Abstract
Objective
For individuals with 1–2 small (<1 cm) low-risk colorectal adenomas, international guidelines range from no surveillance to offering surveillance colonoscopy in 5–10 years. We hypothesised that the risks for metachronous advanced neoplasia (AN) among patients with low-risk adenomas differ based on clinical factors distinct from those currently used.
Design
We pooled data from seven prospective studies to assess the risk of metachronous AN. Two groups with 1–2 small adenomas were defined based on guidelines from the UK (n=4516) or the European Union (EU)/US (n=2477).
Results
Absolute risk of metachronous AN ranged from a low of 2.9% to a high of 12.2%, depending on specific risk factor and guideline used. For the UK group, the highest absolute risks for metachronous AN were found among individuals with a history of prior polyp (12.2%), villous histology (12.2%), age ≥70 years (10.9%), high-grade dysplasia (10.9%), any proximal adenoma (10.2%), distal and proximal adenoma (10.8%) or two adenomas (10.1%). For the EU/US group, the highest absolute risks for metachronous AN were among individuals with a history of prior polyp (11.5%) or the presence of both proximal and distal adenomas (11.0%). In multivariate analyses, strong associations for increasing age and history of prior polyps and odds of metachronous AN were observed, whereas more modest associations were shown for baseline proximal adenomas and those with villous features.
Conclusions
Risks of metachronous AN among individuals with 1–2 small adenomas vary according to readily available clinical characteristics. These characteristics may be considered for recommending colonoscopy surveillance and require further investigation.
INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of cancer death worldwide1 and can be prevented by detection and removal of colorectal polyps.2–11 Surveillance colonoscopy is often recommended after polypectomy to further reduce the risk of subsequent CRC. Practice guidelines generally base recommendations for surveillance on baseline polyp characteristics, with differing recommendations for low-risk versus high-risk adenomas. Importantly, there are significant variations by geographical region in both the definition of baseline low risk, as well as recommendations for follow-up among individuals with 1–2 small adenomas <1 cm in size12–15 (table 1). Specifically, for definition of low-risk groups, United States Multisociety Task Force on Colorectal Cancer (USMSTF) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines exclude individuals with high-grade dysplasia or tubulovillous or villous features within adenomas, European Union (EU) guidelines offer the option of excluding individuals with these criteria from the lowest risk group, while the UK National Institute for Health and Care guidelines include these individuals. For follow-up of their respective low-risk groups, USMSTF guidelines recommend offering surveillance colonoscopy in 5–10 years, ESGE guidelines recommend return to screening in 10 years, EU guidelines recommend against routine surveillance colonoscopy, while the UK guidelines recommend consideration of surveillance colonoscopy at 5 years. Few data exist to guide identification of patients with 1–2 small adenomas who might benefit from early (eg, 5 year) surveillance, versus those who might either defer surveillance colonoscopy for 10 years, return to routine screening in 10 years or avoid further screening or surveillance altogether.
Table 1.
Guideline definitions of individuals with low-risk baseline adenomas and recommended management
| Guideline | Definition of low risk | Recommended management |
|---|---|---|
| USMSTF 201213 | 1–2 adenomas, both smaller than 1 cm, without villous histology or high-grade dysplasia | Offer repeat colonoscopy in 5–10 years |
| ESGE15 2013 | 1–2 adenomas, both smaller than 1 cm, without villous histology or high-grade dysplasia | Return to screening after 10 years if screening programme available, otherwise repeat colonoscopy in 10 years |
| EU 201012 | 1–2 adenomas, both smaller than 1 cm, without villous histology or high-grade dysplasia* | Routine screening (no routine surveillance colonoscopy) |
| UK National Institute for Health and Care Excellence (NICE) guideline 201114 | 1 –2 adenomas, both smaller than 1 cm | Consider repeat colonoscopy in 5 years |
European Union guidelines offer the option of including patients with villous histology or high-grade dysplasia in the low-risk group.
ESGE, European Society of Gastrointestinal Endoscopy; EU, European Union; USMSTF, United States Multisociety Task Force on Colorectal Cancer.
From both a patient and a societal perspective, the implications of having a colonoscopy in 5 years vs 10 years, or not at all, are substantial with respect to risk exposure, time burden and costs.16 Therefore, additional evidence on which to base recommendations for individuals meeting current low-risk criteria at baseline colonoscopy is needed.
We hypothesised that risk for metachronous advanced neoplasia (AN) among patients with 1–2 small adenomas differs based on clinical factors beyond those used in current guidelines. Accordingly, we conducted analyses using a large, pooled data set of individuals who received surveillance colonoscopy to determine variation in risk for metachronous AN among individuals with 1–2 small adenomas at baseline across a range of clinical factors.
