Abstract
Prairie voles have emerged as an important rodent model for understanding the neuroscience of social behavior. Prairie voles are well known for their capacity for pair bonding and alloparental care. These behavioral phenomena overlap with human social behavior but are not commonly observed in traditional rodent models. In this review, we highlight the many benefits of using prairie voles in neuroscience research. We begin by describing the advantages of using diverse and non-traditional study models. We then focus on social behaviors including pair bonding, alloparental care, and peer interactions that have brought voles to the forefront of social neuroscience. We then describe many additional features of prairie vole biology and behavior that provide researchers with opportunities to address an array of research questions. We survey neuroethological methods that have been used with prairie voles, spanning from classic to modern techniques. Finally, we conclude with a discussion of other vole species, particularly meadow voles, and their own unique advantages for neuroscience studies. Together, this review provides a foundation for researchers who are new to working with voles, as well as for experienced neuroscientists who want to expand their research scope.
Keywords: Prairie vole, vole, meadow vole, research model, neuroscience, social behavior
INTRODUCTION
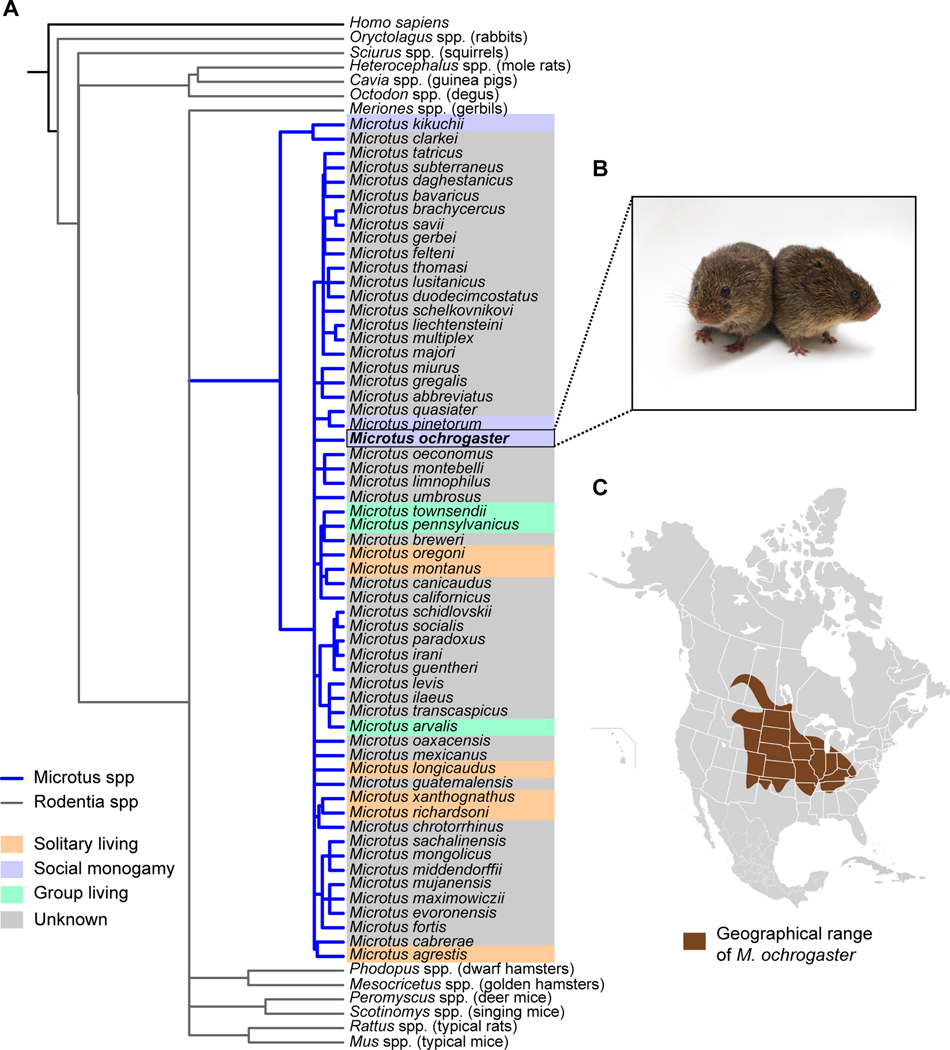
Prairie voles are small herbivorous rodents native to alfalfa, bluegrass, and tallgrass prairie found in eastern and central North America (~40 to 70˚N) (Figure 1) (Getz et al., 1979) (Cole and Batzli, 1979). Prairie voles have become increasingly prominent in neuroscience research since the discovery in the early 1980s by Lowell Getz and colleagues that wild prairie voles exhibit social monogamy—i.e. stable behavioral associations between mates (Getz et al., 1981). Early investigations into pair bonding established that prairie voles preferred to interact with familiar mates in dyadic or group social interactions (Thomas and Birney, 1979; Getz et al., 1981). These social choices were subsequently assessed using the Partner Preference Test (PPT; Williams et al., 1992b, 1992a), a choice test that assesses the extent of preference for a familiar mate over an unfamiliar stranger, and that remains the predominant assay of social behavior in voles today (see Techniques below and protocol: Beery, 2021 this issue). Although not genetically exclusive, prairie vole partnerships nonetheless represent long-term social relationships, or ‘pair bonds’, with males and females both taking part in direct parental care of young.
Figure 1.
Prairie voles have become popular for studies of social neuroscience, and have many additional advantages and features described in this review. A. Phylogeny of Microtus (blue lines), representative species from other Rodentia groups (gray lines), and Homo sapiens as an outgroup. Phylogenetic data are from a published mammalian supertree (Fritz et al., 2009), and the tree was plotted with R software (R v3.5.3) using the ‘ape’ package (Paradis and Schliep, 2019). Social systems for Microtus spp are color coded based on previously published descriptions (Lukas and Clutton-Brock, 2013). B. Laboratory bred prairie voles (Image ©Beery Lab). C. Distribution of prairie voles in North America. (Image by Simon Pierre Barrette / CC BY-SA 4.0, adapted from (Naughton et al., 2012).
While monogamy is prevalent in some taxonomic groups, it is rare in rodents, and the discovery of pair bonding in prairie voles has launched four decades of exploration into the causes and consequences of social bond formation between mates. Prairie voles (Figure 1B) are now the mostly widely studied monogamous rodent, and have provided numerous insights into the mechanisms that underlie pair bond formation. Prairie vole research has also shed light on several social phenomena from alloparental care to social buffering. Moreover, their social behavior and physiology has translational value for mental and physical health, from the effects of endocrine disruptor exposure to long-term developmental impacts of pharmaceuticals such as antidepressants (Sullivan et al., 2014; Lawrence et al., 2020).
In this review, we highlight the advantages of using prairie voles and other vole species (Figure 1A) in neuroscience research. An exhaustive survey of prairie vole neuroscience is beyond the scope of the present review. Instead, we describe the benefits of using diverse and non-traditional rodent models, then discuss the uncommon social behavioral traits of prairie voles that exhibit substantial overlap with human behavior. We provide a foundation for researchers new to working with voles by detailing a host of other physiological characteristics, from autonomic nervous system regulation to reproductive biology, and survey the variety of neuroethological methods employed in vole research. Finally, we discuss research in sister vole taxa, particularly meadow voles, with behaviors of interest to comparative neuroscience.
Benefits of non-traditional model organisms
Contemporary biological research has focused primarily on rats and mice, in part because of their ease of use in laboratory settings, availability of genetic engineering tools, and the accumulated bodies of knowledge in these species. There is no particular homology between rats or mice and humans relative to other rodent species, however (Figure 1C), and despite advantages of “drilling deep” within a few model systems, there are pitfalls of relying on one or two species to model human biology. Use of diverse species provides opportunities to a) examine species with specific traits of interests, which may not be present or easily studied in rats and mice, and to b) shed light on the extent to which physiological and behavioral processes are conserved or vary across species. There is a growing movement to study the neural basis of diverse, species-specific natural behaviors within the context of natural ecology, uniting mechanistic and evolutionary explanations for behavior (Phelps et al., 2010; Hofmann et al., 2014; Taborsky et al., 2015; Hale, 2019).
Neuroscience is rife with examples of species studied for particular features of interest, from the giant axon of the longfin inshore squid to impressive sound-triangulation in barn owls. The idea that “for many problems there is an animal on which it can be most conveniently studied” was dubbed Krogh’s principle (Krebs, 1975; Krogh, 1929). Prairie voles, other monogamous rodents (such as California mice: Peromyscus californicus), and rodents with varied social systems provide this sort of opportunity to study social behavioral traits not present in mice and rats.
Krogh also believed that comparative studies across multiple species are critical for obtaining a complete understanding of mammalian physiology, and others have added the sentiment that no single species can stand as a model for others (Jørgensen, 2001; Wayne and Staves, 1996). A diversity of model organisms enables the discovery and empirical exploration of a wide range of distinct phenomena (Hodgkin, 2019). Comparative studies allow researchers to test questions about conserved and derived mechanisms that underlie physiological and behavioral traits. Such comparative work has the potential to yield insights into the evolution of mechanisms underlying behaviors, including monogamy in voles (described below in Neuroendocrinology of monogamy across voles).
BENEFITS OF PRAIRIE VOLES
The natural history of prairie voles renders them an excellent model for investigating the connections between social behavior and health. Here, we review some of these behavioral traits, particularly in regards to selective social bonding, biparenting and alloparental care (Young et al., 2011). Finally, we discuss some lesser-known behavioral traits for which prairie voles are a useful model species for neuroscience.
Field-lab crossover
Although the bulk of recent prairie vole research has taken place in the lab, a complementary line of work has explored their behavior and physiology in naturalistic field environments (Madrid et al., 2020; Sabol et al., 2018). This field-lab crossover is possible because prairie voles behave similarly across research settings. Prairie voles in the field are frequently trapped in opposite-sex pairs that remain stable across seasons (Getz et al., 1981, 1993). Voles brought into the lab environment readily engage in species-typical behaviors like intra-pair affiliation, extra-pair aggression, nest building and direct biparental care (Getz et al., 1981; Thomas and Birney, 1979). This ease of translation from field to lab and back is an important advantage when using voles over traditional rodent models in neuroscience studies. Researchers can be confident that lab paradigms to investigate social bonding, alloparental care (with some caveats, discussed below), and aggression have high ecological validity.
An additional benefit to studying prairie voles living in (or derived from) the field is that their behavior naturally varies between and within populations (Madrid et al., 2020). Inter-group variation in genetic monogamy, biparental care, and aggression has been documented in voles from Kansas, Illinois and Indiana (Mabry et al., 2011; Roberts et al., 1998b). Behavioral variation amongst these populations corresponds to neural characteristics such as resting state functional connectivity (Ortiz et al. 2021). Variation also occurs within populations, where reproductive behavior and fitness depends on habitat fluctuations and predation risk (Desy and Batzli, 1989; Getz et al., 1990) as well as population demographics and individual strategies (Getz et al., 1993; Sabol et al., 2020; Shuster et al., 2019; Madrid et al., 2020). For instance, many males express a “resident” strategy and align their home ranges with a single female, while other “wanderer” males have home ranges that overlap with multiple females (Getz and Hofmann, 1986; McGuire and Getz, 1998). These distinct male strategies correspond to neuropeptide (i.e., vasopressin) receptor expression in the retrosplenial cortex, a brain area involved in spatial memory (Okhovat et al., 2015; Ophir et al., 2008). Collectively, these studies show that prairie vole behavior is flexible. Researchers can capitalize on this intraspecific flexibility to model interactions between ecology, behavior, and physiology in both field and laboratory settings.
Pair bonding
Romantic attachment is a cross cultural human universal (Fletcher et al., 2015) and social monogamy is the most common human reproductive tactic (Schacht and Kramer, 2019). These reproductive behaviors are critical to study because of their relevance to health and well-being in humans. In contemporary, western societies, married individuals report higher happiness and life satisfaction than those who have never married, are widowed, or are divorced (Gove et al., 1990; Lawrence et al., 2019; Myers and Diener, 1995), a pattern also seen in terms of improved mental and physical health among the married (Holt-Lunstad et al., 2008; Robles et al., 2014; Sbarra et al., 2014). Romantic attachment is particularly relevant to affective state, with associations to mood, anxiety, depression, and substance use. This suggests that while better mental health facilitates romantic attachment, the reverse is also true, with romantic attachment promoting mental health (Braithwaite and Holt-Lunstad, 2017).
