Abstract
This review aims to serve as an introduction to the solute carrier proteins (SLC) superfamily of transporter proteins and their roles in human cells. The SLC superfamily currently includes 458 transport proteins in 65 families that carry a wide variety of substances across cellular membranes. While members of this superfamily are found throughout cellular organelles, this review focuses on transporters expressed at the plasma membrane. At the cell surface, SLC proteins may be viewed as gatekeepers of the cellular milieu, dynamically responding to different metabolic states. With altered metabolism being one of the hallmarks of cancer, we also briefly review the roles that surface SLC proteins play in the development and progression of cancer through their influence on regulating metabolism and environmental conditions.
Keywords: amino acids, cancer, cell surface, cellular metabolism, cellular transport, glucose, pH, physiology, plasma membrane, solute carrier transporter
In this ‘A Guide to SLC proteins’, we focus on transporter proteins—known as solute carriers (SLCs)—expressed on the plasma membrane. We aim to provide a useful survey of how metabolites and ions are transported between the extracellular and intracellular milieus. With altered metabolism being one of the hallmarks of cancer, we also briefly review roles that surface SLCs play in the development and progression of cancer.
Abbreviations
- 7TMIR
7TM‐inverted repeat
- ABC
ATP‐binding cassette transporters
- ATP
adenosine triphosphate
- HATs
heteromeric amino acid transporters
- LATs
L‐type amino acid transporters
- LeuT
leucine transporter
- MFS
major facilitator superfamily
- PM
plasma membrane
- SLC
solute carrier transporter
- TM
transmembrane
Introduction
In order to survive and maintain proper function, cells must closely monitor and control their intracellular contents. By allowing specific molecules such as metabolites and ions to pass through the lipid bilayer and enter or leave the cell, transport proteins control nutrient levels, remove waste from cells, and regulate cell volume [1]. Mirroring the large range of compounds requiring transport, different types of transporter proteins have evolved that can be subdivided into four main superfamilies: (a) the ATP‐binding cassette (ABC) transporters, (b) ATPases, (c) ion channels, and (d) solute carrier proteins (SLC) [2, 3]. The current review aims to serve as an introduction to the SLC superfamily of transporter proteins and their roles in human cells. Members of this superfamily are found throughout the cell in the membrane of almost every organelle, as well as the plasma membrane. In the first section, we summarize how the family is defined, highlight common structural features, and briefly introduce their roles in human health and diseases. Next, we provide an overview of the various SLC families in humans, focusing on SLC proteins that are expressed on the cell surface and may be considered the gatekeepers of cellular contents. We briefly review their roles in various pathologies and potential therapeutic implications. We decided to focus on cell surface SLC proteins because of considerable evidence to suggest that the composition of surface‐expressed transporters changes in response to the environment and during cellular differentiation and thus reflect cellular and metabolic states. We refer to existing reviews to cover specific topics, organelles, or families of SLC proteins [4, 5, 6, 7, 8, 9].
In this review, we also mention examples of how the transport of specific classes of molecules can affect the development and progression of disease. Hopefully, a deeper understanding of the functional integration of transporters at the interface between cells and their environment will grant a better appreciation for how SLC proteins allow cells use to thrive under physiological conditions and how this changes in disease states (in particular cancer).
Other transport protein families
While this review aims to provide a guide to plasma membrane SLCs, it is important to place these in the context of other transport proteins that play complementary and essential roles in the uptake and secretion of metabolites and ions and are thus part of the ‘general equation’ of cellular metabolism.
There are four types of transporters that are driven by ATP hydrolysis or drive the synthesis of ATP: P, V, or F‐type ATPases and ABC transporters [10]. ABC transporters serve mainly as exporters in eukaryotic cells, using energy derived from ATP hydrolysis to efficiently drive the transport of their substrates out of the cell [11]. ABC transporters generally consist of four domains: two transmembrane domains, which act as the passageway for substrates, and two nucleotide‐binding domains that bind and hydrolyze ATP. While the transmembrane domains have a wide variety of structures and amino acid sequences, the nucleotide‐binding domains show sequence conservation [12]. Many ABC transport proteins are implicated in resistance to cytotoxic drugs and other targeted chemotherapies [13]. For more information regarding ABC transporters, the reader is referred to Liu [14].
The ATPase family encompasses a large variety of proteins that also interact with ATP in order to perform their function. They can be further subdivided into F‐, V‐, or P‐type ATPases. F‐ and V‐type ATPases are both rotary ATPases, meaning they rotate about an axis as they perform their function, but are specialized in opposite functions. F‐type ATPases drive the synthesis of ATP using established ion gradients, whereas the V‐type ATPases use the energy derived from ATP hydrolysis to pump ions across a membrane and build up an electrochemical gradient [15]. P‐type ATPases, on the other hand, drive the transport of ions and lipids across cellular membranes using extensive conformational changes driven by free energy released by ATP hydrolysis [16]. For more information regarding ATPase transporters, the reader is referred to Futai et al., Palmgren et al. and Lippe et al. [16, 17, 18].
Ion channels perform passive transport, meaning substrates are transported down their electrochemical gradient. These proteins provide a pathway to specific ions and allow them to passively move down their concentration gradient [19]. This results in rapid transport, as the channels do not undergo such drastic conformational changes [19]. As their name suggests, the ion channel family transports a wide array of ions across cell membranes, maintaining the membrane potential and playing a critical role in cellular excitation and signaling [20]. For more information regarding ion channels, the reader is referred to Alexander et al. [21].
SLC superfamily
The SLC superfamily currently includes 458 transport proteins in 65 families that transport a wide variety of substances across cell membranes [22]. These families are defined by the HUGO Gene Nomenclature Committee (HGNC) of the Human Genome Organization (HUGO) and organized such that member proteins within each family share at least 20–25% sequence similarity with at least one other member of the family [22, 23]. The average SLC family contains seven members, with eight families containing only one member (SLC32, SLC40, SLC48, SLC50, SLC53, SLC61, SLC62, and SLC64) and the largest, SLC25, containing 53 members. More recently, newer models of classification have emerged based on clustering and phylogenic analysis or a combination of functional and phylogenetic analysis [24, 25, 26]. For example, Höglund et al. analyzed the entire human genome (along with 16 other species) and identified 400 unique SLC genes [25]. They further found that several of the HUGO‐defined families could be organized into four large phylogenic clusters. The largest of these clusters was the α group, containing 13 SLC families. For a comprehensive overview of the naming and classification systems used for SLC proteins, we refer the reader to Perland et al. [27].
Overall, SLC proteins transport a wide array of molecules, including sugars, amino acids, vitamins, nucleotides, metals, inorganic ions, organic anions, oligopeptides, and drugs [23]. General substrate class specificity tends to be consistent within most families. As detailed below and in Table 1, SLC proteins also have a range of substrate specificity, with some proteins transporting a range of biomolecules, while others are currently known to transport only one biomolecule and still others are ‘orphan’—with no known substrate. Recent reviews estimated that as many as 30% of SLC proteins remain such orphan transporters, even as recent technological developments have afforded novel methods to study these transport proteins [27, 28]. The SLC superfamily does not contain active transporters that directly use the energy released by ATP hydrolysis to drive the transport of substances against their concentration gradient. Rather, these proteins act as passive facilitative transporters or secondary active transporters [29]. Facilitative transport is a system of transport in which the SLC acts as a simple gatekeeper for a compound to passively move down its gradient [2]. Facilitative transport refers to systems in which only one molecule is transported in a thermodynamically favorable direction. In secondary active transport, transporters couple the passage of two or more substances. One substrate goes down its electrochemical gradient, which provides the free energy to drive the transport of the other substrate(s). Thus, the thermodynamically favorable transport of one substance provides the necessary free energy to transport the other in an unfavorable direction. In many of these cases, the rate of transport is proportional to the electrochemical gradient of the coupled ion [30]. Such secondary active transporters can either be symporters, which transport their substrates in the same direction, or antiporters, in which the substrates cross the membrane in opposite directions [2]. The substrate specificity of these transporters is determined not only by interactions between amino acid residues and the substrate, but also by intramolecular interactions that regulate gating and/or selectivity elements [31, 32, 33, 34]. Most secondary active transporters are thought to use the ‘alternating access’ transport mechanism, whereby protein domains are arranged to have a ligand binding site available on only one side of the membrane at a time, changing conformations to transport their substrates by shifting to the other side of the membrane [35].
Table 1.
Summary of SLC families, their general structure, and relation to the development or progression of cancer. Proteins in bold are described in more depth in the review. Proteins in italics have been annotated in other parts of the cell in addition to the plasma membrane. Protein folds have been classified as either the MFS, the LeuT or labeled with ‘Other’ and the prokaryotic homolog structure is in parentheses (ND, not determined). The Major substrates column is a representative but not exhaustive list of substrates transported by the proteins in the given SLC family. For more specific information regarding substrates, the reader is referred to the literature reviewed in the article. The number of transmembrane domains was obtained from Uniprot and literature cited in the respective sections. This table was assembled using information from [36] and [559]. For more specific structural information for these protein families, the reader is referred to Bai et al. [36]. A continuously updated resource containing similar information will be available through Meixner, Girardi et al. [562].
| Family | Proteins at cell membrane | Fold type | Range of TM domains | Major substrates | Cancer related genes mentioned in this review |
|---|---|---|---|---|---|
| Sugars | |||||
| SLC2 | SLC2A1, SLC2A2, SLC2A3, SLC2A4, SLC2A5, SLC2A7, SLC2A9, SLC2A10, SLC2A11, SLC2A12, SLC2A13, SLC2A14 | MFS | 12 | Glucose, fructose, mannose, galactose | SLC2A1, SLC2A3 |
| SLC5 | SLC5A1, SLC5A2, SLC5A3, SLC5A4, SLC5A4, SLC5A5, SLC5A6, SLC5A7, SLC5A8, SLC5A12 | LeuT | 11–13 | Glucose, fructose, mannose, galactose | SLC5A1 |
| Amino acids and peptides* | |||||
| SLC1 | SLC1A1, SLC1A2, SLC1A3, SLC1A4, SLC1A5, SLC1A6, SLC1A7 | Other (GltPh) | 8–10 | Ala, Ser, Cys, Thr | SLC1A5 |
| SLC3; SLC7 | SLC3A1, SLC3A2; SLC7A1, SLC7A2, SLC7A3, SLC7A4, SLC7A5, SLC7A7, SLC7A8, SLC7A9, SLC7A10, SLC7A11, SLC7A13 | ND (SLC3); LeuT (SLC7) | 1 (SLC3); 9–14 (SLC7) | Leu, Val, Gly, Ala, Ser, Glu, Cys | LAT1 (SLC7A5 and SLC3A2), xCT (SLC7A11 and SLC3A2) |
| SLC6 | SLC6A1, SLC6A2, SLC6A3, SLC6A4, SLC6A5, SLC6A6, SLC6A7, SLC6A8, SLC6A9, SLC6A11, SLC6A12, SLC6A13, SLC6A14, SLC6A15, SLC6A18, SLC6A19, SLC6A20 | LeuT | 12 | GABA, norepinephrine, dopamine, serotonin, Gly, Leu, Iso, Val, Pro | SLC6A14 |
| SLC38 | SLC38A1, SLC38A2, SLC38A4, SLC38A10 | LeuT | 10–11 | Ala, Glu, Ser, Gly, Met, Thr | |
| SLC43 | SLC43A1, SLC43A2, SLC43A3 | ND | 12 | Leu, Phe, Iso, Val, Met | SLC43A1 |
| Vitamins | |||||
| SLC19 | SLC19A1, SLC19A2, SLC19A3 | MFS | 12 | Folates (e.g., 5‐methyl tetrahydrofolate, 5‐formyl tetrahydrofolate), thiamine | SLC19A1, SLC19A3 |
| SLC23 | SLC23A1, SLC23A2, SLC23A3 | Other (UraA, UapA) | 14 | Ascorbate | SLC23A2 |
| SLC46 | SLC46A1 | ND | 12 | Folates (eg. 5‐methyl tetrahydrofolate, 5‐formyl tetrahydrofolate) | SLC46A1 |
| Nucleotides/nucleosides | |||||
| SLC28 | SLC28A1 , SLC28A2, SLC28A3 | Other (vcCNT) | 13–14 | Nucleotides/nucleosides | SLC28A1 |
| SLC29 | SLC29A1 , SLC29A2, SLC29A3, SLC29A4 | MFS | 10–11 | Nucleotides/nucleosides | SLC29A1, SLC29A2 |
| Bicarbonate ions and protons | |||||
| SLC4 | SLC4A1, SLC4A2, SLC4A3, SLC4A4, SLC4A5, SLC4A7, SLC4A8, SLC4A9, SLC4A10, SLC4A11 | Other (UraA, UapA) | 10–14 | Bicarbonate, carbonate | SLC4A7 |
| SLC9 | SLC9A1, SLC9A2, SLC9A3, SLC9A6, SLC9A7, SLC9B2 | Other (NhaA) | 10–14 | Protons | SLC9A1 |
| SLC26 | SLC26A1, SLC26A2, SLC26A3, SLC26A4, SLC26A4 SLC26A5, SLC26A6, SLC26A7, SLC26A9, SLC26A11 | Other (UraA, UapA) | 8–13 | Bicarbonate, sulfate, formate | SLC26A3 |
| Calcium ions | |||||
| SLC8 | SLC8A1, SLC8A2, SLC8A3 | CaCA | 10–11 | Ca2+ | SLC8A1, SLC8A2, SLC8A3 |
| SLC24 | SLC24A1, SLC24A2, SLC24A3, SLC24A4 | CaCA | 10–11 | Ca2+ | SLC24A4 |
| Inorganic ions | |||||
| SLC12 | SLC12A1, SLC12A2, SLC12A3, SLC12A4, SLC12A5, SLC12A6, SLC12A7 | LeuT | 12 | Cl− | SLC12A2, SLC12A6, SLC12A7 |
| Carboxylates | |||||
| SLC13 | SLC13A1, SLC13A2, SLC13A3, SLC13A4, SLC13A5 | Other (VcINDY) | 11–12 | Sulfates, di‐ and tricarboxylates | SLC13A2, SLC13A3 |
| SLC16 | SLC16A1, SLC16A2, SLC16A3, SLC16A4, SLC16A5, SLC16A6, SLC16A7, SLC16A8, SLC16A9, SLC16A10, SLC16A11, SLC16A12, SLC16A13 | MFS | 12 | Monocarboxylates | SLC16A1, SLC16A3, SLC16A7 |
| Phosphate | |||||
| SLC20 | SLC20A1 , SLC20A2 | ND | 10–12 | HPO4 2−, H2PO4 − | SLC20A1 |
| SLC34 | SLC34A1 , SLC34A2, SLC34A3 | ND | 8 | Inorganic phosphate | SLC34A2 |
| Organic ions | |||||
| SLC14 | SLC14A1, SLC14A2 | Channel like | 10 | Urea | SLC14A1 |
| SLC22 | SLC22A1, SLC22A2, SLC22A3, SLC22A4, SLC22A5, SLC22A6, SLC22A7, SLC22A8, SLC22A9, SLC22A11, SLC22A12, SLC22A13, SLC22A16, SLC22A17, SLC22A24 | MFS; Other (UraA) | 7–12 | Urate, prostaglandins, bile acids, α‐ketoglutarate, amines | SLC22A1, SLC22A2, SLC22A11 |
| Trace metals | |||||
| SLC11 | SLC11A2 | LeuT | 12 | Fe2+, Mn2+, Cu2+, Co2+, Cd2+, Ni2+, Pb2+ | |
| SLC30 | SLC30A1, SLC30A2, SLC30A5, SLC30A10 | Other (YiiP) | 5–16 | Zn2+ | |
| SLC31 | SLC31A1 , SLC31A2 | ND | 3 | Cu2+ | SLC31A1 |
| SLC39 | SLC39A1 , SLC39A2, SLC39A3, SLC39A4, SLC39A5, SLC39A6, SLC39A8, SLC39A10, SLC39A14 | MFS | 6–8 | Zn2+ | |
| SLC40 | SLC40A1 | ND | 10 | Fe2+, Mn2+ | SLC40A1 |
| SLC41 | SLC41A1, SLC41A2, SLC41A3 | ND | 10–11 | Mg2+, Fe2+, Zn2+, Cu2+ | SLC41A1 |
| SLC49 | SLC49A1 | ND | 13 | Heme | |
| Other organic compounds | |||||
| SLC10 | SLC10A1, SLC10A2, SLC10A4, SLC10A5, SLC10A6, SLC10A7 | Other (NhaA) | 8–10 | Bile acids, steroid hormones | SLC10A2 |
| SLCO | SLCO1A2, SLCO1B1, SLCO1B3, SLCO1B7, SLCO1C1, SLCO2A1, SLCO2B1, SLCO3A1, SLCO4A1, SLCO4C1, SLCO5A1 | ND | 11–12 | Wide variety of organic anions and cations | SLCO1A2, SLCO1B1, SLCO1B3, SLC2B1 |
Structure
Since SLCs are grouped together as a ‘superfamily’, SLC proteins belonging to different SLC families have a variety of different three‐dimensional folds that are not all phylogenetically related [29]. Nevertheless, there are certain structural features common to most if not all the SLC transporters. When analyzing hydropathy plots, SLC proteins are predicted to contain between 1 and 16 transmembrane (TM) domains, although most (~ 83%) tend to contain between 7 and 12 TM domains [36, 37]. An overview is provided in Table 1.
SLC proteins with known structures have so far been shown to share a distinct feature: a pseudosymmetry across the core TM domains [38]. Although, as is detailed in Table 1, there are other structural homologues used to classify SLC structures, two of the most common structural folds among SLC proteins are the major facilitator superfamily (MFS) and the leucine transporter (LeuT)‐like folds [36]. The MFS fold consists of two pseudo‐repeats of six TM helices connected by a cytoplasmic loop, while the LeuT‐like fold consists of two five‐TM helices, each of which contains a bundle and a scaffold domain [38]. The specific mechanism that drives alternating access transport depends heavily on the physical structure of proteins [39]. Proteins of the MFS fold utilize a rocker and switch mechanism, while proteins with a LeuT fold utilize a rocking‐bundle approach, and a third ‘elevator’ mechanism is also used by SLC proteins [39, 40]. As mentioned previously, in the alternating access transport mechanism, the substrate‐binding site is available only on one side of the membrane at a time. During the intermediate steps of these transport cycles, the substrate is occluded from access to either side of the membrane. In both the rocker‐switch and the rocking‐bundle model, the substrate binds to the available binding site. Next, the two transmembrane domains shift around the substrate, exposing the binding site to the other side of the membrane and releasing the substrate [40]. The rocking‐bundle mechanism has a similarly centrally localized substrate‐binding site between the two transmembrane domains. However, rather than moving both transmembrane domains around the substrate, only one domain shifts around the binding site and change the side the substrate is exposed to [40]. Finally, in the elevator mechanism, the two domains have distinctly different roles. There is a scaffold domain and a transport domain, which binds the substrate. The transport domain, through rigid body movement, migrates across the membrane and release the substrate [40].
One problem that persists when investigating the structure and function of SLC proteins is how difficult it is to purify and study transmembrane proteins [23]. Thus far, very few high‐resolution structures for human SLC proteins have been determined, exemplified by SLC2A1, SLC2A3, SLC4A1, SLC6A4, and SLC42A3 [41, 42, 43, 44, 45, 46]. The structures of SLC homologues in other species combined with computer modeling have provided insights to human SLC transporters [47]. As an example, the crystal structure of vSGLT, the sodium/glucose cotransporter from Vibrio parahaemolyticus, has been determined, providing insight on the mechanism of similar proteins like SLC5A1 (which has 32% sequence similarity) [48]. Similarly, the high‐resolution atomic structure of a bacterial amino acid transporter has further provided structural context for how the SLC6 family of proteins transports its substrates [49, 50]. However, one of the major limitations of using bacterial transporters as a method to elucidate the structure and function of human transporters is that these crystalized bacterial proteins can lack the longer cytoplasmic tails that play a significant role in transporter activity and specificity [51]. Compounding these difficulties to predict SLC protein behavior a priori is the complex web of regulation resulting from interaction partners, selective gating, and post‐translational modification. Although transporters may have similar binding residues, differences in these factors can change the specificity of a transporter.
Solute carrier proteins proteins are regulated by different post‐translational modifications on the intracellular loops between TM domains and the N and/or C termini. These modifications include phosphorylation, acetylation, and ubiquitination, while the extracellular loops and termini can be heavily glycosylated (reviewed by Czuba et al. [7]). Such modifications have been shown to affect both the rate of transport, affinity for their substrates, and SLC protein activity [52, 53]. As an example of such complex regulatory networks, the activity of every well‐characterized SLC12 transporter is regulated through the phosphorylation and dephosphorylation of serine/threonine residues [54]. The location of these regulatory sites and the effect of modification differs between the different subfamilies of SLC12 [54, 55]. Furthermore and of particular relevance for this review, ubiquitination often regulates the translocation of SLC proteins from vacuoles and/or other intracellular organelles to the plasma membrane and vice versa [56]. As an example, the insulin‐dependent translocation of SLC2A4 to the PM is regulated by ubiquitination and clathrin‐mediated vesicular trafficking [57, 58]. Of note, the activity of SLC proteins is also not only affected by the modification of the transporters themselves, but changes to their intermolecular interactions with other proteins and intramolecular interactions between domains [31, 32, 33, 34]. For example, the substrate specificity of FurE, a fungal transport protein, is regulated by interactions between its terminal cytoplasmic domains that create a gating system for this transporter. As another example, the substrate specificity of the fungal protein UapA is regulated by residues that are not part of the substrate‐binding site [59]. Intermolecular interactions between proteins like PCBP2 and SLC11A2 regulate the proper transport of iron to intracellular sites [60]. SLC16A1, 16A3, and 16A7 also all have their transport function increased in part through their interactions with carbonic anhydrases (CA) (CAII for SLC16A1 and 16A3; CAIV for SLC16A7) [61, 62]. This increase in efficiency is due to a direct supply of protons from the anhydrases to the transporters [63, 64].
For a more comprehensive review on inter‐ and intramolecular interactions and how they impact transporter activity, readers are referred to Mikros et al. [51].
Roles in health and disease
Owing to their role as one of the main regulators of what enters or leaves cells, the transport function of SLC proteins is linked to a wide range of cellular and physiological processes. Some SLC proteins have been found to be tissue specific, performing roles unique to certain cell types, best exemplified by specific transporters of the SLC6 and SLC18 family, which regulate the concentration of neurotransmitters in synapses [65]. Many different SLC families are also involved in transporting nutrients across selective barriers between tissues (like the blood–brain barrier or the gut epithelium) to provide them with the necessary nutrients [66]. Some SLC proteins are now also understood to act as ‘transceptors’, acting as both a transporter and receptor for the cell, allowing transport to also serve as a signaling system within the cell (further reviewed in Hundal et al. [67]). In this vein, some believe that SLC proteins are involved in ‘remote sensing and signaling’, a hypothesis that suggests SLCs and other transporters regulate cell and even tissue function by their altered expression/activity [68]. According to this hypothesis, transporters could play a role in signaling between organs [69]. For example, SLC30A8, a zinc transporter that has been closely linked to diabetes, is highly expressed at the membrane of pancreatic cells [70, 71, 72]. There, the transporter imports zinc from the cytoplasm into insulin secretory granules [71]. The subsequent cosecretion of insulin and zinc will impact not only neighboring endocrine cells, but the zinc serves as a signaling molecule that inhibits downstream hepatic insulin clearance, allowing the delivery of insulin throughout the body [71]. In another transport‐independent role, SLC proteins from several different families contain a virus‐binding site, thus facilitating viral entry into cells, including SLC1A5, SLC3A2, SLC7A1, and SLC52A1 [73, 74, 75, 76, 77].
As a consequence of their importance in physiology, mutations of SLC proteins have been linked to various diseases by contributing to an imbalance in the uptake, disposal, or absorption of metabolites and ions across different tissues resulting in disease states [78, 79]. According to the Online Mendelian Inheritance in Man (OMIM) database, about 190 different SLC genes have been linked to an inherited disease, resulting in phenotypes ranging from deafness (SLC17A8), anemia (SLC25A38), thyroid dyshormonogenesis (SLC5A5), and choreoacanthocytosis (SLC4A1) [28, 80, 81, 82, 83, 84]. Furthermore, genomewide association studies (GWAS) have linked polymorphisms of SLC genes to complex diseases. For example, variants of SLC16A11 have been associated with type 2 diabetes in a GWAS in Mexico, although the exact role that SLC16A11 plays in the development of type 2 diabetes remains unclear [85, 86]. Other examples, such as SLC2A9 (gout), SLC22A4 (inflammatory bowel disease), and SLCOB1 (jaundice), are further reviewed in Lin et al. [78].
Solute carrier proteins proteins are not only transporters of endogenous metabolites and ions, but are also the system by which many drugs are thought to cross the lipid bilayer and gain access to biological systems. Thus, SLC proteins indirectly affect disease outcome by affecting drug pharmacokinetics. In recent drug development guidelines, the FDA emphasizes the importance of screening SLC proteins for potential drug–drug interactions [87]. This further underscores the central role that SLC proteins play in the absorption of drugs in the intestine and across other interfaces [88]. A recent analysis of transport proteins (371 SLC and 46 ABC transporter genes) and their potential as drug interactors linked a total of 493 pharmacological compounds to 107 transporters [89]. The effect can be either via direct transport or indirect, for example, by importing a cofactor needed by an enzyme to convert a prodrug. As an example, SLC proteins play a critical role in the transport of antimetabolites such as 5‐fluorouracil (SLC29A1) and methotrexate (SLC19A1) [90]. Although a wide variety of SLC proteins are known to participate in drug transport, the SLC22 and SLCO families are among the best understood in terms of pharmacokinetics [28]. These findings have led to the hypothesis, which we support, that most drug uptake occurs through transporters rather than simple diffusion through the plasma membrane [91]. Hence, we postulate that more drug transporters will be identified in the future, leading to a better understanding of solute carriers, and particularly those expressed on the cell surface. Unraveling such drug transporters may enable the development of treatments that take advantage of transport discrepancies in disease versus healthy states.
A few SLC proteins are the direct target of approved drugs, most of which are members of the SLC5, SLC6, SLC12, or SLC22 families [92]. The potential afforded by modulating SLC protein activities continues to expand as their significance in pathophysiology continues to unravel, along with structures and transport mechanisms (see review by Rives et al. and Wang et al. [5, 8]). Such potential is best exemplified by the SLC6 family, different members of which are associated with 42 drugs currently approved by the US Food and Drug Administration, mainly for the treatment of psychiatric disorders [92].
An overview of plasma membrane solute carrier proteins
While solute carrier functions cover a broad range of transport between different cellular organelles, about 60% of SLC proteins with known localizations have been annotated at the cell surface on the plasma membrane (PM) [37]. These regulate the transport between the extracellular and intracellular milieus and hence directly control the uptake and efflux of nutrients, drugs, and other biomolecules from/to the environment. However, the expression of surface SLCs is likely to be tuned to the environment as well as the cellular programs of gene/protein expression of growth and differentiation. In this ‘guide to SLC proteins’, we focus on SLC families in which some members are known to be expressed on the PM. Owing to the fact that we do not currently have a complete understanding of the cellular localization for each SLC, this review cannot be a comprehensive list of solute carriers on the PM. We have grouped transporters by broad classes of metabolites and ions that are transported, with the aim to provide the reader with a useful survey of how metabolites and ions are transported in and out of cells. With altered metabolism being one of the hallmarks of cancer, we also briefly review the roles that surface SLC proteins play in the development and progression of cancer through their influence on regulating metabolism and environmental conditions. An overview of the SLC families described in this review is provided in Table 1.
Transporters of sugars
A large source of energy production in cells comes from the breakdown of carbohydrates and in particular glucose. Thus, the uptake of sugars is critical to cell survival. The two major families that drive such transport are SLC2 and SLC5 (Fig. 1).
Fig. 1.
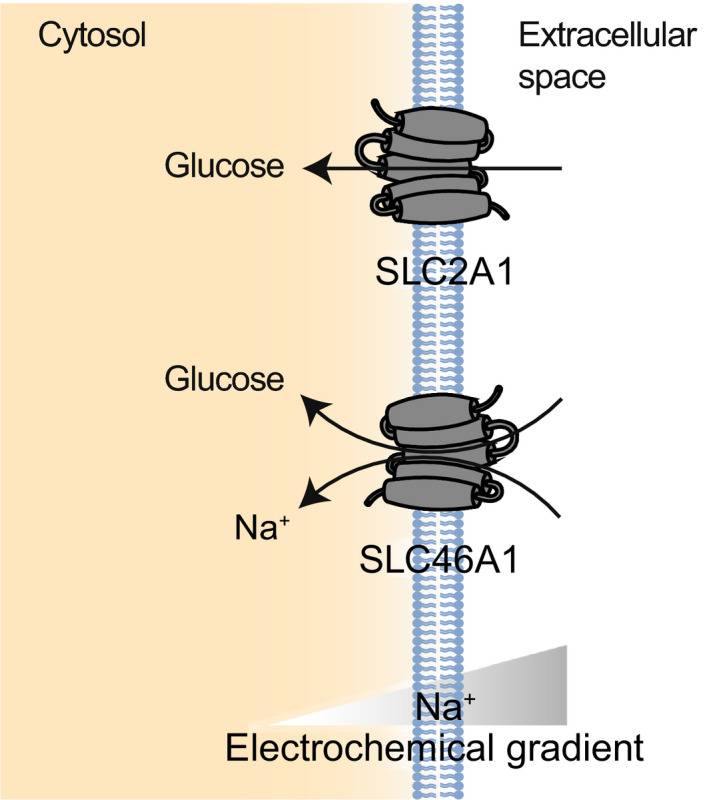
Examples of SLC proteins transporting sugars (SLC2 and SLC5). SLC2A1 is shown as an example of the SLC2 GLUT transporters, while SLC5A1 is shown as an example of the Na+‐dependent SLC5 family of sugar transporters.
SLC2
The SLC2 family transports a wide variety of carbon compounds, including monosaccharides and polyols. These proteins are expressed throughout the body in almost every cell type, underscoring their ubiquitous importance [93]. The subcellular localization of SLC2 proteins can be affected by cellular state and thus allows for very close regulation of monosaccharide concentrations within the cell [6]. Structurally, all SLC2 proteins contain an MFS structural fold and most possess 12 TM domain segments with a cytoplasmic linker domain. These proteins also all have cytoplasmic N and C termini [94]. This family consists of three different subfamilies of transporters based on their sequence similarity. Class 1 consists of SLC2A1, 2A2, 2A3, 2A4, and 2A14 (GLUT1‐4 and 14, respectively) and contains the most closely studied members of the family. Class 2 consists of SLC2A5, 2A7, 2A9, and 2A11 (GLUT5, 7, 9, and 11, respectively) while class 3 contains SLC2A6, 2A8, 2A10, 2A12, and 2A13 (GLUT6, 8, 10, 12, and HMIT, respectively). Members of this family have been implicated in Mendelian disorders like hyperuricemia (SLC2A9), Fanconi–Bickel syndrome (SLC2A2), and GLUT1 deficiency syndrome (SLC2A1) [95, 96, 97].
The main SLC2 proteins expressed on the PM are SLC2A1‐SLC2A5. One of the most well‐understood SLC proteins is SLC2A1 (reviewed by Carruthers et al. and Zambrano et al.) [98, 99]. SLC2A1 has been long investigated for its roles in tumor progression [100]. It is expressed at the PM throughout the human body, and functions primarily as a glucose transporter, although it can also transport mannose, galactose, and glucosamine [98]. SLC2A2 is most highly expressed at the PM in hepatocytes [101]. This transporter has a high affinity for glucosamine and can also transport glucose, galactose, mannose, and fructose. Among other regulatory roles, SLC2A2 functions in the regulation of glucose‐sensitive genes and insulin‐related pathways [101, 102]. One of the primary glucose transporters in brain tissue is SLC2A3, where its higher affinity and maximum turnover number help overcome the lower concentration of glucose in cerebral blood [103]. The cellular distribution of SLC2A3 varies between tissue types and cell states. In neurons, SLC2A3 is found mainly on the cell membrane, but also in vesicles and mitochondria, while in human white blood cells SLC2A3 exists mainly as a cytoplasmic vesicular protein which is then translocated to the cell membrane upon activation of the cells [103, 104]. SLC2A4 is similarly stored in cytoplasmic vesicles, which can then be redistributed to the cell membrane upon activation by insulin signaling [105]. This protein is expressed mainly in skeletal muscle, adipocytes, and cardiomyocytes, where it acts as a glucose transporter after its insulin‐triggered translocation to the cell membrane [106]. SLC2A5 functions mainly to transport fructose across the apical membrane of the small intestine [107]. Interestingly, SLC2A5 is also expressed across a wide array of human tissues, although fructose concentrations are minimal in the blood and thus SLC2A5’s role in more peripheral tissues is uncertain [108].
SLC5
The SLC5 family contains 12 members that perform the concentrative transport of glucose up its own concentration gradient by harnessing the electrochemical Na+ gradient across the cell membrane [109]. Other substrates of SLC5 family include different sugars (mainly galactose, mannose, and fructose), anions, vitamins, and short‐chain fatty acids [110]. One member of this family, SLC5A4, has been found to act not as a transporter, but rather as a glucose sensor in the plasma membrane of tissues [111]. All SLC5 proteins with the exception of SLC5A5 and SLC5A8 are composed of 14 TM helices, with the N terminus located on the extracellular side of the membrane [110].
