Abstract
Mitochondrial dysfunction is a fundamental challenge in septic cardiomyopathy. Mitophagy and the mitochondrial unfolded protein response (UPRmt) are the predominant stress-responsive and protective mechanisms involved in repairing damaged mitochondria. Although mitochondrial homeostasis requires the coordinated actions of mitophagy and UPRmt, their molecular basis and interactive actions are poorly understood in sepsis-induced myocardial injury. Our investigations showed that lipopolysaccharide (LPS)-induced sepsis contributed to cardiac dysfunction and mitochondrial damage. Although both mitophagy and UPRmt were slightly activated by LPS in cardiomyocytes, their endogenous activation failed to prevent sepsis-mediated myocardial injury. However, administration of urolithin A, an inducer of mitophagy, obviously reduced sepsis-mediated cardiac depression by normalizing mitochondrial function. Interestingly, this beneficial action was undetectable in cardiomyocyte-specific FUNDC1 knockout (FUNDC1CKO) mice. Notably, supplementation with a mitophagy inducer had no impact on UPRmt, whereas genetic ablation of FUNDC1 significantly upregulated the expression of genes related to UPRmt in LPS-treated hearts. In contrast, enhancement of endogenous UPRmt through oligomycin administration reduced sepsis-mediated mitochondrial injury and myocardial dysfunction; this cardioprotective effect was imperceptible in FUNDC1CKO mice. Lastly, once UPRmt was inhibited, mitophagy-mediated protection of mitochondria and cardiomyocytes was partly blunted. Taken together, it is plausible that endogenous UPRmt and mitophagy are slightly activated by myocardial stress and they work together to sustain mitochondrial performance and cardiac function. Endogenous UPRmt, a downstream signal of mitophagy, played a compensatory role in maintaining mitochondrial homeostasis in the case of mitophagy inhibition. Although UPRmt activation had no negative impact on mitophagy, UPRmt inhibition compromised the partial cardioprotective actions of mitophagy. This study shows how mitophagy modulates UPRmt to attenuate inflammation-related myocardial injury and suggests the potential application of mitophagy and UPRmt targeting in the treatment of myocardial stress.
Keywords: Mitophagy, Mitochondrial unfolded protein response, Septic cardiomyopathy, Inflammation, FUN14 domain-containing 1
Abbreviations: UPRmt, Mitochondrial unfolded protein response; LPS, lipopolysaccharide; (PBS), PBS, phosphate buffered saline; DMSO, dimethyl sulfoxide; DMEM/F-12, Dulbecco’s modified Eagle’s medium/F-12 nutrient mixture; FS, Fractional shortening; LVEF, left ventricular ejection fraction; LVDd, left ventricular diastolic; siRNAs, small interfering RNAs, 4,6-diamidino-2-phenylindole; LDH, lactate dehydrogenase; EM, electron microscopy
Highlights
-
•
Mitochondrial dysfunction is a fundamental challenge in septic cardiomyopathy.
-
•
LPS-induced sepsis contributes to cardiac dysfunction and mitochondrial damage.
-
•
Endogenous UPRmt and mitophagy could be slightly activated by myocardial stress.
-
•
Mitophagy modulates UPRmt to attenuate inflammation-related myocardial injury.
-
•
Mitophagy and UPRmt targeting can be applied in treatment of myocardial stress.
1. Introduction
Septic cardiomyopathy is characterized by decreased left ventricular dilatation and an impaired ejection fraction resulting from severe sepsis syndrome [1]. In contrast to myocardial infarction, septic cardiomyopathy is a reversible form of myocardial depression. Reportedly, oxidative stress, cytokine overproduction, microvascular damage, and cardiomyocyte ATP metabolism are potential molecular mechanisms underlying sepsis-induced myocardial damage [[2], [3], [4], [5]]. Anti-inflammatory agents and myocardial metabolic therapies have been identified as effective strategies for the treatment of septic cardiomyopathy [6,7]. As the mitochondria are regulators of cardiomyocyte metabolism and inflammation response, their dysfunction is a key event in myocardial depression [8,9]. Mitochondrial dysfunction plays an important role in inducing oxidative stress and promoting energy crisis; these alterations are always followed by cardiomyocyte death through either apoptosis or necrosis [10,11]. Irreversible cardiomyocyte death is a key molecular mechanism in activating the inflammatory response. Despite extensive research focused on understanding the complex impact of mitochondrial damage on sepsis-related myocardial depression, the pathological alterations in the mitochondria in cardiomyocytes are poorly understood.
In order to maintain a functional mitochondrial network, the mitochondria develop specific repair pathways, including mitophagy and the mitochondrial unfolded protein response (UPRmt) [12,13]. UPRmt prevents abnormal protein accumulation within the mitochondria via normalization of mitochondrial protein folding and degradation [14]. Mitophagy selectively removes damaged mitochondria via ubiquitination degradation [15]. In contrast to UPRmt, mitophagy reduces the mitochondrial population, an alteration that is occasionally associated with shortages in ATP and cell death [16]. Unlike mitophagy, UPRmt dynamically controls mitochondrial protein import or export and fine-tunes mitochondrial behaviors [17]. Our previous studies described the features of mitophagy in cardiovascular disorders, such as myocardial infarction, cardiac ischemia-reperfusion injury, and diabetic cardiomyopathy [[18], [19], [20], [21], [22], [23]]. Once moderately activated, mitophagy exerts cardioprotective effects by sustaining mitochondrial quality; however, excessive induction of mitophagy is fatal because most mitochondria are degraded and ATP production is therefore suppressed [24]. Moreover, according to our recent findings, the role of mitophagy in deciding cell fate is also controlled by various mitophagic adaptors, such as FUNDC1, Parkin, and Bnip3 [18,24,25].
Recently, the role of UPRmt in myocardial stress has been reported [26,27]. In a mouse model of chronic pressure overload-mediated cardiac hypertrophy [27], the markers of UPRmt, such as Atf5, CHOP, mtDNAj, ClpP, and LonP1, were significantly elevated at the mRNA level, suggesting that UPRmt is an adaptive response to myocardial stress. Interestingly, further activation of UPRmt through nicotinamide riboside supplementation has been associated with normalized mitochondrial respiration and decreased cardiomyocytes in vitro [27]. Moreover, in response to nicotinamide riboside, cardiomyocyte apoptosis is reduced and cardiac function is improved, suggesting the cardioprotective action of UPRmt activation [27]. In a mouse model of cardiac ischemia-reperfusion (I/R) injury [26], the genes related to UPRmt were also significantly upregulated, as identified using RNA-Seq analysis. Interestingly, genetic ablation of Atf5 has no influence on baseline cardiac I/R injury [26]. However, administration of the UPRmt inducer reduced I/R-mediated myocardial injury in wild-type (WT) mice, but not in Atf5-knockout mice [26]. This result demonstrates that I/R-mediated UPRmt activation is insufficient to protect the heart against acute stress, and that additional induction of UPRmt is a promising target for the treatment of cardiac I/R injury. However, the role of UPRmt in septic cardiomyopathy has not yet been elucidated. Moreover, as the mitochondria repair pathways, the relationship between mitophagy and UPRmt remains unclear, although several studies report that mitophagy inhibition is followed by UPRmt activation [28]. The aim of this study was to explore the role of UPRmt in septic cardiomyopathy and to clarify the interactive effects of UPRmt and mitophagy.
2. Materials and methods
2.1. Animal care
FUNDC1f/f mice and cardiomyocyte-specific FUNDC1 knockout (FUNDC1CKO) mice were generated as described in our previous study [25]. These mice were bred and maintained at the University of Wyoming Institutional Animal Center. All procedures related to animal treatment and surgery were approved by the University of Wyoming Institutional Animal Use and Care Committee (Laramie, WY, USA). In our study, 8-week-old mice were injected intraperitoneally with a single dose of phosphate buffered saline (PBS) or lipopolysaccharide (LPS) (5 mg/kg) to induce septic cardiomyopathy. The animals were euthanized via pentobarbital 48 h after LPS injection. Heart tissues were then isolated and used for subsequent experiments. To induce UPRmt in vivo, mice were intraperitoneally injected with oligomycin (500 μg/kg) in sterile saline with 0.1% dimethyl sulfoxide (DMSO). To activate mitophagy, urolithin A (UA; 30 mg/kg) was intraperitoneally injected in sterile saline with 0.1% DMSO.