Prairie voles are one of the few mammalian models where this human social phenomenon, the formation of long-term social attachments, can be studied experimentally in the lab. Traditional laboratory species do not readily form selective social attachments, even with same-sex peers (Beery and Shambaugh, 2021). For example, mice and rats often prefer social novelty (Moy et al., 2004; Smith et al. 2005; Hackenberg et al. 2021), whereas voles typically prefer familiar social partners. Selective familiarity preferences have been described in both meadow and prairie voles (Lee et al. 2019), which are unique among social rodents tested thus far, including mice (Beery et al., 2018), rats (Beery and Shambaugh, 2021), and degus (Insel et al., 2020).
Prairie voles are well known for their capacity for social monogamy, wherein adult males and females form selective social bonds. These bonds are marked by a preference for the familiar ‘partner’ over novel ‘strangers’ in the Partner Preference Test (described with additional context and variations in the accompanying protocol; Beery, 2021 this issue), as well as defensive territoriality and distress upon separation from the pair mate (Bosch et al., 2008). Research in this area has benefited from the presence of closely related species that do not demonstrate social monogamy (e.g. meadow voles or montane voles), which has allowed for intra- and inter-species comparisons at the behavioral, neuroendocrine and genetic levels (Carter and Getz, 1993; Hammock and Young, 2005b; Lim et al., 2004; Madrid et al., 2020).
Pair bonding in prairie voles also provides an opportunity to study social bonds as a dependent variable in response to the effects of a wide variety of socially relevant early life experiences and exposures. For example, the formation and expression of partner preferences can be shaped by early social experience and developmental exposures (Bales and Perkeybile, 2012). Researchers have also examined how adult prairie vole social behaviors vary in response to early life conditions such as perinatal oxytocin administration during delivery (Kenkel et al., 2019; Kenkel, 2020), or gestational exposure to endocrine disruptors (Sullivan et al., 2014).
Biparental and alloparental care
In order to achieve large, expensive, slowly developing brains, humans have had to rely on biparental care and cooperative breeding (reproduction with the help of others, termed ‘alloparents’ when caring for offspring other than their own) (Fletcher et al., 2015; Isler and van Schaik, 2012; Kenkel et al., 2017; Navarrete et al., 2011). Care from unrelated adults is a universal experience among modern human societies (NICHD Early Child Care Research Network, 2001; Sear and Mace, 2008). The quality of such alloparental care can predict social competence and emotional regulation (Pluess and Belsky, 2009). Likewise, care from fathers is an important contributor to health and development outcomes in children. Human fathers’ presence improves children’s educational achievement (Jeynes, 2015), emotional health and cognitive development (Cherlin et al., 1991; DuBois et al., 1994; Florsheim et al., 1998; Furstenberg and Teitler, 1994; Sarkadi et al., 2008). Furthermore, fathers’ care appears protective against anxiety (Bögels and Phares, 2008; Möller et al., 2016), attention-deficit / hyperactivity disorder (Fabiano, 2007) and substance abuse (Stein et al., 2009). Indeed, in the case of anxiety, meta-analysis suggests fathers’ parenting contributions appear to outweigh mothers’ (Möller et al., 2016). A better understanding of non-maternal caregiving could inform the etiology of its dysfunction, which is a major source of child neglect and maltreatment (Kenkel et al., 2017).
Traditionally, the neurobiological study of caregiving was limited to maternal behavior and its strong endocrine regulation (Glasper et al., 2019). In prairie voles, fathers (Kenkel et al., 2014; Rogers and Bales, 2019; Campbell et al., 2009), virgin adults, and older siblings (Rogers and Bales, 2020) all exhibit care towards pups, allowing vole studies to disentangle the contributions of the hormones of pregnancy and parturition. Prairie vole research has also been able to document the consequences of non-maternal care and its absence (Ahern and Young, 2009; Rogers and Bales, 2020).
Same-sex “peer” social behaviors
While male-female interactions have been the focus of the majority of prairie vole research, study of interactions with same-sex “peers” has contributed to our understanding of peer relationships, the effects of isolation and cohabitation on physiology and behavior, and social interactions that console or buffer stress. Prairie voles exhibit selective preferences for familiar same-sex partners after ~18–24 hours of cohabitation (DeVries et al., 1997; Beery et al., 2018, Lee et al., 2019). These preferences may be of importance by supporting the formation of extended family groups with undispersed offspring in fall and winter months (Getz et al., 1993). While the formation of small groups is seasonal, interestingly, prairie voles exhibit peer partner preferences and extensive huddling behavior in both long (summer-like) and short (winter-like) day lengths (Lee et al., 2019), suggesting familiarity preferences are not conditional on the environment in this species.
Isolation from a same-sex cagemate for extended intervals (2 weeks to 2 months) results in changes in neuroendocrine function, anxiety and depression-like behaviors, and cardiovascular function (Stowe et al., 2005; Grippo et al., 2007a, 2008a). In particular, separation leads to higher heart rate and changes in cardiac function associated with cardiovascular disease (Grippo et al., 2011; Peuler et al., 2012), similar to findings in mice and rats (reviewed in Beery et al., 2020). Just as isolation can act as a stressor, social cohabitation can lead to improved outcomes in the face of stress. Studies across a variety of social mammals show profound effects of cohabitation on the recovery from stressors (Beery and Kaufer, 2015). In prairie voles, familiarity plays a further role in stress buffering: females exposed to a stressed same-sex cagemate but not a stressed stranger exhibit ‘consolation behavior’ in the form of increased allogrooming. They also exhibit several empathy-like behaviors, including matching their cage-mate’s corticosterone levels, showing coordinated freezing to a stressor only their cagemate experienced, and showing elevated fos activity in the anterior cingulate cortex, a brain region associated with empathy in humans (Burkett et al., 2016).
These consolation behaviors and social buffering of stress have also been demonstrated in opposite-sex prairie vole relationships (Smith and Wang, 2014; Burkett et al., 2016), as have behavioral and cardiac consequences of isolation from a mate (Bosch et al., 2009; McNeal et al., 2014). Because prairie voles form selective preferences for both mates and same-sex peers, they can be used to study the effects of relationship type on a wide variety of social behaviors. For example, brief (<1 week) separation from a mate but not a same-sex sibling resulted in increased circulating corticosterone, adrenal weight, and depressive-like behaviors (Bosch et al., 2009). Most studies to date have focused on either peer or mate relationships without such comparisons, but this represents a promising realm for future exploration. Between the variety of social behaviors that can be assessed, the variety of factors that shape them, and the added variable of relationship type (familiar/unfamiliar, same-sex/opposite sex), prairie voles provide many benefits for social neuroscience research.
ANCILLARY FEATURES OF WORKING WITH PRAIRIE VOLES
Prairie vole neuroscience research began with investigations into social behavior, but over the course of such studies, several unique features of their physiology and natural history have become especially useful or noteworthy to researchers. What follows is a list of select features accompanied by either brief explanations as to how they make the prairie vole an attractive model system for research questions beyond the realm of social behavior, or how they can be taken into consideration. These attributes can be thought of as secondary, ancillary features that are an important part of prairie vole biology. Although these features are “ancillary” for now, emphasis on these traits may grow in the future.
Ultradian Rhythms
In contrast to the circadian rhythms displayed by laboratory rodents that are nocturnal, or in some cases diurnal, vole species show rhythms of activity throughout the day and night with periods of 1–4 hours (Gerkema and van der Leest, 1991). These shorter than one day “ultradian” rhythms in physical activity are accompanied by ultradian rhythms in core body temperature, heart rate, and blood pressure (Grippo et al., 2007b; Beery et al., 2008; Nieminen et al., 2013; Lewis and Curtis, 2016). The presence of ultradian rhythmicity can be convenient for researchers, as there is no need to conduct studies during the dark phase in order to coincide with periods of activity. Ultradian rhythms are synchronized by the light cycle and follow consistent patterns over time and across individuals (e.g. Beery et al., 2008), hence testing can be performed at any time that can be held reasonably consistent within a study. As discussed in the next section, prairie voles differ from laboratory rodents not only in activity rhythms, but also in lacking spontaneous estrous cyclicity. These species differences have important implications for husbandry and experimental design.
Reproduction
Prairie vole reproduction differs from that of mice and rats in several important ways, including ovulatory cycle, litter size, and cross-fostering potential—each of which can be leveraged by researchers. Most laboratory rodents undergo spontaneous ovulatory cycles, for example mice, rats, and hamsters are spontaneous ovulators with a mating-induced pseudopregnancy (i.e. luteal phase). In contrast, prairie voles are induced ovulators, meaning that females do not ovulate until exposed to an unfamiliar male, or his urine (Carter et al., 1980). Until this happens, the female hypothalamic-pituitary-gonadal axis is relatively quiescent. This may be of use to researchers interested in the organizational effects of sex hormones separate from their activational effects, as unpaired females do not undergo estrous cycles. Furthermore, it provides more control over reproductive pairings, as mating pairs can be put together at any time. Females can be prepared for mating by advance exposure to a male or soiled bedding, or may be hormonally primed by injection of estradiol benzoate (Roberts et al., 1998b). Unprimed females typically ovulate ~10.5 hours after mating, and females continuously housed near males may remain in persistent estrus for ~1 month (Stalling, 1990).
In continuously cohoused breeding pairs, most matings occurs during post-partum estrus, leading to the birth of a new litter every ~21 days. One noteworthy consideration is that in most lab-housed prairie vole colonies, offspring are separated from parents at 20–21 days of age, prior to the birth of the next litter, whereas in the wild, most vole offspring remain with their parents until at least 6–7 weeks of age (Carter and Roberts, 1997; Getz et al., 1994). This is important because exposure to a pup in early life alters an individual’s developmental trajectory, such that voles exposed to the next litter show higher rates of alloparental responsiveness (Lonstein and Vries, 2001; Roberts et al., 1998a; Stone et al., 2010). Thus, conventional husbandry practices that deviate from what is most common in the wild may have meaningful consequences.
Mate pairs can continue to produce year-round if housed in long day lengths (i.e. >12 hours of light/day). Exposure to short (winter-like) day lengths leads to gonadal regression and cessation or reduction of reproduction in multiple vole species, in conjunction with temperature and dietary cues (Craven and Clarke, 1982; Dark et al., 1983; Nelson et al., 1983; Kriegsfeld et al., 2000). Maternal photoperiodic history can also influence offspring development, and interacts with the availability of compounds found in new vegetation (Lee and Zucker, 1988).
Prairie voles have small litters (~3–5) relative to mice (~5–10) and rats (~8–12), which have each been artificially selected for increased fecundity. Litter size is an important determinant of a number of metabolic characteristics in rodents (Parra-Vargas et al., 2020). Furthermore, litter effects are routinely (>80%) not controlled for in developmental studies of prenatal stress or variation in maternal care (Williams et al., 2017). Although prairie voles’ small litters will necessitate slower subject pool growth, this is offset by the diminished propensity for litter effects and variation in litter size to bias results.
Multiple aspects of development, including stress reactivity and metabolism, are affected by cross-fostering in traditional laboratory species (Bartolomucci et al., 2004; Cox et al., 2010; Curley et al., 2010; Maccari et al., 1995; Matthews et al., 2011; Santangeli et al., 2016; Maxson and Trattner, 1981). For instance, in mice, the experience of being cross-fostered in and of itself increases depressive-like behavior, decreases maternal nest-building (Lerch et al., 2014) and increases appetite, body weight, and abdominal fatness (Matthews et al., 2011), which may relate to differences in maternal behavior on the part of foster dams. While, prairie voles readily and indiscriminately adopt unrelated pups and show no differences in parental behavior toward them (Perkeybile et al., 2015), further research is needed to determine whether prairie voles avoid the developmental consequences of cross-fostering observed in mice and rats.
Domestication
Reproduction in laboratory rodents has been extensively modified by domestication in traditional laboratory species (Chalfin et al., 2014; Perrigo et al., 1993). Recently, conventional wisdom has begun to cede ground to the contention that outbred mice may be more appropriate than inbred strains for biomedical research (Tuttle et al., 2018). Moreover, domestication results in changes to brain and behavior in several domains such as: neuropeptide receptor expression, temperament, social behavior, stress reactivity and reproduction, especially in females (Chalfin et al., 2014). For instance, whereas domesticated female lab mice generally tolerate pups as virgins (70% parental), wild virgin female mice react aggressively to pups (100% infanticidal) (Chalfin et al., 2014). Similar patterns can be found in males (Perrigo et al., 1993; Jakubowski and Terkel, 1982). In contrast, prairie voles are recently descended from wild-caught stock, are often purposefully outbred to wild-caught voles, and have not undergone substantial domestication. Additionally, prairie voles have not had the diversity of their microbiomes artificially reduced via abnormally hygienic housing conditions—conditions which have been shown to reduce the translational relevance of mice (Beura et al., 2016; Rosshart et al., 2019). The prairie vole microbiome is markedly distinct from that of rats and mice, with novel dominant bacterial strains, as well as more variability between individuals (Donovan et al., 2020a, 2020b). Finally, domestication also affects the expression of oxytocin and vasopressin in brain regions of rats and mice known to regulate social behavior and emotion (Ruan and Zhang, 2016). Thus, the use of prairie voles in lab research can avoid issues associated with domestication in traditional study models.
