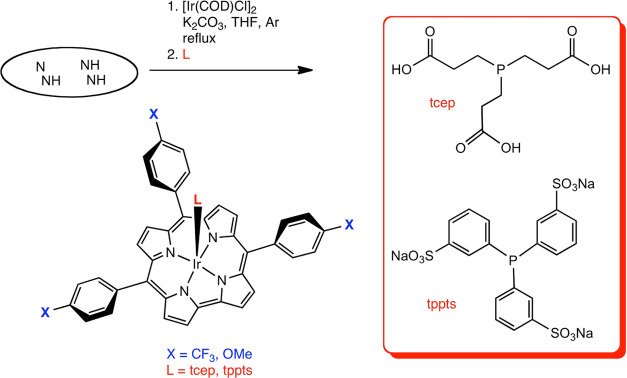
Abstract
The synthesis and purification of water-soluble porphyrin-type compounds for photodynamic therapy and other medical applications is often a tedious exercise. Here, we have investigated the simple stratagem of adding a water-soluble axial ligand to the standard protocol for iridium insertion into simple meso-triarylcorroles. Early results showed that six-coordinate Ir[TpXPC](dna)2 derivatives, in which TpXPC = tris(para-X-phenyl)corrole (X = CF3, CN, H, and OMe) and dna = dinicotinic acid, are highly water-soluble. In the end, however, all axially nitrogen-ligated complexes proved unstable with respect to chromatographic purification and storage. Five-coordinate water-soluble phosphine adducts, fortunately, proved a great improvement. From the point of view of ease of purification and storage, the best products proved to be Ir[TpXPC](L), where X = CF3 and OMe and L = tris(2-carboxyethyl)phosphine (tcep) and trisodium tris(3-sulfonatophenyl)phosphine (tppts); carefully optimized synthetic protocols are presented for these four compounds.
Introduction
Porphyrin-type compounds have long been a cornerstone of photodynamic therapy.1−5 Recently, porphyrin analogues such as corroles6,7 have also proved promising as anticancer compounds.8,9 Several families of 5d metallocorroles (including ReO,10 OsN,11 Ir,12 Pt,13 and Au14−17 corroles) that we and others have studied in recent years are relevant in this connection. Although they were originally of interest primarily as curious, size-mismatched metal–ligand assemblies, their photophysical properties, especially near-infrared (NIR) phosphorescence under ambient conditions, now promise a wide range of practical applications,18,19 such as in oxygen sensors, photodynamic therapy, and dye-sensitized solar cells and for triplet–triplet annihilation upconversion.20−28 A number of these applications, especially in the biomedical sphere, require water-soluble derivatives of the complexes, which are typically accessible via cumbersome synthetic and purification steps.2,29,30 A recent reinvestigation of iridium corroles (in which 4-picolinic acid derivatives were found to be partially water-soluble) suggested that the use of water-soluble axial ligands might afford a simple, one-pot route to water-soluble Ir corroles as a new class of singlet oxygen photosensitizers.31 The beguilingly simple exercise, however, threw up unexpected challenges. Many of the compounds synthesized proved unstable, decomposing upon chromatographic purification or storage. Here, we detail carefully optimized synthetic protocols for four complexes (Scheme 1) that could be readily purified and stored and are therefore suitable for further investigations of potential applications.
Scheme 1. Synthesis of Four Stable, Water-Soluble Iridium Corroles.
