Abstract
OBJECTIVE
Poor cognition has been observed in children and adolescents with youth-onset type 1 (T1D) and type 2 diabetes (T2D) compared with control subjects without diabetes. Differences in cognition between youth-onset T1D and T2D, however, are not known. Thus, using data from SEARCH for Diabetes in Youth, a multicenter, observational cohort study, we tested the association between diabetes type and cognitive function in adolescents and young adults with T1D (n = 1,095) or T2D (n = 285).
RESEARCH DESIGN AND METHODS
Cognition was assessed via the National Institutes of Health Toolbox Cognition Battery, and age-corrected composite Fluid Cognition scores were used as the primary outcome. Confounder-adjusted linear regression models were run. Model 1 included diabetes type and clinical site. Model 2 additionally included sex, race/ethnicity, waist-to-height ratio, diabetes duration, depressive symptoms, glycemic control, any hypoglycemic episode in the past year, parental education, and household income. Model 3 additionally included the Picture Vocabulary score, a measure of receptive language and crystallized cognition.
RESULTS
Having T2D was significantly associated with lower fluid cognitive scores before adjustment for confounders (model 1; P < 0.001). This association was attenuated to nonsignificance with the addition of a priori confounders (model 2; P = 0.06) and Picture Vocabulary scores (model 3; P = 0.49). Receptive language, waist-to-height ratio, and depressive symptoms remained significant in the final model (P < 0.01 for all, respectively).
CONCLUSIONS
These data suggest that while youth with T2D have worse fluid cognition than youth with T1D, these differences are accounted for by differences in crystallized cognition (receptive language), central adiposity, and mental health. These potentially modifiable factors are also independently associated with fluid cognitive health, regardless of diabetes type. Future studies of cognitive health in people with youth-onset diabetes should focus on investigating these significant factors.
Introduction
The Centers for Disease Control and Prevention estimates that 10.5% of the population in the U.S. is living with type 1 diabetes (T1D) or type 2 diabetes (T2D), according to the 2020 National Diabetes Statistics Report (1). T1D and T2D impact major organ systems and specific organs including cardiovascular, gastrointestinal, kidney, eye, liver, and muscle. The brain too has come into focus as a critical system affected by diabetes. Specifically, deficits in cognitive function have been reported in children and adults with diabetes (2–6).
Studies among adults with T1D have demonstrated poorer performance on tests of cognitive functioning in comparison with nondiabetic control groups (7–9). Adults with T2D also show measurable cognitive deficits compared with control subjects (2,4,10). Cognitive function in adults has been shown to decline significantly with longer diabetes disease duration in both T1D (11) and T2D (11,12). Given the well-documented long-term cognitive impact of both T1D and T2D in adults, the neurodevelopmental consequences of extended exposure to diabetes among people with youth-onset T1D or T2D is of concern for health management and quality of life across the life course.
Consistent with the adult literature, compared with their counterparts without diabetes, children and youth with T1D show significantly poorer cognitive performance overall (3,7,13). Importantly, these deficits are most pronounced in specific domains of fluid cognitive functioning, including processing speed (3,13,14), cognitive flexibility (7), attention (3,6,13,14), and memory (14,15). Although limited, research in youth T2D also shows deficits in memory and processing speed compared with youth without diabetes who are matched by obesity status (16,17). It is unclear, however, whether diabetes type differentially impacts neurocognition in youth-onset disease, as no comparison has yet been made between those with T1D and T2D.
In the current study, we compared cognitive performance between youth and young adults with T1D and T2D from the SEARCH for Diabetes in Youth (SEARCH) study. We evaluated fluid cognition specifically, given its impact on self-care and good diabetes management (18–21). Furthermore, we examined the relationship between youth-onset diabetes type and fluid cognition while accounting for potentially confounding clinical and sociodemographic factors. These factors included glycemic control (3,22,23), episodes of hypoglycemia (3,13,24), overweight and obesity (25), depression (26), and socioeconomic variables (e.g., parental education, household income, race/ethnicity) (27,28). Recent evidence has indicated that youth with T2D have a more aggressive clinical course (29,30). Thus, in line with these recent findings we hypothesized that fluid cognitive function in the group with T2D would be worse compared with the T1D group, independent of relevant sociodemographic and clinical factors.
Research Design and Methods
Participants
The SEARCH study cohort is a longitudinal study of individuals with youth-onset (diagnosed <20 years of age) T1D or T2D. The cohort was recruited from the population-based SEARCH Registry, which has ascertained youth-onset T1D and T2D cases from locations throughout the U.S. including Colorado with southwestern American Indian reservations, Ohio, Washington, South Carolina, and California (31) continuously since 2002. Individuals diagnosed with T1D or T2D in 2002–2006 or 2008 were enrolled in the cohort study shortly after diagnosis (baseline). Follow-up visits were conducted in 2011–2015 and 2015–2019 among those with ≥5 years’ diabetes duration. All participants or parent/guardians provided written informed consent and assent as appropriate by age.
In the current study, we used data from participants who completed in-person procedures from the second follow-up visit (2015–2019) of the cohort study (N = 1,673), when participants were, on average, 21.6 (SD 5.1) years old with an average of 11.0 (SD 3.4) years diabetes duration. Only participants with etiologically defined (29) T1D (antibody positive or antibody negative/missing and insulin sensitive) (n = 1,138) or T2D (antibody negative and insulin resistant) (n = 301) having complete data on neurocognitive outcomes (T1D, n = 1,095; T2D, n = 285) were included in the analytic sample, leaving a sample size of 1,380.
