Abstract
Objective
During PREVENT (NCT01892345), eculizumab significantly reduced relapse risk versus placebo in patients with aquaporin‐4 immunoglobulin G‐positive neuromyelitis optica spectrum disorder (AQP4‐IgG+ NMOSD). We report an interim analysis of PREVENT's ongoing open‐label extension (OLE; NCT02003144) evaluating eculizumab's long‐term safety and efficacy.
Methods
Patients who completed PREVENT could enroll in the OLE to receive eculizumab (maintenance dose = 1,200 mg/2 weeks, after a blinded induction phase). Safety and efficacy data from PREVENT and its OLE (interim data cut, July 31, 2019) were combined for this analysis.
Results
Across PREVENT and the OLE, 137 patients received eculizumab and were monitored for a median (range) of 133.3 weeks (5.1–276.9 weeks), for a combined total of 362.3 patient‐years (PY). Treatment‐related adverse event (AE) and serious adverse event (SAE) rates were 183.5 in 100 PY and 8.6 in 100 PY, respectively. Serious infection rates were 10.2 in 100 PY in eculizumab‐treated patients versus 15.1 in 100 PY in the PREVENT placebo group. No patient developed a meningococcal infection. At 192 weeks (3.7 years), 94.4% (95% confidence interval [CI], 88.6–97.3) of patients remained adjudicated relapse‐free. The adjudicated annualized relapse rate was 0.025 (95% CI = 0.013–0.048) in all eculizumab‐treated patients versus 0.350 (95% CI = 0.199–0.616) in the PREVENT placebo group. During the OLE, 37% of patients (44 of 119 patients) stopped or decreased background immunosuppressive therapy use.
Interpretation
This analysis demonstrates that eculizumab's long‐term safety profile in NMOSD is consistent with its established profile across other indications. This analysis also demonstrated the sustained ability of long‐term eculizumab treatment to reduce relapse risk in patients with AQP4‐IgG+ NMOSD. ANN NEUROL 2021;89:1088–1098
Aquaporin‐4 immunoglobulin G‐positive (AQP4‐IgG+) neuromyelitis optica spectrum disorder (NMOSD) is a rare, complement‐mediated autoimmune disease characterized by relapses that can cause significant and irreversible neurologic disability. 1 , 2 , 3 , 4 Unlike multiple sclerosis, a progressive disease course rarely occurs in NMOSD, 5 but risk of clinical relapse persists even after long periods of remission. 3 Effective and tolerable relapse‐preventing treatments are therefore required indefinitely. 6
In preclinical studies, AQP4‐IgG antibodies have been shown to trigger the complement cascade, leading to inflammation and subsequent damage to the central nervous system. 7 , 8 , 9 , 10 Eculizumab, a terminal complement protein (C5) inhibitor, reduces the risk of relapse in patients with AQP4‐IgG+ NMOSD and is the first agent approved for the treatment of this potentially life‐threatening condition; eculizumab is currently approved for this indication in Australia, Canada, Europe, Japan, and the United States. 11 , 12 , 13 , 14 In the phase III PREVENT study, eculizumab reduced the risk of adjudicated relapse by 94% compared with placebo (p < 0.0001) in patients with AQP4‐IgG+ NMOSD. 11 , 12 This benefit of eculizumab was seen across a wide range of patient subgroups based on concomitant immunosuppressive therapy (IST) and past rituximab use, geographical region, age, race, sex, disease duration, relapse history, and disability status. 15 The safety profile of eculizumab in AQP4‐IgG+ NMOSD was consistent with that seen for eculizumab in other approved indications. 11 , 14
We report an interim analysis (data cutoff date was July 31, 2019) of combined data from PREVENT and its ongoing open‐label extension (OLE) on the long‐term safety and efficacy of eculizumab in patients with AQP4‐IgG+ NMOSD.
Methods
Study Design and Participants
In PREVENT (NCT01892345), eligible patients with AQP4‐IgG+ NMOSD were randomized in a 2:1 ratio to receive either eculizumab or placebo. Study design, methodology, and inclusion and exclusion criteria for PREVENT have been reported previously. 11 Briefly, participants were adults with AQP4‐IgG+ NMOSD (2006 and 2007 disease definitions) 1 , 16 and a history of at least 2 relapses in the previous 12 months or 3 in the previous 24 months, including 1 in the past 12 months. Patients also had an Expanded Disability Status Scale (EDSS) score of 7.0 or less. Patients receiving stable dosing with ISTs (including corticosteroids, azathioprine, mycophenolate mofetil, cyclosporine, cyclophosphamide, methotrexate, mizoribine, and tacrolimus) were eligible. 11 Those previously treated with rituximab or mitoxantrone were eligible unless they had received either drug during the 3 months before screening (3 months represents approximately 5 half‐lives of rituximab).
All participants who remained in PREVENT until study end or who experienced a physician‐determined relapse during the study could enroll into the OLE (NCT02003144) and receive eculizumab for up to an additional 5.5 years. Retrospective adjudication of physician‐determined relapses has been described previously. 11 Eligible participants were required to enter the OLE within 14 ± 2 days from the last administration of study treatment in PREVENT, to avoid treatment interruptions. Participants who withdrew from PREVENT owing to adverse events (AEs) related to the study drug were not eligible to enter the OLE.
Both studies were conducted in accordance with the provisions of the Declaration of Helsinki, 17 the International Conference on Harmonization guidelines for good clinical practice, 18 and applicable regulatory requirements, and were approved by the institutional review board at each participating institution. All study participants provided written informed consent.
Study Treatments
All patients were vaccinated against Neisseria meningitidis serotypes A, C, W, and Y before treatment commenced in PREVENT, and could be revaccinated during the trial to provide active coverage, according to vaccine manufacturer or country guidelines (vaccination against serotype B was administered as required by country guidelines).
Participants randomized to eculizumab in PREVENT initiated treatment with four 900 mg once‐weekly induction doses followed by maintenance dosing of 1,200 mg once every 2 weeks, which continued into the OLE. To maintain study blinding, on entering the OLE, all participants underwent a blinded induction phase. During this phase, patients in the PREVENT eculizumab arm continued to receive eculizumab 4 vials/1,200 mg once every 2 weeks (at weeks 1 and 3) and 4 vials placebo at weeks 2 and 4. Patients in the PREVENT placebo arm transitioning to eculizumab received four 900 mg once‐weekly induction doses (3 vials of eculizumab and 1 vial of placebo) before receiving the maintenance dose of 1,200 mg once every 2 weeks at visit 5 onward.
Stable‐dose background ISTs received at screening were continued unchanged during PREVENT unless treating physicians determined that a relapse had occurred or there was a compelling medical need for adjustment. IST use could be changed during the OLE at the discretion of the physician. Medications not permitted in either study were rituximab or other biological agents, mitoxantrone, immunomodulatory therapies for multiple sclerosis (including interferon β‐1a and β‐1b, and glatiramer acetate), and intravenous (i.v.) immunoglobulin and plasma exchange for relapse prevention.