SLC5A1 (SGLT1) is the most well‐understood protein in the SLC5 family. This protein transports one molecule of glucose or galactose with two sodium ions across the brush border of the small intestine and kidney [110, 112, 113]. Due to the extreme sodium gradient across the cell membrane and the stoichiometric ratio of transport, SLC5A1 acts as a concentrative transporter and is the most highly expressed transporter in the small intestine’s brush‐border membrane [114]. SLC5A2 (SGLT2) cotransports one sodium ion with one molecule of glucose and is most highly expressed in the kidney cortex, where it localizes to the brush‐border membrane of the proximal tubule [110]. SLC5A2 plays a critical role in glucose reabsorption, absorbing ~ 90% of the filtered glucose [115]. Both SLC5A1 and SLC5A2 have been closely associated with diabetes and thus are significant targets of approved drugs [116, 117, 118].
SLC5A3 and SLC5A5 do not transport sugars across membranes, despite their sequence similarity to other SLC5 members. SLC5A3 (SMIT1) cotransports Na+ and myoinositol, a critical osmotic regulator for cells, across membranes, thereby playing a role in volume regulation [119]. In hypotonic environments, both the expression and the plasma membrane localization of SLC5A3 are increased, resulting in increased uptake of myoinositol [120]. SLC5A5 (NIS) is a symporter, coupling the import of an anion (primarily I− but other substrates include ClO3 −, SeCN−, and SCN−) with Na+ [121]. This protein is expressed primarily in the thyroid gland, but is also found in the salivary gland, stomach, small intestine, and mammary glands [122]. As the main I—importer in the thyroid, SLC5A5 plays a critical role in thyroid hormone biosynthesis [123].
Sugar transporters and cancer
Cancerous cells are known to often have altered metabolic states, relying more on glycolysis as their source of ATP [124, 125]. This change requires cells to increase their uptake of glucose to fuel these more metabolically active cells [126]. Thus, targeting glucose transporters discussed above would provide a targeted strategy through which to disrupt the energetic supply of cancerous cells. The expression of both SLC2 and SLC5 proteins changes during the development of cancer, although the proteins thought to be the most significant contributors to these changes are SLC2A1, SLC2A3, and SLC5A1 [127, 128, 129]. Both SLC2A1 and SLC2A3 are expressed at significantly higher levels in most cancers, with higher expression of proteins found in more aggressive and proliferative cancers and lower protein expression being linked to higher survival rates of patients [130, 131]. Higher expression of SLC2 proteins has also been linked to the development of chemotherapy resistance [128, 132, 133]. SLC5 proteins have been found expressed at higher levels in colon, lung, head, neck, and pancreatic cancers [127]. SLC5A1, as the main sodium‐glucose transporter in the body, is often overexpressed in cancerous tissues, allowing these higher rates of aerobic glycolysis [129]. These alterations in SLC5A1 expression at the cell membrane may be due to the EGFR increasing expression of the protein while also decreasing its degradation [129, 134]. Imaging studies using a small molecule that is transported specifically by SLC5A2 have shown that also SLC5A2 is expressed at higher levels in certain cancers [135, 136].
Transporters of amino acids and peptides
Amino acids are utilized as the building blocks for proteins and also serve a multitude of different signaling and energetic roles within cells. In this section, we review SLC families 1, 3, 7, 6, 38, and 43 which together drive the majority of transport of these compounds (Fig. 2). SLCs in these families have been in the past annotated also by their transport substrates and mechanism (Table 2). However, these classifications are becoming increasingly difficult to define, as proteins like SLC38A7 possess properties of both systems of transport [137].
Fig. 2.
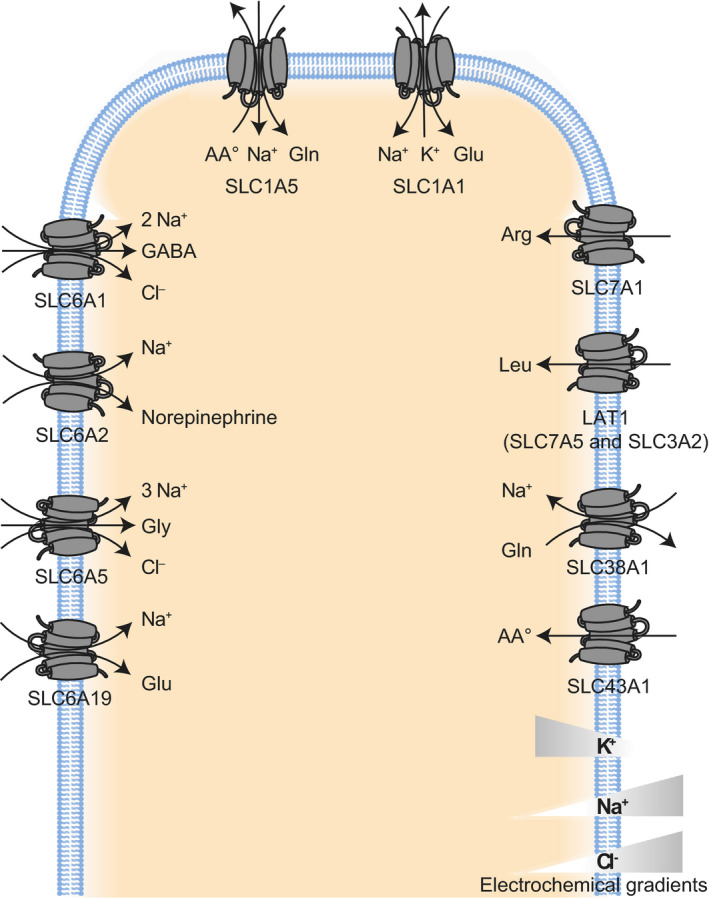
Examples of SLC proteins transporting amino acids and peptides (SLC1, SLC3, SLC6, SLC7, SLC38, and SLC43). SLC1A1 represents the SLC1 subfamily of high‐affinity glutamate transporters, while SLC1A5 represents the subfamily of neutral amino acid transporters. SLC7A1 represents the subfamily of L‐type amino acid transporters (LATs), while LAT1 represents the group of heterodimeric transporters called heteromeric amino acid transporters (HATs). SLC6A1 represents the SLC6 subfamily of GABA transporters. SLC6A2 represents the subfamily of monoamine transporters. SLC6A5 represents the subfamily of Na+‐ and Cl−‐dependent amino acid transporters while SLC6A19 represents the nutrient amino acid transporters. SLC38A1 is shown as an example of SLC38 amino acid transporter, while SLC43A1 is shown as an example of the SLC43 family of system L amino acid transporters.
Table 2.
Different transport systems of amino acids.
| Transport system [182, 560, 561] | Meaning | Transporters from SLC families |
|---|---|---|
| System A | Proteins cotransport Na+ with small, polar amino acids amino acids | SLC38 |
| System N | Proteins cotransport Na+ with His, Gln, or Asn and the simultaneous antiport of protons | SLC38 |
| System L | Proteins that have Na+ independent transport of large hydrophobic neutral amino acids | SLC3/7, SLC43 |
| System ASC | Proteins cotransport Na+ with Ala, Ser, or Cys | SLC1 |
| System asc | Proteins transport small neutral amino acids | SLC3/7 |
| System xC − | Proteins exchange Cys for Glu | SLC3/7 |
| System xAG − | Proteins cotransport Na+ with Asp or Glu and antiport K+ | SLC1 |
| System y+L | Proteins cotransport Na+ with cationic and neutral amino acids | SLC3/7 |
| System y+ | Proteins transport cationic amino acids | SLC7 |
| System b0,+ | Proteins transport cationic amino acids as well as neutral amino acids | SLC3/7 |
| System B0 | Proteins cotransport Na+ with neutral amino acids | SLC6 |
SLC1
The SLC1 family contains seven different glutamate and neutral amino acid transporters expressed throughout the body, although they play a particularly important role in the central nervous system (CNS) [138]. Five of the transporters, SLC1A1 (EAAC1), SLC1A2 (GLT1), SLC1A3 (GLAST), SLC1A6 (EAAT4), and SLC1A7 (EAAT5), are high‐affinity glutamate transporters, while SLC1A4 (ASCT1) and SLC1A5 (ASCT2) are referred to as neutral amino acids transporters (alanine, serine, cysteine, and threonine), although transport is not restricted to these compounds (e.g., SLC1A5’s preferred substrate is glutamine and at lower pHs also readily transports glutamate) [138, 139, 140]. The high‐affinity glutamate transporters cotransport glutamate with 3 Na+ ions against the counter‐transport of 1 K+ ion [138]. This subset of SLC1 transporters functions to maintain a sufficiently low extracellular concentration of glutamate, the major excitatory neurotransmitter in the CNS that is cytotoxic at higher concentrations [141]. These transporters localize to the plasma membrane of astrocytes and neurons [142]. In addition, these high‐affinity glutamate transporters also play a role in the regulation of cellular metabolism because their substrate is involved in the synthesis of glutamine [140]. The two neutral amino acid transporters are expressed throughout the body [138]. SLC1A5 is expressed at cell membranes, where its main function is to import glutamine in exchange for neutral amino acids (e.g., serine, asparagine, or threonine) in a Na+‐dependent manner [77].
SLC3/7
Two SLC families, SLC3 and SLC7, are closely linked in their function. A protein from each of these families can be linked by disulfide bridges in order to form a protein complex known as the heteromeric amino acid transporters (HATs) [143]. These SLC3/SLC7 dimers facilitate a wide range of transport, described in detail below. SLC3 proteins are also known as the heavy subunit of HATs, while SLC7 proteins are also known as the light subunit. Although most SLC7 proteins form these heterodimers to transport amino acids, some members remain functional as monomers.
The SLC7 family of amino acid transporters contains 13 members, which can in turn be further divided into two subfamilies: the cationic amino acid transporters (CATs), which include SLC7A1‐4 and SLC7A14, and the L‐type amino acid transporters (LATs), which include SLC7A5‐13 and the pseudogene SLC7A15P [143]. These two subgroups differ slightly in terms of structure, consisting of 14 and 12 TM segments, respectively. SLC7 proteins can also serve as amino acid sensors and are necessary to initiate the activation of the mTORC1 pathway [144].
SLC7A1‐4 is monomeric transport proteins called CATs. These proteins function as system y+ transporters (Table 2) [145, 146].
LATs act as the light or catalytic subunit of the HATs and determine the transport system (Table 2) and substrate specificity of the heterodimer [143]. There are five transport systems used by the HATs: system L, which is driven by SLC7A5 (LAT1) and SLC7A8 (LAT2); system asc, which is driven by SLC7A10 (Asc‐1) and SLC7A12 (Asc‐2); system xc −, which is driven by SLC7A11 (xCT); system y+L, driven by SLC7A7 (y+LAT1) and SLC7A6 (y+LAT1), and finally system b0,+, driven by SLC7A9 (b0,+AT) [143, 146, 147]. The functional heterodimeric transporter is often simply referred to by the name of its light subunit. The heavy subunit is one of the two members of the SLC3 family: SLC3A1 (rBAT) and SLC3A2 (4F2hc) [148]. These proteins have a single TM domain with an extracellular C terminus and are essential to the proper routing of the light subunits of HATs to the plasma membrane, where the heterodimer drives the sodium‐independent transport of amino acids [143, 149].
Perhaps, the most well‐understood heterodimer is LAT1, which consists of SLC3A2 and SLC7A5 and transports branched‐chain and aromatic amino acids [150, 151]. LAT1 is critical to cell growth due to its role as the main transporter of eight of the nine essential amino acids (leucine, isoleucine, valine, phenylalanine, tyrosine, tryptophan, methionine, and histidine) [152, 153]. LAT2 on the other hand, formed by the dimerization of SLC7A8 and SLC3A2, transports a wider range of amino acids at a lower affinity [151]. Both transport dimers are widely expressed throughout the body at varying levels [146, 152].
SLC7A10 forms a heterodimer with SLC3A2 and localizes to the cell membrane of central nervous system tissue to transport small neutral amino acids like glycine, alanine, serine, threonine, and cysteine [154].
SLC7A7 and SLC7A6 both form complexes with SLC3A2, forming similar transporters called y+LAT1 and y+LAT2, respectively, driving the exchange of cationic amino acids for Na+ and a neutral amino acid [143, 155, 156].
SLC7A11 also forms a complex with SLC3A2 to form xCT, a sodium‐independent exchanger of intracellular glutamate and cystine [157]. This transporter has been shown to play a critical role in maintaining the metabolic and redox balance of cells [158].
SLC7A9 forms a heterodimer with SLC3A1 to form a transporter called rBAT/b0,+AT. This transporter drives the uptake of cystine and dibasic amino acids and is highly expressed at the apical membrane of the renal proximal tubule, where it performs the majority of cystine reabsorption [147, 159]. More recently, SLC3A1 has also been found to interact with SLC7A13 (AGT1) in the sections of the renal proximal tubules where SLC7A9 is not as highly expressed [160]. This newly discovered heterodimer is thought to act as a second cystine transporter on the plasma membrane of the kidney.
SLC6
Transporters of the SLC6 family use the sodium gradient to carry out the secondary active transport of small amino acids or amino acid‐like substrates [30, 161]. Members of this family tend to have very specific localization in cells, owing to their specialized roles [161]. These proteins have 12 TMs with both C and N termini in the cytoplasm. These termini have been shown to play regulatory roles in protein trafficking and the stoichiometry of transport [162]. They are also implicated in the proper function of these proteins, serving as scaffolds upon which some of these proteins form dimers and higher oligomers [163, 164]. Many members of this family are well‐known targets of inhibitory drugs, treating epilepsy and movement disorders like Parkinson for example [162, 165, 166, 167, 168]. This family can be divided into four subfamilies based on their transport substrates: (a) the gamma‐aminobutyric acid (GABA) transporters, (b) the monoamine transporters, (c) the neurotransmitter amino acid transporters, and (d) the nutrient amino acid transporters [30]. There is also one remaining orphan transporter (SLC6A16) [161].
The GABA transporter subfamily includes SLC6A1 (GAT1), 6A6 (TauT), 6A8 (CT1), 6A11 (GAT3), 6A12 (BGT1), and 6A13 (GAT2) [30, 161, 169]. Although referred to widely as the GABA transporter subfamily, members also transport a variety of other substrates, including taurine (SLC6A6), creatine (SLC6A8), and betaine (SLC6A12) [161]. SLC6A1 is the main neuronal GABA transporter and one of the best understood members of this family [169]. SLC6A1 is found primarily in the synapse of presynaptic neurons, while SLC6A11 localizes to the cell membrane of astrocytes in close proximity to the synapses of GABAeric neurons [166, 170]. The transport function of GABA transporters is driven by sodium and chloride cotransport, although the exact stoichiometry of this transport varies [170, 171]. SLC6A13 is not only expressed in the brain but also in peripheral tissues like the liver and kidney [161]. SLC6A13 localizes to the sinusoidal membrane of periportal hepatocytes and to the basolateral membrane of proximal tubules [172]. SLC6A6 and SLC6A12 are also expressed in the brain, as well as the kidney and other tissues [161].
The SLC6 subfamily of monoamine transporters contains SLC6A2 (NET), 6A3 (DAT), and 6A4 (SERT) [30, 168]. These transporters are all primarily expressed in the CNS and localize to the cell membrane of neurons at the presynaptic cleft [168]. There, these proteins perform the reuptake of norepinephrine (SLC6A2), dopamine (SLC6A3), or serotonin (SLC6A4), which is driven by the cotransport of a sodium ion [168].
The subfamily of amino acid transporters includes SLC6A5 (GlyT2), SLC6A7 (PROT), SLC6A9 (GlyT1), and SLC6A14 (ATB0,+) [30]. These transporters are all dependent on both sodium and chloride [162, 173]. Both SLC6A5 and SLC6A9 transport glycine and 1 Cl− along with 3 Na+ or 2 Na+, respectively [162]. The reuptake of glycine functions to prevent the over activity of glycine receptors in synapses [174]. SLC6A5 functions to maintain the concentration gradient between the extracellular space (where glycine is found at submicromolar levels) and the cytosol (millimolar levels) [174]. SLC6A9 has been shown to also reverse its direction of transport in response to dopamine stimulation of neurons, possibly as a way to rapidly transduce dopamine signaling into glycine release [175]. SLC6A7 is expressed primarily in a subset of glutamatergic neurons and functions as a high‐affinity proline transporter [176]. SLC6A14 differs from the other transporters in this subfamily, driving the transporting all essential amino acids as well as glutamine and arginine [173]. Although it is expressed at lower levels in normal tissues, it has been found to be upregulated in different kinds of cancers [177, 178].
The fourth subfamily contains the nutrient amino acid transporters. This subfamily encompasses SLC6A15 (B0AT2), 6A16 (NTT5), 6A17 (NTT4), 6A18 (B0AT3), 6A19 (B0AT1), and 6A20 (SIT1) [30]. SLC6A15 and SLC6A17 both are expressed mainly at the cell membrane of neurons, although also at lower levels in kidney, pituitary, lung, and brain cells [30, 161]. Mouse homologues of SLC6A15 transports leucine, isoleucine, valine, proline, and methionine, while SLC6A17 transports proline, glycine, leucine, and alanine [179, 180]. SLC6A18 (B0AT3) is highly expressed at the luminal membrane of the proximal tubules in the kidney, where it acts as a sodium and chloride‐dependent neutral amino acid transporter [181]. SLC6A19 (B0AT1) and SLC6A20 are brush boarder transport proteins, expressed primarily at the apical membrane of small intestine and kidney cells [182, 183, 184].
SLC38
The SLC38 family contains 11 proteins that transport amino acids, with specificity varying greatly between the different members [137]. SLC38 proteins transport small amino acids like alanine, glutamine, serine, glycine, methionine, and threonine [185]. These transporters are expressed throughout the body [137, 186]. The SLC38 family is considered to possess similar structural features throughout the family, consisting of 11 TM segments with an extracellular C terminus and intracellular N terminus [187].
Members of the SLC38 family are thought to perform either system A transport or system N (Table 2) [186]. Of the characterized proteins, SLC38A1 (SNAT1), SLC38A2 (SNAT2), SLC38A4 (SNAT4) are all thought to be System A transporters, while SLC38A3 (SNAT3), SLC38A5 (SNAT5), and SLC38A7 (SNAT7) are thought to be system N [186]. SLC38A2, among other members of the SLC38 family, is also suggested to behave as a ‘transceptor’, whereby amino acid binding or transport could trigger signaling network within the cell, resulting in both transport of a substrate and activity as a receptor [185, 188, 189]. SLC38A10 has been suggested to provide the bidirectional transport of glutamine, alanine, glutamate, and aspartate and the efflux of serine [190]. This protein is expressed at the plasma membrane of both neuronal cells and astrocytes [190].
SLC43
The SLC43 family of proteins is rather small, consisting of only three members: SLC43A1 (LAT3), SLC43A2 (LAT4), and the orphan transporter SLC43A3 (EEG1) [191]. Both characterized proteins are highly similar plasma membrane system L amino acid transporters (Table 2), sharing ~ 57% amino acid similarity and consist of 12 TM domains [191]. Typical of other system L transporters, both SLC43A1 and SLC43A2 provide sodium‐independent transport [192, 193]. Unlike the SLC7 LATs, the SLC43 family does not require a binding partner to form a heterodimeric transport unit [193]. They act as low affinity facilitated diffusers of neutral amino acids, preferentially transporting leucine, phenylalanine, isoleucine, valine, and methionine [192, 193, 194]. Through their transport of leucine, both SLC43A1 and SLC43A2 play a role in the mTOR signaling pathway [194].
SLC43A1 is most highly expressed at the plasma membrane of liver, skeletal muscle, and pancreatic tissues, although it is also expressed at lower levels in other tissues of the body [193]. Expression of this protein is upregulated in response to starvation states, suggesting that it plays a critical role in interorgan amino acid balance [195]. SLC43A2 has a broader expression profile than SLC43A1, being expressed in the placenta, kidney, leukocytes, and at lower levels in many other tissue types [192]. The localization of this protein to the plasma membrane follows feeding patterns in mice, with increased plasma membrane expression of SLC43A2 occurring cyclically in anticipation of food intake, attesting to a role for SLC43A2 in amino acid absorption [196]. This protein is also required for proper mouse development, with knockout models exhibiting significant growth retardation and low amniotic fluid amino acid levels [197].
Amino acid transporters and cancer
In cancer cells, the dysregulation of amino acid transporters alters amino acid levels, helping drive carcinogenesis [198]. Not only do cancer cells have a higher dependency on amino acids to drive the synthesis of proteins as they proliferate, but altered amino acid levels also contribute to the modulation of mTOR, a key protein kinase in cellular metabolism [198]. Of particular relevance to the etiology of cancer is the dysregulation of leucine and/or glutamine. Leucine serves as the main regulatory mechanism for mTOR in many tissues [199]. Cancer cells often overexpress leucine transporters, leading to higher intracellular leucine concentrations, mTOR activation, and the subsequent proliferation of cancer cells [199, 200]. Glutamine on the other hand serves a multitude of different roles within cells as a carrier for ammonia in tissues, a regulator of the acid/base balance in kidney cells and as the precursor of compounds like glutathione, glutamate, and GABA [201]. It is used as a building block for nucleotides and other amino acids, provides a source of α‐ketoglutarate for the TCA cycle (as a precursor of glutamate), and helps regulate the redox balance in cells [200]. These roles make glutamine a critical metabolite for cancer cells. Five of the major contributors to aberrant leucine and glutamine transport in cancer cells are SLC1A5, LAT1, xCT, SLC6A14, and SLC43A1 [131].
SLC1A5 serves as the primary source of glutamine uptake in cancer cells [131]. Its activity controls the rate of tumor growth in breast cancer and blocking this transporter’s activity has been shown to prevent tumor cell proliferation in different tissues [77, 201]. The function of LAT1 (the heterodimer of SLC7A5 and SLC3A2) is linked to that of SLC1A5, as it uses the efflux of glutamine to drive the uptake of leucine, which subsequently activates mTOR signaling. These two proteins can cooperatively drive the proliferation of tumor cells [201]. xCT (the heterodimer of SLC7A11 and SLC3A2) is essential for maintaining the redox balance of cells and the increased expression of xCT in cancer cells allows cells to reduce their oxidative stress and avoid apoptosis [158, 200]. This dimer has upregulated expression in breast cancers that metastasize and inhibition of SLC7A11 (the functional subunit of xCT) delays lung metastasis, suggesting that xCT plays a significant role in cancer metastasis [202, 203].
SLC43A1, similarly to LAT1, has upregulated expression in cancers as a source of essential amino acids (in particular leucine) [191, 204]. SLC43A1 has also been implicated in cell proliferation and is particularly implicated in the severity of prostate cancers [194].
SLC6A14, a highly concentrative amino acid transporter, is expressed at higher levels in cancers in various tissue systems (e.g., breast, colorectal, cervical) [131, 200]. Owing to the fact that SLC6A14 is generally expressed lower in healthy tissues than in cancerous tissues, it serves as a promising drug target.
Transport of vitamins
Vitamins like ascorbic acid, folates, and thiamine are used in cells as the precursors of various compounds, as cofactors in metabolism and regulators of oxidative stress. Three SLC families drive the transport of these compounds, SLC19, SLC46, and SLC23 (Fig. 3A).
Fig. 3.
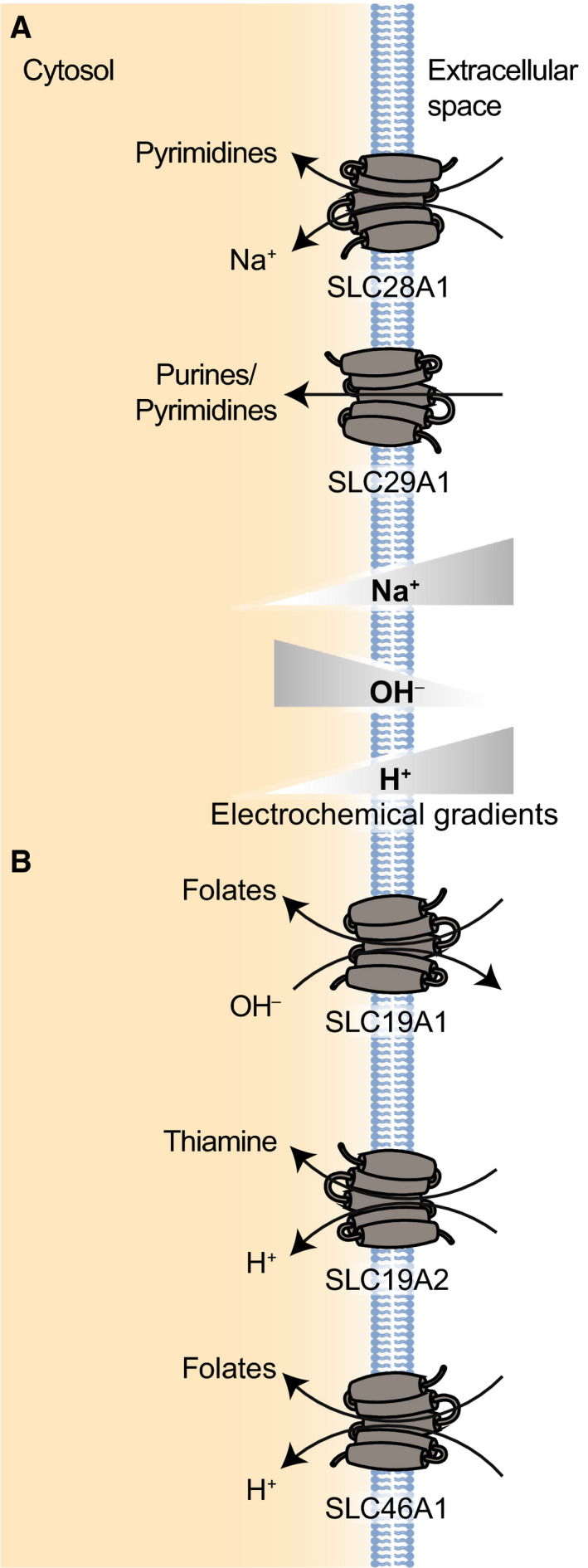
(A) Examples of SLC proteins transporting vitamins (SLC19 and SLC46). SLC19A1 is the folate transporter, while SLC19A2 represents the thiamine transporters. SLC46A1 is a functionally related protein that drives the absorption of folate. (B) Examples of SLC proteins transporting nucleotides and nucleosides (SLC28 and 29). SLC28A1 is shown as an example of a concentrative nucleoside transporter (CNT), while SLC29A1 is shown as an example of an equilibrative nucleoside transporter (ENT).
SLC19/ SLC46
The SLC19 family of proteins contains three members that transport either reduced folates (SLC19A1 [RFC]) or thiamine (SLC19A2 [ThTr1] and SLC19A3 [ThTr2]), driven by the antiport of organic anions [205]. These proteins are expressed throughout the body, ensuring proper levels of these critical vitamins in tissues [206]. The term folates refers to a larger group of water‐soluble B vitamins that are the precursors and substrates for many different compounds related to cell and tissue growth and development [207]. Thiamine, also known as vitamin B1, also plays a critical role in cells as a cofactor in energy metabolism and also as a regulator of oxidative stress [208]. All three proteins perform the most efficient transport at a neutral extracellular pH (around 7.4), with efficiency dropping as pH does [206, 207]. This may be due in part to the changing H+/OH− gradient across cell membranes [205].
SLC19A1 has a MFS structure and consists of 12 TM segments, with both N and C termini localizing to the cytoplasm [209]. The concentrative transport of SLC19A1 is driven by the export of anionic cellular metabolites down their concentration gradients [207]. SLC19A1 was identified in a recent study to also transport a cyclic dinucleotide that is produced when cytosolic DNA binds to cyclic GMP‐AMP synthase, thus playing a role in triggering larger immune responses [210].
SLC19A2 and SLC19A3 share 48% identity and have different expression patterns within cells and affinities for thiamine. SLC19A2 is expressed primarily at basolateral membranes while SLC19A3 localizes to the apical membrane of cells. This localization suggests that SLC19A2 plays a role in thiamine export while the higher affinity transporter SLC19A3 directs the accumulation of thiamine into the cell [211, 212].
A functionally related family of proteins is the SLC46 proteins. This family of proteins contains three members, two of which remain largely orphan transporters (SLC46A2, SLC46A3) [206]. SLC46A1 (PCFT) is a proton‐coupled folate transporter that mediates the absorption of folate in the intestine, as well as transporting it into the CNS [206, 213, 214]. SLC46A1 performs electrogenic transport at a low pH, where it has an affinity for both folic acid and reduced folates [206].
SLC23
The SLC23 family of transporters contains three members that regulate intracellular ascorbate concentrations [215]. SLC23A1 (SVCT1) and SLC23A2 (SVCT2) are both well characterized, while SLC23A3 (SVCT3) remains an orphan transporter [216]. Both characterized transporters are highly selective for L‐ascorbic acid, with SLC23A1 having slightly higher capacity but lower affinity than SLC23A2 [217]. SLC23A1 and SLC23A2 are both sodium‐dependent transporters that have a stoichoimetry of 2 Na+ : 1 ascorbic acid down sodium’s electrochemical gradient [216]. Interestingly, SLC23A2 is also dependent on the presence of Ca2+ and Mg2+ in order to function, with the cations switching the transporter into an active form [218]. SLC23A1 is expressed mainly in intestinal and renal proximal tubule epithelial cells at the apical membrane [215]. SLC23A2 is expressed throughout the body and is expressed at its highest levels in brain, lung, and bone tissue [218]. It serves as the only specific ascorbic acid transporter in neuronal and glial cells, thus far [219]. SLC23A2 has been shown to localize to both the cell membrane and the mitochondria of tissues [218, 220]. Interestingly, when localized to the mitochondria, SLC23A2 does not require the presence of Ca2+ and Mg2+ in order to function [221].
Vitamin transporters in cancer
Nutritional factors, like ascorbate, thiamine, and folates, function as critical cofactors for enzymes are intermediates in carbohydrate metabolism and regulators of the oxidative balance of cells [207, 208, 215, 222]. Thus, the proper transport of ascorbate, folate‐related nutrients, and thiamine are critical to preventing carcinogenesis [222, 223, 224, 225]. Folates are particularly important for one‐carbon metabolism, wherein 5‐methyltetrahydrofolate is used in the production of S‐adenosylmethionine (SAM) [225]. Thiamine is important for several metabolic enzymes [224]. Although the link between thiamine consumption and the development of cancer remains unclear, studies have shown links between carcinogenesis and lower intracellular and blood levels of these vitamins [224]. Ascorbate plays a key role in maintaining the redox balance of cells by reducing reactive oxygen species and quenching free radicals [222]. Ascorbate also helps regulate the function of α‐ketoglutarate‐dependent dioxygenases (α‐KGDDs), a diverse family of proteins that (among other functions) play a role in regulating DNA methylation and are often downregulated in cancers [222, 226]. Perhaps most importantly for its relation to cancer development and progression, ascorbate is a cofactor for HIF hydroxylases, proteins which identify HIF for degradation [227].
Dysregulation of vitamin transporters, and in particular SLC19A1 and SLC19A3, has been linked to carcinogenesis, as well as therapy resistance owing to their role as important drug transporters [223, 224]. SLC19A1 is the primary transport mechanism for the delivery of antifolates to tumor cells, as it is expressed ubiquitously in tumor cells [206]. Polymorphisms of this transporter have been associated with an increase in adverse drug responses [228, 229]. SLC46A1 provides an intriguing opportunity to harness its concentrative transport activity in acidic environments, which are often found in the tumor microenvironment [230]. SLC46A1, which is ubiquitously expressed in solid tumors, could in principle afford a more targeted delivery of antifolate drugs to tumor cells [230]. Expression of SLC19A3 in cancerous cells has been shown to be both increased and decreased and further studies are needed to elucidate the role SLC19A3 plays in cancer cells [224, 231]. SLC19A3, like other transporters that have been discussed in the review, is also regulated by HIF‐1α [232]. This study showed that under hypoxic conditions the expression of SLC19A3 is induced, leading to higher import of thiamine and suggesting an adaptive role for SLC19A3 in hypoxic environments [232].
Various polymorphisms of SLC23A2 have been associated with an increased risk of both colorectal adenoma and gastric cancer, while SLC23A1 polymorphisms have shown conflicting results for their link to cancer risk [227, 233, 234, 235]. Vitamin C has emerged as an intriguing therapeutic tool in oncology, with high concentrations of vitamin C killing colorectal cells by increasing oxidative stress in cells, inactivating GAPDH and reducing tumor size [222, 236, 237]. The varying localization of SLC23A2 in cells may be important for helping increase the therapeutic effect of vitamin C, as its mitochondrial localization in most cancer cell lines has allowed for mitochondria to be targeted in cancer therapeutics [238]. A recent study showed that a combinatorial therapy of doxycycline, azithromycin, and vitamin C effectively eradicates cancer stem cells [239]. Similarly, a combination treatment of vitamin C and cetuximab was recently shown to be an effective treatment to limit the development of acquired resistances in colorectal cancer, slowed the growth of cancerous cells, and impaired the structure of organoids [240].
