Abstract
Exposure to wildfire smoke increases the risk of respiratory and cardiovascular hospital admissions. Health impact assessments, used to inform decision‐making processes, characterize the health impacts of environmental exposures by combining preexisting epidemiological concentration–response functions (CRFs) with estimates of exposure. These two key inputs influence the magnitude and uncertainty of the health impacts estimated, but for wildfire‐related impact assessments the extent of their impact is largely unknown. We first estimated the number of respiratory, cardiovascular, and asthma hospital admissions attributable to fire‐originated PM2.5 exposure in central California during the October 2017 wildfires, using Monte Carlo simulations to quantify uncertainty with respect to the exposure and epidemiological inputs. We next conducted sensitivity analyses, comparing four estimates of fire‐originated PM2.5 and two CRFs, wildfire and nonwildfire specific, to understand their impact on the estimation of excess admissions and sources of uncertainty. We estimate the fires accounted for an excess 240 (95% CI: 114, 404) respiratory, 68 (95% CI: −10, 159) cardiovascular, and 45 (95% CI: 18, 81) asthma hospital admissions, with 56% of admissions occurring in the Bay Area. Although differences between impact assessment methods are not statistically significant, the admissions estimates' magnitude is particularly sensitive to the CRF specified while the uncertainty is most sensitive to estimates of fire‐originated PM2.5. Not accounting for the exposure surface's uncertainty leads to an underestimation of the uncertainty of the health impacts estimated. Employing context‐specific CRFs and using accurate exposure estimates that combine multiple data sets generates more certain estimates of the acute health impacts of wildfires.
Keywords: air pollution, fine particulate matter, wildfire smoke, health impact assessment, population exposure, health impacts
Key Points
PM2.5 exposure during the 2017 California fires accounted for an estimated 308 excess respiratory and cardiovascular hospital admissions
Health impact assessments, used to inform decision‐making processes, are sensitive to the exposure and epidemiologic inputs specified
Accurate exposure estimates and context‐specific health risk functions estimate fire‐attributable health impacts with greater certainty
1. Introduction
In recent years, California has experienced a number of devastating wildfires. These fires adversely impact air quality both locally and regionally for long periods of time (Reisen et al., 2015), elevating concentrations of fine particulate matter (PM2.5) to levels potentially hazardous to human health. The October 2017 northern California wildfires resulted in record high PM2.5 concentrations, with daily average concentrations greater than 190 μg/m3, dangerously exceeding the 24‐h PM2.5 standard of 35 μg/m3 and exposing large populations to unhealthy air (Bay Area Air Quality Management District, 2019; Cleland et al., 2020). Exposure to fire‐originated PM2.5 can cause a variety of adverse health outcomes, with substantial evidence for increased risk of respiratory morbidity and growing evidence for increased risk of cardiovascular morbidity and all‐cause mortality (Jaffe et al., 2020; Liu et al., 2015; Reid et al., 2016). As the frequency, intensity, and spread of wildfires are likely to increase due to climate change (Boegelsack et al., 2018; Liu et al., 2016; Spracklen et al., 2009; Yue et al., 2013), increasing population‐level exposure to fire‐originated PM2.5 (Ford et al., 2018), it is necessary to improve upon existing methods to better identify and quantify the health impacts of wildfire smoke exposure.
Two primary ways of evaluating the health impacts of wildfire smoke are epidemiological studies, which aim to evaluate health risk and health burden based on health outcome data, and health impact assessments, which aim to evaluate the overall health impact using health risk estimates from prior epidemiological studies. There is a growing body of epidemiological evidence that short‐term smoke exposure increases respiratory and cardiovascular hospital and emergency department (ED) admissions (Borchers‐Arriagada et al., 2019; Deflorio‐Barker et al., 2019; Delfino et al., 2009; Gan et al., 2020; Haikerwal et al., 2015; Liu et al., 2017; Rappold et al., 2012). While essential for understanding the public health implications of fire events, epidemiological risk estimates require detailed health outcome data to be available, which can lag in years, limiting the ability to inform decision making during or just prior to wildfire seasons. In contrast, impact assessments rely on preexisting concentration–response functions (CRFs) with estimated health risks from epidemiological studies, which when combined with current information on pollutant exposure, baseline health incidence, and population density can estimate the total number of hospital and/or ED admissions attributable to wildfire smoke exposure (Borchers‐Arriagada, Palmer, Bowman, Morgan, et al., 2020; Borchers‐Arriagada, Palmer, Bowman, Williamson, et al., 2020; Bowman et al., 2019; Broome et al., 2016; Fann et al., 2013, 2018; Jiang & Yoo, 2019; Johnston et al., 2021; Matz et al., 2020; Rittmaster et al., 2006). Each of the inputs into a health impact assessment contributes to the overall precision and accuracy of the wildfire smoke‐attributable health impacts estimated, but their influence is largely unknown and rarely quantified.
In prior assessments, PM2.5 exposure during wildfires has been estimated using three primary data sets: monitoring station observations, chemical transport models (CTMs), and satellite observations (Borchers‐Arriagada, Palmer, Bowman, Morgan, et al., 2020; Borchers‐Arriagada, Palmer, Bowman, Williamson, et al., 2020; Bowman et al., 2019; Broome et al., 2016; Fann et al., 2013, 2018; Jiang & Yoo, 2019; Johnston et al., 2021; Matz et al., 2020; Rittmaster et al., 2006). Fire‐originated PM2.5 has been isolated using either monitoring station observations over the same geographic region during a nonfire period (Borchers‐Arriagada, Palmer, Bowman, Morgan, et al., 2020; Borchers‐Arriagada, Palmer, Bowman, Williamson, et al., 2020; Bowman et al., 2019; Broome et al., 2016) or a CTM run during the fire period without fire emissions (Fann et al., 2013, 2018; Jiang & Yoo, 2019; Matz et al., 2020). The CRFs selected also vary, with many assessments using CRFs for nonwildfire, ambient PM2.5 (Borchers‐Arriagada, Palmer, Bowman, Morgan, et al., 2020; Borchers‐Arriagada, Palmer, Bowman, Williamson, et al., 2020; Bowman et al., 2019; Broome et al., 2016; Fann et al., 2013; Johnston et al., 2021; Matz et al., 2020) and only some employing wildfire‐specific (WF) CRFs (Fann et al., 2018; Jiang & Yoo, 2019). Further, the majority of prior wildfire‐focused health impact assessments did not account for uncertainty beyond the uncertainty of the CRF and only a select few considered more than one exposure surface or CRF. As wildfires become an increasingly prevalent public health issue, it is important to identify which elements of the impact assessment framework have the most influence on the magnitude and uncertainty of the health impacts estimated in order to understand how best to conduct these assessments moving forward.
Here, we calculate the number of daily excess respiratory, cardiovascular, and asthma hospital admissions attributable to fire‐originated PM2.5 exposure during the October 2017 California wildfires and characterize how these estimates change with alternative choices of CRFs and smoke exposure surfaces. More specifically, we evaluate how different epidemiological health risk and PM2.5 exposure estimates and their associated uncertainty influence both the number of attributable admissions estimated and their confidence bounds. To our knowledge, this is the first systematic assessment of how choices of inputs for health impact assessments affect estimates of the acute health impacts of wildfire smoke exposure. By showing how different sources of uncertain data influence health impact estimates for wildfires, our findings can help strengthen future impact assessments and inform public health decision‐making processes before, during, and after fire events.