Data Collection From Second Follow-up Visit
Participants and parent/guardians completed risk factor, health (e.g., clinical complications), and well-being (e.g., depressive symptoms) assessments via standardized reporting forms and underwent laboratory and cognitive testing. Race/ethnicity, household income, and parents’ educational attainment were self-reported. Race/ethnicity of the participant was dichotomized into minority race/ethnicity or non-Hispanic White. Household income was categorized as <$25,000, $25,000–$49,999, $50,000–$74,999, ≥$75,000, or do not know or refused. The highest level of education from either parent was collapsed into a dichotomous variable: high school or less and some college or more. Participants’ depressive symptoms were assessed by self-report using the Center for Epidemiologic Studies Depression Scale (CES-D) (continuous variable), with higher scores indicating more depressive symptoms (32).
Participant height, waist circumference, and weight were measured, and participant waist-to-height ratio (WHtR) was derived. Participants also self-reported on experiencing one or more hypoglycemic events over the past 12 months prior to the study visit (yes/no). Diabetes duration (years) was derived using the date of diabetes diagnosis and date of the second follow-up visit. Glycemic control was quantified by taking the area under the curve (AUC) of repeated laboratory measures of hemoglobin A1c (HbA1c) collected during baseline and follow-up visits. Thus, the measure of HbA1c used in our models represents long-term glycemic control.
National Institutes of Health Toolbox Cognition Battery
The National Institutes of Health Toolbox Cognition Battery (NIHT-CB) was used to assess cognitive function in young adults participating in the second follow-up visit of the SEARCH study cohort. The NIHT-CB was specifically developed to provide a repeatable and rapidly administered assessment of fluid and crystallized cognitive domains (33), as well as generate composite scores for fluid cognition and crystallized cognition. Fluid cognition collectively refers to executive function skills (e.g., cognitive flexibility, working memory) and processing speed and attention skills, all of which allow an individual to solve problems when presented with a novel situation. Crystallized cognition is a measure of acquired knowledge (e.g., vocabulary). The measures have been demonstrated to have good convergent and discriminant validity (34) and concordance with more typically used neuropsychological measures (35). Subdomains of fluid cognition included cognitive flexibility, working and episodic memory, processing speed, and attention/inhibitory control and were measured via the Dimensional Change Card Sort, List Sorting Working Memory, Picture Sequence Memory, Pattern Comparison Processing Speed, and Flanker Inhibitory Control and Attention tests, respectively. The composite score for fluid cognition represents performance across all subdomain tests. Subdomains of crystallized cognition obtained in SEARCH included receptive language measured via the Picture Vocabulary test.
All tests were administered to participants on a tablet computer during the second follow-up visit by trained study staff providing instruction and supervision. Age-corrected standard scores that were based on the normative population were used for the fluid composite score and the subdomain test scores. An age-corrected score of 100 (SD 15) indicates performance equivalent to the national average relative to age-adjusted norms. While the predominant approach to using the NIHT-CB is to use the fully corrected scores, we chose a priori not to use the fully corrected scores because the racial/ethnic distribution of the SEARCH cohort is enriched with people of color, particularly among youth with T2D, where Black, Hispanic, and American Indian youth make up the majority of the T2D group (Table 1). The underlying racial/ethnic distribution (2010 U.S. Census [36]) used to derive the fully corrected NIHT-CB scores does not reflect the racial/ethnic distribution of SEARCH participants. We believe that the full correction would disproportionately and incorrectly adjust the NIHT-CB scores in the youth with T2D, specifically, thus potentially introducing systematic bias into our comparisons between youth with T2D and T1D. For reference, however, fully corrected scores are reported in Supplementary Table 1 by diabetes group.
Table 1.
