Abstract
Background
It has been reported that ozone therapy might be helpful in treating foot ulcers in people with diabetes mellitus (DM).
Objectives
To assess the effects of ozone therapy on the healing of foot ulcers in people with DM.
Search methods
In March 2015 we searched: The Cochrane Wounds Group Specialised Register, The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), Ovid MEDLINE, Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations), Ovid EMBASE, EBSCO CINAHL, Science Citation Index, Chinese Biomedical Literature Database and The Chinese Clinical Registry. There were no restrictions based on language, date or study setting.
Selection criteria
We included randomised controlled trials (RCTs) that compared ozone therapy with sham ozone therapy or any other interventions for foot ulcers in people with DM, irrespective of publication date or language.
Data collection and analysis
Two reviewers independently screened all retrieved citations, selected relevant citations and extracted data. Disagreements were resolved by discussion with a third reviewer. The methodological quality of included studies and the evidence level of outcomes were assessed using the Cochrane risk of bias tool and the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach respectively. Data were expressed using risk ratio (RR) for dichotomous outcomes and mean difference (MD) for continuous outcomes with their 95% confidence interval (95% CI). Review Manager (RevMan) software was used to analyse the data.
Main results
Three studies (212 participants) were included in this review. The overall risk of bias was high for two trials and unclear for one.
One trial (101 participants) compared ozone treatment with antibiotics for foot ulcers in people with DM. The study had a follow‐up period of 20 days. This study showed that ozone treatment was associated with a greater reduction in ulcer area from baseline to the end of the study than treatment with antibiotics (MD ‐20.54 cm2, 95% CI ‐20.61 to ‐20.47), and a shorter duration of hospitalisation (MD ‐8.00 days, 95% CI ‐14.17 to ‐1.83), but did not appear to affect the number of ulcers healed over 20 days (RR 1.10, 95% CI 0.87 to 1.40). No side effects were observed in either group.
The other two trials (111 participants) compared ozone treatment plus usual care with usual care for foot ulcers in people with DM. The meta‐analysis results did not show evidence of a difference between groups for the outcomes of reduction of ulcer area (MD ‐2.11 cm2, 95% CI ‐5.29 to 1.07), the number of ulcers healed (RR 1.69, 95% CI 0.90 to 3.17), adverse events (RR 2.27, 95% CI 0.48 to 10.79), or amputation rate (RR 2.73, 95%CI 0.12, 64.42).
Authors' conclusions
The available evidence was three small RCTs with unclear methodology, so we are unable to draw any firm conclusions regarding the effectiveness of ozone therapy for foot ulcers in people with DM.
Plain language summary
Ozone therapy as a treatment for foot ulcers in people with diabetes
What is diabetes and what is a foot ulcer?
Diabetes mellitus (DM) is a common condition that leads to high sugar concentrations in the blood. People who have had diabetes for a long time often suffer from foot ulcers. Nearly 35% of all hospital admissions from diabetes clinics are due to them, as are nearly 80% of all non‐traumatic amputations of the leg and foot.
What is ozone therapy?
Ozone is a gas, and can be used as a treatment for ulcers in people with diabetes, which can be delivered with ozonised oils (e.g. ozonised sunflower or olive oil), or by a mixture of oxygen and ozone applied directly to the wound, or through rectal insufflation (blown into the final portion of the gut/intestines through the anus).
The purpose of this review
This review tried to find out whether ozone therapy is effective when given alone, or as part of a package of care, to treat foot ulcers in people with DM.
Findings of this review
The review authors searched the medical literature up to March 3 2015, and identified three relevant clinical trials (212 participants) that investigated ozone therapy for the treatment of foot ulcers in people with diabetes. The available evidence was of low quality.
One trial, with 101 participants, compared ozone treatment with antibiotics and followed up for 20 days. The results of this study showed that the reduction in ulcer size was greater, and also the length of hospital stay was shorter, in those receiving ozone treatment, but there was no apparent benefit in terms of the number of foot ulcers healed. No adverse effects (side effects or harms) were observed with either treatment.
The other two trials (111 participants) compared ozone treatment plus usual care with usual care. The results of these two studies showed that there were no apparent differences between the groups for reduction in ulcer size,the number of foot ulcers healed, or occurrence of adverse events and amputation rates.
Quality of life was not reported by either trial.
Conclusion
On the basis of the limited and poor quality information available, the review authors were unable to draw any conclusions about the effectiveness of ozone therapy for treating foot ulcers in people with DM.
Summary of findings
Background
Description of the condition
Diabetes mellitus (DM) (including type 1 and 2) is rapidly emerging as a new global epidemic (Lau 2009; Gupta 2012a).The prevalence of DM for all age‐groups worldwide was estimated to be 2.8% in 2000 (171 million) (Wild 2004) and it is expected to double in the next three decades (4.4%, 366 million) (Gupta 2012a). In the USA, approximately 6% of the population has been diagnosed with DM, which is the seventh leading cause of death (Malhotra 2012). Around 2.8 million people in the UK have DM (approximately 4.3% of the population) (Dumville 2013). Poor control of DM may result in complications such as foot ulcers, which are defined as full‐thickness wounds that penetrate through the dermis (the deep vascular and collagenous inner layer of the skin) and are located below the ankle in a DM patient (Jeffcoate 2004). Foot ulcers are a major complication of long standing DM, and account for nearly 35% of all hospital admissions in diabetic clinics, and nearly 80% of all non‐traumatic amputations of the lower limb (Gupta 2012a). The prevalence of foot ulcers is 4000 to 10,000 per 100,000 people diagnosed with DM (i.e. 4% to 10%) (Singh 2005).
Foot problems are common in people with DM because of their increased risks of peripheral neuropathy (damage to peripheral nerves, e.g. in the feet), peripheral vascular disease (general circulatory problems), abnormal pressure on the foot, and impaired resistance to infection. Failure of ulcers to heal may result in amputation, and people with DM have a 10 to 20‐fold higher risk of losing a lower limb, or part of a lower limb, to non‐traumatic amputation than those without DM (Morris 1998; Wrobel 2001). It is reported that 15% of the USA DM population will develop foot ulceration, and, 14% to 20% of these people will require an amputation (Malhotra 2012). Foot ulcers are an important cause of morbidity and mortality in patients with DM, and have a staggering economic impact, not only on the individual and his/her family, but also on society (Xie 2008; Gupta 2012a). In 2010‐11 the estimated NHS spend on foot ulceration and amputation in people with DM in England was GBP 639 to GBP 662 million (Kerr 2012). For patients treated using biofilm‐based wound management anchored by molecular diagnostics, total charges per patient for the entire course of treatment was $4,756 (total payments $3,060; £1,987) (Wolcott 2015).
Five‐year mortality rates after the onset of new diabetic ulceration have been reported as being between 43% and 55%, while up to 74% require lower‐extremity amputation (Robbins 2008). It has been reported that the risk of death more than doubles in people who develop a foot ulcer (relative risk (RR) 2.39, 95% confidence interval (CI) 1.13 to 4.58) (Boyko 1996). A recent meta‐analysis reported that foot ulcers in people with DM were associated with an increased risk of all‐cause mortality (RR 1.89, 95% CI 1.60 to 2.23), fatal myocardial infarction (2.22, 95% CI 1.09 to 4.53) and fatal stroke (1.41, 95% CI 0.61 to 3.24) (Brownrigg 2012).
Description of the intervention
Foot ulcers in people with DM are challenging to treat and often require the input of a multi‐disciplinary team of health professionals, including: diabetologists, radiologists, dermatologists, vascular surgeons, orthopaedic surgeons, plastic surgeons, podiatrists and the nursing team (van Sloten 2008). The cornerstones of treatment are relief of pressure, the restoration of perfusion (oxygen brought by blood flow) of the foot, treatment of infection, wound care, optimum glucose (sugar) regulation and education (van Sloten 2008). Treatments for foot ulcers in people with DM include debridement of the wound, management of any infection, revascularisation procedures if indicated, off‐loading (removing weight from) the ulcer, hyperbaric oxygen therapy (exposure to high concentrations of oxygen), use of advanced wound care products, and negative‐pressure wound therapy (Caravaggi 2013). Conservative treatment is successful in the majority of severely infected wounds, but the duration of treatment is often prolonged (Pittet 1999,Venkatesan 1997). A large US study of 27630 patients with diabetic neuropathic foot ulcers treated with standard good wound care such as debridement and off‐loading reported that 47% of foot ulcers has healed after 20 weeks of care (Margolis 2003).
In 1834 Schoenbein discovered ozone (O3, a form of oxygen in which three atoms bind together instead of the more normal two), and he considered it to be an oxidant and also a disinfectant (Bocci 2004). Ozone was used to treat gangrene during the First World War, and today it is widely used to sterilise water (Bocci 2004). Currently low‐dose ozone is recognised as an antiviral and bactericidal agent (Valacchi 2005; Kim 2009), and it has been used clinically as a treatment for indications such as coronary artery disease (Martínez‐Sánchez 2012), chronic severe hepatitis (Gu 2010), sudden sensorineural hearing loss (Ragab 2009), hypersensitive teeth (Dähnhardt 2008), periodontics (Gupta 2012b) and chronic low back pain (Magalhaes 2012). It has also been used for healing chronic wounds such as trophic ulcers, ischaemic ulcers and diabetic wounds (Martínez‐Sánchez 2005; de Monte 2005; Kim 2009).
