ABSTRACT
Background
Little is known about the long-term outcome of children treated for severe acute malnutrition (SAM) after nutritional rehabilitation.
Objectives
To explore the association between SAM in childhood, noncommunicable diseases (NCDs), and low human capital in adulthood.
Methods
We identified 524 adults (median age: 22 y) who were treated for SAM during childhood in Eastern Democratic Republic of Congo between 1988 and 2007. They were compared with 407 community unexposed age- and sex-matched subjects with no history of SAM. The variables of interest were cardiometabolic risk markers for NCDs and human capital. For the comparison, we used linear and logistic regressions to estimate the association between SAM in childhood and the risk of NCDs and ordinal logistic regression for the human capital.
Results
Compared with unexposed subjects, the exposed participants had a higher waist circumference [1.2 (0.02, 2.3) cm; P = 0.015], and a larger waist-to-height ratio [0.01 (0.01, 0.02) cm; P < 0.001]. On the other hand, they had a smaller hip circumference [−1.5 (−2.6, −0.5) cm; P = 0.021]. Regarding cardiometabolic markers for NCDs, apart from a higher glycated hemoglobin (HbA1c) [0.4 (0.2, 0.6); P < 0.001], no difference was observed in other cardiometabolic markers for NCD between the 2 groups. Compared with unexposed participants, exposed participants had a higher risk of metabolic syndrome (crude OR: 2.35; 95% CI: 1.22, 4.54; P = 0.010) and visceral obesity [adjusted OR: 1.44 (1.09, 1.89); P = 0.001]. The prevalence of hypertension, diabetes, overweight, and dyslipidaemia was similar in both groups. Last, the proportion of malnutrition survivors with higher socioeconomic status level was lower.
Conclusion
SAM during childhood was associated with a high risk of NCDs and lower human capital in adulthood. Thus, policymakers and funders seeking to fight the global spread of NCDs in adults in low-resource settings should consider the long-term benefit of reducing childhood SAM as a preventive measure to reduce the socioeconomic burden attributable to NCDs.
Keywords: long-term, childhood acute malnutrition, chronic diseases, follow-up, DR Congo
Introduction
Sub-Saharan Africa countries are experiencing a rapid health transition associated with a rapid rise in the prevalence of noncommunicable diseases (NCDs) and their risk factors, such as overweight and obesity in adults (1). The prevalence of overweight and obesity exceeds that of undernutrition (2). However, undernutrition still largely predominates among children in these regions (3).
This growing prevalence of NCDs is partly driven by the nutrition transition (4). However, the role of episodes of undernutrition in the fetal period and in childhood is also increasingly recognized as a risk factor for the development of certain NCDs or for NCD risk factors in adulthood (5–8). This phenomenon, known as the “developmental origins of health and disease,” is well documented today (5). It has been studied mainly in high- and middle-income countries (HMICs) (5–8).
Despite growing evidence on the negative long-term effects of childhood undernutrition observed in HMICs, data related to the long-term outcomes of children treated for severe acute malnutrition (SAM) in low-income countries (LICs) are surprisingly scarce (9–11).
Studies conducted in Uganda and Malawi (LICs) showed that catch-up growth after an episode of SAM or delayed childhood growth, respectively, was associated with increased blood pressure (BP) in adolescence (11), and that prepubescent survivors of childhood SAM were at greater risk of subsequent NCDs, even though no clinical or biological marker was identified up to 7 y after nutritional rehabilitation (10).
While in South Kivu, located in the east region of the Democratic Republic of Congo (DRC), according to the report of the DRC National Nutrition Program (PRONANUT) published in 2019, half of all children younger than 5 y suffer from chronic malnutrition (CM) (12), available evidence indicates that the country is experiencing growing burdens of obesity, hypertension (HTA), diabetes mellitus (DM), metabolic syndrome, and abdominal obesity in both urban and rural settings (13, 14). However, the presumed role of childhood malnutrition in the increased burden of NCDs and related risk factors in the DRC has not been explored.
Our study investigated the long-term outcomes of childhood SAM on the health and human capital of a group of young adults screened 11–30 y after nutritional rehabilitation, with no nutrition transition (an environment with a monotonous, undiversified, and low-quality food situation), living in the east of the DRC. We primarily assessed the association between childhood SAM and NCD prevalence in adulthood based on different clinical and biological markers. We also documented the association between SAM in childhood and low socioeconomic status (SES) in adulthood.
Methods
Study design and population
The study was conducted among young adults who were treated for SAM during childhood at Lwiro Pediatric Hospital (LPH) between 1988 and 2007. At that time, diagnosis of SAM at LPH was based on the weight-for-height ratio plotted on the local child growth curve (established by DeMaeyer in 1959 and unpublished), the presence of nutritional edema, and low serum albumin concentrations (15, 16).
The nutritional status of the study subjects at the time of their admission to the hospital was reassessed in relation to the WHO child growth standard of 2006 (17). A new classification was established according to the following criteria.
Children were classified as having SAM if they met ≥1 of the following criteria: mid–upper arm circumference (MUAC) <115 mm, weight-for-height z-score <−3, and the presence of nutritional edema in the hands and/or feet and/or face. Kwashiorkor was defined by the presence of nutritional edema in the hands and/or feet and/or face, MUAC >115 mm, and weight-for-height z-score >−3. Marasmus was defined by MUAC <115 mm and/or weight-for-height z-score <−3 without nutritional edema. The mixed form was defined by the presence of nutritional edema with an MUAC <115 mm and/or a weight-for-height z-score <−3. Moderate acute malnutrition was defined by a weight-for-height z-score between −3 and −2 and/or an MUAC between 115 and 125 mm without nutritional edema. Last, CM was defined by a height-for-age z-score <−2 (17). The latter classification of nutritional status was used for the analyses in this study.
After a detailed analysis of the Nutrition Department archives for 1988–2007, a total of 2830 medical records of children admitted for SAM according to the criteria at that time were found and examined. Each record contained information on the subject's identity (name of child and parents, date of birth, sex, and health district of origin), vaccination schedule, anthropometric measurements (weight, height, and MUAC), serum albumin concentrations, physical examination on admission, nutritional diagnosis (marasmus, kwashiorkor, and marasmic kwashiorkor), treatment implemented, and status at discharge from the hospital.
The survey included participants with records containing the information cited above, who came from a health district in Miti-Murhesa or Katana and were ≥16 y old at the time of recruitment. The age of 16 was selected as the beginning of adulthood because most individuals are already pubescent at that age.
The exclusion criteria were records of patients who left or died during their hospital stay and those of patients transferred to another facility, along with records of adults admitted for SAM. Based on these criteria, of the 2830 records, 1981 records (70%) were selected for the study (18).
Over the course of 12 mo (December 2017 to November 2018), 20 community health workers (CHWs) and 2 supervisors worked full time, with the participation of community leaders, to trace individuals who had become adults (≥16 y old) and still living in Miti-Murhesa and Katana in the east of the DRC. Of the 1981 subjects who had been hospitalized, 1335 (67.4%) were traced and 646 (32.6%) were considered as lost to follow-up. Among those traced, 1134 subjects (84.9%) were still alive and 201 (15.1%) were deceased. Among those who were still alive, 600 (52.9%) were seen by the CHWs and 534 (47.1%) had moved to other regions. Of the 600 subjects seen, 524 agreed to participate in the study and 76 declined (Figure 1).
