Abstract
Background
Healthcare professionals are often reluctant to deprescribe fall-risk-increasing drugs (FRIDs). Lack of knowledge and skills form a significant barrier and furthermore, there is no consensus on which medications are considered as FRIDs despite several systematic reviews. To support clinicians in the management of FRIDs and to facilitate the deprescribing process, STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk) and a deprescribing tool were developed by a European expert group.
Methods
STOPPFall was created by two facilitators based on evidence from recent meta-analyses and national fall prevention guidelines in Europe. Twenty-four panellists chose their level of agreement on a Likert scale with the items in the STOPPFall in three Delphi panel rounds. A threshold of 70% was selected for consensus a priori. The panellists were asked whether some agents are more fall-risk-increasing than others within the same pharmacological class. In an additional questionnaire, panellists were asked in which cases deprescribing of FRIDs should be considered and how it should be performed.
Results
The panellists agreed on 14 medication classes to be included in the STOPPFall. They were mostly psychotropic medications. The panellists indicated 18 differences between pharmacological subclasses with regard to fall-risk-increasing properties. Practical deprescribing guidance was developed for STOPPFall medication classes.
Conclusion
STOPPFall was created using an expert Delphi consensus process and combined with a practical deprescribing tool designed to optimise medication review. The effectiveness of these tools in falls prevention should be further evaluated in intervention studies.
Keywords: accidental falls, fall-risk-increasing drugs, deprescribing, aged, adverse effects, older people
Key Points
There is no consensus on which medications are considered as fall-risk-increasing drugs despite several systematic reviews.
STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk) was built through a Delphi process.
The STOPPFall is more comprehensive than most national falls prevention guideline listings.
It can provide a first step towards harmonising the practice and guidelines on drug-related falls in Europe.
The STOPPFall has been combined with a practical deprescribing tool designed to assist in clinical decision-making.
Introduction
Falls are the leading cause of injury and injury-related mortality in older adults [1]. They often result from interacting risks, and one of the prominent risk factors is fall-risk-increasing drugs (FRIDs) use [2–4]. FRIDs use is common, but healthcare professionals are often reluctant to deprescribe FRID [5,6]. Furthermore, there is uncertainty about the effectiveness of FRIDs deprescribing as a stand-alone intervention in falls prevention [7,8]. Regarding general medication reviews, Cameron et al. [8] concluded that they may make little or no difference to the rate of falls or risk of falling in a long-term care setting. A Cochrane review in 2012 reported withdrawal of psychotropics and prescribing-modification programme for primary care physicians to be effective among community-dwelling older adults [7]. However, three other included deprescribing trials had negative results in falls prevention [7].
In general, falls prevention guidelines emphasise that older adults at high risk of falling should be assessed for risk factors, including medication use [9]. Therefore, identifying FRIDs is important as it is the starting point for possible FRID deprescribing as part of the multifactorial falls prevention strategy [5]. However, current national falls prevention guidelines in Europe vary considerably in which medications they include as risk factors for falls and some of them have not been updated during the past decade [10–17].
In recent decades, numerous systematic reviews and meta-analyses have summarised the associations between several medication classes and falls risk [2–4]. However, these efforts have some limitations. Firstly, the studies are often limited to investigating the associations between commonly prescribed medication classes and falls to have sufficient statistical power [5]. Secondly, as most studies do not focus on investigating individual treatment effects, more work is warranted to facilitate personalised drug optimization.
Currently, several explicit prescribing tools including STOPP/START (Screening Tool of Older Persons potentially inappropriate Prescriptions/Screening Tool to Alert doctors to Right Treatment), Beers criteria, FORTA (Fit fOR The Aged)-list and TIME (Turkish Inappropriate Medication use in the Elderly) are available to guide professionals in appropriate mediation use [18–21]. These drug-optimization strategies include some aspects of falls prevention, as FRIDs are mostly labelled as potential falls causative factors [18–20]. Although these tools are not comprehensive in their FRIDs listing, their use in intervention studies has been shown to reduce falls [22,23]. As the existing lists do not represent a complete and uniform medication list to be avoided in older adults at risk of falls, a deprescribing tool focusing on purely medication-related falls may be expected to be more effective in falls prevention than general prescribing tools [24]. However, such a tool should be integrated within a multifactorial falls prevention strategy to achieve the best prospects of success.