2. Data and Methods
2.1. PM2.5 Exposure Data
We used three different total PM2.5 and two different background PM2.5 exposure surfaces to generate four estimates of daily average, ground‐level fire‐originated PM2.5 at a 1‐km resolution. In addition to the exposure surface used in our base case impact assessment, three alternative approaches were used in a sensitivity analysis, described in detail below, two evaluating different estimates of total PM2.5 and one evaluating a different background PM2.5 estimate. The three estimates of total PM2.5 exposure, which were previously developed (Cleland et al., 2020), are: space–time (s/t) kriging of monitoring station observations, using the Bayesian Maximum Entropy (BME) Framework (BME kriging); bias‐corrected Community Multiscale Air Quality (CMAQ) model output, using the Constant Air Quality Model Performance (CAMP) method (CC‐CMAQ); and a BME data fusion of observations with CC‐CMAQ and satellite‐derived concentrations (BME data fusion). The two estimates of background PM2.5 exposure are: the percent of PM2.5 attributable to background emissions, obtained from CMAQ output with and without fire emissions (CMAQ percent attributable) and BME kriging of observations from October 2016 (October 2016). The four estimates of fire‐originated PM2.5 are: BME data fusion with CMAQ percent attributable; BME kriging with CMAQ percent attributable; CC‐CMAQ with CMAQ percent attributable; and BME data fusion with October 2016.
To develop the BME kriging and BME data fusion exposure surfaces, daily average PM2.5 observations were used as input. Observations were obtained from permanent Federal Reference Methods (FRM)/Federal Equivalent Methods (FEM) and temporary non‐FRM/FEM monitoring stations across California for October 1–31, 2017. For the CC‐CMAQ and BME data fusion exposure surfaces, we used CMAQ simulations, a widely used CTM developed by the United States (U.S.) Environmental Protection Agency (EPA), as input. The CMAQ simulations, which used estimates of fire emissions derived from satellite observations and estimates of all other anthropogenic and natural emissions, provided estimates of daily average PM2.5 at a 4‐km resolution in central California for October 3–20, 2017. The third input into the BME data fusion was satellite‐derived PM2.5 concentrations, which we generated by converting aerosol optical depth (AOD) observations to PM2.5 estimates using a day‐specific linear mixed effects model. The 3‐km resolution AOD data were acquired from the Moderate Resolution Imaging Spectroradiometer (MODIS) Terra Satellite across California for October 1–31, 2017.
CC‐CMAQ was generated using the CAMP method, which improves the accuracy of CTM output by applying a bias correction that accounts for the nonlinear, heteroscedastic relationship between observed and modeled concentrations (de Nazelle et al., 2010; Reyes et al., 2017). CC‐CMAQ estimates both the bias‐corrected PM2.5 concentration and its associated variance. The BME kriging and BME data fusion surfaces were generated using the BME framework, which uses modern s/t geostatistics to accurately estimate PM2.5 and measures of associated uncertainty at unmonitored locations by combining information on the trends and variability of the data with information on concentrations at a set of known s/t locations (Christakos, 1990; Christakos et al., 2002; Serre & Christakos, 1999). To produce estimates of ground‐level PM2.5 concentrations and their associated variance, BME s/t kriging was used to interpolate monitoring observations and BME data fusion was used to fuse observations with both CC‐CMAQ and the satellite‐derived concentrations. Compared to CC‐CMAQ and BME kriging, BME data fusion provides the most accurate and precise estimates of ground‐level PM2.5 in the fire‐affected region and period (Cleland et al., 2020). While no prior acute health impact assessment has used the fusion of observed, modeled, and satellite‐derived PM2.5 concentrations to assess smoke exposure, both monitoring station observations and CMAQ output, standalone and in combination, have been used as exposure estimates (Borchers‐Arriagada, Palmer, Bowman, Morgan, et al., 2020; Borchers‐Arriagada, Palmer, Bowman, Williamson, et al., 2020; Bowman et al., 2019; Fann et al., 2013, 2018; Jiang & Yoo, 2019; Johnston et al., 2021).
To estimate background PM2.5, concentrations without emissions from the October 2017 fires, and isolate the fire‐originated component from the total PM2.5, we used two different data sets: modeled concentrations when CMAQ is run without fire emissions and FRM/FEM monitoring station observations from October 2016, when California fire activity was identified to be low (Text S1; Figures S1 and S2). For the CMAQ percent attributable approach, we used the ratio between the CMAQ output with only nonfire emissions and with both fire and nonfire emissions to identify the percent of PM2.5 attributable to background emissions at any s/t location. Using this approach, fire‐originated PM2.5, , is calculated as , where is the total PM2.5 concentration at location set on day , obtained from one of the three exposure estimates described above, and , where and are the CMAQ model output without and with fire emissions, respectively. While prior impact assessments have used CTMs run without fire emissions to determine background PM2.5 during fires (Fann et al., 2013, 2018; Matz et al., 2020), only one has used it in a relative manner (Jiang & Yoo, 2019). For the October 2016 approach, we used BME kriging to spatially interpolate the monthly average of monitoring stations observations across California during October 2016. Using this approach, fire‐originated PM2.5, , is calculated as , where is the estimated October 2016 monthly average PM2.5 concentration at location set . We selected October 2016 because no large fires occurred in that time period and the seasonal meteorological conditions and nonfire emissions were likely similar to those during October 2017. Similar approaches have been used to identify fire‐originated PM2.5 in previous impact assessments (Borchers‐Arriagada, Palmer, Bowman, Morgan, et al., 2020; Borchers‐Arriagada, Palmer, Bowman, Williamson, et al., 2020; Bowman et al., 2019). In both approaches, to avoid negative concentrations, is set to zero if or exceeds .
2.2. Concentration–Response Functions
We considered two types of CRFs: WF CRFs and ambient, nonwildfire‐specific (NF) CRFs. The WF CRFs are from an epidemiological study of the 2003 southern California wildfires, which found a 2.8% (95% confidence interval [CI]: 1.4, 4.1) increase in respiratory, a 0.8% (95% CI: −0.1, 1.8) increase in cardiovascular, and a 4.8% (95% CI: 2.1, 7.6) increase in asthma hospital admissions per 10 µg/m3 increase in 2‐day average PM2.5 (Delfino et al., 2009). Risk coefficients were also calculated for age subgroups for respiratory and asthma admissions and for sex subgroups for asthma admissions. These CRFs have been used in previous health impact assessments of wildfire smoke (Fann et al., 2018; Jiang & Yoo, 2019).
The ambient, NF CRFs are from an epidemiological study of 26 U.S. counties between 2000 and 2003, which found a 2.07% (95% CI: 1.20, 2.95) increase in respiratory and 1.89% (95% CI: 1.34, 2.45) increase in cardiovascular hospital admissions per 10 µg/m3 increase in 2‐day average ambient PM2.5 (Zanobetti et al., 2009). These CRFs have been used in a previous health impact assessment of wildfire smoke exposure (Fann et al., 2018).
2.3. Hospital Admission and Population Data
We obtained annual county‐level respiratory, cardiovascular, and asthma hospital admission rates for 2017 across California from the U.S. EPA's Benefits Mapping and Analysis Program‐Community Edition, which calculates hospitalization rates, per 100,000 population, using data from the Healthcare and Cost Utilization Project (Sacks et al., 2018). We then converted these annual rates into daily rates by dividing by 365 (Text S2 and Figure S3). California census tract‐level population data for 2017 were obtained from the U.S. Census Bureau.
2.4. Impact Assessment Methods
We calculated the number of excess respiratory, cardiovascular, and asthma hospital admissions attributable to fire‐originated PM2.5 in central California on each day between October 8 and 20, 2017. We constrained the study area to central California given the geographic extent of the CMAQ model. October 8–20 was identified as the fire period based on recorded fire activity.
