Abstract
Diquat is a widely used herbicide that is substituted for paraquat. With paraquat off the market, cases of diquat poisoning have been gradually increasing. The kidney is the most frequently impaired organ in diquat poisoning. Few cases of multiple organ failure caused by diquat have been reported.
We herein describe a 30-year-old man who orally ingested about 160 mL of enriched diquat. Despite aggressive treatment, the patient’s condition progressed to multiple organ failure and death. The pulmonary lesions in this patient were different from those previously reported. This patient did not die of renal failure but of severe respiratory failure. He exhibited three different stages of pulmonary disease.
The lung lesions in this case were unique. We hope that doctors will pay more attention to the lung lesions in patients with diquat poisoning in future and find new treatment methods to save the lives of such patients.
Keywords: Diquat, poisoning, respiratory failure, multiple organ dysfunction syndrome, herbicide, case report
Introduction
Diquat is a widely used herbicide that is substituted for paraquat. Because of its high fatality rate, paraquat was discontinued from sale and use in China on 1 July 2016. With paraquat off the market, diquat has taken its place. In recent years, reported cases of diquat poisoning have been gradually increasing. 1 , 2 Patients with diquat poisoning develop multiple organ dysfunction and may even die despite diquat requiring a higher dose than paraquat to be lethal. Because of the lack of an effective antidote for diquat poisoning, treatment mainly focuses on symptomatic relief and organ function support; this is also the reason for the poor prognosis in affected patients.
We recently treated an adult patient with high-dose diquat poisoning. Although the patient was not successfully treated in the end, he developed successive failure of multiple organs, which differs from most clinical case reports. We herein report this case of high-dose diquat poisoning. This report conforms to the CARE guideline. 3
Case presentation
A 30-year-old man was admitted to the intensive care unit (ICU) of our hospital with an approximately 12-hour history of oliguria. He was 180 cm tall and weighed 70 kg. The patient and his sister provided the history.
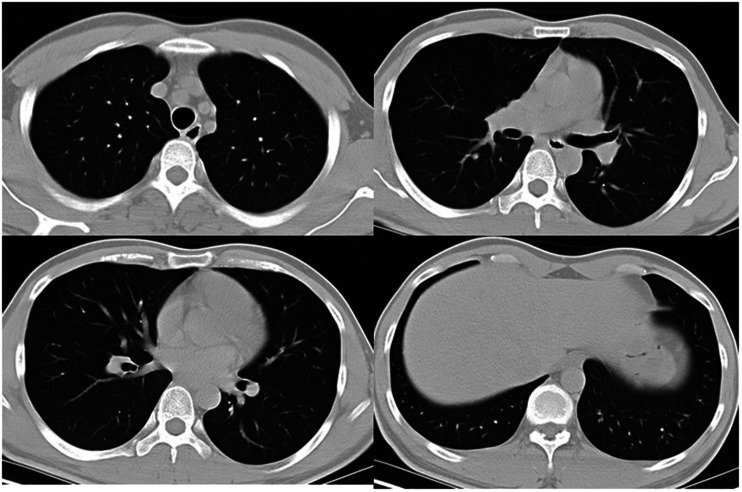
Two days before admission, the patient had orally ingested about 160 mL of enriched diquat (20 g per 100 mL) because of debt-related problems. Three hours later, the patient presented to another hospital for treatment and underwent gastric lavage. At that time, his blood pressure and heart rate were normal, and he had no shortness of breath. His blood creatinine level, routine blood tests, transaminase levels, bilirubin level, coagulation function, troponin level, and electrocardiogram findings were all normal. Chest computed tomography (CT) showed no abnormalities (Figure 1). The patient refused further treatment and went home on his own. Moreover, a history of systematic disease including hypertension, diabetes, respiratory illness, hepatic or renal dysfunction, and mental disorder was denied by the patient and his family member, as was drug or alcohol abuse. His previous health condition was claimed to be quite good.
Figure 1.
Chest computed tomography on the day the patient orally ingested diquat.