2.2. Cell culture and treatments
The AC16 human ventricular cardiomyocyte cell line was maintained in Dulbecco’s modified Eagle’s medium/F-12 nutrient mixture (DMEM/F-12; Invitrogen, Carlsbad, CA) medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C and 5% CO2 [29]. After treating AC16 cells with 1 μM human insulin (Santa Cruz Biotechnology, Inc.) for 1 h, they were harvested for protein purification [30]. To induce sepsis-related cardiomyocyte damage, AC16 cells were treated with filtered (0.2 μ syringe filter; Corning) DMEM/F12 complete medium containing 10 μg/mL of LPS (Sigma-Aldrich).
2.3. Mitochondrial membrane potential and mt-Keima assays
As previously described [31], AC16 cells were incubated with JC-1 dyes to observe changes in the mitochondrial membrane potential. Mitophagy activity was observed using the mt-Keima assay, which was performed using a mitochondria-targeted mKeima-Red expression plasmid (pMT-mKeima-Red, #AM-V-251, MBL Medical & Biological Laboratories, Co., Ltd.. Woburn, MA), as previously described [32,33].
2.4. MTT cell viability assay
The MTT cell viability assay was performed using an MTT assay kit (Abcam, ab211091), according to the manufacturer’s protocol [34,35].
2.5. Echocardiography
Operators blinded to the study groups performed echocardiography on conscious animals using a 20 MHz ultrasound transducer interfaced with a VisualSonics Vevo 2100 imaging system [36]. Two-dimensionally directed M-mode images were obtained from long-axis views. Comprehensive M-mode and two-dimensional imaging were used to determine fractional shortening (FS), wall thickness estimated wall mass, cardiac function, volume, ejection fraction, and cardiac output [37]. Doppler flow imaging was used to assess systolic and diastolic functions [38]. Echocardiographic measurements were performed on three consecutive cardiac cycles using the leading edge-to-leading edge method. Left ventricular ejection fraction (LVEF), left ventricular diastolic dimension (LVDd), and FS were measured or calculated with software [39].
2.6. Quantitative and semi-quantitative
To perform semi-quantitative real-time (RT) PCR, total RNA was extracted from cardiomyocytes using TRIzol (Invitrogen); DNase 1 (Promega) was used to remove genomic DNA. RNA (1 μg) was reverse transcribed into cDNA using the SuperScript IV First-Strand Synthesis System (Invitrogen) with random hexamer primers; 80 μL of ddH2O was added following the reaction at room temperature, and the final cDNA concentration was adjusted to 10 ng/μL [40,41]. A 20 μL regular PCR reaction was performed using human GAPDH primers with 2 μL of cDNA template (20 ng). The following PCR program was used: 95 °C for 20 s (denaturation), 60 °C for 20 s (annealing), 72 °C for 50 s (extension), for a total of 35 cycles. Next, 7 μL of the PCR product was loaded into a 1.5% agarose gel with 1X TAE buffer. Band intensity was digitized and quantified using ImageJet software [42,43].
2.7. Cardiomyocyte transfection
Small interfering RNAs (siRNAs) for FUNDC1 and Atf6 and the negative control (Mission, Sigma-Aldrich Corp., St Louis, MO) were used according to the manufacturer's instructions [44,45]. Following isolation, cardiomyocytes were cultured for 48 h prior to transfection with siRNAs at a concentration of 50 nM using Lipofectamine® RNAiMAX (Invitrogen, Carlsbad, CA) [46,47]. Following 6 h of incubation in Optimem®, the medium was removed and replaced with 5% FBS in DMEM/M199. Changes in FUNDC1 and Atf6 protein and/or transcript levels were assessed 48 h post-transfection [48].
2.8. Immunofluorescence
Cardiomyocytes were seeded onto sterile, acid-treated, 18-mm #1.5 glass coverslips in 24-well plates. They were fixed in 2% paraformaldehyde in PBS for 10 min [49]. Permeabilization was performed by incubating the cells in PBS containing 0.1% Triton X-100 for 5 min at 37 °C. Next, cells were incubated with Tom20 (1:1,000, Abcam, #ab186735) primary antibodies for 1 h at room temperature, followed by three washes to remove unbound antibodies. Next, the cells were incubated with secondary antibodies for 1 h. DNA was stained with 4,6-diamidino-2-phenylindole (DAPI, Sigma, cat no. D9564) at a dilution of 1:5000 for 2 min [50]. Cells were then imaged using a Yokogawa CSU-W1 spinning disk scan head with a 50-μm pinhole disk mounted on a Nikon Ti inverted microscope (Nikon Ti) [51]. Signals from FITC and rhodamine channels were collected using Chroma ET 525/36 and ET 605/52 emission filters, respectively [52].
2.9. ATP and mitochondrial reactive oxygen species (ROS) measurements
The ATP concentration in cells was measured using the Cell Titer-Glo Luminescent Viability Assay (Promega, Madison, WI). Briefly, CellTiter-Glo reagent was added to each well (100 μL); cellular contents were mixed for 2–3 min on an orbital shaker [35]. Next, the plate was incubated for 10 min to stabilize the luminescent signal, and luminescence was read using a Tecan Infinite 200 PRO plate reader (Männedorf, Switzerland) [53]. The mitochondrial ROS concentration in cells was measured using the cell-permeant MitoSOX Red mitochondrial superoxide indicator (Molecular Probes, USA) [54].
2.10. Enzyme-linked immunosorbent assay (ELISA)
ELISA-based assays were performed using kits for lactate dehydrogenase (LDH), troponin T, and CK-MB from InvivoGen (San Diego, CA, USA). Colorimetric and luminescent signals from ELISA kits were measured using a Tecan Infinite 200 PRO plate reader (Männedorf, Switzerland), according to the manufacturer’s instructions [55,56].
2.11. Statistics
All data are presented as the mean ± standard error of the mean (SEM). Independent biological replicates are denoted by an ‘n’ in the figure legends. Statistical analysis was performed in GraphPad (Prizm) using the Student’s t-test (two-tailed). Statistical significance was set at P <0.05.
3. Results
3.1. Activation of FUNDC1-associated mitophagy attenuates sepsis-induced myocardial injury
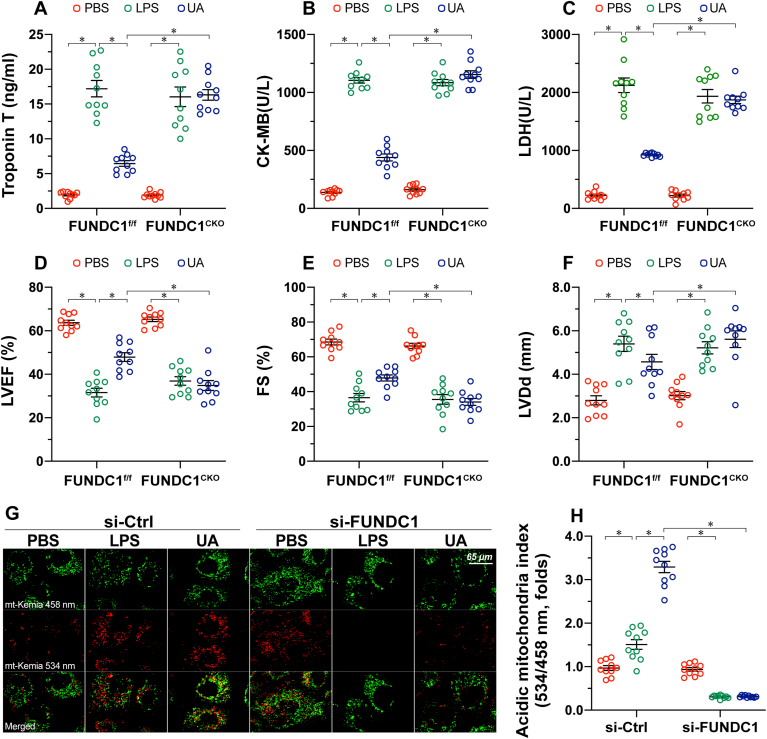
First, to observe the cardioprotective role of mitophagy in regulating septic cardiomyopathy, UA, a mitophagy inducer, was administered to mice prior to LPS treatment. Compared to sham-operated control mice, LPS-mediated sepsis significantly elevated the levels of cardiac injury markers, such as LDH, troponin T, and CK-MB (Fig. 1A–C). In contrast, administration of UA to activate mitophagy suppressed myocardial injury biomarker levels (Fig. 1A–C). In addition, echocardiography of cardiac function also showed that LVEF, LVDd, and FS were disrupted by LPS injection (Fig. 1D–F). However, normalized cardiac function, including LVEF, LVDd, and FS, were observed in mice pretreated with UA (Fig. 1D–F). To determine whether UA-mediated cardioprotection is dependent on FUNDC1, cardiomyocyte-specific FUNDC1CKO mice were used in our subsequent experiments. Interestingly, UA failed to suppress cardiac injury biomarker levels in FUNDC1CKO mice (Fig. 1A–C). Consistent with this finding, LPS-induced cardiac dysfunction could not be markedly reversed by UA in FUNDC1CKO mice (Fig. 1D–F). To validate whether UA and/or FUNDC1 are involved in mitophagy activation in cardiomyocytes, mitophagy activity was detected using an mt-Keima assay in vitro. Our results showed that mitophagy was moderately induced by LPS and robustly activated by UA (Fig. 1G–H). However, deletion of FUNDC1 largely prevented mitophagy activation in the presence of UA (Fig. 1G–H). Taken together, we reasoned that activation of FUNDC1-associated mitophagy confers cardioprotection in a mouse model of septic cardiomyopathy.
