TO THE EDITOR:
Patients with hematologic malignancies have shown an increased risk of morbidity and mortality when infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1, 2, 3, 4, 5 Because of their remarkable activity in preventing severe COVID-19 in clinical trials, 3 SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and Ad26.COV2.S (Johnson and Johnson), have received emergency use approval by the US Food and Drug Administration (FDA)6, 7, 8; however, patients who were actively receiving cancer treatment were excluded from enrollment studies.
Treatment with B-cell–directed therapies may adversely affect the production of antibodies in response to SARS-CoV-2 vaccination in patients with lymphoma because of B-cell depletion and/or disruption of the B-cell receptor signaling pathway.9, 10, 11 The long-term immunologic effects of B-cell depletion and the characteristics of B-cell reconstitution in lymphoma are not well defined, despite the widespread use of B-cell–directed therapies.12 In the lymphoma population the recovery of the memory B-cell pool is delayed compared with normal B-cell ontogeny, remaining below normal controls at 1 year after administration of the anti-CD20 antibody rituximab.13
In this study, we evaluated antibody response to the COVID-19 vaccines in patients with B-cell lymphoma (BCL) who were either actively receiving or were within 3, 3 to 6, 6 to 9, or >9 months of receiving of B-cell–directed therapy, to evaluate the impairment of their antibody production. We hypothesized that the ability to respond to the COVID-19 vaccines could be restored at a certain time after discontinuation of treatment, and we actively tried to define this threshold.
We conducted a prospective noninterventional study. Patients were eligible if they had lymphoma, were actively receiving or had previously completed B-cell-directed therapy, and had received full vaccination with a COVID-19 vaccine. We also studied the vaccines' efficacy in p atients with lymphoid malignancies who were either under observation or receiving non–B-cell–directed therapy, and in individuals without lymphoma. After informed consent, samples from patients with lymphoma, health care personnel (HCP), and nursing home residents were collected under institutional review board–approved protocol I-1151721 from Roswell Park Comprehensive Cancer Center (RPCCC) or in the context of a KSL Diagnostics study conducted in various nursing homes in Western New York (study RD001).
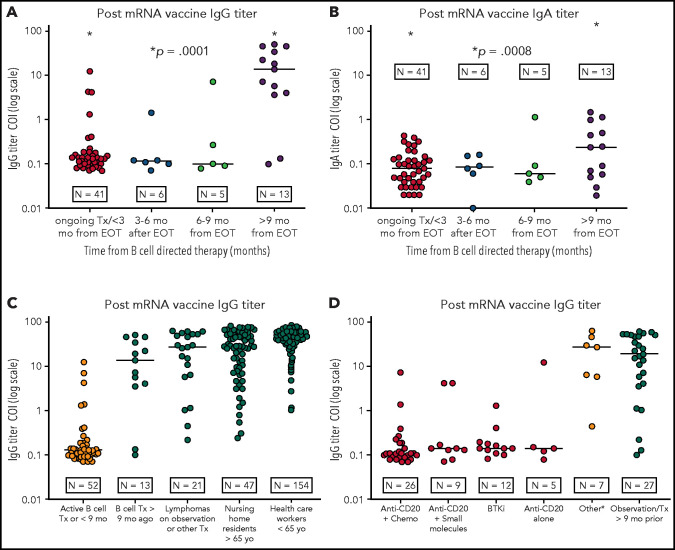
Figure 1.
IgG/IgA titer levels of anti-S protein antibodies after vaccination for SARS-CoV-2. (A-B) IgG titer levels of anti-S protein antibodies in patients with BCL (log10 scale) vaccinated at different time points from the last B-cell–directed therapy. IgG response (A); IgA response (B). (C) Response to the vaccine (IgG titer levels) in patients with BCL receiving active treatment or within 9 months of concluding B-cell–directed treatment vs those treated more than 9 months before the vaccination vs patients with BCL who were under observation or receiving other treatments vs nursing home residents and HCP. (D) IgG titer levels in patients with BCL receiving active treatment or within 9 months from the end of active treatment vs patients under observation or who underwent treatment >9 months before vaccination. The category other includes CHOEP (cyclophosphamide-doxorubicin-vincristine-etoposide-prednisone), ICE (ifosfamide-carboplatin-etoposide), brentuximab vedotin, bexarotene, daratumumab, radiotherapy, or mogamulizumab). Patients undergoing autologous stem cell transplant or CAR T-cell therapy after induction were added to the anti-CD20+chemotherapy category. COI ≥1.0 shows positivity for SARS-CoV-2 IgG antibodies. BTKi, Bruton tyrosine kinase; Tx, treatment.