One day before admission, the patient presented to the emergency department of our hospital because of pharyngeal pain and mild pain in the upper abdomen. At that time, blood tests showed a high creatinine level (267 μmol/L). The patient was given antioxidation and infusion therapy. However, his urine output decreased and his blood creatinine level increased to 541 μmol/L after 24 hours. The doctor recommended a blood purification treatment. However, the patient and his family refused for economic reasons. Seven hours later, the patient and his family agreed to the blood purification treatment. At that time, the patient’s transaminase and bilirubin levels were elevated. He was transferred to the ICU 57 hours after the oral diquat ingestion and developed kidney failure, liver dysfunction, and respiratory failure. He died of respiratory failure on day 13 of hospitalization.
Kidney failure
Diquat is a small molecular substance with molecular weight of 344.05, and it can easily pass through the cell membrane. Hemoperfusion is an important treatment method in the early stage of diquat poisoning. In the present case, hemoperfusion was performed immediately after admission to the ICU; however, only one 2-hour session of hemoperfusion was performed. Oliguria persisted during hospitalization; therefore, continuous renal replacement therapy (CRRT) was initiated following the hemoperfusion treatment. Postdilution continuous venovenous hemodiafiltration with an effluent flow of 30 mL/kg of body weight per hour was implemented until the patient died.
Respiratory failure
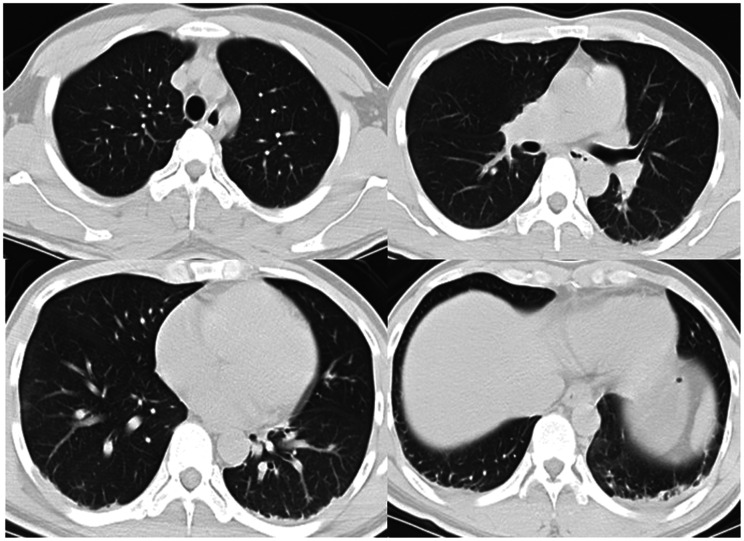
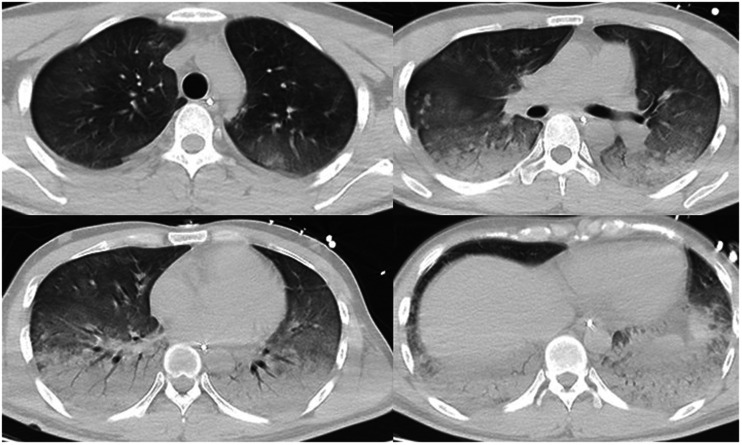
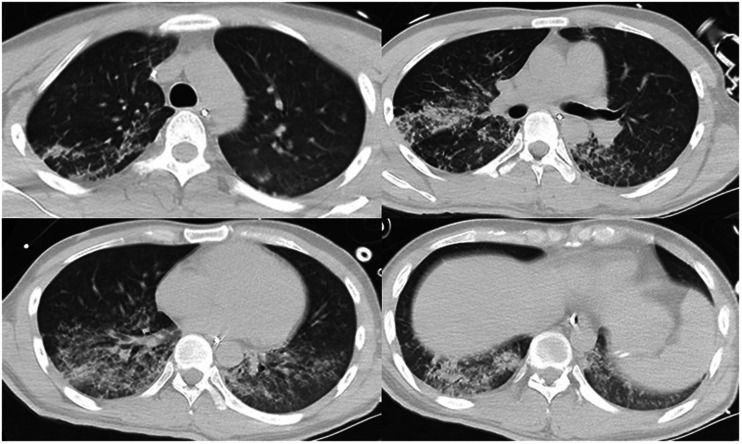
Upon presentation, the patient was conscious, had no shortness of breath, had a percutaneous oxygen saturation (SpO2) of 98% (fraction of inspired oxygen (FiO2), 21%), and had a normal blood pressure and heart rate. His arterial partial pressure of oxygen was acceptable (Table 1). Chest CT findings at that time appeared normal (Figure 2). He was not treated with oxygen therapy because the mechanism of diquat poisoning is mainly the generation of oxygen free radicals leading to cell apoptosis. 