NOSs (whole fruits, milk, and vegetables) [expressed as daily portions calculated by dividing the daily quantity consumed by the reference amount determined by Health Canada for each category of foods) (35)] and cardiometabolic risk factors. Associations at P < 0.05 were considered significant.
Results
Population characteristics
Characteristics of study participants and intakes of key nutrients are presented by quartiles of %E as FSs and NOSs in Tables 1 and 2. Men and women are represented equally across quartiles of %E from FS intake, whereas women represent a lower proportion (35.5%) of the lowest quartile of %E from NOS intake and a higher proportion (65.3%) of the highest quartile of %E from NOS intake. The lowest quartile of %E from FS intake is characterized by a higher proportion of potential energy underreporters (22.9%) and a lower proportion of potential energy overreporters (9.6%) compared with the highest quartile of %E from FS intake. Mean %E from FSs is above the maximum intake of 10%E from FSs recommended by WHO (ISBN: 9789241549028) in the third and fourth quartiles (mean ± SE; quartile 1 = 5.3%E ± 0.1%E; quartile 2 = 9.5%E ± 0.1%E; quartile 3 = 13.1%E ± 0.1%E; quartile 4 = 19.3%E ± 0.2%E).
TABLE 1.
Descriptive characteristics of the study sample of 1019 adults from the province of Quebec, Canada, according to quartiles of FS and NOS intake1
| Quartiles of FS intake, %E | Quartiles of NOS intake, %E | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| %E | <7.8 | 7.8 to <11.3 | 11.3 to <15.2 | ≥15.2 | <4.9 | 4.9 to <7.0 | 7.0 to <9.7 | ≥9.7 |
| Unweighted n | 254 | 255 | 253 | 257 | 258 | 253 | 256 | 252 |
| Sex | ||||||||
| Women | 128 (50.0) | 138 (53.9) | 130 (50.8) | 126 (49.2) | 92 (35.5) | 120 (47.1) | 144 (55.9) | 167 (65.3) |
| Men | 128 (50.0) | 118 (46.1) | 126 (49.2) | 131 (50.8) | 166 (64.5) | 135 (52.9) | 113 (44.1) | 89 (34.7) |
| Administrative region | ||||||||
| Capitale-Nationale/Chaudière-Appalaches | 102 (39.7) | 104 (40.7) | 104 (40.5) | 89 (34.8) | 96 (37.0) | 107 (42.1) | 102 (39.6) | 95 (37.1) |
| Estrie | 30 (11.6) | 25 (9.9) | 23 (8.9) | 22 (8.5) | 29 (11.1) | 25 (9.7) | 22 (8.7) | 24 (9.5) |
| Mauricie | 19 (7.5) | 22 (8.6) | 28 (11.1) | 23 (8.9) | 30 (11.6) | 27 (10.4) | 20 (7.9) | 16 (6.1) |
| Montreal | 90 (35.0) | 83 (32.5) | 76 (29.6) | 83 (32.5) | 80 (31.0) | 66 (25.9) | 87 (33.7) | 100 (39.0) |
| Saguenay-Lac-St-Jean | 16 (6.1) | 21 (8.3) | 25 (9.8) | 39 (15.3) | 24 (9.3) | 30 (12.0) | 26 (10.1) | 21 (8.2) |
| Educational level | ||||||||
| High school or no diploma | 63 (24.5) | 48 (18.8) | 63 (24.7) | 70 (27.1) | 82 (31.6) | 64 (24.9) | 53 (20.6) | 46 (17.9) |
| CEGEP2 | 73 (28.4) | 86 (33.4) | 84 (32.8) | 75 (29.1) | 95 (36.9) | 76 (30.0) | 82 (31.7) | 64 (25.0) |
| University | 121 (47.1) | 123 (47.8) | 109 (42.5) | 113 (43.8) | 81 (31.5) | 115 (45.1) | 122 (47.6) | 146 (57.0) |
| Household income level ($CAD) | ||||||||
| <30,000 | 36 (13.9) | 34 (13.2) | 43 (16.8) | 51 (20.0) | 52 (20.2) | 39 (15.3) | 32 (12.4) | 41 (16.1) |
| ≥30,000 to <60,000 | 81 (31.7) | 67 (26.0) | 66 (25.9) | 82 (31.9) | 85 (32.9) | 68 (26.8) | 70 (27.1) | 73 (28.6) |
| ≥60,000 to <90,000 | 64 (25.0) | 51 (19.8) | 48 (18.9) | 38 (15.0) | 42 (16.3) | 56 (22.0) | 54 (21.1) | 50 (19.4) |
| ≥90,000 | 75 (29.4) | 105 (40.9) | 99 (38.4) | 85 (33.2) | 79 (30.7) | 92 (36.0) | 101 (39.4) | 92 (35.9) |
| Smoking | ||||||||
| Yes | 39 (15.4) | 27 (10.6) | 29 (11.5) | 31 (12.1) | 59 (22.8) | 31 (12.4) | 20 (7.7) | 17 (6.6) |
| No | 111 (43.5) | 137 (53.6) | 148 (57.6) | 159 (61.9) | 115 (44.7) | 130 (51.0) | 156 (60.6) | 154 (60.4) |
| Former smokers | 105 (41.1) | 92 (35.8) | 79 (30.9) | 67 (26.0) | 84 (32.5) | 93 (36.6) | 81 (31.7) | 84 (33.0) |
| Physical activity level3 | ||||||||
| Low | 49 (19.0) | 40 (15.5) | 55 (21.6) | 55 (21.4) | 56 (21.5) | 59 (23.0) | 44 (17.2) | 40 (15.7) |
| Moderate | 106 (41.4) | 89 (34.7) | 105 (40.9) | 98 (38.0) | 115 (44.4) | 96 (37.7) | 102 (39.6) | 85 (33.4) |
| High | 101 (39.6) | 128 (49.8) | 96 (37.5) | 104 (40.6) | 88 (34.1) | 100 (39.4) | 111 (43.2) | 130 (50.9) |
| Plausibility of self-reported energy intakes | ||||||||
| Potential energy underreporters | 59 (22.9) | 42 (16.6) | 29 (11.3) | 25 (9.6) | 38 (14.7) | 44 (17.2) | 29 (11.3) | 44 (17.1) |
| Potential energy plausible reporters | 138 (53.7) | 133 (52.0) | 139 (54.2) | 141 (55.0) | 145 (56.0) | 126 (49.3) | 143 (55.6) | 138 (54.0) |
| Potential energy overreporters | 60 (23.4) | 80 (31.4) | 88 (34.5) | 91 (35.4) | 76 (29.3) | 86 (33.6) | 85 (33.1) | 74 (28.8) |
| Number of 24-h dietary recalls completed on weekend days | ||||||||
| 0 | 49 (19.3) | 45 (17.7) | 37 (14.5) | 44 (17.3) | 36 (14.1) | 35 (13.6) | 44 (17.1) | 61 (24.0) |
| 1 | 118 (46.2) | 113 (44.0) | 121 (47.3) | 124 (48.4) | 117 (45.2) | 125 (49.2) | 127 (49.4) | 107 (42.0) |
| 2 | 78 (30.6) | 85 (33.1) | 86 (33.4) | 75 (29.1) | 94 (36.4) | 80 (31.4) | 72 (28.0) | 78 (30.4) |
| 3 | 10 (4.0) | 13 (5.1) | 12 (4.8) | 14 (5.3) | 11 (4.3) | 15 (5.8) | 14 (5.5) | 9 (3.6) |
| Medication usage4 | ||||||||
| Antidiabetic | 11 (4.4) | 12 (4.9) | 11 (4.3) | 7 (2.8) | 16 (6.3) | 7 (2.8) | 8 (3.2) | 10 (4.0) |
| Antihypertensive | 30 (11.7) | 33 (13.0) | 21 (8.3) | 35 (13.6) | 36 (14.0) | 35 (13.7) | 21 (8.3) | 27 (10.6) |
| Lipid-lowering | 23 (8.9) | 33 (12.8) | 27 (10.6) | 20 (7.6) | 28 (10.9) | 32 (12.7) | 26 (10.1) | 16 (6.1) |
| Heart disease | 12 (4.5) | 4 (1.6) | 7 (2.8) | 7 (2.8) | 9 (3.6) | 10 (4.1) | 8 (3.3) | 2 (0.8) |
| Systemic hormonal contraceptives5 | 16 (6.1) | 22 (8.7) | 21 (8.3) | 20 (7.9) | 18 (7.0) | 17 (6.6) | 31 (12.1) | 14 (5.3) |
| No | 199 (77.8) | 183 (71.4) | 195 (76.0) | 191 (74.1) | 190 (73.5) | 185 (72.7) | 188 (73.2) | 204 (80.0) |
Values are weighted n (%), which might have caused the sample size to be unequal to 1019. CEGEP, Collège d'Enseignement Général et Professionnel; FS, free sugar; NOS, naturally occurring sugar; Q, quartile; %E, percentage of daily energy intake; $CAD, Canadian dollars.