The European Geriatric Medicine Society (EuGMS) Task and Finish Group on FRIDs described in their recent statement paper generic steps for FRIDs withdrawal, from medication review to symptom monitoring after deprescribing [5]. To further support clinicians in FRIDs deprescribing, our first aim was to create a comprehensive STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk) by Delphi consensus for use as a screening tool. Furthermore, we explored possible differences in fall-risk-increasing properties between pharmacological subclasses to gain further insight into medication review. Our second aim was to combine the STOPPFall with a deprescribing tool with practical guidance to simplify and structure FRIDs deprescribing. Thirdly, we aimed at creating consensus to facilitate harmonisation of clinical management of drug-related falls across Europe.
Methods
STOPPFall was built through a consensus effort, using a modified Delphi technique. A principal investigator of STOPP/START (D.O.’M.) permitted us to use the name STOPPFall after finalising this project [18,25]. In an additional questionnaire, we asked (i) when to consider deprescribing STOPPFall medications and (ii) how to monitor patients’ clinical status after deprescribing with a view to developing a deprescribing tool. The internet-based questionnaires were undertaken remotely and anonymously. The Medical Ethics Research Committee of Amsterdam UMC, location AMC declared that the Medical Research Involving Human Subjects Act did not apply to this study. Panellists gave written informed consent during each questionnaire.
A summary of the methods is given below and detailed description is provided in Appendix I.
European expert panel and International advisory board
In total, 24 members EuGMS Task and Finish Group on FRIDs and Special Interest Group (SIG) on Pharmacology accepted the invitation to participate in the Delphi process and to fill in an additional deprescribing tool questionnaire [5,26,27]. A non-European international advisory board was established consisting of experts on geriatric pharmacotherapy.
Initial STOPPFall
Two facilitators created an initial STOPPFall based on evidence from the three recently published systematic reviews and meta-analyses and eight national falls prevention guidelines in Europe [2–4,10–17]. International advisers were consulted regarding the initial STOPPFall.
Delphi rounds
The panellists were asked to indicate to what extent they agreed with the medication classes included in the initial STOPPFall using a Likert scale. Also, the panellists were asked to propose missing medication classes. Furthermore, statements were created based on the answers obtained in round 1 about risk differences between the pharmacological subclasses. The panellists were asked to indicate to what extent they agreed with the statements. If >70% of the panellists agreed (strongly agree/agree) with the proposed STOPPFall medication class or with the statements, this was considered consensus.
Questionnaire to develop a deprescribing tool
The panellists were asked about components of the patient-centred deprescribing process [28]. To develop the questionnaire, a Medline® search was performed (Appendix II). The identified key resources from the literature were mentioned as references, and the panellists were provided with the option to propose resources. The panellists were asked to indicate for every medication class whether a stepwise withdrawal is needed and choose the possible strategy for withdrawal. Furthermore, they were asked in which situations withdrawal should be performed and were requested to indicate those symptoms patients should be monitored for after deprescribing. Finally, panellists were asked to indicate how follow-up checks should be arranged. Also, the results were added to the general decision tree of the FRIDs management separately for every medication class [5].
Results
Each Delphi round questionnaire was completed by 24 panellists from 13 European countries during 2019 and the deprescribing tool questionnaire by 24 panellists in 2020.
STOPPFall
Figure 1 shows the results of the distribution of level of agreements, whether the medication classes should be included in the STOPPFall. In total, 14 classes were included. Consensus for inclusion was reached in round 1 for anticholinergics, diuretics, alpha-blockers used as antihypertensives, opioids, antidepressants, antipsychotics, antiepileptics, benzodiazepines and benzodiazepine-related drugs (Figure 1). Regarding the inclusion in the STOPPFall of centrally-acting antihypertensives, alpha-blockers for prostate hyperplasia, antihistamines and vasodilators used in cardiac diseases, consensus was achieved in round 2 (Figure 1). Finally, in round 3, consensus was reached for inclusion in the STOPPFall of medications for overactive bladder and urge incontinence (Figure 1). For 17 medication classes, no consensus was reached (Figure 1).
Figure 1 .