1 Although the leukocyte count was high, the procalcitonin (PCT) level was within the low range (Table 2). No antimicrobial agent was administered at that time. The patient’s clinical condition rapidly became exacerbated thereafter. On day 3 of hospitalization, the patient developed progressive shortness of breath and hypoxemia, and his SpO2 was maintained at only about 88% with conservative oxygen therapy. The patient was intubated and mechanically ventilated when the severe hypoxemia became unacceptable. After endotracheal intubation, piperacillin/tazobactam was empirically given for anti-infective therapy. Fiberbronchoscopy was performed after intubation, and a large amount of yellow plasma exudate was observed. Sputum was collected for culture (Table 3). Chest CT examination revealed exudation, consolidation, and ground-glass opacity in the bilateral lower lungs, confirming a diagnosis of acute respiratory distress syndrome (ARDS) (Figure 3). The patient’s oxygenation significantly improved after he was moved into the prone position; his FiO2 increased to 30% and his SpO2 was maintained at about 90%. On day 6 of hospitalization, another chest CT examination showed that the lung exudation was significantly reduced (Figure 4).
Table 1.
Blood gas analysis results.
| pH | PaO2 (kPa) | PaCO2 (kPa) | BE (mmol/L) | Lac (mmol/L) | |
|---|---|---|---|---|---|
| Reference range | 7.34–7.45 | 11.1–14.4 | 4.7–6.4 | −3.0–3.0 | 0.5–1.6 |
| Day 1 | 7.41 | 12.7 | 4.1 | −4.7 | 1.3 |
| Day 3 | 7.39 | 7.1 | 4.7 | −3.3 | 1.4 |
| Day 6 | 7.40 | 12.7 | 5.7 | 1.6 | 1.8 |
| Day 8 | 7.44 | 8.1 | 5.5 | 3.8 | 3.9 |
| Day 10 | 7.39 | 11.2 | 6.2 | 2.9 | 2.1 |
| Day 11 am | 7.43 | 10.1 | 5.3 | 1.9 | 1.9 |
| Day 11 pm | 7.16 | 9.7 | 10.4 | −1.3 | 1.4 |
| Day 12 am | 7.30 | 9.3 | 7.4 | 1.1 | 1.4 |
| Day 12 pm | 7.40 | 10.9 | 8.0 | 11.0 | 1.0 |
| Day 13 am | 7.19 | 10.0 | 10.8 | 2.1 | 1.3 |
| Day 13 noon | 7.17 | 9.0 | 13.2 | 6.5 | 1.4 |
PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; BE, base excess; Lac, lactate.
Figure 2.
Chest computed tomography on the third day after the patient orally ingested diquat. This was also the day on which the patient was admitted to the intensive care unit (Day 1).
Table 2.