For patients enrolled at RPCCC, a history of overt infection with SARS-CoV-2 was fully known, and testing for the nucleocapsid antibody had been conducted, to rule out prior asymptomatic exposure. All individuals enrolled in the KSL Diagnostics study (HCP and nursing home residents) were tested for antibody production before the vaccine was administered, to determine prior exposure.
Serum samples were collected within 2 to 8 weeks after the final dose of the vaccine. Detailed description of antibody testing with the ability to discern between response to the vaccine or to SARS-CoV-2 infection are in the supplemental Material (available on the Blood Web site).
The levels of SARS-CoV-2 antibodies were compared between the ongoing therapy cohort and the posttreatment cohort by Fisher's exact test. The association between vaccine titers (immunoglobulin G [IgG] and IgA) and the therapy cohort were evaluated in a 1-way analysis of variance model, with post hoc pairwise comparisons. All analyses were conducted with GraphPad Prism, version 9, and R, version 3.6.3. The sample size calculations were based on a comparing vaccine titers between any 2 cohorts by Bonferroni-adjusted 2-sided t test.
A total of 105 individuals were enrolled at RPCCC, including 95 patients (summarized in supplemental Table 1) and 10 HCP. In addition, 63 aged residents and 183 HCP from nursing homes in western New York were enrolled and tested for antibodies. We excluded from the analysis 2 HCPs and 9 patients from RPCCC who had been infected with the SARS-CoV-2 virus, 8 of whom tested positive in the nucleocapsid antibody assay. All the patients and HCP with previous exposure to SARS-CoV-2 tested positive for IgG against the S protein. We also excluded 47 HCP and 16 residents from the nursing homes who had formed SARS-CoV-2 antibodies before the vaccination. Therefore, the analysis included 86 patients and 7 HCP from RPCCC and the cohorts from the nursing home (47 residents and 147 HCP).
Characteristics of the patients and treatments at the time of vaccination are described in the tables of the supplemental Material. Vaccine-induced antibody responses in patients with lymphoma and HCPs are summarized in Table 1 . Antibodies against the spike SARS-CoV-2 virus protein were detected in all HCP and in 6 of 7 (85.7%) patients without BCL vaccinated during active cancer treatment. One patient with angioimmunoblastic T-cell lymphoma receiving active treatment with chemotherapy did not demonstrate antibody production. Interestingly, another patient with angioimmunoblastic T-cell lymphoma demonstrated an antibody response despite being 88 years of age and receiving active therapy with single-agent brentuximab vedotin. A patient with myeloma receiving long-term therapy with daratumumab also demonstrated antibody response. Only 4 of 41 (9.7%) patients with BCL developed antibodies while actively receiving or within 3 months of completing B-cell depleting therapy.
Table 1.
Humoral response to the SARS-CoV-2 vaccine
| BCL patients on B-cell–directed treatment <9 mo prior | BCL patients with no treatment or treatment >9 mo prior | BCL, TCL, and MM patients receiving other treatments | HCP at RPCCC and from KSL Inc <65 y of age | Nursing home residents >65 y of age* | |
|---|---|---|---|---|---|
| n = 52 | n = 25 | n = 9 | n = 154 | n = 47 | |
| IgG production | |||||
| Yes | 6 (11) | 22 (88) | 8 (61.5) | 154 (100) | 43 (91.5) |
| No | 46 (89) | 3 (12) | 1 (38.5) | 0 | 4 (9.5) |
| P† | .00001 | .00001 | .00001 | .00001 | — |
IgG titer level above COI threshold: COI ≥ 1.0) in BCL patients on active treatment or within 9 mo after treatment with B-cell–directed therapy vs patients with BCL under observation >9 months after B-cell–directed therapy; vs patients with BCL, TCL, or MM receiving other treatments; vs HCP < 65 y of age; vs nursing home residents > 65 y of age. Data are expressed as the number of patients (percentage of total patients in the study group).
MM, multiple myeloma; TCL, T-cell lymphoma.
Data from KSL Inc.
In comparison with patients with BCL receiving B-cell–directed treatment within 9 mo.
We compared antibody production in all patients with BCL who were receiving or had completed B-cell–directed therapy and divided them into 4 temporal groups: receiving the vaccine (1); on active treatment or within 3 months after treatment (2); and 3 to 6 months, (3) 6 to 9 months, or (4) >9 months after B-cell–directed therapy.
None of the patients demonstrated a significant IgM response to vaccination.
