Abstract
Objective
Patients with breast cancer face cognitive impairment that affects their quality of life; partially attributable to treatment. Our aim was to detail the prevalence and change of cognitive impairment during the course of treatment. We also investigated the effect of therapy (chemotherapy [CT]) vs. radiotherapy and/or endocrine therapy vs. healthy controls).
Methods
This article reviews longitudinal cohort studies published to date in Medline and Embase that (i) assess cognition before and after therapy, (ii) report prevalence cognitive impairment or change, and (iii) use standardized and valid neuropsychological tests. We used the original authors' criteria for cognitive impairment.
Results
The title and abstract of 891 articles were screened, resulting in the identification of 90 potentially relevant articles while applying the eligibility criteria. After full‐text examination, 17 studies were included. Prevalence of cognitive impairment range from 25% before therapy, through 24% after therapy to 21% at maximal 1‐year follow‐up (FU). Compared to their pretreatment cognitive functioning, 24% of patients decline after treatment and 24% at 1‐year FU. Some studies also reported cognitive improvement showing that 15% and 31% of patients improve, respectively. In general, patients undergoing CT have a higher chance of cognitive impairment and decline than no‐CT patients and healthy controls.
Conclusions
This study shows that one out of four breast cancer patients shows cognitive impairment prior to treatment administration CT and a significant number of patients decline during the course of disease, suggesting that cognitive impairment is not exclusively related to CT and/or no‐CT therapies. This study shows that assessment of cognitive functioning, ideally over time, is crucial and may help the implementation of personalized rehabilitation pathways.
Keywords: cancer, cancer‐related cognitive impairment, chemotherapy‐related cognitive impairment, longitudinal studies, mamma carcinoma, oncology
1. BACKGROUND
Among women, breast cancer represents the most frequently diagnosed cancer and leading cause of cancer death. 1 Current treatment options consist of surgery with various combinations of chemotherapy (CT), endocrine therapy (ET) and postoperative radiotherapy (RT), depending on disease stage and prognostic factors. With advances in diagnosis and treatment, the long‐term survival rate has increased markedly, therefore allowing an attention shift toward minimizing morbidity. 2 Among available treatment options, CT is most frequently linked to mild to moderate cognitive decline, as illustrated by commonly used terms as “chemobrain” or “chemofog”.3, 4, 5, 6 Cognitive impairment and associated poor quality of life (QOL) is rated as one of the most bothersome symptoms by cancer survivors and has long been reported during and after treatment courses.7, 8, 9 In turn, poor QOL and increased levels of distress are related to increased use of health care and costs. 10 Though standardized neuropsychological tests are considered a golden standard in cognition research, perceived cognitive problems and side effects (e.g., nausea, pain, and fatigue) can also significantly impact QOL and thus are extremely important to assess in clinical practice as well. 11 , 12
The “chemobrain” literature and awareness among the cancer community (including patients) has been increasingly steadily over the past decade. Three meta‐analyses have included studies that examined cognitive effects during and after CT on women diagnosed with breast cancer. All meta‐analyses revealed that breast cancer patients perform more poorly than controls on visuospatial ability and language tests. Additionally, the largest differences in cognitive impairment were found in the cognitive domains of short‐term memory and motor functioning.13, 14, 15 Much less is known about the cognitive effect of no‐CT treatments, such as ET or postoperative RT. One systematic review (N = 12 studies of which one erroneously included, time since treatment onset = 3 – 24 months) on breast cancer patients undergoing ET indicated that decreased cognitive performance is most often seen in the domain of verbal memory. 16 A second systematic review (N = 21 studies, time since treatment divided into ≤2 and >2 years) reports that 80% of the included studies found an association between ET administration and impairment in at least one of the following cognitive domains: processing speed, learning and memory, language, and executive functions. 17 Though evidence is less conclusive for breast cancer patients undergoing RT, longitudinal studies imply induced cognitive impairment mainly in the verbal memory domain.18, 19, 20, 21 Overall, CT has been related to cognitive deficits in the domains of visuospatial ability and language, whereas ET and RT, though less conclusive, have been associated with deficits in verbal memory.
While research on the cognitive consequences of breast cancer treatment has received much attention, the prevalence of cognitive deficits during and after breast cancer treatment remain imprecise. Some patients improve over months or years after completion of CT, whereas others experience long‐term cognitive impairment, up to 21 years after treatment.22, 23, 24, 25 In addition, the review of Wefel and Schagen 6 provides an overview of 53 studies published between 1995 and 2012 on the effect of CT on cognitive impairment. They postulate that between 17% and 75% of patients show cognitive impairment in cross‐sectional studies and that between 19% and 78% of patients show cognitive impairment in longitudinal studies. To our knowledge no reviews have yet bundled cognitive impairment data for patients undergoing ET nor RT. With only broad prevalence estimates available and few studies investigating long‐term consequences knowledge of changes in cognitive impairment and decline over time is needed. Such knowledge could improve the diagnostic process and alert healthcare professionals to the need to identify and support patients with potential decline, thereby facilitating the implementation of personalized rehabilitation pathways with appropriate integrated care after treatment for breast cancer.
The change of cognitive impairment over the course of CT has been illustrated by the landmark study of Wefel et al. 26 First, they revealed that 21% (9 of 42) of breast cancer patients were already impaired before commencement of CT. Therefore, alongside CT other cancer‐related factors are likely to be implicated in cognitive impairment. In fact, Wefel et al. 26 showed that 61% (17 of 28) of patients demonstrated cognitive decline 6 months after CT completion. This underlines that even though one may expect that cognitive impairment would dissipate over time, a large group of patients are cognitively impaired at 6‐months follow‐up (FU). While longitudinal studies such as Wefel et al. 26 shed light on the course of cognitive impairment in patients with breast cancer over time, studies are often solely confided to CT and encompass relatively small sample sizes. Therefore, the effect of the type of adjuvant therapy itself remains unclear and understanding data of larger samples is warranted.
We aim to systematically detail the existing literature to estimate the prevalence of cognitive impairment and cognitive change throughout the breast cancer treatment period. In order to gain knowledge on the effect of therapy type, we compared cognitive consequences of CT with ET and/or RT and/or healthy controls over time.
2. METHODS
The present systematic review was conducted and performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. We registered the review in the International Prospective Register of Systematic Reviews (PROSPERO) to reduce reporting bias and to promote research transparency (registration number = CRD42020123312).
2.1. Eligibility criteria
We reviewed all published manuscripts on the prevalence of cognitive effects in adult patients with breast cancer during and after surgery and treatment period and healthy controls. Treatment was operationalized as CT, ET or postoperative RT. We selected longitudinal studies in which; (i) cognitive functioning was assessed at baseline (defined at any timepoint between surgery and commencement of treatment), and at least once after treatment, (ii) neuropsychological assessment was reported in at least two cognitive domains, (iii) reported prevalence of cognitive impairment and/or cognitive decline in at least one treatment stage, (iv) standardized and valid measurements for objective cognitive functioning were used. We only included objective measurements to assess cognition, because most studies find that cognitive complaints may be more indicative of psychological distress rather than cognitive impairment. 27 , 28 One could argue, however, that the weak association between objective and subjective cognitive impairment could partly be explained because they measure different constructs. 12 We excluded manuscripts that were identified as cross‐sectional studies, case‐studies or series, meta‐analyses or commentaries. Additional reasons for study exclusion were reported childhood or central nervous system malignancies or the sole use of short cognitive screening tools (e.g., Mini‐Mental Status Examination), as they lack sensitivity to detect mild cognitive impairment. 29 For studies that included behavioral interventions, such as cognitive behavioral therapy, we only included the usual care condition in the current systematic review. To maximize study feasibility and generalization towards patients in clinic, we did not consider variation in standard of care treatment regime (e.g., number of cycles, type of CT and type of ET) as an exclusion criterion. If we suspected an overlapping patient sample among the eligible studies, we included the most recent study.30, 31, 32, 33, 34, 35 Reasons for exclusion was documented for each manuscript.