Transporters of nucleotides/nucleosides
As the building blocks for DNA and RNA, as well as fulfilling a multitude of different signaling roles within and between cells, the transport of nucleotides, nucleosides, and related compounds is critical for cell health. In this section, SLC families 28 and 29 are reviewed (Fig. 3B).
SLC28/SLC29
Both SLC28 and SLC29 families provide the transport of nucleotides and nucleosides [241, 242]. Both families also drive the transport of a significant number of nucleoside‐analog‐based drugs, including clofarabine, zebularine, and ribavirin [243]. In general, SLC28 proteins show a higher affinity for their substrates than SLC29 proteins, although SLC28 proteins have a lower turnover number in transport [241].
The SLC28 family of proteins consists of three proteins that provide the concentrative transport of nucleosides and are referred to as concentrative nucleoside transporters (CNT). These proteins link the import of nucleosides with sodium ions [241, 244]. SLC28A1 (CNT1) and SLC28A2 (CNT2) have a transport stoichiometry of 1 Na+ : 1 nucleoside while SLC28A3 has a stoichiometry of 2 Na+ : 1 nucleoside [245]. These proteins are predicted to consist of 13 TM domains, with extracellular C terminus and an intracellular N terminus [244]. These proteins all localize primarily to the apical membrane of cells, where they provide the reabsorption of nucleosides from the extracellular milieu [246].
SLC28A1, unlike SLC28A2 and SLC28A3, does not transport adenosine, instead exhibiting a higher affinity for pyrimidine and pyrimidine‐based analogs [245, 247]. This protein is highly expressed in epithelial tissues, where levels vary as cell progress through the cell cycle [248]. In general, SLC28A1 tends to be upregulated in highly proliferating cells, possibly compensating for insufficient enzymatic synthesis of certain nucleosides with higher uptake [248, 249].
SLC28A2 has a higher affinity for purine nucleosides than SLC28A1 and SLC28A3 [241]. In proliferating cells, this protein has been shown to salvage extracellular adenosine, triggering the activation of AMP‐dependent protein kinase [250]. SLC28A2 has a varied subcellular distribution, localizing to both the cell membrane and intracellular vesicles in certain cell lines. This localization has been shown to be regulated by bile acids (BA), with extracellular BA triggering translocation to the cell membrane [251].
Unlike SLC28A1 and SLC28A2, SLC28A3 has coupled transport of substrates to both Na+ and H+ [243]. Interestingly, the affinity of SLC28A3 toward substrates changes depending on which cation is being cotransported [252]. Na+‐coupled SLC28A3 transports a wider spectrum of pyrimidine and purine nucleosides/nucleoside drugs while H+‐coupled transport is more selective and does not transport guanosine or zidovudine (an antiviral nucleoside drug) [243, 252].
The SLC29 protein family encodes four transport proteins that are referred to as equilibrative nucleoside transporters (ENTs) [242]. These proteins provide the facilitative transport of nucleosides and nucleobases (and monoamines in the case of SLC29A4 (ENT4)), a function that is critical for nucleotide synthesis [241]. SLC29 members are expressed in all tissues [242]. These proteins are predicted to have 11 TMs, with a cytoplasmic N terminus and an extracellular C terminus [241, 253]. These proteins also mediate the transport of nucleoside drugs like gemcitabine and didanosine and are predictors of treatment responses in diseases like pancreatic cancer and gallbladder adenocarcinomas [243, 254, 255].
SLC29A1 (ENT1) and SLC29A2 (ENT2) transport similar substrates across the plasma membrane, although their affinities to purine and pyrimidine nucleosides differ [242]. Through their role in controlling the nucleotide pool available for DNA synthesis, these transporters play a role in regulating the progression of cells through the cell cycle [256].
SLC29A4 (ENT4) localizes to the apical membrane of renal cells, where it provides proton‐driven organic cation reabsorption [257]. This protein’s activity is pH sensitive, with increases in pH resulting in diminished transport and acidic environments activating the protein [257]. SLC29A4 transports mainly adenosine, although it also shows affinity for a wider range of biogenic amines [258].
Nucleotide transporters and cancer
The uptake of nucleosides driven by SLC28 and SLC29 proteins provides cells with the building blocks for nucleotide synthesis. Thus, rapidly dividing cells often rely on these nucleoside/nucleotide transporters, resulting in increased expression of these transport proteins to provide these building blocks [241]. Yet, decreased expression of SLC28 and SLC29 proteins has also been found in hypoxic environments [259]. There, HIF‐1α signaling decreased the expression of SLC29A1 and SLC29A2, resulting in higher extracellular adenosine concentrations [260]. Increased expression of SLC28A1 has also been linked to a decreased proliferation of cancerous cells [261, 262]. A recent study showed that restoring the expression of SLC28A1 reduced the growth of tumors in mice. However, this study showed this effect occurred independent of transport, suggesting that SLC28A1’s antiproliferative effect was signaling related [261].
SLC28 and SLC29 proteins also transport a significant amount of different nucleoside analogs like clofarabine, zebularine, and ribavirin, whose pharmacokinetics are relevant to a wide variety of different diseases (e.g., viral infections, cancer, inflammatory diseases, autoimmune disorders) [243, 263, 264]. Variances in transporter distribution and expression are also linked to differing treatment outcomes, with higher expression of transporters generally resulting in higher sensitivity to nucleoside analogs [264].
Transporters of bicarbonate ions and protons
Through the transport of bicarbonate ions and protons, SLC families 4, 9, and 26 play a major role in the regulation of cellular pH, along with signaling events related to the transport of these compounds (Fig. 4A).
Fig. 4.
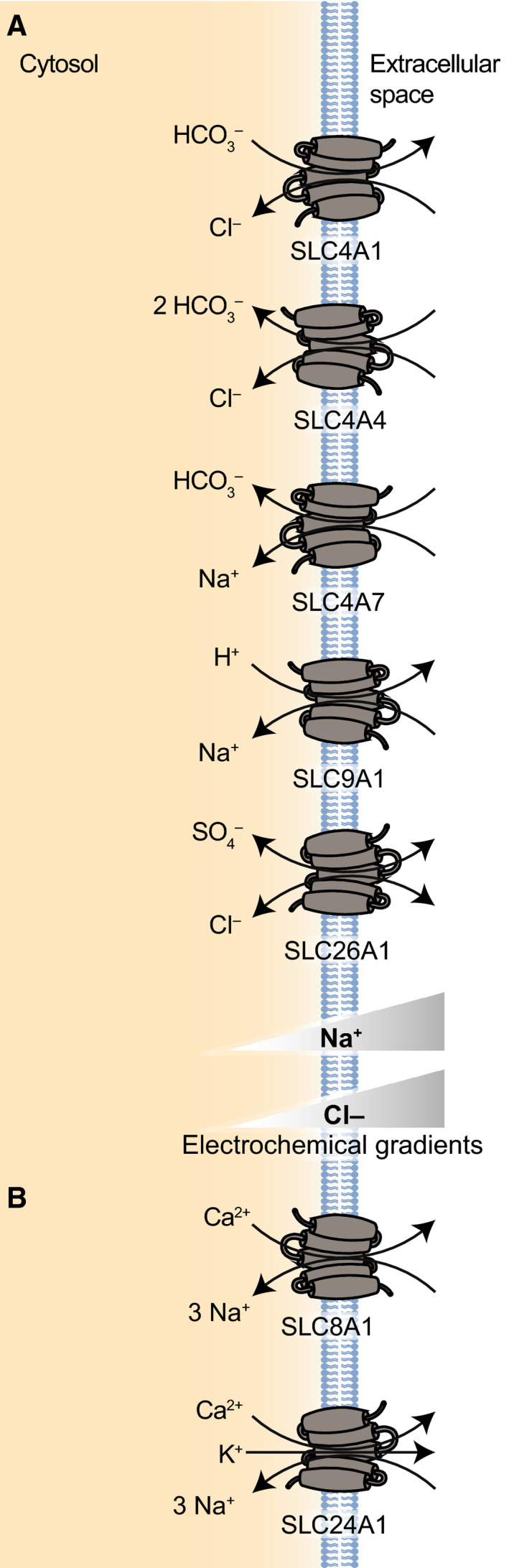
(A) Examples of SLC proteins transporting protons and bicarbonate ions (SLC4, SLC9, SLC26). SLC4A1 represents the subfamily of anion exchangers (AE), SLC4A4 represents the subfamily of electrogenic Na+/HCO3 − cotransporters, while SLC4A7 represents the subfamily of electroneutral Na/HCO3 − cotransporters. SLC9A1 serves as the prototypical Na+/H+ exchanger. SLC26A1 is an example of an SLC26 transporter that cotransports sulfate with Cl−. (B) Examples of SLC proteins transporting divalent ions (SLC8 and SLC24). SLC8A1 is shown as an example of a Ca2+/Na+ exchanger. SLC24A1 is shown as an example of K+‐dependent Na+/Ca2+ exchangers.
SLC4
The SLC4 family has 10 different members that have linked the transport of bicarbonate (or carbonate) with the transport of at least one other ion, Na+ and/or Cl− [265]. These proteins play a critical role in the acid–base homeostasis of the body by acting as either acid loaders or acid extruders, thus regulating both intra‐ and extracellular pH [266, 267, 268]. SLC4 protein topology consists of 10–14 TM domains with hydrophobic N and C termini extending into the cytoplasm [269, 270, 271]. Based on structural similarities between SLC4A1 and a bacterial uracil:proton symporter, SLC4 proteins with 14 TM domains are predicted to be organized in a 7TM‐inverted repeat (7TMIR), meaning that the two structurally related halves span the membrane in opposite orientations and form a functional intertwined structure [272, 273]. More recently, the cryoEM structure of SLC4A4 revealed the structural details of the ion pathways that dictate transport of substrates [274]. Many SLC4 proteins form dimers and oligomers and although the functional relevance of this dimerization is not entirely elucidated, in both bacterial and eukaryotic homologues dimerization is necessary for transport function and/or proper localization to the PM [266, 273, 275, 276].
The SLC4 family includes several functional subfamilies: (a) SLC4A1 (AE1, also commonly referred to as Band 3 protein), SLC4A2 (AE2), and SLC4A3 (AE3) are all Cl−/HCO3 − anion exchangers (AE); (b) SLC4A4 (NBCe1) and SLC4A5 (NBCe2) are electrogenic Na+/HCO3 − cotransporters (NBCs); (c) SLC4A7 (NBCn1) and SLC4A10 (NBCn2) are electroneutral NBCs [265]. For SLC4A8 (NDCBE), SLC4A9 (AE4) and SLC4A11 (BTR1), substrates have not been fully resolved.
SLC4A1 is the predominant transport protein in the cell membranes of erythrocytes, where it participates in clearing CO2 from tissues to the lungs [265]. SLC4A1 is also highly expressed in the basolateral membrane of renal cells, where it functions to reabsorb HCO3 − into the blood [265]. SLC4A2 is the most widely expressed of the anion exchangers, localizing to the basolateral membrane of most epithelial cells [265]. SLC4A2 and SLC4A3 are both regulated by pH, with increases in either intra‐ or extracellular pH resulting in increased activity [277, 278].
SLC4A4 (NBCe1) acts as an electrogenic Na+/HCO3 − cotransporter at the basolateral membrane of renal proximal tubules, pancreatic ducts, and epididymis and is also expressed at lower levels throughout the body [265]. SLC4A5 (NBCe2) generally localizes to the Golgi apparatus within cells, but when intracellular sodium concentrations increase, the transporter is moved to the apical cell membrane [279]. SLC4A7 (NBCn1) is an electroneutral Na+/HCO3 − cotransporter expressed throughout the body but particularly in the spleen and testis [280]. SLC4A10 (NBCn2) is primarily found in the brain, where it functions as an electroneutral Na+/HCO3 − cotransporter [265]. SLC4A10 is expressed on the basolateral membrane of the choroid plexus epithelium, where its activity contributes to CSF secretion [281].
SLC4A8 encodes a Na+‐driven Cl−/HCO3 − exchanger (NDCBE), importing Na+ and HCO3 − in exchange for intracellular Cl− [282]. However, the substrate specificity is not entirely clear, as some data suggest Cl‐ is not necessary for transport [266, 283]. This protein is primarily expressed at the plasma membrane throughout the CNS, where it may play a role in the pH regulation of neurons [284]. Although the protein encoded by SLC4A9 is called AE4, it was recently found to be an electroneutral Na+‐dependent Cl−/HCO3 − exchanger that localizes to the basolateral membrane in cortical collecting ducts [285, 286].
Finally, SLC4A11 (BTR1) is expressed throughout the body and does not transport bicarbonate. Rather, it was recently proposed to be an NH3/2H+ cotransporter, as well as acting as a sodium‐independent borate transporter [287, 288]. Of its three main splice variants, only SLC4A11‐B and SLC4A11‐C are expressed as plasma membrane proteins, with SLC4A11‐A localizing intracellularly [288].
SLC9
The SLC9 family of proteins consists of 13 proteins that mainly act as Na+/H+ exchangers, using the Na+ gradient to transport H+ (or Li+) across membranes and thus contribute greatly to the pH homeostasis of both cells and organelles [289, 290]. These 13 proteins are organized into three different families, SLC9A (the Na+/H+ exchangers), SLC9B (two Na+ or Li+/H+ exchangers), and SLC9C (two Na+/H+ exchangers expressed primarily in sperm tissue) [290, 291]. Interestingly, this family of proteins has relatively large differences in their protein sequences, with the two most disparate transporters (SLC9A1 and SLC9A9) having only 12% amino acid identity [292]. Despite these differences in sequence however, these proteins are predicted to have relatively similar architecture, with 10 to 12 TM segments and a cytoplasmic C‐terminal tail that acts as a regulatory domain [289, 293]. Although all SLC9 members are functional monomers, these proteins contain dimerization domains and some members of the SLC9 family have been shown to form homodimers as a way to increase their stability [289, 290, 294, 295]. Studies investigating the dimerization of SLC9A1 (NHE1) showed that this dimerization is critical to the proper transport function of these proteins [296]. Expression patterns of these proteins vary greatly between the 13 proteins. SLC9A1 is expressed in almost every tissue in the body, although the transporter is often localized to specialized membrane domains in tissues [297, 298]. Other SLC9 transporters are expressed in a more tissue or temporally dependent manner, like SLC9A4, which is expressed primarily in the stomach [292]. Cellular localization of these proteins varies greatly within the family as well, with SLC9A1, SLC9A2, and SLC9A4 (NHE4) all localizing to the plasma membrane of cells [289]. SLC9A3 and SLC9A5 (NHE5) are both trafficked between the plasma membrane and endosomes [299]. SLC9A5 regulates the pH of both endolysosomes (when it is internalized) and locally at the cell membrane, which regulates cell motility, neuronal differentiation, and synaptic plasticity [300, 301]. SLC9A6 (NHE6) is primarily an endosomal transporter, although it localizes to cell membranes after certain stimuli [290]. This Na+ or K+/H+ exchanger helps regulate intraluminal pH and dysfunction of this transporter has been associated with severe neurological disorders like Christianson syndrome, X‐linked intellectual disability, and Angelman‐like syndrome [302, 303]. The transport activity of the two SLC9B proteins, SLC9B1 (NHA1) and SLC9B2 (NHA2), remains relatively unclear, although they are thought to drive Na+/Li+ counter‐transport, as well as (Na+ or Li+)/H+ transport [290, 291, 304]. Depletion of these proteins has been linked to improper sperm motility and osteoclast differentiation, respectively [290].
SLC26
The SLC26 family of transport proteins has 10 members that transport, with different specificity, a variety of anions, including Cl−, HCO3 −, sulfate, oxalate, I−, and formate [305, 306]. The transport system of this family varies, with members being characterized as channels or electrogenic/electroneutral exchangers [307]. SLC26 proteins share similar structural features, all consisting of 14 TM segments. Like SLC4 proteins, these are arranged in a 7TMIR architecture [308, 309]. These two halves have slightly different functional roles, with one being the core, substrate‐binding domain while the other acts as an elongated gate domain shielding the core binding domain [273]. As with all other 7TMIR proteins, SLC26 family members form dimers in the membrane, although the exact physiological role for dimerization is not yet clear [308].
These proteins are of particular importance in the gastrointestinal tract, where they regulate pH, moderate water absorption, and the absorption and secretion of specific substrates [310]. For example, SLC26A1 and SLC26A2 provide the largest source of SO4 2− uptake in the intestine, localizing to the basolateral and apical membranes of cells, respectively [306, 311]. SLC26A3 meanwhile is a Cl−/HCO3 − transporter that is critical to the formation of mucus membranes and epithelial tissues [312, 313, 314]. The role of SLC26 proteins in the gut environment has also implicated these proteins in the composition of the microbiome [315, 316].
pH regulation and cancer
Aberrant regulation of pH in cells has long been linked to the development and progression of cancer [317, 318, 319]. The ability of cell to regulate their pH balance is critical to maintaining a healthy metabolic balance in cells. The regulation of pH can also be weaponized as a way to treat cancerous cells in a more targeted fashion [317]. Understanding the role these transporters play in the development and progression of cancer could potentially unveil vulnerabilities allowing for more targeted treatments. SLC9A1, SLC4A7, and SLC26A3 are particularly well‐studied examples of proton or bicarbonate transporters having an influence on cancer development and progression. SLC9A1, as the primary proton transporter of cells, has been extensively studied as the target of cancer therapeutics [320, 321]. In brief, the over activity of SLC9A1 leads to both cellular alkanization and the acidification of the microenvironment around cells. These changes in cellular state and environment result in increased cellular proliferation and loss of cell–cell contact, which increases the possibility of cellular migration, invasion, and metastasis [322]. SLC9A1 and SLC4A7 have been suggested to work in conjunction to control changes in pH and thus act as cell cycle regulators [323]. SLC4A7 also contributes to the progression of cancer by driving the efflux of acidic waste products that are by‐product of increased cellular metabolism, a hallmark of carcinogenesis [324]. SLC26A3 is another bicarbonate transporter studied in the context of cancer, as its expression is often downregulated in adenomas [325]. However, its specific role in relation to cancer development is not clear [326]. In healthy gut cells, this transporter has been found to interact with tight junction proteins and play a role in protecting the epithelial barrier, suggesting that this transporter may play a role in cellular growth or motility [312, 327].
Transport of calcium ions
The Ca2+ ion is a critical signaling compound in cells, such as playing a role in neuronal action potentials, the restructuring of cytoskeletal components and cell death. In order to lower the intracellular concentration of Ca2+ after a signaling event that resulted in an influx of free Ca2+, cells rely on two different SLC families of proteins to return to their baseline levels: SLC8 and SLC24 (Fig. 4B) [328]. Another significant source of calcium transport is provided by ATPase pumps, but will not be covered here [329].
SLC8/SLC24
The SLC8 family of proteins includes four Na+/Ca2+ exchangers that consist of 10 TM domains, binding Ca2+ ions with a large cytosolic loop between TM5 and TM6 [330, 331, 332, 333]. These proteins are widely expressed throughout the human body and restore calcium concentrations after a signaling cascade triggered either the release or uptake of this ion [334]. With a transport stoichiometry of 3 Na+ : 1 Ca2+, these proteins perform electrogenic transport, with the direction of transport depending on the membrane potential, as well as the concentration gradient of both Na+ and Ca2+ [330, 335]. Due to this critical role in cellular signaling and homeostasis, the SLC8 family has been implicated in different diseases, such as increased invasion of carcinomas, diabetes, and aberrant cardiac signaling [336, 337, 338, 339]. The four characterized members of this family are SLC8A1 (NCX1), SLC8A2 (NCX2), SLC8A3 (NCX3), and SLC8B1 (NCLX) [330]. SLC8 proteins are regulated by the binding of Ca2+ and Na+ ions [330]. A critical component of SLC8 structure, calcium‐binding domains (CBDs) have differing roles in these transporters [332]. For example, in SLC8A1, CBD1 serves as the primary Ca2+ sensor and activates the protein while the second CBD stimulates higher Ca2+ flux when unbound intracellular calcium concentrations are at higher levels [332]. This regulation of SLC8 proteins allows cells to precisely respond to calcium concentrations by changing protein localization and function in cells [340, 341].
The SLC24 family of proteins contains 5 different K+‐dependent Na+/Ca2+ exchangers [342]. SLC24 uses the electrochemical gradients of both Na+ and K+ to drive the efflux of Ca2+ [343]. These proteins are expressed in many different tissue types, although their roles are best understood in photoreceptor, neuronal, and smooth muscle cells [342, 343]. Both SLC24A1 (NCKX1) and SLC24A2 (NCKX2) have been shown to transport these ions at a stoichiometry of 4 : 1 : 1 (Na+ : Ca2+ : K+) [342]. A recent study showed that SLC24A1, SLC24A2, SLC24A3 (NCKX3), and SLC24A4 (NCKX4) all localize to the plasma membrane [344]. This study also suggested that these proteins all share similar topology, consisting of two sets of five‐TM domains connected by a large intracellular loop [344].
Transport of calcium ions in cancer
Among its many roles, calcium signaling is involved in angiogenesis (the generation of new blood vessels), neuronal signaling, and cell migration and can be altered in some cells, resulting in adaptive advantages over healthy cells and thereby promoting carcinogenesis [345, 346]. SLC8A1 has been shown to play a role in angiogenesis [346, 347]. As sodium and calcium concentrations change, this transporter has its transport direction reversed, resulting in ERK1/2 activation and subsequent enhanced angiogenesis [347]. Interestingly, SLC8A2 has been shown to act as a tumor suppressor in the brain, as increased expression of the transporter inhibits angiogenesis and slows tumor growth and invasion [348]. Lower expression of SLC8A1 has also been detected in certain cancers, and the resulting decrease in intracellular calcium consequently suppresses apoptosis [349]. SLC24A4, as well as SLC8A3, has been shown to be overexpressed in therapy‐resistant ovarian carcinoma cells [350]. However, the specific role that SLC24 proteins may play in carcinogenesis remains unclear.
Transport of Inorganic Ions by SLC12 transporters
In this section, SLC12 family is reviewed, a family of SLC proteins that transport ions closely related to cell volume regulation. These proteins drive the transport of Cl− with either Na+ and/or K+, resulting in electroneutral transport (Fig. 5A). The flux of K+ and Cl− ions and the resulting movement of water across the membrane largely regulate changes to cell volume and concurrent cell movement [351].
Fig. 5.
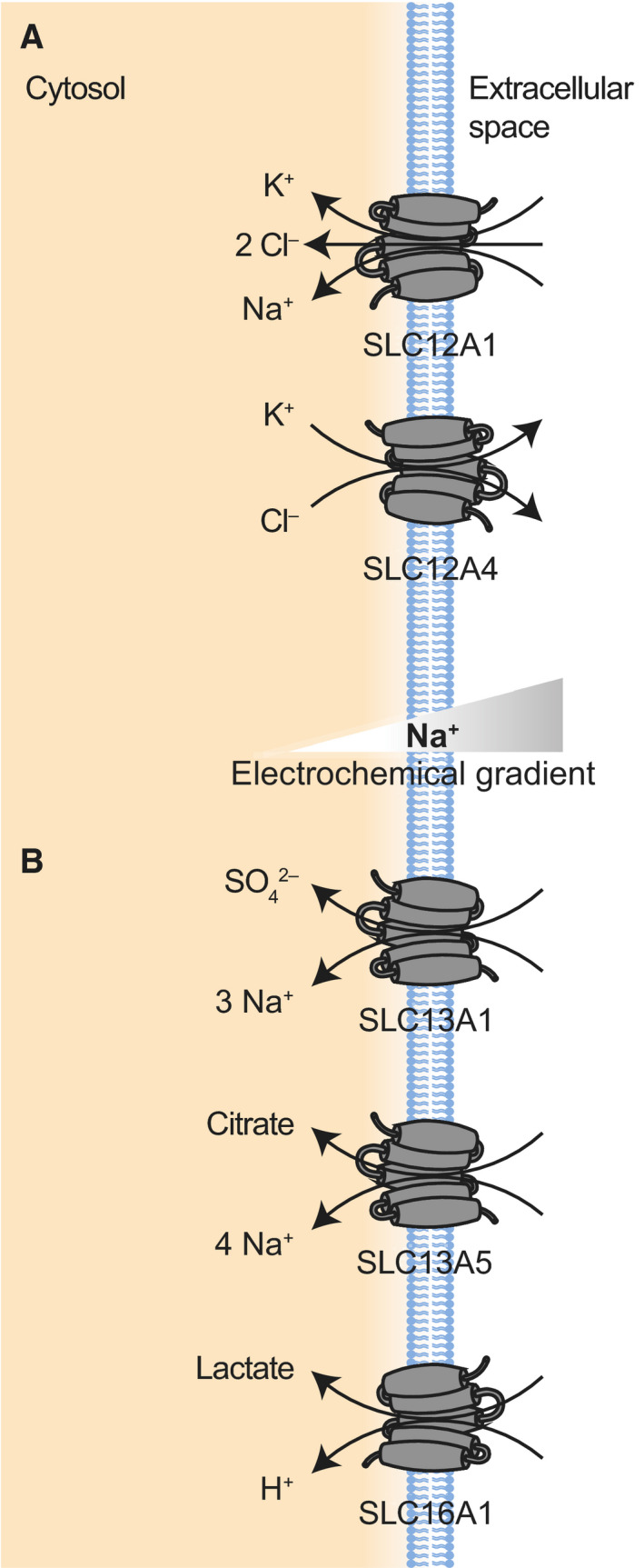
(A) Examples of SLC proteins transporting inorganic ions (SLC12). SLC12A1 represents the subfamily of Na+‐dependent cation‐Cl− cotransporters (NCC), while SLC12A4 represents the subfamily of Na+‐independent cation‐ Cl− cotransporters (KCC). (B) Examples of SLC proteins transporting carboxylates (SLC13, and SLC16). SLC13A1 represents the subfamily of Na+‐dependent sulfate transporters (NaS), while SLC13A5 represents the subfamily of Na+‐dependent di‐ and tricarboxylate transporters (NaDC). SLC16A1 is shown as an example of a monocarboxylate transporter (MCT).
SLC12
The SLC12 family contains 9 proteins, which encode for electroneutral cation‐chloride cotransporters [352, 353]. This family includes two main subfamilies: transporters that are sodium‐dependent and those that are sodium‐independent [353]. These proteins have a common structure, consisting of 12 TM segments with intracellular N and C termini [352]. There are three sodium‐dependent cotransporters—SLC12A1 (NKCC2), SLC12A2 (NKCC1), and SLC12A3 (NCC)—and four sodium‐independent cotransporters, known as the Na+‐independent K‐Cl cotransporters (KCC)—SLC12A4 (KCC1), SLC12A5 (KCC2), SLC12A6 (KCC3), and SLC12A7 (KCC4) [55].
SLC12 transport proteins in cancer
In tumors like gliomas, SLC12A2 takes up Cl−, creating the energetic driving force for the resulting volume increase through water uptake [351]. In addition to regulating cell volume, SLC12A2 impacts cellular migration, with higher expression of this transporter resulting in greater tumor growth, invasive phenotypes, and overall poorer clinical outcomes [354]. SLC12A6 and SLC12A7 are both upregulated in cancer, developing a more invasive phenotype of tumors [355]. Increased SLC12A6 expression plays an important role in triggering the epithelial–mesenchymal transition by downregulating E‐cadherin/β‐catenin complex formation and weakening cell–cell association, while SLC12A7 overexpression results in increased filopodia formation and can act as a cytoskeletal scaffold allowing for higher rates of invasive migration [355, 356]. Thus, the increased expression of these transport proteins results in more aggressive and invasive behavior [355, 356].
Transport of carboxylates
SLC13 and SLC16 are the primarily drivers of carboxylate transport in cells (Fig. 5B). The transport of these substances is critical to regulating cellular volume and maintaining sufficient energy production.
SLC13
The SLC13 family of proteins contains five members that perform Na+‐dependent, electrogenic transport of anionic substances like sulfates and di‐ and tricarboxylates used in the Krebs cycle (e.g., succinate, citrate, α‐ketoglutarate) [357]. These transporters are expressed throughout the body in different kinds of tissue, with the highest expression of these proteins in the kidney, liver, small intestine, placenta, or brain [357]. SLC13 proteins transport their substrates with a stoichiometry of 3 Na+ : 1 substrate. This family can be separated into two subfamilies with distinctly different substrates: the sulfate transporters (SLC13A1 [NaS1] and SLC13A4 [NaS2]) and the di‐ and tricarboxylate transporters (SLC13A2 [NaDC1], SLC13A3 [NaDC3], and SLC13A5 [NaCT]) [358].
SLC13A1, which localizes to the apical membrane of brush‐border membrane in the epithelial cells of the kidney, directs the reabsorption of 90% of filtered sulfates [359, 360]. SLC13A4 is expressed at lower levels throughout the body and at higher levels in the placenta and the brain [361]. Its functional role in tissues is not yet fully understood [357].
The SLC13 di‐ and tricarboxylate transporters regulate and maintain the proper levels of citric acid cycle intermediates like succinate, citrate, and malate in cells [362] and have hence been considered as potential drug targets in metabolic disorders [358, 362]. SLC13A2 is expressed at the apical membrane of renal proximal tubule cells and small intestine cells, which it absorbs all major dicarboxylate ions [357, 363]. This protein plays a major role in facilitating cell’s oxidative metabolism by taking up citric acid cycle intermediates [357]. SLC13A3 transports the same substrates as SLC13A2 across the basolateral membrane of kidney cells, although at higher affinities [358]. SLC13A5 is a Na+‐dependent transporter that, unlike the other members of the SLC13 family, transports 4 Na+ ions with each substrate molecule [364]. This protein drives the transport of primarily citrate and, at a lower affinity, succinate across the plasma membrane of cells [357]. This protein is most highly expressed in liver and brain tissues [358].
SLC16
The SLC16 family of proteins contains 14 transport proteins that are also known as monocarboxylate transporters (MCTs) [365]. The characterized members of the SLC16 family passively transport protons and different groups of monocarboxylates across the cell membrane [366, 367]. These proteins are predicted to be MFS structure proteins, consisting of 12 TM helices with intracellular C and N termini [368]. SLC16A1, 16A8, and 16A3 (MCT1, 3, and 4, respectively) all interact with a chaperone protein, CD147 (basigin), in order to properly translocate to the cell membrane, where the transporter and chaperone remain closely associated [369, 370]. SLC16A7 (MCT2) requires a different chaperone, gp70 (embigin), to properly localize to the plasma membrane [371].
SLC16A1, SLC16A7, SLC16A8, and SLC16A3 can all perform either efflux or import of their substrates depending on the concentration gradients of their substrates and the pH gradient across the membrane [367]. Of these four proteins, SLC16A1 and SLC16A3 are two proteins that are closely linked in both their function and role in diseases, in particular in cancer [372]. SLC16A1 is expressed at the plasma membrane in most tissues of the body, where it provides proton‐coupled passive transport of lactate, pyruvate, and ketone bodies [368]. SLC16A3, on the other hand, is expressed primarily in tissues that rely heavily on glycolysis as a source of energy and transports both lactate and ketone bodies [368]. Within a given microenvironment, primary role of SLC16A1 and SLC16A3 within cells are lactate import and lactate export, respectively [372]. Well‐perfused cells have higher expression of SLC16A1, which imports lactate, helping fuel oxidative phosphorylation. Poorly perfused cells are in more hypoxic environments and rely on glycolytic pathways to generate energy and express SLC16A3 to export lactate [373].
SLC16A7 is expressed throughout the body, with high expression levels in the liver, kidneys, testis, and brain [367]. This protein localizes to the plasma membrane where it functions as an importer of lactate, although it can also transport other monocarboxylates like pyruvate or ketone bodies [374]. Among all family members, SLC16A7 has the highest affinity for monocarboxylates [375].
Unlike the more widely expressed family members, SLC16A8 is expressed primarily in retinal pigmented epithelial cells and choroid plexus epithelial cells where it transports protons and lactate, potentially inhibiting photoreceptor signaling [376, 377]. SLC16A2 (MCT8) is expressed in various tissues and is a very specific high‐affinity thyroid hormone (TH) transporter [378]. SLC16A10 (MCT10) shares high amino acid similarity (58%) to SLC16A2, but functions as a sodium‐independent transporter of aromatic amines and localizes to the basolateral membrane [379, 380, 381].
Transport of carboxylates in cancer
In cancerous cells, the transport of carboxylates is often dysregulated, driving more invasive phenotypes or enabling metabolic changes [131, 351].
Due to the increase in anaerobic metabolism, which produces large quantities of lactate, SLC16 transporters serve as intriguing therapeutic targets [373]. Both SLC16A1 and SLC16A3 protein expression is modulated in cells via CD147, a cell surface glycoprotein often overexpressed in cancers that stimulate cellular proliferation, invasiveness of cancer cells, and angiogenesis [373, 382]. As detailed above, the two transporters work in conjunction and thus increased expression of these transporters does not usually happen in the same cells. In fact, targeting this relationship using SLC16A1 inhibitors to prevent lactate‐based respiration slowed tumor growth and made cancer cells more sensitive to irradiation [383].
SLC16A7’s subcellular localization has been shown to change in cancerous tissues, where there is a dramatic decrease in plasma membrane expression [384]. SLC16A7 was found to instead localize to peroxisomes, where it may increase β‐oxidation levels and contribute to malignant transformation [385]. A recent study showed that SLC16A7 is directly involved in forming the premetastatic niche of breast cancer by inducing the activity of collagen hydroxylase enyzmes, allowing for collagen remodeling [386].