Results of routine blood testing, liver and renal function testing, myocardial enzymes, and coagulation function of the patient.
| WBC (×109/L) | Hb (g/L) | PLT (×109/L) | CRP (mg/L) | PCT (ng/ml) | Pre-albumin (mg/L) | ALT (IU/L) | AST (IU/L) | TB (μmol/L) | DB (μmol/L) | Albumin (g/L) | Cr (μmol/L) | BUN (mmol/L) | Amylase (U/L) | cTnI (ng/mL) | BNP (pg/mL) | PT (s) | APTT (s) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference range | 3.97–9.15 | 131–172 | 85–303 | <10 | <0.5 | 180–380 | <50 | <50 | 5–21 | <3.4 | 35–52 | 74–110 | 2.8–7.2 | 28–100 | <0.03 | 0–100 | 9.9–15 | 25.1–39.5 |
| Day 1 | 17.66 | 148 | 155 | 33 | 0.19 | 301 | 184 | 275 | 60.7 | 41.0 | 39 | 639 | 20.9 | 247 | 0.02 | 144 | 13.2 | 29.0 |
| Day 3 | 17.85 | 150 | 117 | 91 | 0.25 | 207 | 109 | 79 | 44.8 | 42.1 | 32 | 492 | 15.5 | N/A | 0.15 | 473 | N/A | N/A |
| Day 6 | 14.38 | 134 | 208 | 72 | 0.33 | 234 | 326 | 279 | 103.1 | 80.6 | 31 | 294 | 12.5 | N/A | 0.05 | 97 | 12.8 | 26.0 |
| Day 8 | 25.57 | 148 | 280 | 49 | 0.50 | 334 | 777 | 218 | 172.2 | 127.7 | 32 | 303 | 7.9 | 305 | N/A | N/A | N/A | N/A |
| Day 10 | 23.60 | 119 | 264 | 133 | 0.83 | 316 | 287 | 74 | 220.1 | 159.4 | 28 | 333 | 8.4 | N/A | 0.22 | 102 | N/A | N/A |
| Day 11 | 29.28 | 117 | 309 | 161 | 0.92 | 280 | 241 | 75 | 352.5 | 281.3 | 36 | 312 | 8.8 | N/A | 0.36 | 203 | 13.8 | 30.3 |
| Day 12 | 26.77 | 90 | 301 | >200 | 3.39 | 239 | 184 | 60 | 441.3 | 361.0 | 37 | 291 | 10.5 | N/A | 0.19 | 162 | 15.5 | 28.8 |
| Day 13 | 30.54 | 80 | 330 | >200 | 4.85 | 204 | 174 | 67 | 436.7 | 359.3 | 32 | 306 | 10.7 | N/A | N/A | N/A | 13.3 | 29.3 |
WBC, white blood cells; Hb, hemoglobin; PLT, platelets; CRP, C-reactive protein; PCT, procalcitonin; ALT, alanine aminotransaminase; AST, aspartate aminotransaminase; TB, total bilirubin; DB, direct bilirubin; Cr, creatinine; BUN, blood urea nitrogen; cTnI, cardiac troponin I; BNP, brain natriuretic peptide; PT, prothrombin time; APTT, activated partial thromboplastin time; N/A, not available.
Table 3.
Sputum and blood culture results.
| Sputum | Blood | |
|---|---|---|
| Day 3 | – | N/A |
| Day 4 | – | N/A |
| Day 6 | – | N/A |
| Day 8 | Acinetobacter baumannii | N/A |
| Day 9 | – | N/A |
| Day 10 | – | – |
| Day 12 | – | N/A |
N/A, not available.
Figure 3.
Chest computed tomography on the third day after the patient was admitted to the intensive care unit (Day 3).
Figure 4.
Chest computed tomography on the sixth day after the patient was admitted to the intensive care unit (Day 6).