SEARCH study cohort participant characteristics and NIHT-CB scores by diabetes type
| Total (N = 1,380) | T1D (n = 1,095) | T2D (n = 285) | P value* | |
|---|---|---|---|---|
| Participant characteristics | ||||
| Age at SEARCH 4 visit (years), mean (SD) | 1,380 | 21.0 (5.0) | 24.6 (4.4) | <0.001 |
| Female sex, n (%) | 1,380 | 583 (53.2) | 201 (70.5) | <0.001 |
| Race/ethnicity, n (%) | 1,379 | <0.001 | ||
| Non-Hispanic White | 619 (56.6) | 51 (17.9) | ||
| Non-Hispanic Black | 181 (16.5) | 142 (49.8) | ||
| Hispanic | 241 (22.0) | 60 (21.1) | ||
| Asian/Pacific Islander | 37 (3.4) | 6 (2.1) | ||
| American Indian | 11 (1.0) | 25 (8.8) | ||
| Other | 5 (0.5) | 1 (0.4) | ||
| WHtR, mean (SD) | 1,376 | 0.49 (0.07) | 0.64 (0.10) | <0.001 |
| Duration of diabetes (years), mean (SD) | 1,380 | 10.8 (3.4) | 10.2 (3.6) | 0.01 |
| HbA1c (%) AUC, mean (SD) | 1,361 | 8.7 (1.5) | 8.7 (2.3) | 0.83 |
| HbA1c (mmol/mol), mean (SD) | 72 (4.5) | 72 (6.9) | ||
| Any hypoglycemic events in past 12 months, n (%) | 1,356 | 106 (9.8) | 10 (3.6) | <0.001 |
| CES-D score, median (IQR) | 1,372 | 7.0 (4.0, 14.0) | 11.0 (6.0, 19.0) | <0.001 |
| Highest participant education, n (%) | 1,362 | <0.001 | ||
| No high school diploma | 363 (33.7) | 50 (17.6) | ||
| High school graduate | 218 (20.2) | 101 (35.6) | ||
| Some college or associate’s degree | 341 (31.6) | 107 (37.7) | ||
| Bachelor’s degree or higher | 156 (14.5) | 26 (9.2) | ||
| Highest parent education, n (%) | 1,319 | <0.001 | ||
| No high school diploma | 65 (6.2) | 25 (9.4) | ||
| High school graduate | 154 (14.6) | 94 (35.3) | ||
| Some college or associate’s degree | 311 (29.5) | 103 (38.7) | ||
| Bachelor’s degree or higher | 523 (49.7) | 44 (16.5) | ||
| Household income ($), n (%) | 1,365 | <0.001 | ||
| <25,000 | 169 (15.6) | 94 (33.1) | ||
| 25,000–49,999 | 185 (17.1) | 68 (23.9) | ||
| 50,000–74,999 | 127 (11.7) | 12 (4.2) | ||
| ≥75,000 | 326 (30.2) | 13 (4.6) | ||
| Do not know or refused | 274 (25.3) | 97 (34.2) | ||
| NIHT-CB test scores, mean (SD)‡ | ||||
| Composite fluid cognition | 1,380 | 95.5 (16.7) | 84.7 (17.1) | <0.001 |
| Pattern Comparison Processing Speed (fluid) | 1,380 | 100.4 (21.0) | 92.8 (20.5) | <0.001 |
| Flanker Inhibitory Control and Attention (fluid) | 1,380 | 84.7 (12.8) | 79.2 (13.1) | <0.001 |
| List Sorting Working Memory (fluid) | 1,380 | 100.1 (13.8) | 91.2 (15.1) | <0.001 |
| Picture Sequence Memory (fluid) | 1,380 | 102.7 (16.8) | 95.7 (15.7) | <0.001 |
| Dimensional Change Card Sort (fluid) | 1,380 | 98.0 (16.8) | 90.3 (17.5) | <0.001 |
| Picture Vocabulary (crystallized) | 1,380 | 103.6 (15.0) | 91.5 (14.1) | <0.001 |
IQR, interquartile range.
P value from t test or Wilcoxon test (continuous) or χ2 test (categorical).
NIHT age-corrected cognition test scores (mean 100 [SD 15]; lower scores indicate poorer performance on cognitive test in comparison with national average for age).
The primary outcome of interest was the age-corrected composite score for fluid cognition. We included receptive language (Picture Vocabulary test) as a covariate in our analysis because it is considered a measure of crystallized cognition, which is an indicator of premorbid cognitive health (37,38).
Statistical Analyses
Characteristics of SEARCH participants were described using mean (SD) or median (interquartile range) for continuous variables and count (%) for categorical variables. Comparisons (T2D vs. T1D) were evaluated using t tests or Wilcoxon tests for continuous variables and χ2 tests for categorical variables.
We performed a complete case analysis (no missing covariate data, n = 1,277) using a sequence of multivariable linear regression models to test the relationship between diabetes type (T2D vs. T1D) and age-corrected composite fluid cognition score. Model 1 tested the direct association between diabetes type and age-corrected composite fluid cognition score, while adjusting for clinic site. Model 2 included model 1 variables and added relevant covariates: participant sex, race/ethnicity, WHtR, duration of diabetes, CES-D score, HbA1c AUC, having any hypoglycemic episode in the past 12 months, parental highest level of education, and household income. Model 3 included model 2 variables and added participant age-corrected scores on the Picture Vocabulary test, a surrogate for crystallized cognition. Secondary models included model 3 covariates and were run with each fluid cognition subdomain age-corrected score individually (e.g., Dimensional Card Sort, Flanker Inhibitory Control and Attention, etc.). Estimates from the linear regression models were summarized using β-estimates and 95% CIs. All statistical analyses were conducted using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Participants with T2D differed significantly from participants with T1D across all sociodemographic and clinical characteristics (P < 0.05 for all) except for HbA1c AUC (Table 1). Briefly, participants with T2D were, on average, older with higher WHtR and depressive symptoms scores, disproportionately female and of minority race/ethnicity, and from lower-income households with parents having lower educational attainment.
Participants with T2D performed at a full SD below the national average with respect to age-corrected composite fluid cognition score (mean [SD] 84.7 [17.1]). Both T2D and T1D groups performed a full SD below the national average with respect to age-corrected inhibitory control and sustained attention scores (79.2 [13.1] and 84.7 [12.8], respectively).