METHODS
Study participants and data sources
Data from patients with previously resected colorectal adenomas participating in six chemoprevention trials and one cohort study conducted in North America were available for pooling.17–23 As previously reported,24 the pooling study protocol required a complete baseline colonoscopy with the removal of one or more adenomas and all visualised lesions, a specified schedule of surveillance follow-up colonoscopies, and availability of end point data about the adenoma features and CRC detected at follow-up. Patients with a history of prior polyps were eligible for inclusion in the chemoprevention trials but not in the Veterans Affairs Study. The studies used self-administered questionnaires to ascertain patient-level characteristics (age, sex, smoking history, history of CRC in first-degree relatives and history of colorectal polyps preceding the baseline colonoscopy). Data on the number, size, location and histological characteristics of baseline adenomas were abstracted from endoscopy and pathology reports. Adenoma size was ascertained from the pathology report and when this was missing, we used size from the endoscopy report. The coordinating centres for each study obtained approval from their respective institutional review boards for the current protocol.
In the seven studies, 4711 patients had 1–2 small adenomas at baseline. Of these, 195 could not be classified as having metachronous AN due to missing data on histology or high-grade dysplasia, resulting in 4516 patients who met the UK criteria for low risk. We recognise that the EU guidelines offer the option of excluding patients with villous component or high-grade dysplasia from the lowest risk group, and in this analysis have assumed that patients with these features were treated as high risk, similar to USMSTF and ESGE guidelines. Therefore, in this paper, the EU/USMSTF/ESGE group (hereafter referred to as the EU/US group) included individuals with adenomas 1 to 2 <1 cm in size, without the presence of high-grade dysplasia or villous features. To arrive at the EU/US patient group, we excluded 540 and 1133 participants with missing histology and high-grade dysplasia data, respectively. We further excluded 366 patients classified as high risk by having adenomas with villous features or high-grade dysplasia from the larger UK group, which resulted in a total of 2477 participants in the EU/US group. Details regarding the exclusions are found in the online supplementary table.
Outcome variable and statistical analysis
The outcome variable for this study was AN at 3–5 years after qualifying colonoscopy, defined as the presence of CRC, or the presence of an adenoma containing one or more of the following three features: (1) size ≥1 cm, (2) villous histology and (3) high grade dysplasia. We conducted analyses considering two groups with 1–2 small adenomas defined as those classified as low risk at baseline based on guidelines from the UK (n=4516) or the EU/US (n=2477), as summarised above and in table 1.
The outcome categories for the present analyses were advanced metachronous neoplasia versus no metachronous neoplasia. Results were summarised with two approaches. First, absolute risks and 95% CIs of metachronous AN were calculated to quantify the risk associated with each patient and clinical factor. Second, we employed logistic regression to conduct univariate and multivariate analyses between participant and adenoma characteristics and odds of metachronous AN, expressed as crude and adjusted ORs and 95% CIs. Multivariate modelling was conducted by including all variables that were significant in univariate analysis and all the baseline adenoma characteristics. We provide absolute risks as well as multivariate odds for AN because the two approaches provide complimentary information on risk. Analyses were conducted with STATA V.10.0 (College Station, Texas, USA) and SAS V.9.0 (Cary, North Carolina, USA).
RESULTS
As shown in table 2, demographic and clinical characteristics were similar between the UK and EU/US groups, except for the presence of tubulovillous/villous histology or adenoma with high-grade dysplasia at baseline, which was expected as they were the primary characteristics that differentiate between the UK and the EU/US guidelines for the classification of individuals with 1–2 small adenomas.
Table 2.
Baseline characteristics of individuals with low-risk adenomas as defined by UK and EU/US guidelines
| Characteristic | UK definition* n=4516 | EU/US definition† n=2477 |
|---|---|---|
| Age (years, mean±SD) | 61.7±9.5 | 62.3±9.3 |
| Male (n, %) | 3205 (71.0) | 1825 (73.7) |
| Race (n, %) | ||
| White | 4034 (89.3) | 2227 (89.9) |
| Black | 237 (5.3) | 147 (5.9) |
| Other | 245 (5.4) | 103 (4.2) |
| Family history of colorectal cancer‡ (n, %) | ||
| Yes | 1054 (23.3) | 525 (21.2) |
| No | 3174 (70.3) | 1875 (75.7) |
| Unknown | 288 (6.4) | 77 (3.1) |
| Cigarette smoking status (n, %) | ||
| Never | 1562 (34.6) | 814 (32.9) |
| Former | 2241 (49.6) | 1261 (50.9) |
| Current | 691 (15.3) | 387 (15.6) |
| Unknown | 22 (0.5) | 15 (0.6) |
| Body mass index in kg/m2 (n, %)§ | ||
| <25 | 1297 (28.7) | 679 (27.4) |
| 25 to <30 | 2049 (45.4) | 1145 (46.2) |
| ≥30 | 1161 (25.7) | 650 (26.2) |
| Previous polyp (n, %)¶ | ||
| Yes | 1313 (29.1) | 641 (25.9) |
| No | 3113 (68.9) | 1780 (71.9) |
| Unknown | 90 (2.0) | 56 (2.3) |
| Adenoma characteristics | ||
| Number of adenomas (mean±SD) | 1.3±0.4 | 1.2±0.4 |
| Location of adenomas (n, %) | ||
| Distal/rectal only | 2201 (48.7) | 1224 (49.4) |
| Proximal only | 1754 (38.8) | 982 (39.6) |
| Proximal and distal | 397 (8.8) | 218 (8.8) |
| Unknown | 164 (3.6) | 53 (2.1) |
| Size of largest adenoma (mm, mean±SD) | 4.8±1.9 | 4.7±1.9 |
| Adenoma histology (n, %) | ||
| Tubular | 3579 (79.3) | 2477 (100.0) |
| Tubulovillous/villous | 386 (8.6) | 0 (0.0) |
| Unspecified | 551 (12.2)** | 0 (0.0) |
| High-grade dysplasia (%)†† | ||
| Yes | 119 (2.6) | 0 (0.0) |
| No | 3264 (72.4) | 2477 (100.0) |
Low-risk adenomas defined by UK guidelines as 1–2 adenomas <1 cm in diameter, regardless of histology or dysplasia grade.