FIGURE 1.
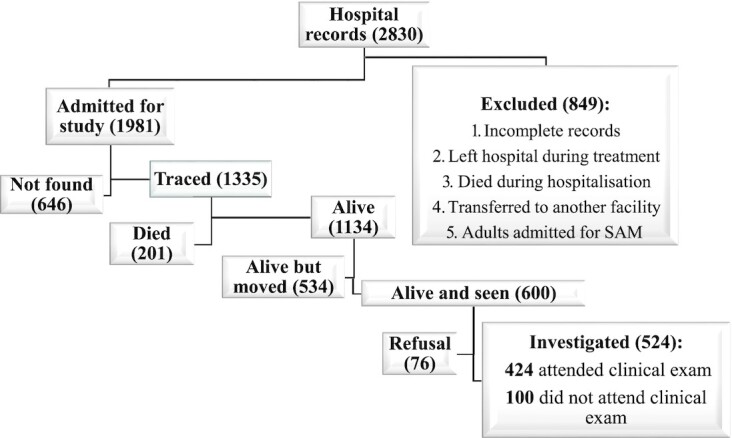
Recruitment of exposed group. SAM, severe acute malnutrition.
The malnutrition survivors who agreed to participate made up the exposed group. For each exposed subject, an unexposed community subject was randomly selected for comparison. An unexposed comparison subject was defined as a subject who had no hospital history of SAM, had the same sex, was living in the same community, and was ≤24 mo older or younger than the exposed subject. We selected community unexposed subjects randomly by spinning a bottle at the exposed adult's home and enquiring door to door, starting from the nearest house to where the bottle pointed (18).
However, unexposed subjects proved harder to recruit than exposed subjects, as many feared being associated with childhood malnutrition and its social stigma. Ultimately, we found unexposed subjects for 407 exposed subjects (18). Of the 524 exposed and 407 unexposed subjects enrolled, 100 exposed and 85 unexposed subjects did not attend the appointments at the health facilities for the various clinical examinations, and for these we therefore only analyzed questionnaire data. Respondents provided signed informed consent for participation in the study, either by written signature or by fingerprints, depending on literacy. For children <18 y of age, consent was obtained from the children's parents or guardians.
Study framework
The study was conducted at the “Centre de Recherche en Science Naturelle de Lwiro (CRSN-Lwiro),” in the health zones of Katana and Miti-Murhesa in South Kivu, DRC. The Nutrition Department of this center has a pediatric hospital and 4 integrated health centers which monitor the state of the health and nutrition of children in the community (19).
Data collection
Data collection was carried out in 2 phases between August 2018 and December 2018 (5 mo). It was conducted by 20 trained CHWs and 2 supervisors and assisted by neighborhood leaders, licensed nurses, and community relays. The CHWs were the same as those who had helped identify the subjects during the creation of the cohort (18).
In the first phase home visits were performed. During these visits, the CHWs administered a questionnaire translated into Kiswahili to the participants, took their anthropometric measurements, and gave them an appointment, scheduled 24 to 48 h after their visit at the nearest hospital, for the second phase. This appointment involved venous and capillary blood samples and BP measurements taken by properly trained nurses working in the various health facilities in the 2 zones. Unlike the nurses and laboratory technicians who did not know whether a participant was an exposed or an unexposed, the CHWs knew this information.
The questionnaire covered variables relating to the participant's identity, their lifestyle (alcohol and tobacco consumption as well as dietary habits), their medical history, and known cardiovascular risk factors (family or personal), as well as their socioeconomic status (education, occupation, and living conditions) (20).
The anthropometric measurements considered were weight, height, waist circumference, and hip circumference. Body weight was measured to the nearest 100 g using electronic scales (Tanita Digital HD-325®), with the subject wearing only light clothing. Height was determined to the nearest 0.1 cm using a SECA 206 cm® measuring device with the subject wearing no shoes. Waist circumference was measured to the nearest 0.1 cm using a measuring tape between the 12th rib and the iliac crest after a breath exhalation. For hip circumference, we used the same measuring tape with the 2 trochanters as a guide. The anthropometric measurements were carried out in accordance with WHO directives (17) and were quality controlled with 2 members of the team taking independent measurements. The final measurement was the average of the 2 independent measurements. In the event of a discrepancy between the independent measurements of >300 g for the weight, >0.5 cm for the height, or >1 cm for the waist and hip circumferences, a third measurement was taken. The average of the 2 closest measurements was used.
Muscle strength in kilograms was measured using a hand dynamometer (Takei Grip-D) twice for each hand (alternating). The participants carried out the test standing with their feet slightly apart, holding the dynamometer at thigh height, with the arm straight and held away from the body. They were asked to squeeze the dynamometer as hard as possible on an exhale. The highest values obtained for each hand were retained for the assessment of the muscle strength of the right and left hands. The maximum muscle strength corresponded to the highest value obtained, regardless of the hand.
BP was measured using an electronic device (OMRON Hem 7001E®). Three measurements were taken with the left arm held at heart level, at 5-min intervals, with the subject seated and relaxed for ≥5 min. The nurses used 1 of the 3 cuff sizes according to the size of each participant's arm. If the systolic blood pressure (SBP) or diastolic blood pressure (DBP) measurements differed by >10 mm Hg, a fourth measurement was taken and the average of the 2 nearest measurements was used; otherwise, the average of the second and third measurements were used.
Capillary glycemia from finger-prick capillary blood sample was immediately analyzed by a glucose oxidase method using a portable electronic glucose meter (Code free®) with reactive strips that could be sourced locally. The method was calibrated in a hospital setting with a laboratory method prior to being implemented. Where fasting glycemia (last meal >8 h previously) or postprandial glycemia (last meal <8 h previously) were ≥126 mg/dL and ≥140 mg/dL, respectively, a fasting control test was carried out the following day.
Last, 4 mL of blood was taken using an antecubital venipuncture after 12 h of fasting to determine the lipid profile (HDL and LDL cholesterol, calculated according to the Friedewald equation (21, triglycerides (TG) and total cholesterol, glycated hemoglobin (HbA1c), and serum creatinine and albumin using standard calorimetric enzymatic methods (CYAN smart CY009) in the laboratory of the general provincial referral hospital in Bukavu (a tertiary hospital). HbA1c analysis was done in a subgroup of 116 subjects (58 exposed and 58 unexposed) due to limited financial means. Quality control of the blood analyses was performed using lyophilized human serum (normal and pathological control) by Cypress Diagnostics.
Outcomes
Our main outcomes of interest were NCDs, including, first and foremost, metabolic syndrome, hypertension, overall obesity, visceral obesity, DM, and dyslipidemia, assessed by their various clinical and biological markers, and second, serum creatinine. We assessed human capital defined by education, occupation, and SES in adulthood.