2.2. Search strategy
The literature search strategy was developed for PubMed and modified for Embase. No limits were placed on publication date. The search was performed on 20 July 2020 using the following search strategy in PubMed (OvidSP) (((((((((((("Neurocognitive Disorders"[Mesh:NoExp] OR "Cognition Disorders"[Mesh:NoExp] OR "Cognitive Dysfunction"[Mesh] OR cognit*[Title/Abstract] OR “Neuropsychological Tests”[Mesh])))) AND ((((((mamma*[Title/Abstract]) OR breast[Title/Abstract])) AND ((("Neoplasms"[Mesh] OR cancer[Title/Abstract] OR cancers[Title/Abstract] OR neoplasm*[Title/Abstract] OR tumor*[Title/Abstract] OR tumour*[Title/Abstract] OR carcinom*[Title/Abstract] OR malign*[Title/Abstract]))))) AND (((longitudinal[Title/Abstract] OR Longitudinal Studies [MeSH] OR prospective[Title/Abstract] OR Prospective studies[MeSH] OR course[Title/Abstract] OR “over time”[Title/Abstract] OR “Time Factors”[MeSH])))))))) NOT ((animals[MeSH]) NOT humans[MeSH]).
2.3. Study selection
Two authors (A. D. and H. S.) independently reviewed all abstracts, after removal of duplicates (N = 891), while assessing the inclusion and exclusion criteria. Disagreement between authors regarding study selection was resolved through consensus meetings or by the involvement of a third author (V. S.) After completing the review process, Scopus was searched with our selected articles for additional keywords or articles.
2.4. Data extraction
We originally aspired to examine the contribution of different types of systemic therapy (CT or ET) or local therapy (RT) on cognition. Because of the unexpected large imbalance of reported RT in the absence of CT (two in seven studies separately reported nonsystemic therapy) this was not deemed possible. 36 Accordingly, patients were divided into two groups; the first group consisted of patients undergoing CT, and if necessary, ET and/or postoperative RT (see Figure 1). The other patient group received ET and/or postoperative RT only (i.e., “the no‐CT group”). Where possible, we also included healthy controls. We labeled patients as cognitively impaired according to the definition criteria of the original authors (see Table 1). A timeline of the breast cancer treatment stages and the time points of data assessment is visualized in Figure 1. To aid comparability across studies, time points of neuropsychological assessment were clustered together. Baseline or T0 refers to any timepoint between surgery and commencement of CT (or comparable time interval for “the no‐CT group”). Posttreatment or T1 was indicated within 1 month of treatment completion (last chemo cycle injection), short‐term follow‐up (FU) or T2 was used within 1‐year after treatment completion (last chemo cycle injection), and long‐term FU or T3 was used for at least 1‐year after treatment completion (last chemo cycle injection). Two separate analyses were performed for the evaluation of the cognitive test data: (i) dichotomous outcome to differentiate between impaired and nonimpaired patients at different treatment stages (i.e., cognitive impairment or not) and (ii) significant changes in test scores over time irrespective of this classification (i.e., cognitive decline and/or cognitive improvement).
FIGURE 1.
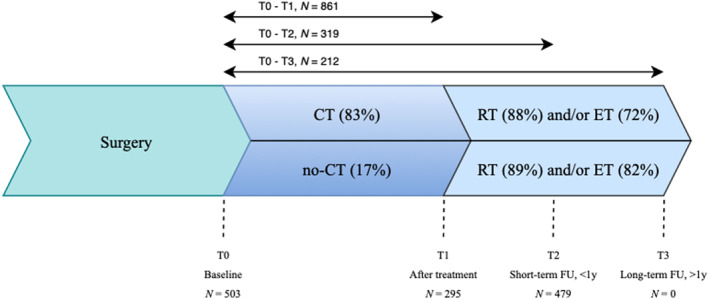
Timeline of the breast cancer treatment stages and the cross‐sectional and longitudinal time points of data assessment. The “CT‐group” consists of patients indicated for chemotherapy and if necessary, radiotherapy and/or endocrine therapy. The “no‐CT group” consists of patients indicated for radiotherapy and/or endocrine therapy. CT, chemotherapy; ET, endocrine therapy; FU, follow up; N, number of patients; RT, radiotherapy
TABLE 1.
Study characteristics included studies
| Authors | Treatment groups | Time points, T | Objective tests, N | Criteria cognitive impairment | Criteria cognitive decline | Risk of bias individual studies | Conclusion |
|---|---|---|---|---|---|---|---|
| Collins et al. (2009) 32 | CT (N = 53) | T0: before CT | 19 | N/A | Standardized regression‐based scores of −2 or less on at least two tests. 75 | Low | From T0 to T1, 34% of CT patients and 13% of no‐CT patients declined. From T0‐T3, 11% of CT patients and 10% of no‐CT patients declined. |
| No‐CT (N = 40) | T1: after CT | ||||||
| T2: 12 months FU | |||||||
| Collins et al. (2013) 3 | CT (N = 60) | T0: before CT | 17 | Scores lower than 2 SD on at least two tests. | N/A | Low | At T0, 12% of CT patients scored impaired, and at T1, 37%. |
| HC (N = 60) | T1–T6: after each CT cycle | ||||||
| Collins et al. (2014) 45 | CT (N = 56) | T0: before CT | 17 | N/A | Standardized regression‐based scores of −2 or less on at least two tests. 75 | Low | 48% of the CT patients declined. From T0‐T3, 22% declined. |
| HC (N = 56) | T1–T6: after each CT cycle | ||||||
| T7: 12 months FU | |||||||
| Debess et al. (2010) 46 | CT (N = 75) | T0: before CT | 4 | N/A | Significant changes (between 5th and 95th percentile in controls) on at least two tests. 76 | Low | From T0 to T1, 4% of CT patients and 15% of no‐CT patients declined. |
| No‐CT (N = 26) | T1: after CT | ||||||
| No‐T (N = 19) | |||||||
| HC (N = 208) a | |||||||
| Hermelink et al. (2007) 37 | CT (N = 101) | T0: before CT | 7 | Scores in the lower 5% range on two or more tests. | Reliable change index, with a probability error of 10%. 77 | Medium | At T0, 31% of CT‐patients scored impaired. From T0‐T1, 27% declined. |
| T1: after CT | |||||||
| Hermelink et al. (2017) 43 | CT (N = 91) | T0: before CT | 8 | Scores below 1.5 SD on five or more tests or lower than 2 SD on at least four tests. | N/A | Low | At T1, 6% of CT patients and 3% of no‐CT patients scored impaired. At T2, 18% of CT patients and 18% of no‐CT patients scored impaired. |
| No‐CT (N = 66) | T1: after CT | ||||||