Low-risk adenomas defined by US guidelines as 1–2 tubular adenomas <1 cm in diameter, without villous/tubulovillous histology and without high-grade dysplasia.
Family history of colorectal cancer in at least one first-degree relative. Participants with familial syndromes such as FAP or Lynch were excluded from the parent studies.
Body mass index (BMI) data not available (n=9).
History of polyps prior to qualifying exam.
Adenoma histology data not available for a subset of UK group patients; these were included in analysis because histology beyond presence of adenoma is not required to be part of UK low risk category.
HGD data not available (n=1133).
EU, European Union; HGD, high-grade dysplasia.
Absolute risk of metachronous AN
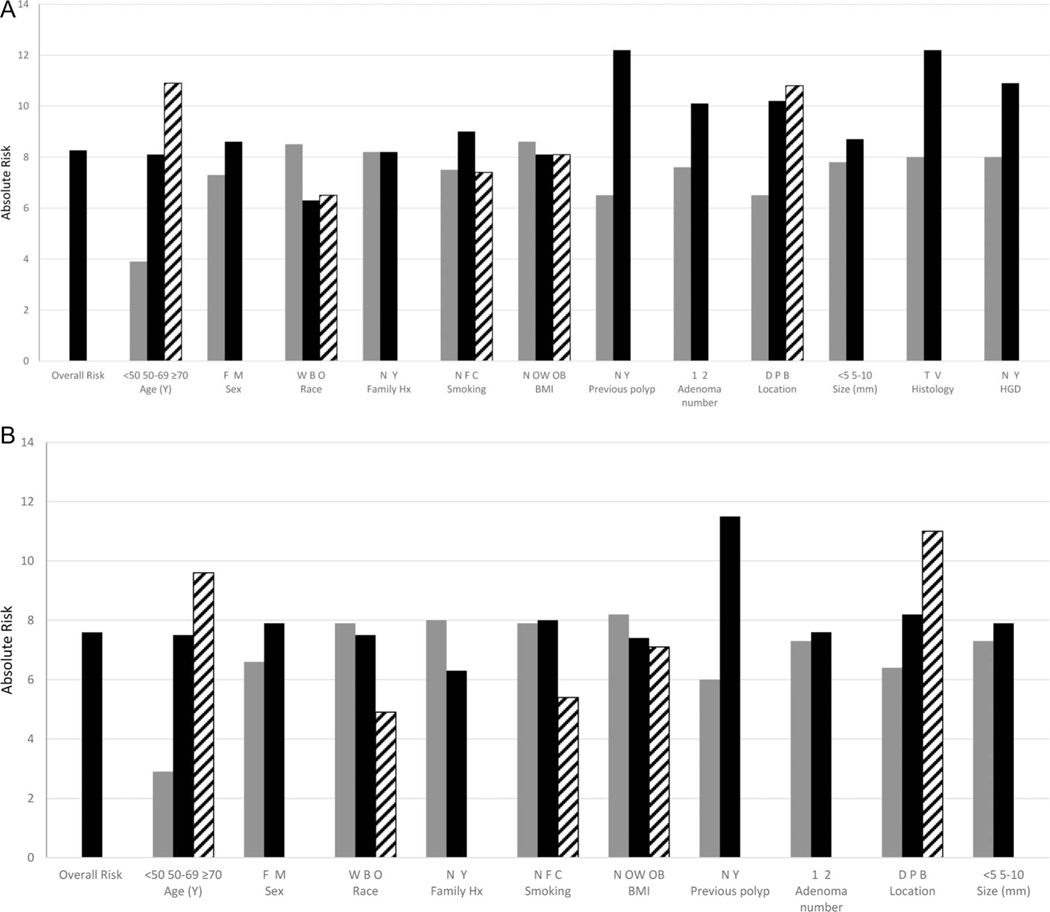
For all low-risk patients, the absolute risk of metachronous AN was 8.3% for the UK group and 7.6% for the EU/US group. Marked variation in the absolute risk of AN across baseline predictors was noted. For example, for the low-risk group defined by the UK guidelines, the absolute risks of metachronous AN ranged from a low of 3.9% (95% CI 2.5% to 6.0%) for persons under 50 years of age to a high of 12.2% (95% CI 9.3% to 15.9%) for those with a tubulovillous or villous adenoma at baseline, and 12.2% (95% CI 10.5% to 14.1%) for those who reported having polyps prior to the index colonoscopy (table 3 and figure 1). For the low-risk group defined by the EU and US guidelines, the absolute risks of metachronous AN ranged from a low of 2.9% (95% CI 1.4% to 5.9%) for persons under 50 years of age to a high of 11.5% (95% CI 9.3% to 14.3%) for those who reported a history of polyps before the index colonoscopy (table 3 and figure 1). When we restricted the analyses to patients who did not report having a prior polyp, the absolute risks were generally attenuated but the magnitude of the differences persisted (data not shown).