Oxytocin and Vasopressin
The impact of domestication on oxytocin and vasopressin is important because these two neuropeptides have been central to the study of sociality (Caldwell, 2017). Indeed, much of the prairie vole research to date has focused on these pleiotropic hormones. The distributions of oxytocin and vasopressin receptors in prairie voles have been extensively studied, although most often in subcortical brain regions related to affect and social behavior (McGraw and Young, 2010). Recently, characterization of oxytocin receptor localization has been extended to the periphery of neonates (Greenwood and Hammock, 2019) and cortex of adults (Duchemin et al., 2017) (see also Cortical Organization, below). It was recently observed that the promoter region of the oxytocin receptor has greater sequence homology to humans in prairie voles than either rats or mice (Perkeybile et al., 2019). The methylation of CpGs within the promoter is relevant for epigenetic investigations of how oxytocin’s actions in early life can steer brain and behavior throughout development (Perkeybile et al., 2019; Kenkel et al., 2019).
Early studies noted the high levels of plasma oxytocin concentrations in prairie voles (2–4x higher than in rats) (Kramer et al., 2004). This was originally taken as evidence that prairie voles have high, human-like levels of circulating oxytocin. However, readers should be cautioned that measurement of oxytocin levels in the blood is an unresolved topic of debate (Leng and Sabatier, 2016; Brandtzaeg et al., 2016; MacLean et al., 2019). In the central nervous system, the prairie vole paraventricular nucleus (a major source nucleus for oxytocin) does not show the same clear distinction between magnocellular and parvocellular neurons as seen in rats and mice. In other species, magnocellular neurons project to the forebrain and pituitary, while parvocellular neurons project to the hindbrain and spinal cord.
Corticosteroids
The hypothalamic-pituitary-adrenal (HPA) axis is intimately tied to oxytocin and vasopressin via the regulation of stress, metabolism, and affect. Prairie voles have much higher circulating levels of two major HPA-axis signaling molecules, the glucocorticoid corticosterone and adrenocorticotropic hormone (ACTH) (Taymans et al., 1997) than either rats or mice, even after accounting for the effects of domestication (Chalfin et al., 2014) (Table 1). Prairie voles are also resistant to dexamethasone induced suppression of corticosterone, which, together with their higher basal set-point, has led to prairie voles being characterized as ‘glucocorticoid resistant’ (Taymans et al., 1997).
Table 1.
Baseline plasma corticosterone concentrations across voles and traditional laboratory rodent species considering domestication status.
| Species | Plasma Corticosterone (ng/ml) | Source |
|---|---|---|
| Rats (domesticated Sprague-Dawley) | 72 ± 7 | (Taymans et al., 1997) |
| Mice (domesticated C57BL/6J) | 19 ± 3 | (Chalfin et al., 2014) |
| Mice (wild-derived) | 165 ± 35 | (Chalfin et al., 2014) |
| Meadow voles (wild-derived) | 130 ± 34 | (Taymans et al., 1997) |
| Prairie voles (wild-derived) | 666 ± 90 | (Taymans et al., 1997) |
Thermoregulation & Metabolism
Oxytocin, vasopressin and the HPA-axis are all important regulators of not only social behavior but also more fundamental processes such as thermoregulation and metabolism, two domains where voles again show important differences from traditional laboratory species. Across the Microtus genus, vole species have 20%−40% greater basal metabolic rates than is expected from allometric predictions (Wunder, 1985). In traditional laboratory housing, prairie voles also appear to have relatively low fat mass (~5% body weight, (Seelke et al., 2018)) compared to rats (25–40% (Martin et al., 2010)) or mice (23–26% (Martin et al., 2010; Reed et al., 2007)). Because human environments require very little energy expenditure on thermogenesis (Brychta and Chen, 2016), such conditions are important when modeling human health in animal studies (Bastías-Pérez et al., 2020; Maloney et al., 2014). Housing temperature is one the largest determinants of energy expenditure in mice (Corrigan et al., 2020). Indeed, basal metabolic rate and food intake of mice housed at room temperature 20°C are both ~50% higher than those housed at mouse-thermoneutral 30°C, which speaks to the magnitude of the chronic cold stress lab mice experience (Maloney et al., 2014; Hylander and Repasky, 2016; Hankenson et al., 2018). As detailed below, voles are better suited to room temperature housing and thereby avoid these effects.
Voles are adapted to boreal habitats, have thick coats of fur, and tolerate cool temperatures (Wunder, 1985; Wunder et al., 1977). Voles’ short tails likely help reduce heat loss compared to rats or mice (Škop et al., 2020) and presumably, so too would the Microtus genus’ eponymous small ears. Standard room temperature housing therefore represents a less stress-confounded approach to housing prairie voles, avoiding the chronic cold stress experienced by mice housed at room temperature (Maloney et al., 2014). Thermoneutrality in mice is 30–32°C (Cannon and Nedergaard, 2011; Hylander and Repasky, 2016), whereas prairie voles tolerate a wide range of temperature (Wunder et al., 1977). Thermoneutrality in voles is ~25–30°C (Beck and Anthony, 1971; Packard, 1968), which expands to 20–30°C when nesting is considered (Beck and Anthony, 1971). The prairie vole has the added benefit of biparental care, where fathers routinely huddle their litters, providing added warmth for vulnerable pups. Group housing and nesting only slightly ameliorates the chronic cold stress experienced by mice at 20°C (Maher et al., 2015).
Autonomic ‘balance’, the relative contributions of sympathetic and parasympathetic tone, is both a window into metabolic state (Green, 2011) and a fundamental aspect of stress and emotional reactivity that underlies much of social behavior (Grippo, 2017). The previously held conventional wisdom, that mice have high heart rates driven predominantly by sympathetic tone, has been overturned by work on mice housed in thermoneutral conditions. In these conditions, mice have lower heart rates and higher levels of parasympathetic cardiac tone (Swoap et al., 2008). Prairie voles show similarly low heart rates and high parasympathetic cardiac tone when housed at 22°C (Grippo et al., 2007b). Thus, it would appear that prairie voles avoid the chronic cold stress that traditional housing conditions present to mice.
Alcohol consumption
Drug use and social relationships are inter-related in humans, and prairie voles have emerged as a model system to study these interactions (Potretzke and Ryabinin, 2019). Voles consume alcohol at high rates and show robust preferences for ethanol over pure water (Anacker et al., 2011, 2012). Prairie vole alcohol use is particularly sensitive to peers. Same-sex cagemates will adjust their drinking levels to match each other (Anacker et al., 2011; Anacker and Ryabinin, 2013), and having a peer around appears to attenuate relapse drinking behavior (Hostetler and Ryabinin, 2014). Interactions between social environment and alcohol use also occur with respect to pair bonding. Partner preferences decrease when males consume alcohol during bond formation, and the opposite effect occurs for females (Anacker et al., 2014). Moreover, bonded males show decreased partner preferences when a pair shows discordant patterns of alcohol consumption, and this effect is absent in females (Walcott and Ryabinin, 2017, 2019). Thus, prairie voles are an interesting model system to study social facilitation of drinking, and in reverse, how drinking behavior influences social bonding.
Vocal communication
Prairie vole social interaction involves multiple sensory modalities, and yet, research tends to be biased towards conspicuous modalities, including touch (e.g., huddling) and olfactory cues (e.g., anogenital investigation). Another important sensory modality is acoustic signaling. Early observations showed that prairie vole adult males produce high rates of ultrasonic vocalizations (USVs) when exposed to novel females (Lepri et al., 1988), but only a couple studies have followed up on these early observations. This recent work shows that prairie voles produce a diverse repertoire of USVs (25–80 kHz) that are spectrally similar to mouse and rat USVs (Ma et al., 2014; Stewart et al., 2015). Both sexes vocalize when exposed to conspecifics, producing different USVs depending on partner traits like familiarity, sex, and reproductive state (Ma et al., 2014). The most elaborate calls are made by males exposed to estrus females, and these elaborate calls also occur when adults are given the dopamine agonist amphetamine. Another context where vocalizations occur is when adults are physically separated from, but in audiovisual contact with, familiar cagemates (Stewart et al., 2015). In this context, USV acoustic structure (e.g., fundamental frequency) is tightly linked with heart rate. Together, these findings indicate that prairie vole USVs are involved in the formation and maintenance of adult social bonds. Underlying mechanisms likely involve, but are not limited to, the dopaminergic system and autonomic nervous system.
Vocalizations also occur in parent-offspring interactions. Prairie vole pups are incredibly vocal when isolated from their parents (Robison et al., 2016), calling at rates that are several times higher than the pups of sister taxa (Blake, 2002; Rabon Jr. et al., 2001; Shapiro and Insel, 1990). These calling rates are positively correlated with corticosterone levels during separation (Shapiro and Insel, 1990). Calling rates peak in the first 1–2 weeks after birth, and then decrease quickly thereafter (Kenkel et al., 2015). Pup vocalizations span both audible (~12 khz) to ultrasonic (~36 kHz) frequencies, with more acoustically complex calls occurring in early infancy (Terleph, 2011). Playback experiments show that USVs are particularly attractive to postpartum and late gestation parents (Terleph, 2011). Therefore, pup vocalizations may help solicit parental attention during early infancy. This vocal response to separation could be mediated by the “stress” system (i.e., HPA axis). Our understanding of prairie vole vocalizations has only just begun. Future research is needed to determine the precise social functions of juvenile and adult vocalizations, and to identify neural substrates that govern this complex behavior.
Cortical organization
Prairie vole neuroanatomy is highly conserved with other mammals, yet it has some distinctive features that differ from sister taxa and other rodents. One of the most distinctive features is the size and structural connectivity of sensorimotor areas. Prairie voles are known to have a strikingly large auditory cortex and auditory-responsive areas (relative to neocortex) compared to mice and mammals with similarly sized neocortex (Campi et al., 2007; Krubitzer et al., 2011). In somatosensory cortex, there is a disproportionate area devoted to perioral facial hairs and the snout as compared with non-vole species (Campi et al., 2007). Another unique feature of prairie vole somatosensory areas is that they are broadly inter-connected with each other (Campi et al., 2010). This cross-modality connectivity differs from rats, mice and squirrels, for which connections are focused within a single sensory modality. Both of these features – sensorimotor organization and connectivity – are highly sensitive to early life experience. Prairie voles that received high parental care have larger primary motor and visual areas, but smaller and more intrinsically connected somatosensory areas, than do voles that received low parental care in infancy (Seelke et al., 2016a, 2016b). Thus, strong multisensory integration appears to be an important component of prairie vole physiology and developmental plasticity.
Prairie vole limbic-associated frontal cortex and extended amygdala also have unique features related to size and structural connectivity. Prairie vole medial prefrontal cortex (mPFC) takes up a smaller proportion of cortex than does meadow vole mPFC, while other parts of cortex (e.g., retrosplenial cortex) are similarly sized (Kingsbury et al., 2012). These frontal regions also are sensitive to early life experience. Infants with high parental care exhibit thicker cortex in prelimbic and anterior cingulate regions, compared to infants with low parental contact (Bottom et al., 2020). As for structural connectivity, the prairie vole medial agranular cortex has pronounced corticocortical connections as compared to rats (Reep and Kirkpatrick, 1999). Another region where prairie voles show unique connectivity is in extended amygdala. Males have a species-specific network of TH-ir cell projections involving principal nucleus of the BNST, posterodorsal medial amygdala, and medial preoptic area. These TH-ir projections are not commonly found in rats, hamsters, or meadow voles (Ahmed et al., 2012; Northcutt et al., 2007; Northcutt and Lonstein, 2011). Taken together, this body of work shows that the organization of the prairie vole brain is distinctive from other rodents, and these differences may be partially influenced by early life experiences.