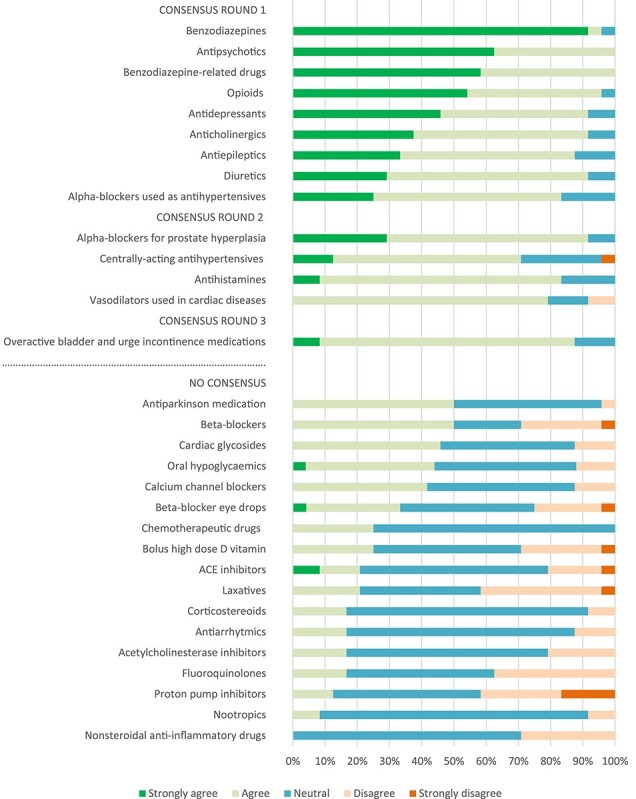
Distributions of level of agreements for the medication classes included in the STOPPFall and for the classes that reached no consensus.
Statements regarding subclass differences
Supplementary Figure 1 shows the distribution of levels of agreements concerning statements about risk differences within the pharmacological classes (Appendix III). Consensus was achieved for the 18 statements shown in Table 1.
Table 1 .
Statements about possible risk differences within the pharmacological classes that reached consensus
| Antipsychotics | • Risk difference is related to variation in (i) sedative, (ii) anticholinergic and (iii) alpha-receptor properties |
| Opioids | • Strong opioids are more fall-risk-increasing than weak opioids |
| Antidepressants | • Tricyclic antidepressants (TCA’s) are more fall-risk-increasing than others |
| • Risk difference is related to the variation in (i) sedative effects, (ii) propensity to cause orthostatic hypotension and (iii) anticholinergic activity | |
| Anticholinergics | • Medications with high anticholinergic activity are more fall-risk-increasing than weak anticholinergics |
| Antiepileptics | • Older generation antiepileptics are more fall-risk-increasing than newer antiepileptics |
| • Risk difference is related to the variation in sedative effects | |
| Diuretics | • Loop diuretics are more fall-risk-increasing than other diuretics |
| Alpha-blockers for benign prostatic hyperplasia | • Non-selective alpha-blockers are more fall-risk-increasing than selective |
| Antihistamines | • First-generation antihistamines are more fall-risk-increasing than second-generation antihistamines |
| • Risk difference is related to variation in (i) sedative effects and (ii) anticholinergic activity | |
| Medications for overactive bladder and urge incontinence | • Risk difference is related to variation in anticholinergic activity |
| Oral hypoglycaemics | • Oral hypoglycaemic agents that can cause hypoglycaemia, sulfonylureas, are more risk-increasing than other agents |
Deprescribing tool
A summary of the deprescribing guidance for STOPPFall items can be found in Table 2 and the detailed results and the deprescribing decision trees in Appendix IV and Appendix V. Also, the decision trees are available as online tools; https://kik.amc.nl/falls/decision-tree/.
Table 2 .
Deprescribing guidance for STOPPFall items
| Fall-risk assessment: In which cases to consider withdrawal?a |
Is stepwise withdrawal needed?b | Monitoring after deprescribingc | |
|---|---|---|---|
| Always | -If no indication for prescribing-If safer alternative available | -Fall incidence and change in symptoms e.g. OH, blurred vision, dizziness-Organise follow-ups on individual basis | |
| Benzodiazepines (BZD) and BZD-related drugs | -If daytime sedation, cognitive impairment, or psychomotor impairments -In case of both indications: sleep and anxiety disorder |
In general needed | -Monitor: anxiety, insomnia, agitation -Consider monitoring: delirium, seizures, confusion |