Table 3.
Absolute risk of advanced* neoplasia at follow-up evaluation, according to baseline patient and adenoma characteristics among individuals with low-risk adenomas according to UK guidelines† and EU/US guidelines‡
| UK guidelines (373/4516) |
EU/US guidelines (188/2477) |
|||
|---|---|---|---|---|
| No events/no at risk | Absolute risk (95% CI) | No events/no. at risk | Absolute risk (95% CI) | |
| Age (years) | ||||
| <50 | 19/490 | 3.9 (2.5 to 6.0) | 7/243 | 2.9 (1.4 to 5.9) |
| ≥50 and <70 | 241/2991 | 8.1 (7.1 to 9.1) | 122/1620 | 7.5 (6.3 to 8.9) |
| ≥70 | 114/1035 | 10.9 (9.2 to 13.0) | 59/614 | 9.6 (7.5 to 12.2) |
| Sex | ||||
| Female | 96/1311 | 7.3 (6.0 to 8.9) | 43/652 | 6.6 (4.9 to 8.8) |
| Male | 277/3205 | 8.6 (7.7 to 9.7) | 145/1825 | 7.9 (6.8 to 9.3) |
| Race | ||||
| White | 342/4034 | 8.5 (7.7 to 9.7) | 172/2227 | 7.7 (6.7 to 8.9) |
| Black | 15/237 | 6.3 (3.8 to 10.3) | 11/147 | 7.5 (4.3 to 13.1) |
| Other | 16/245 | 6.5 (4.0 to 10.4) | 5/103 | 4.9 (2.0 to 11.3) |
| Family history of colorectal cancer§ | ||||
| No | 260/3174 | 8.2 (7.3 to 9.2) | 150/1875 | 8.0 (4.5 to 8.7) |
| Yes | 86/1054 | 8.2 (6.6 to 10.0) | 33/525 | 6.3 (4.5 to 8.7) |
| Cigarette smoking status¶ | ||||
| Never | 117/1562 | 7.5 (6.3 to 8.9) | 64/814 | 7.9 (6.2 to 9.9) |
| Former | 203/2241 | 9.0 (7.9 to 10.3) | 102/1261 | 8.0 (6.7 to 9.7) |
| Current | 51/691 | 7.4 (5.6 to 9.6) | 21/387 | 5.4 (3.6 to 8.2) |
| Body mass index in kg/m2** | ||||
| <25 | 111/1297 | 8.6 (7.2 to 10.2) | 56/679 | 8.2 (6.3 to 10.6) |
| 25 to <30 | 166/2049 | 8.1 (7.0 to 9.4) | 85/1145 | 7.4 (6.0 to 9.1) |
| 30+ | 94/1161 | 8.1 (6.7 to 9.8) | 46/650 | 7.1 (5.3 to 9.3) |
| Previous polyp†† | ||||
| No | 202/3113 | 6.5 (5.7 to 7.4) | 107/1780 | 6.0 (5.0 to 7.2) |
| Yes | 160/1313 | 12.2 (10.5 to 14.1) | 74/641 | 11.5 (9.3 to 14.3) |
| Adenoma characteristics | ||||
| Adenoma number | ||||
| 1 | 257/3369 | 7.6 (6.8 to 8.6) | 137/1887 | 7.3 (6.2 to 8.5) |
| 2 | 116/1147 | 10.1 (8.5 to 12.0) | 51/590 | 8.6 (6.6 to 11.2) |
| Adenoma locations‡‡ | ||||
| Distal | 143/2201 | 6.5 (5.5 to 7.6) | 79/1224 | 6.4 (5.2 to 8.0) |
| Proximal | 179/1754 | 10.2 (8.9 to 11.7) | 81/982 | 8.2 (6.7 to 10.1) |
| Distal and proximal | 43/397 | 10.8 (8.1 to 14.3) | 24/218 | 11.0 (7.5 to 15.9) |
| Adenoma size (mm) | ||||
| <5 | 170/2179 | 7.8 (6.7 to 9.0) | 91/1255 | 7.3 (5.9 to 8.8) |
| 5 to <10 | 203/2337 | 8.7 (7.6 to 9.9) | 97/1222 | 7.9 (6.5 to 9.6) |
| Adenoma histology§§ | ||||
| Tubular | 285/3579 | 8.0 (7.1 to 8.9) | 188/2477 | 7.6 (6.6 to 8.7) |
| Tubulovillous/villous | 47/386 | 12.2 (9.3 to 15.9) | 0 | 0 |
| High-grade dysplasia¶¶ | ||||
| No | 263/3264 | 8.0 (7.1 to 9.0) | 188/2477 | 7.6 (6.6 to 8.7) |
| Yes | 13/119 | 10.9 (6.4 to 18.0) | 0 | 0 |
Adenoma ≥1 cm in size; or containing >25% villous or tubulovillous histology; or the presence of high-grade dysplasia; or colorectal cancer.