| HC (N = 60) | T2: 6 months FU | ||||||
| Hurria et al. (2006) 38 | CT (N = 31) | T0: before CT | 10 | Scores lower than 2 SD on at least two tests. | A decrease of one or more SD on at least two tests in at least two domains. | Low | At T0, 11% of CT‐patients scored impaired and at T2, 29%. From T0‐T1, 39% declined and 11% improved. |
| T1: 6 months FU | |||||||
| Jansen et al. (2011) 27 | CT (N = 71) | T0: before CT | 7 | Scores below 1.5 SD on at least two tests or lower than 2 SD on at least one test. | Indicated on each cognitive domain by using hierarchical linear modelling. 78 | Low | At T0, 23% of CT patients scored impaired, at T1, 52%, and at T2, 20%. From T0‐T1, 29% declined and from T0 to T2, 3% declined. |
| T1: mid‐CT | |||||||
| T2: after CT | |||||||
| T3: 6 months FU | |||||||
| Jenkins et al. (2006) 47 | CT (N = 85) | T0: before CT | 8 | N/A | Reliable change index on at least two measurements. 77 | Low | From T0 to T1, 20% of CT patients and 26% of no‐CT patients declined. From T0–T3, 18% of CT‐patients and 14% of no‐CT patients declined. |
| No‐CT (N = 43) | T1: after CT | ||||||
| HC (N = 49) | T2: 12 months FU | ||||||
| Kesler et al. (2017) 39 | CT (N = 31) | T0: before CT | 4 | Scores below 1.5 SD on at least two tests or lower than 2 SD on at least one test. | N/A | Medium | At T0, 32% of the CT patients scored impaired and at T3, 55%. |
| T1: after CT | |||||||
| T2: 12 months FU | |||||||
| Mehlsen et al. (2009) 48 | CT (N = 34) | T0: before CT | 13 | N/A | Reliable change index on at least three measurements. 77 | Medium | From T0 to T1, 28% of the CT patients declined. |
| Cardio (N = 12) | T1: after CT | ||||||
| HC (N = 12) | |||||||
| Menning et al. (2016) 44 | CT (N = 31) | T0: before CT | 11 | Multivariate normative comparison with a probability error of 5%. | N/A | Low | At T2, 16% of systemic patients (CT and/or ET) and 4% of non‐systemic patients scored impaired. |
| No‐CT (N = 24) | T1: 6 months FU | ||||||
| HC (N = 33) | |||||||
| Ruzich et al. (2007) 40 | CT (N = 35) | T0: before CT | 15 | Scores below 1 SD on at least two tests. | N/A | Low | At T0, 51% of CT patients scored impaired, at T1, 31%, and at T2, 30%. |
| T1: mid‐CT | |||||||
| T2: after CT | |||||||
| T3: 6 months FU | |||||||
| Schagen et al. (2006) 36 | CT (N = 67) | T0: before CT | 10 | Scores below 2 SD on at least three tests. | Reliable change index on at least four measurements. 77 | Low | At T0, 16% of systemic patients (CT and/or ET) and 30% of non‐systemic patients scored impaired. At T2, 15% and 23% scored impaired, respectively. From T0 to T2, 18% of systemic patients and 18% of nonsystemic patients declined. |
| No‐CT (N = 57) | T1: 6 months FU | ||||||
| HC (N = 60) | |||||||
| Schrauwen et al. 2020 49 | CT (N = 69) | T0: before CT | 5 | N/A | Standardized difference score exceededing ‐2.5 SD on at least one test. | Low | From T0 to T1, 24% of CT patients, 15% of no‐CT patients declined, and 7% of healthy controls declined. |
| No‐CT (N = 41) | T1: after CT | ||||||
| HC (N = 56) | |||||||
| Vearncombe et al. (2009) 79 | CT (N = 136) | T0: before CT | 10 | N/A | Reliable change index on at least two measurements. 77 | Low | From T0 to T1, 17% of CT patients declined. |
| T1: after CT | |||||||
| Wefel et al. (2010) 26 | CT (N = 42) | T0: before CT | 6 | Scores below 2 SD on at least one test. | Reliable change index. 77 | Low | At T0, 21% of CT patients scored impaired. From T0 to T2, 65% declined and from T0 to T3, 61% declined. |
| T1: mid‐CT | |||||||
| T2: after CT | |||||||
| T3: 12 months FU |
Note: The “CT‐group” consists of patients indicated for chemotherapy and if necessary, radiotherapy and/or endocrine therapy. The “no‐CT group” consists of patients indicated for radiotherapy and/or endocrine therapy.
Abbreviations: CT, chemotherapy; FU, follow‐up; HC, healthy control group; N, sample size; NA, not assessed; no‐CT, endocrine therapy and/or radiation therapy.
The authors did not report the prevalence of cognitive decline or cognitive improvement for the healthy controls.
2.5. Risk of bias in individual studies and across studies
The quality of studies was independently assessed by two reviewers (A. D. and H. S.) using a 17‐item predefined checklist adapted from Pullens et al. 12 For each item on the checklist studies could be assigned 0 (criteria not met or insufficient information) or 1 point. Disagreement between authors regarding development and evaluation of the criteria was resolved through consensus meetings or by the involvement of a third author (V. S.). Studies scoring 70% or more of the maximum score (12 or more points) were labeled as “high quality.” Studies scoring between 50% and 70% were considered to be “moderate quality” (between 9 and 12 points). Studies scoring below 50% were categorized as “low quality” (8 or less points). See Table 1 for the risk of bias in individual studies and Figure 2 for the risk of bias across studies.
FIGURE 2.
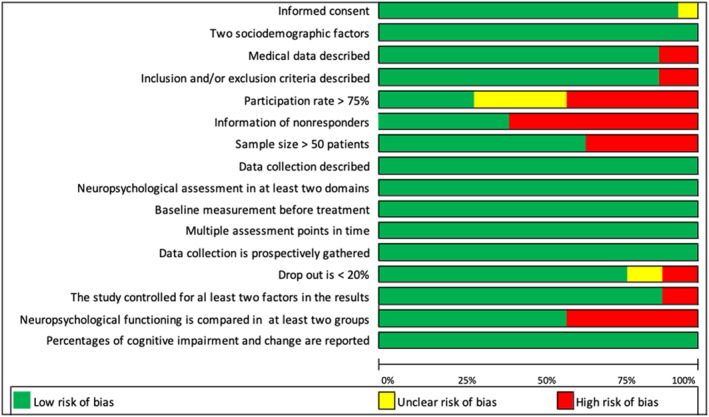
Assessment of risk of the risk of bias across studies
3. RESULTS
3.1. Study selection
The initial search in Medline and Embase yielded 1194 articles. After removal of duplicates, the titles and abstracts of 891 articles remained. The title and abstract screening resulted in the identification of 90 articles as potentially relevant while applying the eligibility criteria. After full‐text examination 17 studies were included for review. The PRISMA flow diagram of the selection process is provided in Figure 3. Risk of bias of included studies indicated high quality for 15 studies and moderate quality for 3 studies (Table 1). The scope through Scopus did not provide further keywords or articles. Table 1 provides an overview of the study characteristics of the 17 included studies at baseline.
FIGURE 3.