Assessments and End Points
The primary objective of the OLE was to evaluate the long‐term safety of eculizumab in patients with relapsing NMOSD, including the occurrence of AEs and serious AEs (SAEs).
Secondary objectives included the evaluation of the long‐term efficacy of eculizumab, assessed according to annualized relapse rate (ARR), EDSS score, Hauser Ambulation Index (HAI) score, modified Rankin Scale (mRS) score, and patient‐reported outcomes, including 3‐level (EQ‐5D‐3L) visual analog scale (VAS) and EQ‐5D‐3L index scores.
Relapse types and acute treatment for relapses throughout PREVENT and the OLE, and changes in IST use during the OLE were also recorded. Optic spinal impairment scale (OSIS) visual acuity (VA) scores were recorded following relapses. Although magnetic resonance imaging (MRI) was performed in many cases, both prior to study entry and at the time of relapse, it was not performed systematically for every patient or at prespecified time points.
Statistical Analysis
The data cutoff date for this interim OLE analysis was July 31, 2019. Safety analyses included all participants who had received at least one dose of eculizumab. Treatment adherence was calculated as the ratio between the actual number of eculizumab doses taken by a patient and the number of doses required. Efficacy analyses were performed using the combined PREVENT and OLE data, which included all participants who had received at least one dose of eculizumab in either study. ARR during study treatment was calculated as the total number of relapses (physician‐determined or adjudicated) for all patients divided by the number of patient‐years (PY) in the study period. Confidence intervals (CIs) for ARRs were based on a Poisson regression. Estimated proportions of relapse‐free patients were based on the Kaplan–Meier product limit method; 95% CIs were based on complementary log–log transformation.
The annualized rates of different acute relapse treatments (high‐dose oral steroids, i.v. methylprednisolone, plasma exchange, and i.v. immunoglobulin) were calculated as the total number of adjudicated relapses requiring each treatment during the study period for all patients divided by the total number of PY in the study period. Wald CIs were calculated from Poisson regression.
Changes in disability and health‐related quality of life scores from eculizumab baseline (the last available assessment before the first eculizumab dose in either PREVENT or the OLE) to each scheduled visit up to year 1 were analyzed using mixed models for repeated measures with terms for visit and baseline score.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Study Population
Patient disposition is summarized in Figure 1. In PREVENT, 143 participants were randomized: 96 received eculizumab and 47 received placebo. A total of 124 participants completed PREVENT and 119 continued into the OLE (79 reached the end of PREVENT relapse‐free and 40 entered the OLE following a relapse as determined by the treating physician) and received open‐label eculizumab (eculizumab/eculizumab, n = 78; placebo/eculizumab, n = 41); 14 participants in the OLE (11.8%) discontinued study treatment by the interim analysis data cutoff date. A total of 137 participants received eculizumab during PREVENT and/or the OLE (combined PREVENT and OLE analysis set); of these, 30 participants had discontinued by the interim analysis data cutoff date. The most common reason for study discontinuation was withdrawal by the patient.
FIGURE 1.
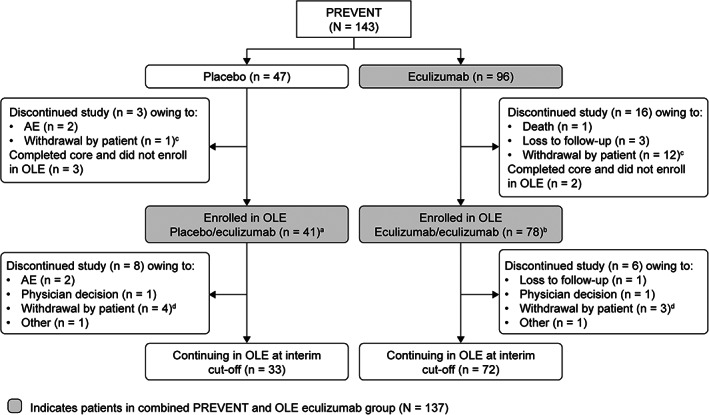
Patient disposition through July 31, 2019. aFifteen patients reached the end of PREVENT relapse‐free, and 26 patients entered the OLE following a relapse as determined by the treating physician. bSixty‐four patients reached the end of PREVENT relapse‐free, and 14 patients entered the OLE following a relapse as determined by the treating physician. cThe 13 patients who withdrew consent (patient decision) during PREVENT included 1 patient in the placebo group who did not wish to continue taking an investigational product, and 12 patients in the eculizumab group who withdrew for the following reasons: a change in life situation or moving to a different area (7 patients); unknown reasons (3 patients); ongoing AEs not related to study drug and difficult venous access (1 patient); and clinical trial fatigue (1 patient). dThe 7 patients who withdrew consent (patient decision) during the OLE included 4 in the placebo/eculizumab group for the following reasons: a change in life situation or moving to a different area (2 patients); to receive treatment with traditional Chinese medicine (1 patient); and unwillingness to continue study visits following an SAE at enrollment and 1 month on study (1 patient). Three patients in the eculizumab/eculizumab group withdrew for the following reasons: study dosing schedule and an AE described as urticaria (1 patient); inability to travel to the study site owing to back pain (1 patient); and a change in life situation (1 patient). AE = adverse event; OLE = open‐label extension; SAE = serious adverse event.
Participants’ baseline demographic and disease characteristics are presented in Table 1. The mean age at first dose for all participants receiving eculizumab was 44.5 years, most participants (90.5%) were women, and most were either White (48.9%) or Asian (36.5%). The mean historical ARR within 24 months before screening for PREVENT was 2.01.
TABLE 1.