Results and Discussion
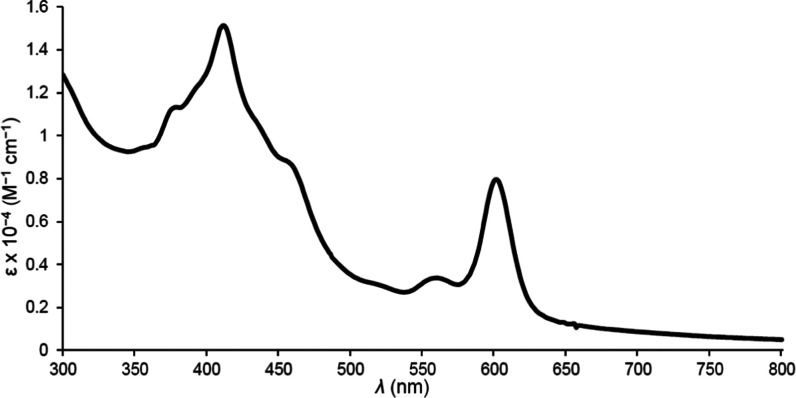
Six different meso-tris(para-X-phenyl)corrole ligands, H3[TpXPC] (X = NO2, CF3, CN, H, Me, and OMe),32−34 as well as meso-tris(pentafluorophenyl)corrole, H3[TPFPC],35 were examined throughout as equatorial ligands. For axial ligands, we initially examined five nitrogen ligands—4-picolinic acid (4pa; Figure 1), 3,5-pyridinedicarboxylic acid (also known as dinicotinic acid, dna), nitrilotriacetic acid (nta), 5-hydroxypyridine-3-carboxylic acid, and 4-pyridylboronic acid. Four different stationary phases were used for chromatographic purification of the complexes—silica gel, basic and neutral alumina, Florisil, and fully endcapped C18 reversed-phase silica gel. Although iridium insertion could be accomplished for all of the corroles except X = NO2, the great majority of the complexes proved unstable; bright green solutions of the freshly prepared complexes frequently turned brown, often with the decomposition product sticking to the glass walls of the reaction vessel. The most promising of the lot proved to be the dna complexes Ir[TpXPC](dna)2 (X = CF3, CN, H, and OMe; Figure 2), exhibiting high water solubility, but these too proved unstable upon chromatographic workup and/or storage, as indicated by the disappearance of the highly characteristic optical spectra. Attempts to avoid chromatography by resorting to solvent extraction and vacuum filtration ultimately also proved unsuccessful.
Figure 1.
UV–vis spectrum of Ir[TpOMePC](4pa)2 in methanol.
Figure 2.
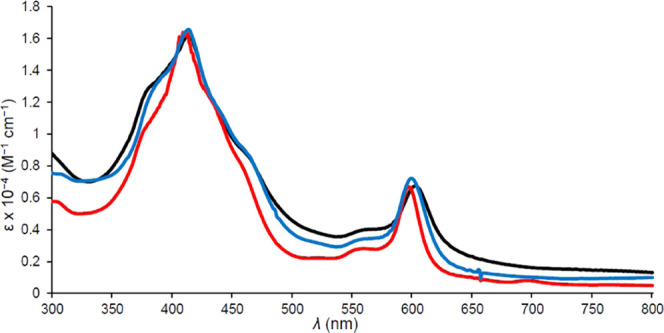
UV–vis comparison of freshly prepared Ir[TpOMePC](dna)2 (black), Ir[TPC](dna)2 (red), and Ir[TpCF3PC](dna)2 (blue) in methanol.
Mass spectrometric analyses of the decomposed complexes generally revealed large quantities of free axial ligands, suggesting that they tend to fall off during chromatographic purification. This observation led us to switch to water-soluble phosphine ligands,36 of which we examined three—tris(2-carboxyethyl)phosphine (tcep), trisodium tris(3-sulfonatophenyl)phosphine (tppts), and tris(hydroxymethyl)phosphine (thp). Like triphenylphosphine,37 these phosphine ligands also led to five-coordinate complexes, which fortunately also proved distinctly more stable than the nitrogen-ligated complexes described above. Of the phosphine complexes, the thp derivatives proved poorly soluble in water (presumably reflecting the lower hydrophilicity of the alcohol functionality relative to carboxylate and sulfonate) and were accordingly excluded from further examination in our study. Fortunately, the tcep and tppts complexes proved fully soluble in distilled water (see Figures 3–5 and Table 1 for representative optical spectra and spectral data). A number of tcep complexes (especially for X = CN, H, and Me), however, proved somewhat hygroscopic, and the optical spectra exhibited broadening upon prolonged standing in water. In contrast, Ir[TpCF3PC](tcep) proved unusually rugged, remaining unchanged in air and both aqueous and nonaqueous solutions for days. Finally, the tppts complexes proved highly stable (albeit slightly hygroscopic, thereby thwarting our attempts at obtaining accurate elemental analyses) as well as readily purifiable via column chromatography on regular silica gel without any problems.