Fig. 1.
Activation of FUNDC1-associated mitophagy attenuates sepsis-induced myocardial injury. FUNDC1f/f mice and cardiomyocyte-specific FUNDC1 knockout (FUNDC1CKO) mice were injected intraperitoneally with a single dose of PBS or LPS (5 mg/kg) to induce septic cardiomyopathy. Blood and heart samples were isolated 48 h after LPS injection. To activate mitophagy, urolithin A (UA, 30 mg/kg) was injected intraperitoneally in sterile saline with 0.1% DMSO. A-C. ELISA analysis of the concentration of cardiac injury markers, including LDH, troponin T, and CK-MB. D-F. Echocardiographic data showing left ventricular ejection fraction (LVEF), left ventricular diastolic dimension (LVDd), and fractional shortening (FS) in FUNDC1CKO and FUNDC1f/f mice in the presence of LPS. G-H. The AC16 human ventricular cardiomyocyte cell line was treated with 10 μg/mL of LPS to induce sepsis-related cardiomyocyte damage. siRNA against FUNDC1 (si-FUNDC1) was transfected into AC15 to inhibit mitophagy activation. UA, an inducer of mitophagy, was used to culture AC16 cells. Next, mitophagy activity was observed using the mt-Keima assay. A yellow signal highlights increased mitophagic flux within cardiomyocytes. Data are presented as mean ± SEM, normalized per 1000 cardiomyocytes. n = 6 per group. *P<0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Loss of FUNDC1-associated mitophagy promotes cardiomyocyte death and mitochondrial dysfunction.
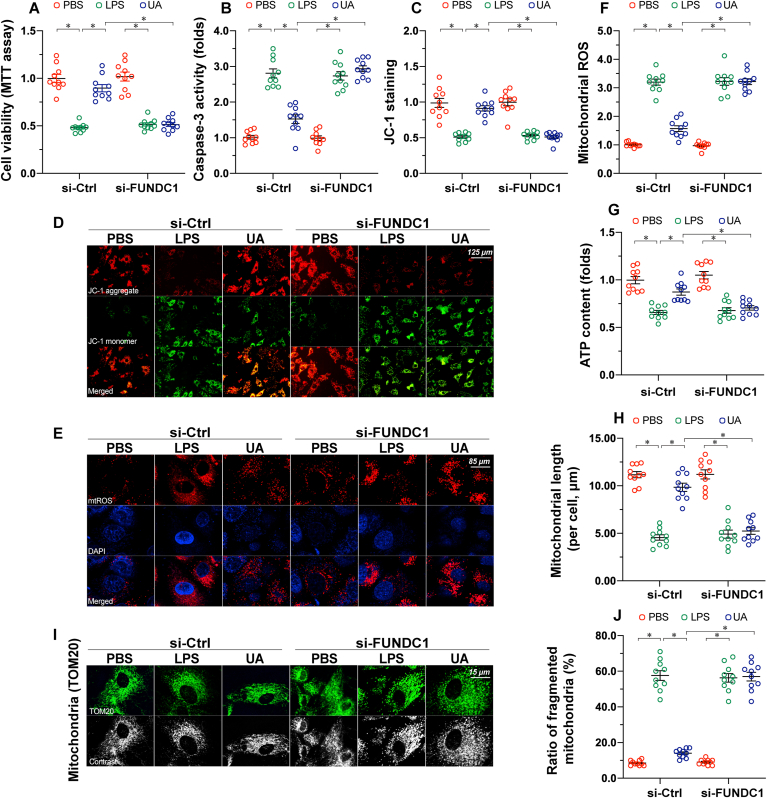
Mitochondrial dysfunction, acting as the downstream of inflammation response, is an essential inducer of septic cardiomyopathy through promoting the death of functional cardiomyocytes [1]. Thus, cardiomyocyte death and mitochondrial function were measured. Firstly, cardiomyocyte viability, as evaluated using an MTT assay, was reduced by LPS, reversing to near-normal levels following UA treatment (Fig. 2A). However, in FUNDC1-deleted cardiomyocytes, UA failed to improve cardiomyocyte viability (Fig. 2A). Similar to this finding, analysis of caspase-3 activity demonstrated that UA prevented LPS-mediated caspase-3 activation in WT cardiomyocytes, but not in FUNDC1-depleted cardiomyocytes (Fig. 2B). Similarly, the LPS-dissipated mitochondrial membrane potential could be stabilized via UA; this effect required FUNDC1 in cardiomyocytes (Fig. 2C–D). Moreover, mitochondrial ROS production levels were also suppressed by UA; this action was detectable in WT cardiomyocytes but not in FUNDC1-depleted cardiomyocytes (Fig. 2E–F). Similar results were also observed in changes to ATP production (Fig. 2G). Moreover, mitochondrial dynamics appeared to be disrupted by LPS, as evidenced by the increased number of fragmented mitochondria (Fig. 2H–J). Furthermore, UA treatment was associated with an elongation of mitochondrial length, resulting in a decreased ratio of fragmented mitochondria; however, these beneficial effects were imperceptible if FUNDC1 was knocked out in cardiomyocytes (Fig. 2H–J). Overall, activation of FUNDC1-associated mitophagy prevented mitochondrial damage and cardiomyocyte death during septic cardiomyopathy.
Fig. 2.
Loss of FUNDC1-associated mitophagy promotes cardiomyocyte death and mitochondrial dysfunction. The AC16 human ventricular cardiomyocyte cell line was treated with 10 μg/mL of LPS to induce sepsis-related cardiomyocyte damage. UA, an inducer of mitophagy, was used to culture AC16 cells. siRNA against FUNDC1 (si-FUNDC1) was transfected into AC15 to inhibit mitophagy activation. A. Cell viability was determined using an MTT assay. B. ELISA analysis of caspase-3 activity. C-D. AC16 cells were stained with JC-1 to observe changes in the mitochondrial membrane potential. E-F. AC16 cells were stained with the MitoSOX red mitochondrial superoxide indicator to show changes in mitochondrial ROS. G. Total ATP production was determined using the Cell Titer-Glo Luminescent Viability assay. H-J. Mitochondrial morphology was revealed using confocal immunofluorescence. TOM20 was used to show the shape of mitochondria in response to LPS or FUNDC1 deletion. Data are presented as mean ± SEM, normalized per 1000 cardiomyocytes. *P<0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. UPRmt is activated in response to FUNDC1 deficiency
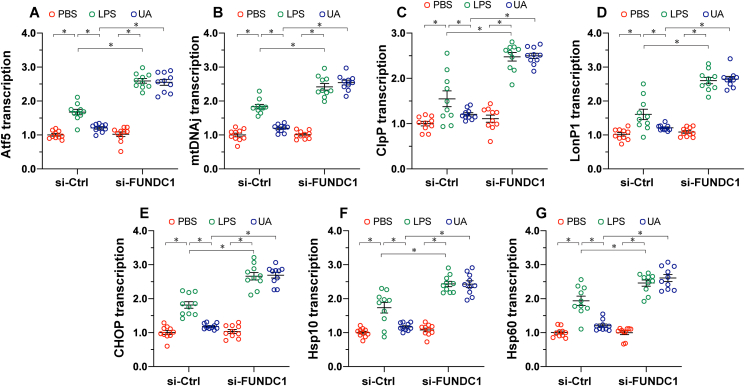
To illustrate the relationship between mitophagy and UPRmt, we measured alterations in UPRmt markers in response to mitophagy activation or inactivation. The qPCR assay demonstrated that the transcription of UPRmt markers, including Atf5, mtDNAj, ClpP, LonP1, CHOP, Hsp10, and Hsp60, was moderately upregulated in LPS-treated heart tissue (Fig. 3A–G), suggesting that UPRmt is induced by the inflammatory response. Interestingly, UA treatment appeared to partly prevent UPRmt activation in LPS-treated mice, suggesting that mitophagy activation is followed by the slight inhibition of UPRmt. In contrast, loss of FUNDC1 significantly upregulated the transcription of UPRmt markers (Fig. 3A–G), indicating that it may act as a compensatory mechanism in response to mitophagy inactivation.
