ABSTRACT
Background
SCFAs are involved in regulation of body weight and bone health.
Objectives
We aimed to examine whether genetic variations related to butyrate modified the relation between dietary fiber intake and changes in bone mineral density (BMD) in response to weight-loss dietary interventions.
Methods
In the 2-y Preventing Overweight Using Novel Dietary Strategies trial, 424 participants with BMD measured by DXA scan were randomly assigned to 1 of 4 diets varying in macronutrient intakes. A polygenic score (PGS) was calculated based on 7 genetic variants related to the production of butyrate for 370 of the 424 participants.
Results
SCFA PGS significantly modified the association between baseline dietary fiber intake and sex on 2-y changes in whole-body BMD (P-interaction = 0.049 and 0.008). In participants with the highest tertile of SCFA PGS, higher dietary fiber intake was related to a greater increase in BMD (β: 0.0022; 95% CI: 0.0009, 0.0035; P = 0.002), whereas no such association was found for participants in the lower tertiles. In the lowest tertiles of SCFA PGS, men showed a significant increase in whole-body BMD (β: 0.0280; 95% CI: 0.0112, 0.0447; P = 0.002) compared with women. In the highest tertile, no significant difference was found for the change in BMD between men and women.
Conclusions
Our data indicate that genetic variants related to butyrate modify the relations of dietary fiber intake and sex with long-term changes in BMD in response to weight-loss diet interventions.
Keywords: Bone mineral density, SCFA, dietary fiber, weight loss, genetic variants
Introduction
Obesity has been closely related to bone health (1–3). Weight-loss diets have shown beneficial effects on reducing cardiometabolic comorbidities among overweight or obese patients (1, 4), but in addition have shown that diet-induced weight loss may lead to bone loss (2, 5–7) in a sex-specific manner (6, 8).
Gut microbiota has been related to both obesity and bone health (9–12). In recent studies, we found that gut microbiota metabolites were associated with weight loss and changes in bone mineral density (BMD) in response to diet interventions. SCFAs are a group of metabolites produced by fermenting fibers in the large intestine by microbiota (13, 14). Circulating SCFAs such as butyrate have been consistently found to be negatively related to obesity (15), stimulate gut hormones during weight loss (16), and regulate osteoclast metabolism and bone homeostasis and protect from pathological bone loss (17–19). A recent genome-wide association study identified 9 gene variants associated with gut production of butyrate (20). However, no study has analyzed whether the SCFA-associated genetic variants affect change in BMD during weight loss.
In the current study, we investigated whether butyrate-related genetic variants were associated with changes in BMD in the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial, to date the longest and largest comparator study of weight-loss diets (21). As the main substrate for microbial fermentation, dietary fiber showed beneficial effects on bone health partly by promoting calcium absorption and calcium retention through the production of SCFAs (22, 23). In addition, the sex-specific difference was observed in both the composition of gut microbiota (24) and changes in BMD during weight loss (i.e., women are more likely to lose bone compared with men) (8). Therefore, we also examined interactions of dietary fiber intake and sex with butyrate-related genetic variants on changes in BMD.
Methods
Study population
POUNDS LOST is a 2-y randomized intervention trial. Detailed information on study design and methods has been described elsewhere (21). In this study, 811 overweight and obese individuals were assigned to 4 energy-reduced diets differing in macronutrient composition of fat [low (20%) or high (40%)], protein [average (15%) or high (25%)], and carbohydrate (range:35–65%) to compare their effects on body weight change over 2 y. Of the 811 participants, 424 were randomly assigned to the DXA substudy, which measured BMD (Supplemental Figure 1). The study had 80% power to detect a 0.7-kg difference in total body fat loss as an effect of the amount of protein or fat in the diet over the 2-y period, with an assumption of a dropout rate of <40% and α = 0.05 (25).
The study was approved by the human participants committee at the Harvard TH Chan School of Public Health, Brigham and Women's Hospital, and the Pennington Biomedical Research Center. The study was also approved by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. Written informed consent was obtained from all participants.
