Abstract
Inducible defences in phytoplankton are often assumed to come at a cost to the organism, but trade-offs have proven hard to establish experimentally. A reason for this may be that some trade-off costs only become evident under resource-limiting conditions. To explore the effect of nutrient limitation on trade-offs in toxin-producing dinoflagellates, we induced toxin production in Alexandrium minutum by chemical cues from copepods under different levels of nitrogen limitation. The effects were both nitrogen- and grazer-concentration dependent. Induced cells had higher cellular toxin content and a larger fraction of the cells was rejected by a copepod, demonstrating the clear benefits of toxin production. Induced cells also had a higher carbon and nitrogen content, despite up to 25% reduction in cell size. Unexpectedly, induced cells seemed to grow faster than controls, likely owing to a higher specific nutrient affinity due to reduced size. We thus found no clear trade-offs, rather the opposite. However, indirect ecological costs that do not manifest under laboratory conditions may be important. Inducing appropriate defence traits in response to threat-specific warning signals may also prevent larger cumulative costs from expressing several defensive traits simultaneously.
Subject terms: Ecology, Microbial ecology
Introduction
Dinoflagellates of the genus Alexandrium produce neurotoxic alkaloids collectively known as paralytic shellfish toxins (PST). The toxins are efficient sodium-channel blockers and among the most potent toxins known [1]. The intracellular toxin content is upregulated in response to the level of threat from zooplankton grazers [2] and, while debated, toxicity as a defence mechanism against grazers is the favoured explanation for the evolution of algal toxins [3–6].
Studies dedicated to defence mechanisms in phytoplankton often focus on the benefits of the defence, but rarely establish potential costs [7]. So far, experimental assessments have suggested toxin production trade-offs to be insignificant. The growth rate of toxic and non-toxic strains of the same species, or grazer-induced versus non-induced cells with very different toxin contents appears to be identical [2, 8, 9]. Blossom et al. [10] compared several species and strains of Alexandrium spp. and did not find any correlation between growth rate and toxin production under light-replete conditions, and even a positive correlation under limiting light. Brown & Kubanek [11] recently demonstrated a negative relation between toxin content and growth rate in Alexandrium minutum exposed to lysed cells of various other species of dinoflagellates, thus suggesting a trade-off. However, the correlation may also result from allelochemical substances in the lysed cells reducing growth [12]. Many dinoflagellates produce such dissolved allelochemicals that reduce the growth rate of other cells [13] and when growth is reduced cells commonly become more toxic [14]. Significant costs of predator-induced toxin production have so far only been convincingly demonstrated in diatoms that produce domoic acid, but here the benefits of the toxins are still debated [15]. However, ecological theory predicts associated costs; otherwise, non-defended species or strains would be outcompeted and only defended species would persist. Also, toxin production is inducible, i.e. it is upregulated in the presence of grazer cues, as seen in Alexandrium spp. dinoflagellates [2, 16, 17] and some toxic strains of the diatom Pseudo-nitzschia [15, 18]. According to optimal defence theory, inducible defences are favoured when predation risks vary in time and defence costs are significant [19, 20]. While these costs have likely been reduced through evolution, the wide variety of inducible defences found in both marine and terrestrial organisms suggests the presence of influential trade-offs to any beneficial defensive trait [21–24].
The failure of experiments to demonstrate costs may be due to the fact that experimental assessments have often been done under resource-replete conditions, while costs may be more significant when resources are limited [7, 20, 25–27]. The PST molecules are high in nitrogen with N:C ratio 4.6 times higher than average phytoplankton materials [28]. Numerous studies have shown cell toxin content to be low in nitrogen-depleted cells [29, 30] even when exposed to a grazer threat [9]. Trade-off costs may be trivial when nutrients and light are plentiful, but when available nitrogen is limiting and grazer biomass high, a fitness-optimization resource-allocation model predicts a significant growth penalty to toxin production [31]. Nitrogen limitation in temperate shelf regions generally occurs during the summer months [32, 33], and coincidentally it is during these months that defence is most needed due to peaking copepod biomass [33, 34].
Here, we quantify the benefits and costs of toxin production in Alexandrium minutum under different degrees of nitrogen limitation using both a chemostat approach and classical batch experiments. The compounds from zooplankton that trigger toxin production are known [35], and we use them for precise manipulation of toxin production without confounding effects of grazing on fitness estimates. The efficiency of the defence is estimated by video recording the response of copepods to induced and non-induced cells. Following the predictions of the model of Chakraborty et al. [31], we hypothesize that the costs of increased toxicity of induced cells will be the highest at intermediate nitrogen limitation, and that cells grown in excess of nitrogen will reap full benefit while paying negligible costs.
Materials and methods
Phytoplankton
Alexandrium minutum strain GUMACC 83 was grown in B1 medium [36] at salinity 26, 18 °C, and an irradiance of ca. 150 µmol photons m−2 s−1 on a 12:12 light:dark cycle. To reduce the carry-over of nitrogen from the stock culture, we diluted cells in B1 with reduced NO3− (80 µM) for two weeks prior to inoculation and used cells that were close to the end of the exponential phase.
Batch-culture experiment
Six replicate batch cultures of A. minutum were initiated at ca. 200 cells mL−1 in 2 L blue-cap glass flasks exposed to ca. 150 µmol photons m−2 s−1 on a 12:12 light:dark cycle and constant temperature of 18 °C. We used modified B1 medium with reduced nitrogen concentration (60 µM NO3−) to make sure that the cells eventually would be limited by nitrogen rather than other resources. The cultures were gently bubbled to avoid high pH-limiting growth. pH was monitored using a PHM220 Lab pH meter (Radiometer Analytical, Lyon, France). Three bottles were treated with copepodamides to induce increased toxin production [35] and three were used as controls. Copepodamides were extracted from freeze-dried Calanus finmarchius (Calanus AS, Tromsø, Norway) as described in Selander et al. [35] and added to the cultures by coating the inside of the bottle with a copepodamide mixture dissolved in methanol to a final concentration of 2 nM. Due to slow release and rapid degradation, the average effective concentration is around 1–2% of the added concentration [18]. The methanol was evaporated using N2 gas and the cultures transferred to the bottles after gentle mixing. Cultures were gently transferred to a freshly coated flasks every 24 h during the treatment period to assure a continuous exposure to the cues [18]. The controls received the same treatment but with methanol without copepodamides. Samples were taken every or every second day for cell abundance and nitrogen concentration, while samples for toxin analysis and cellular carbon and nitrogen were taken at inoculation and in conjunction with the six video experiments (see below) during the course of the experiment. Initial samples of cellular toxin-, carbon-, and nitrogen content were taken from the stock culture.