Fig. 3.
UPRmtis activated in response to FUNDC1 deficiency. FUNDC1f/f and cardiomyocyte-specific FUNDC1 knockout (FUNDC1CKO) mice were injected intraperitoneally with a single dose of PBS or LPS (5 mg/kg) to induce septic cardiomyopathy. Blood and heart samples were isolated 48 h after LPS injection. To activate mitophagy, urolithin A (UA, 30 mg/kg) was injected intraperitoneally in sterile saline with 0.1% DMSO. A-G. Total RNA was isolated from the ventricular tissue of FUNDC1CKO and FUNDC1f/f mice. RT-PCR was conducted to assess genes involved in UPRmt. Data are presented as mean ± SEM. n = 6 per group. *P<0.05.
Activation of UPRmt partly reduces sepsis-induced myocardial injury and mitochondrial dysfunction in FUNDC1-knockout mice.
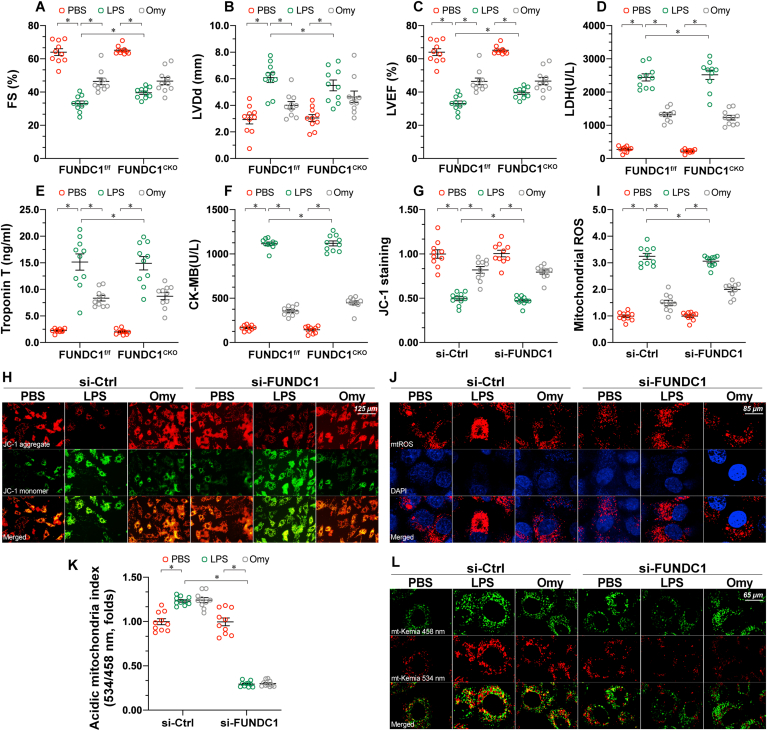
To understand whether UPRmt is a compensatory mechanism during mitophagy inactivation, loss- and gain-of-function assays were performed. First, oligomycin was injected into mice before the induction of septic cardiomyopathy. Compared to LPS-treated mice, oligomycin treatment improved cardiomyocyte function, as indicated by normalized LVEF, LVDd, and FS indices (Fig. 4A–C). In addition, cardiac injury biomarkers were also partly reduced in response to oligomycin treatment (Fig. 4D–F). At the sub-cellular level, the mitochondrial membrane potential was stabilized (Fig. 4G–H), whereas mitochondrial ROS production (Fig. 4I–J) was reduced in the presence of oligomycin, which was associated with increased cardiomyocyte viability. Taken together, these results illustrate that UPRmt activation protects the heart and mitochondria against inflammation injury.
Fig. 4.
Activation of UPRmtpartly reduces sepsis-induced myocardial injury and mitochondrial dysfunction in FUNDC1-knockout mice. FUNDC1f/f and cardiomyocyte-specific FUNDC1 knockout (FUNDC1CKO) mice were injected intraperitoneally with a single dose of PBS or LPS (5 mg/kg) to induce septic cardiomyopathy. Blood and heart samples were isolated 48 h after LPS injection. To induce UPRmtin vivo, mice were injected with oligomycin (500 μg/kg) intraperitoneally in sterile saline with 0.1% DMSO. A-C. Echocardiographic data showing left ventricular ejection fraction (LVEF), left ventricular diastolic dimension (LVDd), and fractional shortening (FS) in FUNDC1CKO and FUNDC1f/f mice in the presence of LPS or oligomycin. D-F. ELISA analysis of the concentration of cardiac injury markers, including LDH, troponin T, and CK-MB. G-H. The AC16 human ventricular cardiomyocyte cell line was treated with 10 μg/mL of LPS to induce sepsis-related cardiomyocyte damage. siRNA against FUNDC1 (si-FUNDC1) was transfected into AC15 to inhibit mitophagy activation. Oligomycin, an inducer of UPRmt, was used to culture AC16 cells. Next, cells were stained with JC-1 to observe changes in the mitochondrial membrane potential. I-J. AC16 cells were stained with the MitoSOX red mitochondrial superoxide indicator to show changes in mitochondrial ROS. K-L. Mitophagy activity was observed using the mt-Keima assay. A yellow signal highlights increased mitophagic flux within cardiomyocytes. Data are presented as mean ± SEM, normalized per 1000 cardiomyocytes. n = 6 per group. *P<0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To understand whether UPRmt acts as a downstream signal of FUNDC1-associated mitophagy, we further examined whether UPRmt-mediated cardioprotection was detectable in FUNDC1CKO mice. Interestingly, in the absence of FUNDC1, oligomycin was able to maintain cardiac function and reduce the levels of cardiac injury biomarkers (Fig. 4A–F). More importantly, mitochondrial function was also partly normalized by oligomycin administration in FUNDC1CKO mice (Fig. 4G–J). Moreover, no statistical significance was observed regarding myocardial injury markers (Fig. 4A–C), cardiac function (Fig. 4D–F) and mitochondrial performance (Fig. 4G–J) between WT and FUNDC1CKO mice in response to oligomycin, suggesting that UPRmt works independently of mitophagy. Therefore, UPRmt serves as a compensatory mechanism to confer cardioprotection in the case of mitophagy inactivation. Lastly, to exclude the influence of UPRmt on mitophagy, the mt-Keima assay was performed. As shown in Fig. 4K-L, although LPS slightly increased mitophagy levels, administration of oligomycin had no effect on mitophagy.