Low-risk adenomas defined by UK guidelines as 1–2 adenomas <1 cm in diameter, regardless of histology or dysplasia grade.
Low-risk adenomas defined by US guidelines as 1–2 tubular adenomas <1 cm in diameter, without villous/tubulovillous histology and without high-grade dysplasia.
Family history data unavailable (n=288).
Smoking data unavailable (n=22).
Body mass index data unavailable (n=9).
Previous polyp data unavailable (n=90).
Adenoma location data unavailable (n=164).
Histology data unavailable (n=551).
High-grade dysplasia data unavailable (n=1133).
EU, European Union.
Figure 1.
Absolute risk of advanced neoplasia at follow-up evaluation, according to baseline patient and adenoma characteristics among individuals with low-risk adenomas according to UK guidelines (A) and European Union (EU)/US (B) guidelines. Age groups (years); sex (F=female, M=male); race (W=white, B=black, O=other); family Hx is family history of colorectal cancer (N=no, Y=yes); smoking (N=never, F=former, C=current); BMI is body mass index (N=normal weight BMI <25 kg/m2; OW=overweight BMI ≥25 kg/m2 and BMI<30 kg/m2; OB=obese BMI ≥30 kg/m2); prev polyp is history of a previous polyp (N=no, Y=yes); number is number of baseline adenomas (<5 mm and ≥5 to <10 mm); histology is histology of baseline adenomas (T=tubular; V=tubulovillous or villous); HGD is high-grade dysplasia in baseline adenomas (N=no, Y=yes).
Multivariate analyses
Table 4 shows crude and multivariate adjusted ORs of the association between patient and adenoma characteristics and odds of AN. Multivariate analyses show general agreement in the relative odds between the UK and the EU/US group (table 4). Strong associations for increasing age and history of prior polyps and odds of metachronous AN were observed in both guideline groups. Statistically significant yet more modest associations for AN were shown for presence of proximal adenomas and those with villous histology at baseline. Although having two adenomas versus one at baseline was significantly associated with metachronous AN, the ORs attenuated and were imprecise in the multivariate model. When we restricted the analysis to patients without a history of polyps, the association for age and metachronous AN continued to be strong and that for baseline proximal lesions was modest but significant; however, the OR for baseline villous histology and AN was attenuated and imprecise (data not shown).
Table 4.
Risk of advanced* neoplasia at follow-up evaluation, according to baseline patient and adenoma characteristics among individuals with low-risk adenomas according to UK guidelines† and EU/US guidelines‡
| UK guidelines (373/4516) |
EU/US guidelines (188/2477) |
|||||
|---|---|---|---|---|---|---|
| n, % | Crude OR (95% CI) | Adjusted OR§ (95% CI) | n, % | Crude OR (95% CI) | Adjusted OR§ (95% CI) | |
| Age (years) | ||||||
| <50 | 19 (3.9) | 1.00 (ref) | 1.00 (ref) | 7 (2.9) | 1.00 (ref) | 1.00 (ref) |
| ≥50 and <70 | 241 (8.1) | 2.46 (1.52 to 3.99) | 2.21 (1.10 to 4.45) | 122 (7.5) | 3.18(1.46 to 6.94) | 2.99 (1.29 to 6.95) |
| ≥70 | 114 (10.9) | 3.78 (2.28 to 6.26) | 3.05 (1.47 to 6.30) | 59 (9.6) | 4.64 (2.07 to 10.38) | 4.08 (1.71 to 9.76) |
| Sex | ||||||
| Female | 96 (7.3) | 1.00 (ref) | 1.00 (ref) | 43 (6.6) | 1.00 (ref) | 1.00 (ref) |
| Male | 277 (8.6) | 1.38 (1.08 to 1.76) | 1.37 (0.98 to 1.91) | 145 (8.0) | 1.42 (0.99 to 2.03) | 1.39 (0.95 to 2.03) |
| Previous polyp | ||||||
| No | 202 (6.5) | 1.00 (ref) | 1.00 (ref) | 107 (6.0) | 1.00 (ref) | 1.00 (ref) |
| Yes | 160 (12.2) | 2.35 (1.88 to 2.95) | 2.34 (1.73 to 3.18) | 74 (11.5) | 2.54 (1.84 to 3.51) | 2.25 (1.61 to 3.15) |
| Race | ||||||
| White | 342 (8.5) | 1.00 (ref) | 172 (7.7) | 1.00 (ref) | ||