One of the most distinctive features of the prairie vole brain is the distribution pattern of neurotransmitter, neuropeptide, and hormone receptors. Among neurotransmitters, dopamine receptors have been studied best in a comparative context. Prairie voles have high densities of D2-like receptors in mPFC but low densities of D1-like receptors in mPFC and nucleus accumbens (NAcc) compared to meadow voles (Aragona et al., 2006; Smeltzer et al., 2006). Among neuropeptides, oxytocin and vasopressin receptors (OXTR and AVPR, respectively) also show distinct species-specific expression patterns (Insel and Shapiro, 1992; Duchemin et al., 2017; Insel et al., 1994; Smeltzer et al., 2006; Wang et al., 1997). For example, prairie voles have higher OXTR and AVPR densities in parts of the mPFC and BNST as compared to non-monogamous vole species. Hormone-related receptors also are well-studied in a comparative context. Corticotropin releasing factor (CRF) has two receptor subtypes (CRFR1 and CRFR2) that differ in neural expression across vole species, especially in NAcc (Lim et al., 2005). Estrogen beta receptors (ERβ) also exhibit species-specific patterns. ERβ expression is focused within distinct hypothalamic subregions in prairie voles but widely distributed across the brains of rats and mice (Ploskonka et al., 2016). Collectively, these studies show that there are a variety of brain regions – notably mPFC, BNST, amygdala, NAcc, lateral septum, and thalamus – for which prairie voles show species-specific expression patterns of dopamine, neuropeptide, and hormone receptors.
Sex Differences
Male and female prairie voles exhibit remarkable similarity in pair bonding and parental care behaviors. Nonetheless this behavioral convergence is built on somewhat different underlying neurochemical pathways. Research on prairie vole pair bond formation has focused on the role of oxytocin in the nucleus accumbens of females, and on arginine vasopressin acting in the ventral pallidum of males (Liu and Wang, 2003; Keebaugh et al., 2015; Pitkow et al., 2001a), although the extent of these sex differences has not been thoroughly explored and oxytocin also plays a role in male pair bond formation (Johnson et al., 2016). Differential modulation of pair bonding in males and females in response to both stress and glucocorticoid administration indicates latent differences in the pathways underlying bond formation (DeVries et al., 1996). The idea that behavioral similarity across the sexes may arise from mechanisms that compensate for differences in gonadal hormone exposure has been espoused by Geert De Vries (De Vries and Boyle, 1998; De Vries, 2004). In essence, while female social bonding builds on female-specific oxytocin pathways involved in lactation and maternal care, male social bonding and parental care has co-opted vasopressin signaling—with strikingly different patterns of expression in males and females—to reach similar ends (Wang et al., 1994). There is a growing appreciation of the importance of including both females and males in preclinical research (Mogil and Chanda, 2005; Beery and Zucker, 2011; Clayton and Collins, 2014; Klein et al., 2015; Woitowich et al., 2020), and multiple guides to practices around inclusion and analysis (Clayton, 2016; Maney, 2016; Beltz et al., 2019). By studying both male and female prairie voles, we will gain particular insights into how parallel mechanisms can support social attachments.
TECHNIQUES
Many neuroethological techniques that were developed in traditional rodent models are broadly applicable to prairie voles (Table 2, Table 3). Despite these similarities, there are notable exceptions where techniques either work differently in voles, were designed specifically for voles, or have yet to be modified for voles. A variety of behavioral assays for socio-cognitive traits like anxiety, depression, parental investment, and social reward, have been applied to prairie voles (Table 2), and yet, voles behave differently in some of these assays than do other rodents. In the Light-Dark Box Test, prairie voles are quick to enter and spend more time in the light chamber than the dark, which is opposite to how mice and rats typically behave (Sun et al., 2014; Lee et al., 2019). Stressors do still lead to voles spending less time in the light, however, similar to other rodents. In the Morris swim test, prairie voles are slow to complete the task relative to rats, possibly due to species differences in cognitive ability, motivation, or physiology (Blankenship et al., 2019). Additionally, voles are not ideal for the Tail Suspension Test of depressive-like behavior because of their short tails, and it is widely reported that prairie voles jump or fall off of Elevated Plus Mazes more frequently than do other rodents (Grippo et al., 2008b).
Table 2.
Overview of ethological techniques used to investigate social and cognitive behavioral traits in prairie voles.
| Socio-cognitive traits and related tests | Example references |
|---|---|
| Anhedonia (e.g., sucrose preference test, forced swim test) | (Grippo et al., 2008b) |
| Anxiety (e.g., elevated plus maze, open field test, light-dark box test) | (Insel et al., 1995; Sun et al., 2014) |
| Empathy and social contagion (e.g., consolation test, social-facilitated drinking) | (Anacker et al., 2011; Burkett et al., 2016) |
| Mating strategies (e.g., radiotelemetry, powder tracking, RFID tagging) | (Jike et al., 1988; Sabol et al., 2018) |
| Pair bond formation and maintenance (e.g., partner preference test, selective aggression “resident-intruder” test) | (Williams et al., 1992b; Insel et al., 1995; PPT protocol Beery, 2021 this issue) |
| Parental care (e.g., pup retrieval) | (Wang et al., 1994) |
| Social reward (e.g., conditioned place preference, operant conditioning) | (Goodwin et al., 2019; Matthews et al., 2013; Lee and Beery, 2021) |
| Spatial memory (e.g., Morris swim task) | (Sawrey et al., 1994) |
Table 3.
Overview of neuroscience tools used to investigate physiological mechanisms in prairie voles.
| Neurophysiological constructs and related tools | Example references |
|---|---|
| Peripheral nervous system functioning (e.g., electrocardiograms of heart rate, hormone assays) | (Blondel et al., 2016; Grippo et al., 2007b; Klein et al., 1997) |
| Genotyping, genomics, and transcriptomics (e.g., PCR and sequencing, genome linkage maps) | (Duclot et al., 2020; Hammock and Young, 2005a; McGraw et al., 2011; Okhovat et al., 2015) |
| Transgenic manipulation (e.g., AAV gene transfer, shRNA-targeted knockdown, CRISPR knockin/knockout) | (Keebaugh et al., 2015; Pitkow et al., 2001b; Horie et al., 2019, 2020) |
| Neural activity (e.g., calcium imaging, electrophysiology, fMRI, immediate early gene expression) | (Amadei et al., 2017; Cushing et al., 2003; Scribner et al., 2020; Yee et al., 2016) |
| Neural manipulation (e.g., optogenetics, peripheral administration and microinjection of compounds) | (Amadei et al., 2017; Winslow et al., 1993; Witt et al., 1990) |
| Neural mapping (e.g., anterograde/retrograde tracing, autoradiography, cell proliferation, electrophysiology, immunohistochemistry) | (Ahmed et al., 2012; Campi et al., 2007; Reep and Kirkpatrick, 1999; Smith et al., 2001; Witt et al., 1991) |
| Neuropeptide and neurotransmitter release (e.g., fast-scan cyclic voltammetry, microdialysis) | (Resendez et al., 2016; Gingrich et al., 2000) |
The Partner Preference Test (PPT) is one behavioral assay that was designed with prairie voles in mind as the primary study species. As described in the introduction, the PPT is used to assess the selective social relationships that so far appear unique to voles among lab rodents (Williams et al., 1992b). In this test, the focal animal is placed in the center chamber of a three-chamber arena. In the opposite-sex (original) version, the focal animal’s familiar mating “partner” is tethered in one of the adjacent chambers, while a novel opposite-sex “stranger” is tethered in the other adjacent chamber. In the same-sex “peer PPT”, the partner and stranger are of the same sex as the focal vole. The focal animal is given a predetermined amount of time (typically ~3h) to freely explore the entire arena and interact with both stimulus animals (see Beery, 2021 this issue). In prairie voles, focal animals that have undergone extended cohabitation (~6–24h) with a mating partner or a familiar peer spend more time in contact with their partner over a stranger. The formation of partner preferences can be enhanced or accelerated by specific experimental manipulations, while other manipulations impair preference formation, providing insight into neural pathways underlying bond formation.
Although many well-established neuroscience tools are used in prairie vole research (Table 3), some of the newest technologies have not yet been adopted. A surge in computational, genetic, and viral tools has greatly advanced rodent research with traditional lab species (reviewed in Navabpour et al., 2020; Nectow and Nestler, 2020; Ueda et al., 2020). Some of these state-of-the-art tools have recently been applied to prairie voles, including calcium imaging (Scribner et al., 2020), free-moving electrophysiology (Amadei et al., 2017), optogenetics (Amadei et al., 2017), and CRISPR knockin/knockouts (Horie et al., 2019, 2020)). Many of these newly designed tools, however, remain untested or are undergoing development, validation and refinement in voles. It is likely that the coming decade will be a golden age for prairie vole research as researchers adopt some of these more specific and nuanced tools.
MEADOW VOLES AND OTHER VOLE SPECIES IN NEUROSCIENCE RESEARCH
Although prairie voles are the best studied Microtus species, other vole species exhibit attributes of interest for neuroscience research. In particular, group-living meadow voles are used to probe the neurobiological basis of life in social groups and peer relationships, and additional monogamous and promiscuous vole species are used in comparative investigations of social monogamy. Non-neuroscience related studies also make use of diverse vole species.
Neuroendocrinology of group living in meadow voles
Meadow voles are closely related to prairie voles, but they exhibit seasonal group living in the absence of monogamy (Getz, 1972; Madison, 1980; Boonstra et al., 1993). This predictable and environmentally inducible variation in sociality provides an opportunity to study the neuroendocrine mechanisms that underlie this change in peer social behavior to support group living, and the biology of affiliation outside the realm of reproductive attachments (Beery, 2019).
Changing environmental conditions lead to variation in meadow vole social behavior in the field and lab. In summer months, females are highly territorial and maintain home-ranges that do not overlap with other females (Edwards et al., 2019; Madison and McShea, 1987). In winter months, these territories collapse, and voles live and sleep in mixed-sex groups of up to 10 individuals (Madison and McShea, 1987). Seasonal changes in sociality in the field are recapitulated in the laboratory under conditions of changing day length. In winter-like, short day lengths (10h of light/day) females form enduring and specific peer partner preferences for same-sex cagemates (e.g. Parker and Lee, 2003; Beery et al., 2008), huddle with conspecifics more, and are less aggressive than long-day (14h light) housed voles (Beery et al., 2008; Lee et al., 2019). Male meadow voles are less territorial than females in the summer (Madison, 1980; Edwards et al., 2019), and do not exhibit changes in peer social preferences with day-length in the laboratory, but also exhibit same-sex partner preferences for cage-mates (Beery et al., 2009; Beery and Shambaugh, 2021).
Peer social interactions are the foundation of life in social groups, and selective peer relationships are of particular importance in human societies. Peer relationships also can be compared and contrasted across meadow and prairie voles. Studies show that peer relationships in both species are less behaviorally rewarding and less dopamine dependent rewarding than mate relationships in prairie voles (Beery and Zucker, 2010; Goodwin et al., 2019; Lee and Beery, 2021). Social tolerance, promoted by both seasonally reduced anxiety and aggression, appears to play an important role in peer affiliation in meadow voles (Beery et al., 2014; Anacker et al., 2016b; Lee et al., 2019). Oxytocin signaling shapes the selectivity of affiliation in meadow voles; oxytocin receptor density changes with day length, and oxytocin administration can increase or eliminate peer partner selectivity, depending on the brain region in which oxytocin is acting (Parker et al., 2001; Beery and Zucker, 2010; Anacker et al., 2016a; Beery, 2019).
Stress and elevated glucocorticoid signaling impairs the formation of social preferences for peers or mates in both meadow and prairie vole females, suggesting common underlying mechanisms for social avoidance among females of these species (DeVries et al., 1996; Anacker et al., 2016b). However, oxytocin appears to alter social selectivity for peers and mates in distinct brain regions and in different ways (Ross and Young, 2009; Beery and Zucker, 2010; Anacker et al., 2016a), indicating that peer relationships must be studied separately. Investigations of the similarities and differences between these types of selective relationships within and across vole species will inform our understanding of how social behaviors and underlying pathways vary or are conserved.
Neuroendocrinology of monogamy across voles
Several studies have examined the comparative basis of monogamy in voles by making use of mating system variation within the genus. Multiple vole species exhibit monogamy or pair-bonding behavior in at least some populations or circumstances, while other species mate promiscuously, as is typical of most rodent species (Figure 1A). Comparative studies have focused primarily on neuropeptide receptor densities in the brains of monogamous (pine and prairie) vole species compared to promiscuous (montane and meadow) vole species (Insel and Shapiro, 1992; Lim et al., 2005; Smeltzer et al., 2006; described in detail above), or on genetic differences in sequences regulating expression of the arginine vasopressin 1a receptor (avpr1a) gene (Young et al., 1996; Hammock and Young, 2002; Fink et al., 2006; Donaldson and Young, 2013). Genetic variation has also been examined across behavioral phenotypes within populations (reviewed in Madrid et al., 2020).