Baseline Patient Demographics and Disease Characteristics
| Variable | PREVENT a | PREVENT OLE b | PREVENT and OLE combined c | |||
|---|---|---|---|---|---|---|
| Placebo (N = 47) | Eculizumab (N = 96) | Placebo/eculizumab d (N = 41) | Eculizumab/eculizumab (N = 78) | Eculizumab (N = 119) | Eculizumab (N = 137) | |
| Age at first dose, mean (SD), yr | 45.0 (13.3) | 43.9 (13.3) | 46.0 (13.8) | 46.6 (13.8) | 46.4 (13.7) | 44.5 (13.5) |
| Sex, n (%) | ||||||
| M | 5 (10.6) | 8 (8.3) | 5 (12.2) | 4 (5.1) | 9 (7.6) | 13 (9.5) |
| F | 42 (89.4) | 88 (91.7) | 36 (87.8) | 74 (94.9) | 110 (92.4) | 124 (90.5) |
| Region, n (%) | ||||||
| Americas | 15 (31.9) | 29 (30.2) | 13 (31.7) | 21 (26.9) | 34 (28.6) | 42 (30.7) |
| Europe | 19 (40.4) | 32 (33.3) | 17 (41.5) | 27 (34.6) | 44 (37.0) | 49 (35.8) |
| Asia‐Pacific | 13 (27.7) | 35 (36.6) | 11 (26.8) | 30 (38.5) | 41 (34.5) | 46 (33.6) |
| Race, n (%) | ||||||
| Asian | 15 (31.9) | 37 (38.5) | 13 (31.7) | 32 (41.0) | 45 (37.8) | 50 (36.5) |
| Black or African American | 8 (17.0) | 9 (9.4) | 7 (17.1) | 3 (3.8) | 10 (8.4) | 16 (11.7) |
| White | 24 (51.1) | 46 (47.9) | 21 (51.2) | 40 (51.3) | 61 (51.3) | 67 (48.9) |
| Other e | 0 (0.0) | 4 (4.2) | 0 (0.0) | 1 (1.3) | 3 (2.5) | 4 (2.9) |
| Historical ARR (within 24 mo before screening for PREVENT), mean (SD) | 2.1 (1.0) | 1.9 (0.9) | 2.2 (1.1) | 1.9 (1.0) | 2.0 (1.0) | 2.0 (1.0) |
| EDSS score | ||||||
| Median (range) | 4.0 (1.0–6.5) | 4.0 (1.0–7.0) | 4.5 (0.0–7.5) | 4.0 (0.0–7.5) | 4.0 (0.0–7.5) | 4.0 (0.0–7.5) |
| Mean (SD) | 4.3 (1.5) | 4.2 (1.6) | 4.3 (1.9) | 4.0 (1.7) | 4.1 (1.8) | 4.2 (1.7) |
| HAI score | ||||||
| Median (range) | 2.0 (0.0–6.0) | 2.0 (0.0–8.0) | 2.0 (0.0–9.0) | 1.0 (0.0–8.0) | 2.0 (0.0–9.0) | 2.0 (0.0–9.0) |
| Mean (SD) | 2.1 (1.4) | 2.4 (2.2) | 2.8 (2.1) | 2.0 (2.2) | 2.3 (2.2) | 2.5 (2.2) |
| mRS score | ||||||
| Median (range) | 2.0 (0.0–4.0) | 2.0 (0.0–4.0) | 2.0 (0.0–6.0) | 2.0 (0.0–4.0) | 2.0 (0.0–6.0) | 2.0 (0.0–6.0) |
| Mean (SD) | 2.1 (1.0) | 2.1 (1.1) | 2.4 (1.4) | 1.9 (1.3) | 2.1 (1.3) | 2.2 (1.2) |
| EQ‐5D‐3L VAS score, median (range) | 60.0 (0.0–9.5) | 70.0 (10.0–100.0) | 65.0 (5.0–100.0) | 79.0 (10.0–100.0) | 70.0 (5.0–100.0) | 68.0 (5.0–100.0) |
| EQ‐5D‐3L index score, median (range) | 0.7 (0.3–1.0) | 0.8 (0.1–1.0) | 0.6 (0.0–1.0) | 0.8 (0.0–1.0) | 0.8 (0.0–1.0) | 0.7 (0.0–1.0) |
Age at first dose and baseline scores at first study drug dose in PREVENT.
Age at first dose and baseline EDSS, HAI, and mRS scores at first study drug dose in the OLE; EQ‐5D‐3L VAS and index scores at last available assessment prior to first study drug dose in the OLE.
Age at first dose and baseline scores at first eculizumab dose in either study.
Patients receiving placebo during PREVENT transitioned to eculizumab in the OLE.
Other includes American Indian, Alaskan native, unknown, and other.
ARR = annualized relapse rate; EDSS = Expanded Disability Status Scale; EQ‐5D‐3L = 3‐level 5‐dimension EuroQol questionnaire; HAI = Hauser Ambulation Index; mRS = modified Rankin Scale; OLE = open‐label extension; SD = standard deviation; VAS = visual analog scale.
The 137 participants who received eculizumab in PREVENT and/or the OLE had a median eculizumab treatment duration of 132.1 weeks (range = 0.1–276.1 weeks), providing a combined total of 355.9 PY of eculizumab treatment.
Safety Analyses
The rates of AEs that were categorized as being related to a trial agent by investigators were similar in the PREVENT placebo group (167.5 AEs in 100 PY) and the combined PREVENT and OLE eculizumab group (183.5 AEs in 100 PY). The rates of these AEs were also similar in the PREVENT eculizumab group (212.9 AEs in 100 PY) and during the OLE (158.6 AEs in 100 PY). Similar proportions of the PREVENT placebo and the combined eculizumab groups experienced a treatment‐related AE (57.4 and 62.0%, respectively; Table 2).
TABLE 2.
AE Summary by Treatment Group
| PREVENT | PREVENT OLE | PREVENT and OLE combined | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N = 47; 53.1 PY) | Eculizumab (N = 96; 172.8 PY) | Eculizumab (N = 119; 187.3 PY) | Eculizumab (N = 137; 362.3 PY) | |||||
| Events, n rate/100 PY) | Patients, n (%) | Events, n (rate/100 PY) | Patients, n (%) | Events, n (rate/100 PY) | Patients, n (%) | Events, n (rate/100 PY) | Patients, n (%) | |
| All AEs | 622 (1170.3) | 45 (95.7) | 1307 (756.3) | 89 (92.7) | 1347 (719.2) | 107 (89.9) | 2654 (732.5) | 131 (95.6) |
| Treatment‐related | 89 (167.5) | 27 (57.4) | 368 (212.9) | 49 (51.0) | 297 (158.6) | 52 (43.7) | 665 (183.5) | 85 (62.0) |
| Leading to withdrawal | 3 (5.6) | 2 (4.3) | 0 (0.0) | 0 (0.0) | 5 (2.7) | 2 (1.7) | 5 (1.4) | 2 (1.5) |
| AEs reported in ≥ 15% of patients in the PREVENT and OLE combined eculizumab group | ||||||||
| Headache | 21 (39.5) | 11 (23.4) | 96 (55.5) | 22 (22.9) | 113 (60.3) | 24 (20.2) | 209 (57.7) | 40 (29.2) |
| Upper respiratory tract infection | 10 (18.8) | 6 (12.8) | 54 (31.2) | 28 (29.2) | 39 (20.8) | 21 (17.6) | 93 (25.7) | 38 (27.7) |
| Nasopharyngitis | 15 (28.2) | 9 (19.1) | 50 (28.9) | 20 (20.8) | 50 (26.7) | 21 (17.6) | 100 (27.6) | 36 (26.3) |
| Urinary tract infection | 13 (24.5) | 10 (21.3) | 45 (26.0) | 13 (13.5) | 48 (25.6) | 19 (16.0) | 93 (25.7) | 30 (21.9) |
| Arthralgia | 10 (18.8) | 5 (10.6) | 12 (6.9) | 11 (11.5) | 20 (10.7) | 15 (12.6) | 32 (8.8) | 25 (18.2) |
| Back pain | 9 (16.9) | 6 (12.8) | 17 (9.8) | 14 (14.6) | 28 (14.9) | 13 (10.9) | 45 (12.4) | 24 (17.5) |
| Diarrhea | 20 (37.6) | 7 (14.9) | 23 (13.3) | 15 (15.6) | 22 (11.7) | 11 (9.2) | 45 (12.4) | 24 (17.5) |
| Nausea | 19 (35.7) | 12 (25.5) | 30 (17.4) | 16 (16.7) | 18 (9.6) | 8 (6.7) | 48 (13.2) | 24 (17.5) |
| Serious AEs excluding NMOSD relapses | 29 (54.6) | 13 (27.7) | 46 (26.6) | 25 (26.0) | 62 (33.1) | 31 (26.1) | 108 (29.8) | 49 (35.8) |
| Treatment‐related | 13 (24.5) | 9 (19.1) | 13 (7.5) | 9 (9.4) | 16 (8.5) | 10 (8.4) | 29 (8.0) | 19 (13.9) |
| Leading to withdrawal | 2 (3.8) | 2 (4.3) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.8) | 1 (0.3) | 1 (0.7) |
| Deaths | – | 0 (0.0) | – | 1 (1.0) | – | 0 (0.0) | – | 1 (0.7) |
AE = adverse event; NMOSD = neuromyelitis optica spectrum disorder; OLE = open‐label extension; PY = patient‐years.