To estimate the total attributable hospitalizations, we used a health impact function that is appropriate when evaluating health impacts attributed to wildfires relative to the long‐term hospital admission rates (Borchers‐Arriagada, Palmer, Bowman, Morgan, et al., 2020; Borchers‐Arriagada, Palmer, Bowman, Williamson, et al., 2020; Bowman et al., 2019; Broome et al., 2016; Fann et al., 2013, 2018; Johnston et al., 2021), rather than relative to the elevated admission rates during the fire period, for which we do not have data. The health impact function, derived from a log linear model for the relationship between the exposure and health outcome
| (1) |
determines the number of attributable hospital admissions per square kilometer, , at location set on day , where . The location set is taken at a set of points, , corresponding to the cells on a regular estimation grid. is the background daily rate of hospital admissions at location set (i.e., the admission rate without emissions from the October 2017 wildfires), estimated from the annual rate and interpolated from each county to the estimation grid. represents the daily rate of hospital admissions including admissions related to pollution from the fires. is the population density in grid cell , interpolated from each census tract to the estimation grid. The quantity is a positive multiplier, where is the rate ratio of admissions at concentration , which determines the percent increase in admissions from due to fire‐originated PM2.5 exposure. is the risk coefficient, where and is the rate ratio for the CRF, either WF or NF, for a 10 µg/m3 increase in 2‐day average PM2.5. and is the 2‐day average fire‐originated PM2.5 concentration at location set on day , where is the 2‐day average total PM2.5 concentration, obtained from one of the three exposure surfaces, and is the background concentration (i.e., concentrations without emissions from the October 2017 wildfires), either or the 2‐day average of . A 2‐day average is used for to match the CRFs. The total number of attributable admissions on day , , is calculated as the sum of excess admissions across all grid cells within central California, .
To identify the most impacted subpopulations using the available CRFs, we stratified the impact assessment results by age for respiratory hospital admissions and by age and sex for asthma hospital admissions. All estimates were rounded to the nearest whole number, instead of two significant figures, given our focus on a small geographic region.
To account for uncertainty in the health impact assessment, we conducted Monte Carlo simulations, using 100,000 iterations, on and . For the Monte Carlo simulations, we assumed that the total PM2.5 estimations () and the CRFs () follow lognormal distributions. For , the uncertainty is defined by the variance of the PM2.5 estimate. For , the uncertainty is defined from the 95% CI of the CRF. We neglect uncertainties in population and background admission rates since they are likely small and not time variant.
2.5. Evaluating Sensitivity of Assessment to Inputs
To determine which inputs have the largest impact on the estimated attributable respiratory and cardiovascular hospital admissions and their CIs, we conducted three sensitivity analyses, creating 10 alternative impact assessment approaches which used a different total PM2.5 surface, background PM2.5 surface, CRF, or sources of uncertainty compared to the base case (Table 1). The base case impact assessment used the following inputs: total exposure estimates from the BME data fusion, CMAQ percent attributable to isolate fire‐originated PM2.5, the WF CRF, and uncertainty from both the total PM2.5 estimate and the CRF (). This was identified as the base case because we believe it incorporates the most accurate and representative information. For the sensitivity analyses, we only changed one input at a time from the base case to understand the individual impact of each.
Table 1.
Definition of Inputs for the Three Sensitivity Analyses and 10 Alternative Impact Assessments to Compare to the Base Case
| Sensitivity analysis | Total PM2.5 estimation | Background PM2.5 estimation | CRF type | Sources of uncertainty |
|---|---|---|---|---|
| Base case | BME data fusion | CMAQ % attributable | WF | CRF and total PM2.5 |
| (1) Sensitivity to fire‐originated PM2.5 estimation | CC‐CMAQ | CMAQ % attributable | WF | CRF and total PM2.5 |
| BME kriging | CMAQ % attributable | WF | CRF and total PM2.5 | |
| BME data fusion | October 2016 | WF | CRF and total PM2.5 | |
| (2) Sensitivity to CRF type | BME data fusion | CMAQ % attributable | NF | CRF and total PM2.5 |
| (3) Sensitivity to sources of uncertainty | BME data fusion | CMAQ % attributable | WF | CRF |
| BME data fusion | CMAQ % attributable | WF | Total PM2.5 | |
| CC‐CMAQ | CMAQ % attributable | WF | CRF | |
| CC‐CMAQ | CMAQ % attributable | WF | Total PM2.5 | |
| BME kriging | CMAQ % attributable | WF | CRF | |
| BME kriging | CMAQ % attributable | WF | Total PM2.5 |
The first sensitivity analysis evaluated the impact of the wildfire PM2.5 exposure estimates on the estimated excess hospital admissions and 95% CIs. For this, we first compared the base case total PM2.5 concentration surface (BME data fusion) to two simpler alternatives: CC‐CMAQ and BME kriging of observations. We next compared the base case background PM2.5 surface (CMAQ percent attributable) to one alternative: the October 2016 approach. The second sensitivity analysis evaluated the impact of the CRF on the estimated excess hospital admissions and their CIs. For this, we compared the WF CRF used in the base case to the ambient NF CRF. The third sensitivity analysis aimed to identify the primary sources of uncertainty in the hospital admissions estimates. For this, we compared the estimated admissions' confidence bounds when uncertainty from both the total PM2.5 concentration surface () and the CRF () was accounted for, to when uncertainty from only or was accounted for. We conducted this sensitivity analysis for all three total PM2.5 surfaces to determine if the primary sources of uncertainty changed depending on the exposure estimate used.
3. Results
3.1. Impact of Wildfire PM2.5 Exposure on Hospital Admissions
Using base case assumptions, we estimate there were an excess 240 (95% CI: 114, 404), 68 (95% CI: −10, 159), and 45 (95% CI: 18, 81) respiratory, cardiovascular, and asthma hospital admissions, respectively, attributable to fire‐originated PM2.5 exposure between October 8 and 20 (Table 2). More than half of the total respiratory and asthma hospital admissions were people over the age of 65, who comprise only 14% of the total population but have higher background rates of hospital admissions. Two thirds of the excess asthma admissions were female, who have a higher risk of asthma‐related hospitalization. The least impacted age groups for both respiratory and asthma admissions were people aged 5–19. While asthma hospital admissions have the highest risk coefficient for wildfire PM2.5 exposure, they account for the lowest number of attributable admissions due to the low background admission rates. It is important to note that the admission counts for the age and sex subgroups do not sum to the all ages, all sexes count because different CRFs are used for each subgroup and are not designed to total to the number calculated using the general population CRF.
Table 2.
Number of Excess Respiratory, Cardiovascular, and Asthma Hospital Admissions Attributable to Wildfire‐Originated PM2.5, October 8–20, 2017, Estimated Using Base Case Assumptions
| RR (95% CI) a | # Admissions (95% CI) | |
|---|---|---|
| Respiratory hospital admissions | ||
| All ages | 1.028 (1.014, 1.041) | 240 (114, 404) |
| Ages 0–4 | 1.045 (1.010, 1.082) | 27 (6, 54) |
| Ages 5–19 | 1.027 (0.984, 1.076) | 15 (−9, 43) |
| Ages 20–64 | 1.024 (1.005, 1.044) | 65 (13, 131) |
| Ages 65–99 | 1.030 (1.011, 1.049) | 126 (44, 232) |
| Cardiovascular hospital admissions | ||
| All ages | 1.008 (0.999, 1.018) | 68 (−10, 159) |
| Asthma hospital admissions | ||
| All ages and sexes | 1.048 (1.021, 1.076) | 45 (18, 81) |
| Male | 1.031 (0.990, 1.073) | 14 (−4, 35) |
| Female | 1.059 (1.022, 1.097) | 29 (10, 55) |
| Ages 0–4 | 1.083 (1.021, 1.149) | 7 (2, 15) |
| Ages 5–19 | 0.999 (0.935, 1.068) | 0 (−10, 11) |
| Ages 20–64 | 1.041 (0.995, 1.090) | 17 (−2, 40) |
| Ages 65–99 | 1.101 (1.030, 1.178) | 27 (7, 57) |
Rate ratio per 10 µg/m3 increase in 2‐day average PM2.5 (Delfino et al., 2009).