Transport of phosphate
Inorganic phosphate is a substrate critical to many cellular metabolites such as nucleic acids and phospholipids and is central to metabolic processes like energy production [387]. The transport of this critical anion is driven by two SLC families, SLC20 and SLC34, which are reviewed in the following section (Fig. 6A).
Fig. 6.
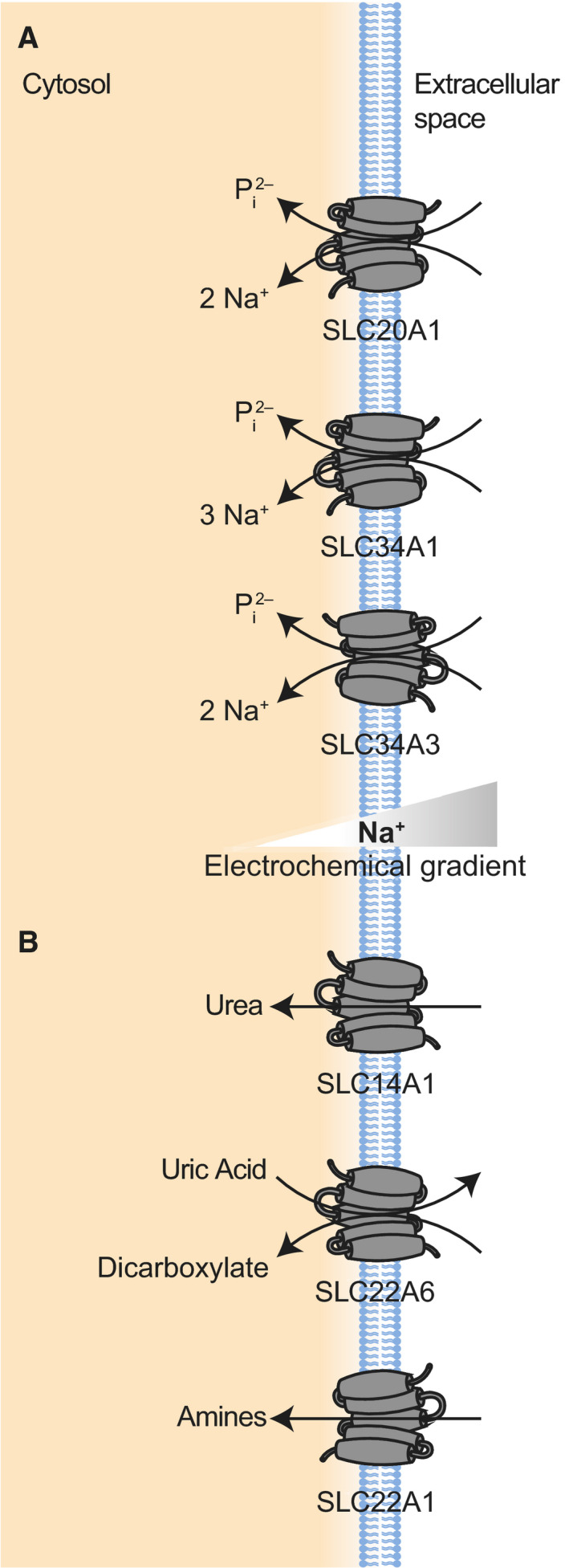
(A) Examples of SLC proteins transporting phosphate (SLC20 and SLC34). SLC20A1 is shown as an example of a Na+‐dependent transporter of inorganic phosphate (Pi). SLC34A1 represents the subfamily of electrogenic Na+‐dependent phosphate transporter, while SLC34A3 is the electroneutral Na+‐dependent phosphate transporter. (B) Examples of SLC proteins transporting organic ions (SLC14 and SLC22). SLC14A1 is shown as an example of the SLC14 family of urea transporters. SLC22A6 represents the organic anion transporter (OAT) major clade of SLC22 proteins, while SLC22A1 represents the organic cation transporter (OCT) major clade of proteins.
SLC20
The SLC20 family contains only two proteins, SLC20A1 (PiT‐1) and SLC20A2 (PiT‐2), that are both Na+‐dependent transporters of inorganic phosphate (Pi) [388]. SLC20 proteins preferentially transport monovalent Pi ions with a transport stoichiometry of 2 Na+ : 1 Pi [389]. These proteins are predicted to consist of 12 TM segments, with extracellular N and C termini, and are expressed at the cell membrane of most tissues [388, 390]. Of note, both SLC20 proteins, through their role as phosphate transporters, also play a role in bone mineralization [391, 392, 393]. The expression of these transporters is inducible, with lower extracellular Pi concentrations resulting in an increase in expression of these proteins [394, 395]. A recent study also suggested that these transporters play a role in sensing and responding to extracellular levels of Pi independently of their transport functions [396].
SLC34
The SLC34 family contains three proteins that couple the transport of Pi with Na+ [388]. The electrogenic SLC34A1 (NaPi‐IIa) and SLC34A2 (NaPi‐IIb) use three Na+ ions to drive the transport of divalent Pi against its concentration gradient, while the electroneutral SLC34A3 (NaPi‐IIc) only uses two Na+ ions [397]. These proteins have 8 TM domains with both N and C termini located in the cytoplasm [398]. Furthermore, these proteins tend to form dimers in the membrane, although they remain functionally independent [398]. These proteins play a critical role in maintaining proper levels of Pi in serum, which can cause severe hypo‐ or hyperphosphatemic states when Pi is dysregulated [399]. These clinical presentations have been linked to complications like cardiovascular diseases and higher mortality in patients with kidney disease [399, 400]. Both SLC34A1 and SLC34A3 are expressed primarily in the kidneys, where they localize to the brush‐border membrane of renal proximal tubule cells [397]. In mice, SLC34A1 provides about 70% of the total renal Pi reabsorption while SLC34A3 performs the remaining 30% [401]. Phosphate balance is maintained by parathyroid hormone (PTH)‐triggered endocytosis of SLC34A1 [402]. Although SLC34A3 internalization is similarly induced by PTH, the response is markedly slower than that of SLC34A1 [403]. SLC34A2 is expressed in many different organs, such as the small intestine, where it provides over 90% of the sodium‐dependent cellular absorption of phosphate [397, 398, 404].
Phosphate transport in cancer
Cancerous cells that are undergoing rapid cell proliferation require a higher amount of phosphate than healthy cells, imparting particular importance to the SLC20 and SLC34 families. Overall, tumor cells both express higher levels of phosphate cotransporters and have higher intracellular stores of inorganic phosphate [405]. SLC20A1 and SLC34A2 have been identified as particularly relevant for the development and progression of cancer [387]. SLC20A1 protein expression has been positively correlated with tumor size, invasiveness of tumor, and reoccurrence of tumors in somatotroph adenomas [406]. In addition to providing import of inorganic phosphate ions, SLC20A1 has been found to also have a transport‐independent role in cells [407, 408]. This potential transceptor plays a role in cellular proliferation and regulating TNF‐induced apoptosis in cell, suggesting that SLC20A1 may have a more elaborate role in the progression of cancer [407, 408]. SLC34A2 expression is similarly correlated with the tumorigenesis and development of cancers, with higher expression of the transporter serving as a prognostic marker for larger tumor size and poorer patient outcomes [409]. Knockdowns of SLC34A2 expression results in decreased cellular proliferation and metastasis [409, 410]. Interestingly, however, a separate study investigating the transporter’s role in lung cancers found that SLC34A2 played a protective role in cells, preventing more aggressive phenotypes from developing [411]. Both SLC20A1 and SLC34A2 have been implicated in activation of the Wnt/β‐catenin signaling pathway resulting in cellular proliferation and carcinogenesis [406, 412].
Transport of organic ions
SLC families 14 and 22 mediate the transport of different organic ions, including urea, amines, bile acids, and α‐ketoglutarate (Fig. 6B). Owing to this wide range of substrates, these two families play a significant role in a variety of processes within the human body, including helping regulate blood pressure and remote signaling between organs. SLC14 is one of the smallest SLC families while SLC22 is one of the largest.
SLC14
Although the SLC14 family contains only 2 distinct genes, SLC14A1 and SLC14A2, these genes are spliced into a total of eight different isoforms that perform different functions within cells [413]. SLC14A1 encodes for transporter proteins also known as UT‐B1 and UT‐B2, while SLC14A2 encodes for proteins known as UT‐A1 through UT‐A6 [414]. The primary function of these transporters is to concentrate urea in the kidneys, allowing for the excretion of urea and also reabsorption of water through passive movement of urea across cell membranes [413]. The majority of these transporters consist of 10 transmembrane segments with intracellular N and C termini [413]. Based on the crystal structure of a bacterial homologue, the selectivity of these transporters is suggested to be driven by a group of phenylalanines surrounding the transport pore [31]. A characteristic property of this family of transporters is their rapid regulation by vasopressin, a hormone synthesized in the hypothalamus [415]. In response to vasopressin signaling, transporters increase their permeability to urea, resulting in increased water resorption and thus increased blood volume, which in turn increases arterial blood pressure [415].
SLC22
The SLC22 family is one of the larger families of SLC transporters, containing roughly 30 members, 13 of which have been localized to plasma membrane [416, 417, 418]. The proteins within this family transport a wide variety of metabolites and signaling molecules with different affinities, resulting in different physiological functions for this family [418, 419]. As examples, substrates include urate, prostaglandins, bile acids, α‐ketoglutarate, β‐hydroxybutyrate, and various amines [416]. SLC22 proteins have been grouped into two major subfamilies (referred to as clades) and based on their phylogenic history in multiple different subclades [420]. The two major clades are called the organic anion transporter (OAT) major clade and the organic cation transporter (OCT) Major clade, both of which are further subdivided into subclades resulting in six final groupings [416]. These groupings are defined by their evolutionary relatedness, apparent common ancestral genes and/or sequence homology [416]. The OAT Major clade is subdivided into the Oat (exemplified by SLC22A6 (OAT1)), Oat‐like (exemplified by SLC22A13 (OAT10)), and Oat‐related (SLC22A17 (BOCT1)) subclades. The OCT Major clade is subdivided into the Oct (exemplified by SLC22A1 (OCT1)), Octn (exemplified by SLC22A5 (OCTN2)), and Oct‐related (exemplified by SLC22A16 (FLIPT2)) subclades [416]. These proteins are predicted to all have similar structural features, consisting of 12 TM domains and have been found to form homo‐oligomers in some cases, although this oligomerization process does not seem to be required for transport function [418, 421].
SLC22A6 is a sodium‐independent organic anion/dicarboxylate exchanger and is one of the most highly expressed proteins in the adult kidney [418]. In the kidney, this protein localizes to the basolateral membrane of the proximal tubules, where it excretes urate, other endogenous anions and anionic drugs [418, 422]. As a urate transporter, this and several other members of the SLC22 family (particularly SLC22A8 but possibly also SLC22A11 and A13) are thought to be involved in remote signaling between the kidney and intestine by regulating uric acid levels in the blood [423, 424]. This transporter has been implicated in the transport of drugs such as nonsteroidal anti‐inflammatory drugs, and differential activity of the protein has been implicated in the efficacy of imatinib in chronic myeloid leukemia patients [425, 426].
SLC22A13 is expressed in the kidneys, as well as in the brain, heart, and intestine, where it localizes to the basolateral membrane [427, 428]. This transporter drives the unidirectional efflux of organic anions like nicotinate, aspartate, and glutamate [427, 428].
SLC22A17 assists in the uptake of iron and does not seem to drive the transport of other usual substrates of the SLC22 family [429]. It also acts as a receptor for lipocalin‐2, a secreted protein that can trigger apoptosis in cells with reduced intracellular iron concentrations [430]. Furthermore, SLC22A17 binds to and mediates the endocytosis of filtered proteins in the kidneys [431].
SLC22A1 is a cation transporter that operates by a mechanism of facilitated diffusion [417]. This protein is expressed throughout the body in epithelial cells of different tissues, at its highest in the liver, kidney, and small intestine, where it localizes to the plasma membrane [432]. SLC22A1 transports a variety of endogenous substrates, such as catecholamines, biogenic mono‐ and polyamines. Furthermore, SLC22A1 transports various drugs like opioids and xenobiotic compounds and hence plays important role in disease management [433].
SLC22A5 is a sodium‐dependent high‐affinity carnitine transporter and otherwise provides sodium‐independent transport of other organic cations, with both transport systems being electrogenic [434]. One of SLC22A5’s main functions within the body is to absorb dietary carnitine at the brush‐border membrane of the epithelial cells in the intestine. Due to its ubiquitous expression in the body, SLC22A5 allows tissues that are unable to synthesize carnitine to take it up [435]. SLC22A5 also provides the transport of organic cations like tetraethylammonium [436].
SLC22A16 is mainly expressed at the plasma membrane of the testis and at lower levels in other tissues [418]. This protein functions as a high‐affinity L‐carnitine transporter and also has transport capabilities for other organic cations like spermidine or the drugs doxorubicin and bleomycin A5 [418, 437, 438]. Similarly to SLC22A5, SLC22A16 has a modular reliance on sodium to drive its transport, as L‐carnitine is at least partially dependent on sodium, while doxorubicin transport is sodium independent [418].
Organic Ion transport in cancer
A variant of SLC14A1 (rs17674580) has been identified as a significant susceptibility gene for urinary bladder cancer [439]. The protein was also found to be expressed at significantly lower levels in lung and prostate cancers, with overexpression of the gene inhibiting further colony formation in lung squamous cell lines [440]. As a urea transporter, deficiency of this protein may cause increased DNA damage in areas where urea concentrations increase [441, 442].
As one of the largest families of solute carriers, SLC22 proteins and their transport substrates have a wide range of roles in the development and progression of cancer. Members of this family have been found to be both downregulated (SLC22A1, SLC22A2, SLC22A11) and upregulated (SLC22A3 and SLC22A18) in the tissue of pancreatic and liver cancer patients with the different transport proteins having varying effects on the long‐term survival of proteins [443, 444, 445, 446]. As organic ion transporters, the SLC22 family of proteins plays a particularly important role in the transport of drugs across cell membranes [416]. The expression of transporters like SLC22A6 is also affected by cancer treatment regiments, with methotrexate downregulating the protein [447]. Similarly, the expression of SLC22A1 is altered by imatinib, a drug used to treat chronic myeloid leukemia [448]. Both SLC22A1 and SLC22A5 expression are also downregulated in carcinomas, which results in higher tumor progression and poorer patient survival [449, 450, 451, 452].
Transport of trace metals
Although found at miniscule levels in cells, trace metals like magnesium, copper, zinc, and nickel play important roles as signaling ions and cofactors in proteins. Cells use SLC families 11, 40, 31, 30, 39, 41, and 49 to drive the influx and efflux of these components and regulate their concentrations (Fig. 7). While many of these SLCs have been studied role transporting a specific metal ion, it has become increasingly clear that these transporters have a much wider specificity to trace metals, as further detailed below.
Fig. 7.
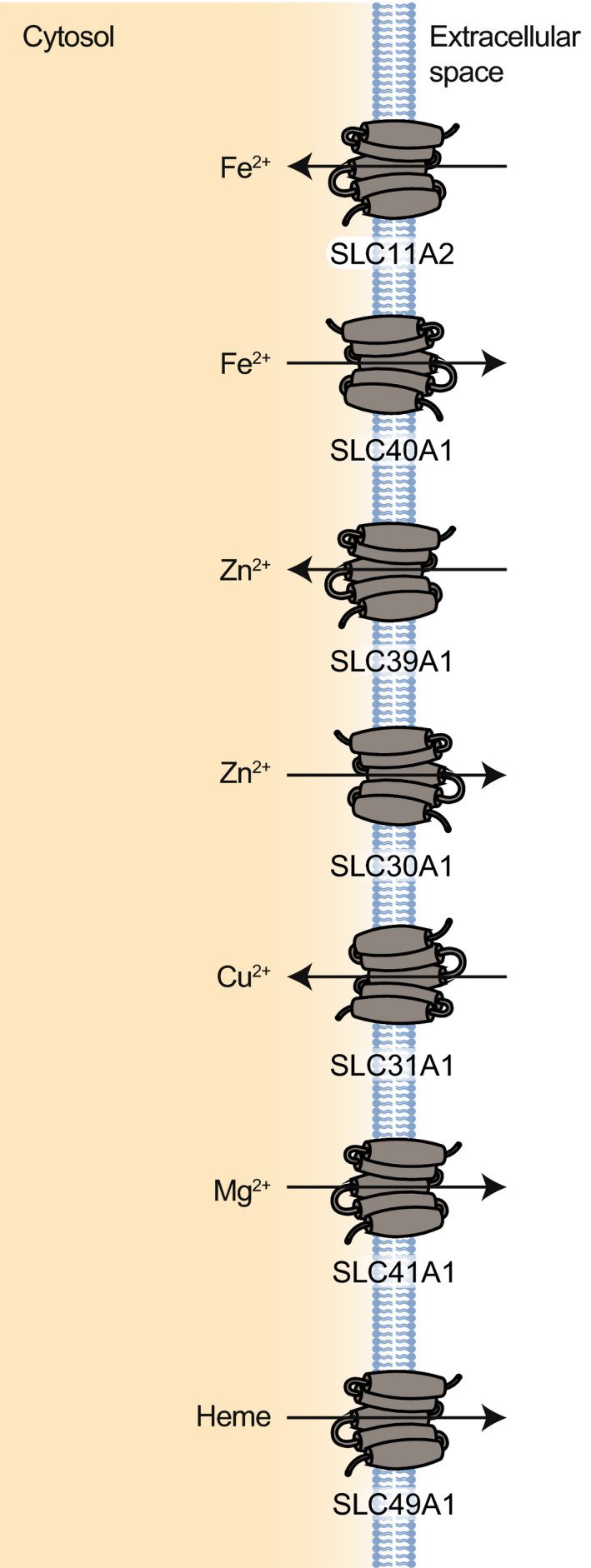
Examples of SLC proteins transporting trace metals (SLC11, SLC30, SLC31, SLC39, SLC40, SLC41, and SLC49). SLC11A2 is shown as an example of a proton‐dependent transporter of metal ions (primarily Fe2+). SLC40A1 is an iron exporter. SLC39A1 is shown as an example of a zinc importer, while SLC30A1 is shown as a zinc exporter. SLC31A1 is shown as an example of a cooper transporter. SLC41A1 is shown as an example of a magnesium transporter. SLC49A1 is shown as an example of a heme transporter.
SLC11/SLC40
The SLC11 family consists of two proteins, SLC11A1 (Nramp1) and SLC11A2 (DMT‐1), that perform the proton‐dependent transport of divalent metal ions and share 66% identity in their amino acid sequence [453]. While SLC11A1 localizes to the membrane of lysosomes and endosomes, SLC11A2 is a plasma membrane transporter [454].
SLC11A2 is expressed throughout the body, as four different cell‐type‐specific splice variants [455, 456]. Expression is found at particularly high levels at the apical plasma membrane of the duodenum, where SLC11A2 is responsible for the absorption of iron [456]. Apart from iron, this protein transports other divalent metal ions (Zn2+, Mn2+, Cu2+, Co2+, Cd2+, Ni2+, and Pb2+), with highest affinity for Mn2+ [457, 458]. Transport is both proton and membrane potential dependent [457]. In order to avoid the release of free ferrous iron into the cytosol, SLC11A2 has been shown to interact with PCBP2 (poly(rC)‐binding protein 2), which removes the iron from the transporter through the formation of a PCBP2‐SLC11A2 complex [60, 455, 459].
The SLC40 family contains only one member, SLC40A1 (FPN1) that functions as an iron exporter [453, 460]. This protein is expressed throughout the body and serves as the major iron efflux pathway [453]. Similarly to SLC11A2, SLC40A1 is also capable of driving Mn2+ transport, suggesting these proteins also work in conjunction to maintain manganese homeostasis [461]. Thus, the SLC11 family primarily transports iron into cells, while SLC40 transports iron out of cells. This is exemplified in enterocytes, where SLC11A2 absorbs dietary iron from the intestine and SLC40A1 facilitates basolateral exit of iron into the circulatory system [453].
SLC30/SLC39
Cells have developed two primary transport mechanisms through which zinc homeostasis is regulated. The SLC30 family functions to decrease cytosolic zinc levels while SLC39 proteins increase concentrations [462, 463]. The SLC30 family of zinc transporters contains 10 members that are expressed throughout the body [463, 464]. With the exception of SLC30A1, these proteins are primarily expressed intracellularly, driving the compartmentalization of zinc, although some members are occasionally translocated to the plasma membrane [463, 465, 466]. SLC30A1 (ZnT1) is expressed at the plasma membrane, where it drives the efflux of zinc into extracellular space. The expression of this transporter is tightly regulated, with both expression and plasma membrane localization increasing in response to higher zinc concentrations in the cytoplasm [467, 468].
SLC39 is a family of 14 proteins that are primarily responsible for transporting zinc across membranes and into the cytoplasm [469]. Although a transport mechanism has not been conclusively determined, kinetics studies suggest that these proteins drive their transport through a secondary active mechanism [462]. However, the driver of this secondary transport remains unclear and has ranged from bicarbonate to protons [470]. In general, these proteins contain between 7 and 9 TM segments with both N and C termini facing away from the cytoplasm, although there is some variation [462, 471]. The SLC39 family is separated into 4 subfamilies based on amino acid sequence similarities [472]. The first subfamily, subfamily I, contains only one protein, SLC39A9 (ZIP9). Subfamily II contains SLC39A1 (ZIP1), SLC39A2 (ZIP2), and SLC39A3 (ZIP3) [469]. The third, and largest with nine members, subfamily is referred to as LIV‐1 and is exemplified by SLC39A4 (ZIP4) [469]. The last family is called gufA and contains only SLC39A11 (ZIP11) [469]. Due to zinc’s role as a secondary messenger molecule, the SLC39 family has also been implicated in signaling pathways, like the PTEN‐Akt signaling axis [473, 474]. SLC39A1 is expressed throughout the human body, and primarily localizes to the plasma membrane [469]. In response to changing zinc concentrations, SLC39A1 can be translocated from intracellular organelles to the plasma membrane or vice versa [475, 476].
SLC39A4 is expressed mainly in the intestine, where its expression and localization is tightly regulated by both transcriptional and post‐transcriptional means [477]. There, SLC39A4 functions mainly to absorb dietary zinc [477].
Thus, as a general rule, the SLC30 and SLC39 families have complementary roles, similar to SLC11 and SLC40. SLC30 proteins transport zinc out of the cytosol, either into organelles or extracellular space, while SLC39 proteins transport zinc into the cytosol [462].
Other SLC families transporting trace metals (SLC31, SLC41, and SLC49)
The SLC31 family contains two copper transporters, SLC31A1 (CTR1) and SLC31A2 (CTR2) [478]. These proteins are expressed ubiquitously and at highest levels in the liver and placenta [479]. SLC31A1 is primarily expressed at the cell membrane and drives the uptake of copper from food in a potassium‐dependent manner [478, 480]. Copper homeostasis is critical for cells, as many enzymes are dependent on it for proper function [481].
The SLC41 family contains three proteins that transport magnesium [482, 483]. SLC41A1 is the best characterized member of the family and is expressed throughout the body [482]. This protein drives the efflux of Mg2+ and also to a lesser degree Fe2+, Zn2+, Cu2+, Co2+, and Cd2+, across the plasma membrane of epithelial cells [484, 485]. The expression and localization of this protein are regulated by extracellular Mg2+ concentrations, which regulates its transport function through endosomal recycling mechanisms allowing for dynamic regulation of the ion [482, 486].
SLC49 is a family of four proteins, two of which, SLC49A1 and SLC49A2, contribute to heme transport across cell membranes [487]. SLC49A1 functions as a heme exporter, protecting cells from heme toxicity [488]. It is expressed at highest levels in the liver and small intestine, where there are higher levels of heme transport and localizes to the plasma membrane of these cells [487].
Transport of trace metals in cancer
Trace metals like magnesium, copper, zinc, and nickel are critical components of enzymes, regulating cellular signaling pathways, DNA replication and repair, apoptosis and much more [489, 490, 491, 492, 493]. Maintaining a proper concentration of these trace metals is important to not only these processes, but also combating the formation and/or accumulation of free radicals [490, 492].
SLC40A1, which as mentioned mediates iron efflux from cells, has been linked to poorer prognoses in both breast cancer and adrenocortical carcinoma [494, 495]. Furthermore, a recent study showed that the downregulation of SLC40A1 results in greater cell proliferation [496, 497]. This downregulation of SLC40A1 by Nrf‐2 has also been shown to induce cisplatin resistance, although this was reversed by increasing iron concentrations within cells [498]. This suggests that iron metabolism plays a large role in determining drug effectiveness.
Mutations, dysregulation, or other perturbations of zinc transporters have been linked to many different diseases [499]. As the cofactor for so many enzymes, zinc deficiency is particularly detrimental to cells and is heavily linked to the development of many different cancers [500]. For example, SLC39A4 was recently shown to be overexpressed in more aggressive types of ovarian and pancreatic cancer, possibly as a response to lower levels of zinc in cancer tissues [501, 502]. For more comprehensive reviews of zinc transport in cancers, the reader is referred to Takatani‐Nakase and Pan et al. [500, 503].
Copper accumulation has been linked to the progression of different cancers and their severity [492]. The knockdown of SLC31A1, and resulting decrease in intracellular copper, has been shown to inhibit the progression of pancreatic cancer [504]. SLC31A1 is also particularly relevant to the toxicity of cisplatin and other similar drugs, as they use this copper transporter as a system to enter cells [462].
Magnesium is required for cell proliferation, membrane stability, and DNA repair/genomic stability [505, 506]. Furthermore, reduced intracellular concentrations have been linked to a suppression or inhibition of Akt/mTOR signaling [505]. SLC41A1, the magnesium efflux transporter, thus acts as a tumor suppressor, with increased expression of the transporter reducing the proliferative effects of mTOR signaling while also helping drive bax‐associated apoptosis [507].
Transport of other organic compounds
The final two SLC families to be covered in this review are SLC10 and SLCO (formerly known as SLC21) (Fig. 8). These two families transport a wide array of organic compounds, including numerous drugs. As transporters of such diverse substrates, members of the SLC10 and SLCO families have wide‐ranging physiological roles in cells.
Fig. 8.
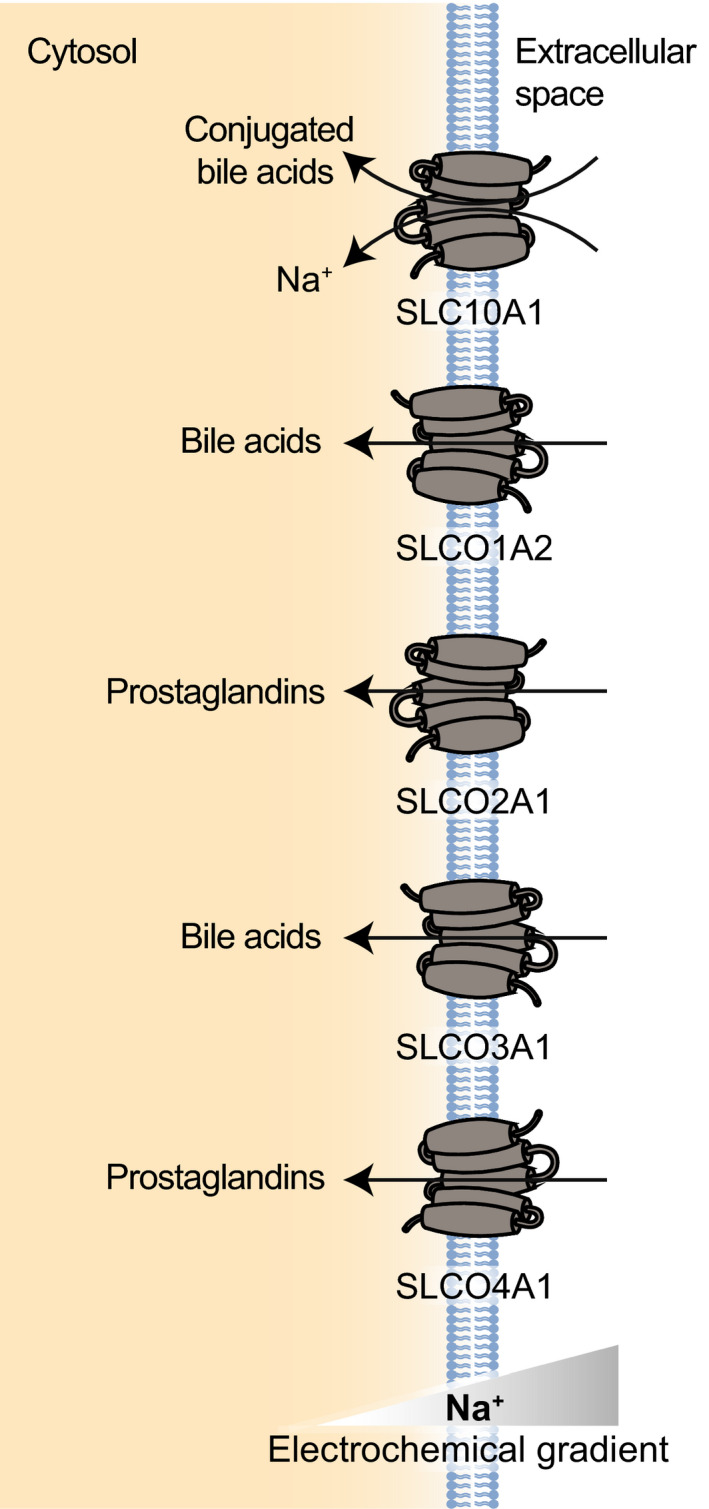
Examples of SLC proteins transporting other organic compounds (SLC10 and SLCO). SLC10A1 is shown as an example of the SLC10 family that transports conjugated bile acids. SLCO1A2 represents the OATP1 subfamily and transports organic anions like bile acids. SLCO2A1 represents the OATP2 subfamily and transports prostaglandins. SLCO3A1 is the only member of the OATP3 subfamily and functions primarily as a bile acid transporter. SLCO4A1 represents the OATP4 subfamily and transports various organic compounds like prostaglandins.
SLC10
The SLC10 family of proteins contains seven members. For a time, it was thought that this family’s primary purpose was to transport bile acid (BA), a family of substances that play a critical role in the solubilization and/or absorption of different nutrients like vitamins and cholesterol as well as different signaling cascades, and endocrine homeostasis [508, 509]. However, it is now clear that these proteins transport a wider variety of molecules, including steroidal hormones, drugs, and other substrates [510].
The best‐studied members of this family, SLC10A1 and SLC10A2, play key roles in the regulation of BA levels in the body, helping reabsorb secreted BAs in the intestine and removing BAs from the liver’s portal circulation [510]. Both SLC10A1 and SLC10A2 have been found to use homo‐ and heterodimeric and higher order oligomers as functional units on the cell membrane [511, 512]. Due to the cytotoxic nature of BAs, both the expression and activity of these two proteins are regulated by their substrates, through the nuclear receptor like farnesoid X receptor [513].
SLC10A1 (NTCP) was found to be a strictly hepatocellular protein, expressed at the basolateral membrane [514, 515]. This symporter couples the uptake of taurocholate or another conjugated BA with the uptake of two Na+ ions (1 conjugated BA: 2 Na+) [516]. The uptake of these BAs is supplemented by the actions of SLCO1B1 and SLCO1B3 (see below), two transporters that uptake BAs in an sodium‐independent manner [513]. Recently, SLC10A1 has also been found to act as a receptor for human hepatitis B virus (HBV) and satellite hepatitis D virus [517, 518]. SLC10A2 (ASBT) is a protein mainly expressed in the intestine, localizing to the ileal brush‐border membrane [519]. SLC10A2 uses the sodium gradient to drive the transport of BA into the cell with a 2 : 1 Na+ : BA stoichiometry [520]. Due to its location in the final portion of the small intestine, SLC10A2’s main function is to reabsorb any BAs that were not already absorbed as a part of mixed micelles. This way, the body takes up to 95% of the BAs and minimizes the amount of BAs that must be synthesized again [512].
SLC10A6 (SOAT) is mainly expressed in the testis, where it localizes to the plasma membrane of primary spermatocytes [521]. Unlike other SLC10 members, this protein does not transport BAs, but mediates sodium‐dependent transport of sulfated steroids like taurolithocholic acid‐2‐sulfate (TLCS), estrone‐3‐sulfate (E1S), and dehydroepiandrosterone sulfate (DHEAS), compounds which can be quickly modified to create active steroids [522, 523].
SLCO
The SLCO family of proteins (formerly SLC21) mediates the transport of organic ions and are also known as organic anion transporting polypeptides (OATPs) [9]. These proteins are predicted to consist of 12 TM segments with cytoplasmic N and C termini [9]. Eleven identified SLCOs have been subdivided into six subfamilies based on amino acid sequence similarity [524, 525]. The largest is the OATP1 subfamily, which contains SLCO1A2 (OATP1A2), SLCO1B1 (OATP1B1), SLCO1B3 (OATP1B3), and SLCO1C1 (OATP1C1). The OATP2 subfamily contains two members, SLCO2A1 (OATP2A1) and SLCO2B1 (OATP2B1). OATP3, OATP5, and OATP6 all contain only one member within their families (SLCO3A1 (OATP3A1), SLCO5A1 (OATP5A1), and SLCO6A1 (OATP6A1), respectively). Finally, OATP4 contains two member proteins, SLCO4A1 (OATP4A1) and SLCO4C1 (OATP4C1) [525].