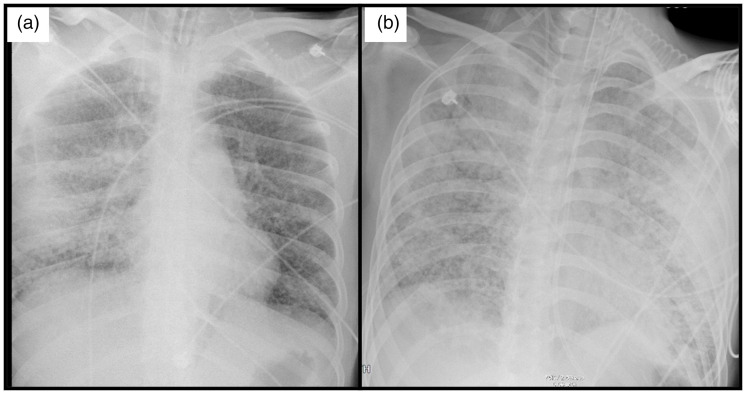
On day 8, however, the SpO2 began to gradually decline, especially after position changes. The FiO2 had to be increased, and higher inspiratory pressure was required to achieve a satisfactory tidal volume because of deterioration in the respiratory compliance. Deep sedation was required to relieve the sensation of asphyxia. At that time, the previous sputum culture revealed ampicillin-sensitive Acinetobacter baumannii. Thus, the treatment with piperacillin/tazobactam was continued. On day 10, a bedside chest radiograph showed interstitial lung lesions (Figure 5). Necrotic mucosal tissue could be seen in the bronchi branches under fiberbronchoscopy. However, the mucosal injury of the oropharynx had healed without mucosal abscission. No plasma exudate was present in the airway, but scattered bleeding was noted after mucosal exfoliation.
Figure 5.
Chest radiograph on the (a) 10th day and (b) 11th day after the patient was admitted to the intensive care unit.
On day 12, the patient’s dyspnea was extremely severe, and his autonomic inspiratory drive was so strong that muscle relaxants were required to maintain deep sedation. However, the respiratory compliance was attenuated and the arterial pressure of carbon dioxide increased rapidly as a result. The patient eventually developed severe carbon dioxide retention, respiratory acidosis, and distributed shock (Table 1), which led to his death on day 13.
Liver dysfunction
When the patient was transferred to the ICU, his serum levels of alanine aminotransferase and aspartate aminotransferase were 184 and 275 IU/L, respectively. His total bilirubin level was 60.7 μmol/L, and his direct bilirubin level was 41.0 μmol/L; both decreased slightly after hemoperfusion treatment. Vitamin C, vitamin B6, N-acetyl-L-cysteine, and glutathione were intravenously infused for antioxidation. Additionally, magnesium isoglycyrrhizinate injection, ademetionine 1,4-butanedisulfonate for injection, and ursodeoxycholic acid capsules were administered for liver protection from the beginning of hospitalization.
The patient’s total bilirubin and direct bilirubin levels increased again during CRRT maintenance (Table 2). An abdominal CT scan showed no reduction in liver volume on days 3 and 6. However, the prealbumin level was independent of the abnormal liver function. Both the prothrombin time and activated partial thromboplastin time were also normal.
Myocardial injury
The troponin level was closely monitored after admission, and the troponin I level slightly increased to a maximum of 0.36 ng/mL. In addition, bedside ultrasound monitoring showed no obvious abnormalities in cardiac function.
Discussion
Diquat has become a widely used herbicide in agriculture since the banning of paraquat in China. The lethal dose of paraquat at a concentration of 20% ranges from 5 to 15 mL. Paraquat has a unique and very unpleasant taste, and most patients spit it out or vomit after oral ingestion. The lethal dose of diquat at a concentration of 20% ranges from 6 to 12 g (30–60 mL). 1 , 4 However, diquat has no peculiar taste, and patients often do not vomit. The patient in this case orally ingested about 160 mL of diquat, which equates to 32 g. This dose is far above the maximum lethal dose. Fourteen days elapsed from the time the patient orally ingested diquat to the time the patient died. He arrived at our ICU 57 hours after ingestion, which was too late to administer effective treatment. Although we extended the patient’s survival time, he unfortunately died.