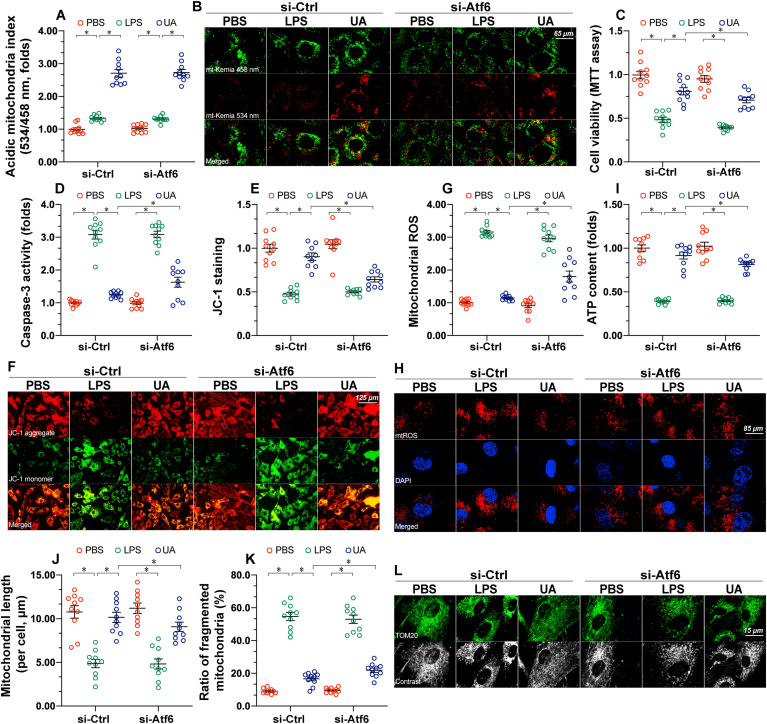
3.3. Inhibition of UPRmt partly abolishes the cardioprotective effect of mitophagy activation
Next, experiments were conducted to analyze the effect of UPRmt inhibition in the presence of mitophagy activation. In vitro, siRNA against Atf6 (si-Atf6) and/or control siRNA (si-Ctrl) was transfected into cardiomyocytes to prevent UPRmt activation. In cardiomyocytes transfected with either si-Atf6 or si-Ctrl, UA was capable of activating mitophagy in the presence of LPS (Fig. 5A–B), confirming that mitophagy activation is independent of UPRmt. However, compared to cardiomyocytes transfected with si-Ctrl, UA-mediated cardiomyocyte survival was partly reduced in cardiomyocytes with si-Atf6 (Fig. 5C). Although UA was able to prevent LPS-mediated caspase-3 activation (Fig. 5D), this action was markedly mitigated in si-Atf6-transfected cardiomyocytes. Similar to these observations, the mitochondrial membrane potential could be improved by UA under LPS challenge (Fig. 5E–F); however, this protective action was weakened in si-Atf6-transfected cardiomyocytes. LPS-induced mitochondrial oxidative stress was mostly suppressed by UA in si-Ctrl-transfected cardiomyocytes (Fig. 5G–H); however, UA partly removed mitochondrial ROS in si-Atf6-transfected cardiomyocytes. Besides, ATP production could be normalized by UA in the presence of LPS treatment, although this effect was partly attenuated by Atf6 knockout (Fig. 5I). Considering mitochondrial structural alterations, mitochondrial fragmentation was rapidly induced by LPS in cardiomyocytes (Fig. 5J-L). Although UA treatment was correlated with an obvious decline in the number of fragmented mitochondria, this protective action was partly retained in si-Atf6-transfected cardiomyocytes (Fig. 5J-L). Collectively, without UPRmt, the cardioprotective action of mitophagy was partly compromised.
Fig. 5.
Inhibition of UPRmtpartly abolishes the cardioprotective effect of mitophagy activation. The AC16 human ventricular cardiomyocyte cell line was treated with 10 μg/mL of LPS to induce sepsis-related cardiomyocyte damage. UA, an inducer of mitophagy, was used to culture AC16 cells. siRNA against FUNDC1 (si-FUNDC1) and Atf6 (si-Atf6) were transfected into AC15 to inhibit the activation of mitophagy and UPRmt, respectively. A-B. Mitophagy activity was observed using the mt-Keima assay. A yellow signal highlights increased mitophagic flux within cardiomyocytes. C. Cell viability was determined using an MTT assay. D. ELISA analysis of caspase-3 activity. E-F. AC16 cells were stained with JC-1 to observe changes in the mitochondrial membrane potential. G-H. AC16 cells were stained with the MitoSOX red mitochondrial superoxide indicator to show changes in mitochondrial ROS. I. Total ATP production was determined using the Cell Titer-Glo Luminescent Viability assay. J-L. Mitochondrial morphology was revealed using confocal immunofluorescence. TOM20 was used to show the shape of mitochondria in response to LPS or FUNDC1 deletion. Data are presented as mean ± SEM, normalized per 1000 cardiomyocytes. *P<0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Inflammation-mediated myocardial damage is a key feature of septic cardiomyopathy. Although the incidence of septic cardiomyopathy ranges from 18 to 29% in patients diagnosed with sepsis [57], the mortality rate is high, and effective therapies are lacking. The pathophysiology of septic cardiomyopathy is reportedly associated with the following mechanisms, cardiotoxic factors (such as TNF-α, CRP, IL-6, complement, and endotoxin), overproduction as a result of inflammation, catecholamine toxicity due to sympathetic nerve activation, decreased calcium sensitivity due to ATP shortage, and microvascular spasm in response to anaerobic metabolite accumulation [58]. The molecular biological mechanisms associated with septic cardiomyopathy include oxidative stress, calcium overload, ATP shortage, autophagy inactivation, metabolic reprogramming, mitochondrial malfunction, endoplasmic reticulum stress, and induction of apoptosis and necrosis [57,58]. In the present study, our data showed that sepsis-mediated myocardial depression is characterized by mitochondrial membrane potential reduction, mitochondrial ROS overloading, and cardiomyocyte apoptosis activation. These alterations contribute to myocardial contractility decline and myocardial injury marker elevation. In addition to functional damage, the structure of the myocardium is disrupted by LPS-induced sepsis. These results are in accordance with previous findings, suggesting that mitochondria could be considered a potential target for the treatment of septic cardiomyopathy.
Damaged mitochondria can be fixed through a complex mechanism involving a series of adaptive responses, including mitochondrial fission/fusion, mitophagy, UPRmt, and mitochondrial biogenesis [59]. These biological events are termed mitochondrial quality control, and have been carefully reviewed in our previous studies [60,61]. Increased mitochondrial quality control activates UPRmt to optimize mitochondrial protein import and export by upregulating gene transcription or promoting protein degradation [62,63]. If UPRmt cannot completely repair the mitochondrial damage, mitochondrial fission is induced to isolate the area of damage from the healthy mitochondrial network [64,65]. Next, mitophagy is employed as a scavenger to eliminate structurally compromised mitochondria [25,66]. As mitophagy-mediated mitochondrial removal is usually followed by a decline in the number of mitochondria, mitochondrial biogenesis is always activated by mitophagy to complement the population and reinforce ATP synthesis [67,68]. Therefore, UPRmt and mitophagy can be considered as distinct mitochondrial repair pathways; the former controlling mitochondrial proteomics and the latter modifying the mitochondrial number. The origin, regulation, and pathophysiological action of mitophagy in cardiovascular disease are well understood [60,61]. Moderate activation of mitophagy attenuates myocardial stress, whereas abnormal mitophagy induction is unexpectedly linked to cardiomyocyte death due to a sharp drop in residual mitochondria and intracellular ATP. In the present study, we explored the involvement of mitophagy in septic cardiomyopathy. Our data demonstrated that activation of FUNDC1-associated mitophagy protected the heart against LPS-induced sepsis by preserving mitochondrial function and structure. Loss of FUNDC1 repressed mitophagy, resulting in mitochondrial dysfunction and cardiomyocyte death; this finding further develops the role of FUNDC1-dependent mitophagy in myocardial stress. Based on this finding, designing and developing approaches targeting FUNDC1-dependent mitophagy may be a promising tactic through which to improve cardiac performance during sepsis.
Unlike mitophagy, evidence of UPRmt in cardiovascular pathophysiology is lacking. Previous studies have observed the cardioprotective action of UPRmt in chronic cardiac hypertrophy and acute cardiac ischemia-reperfusion injury [26,27], concluding that baseline deletion of UPRmt-related genes, such as Atf6, has no significant impact on myocardial function and structure [26]. In response to stress, UPRmt activity rapidly increases, and further enhancement of UPRmt confers cardioprotection against chronic cardiac hypertrophy and acute cardiac ischemia-reperfusion injury [26,27]. These findings suggest that UPRmt, as an adaptive response, could be induced by myocardial stress; although the extent of this activation appears to fail in preventing damage to the myocardium. Administration of a UPRmt chemical agonist further augments UPRmt activity, also exerting additional cardioprotective actions [27]. This finding highlights that endogenous UPRmt is insufficient to benefit the myocardium; similar results were observed in in the present study. Moreover, UPRmt was slightly induced by LPS-associated sepsis; further augmentation of UPRmt activity showed a protective effect on LPS-treated hearts. Our data, combined with previous evidence, provide novel insights into the endogenous physiological mechanisms of UPRmt, also offering potential therapeutic targets for myocardial stress.