Dependent variables
Noncommunicable diseases
Diabetes mellitus (DM) was defined as fasting glycemia ≥126 mg/dL and/or current treatment with hypoglycemic agents (22). Hypertension (HTA) was defined as a SBP ≥140 mm Hg and/or a DBP ≥90 mm Hg and/or current antihypertensive treatment. Mean blood pressure (MBP) was calculated using the formula: MBP = DBP + 1/3 (SBP − DBP) (23).
BMI (in kg/m2) was grouped into 4 categories: <18.5, thinness; 18.5–24.9, normal; 25–29.9, overweight; and ≥30, obese (24). Central obesity was defined by a waist circumference ≥94 cm for men and women (13, 25). Metabolic syndrome was defined as the presence of ≥3 of the 5 following risk factors: 1) central obesity (waist circumference ≥94 cm); 2) fasting hypertriglyceridemia (TGs ≥150 mg/dL or use of medication to reduce TGs); 3) low HDL cholesterol (<40 mg/dL in men and <50 mg/dL in women or use of medication to increase the concentration of HDL cholesterol) 4) hypertension (SBP ≥130 mm Hg and/or DBP ≥85 mm Hg and/or undergoing hypertensive treatment); 5) fasting glycemia ≥100 mg/dL or use of oral antidiabetic drugs (13, 26, 27). Visceral obesity was defined by a waist-to-height ratio (WHtR) >0.5 (28). WHtR and waist-to-hip ratio (WHR) were calculated by dividing the waist circumference (cm) by the height (cm) and the hip circumference (cm), respectively. Dyslipidemia was defined by an LDL cholesterol concentration >200 mg/dL and/or an HDL cholesterol concentration <40 mg/dL (regardless of sex) (27). Last, muscle strength was measured with a Takei Grip-D device (Takei). A value of >16 kg for women and >26 kg for men was considered normal (29).
Socioeconomic level
SES was established based on an empirical score taking into account the subject's level of education and occupation, as well as their living conditions. These living conditions were calculated based on the sum of material possessions owned (in 3 groups with the following ranges: 0–3 = few, 4–6 = average, and 7–9 = many), land ownership (yes/no), and type of housing in which the subject was living (with 3 categories: precarious, average, good) (30). The latter was defined on the basis of the components of the house (walls, roof, presence or otherwise of a cement floor in the house), the type of toilet, and the water supply (30). Good housing was considered for a house with walls made from durable or semidurable materials, a galvanized sheet metal roof, cement floor, inside fitted toilets and the presence of a tap on the plot of land. A home considered average housing had walls made from semidurable materials, a galvanized or recycled sheet metal roof, a cement floor, an outside toilet, and a water supply from a standpipe. Last, precarious housing was considered as a house with cob, plant, or clay walls, recycled sheet metal or thatched roof, no cement flooring, no toilet and a natural water source. The following material possessions were considered for each household: vehicle, motorbike, bike, computer, television, radio, telephone, bed made by a carpenter, and domestic animals (including ≥1 cow or 6 goats). The type of occupation was based on the “Classification Internationale Type des Professions,” the French version of the International Standard Classification of Occupations adapted to the European Union (31). For this classification to apply appropriately in our context, we merged the first 3 occupation groups to give group 1 (manager). The groups 4 and 5 of the standard occupation classification became group 2 (administrative and office worker) and group 3 (farmer, fisher, market vendor), respectively. Last, groups 6 and 7 were merged into group 4 (unskilled workers). Unemployed subjects were put in group 4.
Based on all of this information, subjects were sorted into 3 SES categories. As such, we defined a subject with a low SES as any subject who had poor living conditions (precarious housing and owning few material possessions with or without land ownership), regardless of their occupation or level of education. Subjects with an average SES were those who had an occupation in groups 3 or 4, regardless of their level of education, and had good living conditions (good housing, a lot of material possessions, and owned land), or a subject who had average living conditions (had average housing, an average number of possessions, and owned land), and who also had a secondary level of education and an occupation in groups 2, 3, or 4. Last, a subject with a high SES had an occupation in groups 1 or 2, regardless of their level of education, and had good living conditions (had good housing and a lot of possessions and owned land), or a subject who had average living conditions, an occupation in group 1, and a university level education.
Independent variables
With regard to NCDs, primary exposure was a history of SAM during childhood. Other variables, such as age, sex, SES, anthropometric measurements in adulthood, lifestyle (alcohol, tobacco, and diet diversity), and family history of DM and/or HTA in the parents were added in the modeling as potential confounding factors.
Diet diversity was assessed using a dietary diversity score established by the WHO and the Food and Agriculture Organization of the United Nations (32–34). This score measures the dietary diversity of households, weighted according to frequency of consumption. To achieve this, we asked the head of the household (often the mother) how many days the household had eaten each of the following food groups in the last 7 days: grains, tubers, legumes, vegetables, fruit, meat/fish, milk/dairy products, sugar, oil/fat, and condiments. The frequency with which each food group was consumed was then multiplied by its nutritional value. Last, the scores for each food group were added to obtain the overall score. Depending on the total, the subject was considered as having an insufficient, borderline, or satisfactory diet if their score was 0–28, 28.5–42, or >42, respectively (34, 35). For tobacco exposure, a subject was considered as a smoker if he/she smoked at ≥1 cigarette (including hand-rolled cigarettes), a pipe, or other form of smoking tobacco regularly (>3 d/wk) (36). Lastl, for alcohol consumption, we considered any subject who consumed ≥2 standard glasses per day (women) or ≥3 glasses per day (men) of alcohol on a regular basis (>3 d/wk) (37).
Ethical standards
All procedures performed in this study were approved by the Institutional Ethics Committee of the Université Catholique de Bukavu and were in accordance with the 1964 Helsinki declaration and its later amendments.
Statistical analysis
We used the software Stata, version 13.1. The size of the sample was predetermined by the number of patients admitted for SAM at Lwiro pediatric hospital from 1998 to 2007 who were living in Miti-Murhesa and Katana in 2018, and who were identified as possible subjects and agreed to participate in our study. Categorical variables were summarized in the form of frequency and proportion. Quantitative data were presented as mean ± SD or median and minimum to maximum (min-max), depending on whether the distribution was symmetrical.
Linear and logistic regression models were used, respectively, for the continuous variables (BMI, waist circumference, hip circumference, WHtR, and WHR), muscle strength, TGs, total cholesterol, HDL cholesterol, LDL cholesterol, HbA1c, fasting glycemia, albumin, creatinine, and BP (SBP, DBP, and MBP) and dichotomous variables (overall obesity, thinness, visceral obesity, DM, hypertension, metabolic syndrome and dyslipidemia). However, for the TGs, we made a logarithmic transformation given the usually asymmetrical nature of the distribution, and this variable was shown as geometric mean and dispersion interval. The basic models only included the primary exposure, SAM, giving a crude mean difference between the exposed and unexposed for the quantitative variables, and crude ORs for the categorical variables. The mean differences and ORs are shown with 95% CIs. For the TGs, the exponential of the regression coefficient provided the geometric mean ratio.