Hospital admissions varied daily, reflecting variation in smoke exposure (Figure 1). Between October 8 and 20, October 11 had the highest number of excess respiratory and cardiovascular admissions, 40 (95% CI: 19, 68) and 11 (95% CI: −2, 26), respectively. October 12 had the most excess asthma admissions, with 8 (95% CI: 3, 14) total admissions. The days with the highest number of admissions have the largest uncertainty around the estimates. The peaks in excess admissions occurred the same day as or 1 day after peaks in population‐weighted fire‐originated PM2.5 concentrations, a reflection of the CRFs which report an increase in attributable admissions per increase in 2‐day average PM2.5. There were two peaks in excess admissions, October 11–12 and October 18, reflecting peaks in smoke concentrations. Daily counts of admissions for each health endpoint can be found in supporting information (Text S3 and Table S1).
Figure 1.
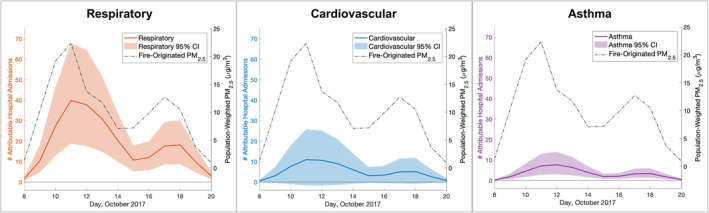
Daily excess respiratory, cardiovascular, and asthma hospital admissions attributable to wildfire PM2.5 and daily population‐weighted average fire‐originated PM2.5 concentrations, October 8–20, 2017, estimated using base case assumptions.
To understand the spatial distribution of hospital admissions across central California during the fires, we mapped the rate of the excess admissions (Figure 2) and the percent increase in hospital admissions due to wildfire PM2.5 exposure (Text S4 and Figure S4) and calculated county‐level attributable admissions (Table 3, Text S5, and Figure S5). Overall, attributable respiratory hospital admissions occurred at a higher rate in comparison to cardiovascular and asthma admissions, aligning with the results in Tables 2 and 3, and were more widespread across multiple counties. Between October 10 and 12, the regions with the highest rates of attributable admissions varied by health outcome. For respiratory hospital admissions, some of the highest rates occurred in Napa, Mendocino, Butte, Madera, and Contra Costa Counties. For cardiovascular and asthma hospital admissions, the highest rates occurred in Napa and Butte Counties and Madera and Alameda Counties, respectively. The counties with the highest number of excess admissions also varied by health outcome, with 56% of all respiratory and cardiovascular admissions occurring in the Bay Area (Alameda, Contra Costa, Marin, Napa, San Francisco, San Mateo, Santa Clara, Solano, and Sonoma Counties). Napa County had the most respiratory (12.9%) and cardiovascular admissions (17.6%), while Alameda County had the most asthma admissions (17.8%).
Figure 2.
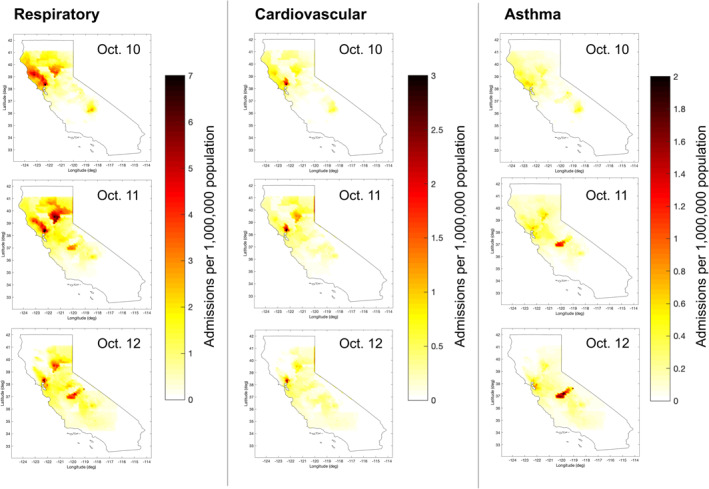
Excess respiratory, cardiovascular, and asthma hospital admissions attributable to wildfire PM2.5, expressed as rate per 1,000,000 person‐days, October 10–12, 2017, estimated using base case assumptions.
Table 3.
Number of Fire‐Attributable Respiratory, Cardiovascular, and Asthma Hospital Admissions, Average Fire‐Originated PM2.5 Concentrations, and Total Population in the 10 Counties With the Most Excess Admissions, October 8–20, 2017, Estimated Using Base Case Assumptions
| County | # Admissions (95% CI) | Average fire‐originated PM2.5 (Std. Dev.) (µg/m3) a | Total population (# persons) b | ||
|---|---|---|---|---|---|
| Respiratory | Cardiovascular | Asthma | |||
| Napa | 31 (15, 48) | 12 (−2, 26) | 3 (1, 5) | 39.57 (17.73) | 141,005 |
| Santa Clara | 25 (12, 42) | 8 (−1, 20) | 5 (2, 10) | 10.50 (0.85) | 1,911,226 |
| Alameda | 25 (12, 42) | 6 (−1, 14) | 8 (3, 14) | 11.94 (2.77) | 1,629,615 |
| Contra Costa | 17 (8, 28) | 4 (−1, 10) | 4 (2, 8) | 14.54 (3.92) | 1,123,678 |
| Sacramento | 12 (6, 21) | 4 (−1, 10) | 3 (1, 5) | 10.00 (1.03) | 1,495,400 |
| Fresno | 11 (5, 18) | 4 (−1, 9) | 2 (1, 4) | 9.03 (2.01) | 971,616 |
| Solano | 11 (5, 19) | 3 (0, 7) | 2 (1, 4) | 19.64 (6.87) | 434,981 |
| Sonoma | 10 (4, 17) | 3 (0, 6) | 2 (1, 3) | 25.54 (5.21) | 500,943 |
| Butte | 10 (5, 18) | 2 (0, 5) | 1 (0, 2) | 9.67 (1.56) | 225,207 |
| San Joaquin | 9 (4, 15) | 2 (0, 5) | 2 (1, 3) | 10.18 (0.46) | 724,153 |
The population‐weighted average and standard deviation of the 1‐km resolution fire‐originated PM2.5 estimations across the county, October 8–20, 2017.
County‐level total population calculated using 2017 census tract‐level population data.
The counties with high excess admission rates and counts are determined by three primary factors: the fire‐originated PM2.5 concentrations, background hospital admission rates, and population density. Since the smoke exposure estimates remain the same for all health outcomes, the differences in where high attributable admission rates are located are driven by the county‐level background admission rates. For example, Napa County was exposed to some of the highest PM2.5 concentrations during the 2017 wildfires (Cleland et al., 2020), with more than a 30% increase in respiratory and asthma admissions due to fire‐originated PM2.5 exposure (Figure S4). While Napa County also had the highest rates of excess respiratory admissions, it had notably lower rates of excess asthma admissions due to a low background rate of asthma hospital admissions in 2017. Further, the differences in total attributable admissions are driven by population density. For example, while sparsely populated Madera County had the highest rates of excess asthma admissions, densely populated Alameda County, which had comparably lower attributable admission rates, had the most excess asthma admissions.
3.2. Sensitivity of Assessment to Inputs
To identify which impact assessment inputs had the most impact on the magnitude and uncertainty of the admissions estimates, we compared four different approaches that used a different total PM2.5 estimation method, background PM2.5 estimation method, or CRF relative to the base case. Overall, the health impact estimates are sensitive to all three inputs, but the overlapping CIs indicate that the sensitivity is not statistically significant (Table 4). Changes in the magnitude and range of uncertainty of the admissions estimated are present both across the entire fire period (Table 4) and on each day (Text S6 and Figure S6). The locations of the excess admissions also change depending on the impact assessment approach used (Text S6 and Figure S7).
Table 4.