This family has become a focus of drug development and pharmacokinetics of novel drugs [526]. Expressed throughout the body, its members are important for drug absorption. Tissue distribution and polymorphisms of these transporters have a significant effect on the efficacy of different drugs such as statins, antibiotics, direct renin inhibitors, and cardiac glycosides [527, 528]. SLCO1B1, SLCO1B3, and SLCO2B1 are of particular interest, contributing to the uptake of drugs across the basolateral membrane of hepatocytes [526].
SLCO1A2 is expressed widely throughout the body, localizing to the plasma membrane of most cells [529, 530]. This protein transports a wide range of organic anions, including bile acids, steroids, thyroid hormones, and various drugs (including imatinib, statins, and methotrexate) [531]. As with other SLCO proteins, SLCO1A2 is trafficked to the plasma membrane and stabilized through its interactions with PDZK1 and NHERF1 [531, 532].
SLCO2A1 is also ubiquitously expressed in the body, localizing to the plasma membrane to transport prostaglandins [9]. This transporter is of particular importance at the blood–brain barrier, where it facilitates the transport of Prostaglandin E2, an essential component of the body temperature regulatory network [533]. SLCO2B1 is expressed at the cell membrane of many different tissues, where it drives the transport of various organic ions like taurocholate, prostaglandins, antifolates, and tyroxine [9]. The transport activity of this protein is stimulated at lower pH levels [534].
SLCO3A1 is expressed in the testis and brain and to a lower extent in the lung, spleen, peripheral blood leukocytes, and thyroid gland [535]. This transporter has two different cell‐specific splice variants. Interestingly, although expression of SLCO3A1 is generally lower in the liver, expression is elevated during cholestatic liver injury—when bile acids accumulate in the blood and liver due to impaired bile formation. There, SLCO3A1 functions as a protective bile acid efflux transporter [536, 537].
SLCO4A1 is expressed throughout the body at the plasma membrane [535]. Substrates of this transporter include prostaglandins, benzylpenicillin, taurocholate, and thyroxine [537]. SLCO4A1 is a prognostic marker for colorectal cancer, with higher expression indicating a poorer prognosis [538].
Transport of organic compounds in cancer
The transport, release, and concentration of organic compounds like bile acids, hormones, and drugs is regulated by the activity of members of the SLC10 and SLCO family [9, 508, 509]. These diverse roles result in a litany of links to the development, progression, spread, and treatment of cancer [538, 539].
Certain SLC10A2 polymorphisms more than double the risk of developing colorectal polyps, which supports the idea that aberrant transport of bile acids increases the risk of colorectal cancer [540]. Furthermore, SLC10A2 knockout mice had a significant twofold increase in the amount of colon adenocarcinomas over the control mice, as well as an increased tumor number and growth rate [541]. These contributions to carcinogenesis are thought to be caused by an increase in fecal bile acids acting as tumor promoters in the colon [539, 541]. The regulation of this transporter remains an intriguing topic, as treatment of solid tumors with irinotecan results in decreased expression of SLC10A2 [542].
The SLCO family of transporters has been increasingly investigated in the past years for their potential role in the transport of chemotherapeutics and their potential impact on efficacy of therapies [543]. This interest has particularly focused on SLCO1A2, SLCO1B1, SLCO1B3, and SLCO2B1 [525, 526, 544]. The expression of many SLCO proteins is altered (both up and down) in many different types of cancers (SLCO1A2, SLCO1B1, and SLCO1B3 tend to expressed at lower levels, while SLCO2B1 are expressed at higher levels in cancer tissue) [525]. These changes in expression patterns of the above transporters have not been fully elucidated, with a range of, at times conflicting, results being reported [525, 545]. The conflicting data on the use of SLCOs as biomarkers highlight the need for a deeper understanding of their biology, especially as expression levels seem to be associated with changes in efficacy for cancer treatments [544, 545]. For more in‐depth reviews of the role that SLCO proteins play in cancer development, progression, and treatment, the reader is referred to Schulte et al. and Obiadat et al. [525, 543].
Discussion
In this review, we have provided a guide to the main families of SLCs that transport sugars, amino acids, peptides, vitamins, nucleotides and nucleosides, different subsets of ions, metals, and organic compounds into cells across their plasma membrane.
In spite of the progress in understanding SLC functions, major gaps remain in our understanding of many members in the SLC protein superfamily. In particular, while we have focused our review on documented plasma membrane SLCs, we must note that there is incomplete understanding of the localization of SLC proteins. Furthermore, some members of this superfamily translocate to different subcellular localizations, as discussed above, or when cellular states change. For example, expression of both SLC9A2 and SLC9A3 has been shown to change in mice depending on their stage in development [546, 547].
As detailed in this review, SLC proteins play important roles in maintaining the proper function of cells and managing their relationship with the environment of specific cell types. The overlapping and redundant transport activity of different SLC proteins allows cells to rapidly respond to changes in their surroundings and maintain close control over their contents. For example, members of both SLC7 and SLC38 provide seemingly redundant transport of small amino acids. However, through ancillary roles like acting as transceptors (SLC38A2) or regulating the redox balance of cells (SLC7A11), these proteins maintain their own unique roles within cells [158, 188]. SLC34A1 and SLC34A3 are another example of the interconnected and complementary roles at the organism level, with the two transporters cooperatively ensuring complete phosphate resorption [401].
As highlighted throughout the review, members of the SLC superfamily are implicated in the development and progression of many diseases. For example, dysregulation of multiple members of the SLC6 family results in disorders related to their altered ability to transport neurotransmitters and other substrates of SLC6 proteins (e.g., mutations of SLC6A3 cause infantile Parkinsonism dystonia, SLC6A8 is linked to X‐linked mental retardation; mutations of SLC6A19 causes Hartnup disorder) [161, 548, 549, 550]. Changes in SLC protein expression and function are also closely linked to the development of diseases such as cancer. In fact, a comparison of healthy and cancerous cell lines suggested that SLC proteins have greater changes in their coexpression networks than protein kinases [28]. Proteins like SLC2A1, SLC39A14, and SLC16A1 may serve as prognostic biomarkers in cancers due to their central role in the development of cancer and responses to treatments [551, 552, 553]. Thus, it is imperative to continue studying transport proteins in a comprehensive manner, focusing not only on individual transport functions, but also unraveling the complex relationships between different transporters and metabolic states.
Furthermore, when aberrant transport of metabolites occurs, entire signaling pathways may be affected due to changing electrochemical gradients and metabolic pathways. For example, the oncogene MYC can induce the expression SLC7A5 and SLC43A1, resulting in the increased transport of essential amino acids and thus supporting increased cell growth and tumorigenesis [554]. There is a critical need to study regulatory networks such as these in order to understand the relationships between transport activities and cellular states.
In the past years, as their roles in cells have been discovered, SLC proteins have increasingly become the target of drugs [8, 90, 92]. However, the vast potential of modulating the activity of SLCs, and in particular plasma membrane expressed SLC proteins, remains largely untapped [5]. Families like SLC39 and SLC30, whose member proteins are recognized as direct regulators of zinc homeostasis, offer great therapeutic potential in combating the progression of cancer [555]. A more complete understanding of substrates, structures, transport mechanisms, interaction networks, and secondary functions within cells may contribute in the future to drug discovery programs targeting such SLCs. A family like SLC16 provides an example for the progression of this field. After understanding the molecular mechanisms that underlie SLC16 proteins and how altered transport may contribute to the development of disease states, SLC16 inhibitors were rapidly developed [556]. Currently, there are multiple potent inhibitors of SLC16 proteins that serve as potent chemical tools to further understand the role of SLC16 proteins in cells, with at least one under clinical evaluation (NCT01791595) [557, 558].
Finally, while this review covered the major classes of substrates transported by plasma membrane SLC proteins, there remain many other substances in the extracellular milieu whose transporter has not been determined or could not be described within the scope of this review. We expect that with as our understanding of the human metabolome grows, more SLCs will be reported to mediate the transport of a growing list of endogenous and exogenous metabolites, further adding to the complex network of transport proteins regulating cellular content.
Conflict of interest
The authors declare a conflict of interest. Ariel Bensimon and Giulio Superti‐Furga have filed patents on SLC pharmacology that will be the object of a commercialization effort in the future.
Author contributions
MDP, AB, and GS‐F conceived the review and wrote the manuscript.
Acknowledgements
We are thankful to all the members of the Superti‐Furga laboratory for discussions and feedback. In particular, we would like to thank Enrico Girardi, Kai‐Chun Li, Gernot Wolf, Vitaly Sedlyarov, Utkarsh Kapoor, Ruth Eichner, Ismet Srndic, Ludwig Bauer, Vojtech Dvorak, Ben Haladik, Patrick Essletzbichler, Giuseppe Fiume, and Manuele Rebsamen for reading through the manuscript and providing with editorial input. We thank the referees for their constructive comments. CeMM and the Superti‐Furga laboratory are supported by the Austrian Academy of Sciences. We acknowledge receipt of third‐party funds from the European Research Council (ERC AdG 695214 GameofGates, AB, MDP). MDP is a recipient of a Fulbright Study/Research Grant.
References
- 1. Okada Y (2004) Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem Biophys 41, 233–258. [DOI] [PubMed] [Google Scholar]
- 2. Stein WD & Litman T. Cambridge, MA: (2014) Channels, Carriers, and Pumps: An Introduction to Membrane Transport, 2nd edn. Cambridge, MA. [Google Scholar]
- 3. Yang NJ & Hinner MJ (2015) Getting across the cell membrane: An overview for small molecules, peptides, and proteins. In Site‐Specific Protein Labeling (Gautier A & Hinner MJ, eds), Vol. 1266, pp. 29–53. Humana Press Inc, Berlin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruprecht JJ & Kunji ERS (2020) The SLC25 mitochondrial carrier family: structure and mechanism. Trends Biochem Sci 45, 244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang W, Gallo L, Jadhav A, Hawkins R & Parker CG (2019) The druggability of solute carriers. J Med Chem 63, 3834–3867. [DOI] [PubMed] [Google Scholar]
- 6. Mueckler M & Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34, 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Czuba LC, Hillgren KM & Swaan PW (2018) Post‐translational modifications of transporters. Pharmacol Ther 192, 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rives ML, Javitch JA & Wickenden AD (2017) Potentiating SLC transporter activity: emerging drug discovery opportunities. Biochem Pharmacol 135, 1–11. [DOI] [PubMed] [Google Scholar]
- 9. Hagenbuch B & Stieger B (2013) The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med 34, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pedersen PL (2005) Transport ATPases: structure, motors, mechanism and medicine: a brief overview. J Bioenerg Biomembr 37, 349–357. [DOI] [PubMed] [Google Scholar]
- 11. Hollenstein K, Dawson R & Locher K (2007) Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol 17, 412–418. [DOI] [PubMed] [Google Scholar]
- 12. Beis K (2015) Structural basis for the mechanism of ABC transporters. Biochem Soc Trans 43, 889–893. [DOI] [PubMed] [Google Scholar]
- 13. Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE & Gottesman MM (2018) Revisiting the role of ABC transporters in multidrug‐resistant cancer. Nat Rev Cancer 18, 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu X (2019) ABC family transporters. In Advances in Experimental Medicine and Biology (Liu X & Pan G, eds), pp. 13–100. Springer, New York. [DOI] [PubMed] [Google Scholar]
- 15. Stewart AG, Laming EM, Sobti M & Stock D (2014) Rotary ATPases—dynamic molecular machines. Curr Opin Struct Biol 25, 40–48. [DOI] [PubMed] [Google Scholar]
- 16. Palmgren MG & Nissen P (2011) P‐Type ATPases. Annu Rev Biophys 40, 243–266. [DOI] [PubMed] [Google Scholar]
- 17. Futai M, Sun‐Wada GH, Wada Y, Matsumoto N & Nakanishi‐Matsui M (2019) Vacuolar‐type ATPase: a proton pump to lysosomal trafficking. Proc Japan Acad Ser B Phys Biol Sci 95, 261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lippe G, Coluccino G, Zancani M, Baratta W & Crusiz P (2019) Mitochondrial F‐ATP synthase and its transition into an energy‐dissipating molecular machine. Oxid Med Cell Longev 2019, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roux B (2017) Ion channels and ion selectivity. Essays Biochem 61, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bates E (2015) Ion channels in development and cancer. Annu Rev Cell Dev Biol 31, 231–247. [DOI] [PubMed] [Google Scholar]
- 21. Alexander S, Mathie A & Peters J (2011) Ion channels. Br J Pharmacol 164, S137–S174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Povey S, Lovering R, Bruford E, Wright M, Lush M & Wain H (2001) The HUGO Gene Nomenclature Committee (HGNC). Hum Genet 109, 678–680. [DOI] [PubMed] [Google Scholar]
- 23. Hediger MA, Clémençon B, Burrier RE & Bruford EA (2013) The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med 34, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fredriksson R, Nordström KJV, Stephansson O, Hägglund MG & Schiöth HB (2008) The solute carrier (SLC) complement of the human genome: phylogenetic classification reveals four major families. FEBS Lett 582, 3811–3816. [DOI] [PubMed] [Google Scholar]
- 25. Höglund PJ, Nordström KJV, Schiöth HB & Fredriksson R (2011) The solute carrier families have a remarkably long evolutionary history with the majority of the human families present before divergence of Bilaterian species. Mol Biol Evol 28, 1531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saier MH Jr (2000) A functional‐phylogenetic classification system for transmembrane solute transporters. Microbiol Mol Biol Rev 64, 354–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perland E & Fredriksson R (2017) Classification systems of secondary active transporters. Trends Pharmacol Sci 38, 305–315. [DOI] [PubMed] [Google Scholar]
- 28. César‐Razquin A, Snijder B, Frappier‐Brinton T, Isserlin R, Gyimesi G, Bai X, Reithmeier RA, Hepworth D, Hediger MA, Edwards AM et al. (2015) A call for systematic research on solute carriers. Cell 162, 478–487. [DOI] [PubMed] [Google Scholar]
- 29. Colas C, Ung PMU & Schlessinger A (2016) SLC transporters: structure, function, and drug discovery. Medchemcomm 7, 1069–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rudnick G, Krämer R, Blakely RD, Murphy DL & Verrey F (2014) The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch 466, 25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levin EJ, Quick M & Zhou M (2009) Crystal structure of a bacterial homologue of the kidney urea transporter. Nature 462, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Papadaki GF, Amillis S & Diallinas G (2017) Substrate specificity of the furE transporter is determined by cytoplasmic terminal domain interactions. Genetics 207, 1387–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krypotou E, Evangelidis T, Bobonis J, Pittis AA, Gabaldón T, Scazzocchio C, Mikros E & Diallinas G (2015) Origin, diversification and substrate specificity in the family of NCS1/FUR transporters. Mol Microbiol 96, 927–950. [DOI] [PubMed] [Google Scholar]
- 34. Papageorgiou I, Gournas C, Vlanti A, Amillis S, Pantazopoulou A & Diallinas G (2008) Specific interdomain synergy in the UapA transporter determines its unique specificity for uric acid among NAT carriers. J Mol Biol 382, 1121–1135. [DOI] [PubMed] [Google Scholar]
- 35. Forrest LR, Zhang YW, Jacobs MT, Gesmonde J, Xie L, Honig BH & Rudnick G (2008) Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci USA 105, 10338–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bai X, Moraes TF & Reithmeier RAF (2017) Structural biology of solute carrier (SLC) membrane transport proteins. Mol Membr Biol 34, 1–32. [DOI] [PubMed] [Google Scholar]
- 37. Bateman A (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47, D506–D515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi Y (2013) Common folds and transport mechanisms of secondary active transporters. Annu Rev Biophys 42, 51–72. [DOI] [PubMed] [Google Scholar]
- 39. Garibsingh R‐AA & Schlessinger A (2019) Advances and challenges in rational drug design for SLCs. Trends Pharmacol Sci 40, 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Drew D & Boudker O (2016) Shared molecular mechanisms of membrane transporters. Annu Rev Biochem 85, 543–572. [DOI] [PubMed] [Google Scholar]
- 41. Deng D, Xu C, Sun P, Wu J, Yan C, Hu M & Yan N (2014) Crystal structure of the human glucose transporter GLUT1. Nature 510, 121–125. [DOI] [PubMed] [Google Scholar]
- 42. Deng D, Sun P, Yan C, Ke M, Jiang X, Xiong L, Ren W, Hirata K, Yamamoto M, Fan S et al. (2015) Molecular basis of ligand recognition and transport by glucose transporters. Nature 526, 391–396. [DOI] [PubMed] [Google Scholar]
- 43. Arakawa T, Kobayashi‐Yurugi T, Alguel Y, Iwanari H, Hatae H, Iwata M, Abe Y, Hino T, Ikeda‐Suno C, Kuma H et al. (2015) Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science 350, 680–684. [DOI] [PubMed] [Google Scholar]
- 44. Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho C‐M, Sali A, Westhoff CM & Stroud RM (2010) Function of human Rh based on structure of RhCG at 2.1 A. Proc Natl Acad Sci USA 107, 9638–9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coleman JA, Yang D, Zhao Z, Wen PC, Yoshioka C, Tajkhorshid E & Gouaux E (2019) Serotonin transporter–ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 569, 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coleman JA, Green EM & Gouaux E (2016) X‐ray structures and mechanism of the human serotonin transporter. Nature 532, 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwede T, Sali A, Honig B, Levitt M, Berman HM, Jones D, Brenner SE, Burley SK, Das R, Dokholyan NV et al. (2009) Outcome of a workshop on applications of protein models in biomedical research. Structure 17, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM & Abramson J (2008) The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321, 810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krishnamurthy H & Gouaux E (2012) X‐ray structures of LeuT in substrate‐free outward‐open and apo inward‐open states. Nature 481, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Joseph D, Pidathala S, Mallela AK & Penmatsa A (2019) Structure and gating dynamics of Na+/Cl– coupled neurotransmitter transporters. Front Mol Biosci 6, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mikros E & Diallinas G (2019) Tales of tails in transporters. Open Biol 9, 190083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seo YA, Kumara R, Wetli H & Marianne WR (2016) Regulation of divalent metal transporter‐1 by serine phosphorylation. Biochem J 473, 4243–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sprowl JA, Ong SS, Gibson AA, Hu S, Du G, Lin W, Li L, Bharill S, Ness RA, Stecula A et al. (2016) A phosphotyrosine switch regulates organic cation transporters. Nat Commun 7, 10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gamba G (2005) Molecular physiology and pathophysiology of electroneutral cation‐chloride cotransporters. Physiol Rev 85, 423–493. [DOI] [PubMed] [Google Scholar]
- 55. Arroyo JP, Kahle KT & Gamba G (2013) The SLC12 family of electroneutral cation‐coupled chloride cotransporters. Mol Aspects Med 34, 288–298. [DOI] [PubMed] [Google Scholar]
- 56. MacGurn JA, Hsu P‐C & Emr SD (2012) Ubiquitin and membrane protein turnover: from cradle to grave. Annu Rev Biochem 81, 231–259. [DOI] [PubMed] [Google Scholar]
- 57. Lamb CA, McCann RK, Stöckli J, James DE & Bryant NJ (2010) Insulin‐regulated trafficking of GLUT4 requires ubiquitination. Traffic 11, 1445–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Camus SM, Camus MD, Figueras‐Novoa C, Boncompain G, Sadacca LA, Esk C, Bigot A, Gould GW, Kioumourtzoglou D, Perez F et al. (2020) CHC22 clathrin mediates traffic from early secretory compartments for human GLUT4 pathway biogenesis. J Cell Biol 219, e201812135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diallinas G (2016) Dissection of transporter function: from genetics to structure. Trends Genet 32, 576–590. [DOI] [PubMed] [Google Scholar]
- 60. Yanatori I, Yasui Y, Tabuchi M & Kishi F (2014) Chaperone protein involved in transmembrane transport of iron. Biochem J 462, 25–37. [DOI] [PubMed] [Google Scholar]
- 61. Forero‐Quintero LS, Ames S, Schneider HP, Thyssen A, Boone CD, Andring JT, McKenna R, Casey JR, Deitmer JW & Becker HM (2019) Membrane‐anchored carbonic anhydrase IV interacts with monocarboxylate transporters via their chaperones CD147 and GP70. J Biol Chem 294, 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ames S, Andring JT, McKenna R & Becker HM (2020) CAIX forms a transport metabolon with monocarboxylate transporters in human breast cancer cells. Oncogene 39, 1710–1723. [DOI] [PubMed] [Google Scholar]
- 63. Becker HM, Hirnet D, Fecher‐Trost C, Sültemeyer D & Deitmer JW (2005) Transport activity of MCT1 expressed in Xenopus oocytes is increased by interaction with carbonic anhydrase. J Biol Chem 280, 39882–39889. [DOI] [PubMed] [Google Scholar]
- 64. Klier M, Schüler C, Halestrap AP, Sly WS, Deitmer JW & Becker HM (2011) Transport activity of the high‐affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II. J Biol Chem 286, 27781–27791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gether U, Andersen PH, Larsson OM & Schousboe A (2006) Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci 27, 375–383. [DOI] [PubMed] [Google Scholar]
- 66. Nałęcz KA (2017) Solute carriers in the blood‐brain barrier: safety in abundance. Neurochem Res 42, 795–809. [DOI] [PubMed] [Google Scholar]
- 67. Hundal HS & Taylor PM (2009) Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 296, E603–E613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ahn S‐Y & Nigam SK (2009) Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol 76, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu W, Dnyanmote AV & Nigam SK (2011) Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol Pharmacol 79, 795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S et al. (2007) A genome‐wide association study identifies novel risk loci for type 2 diabetes. Nature 445, 881–885. [DOI] [PubMed] [Google Scholar]
- 71. Tamaki M, Fujitani Y, Hara A, Uchida T, Tamura Y, Takeno K, Kawaguchi M, Watanabe T, Ogihara T, Fukunaka A et al. (2013) The diabetes‐susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest 123, 4513–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fukunaka A & Fujitani Y (2018) Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int J Mol Sci 19, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Collins JF, Bai L & Ghishan FK (2004) The SLC20 family of proteins: dual functions as sodium‐phosphate cotransporters and viral receptors. Pflugers Arch Eur J Physiol 447, 647–652. [DOI] [PubMed] [Google Scholar]
- 74. Nguyen NNT, Lim Y‐S, Nguyen LP, Tran SC, Luong TTD, Nguyen TTT, Pham HT, Mai HN, Choi J‐W, Han S‐S et al. (2018) Hepatitis C virus modulates solute carrier family 3 member 2 for viral propagation. Sci Rep 8, 15486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bai L, Sato H, Kubo Y, Wada S & Aida Y (2019) CAT1/SLC7A1 acts as a cellular receptor for bovine leukemia virus infection. FASEB J 33, 14516–14527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Colon‐Moran W, Argaw T & Wilson CA (2017) Three cysteine residues of SLC52A1, a receptor for the porcine endogenous retrovirus‐A (PERV‐A), play a critical role in cell surface expression and infectivity. Virology 507, 140–150. [DOI] [PubMed] [Google Scholar]
- 77. Scalise M, Pochini L, Console L, Losso MA & Indiveri C (2018) The human SLC1A5 (ASCT2) amino acid transporter: from function to structure and role in cell biology. Front Cell Dev Biol 6, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin L, Yee SW, Kim RB & Giacomini KM (2015) SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov 14, 543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. El‐Gebali S, Bentz S, Hediger MA & Anderle P (2013) Solute carriers (SLCs) in cancer. Mol Aspects Med 34, 719–734. [DOI] [PubMed] [Google Scholar]
- 80. Amberger JS, Bocchini CA, Schiettecatte F, Scott AF & Hamosh A (2015) OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res 43, D789–D798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B et al. (2008) Impairment of SLC17A8 encoding vesicular glutamate transporter‐3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet 83, 278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kosugi S, Okamoto H, Tamada A & Sanchez‐Franco F (2002) A novel peculiar mutation in the sodium/iodide symporter gene in Spanish siblings with iodide transport defect. J Clin Endocrinol Metab 87, 3830–3836. [DOI] [PubMed] [Google Scholar]
- 83. Flatt JF, Guizouarn H, Burton NM, Borgese F, Tomlinson RJ, Forsyth RJ, Baldwin SA, Levinson BE, Quittet P, Aguilar‐Martinez P et al. (2011) Stomatin‐deficient cryohydrocytosis results from mutations in SLC2A1: a novel form of GLUT1 deficiency syndrome. Blood 118, 5267–5277. [DOI] [PubMed] [Google Scholar]
- 84. Falk MJ, Li D, Gai X, McCormick E, Place E, Lasorsa FM, Otieno FG, Hou C, Kim CE, Abdel‐Magid N et al. (2014) AGC1 deficiency causes infantile epilepsy, abnormal myelination, and reduced N‐acetylaspartate. In JIMD Reports (Morava E, ed), pp. 77–85. Wiley, Hoboken, NJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. SIGMA Type 2 Diabetes Consortium , Williams AL, Jacobs SBR, Moreno‐Macías H, Huerta‐Chagoya A, Churchhouse C, Márquez‐Luna C, García‐Ortíz H, Gómez‐Vázquez MJ, Burtt NP, Aguilar‐Salinas CA et al. (2014) Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature 506, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhao Y, Feng Z, Zhang Y, Sun Y, Chen Y, Liu X, Li S, Zhou T, Chen L, Wei Y et al. (2019) Gain‐of‐function mutations of SLC16A11 contribute to the pathogenesis of type 2 diabetes. Cell Rep 26, 884–892.e4. [DOI] [PubMed] [Google Scholar]
- 87. Center for Drug Evaluation and Research (CDER) (2017) Clinical Drug Interaction Studies — Study Design, Data Analysis, and Clinical Implications Guidance for Industry. Beta.Regulations.Gov. Accessed September 14, 2020. https://beta.regulations.gov/docket/FDA‐2017‐D‐5961
- 88. Sugano K, Kansy M, Artursson P, Avdeef A, Bendels S, Di L, Ecker GF, Faller B, Fischer H, Gerebtzoff G et al. (2010) Coexistence of passive and carrier‐mediated processes in drug transport. Nat Rev Drug Discov 9, 597–614. [DOI] [PubMed] [Google Scholar]
- 89. César‐Razquin A, Girardi E, Yang M, Brehme M, Saez‐Rodriguez J & Superti‐Furga G (2018) In silico prioritization of transporter‐drug relationships from drug sensitivity screens. Front Pharmacol 9, 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nyquist MD, Prasad B & Mostaghel EA (2017) Harnessing solute carrier transporters for precision oncology. Molecules 22, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dobson PD & Kell DB (2008) Carrier‐mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov 7, 205–220. [DOI] [PubMed] [Google Scholar]
- 92. Rask‐Andersen M, Masuram S, Fredriksson R & Schiöth HB (2013) Solute carriers as drug targets: current use, clinical trials and prospective. Mol Aspects Med 34, 702–710. [DOI] [PubMed] [Google Scholar]
- 93. Uldry M & Thorens B (2004) The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch Eur J Physiol 447, 480–489. [DOI] [PubMed] [Google Scholar]
- 94. Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE & Lodish HF (1985) Sequence and structure of a human glucose transporter. Science 229, 941–945. [DOI] [PubMed] [Google Scholar]
- 95. Preitner F, Bonny O, Laverriere A, Rotman S, Firsov D, Da Costa A, Metref S & Thorens B (2009) Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci USA 106, 15501–15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Santer R, Schneppenheim R, Dombrowski A, Götze H, Steinmann B & Schaub J (1997) Mutations in GLUT2, the gene for the liver‐type glucose transporter, in patients with Fanconi‐Bickel syndrome. Nat Genet 17, 324–326. [DOI] [PubMed] [Google Scholar]
- 97. Gras D, Roze E, Caillet S, Méneret A, Doummar D, Billette de Villemeur T, Vidailhet M & Mochel F (2014) GLUT1 deficiency syndrome: An update. Rev Neurol 170, 91–99. [DOI] [PubMed] [Google Scholar]
- 98. Carruthers A, DeZutter J, Ganguly A & Devaskar SU (2009) Will the original glucose transporter isoform please stand up! Am J Physiol Metab 297, E836–E848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zambrano A, Molt M, Uribe E & Salas M (2019) Glut 1 in cancer cells and the inhibitory action of resveratrol as a potential therapeutic strategy. Int J Mol Sci 20, 3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Younes M, Lechago LV, Somoano JR, Mosharaf M & Lechago J (1996) Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res 56, 1164–1167. [PubMed] [Google Scholar]
- 101. Thorens B (2015) GLUT2, glucose sensing and glucose homeostasis. Diabetologia 58, 221–232. [DOI] [PubMed] [Google Scholar]
- 102. Burcelin R, Dolci W & Thorens B (2000) Glucose sensing by the hepatoportal sensor is GLUT2‐dependent: in vivo analysis in GLUT2‐null mice. Diabetes 49, 1643–1648. [DOI] [PubMed] [Google Scholar]
- 103. Leino RL, Gerhart DZ, Van Bueren AM, McCall AL & Drewes LR (1997) Ultrastructural localization of GLUT 1 and GLUT 3 glucose transporters in rat brain. J Neurosci Res 49, 617–626. [DOI] [PubMed] [Google Scholar]
- 104. Maratou E, Dimitriadis G, Kollias A, Boutati E, Lambadiari V, Mitrou P & Raptis SA (2007) Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest 37, 282–290. [DOI] [PubMed] [Google Scholar]
- 105. Muretta JM, Romenskaia I & Mastick CC (2008) Insulin releases Glut4 from static storage compartments into cycling endosomes and increases the rate constant for Glut4 exocytosis. J Biol Chem 283, 311–323. [DOI] [PubMed] [Google Scholar]
- 106. Huang S & Czech MP (2007) The GLUT4 Glucose Transporter. Cell Metab 5, 237–252. [DOI] [PubMed] [Google Scholar]
- 107. Burant CF (1992) Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem 267, 14523–14526. [PubMed] [Google Scholar]
- 108. Douard V & Ferraris RP (2008) Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Metab 295, E227–E237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wright EM, Loo DDF & Hirayama BA (2011) Biology of human sodium glucose transporters. Physiol Rev 91, 733–794. [DOI] [PubMed] [Google Scholar]
- 110. Wright EM (2013) Glucose transport families SLC5 and SLC50. Mol Aspects Med 34, 183–196. [DOI] [PubMed] [Google Scholar]
- 111. Diez‐Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM & Koepsell H (2003) A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci USA 100, 11753–11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lee WS, Kanai Y, Wells RG & Hediger MA (1994) The high affinity Na+/glucose cotransporter. Re‐evaluation of function and distribution of expression. J Biol Chem 269, 12032–12039. [PubMed] [Google Scholar]
- 113. Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, Radović N, Jadrijević S, Aleksic I, Walles T et al. (2015) Localizations of Na+‐d‐glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflügers Arch Eur J Physiol 467, 1881–1898. [DOI] [PubMed] [Google Scholar]