Chemostat experiments
While nutrient concentration declines over time in batch cultures, they are near constant in a continuous culture, thus allowing us to examine costs and benefits of grazer induction at constant concentrations of nutrients. Dinoflagellates do not tolerate vigorous mixing [37], so a classical chemostat cannot be used. Instead, we used exponentially fed batch cultures [38]. The exponentially fed batch culture (hereafter referred to as ‘chemostat’) is similar to a chemostat, except that there is no continuous outflow. The volume is instead reduced to the initial volume at each sampling occasion after gently mixing the culture. Growth medium is added continuously in a constant proportion of the increasing volume of the culture by exponentially increasing the inflow using a computer-controllable multichannel peristaltic pump (IPC 16, Ismatec, Wertheim, Germany).
Six replicate chemostat cultures of A. minutum were set up as in 1-L blue-cap glass bottles as described above. Depending on the dilution rate (DR), the initial culture volume varied between 250 and 500 mL. Four different DR were used to vary cell growth rate: 0.05, 0.10, 0.20, and 0.40 d−1. The inflow medium was B1 with reduced (80 µM) NO3− in all the experiments, except the first one (0.10 d−1 DR) where the NO3− concentration was 30 µM. We increased the nitrogen concentration in the subsequent experiments as this allowed more cells for analyses and because pH limitation turned out not to be a problem. The resulting ambient nitrogen concentration is independent on nitrogen content of the inflow medium (Appendix 1). The cultures were gently bubbled and pH was measured at each sampling occasion. At each DR, the cultures were allowed 7–10 days to achieve steady-state before starting the experimental treatment. In some cases, perfect steady-state was not achieved.
The cultures were exposed to copepodamides daily as described above, using a nominal concentration of 0.63 nM. For the 0.2 d−1 DR, a second experiment was run with higher copepodamide concentration of 6 nM to analyse the effect of increased exposure to grazers. Samples for analysis of cell abundance and size, cell toxin content, cellular carbon and nitrogen, dissolved inorganic nitrogen, and copepod rejection rate were taken daily or every 2‒3 days during the 6‒10-day treatment period. Using the chemostat equations (Appendix 1) and assuming a maximum growth rate of 0.5 d−1 [39] and a half-saturation constant for nitrate of 0.5 μM [40], the resulting nitrate concentration in the cultures should range from severe limitation to saturation. Thus, while nutrient concentration decreases over time in batch culture, it is more constant and controlled by the DR in chemostats.
In the chemostats, the growth rate is controlled by the DR and any growth rate response will manifest as a change in the steady-state concentrations of cells and nutrients. Thus, if induced toxin production causes lower growth or nutrient affinity, the steady-state concentration of nutrients will increase and cell density decrease. The magnitude of the response can be computed from the chemostat equations (Appendix 1).
Cell counts and cell size
Cell concentrations were determined in acid Lugol (1%) fixed samples. All cells in 1 mL or at least 400 cells were counted per replicate in a Sedgewick–Rafter chamber using an inverted microscope (Olympus, Tokyo, Japan). Twenty random cells from each sample were measured at 400× magnification (width–length) and cell volume was estimated by assuming a prolate-spheroid shape [41]. Cell growth was calculated from temporal differences in cell concentration assuming exponential growth. The DR was accounted for when calculating growth in the chemostat experiments.
Nitrate analysis
Subsamples for nitrate analysis were filtered through a 0.2-µm syringe filter, and stored frozen at ‒20 °C until analysis. Nitrate was analysed by reduction to NOx with VCl3 as the reducing reagent [42] on a Smartchem 200 (AMS Alliance, Rome, Italy). Concentrations below 0.5 µM were measured using an extended cuvette (100 mm, FireflySci, New York, New York, USA) by UV–VIS spectrophotometry.
Toxin analysis
Samples (10–120 mL) for cellular toxin contents were filtered onto 25-mm Whatman GF/F glass fibre filters and frozen at −20 °C until extraction. A total of 750 µL of 0.05 M acetic acid was added and samples were subjected to three freeze–thaw cycles to lyse cells. The extracts were filtered (GF/F) and stored at −20 °C until analysis. The samples from the batch experiment and the 0.2 d−1 DR experiments (both low and high dose) were analysed with isocratic ion-pair chromatography followed by post-column derivatization and fluorescent detection [43]. We used a reversed phase C18 column (150 × 4 mm C18, 5 µm, Dr. Maisch GmBH, Ammerbuch, Germany). Samples from the 0.05, 0.10, and 0.40 d−1 DR experiments were separated on an Agilent 1200 HPLC (ZIC-HILIC, 2.1 × 150 mm, 5 µm, Merck KGaA, Darmstadt, Germany) and analysed by tandem mass spectrometry on a triple-quadrupole instrument (Agilent 6470) following the methods described in Ref. [44].
This particular strain of A. minutum is known to only produce gonyautoxins (GTX) 1‒4 [2, 45]. GTX standards 1‒4 were obtained from the Certified Reference Materials Program at National Research Council Canada (Halifax, Canada).