Ozone therapy has been used for many years as an ancillary method to usual treatment for foot ulcers in patients with DM (Bialoszewski 2003), especially in those cases in which conservative treatment methods have not been proven satisfactory. Ozone has been used as a therapeutic method and it may be helpful in promoting complete wound closure. Substances such as olefin (an alkene or unsaturated hydrocarbon) can be treated with gaseous ozone to form an ozonide, which is able to deliver nascent (freshly‐generated) oxygen deep within a lesion (wound or ulcer) without causing any primary skin irritation (Kim 2009). Ozone therapy can be delivered to patients by treating them with ozonised oils (e.g. ozonised sunflower or olive oil); via local application of a mixture of oxygen and ozone (directly to the wound); or through rectal insufflation (delivered into the final portion of the gut/intestines).
How the intervention might work
The mechanism through which ozone achieves healing in chronic wounds is not known. It has been suggested that ozone might be related to growth factors (Kim 2009) and that it can activate the antioxidant system (complex network of antioxidant metabolites and enzymes that work together to prevent oxidative damage to cellular components), and activate superoxide dismutase (enzymes that alternately catalyze the dismutation (or partitioning) of the superoxide (O2−) radical into either ordinary molecular oxygen (O2) or hydrogen peroxide (H2O2)) (Martínez‐Sánchez 2005;Re 2008). In addition, it has been suggested that ozone antioxidant properties preserve ß‐cell functions and reduce hyperglycaemia (high blood glucose levels) (Re 2008).
Why it is important to do this review
Currently, there is no systematic review that evaluates the effects of ozone therapy for foot ulcers in people with DM. It has been recently suggested that ozone therapy might be a helpful treatment (Valacchi 2005), and a systematic review of the available evidence for the therapeutic effects of ozone would help decision making when considering treatment options.
Objectives
To assess the effects of ozone therapy on the healing of foot ulcers in patients with DM.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were considered for inclusion, irrespective of publication status or language.
Types of participants
Adults (over 18 years of age) with type 1 or type 2 DM, and with an active foot ulcer of neuropathic, neuroischaemic or ischaemic aetiology.
Types of interventions
Ozone therapy compared with sham ozone therapy, with or without concomitant interventions such as antibiotics, topical agents or usual care as long as the same concomitant treatment was used in both groups.
Ozone therapy compared with any other intervention used to promote healing, such as antibiotics, topical agents or usual care.
Ozone therapy combined with a concomitant treatment compared with any other intervention used to promote healing, such as antibiotics, topical agents or usual care based, as long as the same concomitant treatment was used in both groups.
Types of outcome measures
Primary outcomes
Time taken to achieve complete ulcer healing.
Number of ulcers healed.
Change in area of ulcer.
Secondary outcomes
Number of complications (adverse events reported), such as toxicity, irritation.
Quality of life (measured using a standardised generic questionnaire such as EQ‐5D, SF‐36, SF‐12 or SF‐6).
Length of hospital stay.
Amputation.
Incidence of wound infection.
Search methods for identification of studies
Electronic searches
We identified relevant randomised clinical trials by searching the following electronic databases:
Cochrane Wounds Group Specialized Register (Searched March 3 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library 2015, Issue 1;
Ovid MEDLINE & Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations ) (1946 to March 2 2015);
Ovid EMBASE (1974 to March 2 2015);
EBSCO CINAHL (1982 to March 3 2015);
Science Citation Index (SCI) (1974 to March 3 2015);
Chinese Biomedical Literature Database (1978 to March 3 2015);
The Chinese Clinical Registry (July 2007 to March 3 2015).
We used the following search strategy for searching The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Diabetic Foot explode all trees #2 MeSH descriptor Foot Ulcer explode all trees #3 diabet* NEAR/3 ulcer*:ti,ab,kw #4 diabet* NEAR/3 (foot or feet):ti,ab,kw #5 diabet* NEAR/3 wound*:ti,ab,kw #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Ozone explode all trees #8 ozon*:ti,ab,kw #9 (#7 OR #8) #10(#6 AND #9)
This strategy was adapted to search Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL (Appendix 1). The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying RCTs in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL search with the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN 2009). There were no restrictions based on language, date or study setting.
Searching other resources
The review authors contacted trial authors and experts in the field about unpublished studies, and searched the reference lists of all potentially relevant study reports for additional relevant studies.
We also searched the following major ongoing clinical trials registries:
International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/)
Clinicaltrials.gov (https://clinicaltrials.gov/)
Current Controlled Trials (http://www.isrctn.com/)
Data collection and analysis
Selection of studies
Two review authors (Zhang and Liu) screened all retrieved citations independently, and selected relevant studies for further assessment on the basis of titles, abstracts and keywords. Full‐text reports for studies that appeared to meet the eligibility criteria were retrieved and considered by at least two review authors independently. Disagreements were resolved by discussion with the other review authors. We corresponded with investigators to clarify study eligibility and request further information, such as missing results or methodological details (e.g. clarification of methods used for randomisation or allocation concealment).
Data extraction and management
Two review authors extracted data independently (Zhang and Tian), and differences were resolved by discussion with a third review author (Yang). The data extracted included:
first author's name;
year of publication;
country/countries of origin of the trial centres;
setting of the trial;
number of participants;
participants' age and gender by treatment group;
duration of the ulcer, number of previous ulcers, grade/severity of ulceration
description of intervention and comparator interventions, including any concomitant treatments;
duration of follow‐up;
outcome measures including methods used to measure outcome.
Assessment of risk of bias in included studies
Two review authors (Liu and Li) assessed each included study independently using the Cochrane Collaboration tool for assessing risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (Higgins 2011a). This tool addresses six specific domains, namely: sequence generation, allocation concealment, blinding (blinding of patient; blinding of care provider; blinding of outcome assessors), incomplete outcome data, selective outcome reporting, and other issues (extreme baseline imbalance and sponsorship bias) (see Appendix 2 for details of criteria on which the judgements were based). We completed a 'Risk of bias' table for each eligible study. Any disagreements were discussed amongst all authors to achieve a consensus. In order to assess the domain of selective outcome reporting as fully as possible, we wrote to the first/corresponding authors of the trials and requested a copy of each study's protocol. If an individual trial achieved adequate sequence generation, adequate allocation concealment and adequate blinding of outcome assessors, we defined it as being at low risk of bias overall; if any one of these key domains was unclear individually but none were high risk we defined the trial as being at overall unclear risk of bias; if any of the key domains were high risk individually the trial was deemed to be at high risk of bias overall.We presented our assessment of risk of bias using a 'Risk of bias' summary figure, which presents all of the judgments in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give to the results of each study.
Measures of treatment effect
We generated measures of treatment effect using Review Manager Software (RevMan). We expressed results for dichotomous outcomes as risk ratio (RR) with 95% confidence intervals (CIs) and continuous outcomes as mean difference (MD) with 95% CIs. If studies reporting time‐to‐event data (e.g. time to healing) did not report a hazard ratio (HR) then, when feasible, we planned to estimate this using other reported outcomes, such as numbers of events, through the application of available statistical methods (Parmar 1998; Tierney 2007) although such an approach was not implemented here as none of the included trials reported time to healing.
Dealing with missing data
Where data were missing, we wrote to the study authors and requested them. If we had not received a response after four weeks, we emailed again. If they still did not respond, we used the estimates based on available data.
Assessment of heterogeneity
We planned to examine heterogeneity among trials using the I2 statistic (Higgins 2003). We planned to regard an I2 statistic estimate greater than 40% as showing substantial or considerable heterogeneity and we planned to investigate its causes by performing subgroup analyses or sensitivity analyses by excluding studies thought to cause the heterogeneity.
Assessment of reporting biases
If sufficient studies (>9) were identified we planned to examine for publication bias using a funnel plot (Sterne 2011). We aimed to minimise the potential impact of reporting bias by ensuring a comprehensive search for eligible studies and by being alert for duplicate publications. We identified only three trials, so we did not investigate the potential for publication bias using a funnel plot.
Data synthesis
A narrative synthesis of findings was presented, with RCTs stratified according to the nature of the interventions and comparators evaluated.
We planned to analyse the data using Review Manager software (version 5.20) for summary estimates. Dichotomous outcomes were expressed as a summary risk ratio (RR), time‐to‐event outcomes as a summary HR and continuous outcomes as a summary mean difference (MD) or standardised mean difference (SMD) if a common concept was measured using different instruments. We planned to generate 95% CIs for all pooled estimates.
We planned to pool data only where groups of RCTs were clinically and statistically homogenous. If RCTs were clinically similar and did not show substantial statistical heterogeneity (defined as I2 less than 40%) then we planned to pool them in a meta‐analysis using a fixed‐effect model. We anticipated using a random‐effects model where there was evidence of substantial statistical heterogeneity (defined as I2 more than 40%) and planned to compare this with a fixed‐effect model to see if the conclusions were altered.
Where included studies were clinically heterogeneous, and a meta‐analysis was precluded, we presented only a narrative synthesis.
Subgroup analysis and investigation of heterogeneity
We planned to undertake subgroup analyses according to differences in trial location and setting, or characteristics of participants.
1) Subgroup analysis according to trial location: Asian vs. European vs. American
2) Subgroup analysis according to the type of diabetes mellitus: type 1 vs. type 2 diabetes mellitus
3) Subgroup analysis according to co‐morbidity.
Sensitivity analysis
We planned a sensitivity analysis by restricting studies with low risk of bias (comparing these to studies classed at unclear or high risk if bias) (adequate sequence generation, adequate allocation concealment and blinding of outcome assessors) to determine whether pooled estimates were robust if possible.
'Summary of findings' tables
We employed the GRADE approach to interpret findings (Guyatt 2008), and the GRADE profiler (GRADEPRO) allowed us to import data from Review Manager 5.3 to create a summary of findings table. This table provides outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes that we considered (Bjelakovic 2014).