| Antipsychotics | -If extrapyramidal or cardiac side effects, sedation, signs of sedation, dizziness, or blurred vision -If given for BPSD or sleep disorder, possibly if given for bipolar disorder |
In general needed | -Monitor: recurrence of symptoms (psychosis, aggression, agitation, delusion, hallucination) -Consider monitoring: insomnia |
| Opioids | -If slow reactions, impaired balance, or sedative symptoms -If given for chronic pain, and possibly if given for acute pain |
In general needed | -Monitor: recurrence of pain -Consider monitoring: musculoskeletal symptoms, restlessness, gastrointestinal symptoms, anxiety, insomnia, diaphoresis, anger, chills |
| Antidepressants | -If hyponatremia, OH, dizziness, sedative symptoms, or tachycardia/arrhythmia -If given for depression but depended on symptom-free time and history of symptoms or given for sleep disorder, and possibly if given for neuropathic pain or anxiety disorder |
In general needed | -Monitor: recurrence of depression, anxiety, irritability and insomnia -Consider monitoring: headache, malaise, gastrointestinal symptoms |
| Antiepileptics | -If ataxia, somnolence, impaired balance, or possibly in case of dizziness -If given for anxiety disorder or neuropathic pain |
Consider | -Monitor: recurrence of seizures -Consider monitoring: anxiety, restlessness, insomnia, headache |
| Diuretics | -If OH, hypotension, or electrolyte disturbance and possibly if urinary incontinence -possibly if given for hypertension |
Consider | -Monitor: heart failure, hypertension, signs of fluid retention |
| Alpha-blockers (AB) used as antihypertensives | -If hypotension, OH, or dizziness | Consider | -Monitor: hypertension -Consider monitoring: palpitations, headache |
| AB for prostate hyperplasia | -If hypotension, OH, or dizziness | In general not needed | -Monitor: return of symptoms |
| Centrally-acting antihypertensives | -If hypotension, OH, or sedative symptoms | Consider | -Monitor: hypertension |
| Sedative antihistamines | -If confusion, drowsiness, dizziness, or blurred vision -In case of all indications: hypnotic/sedative, chronic itch, allergic symptoms |
Consider | -Monitor: return of symptoms -Consider monitoring: insomnia, anxiety |
| Vasodilators used in cardiac diseases | -If hypotension, OH, or dizziness | Consider | -Monitor: symptoms of Angina Pectoris |
| Overactive bladder and incontinence medications | -If dizziness, confusion, blurred vision, drowsiness, or increased QT-interval | Consider | -Monitor: return of symptoms |
aThis column includes answer categories that were chosen by more than 70% of the experts. In addition, after word ‘possibly’ are indicated the categories that were selected by 30–70% of the experts.
b‘In general needed’ indicates that >70% of experts chose categories of yes or depending. ‘Consider’ indicates that 30–70% of experts chose categories of yes or depending. ‘In general not needed’ indicates that <30% of experts chose categories of yes or depending.
c‘Monitor’ refers to >70% of the experts selecting these symptoms. ‘Consider monitoring’ refers to 30–70% of the experts selecting these symptoms. BPSD, behavioural and psychological symptoms of dementia; OH, orthostatic hypotension.