Cellular carbon and nitrogen
Samples for cellular C and N were filtered onto pre-combusted (550 °C, 2 hours) 13-mm GF/C filters, packed in tin capsules and dried for 24 hours at 60 °C. The samples were kept dry at room temperature in a desiccator until analysis with a Thermo Scientific Flash 2000 Organic Elemental Analyzer (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Copepod feeding response
We directly observed individual copepod-cell interactions and recorded the fraction of captured cells that were rejected. We used the feeding-current feeding copepod Temora longicornis from a continuous culture that was maintained on a phytoplankton diet consisting of Rhodamonas salina, Thalassioria weissflogii, Heterocapsa triquetra, and Oxyrrhis marina.
Adult female copepods were tethered to a human hair by their dorsal surface using cyanoacrylate-based super glue [46]. The tethered copepods were starved overnight in darkness at the same temperature (18 °C) and salinity (26) as the cultures before being used for experiments. The tethered copepods are seemingly unaffected by the tethering and can live for many days while feeding, defecating, and producing eggs.
The feeding experiments took place in darkness. The untethered end of the hair was glued to a needle attached to a micromanipulator. The copepod was submerged in a 10 × 10 × 10 cm3 aquarium and Alexandrium cells were added to a final concentration of 100–200 cells mL−1. The experiment started when cells were added. The water was gently mixed by a magnetic stirrer to keep the cells suspended. Three 10-min sequences (0–10 min, 25–35 min, and 50–60 min) of 24-fps footage were recorded during a 1-h period using a high-speed camera (Phantom V210; Vision Research, Wayne, New Jersey, USA) connected to a computer. The camera was equipped with lenses to get a field of view of 1.3 × 1 mm2. The video sequences were analysed to quantify prey capture, ingestion, and the fraction of cells that were rejected by the copepod [46]. A new copepod was used for each replicate culture.
Statistical analysis
The effect of the copepodamide treatment in the batch-culture experiment was analysed using a generalized additive mixed model (GAMM) in the ‘gamm4’ R package [47]. ‘Treatment’ and ‘Time’ were used as fixed effects and ‘Replicate’ as the random effect.
To analyse the effect of the copepodamide treatment in the chemostat experiments, we used a linear mixed-effects model with ‘Time’, ‘Treatment’, and ‘Dilution rate’ as fixed effects, and ‘Replicate’ as the random effect, in the ‘lmerTest’ R package [48]. The analysis of the repeated ‘High’ copepodamide-dose experiment was done separately. The Akaike information criterion was used to select the model that best fits the data. In case of a significant interaction between ‘Dilution rate’ and ‘Treatment’ a posthoc test was made via pairwise comparisons by estimated marginal means using the Satterthwaite degrees-of-freedom method. The random-effect-variance component was close to zero for some variables, but retained in the model to incorporate the dependency of the response variable on the replications. Some variables were log-transformed to homogenize variances. Statistical tests were considered significant at the 0.05 level and are summarized in Appendix Tables S1, S2, S3 and S4.
Results
Batch-culture experiment
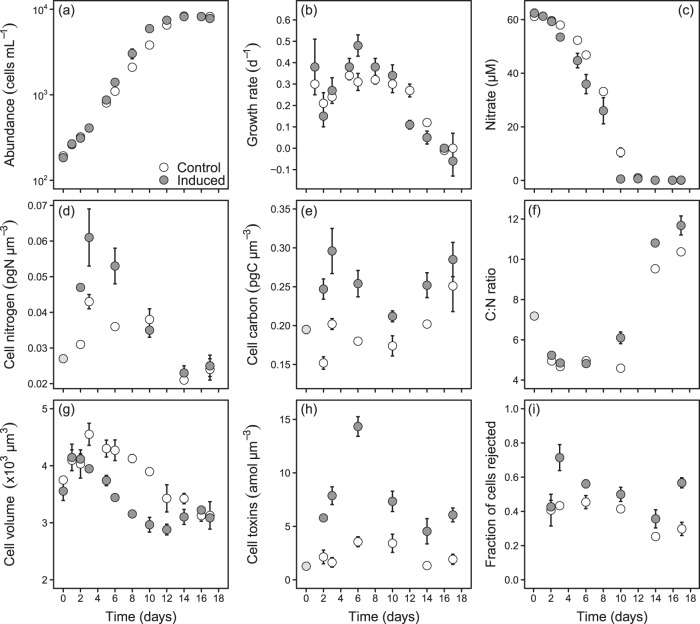
Cell abundance in the grazer-induced cultures increased faster than controls during the exponential phase (days 3‒12; GAMM, F = 14.0, p < 0.001) and reached the stationary phase after around 14 days as the inorganic nitrogen in the cultures became depleted (Fig. 1a, c). The available nitrate in the culture medium was used up at a significantly higher rate in the induced treatment (Fig. 1c; GAMM, F = 9.7, p = 0.003), because cell accumulation rate in terms of cellular nitrogen was also faster in induced than in non-induced cultures (Appendix 2 Fig. S1; GAMM, F = 11.0, p = 0.003). Cellular nitrogen content and cell sizes initially increased and then decreased as nutrients were exhausted and growth ceased, but induced cells had a significantly higher nitrogen content (Fig. 1d) and were significantly smaller (Fig. 1g, Appendix 2 Table S1) than non-induced cells during the exponential growth phase but converged with control cells after 10 and 16 days, respectively. Cellular carbon content was significantly higher in induced than in non-induced cells (Fig. 1e, Appendix 2 Table S1), resulting also in a faster accumulation of biomass during the exponential phase (Appendix 2 Fig. S1; GAMM, F = 61.8, p < 0.001). The differences in cellular C and N contents between induced and non-induced cultures on a per-cell level showed the same pattern, but were less pronounced (Appendix 2 Fig. S1). The carbon-to-nitrogen ratio also increased in response to nitrate depletion but more markedly so in induced treatments (Fig. 1f). Overall, cellular nitrogen content increased and cellular carbon content decreased with increasing growth rate and the contents of both nitrogen and carbon were higher in induced cells (Fig. 2, Appendix Table S2).
Fig. 1. Changes over time in the batch-culture experiment.