We established 'Summary of findings' tables using the following outcomes where available; these seven outcomes were selected and listed according to their priority (Schünemann 2011).
Time taken to achieve complete ulcer healing.
Number of ulcers healed in the trial period.
Change in area of ulcer.
Number of complications (adverse events reported), such as toxicity, irritation.
Quality of life.
Length of hospital stay.
Amputation
Results
Description of studies
See: Characteristics of included studies; and Characteristics of excluded studies
Results of the search
We identified a total of 48 records from databases and reference checking using the search strategies specified earlier. Forty of these were considered irrelevant because they were case reports (19 records), review articles (15 records), duplicates (three records) and other reasons (three records). Eight papers were retrieved for full‐text assessment of which five were excluded because they were not RCTs (Anonymous 2011; Falanga 2006; Gazin 2008a; Gazin 2008b; Kulikov 2002).
The Clinical Trials Registry Platforms were also searched, and we found one ongoing trial (NCT01643967). Finally, three trials (Martínez‐Sánchez 2005; Wainstein 2011; Zhang 2014) were included (Figure 1).
1.
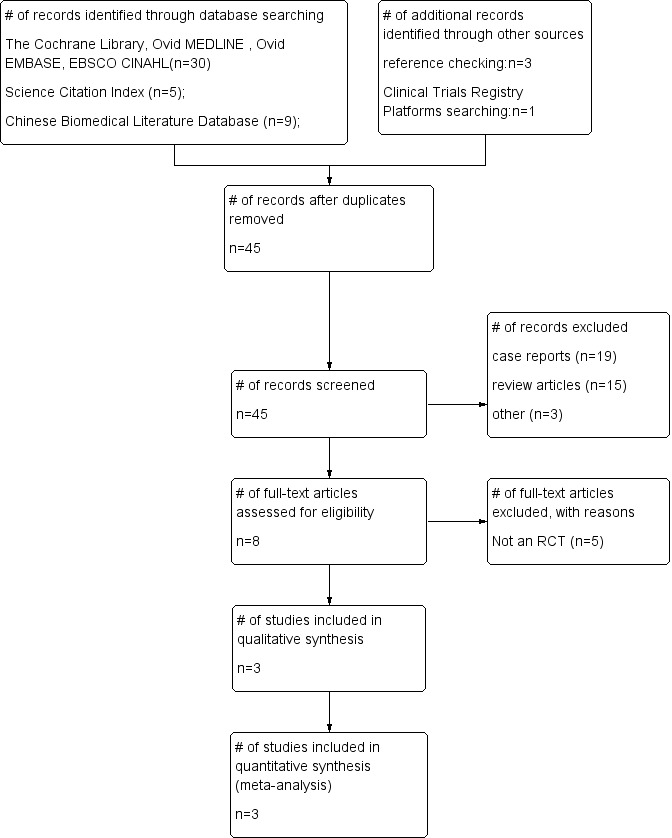
Study flow diagram.
Included studies
Three trials (Martínez‐Sánchez 2005; Wainstein 2011; Zhang 2014) (212 participants) were included in the review. One trial compared ozone treatment (52 participants) with antibiotics (49 participants) for foot ulcers in participants with DM (Martínez‐Sánchez 2005), the other two compared ozone therapy plus usual care (56 participants) with usual care (55 participants) for foot ulcers in participants with DM (Wainstein 2011; Zhang 2014)
Excluded studies
After reading the full texts, five studies were excluded because they were not RCTs (Kulikov 2002; Falanga 2006; Gazin 2008b; Gazin 2008a; Anonymous 2011).
Risk of bias in included studies
See Figure 2. The Wainstein 2011 trial was unclear overall whereas the other two trials (Martínez‐Sánchez 2005; Zhang 2014) were at high risk of bias.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
The three included studies were assessed as being at unclear risk of bias for allocation (selection bias) (Martínez‐Sánchez 2005; Wainstein 2011; Zhang 2014). Whilst they were described as being randomised, randomisation methods and concealment of allocation were not described clearly.
Blinding
Martínez‐Sánchez 2005 did not use blinding and was assessed as being at high risk of bias for blinding of participants and personnel (performance bias) and blinding of outcome assessment (detection bias).
Wainstein 2011 was assessed as being at low risk of bias for blinding of participants and personnel, and for blinding of outcome assessment, as the authors stated that, "The patients and the investigator were blinded to the mode of treatment", and, "The ozone (active treatment) group received ozone treatments using the Ozoter 101 device (OZ Recovery Technologies, Ramat Gan, Israel) in addition to usual care. The control group received sham treatments using the Ozoter 101 device set to the inactive mode in addition to usual care."
Zhang 2014 did not use blinding and was assessed as being at high risk of bias for blinding of participants and personnel (performance bias) and blinding of outcome assessment (detection bias).
Incomplete outcome data
Martínez‐Sánchez 2005 was assessed as being at low risk of bias for incomplete outcome data (attrition bias). Whilst the study abstract reported that 101 participants had been randomised, the paper reported data on 100 participants and the analysis included 100 participants, there was one drop‐out in the ozone group. We deemed that this would not affect the results and assessed the risk of bias for this domain as being low for this study.
Wainstein 2011 was assessed as being at high risk for this domain, as only 34 out of 61 participants completed the study. This will introduce high risk of bias, although they conducted intention to treat analysis.
Zhang 2014 was assessed as being at low risk for this domain, as the authors stated that, "50 patients with DFU were randomized to ozone group (n = 25) and control group (n = 25). All of these patients completed the study visits."
Selective reporting
All included studies were assessed as being at unclear risk of bias for selective reporting (reporting bias) (Martínez‐Sánchez 2005; Wainstein 2011; Zhang 2014). Although we were unable to obtain the study protocols for full assessment of selective reporting bias, all the outcomes listed in the methods section were reported in the results.
Other potential sources of bias
One RCT was judged as being at low risk of bias for this domain (Wainstein 2011) whilst the other two were assessed as unclear because there was insufficient information to assess baseline imbalance and conflict of interest (Martínez‐Sánchez 2005; Zhang 2014).
Effects of interventions
Summary of findings for the main comparison. Ozone versus antibiotic treatments for treating foot ulcers in people with diabetes.
| Ozone versus antibiotic treatments for treating foot ulcers in people with diabetes | ||||||
| Patient or population: patients with treating foot ulcers in people with diabetes Settings: Intervention: Ozone versus antibiotic treatments | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Ozone versus antibiotic treatments | |||||
| Number of ulcers healed Follow‐up: mean 20 days | Study population | RR 1.1 (0.87 to 1.4) | 100 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 694 per 1000 | 763 per 1000 (604 to 971) | |||||
| Moderate | ||||||
| 694 per 1000 | 763 per 1000 (604 to 972) | |||||
| Reduction in ulcer area Follow‐up: mean 20 days | The mean reduction in ulcer area in the intervention groups was 20.54 lower (20.61 to 20.47 lower) | 100 (1 study) | ⊕⊕⊝⊝ low1,2 | |||
| Length of hospitalisation Follow‐up: mean 20 days | The mean length of hospitalisation in the intervention groups was 8 lower (14.17 to 1.83 lower) | 100 (1 study) | ⊕⊕⊝⊝ low1,3 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The included trial was of unclear risk of bias. 2 Total number of events was less than 300 3 Total population size was less than 400
Summary of findings 2. Ozone + usual care versus sham ozone + usual care for treating foot ulcers in people with diabetes.
| Ozone + usual care versus sham ozone + usual care for treating foot ulcers in people with diabetes | ||||||
| Patient or population: patients with treating foot ulcers in people with diabetes Settings: Intervention: Ozone + usual care versus sham ozone + usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Ozone + usual care versus sham ozone + usual care | |||||
| Number of ulcers healed Follow‐up: 20‐168 days | Study population | RR 1.69 (0.9 to 3.17) | 111 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 200 per 1000 | 338 per 1000 (180 to 634) | |||||
| Moderate | ||||||
| 193 per 1000 | 326 per 1000 (174 to 612) | |||||
| Reduction in ulcer area Follow‐up: 20‐168 days | The mean reduction in ulcer area in the intervention groups was 3.48 lower (3.82 to 3.14 lower) | 111 (2 studies) | ⊕⊝⊝⊝ very low1,3,4 | |||
| Adverse events/complications Follow‐up: mean 24 weeks | Study population | RR 2.27 (0.48 to 10.79) | 61 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 69 per 1000 | 157 per 1000 (33 to 744) | |||||
| Moderate | ||||||
| 69 per 1000 | 157 per 1000 (33 to 745) | |||||
| amputation Follow‐up: mean 24 weeks | Study population | RR 2.73 (0.12 to 64.42) | 61 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 These trials were of unclear risk of bias. 2 Total number of events was less than 300 3 Heterogeneity: Chi² = 17.06, df = 1 (P < 0.0001); I² = 94% 4 Total population size was less than 400
The data for outcomes in each trial is shown in Table 3.