Measurements of BMD and other measurements
The primary outcome of the study was the change in body weight, which has already been published (21), and the secondary outcome was the change in BMD. DXA (Hologic QDR-4500A bone densitometer; Hologic) was used to measure the BMD of the spine, total hip, and whole-body BMD as previously described (8). Using calibrated hospital scales, body weight was measured on 2 nonconsecutive days at baseline, 6 mo, and 2 y in the morning before breakfast and after urinating. Height was measured at baseline. Biomarkers of nutrient intake, including urinary nitrogen excretion and respiratory quotient, were measured to validate protein and fat intake, respectively. At baseline, dietary intake information, including dietary fiber and calcium intake, was obtained from 5-d diet records and at 6 and 24 mo from 24-h recalls collected by telephone on 3 nonconsecutive days in a random sample of 50% of the participants (26). Menopausal status was self-reported at enrollment according to the absence of menstrual periods during the past 12 mo or surgical removal of both ovaries (8).
Genotyping
DNA was extracted from the buffy coat fraction of centrifuged blood by using the QIAmp Blood Kit (Qiagen). Genotyping was performed among all 799 participants with the OpenArray SNP Genotyping System (BioTrove) with a sample call rate of 99%. Replicated quality-control samples (10%) were included in every genotyping plate with >99% concordance (27). Michigan Imputation Server was used to impute single nucleotide polymorphisms (SNPs) on 22 autosomes, with 1000G Phase 3 v5 as the reference panel.
In the current study, rs10483112 was excluded because it is a rare variant among whites; another rs2089222 was excluded for not being in Hardy–Weinberg equilibrium (P < 0.001). The polygenic score (PGS) was calculated by [β1 × SNP1 + β2 × SNP2 + … + β7 × SNP7] × [7/sum of the β-coefficients]. The PGS was available for 370 of the 424 participants with available BMD measurements. A higher PGS indicates a higher butyrate concentration.
Statistical analysis
Statistical analyses were performed with SAS version 9.4 (SAS Institute). All reported P values were 2-sided. P < 0.05 was used as the significance level. At baseline, the general linear model for continuous variables and chi-square test for categoric variables were performed for comparison of characteristics by tertiles of the PGS. Paired t test was used to calculate the P value for the change in BMD among men, women, and pooled participants. We used a generalized linear model to test the association between PGS and change in BMD during weight loss, with adjustment for age, sex, race, baseline values of the respective outcomes, baseline BMI, baseline dietary fiber intake, dietary calcium intake, and concurrent weight change. A generalized linear model was also used to test interactions by including a PGS-by-dietary fiber/PGS-by-sex interaction term, adjusted for age, race, sex, baseline dietary fiber intake, baseline dietary calcium intake, baseline BMI, concurrent weight change, and baseline values of the respective outcomes.
Results
Table 1 shows the baseline characteristics of participants. The mean ± SD age was 52 ± 9 y. The distribution of PGS (in tertiles) varied according to race and sex. No significant difference was found for other baseline measurements, nutrient intakes, or biomarkers of nutrient adherence across the tertiles of PGS. The frequency of menopause among women was 67.5% (137/203).
TABLE 1.
Baseline characteristics according to the SCFA PGS1
| Tertiles of the PGS | ||||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | P 2 | |
| n | 124 | 144 | 102 | |
| Age, y | 53 ± 8 | 52 ± 9 | 52 ± 10 | 0.84 |
| Female, n (%) | 79 (64) | 69 (47.9) | 55 (53.9) | 0.03 |
| Race, n (%) | <0.01 | |||
| Non-white | 31 (25) | 15 (10) | 3 (3) | |
| White | 93 (75) | 129 (90) | 99 (97) | |
| BMI, kg/m2 | 33 ± 4 | 33 ± 4 | 33 ± 4 | 0.39 |
| Height, cm | 169 ± 9 | 171 ± 9 | 169 ± 9 | 0.80 |
| Weight, kg | 93 ± 16 | 95 ± 15 | 94 ± 15 | 0.47 |
| Spine BMD, g/cm2 | 1.069 ± 0.135 | 1.066 ± 0.148 | 1.025 ± 0.148 | 0.523 |
| Femoral hip BMD, g/cm2 | 1.024 ± 0.131 | 1.020 ± 0.128 | 1.006 ± 0.144 | 0.963 |
| Whole-body BMD, g/cm2 | 1.134 ± 0.097 | 1.137 ± 0.111 | 1.124 ± 0.114 | 0.463 |
| Dietary intake per day | ||||
| Energy, kcal | 1910 ± 532 | 2037 ± 526 | 1991 ± 598 | 0.09 |
| Carbohydrate, % | 46 ± 8 | 43 ± 7 | 45 ± 7 | 0.19 |
| Fat, % | 36 ± 6 | 38 ± 6 | 37 ± 5 | 0.85 |
| Protein, % | 18 ± 3 | 18 ± 3 | 18 ± 3 | 0.25 |
| Dietary calcium, mg/d | 880 ± 528 | 906 ± 442 | 940 ± 525 | 0.16 |
| Dietary fiber, g/d | 12.4 ± 5.5 | 12.5 ± 5.6 | 13.5 ± 6.7 | 0.10 |
| Physical activity score4 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.82 |
Values are means ± SDs unless otherwise indicated. BMD, bone mineral density; PGS, polygenic score.