One of the key considerations is the relationship between mitophagy and UPRmt [69,70]. As discussed above, UPRmt appears to be activated prior to mitophagy induction [71]. As endogenous UPRmt cannot completely repair mitochondrial damage, mitophagy activation is an inevitable consequence under stress conditions, regardless of UPRmt activation. In the present study, we found that mitophagy activation had no influence on UPRmt activity. Unexpectedly, inhibiting mitophagy through the genetic ablation of FUNDC1 significantly augmented endogenous UPRmt activity, suggesting that UPRmt may act as a compensatory mechanism in response to mitophagy repression. Inconsistent with our theoretical hypothesis, UPRmt was found to be a downstream event of mitophagy. To validate our findings, we inhibited UPRmt in the presence of a mitophagy inducer. In UPRmt-inhibited cardiomyocytes, the mitophagy activation-mediated protection of mitochondria and cardiomyocytes was partly blunted, reconfirming that mitophagy-conferred cardioprotection, in part, requires UPRmt. Overall, we concluded that: 1) endogenous UPRmt and mitophagy can be slightly activated by myocardial stress, working together to sustain mitochondrial performance and cardiac function; 2) endogenous UPRmt, as a downstream signal of mitophagy, plays a compensatory role in maintaining mitochondrial homeostasis during mitophagy inhibition; 3) although UPRmt activation had no influence on mitophagy, its inhibition compromised the partial cardioprotective action of mitophagy.
This study has several limitations. First, as we only observed an association between FUNDC1-dependent mitophagy and UPRmt, it remains unknown whether mitophagy mediated by other adaptors also acts as an upstream inducer of UPRmt. Second, the UPRmt loss-of-function assay was only performed in vitro; animal studies are necessary to further support our results. Third, although we determined the relationship between mitophagy and UPRmt, the regulatory mechanism through which mitophagy controls UPRmt has not been elucidated [17,72].
Author contributions
H.Z. and X.C. involved in conception and design, performance of experiments, data analysis and interpretation, and manuscript writing; Y·W., H.J. and S.T. involved in the development of methodology, D.M. and X.C. involved in the data acquisition, Y·W., H.Z. and X.C. involved data analysis and interpretation; H.Z. involved in study supervision and final approval of manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
All authors declare that they have no conflict of interest.
Contributor Information
Xing Chang, Email: cx931301556@163.com.
Hao Zhou, Email: zhouhao@plagh.org, zhouhao301@outlook.com.
References
- 1.Hollenberg S.M., Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 2021;18(6):424–434. doi: 10.1038/s41569-020-00492-2. [DOI] [PubMed] [Google Scholar]
- 2.Tan Y., Wan H.H., Sun M.M., Zhang W.J., Dong M., Ge W., Ren J., Peng H. Cardamonin protects against lipopolysaccharide-induced myocardial contractile dysfunction in mice through Nrf2-regulated mechanism. Acta Pharmacol. Sin. 2021;42(3):404–413. doi: 10.1038/s41401-020-0397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasques-Nóvoa F., Angélico-Gonçalves A., Bettencourt N., Leite-Moreira A.F., Roncon-Albuquerque R., Jr. Myocardial edema and remodeling: a link between acute myocarditis and septic cardiomyopathy? J. Am. Coll. Cardiol. 2020;75(12):1497–1498. doi: 10.1016/j.jacc.2019.12.071. [DOI] [PubMed] [Google Scholar]
- 4.Sanfilippo F., Orde S., Oliveri F., Scolletta S., Astuto M. The challenging diagnosis of septic cardiomyopathy. Chest. 2019;156(3):635–636. doi: 10.1016/j.chest.2019.04.136. [DOI] [PubMed] [Google Scholar]
- 5.Zhong J., Tan Y., Lu J., Liu J., Xiao X., Zhu P., Chen S., Zheng S., Chen Y., Hu Y., Guo Z. Therapeutic contribution of melatonin to the treatment of septic cardiomyopathy: a novel mechanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function. Redox Biol. 2019;26:101287. doi: 10.1016/j.redox.2019.101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillon A., Preau S., Aboab J., Azabou E., Jung B., Silva S., Textoris J., Uhel F., Vodovar D., Zafrani L., De Prost N., Radermacher P. Preclinical septic shock research: why we need an animal ICU. Ann. Intensive Care. 2019;9(1):66. doi: 10.1186/s13613-019-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk L., Hultman J., Broman L.M. Extracorporeal membrane oxygenation for septic shock. Crit. Care Med. 2019;47(8):1097–1105. doi: 10.1097/CCM.0000000000003819. [DOI] [PubMed] [Google Scholar]
- 8.Honda T., He Q., Wang F., Redington A.N. Acute and chronic remote ischemic conditioning attenuate septic cardiomyopathy, improve cardiac output, protect systemic organs, and improve mortality in a lipopolysaccharide-induced sepsis model. Basic Res. Cardiol. 2019;114(3):15. doi: 10.1007/s00395-019-0724-3. [DOI] [PubMed] [Google Scholar]
- 9.Martin L., Derwall M., Al Zoubi S., Zechendorf E., Reuter D.A., Thiemermann C., Schuerholz T. The septic heart: current understanding of molecular mechanisms and clinical implications. Chest. 2019;155(2):427–437. doi: 10.1016/j.chest.2018.08.1037. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Zhu P., Toan S., Li R., Ren J., Zhou H. Pum2-Mff axis fine-tunes mitochondrial quality control in acute ischemic kidney injury. Cell Biol. Toxicol. 2020;36(4):365–378. doi: 10.1007/s10565-020-09513-9. [DOI] [PubMed] [Google Scholar]
- 11.Kokkinaki D., Hoffman M., Kalliora C., Kyriazis I.D., Maning J., Lucchese A.M., Shanmughapriya S., Tomar D., Park J.Y., Wang H., Yang X.F., Madesh M., Lymperopoulos A., Koch W.J., Christofidou-Solomidou M., Drosatos K. Chemically synthesized Secoisolariciresinol diglucoside (LGM2605) improves mitochondrial function in cardiac myocytes and alleviates septic cardiomyopathy. J. Mol. Cell. Cardiol. 2019;127:232–245. doi: 10.1016/j.yjmcc.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L., Luo X., Fu N., Chen L. Mitochondrial unfolded protein response: a novel pathway in metabolism and immunity. Pharmacol. Res. 2021;168:105603. doi: 10.1016/j.phrs.2021.105603. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb R.A., Piplani H., Sin J., Sawaged S., Hamid S.M., Taylor D.J., De Freitas Germano J. At the heart of mitochondrial quality control: many roads to the top. Cell. Mol. Life Sci. 2021;78(8):3791–3801. doi: 10.1007/s00018-021-03772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shpilka T., Du Y., Yang Q., Melber A., Uma Naresh N., Lavelle J., Kim S., Liu P., Weidberg H., Li R., Yu J., Zhu L.J., Strittmatter L., Haynes C.M. UPR(mt) scales mitochondrial network expansion with protein synthesis via mitochondrial import in Caenorhabditis elegans. Nat. Commun. 2021;12(1):479. doi: 10.1038/s41467-020-20784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y., Zhou L., Li H., Sun T., Wen X., Li X., Meng Y., Li Y., Liu M., Liu S., Kim S.J., Xiao J., Li L., Zhang S., Li W., Cohen P., Hoffman A.R., Hu J.F., Cui J. Nuclear-encoded lncRNA MALAT1 epigenetically controls metabolic reprogramming in HCC cells through the mitophagy pathway. Mol. Ther. Nucleic Acids. 2021;23:264–276. doi: 10.1016/j.omtn.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philp A.M., Saner N.J., Lazarou M., Ganley I.G., Philp A. The influence of aerobic exercise on mitochondrial quality control in skeletal muscle. J. Physiol. 2020 doi: 10.1113/JP279411. [DOI] [PubMed] [Google Scholar]