(a) cell abundance (cells mL−1), (b) growth rate (d−1), (c) culture nitrate concentration (µM), (d) cell nitrogen content (pg N µm−3), (e) cell carbon content (pg C µm−3), (f) C:N ratio, (g) cell volume (µm−3), (h) cell toxin content (amol µm−3), and (i) the fraction of cells rejected by copepods. The light-grey points in (d), (e), (f), and (h) are initial values from the stock culture. Values are means and error bars are standard error (n = 3).
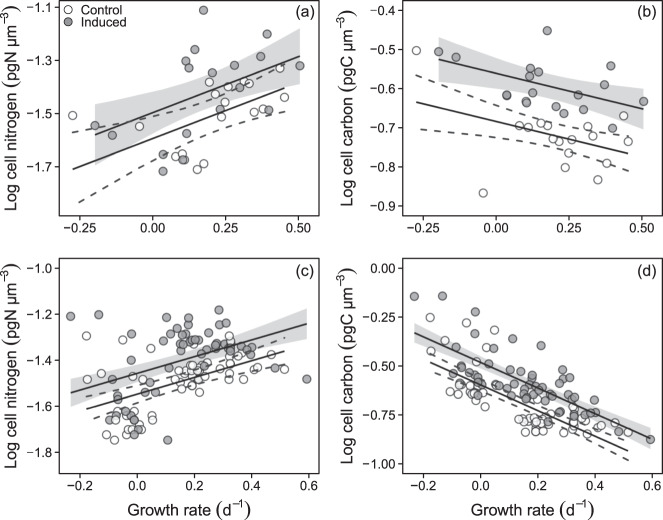
Fig. 2. Relation between cellular nitrogen (N, pg N µm-3) or cellular carbon (C, pg C µm−3), and growth rate (GR, d−1).
(a, b) batch-culture and (c, d) chemostat experiments. Growth rate is calculated from change in biovolume. Multiple linear regression (with 95% confidence intervals) was fit to the data (a) control: log cell N = − 1.594 + 0.420 × GR, induced: log cell N = − 1.497 + 0.420 × GR (R2 = 0.27, p = 0.002); (b) control: log cell C = − 0.684 − 0.181 × GR, induced: −0.561 − 0.181 × GR (R2 = 0.539, p < 0.001); (c) control: log cell N = − 1.546 + 0.363 × GR, induced: −1.456 + 0.363 × GR (R2 = 0.28, p < 0.001); and (d) control: log cell C = − 0.594 – 0.654 × GR, induced: −0.479 – 0.654 × GR (R2 = 0.61, p < 0.001).
Cell toxicity peaked after 6 days of exposure in the induced treatments with 400% higher GTX content than controls, after which it decreased throughout the rest of the exponential phase (Fig. 1h). Toxin production essentially reached zero after 14 days but cell toxicity remained stable at around 5‒7 amol µm−3 due to the low cell division rates. In the control treatment, cell toxicity followed the same temporal patterns but was lower throughout than in induced cells (Fig. 1h). Finally, a significantly higher fraction of induced than non-induced cells was rejected by copepods, demonstrating a clear benefit (Fig. 1i).
Chemostat experiments
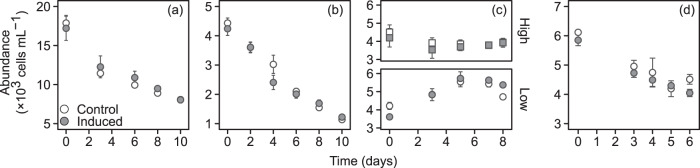
Growth rate in the 0.05, 0.10, and 0.40 d−1 DR experiments was lower than the DR and cell concentrations thus decreased over time (Fig. 3). It was only in the two 0.20 d−1 DR experiments that the cells were able to keep up with the DR (Fig. 3c, Fig. 4a). However, growth rates calculated from cell-bound nitrogen values per culture volume (µg N mL−1) were nearly constant over time at the three lowest DR, and the growth rates were similar to the DR, except at the lowest and highest DR (Fig. 4b, Appendix 2 Fig. S2). The small differences in cell concentrations and the low sensitivity of estimates of affinity and maximum growth rate parameters to changes in cell concentration at low DR makes the estimation of these parameters meaningless (Appendix 1).
Fig. 3. Change in cell abundance (cells mL−1) in the chemostats at the different dilution rates.
(a) 0.05 d−1, (b) 0.10 d−1, (c) 0.20 d−1 with high (6 nM) and low (0.63 nM) dose of copepodamides, (d) 0.40 d−1. The values are means and error bars show standard error (n = 3). Note the different y-axis scales.
Fig. 4. Summary of the chemostat experiments.
(a) cell growth rate (d−1), (b) N-specific growth rate (d−1), (c) cell volume (×103 µm3), (d) cell nitrogen (pg N µm−3), (e) cell carbon (pg C µm−3), (f) C:N ratio, (g) cell toxin content (amol µm−3), and (h) the fraction of cells rejected by copepods. Values are averaged over time during the treatment period and error bars show standard deviation (n = 4 in 0.05, 0.20, and 0.40 d−1; n = 5 in 0.10 d−1; n = 3 in 0.10 d−1 C/N measurements). Asterisks above bars indicate significant differences between treatments within dilution rates (***p < 0.001, **p < 0.01, *p < 0.05). In (h) and all 0.2 d−1 high they indicate significant effect of the ‘Treatment’ factor. Further statistical analysis is reported in the appendix.