- 114. Wiśniewski JR, Friedrich A, Keller T, Mann M & Koepsell H (2015) The impact of high‐fat diet on metabolism and immune defense in small intestine mucosa. J Proteome Res 14, 353–365. [DOI] [PubMed] [Google Scholar]
- 115. Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H & Rieg T (2011) SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Solini A, Rossi C, Mazzanti CM, Proietti A, Koepsell H & Ferrannini E (2017) Sodium‐glucose co‐transporter ( SGLT )2 and SGLT1 renal expression in patients with type 2 diabetes. Diabetes Obes Metab 19, 1289–1294. [DOI] [PubMed] [Google Scholar]
- 117. Spatola L, Finazzi S, Angelini C, Dauriz M & Badalamenti S (2018) SGLT1 and SGLT1 inhibitors: a role to be assessed in the current clinical practice. Diabetes Ther 9, 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lee S (2017) Update on SGLT2 inhibitors—new data released at the American Diabetes Association. Crit Pathw Cardiol 16, 93–95. [DOI] [PubMed] [Google Scholar]
- 119. Chauvin TR & Griswold MD (2004) Characterization of the expression and regulation of genes necessary for myo‐inositol biosynthesis and transport in the seminiferous epithelium 1. Biol Reprod 70, 744–751. [DOI] [PubMed] [Google Scholar]
- 120. Andronic J, Shirakashi R, Pickel SU, Westerling KM, Klein T, Holm T, Sauer M & Sukhorukov VL (2015) Hypotonic activation of the myo‐inositol transporter SLC5A3 in HEK293 cells probed by cell volumetry, confocal and super‐resolution microscopy. PLoS One 10, e0119990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Eskandari S, Loo DDF, Dai G, Levy O, Wright EM & Carrasco N (1997) Thyroid Na + /I − Symporter. J Biol Chem 272, 27230–27238. [DOI] [PubMed] [Google Scholar]
- 122. Vayre L, Sabourin J, Caillou B, Ducreux M, Schlumberger M & Bidart J (1999) Immunohistochemical analysis of Na+/I‐ symporter distribution in human extra‐thyroidal tissues. Eur J Endocrinol 141, 382–386. [DOI] [PubMed] [Google Scholar]
- 123. Ravera S, Reyna‐Neyra A, Ferrandino G, Amzel LM & Carrasco N (2017) The Sodium/Iodide Symporter (NIS): molecular physiology and preclinical and clinical applications. Annu Rev Physiol 79, 261–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Warburg O (1956) On the origin of cancer cells. Science 123, 309–314. [DOI] [PubMed] [Google Scholar]
- 125. Schwartz L, Supuran C & Alfarouk K (2017) The Warburg effect and the hallmarks of cancer. Anticancer Agents Med Chem 17, 164–170. [DOI] [PubMed] [Google Scholar]
- 126. Granja S, Pinheiro C, Reis R, Martinho O & Baltazar F (2015) Glucose addiction in cancer therapy: advances and drawbacks. Curr Drug Metab 16, 221–242. [DOI] [PubMed] [Google Scholar]
- 127. Madunić IV, Madunić J, Breljak D, Karaica D & Sabolić I (2018) Sodium‐glucose cotransporters: new targets of cancer therapy? Arh Hig Rada Toksikol 69, 278–285. [DOI] [PubMed] [Google Scholar]
- 128. Ancey PB, Contat C & Meylan E (2018) Glucose transporters in cancer – from tumor cells to the tumor microenvironment. FEBS J 285, 2926–2943. [DOI] [PubMed] [Google Scholar]
- 129. Koepsell H (2017) The Na+‐D‐glucose cotransporters SGLT1 and SGLT2 are targets for the treatment of diabetes and cancer. Pharmacol Ther 170, 148–165. [DOI] [PubMed] [Google Scholar]
- 130. Macheda ML, Rogers S & Best JD (2005) Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 202, 654–662. [DOI] [PubMed] [Google Scholar]
- 131. Ganapathy V, Thangaraju M & Prasad PD (2009) Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther 121, 29–40. [DOI] [PubMed] [Google Scholar]
- 132. Nakaigawa N, Kondo K, Ueno D, Namura K, Makiyama K, Kobayashi K, Shioi K, Ikeda I, Kishida T, Kaneta T et al. (2017) The acceleration of glucose accumulation in renal cell carcinoma assessed by FDG PET/CT demonstrated acquisition of resistance to tyrosine kinase inhibitor therapy. BMC Cancer 17, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Xu Y‐Y, Wu T‐T, Zhou S‐H, Bao Y‐Y, Wang Q‐Y, Fan J & Huang Y‐P (2014) Apigenin suppresses GLUT‐1 and p‐AKT expression to enhance the chemosensitivity to cisplatin of laryngeal carcinoma Hep‐2 cells: an in vitro study. Int J Clin Exp Pathol 7, 3938–3947. [PMC free article] [PubMed] [Google Scholar]
- 134. Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ & Hung MC (2008) Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 13, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sala‐Rabanal M, Hirayama BA, Ghezzi C, Liu J, Huang SC, Kepe V, Koepsell H, Yu A, Powell DR, Thorens B et al. (2016) Revisiting the physiological roles of SGLTs and GLUTs using positron emission tomography in mice. J Physiol 594, 4425–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wright EM, Ghezzi C & Loo DDF (2017) Novel and unexpected functions of SGLTs. Physiology 32, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Schiöth HB, Roshanbin S, Hägglund MGA & Fredriksson R (2013) Evolutionary origin of amino acid transporter families SLC32, SLC36 and SLC38 and physiological, pathological and therapeutic aspects. Mol Aspects Med 34, 571–585. [DOI] [PubMed] [Google Scholar]
- 138. Kanai Y, Clémençon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M & Hediger MA (2013) The SLC1 high‐affinity glutamate and neutral amino acid transporter family. Mol Aspects Med 34, 108–120. [DOI] [PubMed] [Google Scholar]
- 139. Avissar NE, Ryan CK, Ganapathy V & Sax HC (2001) Na + ‐dependent neutral amino acid transporter ATB 0 is a rabbit epithelial cell brush‐border protein. Am J Physiol Physiol 281, C963–C971. [DOI] [PubMed] [Google Scholar]
- 140. Grewer C, Gameiro A & Rauen T (2014) SLC1 glutamate transporters. Pflugers Arch 466, 3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chi‐Castañeda D, Suárez‐Pozos E & Ortega A (2017) Regulation of glutamate transporter expression in glial cells. Adv Neurobiol 16, 199–224. [DOI] [PubMed] [Google Scholar]
- 142. Kanai Y, Smith CP & Hediger MA (1993) A new family of neurotransmitter transporters: the high‐affinity glutamate transporters. FASEB J 7, 1450–1459. [DOI] [PubMed] [Google Scholar]
- 143. Fotiadis D, Kanai Y & Palacín M (2013) The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34, 139–158. [DOI] [PubMed] [Google Scholar]
- 144. Taylor PM (2014) Role of amino acid transporters in amino acid sensing. Am J Clin Nutr 99, 223S–230S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Devés R & Boyd CAR (1998) Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev 78, 487–545. [DOI] [PubMed] [Google Scholar]
- 146. Verrey F, Closs EI, Wagner CA, Palacin M, Endou H & Kanai Y (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch Eur J Physiol 447, 532–542. [DOI] [PubMed] [Google Scholar]
- 147. Chillarón J, Estévez R, Mora C, Wagner CA, Suessbrich H, Lang F, Gelpí JL, Testar X, Busch AE, Zorzano A et al. (1996) Obligatory amino acid exchange via systems b(o,+)‐like and y+L‐like. A tertiary active transport mechanism for renal reabsorption of cystine and dibasic amino acids. J Biol Chem 271, 17761–17770. [DOI] [PubMed] [Google Scholar]
- 148. Palacín M & Kanai Y (2004) The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers Arch Eur J Physiol 447, 490–494. [DOI] [PubMed] [Google Scholar]
- 149. Wagner CA, Lang F & Bröer S (2001) Function and structure of heterodimeric amino acid transporters. Am J Physiol Physiol 281, C1077–C1093. [DOI] [PubMed] [Google Scholar]
- 150. Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB & Verrey F (1998) Amino‐acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 395, 288–291. [DOI] [PubMed] [Google Scholar]
- 151. Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H & Kanai Y (1999) Identification and functional characterization of a Na + ‐independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem 274, 19745–19751. [DOI] [PubMed] [Google Scholar]
- 152. Scalise M, Galluccio M, Console L, Pochini L & Indiveri C (2018) The Human SLC7A5 (LAT1): the intriguing histidine/large neutral amino acid transporter and its relevance to human health. Front Chem 6, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Chien H‐C, Colas C, Finke K, Springer S, Stoner L, Zur AA, Venteicher B, Campbell J, Hall C, Flint A et al. (2018) Reevaluating the substrate specificity of the L‐Type amino acid transporter (LAT1). J Med Chem 61, 7358–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Fukasawa Y, Segawa H, Kim JY, Chairoungdua A, Kim DK, Matsuo H, Cha SH, Endou H & Kanai Y (2000) Identification and characterization of a Na + ‐independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral d‐ and l‐amino acids. J Biol Chem 275, 9690–9698. [DOI] [PubMed] [Google Scholar]
- 155. Bröer A, Wagner CA, Lang F & Bröer S (2000) The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem J 349(Pt 3), 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kanai Y, Fukasawa Y, Cha SH, Segawa H, Chairoungdua A, Kim DK, Matsuo H, Kim JY, Miyamoto K, Takeda E et al. (2000) Transport properties of a system y + L neutral and basic amino acid transporter. J Biol Chem 275, 20787–20793. [DOI] [PubMed] [Google Scholar]
- 157. Sato H, Tamba M, Ishii T & Bannai S (1999) Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 274, 11455–11458. [DOI] [PubMed] [Google Scholar]
- 158. Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M et al. (2013) The cystine/glutamate antiporter system x(c)(‐) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal 18, 522–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fernández E, Carrascal M, Rousaud F, Abián J, Zorzano A, Palacín M & Chillarón J (2002) rBAT‐b 0,+ AT heterodimer is the main apical reabsorption system for cystine in the kidney. Am J Physiol Physiol 283, F540–F548. [DOI] [PubMed] [Google Scholar]
- 160. Nagamori S, Wiriyasermkul P, Guarch ME, Okuyama H, Nakagomi S, Tadagaki K, Nishinaka Y, Bodoy S, Takafuji K, Okuda S et al. (2016) Novel cystine transporter in renal proximal tubule identified as a missing partner of cystinuria‐related plasma membrane protein rBAT/SLC3A1. Proc Natl Acad Sci USA 113, 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Pramod AB, Foster J, Carvelli L & Henry LK (2013) SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med 34, 197–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K & Gether U (2011) SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev 63, 585–640. [DOI] [PubMed] [Google Scholar]
- 163. Jayaraman K, Morley AN, Szöllősi D, Wassenaar TA, Sitte HH & Stockner T (2018) Dopamine transporter oligomerization involves the scaffold domain, but spares the bundle domain. PLoS Comput Biol 14, e1006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhen J & Reith MEA (2018) Functional properties of dopamine transporter oligomers after copper linking. J Neurochem 144, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Schmitt KC, Rothman RB & Reith MEA (2013) Nonclassical pharmacology of the dopamine transporter: atypical inhibitors, allosteric modulators, and partial substrates. J Pharmacol Exp Ther 346, 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Madsen KK, White HS & Schousboe A (2010) Neuronal and non‐neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol Ther 125, 394–401. [DOI] [PubMed] [Google Scholar]
- 167. Huot P, Fox SH & Brotchie JM (2015) Monoamine reuptake inhibitors in Parkinson’s disease. Parkinsons Dis 2015, 609428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Aggarwal S & Mortensen OV (2017) Overview of monoamine transporters. Curr Protoc Pharmacol 79, 12.16.1–12.16.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Scimemi A (2014) Structure, function, and plasticity of GABA transporters. Front Cell Neurosci 8, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Zhou Y & Danbolt NC (2013) GABA and glutamate transporters in brain. Front Endocrinol (Lausanne) 4, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Eskandari S, Willford SL & Anderson CM (2017) Revised ion/substrate coupling stoichiometry of GABA transporters. Adv Neurobiol 16, 85–116. [DOI] [PubMed] [Google Scholar]
- 172. Zhou Y, Holmseth S, Guo C, Hassel B, Höfner G, Huitfeldt HS, Wanner KT & Danbolt NC (2012) Deletion of the γ‐aminobutyric acid transporter 2 (GAT2 and SLC6A13) gene in mice leads to changes in liver and brain taurine contents. J Biol Chem 287, 35733–35746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Karunakaran S, Ramachandran S, Coothankandaswamy V, Elangovan S, Babu E, Periyasamy‐Thandavan S, Gurav A, Gnanaprakasam JP, Singh N, Schoenlein PV et al. (2011) SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor‐positive breast cancer. J Biol Chem 286, 31830–31838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Eulenburg V & Gomeza J (2010) Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res Rev 63, 103–112. [DOI] [PubMed] [Google Scholar]
- 175. Shibasaki K, Hosoi N, Kaneko R, Tominaga M & Yamada K (2017) Glycine release from astrocytes via functional reversal of GlyT1. J Neurochem 140, 395–403. [DOI] [PubMed] [Google Scholar]
- 176. Crump FT, Fremeau RT & Craig AM (1999) Localization of the brain‐specific high‐affinity l‐proline transporter in cultured hippocampal neurons: molecular heterogeneity of synaptic terminals. Mol Cell Neurosci 13, 25–39. [DOI] [PubMed] [Google Scholar]
- 177. Gupta N, Prasad PD, Ghamande S, Moore‐Martin P, Herdman AV, Martindale RG, Podolsky R, Mager S, Ganapathy ME & Ganapathy V (2006) Up‐regulation of the amino acid transporter ATB0,+ (SLC6A14) in carcinoma of the cervix. Gynecol Oncol 100, 8–13. [DOI] [PubMed] [Google Scholar]
- 178. Gupta N, Miyauchi S, Martindale RG, Herdman AV, Podolsky R, Miyake K, Mager S, Prasad PD, Ganapathy ME & Ganapathy V (2005) Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim Biophys Acta Mol Basis Dis 1741, 215–223. [DOI] [PubMed] [Google Scholar]
- 179. Bröer A, Tietze N, Kowalczuk S, Chubb S, Munzinger M, Bak LK & Bröer S (2006) The orphan transporter v7–3 (slc6a15) is a Na + ‐dependent neutral amino acid transporter (B 0 AT2). Biochem J 393, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Parra LA, Baust T, El Mestikawy S, Quiroz M, Hoffman B, Haflett JM, Yao JK & Torres GE (2008) The orphan transporter Rxt1/NTT4 (SLC6A17) functions as a synaptic vesicle amino acid transporter selective for proline, glycine, leucine, and alanine. Mol Pharmacol 74, 1521–1532. [DOI] [PubMed] [Google Scholar]
- 181. Singer D, Camargo SMR, Huggel K, Romeo E, Danilczyk U, Kuba K, Chesnov S, Caron MG, Penninger JM & Verrey F (2009) Orphan transporter SLC6A18 is renal neutral amino acid transporter B 0 AT3. J Biol Chem 284, 19953–19960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88, 249–286. [DOI] [PubMed] [Google Scholar]
- 183. Kowalczuk S, Bröer A, Munzinger M, Tietze N, Klingel K & Bröer S (2005) Molecular cloning of the mouse IMINO system: An Na+‐ and Cl –dependent proline transporter. Biochem J 386, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Verrey F, Singer D, Ramadan T, Vuille‐Dit‐Bille RN, Mariotta L & Camargo SMR (2009) Kidney amino acid transport. Pflugers Arch Eur J Physiol 458, 53–60. [DOI] [PubMed] [Google Scholar]
- 185. Fan SJ & Goberdhan DCI (2018) PATs and SNATs: amino acid sensors in disguise. Front Pharmacol 9, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Bröer S (2014) The SLC38 family of sodium‐amino acid co‐transporters. Pflugers Arch Eur J Physiol 466, 155–172. [DOI] [PubMed] [Google Scholar]
- 187. Mackenzie B & Erickson JD (2004) Sodium‐coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch 447, 784–95. [DOI] [PubMed] [Google Scholar]
- 188. Pinilla J, Aledo JC, Cwiklinski E, Hyde R, Taylor PM & Hundal HS (2011) SNAT2 transceptor signalling via mTOR: a role in cell growth and proliferation? Front Biosci (Elite Ed) 3, 1289–1299. [DOI] [PubMed] [Google Scholar]
- 189. Rebsamen M, Pochini L, Stasyk T, de Araújo MEG, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M et al. (2015) SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519, 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Hellsten SV, Hägglund MG, Eriksson MM & Fredriksson R (2017) The neuronal and astrocytic protein SLC38A10 transports glutamine, glutamate, and aspartate, suggesting a role in neurotransmission. FEBS Open Bio 7, 730–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bodoy S, Fotiadis D, Stoeger C, Kanai Y & Palacín M (2013) The small SLC43 family: facilitator system l amino acid transporters and the orphan EEG1. Mol Aspects Med 34, 638–645. [DOI] [PubMed] [Google Scholar]
- 192. Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R & Bertran J (2005) Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem 280, 12002–12011. [DOI] [PubMed] [Google Scholar]
- 193. Babu E, Kanai Y, Chairoungdua A, Kim DK, Iribe Y, Tangtrongsup S, Jutabha P, Li Y, Ahmed N, Sakamoto S et al. (2003) Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem 278, 43838–43845. [DOI] [PubMed] [Google Scholar]
- 194. Wang Q & Holst J (2015) L‐type amino acid transport and cancer: targeting the mTORC1 pathway to inhibit neoplasia. Am J Cancer Res 5, 1281–1294. [PMC free article] [PubMed] [Google Scholar]
- 195. Fukuhara D, Kanai Y, Chairoungdua A, Babu E, Bessho F, Kawano T, Akimoto Y, Endou H & Yan K (2007) Protein characterization of Na+‐independent system L amino acid transporter 3 in mice. Am J Pathol 170, 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Oparija L, Rajendran A, Poncet N & Verrey F (2019) Anticipation of food intake induces phosphorylation switch to regulate basolateral amino acid transporter LAT4 (SLC43A2) function. J Physiol 597, 521–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Guetg A, Mariotta L, Bock L, Herzog B, Fingerhut R, Camargo SMR & Verrey F (2015) Essential amino acid transporter Lat4 (Slc43a2) is required for mouse development. J Physiol 593, 1273–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kandasamy P, Gyimesi G, Kanai Y & Hediger MA (2018) Amino acid transporters revisited: new views in health and disease. Trends Biochem Sci 43, 752–789. [DOI] [PubMed] [Google Scholar]
- 199. Avruch J, Long X, Ortiz‐Vega S, Rapley J, Papageorgiou A & Dai N (2009) Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab 296, E592–E602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Cha YJ, Kim ES & Koo JS (2018) Amino acid transporters and glutamine metabolism in breast cancer. Int J Mol Sci 19, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Bhutia YD & Ganapathy V (2016) Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta Mol Cell Res 1863, 2531–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lanzardo S, Conti L, Rooke R, Ruiu R, Accart N, Bolli E, Arigoni M, Macagno M, Barrera G, Pizzimenti S et al. (2016) Immunotargeting of antigen xCT attenuates stem‐like cell behavior and metastatic progression in breast cancer. Cancer Res 76, 62–72. [DOI] [PubMed] [Google Scholar]
- 203. Sato R, Nakano T, Hosonaga M, Sampetrean O, Harigai R, Sasaki T, Koya I, Okano H, Kudoh J, Saya H et al. (2017) RNA sequencing analysis reveals interactions between breast cancer or melanoma cells and the tissue microenvironment during brain metastasis. Biomed Res Int 2017, 8032910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Wang Q, Bailey CG, Ng C, Tiffen J, Thoeng A, Minhas V, Lehman ML, Hendy SC, Buchanan G, Nelson CC et al. (2011) Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res 71, 7525–7536. [DOI] [PubMed] [Google Scholar]
- 205. Ganapathy V, Smith SB & Prasad PD (2004) SLC19: the folate/thiamine transporter family. Pflugers Arch 447, 641–646. [DOI] [PubMed] [Google Scholar]
- 206. Zhao R & Goldman ID (2013) Folate and thiamine transporters mediated by facilitative carriers (SLC19A1‐3 and SLC46A1) and folate receptors. Mol Aspects Med 34, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Hou Z & Matherly LH (2014) Biology of the major facilitative folate transporters SLC19A1 and SLC46A1. Curr Top Membr 73, 175–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Said HM (2015) Nutrition for the primary care provider. In World Review of Nutrition and Dietetics (Bier DM, Mann J, Alpers DH, Vorster HE & Gibney MJ, eds), vol. 111, pp. 30–37. [Google Scholar]
- 209. Ferguson PL & Flintoff WF (1999) Topological and functional analysis of the human reduced folate carrier by hemagglutinin epitope insertion. J Biol Chem 274, 16269–16278. [DOI] [PubMed] [Google Scholar]
- 210. Luteijn RD, Zaver SA, Gowen BG, Wyman SK, Garelis NE, Onia L, McWhirter SM, Katibah GE, Corn JE, Woodward JJ et al. (2019) SLC19A1 transports immunoreactive cyclic dinucleotides. Nature 573, 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Dutta B, Huang W, Molero M, Kekuda R, Leibach FH, Devoe LD, Ganapathy V & Prasad PD (1999) Cloning of the human thiamine transporter, a member of the folate transporter family. J Biol Chem 274, 31925–31929. [DOI] [PubMed] [Google Scholar]
- 212. Said HM, Balamurugan K, Subramanian VS & Marchant JS (2004) Expression and functional contribution of hTHTR‐2 in thiamin absorption in human intestine. Am J Physiol Liver Physiol 286, G491–G498. [DOI] [PubMed] [Google Scholar]
- 213. Zhao R, Min SH, Wang Y, Campanella E, Low PS & Goldman ID (2009) A role for the proton‐coupled folate transporter (PCFT‐SLC46A1) in folate receptor‐mediated endocytosis. J Biol Chem 284, 4267–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Urquhart BL, Gregor JC, Chande N, Knauer MJ, Tirona RG & Kim RB (2010) The human proton‐coupled folate transporter (hPCFT): modulation of intestinal expression and function by drugs. Am J Physiol Liver Physiol 298, G248–G254. [DOI] [PubMed] [Google Scholar]
- 215. May JM (2011) The SLC23 family of ascorbate transporters: ensuring that you get and keep your daily dose of vitamin C. Br J Pharmacol 164, 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Bürzle M, Suzuki Y, Ackermann D, Miyazaki H, Maeda N, Clémençon B, Burrier R & Hediger MA (2013) The sodium‐dependent ascorbic acid transporter family SLC23. Mol Aspects Med 34, 436–454. [DOI] [PubMed] [Google Scholar]
- 217. Savini I, Rossi A, Pierro C, Avigliano L & Catani MV (2008) SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34, 347–355. [DOI] [PubMed] [Google Scholar]
- 218. Godoy A, Ormazabal V, Moraga‐Cid G, Zúñiga FA, Sotomayor P, Barra V, Vasquez O, Montecinos V, Mardones L, Guzmán C et al. (2007) Mechanistic insights and functional determinants of the transport cycle of the ascorbic acid transporter SVCT2: activation by sodium and absolute dependence on bivalent cations. J Biol Chem 282, 615–624. [DOI] [PubMed] [Google Scholar]
- 219. Ballaz SJ & Rebec GV (2019) Neurobiology of vitamin C: expanding the focus from antioxidant to endogenous neuromodulator. Pharmacol Res 146, 104321. [DOI] [PubMed] [Google Scholar]
- 220. Muñoz‐Montesino C, Roa FJ, Peña E, González M, Sotomayor K, Inostroza E, Muñoz CA, González I, Maldonado M, Soliz C et al. (2014) Mitochondrial ascorbic acid transport is mediated by a low‐affinity form of the sodium‐coupled ascorbic acid transporter‐2. Free Radic Biol Med 70, 241–254. [DOI] [PubMed] [Google Scholar]
- 221. Fiorani M, Azzolini C, Cerioni L, Scotti M, Guidarelli A, Ciacci C & Cantoni O (2015) The mitochondrial transporter of ascorbic acid functions with high affinity in the presence of low millimolar concentrations of sodium and in the absence of calcium and magnesium. Biochim Biophys Acta Biomembr 1848, 1393–1401. [DOI] [PubMed] [Google Scholar]
- 222. Cimmino L, Neel BG & Aifantis I (2018) Vitamin C in stem cell reprogramming and cancer. Trends Cell Biol 28, 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Matherly LH, Hou Z & Deng Y (2007) Human reduced folate carrier: Translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev 26, 111–128. [DOI] [PubMed] [Google Scholar]
- 224. Zastre JA, Sweet RL, Hanberry BS & Ye S (2013) Linking vitamin B1 with cancer cell metabolism. Cancer Metab 1, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Pieroth R, Paver S, Day S & Lammersfeld C (2018) Folate and its impact on cancer risk. Curr Nutr Rep 7, 70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Camarena V & Wang G (2016) The epigenetic role of vitamin C in health and disease. Cell Mol Life Sci 73, 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Wohlrab C, Phillips E & Dachs GU (2017) Vitamin c transporters in cancer: Current understanding and gaps in knowledge. Front Oncol 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kotnik BF, Jazbec J, Grabar PB, Rodriguez‐Antona C & Dolzan V (2017) Association between SLC19A1 gene polymorphism and high dose methotrexate toxicity in childhood acute lymphoblastic leukaemia and non Hodgkin malignant lymphoma: introducing a haplotype based approach. Radiol Oncol 51, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Zaïr ZM & Singer DRJ (2016) Influx transporter variants as predictors of cancer chemotherapy‐induced toxicity: systematic review and meta‐analysis. Pharmacogenomics 17, 1189–1205. [DOI] [PubMed] [Google Scholar]
- 230. Zhao R, Najmi M, Aluri S, Spray DC & Goldman ID (2018) Concentrative transport of antifolates mediated by the proton‐coupled folate transporter (slc46a1); augmentation by a hepes buffer. Mol Pharmacol 93, 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Cheuk IW, Shin VY, Siu MT, Tsang JY, Ho JC, Chen J, Tse GM, Wang X & Kwong A (2015) Association of EP2 receptor and SLC19A3 in regulating breast cancer metastasis. Am J Cancer Res 5, 3389–3399. [PMC free article] [PubMed] [Google Scholar]
- 232. Zera K, Sweet R & Zastre J (2016) Role of HIF‐1α in the hypoxia inducible expression of the thiamine transporter, SLC19A3. Gene 595, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Erichsen HC, Peters U, Eck P, Welch R, Schoen RE, Yeager M, Levine M, Hayes RB & Chanock S (2008) Genetic variation in sodium‐dependent vitamin C transporters SLC23A1 and SLC23A2 and risk of advanced colorectal adenoma. Nutr Cancer 60, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Duell EJ, Lujan‐Barroso L, Llivina C, Muñoz X, Jenab M, Boutron‐Ruault MC, Clavel‐Chapelon F, Racine A, Boeing H, Buijsse B et al. (2013) Vitamin C transporter gene (SLC23A1 and SLC23A2) polymorphisms, plasma vitamin C levels, and gastric cancer risk in the EPIC cohort. Genes Nutr 8, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Wright ME, Andreotti G, Lissowska J, Yeager M, Zatonski W, Chanock SJ, Chow WH & Hou L (2009) Genetic variation in sodium‐dependent ascorbic acid transporters and risk of gastric cancer in Poland. Eur J Cancer 45, 1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio IIC, Giannopoulou EG, Rago C et al. (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350, 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Van Der Reest J & Gottlieb E (2016) Anti‐cancer effects of Vitamin C revisited. Cell Res 26, 269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Peña E, Roa FJ, Inostroza E, Sotomayor K, González M, Gutierrez‐Castro FA, Maurin M, Sweet K, Labrousse C, Gatica M et al. (2019) Increased expression of mitochondrial sodium‐coupled ascorbic acid transporter‐2 (mitSVCT2) as a central feature in breast cancer. Free Radic Biol Med 135, 283–292. [DOI] [PubMed] [Google Scholar]
- 239. Fiorillo M, Tóth F, Sotgia F & Lisanti MP (2019) Doxycycline, Azithromycin and vitamin C (DAV): a potent combination therapy for targeting mitochondria and eradicating cancer stem cells (CSCs). Aging (Albany NY) 11, 2202–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Lorenzato A, Magrì A, Matafora V, Audrito V, Arcella P, Lazzari L, Montone M, Lamba S, Deaglio S, Siena S et al. (2020) Vitamin C restricts the emergence of acquired resistance to EGFR‐targeted therapies in colorectal cancer. Cancers (Basel) 12, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Young JD (2016) The SLC28 (CNT) and SLC29 (ENT) nucleoside transporter families: a 30‐year collaborative odyssey. Biochem Soc Trans 44, 869–876. [DOI] [PubMed] [Google Scholar]
- 242. Pastor‐Anglada M & Pérez‐Torras S (2018) Emerging roles of nucleoside transporters. Front Pharmacol 9, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Young JD, Yao SYM, Baldwin JM, Cass CE & Baldwin SA (2013) The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Aspects Med 34, 529–547. [DOI] [PubMed] [Google Scholar]
- 244. Pastor‐Anglada M, Errasti‐Murugarren E, Aymerich I & Casado FJ (2007) Concentrative nucleoside transporters (CNTs) in epithelia: from absorption to cell signaling. J Physiol Biochem 63, 97–110. [DOI] [PubMed] [Google Scholar]
- 245. Pastor‐Anglada M & Pérez‐Torras S (2018) Who is who in adenosine transport. Front Pharmacol 9, 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Rodraguez‐Mulero S, Errasti‐Murugarren E, Ballaran J, Felipe A, Doucet A, Casado F & Pastor‐Anglada M (2005) Expression of concentrative nucleoside transporters SLC28 (CNT1, CNT2, and CNT3) along the rat nephron: Effect of diabetes. Kidney Int 68, 665–672. [DOI] [PubMed] [Google Scholar]
- 247. Gray JH, Owen RP & Giacomini KM (2004) The concentrative nucleoside transporter family, SLC28. Pflugers Arch Eur J Physiol 447, 728–734. [DOI] [PubMed] [Google Scholar]
- 248. Valdés R, Casado FJ & Pastor‐Anglada M (2002) Cell‐cycle‐dependent regulation of CNT1, a concentrative nucleoside transporter involved in the uptake of cell‐cycle‐dependent nucleoside‐derived anticancer drugs. Biochem Biophys Res Commun 296, 575–579. [DOI] [PubMed] [Google Scholar]
- 249. Pastor‐Anglada M, Casado FJ, Valdés R, Mata J, García‐Manteiga J & Molina M (2001) Complex regulation of nucleoside transporter expression in epithelial and immune system cells. Mol Membr Biol 18, 81–85. [DOI] [PubMed] [Google Scholar]
- 250. Aymerich I, Foufelle F, Ferré P, Casado FJ & Pastor‐Anglada M (2006) Extracellular adenosine activates AMP‐dependent protein kinase (AMPK). J Cell Sci 119, 1612–1621. [DOI] [PubMed] [Google Scholar]
- 251. Fernández‐Veledo S, Huber‐Ruano I, Aymerich I, Duflot S, Casado FJ & Pastor‐Anglada M (2006) Bile acids alter the subcellular localization of CNT2 (concentrative nucleoside cotransporter) and increase CNT2‐related transport activity in liver parenchymal cells. Biochem J 395, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Smith KM, Slugoski MD, Loewen SK, Ng AML, Yao SYM, Chen X‐Z, Karpinski E, Cass CE, Baldwin SA & Young JD (2005) The broadly selective human Na + /nucleoside cotransporter (hCNT3) exhibits novel cation‐coupled nucleoside transport characteristics. J Biol Chem 280, 25436–25449. [DOI] [PubMed] [Google Scholar]
- 253. Boswell‐Casteel RC & Hays FA (2017) Equilibrative nucleoside transporters‐a review. Nucleosides Nucleotides Nucleic Acids 36, 7–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Nordh S, Ansari D & Andersson R (2014) hENT1 expression is predictive of gemcitabine outcome in pancreatic cancer: a systematic review. World J Gastroenterol 20, 8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Espinoza JA, García P, Bizama C, Leal JL, Riquelme I, Weber H, Macanas P, Aguayo G, Viñuela E, Roa JC et al. (2016) Low expression of equilibrative nucleoside transporter 1 is associated with poor prognosis in chemotherapy‐naïve pT2 gallbladder adenocarcinoma patients. Histopathology 68, 722–728. [DOI] [PubMed] [Google Scholar]
- 256. Grañé‐Boladeras N, Spring CM, Hanna WJB, Pastor‐Anglada M & Coe IR (2016) Novel nuclear hENT2 isoforms regulate cell cycle progression via controlling nucleoside transport and nuclear reservoir. Cell Mol Life Sci 73, 4559–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Xia L, Engel K, Zhou M & Wang J (2007) Membrane localization and pH‐dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells. Am J Physiol Renal Physiol 292, F682–F690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Li H, Smolen GA, Beers LF, Xia L, Gerald W, Wang J, Haber DA & Lee SB (2008) Adenosine transporter ENT4 is a direct target of EWS/WT1 translocation product and is highly expressed in desmoplastic small round cell tumor. PLoS One 3, e2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Samanta D & Semenza GL (2018) Metabolic adaptation of cancer and immune cells mediated by hypoxia‐inducible factors. Biochim Biophys Acta Rev Cancer 1870, 15–22. [DOI] [PubMed] [Google Scholar]
- 260. Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schönfeld C, Löffler M, Reyes G, Duszenko M, Karhausen J et al. (2005) HIF‐1‐dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med 202, 1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Pérez‐Torras S, Vidal‐Pla A, Cano‐Soldado P, Huber‐Ruano I, Mazo A & Pastor‐Anglada M (2013) Concentrative nucleoside transporter 1 (hCNT1) promotes phenotypic changes relevant to tumor biology in a translocation‐independent manner. Cell Death Dis 4, e648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Bhutia YD, Hung SW, Patel B, Lovin D & Govindarajan R (2011) CNT1 expression influences proliferation and chemosensitivity in drug‐resistant pancreatic cancer cells. Cancer Res 71, 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Errasti‐Murugarren E & Pastor‐Anglada M (2010) Drug transporter pharmacogenetics in nucleoside‐based therapies. Pharmacogenomics 11, 809–841. [DOI] [PubMed] [Google Scholar]