P values were calculated using the chi-square test for categorical variables and general linear models (PROC GLM) for continuous variable.
Data were adjusted for age, race, sex, BMI, baseline dietary fiber intake, baseline calcium intake, and baseline physical activity; PGS was treated as a continuous variable.
Physical activity score was estimated using the Baecke questionnaire.
Two-year change in whole-body BMD was 0.009 ± 0.028 g/cm2 (P = 0.002) in men and −0.006 ± 0.027 g/cm2 (P = 0.027) in women, with P-sex difference = 0.008. No association was found between baseline dietary fiber intake and change in whole-body BMD at 2 y. Furthermore, we did not find an association between the PGS and change in whole-body BMD in response to the dietary intervention.
We found that the PGS significantly modified the association between baseline dietary fiber intake and change in whole-body BMD at 2 y after multivariable adjustment (P-interaction = 0.049). Higher fiber intake was associated with improved BMD in the highest tertile of the PGS (β: 0.0022; 95% CI: 0.0009, 0.0035; P = 0.002), whereas no association was found in the lower tertiles (P = 0.188 and 0.864) (Figure 1, Table 2).
FIGURE 1.
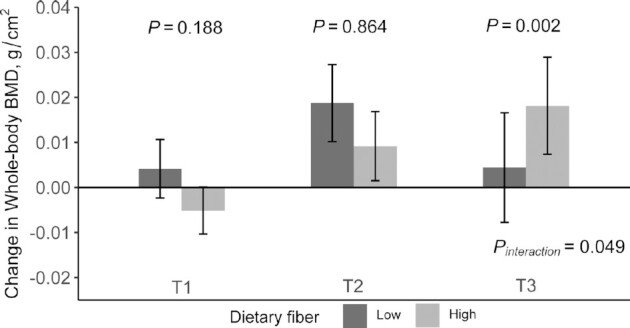
Effect of SCFA PGS and baseline dietary fiber intake on whole-body BMD change during the 2-y intervention. General linear models (PROC GLM) were used to test the effect of PGS and dietary fiber intake on BMD changes. Values are means ± SEs adjusted for age, sex, race, diet group, BMI, physical activity, concurrent weight change, baseline dietary calcium intake, and baseline value for respective outcome. T1–T3 represent the tertiles of SCFA PGS. A higher tertile represents a higher butyrate concentration. T1, n = 66; T2, n = 83; T3, n = 63. BMD, bone mineral density; PGS, polygenic score.
TABLE 2.
Changes in bone mineral density according to baseline dietary fiber and PGS at 24 mo1
| Low dietary fiber (<12.1 g/d) | High dietary fiber (≥12.1 g/d) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | β (SE) | P | Tertile 1 | Tertile 2 | Tertile 3 | β (SE) | P | P-interaction | |
| Spine BMD, g/cm2 | 0.006 ± 0.027 | 0.007 ± 0.045 | 0.002 ± 0.035 | 0.004 (0.003) | 0.136 | −0.002 ± 0.039 | 0.012 ± 0.039 | 0.001 ± 0.039 | 0.002 (0.003) | 0.473 | 0.358 |
| Femoral hip BMD, g/cm2 | −0.020 ± 0.029 | −0.005 ± 0.033 | −0.016 ± 0.030 | 0.000 (0.002) | 0.833 | −0.027 ± 0.034 | −0.014 ± 0.025 | −0.010 ± 0.029 | 0.004 (0.002) | 0.059 | 0.087 |
| Whole-body BMD, g/cm2 | 0.005 ± 0.025 | 0.011 ± 0.031 | −0.005 ± 0.021 | −0.002 (0.002) | 0.417 | −0.005 ± 0.028 | 0.002 ± 0.027 | 0.002 ± 0.035 | 0.002 (0.002) | 0.346 | 0.049 |
Values are means ± SDs unless otherwise indicated. P values were adjusted for age, sex, race, diet group, BMI, physical activity, concurrent weight change, baseline dietary calcium intake, and baseline value for the respective outcome. General linear models (PROC GLM) were used for the analyses. BMD, bone mineral density; PGS, polygenic score.