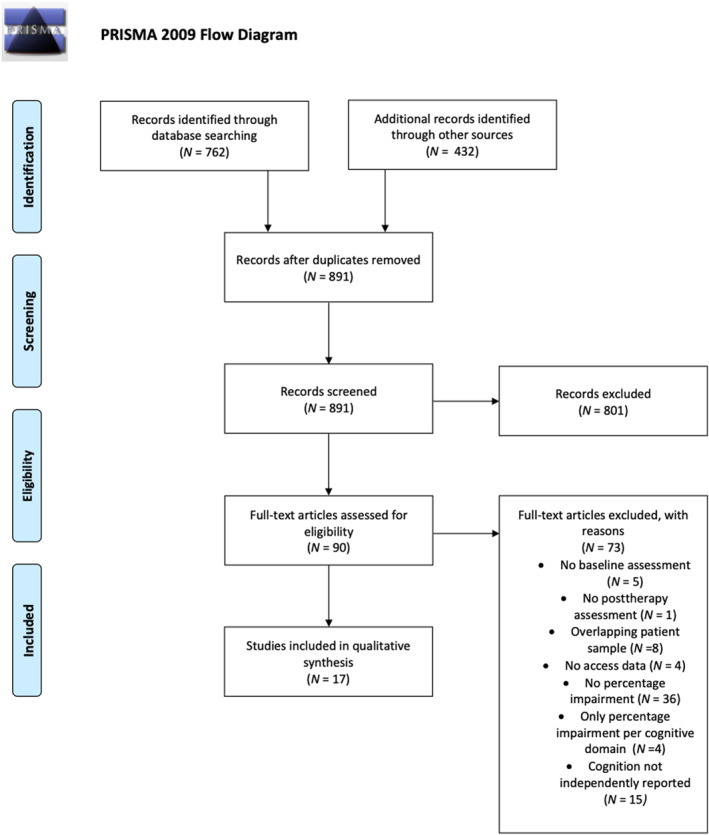
PRISMA (2009) flowchart of study selection
3.2. Prevalence of cognitive impairment at different timepoints before and during treatment period
Ten studies provided prevalence statistics of cognitive impairment at different timepoints before (T0) and throughout breast cancer treatment stages (T1 and T2) in a cross‐sectional analysis as shown in Table 2. At T0, Cognitive impairment was noted in 25% (125 of 503) of the breast cancer patients and 10% (12 of 120) of the healthy controls at T0. 3 , 26 , 27 , 36, 37, 38, 39, 40 Within the patient group, 24% (108 of 446) of the CT patients were labeled as cognitively impaired,, , , , , compared to 30% (17 of 57) of the no‐CT patients., At T1, 24% (70 of 295) of the breast cancer patients and 10% (10 of 102) of the healthy controls scored cognitively impaired. 3 , 27 , 40 , 43 Within the patient group, 30% (68 of 229) of the CT patients were labeled as cognitively impaired compared to 3% (2 of 66) of the no‐CT patients. At T2, 21% (102 of 479) of the breast cancer patients and 7% (10 of 149) of the healthy controls scored cognitively impaired. 27 , 36 , 38, 39, 40 , 43 , 44 Within the patient group, 23% (77 of 336) of the CT patients were labeled as cognitively impaired compared to 18% (25 of 143) of the no‐CT patients. Two studies have separately reported nonsystemic (e.g., RT) treatment showing that 17% (14 of 81) of nonsystemic patients scored cognitively impaired. 36 , 44 None of the studies examined cognitive impairment after at least 1‐year after treatment completion.
TABLE 2.
Overview of prevalence of cognitive impairment at different breast cancer treatment stages
| Treatment stage | Prevalence of cognitive impairments (total sum of patients) | Treatment group | Prevalence of cognitive impairments (total sum of patients per treatment group) |
|---|---|---|---|
| Baseline (T0) | 25% (503) | CT | 24% (446) |
| No‐CT | 30% (57) | ||
| Controls | 10% (120) | ||
| After treatment (T1) | 24% (295) | CT | 30% (229) |
| No‐CT | 3% (66) | ||
| Controls | 10% (102) | ||
| Short‐term FU (T2) | 21% (479) | CT | 23% (336) |
| No‐CT | 18% (143) | ||
| Controls | 7% (149) |
Note: The “CT‐group” consists of patients indicated for chemotherapy and if necessary, radiotherapy and/or endocrine therapy. The “no‐CT grou” consists of patients indicated for radiotherapy and/or endocrine therapy.
Abbreviations: CT, chemotherapy; long‐term FU, follow‐up of at least 1 year after treatment completion; short‐term FU, follow‐up of a maximum of 1 year after treatment completion, no‐CT, endocrine and/or radiotherapy.
3.3. Prevalence of cognitive change before and after treatment period
In order to inquire into the immediate effect (T1 compared to T0) of type of treatment on cognitive change, ten studies described cognitive decline of which six studies also described cognitive improvement as shown in Table 3. From T0 to T1, cognitive decline was noted in 24% (205 of 861) of the breast cancer patients and 12% (20 of 171) of the healthy controls. 26 , 27 , 32 , 43 , 45, 46, 47, 48, 49 Within the patient group, 25% (179 of 713) of the CT patients were labeled as cognitively declined, compared to 18% (26 of 148) of the no‐CT patients. Simultaneously, 15% (55 of 527) of the breast cancer patients and 12% (14 of 115) of the healthy controls were labeled as cognitively improved.32, 43, 46, 47, 48 Within the patient group, 18% (88 of 418) of the CT patients were labeled as cognitively improved, compared to 11% (12 of 109) of the no‐CT patients.
TABLE 3.
Overview of prevalence of cognitive change at different breast cancer treatment stages
| Treatment stage | Prevalence of cognitive change (total sum of patients) | Treatment group | Prevalence of cognitive change (total sum of patients per treatment group) | ||
|---|---|---|---|---|---|
| Improvement | Decline | Improvement | Decline | ||
| After treatment (T1) compared with baseline (T0) | 15% (527) | 24% (861) | CT | 18% (418) | 25% (713) |
| No‐CT | 11% (109) | 18% (148) | |||
| Controls | 12% (115) | 12% (171) | |||
| Short‐term FU (T2) compared with baseline (T0) | 14% (195) | 15% (319) | CT | 16% (155) | 14% (222) |
| No‐CT | 5% (40) | 15% (97) | |||
| Controls | – | – | |||
| Long‐term FU (T3) compared with baseline (T0) | 31% (128) | 24% (212) | CT | 32% (85) | 27% (169) |
| No‐CT | 30% (43) | 14% (43) | |||
| Controls | 17% (54) | 8% (103) | |||
Note: The “CT‐group” consists of patients indicated for chemotherapy and if necessary, radiotherapy and/or endocrine therapy. The “no‐CT group” consists of patients indicated for radiotherapy and/or endocrine therapy.
Abbreviations: CT, chemotherapy; long‐term FU, follow‐up of at least 1 year after treatment completion; short‐term FU, follow‐up of a maximum of 1 year after treatment completion; no‐CT, endocrine and/or radiotherapy.
3.4. Prevalence of cognitive change before treatment and FU period at 6 months
To infer the short‐term outcome effect (T2 at 6‐months FU compared to T0) of type of treatment on cognitive change, four studies described cognitive decline of which one study also described cognitive improvement as shown in Table 3. None of the studies included healthy controls. From T0–T2, cognitive decline was noted in 15% (46 of 319) of the breast cancer patients. 27 , 32 , 36 , 38 Within this group, 14% (32 of 222) of the CT‐patients were labeled as cognitively declined compared to 15% (14 of 97) of the no‐CT patients. Simultaneously, 14% (28 of 195) of the breast cancer patients were labeled as cognitively improved. 27 , 32 , 38 Within this group, 16% (25 of 155) of the CT‐patients were labeled as cognitively improved, compared to 5% (2 of 40) of the no‐CT patients.