1. Outcomes.
| Study | Number of ulcers healed | Reduction in ulcer area | Length of hospitalisation | Adverse events/complications | ||||
| Interventions(n/N) | Control(n/N) | Interventions(MD±SD(N)) | Control(MD±SD(N)) | Interventions(MD±SD(N)) | Control(MD±SD(N)) | Interventions(n/N) | Control(n/N) | |
| Martínez‐Sánchez 2005 | 39/51 | 34/49 | ‐34.66±0.21(51) | ‐14.12±0.14(49) | 26.0±13.0(51) | 34.0±18.0(49) | ||
| Wainstein 2011 | 13/31 | 8/30 | ‐2.0±3.9(31) | ‐1.6±1.7(30) | 5/32 | 2/29 | ||
| Zhang 2014 | 6/25 | 3/25 | ‐6.84±0.62(25) | ‐3.19±0.65(25) | ||||
Comparison one: Ozone compared with antibiotic treatments (one trial with 101 participants)
See Table 1
Martínez‐Sánchez 2005 compared ozone treatment with antibiotics for foot ulcers in people with DM. The trial was variously reported as having 101 or 100 participants; data were presented for 100 participants. The study compared ozone therapy (local and rectal insufflation of the gas) with topical and systemic antibiotic treatment. In addition, all participants received gauze dressings and debridement as required. The follow‐up period was 20 days.
Primary outcomes
Number of ulcers healed
Ulcers in 39 of the 51 (77%) participants in the ozone group and 34 of the 49 (69%) participants in the antibiotic group healed (full wound closure). There was no statistically significant difference between the two groups (RR 1.10, 95% CI 0.87 to 1.40) (Analysis 1.1).
1.1. Analysis.
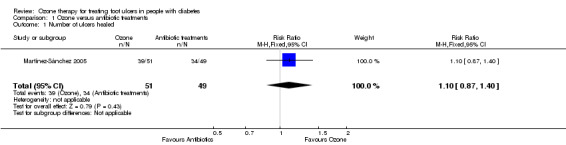
Comparison 1 Ozone versus antibiotic treatments, Outcome 1 Number of ulcers healed.
Reduction in ulcer area
In the ozone group, the mean reduction in ulcer area from baseline to study end was 34.66 cm2 (standard deviation (SD) 0.21 cm2) and the mean reduction in the antibiotic group was 14.12 cm2 (SD 0.14 cm2); SD values were calculated according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Ozone therapy was associated with a greater reduction in ulcer area than antibiotics (MD ‐20.54 cm2, 95% CI ‐20.61 to ‐20.47) (Analysis 1.2).
1.2. Analysis.
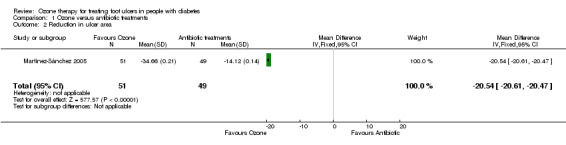
Comparison 1 Ozone versus antibiotic treatments, Outcome 2 Reduction in ulcer area.
Secondary outcomes
Duration of hospitalisation
The mean duration of hospitalisation was reported as 26 days (SD 13 days) in the ozone group compared with 34 days (SD 18 days) in the antibiotic group. There was a evidence of a difference between the two groups in favour of ozone (MD ‐8.00 days, 95% CI ‐14.17 to ‐1.83) (Analysis 1.3).
1.3. Analysis.
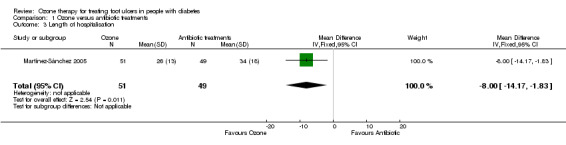
Comparison 1 Ozone versus antibiotic treatments, Outcome 3 Length of hospitalisation.
Adverse events
The study reported that no side effects were observed in either group, but the authors did not explain how the adverse events were assessed.
Quality of life
This trial did not report quality of life.
Amputation
This trial did not report amputation.
Comparison two: Ozone plus usual care versus sham ozone plus usual care or usual care alone (two trials with 111 participants)
See: Table 2
Wainstein 2011 compared ozone treatment plus usual care with sham ozone treatment plus usual care for foot ulcers in people with DM. The trial recruited 61 participants, however, 27 dropped out (16 participants from the ozone group, 11 from the control group). The study had a follow‐up period of 24 weeks.
Zhang 2014 compared ozone treatment plus usual care with usual care alone. A total of 50 patients with type 2 diabetes and DFUs of Wagner stage 2 to 4 were recruited; 25 participants were randomized into each treatment group. All patients completed the study visits. The follow‐up period was 20 days.
Primary outcomes
Number of ulcers healed
Ulcers in 13 of 31 (42%) participants in the ozone plus usual care group and eight of the 30 (27%) participants in the sham ozone plus usual care group healed (full wound closure) in Wainstein 2011, and ulcers in six of the 25 (24%) participants in the ozone plus usual care group and three of the 25 (12%) participants in the usual care alone group healed (full wound closure) in Zhang 2014. The estimate from pooled data suggested no evidence of a difference between treatment groups (RR 1.69, 95% CI 0.90 to 3.17) (Analysis 2.1) without any statistical heterogeneity (I² = 0%, Chi2 test of heterogeneity P = 0.75).
2.1. Analysis.
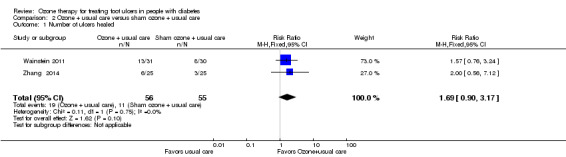
Comparison 2 Ozone + usual care versus sham ozone + usual care, Outcome 1 Number of ulcers healed.
Reduction in ulcer area
The ozone group showed a mean reduction of 2.0 cm2 (SD 0.39 cm2) and the control group showed a mean reduction of 1.6 cm2 (SD 1.7 cm2) in Wainstein 2011; the respective mean reductions were 6.84 cm2 (SD 0.62 cm2) and 3.19 cm2 (SD 0.65 cm2) in Zhang 2014. When data were pooled substantial heterogeneity was detected (I² = 94%, Chi2 test of heterogeneity P<0.0001) therefore a random effects model was selected. This suggested no evidence of a difference between treatment groups in reduction in ulcer area (cm2): MD ‐2.11 (95% CI ‐5.29 to 1.07). (Analysis 2.2). When the analysis was repeated using a fixed effect model a difference was observed in favour of ozone therapy: MD ‐3.48 (95% CI ‐3.82 to ‐3.14). This suggested that the result for this outcome was not stable.
2.2. Analysis.
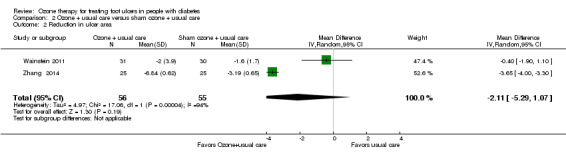
Comparison 2 Ozone + usual care versus sham ozone + usual care, Outcome 2 Reduction in ulcer area.
Secondary outcomes
Adverse events
Two adverse events/complications were reported in the control group (amputation and infection) and five in the ozone group (osteomyelitis, fever, wound infection, and pulmonary congestion) in Wainstein 2011. There was one event per patient, as these patients dropped out because of adverse events. None of the adverse events was judged to have been caused by the study interventions. There was no difference between the two groups for this outcome (RR 2.27, 95% CI 0.48 to 10.79) (Analysis 2.3). Zhang 2014 did not report adverse events.
2.3. Analysis.
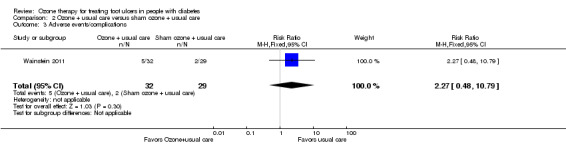
Comparison 2 Ozone + usual care versus sham ozone + usual care, Outcome 3 Adverse events/complications.
Duration of hospitalisation
These trials did not report duration of hospitalisation.
Quality of life
These trials did not report quality of life.
Amputation
One participant in the control group underwent amputation; this was included in the assessment of adverse events in Wainstein 2011. There was no evidence of a clear difference between the two groups (RR 2.73, 95% CI 0.12 to 64.42) (Analysis 2.4). Zhang 2014 did not report amputation rates.
2.4. Analysis.
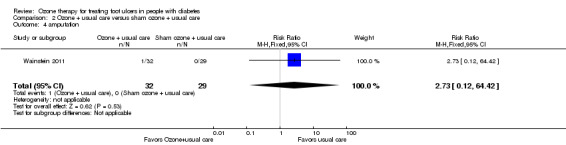
Comparison 2 Ozone + usual care versus sham ozone + usual care, Outcome 4 amputation.
Discussion
Summary of main results
Two main treatment comparisons were identified:
ozone therapy versus antibiotics (101 participants recruited with a follow‐up period of 20 days) (Martínez‐Sánchez 2005) and;
ozone therapy plus usual care compared with sham therapy plus usual care (61 participants with 24 week follow‐up) (Wainstein 2011) or usual care alone (50 participants with 20 day follow‐up) (Zhang 2014).
Evidence from the trial comparing ozone therapy with antibiotics did not suggest a difference between treatment groups for the number of ulcers healed. However, there was evidence of a difference between groups in terms of reduction in ulcer area and duration of hospitalisation that favoured ozone therapy. No side effects were observed in either group. The outcomes of quality of life, adverse events and amputation rates were not reported (Martínez‐Sánchez 2005).
For the second comparison, there was no evidence of a difference between treatment groups when data from two trials were pooled for the outcomes of number of ulcers healed or reduction in ulcer area (Wainstein 2011; Zhang 2014). Data from one trial did not suggest a difference between groups for adverse events or amputation rates (Wainstein 2011). Neither trial reported duration of hospitalisation or quality of life.
All findings should be treated with caution as the overall quality of evidence was low for both comparisons (Table 1; Table 2).
