Abstract
Wound healing complications affect thousands of people each year, thus constituting a profound economic and medical burden. Chronic wounds are a highly complex problem that usually affects elderly patients as well as patients with comorbidities such as diabetes, cancer (surgery, radiotherapy/chemotherapy) or autoimmune diseases. Currently available methods of their treatment are not fully effective, so new solutions are constantly being sought. Cell‐based therapies seem to have great potential for use in stimulating wound healing. In recent years, much effort has been focused on characterizing of adipose‐derived mesenchymal stromal cells (AD‐MSCs) and evaluating their clinical use in regenerative medicine and other medical fields. These cells are easily obtained in large amounts from adipose tissue and show a high proregenerative potential, mainly through paracrine activities.
In this review, the process of healing acute and nonhealing (chronic) wounds is detailed, with a special attention paid to the wounds of patients with diabetes and cancer. In addition, the methods and technical aspects of AD‐MSCs isolation, culture and transplantation in chronic wounds are described, and the characteristics, genetic stability and role of AD‐MSCs in wound healing are also summarized. The biological properties of AD‐MSCs isolated from subcutaneous and visceral adipose tissue are compared. Additionally, methods to increase their therapeutic potential as well as factors that may affect their biological functions are summarized. Finally, their therapeutic potential in the treatment of diabetic and oncological wounds is also discussed.
Keywords: adipose‐derived stromal cells, chronic wounds, diabetic ulcers, fat transfer, oncological wounds, SVF, wound healing
1. INTRODUCTION
Wound healing is a complex process consisting of three main overlapping stages: the inflammatory phase, proliferative phase, and remodeling phase, which occur in temporal sequence (Figure 1). It is regulated by different cell types (e.g., keratinocytes, fibroblasts, stem cells, and immune cells), cytokines, growth factors, and extracellular matrix components (ECM). 1 The inflammatory phase usually lasts for the first 4 days after injury and begins with the formation of a fibrin clot covering the wound which constitutes a temporary matrix enabling the migration of inflammatory cells and protection against pathogens and fluid loss. 2 Neutrophils are attracted to the wound within 24–36 h after injury by various factors secreted from the fibrin clot and damaged tissue, including transforming growth factor beta (TGF‐β), platelet‐derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), and interleukin (IL)‐8. Neutrophils secrete proteases, phagocyte bacteria present in the wound and degrade necrotic tissue.3, 4, 5 They are followed by monocytes, which differentiate into macrophages able to phagocytose cell debris and dead neutrophils. 6 In the late inflammatory phase, macrophages secrete growth factors (TGF‐β, EGF, PDGF, FGF) and the proinflammatory cytokines IL‐1 and IL‐6, thus activating keratinocytes, fibroblasts and endothelial cells.7, 8 The second, proliferative phase, which begins 3–4 days after injury and lasts from 2 to 4 weeks, is stimulated by factors secreted in inflammatory phase. In the proliferative phase, angiogenesis and epithelialization occur and ECM and granulation tissue are formed. 9 Angiogenesis, which is essential for the formation of granulation tissue, is induced by growth factors: vascular endothelial growth factor A (VEGF‐A), FGF‐2, PDGF, and TGF‐β. 10 Collagen secreted by fibroblasts gradually replaces the fibrin matrix. Fibroblasts also differentiate into myofibroblasts expressing α‐smooth muscle actin, which enables wound contraction. 11 Remodeling of the wound and surrounding tissues by fibroblasts, which is the final stage of wound healing, begins approximately 3 weeks after injury and can last up to 2 years. 12 During remodeling, all processes activated in the earlier phases are terminated, and myofibroblasts, macrophages and endothelial cells undergo apoptosis. 13 Collagen III is converted to collagen I by metalloproteinases, which, together with collagen rearrangement into an organized structure, leads to strengthening of the wound. 14 Typically, wounds reach approximately 20% strength of healthy skin after 3 weeks, and 80% after 12 months. 15
Figure 1.
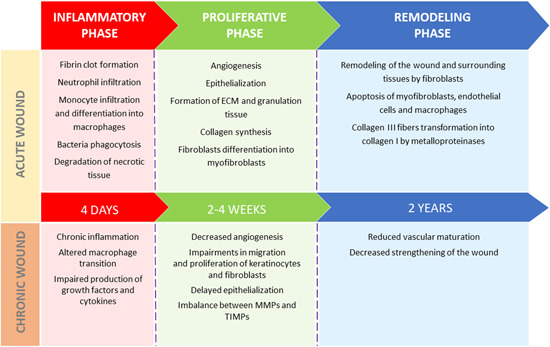
Wound healing process in acute and chronic wounds [Color figure can be viewed at wileyonlinelibrary.com]
Many methods are used in the treatment of nonhealing wounds, including different kinds of dressings (e.g., films, hydrocolloids, foams, hydrogels, alginates, hydrofibers), tissue‐engineered skin substitutes, growth factors, negative pressure therapy, and hyperbaric oxygen. 16 However they are not fully efficient. In recent years, much attention has been paid to the use of cell therapies in the treatment of wounds. For example, epidermal stem cells were first used in wound treatment in 1981 and are now applied to promote healing of burns and chronic wounds.17, 18 Autologous genetically modified cultured epidermal stem cells were also successfully used in a clinical trial for junctional epidermolysis bullosa.19, 20 Recently, therapies with mesenchymal stromal cells (MSCs), especially adipose‐derived MSCs (AD‐MSCs), have been of great interest around the world. 21 For many years, adipose tissue has been considered medical waste but is in fact a great source of stem cells. AD‐MSCs are easy to obtain and have similar properties to bone marrow‐derived MSCs (BM‐MSCs). 22 Adipose tissue is a more effective source of stem cells, which can be extracted in large amounts (500‐fold greater than BM‐MSCs when counted per unit volume of fat) without ethical concerns. Additionally, AD‐MSCs show higher proliferative capacity, longer life‐span and shorter doubling time than BM‐MSCs. 23 Stem cells have great potential for chronic wound healing due to increased cell migration, high proliferative potential and release of cytokines and biological factors that regulate angiogenesis, induce repair processes, and inhibit inflammatory and immune responses.24, 25
There is some inconsistency regarding the nomenclature of MSCs and mesenchymal stem cells. According to the International Society for Cellular Therapy (ISCT) the term “mesenchymal stromal cells” refers to the plastic‐adherent fraction of cells showing immunomodulatory, secretory and homing properties, while “mesenchymal stem cells” refer to the population expressing progenitor properties such as self‐renewal and differentiation potential to multiple cell linages. 26 The abbreviation “MSC” should be used with additional information of the tissue source origin of the cells, for example, AD‐MSCs (adipose tissue‐derived MSCs) and a functional definition should be provided to clarify whether it refers to stem or stromal cells (i.e., stemness confirmation with in vivo and in vitro tests). 27 Additionally, because MSCs act therapeutically by homing in on the injury site and secreting immunomodulatory and regenerative factors, which makes them therapeutic drugs, Caplan 28 suggested naming them “medicinal signaling cells.”
In this review, we summarized current knowledge about chronic wound treatment with the use of AD‐MSCs with a particular focus on wound healing complications in diabetic and oncological patients. Diabetes and cancer are civilization diseases and the number of patients suffering from them is constantly growing. Wound healing problems are a common complication of diabetes and oncological treatment. Intensive research is underway all over the world on new drug compounds and methods of supporting wound healing in cancer patients and patients with diabetes. The review summarizes not only the biological characteristic of AD‐MSCs but also the technical aspects of their isolation, cell culture and transplantation to nonhealing wounds. A comparison of AD‐MSCs from different sources (subcutaneous and visceral adipose tissues [SAT and VAT]) was also made. Additionally, factors that can affect AD‐MSCs as well as ways to enhance their therapeutic potential were described.
2. CHRONIC WOUND CHARACTERISTICS AND CLINICAL NEED
Chronic wounds are wounds that do not heal through normal wound healing phases in an orderly and timely manner for at least 1 month. 29 Medical conditions, for example, diabetes, autoimmune diseases, vascular pathologies, obesity, neuropathy or infections, as well as therapeutics, such as cancer chemotherapeutic agents, radiation therapy, nonsteroidal antiinflammatory agents or glucocorticoids, can affect the wound healing process and lead to the formation of nonhealing or chronic wounds. Patient age, nutrition, smoking status and alcohol consumption are also important extrinsic factors. 30 A distinction is made between wounds of various etiologies: venous and ischemic ulcers, diabetic foot syndromes, posttraumatic and postoperative wounds, pressure sores and burn wounds (Figure 2.).31, 32, 33, 34
Figure 2.
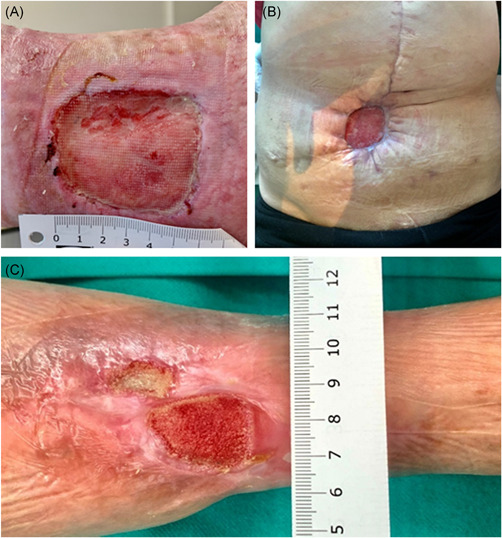
Chronic wounds of different etiology. (A) Posttraumatic chronic wound of lateral ankle, diabetic foot syndrome; (B) Postoperative wound of abdomen, operation of hernia in postoperative scar complicated by infection and necrosis of the abdominal wall, (C) Posttraumatic chronic wound of shank, ischemic wound [Color figure can be viewed at wileyonlinelibrary.com]
Chronic wounds constitute not only a medical issue but also a large economic issue. In developed countries, 1%–2% of the general population has chronic nonhealing wounds. 35 With an aging society and a growing population of patients suffering from diabetes, obesity and vascular diseases, these numbers are expected to rapidly increase. 36 The quality of life of patients with chronic wounds is significantly impaired and they show high morbidity and mortality rates. 37 In addition, treating these wounds is a serious economic burden, estimated to comprise approximately 1%–3% of medical budgets in developed countries. 38
Chronic wounds of different etiologies possess several common features, such as elevated levels of proinflammatory cytokines, proteases, reactive oxygen species (ROS) or senescent cells, dysfunction or deficiency of stem cells, decreased levels of growth factors, abnormalities in ECM functions and weak blood supply.39, 40 Their healing process is mainly arrested in the inflammatory phase, and antimicrobial and phagocytic activity of the immune cells appears to be lower in chronic wounds than in acute wounds, which likely leads to the accumulation of necrotic tissue on the wound edges. 41 The level of growth factors essential for proper wound healing can also be important in the formation of chronic wounds. For example, bFGF, PDGF, TGF‐β, and EGF levels are reduced in chronic pressure ulcers compared to acute wounds. 42 In addition, chronic wounds are often gradually colonized by various bacteria, for example, Staphylococcus aureus, Enterococcus faecalis, and Pseudomonas aeruginosa, all of which form a biofilm enabling them to become not only resistant to antibiotics, other antimicrobial agents and the body's defense mechanisms but also more susceptible to other bacterial and fungal infections.43, 44, 45 The presence of bacteria and their toxins causes excessive inflammatory reactions and tissue damage and results in intensified local pain. 46 In addition, immune cells and bacteria produce proteases that degrade ECM and growth factors in the wound. 47
2.1. Wound healing in diabetes
Diabetes mellitus is a chronic metabolic disease characterized by hyperglycemia. According to the World Health Organization, 422 million adults suffered from diabetes in 2014, and 1.5 million people died of diabetes‐related complications in 2012. 48 It is estimated that the population of diabetic individuals will grow to 592 million by 2035. 49 In 2017, the cost of diabetes in the United States was 237 billion dollars, one‐third was allocated to the treatment of diabetic foot ulcers (DFUs). 50 These wounds are one of the most common and serious complications of diabetes and a major cause of morbidity and mortality in individuals with diabetes. Ischemia, neuropathy and infection, often occurring together, constitute the etiological triad, which leads to complications of DFUs. 51 Approximately 15% of people suffering from diabetes have diabetic ulcer during their lifetime, and 85% of amputations in diabetic patients are caused by foot ulceration, which further deteriorates to severe infection. Besides, 50%–70% of all lower limb amputations performed are the result of diabetes.52, 53
Hyperglycemia can affect wound healing through different mechanisms. Tissue loss may be aggravated by a neuropathic lack of sensation, and wound healing may also be delayed by dysfunctional epithelialization caused by impaired cell proliferation and resistance to growth factors. 54 Impairment in many key processes for proper wound healing including the production of growth factors, the proliferation and migration of keratinocytes and fibroblasts, the angiogenic response, collagen accumulation or the balance between ECM component accumulation and remodeling, plays main roles in the pathophysiology of DFUs (Figure 1). 55 In diabetic wounds, macrophage transition from a proinflammatory to an antiinflammatory state does not occur, and these cells remain mainly in a proinflammatory state. 56 Prolonged inflammation is also caused by IL‐1β and tumor necrosis factor α (TNFα), whose levels are increased during the inflammatory phase and remain elevated in wounds for a longer time. The stability and activity of hypoxia‐inducible factor 1 affected by hyperglycemia results in the suppression of its target genes, for example, VEGF, and impaired in endothelial progenitor cell recruitment caused by decreased production of nitric oxide (NO) contributes to reduced angiogenesis.57, 58, 59 In addition, keratinocytes from the margins of diabetic ulcers are activated and highly proliferative (increased expression of Ki67) and do not express the differentiation markers keratins 2 and 10 (K2, K10). They also do not migrate and show reduced expression of the precursor of the α3 chain of laminin 5 (LM‐3A32), which is a molecule present in migrating epithelial cells. 60 In vitro cell cultures of fibroblasts isolated from DFUs showed reduced motility, altered secretion of cytokines (lower levels of C‐X‐C motif ligand 1, IL‐6, IL‐8, IL‐23, monocyte chemoattractant protein 1 (MCP‐1), and stromal cell‐derived factor 1) and a drop in the maximum mitogenic response to growth factors compared to those of cells from nondiabetic patients.61, 62 Additionally, in diabetic wounds, reduced levels of many growth factors including PDGF, KGF, VEGF, insulin‐like growth factors 1 and 2 (IGF‐1, IGF‐2), nerve growth factor, TGF‐β1 and KGF, were observed, which may contribute to the delayed healing of these wounds. 63 The results from a prospective cohort study on diabetic patients showed that serum levels of TNF‐α, MCP‐1, matrix metalloproteinase 9 (MMP‐9) and FGF‐2 were higher in patients with nonhealing ulcers than in those whose ulcers had healed. Moreover, increased immune cell infiltration and expression of MMP‐9 and protein tyrosine phosphatase 1B (PTP1B) were observed in skin biopsies of diabetic patients. These factors are associated with, for example, increased inflammation. MMP‐9 is involved in the degradation of proteins and growth factors, while PTP1B takes part in negative regulation of insulin, leptin, and growth factors signaling (e.g., PDGF, VEGF, and EGF). Increased expression of extracellular MMP‐9 and intracellular PTP1B may not only lead to local inactivation and resistance to the activity of growth factors but also, in a way similar to elevated levels of insulin in insulin resistance, to an increase in circulating growth factors levels. 64 Additionally, impairment in the regulation of ECM was confirmed in diabetes. For example, reduced levels of collagen and elastin were found in biopsies from DFU edges. This changes probably arose as a result of persistent inflammation and fibroblast senescence as well as poor vascularization of the wound. 65 Hyperglycemic conditions may also directly contribute to increased production of MMPs and a reduction of tissue inhibitors of metalloproteinases (TIMPs), which results in disruption of the structures essential for proper wound healing.66, 67
2.2. Wound healing in oncological patients
Cancer is one of the greatest challenges of modern medicine and the numbers of newly diagnosed cases and cancer‐related deaths are rapidly growing worldwide. It is estimated that 18.1 million new cases of cancer were diagnosed and that 9.6 million patients died because of cancer in 2018. 68 In the treatment of cancer, chemotherapy and/or radiotherapy can be given to patients preoperatively (neoadjuvant therapy) or after surgical resection of the tumor (adjuvant therapy). Despite having many advantages, such as increased 5‐year survival rates and decreased numbers of local recurrence, neoadjuvant treatment may also cause postoperative complications in wound healing, resulting in reduced blood supply to the wound and regenerative potential as well as a higher incidence of infections. Usually, surgery is postponed to 4–6 weeks after neoadjuvant treatment, but it does not fully prevent the risk of such complications.69, 70 In oncological patients, skin manifestations, wound healing complications and tissue loss may either be caused by the tumor itself or result from the method of treatment used (Figure 3). A common problem is also infections at the surgery site, for example, after breast surgery, which can also delay healing. 71
Figure 3.