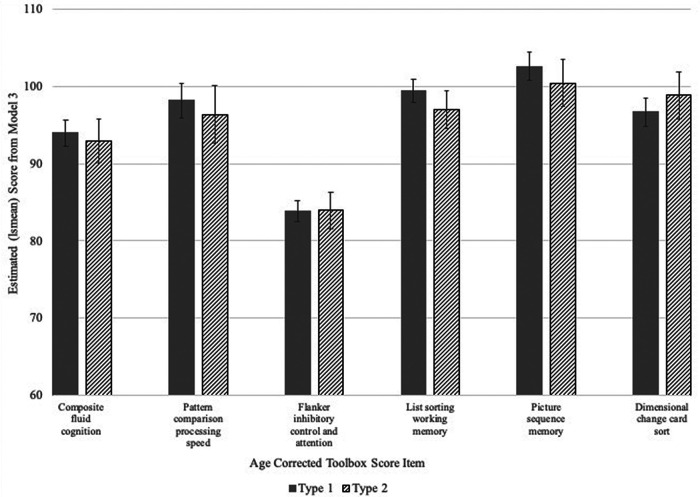
Participants with T2D performed significantly more poorly compared with the T1D group with respect to age-corrected composite fluid cognition scores (β [95% CI] –9.69 [–12.00, –7.39]; P < 0.001), without adjustment for additional covariates in model 1. Independent of the relevant covariates (model 2), however, the effect of diabetes type on age-corrected composite fluid cognition score did not reach statistical significance (β [95% CI] –2.84 [–5.83, 0.14]; P = 0.06). Minority race/ethnicity, lower parental education (high school or less), lower household income (<$25,000 or do not know/refused), higher depressive symptoms score (CES-D), and greater WHtR were significantly associated with lower age-corrected composite fluid cognition scores in this model (P < 0.05 for all). The addition of age-corrected Picture Vocabulary test scores in the final model (model 3) further attenuated the effect of diabetes type on composite fluid cognition (T2D vs. T1D β [95% CI] –0.99 [–3.80, 1.83]; P = 0.49). Greater WHtR and higher depression score remained significantly and inversely associated with age-corrected composite fluid cognition in model 3 (P < 0.05 for both), while female sex was positively associated with age-corrected composite fluid cognition scores (P = 0.04). As expected, better performance on the Picture Vocabulary test (higher score) was significantly associated with higher age-corrected composite fluid cognition scores (1-unit increase β [95% CI] 0.41 [0.35, 0.48]; P < 0.001) (Table 2). After controlling for covariates and age-corrected Picture Vocabulary scores, diabetes type was not significantly associated with performance on any of the fluid cognition subdomains (P ≥ 0.05 for all) (Fig. 1).
Table 2.
Estimates for multivariable regression models 1, 2, and 3 for the composite fluid cognition outcome among SEARCH cohort study participants with complete covariate data (n = 1,277)
| Model 1a | P | Model 2b | P | Model 3c | P | |
|---|---|---|---|---|---|---|
| Diabetes type: T2D vs. T1D | –9.69 (–12.00, –7.39) | <0.001 | –2.84 (–5.83, 0.14) | 0.06 | –0.99 (–3.80, 1.83) | 0.49 |
| Sex | 0.04 | |||||
| Female | — | — | 0.90 (–0.94, 2.75) | 0.33 | 1.81 (0.07, 3.54) | |
| Male | — | — | Ref. | Ref. | ||
| Minority race/ethnicity | 0.07 | |||||
| Yes | — | — | –4.68 (–6.76, –2.59) | <0.001 | –1.81 (–3.82, 0.19) | |
| No | — | — | Ref. | Ref. | ||
| Duration of diabetes (1-year increase) | — | — | –0.16 (–0.43, 0.10) | 0.22 | –0.15 (–0.40, 0.10) | 0.23 |
| WHtR (0.1-unit increase) | — | — | –1.76 (–2.92, –0.60) | <0.01 | –1.50 (–2.59, –0.41) | <0.01 |
| HbA1c AUC (1-unit increase) | — | — | –0.11 (–0.67, 0.45) | 0.70 | 0.24 (–0.29, 0.78) | 0.37 |
| Any hypoglycemic events (12 months) | 0.43 | 0.82 | ||||
| Yes | — | — | –1.29 (–4.49, 1.90) | –0.35 (–3.35, 2.65) | ||
| No | — | — | Ref. | Ref. | ||
| CES-D score (1-unit increase) | — | — | –0.18 (–0.28, –0.08) | <0.001 | –0.20 (–0.30, –0.10) | <0.0001 |
| Parental education | <0.01 | 0.53 | ||||
| High school graduate or less | — | — | –3.22 (–5.38, –1.06) | –0.65 (–2.72, 1.41) | ||
| Some college or more | — | — | Ref. | Ref. | ||
| Household income ($) | <0.01 | 0.14 | ||||
| <25,000 | — | — | –4.63 (–7.60, –1.66) | –2.43 (–5.23, 0.38) | ||
| 25,000–49,999 | — | — | –1.82 (–4.62, 0.98) | –0.49 (–3.13, 2.15) | ||
| 50,000–74,999 | — | — | –0.02 (–3.29, 3.24) | –0.16 (–3.22, 2.91) | ||
| Do not know or refused | — | — | –4.56 (–6.96, –1.75) | –2.69 (–5.15, –0.23) | ||
| ≥75,000 | — | — | Ref. | Ref. | ||
| Picture Vocabulary score (1-unit increase) | — | — | — | — | 0.41 (0.35, 0.48) | <0.0001 |
Data are presented as estimate (95% CI). Ref., referent.
Model 1 included the main effect for diabetes type (T2D vs. T1D). All models also adjusted for SEARCH clinical site.
Model 2 variables included the covariates sex, race/ethnicity, duration of diabetes, WHtR, CES-D, any hypoglycemic events in the past 12 months, parental education, household income, and glycemic control.
Model 3 variables included all model 1 and model 2 variables in addition to age-corrected Picture Vocabulary scores.