CEGEP is a pre-university and technical college institution particular to the province of Quebec educational system, which is considered higher than high school and lower than university.
Physical activity level is based on the 2005 International Physical Activity Questionnaire (IPAQ) categorical scoring rules.
Medication usage that may affect relevant outcomes studied.
Systemic hormonal contraceptives taken by women.
TABLE 2.
Characteristics and key nutrient intakes of the study sample of 1019 adults from the province of Quebec, Canada, according to quartiles of FS and NOS intake1
| Quartiles of FS intake, %E | Quartiles of NOS intake, %E | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 (n = 254) | Q2 (n = 255) | Q3 (n = 253) | Q4 (n = 257) | Q1 (n = 258) | Q2 (n = 253) | Q3 (n = 256) | Q4 (n = 252) | |
| Age, y | 44.7 ± 0.8 | 44.0 ± 0.7 | 43.7 ± 0.8 | 41.4 ± 0.8 | 41.4 ± 0.7 | 43.3 ± 0.7 | 43.5 ± 0.7 | 45.7 ± 0.7 |
| WC, cm | 91.9 ± 1.0 | 92.0 ± 1.0 | 92.1 ± 1.0 | 93.1 ± 1.1 | 95.9 ± 1.0 | 94.2 ± 1.1 | 90.3 ± 1.0 | 88.7 ± 0.9 |
| BMI, kg/m2 | 27.5 ± 0.4 | 27.4 ± 0.4 | 27.4 ± 0.4 | 27.6 ± 0.4 | 28.4 ± 0.4 | 28.1 ± 0.4 | 26.9 ± 0.4 | 26.6 ± 0.3 |
| Fasting glucose,2 mmol/L | 5.2 ± 0.0 | 5.3 ± 0.1 | 5.1 ± 0.0 | 5.2 ± 0.0 | 5.3 ± 0.0 | 5.2 ± 0.1 | 5.1 ± 0.0 | 5.2 ± 0.1 |
| Fasting insulin,2 pmol/L | 91.1 ± 2.9 | 93.0 ± 3.1 | 105.3 ± 5.0 | 106.2 ± 5.2 | 109.0 ± 4.8 | 103.7 ± 4.2 | 89.8 ± 4.1 | 93.2 ± 3.5 |
| HOMA-IR | 3.6 ± 0.2 | 3.8 ± 0.2 | 4.1 ± 0.2 | 4.2 ± 0.2 | 4.4 ± 0.2 | 4.2 ± 0.2 | 3.5 ± 0.2 | 3.7 ± 0.2 |
| CRP,2 mg/L | 2.1 ± 0.2 | 2.1 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.2 | 2.3 ± 0.1 | 2.4 ± 0.2 | 2.0 ± 0.1 | 2.2 ± 0.2 |
| TGs,2 mmol/L | 1.3 ± 0.1 | 1.3 ± 0.0 | 1.4 ± 0.1 | 1.4 ± 0.0 | 1.6 ± 0.1 | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.2 ± 0.0 |
| LDL-C,2 mmol/L | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.1 | 2.9 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 2.9 ± 0.1 |
| HDL-C,2 mmol/L | 1.5 ± 0.0 | 1.5 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.5 ± 0.0 |
| TC:HDL-C,2 mmol/L | 3.6 ± 0.1 | 3.6 ± 0.1 | 3.7 ± 0.1 | 3.8 ± 0.1 | 4.0 ± 0.1 | 3.6 ± 0.1 | 3.5 ± 0.1 | 3.5 ± 0.1 |
| SBP, mm Hg | 117.3 ± 0.9 | 116.4 ± 0.9 | 118.3 ± 0.8 | 119.2 ± 0.9 | 120.0 ± 0.8 | 118.4 ± 0.9 | 116.0 ± 0.8 | 116.7 ± 0.9 |
| DBP, mm Hg | 72.6 ± 0.6 | 72.6 ± 0.6 | 73.9 ± 0.6 | 75.0 ± 0.6 | 74.8 ± 0.7 | 73.9 ± 0.6 | 72.6 ± 0.6 | 72.8 ± 0.6 |
| MS z score3 | -0.1 ± 0.2 | 0.0 ± 0.2 | 0.0 ± 0.2 | 0.3 ± 0.2 | 2.0 ± 0.0 | 12.5 ± 0.0 | -0.4 ± 0.2 | -0.5 ± 0.2 |
| Daily energy intake,4 kcal | 2228 ± 42 | 2341 ± 42 | 2469 ± 44 | 2602 ± 52 | 2542 ± 48 | 2484 ± 49 | 2416 ± 45 | 2199 ± 39 |
| %E as total sugars4 | 13.8 ± 0.3 | 17.4 ± 0.2 | 20.3 ± 0.2 | 25.8 ± 0.3 | 16.5 ± 0.4 | 18.6 ± 0.3 | 20.1 ± 0.3 | 22.1 ± 0.4 |
| %E as FS | 5.3 ± 0.1 | 9.5 ± 0.1 | 13.1 ± 0.1 | 19.3 ± 0.2 | 13.0 ± 0.4 | 12.7 ± 0.3 | 11.8 ± 0.3 | 9.7 ± 0.3 |
| %E FS, drinks | 0.9 ± 0.1 | 2.6 ± 0.1 | 4.4 ± 0.2 | 8.7 ± 0.3 | 5.6 ± 0.3 | 4.6 ± 0.3 | 3.7 ± 0.2 | 2.8 ± 0.2 |
| %E FS, solid foods | 4.3 ± 0.1 | 6.8 ± 0.1 | 8.7 ± 0.2 | 10.6 ± 0.2 | 7.3 ± 0.2 | 8.1 ± 0.2 | 8.1 ± 0.2 | 6.9 ± 0.2 |
| %E as NOS | 8.6 ± 0.3 | 8.0 ± 0.2 | 7.2 ± 0.2 | 6.6 ± 0.2 | 3.6 ± 0.1 | 6.0 ± 0.0 | 8.3 ± 0.0 | 12.5 ± 0.2 |
| %E NOS, drinks | 1.8 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | 1.8 ± 0.1 | 0.7 ± 0.0 | 1.4 ± 0.1 | 2.4 ± 0.1 | 3.2 ± 0.2 |
| %E NOS, solid foods | 6.7 ± 0.3 | 6.0 ± 0.2 | 5.2 ± 0.2 | 4.8 ± 0.2 | 2.9 ± 0.1 | 4.6 ± 0.1 | 6.0 ± 0.1 | 9.3 ± 0.3 |