In addition, a significant interaction was found between the PGS and sex on change in whole-body BMD at 2 y (P-interaction = 0.008). Significant association between sex and change in whole-body BMD was observed in the lower tertiles (β: 0.0280; 95% CI: 0.0112, 0.0447; P = 0.002 and β: 0.0186; 95% CI: 0.0030, 0.0343; P = 0.023) but not in the highest tertile of the PGS (P = 0.216) (Figure 2, Table 3). Further adjustment for menopausal status did not change the results.
FIGURE 2.
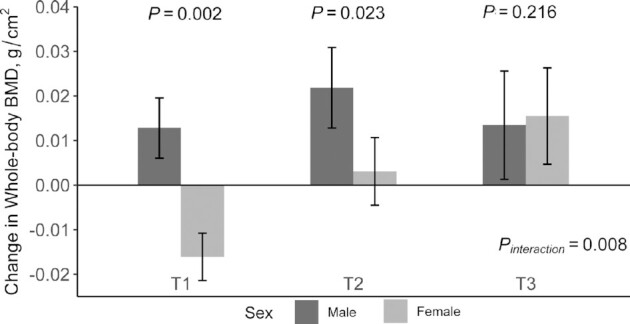
Effect of SCFA PGS and sex on whole-body BMD change during the 2-y intervention. General linear models (PROC GLM) were used to test the effect of PGS and sex on BMD changes. Values are means ± SEs adjusted for age, race, diet group, BMI, physical activity, concurrent weight change, baseline dietary calcium intake, baseline dietary fiber, and baseline value for the respective outcome. T1–T3 represent the tertiles of SCFA PGS. A higher tertile represents a higher butyrate concentration. T1, n = 66; T2, n = 84; T3, n = 63. BMD, bone mineral density; PGS, polygenic score.
TABLE 3.
Changes in BMD according to sex and PGS at 24 mo1
| Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | β (SE) | P | Tertile 1 | Tertile 2 | Tertile 3 | β (SE) | P | P-interaction | |
| Spine BMD, g/cm2 | 0.023 ± 0.021 | 0.023 ± 0.038 | 0.016 ± 0.041 | 0.002 (0.003) | 0.587 | −0.015 ± 0.033 | −0.010 ± 0.037 | −0.008 ± 0.030 | 0.003 (0.002) | 0.232 | 0.588 |
| Femoral hip BMD, g/cm2 | −0.020 ± 0.038 | −0.002 ± 0.027 | −0.016 ± 0.027 | 0.002 (0.003) | 0.567 | −0.027 ± 0.028 | −0.021 ± 0.028 | −0.012 ± 0.031 | 0.003 (0.002) | 0.057 | 0.165 |
| Whole-body BMD, g/cm2 | 0.011 ± 0.024 | 0.014 ± 0.029 | −0.002 ± 0.027 | −0.005 (0.002) | 0.041 | −0.010 ± 0.026 | −0.006 ± 0.023 | −0.002 ± 0.030 | 0.003 (0.002) | 0.092 | 0.008 |
Values are means ± SDs unless otherwise indicated. P values were adjusted for age, sex, race, diet group, BMI, physical activity, concurrent weight change, baseline dietary calcium intake, and baseline value for the respective outcome. General linear models (PROC GLM) were used for the analyses. BMD, bone mineral density; PGS, polygenic score.
No association was found between the PGS and change in BMD in either premenopausal or postmenopausal women. We did not find a significant interaction between the PGS and baseline dietary fiber intake or sex on the change in total hip and spine BMD.