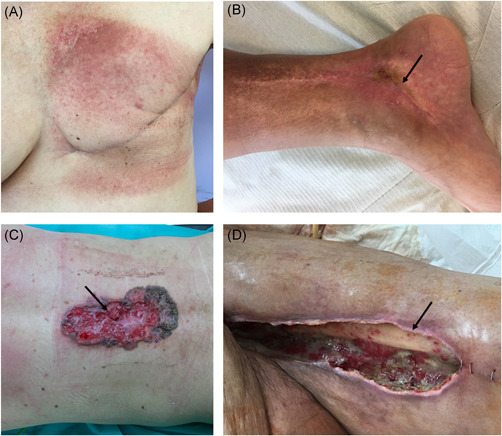
Skin complications in oncological patients. (A) 57‐year‐old patient after a radical mastectomy and postoperative radiotherapy due to breast cancer. The figure shows skin discoloration in the irradiated area, that is, at the breast and armpit. In the postoperative course, features of marginal necrosis of the wound treated in an outpatient setting were observed; (B) 35‐year‐old patient after a radical surgery of soft tissues sarcoma of a right lower leg above the ankle and a postoperative radiotherapy. Due to ischemia, the wound in the lower limb, in the area from the back and below the ankle, was accompanied by prolonged healing. The figure shows the place of impaired healing visible as a depression with a fragment of an atrophic wound (arrow); (C) 47‐year‐old patient with the ulceration of the back (dimensions:17 ×10 cm) resulting from skin cancer before the radical procedure. This type of neoplastic ulcer is associated with infection and necrosis. During radical surgery, the most important element is to protect the operated site from infection of the postoperative wound. The arrow marks the site of ulceration as a place with increased risk of postoperative wound infection; (D) Tissue loss of the left tight in a 63‐year‐old patient after a radical surgery of soft tissues sarcoma and postoperative radiotherapy. A fragment of the thigh bone with a defect in the thigh muscles is visible. It is the most difficult variant to heal, due to the extensive tissue loss and the condition after undergoing oncological treatment. The arrow marks the exposed femur [Color figure can be viewed at wileyonlinelibrary.com]
Chemotherapeutic drugs may interrupt processes responsible for proper wound repair by inhibiting cell division; metabolism and angiogenesis; the synthesis of DNA, RNA, and proteins; cell migration into the wound and the formation of wound matrix. In addition, they impair fibroblast growth, and inhibit collagen production and wound contraction. 72 These drugs are designed to target rapidly dividing cells, so macrophages and fibroblasts are as susceptible to their activity as cancer cells. 73 In addition, chemotherapy weakens patients' immune system which may interfere with the inflammatory phase of wound healing and increase the possibility of wound infections.74, 75 Targeted therapeutics such as epidermal growth factor receptor (EGFR) or VEGF inhibitors should be less toxic to normal cells, but their therapeutic targets involved in cancer growth also participate in wound healing, so the use of these therapeutics is associated with adverse reactions, including skin toxicity. 76 Blocking EGFR signaling in healthy keratinocytes results in inhibition of their growth, proliferation, and migration. 77 The use of bevacizumab, a monoclonal anti‐VEGF antibody, to block angiogenesis, may cause wound dehiscence, bleeding, and infections resulting from the limited delivery of cells, nutrients and oxygen. To avoid wound healing complications, it is recommended to perform surgery 60 days or 6–8 weeks after treatment with this drug and not to take bevacizumab at least 28 days after surgery.78, 79, 80
The side effects of radiotherapy can be categorized as acute (hours to weeks after exposure) and late (months to years after exposure). Acute injury includes hyperpigmentation and early ulceration, while late effects present as tissue fibrosis, necrosis, atrophy, vessel damage, and chronic ulcers. 81 Skin damage is one of the most frequent acute side effects of radiotherapy, with 90% of patients who undergo radiotherapy developing skin reactions. 82 The cytotoxic effect of radiation is a result of direct DNA ionization and interaction of produced ROS with DNA. Base alterations, the formation of dimers and DNA double‐strand breaks result in damage to basal keratinocytes and a reduction in the self‐renewing properties of the epidermis. Overexpression of proinflammatory factors, including TNF‐α, interferon gamma (IFN‐γ), IL‐1, IL‐3, IL‐5, IL‐6, IL‐8, and adhesion molecules, including intercellular adhesion molecule 1, vascular cell adhesion molecule, and E‐selectin sustain the inflammatory phase. Wound strength is decreased by the prevention TNF‐α and IFN‐γ‐mediated collagen deposition, the production of highly disorganized collagen and alterations in the production of ECM proteins resulting from changes in fibroblasts.83, 84, 85, 86 Moreover, elevated levels of TGF‐β in the serum of irradiated patients correlated with a higher risk of radiation‐induced fibrosis. 87 Low levels of angiogenic factors (FGF, HGH, and VEGF) in irradiated skin may be responsible for inappropriate vascularization, disrupted angiogenesis and reduced blood supply. 88 Persistent high concentrations of MMP‐2 and MMP‐9 and an imbalance of the expression of TIMPs and decreased angiogenesis may be the reason why these wounds fail to heal. 89
3. CELLS AND TISSUE SOURCES
Adipose tissue may be obtained in two ways: surgical resection of excess fat tissue (Figure 4) or liposuction of lipoaspirate. The techniques of fat harvesting are summarized in Table 1. Lipoaspirate from liposuction can be directly clinically used without previous AD‐MSCs in vitro culturing. 90 The harvesting procedure uses a tumescent solution of the acidic pH including which includes Klein solution (see below), lidocaine or epinephrine which may induce a perioperative and postoperative burning sensation. The latest studies show that the presence of lidocaine decreases AD‐MSCs survival by an apoptosis increase. The neutralization of the tumescent solution by adding sodium bicarbonate can increase the survival of AD‐MSCs (by an increase in the number of viable cells and apoptosis decrease) and the stromal vascular fraction (SVF, up to 53%) and attenuates perioperative and postoperative pain. 91 Avoiding in vitro manipulation of clinical material seems to be safer and bypasses some legal regulation. 92
Figure 4.
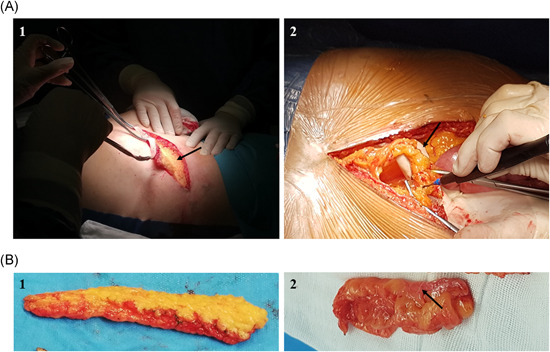
Fat collection through surgical resection. (A, 1) Collection of subcutaneous adipose tissue. Visualization of surgical wound during the process of fat tissue sample collection from subcutaneous tissue. Arrow points at the base of the wound which consists of fascial tissue of abdominal wall. (A, 2) Collection of visceral adipose tissue. Visualization of surgical wound after laparotomy procedure. The fat tissue sample is collected from the round ligament of the liver, arrow points at round ligament of the liver. (B, 1) Subcutaneous adipose tissue. (B, 2) Visceral adipose tissue. The photo shows intraperitoneal adipose tissue, the fragment is coated with the serosa (arrow) [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Techniques of fat harvesting
| Method of fat harvesting | Advantages | Disadvantages |
|---|---|---|
| Vacuum aspiration |
Short time to harvest a larger volume of fat tissue Small scar |
High negative pressure results in trauma to the adipocytes Necessity to have vacuum equipment |
|
Syringe aspiration Coleman technique |
Gentle negative pressure, which minimizes trauma to the adipocytes Small scar The possibility of taking a large amount of fat tissue in local anesthesia |
Longer time to obtain same amount of fat tissue compared to the vacuum aspiration method |
| Surgical excision | Maintains the structure and viability of harvested fat tissue by avoiding damage to the adipocytes |
Bigger scar General anesthesia must be used to excise more fat tissue Lower tissue plasticity |
The type of harvesting procedure influences the yield, viability and proliferation of AD‐MSCs. There are few main isolation methods: mechanical‐assisted liposuction (MAL), power‐assisted liposuction (PAL), laser‐assisted liposuction (LAL), ultrasound‐assisted liposuction (UAL) and surgical resection. PAL has been identified as the best method because cells isolated using this technique show high proliferation potential and low senescence. A comparison between MAL and UAL‐derived AD‐MSCs did not indicate significant changes in the expression of MSC markers (CD13, CD29, CD73, CD90, CD105; only the level of CD166 was higher in UAL‐derived cells). Other comparisons (surgical resection, PAL, and LAL) confirmed that different harvesting methods do not change the expression of basic mesenchymal markers (such as CD90 and CD40).93, 94, 95 AD‐MSCs obtained from the abdomen through resection or liposuction yield more cells than either UAL or fat tissue collected from the hip/thigh district. 96
3.1. Isolation of AD‐MSCs
Cell isolation is a critical step in obtaining cells for experimental and therapeutic procedures. Enzymatic or nonenzymatic methods of AD‐MSCs isolation are described in the literature; the enzymatic method utilizing collagenase (Figure 5) is most frequently used. However, proteolytic enzymes, for example, trypsin‐EDTA, dispase or collagenase may negatively affect the viability and surface antigen expression on AD‐MSCs. 97 Moreover, similar to fetal bovine serum (FBS) utilization, there are safety issues regarding the use of xenogeneic collagenase. To overcome this problem, nonenzymatic methods such as sonication, explant culture, and centrifugation were developed. However, none of the proposed nonenzymatic methods isolated the same number of cells as the enzymatic method. 98 Comparison of the explant culture method and enzymatic method showed that the former had higher hematopoiesis potential, a lower percentage of CD34 expression and better quality, but required a large amount of lipoaspirate and resulted in lower overall yield of recovered cell. 99 The enzymatic method elicited a significantly higher number of cells, higher colony‐forming efficiency (higher Nanog and Oct4 expression) and better differentiation capability. 100 As we believe that the isolation efficacy is critical, we assume that enzymatic isolation is the best way of obtaining cells for clinical use. Therefore, it is important to develop protocols for AD‐MSCs isolation with clinical grade collagenase. Carvalho et al. 101 showed that xeno‐free enzymatic products containing collagenases (e.g., Liberase TM) are as effective as research‐grade products in the isolation of AD‐MSCs. They did not notice statistically significant differences in cell properties (proliferation, differentiation, cell surface markers) among the cells isolated with different enzymatic products (CLS1 [Worthington], CLSAFA [Worthington], NB4 [Serva], Liberase [Roche]). Moreover, in 2013, a clinical trial using ex vivo expanded AD‐MSCs isolated with clinical collagenase NB 6 good manufacturing practice (GMP) grade (SERVA Electrophoresis GmbH) was performed to analyze the effect of fat grafts enrichment with AD‐MSCs on graft survival. The procedure showed good feasibility and safety. 102
Figure 5.
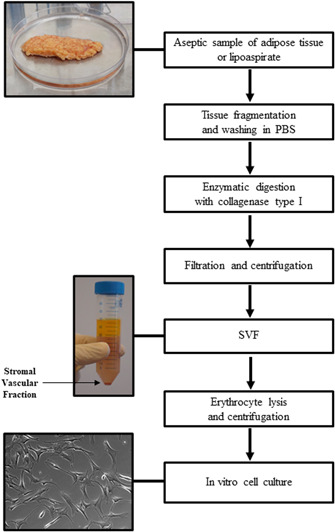
Isolation of AD‐MSCs by enzymatic method. AD‐MSC, adipose‐derived mesenchymal stromal cell [Color figure can be viewed at wileyonlinelibrary.com]
The efficiency, proliferation and pluripotency of AD‐MSCs depends on the donor site area. Analysis of cells from adipose tissue taken by liposuction from the abdomen, flanks, thighs, and medial knees showed that the abdominal region and inner thighs (expanding region during excess caloric intake) produce the highest yield of AD‐MSCs. The superficial abdominal depot is less sensitive to apoptosis and has higher differentiation potential compared to other subcutaneous depots.103, 104 AD‐MSCs can also be obtained from atypical locations, for example, buccal fat pads. Cells from this area express high levels of osteogenic markers; therefore, they seem to be more appropriate for treating bone defects. 105 Comparison of AD‐MSCs lipoaspirates taken from the abdomen and hump showed differences: hump‐derived AD‐MSCs are smaller in size and narrower in overall appearance than are abdominal AD‐MSCs. 106 Additionally, some differences have been observed between subcutaneous AD‐MSCs taken from the medial thigh or trochanteric area and those taken from the upper arm, which express higher levels of peroxisome proliferator‐activated receptor gamma (PPAR‐γ‐2). 107
3.1.1. SVF versus AD‐MSCs
The advantage of SVF over AD‐MSCs is its heterogeneous cellular composition (preadipocytes, fibroblasts, vascular smooth muscle, endothelial cells, macrophages, and lymphocytes), which is responsible for better therapeutic outcomes, comparable safety and less regulatory criteria. During culture expansion, AD‐MSCs can change surface marker expression and cells morphology. However, AD‐MSCs can be used in allogeneic and autologous treatments, while SVF can be used only in autologous treatment (Table 2).108, 109, 110 Allogeneic AD‐MSCs carry minimal rejection risk, and obtaining AD‐MSCs is less deleterious than obtaining other types of MSC. 111 Furthermore, fluids secreted by acute and chronic wounds have an impact on AD‐MSCs and may regulate their regenerative potential. 112
Table 2.
Comparison of AD‐MSCs and SVF
| Treatment | Cell population | Cell types | Properties | Ex vivo exposure | |
|---|---|---|---|---|---|
| AD‐MSCs | Allogenic and autologous | Homogenous | AD‐MSCs | Immunomodulatory, differentiative | High (weeks) |
| SVF | Only autologous | Heterogenous | AD‐MSCs, fibroblasts, vascular smooth muscle, endothelial cells, macrophages, lymphocytes | Immunomodulatory, differentiative, angiogenic | Low (hours) |
Abbreviations: AD‐MSC, adipose‐derived mesenchymal stromal cell; SVF, stromal vascular fraction.
For clinical application, it is important to properly store the SVF and AD‐MSCs for later therapeutic use. Only an elongated period of cryopreservation at −70°C (>2 years) reduces the number of live cells and their viability. 113 Compared to fresh lipoaspirate‐derived SVF, SVF from cryopreserved lipoaspirate has reduced cell viability and a lower colony‐forming‐unit percentage (16‐fold). 114 It was shown that cryopreservation media containing human serum (HS) albumin, HS, or knockout serum replacement has no influence on AD‐MSCs conditions (gene expression, immunophenotype, and differentiation ability) for up to 3–4 freeze‐thaw cycles. However, their proliferation was significantly reduced, and it was suggested to perform no more than two freeze‐thaw cycles on cells for clinical application. 115
4. CHARACTERIZATION OF AD‐MSCs
The characterization of AD‐MSCs is critical before their application in not only laboratory research but also the clinic. Culture conditions can affect the properties of these cells and thus their therapeutic potential.
4.1. Markers and differentiation potential of AD‐MSCs
Since the discovery and isolation of MSCs populations from adipose tissue, much effort has been paid to their characterization, especially in the identification of their surface markers. According to the statement from the ISCT, MSCs are defined by minimal criteria, including adherence to plastic, specific expression pattern of markers (positive [>95%]: CD105, CD73 and CD90, negative [<2% positive]: CD45, CD34, CD14, or CD11b, CD79a or CD19 and HLA Class II) and multipotent differentiation potential (differentiation into adipocytes, osteoblasts and chondroblasts under standard differentiation conditions). 116 Later, the ISCT with the International Federation for Adipose Therapeutics and Science (IFATS) published guidelines for the characterization of AD‐MSCs. According to these guidelines, cells in SVF should be identified by the following markers: CD45−CD235a‐CD31‐CD34+ and additional expression of CD13, CD73, CD90, and CD105. In turn, AD‐MSCs in cell culture should have expression pattern akin to that of other MSCs: positive for CD90, CD73, CD105, and CD44 and negative for CD45 and CD31 (Table 3). Expression of CD36 and a lack of CD106 expression can be used to distinguish AD‐MSCs and BM‐MSCs.
Table 3.
Immunophenotype of SVF and AD‐MSCs according to IFATS and ISCT 117
| SVF | AD‐MSCs | |||
|---|---|---|---|---|
| Positive markers | Primary stable |
CD13, CD29, CD44, CD73, CD90 (>40%), CD34 (>20%) |
Primary stable |
CD13, CD29, CD44, CD73, CD90, CD105 (>80%) |
| Primary unstable | CD34 (present at variable levels) | |||
| Other markers |
CD10, CD26, CD36, CD49d, CD49e |
|||
| Negative markers | Primary negative | CD31 (<20%), CD45 (<50%) | Primary negative | CD31, CD45, CD235a (<2%) |
| Other markers | CD3, CD11b, CD49f, CD106, PODXL | |||
Abbreviations: AD‐MSC, adipose‐derived mesenchymal stromal cell; SVF, stromal vascular fraction; IFATS, International Federation for Adipose Therapeutics and Science; ISCT, International Society for Cellular Therapy.
In addition, the differentiation potential of AD‐MSCs into adipocytes, osteocytes and chondrocytes should be qualitatively analyzed by histological staining, and additional quantitative analyses with biochemical methods (Western blot, enzyme‐linked immunosorbent assay) or RT‐PCR should be considered (Figure 6). 117
Figure 6.