| Black | 15(6.3) | 0.75 (0.43 to 1.29) | 11 (7.5) | 1.07 (0.56 to 2.06) | ||
| Other | 16 (6.5) | 0.64 (0.38 to 1.08) | 5 (4.9) | 0.50 (0.20 to 1.26) | ||
| Family history of colorectal cancer | ||||||
| No | 260 (8.2) | 1.00 (ref) | 150 (8.0) | 1.00 (ref) | ||
| Yes | 86 (8.2) | 1.04 (0.80 to 1.35) | 33 (6.3) | 0.89 (0.56 to 1.25) | ||
| Cigarette smoking status | ||||||
| Never | 117 (7.5) | 1.00 (ref) | 64 (7.9) | 1.00 (ref) | ||
| Former | 203 (9.1) | 1.31 (1.02 to 1.66) | 102 (8.1) | 1.05 (0.75 to 1.47) | ||
| Current | 51 (7.4) | 1.01 (0.71 to 1.44) | 21 (5.4) | 0.67 (0.40 to 1.12) | ||
| Body mass index in kg/m2 | ||||||
| <25 | 111 (8.6) | 1.00 (ref) | 56 (8.3) | 1.00 (ref) | ||
| 25 to <30 | 166 (8.1) | 1.01 (0.78 to 1.30) | 85 (7.4) | 0.98 (0.68 to 1.41) | ||
| 30+ | 94 (8.1) | 1.01 (0.75 to 1.35) | 46 (7.1) | 0.91 (0.60 to 1.37) | ||
| Adenoma characteristics | ||||||
| Adenoma number | ||||||
| 1 | 257 (7.6) | 1.00 (ref) | 1.00 (ref) | 137 (7.3) | 1.00 (ref) | 1.00 (ref) |
| 2 | 116 (10.1) | 1.66 (1.31 to 2.11) | 1.26 (0.85 to 1.87) | 51 (8.6) | 1.54 (1.09 to 2.19) | 1.22 (0.78 to 1.92) |
| Adenoma location | ||||||
| Distal | 143 (6.5) | 1.00 (ref) | 1.00 (ref) | 79 (6.5) | 1.00 (ref) | 1.00 (ref) |
| Proximal | 179 (10.2) | 1.82 (1.44 to 2.30) | 1.60 (1.17 to 2.18) | 81 (8.3) | 1.46 (1.05 to 2.02) | 1.41 (1.00 to 1.99) |
| Distal and proximal | 43 (10.8) | 2.26 (1.55 to 3.28) | 1.74 (0.97 to 3.13) | 24 (11.0) | 2.53 (1.53 to 4.21) | 1.98 (1.04 to 3.79) |
| Adenoma size (mm) | ||||||
| <5 | 170 (7.8) | 1.00 (ref) | 1.00 (ref) | 91 (7.3) | 1.00 (ref) | 1.00 (ref) |
| 5 to <10 | 203 (8.7) | 1.12 (0.90 to 1.39) | 1.17 (0.87 to 1.58) | 97 (7.9) | 1.13 (0.83 to 1.53) | 1.20 (0.87 to 1.66) |
| Adenoma histology | ||||||
| Tubular | 285 (8.0) | 1.00 (ref) | 1.00 (ref) | 188 (7.6) | N/A¶ | N/A |
| Tubulovillous/villous | 47 (12.2) | 1.52 (1.08 to 2.13) | 1.77 (1.17 to 2.66) | 0 (0.0) | ||
| High-grade dysplasia | ||||||
| No | 263 (8.1) | 1.00 (ref) | 1.00 (ref) | 188 (7.6) | N/A | N/A |
| Yes | 13 (10.9) | 1.42 (0.77 to 2.62) | 0.88 (0.41 to 1.88) | 0 (0.0) | ||
Adenoma >1 cm in size, and/or containing >25% villous or tubulovillous histology, presence of high-grade dysplasia or colorectal cancer.
Low-risk adenomas defined by UK guidelines as 1–2 adenomas <1 cm in diameter, regardless of histology or dysplasia.
Low-risk adenomas defined by US guidelines as 1–2 tubular adenomas <1 cm in diameter.
Models include simultaneous adjustment for all variables.
Not applicable given that these characteristics are not part of the guidelines.
EU, European Union.
DISCUSSION
Guideline recommendations for surveillance colonoscopy after removal of 1–2 small adenomas vary considerably, ranging from recommendations for performance of surveillance in 5–10 years (USMSTF), no surveillance/return to routine screening in 10 years (EU/ESGE), to consideration of 5-year surveillance (UK, table 1). Guideline variation, in part, may be due to uncertainty regarding risk for metachronous neoplasia among the large, and probably heterogeneous group of individuals with low-risk adenomas at baseline. Indeed, we found substantial variation in risk for metachronous AN across a number of readily available clinical characteristics. For example, according to the presence of various characteristics, absolute risks varied from a low of 3.9% to a high of 12.2% for the UK group, and from a low of 2.9% to a high of 11.5% for the EU/US group. Results from multivariate analyses, which provide data on relative odds of developing metachronous AN, are generally consistent with the results from absolute risk analyses pointing to increasing age, history of previous polyps and presence of proximal adenomas at prior colonoscopy as criteria associated with increased risk.