The most common AEs in the combined PREVENT and OLE eculizumab treatment group included headache (57.7 events in 100 PY; 40/137 patients), upper respiratory tract infection (25.7 events in 100 PY; 38/137 patients), nasopharyngitis (27.6 events in 100 PY; 36/137 patients), urinary tract infection (25.7 events in 100 PY; 30/137 patients), arthralgia (8.8 events in 100 PY; 25/137 patients), back pain (12.4 events in 100 PY; 24/137 patients), diarrhea (12.4 events in 100 PY; 24/137 patients), and nausea (13.2 events in 100 PY; 24/137 patients).
The rates of treatment‐related SAEs were 24.5 events in 100 PY in the PREVENT placebo group and 8.6 events in 100 PY in the combined eculizumab group. Excluding NMOSD relapses, rates of treatment‐related SAEs were 24.5 events in 100 PY in the PREVENT placebo group and 8.0 events in 100 PY in the combined eculizumab group (Table 2).
SAEs that occurred in more than 2 patients in the combined eculizumab group were pneumonia (5 patients), acute cholecystitis (4 patients), and urinary tract infection (4 patients). There was one death in the eculizumab group during PREVENT (from pulmonary empyema), 11 and no deaths occurred during the OLE through June 1, 2020.
The rates of serious infections were 15.1 events in 100 PY in the PREVENT placebo group (6/47 patients [12.8%]), 9.3 events in 100 PY in the PREVENT eculizumab group (11/96 patients [11.5%]), and 10.2 events in 100 PY in the combined eculizumab group (25/137 patients [18.2%]). Serious infections that occurred in more than one patient in the combined eculizumab group were pneumonia (5 patients), urinary tract infection (4 patients), cellulitis (2 patients), and sepsis (2 patients). During the OLE through June 1, 2020, one patient developed an infection due to Neisseria gonorrhoeae; this infection resolved with antibiotic treatment. No patient experienced a meningococcal infection through June 1, 2020.
Efficacy Analyses
Impact on Relapse Risk
By interim data cutoff, 8 patients in the combined eculizumab group had experienced 9 adjudicated relapses; 5 of these patients experienced an adjudicated relapse during the OLE (Fig 2). Of these 8 patients, 3 had previously received rituximab, and 6 experienced a relapse in the first 60 weeks of eculizumab treatment. Among these 8 patients, the median adherence to eculizumab treatment was 100.0% (range = 81.3–100.0%) during PREVENT and 96.3% (range = 82.9–100.0%) during the OLE. Optic neuritis was the most common type of relapse, accounting for 7 of 9 relapses (77.8%; 4 events were on the left side, 1 event was on the right side, and 2 events were bilateral). The other 2 relapses (2/9; 22.2%) were myelitis. Five optic neuritis events were major (highest post‐relapse OSIS VA scores, 4–7; improvement by 1–2 points by week 6 after 4 of these events), and one myelitis event was major. No further adjudicated relapses have been reported through June 1, 2020.
FIGURE 2.
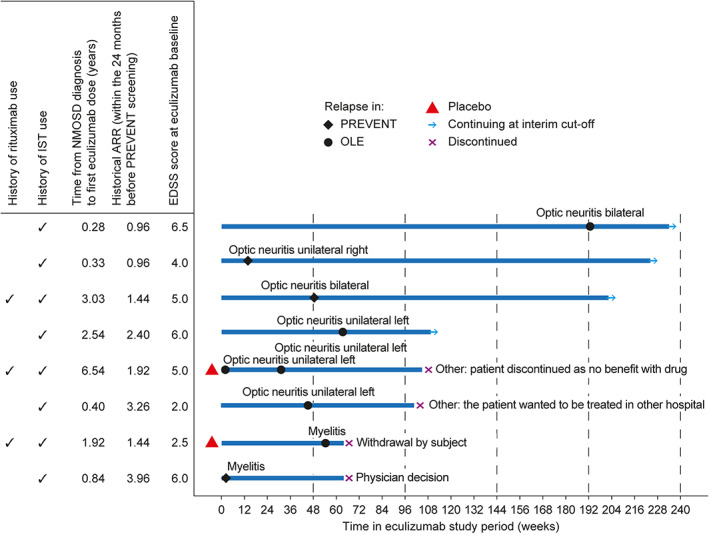
Clinical profiles of patients receiving eculizumab during PREVENT and the OLE who experienced adjudicated relapses. ARR = annualized relapse rate; EDSS = Expanded Disability Status Scale; IST = immunosuppressive therapy; NMOSD = neuromyelitis optics spectrum disorder; OLE = open‐label extension.
In the combined eculizumab group, 94.4% of participants were estimated to be free from adjudicated relapses at weeks 96 and 192 of eculizumab treatment (Kaplan–Meier analysis; 95% CI = 88.6–97.3). The proportions of OLE participants estimated to be free from adjudicated relapses were similarly high in the placebo/eculizumab and the eculizumab/eculizumab groups (94.8% [95% CI = 80.7–98.7] and 96.1% [95% CI = 88.3–98.7], respectively, at OLE week 96; Fig 3). The adjudicated ARR in the combined PREVENT and OLE eculizumab group was 0.025 (95% CI = 0.013–0.048) versus 0.350 (95% CI = 0.199–0.616) in the PREVENT placebo group. 11 The ARR for physician‐determined relapses in this group was 0.089 (95% CI = 0.063–0.126) versus 0.066 (95% CI = 0.036–0.120) in the PREVENT eculizumab group and 0.446 (95% CI = 0.272–0.732) in the PREVENT placebo group.
FIGURE 3.