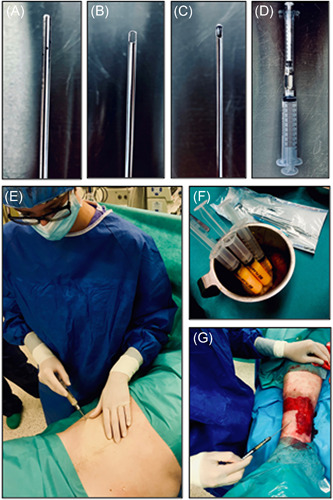
Fat grafting by Coleman technique. (A) Infiltration cannula; (B) Harvesting canula; (C) Fat transfer canula; (D) Syringe for fat harvesting connected with insulin syringe for fat transfer with a Luer‐ Lock connector; (E) Harvesting fat tissue—Coleman technique; (F) Lipoaspirate; (G) Autologous fat grafting into the chronic wound (our preliminary trials in patients, approved by the Independent Bioethics Commission for Research of the Medical University of Gdansk—permission number NKBBN/707/2018‐2019) [Color figure can be viewed at wileyonlinelibrary.com]
There is some controversy regarding the CD34 marker, whose expression on AD‐MSCs is unstable. MSCs should be negative for these markers, however, the results of various studies indicate their differential expression. 118 In the SVF, a large percentage of cells (up to 85%) were shown to be CD34+.119, 120 Some authors confirmed that cultured AD‐MSCs do not express CD34,121, 122, 123 while others reported some fractions of AD‐MSCs to be CD34+.124, 125 Comparison of sorted fractions of early passages of cultured human AD‐MSCs showed that the CD34+ cells had greater proliferative potential and colony‐forming ability whereas CD34− cells were characterized by greater differentiation potential into osteocytes and adipocytes. 126 In our studies, in flow cytometric and quantitative polymerase chain reaction (qPCR) analyses, we observed little or no CD34 expression in AD‐MSCs cultured in vitro up to the sixth passage. 127 CD34 expression was confirmed to be affected by culture conditions (e.g., plating density and culture medium) and decreased during cell culture.128, 129
4.2. Genetic stability of AD‐MSCs and effect of FBS deprivation on AD‐MSCs
The genetic stability of AD‐MSCs during cell culture should be addressed due to their clinical application. In clinical trials, cells up to the second passage are usually used, but sometimes longer in vitro culture is required. During cell culture, the proliferative and differentiation potential of cells can diminish. Reduced DNA synthesis and DNA repair efficiency may lead to the accumulation of DNA damage, genetic instability, cell senescence, and functional changes and consequently affect the therapeutic efficacy and patient safety. 130 Rubio et al. 131 showed that AD‐MSCs can undergo spontaneous transformation in in vitro cell culture. However, the culture was continually passaged for 4–5 months. Our studies showed that in a long‐term (up to sixth passage) in vitro cell culture, AD‐MSCs from different donors (plastic surgery and oncological patients) maintain their differentiation potential (i.e., ability to differentiate into adipocytes, osteocytes and chondrocytes) and retain their phenotype based on the expression of key surface markers at the transcript (qPCR) and protein level (flow cytometry). These results confirm AD‐MSCs stability and safety during long‐term culture. 127 AD‐MSCs stability was also demonstrated by other research teams. Neri et al. 132 observed an expected slowdown of the proliferation rate during long‐term culture but no instances of genetic changes (alterations in chromosome or short repeated sequences), replicative senescence (telomere attrition, expression of significant amounts of active telomerase) or anchorage‐independent growth ability, which indicates the therapeutic safety of AD‐MSCs. It was also indicated that AD‐MSCs do not show signs of senescence up to the seventh passage regardless of culture conditions (oxygen tension and medium supplementation with FBS or human platelet lysate). 133
Another important issue regarding AD‐MSCs application is the evaluation of the effect of FBS deprivation on these cells. Traditionally, cells are cultured in medium supplemented with FBS. However, it is recommended to avoid FBS during the culture of cells for preclinical and therapeutic applications. To be considered safe, cells have to be cultured according to GMP standards, so animal‐related products need to be eliminated. This is due to the risk of cell product contamination with infectious agents. In addition, FBS contains various growth factors, hormones, nutrients and other components, and its composition varies significantly between batches. FBS use may lead to unspecific activation of cell differentiation and proliferation as well as immune responses.134, 135, 136 Accordingly, FBS is removed from cell culture before clinical application and during testing of drug candidates.137, 138 The available results show, that AD‐MSCs maintain their stem cell characteristics in serum‐deprived medium; they survive, proliferate and are able to differentiate.139, 140 Our research on AD‐MSCs after the second passage, which were cultured in the absence of FBS for 48 h, comprised whole transcriptome sequencing followed by gene expression analysis and showed that FBS‐deprived AD‐MSCs, at the transcription level, show lower metabolic and proliferative activity but retain the expression of key surface markers. Additionally, we did not find evidence of apoptosis and necrosis. These observations suggest that FBS deprivation does not induce changes that could preclude the clinical application AD‐MSCs. 127
To overcome the problem of FBS use in cell culture, media containing a combination of recombinant growth factors are used. For example, commercially available chemically modified STK2 medium (DS Pharma Biomedical), which contains FGF2, PDGF, EGF, insulin, lipids, nutrients and minerals, is provided for AD‐MSCs in vitro cell expansion. 141 AD‐MSCs cultured in STK2 medium showed a higher proliferation rate, increased expression of MSC surface markers and reduced senescence than AD‐MSCs grown in DMEM supplemented with FBS. 142 However, serum‐free media are mainly applied in laboratory research and not in clinical trials, and the safety of serum‐free media in the clinic needs to be evaluated. 143 Other supplements, such as pooled human AB serum, platelet lysates, cord blood serum or thrombin‐activated platelet rich plasma (tPRP) were tested as alternatives for FBS in AD‐MSCs cell culture. Dessels et al. 136 proved that compared to cells cultured in medium with FBS, AD‐MSCs expanded in pooled human platelet lysates (pHPLs) were characterized by higher expression levels of genes involved in the cell cycle, proliferation and division. Additionally, pHPL supplementation did not affect the expression levels of genes responsible for the differentiation of specific developmental processes. Similar findings were also presented in other studies.144, 145 In addition, Kocaoemer et al. 146 reported that AD‐MSCs culture in medium supplemented with pooled human AB serum or tPRP increased their proliferative capacity without compromising their immunophenotype and differentiation potential. However, cells cultured in tPRP‐supplemented medium showed decreased adhesion. Additional whole genome gene analysis revealed significantly higher expression levels of 90 genes in AD‐MSCs cultured in FBS‐supplemented medium. Moreover, cells grown in medium containing human supplements showed lower expression of adhesion‐ and ECM‐associated molecules. 147 In turn, Lindroos et al. 148 indicated that AD‐MSCs cultured in allogeneic HS (allo‐HS) and FBS show comparable proliferative potential and phenotypes, but there were differences in the gene expression patterns between those culture methods (overexpression of cell cycle pathway genes in allo‐HS culture and BMP receptor‐mediated signaling in the TGF‐β pathway in FBS culture). Culture conditions can affect AD‐MSCs properties, therefore, there is still a need to look for appropriate xeno‐free culture media that will provide the right number of cells for clinical application without affecting their therapeutic potential.
4.3. SAT versus VAT
Subcutaneous adipose tissue (SAT) and VAT have different characteristics and functional roles in metabolic regulation, for example, VAT has a higher inflammatory response than SAT. 149 Differences between these kinds of fat tissue are probably based on other developmental origins: SAT originates from somatic lateral mesoderm while VAT originates from splanchnic lateral mesoderm. 150 AD‐MSCs obtained from visceral (V‐AD‐MSCs) or subcutaneous fat tissue (S‐AD‐MSCs) exhibit no difference in reconstructive potential, but collecting VAT is more invasive for patients (Table 4). 103 Single‐cell RNA‐seq analysis showed different transcriptomic features between S‐AD‐MSCs and V‐AD‐MSCs (S‐AD‐MSCs have larger DPP4‐positive subpopulation). AD‐MSCs from both tissues show diverse fibrosis, adipogenesis, vascularization and inflammation outcomes and have different, heterogeneous gene expression profiles. V‐AD‐MSCs are more adipogenic and proinflammatory while, S‐AD‐MSCs are more like progenitor cells with antiinflammatory properties.151, 152
Table 4.
Comparison of S‐AD‐MSCs and V‐AD‐MSCs
| Kind of white fat tissue | Developmental origin | Inflammatory cytokines | Type of cell | Differentiation into adipocytes/osteocytes | |
|---|---|---|---|---|---|
| S‐AD‐MSCs | Subcutaneous | Somatic lateral mesoderm | Antiinflammatory | Progenitor | Lower |
| V‐AD‐MSCs | Visceral | Splanchnic lateral mesoderm | Proinflammatory | Adipogenic | Higher |
Abbreviations: AD‐MSC, adipose‐derived mesenchymal stromal cell; V‐AD‐MSC, AD‐MSC obtained from visceral adipose tissue; S‐AD‐MSC, AD‐MSC obtained from subcutaneous adipose tissue.
S‐AD‐MSCs and V‐AD‐MSCs share similar surface markers and cell viability but differ in functions (depending on the surrounding microenvironment), motility, focal adhesion dynamics, secretion of inflammatory cytokines, and expression of stemness‐related genes. 153 Compared to V‐AD‐MSCs, S‐AD‐MSCs have a higher potential to differentiate into adipogenic and osteogenic cells but V‐AD‐MSCs secrete higher levels of inflammatory cytokines (IL‐6, IL‐8) and TNF‐α. 154
Raajendiran et al. 155 identified three distinct human adipocyte progenitor cell (APC) subtypes according to the expression of CD34 (CD34−, CD34low, CD34high). Performed analysis showed that AD‐MSCs in visceral fat tissue depot have an equal number of CD34− and CD34hi, while in abdominal, subcutaneous depot AD‐MSCs are highly enriched with CD34−, followed by CD34hi and CD34lo. S‐AD‐MSCs are specifically characterized by CD10 expression, while V‐AD‐MSCs predominantly by CD200. 156 The adipogenic capacity in cells is correlated with CD10 positivity and CD200 negativity, which agree with better differentiation of S‐AD‐MSCs than V‐AD‐MSCs in response to in vitro adipogenic stimuli.156, 157 V‐AD‐MSCs proliferate slower and need stronger stimulation to differentiate. 157 Its weaker capacity to differentiate into new adipocytes could partly explain the hypertrophy of existing adipocytes in VAT as a response to fat accumulation in obesity. 158 Additionally, V‐AD‐MSCs show a reduction of CD90, which could mediate metabolic disorder. S‐AD‐MSCs express a high level of CD90 and show an increase in proliferation, mitotic clonal expansion, and adipogenic differentiation, together with AKT activation and G1‐S phase transition, which may contribute to metabolic homeostasis via preventing adipocyte hypertrophy in SAT. 159
Wada et al. 160 indicate differences in the angiogenesis and inflammatory cytokines release pattern between S‐AD‐MSCs and V‐AD‐MSCs. The secretion of cytokines, for example, CHI3LI; IL‐1β; EGF; MCP‐1; CST3; IL‐6; IL‐8; PTX3; TGF‐β; PLAUR, and TNF‐α was smaller in the supernatants of the S‐AD‐MSCs. Researchers also show that the S‐AD‐MSCs proliferate 1.75 times faster than the V‐AD‐MSCs. The insulin‐producing cells (IPCs) for glucose stimulation, generated from the V‐AD‐MSCs, and S‐AD‐MSCs, showed that insulin secretion capacity was higher in the subcutaneous IPCs than the visceral IPCs.
Both S‐AD‐MSCs and V‐AD‐MSCs from obese and Type‐2‐diabetic patients show higher migration, invasion, and phagocytosis capacity than those from lean subjects. The weight loss in visceral and subcutaneous fat depots is able to at least partially restore their metabolic homeostasis. 158
The above differences should be considered in regenerative therapies.
4.4. BAT versus WAT AD‐MSCs
Brown adipose tissue (BAT) which develops early in life is most prominent in human newborns and until recently, it was widely believed that BAT disappears by adulthood. 161 In adults human, it is concentrated in the supraclavicular, neck, axillar, and paravertebral regions and it is inversely correlated with body mass index (BMI).162, 163, 164
Brown adipocytes are characterized by the presence of a large number of mitochondria, multilocular lipid droplets, sympathetic innervations, and the expression of uncoupling protein‐1 (UCP1) which allows generating heat with little ATP production.161, 165 Brown adipose develops from paraxial mesoderm (dermomyotome) similar to muscles and dorsal dermis. 166
The Ebf2 (early B‐cell factor 2) transcription factor is a highly specific marker expressed in both BAT and beige/brite precursors, used for BAT identity and efficient brite/beige cell formation.164, 166 WAT can change to BAT in response to cold exposure or other stimuli (i.e., β‐adrenergic) that increase sympathetic tone. 161 Cells from white fat tissue that show Ebf2 expression differentiate into brown‐like (or beige) adipocytes, what's more loss of Ebf2 in brown preadipose cells reduce the expression levels of brown preadipose‐signature genes. These results indicate that Ebf2 specifically marks and regulates the molecular profile of brown preadipose cells. 166 Human abdominal subcutaneous white‐fat preadipocytes have greater brown‐adipocyte lineage commitment potential following BMP‐7 induction than preadipocytes isolated from visceral white fat. 162 The mesenchymal progenitors that give rise to beige adipocytes express a unique set of cytokines and transcriptional regulators involved in immune cell modulation of adipose tissue browning. An iron accumulation and withstanding with oxidative stress suggest that beige/brite adipocytes are adapted to mitochondrial biogenesis and fatty acid oxidation upon thermogenic stimulation. 167
WAT to BAT conversion is induced in response to β‐adrenergic receptor stimulation by β‐adrenergic agonist (e.g., norepinephrine, 168 PPAR agonists, 169 mirabegron, 170 CL‐316,243, 171 BRL 26830A 172 ).
Multipotent metabolically active BAT‐derived stem cells were identified in adult humans mediastinum. 173 BAT‐derived MSCs (BAT‐MSCs) differ from stem cells derived from WAT in the origin and lineage characteristics, particularly, WAT‐stem cells originate from Myf5 (myogenic regulatory factor) negative progenitors, whereas BAT‐MSCs express Myf5 and originate from myogenic lineage.173, 174 BAT‐MSCs are metabolically active, can be expanded in vitro, exhibit multilineage differentiation potential and can be functionally differentiated into metabolically active brown adipocytes. The BAT‐MSCs compared to the WAT stem cells have a unique gene expression profile, especially the higher expression of genes associated with BAT such as PPAR‐γ coactivator 1‐alpha (PGC‐1a), PR domain containing 16 (PRDM16), CAMP responsive element binding protein one (CREB1), and UCP1. Cells exhibit the capacity to undergo osteogenesis, chondrogenesis, and both brown and white adipogenesis. 173
5. FACTORS THAT CAN AFFECT AD‐MSCs
Obesity, age and related chronic diseases can negatively affect AD‐MSCs. The properties of AD‐MSCs differ among fat depots and change with age. 175 Cells from younger patients proliferate faster, are more successful in differentiating into mature adipocytes and have more lipolysis activity. 176 Higher adipogenesis was shown in the middle‐aged group (40–45 years old vs. compared with 25–30 years old and 55–60 years old). The younger group demonstrated lower apoptosis susceptibility, the lowest of which was observed in the superficial abdominal depot. Cells isolated from elderly patients have lower function and adipogenic potential, while those isolated from younger individuals (25–30 years old) have a higher growth rate and paracrine activity. Additional comparisons showed that in the elderly patients, higher differentiation potential stays substantial only in the arm and subcutaneous thigh.107, 177
5.1. Obesity
AD‐MSCs from obese patients show reduced function and differentiation potential compared to those from lean controls. 178 Louwen et al. 179 showed that obesity has an unfavorable impact on AD‐MSCs, that is, defective functionalities and properties (differentiation, angiogenesis, motility, multipotent state, metabolism, and immunomodulation). Additionally, the undifferentiated multipotent state of AD‐MSCs is impaired in obese individuals. AD‐MSCs from obese individuals have upregulated adipogenic and inflammatory genes, enhanced epithelial‐mesenchymal transition, reduced expression of multipotency‐associated genes (e.g., OCT4, SAL4, SOX15, and KLF4), and decreased telomerase activity and telomere length (self‐renewal capacity). A higher BMI decreases AD‐MSCs osteogenesis potential and impairs angiogenic potential. Obesity also alters AD‐MSCs secretome profile due to its association with the proinflammatory environment, which can negatively impact on AD‐MSCs differentiation potential and regenerative capability.180, 181 Compared to obese AD‐MSCs, lean‐derived AD‐MSCs showed reduced immunosuppressive activities and weaker suppression of lymphocyte proliferation, which protects against obesity‐associated inflammation and insulin resistance. 182
5.2. Cancer
AD‐MSCs and cancer cells show bidirectional effects. Tumor cells change the AD‐MSCs phenotype and function in a paracrine way. In coculture with lung cancer cells (H358), AD‐MSCs differentiate into myofibroblasts. 183 Similar differentiation of AD‐MSCs was observed with cells cultured in presence of breast cancer exosomes, breast tumor‐derived factors and ovarian cancer exosomes. 184 Wang et al. 185 showed that AD‐MSCs adipogenesis and adipogenic‐specific genes are strongly inhibited by internalization of lung cancer‐derived exosomes. AD‐MSCs from patients with urological neoplasms show equivalent mesenchymal surface markers, exosome miRNA content, molecular karyotyping and similar growth kinetics to AD‐MSCs from healthy subjects, thus confirming the proper use of autologous stem cell transplantation in clinical treatment. 186 In our experiments, we also did not observe differences in the differentiation potential and expression of key MSC markers at the transcriptome and protein levels in in vitro cultured AD‐MSCs from plastic surgery and oncological patients. 127
Some in vivo and in vitro preclinical studies indicate AD‐MSCs as a factor that increases tumor growth and progression. 187 The interaction between AD‐MSCs and the tumor microenvironment has been confirmed in patients with obesity, colorectal tumors, prostate tumors, melanoma and breast cancer, all of which show a higher quantity of circulating AD‐MSCs. Tumor stroma and inflammatory cells can release factors (e.g., SD‐1 and MCP‐1) that stimulate AD‐MSCs migration to the cancer microenvironment. 188 AD‐MSCs can increase prooncogenic risk but do not differentiate or stimulate angiogenesis in cancer cells. 189 It is suggested that AD‐MSCs promote tumor progression and invasiveness, but clinical trials have failed to demonstrate their prooncogenic potential. 163 By contrast, some studies have shown that ASC exosomes exert anticancer immunomodulatory functions and decrease cancer growth, migration and colony formation. 190
5.3. Chemotherapy/radiotherapy
It is important to evaluate the impact of oncological treatment on AD‐MSCs in the context of their potential autologous transplantation into the wounds in these patients. In mouse model, it was shown that whole‐body irradiation can damage adipose tissue and reduce the cell number and proliferative potential of AD‐MSCs. 191 In vitro external radiation reduced the proliferation of AD‐MSCs, but the effect was smaller than that for normal human fibroblasts (NHFs). In the coculture of these cells, external radiation did not significantly reduce cell proliferation, which suggests that AD‐MSCs may protect NHFs and promote their growth. 192 AD‐MSCs cocultured with NHF and microvascular endothelial cells (HDMECs) showed increased expression of cytokines and adhesion molecules (in NHFs and HDMECs) after radiation, which suggests that AD‐MSCs may have a stabilizing effect on irradiated wounds. 193 The injection of AD‐MSCs is a promising therapeutic strategy in wound healing, especially after laryngectomy combined with radiotherapy. 194 Fat transfer can lead to the healing of chronic ulcers resistant to other forms of postradiation treatment 195
Chemotherapeutic agents may also affect AD‐MSCs. Tamoxifen, a hormonal therapeutic widely used in breast cancer treatment, was shown to inhibit proliferation and induce apoptosis of AD‐MSCs in a time and dose‐dependent manner. Additionally, their ability to differentiate into adipocytes and osteocytes was impeded. 196 On the other hand, AD‐MSCs are relatively resistant to commonly used chemotherapeutic agents (cisplatin, vincristine, and camptothecin) in vitro. After exposure to cisplatin and camptothecin at concentrations that reflect clinically relevant doses, AD‐MSCs maintained their stem characteristics (surface markers and osteogenic and adipogenic differentiation). They were also able to fully recover after treatment with high doses of cisplatin, vincristine, and camptothecin, which indicates that they are resistant to genotoxic damage in vitro. 197
It is worth noting, that most of the research regarding the effect of chemotherapy or radiotherapy on AD‐MSCs was conducted in in vitro cell or in vivo animal models. This research is valuable but does not fully reflect the state of these cells in the human body. Thus, it is important to perform more extensive and detailed analyses of the properties of AD‐MSCs isolated from adipose tissues obtained from patients subjected to oncological treatment.