Comparison of Methods for Estimating Excess Respiratory and Cardiovascular Hospital Admissions and Fire‐Originated PM2.5 Exposure, October 8–20, 2017
| Impact assessment method | # Admissions (95% CI) | Fire‐originated PM2.5 (µg/m3) a | ||||||
|---|---|---|---|---|---|---|---|---|
| Total PM2.5 estimation | Background PM2.5 estimation | CRF type | Respiratory | Cardiovascular | Population‐weighted average (Std. Dev.) | Spatial average (Std. Dev.) | 95th percentile | |
| 1 b | BME data fusion | CMAQ % attributable | WF c | 240 (114, 404) | 68 (−10, 159) | 10.05 (6.58) | 7.05 (9.81) | 26.59 |
| 2 | CC‐CMAQ | CMAQ % attributable | WF | 251 (77, 620) | 70 (−10, 211) | 9.84 (6.10) | 6.56 (9.63) | 27.47 |
| 3 | BME kriging | CMAQ % attributable | WF | 280 (124, 512) | 78 (−12, 192) | 11.02 (7.08) | 8.19 (11.17) | 26.40 |
| 4 | BME data fusion | October 2016 | WF | 299 (126, 544) | 84 (−13, 208) | 8.77 (7.50) | 6.56 (8.48) | 22.65 |
| 5 | BME data fusion | CMAQ % attributable | NF d | 177 (87, 305) | 163 (95, 261) | 10.05 (6.58) | 7.05 (9.81) | 26.59 |
The population‐weighted average and standard deviation, spatial average and standard deviation, and 95th percentile of the 1‐km resolution fire‐originated PM2.5 estimations across central California.
Base case impact assessment.
Wildfire‐specific CRFs (rate ratio per 10 μg/m3 increase in 2‐day average PM2.5)—respiratory: 1.028 (95% CI: 1.014, 1.041); cardiovascular: 1.008 (95% CI: 0.999, 1.018) (Delfino et al., 2009).
Nonwildfire‐specific CRFs (rate ratio per 10 μg/m3 increase in 2‐day average PM2.5)—respiratory: 1.021 (95% CI 1.012, 1.030); cardiovascular: 1.019 (95% CI: 1.013, 1.025) (Zanobetti et al., 2009).
Of the three inputs, the NF CRFs have the largest impact on the magnitude of the estimate compared to the base case, with a 26% decrease and 140% increase in the estimated number of excess respiratory and cardiovascular admissions, respectively. These changes are due to the NF CRFs having a lower risk coefficient for respiratory and considerably higher risk coefficient for cardiovascular hospital admissions compared to the WF CRFs. The widths of confidence bounds shrink slightly when using the NF CRFs, due to their narrower 95% CIs. The CRFs' large impact on the estimated excess admissions emphasizes the importance of using a WF CRF, especially as the PM2.5 composition of smoke affects toxicity (Liu & Peng, 2019).
The method used to estimate background PM2.5 also impacts the magnitude and uncertainty of admissions estimates. When the October 2016 approach is used to isolate fire‐originated PM2.5 instead of the CMAQ percent attributable approach, there is a 25% and 24% increase in the excess respiratory and cardiovascular admissions estimated, respectively, and a 44% and 31% increase in the CI width, respectively. Using the October 2016 surface increases the frequency of low estimated fire‐originated PM2.5 concentrations, shown by the reduction in both the spatial and population‐weighted average and the 95th percentile of concentrations. This shift in the distribution of smoke concentrations across California during the fires increases the number and associated uncertainty of excess admissions estimated.
Changing the method for estimating total exposure to either CC‐CMAQ or BME kriging has the least impact on magnitude but the largest impact on uncertainty, especially for respiratory admissions. CC‐CMAQ increases the estimated excess respiratory and cardiovascular admissions by 5% and 3%, respectively, and the width of the CIs by 87% and 31%, respectively. There is a slight increase in admissions because, compared to BME data fusion, CC‐CMAQ has a higher frequency of high estimated smoke concentrations, shown by the increase in the 95th percentile of fire‐originated PM2.5. BME kriging increases the estimated excess respiratory and cardiovascular admissions by 16% and 15%, respectively, and increases the width of the CIs by 33% and 21%, respectively. This increase in admissions occurs because, compared to BME data fusion, BME kriging on average estimates higher fire‐originated PM2.5 concentrations across central California. The observed decrease in the impact assessment results' precision when either BME kriging or CC‐CMAQ is used occurs because both exposure surfaces have higher estimation variance than BME data fusion (Cleland et al., 2020).
To further understand how estimates of fire‐originated PM2.5 exposure differed between methods, we compared the four exposure surfaces spatially on October 10 (Figure 3) and temporally between October 8 and 20 (Text S6 and Figure S8). Using BME kriging or CC‐CMAQ to estimate total exposure instead of BME data fusion changes the smoke plume characteristics, impacting both the location and magnitude of concentrations and the number of attributable admissions estimated. Both CC‐CMAQ and BME kriging have larger plumes of high fire‐originated concentrations north of San Francisco Bay compared to BME data fusion, partly explaining the increase in estimated admissions. When the October 2016 approach is used instead of the CMAQ percent attributable approach to estimate background concentrations, there appears to be less clear isolation of the smoke plumes, with widespread low fire‐originated PM2.5 concentrations south and east of the Bay Area. The increased area covered by wildfire PM2.5 using this method partly explains the increase in estimated admissions. It is possible that ambient, nonfire PM2.5 across California changed between 2016 and 2017, which would limit the October 2016 surface's ability to accurately isolate smoke concentrations.
Figure 3.
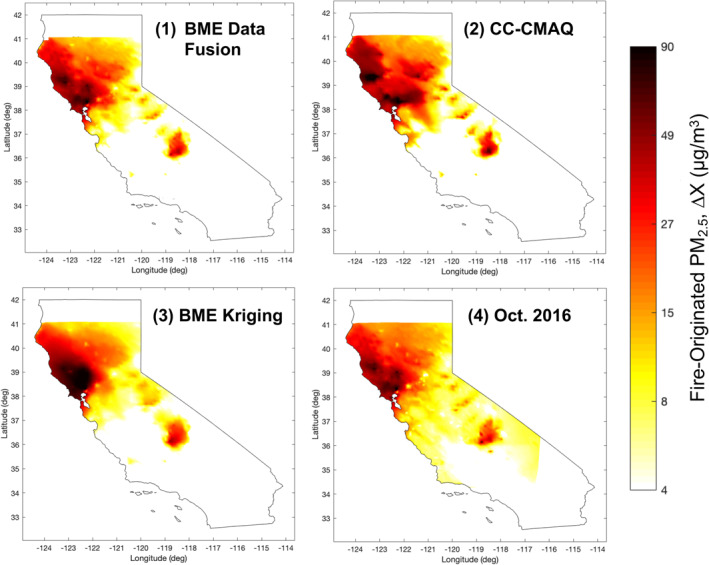
Comparison of methods for estimating exposure to fire‐originated PM2.5 on October 10, 2017. (1) Base case, BME data fusion with CMAQ % attributable as background; (2) CC‐CMAQ with CMAQ % attributable as background; (3) BME kriging with CMAQ % attributable as background; and (4) BME data fusion with October 2016 as background. BME, Bayesian Maximum Entropy; CMAQ, Community Multiscale Air Quality; CC‐CMAQ, corrected Community Multiscale Air Quality model output, using the Constant Air Quality Model Performance.