More recently, studies of the Taiwan vole (Microtus kikuchii) have attempted to establish whether this putatively monogamous species exhibits space use consistent with monogamy, whether they exhibit partner preferences for familiar mates, and how oxytocin receptors are distributed in their brains (Wu et al., 2012; Chappell et al., 2016; Lee et al., 2014). Other closely related species that have been described as monogamous include the European water vole (Arivicola amphibious), Southwestern water vole (Arvicola sapidus), Northern mole-vole (Ellobius talpinus), and the Alai vole (Ellobius alaicus) (Lukas and Clutton-Brock, 2013), although relatively little is known about these species. Study of additional vole species will enhance our understanding of the evolution of mechanisms underlying variation in social behaviors.
CONCLUSION
Neuroscience research benefits from comparative study, which allows researchers to validate prior research outcomes and explore traits that are not found in traditional model systems. Already we have begun to encounter the limits of biasing our translational and preclinical research toward only two species of laboratory rodents. Beyond the value of diversifying model organisms, prairie voles have proven invaluable for neuroscience research because of a distinctive suite of social behaviors including the formation of selective social bonds. Additional differences from the physiology of rats and mice provide further opportunities for study. With the adoption of new tools and technologies, we expect to see the success of the prairie vole model grow, and for the scope of prairie vole research to expand going forward.
ACKNOWLEDGEMENTS
WMK is supported by the National Institute of General Medical Studies of the National Institutes of Health Award Number P20GM103653. MLG is supported by the National Institute of Mental Health of the National Institutes of Health Award Number R01MH115267. AB is supported by the National Institute of Mental Health of the National Institutes of Health Award Number R15MH113085.
LITERATURE CITED:
- Ahern TH, and Young LJ 2009. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster). Frontiers in Behavioral Neuroscience 3. Available at: https://www.frontiersin.org/articles/10.3389/neuro.08.017.2009/full [Accessed December 10, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed EI, Northcutt KV, and Lonstein JS 2012. L-Amino acid decarboxylase- and tyrosine hydroxylase-immunoreactive cells in the extended olfactory amygdala and elsewhere in the adult prairie vole brain. Journal of Chemical Neuroanatomy 43:76–85. [DOI] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Jun Kwon Y, Shpiner AC, Saravanan V, Mays WD, Ryan SJ, Walum H, Rainnie DG, Young LJ, et al. 2017. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Ahern TH, Hostetler CM, Dufour BD, Smith ML, Cocking DL, Li J, Young LJ, Loftis JM, and Ryabinin AE 2014. Drinking alcohol has sex-dependent effects on pair bond formation in prairie voles. Proceedings of the National Academy of Sciences of the United States of America 111:6052–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Ahern TH, Young LJ, and Ryabinin AE 2012. The role of early life experience and species differences in alcohol intake in Microtine rodents. PLoS ONE 7:e39753–e39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Christensen JD, LaFlamme EM, Grunberg DM, and Beery AK 2016a. Septal oxytocin administration impairs peer affiliation via V1a receptors in female meadow voles. Psychoneuroendocrinology 68:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Loftis JM, Kaur S, and Ryabinin AE 2011. Prairie voles as a novel model of socially facilitated excessive drinking. Addiction Biology 16:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Reitz KM, Goodwin NL, and Beery AK 2016b. Stress impairs new but not established relationships in seasonally social voles. Hormones and Behavior 79:52–57. [DOI] [PubMed] [Google Scholar]
- Anacker AMJ, and Ryabinin AE 2013. Identification of subpopulations of prairie voles differentially susceptible to peer influence to decrease high alcohol intake. Frontiers in Pharmacology 4:84–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, and Wang Z. 2006. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature neuroscience 9:133–9. [DOI] [PubMed] [Google Scholar]
- Bastías-Pérez M, Zagmutt S, Soler-Vázquez M, Serra D, Mera P, and Herrero L. 2020. Impact of Adaptive Thermogenesis in Mice on the Treatment of Obesity. Cells 9. Available at: https://europepmc.org/article/pmc/pmc7072509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LR, and Anthony RG 1971. Metabolic and Behavioral Thermoregulation in the Long-Tailed Vole, Microtus longicaudus. Journal of Mammalogy 52:404–412. [PubMed] [Google Scholar]
- Beery AK 2019. Frank Beach award winner: Neuroendocrinology of group living. Hormones and Behavior 107:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK 2021. Familiarity and mate preference assessment with the partner preference test. Current Protocols in Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Christensen JD, Lee NS, and Blandino KL 2018. Specificity in Sociality: Mice and Prairie Voles Exhibit Different Patterns of Peer Affiliation. Frontiers in Behavioral Neuroscience 12. Available at: https://www.frontiersin.org/articles/10.3389/fnbeh.2018.00050/full [Accessed December 16, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Holmes MM, Lee W, and Curley JP 2020. Stress in groups: Lessons from non-traditional rodent species and housing models. Neuroscience & Biobehavioral Reviews 113:354–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, and Kaufer D. 2015. Stress, social behavior, and resilience: insights from rodents. Neurobiology of Stress 1:116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Loo TJ, and Zucker I. 2008. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Hormones and behavior 54:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Routman DM, and Zucker I. 2009. Same-sex social behavior in meadow voles: Multiple and rapid formation of attachments. Physiology & behavior 97:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, and Shambaugh KL 2021. Comparative assessment of familiarity/novelty preferences in rodents. Frontiers in Behavioral Neuroscience 15. Available at: https://www.frontiersin.org/articles/10.3389/fnbeh.2021.648830/abstract [Accessed March 25, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Vahaba DM, and Grunberg DM 2014. Corticotropin-releasing factor receptor densities vary with photoperiod and sociality. Hormones and Behavior 66:779–786. [DOI] [PubMed] [Google Scholar]
- Beery AK, and Zucker I. 2010. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 169:665–673. [DOI] [PubMed] [Google Scholar]
- Beery AK, and Zucker I. 2011. Sex bias in neuroscience and biomedical research. Neuroscience and biobehavioral reviews 35:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz AM, Beery AK, and Becker JB 2019. Analysis of sex differences in pre-clinical and clinical data sets. Neuropsychopharmacology 44:2155–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532:512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake BH 2002. Ultrasonic calling in isolated infant prairie voles (Microtus ochrogaster) and montane voles (M. montanus). Journal of Mammalogy 83:536–545. [Google Scholar]
- Blankenship PA, Normann MC, Donaldson TN, Baumeister J, McNeal N, Grippo AJ, and Wallace DG 2019. Making waves: Comparing Morris water task performance in rats and prairie voles. Behavioural Brain Research 360:7–15. [DOI] [PubMed] [Google Scholar]
- Blondel DV, Wallace GN, Calderone S, Gorinshteyn M, Mary CM St., and Phelps SM 2016. Effects of population density on corticosterone levels of prairie voles in the field. General and Comparative Endocrinology 225:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögels S, and Phares V. 2008. Fathers’ role in the etiology, prevention and treatment of child anxiety: a review and new model. Clinical Psychology Review 28:539–558. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Xia X, and Pavone L. 1993. Mating system of the meadow vole, Microtus pennsylvanicus. Behavioral Ecology 4:83–89. [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, and Young LJ 2008. The CRF System Mediates Increased Passive Stress-Coping Behavior Following the Loss of a Bonded Partner in a Monogamous Rodent. Neuropsychopharmacology 34:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, and Young LJ 2009. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology 34:1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottom RT, Krubitzer LA, and Huffman KJ 2020. Early postnatal gene expression in the developing neocortex of prairie voles (Microtus ochrogaster) is related to parental rearing style. Journal of Comparative Neurology 528:3008–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite S, and Holt-Lunstad J. 2017. Romantic relationships and mental health. Current Opinion in Psychology 13:120–125. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg OK, Johnsen E, Roberg-Larsen H, Seip KF, MacLean EL, Gesquiere LR, Leknes S, Lundanes E, and Wilson SR 2016. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci Rep 6:31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brychta RJ, and Chen KY 2016. Cold-induced thermogenesis in humans. European journal of clinical nutrition 71:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, Waal F. B. M. de, and Young LJ 2016. Oxytocin-dependent consolation behavior in rodents. Science 351:375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK 2017. Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. The Neuroscientist 23:517–528. [DOI] [PubMed] [Google Scholar]
- Campbell JC, Laugero KD, Van Westerhuyzen JA, Hostetler CM, Cohen JD, and Bales KL 2009. Costs of pair-bonding and paternal care in male prairie voles (Microtus ochrogaster). Physiol Behav 98:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Bales KL, Grunewald R, and Krubitzer L. 2010. Connections of auditory and visual cortex in the prairie vole (microtus ochrogaster): Evidence for multisensory processing in primary sensory areas. Cerebral Cortex 20:89–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Karlen SJ, Bales KL, and Krubitzer L. 2007. Organization of sensory neocortex in Prairie voles (Microtus ochrogaster). The Journal of comparative neurology 504:414–426. [DOI] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J. 2011. Nonshivering thermogenesis and its adequate measurement in metabolic studies. The Journal of Experimental Biology 214:242–253. [DOI] [PubMed] [Google Scholar]
- Carter CS, and Getz LL 1993. Monogamy and the prairie vole. Sci Am 268:100–6. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL, Gavish L, Dermott JLM, and Arnold P. 1980. Male-related Pheromones and the Activation of Female Reproduction in the Prairie Vole (Microtus ochrogaster). Biology of Reproduction 23:1038–1045. [DOI] [PubMed] [Google Scholar]
- Carter CS, and Roberts RL 1997. The psychobiological basis of cooperative breeding. In Cooperative Breeding in Mammals (Solomon NG and French JA, eds.) pp. 231–266. Cambridge Press, NY. [Google Scholar]
- Chalfin L, Dayan M, Levy DR, Austad SN, Miller RA, Iraqi FA, Dulac C, and Kimchi T. 2014. Mapping ecologically relevant social behaviours by gene knockout in wild mice. Nature Communications 5:4569. [DOI] [PubMed] [Google Scholar]
- Chappell AR, Freeman SM, Lin YK, LaPrairie JL, Inoue K, Young LJ, and Hayes LD 2016. Distributions of oxytocin and vasopressin 1a receptors in the Taiwan vole and their role in social monogamy. Journal of zoology (London, England : 1987) 299:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherlin AJ, Furstenberg FF, Chase-Lansdale L, Kiernan KE, Robins PK, Morrison DR, and Teitler JO 1991. Longitudinal studies of effects of divorce on children in Great Britain and the United States. Science (New York, N.Y.) 252:1386–1389. [DOI] [PubMed] [Google Scholar]
- Clayton JA 2016. Studying both sexes: a guiding principle for biomedicine. The FASEB Journal 30:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, and Collins FS 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole FR, and Batzli GO 1979. Nutrition and population dynamics of the prairie vole, Microtus ochrogaster, in central illinois. Journal of Animal Ecology 48:455–470. [Google Scholar]
- Corrigan JK, Ramachandran D, He Y, Palmer CJ, Jurczak MJ, Chen R, Li B, Friedline RH, Kim JK, Ramsey JJ, et al. 2020. A big-data approach to understanding metabolic rate and response to obesity in laboratory mice. eLife 9:e53560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RP, and Clarke JR 1982. Gonadotrophin levels in male voles (Microtus agrestis) reared in long and short photoperiods. J Reprod Fertil 66:709–14. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Mogekwu N, Le WW, Hoffman GE, and Carter CS 2003. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Research 965:203–211. [DOI] [PubMed] [Google Scholar]
- Dark J, Zucker I, and Wade GN 1983. Photoperiodic regulation of body mass, food intake, and reproduction in meadow voles. Am J Physiol 245:R334–8. [DOI] [PubMed] [Google Scholar]
- De Vries GJ 2004. Minireview: Sex Differences in Adult and Developing Brains: Compensation, Compensation, Compensation. Endocrinology 145:1063–1068. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, and Boyle PA 1998. Double duty for sex differences in the brain. Behavioural Brain Research 92:205–213. [DOI] [PubMed] [Google Scholar]