Different models were then constructed to obtain adjusted effects of SAM. For each outcome, the adjustment variables were those significantly associated with the outcome and the exposition.
These different variables were chosen in models because nonmodifiable cardiovascular risk factors (gender, age, and parental history of HTA and/or DM) are obviously only predictive and are not predicted by other factors (38). Behavioral factors (alcohol consumption, dietary diversity, and smoking) form very few associations with each other, they predict many clinical factors and are predicted by a very small number of nonmodifiable or clinical factors (39, 38). Clinical factors (HTA, dyslipidemia, DM) form many associations with each other, they predict very few factors but are predicted by a large number of behavioral or nonmodifiable risk factors (38).
Last, ordinal logistic regression was used to analyze the differences between the exposed and their community unexposed as regards socioeconomic, education, and occupation variables and the dietary score. However, for the dichotomous variables, the Pearson chi-square test or Fisher exact test were used for comparison.
Results
Sociodemographic and economic characteristics of the population
The median age in the 2 groups was 22 y (min-max: 16, 40, 17–37, 39, 38), and males accounted for 52.1% and 50.6% of the exposed and unexposed, respectively. Compared with the unexposed, the exposed had a lower level of education, poorer housing, less land, and less satisfactory diet diversity. These differences were statistically significant (all P < 0.05). However, no significant difference was observed as regards material possessions or occupational categories (Table 1). The synthetic indicators for SES, constructed using the variables for living conditions, education, and occupation, were significantly better in the unexposed than in the exposed (Table 1).
TABLE 1.
Sociodemographic and economic characteristics of the 2 groups of the study population
| Exposed | Unexposed | ||||
|---|---|---|---|---|---|
| n (total) | Value1 | n (total) | Value1 | P value2 | |
| Age, y | 524 | 22 (16–40) | 407 | 22 (16–40) | 0.381 |
| Male | 524 | 52.1 | 407 | 50.6 | 0.351 |
| Education level | 515 | 405 | |||
| None | 27.8 | 20.0 | |||
| Primary | 37.1 | 33.6 | <0.001 | ||
| Secondary | 34.2 | 42.0 | |||
| University | 1.0 | 4.4 | |||
| Occupational category | 479 | 359 | |||
| Executive | 3.1 | 7.5 | |||
| Administrative + office worker | 0.8 | 1.1 | 0.137 | ||
| Farmer + fisher + market vendor | 64.9 | 62.1 | |||
| Unskilled workers | 31.1 | 29.3 | |||
| Living conditions | |||||
| A. Housing (wall + roof + cement floor + water + toilet) | 524 | 407 | |||
| Precarious | 33.4 | 21.1 | |||
| Average | 63.5 | 74.4 | <0.001 | ||
| Good | 3.1 | 4.4 | |||
| B. Material possessions (sum of all possessions) | 524 | 407 | |||
| Few (≤3 possessions) | 81.7 | 82.8 | |||
| Average (4–6 possessions) | 18.1 | 17.2 | 0.848 | ||
| Many (>6 possessions) | 0.2 | 0.0 | |||
| C. Land ownership (yes) | 524 | 59.9 | 407 | 67.8 | 0.013 |
| Socioeconomic status (education + living conditions + occupation) | 472 | 357 | |||
| Low | 64.0 | 55.5 | |||
| Average | 33.1 | 37.8 | 0.007 | ||
| High | 3.0 | 6.7 | |||
| Diet diversity score | 524 | 407 | |||
| Insufficient | 11.1 | 6.9 | |||
| Borderline | 39.3 | 31.7 | <0.001 | ||
| Satisfactory | 49.6 | 61.4 | |||
Values are medians (minimum–maximum) or percentages unless otherwise indicated.
P value calculated with ordinal logistic regression for ordinal variables.
Cardiometabolic markers and NCD prevalence in the 2 groups
The prevalence of overweight, DM, HTA, and metabolic syndrome were 13%; 9.6%; 6.5%; and 10.8% in the exposed and 12.9%; 7.5%; 6.4%; and 4.9% in the unexposed respectively. Last, the proportion of thinness was 7.7% and 3.8% in the exposed and unexposed respectively (Table 2).
TABLE 2.
Cardiometabolic markers and NCD prevalence in the 2 groups1
| Exposed | Unexposed | ||||||
|---|---|---|---|---|---|---|---|
| n (total) | % | Mean ± SD | n (total) | % | Mean ± SD | P value | |
| Anthropometry | |||||||
| Weight, kg | 509 | 53.5 ± 7.9 | 396 | 55.1 ± 7.2 | <0.001 | ||
| Height, cm | 520 | 155.9 ± 8.9 | 406 | 157.6 ± 8.9 | 0.002 | ||
| BMI | 509 | 22.0 ± 2.9 | 396 | 22.2 ± 2.5 | 0.24 | ||
| Thinness | 7.7 | 3.8 | 0.049 | ||||
| Normal | 79.4 | 83.3 | |||||
| Overweight | 13.0 | 12.9 | |||||
| Waist circumference, cm | 519 | 79.1 ± 9.2 | 406 | 77.9 ± 8.3 | 0.046 | ||
| Hip circumference, cm | 517 | 84.5 ± 8.6 | 405 | 86.0 ± 7.7 | 0.005 | ||
| Waist-to-hip ratio | 516 | 0.94 ± 0.12 | 405 | 0.91 ± 0.11 | <0.001 | ||
| Waist-to-height ratio | 519 | 0.51 ± 0.06 | 406 | 0.50 ± 0.06 | 0.001 | ||
| Muscle strength, kg | 385 | 29.9 ± 8.6 | 303 | 32.8 ± 8.8 | <0.001 | ||
| BP, mm Hg | |||||||
| Systolic BP | 358 | 121.1 ± 13.5 | 269 | 121.8 ± 12.9 | 0.54 | ||
| Diastolic BP | 386 | 70.9 ± 10.7 | 71.6 ± 10.1 | 0.37 | |||
| Mean BP | 358 | 95.8 ± 10.3 | 96.7 ± 9.8 | 0.29 | |||
| Glucose | |||||||
| Fasting glycemia, mg/dL | 398 | 105.1 ± 16.5 | 319 | 103.7 ± 14.5 | 0.23 | ||
| HbA1c, % | 58 | 4.6 ± 0.6 | 52 | 4.2 ± 0.3 | <0.001 | ||
| Lipid profile, mg/dL | |||||||
| Total cholesterol | 424 | 154.2 ± 35.5 | 331 | 159.1 ± 36.6 | 0.062 | ||
| HDL-C | 424 | 43.8 ± 8.5 | 331 | 44.4 ± 8.4 | 0.37 | ||
| LDL-C | 412 | 90.1 ± 30.5 | 319 | 94.2 ± 31.2 | 0.08 | ||
| TG | 412 | 97.8 (74.7, 128.4)2 | 322 | 97.2 (74.7, 126.4)2 | 0.72 | ||
| Creatinine, mg/dL | 421 | 0.87 ± 0.17 | 331 | 0.88 ± 0.19 | 0.55 | ||
| Albumin, mg/dL | 424 | 4.38 ± 0.34 | 328 | 4.44 ± 0.31 | 0.020 | ||
| Cardiovascular risk factors | |||||||
| 1. Alcohol (yes) | 524 | 35.9 | 407 | 40.3 | 0.17 | ||
| 2. Tobacco (yes) | 524 | 3.1 | 407 | 1.5 | 0.12 | ||
| 3. Parent with HTA and/or DM | 524 | 32.3 | 407 | 32.9 | 0.83 | ||
| 4. Dyslipidemia | |||||||
| High LDL-C | 412 | 2.4 | 319 | 1.6 | 0.42 | ||
| low HDL-C | 424 | 38.4 | 331 | 34.1 | 0.22 | ||
| 5. DM | 398 | 9.6 | 319 | 7.5 | 0.34 | ||
| 6. HTA | 384 | 6.5 | 299 | 6.4 | 0.93 | ||
| 7. Visceral obesity | 492 | 52.4 | 372 | 43.8 | 0.012 | ||
| 8. Metabolic syndrome | 332 | 10.8 | 265 | 4.9 | 0.009 | ||
| 1. Central obesity | 517 | 6.4 | 405 | 4.4 | |||
| 2. Fasting hyperglycemia | 398 | 32.4 | 319 | 25.4 | |||
| 3. Low HDL-C | 424 | 58.5 | 331 | 55.6 | |||
| 4. High TG | 412 | 8.7 | 322 | 7.1 | |||
| 5. HTA or anti-hypertensive therapy | 384 | 19.0 | 299 | 18.4 | |||
BP, blood pressure; DM, diabetes mellitus; HbA1c, glycated hemoglobin; HTA, hypertension; NCD, noncommunicable diseases; TG, triglyceride.