Figure 1.
Predicted means derived from model 3 for composite fluid cognitive scores and NIHT-CB fluid cognition subtest scores by diabetes type. Covariates in model 3 included SEARCH clinical site, sex, race/ethnicity, duration of diabetes, WHtR, CES-D, any hypoglycemic events in the past 12 months, parental education, household income, glycemic control, and Picture Vocabulary score. lsmean, least squares mean.
Conclusions
Among youth and young adults with youth-onset diabetes participating in the SEARCH study, a large, geographically and ethnically diverse longitudinal cohort study, we found significantly poorer fluid cognition in individuals with T2D compared with T1D in unadjusted comparisons. When additionally accounting for confounders and differences in receptive language, however, fluid cognition in the T2D group did not significantly differ from the T1D group. This finding suggests that worse fluid cognition in youth with T2D compared with those with T1D can be attributed, in part, to differences in crystallized cognition, obesity, and depression between the two groups. Moreover, in our analysis, crystallized cognition, obesity, and depression were found to be significant factors contributing to fluid cognitive function across both youth-onset diabetes types.
Crystallized cognition is defined as an individual’s experience-based knowledge accumulated through prior exposure, learning, and interactions with the environment (39,40). While a measure of lifetime experience, early childhood factors that may impact the development of crystallized cognition include, but are not necessarily limited to, child home enrichment (27,41) (e.g., availability of books in the home), quality of child care (42), preschool attendance (27), and lifetime opportunity. Crystallized cognition is related to early-life socioeconomic factors, including parental education and income (28,43). Therefore, crystallized cognitive function may significantly overlap with socioeconomic status in the current analysis. Indeed, in a secondary analysis, we confirmed the significant associations among age-corrected Picture Vocabulary scores and household income, parental education, and race/ethnicity (P < 0.001 for all; data not shown). Variance inflation factor estimates, however, indicated no significant collinearity among age-corrected Picture Vocabulary scores, household income, parental education, and race/ethnicity in the final model (variance inflation factor <2 for all). Thus, we conclude that age-corrected Picture Vocabulary scores or crystallized cognition may also be capturing the effect of early life exposures and cognitive health prior to diabetes onset (premorbid) on fluid cognitive function that are not accounted for by socioeconomic factors in model 3. The significant association between age-corrected Picture Vocabulary scores and composite fluid cognitive scores in model 3 may also be due, in part, to the bidirectional relationship between fluid and crystallized cognitive abilities (28).
Fluid cognition is related to but distinct from crystallized abilities. It involves the effortful maintenance of information in working memory to plan, execute, and evaluate goal-directed behavior. Fluid cognition is sensitive to alterations of neurologic integrity, such as aging and disease processes like diabetes (44,45). While our final model showed no independent effect of diabetes type on fluid cognition, we did observe that WHtR, a surrogate marker of central adiposity and obesity, was significantly and inversely associated with age-corrected composite fluid cognition scores. Thus, independent of diabetes type and other clinical and socioeconomic confounders, obesity is a significant factor in fluid cognitive health in adolescents and young adults with youth-onset diabetes in the SEARCH cohort. Consistent with the sensitivity of fluid cognitive performance to disease processes, obesity, which is part and parcel to the development of T2D specifically, is consistently shown to be associated with poorer cognitive health in adults and youth (25,46–48). The directionality of the relationship between obesity and cognitive health in the SEARCH cohort, however, cannot be determined by the current analysis given the cross-sectional nature of the NIHT-CB data.
Glycemic control is an additional factor that has been linked to poorer cognitive health outcomes in people with diabetes in the extant literature (3,22). We found no association between glycemic control and fluid cognitive performance, independent of diabetes type. This is likely due to the inclusion of both diabetes type and glycemic control in the same model and the overlap in variance explained by each of these variables. The role of glycemic control on these cognitive outcomes within youth with either type of diabetes was not the focus of this report, and it remains to be explored.
It is important to also note our finding of depression as an additional significant factor related to poorer fluid cognitive function regardless of diabetes type. Unfortunately, the relationship between greater depression symptomology and poorer cognition in youth has yielded mixed results, largely owing to which pediatric sample is studied. For example, in a large cohort study of typically developing adolescents, depression was not related to inhibitory control either cross-sectionally or longitudinally (49). In contrast, in a smaller study of children and adolescents who were enrolled in a pediatric neuropsychology program, Kavanaugh et al. (26) found significant associations between executive functions and clinically diagnosed depression and anxiety. In youth with diabetes, the prevalence of mild to moderate depression has been reported in 12–40% of those with T1D (50,51) and ∼20% of youth with T2D (51,52). The relationship between depression and cognitive functioning in youth with diabetes is, however, understudied. Therefore, our study may be the first to report an inverse relationship between depression symptomology and fluid cognition in youth with diabetes. Further research is needed to confirm and expand these findings from the SEARCH cohort.