Table 1 provides an overview of particular cases where withdrawal should be considered at fall-risk assessment. Figure 2 shows the detailed results for this question for each medication class.
Figure 2 .
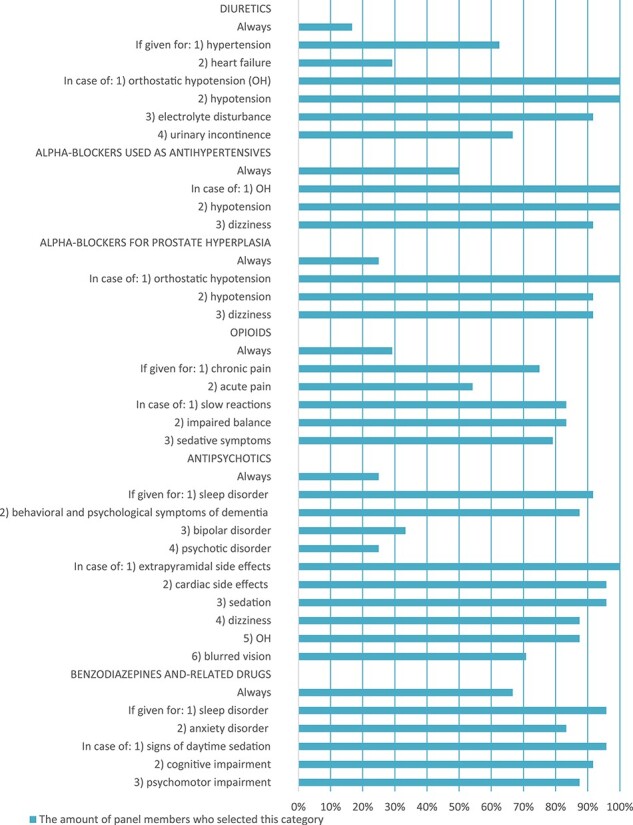
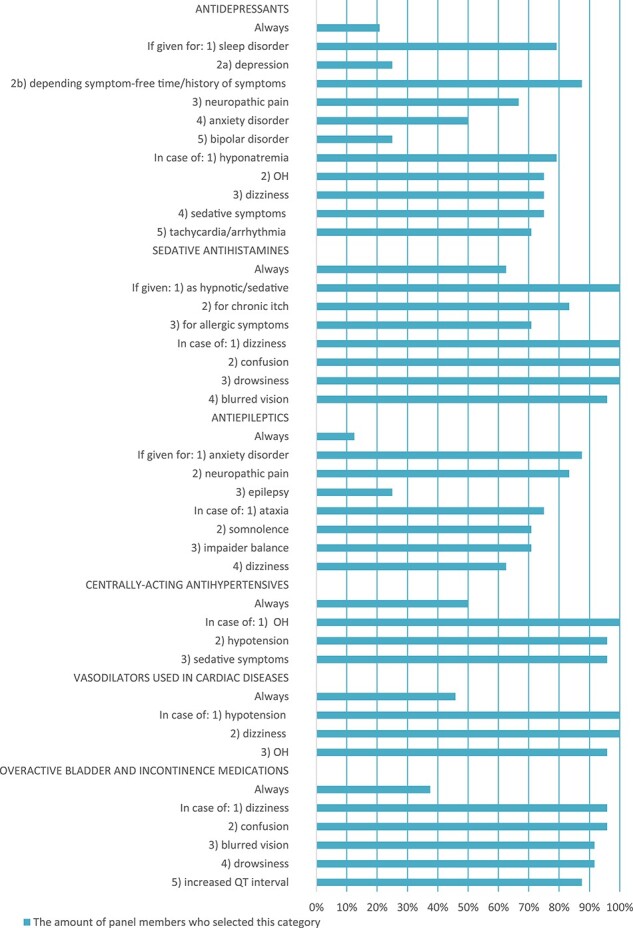
Panel’s answers to ‘in which cases should withdrawal be considered?’
Table 2 shows a summary of how to decide whether a stepwise withdrawal is needed, and the whole spread of the panel’s responses to this question is shown in Figure 3. The most frequently chosen and proposed strategies for tapering can be found in Appendix V. The adverse drug withdrawal effects and recurrence of symptoms for which patients should be monitored are summarised in Table 2.
Figure 3 .
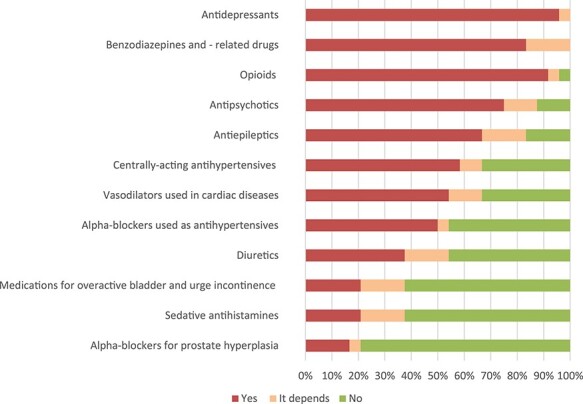
Panel’s answers to ‘whether stepwise withdrawal is needed in general?’.
The panel was divided in their views on how often and for how long follow-up checks should be continued following deprescribing (Appendix V). Additional key deprescribing resources identified from the literature search or proposed by panellists are provided in Appendix V.
Discussion
In this expert effort by the EuGMS Task and Finish Group on FRIDs and SIG on Pharmacology, a consensus was achieved for 14 medication classes to create a comprehensive list of FRIDs, STOPPFall. Many of these were psychotropics, but several less well-established risk medications were also identified. However, the role of numerous medication classes as FRIDs are to be further elucidated, since no consensus was reached for 17 classes. Furthermore, the panellists indicated several differences in fall-risk-increasing properties between pharmacological subclasses, especially for antipsychotics and antidepressants. The STOPPFall was combined with a practical deprescribing tool to facilitate optimal deprescribing.
This is the first European-wide effort to establish a consensus on FRIDs in older adults. It is evident when comparing the STOPPFall to national fall prevention guidelines, that it is more comprehensive than most guideline listings. The STOPPFall contains items such as alpha-blockers, centrally-acting antihypertensives, antihistamines and anticholinergics, which are not regularly included in guidelines. The difference can be explained by the different methodologies used to create these listings. Typically, FRIDs in national guidelines are based purely on associations derived from meta-analyses. As some guidelines have not been updated in recent years, these listings rely on data from older meta-analyses and are therefore often not up-to-date [12–15,17]. In contrast, we asked the panellists to comment also based on their expertise in the field and clinical experience. Furthermore, the STOPPFall is more comprehensive than the section of ‘drugs that predictably increase the risk of falls in older people’ in STOPP/START so that in general, the STOPPFall could be expected to be more suited for falls prevention. In STOPP/START version 2, only benzodiazepines, neuroleptics, vasodilators with persistent postural hypotension and Z-drugs are mentioned under the FRIDs section.