Overall completeness and applicability of evidence
The overall volume of evidence is low, consisting of three RCTs recruiting 212 participants in total, and is of poor quality (see Table 1; Table 2). Two out of the three RCTs had follow‐up periods limited to 20 days (Martínez‐Sánchez 2005; Zhang 2014) and so there is a lack of evidence on the longer term effects of ozone therapy. All three trials reported the number of participants with healed ulcers and the reduction in ulcer area but none reported time to healing which is a more informative healing outcome. Limited data were available on length of hospital stay and amputation rates and none of the trials reported quality of life.
There was also a lack of information relating to adverse events. Although in one included study ozone therapy reduced ulcer size and length of hospital stay without causing adverse events when compared with antibiotics (Martínez‐Sánchez 2005), this study had a short follow‐up (20 days). Recently, a case report confirmed that there were no complications associated with ozone therapy (Shah 2011), however, another trial included in this review suggested that adverse events of ozone therapy can include osteomyelitis (bone infection), fever, wound infection, and pulmonary congestion (collection of fluid in the lungs) (Wainstein 2011). The Wainstein 2011 trial reported that these adverse events may have been caused by the reactivity of O3 (Di Filippo 2010; Elvis 2011). Whilst attention should be paid when prescribing ozone for people with DM, more evidence is required on both the benefits and safety of ozone therapy in order to inform clinical decision making.
Quality of the evidence
The three included studies were described as randomised, but the methodological details were not well reported. The trials did not report the methods used to generate the randomisation sequence, or the methods used to conceal allocation. One RCT (Wainstein 2011) reported that participants and investigators were blind, but the other two did not use blinding (Martínez‐Sánchez 2005; Zhang 2014). In terms of the overall risk of bias, we rated one trial as unclear (Wainstein 2011) and the other two as high risk (Martínez‐Sánchez 2005; Zhang 2014). As a result, the findings of these three studies may be biased. Meanwhile, the sample size for each trial was not large enough and the duration of follow‐up was short. Although there were no significant differences in the wound size between two groups in both studies, different baseline wound size between two studies could introduce clinical heterogeneity, and further statistical heterogeneity. So we considered their quality of evidence to be low due to potential methodological flaws and imprecision. In addition the trials were underpowered and therefore unlikely to detect clinically meaningful differences even if they are present between the interventions, and where differences were detected, these cannot be relied upon. This is the main reason for not being able to draw conclusions even though statistically significant differences were detected in some comparisons.
Potential biases in the review process
The review considered as much evidence as possible, including unpublished studies identified from an on‐line trial registry, and the review authors also contacted as many authors and clinical experts as possible in order to obtain unpublished studies, but we did not receive any unpublished data. This may have led to publication bias.
Agreements and disagreements with other studies or reviews
Ozone therapy has been shown to improve wound healing, modulate the immune system, and act as an antibacterial agent in two recent case reports (Bocci 2009; Shah 2011). One of these case reports also showed the feasibility of using ozone therapy as an adjuvant to the conventional modality for treatment of extensive orthopaedic wounds (Shah 2011). A third case report showed the effectiveness of two‐week ozone therapy for Buruli ulcer, where ozone therapy produced an excellent outcome (Bertolotti 2013). Very little research has been done in this field, and so there is a paucity of results to compare with our findings.
Authors' conclusions
Implications for practice.
The results of this review suggest that ozone therapy, when compared to antibiotics, might reduce ulcer size and length of hospital stay in the short‐term, but there is no evidence to suggest it promotes overall healing or reduces the number of adverse events. When applied together with usual care, ozone may not reduce ulcer size compared with usual care alone, and there was no evidence of an increase in adverse events with ozone therapy and the number of ulcers healed. Whether or not ozone therapy could be an effective option for managing foot ulcers in people with DM could not be judged on the basis of the three studies included in this review, as they were too small to reliably detect clinically meaningful differences and suffered from methodological flaws.
Implications for research.
Since the included trials were at unclear or high risk of bias and of short follow‐up, well‐designed, adequately powered, high quality RCTs with longer duration of follow‐up are needed to determine the effectiveness and safety of ozone therapy on foot ulcers in people with DM. The proportion of participants lost to follow‐up was high in one study (Wainstein 2011), so consideration should be given to promoting participant concordance in future evaluations in order to minimise attrition bias.
Acknowledgements
The authors would like to acknowledge the contribution of Wounds Group editor, Jo Dumville, statistician Gill Worthy, copy editor Elizabeth Royle and peer referees Audrey Demetriou, Shirley Manknell and Nerys Woolacott.
Appendices
Appendix 1. Search Strategies
Ovid Medline
1 exp Foot Ulcer/ 2 exp Diabetic Foot/ 3 (diabet* adj3 ulcer*).tw. 4 (diabet* adj3 (foot or feet)).tw. 5 (diabet* adj3 wound*).tw. 6 or/1‐5 7 exp Ozone/ 8 ozon*.tw. 9 or/7‐8 10 6 and 9 11 randomized controlled trial.pt. 12 controlled clinical trial.pt. 13 randomi?ed.ab. 14 placebo.ab. 15 clinical trials as topic.sh. 16 randomly.ab. 17 trial.ti. 18 or/11‐17 19 exp animals/ not humans.sh. 20 18 not 19 21 10 and 20
Ovid Embase
1 exp foot ulcer/ 2 exp diabetic foot/ 3 (diabet* adj3 ulcer*).tw. 4 (diabet* adj3 (foot or feet)).tw. 5 (diabet* adj3 wound*).tw. 6 or/1‐5 7 exp ozone therapy/ 8 exp ozone/ 9 ozon*.tw. 10 or/7‐9 11 6 and 10 12 Randomized controlled trials/ 13 Single‐Blind Method/ 14 Double‐Blind Method/ 15 Crossover Procedure/ 16 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. 17 (doubl$ adj blind$).ti,ab. 18 (singl$ adj blind$).ti,ab. 19 or/12‐18 20 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 21 human/ or human cell/ 22 and/20‐21 23 20 not 22 24 19 not 23 25 11 and 24
EBSCO CINAHL
S23 S10 AND S22 S22 S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 S21 TX allocat* random* S20 (MH "Quantitative Studies") S19 (MH "Placebos") S18 TX placebo* S17 TX random* allocat* S16 (MH "Random Assignment") S15 TX randomi* control* trial* S14 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) S13 TX clinic* n1 trial* S12 PT Clinical trial S11 (MH "Clinical Trials+") S10 S5 and S9 S9 S6 or S7 or S8 S8 TI ozon* or AB ozon* S7 (MH "Ozone Therapy") S6 (MH "Ozone") S5 S1 or S2 or S3 or S4 S4 TI diabet* N3 wound* or AB diabet* N3 wound* S3 TI ( diabet* N3 foot OR diabet* N3 feet ) or AB ( diabet* N3 foot OR diabet* N3 feet ) S2 TI diabet* N3 ulcer* or AB diabet* N3 ulcer* S1 (MH "Foot Ulcer+")
Clinical Trials Registries
Search terms:
"ozone and diabet*"
Appendix 2. Risk of bias
(1)Random sequence generation
Criteria for a judgement of 'Low risk' of bias.
The investigators describe a random component in the sequence generation process such as:
Referring to a random number table;
Using a computer random number generator;
Coin tossing;
Shuffling cards or envelopes;
Throwing dice;
Drawing of lots;
Minimisation*.
*Minimisation may be implemented without a random element, and this is considered to be equivalent to being random.
Criteria for the judgement of 'High risk' of bias.
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Sequence generated by odd or even date of birth;
Sequence generated by some rule based on date (or day) of admission;
Sequence generated by some rule based on hospital or clinic record number.
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example:
Allocation by judgement of the clinician;
Allocation by preference of the participant;
Allocation based on the results of a laboratory test or a series of tests
Allocation by availability of the intervention.
Criteria for the judgement of 'Unclear risk' of bias.
Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk'.
(2)Allocation concealment
Criteria for a judgement of 'Low risk' of bias.
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
Central allocation (including telephone, web‐based and pharmacy‐controlled randomisation);
Sequentially numbered drug containers of identical appearance;
Sequentially numbered, opaque, sealed envelopes.
Criteria for the judgement of 'High risk' of bias.
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
Using an open random allocation schedule (e.g. a list of random numbers);
Assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered);
Alternation or rotation;
Date of birth;
Case record number;
Any other explicitly unconcealed procedure.
Criteria for the judgement of 'Unclear risk' of bias.
Insufficient information to permit judgement of 'Low risk' or 'High risk'. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
(3)Blinding of participants and personnel
Criteria for a judgement of 'Low risk' of bias.
Any one of the following:
No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding;
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Criteria for the judgement of 'High risk' of bias.
Any one of the following:
No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding;
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Criteria for the judgement of 'Unclear risk' of bias.
Any one of the following:
Insufficient information to permit judgement of 'Low risk' or 'High risk';
The study did not address this outcome.
(4)Blinding of outcome assessment
Criteria for a judgement of 'Low risk' of bias.
Any one of the following:
No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding;
Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Criteria for the judgement of 'High risk' of bias.
Any one of the following:
No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding;
Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Criteria for the judgement of 'Unclear risk' of bias.
Any one of the following:
Insufficient information to permit judgement of 'Low risk' or 'High risk';
The study did not address this outcome.
(5)Incomplete outcome data
Criteria for a judgement of 'Low risk' of bias.
Any one of the following:
No missing outcome data;
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups;
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate;
For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size;
Missing data have been imputed using appropriate methods.
Criteria for the judgement of 'High risk' of bias.
Any one of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups;
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate;
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size;
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation;
Potentially inappropriate application of simple imputation.
Criteria for the judgement of 'Unclear risk' of bias.