Geometric mean ± SD.
Mean differences in clinical and biological markers for NCDs between exposed and unexposed
As shown in Table 3, in regard to anthropometry, compared with the unexposed, the exposed had a significantly higher waist circumference, WHR and WHtR. On the other hand, they exhibited significant lower hip circumference and smaller muscle strength than the unexposed. Importantly, these effects did not diminish after adjustment (for muscle strength).
TABLE 3.
Mean differences (95% CI) in clinical and biological markers for NCDs between exposed and unexposed1
| Crude difference (95% CI) | Adjusted difference (95% CI) | |
|---|---|---|
| Anthropometry | ||
| Waist circumference, cm (n = 925) | 1.2 (0.02, 2.3) | |
| Hip circumference, cm (n = 922) | −1.5 (−2.6, −0.5) | |
| Waist-to-hip ratio (n = 921) | 0.03 (0.02, 0.05) | |
| Waist-to-height ratio (n = 925) | 0.01 (0.01, 0.02) | |
| Muscle strength, kg (n = 688) | −2.9 (−4.2, −1.6) | −3.0 (−4.3, −1.7)a |
| BP, mm Hg | ||
| Systolic BP (n = 627) | −0.7 (−2.8, 1.4) | |
| Diastolic BP (n = 687) | −0.7 (−2.3, 0.9) | |
| Mean BP (n = 627) | −0.9 (−2.5, 0.7) | |
| Glucose | ||
| Glycemia, mg/dL (n = 717) | 1.4 (−0.9, 3.7) | 1.1 (−1.3, 3.4)a |
| HbA1c, % (n = 110) | 0.5 (0.3, 0.6) | 0.4 (0.2, 0.6)a |
| Lipid profile, mg/dL | ||
| Total cholesterol (n = 755) | −4.9 (−10.1, 0.3) | −3.8 (−9.3, 1.8) (n = 672)c |
| HDL-C (n = 755) | −0.6 (−1.8, 0.7) | −0.5 (−1.9, 0.8) (n = 672)c |
| LDL-C (n = 731) | −4.1 (−8.6, 0.4) | −2.8 (−7.6, 2.0) (n = 650)c |
| Triglyceride (n = 734) | 1.01 (0.97, 1.04)2 | 1.00 (0.97, 1.04)2,a |
| Creatinine, mg/dL (n = 752) | −0.01 (−0.03, 0.02) | |
| Albumin, mg/dL (n = 752) | −0.06 (−0.10, −0.01) | −0.04 (−0.09, 0.01)b (n = 669) |
Difference with 95% CI calculated by linear regression. BP, blood pressure, HbA1c, glycated hemoglobin; NCD, noncommunicable diseases; SES, socioeconomic status.
Geometric means ratio.
Adjusted for diet diversity.
Adjusted for SES.
Adjusted for diet diversity and SES.
As regards the different clinical and biological markers for NCDs (Table 3), the exposed had markedly significantly higher HbA1c than the unexposed, even after adjustment. However, BP (SBP, DBP, and MBP), lipid profile (total cholesterol, LDL cholesterol, HDL cholesterol, and TGs), fasting glycemia. and creatinine were similar in the 2 groups, even after adjustment. Compared with unexposed, the exposed exhibited slightly lower concentrations of albumin; however, the observed difference diminished after adjustment.
Risk of developing NCDs in the exposed compared with the unexposed
Table 4 shows that, compared with the unexposed, the exposed had a significantly higher risk of visceral obesity, even after adjustment for confounders. The same effect can be seen for metabolic syndrome. No significant difference was observed for HTA, DM, overweight, and dyslipidemia. Of note, we observed that the exposed had a higher risk of thinness even after adjustment for diet diversity.
TABLE 4.
Risk of developing NCDs (95% CI) in the exposed compared with the unexposed1
| Crude OR (95% CI) | Adjusted OR | |
|---|---|---|
| 1. Dyslipidemia | ||
| High LDL-C (n = 731) | 1.56 (0.53, 4.62) | |
| Low HDL-C (n = 755) | 1.20 (0.89, 1.63) | |
| High triglyceride (n = 734) | 1.24 (0.72, 2.15) | |
| 2. Diabetes (n = 717) | 1.30 (0.76, 2.21) | |
| 3. Hypertension (n = 683) | 1.03 (0.55, 1.90) | 0.98 (0.52, 1.85b (n = 613) |
| 4. Visceral obesity (n = 864) | 1.41 (1.08, 1.85) | 1.44 (1.09, 1.89)a |
| 5. BMI (n = 905) | ||
| Overweight | 1.06 (0.71, 1.57) | 1.11 (0.75, 1.65)a |
| Thinness | 2.12 (1.15, 3.92) | 1.92 (1.03, 3.57)a |
| 7. Metabolic syndrome (n = 597) | 2.35 (1.22, 4.54) |
ORs (95% CIs) calculated by logistic regression. NCD, noncommunicable diseases; SES, socioeconomic status.
Adjusted for diet diversity.
Adjusted for SES.
Discussion
Our findings suggest that SAM during childhood is associated with long-term negative effects on adult anthropometry and muscle strength, but with lesser association on the development of overall obesity. In addition, it is associated with a high risk of NCDs in adulthood, mainly visceral obesity, metabolic syndrome and glucose homeostasis, even in a context of no nutrition transition. Importantly, SAM during childhood is associated with a lower SES than a general population with no history of childhood SAM.