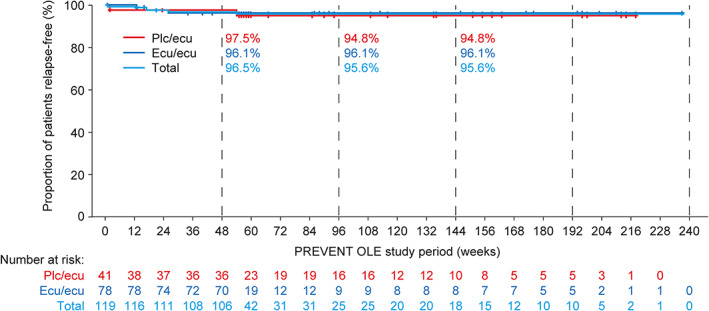
Time to first adjudicated relapse during eculizumab treatment during the PREVENT OLE by treatment arm (OLE participants, N = 119). Patients who did not experience an adjudicated on‐trial relapse were censored at the OLE interim cutoff date. The tick marks indicate censoring of data. Proportions of patients who were relapse‐free at OLE weeks 48, 96, and 144 were estimated using the Kaplan–Meier product limit method. Ecu, eculizumab; OLE = open‐label extension.
In the combined eculizumab group, the annualized rates of adjudicated relapse‐related acute treatments were 0.022 (95% CI = 0.011–0.045) for i.v. methylprednisolone (7 patients), 0.017 (95% CI = 0.008–0.037) for plasma exchange (5 patients), and 0.014 (95% CI = 0.006–0.033) for high‐dose oral corticosteroids (5 patients). This compares with annualized rates of adjudicated relapse‐related acute treatments of 0.012 (95% CI = 0.003–0.047) for each of i.v. methylprednisolone, plasma exchange, and high‐dose oral corticosteroids (2 patients each) in the eculizumab group during PREVENT, and 0.286 (95% CI = 0.173–0.475) for i.v. methylprednisolone (15 patients), 0.134 (95% CI = 0.064–0.280) for plasma exchange (7 patients) and 0.114 (95% CI = 0.051–0.255) for high‐dose oral corticosteroids (6 patients) in the PREVENT placebo group.
Impact on Disability Outcomes and Health‐Related Quality of Life
The trends toward improvement in mean EDSS, HAI, mRS, and EQ‐5D‐3L scores with eculizumab observed during PREVENT were maintained in the eculizumab/eculizumab group and replicated in the placebo/eculizumab group during the OLE (data not shown). In the combined eculizumab group, these trends toward improvement were consistently observed for 1 year from eculizumab baseline (Fig 4 and Fig 5).
FIGURE 4.
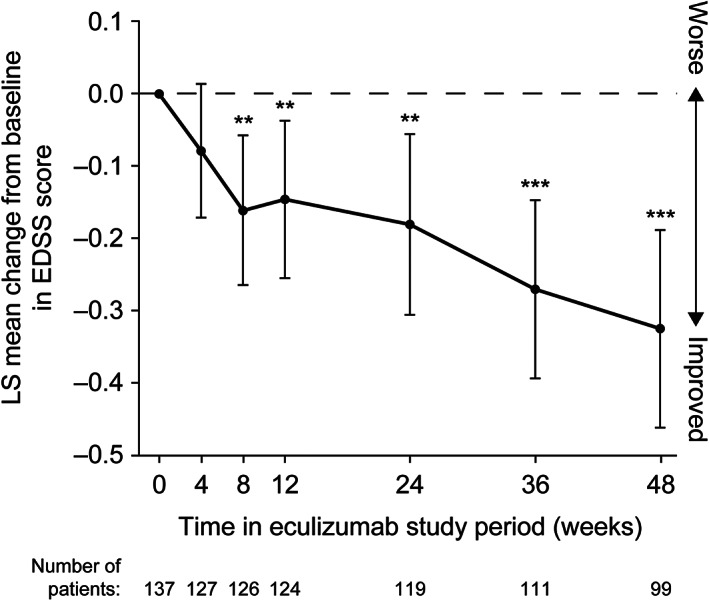
Change from eculizumab baseline in EDSS score over time through 1 year in the combined PREVENT and OLE eculizumab group. *, **, and *** represent the 2‐sided nominal p value of 0.05, 0.01, and 0.001, respectively, testing whether the LS mean change from baseline equals 0. The LS mean, 95% CI, and p value are from a restricted maximum likelihood based repeated‐measures analysis of change from eculizumab baseline. The repeated‐measures model included terms of visit and baseline score. OLE visits at weeks 26, 40, and 52 are shown as weeks 24, 36, and 48, respectively. CI = confidence interval; EDSS = Expanded Disability Status Scale; LS = least‐squares; OLE = open‐label extension.
FIGURE 5.
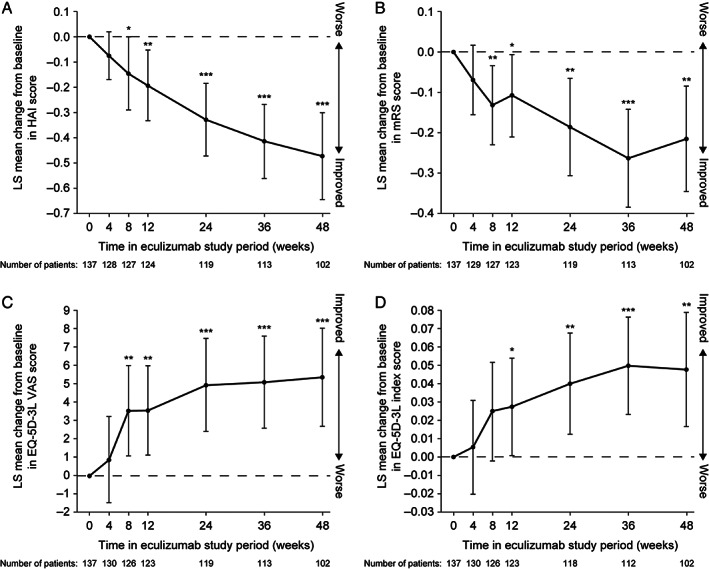
Change from eculizumab baseline in (A) HAI score, (B) mRS score, (C) EQ‐5D‐3L VAS and (D) EQ‐5D‐3L index score over time through 1 year in the combined PREVENT and OLE eculizumab group. *, **, and *** represent the 2‐sided nominal p value of 0.05, 0.01, and 0.001, respectively, testing whether the LS mean change from baseline equals 0. The LS mean, 95% CI, and p value are from a restricted maximum likelihood based repeated‐measures analysis of change from eculizumab baseline. The repeated‐measures model included terms of visit and baseline score. OLE visits at weeks 26, 40, and 52 are shown as weeks 24, 36, and 48, respectively. CI = confidence interval; EQ‐5D‐3L = 3‐level 5‐dimension EuroQol questionnaire; HAI = Hauser Ambulation Index; LS = least‐squares; mRS = modified Rankin Scale; OLE = open‐label extension; VAS = visual analog scale.