6. THE ROLE OF AD‐MSCs IN WOUND HEALING
AD‐MSCs play an essential role in wound healing, however, the mechanism of their action is still under investigation. Endogenous AD‐MSCs may be activated after wounding. During the proliferative phase mature adipocytes and their precursors, together with fibroblasts, populate the wound site. 198 AD‐MSCs can also differentiate into fibroblasts, keratinocytes and endothelial cells and secrete various factors (e.g., cytokines and growth factors) that stimulate the proliferation and migration of these cells. In addition, through the secretion of growth factors (e.g., VEGF, bFGF, EGF, PDGF, hepatocyte growth factor, TGF‐α), cytokines (e.g., IL‐6, and IL‐8) and chemokines in a paracrine manner, AD‐MSCs can promote angiogenesis, the immune response, epithelial regeneration, and wound remodeling. 199 They were also reported to exert antioxidant effects in wound healing. 200
6.1. Fat grafting by the Coleman technique
Fat grafting was first described by Coleman as a cosmetic facial filler in the 1980s. In 1994, Coleman first introduced his technique of processing fat for structural fat grafting. This technique is called the Coleman technique (Figure 6) or structural fat grafting or “lipostructure” and uses syringes, cannulas, centrifuges and centrifugation protocols. Fat can be harvested under local or general anesthesia, depending on patient preference, pain tolerance and the volume of fat needed.201, 202, 203
The first step is to prepare fat tissue for harvesting and transplantation. For this purpose, fat tissue needs to be infiltrated through the miniature holes in skin, with a specialized cannula (Figure 6A) with a solution, known as Klein solution, which consists 0.5% lidocaine, 1:1000 epinephrine, sodium bicarbonate and Ringer's solution.204, 205
The next step is to uptake fat tissue by gentle manual suction of fat with 10 ml Luer‐Lock syringes and the specialized Coleman cannula (Figure 6B). The plunger of the syringe is gently pulled back to create light negative pressure to harvest the fat. This method produces a very low and constant vacuum that minimizes the destruction of adipocytes (Figure 6E). 206
The lipoaspirate is processed for removal of the lipid and aqueous portions to isolate the adipose stroma for grafting. There are a few techniques for this isolation process, such as centrifugation, decantation, sedimentation, filtration, and mesh/gauze rolling; the Coleman protocol recommends centrifugation. Freshly harvested fat is centrifuged using appropriate gravitational force (3000g for 3 min) to separate the fat from unnecessary pollutants and nonviable components. Processed fat is transferred to 1 ml syringes and is ready for placement using the specialized cannulas (Figure 6D). There are several types of cannulas with different diameters, lengths and ends depending on the tissues into which the lipoaspirate is grafted.207, 208, 209
Coleman fat grafts have a greater number of viable adipocytes and sustain better cellular function than fat grafts harvested with other methods, and the Colman technique is currently the most common method of autologous fat transfer.210, 211 This procedure can also be used in wound healing (Figure 6G).
6.2. Clinical application of AD‐MSCs in wound healing
The first evidence of ASC use in regenerative medicine was published in 2004. In this case, AD‐MSCs in a fibrin glue in combination with bone grafts were used to treat widespread traumatic calvarial defects in a 7‐year‐old girl who suffered severe head trauma. 212 Since then, AD‐MSCs have been widely tested for their therapeutic potential in the treatment of numerous diseases. According to ClinicalTrials.gov, 335 clinical trials regarding AD‐MSCs use are registered (https://clinicaltrials.gov/, 12.11.2020, search term: adipose‐derived stem cells) and address a wide range of medical conditions including delayed wound healing, burns, Crohn's disease, diabetes and diabetic wounds, chronic obstructive pulmonary disease, cardiovascular diseases, rheumatoid arthritis, bone and cartilage damage and many others. Interestingly, clinical trials evaluating AD‐MSCs potential in treatment of COVID‐19 are also registered.
To achieve therapeutic potential, AD‐MSCs can be transplanted in various forms: lipoaspirate, SVF, cell suspension of in vitro expanded cells or scaffolded cells. Scaffolds are designed to provide a 3D microenvironment for cell proliferation and differentiation as well as to enhance cell viability, which is beneficial for the regulation of regeneration. Currently much attention is paid to the use of constructs based on AD‐MSCs and scaffolds. Scaffolds should possess several features, such as nontoxicity, nonimmunogenicity, good biodegradability and biocompatibility, and be easy to handle. Additionally, they should also exhibit good chemical and mechanical surface properties (i.e., high porosity) to support cell resistance; promote the adhesion, proliferation, and differentiation of stem cells and allow retention of metabolic futures.213, 214, 215 Clinical trials of fat grafting and SVF application in wound healing are described in Table 5, and clinical trials of AD‐MSCs application in wound healing are summarized in Table 6. Out of 335 identified studies only those specifically regarding the treatment of wounds and additional studies describing the use of fat transfer for wound healing, tissue reconstruction in traumatic injury and oncological patients were summarized. However, another interesting trial—the ACellDREAM II—that is currently recruiting may provide new insight into this topic (NCT03968198). There, Investigators plan to assess the efficacy of use of AD‐MSCs for critical limb ischemia. Inclusion criteria and primary endpoints of the mentioned trial do not require presence of a chronic wounds, as patients only with rest pain and no wounds may be included as well, but this study may provide new evidence on the topic of AD‐MSCs use in wound. Of note, this trial has been processed by a feasibility study (ACellDREAM, NCT01211028) which showed promising results in regard to wound healing. 216 Clinical trials proved the efficiency of AD‐MSCs in stimulating DFU healing. For example, in a randomized, controlled clinical trial in Korea (clinical trial reg. no.: NCT02619877), the effects of an allogeneic AD‐MSCs sheet (allogeneic AD‐MSCs in a hydrogel, ALLO‐ASCs) were evaluated for the treatment of DFUs. Control patients received treatment with polyurethane film. At Week 8, complete wound closure was observed in 47% and 73% of patients in the control and treatment groups, respectively. At the end of the evaluation (12 weeks) 82% of wounds were completely closed in the group receiving the ALLO‐ASCs sheet, while only 53% were closed in the control group. 217
Table 5.
Clinical trials of fat grafting and SVF application in wound healing (clinicaltrials.gov, accessed 12.11.2020)
| Study title | Condition/disease | Procedure | Phase | Status | Number of patients | Identification number/Location |
|---|---|---|---|---|---|---|
| 3‐D Imaging Assessment of Scar Formation and Would Healing in Fat Grafted vs Nonfat Grafted Facial Reconstruction Wound Sites | Scar formation autologous fat grafting | Autologous fat grafting |
Phase 1 Phase 2 |
Withdrawn | 0 | NCT01750424/United States |
| A Study to Evaluate the Results of Facial Soft Tissue Reconstruction in Patients Who Have Suffered Traumatic Injury (BTI) |
Facial injuries Adipose tissue |
Fat grafting | Not applicable | Completed | 20 | NCT01345591/United States |
| Acellular Adipose Tissue (AAT) for Soft Tissue Reconstruction |
Soft tissue injuries Trauma |
Acellular adipose tissue (AAT) | Phase 2 | Active, not recruiting | 15 | NCT03544632/United States |
| Adipose Derived Regenerative Cellular Therapy of Chronic Wounds |
Diabetic foot Venous ulcer Pressure ulcer |
AD‐MSCs | Phase 2 | Completed | 25 | NCT02092870/United States |
| Autologous Growth Factor Effect on Split‐thickness Donor Site Healing: a Comparison of Adipose Tissue Extract and PRP | Wound healing |
Adipose tissue extract Biological: platelet‐rich plasma gel |
Phase 2 | Completed | 24 | NCT02799290/Finland |
| Child's Adipose Cells: Capacity of Tissue Regeneration (cicASChild) | Burns | Adipose tissue sample | Not applicable | Completed | 38 | NCT02779205/France |
| Effect of Autologous Fat Grafting on Acute Burn Wound Healing | Burns |
Autologous fat grafting Drug: topical cream Procedure: split thickness skin grafting |
Phase 3 | Recruiting | 50 | NCT03791710/Egypt |
| Long Term Status of Free Dermal Fat Autografts for Complex Craniofacial Wounds (FTFDT2) | complex craniofacial wounds | Autologous dermal fat grafting | – | Enrolling by invitation | 20 | NCT03880188/United States |
| Platelet Rich Plasma and Autologous Fat Graft for Diabetic Ulcer | DFU |
Fat grafting Fat grafting + platelet rich plasma |
Not applicable | Unknown | 30 | NCT03085550/United Kingdom |
| Safety of Adipose‐Derived Stem Cell Stromal Vascular Fraction |
Abnormally healing Wounds Scars Soft tissue defects |
ADSC‐ SVF‐002 | Phase 1 | Not yet recruiting | 10 | NCT02590042/Canada |
| Short Term Status of Free Dermal Fat Autografts for Complex Craniofacial Wounds (FTFDT3) | Complex craniofacial wounds | Autologous fat grafting | – | Not yet recruiting | 20 | NCT03872544/United States |
| Stromal Vascular Fraction From Lipoaspirate to Treatment of Chronic Non‐healing Wound | Chronic wounds | Antria cell preparation process | Phase 1 | Recruiting | 40 | NCT03882983/United States |
| Structural Fat Grafting for Craniofacial Trauma: Repeat Fat Grafting Injection‐5 Subject Cohort (BTIPlusUp) |
Facial injuries Adipose tissue |
Repeat fat grafting | Not applicable | Completed | 5 | NCT01822301/United States |
| The Role of Lipoaspirate Injection in the Treatment of Diabetic Lower Extremity Wounds and Venous Stasis Ulcers |
Diabetic wounds Venous stasis wounds |
Injection of lipoaspirate | Not applicable | Unknown | 250 | NCT00815217/United States |
| Treatment of Chronic Leg Ulcers With Autologous Stromal Vascular Fraction | Leg ulcer | Liposuction | Not applicable | Unknown | 30 | NCT02987101/Denmark |
| Standard wound care | ||||||
| Adipose‐derived regenerative cells | ||||||
| Treatment of Hypertensive Leg Ulcer by Adipose Tissue Grafting (Angiolipo) | Skin ulcer | Adipose tissue grafting | Not applicable | Unknown | 10 | NCT01932021/France |
| Use of Concentrated Endogenous Autologous Adipose Stromal Cells in Fat Grafts for Craniofacial Trauma (ARM5) | Craniofacial injuries | Fat grafting | Not applicable | Terminated | 5 | NCT01633892/United States |
| Healing Chronic Venous Stasis Wounds With Autologous Cell Therapy | Nonpenetrating wound | Autologous SVF | Not applicable | Active, notrecruiting | 36 | NCT02961699/United States |
| Device: transpose RT System | ||||||
| Other: debridement/dressing of wound | ||||||
| Assessment of the Efficacy and Tolerance of Subcutaneous Reinjection of Autologous Adipose‐derived Regenerative Cells in the Local Treatment of Neuropathic DFUs | DFU | Drug: adipose derived regenerative cells (as SVF) | Phase 2 | Unknown | 45 | NCT02866565/France |
| Nanofat on Wound Healing and Scar Formation | Scars | Nanofat injection (lipoaspirate) | Not applicable | Not yet recruiting | 15 | NCT03850119/Belgium |
| Delayed Wound Healing | ||||||
| Hypertrophic Scar | ||||||
| Postinflammatory | ||||||
| Hyperpigmentation | ||||||
| Donor site complication | ||||||
| Adipose‐Derived Stromal Cells (ASC's) for Pressure Ulcers | Pressure ulcer | Biological: adipose derived stromal cells (SVF) | Phase 1 | Active, not recruiting | 12 | NCT02375802/United States |
| Autologous Adipose derived Regenerative Cells Injection for Treatment of Radiation‐induced Rectovaginal Fistula | Rectovaginal fistula | Injection of autologous regenerative cells of adipose tissue (cells obtained by enzymatic digestion of lipoaspirate) | Phase 1 | Completed | 16 | NCT03643614/Russian Federation |
| 19F Hot Spot MRI of Human Adipose‐derived Stem Cells for Breast Reconstruction | Breast cancer | Drug: CS‐1000 labeled SVF cells | Phase 1 | Recruiting | 6 | NCT02035085/United States |
| Pilot Study of Skin Quality Improvement After AD‐MSCs Transfer in Irradiated Breasts | Breast neoplasms | Biological: adipose SVF cell | Not applicable | Not yet recruiting | 10 | NCT01801878/Republic of Korea |
| Skin abnormalities | Biological: normal saline | |||||
| Study of Autologous Fat Enhanced w/Regenerative Cells Transplanted to Reconstruct Breast Deformities After Lumpectomy | Breast neoplasms | ADRC enhanced autologous | Phase 4 | Completed | 71 | NCT00616135/Belgium, Italy, Spain, United Kingdom |
| Carcinoma, ductal, breast | ||||||
| Mammaplasty | Fat transplant | |||||
| Mastectomy, segmental, lumpectomy, breast reconstruction | ||||||
| Autologous Fat Transfer for Scar Prevention and Remodeling | Wound | Autologous fat transfer | Phase 1 Phase 2 | Completed | 14 | NCT01119326/United States |
| Effect of Concentrating Endogenous Stromal Cells in the Fat Graft | Facial injuries | Fat graft surgical procedure | – | Terminated | 3 | NCT01564524/United States |
| Adipose tssue | ||||||
| Effect of Concentrating Endogenous Stromal Cells in the Fat Graft Using TGI Device | Facial injuries | Device: tissue genesis CellIsolation System (TGI CIS) | Not applicable | Completed | 7 | NCT01924364/United States |
| Tissue injury | Procedure: standard of carefat grafting | |||||
| Fat Grafting in Skin‐grafted Deep Burn Scars | Burn scar | Procedure: lipofilling/fatgrafting | Not applicable | Completed | 15 | NCT03627650 |
| Procedure: placebo injection | ||||||
| Structural Fat Grafting for Craniofacial Trauma Using Manual Technique for Processing Fat Graft Material | Facial injuries | Procedure: fat grafting | Not applicable | Completed | 15 | NCT02267187/United States |
| Drug: general anesthesia | ||||||
| Device: Coleman cannulas | ||||||
| Other: Tefla nonadherent gauze pad | ||||||
| Adipose‐Induced Regeneration of Breast Skin to Treat Postmastectomy Radiation Injury in Breast Cancer Patients | Breast cancer | Fat grafting | Not applicable | Not yet recruiting | 20 | NCT03981718/United States |
| Autologous Adipose Tissue in the Treatment of Systemic Sclerosis Digital Ulcers | Systemic sclerosis | Procedure: autologous fat grafting | Not applicable | Unknown | 46 | NCT03406988/Italy |
| Digital ulcer | Procedure: Sham procedure | |||||
| Autologous Micro‐fragmented Adipose Tissue in the Treatment of Minor Amputations of Diabetic Foot | Diabetic foot | Device: lipogems | Not applicable | Completed | 112 | NCT03276312/Italy |
| Comparative Study Between Fat Injection And Platelet Rich Plasma In Post Burn Facial Scar | Burn scar | Platelet rich plasma injection in post burn facial scar | Not applicable | Not yet recruiting | 60 | NCT04557514 |
| Fat injection in post burn facial scar |
Table 6.