Consistent with the results of the batch-experiment results, induced cells were significantly smaller than non-induced cells at intermediate DR but similar at the lowest and highest rates. (Fig. 4c). Cellular carbon increased and nitrogen contents decreased with growth rate and both were significantly higher in induced than non-induced cells, particularly at intermediate DR (Fig. 4d, e, Fig. 2). Cellular C:N ratio varied inversely with DR and was slightly higher in induced than non-induced cells, all again consistent with the patterns in the batch experiment (Fig. 4f, Fig. 2). As in the batch experiment, effects were similar but less pronounced when expressed on a per-cell basis (Appendix 2 Table S5, S6). The effect of varying the copepodamide dose from low (0.63 nM) to high (6 nM) in the 0.2 d−1 DR experiment had a significant effect on (reduced) cell volume and also further increased toxin content relative to the controls (Fig. 4c, g; Appendix 2 Table S4), consistent with the result of a more comprehensive dose–response experiment (Appendix 3; Appendix 3. Fig. S1).
Cell toxin content increased in all but the 0.05 and 0.10 d−1 DR experiments in response to copepodamides (Fig. 4g). Consequently, the copepods generally rejected a larger fraction of induced cells, except at the lowest DR (Fig. 4h; Appendix 2 Table S3, S4). Overall, the fraction of rejected cells increased with increasing toxin content but the effect saturates at a rather low toxin content of ca. 10 amol GTX µm−3 (Fig. 5).
Fig. 5. Relation between copepod rejection rate and cell toxin content (GTX) normalized by volume (amol µm−3).

Data are from both batch culture and chemostat experiments. The regression line (with 95% confidence intervals) is Rejection = 0.320 + 0.219 × log GTX (R2 = 0.42, p < 0.001). Due to odd behaviour, data from the 0.10 d−1 chemostat experiment (triangles) are not included in the regression. Including them does not change the significant effect (R2 = 0.12, p < 0.001).
Discussion
We set out to quantify the costs and benefits of toxin production in a dinoflagellate by comparing the performance of cells induced to express their defence with those that were not under different degrees of nitrogen limitation. Our experiments produced decreasing growth rates with increasing nitrogen limitation in both batch and chemostat cultures (Fig. 2a, c), and high C:N ratios in N-limited cells (Fig. 1f, Fig. 4f). We have utilized that toxin production in many dinoflagellates, including A. minutum, can be induced by grazer cues [2, 35], thus allowing us to examine the same strain at different toxin-production rates. This is important because different strains of the same species may differ in many traits, including in their toxin profiles [45, 49]. We also note that we examined the ‘private-good’ [50] grazer-deterrent effect of the defence at the level of the individual. That is, we quantify the benefits that only the individual cell that produces the toxin may benefit from. This is different from any toxic effects on the copepod that reduce its ability to graze on further cells, which is a ‘public good’, as also cells not producing the toxin may benefit [50]. We had predicted that both benefits and costs would be small in nutrient-starved cells, that benefits would be large but costs relatively small in nutrient-replete cells and that both benefits and costs would be high at intermediate nitrogen levels. In line with our hypothesis, toxin induction was highest at intermediate nitrogen limitation, but we found no evidence of direct costs in terms of reduced growth rate.
Defence trade-offs
The benefits of toxin production were clear and largely followed the pattern predicted. Moreover, the results were consistent between the two types of experiments: induced cells have up to three times higher chance of being rejected by the copepod than non-induced cells, and the chance of rejection was positively correlated to the toxin content of the cells. This confirms previous reports of reduced grazing on induced dinoflagellates [2], but the demonstration at the individual cell level is novel. It is well established that nitrogen-starved cells produce no or very little toxins [9, 51] and, hence, gain little or no defensive benefits. In the batch experiment, cells remained toxic even in the stationary phase due to low cell division rate, but they did not produce new toxins. With low toxin content but high rejection fraction, the 0.1 d−1 DR experiment shows some odd behaviour. Thus, we cannot exclude the possibility that other defences, such as allelochemicals [52, 53], are also at play.
The efficiency of the defence was not as high as that reported for other strains and species of Alexandrium spp. Thus, Xu and Kiørboe [5] found that >90% of the cells of some toxic Alexandrium species/strains were rejected by a copepod, but also that other strains containing toxins were readily consumed by the copepod. Neither Xu et al. [46] nor Teegarden et al. [54] were consequently able to relate the efficiency of the defence to the composition and concentration of specific toxins in the cells of Alexandrium spp. Here, we have established a direct correlation between the cells’ content of the GTX toxin and the efficiency of the defence in the same strain of Alexandrium minutum.
It has been notoriously difficult to demonstrate the cost part of defence trade-offs in phytoplankton [7], and this study is no exception, despite a novel approach. Ideally, ‘costs’ should be quantified in terms of reduced cell division rate. We found no reduction in the growth rate nor in nutrient affinity of the cells, even at nutrient-deplete conditions. The proportion of cellular nitrogen invested in toxins increased the more toxic the cells were (Appendix 2 Fig. S3), but this did not affect the growth rate.
However, we document a number of very clear effects of induction in addition to enhanced toxin production, i.e. elevated cellular contents of C and N, a reduction in cell size, and even an increase in cell division rate, with the effects being most pronounced at intermediate nutrient levels. The responses are consistent between the batch and the continuous cultures.
The expectation of reduced cell division rate of grazer-induced, nitrogen-limited cells is based on the nitrogen requirements for PST production in Alexandrium tamarense as worked out by Chakraborty et al. [31]. However, A. tamarense produces 1–2 orders-of-magnitude more toxins than the A. minutum used here. Thus, the biochemical syntheses costs and the N requirements for toxins are correspondingly smaller in our experiment and the higher N-uptake of induced cells cannot be explained by direct requirements for toxin production.
It is well established that cell size decreases when nutrient is limited [55, 56], as also seen most clearly in the batch experiment (Fig. 1g). However, cell volume shrinks in response to grazer cues by up to 25% relative to non-induced cells. This has two implications. First, a smaller cell volume results in a higher concentration of toxins in the cells. It is reasonable to assume that the copepods respond to the concentration rather than the contents of toxins, and the shrinking of the cells may therefore be adaptive and part of the defence. A similar consistent response in cell size to grazer cues has been found in four species of diatoms [24]. For the diatoms, smaller cell sizes imply higher concentrations of biogenic silica and, therefore, a stronger protective shell that makes the cells less susceptible to copepod grazing [23].