Impact on IST Use
Of the 119 OLE participants, 49 (41.2%) changed their IST use during open‐label eculizumab treatment: 17 (14.3% of OLE participants) stopped using ISTs (corticosteroids, azathioprine, mycophenolate mofetil, or cyclophosphamide), 27 (22.7%) decreased their IST use, 1 (0.8%) started an IST, and 4 (3.4%) had multiple IST changes (none only increased an IST dose). More than one‐fifth of OLE participants (27/119, 22.7%) used no concomitant IST throughout the OLE.
Discussion
This interim analysis of the OLE provides data on the long‐term safety and efficacy of eculizumab from more than twice the number of PY of eculizumab exposure than in PREVENT (median treatment duration of almost 2.5 years). The combined PREVENT and interim OLE eculizumab safety data reported here are consistent with its known safety profile in patients with AQP4‐IgG+ NMOSD 11 , 15 and with its well‐characterized safety profile in its other approved indications, paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, and generalized myasthenia gravis, based on clinical trial data and more than 10 years of postmarketing experience. 19 , 20 , 21 , 22 , 23 , 24 No new safety signals were observed between the primary analysis of PREVENT and this interim analysis of the OLE. Rates of AEs and serious infections did not change with continued eculizumab exposure. In addition, many patients were able to reduce or stop their use of concomitant IST during the PREVENT OLE, thus relieving the overall treatment burden associated with NMOSD. All OLE participants received N. meningitidis vaccinations and there were no meningococcal infections through June 1, 2020. As reported previously, one patient receiving eculizumab died during PREVENT (pulmonary empyema) 11 and no deaths have been reported during the OLE through June 1, 2020.
During PREVENT and the OLE combined, the proportion of patients free from adjudicated relapse remained high (94.4%) through 192 weeks. Interestingly, most of the adjudicated relapses that occurred during PREVENT and the OLE were optic neuritis (7/9; only 1 of the 5 major optic neuritis events was not followed by an improvement in OSIS VA score) and 2 were myelitis. In contrast, during the 24 months before PREVENT in the overall study population, 56% of patients experienced optic neuritis relapses and 81% experienced transverse myelitis relapses. 11
Both the adjudicated ARR (0.016 in the PREVENT eculizumab group 11 and 0.025 in the combined PREVENT and OLE eculizumab group) and the physician‐determined ARR (0.066 in PREVENT and 0.089 in PREVENT and the OLE) remained low with long‐term eculizumab treatment (median = 2.5 years), demonstrating the durability of eculizumab's efficacy in reducing relapse risk.
Because the effect of rituximab on B cells may be sustained for up to 6 to 12 months, peripheral B cells may still have been depleted during PREVENT in some members of both the eculizumab and placebo groups who had received rituximab more than 3 months (equivalent to approximately 5 half‐lives of rituximab) before study screening. Rates of serious infections were, however, similarly low with eculizumab and placebo, and relapse risks were reduced with eculizumab compared with placebo regardless of prior rituximab use. 15 These results indicate that use of rituximab more than 3 months before study entry did not impact the safety profile or the efficacy of eculizumab during PREVENT.
The trends toward improvements in disability and health‐related quality of life outcomes with eculizumab observed during PREVENT 11 were replicated in the placebo/eculizumab group after eculizumab initiation and were maintained in the eculizumab/eculizumab group during the OLE; these trends were consistent in the combined PREVENT and OLE eculizumab group. This demonstrates that the reduced relapse rate in eculizumab‐treated patients is associated with stabilization of disability.
The limitations of PREVENT have been discussed previously. 11 The main limitation of the OLE study is its open‐label design, which could allow unconscious reporting bias. The blinded induction phase, however, preserved the blinded nature of PREVENT during the first 4 weeks of the OLE. In addition, because MRI was not performed systematically during PREVENT and its OLE, the impact of eculizumab on MRI lesion activity in the brain and spinal cord could not be assessed.
In conclusion, the results of this interim analysis of the PREVENT OLE demonstrate the long‐term tolerability of eculizumab and an additional clinical benefit of reducing dependence on concomitant ISTs. Furthermore, this analysis extends the evidence from PREVENT for eculizumab's substantial efficacy in reducing relapse risk to demonstrate the durability of this effect in patients with AQP4‐IgG+ NMOSD. The trends toward improvements in disability and health‐related quality of life measures observed during PREVENT 11 were maintained through a median of 2.5 years in patients receiving long‐term eculizumab in the OLE. These are the first prospective data that support the hypothesis that there is no disease progression in the absence of relapse, and the disability stability reported here represents a key component of the full clinical benefit provided by long‐term eculizumab treatment for NMOSD. 1 , 5 , 6 These interim data from the ongoing OLE therefore provide robust evidence of a favorable benefit–risk profile for long‐term eculizumab therapy for patients with AQP4‐IgG+ NMOSD.
Author Contributions
S.J.P., L.M., S.S., and A.B. contributed to the conception and design of the study. S.J.P., L.M., and S.S. contributed to the drafting of the manuscript and figures. All authors contributed to the acquisition and analysis of data, and to revising the manuscript critically for important intellectual content. PREVENT Study Group members are listed in Table S1.
Potential Conflicts of Interest
This work was funded by Alexion Pharmaceuticals, which owns patent rights to eculizumab that was used in this study. D.M.W., J.P., A.B., M.L., K.‐C.W., and S.J.P. report grants, clinical trial compensation, or research support from Alexion Pharmaceuticals. K.F., J.P., A.B., M.L., H.J.K., and S.J.P. report personal fees from Alexion Pharmaceuticals. K.F. and S.J.P. report other support from Alexion Pharmaceuticals. All personal compensation for S.J.P. from Alexion Pharmaceuticals is paid to the Mayo Clinic. S.J.P has a patent, Patent #9,891,219B2 (Application #12–573942, Methods for Treating Neuromyelitis Optica [NMO] by Administration of Eculizumab to an individual that is Aquaporin‐4 [AQP4]‐IgG Autoantibody positive) – issued. L.M., S.S., G.S., and M.Y. are employees of and hold stock in Alexion Pharmaceuticals. I.N. and C.O.‐G. have no conflicts of interest to report.
Data Availability
Alexion will consider requests for disclosure of clinical study participant‐level data provided that participant privacy is assured through methods like data de‐identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant‐level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion‐sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at http://alexion.com/research-development. Link to data request form: https://alexion.com/contact-alexion/medical-information.
Supporting information
Table S1 Members of the PREVENT Study Group
Acknowledgments
The authors would like to thank all investigators and collaborators who contributed to PREVENT, as well as the participating patients and their families. We also thank Cynthia Carrillo‐Infante of Alexion Pharmaceuticals for reviewing the safety data. We would like to acknowledge Lisa Hammond‐Marty of Oxford PharmaGenesis, Oxford, UK, who provided editorial support based on authors’ detailed directions in the production of this manuscript (funded by Alexion Pharmaceuticals). This work was funded by Alexion Pharmaceuticals.