Any one of the following:
Insufficient reporting of attrition/exclusions to permit judgement of 'Low risk' or 'High risk' (e.g. number randomised not stated, no reasons for missing data provided);
The study did not address this outcome.
(6)Selective reporting
Criteria for a judgement of 'Low risk' of bias.
Any of the following:
The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way;
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
Criteria for the judgement of 'High risk' of bias.
Any one of the following:
Not all of the study's pre‐specified primary outcomes have been reported;
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified;
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect);
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis;
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Criteria for the judgement of 'Unclear risk' of bias.
Insufficient information to permit judgement of 'Low risk' or 'High risk'. It is likely that the majority of studies will fall into this category.
(7)Other bias
Criteria for a judgement of 'Low risk' of bias.
The study appears to be free of other sources of bias(extreme baseline imbalance and sponsorship bias).
Criteria for the judgement of 'High risk' of bias.
There is at least one important risk of bias. For example, the study:
Had a potential source of bias related to the specific study design used; or
Has been claimed to have been fraudulent; or
Had some other problem.
Criteria for the judgement of 'Unclear risk' of bias.
There may be a risk of bias, but there is either:
Insufficient information to assess whether an important risk of bias exists; or
Insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Ozone versus antibiotic treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ulcers healed | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.87, 1.40] |
| 2 Reduction in ulcer area | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐20.54 [‐20.61, ‐20.47] |
| 3 Length of hospitalisation | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐14.17, ‐1.83] |
Comparison 2. Ozone + usual care versus sham ozone + usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of ulcers healed | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.90, 3.17] |
| 2 Reduction in ulcer area | 2 | 111 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐5.29, 1.07] |
| 3 Adverse events/complications | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.48, 10.79] |
| 4 amputation | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.12, 64.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Martínez‐Sánchez 2005.
| Methods | RCT Randomisation: mentioned Allocation concealment: unclear Blinding: unblinded n = 101 |
|
| Participants | Inclusion criteria: adults of both sexes and any ethnic origins with a diagnosis of neuro‐infectious diabetic foot Exclusion criteria: severe septic conditions, hypersensitivity to planned treatments, hepatic dysfunction, renal failure (serum creatinine level >1.32 μmol/l), pregnancy, cancer or other serious disease, inability to co‐operate with the requirements of the study, recent history of alcohol or drug abuse, current therapy with any immunosuppressive agent or anticonvulsant, concurrent participation in another clinical study, or current treatment with an investigational drug Gender: 26:26 in ozone group (female:male); 19:30 in the control group (female:male) Evolution time of the disease (Mean±SD): 17 ± 11 years vs 18 ± 8 (ozone vs control) Age: > = 20 years |
|
| Interventions |
Intervention: ozone (generated by an OZOMED equipment, Cuba), 20 sessions, by rectal insufflation (with an ozone dose of 10 mg, ozone concentration: 50 mg/l) and locally. For local ozone treatment, the lesion was covered with a plastic bag sealed to the leg, which was then put under vacuum, in order to eliminate the air inside it. Afterwards, the bag was refilled with ozone at a concentration of 60 mg/l. The participant remained with the plastic bag in place for 1 h. After that, the bag was removed and the lesion was covered with ozonized sunflower oil (Oleozon®) (n = 51) Control: antibiotic therapy (systemic antibiotic therapy using the conventional method of treatment, with topical application to the lesion for 20 days) (n = 49) Other details: debridement was indicated for essentially every wound and gauze dressings were used |
|
| Outcomes |
|
|
| Notes | Follow‐up was 20 days there was one drop‐out in the ozone group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised to two different groups of treatment: . . ." "This randomised controlled clinical trial was approved by an institutional review board (Scientific and Ethics Committees of the Institution) in accordance with the principle of the Declaration of Helsinki (1997)." "In relation to the baseline characteristics (Table 1), both groups were similar at randomisation (P>0.05)." The study was described as a randomised clinical trial. Randomisation method was not well described |
| Allocation concealment (selection bias) | Unclear risk | The included study was described as a randomised clinical trial. Method for concealment of allocation was not well described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors stated the number recruited (101), and the fact that there was one drop‐out in the ozone group. The numbers of participants in the analysis were given (100). The authors did not respond to our requests for clarification. This one drop‐out is unlikely to have had a significant impact on the results, as the baseline variables for both groups were comparable |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes mentioned in the methods section were reported in the results. We could not retrieve the protocol |
| Other bias | Unclear risk | The authors did not respond to our requests for clarification. We found no significant statistical differences between groups for baseline variables. We could not judge whether there were any other potential sources of other bias, despite contacting the authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors did not use blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The authors did not use blinding |
Wainstein 2011.
| Methods | Multicentred, placebo‐controlled RCT Randomisation: mentioned Allocation concealment: unclear Blinding: blinded to participants and the investigator |
|
| Participants | Inclusion criteria: adult (18 years of age and over) men and women with type 1 and type 2 diabetes and a Wagner classification stage 2 or 3 or post‐debridement stage 4 foot ulcer. Wound size ≤ 40 cm2, with the wound at least 8 weeks old at study initiation Exclsuon criteria: gangrenous foot ulcers, active osteomyelitis, a history of collagen diseases, hyperthyroidism, pregnant or nursing, haemoglobin A1c (HbA1c) levels > 10.5%, ankle brachial index < 0.65, haemoglobin < 8 g/dL, liver function tests (alanine transaminase, aspartate transaminase, or c‐glutamyl transpeptidase) elevated to more than 3 times the upper normal limit, serum creatinine > 2.5 mg/dL or dialysis, or a known allergy to ozone Age for those who completed the treatment: 40–81 years vs 46–62 years (ozone vs control) Gender for those who completed the treatment: female:male: 13:19 vs 10:19 (ozone vs control) Duration of diabetes (Mean±SD): 15.2 ± 9.7 years vs 16.4 ± 11.0 years (ozone vs control) |
|
| Interventions |
Intervention: ozone treatments using the Ozoter 101 device (OZ Recovery Technologies, Ramat Gan, Israel) in addition to usual care (n=32) Control: sham ozone treatments using the Ozoter 101 device set to the inactive mode in addition to usual care (n=29) Usual care included debridement and daily wound dressings appropriate for the degree of secretion and moisture maintenance of the wound Active ozone treatment was divided into two phases: at first, patients received treatment sessions four times each week for the maximum period of 4 weeks, or until granulation appeared in 50% of the wound area, whichever came first. Intervals between treatments did not exceed 1 day in 5 days a week, and gas concentrations were 96% oxygen and 4% (80lg/mL) ozone. During the second treatment period, session frequency was reduced to twice a week to complete the 12 weeks of treatment, and gas concentration was changed to 98% oxygen and 2% (40lg/mL) ozone. Patients in the control group received sham treatments, and the ozone device circulated room air only. Each treatment session lasted 26 min. |
|
| Outcomes |
Primary end point: the proportion of subjects with complete closure of the wound Secondary end point: wound size and the proportion of participants who had a reduction in wound size Adverse events: deterioration of the target ulcer (manifested as new‐onset erythema, pain, purulent discharge, infection, tissue necrosis, requirement for repeated debridement, or other surgical intervention such as amputation) was considered a serious adverse event, and the participant concerned was then considered a 'non‐responder' even if the ulcer was completely closed prior to the final visit Total area closed: calculated for each participant by subtracting the last available wound size measurement from the first one |
|
| Notes | Follow‐up was 24 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study was a randomised, double‐blind, placebo controlled clinical trial." "Included patients were randomised to one of two treatment groups." "The technician determined the mode of action for each patient according to the randomisations, so that 32 patients were treated in the active model (ozone–oxygen treatment), and 29 in the inactive mode (room air only)." The study was described as a randomised clinical trial. Randomisation method was not well described |
| Allocation concealment (selection bias) | Unclear risk | "The study was a randomised, double‐blind, placebo controlled clinical trial." "Included patients were randomised to one of two treatment groups." "The technician determined the mode of action for each patient according to the randomisations, so that 32 patients were treated in the active model (ozone–oxygen treatment), and 29 in the inactive mode (room air only)." The study was described as a randomised clinical trial. The method for concealment of allocation was not well described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 61 participants with DM were randomised: 32 to the active ozone treatment and 29 to the sham treatment. All participants randomised to treatment were included in the ITT cohort. A total of 34 participants completed the study: 16 in the ozone group and 18 in the sham treatment group |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes mentioned in the methods section were reported in the results. We could not retrieve the protocol |
| Other bias | Low risk | No competing financial interests existed. We found no significant statistical differences between both groups for the baseline variables |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patients and the investigator were blinded to the mode of treatment." "The ozone (active treatment) group received ozone treatments using the Ozoter 101 device (OZ Recovery Technologies, Ramat Gan, Israel) in addition to usual care. The control group received sham treatments using the Ozoter 101 device set to the inactive mode in addition to usual care." "The patients and the investigator were blinded to the mode of treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The patients and the investigator were blinded to the mode of treatment." "The ozone (active treatment) group received ozone treatments using the Ozoter 101 device (OZ Recovery Technologies, Ramat Gan, Israel) in addition to usual care. The control group received sham treatments using the Ozoter 101 device set to the inactive mode in addition to usual care." "The patients and the investigator were blinded to the mode of treatment." |
Zhang 2014.