Figure 3.
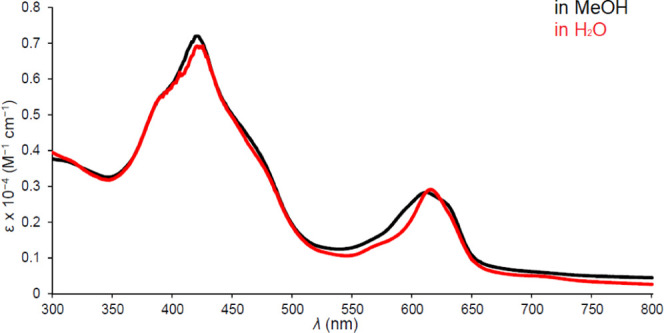
UV–vis spectrum of Ir[TpCF3PC](tcep) in methanol and water.
Figure 5.
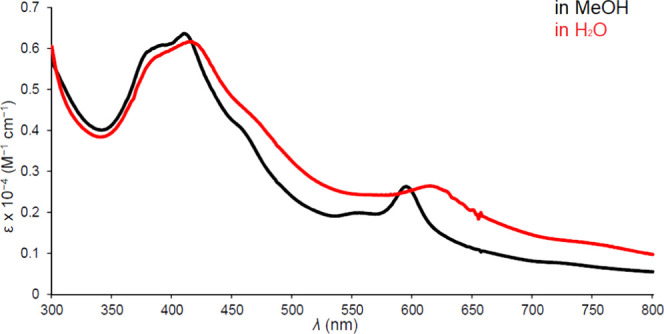
UV–vis spectrum of Ir[TpOMePC](tcep) in methanol and water.
Table 1. Absorption Maxima (λ, nm) for Ir Corrolesa.
| complex | solvent | B | Q |
|---|---|---|---|
| Ir[TpOMePC](tcep) | MeOH | 389, 411* | 609* |
| Ir[TpOMePC](tppts) | MeOH | 391, 413* | 559, 594* |
| Ir[TpCF3PC](tcep) | MeOH | 420* | 610* |
| Ir[TpCF3PC](tppts) | MeOH | 413*, 427 | 556, 594* |
| Ir[TpOMePC](tcep) | H2O | 416* | 615* |
| Ir[TpCF3PC](tcep) | H2O | 425* | 615* |
| Ir[TpCF3PC](tppts) | H2O | 413* | 557, 595* |
The numbers marked with an asterisk indicate the wavelengths with the most intense absorption.
A brief word on the optical spectra of the new compounds may be of interest. The spectra (Figures 1–5) clearly show highly distinctive absorption profiles, including a complex Soret manifold and a Q manifold, whose shape also varies considerably. Thus, the sharp and intense Q band of Ir[TpCF3PC](tppts) (Figure 4) may be distinguished from those of the tcep complexes (Figures 3 and 5). These distinctive spectra provided simple “spectroscopic handles” for assessing the integrity and purity of the compounds studied. A discussion of the electronic origin of the diverse absorption profiles, while of significant theoretical interest, is outside the scope of this study.
Figure 4.
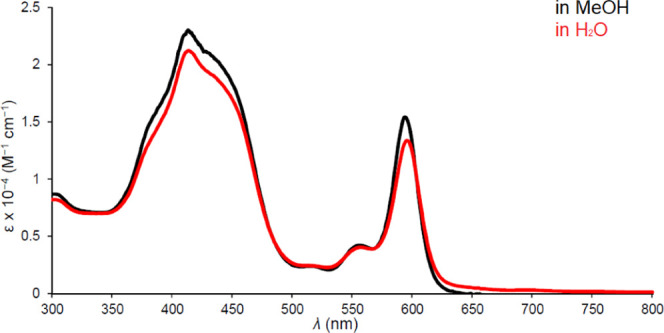
UV–vis spectrum of Ir[TpCF3PC](tppts) in methanol and water.