A major finding of the present study was the high proportion of malnutrition survivors who developed visceral obesity indices (higher WHtR and WHR and a larger waist circumference), with a smaller hip circumference. This suggests an altered distribution between visceral adipose tissue and gluteo-femoral adipose tissue, and/or a gluteo-sarcopenia component. Our findings are consistent with those of other studies which reported that malnutrition survivors are at significantly higher risk of visceral obesity (8, 10, 41). This phenotype of individuals with central obesity puts them at risk of cardiometabolic events to a degree similar to or greater than obese or overweight subjects (42). Indeed, visceral fat is considered metabolically less favorable, particularly as regards the secretion of harmful adipokines. The presence of visceral fat positively correlates with an increased cardiometabolic risk, particularly atherosclerosis and the damaging effects of chronic insulin resistance and reactive hyperinsulinemia that the latter promotes (42–44).
Another important finding of the present survey is that malnutrition survivors had lower muscle strength in adulthood. These data are in keeping with previous observations in malnutrition survivors 7 y later after nutritional rehabilitation in Malawi (10). An initial hypothesis for this lower muscle strength could be that malnutrition survivors have reduced lean body mass in adulthood (45–47). Indeed, it has been shown that undernutrition in childhood is associated with a reduced lean body mass in adulthood independently of the potential catch-up growth that may have occurred afterward (8, 10). This might be due to the fact that after an episode of malnutrition, fat mass primarily accumulates in most parts of the body, which leads to a decrease in lean body mass in adulthood (45). A reduced lean mass in adulthood is linked to lower thermogenesis, insulin resistance/hyperinsulinemia, decreased fasting and postprandial glucose uptake, raised risk of metabolic syndrome and/or type-2 diabetes, and higher incidence of atherothrombotic cardiovascular diseases (41, 42, 46).
As regards the risk of metabolic syndrome, we observed a higher prevalence in the malnutrition survivors than in the unexposed, which suggests increased insulin resistance and visceral obesity (Table 4). This observation is in line with those of other studies carried out in several regions of the world, which reported that subjects exposed to undernutrition in childhood were at greater risk of incident metabolic syndrome than those not exposed, with nearly similar ORs as those observed in our study (OR: 2) (48–51). Malnutrition survivors generally have a low BMI during childhood so, in contrast to the unexposed population, it is possible that those who developed a metabolic syndrome had accelerated BMI gain after childhood, becoming “obese in relation to themselves” but without having a high BMI in absolute terms, which is consistent with the concept of “metabolically obese–normal weight” subjects (52). Consequently, the gain in BMI was based more on fat mass than on lean mass, in line with acquired insulin resistance and visceral obesity underlying the metabolic syndrome phenotype.
The exposed exhibited slightly higher HbA1c. This could probably expose them to a subsequent higher risk of glucose homeostasis abnormalities in comparison to the unexposed. These glucose metabolism abnormalities could be ascribed to inadequate nutrition in childhood leading to decreased number or secretory function of pancreatic β cells, in addition to acquired insulin resistance in malnutrition survivors as a result of reduced lean body mass (53, 54). Another factor that may contribute to increased HbA1c is sarcopenia, which is associated with lower muscle glucose uptake, particularly in the postprandial period.
However, aside from HbA1c, other cardiometabolic markers for NCDs (BP, fasting glycemia, total cholesterol, LDL cholesterol, HDL cholesterol, and TG) were similar in both groups. Similarly, the frequencies of HTA, DM, low HDL cholesterol, and hypertriglyceridemia was comparable between the 2 groups. These findings are consistent with other studies conducted in low and middle-income countries (10, 41, 48, 49).
Last, we observed that, in contrast to unexposed, malnutrition survivors were less likely to achieve an education and a high SES, even after adjustment for age and gender, although no significant difference was observed as regards occupation. Our findings are consistent with those of several studies that show a strong link between undernutrition during childhood and reduced human capital later in life, pertaining to education, occupation, and social status (8, 55–57).
There are several limitations to this study. First, survivorship bias could represent a major limitation. Indeed, only 524 among 1981 subjects in the initial cohort were examined, and they could have had different characteristics than those of the subjects who were not studied. Nevertheless, we estimate that this would not be significantly different with regard to our main findings given that the characteristics on admission did not differ between those lost to follow-up and the subjects who were traced (18). Second, we do not have any infant health information, including gestational age, birth weight and height, or rate of growth in the first 2 y of life, nor on growth between the time of discharge from hospital and the time when our study was conducted. These variables are potential confounding factors because they are linked with both malnutrition and negative long-term effects (58). Third, although not malnourished in the past, the unexposed lived in the same unfavorable conditions as the exposed, and it is difficult to establish whether they were perfectly healthy on a cardiometabolic level. The continued unfavorable situation in which our 2 groups lived probably helped significantly reduce any differences in most cardiometabolic markers studied. Fourth, the study design is incapable of separating mechanisms due to SAM per se from mechanisms due to persistent effects of the child's early environment or persistent living in the same poor environment.
In conclusion, SAM during childhood is associated with a high potential risk of developing NCDs and a lower human capital in adulthood, even in the absence of nutrition transition.
These results underscore the relevance of implementing cost-effective strategies to prevent the onset of NCDs in adulthood and enhancing economic development through child nutrition improvement, BMI screening, and promotion of healthy lifestyle in later. Policymakers and funders seeking to fight the global NCDs epidemic in adults and to reduce poverty rates must be aware of the fact that appropriate investment in child health is a significant, albeit little known, means to improve adult health and economic development.
Acknowledgements
We are thankful to Philippe Hennart and the CEMUBAC team who initiated the system for collecting and computerizing data on malnutrition at Lwiro Hospital since 1988. Our thanks also go to the entire team of doctors and nurses who participated in the treatment of malnourished children between 1988 and 2007. We also thank the Van Buuren Foundation, which contributed to the functioning of Lwiro Hospital. Finally, we express gratitude to Nicolas Kashama, and all community health workers for the data collection and administrative support during this work.
The authors’ responsibilities were as follows—PM, GB, JM, PD: conceived and designed the experiments; PM, GN: did the data collection; MD: contributed to specific areas of the methods, data analysis, statistics, and quality control; PM: analyzed the data and wrote the first draft of the manuscript; GB, GN, MD, JM, MH, DL, PD: contributed to the writing of the manuscript and agree with the manuscript's results and conclusions; and all authors: read and approved the final manuscript. Author disclosures: The authors report no conflicts of interest.
Notes
Funded as part of the research for development project funded by the Belgian Development Cooperation through the Académie de Recherche et d'Enseignement Supérieur (ARES). The views expressed in this study are those of the authors and do not necessarily reflect those of the ARES. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Abbreviations used: BP, blood pressure; CHW, community health worker; CM, chronic malnutrition; DBP, diastolic blood pressure; DM, diabetes mellitus; DRC, Democratic Republic of Congo; HbA1c, glycated hemoglobin; HMIC, high- and middle-income countries; HTA, hypertension; LIC, low-income countries; LPH, Lwiro Pediatric Hospital; MBP, mean blood pressure; MUAC, mid–upper arm circumference; NCD, noncommunicable disease; SAM, severe acute malnutrition; SBP, systolic blood pressure; SES, socioeconomic status; TG, triglyceride; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio.