Discussion
In the current study, genetically determined butyrate concentration showed significant interactions with baseline dietary fiber intake and sex on changes in whole-body BMD at 2 y. In the highest tertile of the butyrate PGS, higher dietary fiber intake was associated with a higher increase in whole-body BMD, whereas no such association was found among the lower tertiles. In addition, men showed more significant improvement in whole-body BMD compared with women in the lower tertiles of the butyrate PGS, whereas such sex difference was not observed among participants in the highest tertile.
Gut microbiota play important roles in regulating adiposity and bone health (19, 28–33). Gut microbiota may affect energy harvesting from the diet and the regulation of fat storage (29), and they may ferment indigestible carbohydrates such as fiber to SCFAs to provide energy. However, evidence also suggests that butyrate may alleviate diet-induced obesity through increasing energy expenditure (34). In addition, gut microbiota metabolites such as SCFAs have shown multiple beneficial effects on bone health (17, 18, 35, 36). For example, treatment of mice with SCFA significantly increases bone mass and prevents postmenopausal and inflammation-induced bone loss (17).
We found that genetically determined butyrate significantly modified the association between dietary fiber intake and change in whole-body BMD during weight loss. Dietary fiber intake significantly promotes bone health in participants with higher genetically determined butyrate but not in those with lower genetically determined butyrate. Dietary fiber has been associated with improved bone health (23, 37). For example, data from the Framingham Offspring cohort indicate that higher dietary fiber may modestly reduce bone loss in men but not in women (23). Dietary fiber is the main source of microbial fermentation to produce SCFAs, and enhancing the fermentation of fibers increases the production of SCFAs (17, 22, 38). SCFAs play a critical role in mediating the effect of dietary fiber on bone health (17) through several pathways (18, 35, 39). In our study, participants with higher genetically determined butyrate might have higher production of butyrate, which might improve the beneficial effects of dietary fiber on bone mass.
Weight-loss diets have shown sex-specific effects on changes in BMD (6, 8). In the current study, the whole-body BMD decreased more in women than in men. Interestingly, we also found that men and women exhibited different changes in BMD according to the genetically determined butyrate. A previous study indicated a sex-specific effect of SCFAs on bone health (35), with a stronger effect observed in male mice. We observed a similar sex difference among participants with lower genetically determined butyrate. However, such differences diminished among participants with the highest genetically determined butyrate. The observed sex difference might be partly related to the potential effects of SCFAs on sex hormones (33). For example, declining estrogen concentrations lead to bone loss via stimulating bone resorption and decreasing bone formation (40). Butyrate increases estradiol secretion and maintains circulating estrogen concentrations (41, 42), which may improve bone health. Taken together, we assumed that the genetically determined higher butyrate improved bone health by affecting sex hormones, thus overwhelming the sex difference in changes in BMD during weight loss. Further studies are warranted to investigate the mechanisms underlying these observations.
Interestingly, butyrate PGS predicted changes in whole-body BMD but not bone sites at the hip and spine with more metabolically active trabecular bone, which were found to be sensitive to weight loss in women (43). The mechanisms underlying such distinct associations are unclear. Approximately 80% of the bone mass is in the cortical compartment, and cortical bone has more calcium content compared with trabecular bone (44). Previous studies suggest that butyrate enhances calcium absorption and bone mineralization (45), which may exert more beneficial effects on cortical bone.
Our study has several strengths. To our knowledge, this is the first study to examine the relations between genetically determined butyrate and changes in BMD during diet-induced weight loss. The PGS used in the current study may capture lifelong butyrate concentration because the genetic variant and score cannot be influenced by environmental factors. Furthermore, compared with observational studies, our study design enabled us to minimize potential confounding bias. However, our study also had several potential limitations. First, we did not measure blood butyrate in the POUNDS LOST study. However, the genetic markers used in our study have been associated with the production of butyrate (20). Second, sex hormone concentrations, serum calcium, 25-hydroxyvitamin D concentration, and other biomarkers related to bone health were not available, which might provide additional information to understand the potential mechanisms underlying our findings. Last, 87% of the participants in our study were white; further studies are warranted to verify our findings in minority groups.