| AHEI score4,5 | 59.7 ± 0.7 | 56.4 ± 0.7 | 51.8 ± 0.7 | 47.2 ± 0.6 | 46.0 ± 0.6 | 51.5 ± 0.6 | 56.0 ± 0.7 | 61.5 ± 0.6 |
| AHEI partial score4,6 | 47.1 ± 0.6 | 46.3 ± 0.5 | 44.7 ± 0.5 | 41.9 ± 0.5 | 40.1 ± 0.5 | 43.9 ± 0.5 | 46.9 ± 0.5 | 49.1 ± 0.5 |
Values are means ± SEs. n is unweighted. n values are listed for each variable: age (n = 1019); WC and BMI (n = 1017); fasting glucose, fasting insulin, and HOMA-IR (n = 1004); CRP (n = 947); TGs, LDL-C, HDL-C, and TC:HDL (n = 1009); SBP and DBP (n = 1018); MS z score (n = 1001); energy and sugar intakes and AHEI (n = 1019). AHEI, Alternative Healthy Eating Index–2010; CRP, C-reactive protein; DBP, diastolic blood pressure; FS, free sugar; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MS z Score, continuous metabolic syndrome risk score; NOS, naturally occurring sugar; Q, quartile; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WC, waist circumference; %E, percentage of daily energy.
Blood tests were performed on serum.
A higher MS z score indicates a stronger presence of the metabolic syndrome.
For each participant, energy intake, sugar intake, AHEI score, and AHEI partial score are based on the average of all available dietary recalls.
A higher AHEI score (maximum: 110) indicates a diet that is associated with a significant reduction in chronic disease risk and all-cause mortality.
AHEI partial score (maximum: 90): whole fruits and sugar-sweetened beverages and fruit juice subscores were removed from the AHEI score.
Food sources of sugars
In our sample, sugars, syrups, and confectionary (20.4%); fruit juices (17.6%); baked products (17.2%); and soft drinks (8.5%) were the main sources of FSs. Top food sources of NOSs were whole fruits (34.7%), milk (24.2%), vegetables (14.6%), and yogurt (4.3%). Top food sources of total sugars, FSs, and NOSs are presented in Supplemental Table 3.
Correlations between the different types of sugars and subscores of the AHEI
Supplemental Table 4 shows correlation coefficients between the different types of sugars and AHEI total score and AHEI partial score and subscores. %E as FS from foods was inversely associated with AHEI total (r = −0.10, P = 0.001) and partial (r = −0.07, P = 0.03) scores. %E as FS from drinks was also inversely associated with AHEI total (r = −0.50, P < 0.0001) and partial (r = −0.23, P < 0.0001) scores and with most of the AHEI subscores. %E as NOS from foods was positively associated with AHEI total (r = 0.59, P < 0.0001) and partial (r = 0.44, P < 0.0001) scores and with nearly all AHEI subscores.
Multivariable linear associations of sugar intake (%E as FS and NOS, from foods and drinks) with cardiometabolic risk factors
Table 3 shows results of the multivariable linear regression analyses assessing the associations between FS intake and cardiometabolic risk factors. In fully adjusted models, %E as FSs from foods was associated positively with LDL cholesterol. For %E as FSs from drinks, positive associations with fasting insulin and HOMA-IR were found in fully adjusted models. Although %E as FSs from drinks was significantly associated with TGs, HDL cholesterol, TC:HDL cholesterol, SBP, DBP, and MS z score in model 1 (which includes only sex, age, and administrative region), these associations were no longer significant in model 2 (all covariates included except for diet quality) and model 3 (fully adjusted). %E as FSs from drinks was positively associated with CRP in model 1 and model 2, but the association was no longer significant in model 3, with further adjustment for diet quality.
TABLE 3.