3.5. Prevalence of cognitive change before treatment and FU period from at least 1 year
To calculate the long‐term outcome effect (T3 at minimum 1‐year FU compared to T0) of type of treatment on cognitive change, four studies provided statistics on cognitive decline of which one study also described cognitive improvement as shown in Table 3. From T0 to T3, cognitive decline was noted in 24% (51 of 212) of the breast cancer patients and 8% (8 of 103) of the healthy controls. 26 , 45 , 47 Within the patient group, 27% (45 of 169) of the CT‐patients were labeled as cognitively declined, compared to 14% (6 of 43) of the no‐CT patients. Simultaneously, 31% (40 of 128) of the breast cancer patients were labeled as cognitively improved. 47 Within this group, 32% (27 of 85) of the CT‐patients were labeled as cognitively improved, compared to 30% (13 of 43) of the no‐CT patients.
4. DISCUSSION
The aim of the current study was to investigate the cognitive impairments throughout the cancer treatment period by systematically reviewing the data. First, our review reveals that a significant number of patients show cognitive impairment before, during and after treatment of breast cancer. Three main findings can be extracted from the results. First of all, the prevalence of cognitive impairments ranges from 25% prior to therapy (10% for healthy controls), 24% after therapy (10% for healthy controls) to 21% at short‐term FU (7% for healthy controls). Our findings suggest that one in four patients with breast cancer are already cognitively impaired before initiation of treatment (CT and/or ET and/or RT), therefore suggesting that cognitive impairment is not exclusively related to (nonsurgical) treatment. Second, the onset of cognitive decline and improvement varies between patients. That is, a significant number of patients show cognitive decline during the course of the disease while some studies also report that other patients show cognitive improvement. When patients are compared to their pretreatment level of cognitive functioning, 24% demonstrated cognitive decline after completion of therapy and 24% at long‐term FU. Simultaneously, even though it is based on fewer studies, a significant number of patients seem to improve (15% after therapy completion, 31% at long‐term FU). Although the percentages of impairments remain largely stable over the course of the disease, it can be assumed that there is a great variation, in that some patients become worse over time while others improve. Third, in order to gain more understanding of the effect of treatment and changes within patients during treatment of breast cancer, we compared cognitive consequences of patients undergoing CT (plus or minus ET and/or RT) versus patients undergoing ET and/or RT (i.e., “the no‐CT group”) and healthy controls over time. Our data suggests that CT might impact cognitive dysfunction more heavily compared to ET and/or RT (25% and 18% after treatment completion, 27% and 14% at long‐term FU, respectively). At similar time intervals 12% and 8% of the healthy controls demonstrated cognitive decline. Only two studies have separately reported nonsystemic breast cancer treatment (mostly RT) showing that 14% of patients scored cognitively impaired at short‐term FU.
For a long time, research has focused on the effect of CT on cognition, as emphasized by the commonly used terms “chemobrain” and “chemofog”.3, 4, 5, 6 Our results indicate that CT plays a role in the course of cognitive impairment and that patients who are scheduled to undergo CT more often score impaired on cognitive tests during and after treatment period. Compelling evidence for the association between CT and reduced cognitive functioning is derived from randomized control studies demonstrating a dose‐response relationship. 3 , 4 There are several possible mechanisms through which CT can result in reduced cognitive functioning. Preclinical animal models have shown that delayed effects of chemotherapeutic agents include diminished proliferation and apoptosis in myelinated tracts. Evidence from neuroimaging studies has correlated CT with cerebral changes in terms of gray matter volume loss, reduced white matter integrity and altered cerebral network organization, cortical calcification and decreased metabolic activity.50, 51, 52, 53, 54 Although most studies focus on cognitive functioning in the first years after CT, outcomes over longer survival periods are starting to accumulate as well. Of note, up to 21 years after CT treatment microstructural white matter alternations and smaller overall brain volume have been found. 24 , 55
Nonetheless, our results suggest that one in four patients already have cognitive impairment prior to the initiation of (nonsurgical) treatment suggesting that cancer (related) and personal factors make a significant contribution to cognitive functioning. In contrast to our study, some studies have assessed their patients prior to surgery to retrieve a more reflective baseline performance. 39 , 56 , 57 Sato et al. 57 found that the breast cancer patients showed a decreased learning effect (2 days after surgery compared to 1 day before surgery) on the attention domain task compared to controls, even after controlling for pre‐existing cognitive impairment, systemic inflammation and the occurrence of complications. However, recent evidence shows that patients diagnosed with cancer may already perform lower than expected on cognitive tests prior to surgical treatment. 58 Although these patients have been confronted with cancer diagnosis, cognitive impairment persists after statistical correction for fatigue and psychological distress. 58 This suggests that risk factors for both cancer and cognitive impairment, such as genetic susceptibility, older age and lifestyle might be implicated. 28 , 43 , 49 , 59, 60, 61, 62 The study of van der Willik et al. 58 evaluated the longitudinal change of cognitive function prior to disease manifestation using a population‐based cohort that consisted of 2059 cancer patients and 7403 control subjects. They found no evidence that cognitive function declines differently over time among individuals who are later diagnosed with cancer than among individuals who remain cancer free. They conclude that the role of shared risk factors (genetic susceptibility, older age and life factors) is limited in the weeks directly preceding cancer diagnosis. In addition, the cancer growth itself might cause cognitive impairment, for instance, through vascular processes or inflammation. 63 , 64 Olson and Marks 65 have put forward a model of putative mechanisms by which peripheral tumors interface with the CNS to initiate and sustain cognitive decline. In short, they argue that peripheral cancers are associated with increased circulating inflammatory cytokines that can enter the blood stream and interface with the brain by passing the blood–brain barrier. After crossing the blood–brain barrier the inflammatory cytokines can activate glial cells and antigen‐presenting cells therefore promoting further inflammatory responses. The constant production of cytokines will ultimately inflect pathological structural and biochemical changes in neuron populations central to cognitive function.
In addition, our results show that treatment other than CT (the “no‐CT‐group”) may hamper the cognitive functions. Aside from chemo‐induced toxicity, inflammatory processes and hormonal changes are expected to play a role in the development of cognitive dysfunction. 6 , 59 , 66 , 67 Studies indicate that estrogen acts on the brain and influences cognitive performance, as data to suggests that anti‐estrogen treatments may themselves have adverse effects on cognition.68, 69, 70, 71, 72, 73 Less is known about the effect of localized RT on the development of cognitive impairment in breast cancer. Because RT is a non‐systemic therapy one might hypothesize that chances of developing cognitive impairment might be lower. It remains difficult to disentangle the unique effects of CT, ET, and RT as many patients received multiple treatments throughout disease course. However, receiving CT seem to result in more cognitive impairments during the disease and more cognitive decline, especially in long‐term FU.