Furthermore, a consensus was reached for 18 statements concerning differences in the fall-risk-increasing properties within pharmacological classes. The lack of knowledge of the risk related to pharmacological subclasses and individual agents was identified by the Task and Finish Group as a gap in the current literature [5]. The panellists frequently identified variation in sedative effects, anticholinergic activity and propensity to cause orthostatic hypotension as features causing differences in medication-related falls risk. These differences emphasise the need for critically evaluating the choice of individual agents when prescribing FRIDs. Moreover, to gain further insights into these risk differences, the evaluation of specific pharmacological agents should be a key item in the future FRIDs research agenda. Such studies will enable the identification of safer prescription alternatives.
It has been reported that deprescribing can be performed safely in older people [29]. However, despite the growing evidence on falls as an adverse drug reaction, deprescribing FRIDs is often difficult and infrequently performed [5]. To support healthcare professionals in their decision-making, we developed a practical deprescribing tool, including important components of the deprescribing process [28]. This practical guide can help overcome current reluctance in clinical practice by providing an up-to-date and straightforward source of expert knowledge. However, for successful implementation, national dissemination of this tool among healthcare professionals is essential. The Task and Finish Group members intend to take a role in this spread of knowledge e.g. through national conferences, seminars and webpages. A link to the online decision trees is available at the Task and Finish Group webpage [30]. Finally, patient-centred care has been found to improve patient satisfaction, adherence, quality of life and overall health outcomes [28]. Therefore, patients should be engaged throughout the process and their personalised needs and concerns should be taken into account [28,31].
Future research and clinical implications
Since panellists could not reach consensus on 17 medication classes regarding whether they should be classified as FRIDs, it is apparent that more research is warranted in the future. Firstly, only a few studies have investigated falls risk related to several of these medication classes to date. Secondly, considering the quality issues in the published observational studies including medication and falls ascertainment and controlling for confounding, advocating better research quality is important. Another explanation for lack of consensus could be heterogeneous treatment effects due to different patient characteristics. Disentangling a single drug effect in the context of drug–drug interactions and drug–disease interactions is difficult, given the multiple causes for a fall and high prevalence of polypharmacy in older patients. Furthermore, it is challenging to label these medication classes, such as beta-blockers, as purely FRIDs as they have known benefits regarding prevention of cardiovascular disease and symptom improvement. Thus, investigating heterogeneous treatment effects and attempts to identify the older persons at risk of medication-related falls are necessary to gain improved insight. Furthermore, assessing effect of dosages, combination therapies and drug–drug interactions on falls risk was beyond the scope of this study and should be addressed in the future.
The EuGMS supports the use of STOPPFall as a screening tool to identify FRIDs when performing a medication review in older fallers. Also, in accordance with our position paper, we recommend systematically checking for a history of falls and a high risk of falling before prescribing STOPPFall medications for older people [5]. Furthermore, the deprescribing tool is not aimed to be used as a stand-alone strategy to reduce fall incidents but should be implemented in a multifactorial strategy to achieve the best chance of success. Finally, general interventions focusing on minimising polypharmacy are unlikely to be effective at patient-level in falls prevention since the population attributable risk fraction related to polypharmacy alone is low [32]. Therefore, in falls clinics and falls prevention programmes, deprescribing strategies targeting FRIDs like the STOPPFall are warranted. However, in a general geriatric setting, specific deprescribing tools for every geriatric syndrome are undesirable. Therefore, we have teamed up with the STOPP/START tools, and the STOPPFall results will be included in the draft criteria of the anticipated STOPP/START version 3 to be further validated by the STOPP/START panellists.
Limitations
There are several limitations to this study related to the Delphi process and to the use of online surveys. Firstly, the formulation of the questions by the facilitators might have influenced the responses, and individual panellists might have interpreted the questions somewhat differently, even though the purpose of the study was described in detail in the invitation letters. Secondly, no face-to-face meetings were organised during the rounds due to lack of feasibility and inclusion of panellists from the whole continent. Such meetings could have utilised the expertise better, but in contrast, the anonymous process probably avoided domination by the substantial number of panellists or by the strength of individual personalities. In the future, the STOPPFall should be updated to maintain constant review. Thirdly, the evidence given for panellists was based on heterogeneous observational studies that have typically quality issues such as accounting for confounding by indication. However, the panellists were asked to indicate their view also based on their own experience and thus consider these issues. Fourthly, a general standard of consensus measurement and an agreement concerning the declaration of consensus in Delphi studies do not exist to date, and various methods have been used. Furthermore, we did not formally evaluate the stability of the consensus reached when applying the Delphi process. However, there was a strong consensus regarding the medication classes that reached consensus in round 1. Due to the strong consensus, we do not expect that these medication classes would not have reached consensus in the following rounds if re-evaluated. Moreover, the medication classes that reached consensus in round 2 or 3 had almost reached consensus in the previous rounds. Finally, the data regarding deprescribing as a single intervention in falls prevention is inconclusive to date. The effectiveness of the STOPPFall and accompanying deprescribing tool should still be evaluated in different settings including community and nursing homes.