The second potential implication of cell shrinking is a higher specific affinity for dissolved nutrients. To the first order, specific affinity scales inversely with cell radius due to the nature of molecular diffusion [57], and it is well established experimentally that the volume-specific nutrient uptake indeed increases with decreasing cell size [58, 59]. Thus, a 25% decrease in cell volume corresponds to an 8% decrease in radius and a corresponding increase in specific affinity. This, in fact, may account for the elevated nitrogen uptake, nitrogen content, and growth rate of induced cells when cells start being nutrient limited, as most clearly seen in the batch experiment (Fig. 1). If the decrease in cell size is an adaptation to increased toxin concentration, then the elevated nitrogen assimilation and growth rate of induced cells is a secondary and beneficiary effect.
The increased carbon content found in induced cells may be due to thickening of their thecal plates, providing them with another possible defence. It is unclear if this has an effect on the copepods, but it has been shown that diatoms that increase their silica shell thickness experience reduced grazing from both juvenile and adult copepods [23].
Ecological and indirect costs of defence
While we were unable to demonstrate direct costs of toxin production even in nitrogen-starved cells, defences may come with indirect ecological costs that do not manifest in simplified laboratory settings [21]. This includes, e.g. increased sinking rate or reduced swimming speed that may inflict fitness costs in nature [60, 61]. A possible ecological cost of the reduced cell size recorded here is elevated predation risk. In general, mortality rate of plankton organisms scales inversely with their volume to power 0.25 [62], and a 25% decrease in volume thus implies a 7.5% increase in predation mortality from other predators than copepods. Copepods and other larger herbivores are probably the most important grazers on dinoflagellates, thus >50% decrease in copepod grazing pressure of induced cells more than outweighs the cost in most situations, and toxin production increases the fitness of the cell.
Theory of defences predicts that defences should only be inducible if they are associated with a cost [63]. The number of studies unable to detect costs associated with induced toxin production in phytoplankton suggests that additional factors may be at play. Recent advancements in genome sequencing reveal that a substantial part of the genome may be dedicated to secondary metabolism, up to one-fifth in some cyanobacteria [64]. Keeping a single biosynthetic pathway active may inflict a very limited cost, whereas the cost for constant activation of one-fifth of the genome will be substantial [65]. Thus, the evolution of inducible defence may be driven not by the allocation of resources to a single pathway, but the necessity to avoid allocation to all defence systems simultaneously. This is but a corollary hypothesis to the optimal defence theory, but one that may explain the lack of detectable costs in some induced responses to herbivory.
In conclusion, we found a diverse nutrient-dependent response of a dinoflagellate to copepod cues: increased toxicity with implied lower predation risk, higher cellular contents of carbon and nitrogen, reduced cell size, and higher growth rate. Most of these responses may be beneficial to the cells, while we found no indication of direct costs. Because dinoflagellates are not Darwinian demons, the necessary costs are most likely indirect or ecological that are apparent only in nature.
Supplementary information
Acknowledgements
The Centre for Ocean Life is supported by the Villum Foundation. ES was funded by Swedish Research Council VR no. 2019-05238. We thank Benni Winding Hansen for CHN measurements, Jack Melbye for maintaining copepod cultures, Colin Stedmon for assistance with NO3- measurements, Josephine Grønning for copepodamide extractions, Aurore Maureaud for providing the script for the mixed model, Daniël van Denderen for providing statistical assistance, and Per Juel Hansen for rewarding discussions.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41396-021-00908-y.
References
- 1.Llewellyn LE. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat Prod Rep. 2006;23:200–18. doi: 10.1039/b501296c. [DOI] [PubMed] [Google Scholar]
- 2.Selander E, Thor P, Toth G, Pavia H. Copepods induce paralytic shellfish toxin production in marine dinoflagellates. Proc R Soc B. 2006;273:1673–80. doi: 10.1098/rspb.2006.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner JT, Tester PA. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol Oceanogr. 1997;42:1203–13. [Google Scholar]
- 4.Smetacek V. A watery arms race. Nature. 2001;441:745. doi: 10.1038/35081210. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Kiørboe T. Toxic dinoflagellates produce true grazer deterrents. Ecology. 2018;99:2240–9. doi: 10.1002/ecy.2479. [DOI] [PubMed] [Google Scholar]
- 6.Cusick KD, Widder EA. Bioluminescence and toxicity as driving factors in harmful algal blooms: ecological functions and genetic variability. Harmful Algae. 2020;98:101850. doi: 10.1016/j.hal.2020.101850. [DOI] [PubMed] [Google Scholar]
- 7.Pančić M, Kiørboe T. Phytoplankton defence mechanisms: traits and trade-offs: defensive traits and trade-offs. Biol Rev. 2018;93:1269–303. doi: 10.1111/brv.12395. [DOI] [PubMed] [Google Scholar]
- 8.John EH, Flynn KJ. Growth dynamics and toxicity of Alexandrium fundyense (Dinophyceae): the effect of changing N:P supply ratios on internal toxin and nutrient levels. Eur J Phycol. 2000;35:11–23. [Google Scholar]
- 9.Selander E, Cervin G, Pavia H. Effects of nitrate and phosphate on grazer-induced toxin production in Alexandrium minutum. Limnol Oceanogr. 2008;53:523–30. [Google Scholar]
- 10.Blossom HE, Markussen B, Daugbjerg N, Krock B, Norlin A, Hansen PJ. The cost of toxicity in microalgae: direct evidence from the dinoflagellate Alexandrium. Front Microbiol. 2019;10:1065. doi: 10.3389/fmicb.2019.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown ER, Kubanek J. Harmful alga trades off growth and toxicity in response to cues from dead phytoplankton. Limnol Oceanogr. 2020;65:1723–33.