4.1. Study limitations
This is the first study to systematically review the prevalence of cognitive impairment and change over time in patients with breast cancer undergoing CT compared to patients scheduled for ET and/or RT and to also include healthy controls. Because we combined cross‐sectional and longitudinal analyses, we were able to detail the prevalence of cognitive impairment at different treatment stages and investigate how many patients declined at different time points compared to their own pretreatment level of cognitive functioning. Our systematic review has some limitations. First of all, as we have included studies based on a predefined list of criteria which may or may not have been used or reported by previous studies, we cannot rule out any selection bias. Second, because the neuropsychological tests are mostly developed and used to detect major cognitive deficits in patients with acquired brain injury, our results might be underrepresenting the number and degree of cognitive problems in patients with breast cancer. Third, with our study setup we only had access to reported data on cognitive change in comparison to baseline cognitive functioning, therefore we were not able to detect subgroups of clinical patients, such as late‐onset cognitive decliners. The quantification of any variance between the treatment stages was beyond the scope of this review but would be important in identifying modifiable treatment factors to protect cognitive function. Fourth, with a median FU period of 6 months and a maximum FU period of 13 months we gained limited insight into the long‐term effects of cognitive impairment. This is of particularly importance since cognitive impairment is prevalent and since breast cancer patients have excellent long‐term survival rates. 74 Fifth, our review did not have access to the original data and we therefore we labeled patients as cognitively impaired or declined according to the definition criteria of the original authors. The included studies may have used different criteria for cognitive impairment and cognitive decline (numbers of) neuropsychological tests and time points of assessment, and solutions to account for repeated testing, therefore leading to data heterogeneity. As a result, this data serves as a first indication of prevalence of cognitive impairment and change in patients with breast cancer and cannot be blindly generalized to specific therapies or individual outcome. Finally, the quantification of affected cognitive domains was beyond the scope of this study but it would be important to identify cognitive domains that might be vulnerable to breast cancer treatment regimes.
5. CONCLUSION
This is the first study to systematically review the prevalence of cognitive impairment and cognitive change in breast cancer patients prior to CT or ”no‐CT” treatment and healthy controls. Our study revealed that one in four patients already demonstrates cognitive deficits prior to breast cancer treatment initiation of CT or ET and/or RT at comparable time intervals. Therefore, our data suggests that cognitive impairment is not exclusively related to CT or no‐CT treatment. Second, a significant number of patients show cognitive decline during the disease course (24% after therapy, 24% at 1‐year FU). Third, our data suggests that CT might impact cognitive functioning more heavily compared to ET and/or RT. In conclusion, this study shows that assessment of cognitive functioning, ideally over time, in breast cancer patients undergoing treatment is crucial. Active screening may serve as a first step to identifying and supporting patients with potential decline, thereby implementing patient‐tailored cognitive rehabilitation pathways. FU care for cognitive problems can include referral to a rehabilitation physician for treatment such as cognitive rehabilitation. To enable comparability between studies a more homogenous approach in test selection, data analyzation and reporting is warranted.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
Supporting information
Supplementary Material
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. http://doi.wiley.com/10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Bodai B. Breast cancer survivorship: a comprehensive review of long‐term medical issues and lifestyle recommendations. Perm J. 2015;19(2):48‐79. http://www.thepermanentejournal.org/issues/2015/spring/5831-breast-cancer.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins B, MacKenzie J, Tasca GA, Scherling C, Smith A. Cognitive effects of chemotherapy in breast cancer patients: a dose‐response study. Psychooncology. 2013;22(7):1517‐1527. http://doi.wiley.com/10.1002/pon.3163 [DOI] [PubMed] [Google Scholar]
- 4. van Dam FSAM, Boogerd W, Schagen SB, et al. Impairment of cognitive function in women receiving adjuvant treatment for high‐risk breast cancer: high‐dose versus standard‐dose chemotherapy. J Natl Cancer Inst. 1998;90(3):210‐218. https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/90.3.210 [DOI] [PubMed] [Google Scholar]
- 5. O’Farrell E, Smith A, Collins B. Objective‐subjective disparity in cancer‐related cognitive impairment: does the use of change measures help reconcile the difference? Psychooncology. 2017;26(10):1667‐1674. http://doi.wiley.com/10.1002/pon.4190 [DOI] [PubMed] [Google Scholar]
- 6. Wefel JS, Schagen SB. Chemotherapy‐related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12(3):267‐275. http://link.springer.com/10.1007/s11910-012-0264-9 [DOI] [PubMed] [Google Scholar]
- 7. Bolton G, Isaacs A. Women’s experiences of cancer‐related cognitive impairment, its impact on daily life and care received for it following treatment for breast cancer. Psychol Health Med. 2018;23(10):1261‐1274. 10.1080/13548506.2018.1500023 [DOI] [PubMed] [Google Scholar]
- 8. Henderson FME, Cross AJ, Baraniak AR. ‘A new normal with chemobrain’: experiences of the impact of chemotherapy‐related cognitive deficits in long‐term breast cancer survivors. Health Psychol Open. 2019;6(1):205510291983223. http://journals.sagepub.com/doi/10.1177/2055102919832234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jim HSL, Phillips KM, Chait S, et al. Meta‐analysis of cognitive functioning in breast cancer survivors previously treated with standard‐dose chemotherapy. J Clin Oncol. 2012;30(29):3578‐3587. http://ascopubs.org/doi/10.1200/JCO.2011.39.5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park BW, Hwang SY. Unmet needs and their relationship with quality of life among women with recurrent breast cancer. J Breast Cancer. 2012;15(4):454‐461. https://ejbc.kr/DOIx.php?id=10.4048/jbc.2012.15.4.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sleight AG, Duker LIS. Toward a broader role for occupational therapy in supportive oncology care. Am J Occup Ther. 2016;70(4):7004360030p1. http://ajot.aota.org/article.aspx?doi=10.5014/ajot.2016.018101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pullens MJJ, De Vries J, Roukema Ja. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology. 2010;19(11):1127‐1138. http://doi.wiley.com/10.1002/pon.1673 [DOI] [PubMed] [Google Scholar]
- 13. Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A meta‐analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006;20(1):76‐89. http://www.tandfonline.com/doi/abs/10.1080/138540491005875 [DOI] [PubMed] [Google Scholar]
- 14. Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips K‐A. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta‐analysis of the current literature. Brain Cogn. 2005;59(1):60‐70. https://linkinghub.elsevier.com/retrieve/pii/S0278262605000667 [DOI] [PubMed] [Google Scholar]