Multivariable linear associations of %E as FS from solid foods and drinks with cardiometabolic risk factors in an adult sample (n = 1019) from the province of Quebec, Canada1
| Model 12 | Model 23 | Model 34 | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| WC, cm | ||||||
| %E FS, solid foods | –0.064 (–0.324, 0.197) | 0.63 | 0.026 (–0.203, 0.255) | 0.83 | –0.042 (–0.268, 0.183) | 0.71 |
| %E FS, drinks | 0.219 (–0.004, 0.441) | 0.05 | 0.154 (–0.041, 0.350) | 0.12 | 0.032 (–0.163, 0.228) | 0.75 |
| BMI, kg/m2 | ||||||
| %E FS, solid foods | –0.015 (–0.126, 0.097) | 0.80 | 0.015 (–0.082, 0.113) | 0.76 | –0.008 (–0.105, 0.090) | 0.88 |
| %E FS, drinks | 0.043 (–0.049, 0.135) | 0.36 | 0.015 (–0.064, 0.095) | 0.71 | –0.027 (–0.106, 0.051) | 0.50 |
| Fasting glucose,5,6 % | ||||||
| %E FS, solid foods | –0.08 (–0.34, 0.19) | 0.57 | –0.04 (–0.32, 0.25) | 0.81 | –0.03 (–0.31, 0.26) | 0.86 |
| %E FS, drinks | –0.03 (–0.22, 0.16) | 0.78 | –0.07 (–0.26, 0.12) | 0.46 | –0.05 (–0.25, 0.14) | 0.58 |
| Fasting insulin,5,6 % | ||||||
| %E FS, solid foods | –0.53 (–1.52, 0.48) | 0.30 | –0.38 (–1.38, 0.62) | 0.45 | –0.42 (–1.41, 0.59) | 0.41 |
| %E FS, drinks | 1.39 (0.58, 2.21) | 0.0008 | 1.08 (0.33, 1.83) | 0.005 | 1.06 (0.30, 1.84) | 0.006 |
| HOMA-IR,6 % | ||||||
| %E FS, solid foods | –0.60 (–1.58, 0.39) | 0.23 | –0.42 (–1.34, 0.51) | 0.37 | –0.44 (–1.37, 0.49) | 0.35 |
| %E FS, drinks | 1.36 (0.50, 2.23) | 0.002 | 1.01 (0.20, 1.82) | 0.01 | 1.01 (0.19, 1.84) | 0.02 |
| CRP,5,6 % | ||||||
| %E FS, solid foods | –0.51 (–2.31, 1.32) | 0.58 | 0.09 (–1.50, 1.70) | 0.92 | –0.04 (–1.65, 1.60) | 0.96 |
| %E FS, drinks | 2.49 (0.77, 4.25) | 0.005 | 1.59 (0.14, 3.05) | 0.03 | 1.42 (–0.05, 2.90) | 0.06 |
| TGs,5,6 % | ||||||
| %E FS, solid foods | –0.10 (–1.00, 0.82) | 0.83 | 0.02 (–0.85, 0.89) | 0.97 | –0.06 (–0.94, 0.83) | 0.89 |
| %E FS, drinks | 0.87 (0.12, 1.63) | 0.02 | 0.63 (–0.13, 1.40) | 0.10 | 0.50 (–0.26, 1.27) | 0.20 |
| LDL-C,5 mmol/L | ||||||
| %E FS, solid foods | 0.0144 (–0.0004, 0.0292) | 0.06 | 0.0176 (0.0025, 0.0327) | 0.02 | 0.0174 (0.0022, 0.0327) | 0.02 |
| %E FS, drinks | 0.0033 (–0.0093, 0.0160) | 0.61 | 0.0027 (–0.0102, 0.0156) | 0.68 | 0.0022 (–0.0111, 0.0154) | 0.75 |
| HDL-C,5 mmol/L | ||||||
| %E FS, solid foods | –0.0048 (–0.0120, 0.0024) | 0.19 | –0.0055 (–0.0127, 0.0016) | 0.13 | –0.0053 (–0.0125, 0.0020) | 0.15 |
| %E FS, drinks | –0.0080 (–0.0141, –0.0019) | 0.01 | –0.0054 (–0.0113, 0.0005) | 0.07 | –0.0051 (–0.0110, 0.0008) | 0.09 |
| TC:HDL-C,5 mmol/L | ||||||
| %E FS, solid foods | 0.012 (–0.013, 0.034) | 0.35 | 0.016 (–0.008, 0.040) | 0.19 | 0.014 (–0.011, 0.038) | 0.26 |
| %E FS, drinks | 0.023 (0.004, 0.042) | 0.02 | 0.018 (–0.002, 0.038) | 0.07 | 0.014 (–0.005, 0.034) | 0.15 |
| SBP, mm Hg | ||||||
| %E FS, solid foods | 0.14 (–0.09, 0.36) | 0.24 | 0.08 (–0.14, 0.30) | 0.48 | 0.08 (–0.14, 0.30) | 0.47 |
| %E FS, drinks | 0.23 (0.03, 0.43) | 0.02 | 0.11 (–0.08, 0.31) | 0.25 | 0.12 (–0.08, 0.32) | 0.23 |
| DBP, mm Hg | ||||||
| %E FS, solid foods | 0.07 (–0.11, 0.24) | 0.44 | 0.02 (–0.14, 0.19) | 0.77 | 0.01 (–0.15, 0.18) | 0.88 |
| %E FS, drinks | 0.21 (0.06, 0.36) | 0.01 | 0.13 (–0.01, 0.28) | 0.08 | 0.11 (–0.04, 0.26) | 0.14 |
| MS z score7 | ||||||
| %E FS, solid foods | 0.014 (–0.044 , 0.071) | 0.65 | 0.014 (–0.033, 0.062) | 0.56 | 0.012 (–0.037, 0.061) | 0.63 |
| %E FS, drinks | 0.053 (0.010 , 0.097) | 0.02 | 0.028 (–0.004, 0.060) | 0.09 | 0.024 (–0.010, 0.057) | 0.17 |
Values are estimates of the change in the outcome (95% CI) for every unit of %E FS from solid foods or drinks. Individuals taking medication that could affect the studied outcome were removed from analyses. See the Methods section for details. n is listed for each variable: WC and BMI (n = 1017); fasting glucose, fasting insulin, and HOMA-IR (n = 963); CRP (n = 868); TGs, LDL-C, HDL-C, and TC:HDL-C (n = 911); SBP and DBP (n = 902); and MS z score (n = 816). AHEI, Alternative Healthy Eating Index–2010; CRP, C-reactive protein; DBP, diastolic blood pressure; FS, free sugar; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MS z score, continuous metabolic syndrome risk score; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WC, waist circumference; %E, percentage of daily energy.
Model 1 is adjusted for sex, age, and administrative region.
Model 2 is adjusted for model 1 + WC (except when WC or BMI is the studied outcome), educational and household income levels, smoking, physical activity [based on the 2005 International Physical Activity Questionnaire (IPAQ) categorical scoring rules], mean daily energy intake, plausibility of self-reported energy intake, and the number of 24-h dietary recalls completed on weekend days.
Model 3 is adjusted for model 2 + AHEI partial score (AHEI score from which whole fruits and sugar-sweetened beverages and fruit juice subscores were removed).
Blood tests were performed on serum.
Log-transformed data; β values (95% CI) are expressed as % change upon back-transformation calculated as 100 × exponential (logB) − 100.
A higher MS z score indicates a stronger presence of the metabolic syndrome.
Table 4 shows results of the multivariable linear regression analyses assessing the associations between NOS intake and cardiometabolic risk factors. Although %E as NOSs from foods was inversely associated with fasting insulin, HOMA-IR, CRP, TGs, TC:HDL cholesterol, DBP and MS z score in Model 1, these associations were no longer significant in model 2 and model 3. For WC and BMI, the inverse association observed in model 1 and model 2 was no longer significant in model 3. %E as NOSs from drinks was associated only with fasting insulin (positive association observed in all models).
TABLE 4.