Conclusion
A new screening tool STOPPFall was created with a consensus Delphi effort. The STOPPFall contains mainly psychotropic medications, but also several other pharmacological classes were recognised as risk factors. Therefore, the STOPPFall is more comprehensive than most national falls prevention guideline listings and can help harmonise the practice and guidelines on drug-related falls in Europe. The STOPPFall has been combined with a practical deprescribing tool designed to assist in clinical decision-making and simplify FRIDs management, and thereby optimise care. Furthermore, it is mandatory to promote high-quality research in defining the effects of FRID and identifying best strategies to promote greater awareness and knowledge among healthcare professionals on this topic.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the contribution of the international expert board. The board consisted following members: Allen Huang (A.H.) from Canada, Mike Steinman (M.S.) from the United States, Paula Rochon (P.R.) from Canada, Dee Mangin (D.M.) from Canada, Danijela Gnijdic (D.G.) from Australia, Stephen Lord (S.L.) from Australia and Jerry Gurwitz (J.G.) from the United States.
Declaration of Conflicts of Interest
Martin Wehling was employed by AstraZeneca R&D, Mölndal, as director of discovery medicine (=translational medicine) from 2003 to 2006, while on sabbatical leave from his professorship at the University of Heidelberg. Since returning to this position in January 2007, he has received lecturing and consulting fees from Sanofi-Aventis, Bayer, Berlin-Chemie, Boehringer-Ingelheim, Aspen, Novartis, Takeda, Roche, Pfizer, Bristol-Myers, Daichii-Sankyo, Lilly, Otsuka, Novo-Nordisk, Shire and LEO Pharma. Sirpa Hartikainen has received lecturing fees from Astellas Pharma. Gülistan Bahat has received financial support for symposia/educational programmes or given lectures arranged by Abbott, Astellas, Lilly, Nestle and Nutricia. Rest of the authors have no conflicts of interest to state.
Declaration of Sources of Funding
This work was supported by the Amsterdam Public Health Aging and Later Life Innovation Price and Clementine Brigitta Maria Dalderup fund, which is an Amsterdam University fund. The sponsors played no part in the design, execution, analysis and interpretation of data, or writing of the study.
References
- 1. Centers for Disease Control and Prevention . Injury Prevention & Control. Keep on your feet - Preventing Older Adult Falls. https://www.cdc.gov/injury/features/older-adult-falls/index.html. (20 April 2020, date last accessed).
- 2. de Vries M, Seppala LJ, Daams JG et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: I. cardiovascular drugs. J Am Med Dir Assoc 2018; 19: 371 e1–9. [DOI] [PubMed] [Google Scholar]
- 3. Seppala LJ, Wermelink A, de Vries M et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics. J Am Med Dir Assoc 2018; 19: 371 e11–7. [DOI] [PubMed] [Google Scholar]
- 4. Seppala LJ, van de Glind EMM, Daams JG et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc 2018; 19: 372 e1–8. [DOI] [PubMed] [Google Scholar]
- 5. Seppala LJ, van der Velde N, Masud T et al. EuGMS task and finish group on fall-risk-increasing drugs (FRIDs): position on knowledge dissemination, management, and future research. Drugs Aging 2019; 36: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laflamme L, Monarrez-Espino J, Johnell K et al. Type, number or both? A population-based matched case-control study on the risk of fall injuries among older people and number of medications beyond fall-inducing drugs. PLoS One 2015; 10: e0123390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gillespie LD, Robertson MC, Gillespie WJ et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012; Cd007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cameron ID, Dyer SM, Panagoda CE et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev 2018; 9: CD005465. doi: 10.1002/14651858.CD005465.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Summary of the Updated American Geriatrics Society . British geriatrics society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc 2011; 59: 148–57. [DOI] [PubMed] [Google Scholar]
- 10. Valbreventie.be. Expertisecentrum Valpreventie Vlaanderen . Valpreventie bij thuiswonende ouderen. In: Flamish guideline Falls prevention in older people living at home, 2017, Leuven, http://www.valpreventie.be.