- Desy EA, and Batzli GO 1989. Effects of food availability and predation on prairie vole demography: a field experiment. Ecology 70:411–421. [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, and Carter CS 1996. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences 93:11980–11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, and Carter CS 1997. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can J Zool 75:295–301. [Google Scholar]
- Donaldson ZR, and Young LJ 2013. The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor (Avpr1a) Gene Expression and Behavior. PLoS Genet 9:e1003729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan M, Lynch MDJ, Mackey CS, Platt GN, Washburn BK, Vera DL, Trickey DJ, Charles TC, Wang Z, and Jones KM 2020a. Metagenome-Assembled Genome Sequences of Five Strains from the Microtus ochrogaster (Prairie Vole) Fecal Microbiome. Microbiology Resource Announcements 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan M, Mackey CS, Platt GN, Rounds J, Brown AN, Trickey DJ, Liu Y, Jones KM, and Wang Z. 2020b. Social isolation alters behavior, the gut-immune-brain axis, and neurochemical circuits in male and female prairie voles. Neurobiology of Stress 13. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7739176/ [Accessed December 30, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois DL, Eitel SK, and Felner RD 1994. Effects of Family Environment and Parent-Child Relationships on School Adjustment during the Transition to Early Adolescence. Journal of Marriage and Family 56:405–414. [Google Scholar]
- Duchemin A, Seelke AMH, Simmons TC, Freeman SM, and Bales KL 2017. Localization of oxytocin receptors in the prairie vole (Microtus ochrogaster) neocortex. Neuroscience 348:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclot F, Sailer L, Koutakis P, Wang Z, and Kabbaj M. 2020. Transcriptomic regulations underlying pair-bond formation and maintenance in the socially monogamous male and female prairie vole. Biological Psychiatry:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards PD, Dean EK, Palme R, and Boonstra R. 2019. Assessing space use in meadow voles: the relationship to reproduction and the stress axis. Journal of Mammalogy 100:4–12. [Google Scholar]
- Fabiano GA 2007. Father participation in behavioral parent training for ADHD: review and recommendations for increasing inclusion and engagement. Journal of family psychology: JFP: journal of the Division of Family Psychology of the American Psychological Association (Division 43) 21:683–693. [DOI] [PubMed] [Google Scholar]
- Fink S, Excoffier L, and Heckel G. 2006. Mammalian monogamy is not controlled by a single gene. Proceedings of the National Academy of Sciences 103:10956–10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GJO, Simpson JA, Campbell L, and Overall NC 2015. Pair-Bonding, Romantic Love, and Evolution: The Curious Case of Homo sapiens. Perspectives on Psychological Science 10:20–36. [DOI] [PubMed] [Google Scholar]
- Florsheim P, Tolan P, and Gorman‐Smith D. 1998. Family Relationships, Parenting Practices, the Availability of Male Family Members, and the Behavior of Inner-City Boys in Single-Mother and Two-Parent Families. Child Development 69:1437–1447. [PubMed] [Google Scholar]
- Fritz SA, Bininda-Emonds ORP, and Purvis A. 2009. Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecology Letters 12:538–549. [DOI] [PubMed] [Google Scholar]
- Furstenberg FF, and Teitler JO 1994. Reconsidering the Effects of Marital Disruption: What Happens to Children of Divorce in Early Adulthood? Journal of Family Issues 15:173–190. [Google Scholar]
- Gerkema MP, and van der Leest F. 1991. Ongoing ultradian activity rhythms in the common vole, Microtus arvalis, during deprivations of food, water and rest. Journal of Comparative Physiology A 168:591–597. [DOI] [PubMed] [Google Scholar]
- Getz LL 1972. Social structure and aggressive behavior in a population of Microtus pennsylvanicus. Journal of Mammalogy 53:310–317. [Google Scholar]
- Getz LL, Carter CS, and Gavish L. 1981. The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol 8:189–194. [Google Scholar]
- Getz LL, and Hofmann JE 1986. Social organization in free-living prairie voles, Microtus ochrogaster. Behavioral Ecology and Sociobiology 18:275–282. [Google Scholar]
- Getz LL, McGuire B, Hofmann J, Pizzuto T, and Frase B. 1994. Natal dispersal and philopatry in the prairie vole (Microtus ochrogaster): settlement, survival, and potential reproductive success. Ethology, Ecology, and Evolution 6:267–284. [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann JE, and Frase B. 1993. Social organization of the prairie vole (Microtus ochrogaster). Journal of Mammalogy 74:44–58. [Google Scholar]
- Getz LL, Solomon NG, and Pizzuto TM 1990. The effects of predation of snakes on social organization of the prairie vole, Microtus ochrogaster. The American Midland Naturalist 123:365–371. [Google Scholar]
- Getz LL, Verner L, Cole FR, Ann JEH, and Avalos DE 1979. Comparisons of population demography of Microtus ochrogaster and M. pennsylvanicus. Acta Theriologica 24:319–349. [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, and Insel TR 2000. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behavioral Neuroscience 114:173–183. [DOI] [PubMed] [Google Scholar]
- Glasper ER, Kenkel WM, Bick J, and Rilling JK 2019. More than just mothers: The neurobiological and neuroendocrine underpinnings of allomaternal caregiving. Frontiers in Neuroendocrinology 53:100741. [DOI] [PubMed] [Google Scholar]
- Goodwin NL, Lopez SA, Lee NS, and Beery AK 2019. Comparative role of reward in long-term peer and mate relationships in voles. Hormones and Behavior 111:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gove WR, Style CB, and Hughes M. 1990. The Effect of Marriage on the Well-Being of Adults: A Theoretical Analysis. Journal of Family Issues 11:4–35. [Google Scholar]
- Green JA 2011. The heart rate method for estimating metabolic rate: Review and recommendations. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 158:287–304. [DOI] [PubMed] [Google Scholar]
- Greenwood MA, and Hammock EAD 2019. Oxytocin Receptor Binding Sites in the Periphery of the Neonatal Prairie Vole. Frontiers in Neuroscience, 13. Available at: https://www.frontiersin.org/articles/10.3389/fnins.2019.00474/full [Accessed December 29, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ 2017. Opinion: “Heart Rate Variability, Health and Well-Being: A Systems Perspective” Research Topic. Frontiers in Public Health 5. Available at: https://doi.org/10.3389%2Ffpubh.2017.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Carter CS, McNeal N, Chandler DL, LaRocca MA, Bates SL, and Porges SW 2011. 24-Hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: Implications for the study of depression and cardiovascular disease. Psychosomatic Medicine 73:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, and Carter CS 2007a. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32:966–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, and Porges SW 2007b. Cardiac regulation in the socially monogamous prairie vole. Physiol Behav 90:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, and Carter CS 2008a. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depression and anxiety 25:E17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, and Carter CS 2008b. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depression and Anxiety 25:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenberg TD, Vanderhooft L, Huang J, Wagar M, Alexander J, and Tan L. 2021. Social preference in rats. Journal of the Experimental Analysis of Behavior n/a:1–16. [DOI] [PubMed] [Google Scholar]
- Hale ME 2019. Toward Diversification of Species Models in Neuroscience. Brain, Behavior and Evolution 93:166–168. [DOI] [PubMed] [Google Scholar]
- Hammock EAD, and Young LJ 2005a. Genetics: Microsatellite instability generates diversity in brain and sociobehavioral traits. Science 308:1630–1634. [DOI] [PubMed] [Google Scholar]
- Hammock EAD, and Young LJ 2005b. Microsatellite Instability Generates Diversity in Brain and Sociobehavioral Traits. Science 308:1630–1634. [DOI] [PubMed] [Google Scholar]
- Hammock EAD, and Young LJ 2002. Variation in the vasopressin V1a receptor promoter and expression: implications for inter- and intraspecific variation in social behaviour*: Variation in gene structure, expression and behaviour. European Journal of Neuroscience 16:399–402. [DOI] [PubMed] [Google Scholar]
- Hankenson FC, Marx JO, Gordon CJ, and David JM 2018. Effects of Rodent Thermoregulation on Animal Models in the Research Environment. Comparative Medicine 68:425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J. 2019. The model organism diaspora. Heredity 123:14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann HA, Beery AK, Blumstein DT, Couzin ID, Earley RL, Hayes LD, Hurd PL, Lacey EA, Phelps SM, Solomon NG, et al. 2014. An evolutionary framework for studying mechanisms of social behavior. Trends in Ecology & Evolution 29:581–589. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham W, and Jones BQ 2008. Is There Something Unique about Marriage? The Relative Impact of Marital Status, Relationship Quality, and Network Social Support on Ambulatory Blood Pressure and Mental Health. Annals of Behavioral Medicine 35:239–244. [DOI] [PubMed] [Google Scholar]
- Horie K, Inoue K, Nishimori K, and Young LJ 2020. Investigation of Oxtr-expressing neurons projecting to nucleus accumbens using Oxtr-ires-Cre knock-in prairie voles (Microtus ochrogaster). Neuroscience 448:312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Inoue K, Suzuki S, Adachi S, Yada S, Hirayama T, Hidema S, Young LJ, and Nishimori K. 2019. Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Hormones and Behavior 111:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, and Ryabinin AE 2014. Social partners prevent alcohol relapse behavior in prairie voles. Psychoneuroendocrinology 39:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylander BL, and Repasky EA 2016. Thermoneutrality, Mice, and Cancer: A Heated Opinion. Trends in Cancer 2:166–175. [DOI] [PubMed] [Google Scholar]
- Insel N, Shambaugh KL, and Beery AK 2020. Female degus show high sociality but no preference for familiar peers. Behavioural Processes 174:104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Preston S, and Winslow JT 1995. Mating in the monogamous male: behavioral consequences. Physiology & Behavior 57:615–27. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, and Ferris CF 1994. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. Journal of Neuroscience 14:5381–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, and Shapiro L. 1992. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences 89:5981–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler K, and van Schaik CP 2012. Allomaternal care, life history and brain size evolution in mammals. Journal of Human Evolution 63:52–63. [DOI] [PubMed] [Google Scholar]
- Jakubowski M, and Terkel J. 1982. Infanticide and caretaking in non-lactating Mus musculus: Influence of genotype, family group and sex. Animal Behaviour 30:1029–1035. [Google Scholar]
- Jeynes WH 2015. A Meta-Analysis: The Relationship between Father Involvement and Student Academic Achievement. Urban Education 50:387–423. [Google Scholar]
- Jike L, Batzli GO, and Getz LL 1988. Home ranges of prairie voles as determined by radiotracking and my powertracking. Journal of Mammalogy 69:183–186. [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, and Young LJ 2016. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Hormones and Behavior 79:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen CB 2001. August Krogh and Claude Bernard on Basic Principles in Experimental Physiology. Bioscience 51:59–61. [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, and Young LJ 2015. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Social Neuroscience 10:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Perkeybile AM, and Carter CS 2017. The neurobiological causes and effects of alloparenting. Dev Neurobiol 77:214–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Perkeybile AM, Yee JR, Pournajafi-Nazarloo H, Lillard TS, Ferguson EF, Wroblewski KL, Ferris CF, Carter CS, and Connelly JJ 2019. Behavioral and epigenetic consequences of oxytocin treatment at birth. Science Advances 5:eaav2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Suboc G, and Sue Carter C. 2014. Autonomic, behavioral and neuroendocrine correlates of paternal behavior in male prairie voles. Physiol Behav 128:252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Yee JR, Porges SW, Ferris CF, and Carter CS 2015. Cardioacceleration in alloparents in response to stimuli from prairie vole pups: The significance of thermoregulation. Behav Brain Res 286:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Gleason ED, Ophir AG, Phelps SM, Young LJ, and Marler CA 2012. Monogamous and promiscuous rodent species exhibit discrete variation in the size of the medial prefrontal cortex. Brain, Behavior and Evolution 80:4–14. [DOI] [PubMed] [Google Scholar]
- Klein SL, Hairston JE, Devries AC, and Nelson RJ 1997. Social environment and steroid hormones affect species and sex differences in immune function among voles. Hormones and Behavior 32:30–39. [DOI] [PubMed] [Google Scholar]
- Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, et al. 2015. Opinion: Sex inclusion in basic research drives discovery. Proceedings of the National Academy of Sciences:201502843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS, Wu J, and Ottinger MA 2004. Sex and Species Differences in Plasma Oxytocin Using an Enzyme Immunoassay. Canadian Journal of Zoology 82:1194–1200. [Google Scholar]
- Krebs HA 1975. The August Krogh principle: “For many problems there is an animal on which it can be most conveniently studied”. J Exp Zool 194:221–6. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Trasy AG, and Nelson RJ 2000. Temperature and photoperiod interact to affect reproduction and GnRH synthesis in male prairie voles. Journal of Neuroendocrinology 12:553–558. [DOI] [PubMed] [Google Scholar]
- Krogh A. 1929. THE PROGRESS OF PHYSIOLOGY. Science (New York, N.Y.) 70:200–204. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Campi KL, and Cooke DF 2011. All rodents are not the same: A modern synthesis of cortical organization. Brain, Behavior and Evolution 78:51–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence EM, Rogers RG, Zajacova A, and Wadsworth T. 2019. Marital Happiness, Marital Status, Health, and Longevity. Journal of Happiness Studies 20:1539–1561. [Google Scholar]
- Lawrence RH, Palumbo MC, Freeman SM, Guoynes CD, and Bales KL 2020. Developmental Fluoxetine Exposure Alters Behavior and Neuropeptide Receptors in the Prairie Vole. Frontiers in Behavioral Neuroscience 14. Available at: https://www.frontiersin.org/articles/10.3389/fnbeh.2020.584731/full?&utm_source=Email_to_authors_&utm_medium=Email&utm_content=T1_11.5e1_author&utm_campaign=Email_publication&field=&journalName=Frontiers_in_Behavioral_Neuroscience&id=584731#h4 [Accessed December 29, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-C, Chiu C-H, Lin L-K, and Lin YK 2014. Partner Preference and Mating System of the Taiwan Field Vole. 59:12. [Google Scholar]
- Lee NS, and Beery AK 2021. The role of dopamine signaling in prairie vole peer relationships. Hormones and Behavior 127:104876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NS, Goodwin NL, Freitas KE, and Beery AK 2019. Affiliation, Aggression, and Selectivity of Peer Relationships in Meadow and Prairie Voles. Frontiers in Behavioral Neuroscience 13. Available at: https://www.frontiersin.org/articles/10.3389/fnbeh.2019.00052/full?&utm_source=Email_to_authors_&utm_medium=Email&utm_content=T1_11.5e1_author&utm_campaign=Email_publication&field=&journalName=Frontiers_in_Behavioral_Neuroscience&id=447143 [Accessed March 19, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, and Zucker I. 1988. Vole infant development is influenced perinatally by maternal photoperiodic history. Am J Physiol 255:R831–8. [DOI] [PubMed] [Google Scholar]
- Leng G, and Sabatier N. 2016. Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. Journal of Neuroendocrinology 28. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5096068/ [Accessed December 29, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepri JJ, Theodorides M, and Wysocki CJ 1988. Ultrasonic vocalizations by adult prairie voles, Microtus ochrogaster. Experientia 44:271–273. [DOI] [PubMed] [Google Scholar]
- Lerch S, Brandwein C, Dormann C, Gass P, and Chourbaji S 2014. What makes a good mother? Implication of inter-, and intrastrain strain “cross fostering” for emotional changes in mouse offspring. Behavioural Brain Research 274:270–281. [DOI] [PubMed] [Google Scholar]
- Lewis R, and Curtis JT 2016. Male prairie voles display cardiovascular dipping associated with an ultradian activity cycle. Physiology & Behavior 156:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Nair HP, and Young LJ 2005. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. Journal of Comparative Neurology 487:75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, and Young LJ 2004. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429:754–7. [DOI] [PubMed] [Google Scholar]
- Liu Y, and Wang ZX 2003. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121:537–44. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, and Vries GJD 2001. Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster). Journal of Comparative Psychology 115:53–61. [DOI] [PubMed] [Google Scholar]
- Lukas D, and Clutton-Brock TH 2013. The Evolution of Social Monogamy in Mammals. Science 341:526–530. [DOI] [PubMed] [Google Scholar]
- Ma ST, Resendez SL, and Aragona BJ 2014. Sex differences in the influence of social context, salient social stimulation and amphetamine on ultrasonic vocalizations in prairie voles. Integrative zoology 9:280–93. [DOI] [PubMed] [Google Scholar]
- Mabry KE, Streatfeild CA, Keane B, and Solomon NG 2011. Avpr1a length polymorphism is not associated with either social or genetic monogamy in free-living prairie voles. Animal Behaviour 81:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, and Carter CS 2019. Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology 107:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DM 1980. Space use and social structure in meadow voles, Microtus pennsylvanicus. Behav Ecol Sociobiol 7:65–71. [Google Scholar]
- Madison DM, and McShea W. 1987. Seasonal changes in reproductive tolerance, spacing, and social organization in meadow voles: a microtine model. American zoologist 27:899–908. [Google Scholar]
- Madrid JE, Parker KJ, and Ophir AG 2020. Variation, plasticity, and alternative mating tactics: Revisiting what we know about the socially monogamous prairie vole. In Advances in the Study of Behavior pp. 203–242. Elsevier Available at: https://linkinghub.elsevier.com/retrieve/pii/S0065345420300036 [Accessed December 16, 2020]. [Google Scholar]
- Maher RL, Barbash SM, Lynch DV, and Swoap SJ 2015. Group housing and nest building only slightly ameliorate the cold stress of typical housing in female C57BL/6J mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 308:R1070–R1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney SK, Fuller A, Mitchell D, Gordon C, and Overton JM 2014. Translating Animal Model Research: Does It Matter That Our Rodents Are Cold? Physiology 29:413–420. [DOI] [PubMed] [Google Scholar]
- Maney DL 2016. Perils and pitfalls of reporting sex differences. Philosophical Transactions of the Royal Society B: Biological Sciences 371:20150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, and Mattson MP 2010. “Control” laboratory rodents are metabolically morbid: Why it matters. Proceedings of the National Academy of Sciences 107:6127–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews TJ, Williams DA, and Schweiger L. 2013. Social motivation and residential style in prairie and meadow voles. The Open Behavioral Science Journal 7:16–23. [Google Scholar]
- McGraw LA, Davis JK, Young LJ, and Thomas JW 2011. A genetic linkage map and comparative mapping of the prairie vole (Microtus ochrogaster) genome. BMC Genetics 12:60–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, and Young LJ 2010. The prairie vole: an emerging model organism for understanding the social brain. Trends in Neurosciences 33:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B, and Getz LL 1998. The nature and frequency of social interactions among free-living prairie voles (Microtus ochrogaster). Behavioral Ecology and Sociobiology 43:271–279. [Google Scholar]
- McNeal N, Scotti M-AL, Wardwell J, Chandler DL, Bates SL, LaRocca M, Trahanas DM, and Grippo AJ 2014. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Autonomic Neuroscience 180:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, and Chanda ML 2005. The case for the inclusion of female subjects in basic science studies of pain. Pain 117:1–5. [DOI] [PubMed] [Google Scholar]
- Möller EL, Nikolić M, Majdandžić M, and Bögels SM 2016. Associations between maternal and paternal parenting behaviors, anxiety and its precursors in early childhood: A meta-analysis. Clinical Psychology Review 45:17–33. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, and Crawley JN 2004. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain and Behavior 3:287–302. [DOI] [PubMed] [Google Scholar]
- Myers DG, and Diener E. 1995. Who Is Happy? Psychological Science 6:10–19. [Google Scholar]
- Naughton D, Geraghty P, Csotonyi J, Carter B, Beaulieu-Bouchard M, and McDonald A. 2012. FAMILY CRICETIDAE: rats, mice, voles, and lemmings. In The Natural History of Canadian Mammals pp. 118–201. University of Toronto Press; Available at: https://www.jstor.org/stable/10.3138/j.ctt5hjx9d.16 [Accessed January 23, 2021]. [Google Scholar]
- Navabpour S, Kwapis JL, and Jarome TJ 2020. A neuroscientist’s guide to transgenic mice and other genetic tools. Neuroscience and Biobehavioral Reviews 108:732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete A, van Schaik CP, and Isler K 2011. Energetics and the evolution of human brain size. Nature 480:91–93. [DOI] [PubMed] [Google Scholar]
- Nectow AR, and Nestler EJ 2020. Viral tools for neuroscience. Nature Reviews Neuroscience 21:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Dark J, and Zucker I. 1983. Influence of photoperiod, nutrition and water availability on reproduction of male California voles (Microtus californicus). Reproduction 69:473–477. [DOI] [PubMed] [Google Scholar]
- Nieminen P, Hohtola E, and Mustonen A-M 2013. Body temperature rhythms in Microtus voles during feeding, food deprivation, and winter acclimatization. Journal of Mammalogy 94:591–600. [Google Scholar]
- NICHD Early Child Care Research Network. 2001. Nonmaternal care and family factors in early development: An overview of the NICHD Study of Early Child Care. Journal of Applied Developmental Psychology 22:457–492. [Google Scholar]
- Northcutt KV, and Lonstein JS 2011. Neuroanatomical projections of the species-specific tyrosine hydroxylase-immunoreactive cells of the male prairie vole bed nucleus of the stria terminalis and medial amygdala. Brain, Behavior and Evolution 77:176–192. [DOI] [PubMed] [Google Scholar]
- Northcutt KV, Wang Z, and Lonstein JS 2007. Sex and species differences in tyrosine hydroxylase-synthesizing cells of the rodent olfactory extended amygdala. Journal of Comparative Neurology 600:103–115. [DOI] [PubMed] [Google Scholar]
- Okhovat M, Berrio A, Wallace G, Ophir AG, and Phelps SM 2015. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science 350:1371–1374. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, and Phelps SM 2008. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proceedings of the National Academy of Sciences 105:1249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard GC 1968. Oxygen Consumption of Microtus Montanus in Relation to Ambient Temperature. Journal of Mammalogy 49:215–220. [PubMed] [Google Scholar]
- Paradis E, and Schliep K. 2019. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528. [DOI] [PubMed] [Google Scholar]
- Parker KJ, and Lee TM 2003. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. Journal of comparative psychology 117:283–9. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Kinney LF, and Lee TM 2001. Day length and sociosexual cohabitation alter central oxytocin receptor binding in female meadow voles (Microtus pennsylvanicus). Behavioral neuroscience 115:1349–1356. [PubMed] [Google Scholar]
- Parra-Vargas M, Ramon-Krauel M, Lerin C, and Jimenez-Chillaron JC 2020. Size Does Matter: Litter Size Strongly Determines Adult Metabolism in Rodents. Cell Metabolism 32:334–340. [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, Carter CS, Wroblewski KL, Puglia MH, Kenkel WM, Lillard TS, Karaoli T, Gregory SG, Mohammadi N, Epstein L, et al. 2019. Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology 99:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrigo G, Belvin L, Quindry P, Kadir T, Becker J, van Look C, Niewoehner J, and vom Saal FS 1993. Genetic mediation of infanticide and parental behavior in male and female domestic and wild stock house mice. Behavior Genetics 23:525–531. [DOI] [PubMed] [Google Scholar]
- Peuler JD, Scotti M-AL, Phelps LE, McNeal N, and Grippo AJ 2012. Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiology & Behavior 106:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps SM, Campbell P, Zheng D-J, and Ophir AG 2010. Beating the boojum: Comparative approaches to the neurobiology of social behavior. Neuropharmacology 58:17–28. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, and Young LJ 2001a. Facilitation of Affiliation and Pair-Bond Formation by Vasopressin Receptor Gene Transfer into the Ventral Forebrain of a Monogamous Vole. The Journal of Neuroscience 21:7392–7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, and Young LJ 2001b. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. Journal of Neuroscience 21:7392–7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploskonka SD, Eaton JL, Carr MS, Schmidt JV, and Cushing BS 2016. Developmental expression of estrogen receptor beta in the brain of prairie voles (Microtus ochrogaster). Developmental Psychobiology 58:223–230. [DOI] [PubMed] [Google Scholar]
- Pluess M, and Belsky J. 2009. Differential susceptibility to rearing experience: the case of childcare. Journal of Child Psychology and Psychiatry 50:396–404. [DOI] [PubMed] [Google Scholar]
- Potretzke S, and Ryabinin AE 2019. The prairie vole model of pair-bonding and its sensitivity to addictive substances. Frontiers in Psychology 10:2477–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabon DR Jr., Sawrey DK, and Webster, Wm D. 2001. Infant ultrasonic vocalizations and parental responses in two species of voles (Microtus). Canadian Journal of Zoology 79:830–837. [Google Scholar]