Clinical trials of AD‐MSCs application in wound healing (clinicaltrials.gov, accessed 12.11.2020)
| Study title | Condition/disease | Procedure | Phase | Status | Number of patients | Identification number |
|---|---|---|---|---|---|---|
| Treatment of Chronic Wounds in Diabetic Foot Syndrome With Autologous Adipose Derived MSCs | DFU |
Biological: application of autologous ADSC stem cells in fibrin gel Procedure: standard care in DFU |
Phase 1 Phase 2 |
Recruiting | 20 | NCT03865394/Poland |
| Autologous Keratinocyte Suspension Versus ASC‐Keratinocyte Suspension for Postburn Raw Area |
Burn with full‐thickness skin loss |
Procedure: noncultured autologous keratinocyte suspension Procedure: ASC‐keratinocyte suspension Procedure: split skin graft |
Not applicable | Not yet recuiting | 33 | NCT03686449/Egypt |
| A Follow‐up Study to Evaluate the Safety of ALLO‐ASC‐DFU in ALLO‐ASC‐BI‐101 Clinical Trial | Burn |
Biological: ALLOASC‐ DFU |
– | Completed | 5 | NCT03183622/Republic of Korea |
| Allogeneic ADSCs and Platelet‐ Poor Plasma Fibrin Hydrogel to Treat the Patients With Burn Wounds (ADSCs‐BWs) | Second‐ or third‐degree Burns | Biological: ALLOASCs |
Phase 1 Phase 2 |
Unknown status | 20 | NCT03113747/Ukraine |
| A Study to Evaluate the Safety of ALLO‐ASC‐DFU in the Subjects With Deep Second degree Burn Wound | Burn | Biological: ALLOASC‐DFU | Phase 1 | Completed | 5 | NCT02394873/Republic of Korea |
| Clinical Study of AD‐MSCs in the Treatment of Diabetic Foot | DFU | Biological: MSCs treatment | Phase 1 | Not yet recriuting | 60 | NCT03916211/China |
| Clinical Study of ALLO‐ASC SHEET in Subjects With DFUs | DFU |
Biological: ALLOASC‐DFU Procedure: hydrogel SHEET (vehicle control) |
Phase 2 | Recriuting | 44 | NCT03754465/United States |
|
Clinical Study to Evaluate Efficacy and Safety of ALLOASC‐ DFU in Patients With DFUs |
DFU |
Biological: ALLOASC‐DFU Procedure: vehicle sheet |
Phase 3 | Unknown status | 164 | NCT03370874/Republic of Korea |
| A Follow‐up Study to Evaluate the Safety of ALLO‐ASC‐DFU in ALLO‐ASC‐DFU‐101 Clinical Trial | DFU | Biological: ALLOASC‐DFU | – | Completed | 4 | NCT03183726 |
| A Clinical Study Using ASCs for Diabetic Foot | Peripheral vascular diseasei | Biological: AD‐MSCs | Phase 1 | Unknown status | 240 | NCT02831075/China |
| Ischemia | ||||||
| Diabetic foot | Biological: saline | |||||
| Safety of ALLO‐ASC‐DFU in the Patients With DFUs | DFU | Biological: ALLOASC‐ DFU | Phase 1 | Completed | 5 | NCT02394886/Republic of Korea |
| A Follow‐up Study to Evaluate the Safety of ALLO‐ASC‐DFU in ALLO‐ASC‐BI‐201 Clinical Trial | Burn | Biological: ALLOASC‐DFU | – | Enrolling by invitation | 30 | NCT03183648/Republic of Korea |
| A Follow‐up Study to Evaluate the Efficacy and Safety for the Patients With ALLO‐ASC‐DFU Treatment in Phase 1/2 Clinical Trial of ALLO‐ASC‐EB‐101 | Dystrophic epidermolysis bullosa | Biological: ALLO‐ASC‐DFU | – | Not yet recruiting | 5 | NCT03183934/Republic of Korea |
| Clinical Application of Mesenchymal Stem Cells Seeded in Chitosan Scaffold for Diabetic Foot Ulcers | Stem cell transplant | Drug: stem cell product | Phase 1 | Unknown | 40 | NCT03259217 |
| Subcutaneous Injections of Autologous ASC to Heal Digital Ulcers in Patients With Scleroderma. | Systemic slerosis | Procedure: adipose tissue harvest | Phase 2 | Recruiting | 32 | NCT04356755/France |
| Drug: autologous ASC | ||||||
| Drug: Placebo | ||||||
| Treatment of Patients With Trophic Ulcers Using Mesenchymal Stem Cells | Trophic ulcer | Biological: autologous adipose‐derived mesenchymal stem cells | Phase 1 Phase 2 | Completed | 18 | NCT04457037/Belarus |
| Safety and Efficacy of Allogeneic Adipose Tissue Mesenchymal Stem Cells in Diabetic Patients With Critical Limb Ischemia | Limb ischemia | Drug: high dose allogeneic mesenchymal stromal cells | Phase 2 | Not yet recruiting | 90 | NCT04466007 |
| Diabetic foot | Drug: low dose allogeneic mesenchymal stromal cells | |||||
| Drug: placebos | ||||||
| Clinical Study of ALLO‐ASC‐SHEET in Subjects With Diabetic Wagner Grade II Foot Ulcers | DFU | Biological: ALLO‐ASC SHEET | Phase 2 | Not yet recruiting | 64 | NCT04497805 |
| Clinical Study to Evaluate Efficacy and Safety of ALLO‐ASC‐DFU in Patients With Diabetic Wagner Grade 2 Foot Ulcers | DFU | Biological: ALLO‐ASC‐DFU | Phase 3 | Recruiting | 104 | NCT04569409/Republic of Korea |
| Procedure: vehicle sheet |
Attempts to transplant AD‐MSCs into radiotherapy‐ or cancer‐damaged tissues were also made. For example, fat transfer followed by split‐thickness skin grafting was performed in a 67‐year‐old woman with a chronic, nonhealing ulcer on her leg resulting from squamous cell carcinoma excision and radiotherapy, this resulted in complete healing of the ulcer. 195 Fat transfer was also used in patients after radiotherapy for breast or head and neck cancers (Table 7).
Table 7.
Examples of AD‐MSCs therapy in patients with skin complications after oncological treatment
| Procedure | Number of patients | Results | Reference | |
|---|---|---|---|---|
| Effects of lipofilling on the functional and the esthetic aspects of irradiated breast reconstruction | Serial autologous fat grafting |
Study group: 20 patients Control group: 41 patients (42 breasts) |
Significant improvement in LENT‐SOMA scores after treatment compared to control and those before treatment Significantly enhanced cosmetic outcomes Implant exposure in two cases in the active branch with severe flap thinning in control group, while in study Group 4 cases with this problem resulted with no implant exposure |
Panettiere et al. 218 |
| Lipofilling on irradiated expanders in patients undergoing postmastectomy radiotherapy | Autologous fat grafting by Coleman technique |
Study group: 16 patients Control group: 16 patients |
No ulceration and implant exposure in the irradiated area in the study group compared to 31.25% extrusion rate of the implant in control group The shape and symmetry were significantly better in study group |
Ribuffo et al. 219 |
| Lipoaspirate transplant into tissue damaged by radiotherapy | Autologous transplantation of purified lipoaspirate | 20 Patients tissue damage after radiotherapy for breast cancer— LENT‐SOMA Grade 3 (severe symptoms) or Grade 4 (irreversible functional damage) |
Profound improvement of symptoms in 19 of 20 patients Progressive regeneration (neovessel formation and improved hydration) observed in tissue ultrastructure |
Rigotti et al. 220 |
| Fat grafting in irradiated head and neck tissues | Autologous fat transplants similar to Coleman technique | 11 Patients in cancer remission after radiotherapy requiring aesthetic subcutaneous or submucous head and neck reconstruction |
Esthetic and functional rehabilitation in 10 of 11 patients Improvement in patients’ quality of life Normal histologic structure, absence of necrotic areas and a highly dense vascular network |
Phulpin et al. 221 |
Abbreviation: AD‐MSC, adipose‐derived mesenchymal stromal cell.
6.3. Allogeneic versus autologous therapy and the immunological properties of AD‐MSCs
The use of allogeneic cells gives rise to the possibility of rapid application of cells to the patient and enables full cell characterization before therapy. Additionally, a small number of donors would provide treatment for many patients; hence, immunogenicity tests and analyses of transplantation safety in allogeneic systems are very important. Allogeneic transplants allow the pooling of cells from many donors and standardization of the cell product. 222 The application of allogeneic, stored cells is already in use. However, there are still some issue to be considered, for example, a lack of standardized parameters for handling frozen cells. Another issue is the lack of uniform guidelines for all AD‐MSCs banking processes, such as donor recruitment, manipulation, banking procedures, exemption and specific postthaw qualification tests. It would ensure multiple treatment of the patients, avoiding recurrent liposuction.223, 224
AD‐MSCs are suitable candidates for allogeneic cell therapies due to their low immunogenic profile, which was demonstrated by low expression of major histocompatibility complex (MHC) Class II molecules, and T and B cell costimulatory molecules CD80, CD86, and CD40 in vitro. 225 However, in vivo studies have shown that AD‐MSCs can induce a humoral and cellular immune response; hence, they are not fully immunologically privileged cells. AD‐MSCs may also be under the influence of inflammation‐based signaling and induce the expression of MHC II molecules and Toll‐like receptors. In addition, AD‐MSCs may also increase first‐ and second‐class MHC expression as a result of differentiation.226, 227, 228 In vitro cell culture can also influence immunogenicity; for example, cell culture with bovine serum slightly reduces cell immunogenicity compared to that in culture without serum. 229
Following modulation of the local inflammatory environment, it is believed that the final determination of immunomodulatory responses is likely elicited by the differentiation of cells, a combined action of direct cell–cell contacts and the secretion of soluble factors.229, 230 The immunological properties of AD‐MSCs are also dependent on the donor's concomitant diseases. Cells derived from obese Type 2 diabetes (T2D) patients show increased expression of inflammatory markers, activation of the NLRP3 inflammasome, and a greater capacity for migration and phagocytosis than those derived from lean donors. Interestingly, AD‐MSCs derived from obese and T2D individuals also show a reduction in typical immunosuppressive activities attributed to MSCs. 231 Moreover, it has recently been proven that Crohn's disease disturbs the immune activities of AD‐MSCs, which is related to inflammasome activation. 232
7. MSCs INTERACTIONS WITH IMMUNE CELLS
It should be noted that MSCs also take part in regenerative processes by affecting the immune system. MSCs cells produce number of modulating and immunosuppressive cytokines as well as they can affect immune cells through direct interactions.233, 234 MSCs may inhibit the activity of natural killer (NK) cells, lymphocytes Tc and dendritic cells (DCs). 235 MSCs can also induce the transition of M1 macrophages into M2 macrophages, thus promoting tissue regeneration. M2 produce various cytokines and growth factors such as IL‐10, TGF‐β1, PDGF, IGF‐1, and VEGF, which may stimulate cell proliferation, granulation tissue formation, angiogenesis and wound healing.7, 8 One of the immunosuppressive mechanisms of MSCs activity is also the production of indoleamine 2,3‐dioxygenase, which is involved in the l‐tryptophan catabolism. This process leads to a reduction in the level of tryptophan in the microenvironment at the same time increasing the level of kynurenine which inhibits the activity of T cells, NK cells, and DCs. 236 MSCs also use other immunosuppressive and antiinflammatory agents such as nitric oxide synthase (iNOS), TNF‐α‐stimulated gene‐6 (TSG6) and prostaglandin E2 (PGE2). MSCs have been shown to increase the expression of iNOS in response to proinflammatory cytokines (TNF‐α, IL‐1) and IFN‐γ.237, 238 Another factor produced by MSCs is a glycoprotein TSG‐6 (35 kDa). It is believed that TSG‐6 counteract inflammatory effects of TNF and IL‐1 thus inhibiting inflammation. Interestingly, proper expression of the TSG‐6 is crucial to achieve the therapeutic effect of MSC cells. 239 PGE2 is also involved in modulating the activity of the immune system by inhibiting the inflammatory response and stimulating Treg lymphocytes.233, 240
8. STRATEGIES FOR STIMULATING OF AD‐MSCs ACTIVITIES IN WOUND HEALING
Numerous clinical trials investigating the therapeutic potential of autologous or allogenic AD‐MSCs in the treatment of different diseases have been conducted. However, their results are unsatisfactory, which creates a need to search for new strategies of AD‐MSCs stimulation to enhance their activity and translate AD‐MSCs‐based therapies to everyday clinical practice. Several strategies, including preconditioning with bioactive molecules, genetic engineering of AD‐MSCs, modification of culture conditions and direct application of exosomes or extracellular vesicles, were established to overcome these problems. The impact of these strategies on improving of AD‐MSCs potential in wound healing was evaluated in in vitro and in vivo experiments. 241
It was shown that AD‐MSCs cultured in endothelial growth medium enhance their proangiogenic properties, which may increase their therapeutic potential in ischemic diseases, for example, ischemic wounds. 242 In another study, AD‐MSCs administrated with bFGF via sustained release from a gelatin hydrogel showed increased secretion of angiogenic growth factors and vessel maturation in a murine ischemic hind limb model. 243 Moreover, priming of AD‐MSCs with deferoxamine upregulated VEGF expression in a time and dose‐dependent manner, which was mediated by augmentation of HIF‐1 activity in AD‐MSCs, and increased their paracrine effect on endothelial cells (i.e., increase in migration). 244 PDGF‐D can also be used for AD‐MSCs preconditioning before transplantation because it increases their proliferation and migration; upregulates expression of growth factors (such as VEGFA, FGF1, FGF5, bone morphogenetic protein 8B [BMP8B], leukemia inhibitory factor [LIF], inhibin beta A [INHBA], IL11, and heparin‐binding EGF‐like growth factor [HBEGF]) in AD‐MSCs and enhances their regenerative potential. 245
The preconditioning of AD‐MSCs and modifications of other culture conditions can affect the effectiveness of AD‐MSCs in wound healing. In traditional 2D cell culture cells are grown in a monolayer; however, this method does not fully reflect conditions prevailing in normal tissue, such as cell‐to‐cell or extracellular interactions. In recent years, 3D spheroid cell culture was developed, which better reflects these interactions and can be helpful in improving of AD‐MSCs the therapeutic potential. 246 Cheng et al. 247 cultured AD‐MSCs in spheroids and then expanded them in monolayers to determine whether they exhibit superior pro‐regenerative activity. They found that compared to 2D‐cultured AD‐MSCs, spheroid‐cultured AD‐MSCs show higher expansion, less senescence, higher levels of pluripotency markers, C‐X‐C chemokine receptor type 4 (CXCR4) and angiogenic growth factors and enhanced migration and expression of MMP‐9 and MMP‐13. Moreover, in a murine model of impaired wound healing, the spheroid‐cultured cells caused faster healing and increased angiogenesis with better cell engraftment in the wound as well as evidence of their differentiation into epidermal and endothelial lineages. Amos et al. 248 showed that AD‐MSCs formulated in multicellular aggregates had better acceleration of diabetic wound closure than did an equal number of cells delivered via suspension. In addition, AD‐MSCs cultured in hypoxic conditions were shown to have enhanced proregenerative potential owing to their increased proliferation rate and secretion of growth factors (VEGF, bFGF). It was also proved that hypoxia upregulated VEGF and bFGF expression on messenger RNA and protein level. Additionally, the conditioned medium from AD‐MSCs cultured in hypoxic conditions compared to normoxia‐cultured AD‐MSCs conditioned medium, stimulated collagen synthesis and migration of human dermal fibroblasts as well as accelerated wound closure in a mice full‐thickness wound model. 249 Culturing MSCs under hypoxic conditions also helps to improve their self‐renewal capacity and retain undifferentiated phenotype. 250
Genetic modifications were also shown to increase AD‐MSCs activity in wound healing. Kim et al. 251 proved that overexpression of CXCR4 in AD‐MSCs leads to an increase in their homing and engraftment into ischemic areas after transplantation. These cells also promoted long‐term engraftment and muscle tissue regeneration in diabetic mice with hindlimb ischemia. Similarly, AD‐MSCs with gene transfer of manganese superoxide dismutase (SOD2) that were transplanted into syngeneic mice elicited cytoprotective effects and improved survival and engraftment rates. 252 Additionally, overexpression of Oct4 and Sox2 in AD‐MSCs increases their proliferation and differentiation and can be helpful in expanding and enhancing their stemness. 253
Direct application of AD‐MSCs‐derived exosomes may also have therapeutic potential in cutaneous wound healing. Conducted studies revealed that their application accelerates wound healing in a murine model by optimizing fibroblast functions. AD‐MSCs exosomes stimulate the production of collagen I and III in the early stages of wound healing and reduce scar formation by decreasing collagen expression in the late stages. 254 In a continued study, it was found that AD‐MSCs‐derived exosomes promote scarless wound healing through regulation of ECM remodeling (increases in TGFβ3:TGFβ1 and MMP3:TIMP1 ratios and prevention of myofibroblast differentiation), and the mechanism involves activation of the ERK/MAPK pathway. 255 It was also demonstrated that stimulation with inflammatory cytokines (TNF‐α/IFN‐γ) can enhance the immunosuppressive and antiinflammatory potential of AD‐MSCs‐derived exosomes. 256
9. CONCLUSIONS
AD‐MSCs seem to be a promising tool for the treatment of various types of diseases. They are successfully used, among others, in the treatment of chronic or complicated wounds of various etiologies, for example, diabetic wounds. These cells secrete a number of cytokines and growth factors that stimulate the processes responsible for proper wound healing. They are also a good model for testing the activity of new proregenerative compounds. However, despite the large number of studies on the biology and characterization of AD‐MSCs and the existence of clinical trials evaluating their therapeutic potential, the topic of their safety is still widely discussed, particularly in relation to cancer patients. There is still a lack of an adequate number of clinical trials on the effectiveness of AD‐MSCs in the treatment of oncological wounds. The number of complicated wounds and tissue defects arising as a result of cancer and oncological treatment is a major problem in the daily work of oncologists, these adverse event significantly worsen the patients' quality of life. However, it seems that these cells should be used with extreme caution in cancer patients, once the patient is made aware of the risk. The issue of their safety and effectiveness in stimulating wound healing in cancer patients should certainly be further investigated and relevant clinical trials should be performed. It is also worth mentioning that some limitations of cellular therapies are their high costs, restrictive legal regulations and the time needed for cell expansion. In conclusion, the applications of these cells are very attractive, but they still require more basic and clinical investigations.