Multivariable linear associations of %E as NOS from solid foods and drinks with cardiometabolic risk factors and in an adult sample (n = 1019) from the province of Quebec, Canada1
| Model 12 | Model 23 | Model 34 | ||||
|---|---|---|---|---|---|---|
| β (95%CI) | P | β (95%CI) | P | β (95%CI) | P | |
| WC, cm | ||||||
| %E NOS, solid foods | –0.63 (–0.96, –0.29) | 0.0002 | –0.43 (–0.78, –0.08) | 0.01 | –0.22 (–0.50, 0.06) | 0.13 |
| %E NOS, drinks | 0.04 (–0.46, 0.54) | 0.88 | 0.20 (–0.25, 0.64) | 0.38 | 0.16 (–0.27, 0.59) | 0.47 |
| BMI , kg/m2 | ||||||
| %E NOS, solid foods | –0.226 (–0.369, –0.083) | 0.002 | –0.152 (–0.298, –0.006) | 0.04 | –0.080 (–0.205, 0.045) | 0.21 |
| %E NOS, drinks | 0.037 (–0.162, 0.236) | 0.71 | 0.088 (–0.083, 0.259) | 0.31 | 0.076 (–0.090, 0.242) | 0.37 |
| Fasting glucose,5,6 % | ||||||
| %E NOS, solid foods | –0.061 (–0.268, 0.146) | 0.56 | 0.057 (–0.128, 0.243) | 0.55 | 0.026 (–0.182, 0.235) | 0.81 |
| %E NOS, drinks | –0.371 (–0.743, 0.003) | 0.05 | –0.360 (–0.742, 0.024) | 0.07 | –0.355 (–0.737, 0.029) | 0.07 |
| Fasting insulin,5,6 % | ||||||
| %E NOS, solid foods | –1.46 (–2.70, –0.20) | 0.02 | –0.67 (–1.70, 0.36) | 0.20 | –0.63 (–1.72, 0.49) | 0.27 |
| %E NOS, drinks | 1.78 (0.18, 3.41) | 0.03 | 1.86 (0.36, 3.38) | 0.02 | 1.84 (0.34, 3.36) | 0.02 |
| HOMA-IR,6 % | ||||||
| %E NOS, solid foods | –1.52 (–2.89, –0.13) | 0.03 | –0.62 (–1.73, 0.50) | 0.28 | –0.60 (–1.81, 0.62) | 0.33 |
| %E NOS, drinks | 1.40 (–0.31, 3.15) | 0.11 | 1.49 (–0.13, 3.14) | 0.07 | 1.48 (–0.14, 3.13) | 0.07 |
| CRP,5,6 % | ||||||
| %E NOS, solid foods | –3.09 (–4.77, –1.39) | 0.0004 | –1.43 (–2.94, 0.10) | 0.07 | –1.14 (–2.76, 0.52) | 0.18 |
| %E NOS, drinks | –0.10 (–3.51, 3.42) | 0.95 | 1.42 (–1.62, 4.56) | 0.36 | 1.34 (–1.68, 4.44) | 0.39 |
| TGs,5,6 % | ||||||
| %E NOS, solid foods | –1.91 (–3.71, –0.08) | 0.04 | –1.14 (–2.71, 0.44) | 0.16 | –0.95 (–2.56, 0.68) | 0.25 |
| %E NOS, drinks | –0.66 (–2.33, 1.04) | 0.44 | –0.33 (–1.91, 1.28) | 0.69 | –0.37 (–1.95, 1.23) | 0.65 |
| LDL-C5, mmol/L | ||||||
| %E NOS, solid foods | –0.010 (–0.025, 0.005) | 0.17 | –0.004 (–0.018, 0.011) | 0.63 | –0.003 (–0.017, 0.012) | 0.72 |
| %E NOS, drinks | –0.009 (–0.037, 0.020) | 0.55 | –0.006 (–0.036, 0.023) | 0.68 | –0.006 (–0.036, 0.023) | 0.67 |
| HDL-C,5 mmol/L | ||||||
| %E NOS, solid foods | 0.0083 (–0.0028, 0.0193) | 0.14 | 0.0011 (–0.0079, 0.0101) | 0.81 | 0.0003 (–0.0089, 0.0095) | 0.95 |
| %E NOS, drinks | –0.0101 (–0.0229, 0.0028) | 0.12 | –0.0107 (–0.0225, 0.0011) | 0.08 | –0.0105 (–0.0224, 0.0013) | 0.08 |
| TC:HDL-C,5 mmol/L | ||||||
| %E NOS, solid foods | –0.036 (–0.069, –0.003) | 0.03 | –0.016 (–0.044, 0.012) | 0.25 | –0.010 (–0.036, 0.016) | 0.46 |
| %E NOS, drinks | –0.013 (–0.056, 0.030) | 0.55 | –0.008 (–0.050, 0.033) | 0.69 | –0.010 (–0.051, 0.032) | 0.65 |
| SBP, mm Hg | ||||||
| %E NOS, solid foods | –0.12 (–0.38, 0.13) | 0.35 | 0.09 (–0.17, 0.36) | 0.50 | 0.10 (–0.19, 0.38) | 0.50 |
| %E NOS, drinks | 0.18 (–0.22, 0.57) | 0.39 | 0.21 (–0.18, 0.60) | 0.29 | 0.21 (–0.18, 0.61) | 0.29 |
| DBP, mm Hg | ||||||
| %E NOS, solid foods | –0.25 (–0.42, –0.09) | 0.003 | –0.11 (–0.27, 0.06) | 0.21 | –0.07 (–0.25, 0.12) | 0.47 |
| %E NOS, drinks | 0.21 (–0.07, 0.50) | 0.13 | 0.22 (–0.04, 0.48) | 0.10 | 0.21 (–0.05, 0.46) | 0.12 |
| MS z score7 | ||||||
| %E NOS, solid foods | –0.106 (–0.189, –0.022) | 0.01 | –0.021 (–0.071, 0.029) | 0.40 | –0.014 (–0.064, 0.036) | 0.59 |
| %E NOS, drinks | –0.034 (–0.133, 0.064) | 0.49 | –0.005 (–0.074, 0.065) | 0.89 | –0.007 (–0.077, 0.063) | 0.85 |
Values are estimates of the change in the outcome (95% CI) for every unit of %E NOS from solid foods or drinks. Individuals taking medication that could affect the studied outcome were removed from analyses. See the Methods section for details. n is listed for each variable: WC and BMI (n = 1017); fasting glucose, fasting insulin, and HOMA-IR (n = 963); CRP (n = 868); TGs, LDL-C, HDL-C, and TC:HDL-C (n = 911); SBP and DBP (n = 902); and MS z score (n = 816). AHEI, Alternative Healthy Eating Index–2010; CRP, C-reactive protein; DBP, diastolic blood pressure; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MS z score, continuous metabolic syndrome risk score; NOS; naturally occurring sugar; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WC, waist circumference; %E, percentage of daily energy.
Model 1 is adjusted for sex, age, and administrative region.
Model 2 is adjusted for model 1 + WC (except when WC or BMI is the studied outcome), educational and household income levels, smoking, physical activity [based on the 2005 International Physical Activity Questionnaire (IPAQ) categorical scoring rules], mean daily energy intake, plausibility of self-reported energy intake, and the number of 24-h dietary recalls completed on weekend days.
Model 3 is adjusted for model 2 + AHEI partial score (AHEI score from which whole fruits and sugar-sweetened beverages and fruit juice subscores were removed).
Blood tests were performed on serum.
Log-transformed data; β values (95% CI) are expressed as % change upon back-transformation calculated as 100 × exponential (logB) − 100.
A higher MS z score indicates a stronger presence of the metabolic syndrome.