- 15. Bernstein LJ, McCreath GA, Komeylian Z, Rich JB. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: a multilevel meta‐analysis. Neurosci Biobehav Rev. 2017;83(October):417‐428. 10.1016/j.neubiorev.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 16. Bakoyiannis I, Tsigka E‐A, Perrea D, Pergialiotis V. The impact of endocrine therapy on cognitive functions of breast cancer patients: a systematic review. Clin Drug Investig. 2016;36(2):109‐118. http://link.springer.com/10.1007/s40261-015-0364-9 [DOI] [PubMed] [Google Scholar]
- 17. Lee PE, Tierney MC, Wu W, Pritchard KI, Rochon PA. Endocrine treatment‐associated cognitive impairment in breast cancer survivors: evidence from published studies. Breast Cancer Res Treat. 2016;158(3):407‐420. http://link.springer.com/10.1007/s10549-016-3906-9 [DOI] [PubMed] [Google Scholar]
- 18. Shibayama O, Yoshiuchi K, Inagaki M, et al. Association between adjuvant regional radiotherapy and cognitive function in breast cancer patients treated with conservation therapy. Cancer Med 2014;3(3):702‐709. https://onlinelibrary.wiley.com/doi/10.1002/cam4.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noal S, Levy C, Hardouin A, et al. One‐year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J Radiat Oncol. 2011;81(3):795‐803. https://linkinghub.elsevier.com/retrieve/pii/S0360301610008886 [DOI] [PubMed] [Google Scholar]
- 20. Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res Treat. 2009;116(1):113‐123. http://link.springer.com/10.1007/s10549-008-0114-2 [DOI] [PubMed] [Google Scholar]
- 21. Phillips KM, Jim HS, Small BJ, Laronga C, Andrykowski MA, Jacobsen PB. Cognitive functioning after cancer treatment. Cancer. 2012;118(7):1925‐1932. http://doi.wiley.com/10.1002/cncr.26432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahles TA. Neuropsychologic impact of standard‐dose systemic chemotherapy in long‐term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20(2):485‐493. http://www.jco.org/cgi/doi/10.1200/JCO.20.2.485 [DOI] [PubMed] [Google Scholar]
- 23. Koppelmans V, Breteler MMBB, Boogerd W, et al. Cognitive functions in long‐term survivors of ovarian cancer. J Pain Symptom Manage. 2012;50(2):830‐841. http://linkinghub.elsevier.com/retrieve/pii/S0885392415004510 [Google Scholar]
- 24. de Ruiter MB, Reneman L, Boogerd W, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32(8):1206‐1219. http://doi.wiley.com/10.1002/hbm.21102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamada TH, Denburg NL, Beglinger LJ, Schultz SK. Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22(1):48‐54. http://psychiatryonline.org/doi/abs/10.1176/jnp.2010.22.1.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer Cancer. 2010;116:3348‐3356. http://doi.wiley.com/10.1002/cncr.25098 [DOI] [PubMed] [Google Scholar]
- 27. Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy‐induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19(10):1647‐1656. http://link.springer.com/10.1007/s00520-010-0997-4 [DOI] [PubMed] [Google Scholar]
- 28. Janelsins MC, Heckler CE, Peppone LJ, et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age‐matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol. 2017;35(5):506‐514. http://ascopubs.org/doi/10.1200/JCO.2016.68.5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tombaugh TN, McIntyre NJ. The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922‐935. http://doi.wiley.com/10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- 30. Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard‐dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292‐2299. http://doi.wiley.com/10.1002/cncr.20272 [DOI] [PubMed] [Google Scholar]
- 31. Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D. The effects of adjuvant chemotherapy on cognition in women with breast cancer—preliminary results of an observational longitudinal study. The Breast. 2005;14(2):142‐150. https://linkinghub.elsevier.com/retrieve/pii/S0960977604002152 [DOI] [PubMed] [Google Scholar]
- 32. Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post‐menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18(2):134‐143. http://doi.wiley.com/10.1002/pon.1379 [DOI] [PubMed] [Google Scholar]
- 33. Stewart A, Collins B, Mackenzie J, Tomiak E, Verma S, Bielajew C. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 2008;17(2):122‐130. http://doi.wiley.com/10.1002/pon.1210 [DOI] [PubMed] [Google Scholar]
- 34. Jansen CE, Dodd MJ, Miaskowski CA, Dowling GA, Kramer J. Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology. 2008;17:1189‐1195. http://doi.wiley.com/10.1002/pon.1342 [DOI] [PubMed] [Google Scholar]
- 35. Schilder CM, Seynaeve C, Linn SC, et al. The impact of different definitions and reference groups on the prevalence of cognitive impairment: a study in postmenopausal breast cancer patients before the start of adjuvant systemic therapy. Psychooncology. 2010;19(4):415‐422. http://doi.wiley.com/10.1002/pon.1595 [DOI] [PubMed] [Google Scholar]
- 36. Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FSAM. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98(23):1742‐1745. http://academic.oup.com/jnci/article/98/23/1742/2521918/Change-in-Cognitive-Function-After-Chemotherapy-a [DOI] [PubMed] [Google Scholar]
- 37. Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer Cancer. 2007;109(9):1905‐1913. http://doi.wiley.com/10.1002/cncr.22610 [DOI] [PubMed] [Google Scholar]
- 38. Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54(6):925‐931. http://doi.wiley.com/10.1111/j.1532-5415.2006.00732.x [DOI] [PubMed] [Google Scholar]
- 39. Kesler SR, Rao A, Blayney DW, Oakley‐Girvan IA, Karuturi M, Palesh O. Predicting long‐term cognitive outcome following breast cancer with pre‐treatment resting state fMRI and random forest machine learning. Front Hum Neurosci. 2017;11:1‐10. http://journal.frontiersin.org/article/10.3389/fnhum.2017.00555/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ruzich M, Ryan B, Owen C, Delahunty A, Stuart‐harris R. Prospective evaluation of cognitive function in patients with early breast cancer receiving adjuvant chemotherapy. Asia Pac J Clin Oncol. 2007;3(3):125‐133. http://doi.wiley.com/10.1111/j.1743-7563.2007.00109.x [Google Scholar]
- 41. Hurria A, Lachs M. Is cognitive dysfunction a complication of adjuvant chemotherapy in the older patient with breast cancer? Breast Cancer Res Treat. 2007;103(3):259‐268. http://link.springer.com/10.1007/s10549-006-9383-9 [DOI] [PubMed] [Google Scholar]
- 42. Schagen SB. Late effects of adjuvant chemotherapy on cognitive function: a follow‐up study in breast cancer patients. Ann Oncol. 2002;13(9):1387‐1397. https://academic.oup.com/annonc/article-lookup/doi/10.1093/annonc/mdf241 [DOI] [PubMed] [Google Scholar]
- 43. Hermelink K, Bühner M, Sckopke P, et al. Chemotherapy and post‐traumatic stress in the causation of cognitive dysfunction in breast cancer patients. J Natl Cancer Ins. 2017;109:1‐15. https://academic.oup.com/jnci/article/doi/10.1093/jnci/djx057/3795524 [DOI] [PubMed] [Google Scholar]
- 44. Menning S, de Ruiter MB, Kieffer JM, et al. Cognitive impairment in a subset of breast cancer patients after systemic therapy—results from a longitudinal study. J Pain Symptom Manage. 2016;52(4):560‐569.e1. https://linkinghub.elsevier.com/retrieve/pii/S0885392416303013 [DOI] [PubMed] [Google Scholar]
- 45. Collins B, Mackenzie J, Tasca GA, Scherling C, Smith A. Persistent cognitive changes in breast cancer patients 1 year following completion of chemotherapy. J Int Neuropsychol Soc. 2014;20(2):370‐379. http://www.journals.cambridge.org/abstract_S1355617713001215 [DOI] [PubMed] [Google Scholar]
- 46. Debess J, Riis JØ, Engebjerg MC, Ewertz M. Cognitive function after adjuvant treatment for early breast cancer: a population‐based longitudinal study. Breast Cancer Res Treat. 2010;121(1):91‐100. http://link.springer.com/10.1007/s10549-010-0756-8 [DOI] [PubMed] [Google Scholar]
- 47. Jenkins V, Shilling V, Deutsch G, et al. A 3‐year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94(6):828‐834. http://www.nature.com/articles/6603029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mehlsen M, Pedersen AD, Jensen AB, Zachariae R. No indications of cognitive side‐effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psychooncology. 2009;18(3):248‐257. http://doi.wiley.com/10.1002/pon.1398 [DOI] [PubMed] [Google Scholar]