We then quantified, for each of the three total exposure surfaces, the contributions of uncertainties in the total PM2.5 estimate and CRF to the overall uncertainty of the number of excess respiratory admissions estimated (Figure 4). Only accounting for one source of uncertainty, regardless of the exposure surface used, produces optimistically small confidence bounds and underestimates the admissions estimates' uncertainty. When uncertainty from both the total exposure estimate and CRF is accounted for, CC‐CMAQ, the least precise PM2.5 estimate, produces the least precise admissions estimates. BME data fusion, the most precise PM2.5 estimate, produces the most precise admissions estimates. With BME data fusion, the majority of uncertainty in the admissions estimate comes from the CRF; accounting for the CRF's uncertainty, when uncertainty from the PM2.5 exposure surface is already accounted for, increases the CI width by 121%. In comparison, the primary source of uncertainty when using CC‐CMAQ is the exposure surface; accounting for the CRF's uncertainty, when uncertainty from the PM2.5 exposure surface is already accounted for, only increases the CI width by 14%. The exposure surface and the CRF contribute comparably to uncertainty when BME kriging is used. In health impact assessments, when using CRFs from previously published literature, the uncertainty of the CRF is predefined. In this case, when there is not control over the CRF's uncertainty, there is often control over the uncertainty of the exposure surface, especially if it is developed for the purpose of the assessment. Reducing the variance of the exposure estimate, which BME data fusion does by incorporating three different data sets into the PM2.5 estimation surface, not only changes the primary source of uncertainty but also increases the overall precision of the final respiratory admissions estimate. Similarly, when estimating excess cardiovascular hospital admissions using BME data fusion, the CRF contributes the vast majority of uncertainty to the final admissions estimate (Text S6 and Figure S9).
Figure 4.
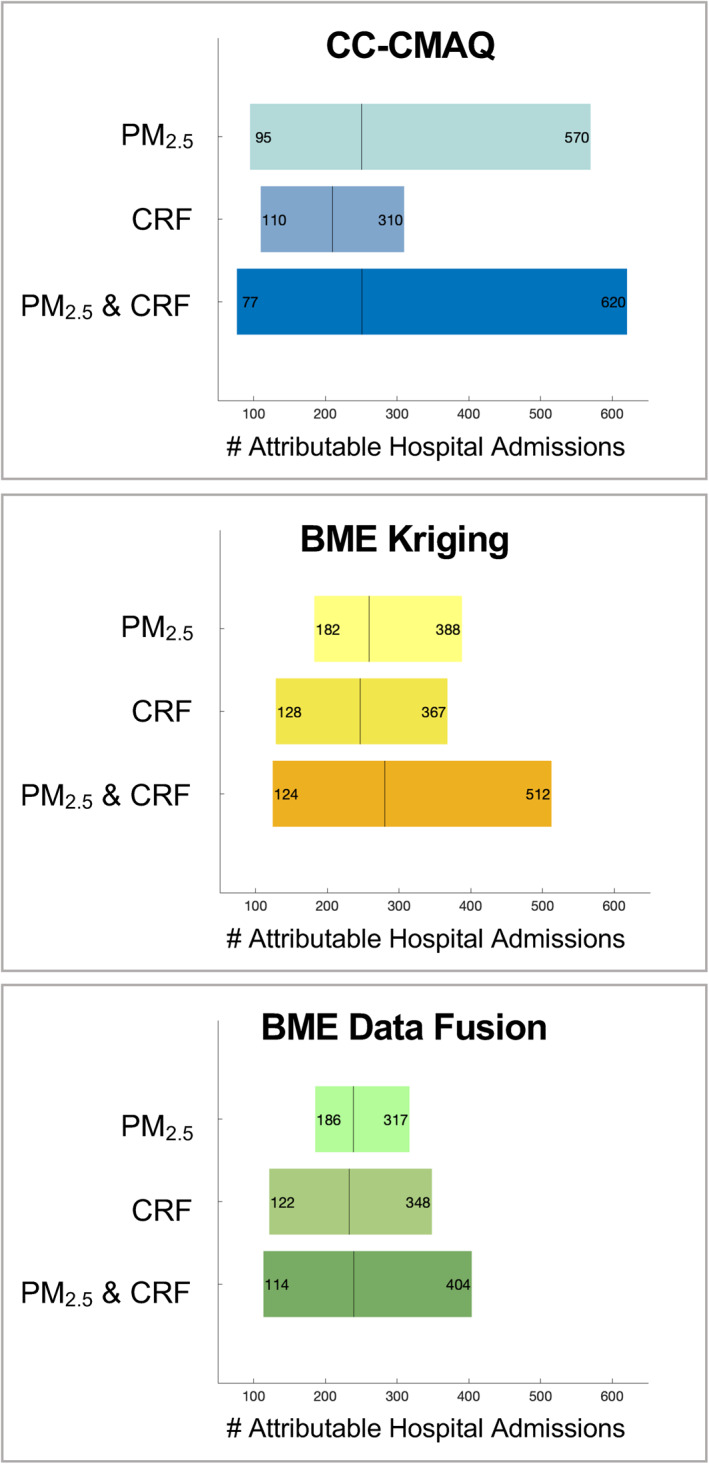
The individual contributions of uncertainties in the CRF and total PM2.5 surface to the total uncertainty in estimated respiratory hospital admissions when uncertainties in both the CRF and total PM2.5 surface are accounted for. Uncertainties are shown as 95% confidence intervals with the vertical line marking the mean estimate. Results are shown using the three total PM2.5 exposure estimates (CC‐CMAQ, BME kriging, and BME data fusion), which all use CMAQ % attributable for the background concentrations and the WF CRF. CRF, concentration–response function; CC‐CMAQ, Constant Air Quality Model Performance‐corrected Community Multiscale Air Quality model output; BME, Bayesian Maximum Entropy; WF, wildfire specific.
4. Discussion and Conclusions
Using the base case impact assessment, we estimate that exposure to fire‐originated PM2.5 during the October 2017 wildfires accounted for over 300 excess respiratory and cardiovascular hospital admissions, with the majority of admissions occurring in the Bay Area. The regions with the highest estimated rates and number of attributable admissions were those with extreme smoke concentrations and high background admission rates and population density, such as Napa and Alameda County, where 14% and 10% of the total estimated respiratory and cardiovascular admissions were associated, respectively. While there is only an estimated 1%–4% increase in respiratory and cardiovascular hospital admissions per 10 µg/m3 increase in PM2.5, since wildfires elevate PM2.5 concentrations to hazardous levels across large geographic regions for multiple weeks, the overall health impact of fire events can be noticeable and widespread.
We also show that choices of inputs used in the health impact assessment have an impact on both the magnitude and uncertainty of the number of attributable admissions estimated. In our assessment, the CRF had the greatest impact on the number of excess admissions estimated, while the uncertainty was most impacted by the exposure surface. Regardless of the health effect being studied, the impact of the CRF will depend on which CRFs are selected. Our findings indicate that using a NF CRF may underrepresent respiratory admissions and overrepresent cardiovascular admissions attributable to fire‐originated PM2.5. Since there is often variety between CRFs for ambient versus wildfire PM2.5 exposure, we recommend that future impact assessments of smoke exposure use a WF CRF when available.
The method used to isolate fire‐originated PM2.5 also has implications when assessing the acute health impacts of smoke exposure. Since ambient PM2.5 and other meteorological conditions in the same geographic region can change over time, using a CTM run during the fire period without fire emissions, rather monitoring station observations in the study area during a nonfire period, may be able to more realistically isolate fire‐originated PM2.5. Additionally, while prior acute health impacts assessments of wildfires have primarily used either monitoring station observations or CTM output to estimate total exposure, we show that combining multiple PM2.5 data sets through data fusion reduces the variance of the exposure estimate and increases the precision of the excess admissions estimates. Using multiple PM2.5 data sets also often increases the accuracy of the exposure surface (Cleland et al., 2020; Lassman et al., 2017; Reid et al., 2015), in turn likely increasing the accuracy of the estimated acute health impacts.
We further show that accounting for uncertainty from only the CRF or the exposure estimate leads to an underestimation of the health impact estimates' uncertainty, emphasizing the importance of accounting for multiple sources of uncertainty. In many prior assessments that only propagated uncertainty using the CRF's distribution (Borchers‐Arriagada, Palmer, Bowman, Morgan, et al., 2020; Borchers‐Arriagada, Palmer, Bowman, Williamson, et al., 2020; Broome et al., 2016; Fann et al., 2013, 2018; Matz et al., 2020), the confidence bounds around the admissions estimates may be optimistically small. Since PM2.5 exposure surfaces are inherently uncertain, it is important to include them as a source of uncertainty to obtain more realistic CIs.