- 17.Lim Y., Berry B., Viteri S., Mccall M., Park E.C., Rongo C., Brookes P.S., Nehrke K. FNDC-1-mediated mitophagy and ATFS-1 coordinate to protect against hypoxia-reoxygenation. Autophagy. 2021:1–13. doi: 10.1080/15548627.2021.1872885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Q., Li R., Hu N., Xin T., Zhu P., Hu S., Ma S., Zhu H., Ren J., Zhou H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576–587. doi: 10.1016/j.redox.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R., Xin T., Li D., Wang C., Zhu H., Zhou H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018;18:229–243. doi: 10.1016/j.redox.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H., Shi C., Hu S., Zhu H., Ren J., Chen Y. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis. 2018;21(3):599–615. doi: 10.1007/s10456-018-9611-z. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H., Zhu P., Wang J., Toan S., Ren J. DNA-PKcs promotes alcohol-related liver disease by activating Drp1-related mitochondrial fission and repressing FUNDC1-required mitophagy. Signal Transduct Target Ther. 2019;4(1):56. doi: 10.1038/s41392-019-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Zhu P., Li R., Ren J., Zhou H. Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biol. 2020;30:101415. doi: 10.1016/j.redox.2019.101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H., Wang S., Zhu P., Hu S., Chen Y., Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–346. doi: 10.1016/j.redox.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H., Zhang Y., Hu S., Shi C., Zhu P., Ma Q., Jin Q., Cao F., Tian F., Chen Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 2017;63(1) doi: 10.1111/jpi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H., Zhu P., Wang J., Zhu H., Ren J., Chen Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25(6):1080–1093. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y.T., Lim Y., Mccall M.N., Huang K.T., Haynes C.M., Nehrke K., Brookes P.S. Cardioprotection by the mitochondrial unfolded protein response requires ATF5. Am. J. Physiol. Heart Circ. Physiol. 2019;317(2):H472–H478. doi: 10.1152/ajpheart.00244.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smyrnias I., Gray S.P., Okonko D.O., Sawyer G., Zoccarato A., Catibog N., Lopez B., Gonzalez A., Ravassa S., Diez J., Shah A.M. Cardioprotective effect of the mitochondrial unfolded protein response during chronic pressure overload. J. Am. Coll. Cardiol. 2019;73(14):1795–1806. doi: 10.1016/j.jacc.2018.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh K.H., Sheoran S., Richmond J.E., Kim H. Alcohol induces mitochondrial fragmentation and stress responses to maintain normal muscle function in Caenorhabditis elegans. Faseb. J. 2020;34(6):8204–8216. doi: 10.1096/fj.201903166R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adapala R.K., Kanugula A.K., Paruchuri S., Chilian W.M., Thodeti C.K. TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic Res. Cardiol. 2020;115(2):14. doi: 10.1007/s00395-020-0775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakhta O., Pascaud A., Dieu X., Beaumont J., Kouassi Nzoughet J., Kamel R., Croyal M., Tamareille S., Simard G., Chao De La Barca J.M., Reynier P., Prunier F., Mirebeau-Prunier D. Tryptophane-kynurenine pathway in the remote ischemic conditioning mechanism. Basic Res. Cardiol. 2020;115(2):13. doi: 10.1007/s00395-019-0770-x. [DOI] [PubMed] [Google Scholar]
- 31.Cao F., Maguire M.L., Mcandrew D.J., Lake H.A., Neubauer S., Zervou S., Schneider J.E., Lygate C.A. Overexpression of mitochondrial creatine kinase preserves cardiac energetics without ameliorating murine chronic heart failure. Basic Res. Cardiol. 2020;115(2):12. doi: 10.1007/s00395-020-0777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou H., Toan S., Zhu P., Wang J., Ren J., Zhang Y. DNA-PKcs promotes cardiac ischemia reperfusion injury through mitigating BI-1-governed mitochondrial homeostasis. Basic Res. Cardiol. 2020;115(2):11. doi: 10.1007/s00395-019-0773-7. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Zhu P., Li R., Ren J., Zhang Y., Zhou H. Bax inhibitor 1 preserves mitochondrial homeostasis in acute kidney injury through promoting mitochondrial retention of PHB2. Theranostics. 2020;10(1):384–397. doi: 10.7150/thno.40098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lugassy C., Kleinman H.K., Vermeulen P.B., Barnhill R.L. Angiotropism, pericytic mimicry and extravascular migratory metastasis: an embryogenesis-derived program of tumor spread. Angiogenesis. 2020;23(1):27–41. doi: 10.1007/s10456-019-09695-9. [DOI] [PubMed] [Google Scholar]
- 35.Pabel S., Ahmad S., Tirilomis P., Stehle T., Mustroph J., Knierim M., Dybkova N., Bengel P., Holzamer A., Hilker M., Streckfuss-Bömeke K., Hasenfuss G., Maier L.S., Sossalla S. Inhibition of Na(V)1.8 prevents atrial arrhythmogenesis in human and mice. Basic Res. Cardiol. 2020;115(2):20. doi: 10.1007/s00395-020-0780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pflüger-Müller B., Oo J.A., Heering J., Warwick T., Proschak E., Günther S., Looso M., Rezende F., Fork C., Geisslinger G., Thomas D., Gurke R., Steinhilber D., Schulz M., Leisegang M.S., Brandes R.P. The endocannabinoid anandamide has an anti-inflammatory effect on CCL2 expression in vascular smooth muscle cells. Basic Res. Cardiol. 2020;115(3):34. doi: 10.1007/s00395-020-0793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vatner D.E., Oydanich M., Zhang J., Babici D., Vatner S.F. Secreted frizzled-related protein 2, a novel mechanism to induce myocardial ischemic protection through angiogenesis. Basic Res. Cardiol. 2020;115(4):48. doi: 10.1007/s00395-020-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y., Liang P., Jiang B., Tang Y., Liu X., Liu M., Sun H., Chen C., Hao H., Liu Z., Xiao X. CARD9 promotes autophagy in cardiomyocytes in myocardial ischemia/reperfusion injury via interacting with Rubicon directly. Basic Res. Cardiol. 2020;115(3):29. doi: 10.1007/s00395-020-0790-6. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y.J., Zhang M., Zhao X., Shi K., Ye M., Tian J., Guan S., Ying W., Qu X. NAD(+) administration decreases microvascular damage following cardiac ischemia/reperfusion by restoring autophagic flux. Basic Res. Cardiol. 2020;115(5):57. doi: 10.1007/s00395-020-0817-z. [DOI] [PubMed] [Google Scholar]
- 40.Le Cras T.D., Goines J., Lakes N., Pastura P., Hammill A.M., Adams D.M., Boscolo E. Constitutively active PIK3CA mutations are expressed by lymphatic and vascular endothelial cells in capillary lymphatic venous malformation. Angiogenesis. 2020;23(3):425–442. doi: 10.1007/s10456-020-09722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fournier P., Viallard C., Dejda A., Sapieha P., Larrivée B., Royal I. The protein tyrosine phosphatase PTPRJ/DEP-1 contributes to the regulation of the Notch-signaling pathway and sprouting angiogenesis. Angiogenesis. 2020;23(2):145–157. doi: 10.1007/s10456-019-09683-z. [DOI] [PubMed] [Google Scholar]
- 42.Lamprou M., Kastana P., Kofina F., Tzoupis Η., Barmpoutsi S., Sajib M.S., Koutsioumpa M., Poimenidi E., Zompra A.A., Tassopoulos D., Choleva E., Tselios T., Mikelis C.M., Papadimitriou E. Angiogenesis; 2020. Pleiotrophin Selectively Binds to Vascular Endothelial Growth Factor Receptor 2 and Inhibits or Stimulates Cell Migration Depending on α(ν)β(3) Integrin Expression. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig N., Yerneni S.S., Azambuja J.H., Gillespie D.G., Menshikova E.V., Jackson E.K., Whiteside T.L. Angiogenesis; 2020. Tumor-derived Exosomes Promote Angiogenesis via Adenosine A(2B) Receptor Signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J., Toan S., Li R., Zhou H. Melatonin fine-tunes intracellular calcium signals and eliminates myocardial damage through the IP3R/MCU pathways in cardiorenal syndrome type 3. Biochem. Pharmacol. 2020;174:113832. doi: 10.1016/j.bcp.2020.113832. [DOI] [PubMed] [Google Scholar]