In summary, our results indicate that genetically determined butyrate might modify the effect of dietary fiber and sex on change in BMD during weight loss. Participants with a higher genetically determined butyrate might have better improvement in bone health during weight loss when taking more dietary fiber. In addition, women with higher genetically determined butyrate may benefit more in maintaining BMD by following weight-loss diets.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows–TZ, FMS, and LQ: designed the research; TZ, MSL, GAB, FMS, and LQ: conducted the research; TZ, DS, YH, and LQ: analyzed the data or performed statistical analysis; TZ and LQ: wrote the manuscript; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
This study was funded by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, and HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK115679, and DK078616), the Boston Obesity Nutrition Research Center (DK46200), and the United States–Israel Binational Science Foundation (2011036). LQ was a recipient of an American Heart Association Scientist Development Award (0730094N). The funders had no role in the design, implementation, analysis, and interpretation of the data.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviation used: BMD, bone mineral density; PGS, polygenic score; POUNDS LOST, Preventing Overweight Using Novel Dietary Strategies; SNP, single nucleotide polymorphism.
Contributor Information
Tao Zhou, School of Public Health (Shenzhen), Sun Yat-sen University, Guangzhou, China; Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA.
Dianjianyi Sun, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA; Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China.
Xiang Li, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA.
Yoriko Heianza, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA.
Meryl S LeBoff, Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA.
George A Bray, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA, USA.
Frank M Sacks, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Lu Qi, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA; Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.
References
- 1. Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang BC, Villareal DT. Weight loss–induced reduction of bone mineral density in older adults with obesity. J Nutr Gerontol Geriatr. 2019;38:100–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter GR, Plaisance EP, Fisher G. Weight loss and bone mineral density. Curr Opin Endocrinol Diabetes Obes. 2014;21:358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41. [DOI] [PubMed] [Google Scholar]
- 5. Pop LC, Sukumar D, Schneider SH, Schlussel Y, Stahl T, Gordon C, Wang X, Papathomas TV, Shapses SA. Three doses of vitamin D, bone mineral density, and geometry in older women during modest weight control in a 1-year randomized controlled trial. Osteoporos Int. 2017;28:377–88. [DOI] [PubMed] [Google Scholar]
- 6. Choksi P, Rothberg A, Kraftson A, Miller N, Zurales K, Burant C, Van Poznak C, Peterson M. Weight loss and bone mineral density in obese adults: a longitudinal analysis of the influence of very low energy diets. Clin Diabetes Endocrinol. 2018;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2004;20:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tirosh A, de Souza RJ, Sacks F, Bray GA, Smith SR, LeBoff MS. Sex differences in the effects of weight loss diets on bone mineral density and body composition: POUNDS LOST trial. J Clin Endocrinol Metab. 2015;100:2463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guss JD, Horsfield MW, Fontenele FF, Sandoval TN, Luna M, Apoorva F, Lima SF, Bicalho RC, Singh A, Ley REet al. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res. 2017;32:1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2015;26:69–74. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y-CC, Greenbaum J, Shen H, Deng H-WW. Association between gut microbiota and bone health: potential mechanisms and prospective. J Clin Endocrinol Metab. 2017;102:3635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCabe L, Britton RA, Parameswaran N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep. 2015;13:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol. 2014;10:416–24. [DOI] [PubMed] [Google Scholar]
- 14. Rizzoli R. Nutritional influence on bone: role of gut microbiota. Aging Clin Exp Res. 2019;31:743–51. [DOI] [PubMed] [Google Scholar]