A couple of phosphine complexes exhibited a small quantity of impurity in their NMR spectra (see Figures S9–S16 in the Supporting Information); we have not identified this species as of yet but view a six-coordinate water or solvent adduct as a plausible candidate.
Conclusions
The present study was motivated by a desire to synthesize water-soluble iridium corroles for photodynamic therapy and other biomedical applications via the simple stratagem of employing a water-soluble axial ligand. Although ultimately successful, the exercise entailed unexpected challenges. Thus, the complexes with axial amine ligands such as 4-picolinic acid and dinicotinic acid proved unstable, decomposing over hours to days upon standing in water. Five-coordinate phosphine complexes, in contrast, proved much more stable and readily purifiable with reversed-phase column chromatography, with tcep and tppts emerging as the most promising axial ligands. Of the various complexes synthesized, Ir[TpCF3PC](tppts) is arguably the most attractive, considering its high water solubility, long-term stability in solution, and distinctive optical signature. The performance of the new compounds in photocytotoxicity measurements is currently under evaluation and will be reported in due course.
Experimental Section
Materials
Tris(2-carboxyethyl)phosphine hydrochloride (99%), trisodium tris(3-sulfonatophenyl)phosphine (<10% phosphine oxide), and tris(hydroxymethyl)phosphine (95%) were purchased from Strem Chemicals, Inc. Free-base corroles were prepared as previously reported.33−35 Unless otherwise mentioned, all other chemicals were obtained from Sigma-Millipore (Merck).
Instrumental Methods
UV–visible spectra were recorded on an HP 8453 spectrophotometer. 1H NMR, 19F NMR, and 31P NMR spectra were recorded on a 400 MHz Bruker Avance III HD spectrometer equipped with a 5 mm BB/1H SmartProbe in CD3OD or (CD3)2SO. 1H NMR spectra were referenced to residual CH3OH (3.31 ppm) or to (CH3)2SO (2.50 ppm). High-resolution electrospray-ionization (HR-ESI) mass spectra were recorded from methanolic solution on an LTQ Orbitrap XL spectrometer.
General Procedure for the Synthesis of Ir[TpXPC](L) (X = OMe, CF3; L = tcep, tppts)
The iridium complexes were prepared according to a modified version of a previously reported procedure.12 Bis(1,5-cyclooctadiene)diiridium(I) dichloride (1.5 equiv) and potassium carbonate (10 equiv) were dissolved in an anhydrous tetrahydrofuran (THF) solution (20 mL) of a free-base corrole (∼0.025–0.1 mmol, 1 equiv). After degassing with argon for a few minutes, the solution was brought to reflux under an inert atmosphere. Heating was discontinued after 90 min, and the phosphine (1 equiv) was added as a solution of anhydrous methanol (10 mL); the reaction mixture was then left to stir for 30 min. As alluded to above, four products were purified and fully characterized, as described below. Because of the hygroscopic nature of the compounds, satisfactory elemental analyses, in general, could not be obtained; the yields accordingly should be regarded as upper limits.