Our results may have clinical and research implications. Some decision makers may believe that a high threshold should be set for recommending early 5 years or any surveillance at all for individuals with 1–2 small adenomas. In this scenario, it might be noted that none of the clinical characteristics considered in our study was associated with a more than 12% absolute risk of metachronous AN, in which case, our data would support EU guidelines that recommend no surveillance for individuals with low-risk adenomas. Other decision makers may be concerned that current guideline-defined low-risk groups include individuals who might benefit from early surveillance, and note twofold or higher increased odds of metachronous neoplasia, and ≥10% risk of absolute neoplasia were present for individuals with baseline proximal adenoma, age >70 years and history of prior polyp. Of note, even without the ability to confirm histology of prior polyps for all individuals in our analysis, history of prior polyps showed the strongest association with metachronous AN. Our observation of 1.7-fold increased risk for metachronous advanced adenoma among individuals with adenoma containing villous features within the UK group might sway some to argue that the EU/US definitions of low risk (which exclude villous histology) are superior to the UK guideline definition. The clinical impact of our observations, if replicated, depends highly on the risk thresholds set to change management, which currently do not exist. For example, in the US, if a 10% or higher threshold of absolute metachronous AN were set to justify special management, patients with low risk adenomas at baseline might be advised to have surveillance colonoscopy in 5 years only if a history of prior polyp or presence of both a distal and proximal adenoma were noted. Such a shift could have significant practice implications by reducing the demand for early, 5-year surveillance colonoscopy. Conversely, if decision makers in the US deemed a 15% risk for metachronous AN (similar to what is observed among individuals with advanced adenoma at baseline16,24), then guidelines might be changed to advise that no patients with low-risk adenoma receive early 5-year surveillance. To make full use of our observation of substantial variation in metachronous AN risk among individuals with low-risk adenomas at baseline across multiple clinical characteristics, future research is required to replicate our risk findings, and, importantly, determine from patients, providers, and policy makers the optimal risk thresholds that should trigger altered clinical management. Criteria that might be considered for determining the best minimum risk threshold include benefits such as early detection and removal of neoplasia, risks of surveillance colonoscopy, time horizon for benefit (especially relevant for older individuals who may have competing health risks), proportion of individuals requiring specialised surveillance, expected downstream impact of modifying recommendations on colonoscopy resources, baseline risks for AN in a screening population (such as for individuals of similar age) and costs. Overall, our results indicate that considering additional clinical factors, beyond polyp number, size and histology has the potential to contribute to more precise risk stratification and management of individuals with colorectal polyps.
We are not aware of previous reports focused on identifying individuals at increased risk for metachronous AN with only baseline low risk adenomas. Therefore, it is difficult to place our results directly in the context of prior work. In a meta-analysis comparing the risk for metachronous AN among individuals with low-risk adenomas versus normal colonoscopy at baseline, Hassan et al reported that the overall risk for metachronous AN was 3.6% for the group with low-risk adenomas versus 1.6% for those with normal colonoscopy.16 However, an analysis of subgroups of patients who might be at increased risk for AN within either group was not conducted. Our work is consistent with this meta-analysis in confirming that most individuals with low-risk adenomas at baseline have a low risk for metachronous AN, but add to the literature by pointing out several readily available clinical characteristics that may help to identify the subset of patients who might benefit from repeat and/or early colonoscopy surveillance.
Several limitations must be considered in interpreting our results. Our analysis could not consider factors such as type of prior polyp or relationship and age of family members with CRC. In addition, we had incomplete information on variation in bowel preparation across studies, especially those conducted in earlier years. Colonoscopies contributing data were performed between 1984 and 1998. Changes in colonoscopy quality may have occurred over time, such that individuals with only 1–2 small adenomas encountered in clinical practice now might be at even lower risk for metachronous AN than the individuals with 1–2 small adenomas included in this study due to increases in awareness of the importance of polyp detection and complete removal. We hypothesise that such a reduction in risk could result from a lower risk of missed lesions, and the potential for higher detection of diminutive lesions of minimal significance with today’s high definition colonoscopes. Indeed, our overall rate of metachronous AN for the US/EU group was substantially higher than reported in a recent meta-analysis that included more modern studies16 (7.6% vs 3.6%). Another limitation is that six of the seven studies contributing to this analysis were from prevention trials, such that the patients contributing data may not be representative of the general population of individuals with 1–2 small adenomas. Furthermore, the primary outcome used was metachronous AN detected at 3–5 years. While metachronous AN is an accepted surrogate marker for CRC risk, the ideal outcome for comparing surveillance intervals would be CRC -related mortality.25 In practice, it is unclear whether there is a substantial clinical benefit to detecting metachronous AN at 5 vs 10 years among individuals with only 1–2 small adenomas at baseline. We did not have data on sessile serrated adenomas/polyps, which were not commonly recognised during the timeframe studies contributing data were completed.