- 12.Windust AJ, Wright JLC, McLachlan JL. The effects of the diarrhetic shellfish poisoning toxins, okadaic acid and dinophysistoxin-1, on the growth of microalgae. Mar Biol. 1996;126:19–25. [Google Scholar]
- 13.Legrand C, Rengefors K, Fistarol GO, Granéli E. Allelopathy in phytoplankton—biochemical, ecological and evolutionary aspects. Phycologia. 2003;42:406–19. [Google Scholar]
- 14.John E, Flynn K. Modelling changes in paralytic shellfish toxin content of dinoflagellates in response to nitrogen and phosphorus supply. Mar Ecol Prog Ser. 2002;225:147–60. [Google Scholar]
- 15.Lundholm N, Krock B, John U, Skov J, Cheng J, Pančić M, et al. Induction of domoic acid production in diatoms—types of grazers and diatoms are important. Harmful Algae. 2018;79:64–73. doi: 10.1016/j.hal.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Bergkvist J, Selander E, Pavia H. Induction of toxin production in dinoflagellates: the grazer makes a difference. Oecologia. 2008;156:147–54. doi: 10.1007/s00442-008-0981-6. [DOI] [PubMed] [Google Scholar]
- 17.Griffin JE, Park G, Dam HG. Relative importance of nitrogen sources, algal alarm cues and grazer exposure to toxin production of the marine dinoflagellate Alexandrium catenella. Harmful Algae. 2019;84:181–7. doi: 10.1016/j.hal.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Selander E, Berglund EC, Engström P, Berggren F, Eklund J, Harðardóttir S, et al. Copepods drive large-scale trait-mediated effects in marine plankton. Sci Adv. 2019;5:eaat5096. doi: 10.1126/sciadv.aat5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhoades DF. Evolution of plant chemical defense against herbivores. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press;1979. p 1–55.
- 20.Karban R. The ecology and evolution of induced resistance against herbivores: induced resistance against herbivores. Funct Ecol. 2011;25:339–47. [Google Scholar]
- 21.Strauss SY, Rudgers JA, Lau JA, Irwin RE. Direct and ecological costs of resistance to herbivory. TREE. 2002;17:278–85. [Google Scholar]
- 22.Agrawal AA. Current trends in the evolutionary ecology of plant defence. Funct Ecol. 2011;25:420–32. [Google Scholar]
- 23.Pančić M, Torres RR, Almeda R, Kiørboe T. Silicified cell walls as a defensive trait in diatoms. Proc R Soc B. 2019;286:20190184. doi: 10.1098/rspb.2019.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grønning J, Kiørboe T. Diatom defence: grazer induction and cost of shell‐thickening. Funct Ecol. 2020;34:1790–1801.
- 25.Kiørboe T, Andersen KH. Nutrient affinity, half-saturation constants and the cost of toxin production in dinoflagellates. Ecol Lett. 2019;22:558–60. doi: 10.1111/ele.13208. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Wang Y, Ou L, He X, Chen D. Allocation costs associated with induced defense in Phaeocystis globosa (Prymnesiophyceae): the effects of nutrient availability. Sci Rep. 2015;5:10850. doi: 10.1038/srep10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu X, Wang J, Chen Q, Chen G, Huang Y, Yang Z. Costs and trade-offs of grazer-induced defenses in Scenedesmus under deficient resource. Sci Rep. 2016;6:22594. doi: 10.1038/srep22594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redfield AC. The biological control of chemical factors in the environment. Am Sci. 1958;46:205–21. [PubMed] [Google Scholar]
- 29.Boyer GL, Sullivan JJ, Andersen RJ, Harrison PJ, Taylor FJR. Effects of nutrient limitation on toxin production and composition in the marine dinoflagellate Protogonyaulax tamarensis. Mar Biol. 1987;96:123–8. [Google Scholar]
- 30.Leong SCY, Murata A, Nagashima Y, Taguchi S. Variability in toxicity of the dinoflagellate Alexandrium tamarense in response to different nitrogen sources and concentrations. Toxicon. 2004;43:407–15. doi: 10.1016/j.toxicon.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Chakraborty S, Pančić M, Andersen KH, Kiørboe T. The cost of toxin production in phytoplankton: the case of PST producing dinoflagellates. ISME J. 2019;13:64–75. doi: 10.1038/s41396-018-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson L. Trends in nutrient and oxygen concentrations in the Skagerrak-Kattegat. J Sea Res. 1996;35:63–71. [Google Scholar]
- 33.Tiselius P, Belgrano A, Andersson L, Lindahl O. Primary productivity in a coastal ecosystem: a trophic perspective on a long-term time series. J Plankton Res. 2016;38:1092–102. [Google Scholar]
- 34.Kiørboe T, Nielsen TG. Regulation of zooplankton biomass and production in a temperate, coastal ecosystem. 1. Copepods. Limnol Oceanogr. 1994;39:493–507. [Google Scholar]
- 35.Selander E, Kubanek J, Hamberg M, Andersson MX, Cervin G, Pavia H. Predator lipids induce paralytic shellfish toxins in bloom-forming algae. Proc Natl Acad Sci USA. 2015;112:6395–400. doi: 10.1073/pnas.1420154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen PJ. The red tide dinoflagellate Alexandrium tamarense: effects on behaviour and growth of a tintinnid ciliate. Mar Ecol Prog Ser. 1989;53:105–16. [Google Scholar]
- 37.Berdalet E, Peters F, Koumandou VL, Roldán C, Guadayol Ò, Estrada M. Species-specific physiological response of dinoflagellates to quantified small-scale turbulence 1. J Phycol. 2007;43:965–77. [Google Scholar]
- 38.Fischer R, Andersen T, Hillebrand H, Ptacnik R. The exponentially fed batch culture as a reliable alternative to conventional chemostats. Limnol Oceanogr Meth. 2014;12:432–40. [Google Scholar]
- 39.Flynn K, Jones KJ, Flynn KJ. Comparisons among species of Alexandrium (Dinophyceae) grown in nitrogen- or phosphorus-limiting batch culture. Mar Biol. 1996;126:9–18. [Google Scholar]
- 40.Brandenburg KM, Wohlrab S, John U, Kremp A, Jerney J, Krock B, et al. Intraspecific trait variation and trade-offs within and across populations of a toxic dinoflagellate. Ecol Lett. 2018;21:1561–71. doi: 10.1111/ele.13138. [DOI] [PubMed] [Google Scholar]
- 41.Hillebrand H, Dürselen C-D, Kirschtel D, Pollingher U, Zohary T. Biovolume calculation for pelagic and benthic microalgae. J Phycol. 1999;35:403–24. [Google Scholar]
- 42.Schnetger B, Lehners C. Determination of nitrate plus nitrite in small volume marine water samples using vanadium(III)chloride as a reduction agent. Mar Chem. 2014;160:91–8. [Google Scholar]
- 43.Asp TN, Larsen S, Aune T. Analysis of PSP toxins in Norwegian mussels by a post-column derivatization HPLC method. Toxicon. 2004;43:319–27. doi: 10.1016/j.toxicon.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Turner A, Tölgyesi L. Determination of Paralytic Shellfish Toxins and Tetrodotoxin in Shellfish using HILIC/MS/MS. Application Note No. 5994-0967EN. [Internet]. 2019. Available from: https://www.agilent.com/en/solutions/food-testing-agriculture/seafood-testing.