Ir[TpOMePC](tcep)
The reaction mixture was rotary-evaporated to dryness. The dark solid residue was suspended in dichloromethane and thoroughly shaken; the solvent was decanted off to remove unreacted free-base corrole and other nonpolar impurities. This step was repeated with ethyl acetate and acetonitrile, finally leaving behind a dark green solid, which was dissolved in methanol. The solution was filtered to remove any remaining salts, and the filtrate was evaporated to dryness. The resulting solid was dissolved in a minimum amount of methanol and chromatographed on a fully C18-endcapped reversed-phase silica gel column with different MeCN/MeOH mixed solvents as the mobile phase (as detailed below), yielding the expected product along with some silica particles as a light green solid. The silica particles were removed by suspending the solid in pentane, sonicating the suspension briefly, and filtering off the pentane solution containing the dissolved/micro-suspended silica. The dark green solid residue was dissolved in methanol and transferred to a new vessel; upon removal of the solvent under vacuum, the product was obtained as a deep green, hygroscopic solid. Yield 98 mg (85%). UV–vis (CH3OH) λmax (nm) [ϵ × 10–4 (M–1 cm–1)]: 389 (sh, 0.56), 411 (0.62), 609 (0.25). 1H NMR (400 MHz, methanol-d4) δ 8.63 (d, J = 4.0 Hz, 2H), 8.48 (d, J = 4.0 Hz, 2H), 8.29 (d, J = 4.7 Hz, 2H), 8.09 (t, J = 4.8 Hz, 2H), 7.91–7.80 (m, 4H), 7.32–7.14 (m, 8H), 4.00 (d, J = 4.6 Hz, 9H), −0.19 (t, J = 8.2 Hz, 6H), −1.58 (t, J = 8.2 Hz, 6H). 31P NMR (162 MHz, composite pulse-decoupled, methanol-d4) δ −22.43. MS (ESI): [M–] = 1055.2415 (expt), 1055.2406 (calcd for IrC49H43N4O9P).
Ir[TpOMePC](tppts)
The reaction mixture was rotary-evaporated to dryness, yielding a dark green solid. The residue was dissolved in a minimum amount of methanol and subjected to column chromatography (regular silica gel, 10:1 MeCN/MeOH, then 4:1 MeCN/MeOH) to obtain the title compound as a dichroic brown-green solid. Yield 16.6 mg (22.5%). UV–vis (CH3OH) λmax (nm) [ϵ × 10–4 (M–1 cm–1)]: 391 (sh, 1.82), 413 (2.02), 559 (sh, 0.72), 594 (0.84). 1H NMR (400 MHz, DMSO-d6) δ 7.94 (d, J = 1.8 Hz, 4H), 7.83 (dt, J = 7.6, 1.5 Hz, 4H), 7.52 (t, J = 7.6 Hz, 6H), 7.48–7.43 (m, 6H), 4.19–3.93 (m, 9H), 3.38 (t, J = 6.4 Hz, 3H), 2.23 (t, J = 7.4 Hz, 3H), 1.63 (p, J = 6.9 Hz, 3H), 1.29–1.17 (m, 3H). 31P NMR (162 MHz, composite pulse-decoupled, DMSO-d6) δ 26.36. MS (ESI): [M–] = 1328.1142 (expt), 1328.1154 (calcd for IrC58H44N4O12S3PNa).
Ir[TpCF3PC](tcep)
The purification was carried out as for Ir[TpOMePC](tcep) to afford the title compound as a deep green, hygroscopic solid. Yield 26 mg (90%). UV–vis (CH3OH) λmax (nm) [ϵ × 10–4 (M–1 cm–1)]: 420 (0.70), 610 (0.26). 1H NMR (400 MHz, methanol-d4) δ 8.73 (t, J = 3.8 Hz, 2H), 8.62 (d, J = 7.9 Hz, 4H), 8.55 (d, J = 7.9 Hz, 2H), 8.48 (d, J = 4.8 Hz, 2H), 8.33 (dd, J = 4.8, 1.5 Hz, 2H), 8.13–8.11 (m, 2H), 7.98 (d, J = 8.0 Hz, 4H), 7.94 (d, J = 8.0 Hz, 2H), −0.28 to −0.33 (m, 6H), −2.13 (s, 6H). 19F NMR (377 MHz, methanol-d4) δ −63.27. 31P NMR (162 MHz, composite pulse-decoupled, methanol-d4) δ −22.66. MS (ESI): [M–] = 1169.1697 (expt), 1169.1710 (calcd for IrC49H34N4F9O6P).