To our knowledge, this study is the first to compare cognitive function between adolescents and young adults with T1D or T2D. Our results are generally consistent with prior evidence of cognitive dysfunction in both youth-onset diabetes types compared with control subjects without diabetes; meta-analyses have demonstrated consistently poorer performance on tests of fluid cognition in children and youth with T1D compared with control subjects (3,6). On tests of crystallized cognition, however, these meta-analyses did not observe differences between the T1D group and control group. This is supported by our descriptive findings; the T1D group scored, on average, at the population mean for picture vocabulary but showed some deficits in inhibitory control (Flanker test) compared with the age-matched NIHT-CB normative population. In a small clinical study of adolescents with obesity and T2D, Yau et al. (17) found significantly poorer performance across all cognitive function assessments, including tests of crystallized cognition, administered to the T2D group compared with the obesity-matched control groups. Similar to Yau et al., Brady et al. (16) showed that adolescents with obesity and T2D scored significantly lower on tests of fluid cognitive function (e.g., processing speed) than control subjects. Brady et al. also showed significantly poorer performance on tests of crystallized cognition among the T2D group. In our descriptive comparisons, we too found significantly poorer performance on the Picture Vocabulary test in T2D compared with the NIHT-CB normative population. Of note, however, Brady et al. included a limited set of covariates in their models and Yau et al. matched youth with T2D to control subjects on a limited set of socioeconomic and clinical factors compared with our comprehensive a priori–defined list of confounders. Moreover, Brady et al. and Yau et al. both matched the T2D group to the control group by obesity status, thus removing the potential effect of obesity on cognition in youth-onset diabetes. Despite not having a nondiabetic control group in the current study (by design), our findings provide evidence that youth with T2D have significantly worse fluid cognitive function compared with youth with T1D; however, this T2D effect is fully accounted for by differences between the two diabetes groups in crystallized cognition and other important clinical factors like obesity and depression.
The importance and novelty of the current study’s results are bolstered by the strength of the study design and the study population. Specifically, following each participant’s diabetes diagnosis and subsequent enrollment in SEARCH, prospective data were collected via in-person visits and medical record abstraction on clinical factors considered etiologic for diabetes severity and progression, including those thought relevant to cognition (e.g., glycemic control). While the cross-sectional nature of the cognitive data limits our ability to account for baseline cognition, the prospective data collection of the SEARCH cohort, overall, facilitated precise confounder adjustment in the current study. Our results remain limited, however, in that the descriptive differences in the NIHT-CB age-corrected scores observed between the youth-onset diabetes groups cannot be corroborated with potential day-to-day functional difficulties, such as with academic performance, as this was not measured in SEARCH. Thus, the results reported in this study should not be interpreted to mean that all young adults with diabetes are cognitively impaired or that the deficits reported here prevent them from being successful. Further, by design, the SEARCH study does not include a control group without diabetes and, therefore, our comparisons are limited to youth with diabetes. However, the strength of the NIHT-CB is its normative scoring, which allowed us to indirectly compare the two diabetes groups to the age-matched national averages for all cognitive test scores included in the current study.
In conclusion, the current study provides new evidence that worse fluid cognitive functioning among adolescents and young adults with T2D compared with those with T1D is completely accounted for by differences in relevant socioeconomic and clinical variables between the two groups, including crystallized cognition, obesity, and depression. Importantly, crystallized cognition, obesity, and depression are associated with fluid cognition regardless of diabetes type and are potentially modifiable factors. Moreover, obesity and depressive symptoms, as well as crystallized cognition and its potential underlying socioeconomic contributors, may be important for understanding the clinical and quality-of-life ramifications and modifications of care that might address disparities in fluid cognition in youth with diabetes. Future research is needed to explore these factors further across the life course of youth with diabetes.
Article Information
Acknowledgments. The SEARCH study is indebted to the many youth and their families and health care providers whose participation made this study possible. The authors acknowledge the involvement of the Kaiser Permanente Southern California Clinical Research Center (funded by Kaiser Foundation Health Plan and supported in part by the Southern California Permanente Medical Group), the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences (NCATS) grants UL1-TR-000062 and UL1-TR-001450; Seattle Children’s Hospital and the University of Washington, NIH/NCATS grant UL1-TR-00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant UL1-TR-000154; the Barbara Davis Center at the University of Colorado at Denver, Diabetes Endocrinology Research Center NIH grant P30-DK-57516; the University of Cincinnati, NIH/NCATS grants UL1-TR-000077 and UL1-TR-001425; and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions.
Funding. Grant support for SEARCH 1, 2, and 3 is provided by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. SEARCH 4 is supported by National Institute of Diabetes and Kidney Diseases grant 1UC4-DK-108173 and by the Centers for Disease Control and Prevention. The Population Based Registry of Diabetes in Youth Study is funded by the Centers for Disease Control and Prevention and supported by NIH, National Institute of Diabetes and Digestive and Kidney Diseases (1U18-DP-006131, U18-DP-006133, U18-DP-006134, U18-DP-006136, U18-DP-006138, and U18-DP-006139). SEARCH sites 1–4 are supported by Kaiser Permanente Southern California (U18-DP-006133, U48/CCU919219, U01-DP-000246, and U18-DP-002714), University of Colorado Denver (U18-DP-006139, U48/CCU819241-3, U01-DP-000247, and U18-DP-000247-06A1), Cincinnati Children’s Hospital Medical Center (U18-DP-006134, U48/CCU519239, U01-DP-000248, and 1U18-DP-002709), University of North Carolina at Chapel Hill (U18-DP-006138, U48/CCU419249, U01-DP-000254, and U18-DP-002708), Seattle Children’s Hospital (U18-DP-006136, U58/CCU019235-4, U01-DP-000244, and U18-DP-002710-01), and Wake Forest University School of Medicine (U18-DP-006131, U48/CCU919219, U01-DP-000250, and 200-2010-35171).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.L.B.S., D.D., and G.W. conceptualized the idea for this manuscript and produced the full draft. J.M.S. and R.D’A. analyzed the data. All authors contributed significant scientific review and suggested revisions for the manuscript and approved the final manuscript. A.L.B.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, Virtual, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14207852.