- 45.Tan Y., Mui D., Toan S., Zhu P., Li R., Zhou H. SERCA overexpression improves mitochondrial quality control and attenuates cardiac microvascular ischemia-reperfusion injury. Mol. Ther. Nucleic Acids. 2020;22:696–707. doi: 10.1016/j.omtn.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh E., Redgrave R.E., Phillips H.M., Arthur H.M. Angiogenesis; 2020. Arterial Endoglin Does Not Protect against Arteriovenous Malformations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van De Wouw J., Sorop O., Van Drie R.W.A., Van Duin R.W.B., Nguyen I.T.N., Joles J.A., Verhaar M.C., Merkus D., Duncker D.J. Perturbations in myocardial perfusion and oxygen balance in swine with multiple risk factors: a novel model of ischemia and no obstructive coronary artery disease. Basic Res. Cardiol. 2020;115(2):21. doi: 10.1007/s00395-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y., Ma L., Wang C., Song M., Li C., Chen M., Zhou J., Mei C. Matrix metalloproteinase-7 in platelet-activated macrophages accounts for cardiac remodeling in uremic mice. Basic Res. Cardiol. 2020;115(3):30. doi: 10.1007/s00395-020-0789-z. [DOI] [PubMed] [Google Scholar]
- 49.Szaraz P., Mander P., Gasner N., Librach M., Iqbal F., Librach C. Glucose withdrawal induces Endothelin 1 release with significant angiogenic effect from first trimester (FTM), but not term human umbilical cord perivascular cells (HUCPVC) Angiogenesis. 2020;23(2):131–144. doi: 10.1007/s10456-019-09682-0. [DOI] [PubMed] [Google Scholar]
- 50.Steffen E., Mayer Von Wittgenstein W.B.E., Hennig M., Niepmann S.T., Zietzer A., Werner N., Rassaf T., Nickenig G., Wassmann S., Zimmer S., Steinmetz M. Murine sca1/flk1-positive cells are not endothelial progenitor cells, but B2 lymphocytes. Basic Res. Cardiol. 2020;115(2):18. doi: 10.1007/s00395-020-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou H., Wang J., Zhu P., Hu S., Ren J. Ripk3 regulates cardiac microvascular reperfusion injury: the role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell. Signal. 2018;45:12–22. doi: 10.1016/j.cellsig.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Zhu H., Jin Q., Li Y., Ma Q., Wang J., Li D., Zhou H., Chen Y. Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones. 2018;23(1):101–113. doi: 10.1007/s12192-017-0827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veith C., Neghabian D., Luitel H., Wilhelm J., Egemnazarov B., Muntanjohl C., Fischer J.H., Dahal B.K., Schermuly R.T., Ghofrani H.A., Grimminger F., Fink L., Kwapiszewska G., Weissmann N., Sydykov A. FHL-1 is not involved in pressure overload-induced maladaptive right ventricular remodeling and dysfunction. Basic Res. Cardiol. 2020;115(2):17. doi: 10.1007/s00395-019-0767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winter M.P., Sharma S., Altmann J., Seidl V., Panzenböck A., Alimohammadi A., Zelniker T., Redwan B., Nagel F., Santer D., Stieglbauer A., Podesser B., Sibilia M., Helbich T., Prager G., Ilhan-Mutlu A., Preusser M., Lang I.M. Interruption of vascular endothelial growth factor receptor 2 signaling induces a proliferative pulmonary vasculopathy and pulmonary hypertension. Basic Res. Cardiol. 2020;115(6):58. doi: 10.1007/s00395-020-0811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Unterleuthner D., Neuhold P., Schwarz K., Janker L., Neuditschko B., Nivarthi H., Crncec I., Kramer N., Unger C., Hengstschläger M., Eferl R., Moriggl R., Sommergruber W., Gerner C., Dolznig H. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23(2):159–177. doi: 10.1007/s10456-019-09688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou H., Li D., Zhu P., Ma Q., Toan S., Wang J., Hu S., Chen Y., Zhang Y. Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J. Pineal Res. 2018;65(3) doi: 10.1111/jpi.12503. [DOI] [PubMed] [Google Scholar]
- 57.Beesley S.J., Weber G., Sarge T., Nikravan S., Grissom C.K., Lanspa M.J., Shahul S., Brown S.M. Septic cardiomyopathy. Crit. Care Med. 2018;46(4):625–634. doi: 10.1097/CCM.0000000000002851. [DOI] [PubMed] [Google Scholar]
- 58.Ehrman R.R., Sullivan A.N., Favot M.J., Sherwin R.L., Reynolds C.A., Abidov A., Levy P.D. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: a review of the literature. Crit. Care. 2018;22(1):112. doi: 10.1186/s13054-018-2043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suliman H.B., Piantadosi C.A. Mitochondrial quality control as a therapeutic target. Pharmacol. Rev. 2016;68(1):20–48. doi: 10.1124/pr.115.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou H., Ren J., Toan S., Mui D. Role of mitochondrial quality surveillance in myocardial infarction: from bench to bedside. Ageing Res. Rev. 2021;66:101250. doi: 10.1016/j.arr.2020.101250. [DOI] [PubMed] [Google Scholar]
- 61.Zhu H., Toan S., Mui D., Zhou H. Mitochondrial quality surveillance as a therapeutic target in myocardial infarction. Acta Physiol (Oxf) 2021;231(3) doi: 10.1111/apha.13590. [DOI] [PubMed] [Google Scholar]
- 62.Wang J., Zhou H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharm. Sin. B. 2020;10(10):1866–1879. doi: 10.1016/j.apsb.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J., Toan S., Zhou H. Mitochondrial quality control in cardiac microvascular ischemia-reperfusion injury: new insights into the mechanisms and therapeutic potentials. Pharmacol. Res. 2020;156:104771. doi: 10.1016/j.phrs.2020.104771. [DOI] [PubMed] [Google Scholar]
- 64.Wang J., Toan S., Zhou H. New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury. Angiogenesis. 2020;23(3):299–314. doi: 10.1007/s10456-020-09720-2. [DOI] [PubMed] [Google Scholar]
- 65.Zhou H., Wang J., Zhu P., Zhu H., Toan S., Hu S., Ren J., Chen Y. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res. Cardiol. 2018;113(4):23. doi: 10.1007/s00395-018-0682-1. [DOI] [PubMed] [Google Scholar]
- 66.Zhou H., Zhu P., Guo J., Hu N., Wang S., Li D., Hu S., Ren J., Cao F., Chen Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang X., Lochner A., Wang H.-H., Wang S., Zhu H., Ren J., Zhou H. Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Theranostics. 2021;11(14):6766–6785. doi: 10.7150/thno.60143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou H., Toan S. Pathological roles of mitochondrial oxidative stress and mitochondrial dynamics in cardiac microvascular ischemia/reperfusion injury. Biomolecules. 2020;10(1) doi: 10.3390/biom10010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pellegrino M.W., Haynes C.M. Mitophagy and the mitochondrial unfolded protein response in neurodegeneration and bacterial infection. BMC Biol. 2015;13:22. doi: 10.1186/s12915-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De La Cruz-Ruiz P., Hernando-Rodríguez B., Pérez-Jiménez M.M., Rodríguez-Palero M.J., Martínez-Bueno M.D., Pla A., Gatsi R., Artal-Sanz M. Prohibitin depletion extends lifespan of a TORC2/SGK-1 mutant through autophagy and the mitochondrial UPR. Aging Cell. 2021;20(5) doi: 10.1111/acel.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Svaguša T., Martinić M., Martinić M., Kovačević L., Šepac A., Miličić D., Bulum J., Starčević B., Sirotković-Skerlev M., Seiwerth F., Kulić A., Sedlić F. Mitochondrial unfolded protein response, mitophagy and other mitochondrial quality control mechanisms in heart disease and aged heart. Croat. Med. J. 2020;61(2):126–138. doi: 10.3325/cmj.2020.61.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smyrnias I. The mitochondrial unfolded protein response and its diverse roles in cellular stress. Int. J. Biochem. Cell Biol. 2021;133:105934. doi: 10.1016/j.biocel.2021.105934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.