Ir[TpCF3PC](tppts)
The reaction mixture was rotary-evaporated to dryness, yielding a dark green solid. The residue was suspended in dichloromethane and shaken thoroughly, and the solvent was decanted off to remove unreacted free-base corrole and other less polar impurities. The solid residue was dissolved in a minimum amount of methanol and subjected to column chromatography (regular silica gel, 5:1 CH2Cl2/MeOH, then 1:1 CH2Cl2/MeOH), affording the product as a dichroic red-green solid. Yield 26 mg (52%). UV–vis (CH3OH) λmax (nm) [ϵ × 10–4 (M–1 cm–1)]: 413 (2.68), 427 (sh, 2.47), 556 (sh, 0.49), 594 (1.79). 1H NMR (400 MHz, methanol-d4) δ 8.64 (d, J = 4.3 Hz, 2H), 8.43 (d, J = 4.8 Hz, 2H), 8.16 (d, J = 4.8 Hz, 2H), 8.03–7.95 (m, 4H), 7.90 (d, J = 4.3 Hz, 2H), 7.88–7.51 (m, 8H), 7.39–7.34 (m, 3H), 6.76 (t, J = 7.8 Hz, 3H), 5.33 (d, J = 1.8 Hz, 3H), 3.89 (dd, J = 7.9, 1.6 Hz, 3H). 19F NMR (377 MHz, methanol-d4) δ −63.30. 31P NMR (162 MHz, composite pulse-decoupled, methanol-d4) δ −25.59. MS (ESI): [M–] = 1421.0710 (expt), 1421.0716 (calcd for IrC58H34N4F9O9S3P).
Acknowledgments
This work was supported by the Research Council of Norway (grant no. 262229 to A.G.) and the Arctic Center for Sustainable Energy at UiT—The Arctic University of Norway.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02399.
UV–vis, HR-ESI mass, and 1H NMR spectra (PDF)
Author Contributions
I.K.T. carried out the majority of the experimental work; D.R. and R.F.E. provided significant assistance. A.G. planned and coordinated the research. I.K.T. and A.G. together wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Bonnett R.Chemical Aspects of Photodynamic Therapy; CRC Press, 2000. [Google Scholar]
- Pandey R. K.; Kessel D.; Dougherty T. J.. Handbook of Photodynamic Therapy: Updates on Recent Applications of Porphyrin-Based Compounds; World Scientific, 2016. [Google Scholar]
- Amos-Tautua B. M.; Songca S. P.; Oluwafemi O. S. Application of porphyrins in antibacterial photodynamic therapy. Molecules 2019, 24, 2456 10.3390/molecules24132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.; Zhou T.; Bai R.; Xie Y. Chemical approaches for the enhancement of porphyrin skeleton-based photodynamic therapy. J. Enzyme Inhib. Med. Chem. 2020, 35, 1080–1099. 10.1080/14756366.2020.1755669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J.; Huang B.; Nawaz M. H.; Zhang W. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Coord. Chem. Rev. 2020, 420, 213410 10.1016/j.ccr.2020.213410. [DOI] [Google Scholar]
- Ghosh A. Electronic Structure of Corrole Derivatives: Insights from Molecular Structures, Spectroscopy, Electrochemistry, and Quantum Chemical Calculations. Chem. Rev. 2017, 117, 3798–3881. 10.1021/acs.chemrev.6b00590. [DOI] [PubMed] [Google Scholar]
- Nardis S.; Mandoj F.; Stefanelli M.; Paolesse R. Metal complexes of corrole. Coord. Chem. Rev. 2019, 388, 360–405. 10.1016/j.ccr.2019.02.034. [DOI] [Google Scholar]
- Teo R. D.; Hwang J. Y.; Termini J.; Gross Z.; Gray H. B. Fighting Cancer with Corroles. Chem. Rev. 2017, 117, 2711–2729. 10.1021/acs.chemrev.6b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.; Liu R.-X.; Hai-Yang L.; Chang C. K. Corrole-based photodynamic antitumor therapy. J. Chin. Chem. Soc. 2019, 66, 1090–1099. 10.1002/jccs.201900176. [DOI] [Google Scholar]