- 11. Federatie Medisch Specialisten . Richtlijn Preventie van valincidenten bij ouderen. In: Dutch guideline prevention of falls in older people, 2017, https://richtlijnendatabase.nl/richtlijn/preventie_van_valincidenten_bij_ouderen/startpagina_-_preventie_van_valincidenten.html (20 March 2020, date last accessed).
- 12. NICE . NICE guidelines: falls assessment and prevention of falls in older people (CG161), National Institute for Health and Care Excellence: Manchester, 2013. [PubMed]
- 13. Prevenzione delle cadute da incidente domestico negli anziani (Italian guideline Falls prevention in older people), PROGRAMMA NAZIONALE LINEE GUIDA: Roma, 2009.
- 14. Strategy to Prevent Falls and Fractures in Ireland’s Ageing Population . Report of the National Steering Group on the Prevention of Falls in Older People and the Prevention and Management of Osteoporosis throughout Life, The Health Service Executive, Department of Health and Children and National Council on Ageing and Older people: Dublin, 2008.
- 15. Documento de consenso sobre prevención de fragilidad y caídas en la persona mayor (Spanish consensus Document on prevention of frailty and falls in the older person), MINISTERIO DE SANIDAD, SERVICIOS SOCIALES E IGUALDAD CENTRO DE PUBLICACIONES: Madrid, 2014.
- 16. Sundhedstyrelssen . National klinisk retninglijne for forebyggelse af fald hos ældre (Danish national clinical guideline for the prevention of falls in elderly people), Sundhedsstyrelsen: København, 2018.
- 17. Évaluation et prise en charge des personnes âgées faisant des chutes répétées . French guideline Assessment and management of elderly people having recurrent falls, Haute Autorité de Santé: Saint-Denis, 2009.
- 18. O'Mahony D, O'Sullivan D, Byrne S et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44: 213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Geriatrics Society . American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63: 2227–46. [DOI] [PubMed] [Google Scholar]
- 20. Pazan F, Wehling M. The FORTA (fit fOR the aged) app as a clinical tool to optimize complex medications in older people. J Am Med Dir Assoc 2017; 18: 893. [DOI] [PubMed] [Google Scholar]
- 21. Bahat G, Ilhan B, Erdogan T et al. Turkish inappropriate medication use in the elderly (TIME) criteria to improve prescribing in older adults: TIME-to-STOP/TIME-to-START. European Geriatric Medicine 2020; 11: 491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michalek C, Wehling M, Schlitzer J et al. Effects of “fit fOR the aged” (FORTA) on pharmacotherapy and clinical endpoints—a pilot randomized controlled study. Eur J Clin Pharmacol 2014; 70: 1261–7. [DOI] [PubMed] [Google Scholar]
- 23. Frankenthal D, Lerman Y, Kalendaryev E et al. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc 2014; 62: 1658–65. [DOI] [PubMed] [Google Scholar]
- 24. Curtin D, Byrne S, O’Mahony D. Identifying Explicit Criteria for the Prevention of Falls. In: Huang AR, Mallet L, eds. Medication-related falls in older people; Springer International Publishing: Switzerland, 179–88.
- 25. Gallagher P, Ryan C, Byrne S et al. STOPP (screening tool of older Person's prescriptions) and START (screening tool to alert doctors to right treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46: 72–83. [DOI] [PubMed] [Google Scholar]
- 26. EuGMS . Research & Cooperation. Special Interest Groups. Pharmacology. https://www.eugms.org/research-cooperation/special-interest-groups/pharmacology.html. (20 March 2020, date last accessed).
- 27. EuGMS . Research & Cooperation. Special Interest Groups. Falls and Fractures. https://www.eugms.org/research-cooperation/special-interest-groups/falls-and-fractures.html. 20 March 2020, date last accessed.
- 28. Reeve E, Shakib S, Hendrix I et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmacol 2014; 78: 738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iyer S, Naganathan V, McLachlan AJ et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging 2008; 25: 1021–31. [DOI] [PubMed] [Google Scholar]
- 30. EuGMS . Research & Cooperation. Task & Finish Groups. FRID - Fall Risk Increasing Drugs. https://www.eugms.org/research-cooperation/task-finish-groups/frid-fall-risk-increasing-drugs.html. 20 August 2020, date last accessed.
- 31. Reeve E, Low L-F, Shakib S et al. Development and validation of the revised patients’ attitudes towards Deprescribing (rPATD) questionnaire: versions for older adults and caregivers. Drugs Aging 2016; 33: 913–28. [DOI] [PubMed] [Google Scholar]
- 32. Morin L, Calderon Larrañaga A, Welmer A-K et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol 2019; 11: 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