- 49. Schrauwen W, Van de Cavey J, Vingerhoets G, Vanheule S, Van den Broecke R, Denys H. Heterogeneous response of chemotherapy‐related cognitive decline in patients with breast cancer: a prospective study. J Int Neuropsychol Soc. 2020;26(8):806‐814. https://www.cambridge.org/core/product/identifier/S1355617720000296/type/journal_article [DOI] [PubMed] [Google Scholar]
- 50. McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30(20):2500‐2508. http://ascopubs.org/doi/10.1200/JCO.2011.38.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30:S117‐25. https://linkinghub.elsevier.com/retrieve/pii/S0889159112001146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Ruiter MB, Reneman L, Boogerd W, et al. Late effects of high‐dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33(12):2971‐2983. http://doi.wiley.com/10.1002/hbm.21422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koppelmans V, Breteler MMB, Boogerd W, Seynaeve C, Schagen SB. Late effects of adjuvant chemotherapy for adult onset non‐CNS cancer; cognitive impairment, brain structure and risk of dementia. Crit Rev Oncol Hematol. 2013;88(1):87‐101. https://linkinghub.elsevier.com/retrieve/pii/S1040842813000826 [DOI] [PubMed] [Google Scholar]
- 54. Bruno J, Hosseini SMH, Kesler S. Altered resting state functional brain network topology in chemotherapy‐treated breast cancer survivors. Neurobiol Dis. 2012;48(3):329‐338. https://linkinghub.elsevier.com/retrieve/pii/S0969996112002501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koppelmans V, Breteler MMB, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30(10):1080‐1086. http://ascopubs.org/doi/10.1200/JCO.2011.37.0189 [DOI] [PubMed] [Google Scholar]
- 56. Yao C, Bernstein LJ, Rich JB. Executive functioning impairment in women treated with chemotherapy for breast cancer: a systematic review. Breast Cancer Res Treat. 2017;166(1):15‐28. http://doi.wiley.com/10.1002/pon.4351 [DOI] [PubMed] [Google Scholar]
- 57. Sato C, Sekiguchi A, Kawai M, et al. Postoperative structural brain changes and cognitive dysfunction in patients with breast cancer. PLoS One. 2015;10(11):e0140655. https://dx.plos.org/10.1371/journal.pone.0140655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van der Willik KD, Hauptmann M, Jóźwiak K, et al. Trajectories of cognitive function prior to cancer diagnosis: a population‐based study. J Natl Cancer Inst. 2020;112:480‐488. https://academic.oup.com/jnci/article/112/5/480/5566247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy‐induced cognitive changes. Nat Rev Cancer. 2007;7(3):192‐201. http://www.nature.com/articles/nrc2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Joly F, Giffard B, Rigal O, et al. Impact of cancer and its treatments on cognitive function: advances in research from the Paris International Cognition and Cancer Task Force Symposium and update since 2012. J Pain Symptom Manage. 2015;50(6):830‐841. http://linkinghub.elsevier.com/retrieve/pii/S0885392415004510 [DOI] [PubMed] [Google Scholar]
- 61. Merriman JD, Sereika SM, Brufsky AM, et al. Trajectories of self‐reported cognitive function in postmenopausal women during adjuvant systemic therapy for breast cancer. Psychooncology. 2017;26(1):44‐52. http://doi.wiley.com/10.1002/pon.4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vearncombe KJ, Rolfe M, Andrew B, Pachana NA, Wright M, Beadle G. Cognitive effects of chemotherapy‐induced menopause in breast cancer. Clin Neuropsychol. 2011;25(8):1295‐1313. http://www.tandfonline.com/doi/abs/10.1080/13854046.2011.631586 [DOI] [PubMed] [Google Scholar]
- 63. Patel SK, Wong AL, Wong FL, et al. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst. 2015;107(8):djv131. https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/djv131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lyon DE, Cohen R, Chen H, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol. 2016;301:74‐82. https://linkinghub.elsevier.com/retrieve/pii/S016557281630399X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Olson B, Marks DL. Pretreatment cancer‐related cognitive impairment—mechanisms and outlook. Cancers. 2019;11(5):1‐18. https://www.mdpi.com/2072-6694/11/5/687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nelson WL, Suls J. New approaches to understand cognitive changes associated with chemotherapy for non‐central nervous system tumors. J Pain Symptom Manage. 2013;46(5):707‐721. https://linkinghub.elsevier.com/retrieve/pii/S0885392413001085 [DOI] [PubMed] [Google Scholar]
- 67. Scherling C, Collins B, MacKenzie J, Bielajew C, Smith A. Pre‐chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an fMRI study. Front Hum Neurosci. 2011;5:122. http://journal.frontiersin.org/article/10.3389/fnhum.2011.00122/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bender CM, Paraska KK, Sereika SM, Ryan CM, Berga SL. Cognitive function and reproductive hormones in adjuvant therapy for breast cancer. J Pain Symptom Manage. 2001;21(5):407‐424. https://linkinghub.elsevier.com/retrieve/pii/S0885392401002688 [DOI] [PubMed] [Google Scholar]
- 69. Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15(5):422‐430. http://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=106155091&site=ehost-live [DOI] [PubMed] [Google Scholar]
- 70. Eberling JL, Wu C, Tong‐Turnbeaugh R, Jagust WJ. Estrogen‐ and tamoxifen‐associated effects on brain structure and function. Neuroimage. 2004;21(1):364‐371. https://linkinghub.elsevier.com/retrieve/pii/S1053811903005457 [DOI] [PubMed] [Google Scholar]
- 71. Gervais NJ, Remage‐Healey L, Starrett JR, Pollak DJ, Mong JA, Lacreuse A. Adverse effects of aromatase inhibition on the brain and behavior a nonhuman primate. J Neurosci. 2019;39(5):918‐928. http://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.0353-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16(4):248‐266. http://learnmem.cshlp.org/cgi/doi/10.1101/lm.918309 [DOI] [PubMed] [Google Scholar]
- 73. Chen X, Li J, Chen J, et al. Decision‐making impairments in breast cancer patients treated with tamoxifen. Horm Behav. 2014;66(2):449‐456. https://linkinghub.elsevier.com/retrieve/pii/S0018506X14001408 [DOI] [PubMed] [Google Scholar]
- 74. Soerjomataram I, Louwman MWJ, Ribot JG, Roukema JA, Coebergh JWW. An overview of prognostic factors for long‐term survivors of breast cancer. Breast Cancer Res Treat. 2008 22;107(3):309‐330. http://link.springer.com/10.1007/s10549-007-9556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McSweeny AJ, Naugle RI, Chelune GJ, Lüders H. “T Scores for Change”: an illustration of a regression approach to depicting change in clinical neuropsychology. Clin Neuropsychol. 1993;7(3):300‐312. http://www.tandfonline.com/doi/abs/10.1080/13854049308401901 [Google Scholar]
- 76. Temkin NR, Heaton RK, Grant I, Dikmen SS. Detecting significant change in neuropsychological test performance: a comparison of four models. J Int Neuropsychol Soc. 1999;5(4):357‐369. https://www.cambridge.org/core/product/identifier/S1355617799544068/type/journal_article [DOI] [PubMed] [Google Scholar]
- 77. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12‐9. http://doi.apa.org/getdoi.cfm?doi=10.1037/0022-006X.59.1.12 [DOI] [PubMed] [Google Scholar]
- 78. Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. https://oxford.universitypressscholarship.com/view/10.1093/acprof:oso/9780195152968.001.0001/acprof-9780195152968 [Google Scholar]
- 79. Vearncombe KJ, Rolfe M, Wright M, Pachana NA, Andrew B, Beadle G. Predictors of cognitive decline after chemotherapy in breast cancer patients. J Int Neuropsychol Soc 2009 1;15(6):951‐962. https://www.cambridge.org/core/product/identifier/S1355617709990567/type/journal_article [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