- Einrem R. F.; Gagnon K. J.; Alemayehu A. B.; Ghosh A. Metal-Ligand Misfits: Facile Access to Rhenium-Oxo Corroles by Oxidative Metalation. Chem. - Eur. J. 2016, 22, 517–520. 10.1002/chem.201504307. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Gagnon K. J.; Terner J.; Ghosh A. Oxidative Metalation as a Route to Size-Mismatched Macrocyclic Complexes: Osmium Corroles. Angew. Chem., Int. Ed. 2014, 53, 14411–14414. 10.1002/anie.201405890. [DOI] [PubMed] [Google Scholar]
- Palmer J. H.; Durrell A. C.; Gross Z.; Winkler J. R.; Gray H. B. Iridium Corroles. J. Am. Chem. Soc. 2008, 130, 7786–7787. 10.1021/ja801049t. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Vazquez-Lima H.; Beavers C. M.; Gagnon K. J.; Bendix J.; Ghosh A. Platinum Corroles. Chem. Commun. 2014, 50, 11093–11096. 10.1039/C4CC02548B. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Ghosh A. Gold Corroles. J. Porphyrins Phthalocyanines 2011, 15, 106–110. 10.1142/S1088424611003045. [DOI] [Google Scholar]
- Rabinovich E.; Goldberg I.; Gross Z. Gold(I) and Gold(III) Corroles. Chem. Eur. J. 2011, 17, 12294–12301. 10.1002/chem.201102348. [DOI] [PubMed] [Google Scholar]
- Thomas K. E.; Alemayehu A. B.; Conradie J.; Beavers C.; Ghosh A. Synthesis and Molecular Structure of Gold Triarylcorroles. Inorg. Chem. 2011, 50, 12844–12851. 10.1021/ic202023r. [DOI] [PubMed] [Google Scholar]
- Thomas K. E.; Vazquez-Lima H.; Fang Y.; Song Y.; Gagnon K. J.; Beavers C. M.; Kadish K. M.; Ghosh A. Ligand Noninnocence in Coinage Metal Corroles: A Silver Knife-Edge. Chem. - Eur. J. 2015, 21, 16839–16847. 10.1002/chem.201502150. [DOI] [PubMed] [Google Scholar]
- Mahammed A.; Gross Z. Corroles as triplet photosensitizers. Coord. Chem. Rev. 2019, 379, 121–132. 10.1016/j.ccr.2017.08.028. [DOI] [Google Scholar]
- Lemon C. M. Corrole photochemistry. Pure Appl. Chem. 2019, 92, 1901–1919. 10.1515/pac-2020-0703. [DOI] [Google Scholar]
- Palmer J. H.; Durrell A. C.; Gross Z.; Winkler J. R.; Gray H. B. Near-IR Phosphorescence of Iridium(III) Corroles at Ambient Temperature. J. Am. Chem. Soc. 2010, 132, 9230–9231. 10.1021/ja101647t. [DOI] [PubMed] [Google Scholar]
- Sinha W.; Ravotto L.; Ceroni P.; Kar S. NIR-Emissive Iridium(III) Corrole Complexes as Efficient Singlet Oxygen Sensitizers. Dalton Trans. 2015, 44, 17767–17773. 10.1039/C5DT03041B. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Day J.; Mani N. U.; Rudine T.; Thomas A. B.; Gederaas K. E.; Vinogradov O. A.; Wamser S. A.; Ghosh C. C.; Gold A. Tris(carboxyphenyl)corroles as Multifunctional Materials: Room Temperature Near-IR Phosphorescence and Applications to Photodynamic Therapy and Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 18935–18942. 10.1021/acsami.6b04269. [DOI] [PubMed] [Google Scholar]
- Borisov S. M.; Alemayehu A.; Ghosh A. Osmium-Nitrido Corroles as NIR Indicators for Oxygen Sensors and Triplet Sensitizers for Organic Upconversion and Singlet Oxygen Generation. J. Mater. Chem. C 2016, 4, 5822–5828. 10.1039/C6TC01126H. [DOI] [Google Scholar]