- 264. Zhang J, Visser F, King KM, Baldwin SA, Young JD & Cass CE (2007) The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev 26, 85–110. [DOI] [PubMed] [Google Scholar]
- 265. Romero MF, Chen A‐P, Parker MD & Boron WF (2013) The SLC4 family of bicarbonate (HCO₃−) transporters. Mol Aspects Med 34, 159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Parker MD & Boron WF (2013) The divergence, actions, roles, and relatives of sodium‐coupled bicarbonate transporters. Physiol Rev 93, 803–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Christensen HL, Nguyen AT, Pedersen FD & Damkier HH (2013) Na(+) dependent acid‐base transporters in the choroid plexus; insights from slc4 and slc9 gene deletion studies. Front Physiol 4, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Pushkin A & Kurtz I (2006) SLC4 base (HCO3‐, CO32‐) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Ren Physiol 290, F580–F599. [DOI] [PubMed] [Google Scholar]
- 269. Zhu Q, Lee DWK & Casey JR (2003) Novel topology in C‐terminal region of the human plasma membrane anion exchanger, AE1. J Biol Chem 278, 3112–3120. [DOI] [PubMed] [Google Scholar]
- 270. Abuladze N, Azimov R, Newman D, Sassani P, Liu W, Tatishchev S, Pushkin A & Kurtz I (2005) Critical amino acid residues involved in the electrogenic sodium‐bicarbonate cotransporter kNBC1‐mediated transport. J Physiol 565, 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Zhu Q, Kao L, Azimov R, Newman D, Liu W, Pushkin A, Abuladze N & Kurtz I (2010) Topological location and structural importance of the NBCe1‐A residues mutated in proximal renal tubular acidosis. J Biol Chem 285, 13416–13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Cordat E & Reithmeier RAF (2014) Structure, function, and trafficking of SLC4 and SLC26 anion transporters. Curr Top Membr 73, 1–67. [DOI] [PubMed] [Google Scholar]
- 273. Yu X, Yang G, Yan C, Baylon JL, Jiang J, Fan H, Lu G, Hasegawa K, Okumura H, Wang T et al. (2017) Dimeric structure of the uracil:proton symporter UraA provides mechanistic insights into the SLC4/23/26 transporters. Cell Res 27, 1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Huynh KW, Jiang J, Abuladze N, Tsirulnikov K, Kao L, Shao X, Newman D, Azimov R, Pushkin A, Zhou ZH et al. (2018) CryoEM structure of the human SLC4A4 sodium‐coupled acid‐base transporter NBCe1. Nat Commun 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Martzoukou O, Karachaliou M, Yalelis V, Leung J, Byrne B, Amillis S & Diallinas G (2015) Oligomerization of the UapA purine transporter is critical for ER‐Exit, plasma membrane localization and turnover. J Mol Biol 427, 2679–2696. [DOI] [PubMed] [Google Scholar]
- 276. Alguel Y, Amillis S, Leung J, Lambrinidis G, Capaldi S, Scull NJ, Craven G, Iwata S, Armstrong A, Mikros E et al. (2016) Structure of eukaryotic purine/H+ symporter UapA suggests a role for homodimerization in transport activity. Nat Commun 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Stewart AK, Chernova MN, Shmukler BE, Wilhelm S & Alper SL (2002) Regulation of AE2‐mediated Cl‐ transport by intracellular or by extracellular pH requires highly conserved amino acid residues of the AE2 NH2‐terminal cytoplasmic domain. J Gen Physiol 120, 707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Sterling D & Casey JR (1999) Transport activity of AE3 chloride/bicarbonate anion‐exchange proteins and their regulation by intracellular pH. Biochem J 344(Pt 1), 221–229. [PMC free article] [PubMed] [Google Scholar]
- 279. Gildea JJ, Xu P, Carlson JM, Gaglione RT, Bigler Wang D, Kemp BA, Reyes CM, McGrath HE, Carey RM, Jose PA et al. (2015) The sodium‐bicarbonate cotransporter NBCe2 ( slc4a5) expressed in human renal proximal tubules shows increased apical expression under high‐salt conditions. Am J Physiol Integr Comp Physiol 309, R1447–R1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Choi I, Aalkjaer C, Boulpaep EL & Boron WF (2000) An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405, 571–575. [DOI] [PubMed] [Google Scholar]
- 281. Jacobs S, Ruusuvuori E, Sipilä ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM et al. (2008) Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci USA 105, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Parker MD, Bouyer P, Daly CM & Boron WF (2008) Cloning and characterization of novel human SLC4A8 gene products encoding Na+‐driven Cl‐/HCO3(‐) exchanger variants NDCBE‐A, ‐C, and ‐D. Physiol Genomics 34, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Wang Z, Conforti L, Petrovic S, Amlal H, Burnham CE & Soleimani M (2001) Mouse Na+:HCO3‐ cotransporter isoform NBC‐3 (kNBC‐3): Cloning, expression, and renal distribution. Kidney Int 59, 1405–1414. [DOI] [PubMed] [Google Scholar]
- 284. Chen L‐M, Kelly ML, Parker MD, Bouyer P, Gill HS, Felie JM, Davis BA & Boron WF (2008) Expression and localization of Na‐driven Cl‐HCO(3)(‐) exchanger (SLC4A8) in rodent CNS. Neuroscience 153, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Peña‐Münzenmayer G, George AT, Shull GE, Melvin JE & Catalán MA (2016) Ae4 (Slc4a9) is an electroneutral monovalent cation‐dependent Cl − /HCO 3 − exchanger. J Gen Physiol 147, 423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Purkerson JM, Heintz EV, Nakamori A & Schwartz GJ (2014) Insights into acidosis‐induced regulation of SLC26A4 (pendrin) and SLC4A9 (AE4) transporters using three‐dimensional morphometric analysis of β‐intercalated cells. Am J Physiol Physiol 307, F601–F611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zhang W, Ogando DG, Bonanno JA & Obukhov AG (2015) Human SLC4A11 is a novel NH3/H+ co‐transporter. J Biol Chem 290, 16894–16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Kao L, Azimov R, Shao XM, Frausto RF, Abuladze N, Newman D, Aldave AJ & Kurtz I (2016) Multifunctional ion transport properties of human SLC4A11: comparison of the SLC4A11‐B and SLC4A11‐C variants. Am J Physiol Physiol 311, C820–C830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Donowitz M, Ming Tse C & Fuster D (2013) SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol Aspects Med 34, 236–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Pedersen SF & Counillon L (2019) The SLC9A‐C mammalian Na+/H+ exchanger family: molecules, mechanisms, and physiology. Physiol Rev 99, 2015–2113. [DOI] [PubMed] [Google Scholar]
- 291. Holmes RS & Spradling Reeves KD (2016) Evolution of vertebrate solute carrier family 9B genes and proteins (SLC9B): evidence for a marsupial origin for testis specific SLC9B1 from an ancestral vertebrate SLC9B2 gene. J Phylogenetics Evol Biol 4, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Xu H, Ghishan FK & Kiela PR (2018) SLC9 gene family: function, expression, and regulation. Compr Physiol 8, 555–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Donowitz M, Mohan S, Zhu CX, Chen T‐E, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R & Li X (2009) NHE3 regulatory complexes. J Exp Biol 212, 1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Padan E, Kozachkov L, Herz K & Rimon A (2009) NhaA crystal structure: functional‐structural insights. J Exp Biol 212, 1593–1603. [DOI] [PubMed] [Google Scholar]
- 295. Fafournoux P, Noël J & Pouysségur J (1994) Evidence that Na+/H+ exchanger isoforms NHE1 and NHE3 exist as stable dimers in membranes with a high degree of specificity for homodimers. J Biol Chem 269, 2589–2596. [PubMed] [Google Scholar]
- 296. Hisamitsu T, Ben Ammar Y, Nakamura TY & Wakabayashi S (2006) Dimerization is crucial for the function of the Na+/H + exchanger NHE1. Biochemistry 45, 13346–13355. [DOI] [PubMed] [Google Scholar]
- 297. Parker MD, Myers EJ & Schelling JR (2015) Na+‐H+ exchanger‐1 (NHE1) regulation in kidney proximal tubule. Cell Mol Life Sci 72, 2061–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Baumgartner M, Patel H & Barber DL (2004) Na + /H + exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Physiol 287, C844–C850. [DOI] [PubMed] [Google Scholar]
- 299. Szabó EZ, Numata M, Lukashova V, Iannuzzi P & Orlowski J (2005) beta‐Arrestins bind and decrease cell‐surface abundance of the Na+/H+ exchanger NHE5 isoform. Proc Natl Acad Sci USA 102, 2790–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Diering GH, Numata Y, Fan S, Church J & Numata M (2013) Endosomal acidification by Na+/H+ exchanger NHE5 regulates TrkA cell‐surface targeting and NGF‐induced PI3K signaling. Mol Biol Cell 24, 3435–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Diering GH & Numata M (2014) Endosomal pH in neuronal signaling and synaptic transmission: role of Na(+)/H(+) exchanger NHE5. Front Physiol 4, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Kondapalli KC, Prasad H & Rao R (2014) An inside job: how endosomal Na+ /H+ exchangers link to autism and neurological disease. Front Cell Neurosci 8, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Ilie A, Boucher A, Park J, Berghuis AM, McKinney RA & Orlowski J (2020) Assorted dysfunctions of endosomal alkali cation/proton exchanger SLC9A6 variants linked to Christianson syndrome. J Biol Chem 295, 7075–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Kondapalli KC, Kallay LM, Muszelik M & Rao R (2012) Unconventional chemiosmotic coupling of NHA2, a mammalian Na +/H+ antiporter, to a plasma membrane H+ gradient. J Biol Chem 287, 36239–36250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Soleimani M (2013) SLC26 Cl‐/HCO3‐ exchangers in the kidney: roles in health and disease. Kidney Int 84, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Alper SL & Sharma AK (2013) The SLC26 gene family of anion transporters and channels. Mol Aspects Med 34, 494–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Ohana E, Yang D, Shcheynikov N & Muallem S (2009) Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol 587, 2179–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Chang YN, Jaumann EA, Reichel K, Hartmann J, Oliver D, Hummer G, Joseph B & Geertsma ER (2019) Structural basis for functional interactions in dimers of SLC26 transporters. Nat Commun 10, 2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Chang Y‐N & Geertsma ER (2017) The novel class of seven transmembrane segment inverted repeat carriers. Biol Chem 398, 165–174. [DOI] [PubMed] [Google Scholar]
- 310. Seidler U & Nikolovska K (2019) Slc26 family of anion transporters in the gastrointestinal tract: expression, function, regulation, and role in disease. Compr Physiol 9, 839–872. [DOI] [PubMed] [Google Scholar]
- 311. Ohana E, Shcheynikov N, Park M & Muallem S (2012) Solute carrier family 26 member a2 (Slc26a2) protein functions as an electroneutral SOFormula/OH‐/Cl‐ exchanger regulated by extracellular Cl‐. J Biol Chem 287, 5122–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Ding X, Li D, Li M, Wang H, He Q, Wang Y, Yu H, Tian D & Yu Q (2018) SLC26A3 (DRA) prevents TNF‐alpha‐induced barrier dysfunction and dextran sulfate sodium‐induced acute colitis. Lab Investig 98, 462–476. [DOI] [PubMed] [Google Scholar]
- 313. Xiao F, Yu Q, Li J, Johansson MEV, Singh AK, Xia W, Riederer B, Engelhardt R, Montrose M, Soleimani M et al. (2014) Slc26a3 deficiency is associated with loss of colonic HCO3‐ secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol 211, 161–175. [DOI] [PubMed] [Google Scholar]
- 314. Ishiguro H (2014) HCO3 ‐ secretion by SLC26A3 and mucosal defence in the colon. Acta Physiol 211, 17–19. [DOI] [PubMed] [Google Scholar]
- 315. Cremer J, Arnoldini M & Hwa T (2017) Effect of water flow and chemical environment on microbiota growth and composition in the human colon. Proc Natl Acad Sci USA 114, 6438–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Engevik MA, Hickerson A, Shull GE & Worrell RT (2013) Acidic conditions in the NHE2 −/− mouse intestine result in an altered mucosa‐associated bacterial population with changes in mucus oligosaccharides. Cell Physiol Biochem 32, 111–128. [DOI] [PubMed] [Google Scholar]
- 317. Parks SK, Chiche J & Pouysségur J (2013) Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer 13, 611–623. [DOI] [PubMed] [Google Scholar]
- 318. Wike‐Hooley JL, Haveman J & Reinhold HS (1984) The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol 2, 343–366. [DOI] [PubMed] [Google Scholar]
- 319. Tannock IF & Rotin D (1989) Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res 49, 4373–4384. [PubMed] [Google Scholar]
- 320. Boedtkjer E, Bunch L & Pedersen SF (2012) Physiology, pharmacology and pathophysiology of the pH regulatory transport proteins NHE1 and NBCn1: similarities, differences, and implications for cancer therapy. Curr Pharm Des 18, 1345–1371. [DOI] [PubMed] [Google Scholar]
- 321. Harguindey S, Orozco JP, Alfarouk KO & Devesa J (2019) Hydrogen ion dynamics of cancer and a new molecular, biochemical and metabolic approach to the etiopathogenesis and treatment of brain malignancies. Int J Mol Sci 20, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Amith SR & Fliegel L (2013) Regulation of the Na/H exchanger (NHE1) in breast cancer metastasis. Cancer Res 73, 1259–1264. [DOI] [PubMed] [Google Scholar]
- 323. Flinck M, Kramer SH, Schnipper J, Andersen AP & Pedersen SF (2018) The acid‐base transport proteins NHE1 and NBCn1 regulate cell cycle progression in human breast cancer cells. Cell Cycle 17, 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Boedtkjer E (2019) Na+, HCO3− cotransporter NBCn1 accelerates breast carcinogenesis. Cancer Metastasis Rev 38, 165–178. [DOI] [PubMed] [Google Scholar]
- 325. Chapman JM, Knoepp SM, Byeon MK, Henderson KW & Schweinfest CW (2002) The colon anion transporter, down‐regulated in adenoma, induces growth suppression that is abrogated by E1A. Cancer Res 62, 5083–5088. [PubMed] [Google Scholar]
- 326. Gorbatenko A, Olesen CW, Boedtkjer E & Pedersen SF (2014) Regulation and roles of bicarbonate transporters in cancer. Front Physiol 5, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z & Soleimani M (2006) slc26a3 (dra)‐deficient mice display chloride‐losing diarrhea, enhanced colonic proliferation, and distinct up‐regulation of ion transporters in the colon. J Biol Chem 281, 37962–37971. [DOI] [PubMed] [Google Scholar]
- 328. Lytton J (2007) Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 406, 365–382. [DOI] [PubMed] [Google Scholar]
- 329. Monteith GR, Davis FM & Roberts‐Thomson SJ (2012) Calcium channels and pumps in cancer: changes and consequences. J Biol Chem 287, 31666–31673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Khananshvili D (2013) The SLC8 gene family of sodium–calcium exchangers (NCX) – structure, function, and regulation in health and disease. Mol Aspects Med 34, 220–235. [DOI] [PubMed] [Google Scholar]
- 331. Besserer GM, Ottolia M, Nicoll DA, Chaptal V, Cascio D, Philipson KD & Abramson J (2007) The second Ca2+‐binding domain of the Na+ Ca2+ exchanger is essential for regulation: crystal structures and mutational analysis. Proc Natl Acad Sci USA 104, 18467–18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Hilge M, Aelen J & Vuister GW (2006) Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol Cell 22, 15–25. [DOI] [PubMed] [Google Scholar]
- 333. Ren X & Philipson KD (2013) The topology of the cardiac Na+/Ca2+ exchanger, NCX1. J Mol Cell Cardiol 57, 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Brini M & Carafoli E (2011) The plasma membrane Ca2+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb Perspect Biol 3, a004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Quednau BD, Nicoll DA & Philipson KD (2004) The sodium/calcium exchanger family‐SLC8. Pflugers Arch 447, 543–548. [DOI] [PubMed] [Google Scholar]
- 336. Xu J, Yang Y, Xie R, Liu J, Nie X, An J, Wen G, Liu X, Jin H & Tuo B (2018) The NCX1/TRPC6 complex mediates TGFβ‐driven migration and invasion of human hepatocellular carcinoma cells. Cancer Res 78, 2564–2576. [DOI] [PubMed] [Google Scholar]
- 337. Herchuelz A & Pachera N (2018) The Na + /Ca 2+ exchanger and the plasma membrane Ca 2+ ‐ATPase in β‐cell function and diabetes. Neurosci Lett 663, 72–78. [DOI] [PubMed] [Google Scholar]
- 338. Hong K‐W, Lim JE, Kim JW, Tabara Y, Ueshima H, Miki T, Matsuda F, Cho YS, Kim Y & Oh B (2014) Identification of three novel genetic variations associated with electrocardiographic traits (QRS duration and PR interval) in East Asians. Hum Mol Genet 23, 6659–6667. [DOI] [PubMed] [Google Scholar]
- 339. Kim JW, Hong K‐W, Go MJ, Kim SS, Tabara Y, Kita Y, Tanigawa T, Cho YS, Han B‐G & Oh B (2012) A common variant in SLC8A1 is associated with the duration of the electrocardiographic QT interval. Am J Hum Genet 91, 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Hilgemann DW, Collins A & Matsuoka S (1992) Steady‐state and dynamic properties of cardiac sodium‐calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol 100, 933–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Boyman L, Hagen BM, Giladi M, Hiller R, Lederer WJ & Khananshvili D (2011) Proton‐sensing Ca2+ binding domains regulate the cardiac Na+/Ca2+ exchanger. J Biol Chem 286, 28811–28820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Jalloul AH, Szerencsei RT, Rogasevskaia TP & Schnetkamp PPM (2018) SLC24A family (K+‐dependent Na+‐Ca2+ exchanger, NCKX). In Encyclopedia of Signaling Molecules (Choi S, ed.), pp. 4994–5002. Springer International Publishing, Cham. [Google Scholar]
- 343. Dong H, Jiang Y, Triggle CR, Li X & Lytton J (2006) Novel role for K+‐dependent Na+/Ca2+ exchangers in regulation of cytoplasmic free Ca2+ and contractility in arterial smooth muscle. Am J Physiol Heart Circ Physiol 291, H1226–H1235. [DOI] [PubMed] [Google Scholar]
- 344. Jalloul AH, Szerencsei RT & Schnetkamp PPM (2016) Cation dependencies and turnover rates of the human K+‐dependent Na+‐Ca2+ exchangers NCKX1, NCKX2, NCKX3 and NCKX4. Cell Calcium 59, 1–11. [DOI] [PubMed] [Google Scholar]
- 345. Prevarskaya N, Ouadid‐Ahidouch H, Skryma R & Shuba Y (2014) Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks? Philos Trans R Soc B Biol Sci 369, 20130097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Andrikopoulos P, Baba A, Matsuda T, Djamgoz MBA, Yaqoob MM & Eccles SA (2011) Ca 2+ influx through reverse mode Na +/Ca 2+ exchange is critical for vascular endothelial growth factor‐mediated extracellular signal‐regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J Biol Chem 286, 37919–37931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Andrikopoulos P, Eccles SA & Yaqoob MM (2017) Coupling between the TRPC3 ion channel and the NCX1 transporter contributed to VEGF‐induced ERK1/2 activation and angiogenesis in human primary endothelial cells. Cell Signal 37, 12–30. [DOI] [PubMed] [Google Scholar]
- 348. Qu M, Yu J, Liu H, Ren Y, Ma C, Bu X & Lan Q (2017) The candidate tumor suppressor gene SLC8A2 inhibits invasion, angiogenesis and growth of glioblastoma. Mol Cells 40, 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Muñoz JJ, Drigo SA, Barros‐Filho MC, Marchi FA, Scapulatempo‐Neto C, Pessoa GS, Guimarães GC, Trindade Filho JCS, Lopes A, Arruda MAZ et al. (2015) Down‐regulation of SLC8A1 as a putative apoptosis evasion mechanism by modulation of calcium levels in penile carcinoma. J Urol 194, 245–251. [DOI] [PubMed] [Google Scholar]
- 350. Pelzl L, Hosseinzadeh Z, Alzoubi K, Al‐Maghout T, Schmidt S, Stournaras C & Lang F (2015) Impact of Na + /Ca 2+ exchangers on therapy resistance of ovary carcinoma cells. Cell Physiol Biochem 37, 1857–1868. [DOI] [PubMed] [Google Scholar]
- 351. Turner KL & Sontheimer H (2014) Cl‐ and K+ channels and their role in primary brain tumour biology. Philos Trans R Soc B Biol Sci 369, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Gagnon KB & Delpire E (2013) Physiology of SLC12 transporters: lessons from inherited human genetic mutations and genetically engineered mouse knockouts. Am J Physiol Cell Physiol 304, C693–C714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Bazúa‐Valenti S, Castañeda‐Bueno M & Gamba G (2016) Physiological role of SLC12 family members in the kidney. Am J Physiol Physiol 311, F131–F144. [DOI] [PubMed] [Google Scholar]
- 354. Sun PL, Jin Y, Park SY, Kim H, Park E, Jheon S, Kim K, Lee CT & Chung JH (2016) Expression of Na+‐K+‐2Cl‐ cotransporter isoform 1 (NKCC1) predicts poor prognosis in lung adenocarcinoma and EGFR‐mutated adenocarcinoma patients. QJM 109, 237–244. [DOI] [PubMed] [Google Scholar]
- 355. Chen Y‐F, Chou C‐Y, Ellory JC & Shen M‐R (2010) The emerging role of KCl cotransport in tumor biology. Am J Transl Res 2, 345–355. [PMC free article] [PubMed] [Google Scholar]
- 356. Brown TC, Murtha TD, Rubinstein JC, Korah R & Carling T (2018) SLC12A7 alters adrenocortical carcinoma cell adhesion properties to promote an aggressive invasive behavior. Cell Commun Signal 16, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Bergeron MJ, Clémençon B, Hediger MA & Markovich D (2013) SLC13 family of Na+‐coupled di‐ and tri‐carboxylate/sulfate transporters. Mol Aspects Med 34, 299–312. [DOI] [PubMed] [Google Scholar]
- 358. Pajor AM (2014) Sodium‐coupled dicarboxylate and citrate transporters from the SLC13 family. Pflügers Arch Eur J Physiol 466, 119–130. [DOI] [PubMed] [Google Scholar]
- 359. Markovich D (2014) Na+–sulfate cotransporter SLC13A1. Pflügers Arch Eur J Physiol 466, 131–137. [DOI] [PubMed] [Google Scholar]
- 360. Biber J, Custer M, Quabius ES, Murer H, Lötscher M & Kaissling B (1996) Immunolocalization of Na/SO4‐cotransport (NaSi‐1) in rat kidney. Pflügers Arch Eur J Physiol 432, 373–378. [DOI] [PubMed] [Google Scholar]
- 361. Markovich D, Regeer RR, Kunzelmann K & Dawson PA (2005) Functional characterization and genomic organization of the human Na+‐sulfate cotransporter hNaS2 gene (SLC13A4). Biochem Biophys Res Commun 326, 729–734. [DOI] [PubMed] [Google Scholar]
- 362. Colas C, Pajor AM & Schlessinger A (2015) Structure‐based identification of inhibitors for the SLC13 family of Na(+)/dicarboxylate cotransporters. Biochemistry 54, 4900–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Lee HW, Handlogten ME, Osis G, Clapp WL, Wakefield DN, Verlander JW & Weiner ID (2017) Expression of sodium‐dependent dicarboxylate transporter 1 (NaDC1/SLC13A2) in normal and neoplastic human kidney. Am J Physiol Ren Physiol 312, F427–F435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Bhutia YD, Kopel JJ, Lawrence JJ, Neugebauer V & Ganapathy V (2017) Plasma membrane Na+‐coupled citrate transporter (SLC13A5) and neonatal epileptic encephalopathy. Molecules 22, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Jones R & Morris M (2016) Monocarboxylate transporters: therapeutic targets and prognostic factors in disease. Clin Pharmacol Ther 100, 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Pérez‐Escuredo J, Van Hée VF, Sboarina M, Falces J, Payen VL, Pellerin L & Sonveaux P (2016) Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta 1863, 2481–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Halestrap AP (2013) The SLC16 gene family – structure, role and regulation in health and disease. Mol Aspects Med 34, 337–349. [DOI] [PubMed] [Google Scholar]
- 368. Halestrap AP (2012) The monocarboxylate transporter family—Structure and functional characterization. IUBMB Life 64, 1–9. [DOI] [PubMed] [Google Scholar]
- 369. Poole RC & Halestrap AP (1997) Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70‐kDa membrane glycoprotein of the immunoglobulin superfamily. J Biol Chem 272, 14624–14628. [DOI] [PubMed] [Google Scholar]
- 370. Muramatsu T (2016) Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem 159, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Wilson MC, Meredith D, Fox JEM, Manoharan C, Davies AJ & Halestrap AP (2005) Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4. J Biol Chem 280, 27213–27221. [DOI] [PubMed] [Google Scholar]
- 372. Park SJ, Smith CP, Wilbur RR, Cain CP, Kallu SR, Valasapalli S, Sahoo A, Guda MR, Tsung AJ & Velpula KK (2018) An overview of MCT1 and MCT4 in GBM: small molecule transporters with large implications. Am J Cancer Res 8, 1967–1976. [PMC free article] [PubMed] [Google Scholar]
- 373. Marchiq I & Pouysségur J (2016) Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H+ symporters. J Mol Med 94, 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Garcia CK, Brown MS, Pathak RK & Goldstein JL (1995) cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem 270, 1843–1849. [DOI] [PubMed] [Google Scholar]
- 375. Bröer S, Bröer A, Schneider H‐P, Stegen C, Halestrap AP & Deitmer JW (1999) Characterization of the high‐affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J 341, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Daniele LL, Sauer B, Gallagher SM, Pugh EN, Philp NJ & Philp NJ (2008) Altered visual function in monocarboxylate transporter 3 (Slc16a8) knockout mice. Am J Physiol Cell Physiol 295, C451–C457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Philp NJ, Yoon H & Lombardi L (2001) Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am J Physiol Physiol 280, C1319–C1326. [DOI] [PubMed] [Google Scholar]
- 378. Dong H & Wade MG (2017) Application of a nonradioactive assay for high throughput screening for inhibition of thyroid hormone uptake via the transmembrane transporter MCT8. Toxicol Vitr 40, 234–242. [DOI] [PubMed] [Google Scholar]
- 379. Johannes J, Braun D, Kinne A, Rathmann D, Köhrle J & Schweizer U (2016) Few amino acid exchanges expand the substrate spectrum of monocarboxylate transporter 10. Mol Endocrinol 30, 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Kim DK, Kanai Y, Chairoungdua A, Matsuo H, Cha SH & Endou H (2001) Expression cloning of a Na + ‐independent aromatic amino acid transporter with structural similarity to H + /monocarboxylate transporters. J Biol Chem 276, 17221–17228. [DOI] [PubMed] [Google Scholar]
- 381. Ramadan T, Camargo SMR, Summa V, Hunziker P, Chesnov S, Pos KM & Verrey F (2006) Basolateral aromatic amino acid transporter TAT1 (Slc16a10) functions as an efflux pathway. J Cell Physiol 206, 771–779. [DOI] [PubMed] [Google Scholar]
- 382. Yan L, Zucker S & Toole BP (2005) Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost 93, 199–204. [DOI] [PubMed] [Google Scholar]
- 383. Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF et al. (2008) Targeting lactate‐fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118, 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Pinheiro C, Longatto‐Filho A, Azevedo‐Silva J, Casal M, Schmitt FC & Baltazar F (2012) Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr 44, 127–139. [DOI] [PubMed] [Google Scholar]
- 385. Valença I, Pértega‐Gomes N, Vizcaino JR, Henrique RM, Lopes C, Baltazar F & Ribeiro D (2015) Localization of MCT2 at peroxisomes is associated with malignant transformation in prostate cancer. J Cell Mol Med 19, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Elia I, Rossi M, Stegen S, Broekaert D, Doglioni G, van Gorsel M, Boon R, Escalona‐Noguero C, Torrekens S, Verfaillie C et al. (2019) Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature 568, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Lacerda‐Abreu MA, Russo‐Abrahão T, Monteiro RQ, Rumjanek FD & Meyer‐Fernandes JR (2018) Inorganic phosphate transporters in cancer: Functions, molecular mechanisms and possible clinical applications. Biochim Biophys Acta Rev Cancer 1870, 291–298. [DOI] [PubMed] [Google Scholar]
- 388. Forster IC, Hernando N, Biber J & Murer H (2013) Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med 34, 386–395. [DOI] [PubMed] [Google Scholar]
- 389. Ravera S, Virkki LV, Murer H & Forster IC (2007) Deciphering PiT transport kinetics and substrate specificity using electrophysiology and flux measurements. Am J Physiol Cell Physiol 293, C606–C620. [DOI] [PubMed] [Google Scholar]
- 390. Salaun C, Rodrigues P & Heard JM (2001) Transmembrane topology of PiT‐2, a phosphate transporter‐retrovirus receptor. J Virol 75, 5584–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Couasnay G, Bon N, Devignes CS, Sourice S, Bianchi A, Véziers J, Weiss P, Elefteriou F, Provot S, Guicheux J et al. (2019) PiT1/Slc20a1 is required for endoplasmic reticulum homeostasis, chondrocyte survival, and skeletal development. J Bone Miner Res 34, 387–398. [DOI] [PubMed] [Google Scholar]
- 392. Yoshiko Y, Candeliere GA, Maeda N & Aubin JE (2007) Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol 27, 4465–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Beck‐Cormier S, Lelliott CJ, Logan JG, Lafont DT, Merametdjian L, Leitch VD, Butterfield NC, Protheroe HJ, Croucher PI, Baldock PA et al. (2019) Slc20a2, encoding the phosphate transporter PiT2, is an important genetic determinant of bone quality and strength. J Bone Miner Res 34, 1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Zoidis E, Ghirlanda‐Keller C, Gosteli‐Peter M, Zapf J & Schmid C (2004) Regulation of phosphate (Pi) transport and NaPi‐III transporter (Pit‐1) mRNA in rat osteoblasts. J Endocrinol 181, 531–540. [DOI] [PubMed] [Google Scholar]
- 395. Chien ML, Foster JL, Douglas JL & Garcia JV (1997) The amphotropic murine leukemia virus receptor gene encodes a 71‐kilodalton protein that is induced by phosphate depletion. J Virol 71, 4564–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Bon N, Couasnay G, Bourgine A, Sourice S, Beck‐Cormier S, Guicheux J & Beck L (2018) Phosphate (Pi)‐regulated heterodimerization of the high‐affinity sodium‐dependent Pi transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular Pi sensing independently of Pi uptake. J Biol Chem 293, 2102–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Wagner CA, Hernando N, Forster IC & Biber J (2014) The SLC34 family of sodium‐dependent phosphate transporters. Pflügers Arch Eur J Physiol 466, 139–153. [DOI] [PubMed] [Google Scholar]
- 398. Forster IC (2019) The molecular mechanism of SLC34 proteins: insights from two decades of transport assays and structure‐function studies. Pflügers Arch Eur J Physiol 471, 15–42. [DOI] [PubMed] [Google Scholar]
- 399. Miyamoto K‐I, Haito‐Sugino S, Kuwahara S, Ohi A, Nomura K, Ito M, Kuwahata M, Kido S, Tatsumi S, Kaneko I et al. (2011) Sodium‐dependent phosphate cotransporters: lessons from gene knockout and mutation studies. J Pharm Sci 100, 3719–3730. [DOI] [PubMed] [Google Scholar]
- 400. Leung J & Crook M (2019) Disorders of phosphate metabolism. J Clin Pathol 72, 741–747. [DOI] [PubMed] [Google Scholar]
- 401. Levi M & Gratton E (2019) Visualizing the regulation of SLC34 proteins at the apical membrane. Pflügers Arch Eur J Physiol 471, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Blaine J, Okamura K, Giral H, Breusegem S, Caldas Y, Millard A, Barry N & Levi M (2009) PTH‐induced internalization of apical membrane NaPi2a: role of actin and myosin VI. Am J Physiol Physiol 297, C1339–C1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Lanzano L, Lei T, Okamura K, Giral H, Caldas Y, Masihzadeh O, Gratton E, Levi M & Blaine J (2011) Differential modulation of the molecular dynamics of the type IIa and IIc sodium phosphate cotransporters by parathyroid hormone. Am J Physiol Physiol 301, C850–C861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Marks J (2019) The role of SLC34A2 in intestinal phosphate absorption and phosphate homeostasis. Pflugers Arch 471, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Brown RB & Razzaque MS (2018) Phosphate toxicity and tumorigenesis. Biochim Biophys Acta Rev Cancer 1869, 303–309. [DOI] [PubMed] [Google Scholar]
- 406. Li J, Dong W, Li Z, Wang H, Gao H & Zhang Y (2019) Impact of SLC20A1 on the Wnt/β‐catenin signaling pathway in somatotroph adenomas. Mol Med Rep 20, 3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Salaün C, Leroy C, Rousseau A, Boitez V, Beck L & Friedlander G (2010) Identification of a novel transport‐independent function of PiT1/SLC20A1 in the regulation of TNF‐induced apoptosis. J Biol Chem 285, 34408–34418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Beck L, Leroy C, Salaün C, Margall‐Ducos G, Desdouets C & Friedlander G (2009) Identification of a novel function of PiT1 critical for cell proliferation and independent of its phosphate transport activity. J Biol Chem 284, 31363–31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Ye W, Chen C, Gao Y, Zheng ZS, Xu Y, Yun M, Weng HW, Xie D, Ye S & Zhang JX (2017) Overexpression of SLC34A2 is an independent prognostic indicator in bladder cancer and its depletion suppresses tumor growth via decreasing c‐Myc expression and transcriptional activity. Cell Death Dis 8, e2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Bao Z, Chen L & Guo S (2019) Knockdown of SLC34A2 inhibits cell proliferation, metastasis, and elevates chemosensitivity in glioma. J Cell Biochem 120, 10205–10214. [DOI] [PubMed] [Google Scholar]