Once uncertainties from both are accounted for, the importance of a good exposure surface becomes more evident. A less precise estimate of total PM2.5, like CC‐CMAQ, contributes far more uncertainty to the final health impact estimates than a more precise exposure estimate, like the BME data fusion output. A prior health impact assessment similarly found that when using a calibrated CMAQ model to estimate WF PM2.5 exposure, the PM2.5 surface contributed more uncertainty than the CRF to the final health impact estimate (Jiang & Yoo, 2019). Since CRFs and their parameterized uncertainty are often drawn from published epidemiological studies, one simple way to reduce the overall uncertainty of health impact assessments is to improve the quality of the exposure surfaces used. To further improve impact assessments and obtain a clearer understanding of the health impacts of wildfires, we recommend that epidemiological studies also use more accurate and informed exposure estimates that integrate multiple PM2.5 data sets to increase the accuracy and precision of CRFs. In order to improve access to more advanced smoke exposure estimates, it is necessary to increase collaboration and data sharing among those investigating the air quality and health impacts of fires.
While our study has many strengths, there are also some important limitations. First, we are unable to validate the accuracy of our attributable admissions estimates because we do not know the number of daily hospital admissions that occurred during October 2017, nor do we know how many were attributable to smoke exposure. Further, our analyses only focused on the central California region, given the bounds of the CMAQ model. By limiting the geographical bounds of our analysis, we likely underestimate the total number of admissions attributable to the October 2017 wildfires, since smoke from the fires impacted regions beyond central California. We also do not account for locations that were evacuated due to the fires, nor for time‐activity patterns among the exposed population, and as a result may have under or overestimated population exposure and the number of excess admissions. Additionally, the exposure, background admission rate, and population data all have different geographic scales which may introduce additional uncertainty into our analyses. Finally, we do not account for uncertainty from the estimation of background concentrations.
By showing that health impact estimates are sensitive to different exposure and epidemiological inputs, and by demonstrating the importance of accounting for multiple sources of uncertainty, employing context‐specific CRFs, and using more advanced PM2.5 exposure estimates, our work can help improve the quality of health impact assessments, for wildfire smoke and for other exposures, moving forward. Using these insights in combination with available resources, future impact assessments can better identify the appropriate data and methods for estimating, with greater certainty, how extreme air pollution events impact hospitals, EDs, and the population's health. These more informed and realistic estimates of the health impacts of wildfires can better inform decision‐making processes and improve public health evaluation before, during, and in the aftermath of fire events.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Acknowledgments
This project was supported by the National Aeronautics and Space Administration Health and Air Quality Applied Sciences Team (grant #NNX16AQ30G), the National Institute of Occupational Safety and Health (T42‐OH008673), and an appointment to the Research Participation Program at the Center for Public Health and Environmental Assessment, U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Environmental Protection Agency. Although this work has been reviewed for publication by the U.S. Environmental Protection Agency it does not necessarily reflect the views and policies of the Agency. We thank Patrick Kinney for helpful discussion, and Susan O'Neill and Minghui Diao for their leadership of the NASA HAQAST California Wildfires Tiger Team project.
Cleland, S. E. , Serre, M. L. , Rappold, A. G. , & West, J. J. (2021). Estimating the acute health impacts of fire‐originated PM2.5 exposure during the 2017 California wildfires: Sensitivity to choices of inputs. GeoHealth, 5, e2021GH000414. 10.1029/2021GH000414
Data Availability Statement
The PM2.5 data sets used in this research are available at https://doi.org/10.15139/S3/1SRKDN.
References
- Bay Area Air Quality Management District . (2019). Annual Bay Area air quality summaries. Retrieved from https://www.baaqmd.gov/about-air-quality/air-quality-summaries (January 23, 2020). [Google Scholar]
- Boegelsack, N. , Withey, J. , O'Sullivan, G. , & McMartin, D. (2018). A critical examination of the relationship between wildfires and climate change with consideration of the human impact. Journal of Environmental Protection, 9(5), 461–467. 10.4236/jep.2018.95028 [DOI] [Google Scholar]
- Borchers‐Arriagada, N. , Horsley, J. A. , Palmer, A. J. , Morgan, G. G. , Tham, R. , & Johnston, F. H. (2019). Association between fire smoke fine particulate matter and asthma‐related outcomes: Systematic review and meta‐analysis. Environmental Research, 179, 108777. 10.1016/j.envres.2019.108777 [DOI] [PubMed] [Google Scholar]
- Borchers‐Arriagada, N. , Palmer, A. J. , Bowman, D. M. J. S. , Williamson, G. J. , & Johnston, F. H. (2020). Health impacts of ambient biomass smoke in Tasmania, Australia. International Journal of Environmental Research and Public Health, 17(9), 3264. 10.3390/ijerph17093264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers‐Arriagada, N. , Palmer, A. J. , Bowman, D. M. , Morgan, G. G. , Jalaludin, B. B. , & Johnston, F. H. (2020). Unprecedented smoke‐related health burden associated with the 2019–20 bushfires in eastern Australia. Medical Journal of Australia, 213(6), 282–283. 10.5694/mja2.50545 [DOI] [PubMed] [Google Scholar]
- Bowman, D. M. J. S. , Moreira‐Muñoz, A. , Kolden, C. A. , Chávez, R. O. , Muñoz, A. A. , Salinas, F. , et al. (2019). Human–environmental drivers and impacts of the globally extreme 2017 Chilean fires. Ambio, 48(4), 350–362. 10.1007/s13280-018-1084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome, R. A. , Johnston, F. H. , Horsley, J. , & Morgan, G. G. (2016). A rapid assessment of the impact of hazard reduction burning around Sydney, May 2016. Medical Journal of Australia, 205(9), 407–408. 10.5694/mja16.00895 [DOI] [PubMed] [Google Scholar]
- Christakos, G. (1990). A Bayesian/maximum‐entropy view to the spatial estimation problem. Mathematical Geology, 22(7), 763–777. 10.1007/BF00890661 [DOI] [Google Scholar]
- Christakos, G. , Bogaert, P. , & Serre, M. L. (2002). Temporal GIS: Advanced functions for field‐based applications. New York, NY: Springer‐Verlag. [Google Scholar]
- Cleland, S. E. , West, J. J. , Jia, Y. , Reid, S. , Raffuse, S. , O'Neill, S. , & Serre, M. L. (2020). Estimating wildfire smoke concentrations during the October 2017 California fires through BME space/time data fusion of observed, modeled, and satellite‐derived PM2.5 . Environmental Science and Technology, 54(21), 13439–13447. 10.1021/acs.est.0c03761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deflorio‐Barker, S. , Crooks, J. , Reyes, J. , & Rappold, A. G. (2019). Cardiopulmonary effects of fine particulate matter exposure among older adults, during wildfire and non‐wildfire periods, in the United States 2008–2010. Environmental Health Perspectives, 127(3), 37006. 10.1289/EHP3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino, R. J. , Brummel, S. , Wu, J. , Stern, H. , Ostro, B. , Lipsett, M. , et al. (2009). The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occupational and Environmental Medicine, 66(3), 189–197. 10.1136/oem.2008.041376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nazelle, A. , Arunachalam, S. , & Serre, M. L. (2010). Bayesian maximum entropy integration of ozone observations and model predictions: An application for attainment demonstration in North Carolina. Environmental Science & Technology, 44(15), 5707–5713. 10.1021/es100228w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann, N. , Alman, B. , Broome, R. A. , Morgan, G. G. , Johnston, F. H. , Pouliot, G. , & Rappold, A. G. (2018). The health impacts and economic value of wildland fire episodes in the U.S. 2008–2012. Science of the Total Environment, 610–611, 802–809. 10.1016/j.scitotenv.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann, N. , Fulcher, C. M. , & Baker, K. (2013). The recent and future health burden of air pollution apportioned across U.S. sectors. Environmental Science and Technology, 47(8), 3580–3589. 10.1021/es304831q [DOI] [PubMed] [Google Scholar]