AUTHOR CONTRIBUTIONS
Milena Deptuła: participated in manuscript conceptualization, drafted the manuscript, described wound healing process of acute and chronic wounds including wound healing in diabetes and oncological patients, AD‐MSCs characteristics (markers, differentiation, genetic stability, effect of FBS deprivation), AD‐MSCs role in wound healing and their clinical application (Tables 6 and 7) and strategies of stimulation of their activities, prepared figures, conducted part of the clinical trials research, revised the manuscript; Agnieszka Brzezicka: provided photos of fat grafting by Coleman technique, conducted part of clinical trials research, described Coleman technique and prepared Table 1; Aneta Skoniecka: provided photos of cell isolation, described cell isolation and cells and tissue sources, compared SAT and VAT (with Table 4), compared AD‐MSCs and SVF (with Table 2) and described factors affecting AD‐MSCs and BAT and WAT AD‐MSCs; Jacek Zieliński: provided photos of fat harvesting through surgical resection and wound healing complications in oncological patients and critically revised the manuscript; Michał Pikuła: participated in manuscript conceptualization, described immunological properties of AD‐MSCs, MSCs interactions with immune cells, supervised work, critically revised the manuscript and provided financial support.
All authors approved final version of the manuscript. The authors declare that there are no conflict of interests.
ACKNOWLEDGEMENTS
This work was supported by the funds of the National Science Centre (NCN, Poland), grant no.: 2019/33/B/NZ7/02676 [Michał Pikuła] and grant no.: 2019/03/X/NZ3/00276 [Milena Deptuła]).
Biographies
Milena Deptuła, Eng, MSc, PhD is a research specialist in the Laboratory of Tissue Engineering and Regenerative Medicine in the Department of Embryology, at the Medical University of Gdansk, Poland. She has experience in cell culture, isolation and biology. She has been involved in projects related to the search for new compounds that stimulate wound healing. Her research interests are focused on biology of adipose‐derived stromal cells and their effect of skin cells form diabetic (she is a Principal Investigator in the project) and oncological patients as well as the effect of neoadjuvant therapy on skin cell from oncological patients. She is also working on new bioactive compounds and biomaterials for regenerative medicine.
Agnieszka Brzezica, MD is a resident in Department of Plastic Surgery, Medical University of Gdansk, Poland. She is preparing her PhD thesis under the supervision of Prof. Pikuła. She has experience in fat transplantation into chronic wounds.
Aneta Skoniecka, MSc, PhD is a teaching assistant in a Department of Embryology at Medical University of Gdansk, Poland. She is experienced in cell culture, isolation and biology. Her research is now focused on biology of adipose‐derived stromal cells and comparison of biological functions of AD‐MSCs derived from subcutaneous and visceral adipose tissue.
Jacek Zieliński, MD, PhD is a Professor in Department of Oncologic Surgery, Medical University of Gdansk. He has years of experience in oncological surgery and wound healing. He is also interested in the use of 3D imaging in surgery.
Michał Pikuła, MSc, PhD is a Full Professor at the Laboratory of Tissue Engineering and Regenerative Medicine, Department of Embryology, at the Medical University of Gdansk (MUG). He completed his M.Sc. from University of Gdansk (Molecular biology), M. Pharm. from Medical University of Gdansk (Pharmacy Practice), and his PhD in medical biology from Medical University of Gdansk (specialty cell biology). He is a specialist in regenerative medicine, tissue engineering, and experimental dermatology. Prof. Pikuła is currently responsible for several projects focused on stem cells, wound healing, new biomaterials, and biologically active peptides. Prof. Pikuła is a group leader in the interdisciplinary consortium grant BIONANOVA and a Principal Investigator in the project “Effect of adipose‐derived stem cells on in vitro cultured skin cells obtained from patients undergoing chemotherapy” (Research grant founded by National Science Centre, Poland). He also conducts didactic courses for medical students and PhD students of the Medical University of Gdańsk (MUG). Prof. Pikuła is a Deputy Director of the First MUG Doctoral School.
Deptuła M, Brzezicka A, Skoniecka A, Zieliński J, Pikuła M. Adipose‐derived stromal cells for nonhealing wounds: Emerging opportunities and challenges Med Res Rev. 2021;41:2130‐2171. 10.1002/med.21789
Contributor Information
Milena Deptuła, Email: milenadeptula@gumed.edu.pl, Email: ikula@gumed.edu.pl.
Michał Pikuła, Email: milenadeptula@gumed.edu.pl, Email: ikula@gumed.edu.pl.
REFERENCES
- 1. Pikuła M, Langa P, Kosikowska P, Trzonkowski P. Stem cells and growth factors in wound healing. Postepy Hig Med Dosw. 2015;69:874‐885. [DOI] [PubMed] [Google Scholar]
- 2. Bielefeld KA, Amini‐Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2013;70(12):2059‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 4. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528‐1542. [DOI] [PubMed] [Google Scholar]
- 5. Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem‐cell‐based therapeutic strategies. Stem Cell Res Ther. 2019;10(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delavary BM, van der Veer WM, van Egmond M, Niessen FB, Beelen RHJ. Macrophages in skin injury and repair. Immunobiology. 2011;216(7):753‐762. [DOI] [PubMed] [Google Scholar]
- 7. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro‐wound healing phenotypes. Front Physiol. 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79(1):593‐617. [DOI] [PubMed] [Google Scholar]
- 9. Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci. 2013;72(3):206‐217. [DOI] [PubMed] [Google Scholar]
- 10. Saghazadeh S, Rinoldi C, Schot M, et al. Drug delivery systems and materials for wound healing applications. Adv Drug Deliv Rev. 2018;127:138‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen M, Przyborowski M, Berthiaume F. Stem cells for skin tissue engineering and wound healing. Crit Rev Biomed Eng. 2009;37(4–5):399‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Das S, Baker AB. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front Bioeng Biotechnol. 2016;4:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol. 2010;3(7):643‐653. [PMC free article] [PubMed] [Google Scholar]
- 14. Park J, Hwang S, Yoon I‐S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules. 2017;22(8):1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morton LM, Phillips TJ. Wound healing and treating wounds Differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74(4):589‐605. [DOI] [PubMed] [Google Scholar]
- 16. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Connor N, Mulliken J, Banks‐Schlegel S, Kehinde O, Green H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;317(8211):75‐78. [PubMed] [Google Scholar]
- 18. Langa P, Wardowska A, Zieliński J, et al. Transcriptional profile of in vitro expanded human epidermal progenitor cells for the treatment of non‐healing wounds. J Dermatol Sci. 2018;89(3):272‐281. [DOI] [PubMed] [Google Scholar]
- 19. Hirsch T, Rothoeft T, Teig N, et al. Regeneration of the entire human epidermis by transgenic stem cells. Nature. 2017;551(7680):327‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Rosa L, Carulli S, Cocchiarella F, et al. Long‐term stability and safety of transgenic cultured epidermal stem cells in gene therapy of junctional epidermolysis bullosa. Stem Cell Reports. 2014;2(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schumacher A, Cichorek M, Pikuła M. Adipose‐derived stem cells for tissue engineering and therapy of non‐healing wounds. Postepy Hig Med Dosw. 2018;72:806‐821. [Google Scholar]
- 22. Mohamed‐Ahmed S, Fristad I, Lie SA, et al. Adipose‐derived and bone marrow mesenchymal stem cells: a donor‐matched comparison. Stem Cell Res Ther. 2018;9(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bacakova L, Zarubova J, Travnickova M, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose‐derived stem cells—a review. Biotechnol Adv. 2018;35(4):1111‐1126. [DOI] [PubMed] [Google Scholar]
- 24. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648‐2659. [DOI] [PubMed] [Google Scholar]
- 25. Brzezicka A, Kondej K, Błażyńska‐Spychalska A, Spychalski P, Jankau J, Pikuła M. Medical applications of adipose‐derived stem cells—the latest trends. Chir Plast i Oparzenia/Plast Surg Burn. 2018;6(3):79‐85. [Google Scholar]
- 26. Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393‐395. [DOI] [PubMed] [Google Scholar]
- 27. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019‐1024. [DOI] [PubMed] [Google Scholar]
- 28. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6(6):1445‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larouche J, Sheoran S, Maruyama K, Martino MM. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care. 2018;7(7):209‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346(6212):941‐945. [DOI] [PubMed] [Google Scholar]
- 31. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell‐laden anti‐inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2016;5(1):18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jayaram P, Ikpeama U, Rothenberg JB, Malanga GA. Bone marrow‐derived and adipose‐derived mesenchymal stem cell therapy in primary knee osteoarthritis: a narrative review. PM&R. 2019;11(2):177‐191. [DOI] [PubMed] [Google Scholar]
- 34. Ojeh N, Pastar I, Tomic‐Canic M, Stojadinovic O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci. 2015;16(10):25476‐25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Heal. 2018;21(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 36. Otero‐Viñas M, Falanga V. Mesenchymal stem cells in chronic wounds: the spectrum from basic to advanced therapy. Adv Wound Care. 2016;5(4):149‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heyer K, Herberger K, Protz K, Glaeske G, Augustin M. Epidemiology of chronic wounds in Germany: analysis of statutory health insurance data. Wound Repair Regen. 2016;24(2):434‐442. [DOI] [PubMed] [Google Scholar]
- 38. Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen. 2019;27(1):114‐125. [DOI] [PubMed] [Google Scholar]
- 39. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ho J, Walsh C, Yue D, Dardik A, Cheema U. Current advancements and strategies in tissue engineering for wound healing: a comprehensive review. Adv Wound Care. 2017;6(6):191‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cooper DM, Yu EZ, Hennessey P, et al. Determination of endogenous cytokines in chronic wounds. Ann Surg. 1994;219(6):688‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, Krogfelt KA. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J. 2006;3(3):225‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suleman L. Extracellular bacterial proteases in chronic wounds: a potential therapeutic target? Adv Wound Care. 2016;5(10):455‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trostrup H, Bjarnsholt T, Kirketerp‐Moller K, et al. What is new in the understanding of non healing wounds epidemiology, pathophysiology, and therapies. Ulcers. 2013;2013:625934‐625938. [Google Scholar]
- 46. Negut I, Grumezescu V, Grumezescu A. Treatment strategies for infected wounds. Molecules. 2018;23(9):2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Edwards R, Harding KGG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91‐96. [DOI] [PubMed] [Google Scholar]
- 48. World Health Organization . Global Report on Diabeties 2016;6p.