- Lemon C. M.; Powers D. C.; Brothers P. J.; Nocera D. G. Gold Corroles as Near-IR Phosphors for Oxygen Sensing. Inorg. Chem. 2017, 56, 10991–10997. 10.1021/acs.inorgchem.7b01302. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; McCormick L. J.; Gagnon K. J.; Borisov S. M.; Ghosh A. Stable Platinum(IV) Corroles: Synthesis, Molecular Structure, and Room-Temperature Near-IR Phosphorescence. ACS Omega 2018, 3, 9360–9368. 10.1021/acsomega.8b01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisov S. M.; Einrem R. F.; Alemayehu A. B.; Ghosh A. Ambient-temperature near-IR phosphorescence and potential applications of rhenium-oxo corroles. Photochem. Photobiol. Sci. 2019, 18, 1166–1170. 10.1039/C8PP00473K. [DOI] [PubMed] [Google Scholar]
- Einrem R. F.; Alemayehu A. B.; Borisov S. M.; Ghosh A.; Gederaas O. A. Amphiphilic Rhenium-Oxo Corroles as a New Class of Sensitizers for Photodynamic Therapy. ACS Omega 2020, 5, 10596–10601. 10.1021/acsomega.0c01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashino T.; Kurumisawa Y.; Alemayehu A. B.; Einrem R. F.; Sahu D.; Packwood D.; Kato K.; Yamakata A.; Ghosh A.; Imahori H. Heavy Metal Effects on the Photovoltaic Properties of Metallocorroles in Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2020, 3, 12460–12467. 10.1021/acsaem.0c02427. [DOI] [Google Scholar]
- Singh S.; Aggarwal A.; Bhupathiraju N. V. S. D. K.; Arianna G.; Tiwari K.; Drain C. M. Glycosylated Porphyrins, Phthalocyanines, and Other Porphyrinoids for Diagnostics and Therapeutics. Chem. Rev. 2015, 115, 10261–10306. 10.1021/acs.chemrev.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M.; Brückner C. Modifications of Porphyrins and Hydroporphyrins for Their Solubilization in Aqueous Media. Molecules 2017, 22, 980 10.3390/molecules22060980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen I. K.; McCormick-McPherson L. J.; Borisov S. M.; Ghosh A. Iridium Corroles Exhibit Weak Near-Infrared Phosphorescence but Efficiently Sensitize Singlet Oxygen Formation. Sci. Rep. 2020, 10, 7551 10.1038/s41598-020-64389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasbotten I. H.; Wondimagegn T.; Ghosh A. Electronic Absorption, Resonance Raman, and Electrochemical Studies of Planar and Saddled Copper(III) Meso-Triarylcorroles. Highly Substituent-Sensitive Soret Bands as a Distinctive Feature of High-Valent Transition Metal Corroles. J. Am. Chem. Soc. 2002, 124, 8104–8116. 10.1021/ja0113697. [DOI] [PubMed] [Google Scholar]
- Gryko D. T.; Koszarna B. Refined methods for the synthesis of meso-substituted A3- and trans-A2B-corroles. Org. Biomol. Chem. 2003, 1, 350–357. 10.1039/b208950e. [DOI] [PubMed] [Google Scholar]
- Koszarna B.; Gryko D. T. Efficient Synthesis of meso-Substituted Corroles in a H2O–MeOH Mixture. J. Org. Chem. 2006, 71, 3707–3717. 10.1021/jo060007k. [DOI] [PubMed] [Google Scholar]
- Gross Z.; Galili N.; Saltsman I. The First Direct Synthesis of Corroles from Pyrrole. Angew. Chem., Int. Ed. 1999, 38, 1427–1429. . [DOI] [PubMed] [Google Scholar]
- Pinault N.; Bruce D. W. Homogeneous catalysts based on water-soluble phosphines. Coord. Chem. Rev. 2003, 241, 1–25. 10.1016/S0010-8545(02)00306-5. [DOI] [Google Scholar]
- Palmer J. H.; Mahammed A.; Lancaster K. M.; Gross Z.; Gray H. B. Structures and Reactivity Patterns of Group 9 Metallocorroles. Inorg. Chem. 2009, 48, 9308–9315. 10.1021/ic901164r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.