Contributor Information
Dean M. Wingerchuk, Email: wingerchuk.dean@mayo.edu.
PREVENT Study Group:
Andres Villa, Orlando Garcea, Analisa Manin, Luciana Melamud, Florencia Aguirre, Victoria Fernandez, Daniel Julio Muñoz, Jorge Amor, Carolina Bocchiardo, Carolina Daniela Diaz Obregon, Alfredo Laffue, Maria Fernanda Paez, Roberto Martín Perez, Viviana Rocchi, Loreley Teijeiro, Mariana De Virgiliis, Marta Cordoba, Mariana Ingolotti, Anahi Lupinacci, Carlos Ballario, Ana Chiesa, Hernan Gomez, Carolina Mainella, Hernan Lattini, Michael Barnett, Joshua Barton, Heidi Beadnall, Justin Garber, Todd Hardy, John Pollard, Benjamin Trewin, Neil Shuey, Alex Bryson, Ann French, Joshua Laing, Lai Yin Law, Christopher Plummer, Lauren Sanders, Leslie Sedal, Anthony Winkel, Andrew Neal, Mario Habek, Ivan Adamec, Barbara Barun, Luka Crnosija, Tereza Gabelic, Jiri Pitha, Petra Nytrova, Iveta Novakova, Michaela Tyblova, Eva Krasulova, Jana Pavlickova, Thor Petersen, Peter Rasmussen, Morten Stilund, Kristina Svendsen, Ricarda Diem, Michael Platten, Anne Berberich, Hannah Jaschonek, Brigitte Wildemann, Viola Maria Biberacher, Kirsten Brinkhoff, Daniel Golkowski, Markus Kowarik, Klaus Lehmann‐Horn, Viola Pongratz, Uwe Zettl, Jan Klinke, Micha Loebermann, Stefanie Meister, Florian Rimmele, Alexander Winkelmann, Alexander Yuk Lun Lau, Lisa Wing Chi Au, Sin Ying Florence Fan, Vincent Hing Lung Ip, Sze Ho Ma, Ka Yan Karen Ma, Chung Tong Vincent Mok, Francesco Patti, Silvia Messina, Maria Proietto, Allessandro Filla, Vincenzo Brescia Morra, Teresa Costabile, Agostino Nozzolillo, Francesco Saccà, Claudio Gasperini, Vento Claudio, Haggiag Shalom, Simonetta Simonetta, Serena Ruggeri, Carlo Pozzilli, Giovanna Boriello, Floriana De Angelis, Fabiana Marinelli, Satoru Ishibashi, Takanori Yokota, Yoichiro Nishida, Kokoro Ozaki, Nobuo Sanjo, Nozomu Sato, Masahiro Mori, Ryohei Otani, Yukari Sekiguchi, Kazumoto Shibuya, Akiyuki Uzawa, Takuya Matsushita, Hiroyuki Murai, Shintaro Hayashi, Hidenori Ogata, Koji Shinoda, Mitsuru Watanabe, Hiroo Yamaguchi, Ryo Yamasaki, Youwei Lin, Manabu Araki, Madoka Mori, Yohei Mukai, Tomoko Okamoto, Terunori Sano, Wakiro Sato, Yuji Takahashi, Takahiko Saida, Shinichi Nakamura, Tetsuya Nasu, Kyoko Saida, Yuko Shikata, Shinichiro Ukon, Hiroo Yoshikawa, Takashi Kimura, Masamitsu Nishi, Shun Sakamoto, Shohei Watanabe, Tatsuro Misu, Kimihiko Kaneko, Hiroshi Kuroda, Yuki Matsumoto, Chihiro Namatame, Shuhei Nishiyama, Hirohiko Ono, Yoshiki Takai, Takashi Kanda, Masaya Honda, Motoharu Kawai, Michiaki Koga, Toshihiko Maeda, Masatoshi Omoto, Yasuteru Sano, Jae‐Won Hyun, In Hye Jeong, Su‐Hyun Kim, Hyun‐June Shin, JiSung Yoo, Byung‐Jo Kim, SeolHee Baek, YooHwan Kim, Jung Bin Kim, Yong Seo Koo, Chan Nyoung Lee, Hung Youl Seok, Byoung Joon Kim, Eun Bin Cho, Hye‐Jin Cho, MiSong Choi, Ju Hyeon Kim, SeungJu Kim, Dong‐Sun Kim, HyeLim Lee, Kwang‐Ho Lee, Ju‐Hong Min, Ji‐Hyung Park, Jinmyoung Seok, Sung Min Kim, So‐Hyun Ahn, SeolHee Baek, SeokJin Choi, Kyomin Choi, SunYoung Im, Ji‐Sun Kim, BongJe Kim, JunSoon Kim, Young Nam Kwon, Je‐Young Shin, Sung‐Yeon Sohn, Seung Woo Kim, Ha‐Neul Jeong, JinWoo Jung, Yool‐hee Kim, Hyung Seok Lee, Ha Young Shin, Shanthi Viswanathan, Mohd Sufian Adenan, Ahmad Shahir Mawardi, Nantini Muthusamy, Siti Fadhilah Agos, Hazfadzila Mohd Unit, Farit Khabirov, Yuriy Milovanov, Lyudmila Averyanova, Natalya Babicheva, Eugenii Granatov, Sergey Kazarov, Dmitry Pokhabov, Yulia Nesterova, Anastasiya Amelina, Tatyana Bozhenkina, Denis Sazonov, Larisa Babenko, Asya Yarmoschuk, Victor Balyazin, Elena Balyazina, Elena Budaeva, Zoya Goncharova, Natalia Totolyan, Vladimir Krasnov, Marina Myatleva, Alla Timofeeva, Iuliana Samulyzhko, Sabas Boyero Duran, Jose Eulalio Barcena Llona, Maria del Mar Mendibe Bilbao, Amaia Gonzalez Eizaguirre, Jose Maria Losada Domingo, Roberto Valverde Moyano, Carmen Bahamonde Roman, Raquel Piñar Morales, Eduardo Agüera‐Morales, Maria Del Carmen Blanco Valero, Lucia Forero Diaz, Juan Jose Ochoa Sepulveda, Nazaret Pelaez Viña, Judit Diaz Diaz, Marta Fernandez Matarrubia, Irene Gomez Estevez, Nuria Gonzalez Garcia, Mariana Hernandez‐Gonzalez Monje, Diego Mayo Canalejo, Elena Minano Guillamon, Fernando Romero Delgado, Jiu‐Haw Yin, Yu‐Hua Lai, Ching‐Piao Tsai, Suwat Srisuwannanukorn, Thanatat Boonmongkol, Krittika Siritanan, Chidchanoke Thearapati, Duangkamol Singwicha, Arkhom Arayawichanont, Phanpaphon Konpan, Nathapol Riablershirun, Thaddao Wiroteurai, Kongkiat Kulkantrakorn, Praween Lolekha, Artit Potigumjon, Puchit Sukphulloprat, Dararat Suksasunee, Meryem Tuncer, Can Ebru Bekircan Kurt, Ece Gok Dursun, Gokce Ayhan, Gulsen Akman Demir, Burcu Altunrende, Zeliha Matur, Baris Topcular, Melih Tutuncu, Ayse Altintas, Abdulsamed Cam, Sabahattin Saip, Aksel Siva, Uygur Tanrıverdi, Damla Cetinkaya, Ihsan Sengun, Egemen Idiman, Rahmi Tumay Ala, Utku Bulut, Derya Kaya, Husnu Efendi, Seda Aydinlik, Cansu Egilmez Sarikaya, Yunus Emre Gorkem, Hakan Cavus, Murat Terzi, Sehriban Ayer, Musa Kazim Onar, Yanki Sahin, Sedat Sen, Saif Huda, Anu Jacob, Heike Arndt, Damian Jenkins, Maria Isabel Leite, Pedro Rodriguez, George Tackley, Carlos Pardo‐Villamizar, Scott Newsome, Shiv Saidha, Paula Cortes, Walter Royal, Robert Shin, Christopher Bever, Daniel Harrison, Horea Rus, Benjamin Frishberg, Irene Oh, Jay Rosenberg, Mark Sadoff, Gregory Sahagian, Anchi Wang, Annette Okai, Rashedul Hasan, Chaouki Khoury, James Stevens, Thomas Banas, Marlene Bultemeyer, Andrea Haller, Sharon Lynch, Nancy Hammond, Manoj Mittal, Muhammad Nashatizadeh, Michael Rippee, Utku Uysal, Yunxia Wang, James Winkley, Timothy Coleman, Gregory Cooper, Stephanie Sheffield, Kottil Rammohan, William Sheremata, Alexis Lizarraga, Leticia Tornes, Ilya Kister, Erik Charlson, Jonathan Howard, Lana Zhovtis‐Ryerson, Daniel Jacobs, Constance Easterling, Jennifer Fairbank, Revathi Iyengar, Mark Klafter, Heng Lai, Justin Lindquist, Ahmed Sadek, Beatriz Toro, Navin Verma, Joseph Berger, Eric Williamson, Amit Bar‐Or, Dina Jacobs, Clyde Markowitz, Islam Zaydan, Galen Mitchell, Rock Heyman, Valerie Suski, Ryan Orie, Eoin Flanagan, Andrew McKeon, Oliver Tobin, Anastasia Zekeridou, Stacey Clardy, Melissa Cortez, John Rose, Mateo Soldon, Jonathan Carter, Bachir Dajdaj, Charlene Snyder, Pavle Repovic, James Bowen, Angeli Mayadev, Peiqing Qian, Robert Naismith, Dorothy Anne Cross, Megan Orchard, Gregory Wu, Faria Amjad, Carlo Tornatore, Erika Mitchell, Carlos Mora, Bethany Schreiber, and Susan Stuart