| Methods | RCT Randomisation: mentioned Allocation concealment: unclear Blinding: unclear |
|
| Participants |
Inclusion criteria: Hospitalized patients with type 2 diabetes mellitus (T2DM) aged 18 yrs or older, with Diabetic foot ulcer of Wagner classification stages 2, 3, or 4 Patients were excluded from the study if they had one or more of the following conditions: (1) gangrenous ulcers in whole foot, (2) active osteomyelitis, (3) a history of collagen diseases, (4) hyperthyroidism, (5) pregnancy or nursing, (6) hemoglobin A1c (HbA1c) levels >10.5%, (7) ankle brachial index (ABI) <0.70, (8) hemoglobin less than 90 g/L, (9) liver function tests (alanine transaminase, aspartate transaminase, or c‐glutamyl transpeptidase) elevated to more than three times the upper normal limit, (10) serum creatinine >133 mol/L or dialysis, and (11) a known allergy to ozone. |
|
| Interventions | After debridement, the ozone group received noninvasive oxygen‐ozone treatments with 52 μg/mL ozone (total volume: 20‐50 mL) in a special bag for 30 min per day for 20 days using the ozone generator device (Humazon Promedic, German) in addition to standard treatment.(n=25) The control group received only standard treatment which included debridement once every two days and wound dressings appropriate for the degree of exudate and moisture maintenance of the wound.(n=25) | |
| Outcomes | The proportion of subjects with complete closure of the wound Change in area of ulcer |
|
| Notes | Follow‐up was 20 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Included patients were randomized into two groups." "As indicated in Figure 3, 50 patients with DFU were randomized to ozone group (n = 25) and control group (n = 25)." The study was described as a randomised clinical trial. Randomisation method was not well described |
| Allocation concealment (selection bias) | Unclear risk | "Included patients were randomized into two groups." "As indicated in Figure 3, 50 patients with DFU were randomized to ozone group (n = 25) and control group (n = 25)." The study was described as a randomised clinical trial. Allocation concealment method was not well described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "50 patients with DFU were randomized to ozone group (n = 25) and control group (n = 25). All of these patients completed the study visits." |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes mentioned in the methods section were reported in the results. We could not retrieve the protocol. |
| Other bias | Unclear risk | Competing financial interests existed. We found no significant statistical differences between both groups for the baseline variables. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors did not use blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The authors did not use blinding. |
Abbreviations
< = less than ≤ = less than or equal to DM = diabetes mellitus h = hour(s) ITT = intention‐to‐treat analysis RCT = randomised controlled trial vs = versus
DFU=Diabetic foot ulcer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 2011 | Not an RCT |
| Falanga 2006 | Not an RCT |
| Gazin 2008a | Not an RCT |
| Gazin 2008b | Not an RCT |
| Kulikov 2002 | Not an RCT |
Abbreviations
RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01643967.
| Trial name or title | Clinical trial to evaluate the efficacy and safety of the use of ozone versus sunflower oil in treating diabetic foot (PHIOZO0110) |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Ages eligible for study: 18 years and older Genders eligible for study: both Accepts healthy volunteers: no Inclusion criteria: consent form signed; people with DM type I or type II trophic lesions presenting in the lower limbs; injury whose largest diameter is less than 5 cm; heart rate 60‐100 bpm |
| Interventions | Experimental arm: ozone therapy; participants received topical and rectal insufflation of ozone 3 times a week on alternate days for 2 months or at least 12 sessions Other arm: sunflower oil; participants in this arm received sunflower oil supplied by Philozon. Sunflower oil should be applied once daily for 60 days immediately after cleansing the lesion site |
| Outcomes | Primary outcome measures: Evaluate the efficacy and safety of ozone released by the Philozon Medplus device in the treatment of patients with diabetic foot [time frame: 65 days] [designated as safety issue: yes] Evaluate the efficacy and safety of the release of ozone by the Philozon Medplus device in the treatment of patients with diabetic foot, through clinical evaluation based on the time needed for healing, photographing and measuring the largest diameter of the lesion, in order to calculate the size of the lesion in cm2 Secondary outcome measures: Evaluate the release of ozone in the environment by Medplus equipment after rectal insufflation in patients with diabetic foot [time frame: 65 days] [designated as safety issue: yes] Evaluate environmental ozone released by the device Philozon Medplus using ozone analyser in the clinical setting after using the device for rectal insufflation in a patient with diabetic foot |
| Starting date | June 2012 |
| Contact information | Contact: Renato T Santos, Investigator 5554‐3312‐2099 renatotadeus@gmail.com Contact: Keyla L Deucher, Coordinator 5554‐2103‐4064 pesquisaclinica.hsvp@gmail.com |
| Notes |
Differences between protocol and review
We have added 'Summary of finding' tables in both the Methods and Results sections. Meanwhile, we changed the inclusion criteria, as we added "Ozone therapy combined with a concomitant treatment compared with any other intervention used to promote healing, such as antibiotics, topical agents or usual care based, as long as the same concomitant treatment was used in both groups." in the inclusion criteria. We also specified that sham acupuncture in combination with any of those other interventions used to promote healing was eligible as a control.
Contributions of authors
KeHu Yang: conceived, designed and coordinated the review; checked the quality of data extraction; checked quality assessment; performed part of data analysis/ interpretation; performed statistical analysis; checked the quality of the statistical analysis; completed the first draft of the review; performed part of writing/editing the review; advised on, and made an intellectual contribution to the review; approved the final review prior to submission; secured funding; and is a guarantor of the review.
Peng Zhang: conceived, designed and coordinated the review; extracted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; completed the first draft of the review; advised on and made an intellectual contribution to the review; approved the final review prior to submission; and secured funding.
Jian Liu: conceived the review; checked the quality of data extraction; analysed/ interpreted data; performed statistical analysis; completed the first draft of the review; made an intellectual contribution to the review; advised on part of the review; approved the final review prior to submission;
Lun Li: conceived and coordinated the review; extracted data; undertook quality assessment; analysed/interpreted data; performed statistical analysis; checked the quality of the statistical analysis; completed the first draft of the review; advised on and made an intellectual contribution to the review; approved the final review prior to submission.
Jing Tian: designed and coordinated the review; extracted data; undertook quality assessment; checked the quality of the statistical analysis; performed part of writing or editing the review; and approved the final review prior to submission.
Jin Hui Tian: extracted data; undertook quality assessment; analysed/interpreted data; performed statistical analysis; completed the first draft of the review; advised on and made an intellectual contribution to the review; approved the final review prior to submission.
Jun Li: checked the quality of data extraction; analysed/interpreted data; checked quality assessment; completed the first draft of the review; advised on and approved the final review prior to submission; and wrote to study author / experts / companies.
Contributions of editorial base
Nicky Cullum, editor: edited the protocol; advised on methodology; approved the final protocol prior to submission. Susan O'Meara, editor: edited the review; advised on methodology; approved the final review prior to submission. Sally Bell‐Syer: co‐ordinated the editorial process; advised on methodology, interpretation and content. Edited the review. Amanda Briant: designed the search strategy and edited the search methods section.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This project was funded by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or Department of Health.
Declarations of interest
All authors in this review do not have any present or past affiliations or other involvement in any organisation or entity with an interest in the outcome of the review that might lead to a real or perceived conflict of interest (including acting as an investigator of a study that might be included in this review). All the judgements during the review making were made in the absence of any potential conflicts.
KeHu Yang: nothing to declare. Peng Zhang: nothing to declare. Jian Liu: nothing to declare. Lun Li: nothing to declare. Jing Tian: nothing to declare. Jin Hui Tian: nothing to declare. Jun Li: nothing to declare.
New
References
References to studies included in this review
Martínez‐Sánchez 2005 {published data only}
- Martinez‐Sanchez G, Al‐Dalain SM, Menendez S, Re L, Giuliani A, Candelario‐Jalil E, et al. Therapeutic efficacy of ozone in patients with diabetic foot. European Journal of Pharmacology 2005;523:151‐61. [DOI] [PubMed] [Google Scholar]
Wainstein 2011 {published data only}
- Wainstein J, Feldbrin Z, Boaz M, Harman‐Boehm I. Efficacy of ozone‐oxygen therapy for the treatment of diabetic foot ulcers. Diabetes Technology & Therapeutics 2011;13(12):1255‐60. [DOI: 10.1089/dia.2011.0018] [DOI] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang J, Guan M, Xie C, Luo X, Zhang Q, Xue Y. Increased growth factors play a role in wound healing promoted by noninvasive oxygen‐ozone therapy in diabetic patients with foot ulcers. Oxidative Medicine and Cellular Longevity 2014 Jun 24 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 2011 {published data only}
- Anonymous. Ultrasonic cavitation and ozonization in treatment of patients with pyo‐necrotic complications of diabetic foot syndrome. Vestnik Khirurgii Imeni I. I. Grekova 2011;170(1):48‐53. [PubMed] [Google Scholar]
Falanga 2006 {published data only}
- Falanga V, Saap LJ, Ozonoff A. Wound bed score and its correlation with healing of chronic wounds. Dermatologic Therapy 2006;19(6):383‐90. [DOI] [PubMed] [Google Scholar]
Gazin 2008a {published data only}
- Gazin IK. Criteria for intoxication in the evaluation of severity of endotoxicosis, the efficiency of ozone therapy and traditional treatment in patients with diabetes mellitus complicated by pyonecrotic lesions of the lower extremities. Klinicheskaia Laboratornaia Diagnostika 2008;6:21‐3. [PubMed] [Google Scholar]
Gazin 2008b {published data only}
- Gazin IK. Pathophysiological aspects of endotoxicosis complicated with purulent infection of the foot and correction of endotoxicosis with conventional treatment and with application of ozonized physiological solution in patients suffering from diabetes mellitus. Patologicheskaia Fiziologiia i Eksperimentalnaia Terapiia 2008;4:23‐5. [PubMed] [Google Scholar]
Kulikov 2002 {published data only}
- Kulikov AG, Turova EA, Shcherbina TM, Kisileva OM. Efficacy of different methods of ozone therapy in vascular complications of diabetes mellitus. Voprosy Kurortologii, Fizioterapii, i Lechebnoi Fizicheskoi Kultury 2002;5:17‐20. [PubMed] [Google Scholar]
References to ongoing studies
NCT01643967 {published data only}
- NCT01643967. Clinical trial to evaluate the efficacy and safety of the use of ozone versus sunflower oil in treating diabetic foot (PHIOZO0110). http://clinicaltrials.gov/ct2/show/NCT01643967 (date of access needs to be added).