As a hydrophilic substance, diquat can enter the cell and constantly produce oxygen free radicals, leading to multiple organ damage. This is the main mechanism of organ dysfunction caused by diquat. 1 , 5 , 6 As an effective pro-oxidant, diquat has been used to induce oxidative stress in various in vivo and in vitro studies in the fields of toxicology and cellular biology. 5 , 7 , 8 The kidney is the most frequently impaired organ in diquat poisoning; however, there have also been reports of injuries to the liver, lungs, and pancreas. Patients develop multiple organ dysfunction before they die of diquat poisoning.
Unlike patients in previous case reports, our patient did not die of renal failure but of severe respiratory failure. He developed three stages of pulmonary disease: exudation, fibrosis and airway mucous membrane necrosis and exfoliation. In stage 1 (exudation), bilateral diffuse infiltration was found on the CT scan, exhibiting typical characteristics of ARDS. The prone position was very effective, and when combined with CRRT for fluid load balancing, the patient’s condition was significantly improved. In stage 2 (fibrosis), the patient developed bilateral diffuse interstitial lung lesions similar to those seen in paraquat poisoning. Respiratory compliance decreased sharply while the airway pressure increased significantly; this was combined with the onset of dyspnea, which explains the mechanism of recurrent respiratory failure. Very high doses of sedatives were required along with muscle relaxants to maintain oxygenation and ventilation, but the patient’s respiratory function had irrepressibly deteriorated. The changes that occurred in stage 3 (airway mucous membrane necrosis and exfoliation) have not been reported in previous cases. In our patient, diffuse bronchial mucosal exfoliation and intratracheal hemorrhage were initiated in the later stage of the disease course after the development of interstitial lesions, which were identified by bronchoscopy. During this stage of treatment, successive sputum and bronchoalveolar lavage fluid culture results were all negative despite the previous isolation of ampicillin-sensitive Acinetobacter baumannii. Although the patient had airway bleeding, the blood culture was negative. The PCT level was higher than the reference range; however, considering the presence of acute kidney injury and oliguria at the time of admission, the PCT could have been disturbed by renal dysfunction with no signs of infection. Multiple organ failure was present in the last few days of the patient’s life, which could have also affected the PCT level. The serum lactate level was always within the reference range, allowing us to rule out the possibility of septic shock. Notably, however, the patient developed shock with the decompensated respiratory acidosis caused by the sharp increase in the partial pressure of carbon dioxide. Because no reports have described diffuse mucosal exfoliation secondary to infection of Acinetobacter baumannii, we speculated that the mucosal exfoliation in this patient was associated with diquat poisoning.
Varga and Rákóczy 9 reported the highest concentration of diquat in lung tissue on the first day after diquat administration in rats. Under the mechanism of alveolar surfactant impairment and increased capillary permeability, degeneration of alveolar epithelial cells was seen in biopsy specimens, along with focal hemorrhagic edema and alveolar macrophages. Slight pulmonary fibrosis was observed around 2 to 4 weeks after poisoning, implying damage to the lung. 9 Another recent study indicated that the deterioration of the lung was associated with the dose of diquat. 10 These findings might partially explain the pathophysiologic course of our patient with the exception of the end-stage bronchial mucosal exfoliation.
We deduced that the mechanism underlying these pathological changes was the rich blood flow in the lung contributing to the large amount of diquat accumulation within the lung tissue. The pulmonary vascular endothelium was subsequently impaired, leading to pulmonary edema and ARDS. The vascular endothelium was spontaneously repaired during the next few days, and the ARDS gradually improved. However, with the exudation of diquat, large numbers of oxygen free radicals were distributed in the lung interstitium, causing pulmonary fibrosis and damage to the epithelial cells and bronchial mucosa. Apoptosed epithelial and mucosal cells were exfoliated along with the intratracheal hemorrhage. The alveolar collapse and bronchial obstruction were irrecoverably exacerbated, resulting in deteriorated alveolar ventilation and fatal carbon dioxide retention. Because the patient’s previous health condition was good and he had no history of respiratory disease, the pathophysiologic course was induced and exacerbated by the diquat poisoning.