- 15. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest Get al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lucas S, Omata Y, Hofmann J, Böttcher M, Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B, Krönke Get al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan J, Takakura A, Zandi-Nejad K, Charles JF. Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes. 2017;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaiss MM, Jones RM, Schett G, Pacifici R. The gut–bone axis: how bacterial metabolites bridge the distance. J Clin Invest. 2019;129:3018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, Mujagic Z, Masclee AAM, Jonkers D, Oosting Met al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, Mcmanus K, Champagne CM, Bishop LM, Laranjo Net al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wallace TC, Marzorati M, Spence L, Weaver CM, Williamson PS. New frontiers in fibers: innovative and emerging research on the gut microbiome and bone health. J Am Coll Nutr. 2017;36:218–22. [DOI] [PubMed] [Google Scholar]
- 23. Dai Z, Zhang Y, Lu N, Felson DT, Kiel DP, Sahni S. Association between dietary fiber intake and bone loss in the Framingham Offspring Study. J Bone Miner Res. 2018;33:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santos-Marcos JA, Haro C, Vega-Rojas A, Alcala-Diaz JF, Molina-Abril H, Leon-Acuña A, Lopez-Moreno J, Landa BB, Tena-Sempere M, Perez-Martinez Pet al. Sex differences in the gut microbiota as potential determinants of gender predisposition to disease. Mol Nutr Food Res. 2019;63:1800870. [DOI] [PubMed] [Google Scholar]
- 25. de Souza RJ, Bray GA, Carey VJ, Hall KD, Leboff MS, Loria CM, Laranjo NM, Sacks FM, Smith SR. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results. Am J Clin Nutr. 2012;95:614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Champagne CM, Bogle ML, McGee BB, Yadrick K, Allen HR, Kramer TR, Simpson P, Gossett J, Weber J. Dietary intake in the lower Mississippi delta region: results from the Foods of Our Delta study. J Am Diet Assoc. 2004;104:199–207. [DOI] [PubMed] [Google Scholar]
- 27. Xu M, Qi Q, Liang J, Bray Ga, Hu FB, Sacks FM, Qi L. Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: The Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation. 2013;127:1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ley R, Turnbaugh P, Klein S, Gordon J. Human gut microbes associated with obesity. Nature. 2006;444:1022–3. [DOI] [PubMed] [Google Scholar]
- 29. Muscogiuri G, Cantone E, Cassarano S, Tuccinardi D, Barrea L, Savastano S, Colao A. Gut microbiota: a new path to treat obesity. Int J Obes Supp. 2019;9:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008;83:460–9. [DOI] [PubMed] [Google Scholar]
- 32. Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana Det al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yan J, Charles JF. Gut microbiome and bone: to build, destroy, or both?. Curr Osteoporos Rep. 2017;15:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jia Y, Hong J, Li H, Hu Y, Jia L, Cai D, Zhao R. Butyrate stimulates adipose lipolysis and mitochondrial oxidative phosphorylation through histone hyperacetylation-associated β3-adrenergic receptor activation in high-fat diet-induced obese mice. Exp Physiol. 2017;102:273–81. [DOI] [PubMed] [Google Scholar]
- 35. Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA. 2016;113:E7554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wallace TC, Marzorati M, Spence L, Weaver CM, Williamson PS. New frontiers in fibers: innovative and emerging research on the gut microbiome and bone health. J Am Coll Nutr. 2017;36:218–22. [DOI] [PubMed] [Google Scholar]
- 37. Jakeman SA, Henry CN, Martin BR, McCabe GP, McCabe LD, Jackson GS, Peacock M, Weaver CM. Soluble corn fiber increases bone calcium retention in postmenopausal women in a dose-dependent manner: a randomized crossover trial. Am J Clin Nutr. 2016;104:837–43. [DOI] [PubMed] [Google Scholar]
- 38. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rahman M, Kukita A, Kukita T, Shobuike T, Nakamura T, Kohashi O. Two histone deacetylase inhibitors, trichostatin A and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages. Blood. 2003;101:3451–9. [DOI] [PubMed] [Google Scholar]
- 40. Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. [DOI] [PubMed] [Google Scholar]
- 41. Lu N, Li M, Lei H, Jiang X, Tu W, Lu Y, Xia D. Butyric acid regulates progesterone and estradiol secretion via cAMP signaling pathway in porcine granulosa cells. J Steroid Biochem Mol Biol. 2017;172:89–97. [DOI] [PubMed] [Google Scholar]
- 42. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen–gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45–53. [DOI] [PubMed] [Google Scholar]
- 43. Tirosh A, de Souza RJ, Sacks F, Bray GA, Smith SR, LeBoff MS. Sex differences in the effects of weight loss diets on bone mineral density and body composition: POUNDS LOST trial. J Clin Endocrinol Metab. 2015;100:2463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ott SM. Cortical or trabecular bone: what's the difference?. Am J Nephrol. 2018;47:373–5. [DOI] [PubMed] [Google Scholar]
- 45. Katono T, Kawato T, Tanabe N, Suzuki N, Iida T, Morozumi A, Ochiai K, Maeno M. Sodium butyrate stimulates mineralized nodule formation and osteoprotegerin expression by human osteoblasts. Arch Oral Biol. 2008;53:903–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.