- 49. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137‐149. [DOI] [PubMed] [Google Scholar]
- 50. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lepäntalo M, Apelqvist J, Setacci C, et al. Chapter V: diabetic foot. Eur J Vasc Endovasc Surg. 2011;42:S60‐S74. [DOI] [PubMed] [Google Scholar]
- 52. Seidel D, Storck M, Lawall H, et al. Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real‐life clinical practice: results of the German DiaFu‐RCT. BMJ Open. 2020;10(3):e026345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta‐analysis. Ann Med. 2017;49(2):106‐116. [DOI] [PubMed] [Google Scholar]
- 54. Gadelkarim M, Abushouk AI, Ghanem E, Hamaad AM, Saad AM, Abdel‐Daim MM. Adipose‐derived stem cells: effectiveness and advances in delivery in diabetic wound healing. Biomed Pharmacother. 2018;107:625‐633. [DOI] [PubMed] [Google Scholar]
- 55. Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr Diab Rep. 2018;18(1):2. [DOI] [PubMed] [Google Scholar]
- 56. Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage‐mediated inflammation in normal and diabetic wound healing. J Immunol. 2017;199(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 57. Okonkwo U, DiPietro L. Diabetes and Wound Angiogenesis. International Journal of Molecular Sciences. 2017;18(7):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The treatment of impaired wound healing in diabetes: looking among old drugs. Pharmaceuticals. 2020;13(4):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gallagher KA, Liu Z‐J, Xiao M, et al. Diabetic impairments in NO‐mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF‐1α. J Clin Invest. 2007;117(5):1249‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem. 2008;56(7):687‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maione AG, Brudno Y, Stojadinovic O, et al. Three‐dimensional human tissue models that incorporate diabetic foot ulcer‐derived fibroblasts mimic in vivo features of chronic wounds. Tissue Eng Part C Methods. 2015;21(5):499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Loots MAM, Kenter SB, Au FL, et al. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF‐I, bFGF and PDGF‐AB compared to controls. Eur J Cell Biol. 2002;81(3):153‐160. [DOI] [PubMed] [Google Scholar]
- 63. Bruhn‐Olszewska B, Korzon‐Burakowska A, Gabig‐Cimińska M, Olszewski P, Węgrzyn A, Jakóbkiewicz‐Banecka J. Molecular factors involved in the development of diabetic foot syndrome. Acta Biochim Pol. 2012;59(4):507‐513. [PubMed] [Google Scholar]
- 64. Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61(11):2937‐2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sutcliffe JES, Thrasivoulou C, Serena TE, et al. Changes in the extracellular matrix surrounding human chronic wounds revealed by 2‐photon imaging. Int Wound J. 2017;14(6):1225‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP‐1 to TIMP‐1 is a predictor of wound healing. Diabet Med. 2008;25(4):419‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li Z, Guo S, Yao F, Zhang Y, Li T. Increased ratio of serum matrix metalloproteinase‐9 against TIMP‐1 predicts poor wound healing in diabetic foot ulcers. J Diabetes Complications. 2013;27(4):380‐382. [DOI] [PubMed] [Google Scholar]
- 68. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 69. Deptuła M, Zieliński J, Wardowska A, Pikuła M. Wound healing complications in oncological patients: perspectives for cellular therapy. Adv Dermatology Allergol. 2019;XXXVI(2):139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hoare D, Maw A, Gollins S. Does pre‐operative chemoradiotherapy cause wound complications after abdominoperineal excision for rectal cancer? An observational study. Int J Surg. 2013;11(5):395‐399. [DOI] [PubMed] [Google Scholar]
- 71. Palubicka A, Jaworski R, Wekwejt M, et al. Surgical site infection after breast surgery: a retrospective analysis of 5‐year postoperative data from a single center in Poland. Medicina (B Aires). 2019;55(9):512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Payne WG, Naidu DK, Wheeler CK, et al. Wound healing in patients with cancer. Eplasty. 2008;8:e9. [PMC free article] [PubMed] [Google Scholar]
- 74. Kang D‐H, Weaver MT, Park N‐J, Smith B, McArdle T, Carpenter J. Significant impairment in immune recovery after cancer treatment. Nurs Res. 2009;58(2):105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Verma R, Foster RE, Horgan K, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Reyes‐Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: Part I. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014;71(2):203.e1‐203.e12 [DOI] [PubMed] [Google Scholar]
- 77. Fabbrocini G, Cameli N, Romano MC, et al. Chemotherapy and skin reactions. J Exp Clin Cancer Res. 2012;31(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anderson K, Hamm RL. Factors that impair wound healing. J Am Coll Clin Wound Spec. 2012;4(4):84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ahn JW, Shalabi D, Correa‐Selm LM, Dasgeb B, Nikbakht N, Cha J. Impaired wound healing secondary to bevacizumab. Int Wound J. 2019;16(4):1009‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang H, Huang Z, Zou X, Liu T. Bevacizumab and wound‐healing complications: a systematic review and meta‐analysis of randomized controlled trials. Oncotarget. 2016;7(50):82473‐82881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jacobson LK, Johnson MB, Dedhia RD, Niknam‐Bienia S, Wong AK. Impaired wound healing after radiation therapy: a systematic review of pathogenesis and treatment. JPRAS Open. 2017;13:92‐105. [Google Scholar]
- 82. Gieringer M, Gosepath J, Naim R. Radiotherapy and wound healing: principles, management and prospects (review). Oncol Rep. 2011;26(2):299‐307. [DOI] [PubMed] [Google Scholar]
- 83. Johnson MB, Pang B, Gardner DJ, et al. Topical fibronectin improves wound healing of irradiated skin. Sci Rep. 2017;7(1):3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jung Kim D, Lee YW, Park MK, et al. Efficacy of the designer antimicrobial peptide SHAP1 in wound healing and wound infection. Amino Acids. 2014;46(10):2333‐2343. [DOI] [PubMed] [Google Scholar]
- 85. Bray FN, Simmons BJ, Wolfson AH, Nouri K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther (Heidelb). 2016;6(2):185‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Córdoba EE, Abba MC, Lacunza E, et al. Polymorphic variants in oxidative stress genes and acute toxicity in breast cancer patients receiving radiotherapy. Cancer Res Treat. 2016;48(3):948‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pohlers D, Brenmoehl J, Löffler I, et al. TGF‐β and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta—Mol Basis Dis. 2009;1792(8):746‐756. [DOI] [PubMed] [Google Scholar]
- 88. Goessler UR, Bugert P, Kassner S, et al. In vitro analysis of radiation‐induced dermal wounds. Otolaryngol—Head Neck Surg. 2010;142(6):845‐850. [DOI] [PubMed] [Google Scholar]
- 89. Riedel F, Philipp K, Sadick H, Goessler U, Hörmann K, Verse T. Immunohistochemical analysis of radiation‐induced non‐healing dermal wounds of the head and neck. In Vivo. 2005;19(2):343‐350. [PubMed] [Google Scholar]
- 90. Grambow F, Rutkowski R, Podmelle F, et al. The impact of lidocaine on adipose‐derived stem cells in human adipose tissue harvested by liposuction and used for lipotransfer. Int J Mol Sci. 2020;21(8):2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Francis A, Wang WZ, Goldman JJ, Fang X‐H, Williams SJ, Baynosa RC. Enhancement of viable adipose‐derived stem cells in lipoaspirate by buffering tumescent with sodium bicarbonate. Plast Reconstr Surg—Glob Open. 2019;7(3):e2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hassan WU, Greiser U, Wang W. Role of adipose‐derived stem cells in wound healing. Wound Repair Regen. 2014;22(3):313‐325. [DOI] [PubMed] [Google Scholar]
- 93. Bajek A, Gurtowska N, Olkowska J, et al. Does the harvesting technique affect the properties of adipose‐derived stem cells?‐The comparative biological characterization. J Cell Biochem. 2017;118(5):1097‐1107. [DOI] [PubMed] [Google Scholar]
- 94. Bajek A, Gurtowska N, Gackowska L, et al. Does the liposuction method influence the phenotypic characteristic of human adipose‐derived stem cells? Biosci Rep. 2015;35(3):e00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Palumbo P, Lombardi F, Siragusa G, Cifone M, Cinque B, Giuliani M. Methods of isolation, characterization and expansion of human adipose‐derived stem cells (ASCs): an overview. Int J Mol Sci. 2018;19(7):1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dubey N, Mishra V, Dubey R, Deng Y‐H, Tsai F‐C, Deng W‐P. Revisiting the advances in isolation, characterization and secretome of adipose‐derived stromal/stem cells. Int J Mol Sci. 2018;19(8):2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Winnier GE, Valenzuela N, Peters‐Hall J, Kellner J, Alt C, Alt EU. Isolation of adipose tissue derived regenerative cells from human subcutaneous tissue with or without the use of an enzymatic reagent. PLoS One. 2019;14(9):e0221457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Doornaert M, De Maere E, Colle J, et al. Xenogen‐free isolation and culture of human adipose mesenchymal stem cells. Stem Cell Res. 2019;40:101532. [DOI] [PubMed] [Google Scholar]
- 99. Busser H, De Bruyn C, Urbain F, et al. Isolation of adipose‐derived stromal cells without enzymatic treatment: expansion, phenotypical, and functional characterization. Stem Cells Dev. 2014;23(19):2390‐2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Domenis R, Lazzaro L, Calabrese S, et al. Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell‐assisted lipotransfer techniques. Stem Cell Res Ther. 2015;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Carvalho PP, Gimble JM, Dias IR, Gomes ME, Reis RL. Xenofree enzymatic products for the isolation of human adipose‐derived stromal/stem cells. Tissue Eng Part C Methods. 2013;19(6):473‐478. [DOI] [PubMed] [Google Scholar]
- 102. Kølle S‐FT, Fischer‐Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex‐vivo expanded adipose tissue‐derived stem cells for graft survival: a randomised placebo‐controlled trial. Lancet. 2013;382(9898):1113‐1120. [DOI] [PubMed] [Google Scholar]
- 103. Doornaert M, Colle J, De Maere E, Declercq H, Blondeel P. Autologous fat grafting: latest insights. Ann Med Surg. 2019;37:47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Si Z, Wang X, Sun C, et al. Adipose‐derived stem cells: sources, potency, and implications for regenerative therapies. Biomed Pharmacother. 2019;114:108765. [DOI] [PubMed] [Google Scholar]
- 105. Salehi‐Nik N, Rezai Rad M, Kheiri L, Nazeman P, Nadjmi N, Khojasteh A. Buccal fat pad as a potential source of stem cells for bone regeneration: a literature review. Stem Cells Int. 2017;2017:8354640‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ardeshirylajimi A, Rafeie F, Zandi‐Karimi A, et al. Fat harvesting site is an important determinant of proliferation and pluripotency of adipose‐derived stem cells. Biologicals. 2016;44(1):12‐18. [DOI] [PubMed] [Google Scholar]
- 107. Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose‐derived stem cells. Ann Plast Surg. 2008;60(5):538‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bora P, Majumdar AS. Adipose tissue‐derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee JW, Park SH, Lee SJ, Kim SH, Suh IS, Jeong HS. Clinical Impact of Highly condensed stromal vascular fraction injection in surgical management of depressed and contracted scars. Aesthetic Plast Surg. 2018;42(6):1689‐1698. [DOI] [PubMed] [Google Scholar]
- 110. Kilinc MO, Santidrian A, Minev I, et al. The ratio of ADSCs to HSC‐progenitors in adipose tissue derived SVF may provide the key to predict the outcome of stem‐cell therapy. Clin Transl Med. 2018;7(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Patrikoski M, Mannerström B, Miettinen S. Perspectives for clinical translation of adipose stromal/stem cells. Stem Cells Int. 2019;2019:5858247‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Koenen P, Spanholtz TA, Maegele M, et al. Acute and chronic wound fluids inversely influence adipose‐derived stem cell function: molecular insights into impaired wound healing. Int Wound J. 2015;12(1):10‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Devitt SM, Carter CM, Dierov R, Weiss S, Gersch RP, Percec I. Successful isolation of viable adipose‐derived stem cells from human adipose tissue subject to long‐term cryopreservation: positive implications for adult stem cell‐based therapeutics in patients of advanced age. Stem Cells Int. 2015;2015:146421‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zanata F, Bowles A, Frazier T, et al. Effect of cryopreservation on human adipose tissue and isolated stromal vascular fraction cells. Plast Reconstr Surg. 2018;141(2):232e‐243e. [DOI] [PubMed] [Google Scholar]
- 115. Park S, Lee DR, Nam JS, Ahn CW, Kim H. Fetal bovine serum‐free cryopreservation methods for clinical banking of human adipose‐derived stem cells. Cryobiology. 2018;81:65‐73. [DOI] [PubMed] [Google Scholar]
- 116. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315‐317. [DOI] [PubMed] [Google Scholar]
- 117. Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue‐derived stromal vascular fraction and culture expanded adipose tissue‐derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pikuła M, Marek‐Trzonkowska N, Wardowska A, Renkielska A, Trzonkowski P. Adipose tissue‐derived stem cells in clinical applications. Expert Opin Biol Ther. 2013;13(10):1357‐1370. [DOI] [PubMed] [Google Scholar]
- 119. Baer PC. Adipose‐derived mesenchymal stromal/stem cells: an update on their phenotype in vivo and in vitro. World J Stem Cells. 2014;6(3):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Varma MJO, Breuls RGM, Schouten TE, et al. Phenotypical and Functional characterization of freshly isolated adipose tissue‐derived stem cells. Stem Cells Dev. 2007;16(1):91‐104. [DOI] [PubMed] [Google Scholar]
- 121. Cheng K‐H, Kuo T‐L, Kuo K‐K, Hsiao C‐C. Human adipose‐derived stem cells: isolation, characterization and current application in regeneration medicine. Genomic Med Biomarkers, Heal Sci. 2011;3(2):53‐62. [Google Scholar]
- 122. Gaiba S, França LP, , de França JP , , de Ferreira LM . Characterization of human adipose‐derived stem cells. Acta Cir Bras. 2012;27(7):471‐476. [DOI] [PubMed] [Google Scholar]
- 123. Yañez R, Lamana ML, García‐Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue‐derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft‐versus‐host disease. Stem Cells. 2006;24(11):2582‐2591. [DOI] [PubMed] [Google Scholar]
- 124. Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue‐derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118‐129. [DOI] [PubMed] [Google Scholar]
- 125. Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue‐derived stromal cells. J Cell Physiol. 2001;189(1):54‐63. [DOI] [PubMed] [Google Scholar]
- 126. Suga H, Matsumoto D, Eto H, et al. Functional implications of CD34 expression in human adipose–derived stem/progenitor cells. Stem Cells Dev. 2009;18(8):1201‐1210. [DOI] [PubMed] [Google Scholar]
- 127. Mieczkowska A, Schumacher A, Filipowicz N, et al. Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells. Sci Rep. 2018;8(1):11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lin C‐S, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14(10):1159‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Scherberich A. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J Stem Cells. 2013;5(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Neri S. Genetic stability of mesenchymal stromal cells for regenerative medicine applications: a fundamental biosafety aspect. Int J Mol Sci. 2019;20(10):2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rubio D, Garcia‐Castro J, Martín MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65(8):3035‐3039. [DOI] [PubMed] [Google Scholar]
- 132. Neri S, Bourin P, Peyrafitte J‐A, Cattini L, Facchini A, Mariani E. Human adipose stromal cells (ASC) for the regeneration of injured cartilage display genetic stability after in vitro culture expansion. PLoS One. 2013;8(10):e77895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Søndergaard RH, Follin B, Lund LD, et al. Senescence and quiescence in adipose‐derived stromal cells: Effects of human platelet lysate, fetal bovine serum and hypoxia. Cytotherapy. 2017;19(1):95‐106. [DOI] [PubMed] [Google Scholar]
- 134. Rauch C, Feifel E, Amann E‐MM, et al. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011;28(4):305‐316. [DOI] [PubMed] [Google Scholar]
- 135. Kamińska J, Langa P, Deptuła M, et al. Transcriptional activity of epigenetic remodeling genes declines in keratinocytes after in vitro expansion. Adv Med Sci. 2019;64(2):274‐279. [DOI] [PubMed] [Google Scholar]
- 136. Dessels C, Ambele MA, Pepper MS. The effect of medium supplementation and serial passaging on the transcriptome of human adipose‐derived stromal cells expanded in vitro. Stem Cell Res Ther. 2019;10(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. van der Valk J. Fetal bovine serum (FBS): Past–present–future. ALTEX. 2018:99‐118. [DOI] [PubMed] [Google Scholar]
- 138. Deptuła M, Karpowicz P, Wardowska A, et al. Development of a Peptide derived from platelet‐derived growth factor (PDGF‐BB) into a potential drug candidate for the treatment of wounds. Adv Wound Care. 2020;9(12):657‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Follin B, Tratwal J, Haack‐Sørensen M, Elberg J, Kastrup J, Ekblond A. Identical effects of VEGF and serum‐deprivation on phenotype and function of adipose‐derived stromal cells from healthy donors and patients with ischemic heart disease. J Transl Med. 2013;11(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wan Safwani WKZ, Wong CW, Yong KW, et al. The effects of hypoxia and serum‐free conditions on the stemness properties of human adipose‐derived stem cells. Cytotechnology. 2016;68(5):1859‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yoshida K, Nakashima A, Doi S, et al. Serum‐free medium enhances the immunosuppressive and antifibrotic abilities of mesenchymal stem cells utilized in experimental renal fibrosis. Stem Cells Transl Med. 2018;7(12):893‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lee M‐S, Youn C, Kim J, et al. Enhanced cell growth of adipocyte‐derived mesenchymal stem cells using chemically‐defined serum‐free media. Int J Mol Sci. 2017;18(8):1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chu D‐T, Nguyen Thi Phuong T, Tien NLB, et al. Adipose tissue stem cells for therapy: an update on the progress of isolation, culture, storage, and clinical application. J Clin Med. 2019;8(7):917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Trojahn Kølle S‐F, Oliveri RS, Glovinski PV, et al. Pooled human platelet lysate versus fetal bovine serum—investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue‐derived stem cells intended for clinical use. Cytotherapy. 2013;15(9):1086‐1097. [DOI] [PubMed] [Google Scholar]
- 145. Glovinski PV, Herly M, Mathiasen AB, et al. Overcoming the bottleneck of platelet lysate supply in large‐scale clinical expansion of adipose‐derived stem cells: a comparison of fresh versus three types of platelet lysates from outdated buffy coat‐derived platelet concentrates. Cytotherapy. 2017;19(2):222‐234. [DOI] [PubMed] [Google Scholar]
- 146. Kocaoemer A, Kern S, Klüter H, Bieback K. Human AB Serum and Thrombin‐activated platelet‐rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25(5):1270‐1278. [DOI] [PubMed] [Google Scholar]
- 147. Bieback K, Ha VA‐T, Hecker A, et al. Altered gene expression in human adipose stem cells cultured with fetal bovine serum compared to human supplements. Tissue Eng Part A. 2010;16(11):3467‐3484. [DOI] [PubMed] [Google Scholar]
- 148. Lindroos B, Aho K‐L, Kuokkanen H, et al. Differential gene expression in adipose stem cells cultured in allogeneic human serum versus fetal bovine serum. Tissue Eng Part A. 2010;16(7):2281‐2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. 2018;221(Suppl 1):jeb162958. [DOI] [PubMed] [Google Scholar]
- 150. Moore KL, Persaud TVN, Torchia M Body Cavities and Diaphragm. In: THE DEVELOPING HUMAN: CLINICALLY ORIENTED EMBRYOLOGY. Saunders; 2013. p 145.