- 411. Wang Y, Yang W, Pu Q, Yang Y, Ye S, Ma Q, Ren J, Cao Z, Zhong G, Zhang X et al. (2015) The effects and mechanisms of SLC34A2 in tumorigenesis and progression of human non‐small cell lung cancer. J Biomed Sci 22, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Chen J, Wang P, Cai R, Peng H, Zhang C & Zhang M (2019) SLC34A2 promotes neuroblastoma cell stemness via enhancement of miR‐25/Gsk3β‐mediated activation of Wnt/β‐catenin signaling. FEBS Open Bio 9, 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Shayakul C, Clémençon B & Hediger MA (2013) The urea transporter family (SLC14): physiological, pathological and structural aspects. Mol Aspects Med 34, 313–322. [DOI] [PubMed] [Google Scholar]
- 414. Klein JD (2014) Expression of urea transporters and their regulation. Subcell Biochem 73, 79–107. [DOI] [PubMed] [Google Scholar]
- 415. Knepper MA & Star RA (1990) The vasopressin‐regulated urea transporter in renal inner medullary collecting duct. Am J Physiol Physiol 259, F393–F401. [DOI] [PubMed] [Google Scholar]
- 416. Nigam SK (2018) The SLC22 transporter family: a paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu Rev Pharmacol Toxicol 58, 663–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Lai RE, Jay CE & Sweet DH (2018) Organic solute carrier 22 (SLC22) family: potential for interactions with food, herbal/dietary supplements, endogenous compounds, and drugs. J Food Drug Anal 26, S45–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Koepsell H (2013) The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Aspects Med 34, 413–435. [DOI] [PubMed] [Google Scholar]
- 419. Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC & Bhatnagar V (2015) Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10, 2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Zhu C, Nigam KB, Date RC, Bush KT, Springer SA, Saier MH, Wu W, Nigam SK & Nigam SK (2015) Evolutionary analysis and classification of OATs, OCTs, OCTNs, and other SLC22 transporters: structure‐function implications and analysis of sequence motifs. PLoS One 10, e0140569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Keller T, Egenberger B, Gorboulev V, Bernhard F, Uzelac Z, Gorbunov D, Wirth C, Koppatz S, Dötsch V, Hunte C et al. (2011) The large extracellular loop of organic cation transporter 1 influences substrate affinity and is pivotal for oligomerization. J Biol Chem 286, 37874–37886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Nigam SK, Bush KT, Martovetsky G, Ahn S‐Y, Liu HC, Richard E, Bhatnagar V & Wu W (2015) The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev 95, 83–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Nigam SK & Bhatnagar V (2018) The systems biology of uric acid transporters: the role of remote sensing and signaling. Curr Opin Nephrol Hypertens 27, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Bush KT, Wu W, Lun C & Nigam SK (2017) The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut–liver– kidney axis. J Biol Chem 292, 15789–15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. International Transporter Consortium , Giacomini KM, Huang S‐M, Tweedie DJ, Benet LZ, Brouwer KLR, Chu X, Dahlin A, Evers R, Fischer V et al. (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9, 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. White DL, Saunders VA, Dang P, Engler J, Zannettino ACW, Cambareri AC, Quinn SR, Manley PW & Hughes TP (2006) OCT‐1‐mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT‐1 activity is the cause of low in vitro sensitivity to imatinib. Blood 108, 697–704. [DOI] [PubMed] [Google Scholar]
- 427. Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, Burckhardt BC, Sabolić I & Burckhardt G (2008) Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem 283, 16332–16341. [DOI] [PubMed] [Google Scholar]
- 428. Schulz C, Fork C, Bauer T, Golz S, Geerts A, Schömig E & Gründemann D (2014) SLC22A13 catalyses unidirectional efflux of aspartate and glutamate at the basolateral membrane of type A intercalated cells in the renal collecting duct. Biochem J 457, 243–251. [DOI] [PubMed] [Google Scholar]
- 429. Bennett KM, Liu J, Hoelting C & Stoll J (2011) Expression and analysis of two novel rat organic cation transporter homologs, SLC22A17 and SLC22A23. Mol Cell Biochem 352, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Devireddy LR, Gazin C, Zhu X & Green MR (2005) A cell‐surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 123, 1293–1305. [DOI] [PubMed] [Google Scholar]
- 431. Langelueddecke C, Roussa E, Fenton RA, Wolff NA, Lee W‐K & Thévenod F (2012) Lipocalin‐2 (24p3/Neutrophil Gelatinase‐associated Lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J Biol Chem 287, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Koepsell H, Lips K & Volk C (2007) Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24, 1227–1251. [DOI] [PubMed] [Google Scholar]
- 433. Lozano E, Herraez E, Briz O, Robledo VS, Hernandez‐Iglesias J, Gonzalez‐Hernandez A & Marin JJG (2013) Role of the plasma membrane transporter of organic cations OCT1 and its genetic variants in modern liver pharmacology. Biomed Res Int 2013, 692071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Kou L, Sun R, Ganapathy V, Yao Q & Chen R (2018) Recent advances in drug delivery via the organic cation/carnitine transporter 2 (OCTN2/SLC22A5). Expert Opin Ther Targets 22, 715–726. [DOI] [PubMed] [Google Scholar]
- 435. Pochini L, Galluccio M, Scalise M, Console L & Indiveri C (2019) OCTN: a small transporter subfamily with great relevance to human pathophysiology, drug discovery, and diagnostics. SLAS Discov Adv Life Sci R&D 24, 89–110. [DOI] [PubMed] [Google Scholar]
- 436. Wu X, Prasad PD, Leibach FH & Ganapathy V (1998) cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochem Biophys Res Commun 246, 589–595. [DOI] [PubMed] [Google Scholar]
- 437. Aouida M, Poulin R & Ramotar D (2010) The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin‐A5. J Biol Chem 285, 6275–6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Okabe M, Unno M, Harigae H, Kaku M, Okitsu Y, Sasaki T, Mizoi T, Shiiba K, Takanaga H, Terasaki T et al. (2005) Characterization of the organic cation transporter SLC22A16: a doxorubicin importer. Biochem Biophys Res Commun 333, 754–762. [DOI] [PubMed] [Google Scholar]
- 439. Rafnar T, Vermeulen SH, Sulem P, Thorleifsson G, Aben KK, Witjes JA, Grotenhuis AJ, Verhaegh GW, van de Kaa CAH, Besenbacher S et al. (2011) European genome‐wide association study identifies SLC14A1 as a new urinary bladder cancer susceptibility gene. Hum Mol Genet 20, 4268–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Frullanti E, Colombo F, Falvella FS, Galvan A, Noci S, De Cecco L, Incarbone M, Alloisio M, Santambrogio L, Nosotti M et al. (2012) Association of lung adenocarcinoma clinical stage with gene expression pattern in noninvolved lung tissue. Int J cancer 131, E643–E648. [DOI] [PubMed] [Google Scholar]
- 441. Dong Z, Ran J, Zhou H, Chen J, Lei T, Wang W, Sun Y, Lin G, Bankir L & Yang B (2013) Urea transporter UT‐B deletion induces DNA damage and apoptosis in mouse bladder urothelium. PLoS One 8, e76952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Hou R, Kong X, Yang B, Xie Y & Chen G (2017) SLC14A1: a novel target for human urothelial cancer. Clin Transl Oncol 19, 1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Mohelnikova‐Duchonova B, Brynychova V, Hlavac V, Kocik M, Oliverius M, Hlavsa J, Honsova E, Mazanec J, Kala Z, Melichar B et al. (2013) The association between the expression of solute carrier transporters and the prognosis of pancreatic cancer. Cancer Chemother Pharmacol 72, 669–682. [DOI] [PubMed] [Google Scholar]
- 444. Zhao W, Wang Y & Yue X (2018) SLC22A16 upregulation is an independent unfavorable prognostic indicator in gastric cancer. Futur Oncol 14, 2139–2148. [DOI] [PubMed] [Google Scholar]
- 445. Wang X, Liao X, Yang C, Huang K, Yu T, Yu L, Han C, Zhu G, Zeng X, Liu Z et al. (2019) Identification of prognostic biomarkers for patients with hepatocellular carcinoma after hepatectomy. Oncol Rep 41, 1586–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Heise M, Lautem A, Knapstein J, Schattenberg JM, Hoppe‐Lotichius M, Foltys D, Weiler N, Zimmermann A, Schad A, Gründemann D et al. (2012) Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer 12, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Shibayama Y, Ushinohama K, Ikeda R, Yoshikawa Y, Motoya T, Takeda Y & Yamada K (2006) Effect of methotrexate treatment on expression levels of multidrug resistance protein 2, breast cancer resistance protein and organic anion transporters Oat1, Oat2 and Oat3 in rats. Cancer Sci 97, 1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Sreenivasan Tantuan S & Viljoen CD (2018) Imatinib affects the expression of SLC22A1 in a non‐linear concentration‐dependent manner within 24 hours. Med Sci Monit Basic Res 24, 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Schaeffeler E, Hellerbrand C, Nies AT, Winter S, Kruck S, Hofmann U, van der Kuip H, Zanger UM, Koepsell H & Schwab M (2011) DNA methylation is associated with downregulation of the organic cation transporter OCT1 (SLC22A1) in human hepatocellular carcinoma. Genome Med 3, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Lautem A, Heise M, Gräsel A, Hoppe‐Lotichius M, Weiler N, Foltys D, Knapstein J, Schattenberg JM, Schad A, Zimmermann A et al. (2013) Downregulation of organic cation transporter 1 (SLC22A1) is associated with tumor progression and reduced patient survival in human cholangiocellular carcinoma. Int J Oncol 42, 1297–1304. [DOI] [PubMed] [Google Scholar]
- 451. Qu Q, Qu J, Zhan M, Wu LX, Zhang YW, Lou XY, Fu LJ & Zhou HH (2013) Different involvement of promoter methylation in the expression of organic cation/carnitine transporter 2 (OCTN2) in cancer cell lines. PLoS One 8, e76474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Scalise M, Galluccio M, Accardi R, Cornet I, Tommasino M & Indiveri C (2012) Human OCTN2 (SLC22A5) is down‐regulated in virus‐ and nonvirus‐mediated cancer. Cell Biochem Funct 30, 419–425. [DOI] [PubMed] [Google Scholar]
- 453. Montalbetti N, Simonin A, Kovacs G & Hediger MA (2013) Mammalian iron transporters: families SLC11 and SLC40. Mol Aspects Med 34, 270–287. [DOI] [PubMed] [Google Scholar]
- 454. Blackwell JM, Goswami T, Evans CA, Sibthorpe D, Papo N, White JK, Searle S, Miller EN, Peacock CS, Mohammed H et al. (2001) SLC11A1 (formerly NRAMP1) and disease resistance. Cell Microbiol 3, 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Yanatori I & Kishi F (2019) DMT1 and iron transport. Free Radic Biol Med 133, 55–63. [DOI] [PubMed] [Google Scholar]
- 456. Tabuchi M, Tanaka N, Nishida‐Kitayama J, Ohno H & Kishi F (2002) Alternative splicing regulates the subcellular localization of divalent metal transporter 1 isoforms. Mol Biol Cell 13, 4371–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL & Hediger MA (1997) Cloning and characterization of a mammalian proton‐coupled metal‐ion transporter. Nature 388, 482–488. [DOI] [PubMed] [Google Scholar]
- 458. Garrick MD, Singleton ST, Vargas F, Kuo H‐C, Zhao L, Knöpfel M, Davidson T, Costa M, Paradkar P, Roth JA et al. (2006) DMT1: which metals does it transport? Biol Res 39, 79–85. [DOI] [PubMed] [Google Scholar]
- 459. Toyokuni S (2009) Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci 100, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S & Andrews NC (2005) The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1, 191–200. [DOI] [PubMed] [Google Scholar]
- 461. Madejczyk MS & Ballatori N (2012) The iron transporter ferroportin can also function as a manganese exporter. Biochim Biophys Acta Biomembr 1818, 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Schweigel‐Röntgen M (2014) The families of zinc (SLC30 and SLC39) and copper (SLC31) transporters. Curr Top Membr 73, 321–355. [DOI] [PubMed] [Google Scholar]
- 463. Huang L & Tepaamorndech S (2013) The SLC30 family of zinc transporters – a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med 34, 548–560. [DOI] [PubMed] [Google Scholar]
- 464. Yu YY, Kirschke CP & Huang L (2007) Immunohistochemical analysis of ZnT1, 4, 5, 6, and 7 in the mouse gastrointestinal tract. J Histochem Cytochem 55, 223–234. [DOI] [PubMed] [Google Scholar]
- 465. Palmiter RD & Huang L (2004) Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch 447, 744–751. [DOI] [PubMed] [Google Scholar]
- 466. Palmiter RD & Findley SD (1995) Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J 14, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Langmade SJ, Ravindra R, Daniels PJ & Andrews GK (2000) The transcription factor MTF‐1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem 275, 34803–34809. [DOI] [PubMed] [Google Scholar]
- 468. Nishito Y & Kambe T (2019) Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J Biol Chem 294, 15686–15697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Jeong J & Eide DJ (2013) The SLC39 family of zinc transporters. Mol Aspects Med 34, 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Bafaro E, Liu Y, Xu Y & Dempski RE (2017) The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct Target Ther 2, 17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Eide DJ (2006) Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta Mol Cell Res 1763, 711–722. [DOI] [PubMed] [Google Scholar]
- 472. Bowers K & Srai SKS (2018) The trafficking of metal ion transporters of the Zrt‐ and Irt‐like protein family. Traffic 19, 813–822. [DOI] [PubMed] [Google Scholar]
- 473. Yamasaki S, Sakata‐Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K et al. (2007) Zinc is a novel intracellular second messenger. J Cell Biol 177, 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Han C‐T, Schoene NW & Lei KY (2009) Influence of zinc deficiency on Akt‐Mdm2‐p53 and Akt‐p21 signaling axes in normal and malignant human prostate cells. Am J Physiol Physiol 297, C1188–C1199. [DOI] [PubMed] [Google Scholar]
- 475. Milon B, Dhermy D, Pountney D, Bourgeois M & Beaumont C (2001) Differential subcellular localization of hZip1 in adherent and non‐adherent cells. FEBS Lett 507, 241–246. [DOI] [PubMed] [Google Scholar]
- 476. Wang F, Dufner‐Beattie J, Kim B‐E, Petris MJ, Andrews G & Eide DJ (2004) Zinc‐stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J Biol Chem 279, 24631–24639. [DOI] [PubMed] [Google Scholar]
- 477. Cousins RJ (2010) Gastrointestinal factors influencing zinc absorption and homeostasis. Int J Vitam Nutr Res 80, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Kim H, Wu X & Lee J (2013) SLC31 (CTR) family of copper transporters in health and disease. Mol Aspects Med 34, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Zhou B & Gitschier J (1997) hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA 94, 7481–7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Lee J, Peña MMO, Nose Y & Thiele DJ (2002) Biochemical characterization of the human copper transporter Ctr1. J Biol Chem 277, 4380–4387. [DOI] [PubMed] [Google Scholar]
- 481. Magistrato A, Pavlin M, Qasem Z & Ruthstein S (2019) Copper trafficking in eukaryotic systems: current knowledge from experimental and computational efforts. Curr Opin Struct Biol 58, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Sahni J & Scharenberg AM (2013) The SLC41 family of MgtE‐like magnesium transporters. Mol Aspects Med 34, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483. de Baaij JHF, Arjona FJ, van den Brand M, Lavrijsen M, Lameris ALL, Bindels RJM & Hoenderop JGJ (2016) Identification of SLC41A3 as a novel player in magnesium homeostasis. Sci Rep 6, 28565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Kolisek M, Launay P, Beck A, Sponder G, Serafini N, Brenkus M, Froschauer EM, Martens H, Fleig A & Schweigel M (2008) SLC41A1 is a novel mammalian Mg 2+ carrier. J Biol Chem 283, 16235–16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Goytain A & Quamme GA (2005) Functional characterization of human SLC41A1, a Mg 2+ transporter with similarity to prokaryotic MgtE Mg 2+ transporters. Physiol Genomics 21, 337–342. [DOI] [PubMed] [Google Scholar]
- 486. Mandt T, Song Y, Scharenberg AM & Sahni J (2011) SLC41A1 Mg2+ transport is regulated via Mg2+‐dependent endosomal recycling through its N‐terminal cytoplasmic domain. Biochem J 439, 129–139. [DOI] [PubMed] [Google Scholar]
- 487. Khan AA & Quigley JG (2013) Heme and FLVCR‐related transporter families SLC48 and SLC49. Mol Aspects Med 34, 669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, Berg CL, Sassa S, Wood BL & Abkowitz JL (2004) Identification of a human heme exporter that is essential for erythropoiesis. Cell 118, 757–766. [DOI] [PubMed] [Google Scholar]
- 489. Ressnerova A, Raudenska M, Holubova M, Svobodova M, Polanska H, Babula P, Masarik M & Gumulec J (2016) Zinc and copper homeostasis in head and neck cancer: review and meta‐analysis. Curr Med Chem 23, 1304–1330. [DOI] [PubMed] [Google Scholar]
- 490. Blaszczyk U & Duda‐Chodak A (2013) Magnesium: its role in nutrition and carcinogenesis. Rocz Państwowego Zakładu Hig 64, 165–171. [PubMed] [Google Scholar]
- 491. Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K & Librowski T (2017) Antioxidant and anti‐inflammatory effects of zinc. Zinc‐dependent NF‐κB signaling. Inflammopharmacology 25, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Arredondo M & Núñez MT (2005) Iron and copper metabolism. Mol Aspects Med 26, 313–327. [DOI] [PubMed] [Google Scholar]
- 493. Boer JL, Mulrooney SB & Hausinger RP (2014) Nickel‐dependent metalloenzymes. Arch Biochem Biophys 544, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Zhu B, Zhi Q, Xie Q, Wu X, Gao Y, Chen X & Shi L (2019) Reduced expression of ferroportin1 and ceruloplasmin predicts poor prognosis in adrenocortical carcinoma. J Trace Elem Med Biol 56, 52–59. [DOI] [PubMed] [Google Scholar]
- 495. Miller LD, Coffman LG, Chou JW, Black MA, Bergh J, D’Agostino R, Torti SV & Torti FM (2011) An iron regulatory gene signature predicts outcome in breast cancer. Cancer Res 71, 6728–6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496. Kong Y, Hu L, Lu K, Wang Y, Xie Y, Gao L, Yang G, Xie B, He W, Chen G et al. (2019) Ferroportin downregulation promotes cell proliferation by modulating the Nrf2–miR‐17‐5p axis in multiple myeloma. Cell Death Dis 10, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Cloonan N, Brown MK, Steptoe AL, Wani S, Forrest ARR, Kolle G, Gabrielli B & Grimmond SM (2008) The miR‐17‐5p microRNA is a key regulator of the G1/S phase cell cycle transition. Genome Biol 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Wu J, Bao L, Zhang Z & Yi X (2017) Nrf2 induces cisplatin resistance via suppressing the iron export related gene SLC40A1 in ovarian cancer cells. Oncotarget 8, 93502–93515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Baltaci AK & Yuce K (2018) Zinc transporter proteins. Neurochem Res 43, 517–530. [DOI] [PubMed] [Google Scholar]
- 500. Pan Z, Choi S, Ouadid‐Ahidouch H, Yang JM, Beattie JH & Korichneva I (2017) Zinc transporters and dysregulated channels in cancers. Front Biosci 22, 623–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Fan Q, Cai Q, Li P, Wang W, Wang J, Gerry E, Wang T‐L, Shih I‐M, Nephew KP & Xu Y (2017) The novel ZIP4 regulation and its role in ovarian cancer. Oncotarget 8, 90090–90107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang S‐M, Cousins RJ et al. (2007) Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci USA 104, 18636–18641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Takatani‐Nakase T (2018) Zinc transporters and the progression of breast cancers. Biol Pharm Bull 41, 1517–1522. [DOI] [PubMed] [Google Scholar]
- 504. Yu Z, Zhou R, Zhao Y, Pan Y, Liang H, Zhang J, Tai S, Jin L & Teng C (2019) Blockage of SLC31A1‐dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death. Cell Prolif 52, e12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505. Wolf FI & Trapani V (2012) Magnesium and its transporters in cancer: a novel paradigm in tumour development. Clin Sci 123, 417–427. [DOI] [PubMed] [Google Scholar]
- 506. Mendes PMV, Bezerra DLC, dos Santos LR, de Oliveira Santos R, de Sousa Melo SR, Morais JBS, Severo JS, Vieira SC & do Nascimento Marreiro Dilina (2018) Magnesium in breast cancer: what is its influence on the progression of this disease? Biol Trace Elem Res 184, 334–339. [DOI] [PubMed] [Google Scholar]
- 507. Xie J, Cheng C‐s, Zhu XY, Shen YH, Song LB, Chen H, Chen Z, Liu LM & Meng ZQ (2019) Magnesium transporter protein solute carrier family 41 member 1 suppresses human pancreatic ductal adenocarcinoma through magnesium‐dependent Akt/mTOR inhibition and bax‐associated mitochondrial apoptosis. Aging 11, 2681–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Pols TWH, Noriega LG, Nomura M, Auwerx J & Schoonjans K (2011) The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol 54, 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. Claudel T, Zollner G, Wagner M & Trauner M (2011) Role of nuclear receptors for bile acid metabolism, bile secretion, cholestasis, and gallstone disease. Biochim Biophys Acta Mol Basis Dis 1812, 867–878. [DOI] [PubMed] [Google Scholar]
- 510. Claro Da Silva T, Polli JE & Swaan PW (2013) The solute carrier family 10 (SLC10): beyond bile acid transport. Mol Aspects Med 34, 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Bijsmans ITGW, Bouwmeester RAM, Geyer J, Faber KN & van de Graaf SFJ (2012) Homo‐ and hetero‐dimeric architecture of the human liver Na + ‐dependent taurocholate co‐transporting protein. Biochem J 441, 1007–1016. [DOI] [PubMed] [Google Scholar]
- 512. Chothe PP, Czuba LC, Moore RH & Swaan PW (2018) Human Bile Acid Transporter ASBT (SLC10A2) forms functional non‐covalent homodimers and higher order oligomers. Biochim Biophys Acta 1860, 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513. Slijepcevic D, Roscam Abbing RLP, Katafuchi T, Blank A, Donkers JM, van Hoppe S, de Waart DR, Tolenaars D, van der Meer JHM, Wildenberg M et al. (2017) Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice. Hepatology 66, 1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Ananthanarayanan M, Ng OC, Boyer JL & Suchy FJ (1994) Characterization of cloned rat liver Na(+)‐bile acid cotransporter using peptide and fusion protein antibodies. Am J Physiol 267, G637–G643. [DOI] [PubMed] [Google Scholar]
- 515. Wang L, Prasad B, Salphati L, Chu X, Gupta A, Hop CECA, Evers R & Unadkat JD (2015) Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics. Drug Metab Dispos 43, 367–374. [DOI] [PubMed] [Google Scholar]
- 516. Weinman SA. Electrogenicity of Na(+)‐coupled bile acid transporters. Yale J Biol Med 70, 331–340. [PMC free article] [PubMed] [Google Scholar]
- 517. Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H et al. (2012) Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1, e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. Li W & Urban S (2016) Entry of hepatitis B and hepatitis D virus into hepatocytes: basic insights and clinical implications. J Hepatol 64, S32–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Dawson PA, Lan T & Rao A (2009) Bile acid transporters. J Lipid Res 50, 2340–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Weinman SA, Carruth MW & Dawson PA (1998) Bile acid uptake via the human apical sodium‐bile acid cotransporter is electrogenic. J Biol Chem 273, 34691–34695. [DOI] [PubMed] [Google Scholar]
- 521. Bakhaus K, Fietz D, Kliesch S, Weidner W, Bergmann M & Geyer J (2018) The polymorphism L204F affects transport and membrane expression of the sodium‐dependent organic anion transporter SOAT (SLC10A6). J Steroid Biochem Mol Biol 179, 36–44. [DOI] [PubMed] [Google Scholar]
- 522. Grosser G, Bennien J, Sánchez‐Guijo A, Bakhaus K, Döring B, Hartmann M, Wudy SA & Geyer J (2018) Transport of steroid 3‐sulfates and steroid 17‐sulfates by the sodium‐dependent organic anion transporter SOAT (SLC10A6). J Steroid Biochem Mol Biol 179, 20–25. [DOI] [PubMed] [Google Scholar]
- 523. Geyer J, Bakhaus K, Bernhardt R, Blaschka C, Dezhkam Y, Fietz D, Grosser G, Hartmann K, Hartmann MF, Neunzig J et al. (2017) The role of sulfated steroid hormones in reproductive processes. J Steroid Biochem Mol Biol 172, 207–221. [DOI] [PubMed] [Google Scholar]
- 524. Hagenbuch B & Meier PJ (2004) Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO super‐family, new nomenclature and molecular/functional properties. Pflugers Arch Eur J Physiol 447, 653–665. [DOI] [PubMed] [Google Scholar]
- 525. Obaidat A, Roth M & Hagenbuch B (2012) The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol 52, 135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Zaïr ZM, Eloranta JJ, Stieger B & Kullak‐Ublick GA (2008) Pharmacogenetics of OATP (SLC21/SLCO), OAT and OCT (SLC22) and PEPT (SLC15) transporters in the intestine, liver and kidney. Pharmacogenomics 9, 597–624. [DOI] [PubMed] [Google Scholar]
- 527. König J (2011) Uptake transporters of the human OATP family. Handb Exp Pharmacol 201, 1–28. [DOI] [PubMed] [Google Scholar]
- 528. Rebello S, Zhao S, Hariry S, Dahlke M, Alexander N, Vapurcuyan A, Hanna I & Jarugula V (2012) Intestinal OATP1A2 inhibition as a potential mechanism for the effect of grapefruit juice on aliskiren pharmacokinetics in healthy subjects. Eur J Clin Pharmacol 68, 697–708. [DOI] [PubMed] [Google Scholar]
- 529. Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG et al. (2007) Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther 81, 362–370. [DOI] [PubMed] [Google Scholar]
- 530. Eloranta JJ, Hiller C, Jüttner M & Kullak‐Ublick GA (2012) The SLCO1A2 gene, encoding human organic anion‐transporting polypeptide 1A2, is transactivated by the vitamin D receptor. Mol Pharmacol 82, 37–46. [DOI] [PubMed] [Google Scholar]
- 531. Zheng J, Chan T, Cheung FSG, Zhu L, Murray M & Zhou F (2014) PDZK1 and NHERF1 regulate the function of human organic anion transporting polypeptide 1A2 (OATP1A2) by modulating its subcellular trafficking and stability. PLoS One 9, e94712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Choi JH, Murray JW & Wolkoff AW (2011) PDZK1 binding and serine phosphorylation regulate subcellular trafficking of organic anion transport protein 1a1. Am J Physiol Liver Physiol 300, G384–G393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Nakamura Y, Nakanishi T, Shimada H, Shimizu J, Aotani R, Maruyama S, Higuchi K, Okura T, Deguchi Y & Tamai I (2018) Prostaglandin transporter OATP2A1/ SLCO2A1 is essential for body temperature regulation during fever. J Neurosci 38, 5584–5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Visentin M, Chang MH, Romero MF, Zhao R & Goldman ID (2012) Substrate‐ and pH‐specific antifolate transport mediated by organic anion‐transporting polypeptide 2B1 (OATP2B1‐SLCO2B1). Mol Pharmacol 81, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Roth M, Obaidat A & Hagenbuch B (2012) OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 165, 1260–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Wagner M & Trauner M (2016) Recent advances in understanding and managing cholestasis. F1000Research 5, 705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537. Pan Q, Zhang X, Zhang L, Cheng Y, Zhao N, Li F, Zhou X, Chen S, Li J, Xu S et al. (2018) Solute carrier organic anion transporter family member 3A1 is a bile acid efflux transporter in cholestasis. Gastroenterology 155, 1578–1592.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538. Ban MJ, Ji SH, Lee C‐K, Bae SB, Kim HJ, Ahn TS, Lee MS, Baek M‐J & Jeong D (2017) Solute carrier organic anion transporter family member 4A1 (SLCO4A1) as a prognosis marker of colorectal cancer. J Cancer Res Clin Oncol 143, 1437–1447. [DOI] [PubMed] [Google Scholar]
- 539. Li T & Apte U (2015) Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv Pharmacol 74, 263–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Wang W, Xue S, Ingles SA, Chen Q, Diep AT, Frankl HD, Stolz A & Haile RW (2001) An association between genetic polymorphisms in the ileal sodium‐dependent bile acid transporter gene and the risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 10, 931–936. [PubMed] [Google Scholar]
- 541. Jean‐Pierre R, Dawson PA, Rao A, Drachenberg CB, Heath J, Shang AC, Hu S, Zhan M, Polli JE & Cheng K (2015) Slc10a2‐null mice uncover colon cancer‐promoting actions of endogenous fecal bile acids. Carcinogenesis 36, 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Shi A‐x, Zhou Y, Zhang X‐y, Zhao Y‐s, Qin H‐y, Wang Y‐p & Wu X‐a (2017) Irinotecan‐induced bile acid malabsorption is associated with down‐regulation of ileal Asbt (Slc10a2) in mice. Eur J Pharm Sci 102, 220–229. [DOI] [PubMed] [Google Scholar]
- 543. Schulte RR & Ho RH (2019) Organic anion transporting polypeptides: emerging roles in cancer pharmacology. Mol Pharmacol 95, 490–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Niemi M (2007) Role of OATP transporters in the disposition of drugs. Pharmacogenomics 8, 787–802. [DOI] [PubMed] [Google Scholar]
- 545. Thakkar N, Lockhart AC & Lee W (2015) Role of Organic Anion‐Transporting Polypeptides (OATPs) in cancer therapy. AAPS J 17, 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546. Collins JF, Kiela PR, Xu H, Zeng J & Ghishan FK (1998) Increased NHE2 expression in rat intestinal epithelium during ontogeny is transcriptionally mediated. Am J Physiol Physiol 275, C1143–C1150. [DOI] [PubMed] [Google Scholar]
- 547. Collins JF, Xu H, Kiela PR, Zeng J & Ghishan FK (1997) Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Physiol 273, C1937–C1946. [DOI] [PubMed] [Google Scholar]
- 548. Hahn MK & Blakely RD (2007) The functional impact of SLC6 transporter genetic variation. Annu Rev Pharmacol Toxicol 47, 401–441. [DOI] [PubMed] [Google Scholar]
- 549. Kurian MA, Gissen P, Smith M, Heales SJ & Clayton PT (2011) The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol 10, 721–733. [DOI] [PubMed] [Google Scholar]
- 550. Seow HF, Bröer S, Bröer A, Bailey CG, Potter SJ, Cavanaugh JA & Rasko JEJ (2004) Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet 36, 1003–1007. [DOI] [PubMed] [Google Scholar]
- 551. Yu M, Yongzhi H, Chen S, Luo X, Lin Y, Zhou Y, Jin H, Hou B, Deng Y, Tu L et al. (2017) The prognostic value of GLUT1 in cancers: a systematic review and meta‐analysis. Oncotarget 8, 43356–43367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552. Liao Q‐D, Wang C‐G, Zhu Y‐D, Chen W‐H, Shao S‐L, Jiang F‐N & Xu X‐M (2016) Decreased expression of SLC39A14 is associated with tumor aggressiveness and biochemical recurrence of human prostate cancer. Onco Targets Ther 9, 4197–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553. Latif A, Chadwick AL, Kitson SJ, Gregson HJ, Sivalingam VN, Bolton J, McVey RJ, Roberts SA, Marshall KM, Williams KJ et al. (2017) Monocarboxylate transporter 1 (MCT1) is an independent prognostic biomarker in endometrial cancer. BMC Clin Pathol 17, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554. Yue M, Jiang J, Gao P, Liu H & Qing G (2017) Oncogenic MYC activates a feedforward regulatory loop promoting essential amino acid metabolism and tumorigenesis. Cell Rep 21, 3819–3832. [DOI] [PubMed] [Google Scholar]
- 555. Bin B‐H, Seo J & Kim ST (2018) Function, structure, and transport aspects of ZIP and ZnT zinc transporters in immune cells. J Immunol Res 2018, 9365747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556. Fisel P, Schaeffeler E & Schwab M (2018) Clinical and functional relevance of the monocarboxylate transporter family in disease pathophysiology and drug therapy. Clin Transl Sci 11, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557. Futagi Y, Kobayashi M, Narumi K, Furugen A & Iseki K (2018) Identification of a selective inhibitor of human monocarboxylate transporter 4. Biochem Biophys Res Commun 495, 427–432. [DOI] [PubMed] [Google Scholar]
- 558. Polański R, Hodgkinson CL, Fusi A, Nonaka D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE et al. (2014) Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin Cancer Res 20, 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559. Vishwakarma P, Banerjee A, Pasrija R, Prasad R & Lynn AM (2018) Phylogenetic and conservation analyses of MFS transporters. 3 Biotech 8, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Saier MH, Daniels GA, Boerner P & Lin J (1988) Neutral amino acid transport systems in animal cells: potential targets of oncogene action and regulators of cellular growth. J Membr Biol 104, 1–20. [DOI] [PubMed] [Google Scholar]
- 561. Palacín M, Estévez R, Bertran J & Zorzano A (1998) Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev 78, 969–1054. [DOI] [PubMed] [Google Scholar]
- 562. Meixner E, Goldmann U, Sedlyarov V, Scorzoni S, Rebsamen M, Girardi E & Superti‐Furga G (2020) A substrate‐based ontology for human solute carriers. Mol Syst Biol 16, e9652. [DOI] [PMC free article] [PubMed] [Google Scholar]