Multivariable linear associations of mean daily portions of top 3 sources of FSs and NOSs with cardiometabolic risk factors
Main sources of FSs were 1) sugars, syrups, and confectionary; 2) juice; and 3) baked products. Only a few significant associations were observed in fully adjusted models. Mean daily portions of juice were inversely associated with HDL cholesterol (−0.04 mmol/L; 95% CI: −0.07, −0.01 mmol/L; P = 0.01). Main sources of NOSs were 1) whole fruits, 2) milk, and 3) vegetables. As with main sources of FSs, only a few associations were observed in fully adjusted models. Mean daily intake of milk was positively associated with fasting insulin (3.26%; 95% CI: 0.10%, 6.53%; P = 0.04). Mean daily intake of vegetables was inversely associated with fasting insulin (−2.01%; 95% CI: −3.67%, −0.34%; P = 0.02) and HOMA-IR (−2.05%; 95% CI: −3.76%, −0.31%; P = 0.02) (results not shown).
Discussion
Results from multivariable linear regression models assessing the associations between sugar intakes and cardiometabolic risk factors showed that, after adjustment for confounding variables, FS intake, especially from drinks, was associated with some impaired cardiometabolic risk factors related to insulin homeostasis, whereas NOS intake from solid foods was no longer associated with a favorable cardiometabolic risk profile. This suggests that both the state of the sugars (either free or naturally occurring) and their physical form in foods (either liquid or solid; i.e., drinks or solid foods) partly influence the association between sugars and cardiometabolic risk factors.
The only metabolic variables that were significantly associated with FSs from drinks in fully adjusted models were fasting insulin and insulin resistance (HOMA-IR). It has been suggested that FS molecules in drinks, which are rapidly absorbed in the bloodstream, may affect directly glucose-insulin metabolism (36). Some authors came to the conclusion that deleterious effects of FSs (or added sugars) on health markers could be mostly driven by beverages (11, 37–39). Compared with liquid sources of FSs, solid-food sources require chewing and a greater amount of time to be consumed and may thus engage a more efficient energy compensation mechanism, meaning that subsequent intakes are better adjusted to reach a state of energy balance which might contribute to a better insulin homeostasis (40, 41). This may partly explain why %E as FS from solid foods was not associated with elevated insulin concentrations in the present study. Our results showing that NOS intake from drinks as well as milk intake are associated with a higher fasting insulin concentration are also consistent with this potential explanation.
In minimally adjusted models, FSs and NOSs displayed opposite associations with the cardiometabolic risk factors studied. However, results from moderately and fully adjusted models suggest that most of the associations observed between intakes of FSs or NOSs and cardiometabolic risk factors were largely attenuated when including sociodemographic characteristics, markers of adiposity, smoking, physical activity, plausibility of self-reported energy intake, the number of recalls completed on weekend days (model 2), and diet quality (model 3). This was especially true for the intake of NOSs, for which none of the beneficial associations observed with cardiometabolic variables in minimally adjusted models remained significant in fully adjusted models. Our results emphasize that the consumption of sugars might be a marker of a constellation of sociodemographic and lifestyle variables and diet quality known to be associated with cardiometabolic risk (26, 27, 42–47), which, in turn, may greatly influence the association between sugars and health. Indeed, in many studies, fruit intake (which is the main source of NOS in our sample) has been associated with higher socioeconomic status, educational levels, and physical activity, and was higher among nonsmokers (42, 43, 48, 49). In contrast, a higher consumption of SSBs has been frequently associated with lower educational and income levels, inactivity, and smoking (50–52). In a Portuguese sample, NOS and FS intakes showed similar associations with sociodemographic variables than those mentioned above for fruit and SSB intakes, respectively (53).
We cannot exclude that other factors currently unknown or whose scientific interest is growing may also have an impact on the diet–disease and the sugar–disease associations. For example, the gut microbiota, which is itself impacted by the composition of the diet, has been shown to be associated with inflammatory and metabolic disorders, cardiovascular diseases, obesity, and the risk of type 2 diabetes (54).
Our results support the idea that sugar intake is part of a complex dietary scheme. In that context, it becomes difficult to isolate its specific contribution to cardiometabolic risk from that of the overall diet quality and its determinants. Accordingly, some authors have argued that it is preferable to study the link between diet and disease with the perspective of overall diet quality instead of focusing only on 1 single nutrient such as sugar (13, 55). Overall, our results also suggest that sugar intakes are not strongly associated with cardiometabolic risk factors when diet quality and other biological, sociodemographic, and lifestyle factors are taken into account, as suggested by other authors (10, 56).
The strengths of our study were that participants were recruited with random-digit dialing in order to be representative in terms of age and sex of the adult French-speaking population of the province of Quebec and that they completed 3 self-administered, web-based, validated 24-h dietary recalls on randomly allocated days. Also, FSs and NOSs were rigorously differentiated using a systematic algorithm (22).
Our study also has some limitations. Due to its cross-sectional design, causality cannot be established and caution must be used in the interpretation of the results. Results could be different in an older sample or in a sample defined by different sociodemographic characteristics. The fact that our sample was slightly more educated than the general Quebec population might have influenced the results [in our sample, 45% of the participants hold a university degree vs. 33% in the general population of the province of Quebec (57)]. However, it has to be emphasized that the educational level was a covariate in regression models tested. We also have to keep in mind that reverse causation is possible. As such, some participants at risk of chronic diseases may have improved their eating habits, which may possibly influence or hide cross-sectional associations. Also, as in any other observational study examining the association between diet and health risk factors, residual confounding due to factors and variables not measured in this study is possible. Furthermore, despite using repeated 24-h dietary recalls, within-person random error may have biased associations observed. Finally, even if it was suggested that the web-based nature of the 24-h dietary recall tool may reduce the risk of social desirability bias compared with in-person completion (18), it is possible that underreporting of FS intake is more prevalent in people suffering from chronic diseases typically associated with obesity, which may also have biased the results. Plausibility of self-reported energy intakes was, however, added as a covariate in multivariable linear regression models to mitigate this issue.
In conclusion, in addition to being the first study on the topic in Canada, to the best of our knowledge, this study is the first to assess how FSs and NOSs from solid- and liquid-food sources are related to multiple cardiometabolic risk factors, and how sociodemographic characteristics, lifestyle variables, and diet quality affect these associations in an adult sample.
Our results revealed that many of the associations between sugar intake and cardiometabolic variables were attenuated or were no longer significant when considering overall diet quality and other relevant covariates, such as sociodemographic variables. This was true for both the unfavorable associations implicating FSs from drinks and the favorable ones implicating NOSs from solid foods. Other authors also concluded that associations between sugar intakes and cardiometabolic health are either inconsistent, not systematically observed, small, or absent (5, 39, 55, 58–62). In future studies, it would be interesting to study the impact of free and naturally occurring sugar intakes from solid- and liquid-food sources on cardiometabolic health outcomes in a controlled interventional context to be able to draw causal relations. Finally, and more importantly, our results, supported by conclusions from other authors (13, 55), suggest that caution must be taken not to overestimate the sole impact of sugar intake on health outcomes without considering other health determinants (44) and the overall quality of eating habits (63).