- 151. Castro JP, Grune T, Speckmann B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol Chem. 2016;397(8):709‐724. [DOI] [PubMed] [Google Scholar]
- 152. Ritter A, Friemel A, Fornoff F, et al. Characterization of adipose‐derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget. 2015;6(33):34475‐34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ritter A, Friemel A, Roth S, et al. Subcutaneous and visceral adipose‐derived mesenchymal stem cells: commonality and diversity. Cells. 2019;8(10):1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tang Y, Pan Z, Zou Y, et al. A comparative assessment of adipose‐derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017;21(9):2153‐2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Raajendiran A, Ooi G, Bayliss J, et al. Identification of metabolically distinct adipocyte progenitor cells in human adipose tissues. Cell Rep. 2019;27(5):1528‐1540 e7. [DOI] [PubMed] [Google Scholar]
- 156. Ong WK, Tan CS, Chan KL, et al. Identification of specific cell‐surface markers of adipose‐derived stem cells from subcutaneous and visceral fat depots. Stem Cell Reports. 2014;2(2):171‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Macotela Y, Emanuelli B, Mori MA, et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61(7):1691‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Badimon L, Cubedo J. Adipose tissue depots and inflammation: effects on plasticity and resident mesenchymal stem cell function. Cardiovasc Res. 2017;113(9):1064‐1073. [DOI] [PubMed] [Google Scholar]
- 159. Pan Z, Zhou Z, Zhang H, et al. CD90 serves as differential modulator of subcutaneous and visceral adipose‐derived stem cells by regulating AKT activation that influences adipose tissue and metabolic homeostasis. Stem Cell Res Ther. 2019;10(1):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wada Y, Ikemoto T, Morine Y, et al. The differences in the characteristics of insulin‐producing cells using human adipose‐tissue derived mesenchymal stem cells from subcutaneous and visceral tissues. Sci Rep. 2019;9:13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Berry R, Rodeheffer MS, Rosen CJ, Horowitz MC. Adipose tissue‐residing progenitors (adipocyte lineage progenitors and adipose‐derived stem cells (ADSC)). Curr Mol Biol Reports. 2015;1(3):101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Tchkonia T, Thomou T, Zhu Y, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Enerbäck S. Human brown adipose tissue. Cell Metab. 2010;11(4):248‐252. [DOI] [PubMed] [Google Scholar]
- 164. Jung SM, Sanchez‐Gurmaches J, Guertin DA. Brown adipose tissue development and metabolism. Handb Exp Pharmacol. 2019;251:3‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lu K‐Y, Primus Dass KT, Lin S‐Z, Harn H‐J, Liu S‐P. The application of stem cell therapy and brown adipose tissue transplantation in metabolic disorders. Cytotherapy. 2020;22(10):521‐528. [DOI] [PubMed] [Google Scholar]
- 166. Wang W, Kissig M, Rajakumari S, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci. 2014;111(40):14466‐14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Min SY, Desai A, Yang Z, et al. Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc Natl Acad Sci. 2019;116(36):17970‐17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Tsuji T, Ikado K, Koizumi H, Uchiyama S, Kajimoto K. Difference in intracellular temperature rise between matured and precursor brown adipocytes in response to uncoupler and β‐adrenergic agonist stimuli. Sci Rep. 2017;7(1):12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white‐to‐brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15(3):395‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Cypess AM, Weiner LS, Roberts‐Toler C, et al. Activation of human brown adipose tissue by a β3‐adrenergic receptor agonist. Cell Metab. 2015;21(1):33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. de Jong JMA, Wouters RTF, Boulet N, Cannon B, Nedergaard J, Petrovic N. The β3‐adrenergic receptor is dispensable for browning of adipose tissues. Am J Physiol—Endocrinol Metab. 2017;312(6):E508‐E518. [DOI] [PubMed] [Google Scholar]
- 172. Cousin B, Cinti S, Morroni M, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931‐942. [DOI] [PubMed] [Google Scholar]
- 173. Silva FJ, Holt DJ, Vargas V, et al. Metabolically active human brown adipose tissue derived stem cells. Stem Cells. 2013;32:572‐581. [DOI] [PubMed] [Google Scholar]
- 174. Panina YA, Yakimov AS, Komleva YK, et al. Plasticity of adipose tissue‐derived stem cells and regulation of angiogenesis. Front Physiol. 2018;9:1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Efimenko AY, Kochegura TN, Akopyan ZA, Parfyonova YV. Autologous Stem cell therapy: how aging and chronic diseases affect stem and progenitor cells. Biores Open Access. 2015;4(1):26‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM. The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int. 2016;2016:2152435‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Cervelli V. Concise review: adipose‐derived stromal vascular fraction cells and platelet‐rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med. 2012;1(3):230‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging cell. 2010;9(5):667‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Louwen F, Ritter A, Kreis NN, Yuan J. Insight into the development of obesity: functional alterations of adipose‐derived mesenchymal stem cells. Obes Rev. 2018;19(7):888‐904. [DOI] [PubMed] [Google Scholar]
- 180. Oñate B, Vilahur G, Camino‐López S, et al. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte‐like phenotype. BMC Genomics. 2013;14(1):625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Frazier TP, Gimble JM, Devay JW, Tucker HA, Chiu ES, Rowan BG. Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue‐derived stem cells. BMC Cell Biol. 2013;14(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Conley SM, Hickson LJ, Kellogg TA, et al. Human obesity induces dysfunction and early senescence in adipose tissue‐derived mesenchymal stromal/stem cells. Front Cell Dev Biol. 2020;8:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Park YM, Yoo SH, Kim S‐H. Adipose‐derived stem cells induced EMT‐like changes in H358 lung cancer cells. Anticancer Res. 2013;33(10):4421‐4430. [PubMed] [Google Scholar]
- 184. Scioli MG, Storti G, D'Amico F, et al. Adipose‐derived stem cells in cancer progression: new perspectives and opportunities. Int J Mol Sci. 2019;20(13):3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wang S, Li X, Xu M, Wang J, Zhao RC. Reduced adipogenesis after lung tumor exosomes priming in human mesenchymal stem cells via TGFβ signaling pathway. Mol Cell Biochem. 2017;435(1–2):59‐66. [DOI] [PubMed] [Google Scholar]
- 186. García‐Contreras M, Vera‐Donoso CD, Hernández‐Andreu JM, García‐Verdugo JM, Oltra E. Therapeutic potential of human adipose‐derived stem cells (adscs) from cancer patients: a pilot study. PLoS One. 2014;9(11):e113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Freese KE, Kokai L, Edwards RP, et al. Adipose‐derived stems cells and their role in human cancer development, growth, progression, and metastasis: a systematic review. Cancer Res. 2015;75(7):1161‐1168. [DOI] [PubMed] [Google Scholar]
- 188. Gentile P, Calabrese C, De Angelis B, Pizzicannella J, Kothari A, Garcovich S. Impact of the different preparation methods to obtain human adipose‐derived stromal vascular fraction cells (AD‐SVFs) and human adipose‐derived mesenchymal stem cells (AD‐MSCs): enzymatic digestion versus mechanical centrifugation. Int J Mol Sci. 2019;20(21):5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Stamatopoulos A, Stamatopoulos T, Gamie Z, et al. Mesenchymal stromal cells for bone sarcoma treatment: roadmap to clinical practice. J Bone Oncol. 2019;16:100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Ko S‐F, Yip H‐K, Zhen Y‐Y, et al. Adipose‐derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: apparent diffusion coefficient, natural killer T‐cell responses, and histopathological features. Stem Cells Int. 2015;2015:853506‐853511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Poglio S, Galvani S, Bour S, et al. Adipose tissue sensitivity to radiation exposure. Am J Pathol. 2009;174(1):44‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Haubner F, Muschter D, Pohl F, Schreml S, Prantl L, Gassner H. A co‐culture model of fibroblasts and adipose tissue‐derived stem cells reveals new insights into impaired wound healing after radiotherapy. Int J Mol Sci. 2015;16(11):25947‐25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Haubner F, Gassner HG. Potential of adipose‐derived stem cells concerning the treatment of wound healing complications after radiotherapy. HNO. 2015;63(2):111‐117. [DOI] [PubMed] [Google Scholar]
- 194. Haubner F, Gassner HG, Pérez, Álvarez J. Injection of Lipotransplants for wound healing complications after radiotherapy. Laryngorhinootologie. 2016;95(04):242‐244. [DOI] [PubMed] [Google Scholar]
- 195. Mohan A, Singh S. Use of fat transfer to treat a chronic, non‐healing, post‐radiation ulcer: a case study. J Wound Care. 2017;26(5):272‐273. [DOI] [PubMed] [Google Scholar]
- 196. Pike S, Zhang P, Wei Z, et al. In vitro effects of tamoxifen on adipose‐derived stem cells. Wound Repair Regen. 2015;23(5):728‐736. [DOI] [PubMed] [Google Scholar]
- 197. Liang W, Xia H, Li J, Zhao RC. Human adipose tissue derived mesenchymal stem cells are resistant to several chemotherapeutic agents. Cytotechnology. 2011;63(5):523‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hu MS, Borrelli MR, Lorenz HP, Longaker MT, Wan DC. Mesenchymal stromal cells and cutaneous wound healing: a comprehensive review of the background, role, and therapeutic potential. Stem Cells Int. 2018;2018:6901983‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Li P, Guo X. A review: therapeutic potential of adipose‐derived stem cells in cutaneous wound healing and regeneration. Stem Cell Res Ther. 2018;9(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Kim W‐S, Park B‐S, Sung J‐H. The wound‐healing and antioxidant effects of adipose‐derived stem cells. Expert Opin Biol Ther. 2009;9(7):879‐887. [DOI] [PubMed] [Google Scholar]
- 201. Coleman SR, Katzel EB. Fat grafting for facial filling and regeneration. Clin Plast Surg. 2015;42(3):289‐300. [DOI] [PubMed] [Google Scholar]
- 202. Zielins ER, Brett EA, Longaker MT, Wan DC. Autologous fat grafting: the science behind the surgery. Aesthetic Surg J. 2016;36(4):488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Billings EJ, May JWJ. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83(2):368‐381. [DOI] [PubMed] [Google Scholar]
- 204. Pu LLQ, Coleman SR, Cui X. Ferguson REHJ, Vasconez HC. Autologous fat grafts harvested and refined by the Coleman technique: a comparative study. Plast Reconstr Surg. 2008;122(3):932‐937. [DOI] [PubMed] [Google Scholar]
- 205. Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg. 1997;24(2):347‐367. [PubMed] [Google Scholar]
- 206. Strong AL, Cederna PS, Rubin JP, Coleman SR, Levi B. The current state of fat grafting. Plast Reconstr Surg. 2015;136(4):897‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Coleman SR. Hand rejuvenation with structural fat grafting. Plast Reconstr Surg. 2002;110(7):1731‐1737. [DOI] [PubMed] [Google Scholar]
- 208. Loder S, Peterson JR, Agarwal S, et al. Wound healing after thermal injury is improved by fat and adipose‐derived stem cell isografts. J Burn Care Res. 2015;36(1):70‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(3 Suppl):108S‐120S. [DOI] [PubMed] [Google Scholar]
- 210. Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg. 2017;20:2049‐2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Egro FM, Coleman SR. Facial fat grafting: the past, present, and future. Clin Plast Surg. 2020;47(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 212. Lendeckel S, Jödicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Cranio‐Maxillofacial Surg. 2004;32(6):370‐373. [DOI] [PubMed] [Google Scholar]
- 213. Dai R, Wang Z, Samanipour R, Koo K‐I, Kim K. Adipose‐derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016;2016:6737345‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Sun J, Tan H. Alginate‐based biomaterials for regenerative medicine applications. Materials(Basel). 2013;6(4):1285‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88‐95. [Google Scholar]
- 216. Bura A, Planat‐Benard V, Bourin P, et al. Phase I trial: the use of autologous cultured adipose‐derived stroma/stem cells to treat patients with non‐revascularizable critical limb ischemia. Cytotherapy. 2014;16(2):245‐257. [DOI] [PubMed] [Google Scholar]
- 217. Moon K‐C, Suh H‐S, Kim K‐B, Han S‐K, Young K‐W, Lee J‐W. Potential of allogeneic adipose‐derived stem cell‐hydrogel complex for treating diabetic foot ulcers. Diabetes. 2019;68(4):837‐846. [DOI] [PubMed] [Google Scholar]
- 218. Panettiere P, Marchetti L, Accorsi D. The Serial Free Fat Transfer In Irradiated Prosthetic Breast Reconstructions. Aesthetic Plast Surg. 2009;33(5):695‐700. [DOI] [PubMed] [Google Scholar]
- 219. Ribuffo D, Atzeni M, Guerra M, et al. Treatment of irradiated expanders: protective lipofilling allows immediate prosthetic breast reconstruction in the setting of postoperative radiotherapy. Aesthetic Plast Surg. 2013;37(6):1146‐1152. [DOI] [PubMed] [Google Scholar]
- 220. Rigotti G, Marchi A, Galiè M, et al. Therapeutic Healing of Radiolesions by Autologous Lipoaspirate Transplant: a Process Mediated by Adipose‐Derived Adult Stem Cells. Innovations in Plastic and Aesthetic Surgery. Berlin, Heidelberg: Springer Berlin Heidelberg; 2008:49‐63. [Google Scholar]
- 221. Phulpin B, Gangloff P, Tran N, Bravetti P, Merlin J‐L, Dolivet G. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast Reconstr Surg. 2009;123(4):1187‐1197. [DOI] [PubMed] [Google Scholar]
- 222. Widholz B, Tsitlakidis S, Reible B, Moghaddam A, Westhauser F. Pooling of patient‐derived mesenchymal stromal cells reduces inter‐individual confounder‐associated variation without negative impact on cell viability, proliferation and osteogenic differentiation. Cells. 2019;8(6):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Petricciani J, Hayakawa T, Stacey G, Trouvin J‐H, Knezevic I. Scientific considerations for the regulatory evaluation of cell therapy products. Biologicals. 2017;50:5020‐5026. [DOI] [PubMed] [Google Scholar]
- 224. Roh K‐H, Nerem RM, Roy K. Biomanufacturing of therapeutic cells: state of the art, current challenges, and future perspectives. Annu Rev Chem Biomol Eng. 2016;7(1):455‐478. [DOI] [PubMed] [Google Scholar]
- 225. McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose‐derived cells: temporal changes in vitro. Stem Cells. 2006;24(5):1246‐1253. [DOI] [PubMed] [Google Scholar]
- 226. Lohan P, Treacy O, Griffin MD, Ritter T, Ryan AE. Anti‐donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: are we still learning? Front Immunol. 2017;8:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Ryan AE, Lohan P, O'Flynn L, et al. Chondrogenic differentiation increases antidonor immune response to allogeneic mesenchymal stem cell transplantation. Mol Ther. 2014;22(3):655‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Lohan P, Coleman CM, Murphy J, Griffin MD, Ritter T, Ryan AE. Changes in immunological profile of allogeneic mesenchymal stem cells after differentiation: should we be concerned? Stem Cell Res Ther. 2014;5(4):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Patrikoski M, Sivula J, Huhtala H, et al. Different culture conditions modulate the immunological properties of adipose stem cells. Stem Cells Transl Med. 2014;3(10):1220‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Lin L, Du L. The role of secreted factors in stem cells‐mediated immune regulation. Cell Immunol. 2018;326:32624‐32632. [DOI] [PubMed] [Google Scholar]
- 231. Serena C, Keiran N, Ceperuelo‐Mallafre V, et al. Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells. 2016;34(10):2559‐2573. [DOI] [PubMed] [Google Scholar]
- 232. Serena C, Keiran N, Madeira A, et al. Crohn's disease disturbs the immune properties of human adipose‐derived stem cells related to inflammasome activation. Stem Cell Reports. 2017;9(4):1109‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Najar M, Raicevic G, Fayyad‐Kazan H, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy. 2016;18(2):160‐171. [DOI] [PubMed] [Google Scholar]
- 234. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Zachar L, Bačenková D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res. 2016;9:231‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141‐150. [DOI] [PubMed] [Google Scholar]
- 238. Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T‐cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228‐234. [DOI] [PubMed] [Google Scholar]
- 239. Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs Improve Myocardial infarction in mice because cells embolized in lung are activated to secrete the anti‐inflammatory protein TSG‐6. Cell Stem Cell. 2009;5(1):54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Ortiz‐Virumbrales M, Menta R, Pérez LM, et al. Human adipose mesenchymal stem cells modulate myeloid cells toward an anti‐inflammatory and reparative phenotype: role of IL‐6 and PGE2. Stem Cell Res Ther. 2020;11(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Seo Y, Shin T‐H, Kim H‐S. Current strategies to enhance adipose stem cell function: an update. Int J Mol Sci. 2019;20(15):3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Souza LEB, Beckenkamp LR, Sobral LM, et al. Pre‐culture in endothelial growth medium enhances the angiogenic properties of adipose‐derived stem/stromal cells. Angiogenesis. 2018;21(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 243. Horikoshi‐Ishihara H, Tobita M, Tajima S, et al. Coadministration of adipose‐derived stem cells and control‐released basic fibroblast growth factor facilitates angiogenesis in a murine ischemic hind limb model. J Vasc Surg. 2016;64(6):1825‐1834 e1. [DOI] [PubMed] [Google Scholar]
- 244. Liu G‐S, Peshavariya HM, Higuchi M, Chan EC, Dusting GJ, Jiang F. Pharmacological priming of adipose‐derived stem cells for paracrine VEGF production with deferoxamine. J Tissue Eng Regen Med. 2016;10(3):E167‐E176. [DOI] [PubMed] [Google Scholar]
- 245. Hye Kim J, Gyu Park S, Kim W‐K, Song SU, Sung J‐H. Functional regulation of adipose‐derived stem cells by PDGF‐D. Stem Cells. 2015;33(2):542‐556. [DOI] [PubMed] [Google Scholar]
- 246. Frith JE, Thomson B, Genever PG. Dynamic three‐dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16(4):735‐749. [DOI] [PubMed] [Google Scholar]
- 247. Cheng N‐C, Chen S‐Y, Li J‐R, Young T‐H. Short‐term spheroid formation enhances the regenerative capacity of adipose‐derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl Med. 2013;2(8):584‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Amos PJ, Kapur SK, Stapor PC, et al. Human adipose‐derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A. 2010;16(5):1595‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Lee EY, Xia Y, Kim W‐SS, et al. Hypoxia‐enhanced wound‐healing function of adipose‐derived stem cells: increase in stem cell proliferation and up‐regulation of VEGF and bFGF. Wound Repair Regen. 2009;17(4):540‐547. [DOI] [PubMed] [Google Scholar]
- 250. Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Kim M, Kim D‐I, Kim EK, Kim C‐W. CXCR4 Overexpression in human adipose tissue‐derived stem cells improves homing and engraftment in an animal limb ischemia model. Cell Transplant. 2017;26(2):191‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Baldari S, Di Rocco G, Trivisonno A, Samengo D, Pani G, Toietta G. Promotion of survival and engraftment of transplanted adipose tissue‐derived stromal and vascular cells by overexpression of manganese superoxide dismutase. Int J Mol Sci. 2016;17(7):1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Han S‐M, Han S‐H, Coh Y‐R, et al. Enhanced proliferation and differentiation of Oct4‐ and Sox2‐overexpressing human adipose tissue mesenchymal stem cells. Exp Mol Med. 2014;46(6):e101‐e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6(1):32993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Wang L, Hu L, Zhou X, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7(1):13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Domenis R, Cifù A, Quaglia S, et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells‐derived exosomes. Sci Rep. 2018;8(1):13325. [DOI] [PMC free article] [PubMed] [Google Scholar]