- Ford, B. , Val Martin, M. , Zelasky, S. E. , Fischer, E. V. , Anenberg, S. C. , Heald, C. L. , & Pierce, J. R. (2018). Future fire impacts on smoke concentrations, visibility, and health in the contiguous United States. GeoHealth, 2, 229–247. 10.1029/2018GH000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, R. W. , Liu, J. , Ford, B. , O'Dell, K. , Vaidyanathan, A. , Wilson, A. , et al. (2020). The association between wildfire smoke exposure and asthma‐specific medical care utilization in Oregon during the 2013 wildfire season. Journal of Exposure Science and Environmental Epidemiology, 30(4), 618–628. 10.1038/s41370-020-0210-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikerwal, A. , Akram, M. , Monaco, A. D. , Smith, K. , Sim, M. R. , Meyer, M. , et al. (2015). Impact of fine particulate matter (PM2.5) exposure during wildfires on cardiovascular health outcomes. Journal of the American Heart Association, 4(7), e001653. 10.1161/JAHA.114.001653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe, D. A. , O'Neill, S. M. , Larkin, N. K. , Holder, A. L. , Peterson, D. L. , Halofsky, J. E. , & Rappold, A. G. (2020). Wildfire and prescribed burning impacts on air quality in the United States. Journal of the Air and Waste Management Association, 70(6), 583–615. 10.1080/10962247.2020.1749731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X. , & Yoo, E. H. (2019). Modeling wildland fire‐specific PM2.5 concentrations for uncertainty‐aware health impact assessments. Environmental Science and Technology, 53(20), 11828–11839. 10.1021/acs.est.9b02660 [DOI] [PubMed] [Google Scholar]
- Johnston, F. H. , Borchers‐Arriagada, N. , Morgan, G. G. , Jalaludin, B. , Palmer, A. J. , Williamson, G. J. , & Bowman, D. M. J. S. (2021). Unprecedented health costs of smoke‐related PM2.5 from the 2019–20 Australian megafires. Nature Sustainability, 4, 42–47. 10.1038/s41893-020-00610-5 [DOI] [Google Scholar]
- Lassman, W. , Ford, B. , Gan, R. W. , Pfister, G. , Magzamen, S. , Fischer, E. V. , & Pierce, J. R. (2017). Spatial and temporal estimates of population exposure to wildfire smoke during the Washington state 2012 wildfire season using blended model, satellite, and in situ data. GeoHealth, 1, 106–121. 10.1002/2017GH000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. C. , Mickley, L. J. , Sulprizio, M. P. , Dominici, F. , Yue, X. , Ebisu, K. , et al. (2016). Particulate air pollution from wildfires in the Western US under climate change. Climatic Change, 138(3–4), 655–666. 10.1007/s10584-016-1762-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. C. , & Peng, R. D. (2019). The impact of wildfire smoke on compositions of fine particulate matter by ecoregion in the Western US. Journal of Exposure Science and Environmental Epidemiology, 29(6), 765–776. 10.1038/s41370-018-0064-7 [DOI] [PubMed] [Google Scholar]
- Liu, J. C. , Pereira, G. , Uhl, S. A. , Bravo, M. A. , & Bell, M. L. (2015). A systematic review of the physical health impacts from non‐occupational exposure to wildfire smoke. Environmental Research, 136, 120–132. 10.1016/j.envres.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. C. , Wilson, A. , Mickley, L. J. , Dominici, F. , Ebisu, K. , Wang, Y. , et al. (2017). Wildfire‐specific fine particulate matter and risk of hospital admissions in urban and rural counties. Epidemiology, 28(1), 77–85. 10.1097/EDE.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz, C. J. , Egyed, M. , Xi, G. , Racine, J. , Pavlovic, R. , Rittmaster, R. , et al. (2020). Health impact analysis of PM2.5 from wildfire smoke in Canada (2013–2015, 2017–2018). The Science of the Total Environment, 725, 138506. 10.1016/j.scitotenv.2020.138506 [DOI] [PubMed] [Google Scholar]
- Rappold, A. G. , Cascio, W. E. , Kilaru, V. J. , Stone, S. L. , Neas, L. M. , Devlin, R. B. , & Diaz‐Sanchez, D. (2012). Cardio‐respiratory outcomes associated with exposure to wildfire smoke are modified by measures of community health. Environmental Health, 11(1), 71. 10.1186/1476-069X-11-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, C. E. , Brauer, M. , Johnston, F. H. , Jerrett, M. , Balmes, J. R. , & Elliott, C. T. (2016). Critical review of health impacts of wildfire smoke exposure. Environmental Health Perspectives, 124(9), 1334–1343. 10.1289/ehp.1409277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, C. E. , Jerrett, M. , Petersen, M. L. , Pfister, G. G. , Morefield, P. E. , Tager, I. B. , et al. (2015). Spatiotemporal prediction of fine particulate matter during the 2008 northern California wildfires using machine learning. Environmental Science and Technology, 49(6), 3887–3896. 10.1021/es505846r [DOI] [PubMed] [Google Scholar]
- Reisen, F. , Duran, S. M. , Flannigan, M. , Elliott, C. , & Rideout, K. (2015). Wildfire smoke and public health risk. International Journal of Wildland Fire, 24(8), 1029–1044. 10.1071/WF15034 [DOI] [Google Scholar]
- Reyes, J. M. , Xu, Y. , Vizuete, W. , & Serre, M. L. (2017). Regionalized PM2.5 Community Multiscale Air Quality model performance evaluation across a continuous spatiotemporal domain. Atmospheric Environment, 148, 258–265. 10.1016/J.ATMOSENV.2016.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittmaster, R. , Adamowicz, W. L. , Amiro, B. , & Pelletier, R. T. (2006). Economic analysis of health effects from forest fires. Canadian Journal of Forest Research, 36(4), 868–877. 10.1139/X05-293 [DOI] [Google Scholar]
- Sacks, J. D. , Lloyd, J. M. , Zhu, Y. , Anderton, J. , Jang, C. J. , Hubbell, B. , & Fann, N. (2018). The Environmental Benefits Mapping and Analysis Program‐Community Edition (BenMAP‐CE): A tool to estimate the health and economic benefits of reducing air pollution. Environmental Modelling & Software, 104, 118–129. 10.1016/j.envsoft.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre, M. L. , & Christakos, G. (1999). Modern geostatistics: Computational BME analysis in the light of uncertain physical knowledge—The Equus Beds study. Stochastic Environmental Research and Risk Assessment, 13(1–2), 1–26. 10.1007/s004770050029 [DOI] [Google Scholar]
- Spracklen, D. V. , Mickley, L. J. , Logan, J. A. , Hudman, R. C. , Yevich, R. , Flannigan, M. D. , & Westerling, A. L. (2009). Impacts of climate change from 2000 to 2050 on wildfire activity and carbonaceous aerosol concentrations in the western United States. Journal of Geophysical Research, 114, D20301. 10.1029/2008JD010966 [DOI] [Google Scholar]
- Yue, X. , Mickley, L. J. , Logan, J. A. , & Kaplan, J. O. (2013). Ensemble projections of wildfire activity and carbonaceous aerosol concentrations over the western United States in the mid‐21st century. Atmospheric Environment, 77, 767–780. 10.1016/j.atmosenv.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti, A. , Franklin, M. , Koutrakis, P. , & Schwartz, J. (2009). Fine particulate air pollution and its components in association with cause‐specific emergency admissions. Environmental Health, 8(1), 1–12. 10.1186/1476-069X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The PM2.5 data sets used in this research are available at https://doi.org/10.15139/S3/1SRKDN.