The IgG response in the 4 BCL groups was significantly different (P = .0001). The comparison of patients with ongoing treatment or vaccinated within 3 months from the last treatment with patients vaccinated more than 9 months after the last treatment showed a marked difference in the IgG response (P = .0001; 95% confidence interval [CI], 12.98-24.78). Median cutoff index (COI) IgG production was 0.13 (range, 0.0-12.4) for the recent treatment group vs 20.7 (range, 0.1-63.8) for the second group (P = .0001; 95% CI, 18.8-30.92). As expected,14, 15, 16 the IgA response was less pronounced for all groups of patients. The comparisons of IgA levels of patients on active treatment or vaccinated within 3 months from the last treatment vs patients with BCL vaccinated >9 months after the last treatment also provided a significantly different result (P = .0005; 95% CI, 0.14-0.46).
In our cohort of patients with BCL who were actively receiving B-cell–depleting agents or were within 9 months of completing B-cell–directed therapy, only 6 developed antibodies against the SARS-CoV-2 virus, regardless of the type of vaccine used.
Our findings, similar to those reported in chronic lymphocytic leukemia17 and multiple myeloma,18 raised concerns about the efficacy of the COVID-19 vaccines in generating humoral immunity at the current dose schedule for BCL, and prompted us to notify our patients who had negative results and counsel them to continue protective measures against SARS-CoV-2.
Our data suggest that SARS-CoV-2 vaccination at least 9 months from the last B-cell–directed treatment may result in improved antibody titers. The higher titer of IgA in this latter group also reflects B-cell reconstitution and active isotype class switching. This finding is important for further establishing a possible timeline for revaccination of our patients. This revaccination approach warrants evaluation in a research protocol, with further thoughts for patients receiving long-term therapy with B-cell–depleting agents. Studies are also needed to further evaluate the T-cell repertoires in this population and to expand the number of patients with lymphoma who are analyzed.19 Our data highlight the importance for household members and other close contacts, as well as the community at large, to be immunized to establish the herd immunity that will protect immunocompromised patients.
Acknowledgments
The authors thank Suzanne Hess for excellent editorial work on the draft of the manuscript.
Footnotes
Original data are available by e-mail request to the corresponding author (francisco.hernandez@roswellpark.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
Following a similar report on multiple myeloma, Ghione and colleagues report the expected observation that patients with non-Hodgkin lymphoma (NHL) receiving anti-B cell therapies have markedly reduced antibody responses to COVID-19 immunization. Although there is no information regarding T-cell immunity, this suggests that while vaccination is certainly still recommended for this population, patients should be strongly encouraged to maintain social distancing precautions and should be revaccinated after an appropriate interval from the end of their antilymphoma therapy.
Authorship
Contribution: P.G., F.J.H.-I., B.H.S., and E.A.G. designed the study; P.G., F.J.H.-I., P.T., S.S., M.J., R.T., K.M., A.D., J.D., J.K., A.M., and L.R.. enrolled the patients in the study; J.J.G., C.M., M.N., L. Suresh, V.R., L. Shen performed the laboratory analyses; P.G. and K.A. analyzed the data; P.G., F.J.H.-I., P.T., E.A.G., V.R., B.H.S. wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Supplementary Material
REFERENCES
- 1.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 2.Matsushita K, Ding N, Kou M, et al. The relationship of COVID-19 severity with cardiovascular disease and Its traditional risk factors: a systematic review and meta-analysis. Glob Heart. 2020;15(1):64. doi: 10.5334/gh.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passamonti F, Cattaneo C, Arcaini L, et al. ITA-HEMA-COV Investigators Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383(23):2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadoff J, Gray G, Vandebosch A, et al. ENSEMBLE Study Group Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenig A, Ishay Y, Kharouf F, Rubin L. Treatment of B-cell depleted COVID-19 patients with convalescent plasma and plasma-based products. Clin Immunol. 2021;227:108723. doi: 10.1016/j.clim.2021.108723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kridin K, Ahmed AR. Post-rituximab immunoglobulin M (IgM) hypogammaglobulinemia. Autoimmun Rev. 2020;19(3):102466. doi: 10.1016/j.autrev.2020.102466. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N Engl J Med. 2008;359(6):613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 12.Kessel A, Rosner I, Toubi E. Rituximab: beyond simple B cell depletion. Clin Rev Allergy Immunol. 2008;34(1):74–79. doi: 10.1007/s12016-008-8074-1. [DOI] [PubMed] [Google Scholar]
- 13.Anolik JH, Friedberg JW, Zheng B, et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122(2):139–145. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Muramatsu M, Yoshida R, Yokoyama A, et al. Comparison of antiviral activity between IgA and IgG specific to influenza virus hemagglutinin: increased potential of IgA for heterosubtypic immunity. PLoS One. 2014;9(1):e85582. doi: 10.1371/journal.pone.0085582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Lorenzi JCC, Muecksch F, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021;13(577):eabf1555. doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577):eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in elderly myeloma patients after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674–3676. doi: 10.1182/blood.2021011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.