With respect to respiratory failure caused by diquat poisoning, one patient was successfully saved by venovenous extracorporeal membrane oxygenation (ECMO) 11 in a previous report. That patient had ingested about 100 mL of diquat (20 g per 100 mL) and 400 mL of glyphosate (41 g per 100 mL); this dose was much lower than that in our patient, and the previous patient vomited the herbicides soon after ingestion. No interstitial lesions were observed, and the pulmonary impairment was reversible. ECMO treatment was not feasible in our patient for three reasons. First, the patient exhibited significant interstitial changes in the lungs, significant deterioration of respiratory compliance, and poor reversibility of pulmonary lesions. Second, the patient had obvious internal airway bleeding that may have been worsened by anticoagulation for ECMO. Third, the patient had economic limitations that prevented such treatment.
Conclusion
Although diquat requires a higher dose than paraquat to be lethal, oral ingestion of diquat is more easily tolerated than oral ingestion of paraquat because of the taste. Patients with diquat poisoning are more likely to develop multiple organ failure, and clinical treatment is very difficult.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211026117 for High-dose diquat poisoning: a case report by Yanxia Huang, Renjing Zhang, Mei Meng, Dechang Chen and Yunxin Deng in Journal of International Medical Research
Acknowledgment
The authors are grateful for the extensive and knowledgeable feedback provided by the anonymous peer reviewers selected by the editor of the journal.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Yanxia Huang https://orcid.org/0000-0002-5690-9649
Yunxin Deng https://orcid.org/0000-0003-2020-4476
Ethics
Our study was not approved by the local ethics review committee of our institution because the study was a retrospective case analysis and did not involve any interventional experiments. All examinations and treatment measures were implemented according to clinical routines and the decision of the medical team. The ethics review committee of our institution exempts the approval requirements for this research situation.
Patients or their families are routinely asked for authorization to anonymously use the patients’ case data when they are admitted to our teaching hospital. This patient’s family provided written informed consent for publication.
References
- 1.Magalhães N, Carvalho F, Dinis-Oliveira RJ. Human and experimental toxicology of diquat poisoning: toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum Exp Toxicol 2018; 37: 1131–1160. [DOI] [PubMed] [Google Scholar]
- 2.Fortenberry GZ, Beckman J, Schwartz A, et al. Magnitude and characteristics of acute paraquat- and diquat-related illnesses in the US: 1998-2013. Environ Res 2016; 146: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 4.Xing J, Chu Z, Han D, et al. Lethal diquat poisoning manifesting as central pontine myelinolysis and acute kidney injury: a case report and literature review. J Int Med Res 2020; 48: 300060520943824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao S, Wu H, Wang C, et al. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets. J Anim Sci 2018; 96: 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SE, Park YS, Koh HC. NF-κB/p53-activated inflammatory response involves in diquat-induced mitochondrial dysfunction and apoptosis. Environ Toxicol 2018; 33: 1005–1018. [DOI] [PubMed] [Google Scholar]
- 7.Jia P, Ji S, Zhang H, et al. Piceatannol ameliorates hepatic oxidative damage and mitochondrial dysfunction of weaned piglets challenged with diquat. Animals (Basel) 2020; 10: 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen C, Guo Q, Wang W, et al. Taurine alleviates intestinal injury by mediating tight junction barriers in diquat-challenged piglet models. Front Physiol 2020; 11: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varga T, Rákóczy I. Kísérletes diquat mérgezés morphológiai és toxikológiai vizsgálata [Morphological and toxicological studies of experimental diquat poisoning]. Morphol Igazsagugyi Orv Sz 1975; 15: 287–295. [PubMed] [Google Scholar]
- 10.Wu YZ, Kan BT, Wang WJ, et al. [ The experimental study of diquat on the half-lethal dose and pathological injury of related organs in Wistar rats]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2018; 36: 813–818. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Zhang G, Zhang W, et al. Successful extracorporeal membrane oxygenation support for severe acute diquat and glyphosate poisoning: a case report. Medicine (Baltimore) 2019; 98: e14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211026117 for High-dose diquat poisoning: a case report by Yanxia Huang, Renjing Zhang, Mei Meng, Dechang Chen and Yunxin Deng in Journal of International Medical Research