A complete list of the SEARCH for Diabetes in Youth Study Group can be found in the supplementary material online.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2020 [Google Scholar]
- 2. Pelimanni E, Jehkonen M. Type 2 diabetes and cognitive functions in middle age: a meta-analysis. J Int Neuropsychol Soc 2018;25:1–16 [DOI] [PubMed] [Google Scholar]
- 3. He J, Ryder AG, Li S, Liu W, Zhu X. Glycemic extremes are related to cognitive dysfunction in children with type 1 diabetes: a meta-analysis. J Diabetes Investig 2018;9:1342–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadanand S, Balachandar R, Bharath S. Memory and executive functions in persons with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev 2016;32:132–142 [DOI] [PubMed] [Google Scholar]
- 5. Vincent C, Hall PA. Executive function in adults with type 2 diabetes: a meta-analytic review. Psychosom Med 2015;77:631–642 [DOI] [PubMed] [Google Scholar]
- 6. Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive performance in children with type 1 diabetes--a meta-analysis. J Pediatr Psychol 2009;34:271–282 [DOI] [PubMed] [Google Scholar]
- 7. Abo-El-Asrar M, Andrawes NG, Rabie MA, et al. Cognitive functions in children and adolescents with early-onset diabetes mellitus in Egypt. Appl Neuropsychol Child 2018;7:21–30 [DOI] [PubMed] [Google Scholar]
- 8. Awad A, Lundqvist R, Rolandsson O, Sundström A, Eliasson M. Lower cognitive performance among long-term type 1 diabetes survivors: a case-control study. J Diabetes Complications 2017;31:1328–1331 [DOI] [PubMed] [Google Scholar]
- 9. Broadley MM, White MJ, Andrew B. A systematic review and meta-analysis of executive function performance in type 1 diabetes mellitus. Psychosom Med 2017;79:684–696 [DOI] [PubMed] [Google Scholar]
- 10. van Gemert T, Wölwer W, Weber KS, et al. Cognitive function is impaired in patients with recently diagnosed type 2 diabetes, but not type 1 diabetes. J Diabetes Res 2018;2018:1470476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Musen G, Tinsley LJ, Marcinkowski KA, et al. Cognitive function deficits associated with long-duration type 1 diabetes and vascular complications. Diabetes Care 2018;41:1749–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wennberg AMV, Hagen CE, Gottesman RF, et al. Longitudinal association between diabetes and cognitive decline: the National Health and Aging Trends Study. Arch Gerontol Geriatr 2017;72:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He J, Li S, Liu F, et al. Glycemic control is related to cognitive dysfunction in Chinese children with type 1 diabetes mellitus. J Diabetes 2018;10:948–957 [DOI] [PubMed] [Google Scholar]
- 14. Lin A, Northam EA, Rankins D, Werther GA, Cameron FJ. Neuropsychological profiles of young people with type 1 diabetes 12 yr after disease onset. Pediatr Diabetes 2010;11:235–243 [DOI] [PubMed] [Google Scholar]
- 15. Perantie DC, Lim A, Wu J, et al. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes 2008;9:87–95 [DOI] [PubMed] [Google Scholar]
- 16. Brady CC, Vannest JJ, Dolan LM, et al. Obese adolescents with type 2 diabetes perform worse than controls on cognitive and behavioral assessments. Pediatr Diabetes 2017;18:297–303 [DOI] [PubMed] [Google Scholar]
- 17. Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia 2010;53:2298–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Natovich R, Kushnir T, Harman-Boehm I, et al. Cognitive dysfunction: part and parcel of the diabetic foot. Diabetes Care 2016;39:1202–1207 [DOI] [PubMed] [Google Scholar]
- 19. Perez KM, Patel NJ, Lord JH, et al. Executive function in adolescents with type 1 diabetes: relationship to adherence, glycemic control, and psychosocial outcomes. J Pediatr Psychol 2017;42:636–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vloemans AF, Eilander MMA, Rotteveel J, et al. Youth with type 1 diabetes taking responsibility for self-management: the importance of executive functioning in achieving glycemic control: results from the longitudinal DINO study. Diabetes Care 2018;42:225–231 [DOI] [PubMed] [Google Scholar]
- 21. Wiebe DJ, Baker AC, Suchy Y, Stump TK, Berg CA. Individual differences and day-to-day fluctuations in goal planning and type 1 diabetes management. Health Psychol 2018;37:638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mansur RB, Lee Y, Zhou AJ, et al. Determinants of cognitive function in individuals with type 2 diabetes mellitus: a meta-analysis. Ann Clin Psychiatry 2018;30:38–50 [PubMed] [Google Scholar]
- 23. Zheng F, Yan L, Yang Z, Zhong B, Xie W. HbA1c, diabetes and cognitive decline: the English Longitudinal Study of Ageing. Diabetologia 2018;61:839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graveling AJ, Deary IJ, Frier BM. Acute hypoglycemia impairs executive cognitive function in adults with and without type 1 diabetes. Diabetes Care 2013;36:3240–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borkertienė V, Stasiulis A, Zacharienė B, Kyguolienė L, Bacevičienė R. Association among executive function, physical activity, and weight status in youth. Medicina (Kaunas). 2019;55:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kavanaugh BC, Cancilliere MK, Fryc A, et al. Measurement of executive functioning with the National Institute of Health Toolbox and the association to anxiety/depressive symptomatology in childhood/adolescence. Child Neuropsychol 2020;26:754–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larson K, Russ SA, Nelson BB, Olson LM, Halfon N. Cognitive ability at kindergarten entry and socioeconomic status. Pediatrics 2015;135:e440–e448 [DOI] [PubMed] [Google Scholar]