Supplementary Material
Acknowledgments
We thank Louise Corneau for her work in conducting the study and the participants for their involvement in the PREDISE study. We also express our gratitude to Drs. Luigi Bouchard, Julie Houle, Marie-France Langlois, and Rémi Rabasa-Lhoret for their collaboration in the testing of participants in the regions of Saguenay-Lac-St-Jean, Mauricie, Estrie, and Montreal. The authors’ responsibilities were as follows—SL, BL, MB, M-CV, CC, VP, SD, JR, and M-EL: designed the research; CL: contributed to conducting the research and contributed to the elaboration of the methodology for differentiating sugars; AB: analyzed data and wrote the manuscript; AB and SL: had primary responsibility for final content; DB: helped with the statistical analyses; and all authors: read and approved the final manuscript.
Notes
The present study was supported by the Canadian Institutes of Health Research (CIHR; grant number FHG 129921). AB is a recipient of a scholarship from the Fonds de Recherche du Québec–Santé and from the CIHR. DB holds a doctoral training award from the Fonds de Recherche du Québec–Santé.
Author disclosures: DB received a speaking honorarium from the Dairy Farmers of Canada in 2018. M-CV is the Canada Research Chair in Genomics Applied to Nutrition and Metabolic Health. BL is Chair of Nutrition at Université Laval, which is supported by private endowments from Pfizer, La Banque Royale du Canada, and Provigo-Loblaws. The other authors report no conflicts of interest.
The funder had no role in the design, implementation, analysis, interpretation of the data, or writing of this article.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AHEI, Alternative Healthy Eating Index–2010; CRP, C-reactive protein; DBP, diastolic blood pressure; FS, free sugar; MS z score, continuous metabolic syndrome risk score; NOS, naturally occurring sugar; PREDISE, PRÉDicteurs Individuels, Sociaux et Environnementaux; SBP, systolic blood pressure; SSB, sugar-sweetened beverage; TC, total cholesterol; TG, triglyceride; WC, waist circumference; %E, percentage of daily energy.
Contributor Information
Amélie Bergeron, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Marie-Ève Labonté, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Didier Brassard, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Catherine Laramée, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada.
Julie Robitaille, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Sophie Desroches, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Véronique Provencher, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Charles Couillard, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Marie-Claude Vohl, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Mathieu Bélanger, Department of Family Medicine, Université de Sherbrooke, Moncton, Canada.
Benoît Lamarche, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Simone Lemieux, Centre Nutrition, Santé et Société (NUTRISS), Institute of Nutrition and Functional Foods, Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
References
- 1.Tybor DJ, Beauchesne AR, Niu R, Shams-White MM, Chung M. An evidence map of research linking dietary sugars to potentially related health outcomes. Curr Dev Nutr. 2018;2:nzy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson J, Sadeghirad B, Lytvyn L, Slavin J, Johnston BC. The scientific basis of guideline recommendations on sugar intake: a systematic review. Ann Intern Med. 2017;166:257–67. [DOI] [PubMed] [Google Scholar]
- 3.Bowman SA. Added sugars: definition and estimation in the USDA Food Patterns Equivalents Databases. J Food Compos Anal. 2017;64:64–7. [Google Scholar]
- 4.World Health Organization . Guideline: sugars intake for adults and children. Geneva (Switzerland): WHO; 2015. [PubMed] [Google Scholar]
- 5.Scientific Advisory Committee on Nutrition (SACN) . Carbohydrates and health report. London (UK): Public Health England; 2015. [Google Scholar]
- 6.Health Canada . Canada's Dietary Guidelines for Health Professionals and Policy Makers. Ottawa (Canada): Health Canada; 2019. [Google Scholar]
- 7.Sundborn G, Thornley S, Merriman TR, Lang B, King C, Lanaspa MA, Johnson RJ. Are liquid sugars different from solid sugar in their ability to cause metabolic syndrome?. Obesity. 2019;27:879–87. [DOI] [PubMed] [Google Scholar]
- 8.Pepin A, Stanhope KL, Imbeault P. Are fruit juices healthier than sugar-sweetened beverages? A review. Nutrients. 2019;11;1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik VS, Hu FB. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11;1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lean ME, Te Morenga L. Sugar and type 2 diabetes. Br Med Bull. 2016;120:43–53. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Light K, Henderson M, O'Loughlin J, Mathieu ME, Paradis G, Gray-Donald K. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J Nutr. 2014;144:81–6. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 13.Khan TA, Sievenpiper JL. Controversies about sugars: results from systematic reviews and meta-analyses on obesity, cardiometabolic disease and diabetes. Eur J Nutr. 2016;55:25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louie JC, Tapsell LC. Association between intake of total vs added sugar on diet quality: a systematic review. Nutr Rev. 2015;73:837–57. [DOI] [PubMed] [Google Scholar]
- 15.Mok A, Ahmad R, Rangan A, Louie JCY. Intake of free sugars and micronutrient dilution in Australian adults. Am J Clin Nutr. 2018;107:94–104. [DOI] [PubMed] [Google Scholar]
- 16.Health Canada . Eating well with Canada's Food Guide. Ottawa (Canada): Health Canada; 2007. [Google Scholar]
- 17.Brassard D, Laramee C, Corneau L, Begin C, Belanger M, Bouchard L, Couillard C, Desroches S, Houle J, Langlois MFet al. . Poor adherence to dietary guidelines among French-speaking adults in the province of Quebec, Canada: the PREDISE study. Can J Cardiol. 2018;34:1665–73. [DOI] [PubMed] [Google Scholar]
- 18.Lafrenière J, Lamarche B, Laramée C, Robitaille J, Lemieux S. Validation of a newly automated web-based 24-hour dietary recall using fully controlled feeding studies. BMC Nutr. 2017;3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafreniere J, Laramee C, Robitaille J, Lamarche B, Lemieux S. Assessing the relative validity of a new, web-based, self-administered 24 h dietary recall in a French-Canadian population. Public Health Nutr. 2018;21:2744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lafreniere J, Laramee C, Robitaille J, Lamarche B, Lemieux S. Relative validity of a web-based, self-administered, 24-h dietary recall to evaluate adherence to Canadian dietary guidelines. Nutrition. 2019;57:252–6. [DOI] [PubMed] [Google Scholar]
- 21.Health Canada . Canadian nutrient file. Ottawa (Canada): Health Canada; 2015. [Google Scholar]
- 22.Bergeron A, Labonté M-È, Brassard D, Bédard A, Laramée C, Robitaille J, Desroches S, Provencher V, Couillard C, Vohl M-Cet al. . Intakes of total, free, and naturally occurring sugars in the French-speaking adult population of the province of Québec, Canada: the PREDISE study. Nutrients. 2019;11:2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louie JC, Moshtaghian H, Boylan S, Flood VM, Rangan AM, Barclay AW, Brand-Miller JC, Gill TP. A systematic methodology to estimate added sugar content of foods. Eur J Clin Nutr. 2015;69:154–61. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein JT, Schermel A, Mills CM, L'Abbe MR. Total and free sugar content of canadian prepackaged foods and beverages. Nutrients. 2016;8;582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langlois K, Garriguet D, Gonzalez A, Sinclair S, Colapinto CK. Change in total sugars consumption among Canadian children and adults. Health Rep. 2019;30:10–9. [PubMed] [Google Scholar]