References
- 1. Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. [DOI] [PubMed] [Google Scholar]
- 2. McCreary M, Mealy MA, Wingerchuk DM, et al. Updated diagnostic criteria for neuromyelitis optica spectrum disorder: similar outcomes of previously separate cohorts. Mult Scler J Exp Transl Clin 2018;4:2055217318815925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borisow N, Mori M, Kuwabara S, et al. Diagnosis and treatment of NMO spectrum disorder and MOG‐encephalomyelitis. Front Neurol 2018;9:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wingerchuk DM, Pittock SJ, Lucchinetti CF, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology 2007;68:603–605. [DOI] [PubMed] [Google Scholar]
- 6. Huda S, Whittam D, Bhojak M, et al. Neuromyelitis optica spectrum disorders. Clin Med (Lond) 2019;19:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waters P, Reindl M, Saiz A, et al. Multicentre comparison of a diagnostic assay: aquaporin‐4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2016;87:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiao Y, Fryer JP, Lennon VA, et al. Updated estimate of AQP4‐IgG serostatus and disability outcome in neuromyelitis optica. Neurology 2013;81:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinson SR, Romero MF, Popescu BF, et al. Molecular outcomes of neuromyelitis optica (NMO)‐IgG binding to aquaporin‐4 in astrocytes. Proc Natl Acad Sci U S A 2012;109:1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saadoun S, Waters P, Bell BA, et al. Intra‐cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 2010;133:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin‐4‐positive neuromyelitis optica spectrum disorder. N Engl J Med 2019;381:614–625. [DOI] [PubMed] [Google Scholar]
- 12. Soliris® (eculizumab) US Prescribing Information 2019. Alexion Pharmaceuticals, Inc., USA, June 27. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125166s431lbl.pdf. Accessed November 20, 2019.
- 13. Soliris® (eculizumab) Australian product information , 2020. Alexion Pharmaceuticals Australasia Pty Ltd, July 1. Available at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-02947-3&d=202007241016933. Accessed July 24, 2020.
- 14. Frampton JE. Eculizumab: a review in neuromyelitis optica spectrum disorder. Drugs 2020;80:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palace J, Wingerchuk DM, Fujihara K, et al. Benefits of eculizumab in AQP4+ neuromyelitis optica spectrum disorder: subgroup analyses of the randomized controlled phase 3 PREVENT trial. Mult Scler Relat Disord 2020;47:102641. [DOI] [PubMed] [Google Scholar]
- 16. Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 17. World Medical Association . WMA declaration of Helsinki — ethical principles for medical research involving human subjects. Ferney‐Voltaire: World Medical Association, 2013. Available at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. [DOI] [PubMed] [Google Scholar]
- 18. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . ICH harmonized tripartite guideline: guideline for good clinical practice. J Postgrad Med 2001;47:45–50. [PubMed] [Google Scholar]
- 19. Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 2006;355:1233–1243. [DOI] [PubMed] [Google Scholar]
- 20. Legendre CM, Licht C, Loirat C. Eculizumab in atypical hemolytic‐uremic syndrome. N Engl J Med 2013;369:1379–1380. [DOI] [PubMed] [Google Scholar]
- 21. Licht C, Greenbaum LA, Muus P, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2‐year extensions of phase 2 studies. Kidney Int 2015;87:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zuber J, Le Quintrec M, Krid S, et al. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am J Transplant 2012;12:3337–3354. [DOI] [PubMed] [Google Scholar]
- 23. Socie G, Caby‐Tosi MP, Marantz JL, et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10‐year pharmacovigilance analysis. Br J Haematol 2019;185:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howard JF Jr, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti‐acetylcholine receptor antibody‐positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double‐blind, placebo‐controlled, multicentre study. Lancet Neurol 2017;16:976–986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Members of the PREVENT Study Group
Data Availability Statement
Alexion will consider requests for disclosure of clinical study participant‐level data provided that participant privacy is assured through methods like data de‐identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant‐level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion‐sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at http://alexion.com/research-development. Link to data request form: https://alexion.com/contact-alexion/medical-information.