- 28. Rindermann H, Flores-Mendoza C, Mansur-Alves M. Reciprocal effects between fluid and crystallized intelligence and their dependence on parents’ socioeconomic status and education. Learn Individ Differ 2010;20:544–548 [Google Scholar]
- 29. Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care 2014;37:436–443 [DOI] [PubMed] [Google Scholar]
- 31. Dabelea D, Mayer-Davis EJ, Imperatore G. The value of national diabetes registries: SEARCH for Diabetes in Youth study. Curr Diab Rep 2010;10:362–369 [DOI] [PubMed] [Google Scholar]
- 32. Siddaway AP, Wood AM, Taylor PJ. The Center for Epidemiologic Studies-Depression (CES-D) scale measures a continuum from well-being to depression: testing two key predictions of positive clinical psychology. J Affect Disord 2017;213:180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology 2013;80(Suppl. 3):S54–S64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mungas D, Widaman K, Zelazo PD, et al. VII. NIH Toolbox Cognition Battery (CB): factor structure for 3 to 15 year olds. Monogr Soc Res Child Dev 2013;78:103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scott EP, Sorrell A, Benitez A. Psychometric properties of the NIH Toolbox Cognition Battery in healthy older adults: reliability, validity, and agreement with standard neuropsychological tests. J Int Neuropsychol Soc 2019;25:857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beaumont JL, Havlik R, Cook KF, et al. Norming plans for the NIH Toolbox. Neurology 2013;80(11 Suppl 3):S87–S92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Oliveira MO, Nitrini R, Yassuda MS, Brucki SM. Vocabulary is an appropriate measure of premorbid intelligence in a sample with heterogeneous educational level in Brazil. Behav Neurol 2014;2014:875960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Snitz BE, Bieliauskas LA, Crossland AR, Basso MR, Roper B. PPVT-R as an estimate of premorbid intelligence in older adults. Clin Neuropsychol 2000;14:181–186 [DOI] [PubMed] [Google Scholar]
- 39. Lindenberger U. Lifespan theories of cognitive development. In International Encyclopedia of the Social & Behavioral Sciences. Smelser NJ, Baltes PB, Eds. Oxford, Pergamon, 2001, pp. 8848–8854 [Google Scholar]
- 40. Zaval L, Li Y, Johnson EJ, Weber EU. Chapter 8: complementary contributions of fluid and crystallized intelligence to decision making across the life span. In Aging and Decision Making. Hess TM, Strough J, Löckenhoff CE, Eds. San Diego, Academic Press, 2015, pp. 149–168 [Google Scholar]
- 41. Martins AA, Gomes CMA, Alves AF, Almeida LDS. The structure of intelligence in childhood: age and socio-familiar impact on cognitive differentiation. Psychol Rep 2018;121:79–92 [DOI] [PubMed] [Google Scholar]
- 42. Dearing E, McCartney K, Taylor BA. Does higher quality early child care promote low-income children’s math and reading achievement in middle childhood? Child Dev 2009;80:1329–1349 [DOI] [PubMed] [Google Scholar]
- 43. Reuben A, Arseneault L, Belsky DW, et al. Residential neighborhood greenery and children’s cognitive development. Soc Sci Med 2019;230:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischer AL, de Frias CM, Yeung SE, Dixon RA. Short-term longitudinal trends in cognitive performance in older adults with type 2 diabetes. J Clin Exp Neuropsychol 2009;31:809–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kirchhoff BA, Jundt DK, Doty T, Hershey T. A longitudinal investigation of cognitive function in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes 2017;18:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laurent JS, Watts R, Adise S, et al. Associations among body mass index, cortical thickness, and executive function in children. JAMA Pediatr 2020;174:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fellows RP, Schmitter-Edgecombe M. Independent and differential effects of obesity and hypertension on cognitive and functional abilities. Arch Clin Neuropsychol 2018;33:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang Y, Shields GS, Guo C, Liu Y. Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci Biobehav Rev 2018;84:225–244 [DOI] [PubMed] [Google Scholar]
- 49. Lewis G, Button KS, Pearson RM, Munafò MR, Lewis G. Inhibitory control of positive and negative information and adolescent depressive symptoms: a population-based cohort study. Psychol Med 7 July 2020 [Epub ahead of print]. DOI: 10.1017/s0033291720002469 [DOI] [PubMed] [Google Scholar]
- 50. Hamburger ER, Goethals ER, Choudhary A, Jaser SS. Sleep and depressive symptoms in adolescents with type 1 diabetes not meeting glycemic targets. Diabetes Res Clin Pract 2020;169:108442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hood KK, Lawrence JM, Anderson A, et al. Metabolic and inflammatory links to depression in youth with diabetes. Diabetes Care 2012;35:2443–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gulley LD, Shomaker LB. Depression in youth-onset type 2 diabetes. Curr Diab Rep 2020;20:51. [DOI] [PubMed] [Google Scholar]