- Reed DR, Bachmanov AA, and Tordoff MG 2007. Forty mouse strain survey of body composition. Physiology & behavior 91:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, and Kirkpatrick B. 1999. Forebrain connections of medial agranular cortex in the prairie vole, Microtus ochrogaster. Experimental Brain Research 126:336–350. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, Eidson LN, Porter-stransky KA, McLean JW, Kuhnmuench MA, et al. 2016. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife 5:e15325–e15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Miller AK, Taymans SE, and Carter CS 1998a. Role of social and endocrine factors in alloparental behavior of prairie voles (Microtus ochrogaster). Canadian Journal of Zoology:1862–1868. [Google Scholar]
- Roberts RL, Williams JR, Wang AK, and Carter CS 1998b. Cooperative breeding and monogamy in prairie voles: Influence of the sire and geographical variation. Animal Behaviour 55:1131–1140. [DOI] [PubMed] [Google Scholar]
- Robison WT, Myers MM, Hofer MA, Shair HN, and Welch MG 2016. Prairie vole pups show potentiated isolation-induced vocalizations following isolation from their mother, but not their father. Developmental Psychobiology 58:687–699. [DOI] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, and McGinn MM 2014. Marital quality and health: A meta-analytic review. Psychological Bulletin 140:140–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers FD, and Bales KL 2019. Mothers, Fathers, and Others: Neural Substrates of Parental Care. Trends in Neurosciences 42:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers FD, and Bales KL 2020. Revisiting paternal absence: Female alloparental replacement of fathers recovers partner preference formation in female, but not male prairie voles (Microtus ochrogaster). Developmental Psychobiology 62:573–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, and Young LJ 2009. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol 30:534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosshart SP, Herz J, Vassallo BG, Hunter A, Wall MK, Badger JH, McCulloch JA, Anastasakis DG, Sarshad AA, Leonardi I, et al. 2019. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365:eaaw4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan C, and Zhang Z. 2016. Laboratory domestication changed the expression patterns of oxytocin and vasopressin in brains of rats and mice. Anatomical Science International 91:358–370. [DOI] [PubMed] [Google Scholar]
- Sabol AC, Lambert CT, Keane B, Solomon NG, and Dantzer B. 2020. How does individual variation in sociality influence fitness in prairie voles? Animal Behaviour 163:39–49. [Google Scholar]
- Sabol AC, Solomon NG, and Dantzer B. 2018. How to study socially monogamous behavior in secretive animals? Using social network analyses and automated tracking systems to study the social behavior of prairie voles. Frontiers in Ecology and Evolution 6:178–178. [Google Scholar]
- Sarkadi A, Kristiansson R, Oberklaid F, and Bremberg S. 2008. Fathers’ involvement and children’s developmental outcomes: a systematic review of longitudinal studies. Acta Paediatrica 97:153–158. [DOI] [PubMed] [Google Scholar]
- Sawrey DK, Keith JR, and Backes RC 1994. Place learning by three vole species (Microtus ochrogaster, M. montanus, and M. pennsylvanicus) in the Morris swim task. Journal of Comparative Psychology 108:179–188. [DOI] [PubMed] [Google Scholar]
- Sbarra DA, Emery RE, Beam CR, and Ocker BL 2014. Marital Dissolution and Major Depression in Midlife: A Propensity Score Analysis. Clinical Psychological Science 2:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht R, and Kramer KL 2019. Are We Monogamous? A Review of the Evolution of Pair-Bonding in Humans and Its Contemporary Variation Cross-Culturally. Frontiers in Ecology and Evolution 7. Available at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00230/full [Accessed December 16, 2020]. [Google Scholar]
- Schweinfurth MK, Neuenschwander J, Engqvist L, Schneeberger K, Rentsch AK, Gygax M, and Taborsky M. 2017. Do female Norway rats form social bonds? Behavioral Ecology and Sociobiology 71:98. [Google Scholar]
- Scribner JL, Vance EA, Protter DSW, Sheeran WM, Saslow E, Cameron RT, Klein EM, Jimenez JC, Kheirbek MA, and Donaldson ZR 2020. A neuronal signature for monogamous reunion. Proceedings of the National Academy of Sciences 117:11076–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sear R, and Mace R. 2008. Who keeps children alive? A review of the effects of kin on child survival. Evolution and Human Behavior 29:1–18. [Google Scholar]
- Seelke AMH, Perkeybile AM, Grunewald R, Bales KL, and Krubitzer LA 2016a. Individual differences in cortical connections of somatosensory cortex are associated with parental rearing style in prairie voles (Microtus ochrogaster). Journal of Comparative Neurology 524:564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Yuan S-M, Perkeybile AM, Krubitzer LA, and Bales KL 2016b. Early experiences can alter the size of cortical fields in prairie voles (Microtus ochrogaster ). Environmental Epigenetics 2:dvw019–dvw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AM, Rhine MA, Khun K, Shweyk AN, Scott AM, Bond JM, Graham JL, Havel PJ, Wolden-Hanson T, Bales KL, et al. 2018. Intranasal Oxytocin Reduces Weight Gain in Diet-Induced Obese Prairie Voles. Physiology & behavior 196:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LE, and Insel TR 1990. Infant’s response to social separation reflects adult differences in affiliative behavior: A comparative developmetal study in prairie and montane voles. Developmental Psychobiology 23:375–393. [DOI] [PubMed] [Google Scholar]
- Shuster SM, Willen RM, Keane B, and Solomon NG 2019. Alternative mating tactics in socially monogamous prairie voles, Microtus ochrogaster. Frontiers in Ecology and Evolution 7:7–7. [Google Scholar]
- Škop V, Liu N, Guo J, Gavrilova O, and Reitman ML 2020. The contribution of the mouse tail to thermoregulation is modest. American Journal of Physiology-Endocrinology and Metabolism 319:E438–E446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, and Wang Z. 2006. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neuroscience Letters 394:146–151. [DOI] [PubMed] [Google Scholar]
- Smith CJW, Wilkins KB, Mogavero JN, and Veenema AH 2015. Social Novelty Investigation in the Juvenile Rat: Modulation by the μ-Opioid System. Journal of Neuroendocrinology 27:752–764. [DOI] [PubMed] [Google Scholar]
- Smith AS, and Wang Z. 2014. Hypothalamic Oxytocin Mediates Social Buffering of the Stress Response. Biological Psychiatry 76:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Pencea V, Wang Z, Luskin MB, and Insel TR 2001. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Hormones and Behavior 39:11–21. [DOI] [PubMed] [Google Scholar]
- Stalling DT 1990. Microtus ochrogaster. Mammalian Species:1–9. [Google Scholar]
- Stein JA, Milburn NG, Zane JI, and Rotheram-Borus MJ 2009. Paternal and maternal influences on problem behaviors among homeless and runaway youth. The American Journal of Orthopsychiatry 79:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Lewis GF, Yee JR, Kenkel WM, Davila MI, Sue Carter C, and Porges SW 2015. Acoustic features of prairie vole (Microtus ochrogaster) ultrasonic vocalizations covary with heart rate. Physiology & Behavior 138:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AI, Mathieu D, Griffin L, and Bales KL 2010. Alloparenting experience affects future parental behavior and reproductive success in prairie voles (Microtus ochrogaster). Behav Processes 83:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe JR, Liu Y, Curtis JT, Freeman ME, and Wang Z. 2005. Species differences in anxiety-related responses in male prairie and meadow voles: the effects of social isolation. Physiology & Behavior 86:369–78. [DOI] [PubMed] [Google Scholar]
- Sullivan AW, Beach EC, Stetzik LA, Perry A, D’Addezio AS, Cushing BS, and Patisaul HB 2014. A Novel Model for Neuroendocrine Toxicology: Neurobehavioral Effects of BPA Exposure in a Prosocial Species, the Prairie Vole (Microtus ochrogaster). Endocrinology 155:3867–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, and Wang Z. 2014. Breaking bonds in male prairie vole: Long-term effects on emotional and social behavior, physiology, and neurochemistry. Behavioural Brain Research 265:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, and Overton JM 2008. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. American Journal of Physiology-Heart and Circulatory Physiology 294:H1581–H1588. [DOI] [PubMed] [Google Scholar]
- Taborsky M, Hofmann HA, Beery AK, Blumstein DT, Hayes LD, Lacey EA, Martins EP, Phelps SM, Solomon NG, and Rubenstein DR 2015. Taxon matters: promoting integrative studies of social behavior: NESCent Working Group on Integrative Models of Vertebrate Sociality: Evolution, Mechanisms, and Emergent Properties. Trends in Neurosciences 38:189–191. [DOI] [PubMed] [Google Scholar]
- Taymans SE, DeVries AC, DeVries MB, Nelson RJ, Friedman TC, Castro M, Detera-Wadleigh S, Carter CS, and Chrousos GP 1997. The hypothalamic-pituitary-adrenal axis of prairie voles (Microtus ochrogaster): evidence for target tissue glucocorticoid resistance. Gen Comp Endocrinol 106:48–61. [DOI] [PubMed] [Google Scholar]
- Terleph T. a. 2011. A comparison of prairie vole audible and ultrasonic pup calls and attraction to them by adults of each sex. Behaviour 148:1275–1294. [Google Scholar]
- Thomas J, and Birney E. 1979. Parental care and mating system of the prairie vole, Microtus ochrogaster. Behavioral Ecology and Sociobiology 5:171–186. [Google Scholar]
- Tuttle AH, Philip VM, Chesler EJ, and Mogil JS 2018. Comparing phenotypic variation between inbred and outbred mice. Nature Methods 15:994–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda HR, Ertürk A, Chung K, Gradinaru V, Chédotal A, Tomancak P, and Keller PJ 2020. Tissue clearing and its applications in neuroscience. Nature Reviews Neuroscience 21:61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott AT, and Ryabinin AE 2017. Alcohol’s effects on pair-bond maintenance in male prairie voles. Frontiers in Psychiatry 8:226–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott AT, and Ryabinin AE 2019. Effects of Alcohol Consumption on Pair Bond Maintenance and Potential Neural Substrates in Female Prairie Voles. Alcohol and Alcoholism 54:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, and De Vries GJ 1994. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster). Proceedings of the National Academy of Sciences 91:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Young LJ, Liu Y, and Insel TR 1997. Species differences in vasopressin receptor binding are evident early in development: Comparative anatomic studies in prairie and montane voles. Journal of Comparative Neurology 378:535–546. [PubMed] [Google Scholar]
- Wayne R, and Staves MP 1996. The August Krogh principle applies to plants. Bioscience 46:365–9. [PubMed] [Google Scholar]
- Williams DR, Carlsson R, and Bürkner P-C 2017. Between-litter variation in developmental studies of hormones and behavior: Inflated false positives and diminished power. Frontiers in Neuroendocrinology 47:154–166. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, and Insel T 1992a. Partner Preference Development in Female Prairie Voles Is Facilitated by Mating or the Central Infusion of Oxytocina. Annals of the New York Academy of Sciences 652:487–489. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, and Carter CS 1992b. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav 26:339–49. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, and Insel TR 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365:545–548. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter SC, and Insel TR 1991. Oxytocin receptor binding in female prairie voles: Endogenous and exogenous oestradiol stimulation. Journal of Neuroendocrinology 3:155–161. [DOI] [PubMed] [Google Scholar]
- Witt DM, Sue Carter C, and Walton DM 1990. Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster). Pharmacology, Biochemistry and Behavior 37:63–69. [DOI] [PubMed] [Google Scholar]
- Woitowich NC, Beery A, and Woodruff T. 2020. A 10-year follow-up study of sex inclusion in the biological sciences. eLife 9:e56344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-S, Chiang P-J, and Lin L-K 2012. Monogamous System in the Taiwan Vole Microtus kikuchii Inferred from Microsatellite DNA and Home Ranges. Zoological Studies:9. [Google Scholar]
- Wunder BA 1985. Energetics and thermoregulation. In Biology of new world Microtus pp. 812–844. Am. Soc. Mammalogist Lawrence, Kansas. [Google Scholar]
- Wunder BA, Dobkin DS, and Gettinger RD 1977. Shifts of thermogenesis in the prairie vole (Microtus ochrogaster). Oecologia 29:11–26. [DOI] [PubMed] [Google Scholar]
- Yee JR, Kenkel WM, Kulkarni P, Moore K, Perkeybile AM, Toddes S, Amacker JA, Carter CS, and Ferris CF 2016. BOLD fMRI in awake prairie voles: A platform for translational social and affective neuroscience. NeuroImage 138:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, and Wang Z. 2011. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol 32:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Huot B, Nilsen R, Wang Z, and Insel TR 1996. Species differences in central oxytocin receptor gene expression: comparative analysis of promoter sequences. J Neuroendocrinol 8:777–83. [DOI] [PubMed] [Google Scholar]