Additional references
Bertolotti 2013
- Bertolotti A, Izzo A, Grigolato PG, Iabichella ML. The use of ozone therapy in Buruli ulcer had an excellent outcome. BMJ Case Reports 2013 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bialoszewski 2003
- Bialoszewski D, Kowalewski M. Superficially, longer, intermittent ozone therapy in the treatment of the chronic, infected wounds. Ortopedia, Traumatologia, Rehabilitacja 2003;5(5):652‐8. [PubMed] [Google Scholar]
Bjelakovic 2014
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD007469.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bocci 2004
- Bocci V. Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediators of Inflammation 2004;13(1):3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bocci 2009
- Bocci V, Paolo N. Oxygen‐ozone therapy in medicine: an update. Blood purification 2009;28(4):373‐6. [DOI] [PubMed] [Google Scholar]
Boyko 1996
- Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased mortality associated with diabetic foot ulcer. Diabetic Medicine 1996;13(11):967‐72. [DOI] [PubMed] [Google Scholar]
Brownrigg 2012
- Brownrigg JR, Davey J, Holt PJ, Davis WA, Thompson MM, Ray KK, et al. The association of ulceration of the foot with cardiovascular and all‐cause mortality in patients with diabetes: a meta‐analysis. Diabetologia 2012;55(11):2906‐12. [DOI] [PubMed] [Google Scholar]
Caravaggi 2013
- Caravaggi C, Ferraresi R, Bassetti M, Sganzaroli AB, Galenda P, Fattori S, et al. Management of ischemic diabetic foot. The Journal of Cardiovascular Surgery 2013;54(6):737‐54. [PubMed] [Google Scholar]
de Monte 2005
- Monte A, Zee H, Bocci V. Major ozonated autohemotherapy in chronic limb ischemia with ulcerations. Journal of Alternative and Complementary Medicine 2005;11:363‐7. [DOI] [PubMed] [Google Scholar]
Di Filippo 2010
- Filippo C, Cervone C, Rossi C, Ronza C, Marfella R, Capodanno P, et al. Antiarrhythmic effect of acute oxygen‐ozone administration to rats. European Journal of Pharmacology 2010;629(1‐3):89‐95. [DOI] [PubMed] [Google Scholar]
Dumville 2013
- Dumville JC, Hinchliffe RJ, Cullum N, Game F, Stubbs N, Sweeting M, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010318.pub2] [DOI] [PubMed] [Google Scholar]
Dähnhardt 2008
- Dähnhardt JE, Gygax M, Martignoni B, Suter P, Lussi A. Treating sensitive cervical areas with ozone: a prospective controlled clinical trial. American Journal of Dentistry 2008;21(2):74‐6. [PubMed] [Google Scholar]
Elvis 2011
- Elvis AM, Ekta JS. Ozone therapy: a clinical review. Journal of Natural Science, Biology and Medicine 2011;2(1):66‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gu 2010
- Gu XB, Yang XJ, Zhu HY, Xu YQ, Liu XY. Effect of medical ozone therapy on renal blood flow and renal function of patients with chronic severe hepatitis. Chinese Medical Journal 2010;123(18):2510‐3. [PubMed] [Google Scholar]
Gupta 2012a
- Gupta SK, Singh SK. Diabetic foot: a continuing challenge. Advances in Experimental Medicine and Biology 2012;771:123‐38. [PubMed] [Google Scholar]
Gupta 2012b
- Gupta G, Mansi B. Ozone therapy in periodontics. Journal of Medicine and Life 2012;5(1):59‐67. [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8. Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Jeffcoate 2004
- Jeffcoate WJ, Price P, Harding KG, International Working Group on Wound Healing and Treatments for People with Diabetic Foot Ulcers. Wound healing and treatments for people with diabetic foot ulcers. Diabetes/Metabolism Research and Reviews 2004;20(Suppl 1):S78‐89. [DOI] [PubMed] [Google Scholar]
Kerr 2012
- Kerr M. Foot care for people with diabetes: the economic case for change. https://www.diabetes.org.uk/Documents/nhs‐diabetes/footcare/footcare‐for‐people‐with‐diabetes.pdf (date of access needs to be added).
Kim 2009
- Kim HS, Noh SU, Han YW, Kim KM, Kang H, Kim HO, et al. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. Journal of Korean Medical Science 2009;24(3):368‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2009
- Lau TW, Chan YW, Lau CP, Lau KM, Lau CB, Fung KP, et al. Radix Astragali and Radix Rehmanniae, the principal components of two antidiabetic foot ulcer herbal formulae, elicit viability‐promoting effects on primary fibroblasts cultured from diabetic foot ulcer tissues. Phytotherapy Research 2009;23(6):809‐15. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Magalhaes 2012
- Magalhaes FN, Dotta L, Sasse A, Teixera MJ, Fonoff ET. Ozone therapy as a treatment for low back pain secondary to herniated disc: a systematic review and meta‐analysis of randomized controlled trials. Pain Physician 2012;15(2):E115‐29. [PubMed] [Google Scholar]
Malhotra 2012
- Malhotra S, Bello E, Kominsky S. Diabetic foot ulcerations: biomechanics, charcot foot, and total contact cast. Seminars in Vascular Surgery 2012;25(2):66‐9. [DOI] [PubMed] [Google Scholar]
Margolis 2003
- Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. American Journal of Medicine 2003;115:627‐31. [DOI] [PubMed] [Google Scholar]
Martínez‐Sánchez 2012
- Martínez‐Sánchez G, Delgado‐Roche L, Díaz‐Batista A, Pérez‐Davison G, Re L. Effects of ozone therapy on haemostatic and oxidative stress index in coronary artery disease. European Journal of Pharmacology 2012;691(1‐3):156‐62. [DOI] [PubMed] [Google Scholar]
Morris 1998
- Morris AD, McAlpine R, Steinke D, Boyle DI, Ebrahim AR, Vasudev N, et al. Diabetes and lower limb amputations in the community. A retrospective cohort study. DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland/Medicines Monitoring Unit. Diabetes Care 1998;21(5):738‐43. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Pittet 1999
- Pittet D, Wyssa B, Herter‐Clavel C, Kursteiner K, Vaucher J, Lew PD. Outcome of diabetic foot infections treated conservatively: a retrospective cohort study with long‐term follow‐up. Archives of Internal Medicine 1999;159(8):851‐6. [DOI] [PubMed] [Google Scholar]
Ragab 2009
- Ragab A, Shreef E, Behiry E, Zalat S, Noaman M. Randomised, double‐blinded, placebo‐controlled, clinical trial of ozone therapy as treatment of sudden sensorineural hearing loss. The Journal of Laryngology & Otology 2009;123(1):54‐60. [DOI] [PubMed] [Google Scholar]
Re 2008
- Re L, Mawsouf MN, Menéndez S, León OS, Sánchez GM, Hernández F. Ozone therapy: clinical and basic evidence of its therapeutic potential. Archives of Medical Research 2008;39(1):17‐26. [DOI] [PubMed] [Google Scholar]
Robbins 2008
- Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y. Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration?. Journal of the American Podiatric Medical Association 2008;98(6):489‐93. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Shah 2011
- Shah P, Shyam AK, Shah S. Adjuvant combined ozone therapy for extensive wound over tibia. Indian Journal of Orthopaedics 2011;45(4):376‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2009
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 15 June 2009).
Singh 2005
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217‐28. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.. Available from www.cochrane‐handbook.org. [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;7(8):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valacchi 2005
- Valacchi G, Fortino V, Bocci V. The dual action of ozone on the skin. British Journal of Dermatology 2005;153(6):1096‐100. [DOI] [PubMed] [Google Scholar]
van Sloten 2008
- Sloten TT, Friederichs SA, Huijberts MS, Schaper NC. Diabetic foot: new insights into pathophysiology and treatment. Nederlands Tijdschrift voor Geneeskunde 2008;152(44):2400‐5. [PubMed] [Google Scholar]
Venkatesan 1997
- Venkatesan P, Lawn S, Macfarlane RM, Fletcher EM, Finch RG, Jeffcoate WJ. Conservative management of osteomyelitis in the feet of diabetic patients. Diabetic Medicine 1997;14(6):487‐90. [DOI] [PubMed] [Google Scholar]
Wild 2004
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047‐53. [DOI] [PubMed] [Google Scholar]
Wolcott 2015
- Wolcott R. Economic aspects of biofilm‐based wound care in diabetic foot ulcers. Journal of Wound Care 2015;24(5):189‐90, 192‐4. [DOI] [PubMed] [Google Scholar]
Wrobel 2001
- Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower limb extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes Care 2001;24(5):860‐4. [DOI] [PubMed] [Google Scholar]
Xie 2008
- Xie XS, Wang YJ, Zuo C, Fan JM, Li XJ. A case report of an effective treatment for diabetic foot ulcers with integration of traditional Chinese medicine and Western medicine. Journal of Diabetes and its Complications 2008;23(5):360‐4. [DOI] [PubMed] [Google Scholar]