Contributor Information
Pacifique Mwene-Batu, Ecole Régionale de Santé Publique, Université Catholique de Bukavu, Bukavu, Democratic Republic of Congo; Ecole de Santé Publique, Université Libre de Bruxelles, Brussels, Belgium; Nutritional department, Centre de Recherche en Sciences Naturelles, Lwiro, Democratic Republic of Congo; Hôpital Provincial General de Reference de Bukavu, Université Catholique de Bukavu, Bukavu, Democratic Republic of Congo.
Ghislain Bisimwa, Ecole Régionale de Santé Publique, Université Catholique de Bukavu, Bukavu, Democratic Republic of Congo; Nutritional department, Centre de Recherche en Sciences Naturelles, Lwiro, Democratic Republic of Congo.
Gaylord Ngaboyeka, Ecole Régionale de Santé Publique, Université Catholique de Bukavu, Bukavu, Democratic Republic of Congo; Nutritional department, Centre de Recherche en Sciences Naturelles, Lwiro, Democratic Republic of Congo.
Michèle Dramaix, Ecole de Santé Publique, Université Libre de Bruxelles, Brussels, Belgium.
Jean Macq, Institute of Health and Society, Université Catholique de Louvain, Brussels, Belgium.
Michel P Hermans, Division of Endocrinology & Nutrition, Cliniques universitaires St-Luc, Université Catholique de Louvain, Brussels, Belgium.
Daniel Lemogoum, Hôpital ULB-Erasme, Université Libre de Bruxelles, Brussels, Belgium.
Philippe Donnen, Ecole de Santé Publique, Université Libre de Bruxelles, Brussels, Belgium.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application to the corresponding author.
References
- 1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N. Global, regional and national prevalence of overweight and obesity in children and adults 1980–2013: a systematic analysis. Lancet North Am Ed. 2014;384(9945):766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caleyachetty R, Thomas GN, Kengne AP, Echouffo-tcheugui JB, Schilsky S. The double burden of malnutrition among adolescents: analysis of data from the Global School-Based Student Health and Health Behavior in School-Aged Children surveys in 57 low- and middle-income countries. Am J Clin Nutr. 2018;108:414–24. [DOI] [PubMed] [Google Scholar]
- 3. UNICEF, WHO & GROUP WB . Levels and trends in child malnutrition. New York; 2017. [Google Scholar]
- 4. Wells JC, Sawaya AL, Wibaek R, Mwangome M, Poullas MS, Yajnik CS, Demaio A. The double burden of malnutrition: aetiological pathways and consequences for health. Lancet. 2020;395(10217):75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 Suppl):5885–995. [DOI] [PubMed] [Google Scholar]
- 6. Roseboom TJ, van der Meulen JHP, Ravelli ACJ, Osmond C, Barker DJP, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185(5):93–8. [DOI] [PubMed] [Google Scholar]
- 7. Sawaya AL, Sesso R, Florencio TM, Fernandes MT MP. Association between chronic undernutrition and hypertension. Matern Child Nutr. 2005;1:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev H S. Maternal and child undernutrition: consequences for adult health and human capital. Lancet North Am Ed. 2008;371(9609):340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bahwere P, Mtimuni A, Kate Sadler TB, SC . Long term mortality after community and facility-based treatment of severe acute malnutrition: analysis of data. J Public Health Epidemiol. 2012;4(8):215–25. [Google Scholar]
- 10. Lelijveld N, Seal A, Wells JC, Kirkby J, Opondo C, Chimwezi E, Bunn J, Bandsma R, Heyderman R S, Nyirenda M Jet al. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): a cohort study.Lancet Global Health. 2016;4(9):e654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asiki G, Newton R, Marions L, Kamali A, Smedman L. The effect of childhood stunting and wasting on adolescent cardiovascular diseases risk and educational achievement in rural Uganda: a retrospective cohort study. Glob Health Action. 2019;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. PRONANUT (RDC) . Enquêtes nutritionnelles dans les territoires de Kabare et Walungu, Kinshasa, RDC: PRONANUT. 2019. [Google Scholar]
- 13. Katchunga PB, Cikomola J, Tshongo C, Baleke A, Kaishusha D, Mirindi P, Tamburhe T, Kluyskens Y, Sadiki A, Bwanamudogo Set al. Obesity and diabetes mellitus association in rural community of Katana, South Kivu, in Eastern Democratic Republic of Congo: Bukavu Observ Cohort Study Results. BMC Endocr Disord. 2016;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katchunga PB, Mirindi P, Baleke A, Twagirumukiza M. The trend in blood pressure and hypertension prevalence in the general population of South Kivu between 2012 and 2016: results from two representative cross-sectional surveys—the Bukavu observational study. PLoS One. 2019;14:e0219377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lemonnier D, ingenbleek Y. les carences nutritionnelles dans les pays en voie de développement. In: Les malnutritions dans les pays du tiers-monde, Les éditions INSERM, Paris. 1989. [Google Scholar]
- 16. Paluku B. Contribution à l'amelioration et à l’évaluation de la prise en charge globale de l'enfant hospitalisé en afrique centrale (Sud-Kivu). Université Libre de Bruxelles; 2002. [Google Scholar]
- 17. WHO Multicentre Growth Reference Study Group . WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight- for-height and body mass index-for-age: methods and development. Geneva; 2006. [Google Scholar]
- 18. Mwene-batu P, Bisimwa G, Ngaboyeka G, Dramaix M, Macq J, Lemogoum D, Donnen P, Follow-up of a historic cohort of children treated for severe acute malnutrition between 1988 and 2007 in Eastern Democratic Republic of Congo. PLoS One. 2020;15(3):e0229675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Action Contre la Faim. Enquête Nutritionnelle Anthropométrique dans la Zone de Santé de Miti-Murhesa, Province du Sud-Kivu,République Démocratique du Congo . Bukavu, RDC: Action contre la faim; 2011. [Google Scholar]
- 20. Ministère du Plan et Suivi de la Mise en œuvre de la Révolution de la Modernité, Ministère de la Santé Publique, ICF International . Enquête Démographique et de Santé en République Démocratique du Congo 2013–2014. Rockville, MD: 2014; [Google Scholar]
- 21. Friedewald WT, Levy RI FD. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 22. American Diabetes Association (ADA) . Standards of medical care in diabetes-2010. Diabetes Care. 2010; 33:S11.20042772 [Google Scholar]
- 23. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, Backer G, Dominiczak Aet al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–357. [DOI] [PubMed] [Google Scholar]
- 24. National Heart, Lung, and Blood Institute Obesity Education Initiative . The practical guide to identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Heart, Lung, and Blood Institute; 2000. [Google Scholar]