- 26.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118:74–100, e11. [DOI] [PubMed] [Google Scholar]
- 27.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang TT, Roberts SB, Howarth NC, McCrory MA. Effect of screening out implausible energy intake reports on relationships between diet and BMI. Obes Res. 2005;13:1205–17. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine . Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington (DC): National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 31.Ramos JS, Dalleck LC, Borrani F, Beetham KS, Wallen MP, Mallard AR, Clark B, Gomersall S, Keating SE, Fassett RGet al. . Low-volume high-intensity interval training is sufficient to ameliorate the severity of metabolic syndrome. Metab Syndr Relat Disord. 2017;15:319–28. [DOI] [PubMed] [Google Scholar]
- 32. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 33.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. [DOI] [PubMed] [Google Scholar]
- 34.IPAQ Group . IPAQ scoring protocol. [Internet]. 2005; [cited 2020 Jan 11]. Available from: https://sites.google.com/site/theipaq/scoring-protocol. [Google Scholar]
- 35.Health Canada . Nutrition labelling table of reference amounts for food. Ottawa (Canada); Health Canada; 2016. [Google Scholar]
- 36.Malik VS, Hu FB. Fructose and cardiometabolic health: what the evidence from sugar-sweetened beverages tells us. J Am Coll Cardiol. 2015;66:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Connor L, Imamura F, Brage S, Griffin SJ, Wareham NJ, Forouhi NG. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin Nutr. 2018;37:1313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad R, Mok A, Rangan AM, Louie JCY. Association of free sugar intake with blood pressure and obesity measures in Australian adults. Eur J Nutr. 2020;59:651-9. [DOI] [PubMed] [Google Scholar]
- 39.Ramne S, Alves Dias J, González-Padilla E, Olsson K, Lindahl B, Engström G, Ericson U, Johansson I, Sonestedt E. Association between added sugar intake and mortality is nonlinear and dependent on sugar source in 2 Swedish population–based prospective cohorts. Am J Clin Nutr. 2019;109:411–23. [DOI] [PubMed] [Google Scholar]
- 40.Hur YI, Park H, Kang JH, Lee HA, Song HJ, Lee HJ, Kim OH. Associations between sugar intake from different food sources and adiposity or cardio-metabolic risk in childhood and adolescence: the Korean Child-Adolescent Cohort Study. Nutrients. 2015;8;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes. 2000;24:794–800. [DOI] [PubMed] [Google Scholar]
- 42.Psaltopoulou T, Hatzis G, Papageorgiou N, Androulakis E, Briasoulis A, Tousoulis D. Socioeconomic status and risk factors for cardiovascular disease: impact of dietary mediators. Hellenic J Cardiol. 2017;58:32–42. [DOI] [PubMed] [Google Scholar]
- 43.Carroll SJ, Dale MJ, Niyonsenga T, Taylor AW, Daniel M. Associations between area socioeconomic status, individual mental health, physical activity, diet and change in cardiometabolic risk amongst a cohort of Australian adults: a longitudinal path analysis. PLoS One. 2020;15:e0233793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DWet al. . Social determinants of risk and outcomes for cardiovascular disease. Circulation. 2015;132:873–98. [DOI] [PubMed] [Google Scholar]
- 45.Lanier JB, Bury DC, Richardson SW. Diet and physical activity for cardiovascular disease prevention. Am Fam Physician. 2016;93:919–24. [PubMed] [Google Scholar]
- 46.Xu Z, Steffen LM, Selvin E, Rebholz CM. Diet quality, change in diet quality and risk of incident CVD and diabetes. Public Health Nutr. 2020;23:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu EA, Steffen LM, Coresh J, Appel LJ, Rebholz CM. Adherence to the Healthy Eating Index-2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J Nutr. 2020;150:312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tohill BC, Seymour J, Serdula M, Kettel-Khan L, Rolls BJ. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr Rev. 2004;62:365–74. [DOI] [PubMed] [Google Scholar]
- 49.Grosso G, Micek A, Godos J, Pajak A, Sciacca S, Galvano F, Boffetta P. Health risk factors associated with meat, fruit and vegetable consumption in cohort studies: a comprehensive meta-analysis. PLoS One. 2017;12:e0183787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park S, Pan L, Sherry B, Blanck HM. Consumption of sugar-sweetened beverages among US adults in 6 states: Behavioral Risk Factor Surveillance System, 2011. Prev Chronic Dis. 2014;11:E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundeen EA, Park S, Pan L, Blanck HM. Daily intake of sugar-sweetened beverages among US adults in 9 states, by state and sociodemographic and behavioral characteristics, 2016. Prev Chronic Dis. 2018;15;E154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller C, Wakefield M, Braunack-Mayer A, Roder D, O'Dea K, Ettridge K, Dono J. Who drinks sugar sweetened beverages and juice? An Australian population study of behaviour, awareness and attitudes. BMC Obes. 2019;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marinho AR, Severo M, Correia D, Lobato L, Vilela S, Oliveira A, Ramos E, Torres D, Lopes C. Total, added and free sugar intakes, dietary sources and determinants of consumption in Portugal: the National Food, Nutrition and Physical Activity Survey (IAN-AF 2015–2016). Public Health Nutr. 2020;23:869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: causes, consequences, and solutions—but do we have the will?. Fertil Steril. 2017;107:833–9. [DOI] [PubMed] [Google Scholar]
- 55.Rippe JM, Angelopoulos TJ. Relationship between added sugars consumption and chronic disease risk factors: current understanding. Nutrients. 2016;8;697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sneed NM, Patrician PA, Morrison SA. Influences of added sugar consumption in adults with type 2 diabetes risk: a principle-based concept analysis. Nurs Forum. 2019;54:698. [DOI] [PubMed] [Google Scholar]
- 57.Gouvernement du Québec . Institut de la statistique du Québec. Panorama des régions du Québec. 2020 ed. Quebec (Canada); Government of Quebec; 2020. [Google Scholar]
- 58.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100:65–79. [DOI] [PubMed] [Google Scholar]
- 59.Tappy L, Morio B, Azzout-Marniche D, Champ M, Gerber M, Houdart S, Mas E, Rizkalla S, Slama G, Mariotti Fet al. . French recommendations for sugar intake in adults: a novel approach chosen by ANSES. Nutrients. 2018;10;989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Austin GL, Krueger PM. Increasing the percentage of energy from dietary sugar, fats, and alcohol in adults is associated with increased energy intake but has minimal association with biomarkers of cardiovascular risk. J Nutr. 2013;143:1651–8. [DOI] [PubMed] [Google Scholar]
- 61.Prinz P. The role of dietary sugars in health: molecular composition or just calories?. Eur J Clin Nutr. 2019;73:1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rippe JM, Marcos A. Controversies about sugars consumption: state of the science. Eur J Nutr. 2016;55:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tapsell LC, Neale EP, Satija A, Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. 2016;7:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