- 45.Franco JM, Fernández P, Reguera B. Toxin profiles of natural populations and cultures of Alexandrium minutum Halim from Galician (Spain) coastal waters. J Appl Phycol. 1994;6:275–9. [Google Scholar]
- 46.Xu J, Hansen PJ, Nielsen LT, Krock B, Tillmann U, Kiørboe T. Distinctly different behavioral responses of a copepod, Temora longicornis, to different strains of toxic dinoflagellates, Alexandrium spp. Harmful Algae. 2017;62:1–9. doi: 10.1016/j.hal.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 47.Wood S, Scheipl F. gamm4: Generalized additive mixed models using mgcv and lme4. R package version 0.2-6. [Internet]. 2020. Available from: https://cran.r-project.org/.
- 48.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Soft 2017;82:1–26.
- 49.Wohlrab S, Selander E, John U. Predator cues reduce intraspecific trait variability in a marine dinoflagellate. BMC Ecol. 2017;17:8. doi: 10.1186/s12898-017-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Driscoll WW, Hackett JD, Ferrière R. Eco-evolutionary feedbacks between private and public goods: evidence from toxic algal blooms. Ecol Lett. 2016;19:81–97. doi: 10.1111/ele.12533. [DOI] [PubMed] [Google Scholar]
- 51.Flynn K, Franco J, Fernandez P, Reguera B, Zapata M, Wood G, et al. Changes in toxin content, biomass and pigments of the dinoflagellate Alexandrium minutum during nitrogen refeeding and growth into nitrogen or phosphorus stress. Mar Ecol Prog Ser. 1994;111:99–109. [Google Scholar]
- 52.Tillmann U, John U. Toxic effects of Alexandrium spp. on heterotrophic dinoflagellates: an allelochemical defence mechanism independent of PSP-toxin content. Mar Ecol Prog Ser. 2002;230:47–58. [Google Scholar]
- 53.Tillmann U, Hansen PJ. Allelopathic effects of Alexandrium tamarense on other algae: evidence from mixed growth experiments. Aquat Micro Ecol. 2009;57:101–12. [Google Scholar]
- 54.Teegarden GJ, Campbell RG, Anson DT, Ouellett A, Westman BA, Durbin EG. Copepod feeding response to varying Alexandrium spp. cellular toxicity and cell concentration among natural plankton samples. Harmful Algae. 2008;7:33–44. [Google Scholar]
- 55.Peter KH, Sommer U. Interactive effect of warming, nitrogen and phosphorus limitation on phytoplankton cell size. Ecol Evol. 2015;5:1011–24. doi: 10.1002/ece3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia NS, Bonachela JA, Martiny AC. Interactions between growth-dependent changes in cell size, nutrient supply and cellular elemental stoichiometry of marine. Synechococcus ISME J. 2016;10:2715–24. doi: 10.1038/ismej.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiørboe T. Turbulence, phytoplankton cell size, and the structure of pelagic food webs. Adv Mar Biol. 1993;29:1–72. [Google Scholar]
- 58.Lindemann C, Fiksen Ø, Andersen KH, Aksnes DL. Scaling laws in phytoplankton nutrient uptake affinity. Front Microbiol. 2016;3:1–6. [Google Scholar]
- 59.Edwards KF, Thomas MK, Klausmeier CA, Litchman E. Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton. Limnol Oceanogr. 2012;57:554–66. [Google Scholar]
- 60.Lürling M, Van Donk E. Grazer-induced colony formation in Scenedesmus: are there costs to being colonial? Oikos. 2000;88:111–8. [Google Scholar]
- 61.Selander E, Jakobsen HH, Lombard F, Kiorboe T. Grazer cues induce stealth behavior in marine dinoflagellates. Proc Natl Acad Sci USA. 2011;108:4030–4. doi: 10.1073/pnas.1011870108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiørboe T. A mechanistic approach to plankton ecology. Princeton, NJ: Princeton University Press; 2008. pp. 128–129.
- 63.Tollrian R, Harvell CD. The ecology and evolution of inducible defenses. Princeton, NJ: Princeton University Press; 1999. pp. 1–383.
- 64.Leao T, Castelão G, Korobeynikov A, Monroe EA, Podell S, Glukhov E, et al. Comparative genomics uncovers the prolific and distinctive metabolic potential of the cyanobacterial genus Moorea. Proc Natl Acad Sci USA. 2017;114:3198–203. doi: 10.1073/pnas.1618556114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Züst T, Agrawal AA. Trade-Offs Between Plant Growth and Defense Against Insect Herbivory: An Emerging Mechanistic Synthesis. Annu Rev Plant Biol. 2017;68:513–34. doi: 10.1146/annurev-arplant-042916-040856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.