- 25. Longo-mbenza B, Lasi JBK, Okwe AN, Kabangu NK. The metabolic syndrome in a Congolese population and its implications for metabolic syndrome definitions. Diabetes Metab Syndr. 2011;5(1):17–24. [DOI] [PubMed] [Google Scholar]
- 26. Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WP T, Loria CM, Sidney C. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 27. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and T of HBC in A (Adult TPI. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection . USA: JAMA; 2002. [Google Scholar]
- 28. Ashwell M, Gibson S. Waist to height ratio is a simple and effective obesity screening tool for cardiovascular risk factors: analysis of data from the British National Diet and Nutrition Survey of adults aged 19–64 years. Obes Facts. 2009;2:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Celis-morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, Iliodromiti S, Sillars A, Graham N, Mackay DFet al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. Br J Sports Med. 2019;53(21):1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ministère du Plan de la R.D.Congo et UNICEF . Enquête Nationale sur la Situation des Enfants et des Femmes MICS2. Kinshasa; 2010. [Google Scholar]
- 31. Genoud PA. Indice de position socioéconomique (IPSE): un calcul simplifié. 2011; [Internet]. Retrieved from: www.unifr.ch/ipg/assets/files/DocGenoud/IPSE.pdf [Google Scholar]
- 32. IFPRI . Validation du score de consommation alimentaire du PAM par IFPRI. [Internet]. Available from: 2014. http://www.ifpri.org/sites/default/files/publications/ifpridp00870.pdf [Google Scholar]
- 33. Programme Alimentaire Mondial . Indicateurs de la sécurité alimentaire, PAM, Genève; 2014. [Google Scholar]
- 34. ACF International . Enquête Multisectorielle Nutrition et Sécurité Alimentaire Zone de Santé de Nzanza. Matadi,RDC; 2012. [Google Scholar]
- 35. ACF . Rapport d'enquête nutritionnelle sud-kivu, Bukavu, RDC: ACF; 2004. [Google Scholar]
- 36. Convention Cadre de l'OMS pour la lutte Antitabac . Recueil des indicateurs. Genève; 2015. [Google Scholar]
- 37. Belgherbi S, Mutatayi C, Palle C. Les repères de consommation d'alcool : les standards mis en question. Obs français des Drog des Toxicom, Paris, France; 2015:1–160. [Google Scholar]
- 38. Meneton P, Lemogne C, Herquelot E, Bonenfant S, Larson MG, Vasan RS, Ménard J, Goldberg M, Zins M. A global view of the relationships between the main behavioural and clinical cardiovascular risk factors in the GAZEL prospective cohort. PLoS One. 2016;11(9):e0162386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ford ND, Behrman JR, Hoddinott JF, Maluccio JA, Martorell R, Ramirez-zea M, Stein A D. Exposure to improved nutrition from conception to age 2 years and adult cardiometabolic disease risk: a modelling study. Lancet Global Health. 2018;6(8):e875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dramaix M, Hennart M, Brasseur D, Bahwere P, Mudjene O, Tonglet R DP, Smets R. Serum albumin concentration, arm circumference, and oedema and subsequent risk of dying in children in central Africa. BMJ. 1993;307:710–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, Ezzati M, Grantham-Mcgregor S, Katz J, Martorell Ret al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet North Am Ed. 2013;382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 42. Bosomworth NJ. Obésité centrale malgré un poids normal. Le médecin de famille canadien,2019;65:251–60. [PMC free article] [PubMed] [Google Scholar]
- 43. Lee CM, Huxley RR, Wildman RP WM. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61(7):646–53. [DOI] [PubMed] [Google Scholar]
- 44. Seidell JC, Pérusse L, Després JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74(3):315–21. [DOI] [PubMed] [Google Scholar]
- 45. Golden MH. Specific deficiencies versus growth failure: type I and type II nutrients. Journal of Nutritional and environmental Medicine. 1995;(12):10–4. [PubMed] [Google Scholar]
- 46. Van Abeelen AFM, Elias SG, Bossuyt PMM, Grobbee DE, Van Der Schouw YT, Roseboom TJ, Uiterwaal C. Famine exposure in the young and the risk of type 2 diabetes in adulthood. Diabetes. 2012;61(9):2255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Norman K, Stobäus N, Gonzalez MC, Schulzke J-D, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30:135–42. [DOI] [PubMed] [Google Scholar]
- 48. Li Y, Jaddoe VW, Lu qi YH, Wang D, Lai J, Zhang J, FU P, YANG X, HU FB. Exposure to the Chinese famine in early life and the risk of metabolic syndrome in adulthood. Diabetes Care. 2011;34:1014–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lizárraga-mollinedo E, Fernández-millán E, Frutos MG, De Toro-martín J, Fernández-agulló T, Ros M, Álvarez C, Escrivá F. Early and long-term undernutrition in female rats exacerbates the metabolic risk associated with nutritional rehabilitation. J Biol Chem. 2015;290(31):19353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang N, Wang X, Li Q, Han B, Chen Y, Zhu C, Chen Y, Lin D, Wang B, Jensen MDet al. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr. 2017;36(1):253–9. [DOI] [PubMed] [Google Scholar]
- 51. Zheng X, Wang Y, Ren W, Luo R, Zhang S, Zhang JH, Zeng Q. Risk of metabolic syndrome in adults exposed to the great Chinese famine during the fetal life and early childhood. Eur J Clin Nutr. 2012;66(2):231–6. [DOI] [PubMed] [Google Scholar]
- 52. Hermans MP, Amoussou-guenou KD, Bouenizabila E, Sadikot SS, Ahn SA, Rousseau MF. The normal-weight type 2 diabetes phenotype revisited. Diabetes Metab Syndr Clin Res Rev. 2016;10:82–8. [DOI] [PubMed] [Google Scholar]
- 53. Li Y, He Y, Qi L, Jaddoe VW, Feskens EJM, Yang X, Ma G, Hu FB. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59(10):2400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fowden AL HD. Intra-uterine programming of the endocrine pancreas. Br Med Bull. 2001;60:123–42. [DOI] [PubMed] [Google Scholar]
- 55. Martorell R, Melgar P, Maluccio JA, Stein AD, Rivera JA. The nutrition intervention improved adult human capital and economic productivity. J Nutr. 2010.;140:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoddinott J, Behrman JR, Maluccio JA, Melgar P, Quisumbing AR, Ramirez-zea M, Stein A D, Yount K M, Martorell R. Adult consequences of growth failure in early childhood. Am J Clin Nutr. 2013;98:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ampaabeng SK, Tan CM. The long-term cognitive consequences of early childhood malnutrition: the case of famine in Ghana. J Health Econ. 2013;32(6):1013–27. [DOI] [PubMed] [Google Scholar]
- 58. McCarthy A, Hughes R, Tilling K, Davies D, Smith GD, Ben-Shlomo Y. Birth weight; postnatal, infant, and childhood growth; and obesity in young adulthood: evidence from the Barry Caerphilly Growth Study. Am J Clin Nutr. 2007;86(4):907–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application to the corresponding author.