Our pooled study also has considerable strengths, including the prospective design of studies contributing data, pooling of individual patient-level data and consideration of four different international guidelines for postpolypectomy surveillance of individuals with low-risk adenomas.
In conclusion, substantial variation in the risk of metachronous AN among individuals with 1–2 small adenomas exists according to readily available clinical characteristics. Considering these characteristics for risk stratification might reduce both underuse and overuse of surveillance colonoscopy, and ultimately optimise the risks and benefits of programmatic postpolypectomy surveillance. Additionally, the limitations of currently available predictors for metachronous AN call for research on novel approaches for risk stratification of individuals with colorectal polyps. Specifically, future work should investigate whether additional patient, polyp and/or quality factors (such as the adenoma detection rate of the colonoscopist who performed the polypectomy) can offer more precise post polypectomy risk stratification.
Supplementary Material
Significance of this study.
What is already known on this subject?
International recommendations for follow-up surveillance of individuals with 1–2 small colorectal adenomas are highly variable.
Recommendations range from promoting no surveillance to offering colonoscopy in 5–10 years.
Data to support current recommendations are sparse.
What are the new findings?
Absolute risk of metachronous advanced neoplasia (AN) ranged from a low of 2.9% to a high of 12.2%, depending on specific risk factors and guidelines used.
Multivariate relative risks also confirmed variation in risk for metachronous AN across multiple different clinical characteristics.
Several factors not consistently used across guidelines (presence of villous histology, presence of high-grade dysplasia and prior polyp history) or currently used within guidelines (presence of proximal adenoma and two adenomas) were associated with an increased risk for metachronous AN.
How might it impact on clinical practice in the foreseeable future?
Among individuals with 1–2 small adenomas at baseline, characteristics such as presence of proximal adenoma, adenoma with villous or high-grade histology, two adenomas, or history of prior polyp may be considered for recommending colonoscopy surveillance.
Acknowledgements
We would also like to thank the Colorectal Cancer Screening Committee of the World Endoscopy Organization, which generously provided support for the expert working group on surveillance after colonic neoplasm, from which this work originated.
Funding This work was supported by Public Health Service grants CA-41108, CA-23074, CA95060, CA37287, CA104869, CA23108, CA59005 and CA26852 from the National Cancer Institute. Funding for the Veteran’s Affairs Study was supported by the Cooperative Studies Program, Department of Veterans Affairs. Support was also provided in part by Merit Review Award number 1 I01 HX001574–01A1 (Gupta, PI) from the USA Department of Veterans Affairs Health Services Research & Development Service of the VA Office of Research and Development. The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs. Additional support was provide by Instituto de Salud Carlos III and Fondos FEDER (PI11/2630, INT-13–078, INT-14–196, UGP-13–221, PI14/01386)..
Footnotes
Competing interests None declared.
Ethics approval Institutional Review Board of the National Cancer Institute; Committee for the Protection of Human Subjects, Darmouth Medical School; Institutional Review Board, VA Portland Health Care System; University of Arizona Human Subjects Protection Program; University of California, San Diego Human Research Protections Program.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Kahi CJ, Imperiale TF, Juliar BE, et al. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol 2009;7:770–5; quiz 11. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case–control study. Ann Intern Med 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 4.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013;369:1106–14. [DOI] [PubMed] [Google Scholar]
- 6.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National PolypStudy Workgroup. N Engl J Med 1993;328:901–6. [DOI] [PubMed] [Google Scholar]
- 8.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, et al. Evaluating test strategies for colorectal cancer screening: a decision analysis for the u.S. Preventive services task force. Ann Intern Med 2008;149:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33. [DOI] [PubMed] [Google Scholar]
- 10.Holme Ø, Løberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 2014;312:606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial—SCORE. J Natl Cancer Inst 2011;103:1310–22. [DOI] [PubMed] [Google Scholar]
- 12.Atkin WS, Valori R, Kuipers EJ, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—colonoscopic surveillance following adenoma removal. Endoscopy 2012;44(Suppl 3):SE151–63. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the us multi-society task force on colorectal cancer. Gastroenterology 2012;143:844–57. [DOI] [PubMed] [Google Scholar]
- 14.Centre for Clinical Practice at N. National institute for health and clinical excellence: guidance. Colonoscopic surveillance for prevention of colorectal cancer in people with ulcerative colitis, crohn’s disease or adenomas. London, UK: National Institute for Health and Clinical Excellence, 2011. [PubMed] [Google Scholar]
- 15.Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013;45:842–51. [DOI] [PubMed] [Google Scholar]
- 16.Hassan C, Gimeno-García A, Kalager M, et al. Systematic review with meta-analysis: the incidence of advanced neoplasia after polypectomy in patients with and without low-risk adenomas. Aliment Pharmacol Ther 2014;39:905–12. [DOI] [PubMed] [Google Scholar]
- 17.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp prevention trial study group. N Engl J Med 2000;342:1149–55. [DOI] [PubMed] [Google Scholar]
- 18.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 2003;348:891–9. [DOI] [PubMed] [Google Scholar]
- 19.Alberts DS, Martínez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix colon cancer prevention physicians’ network. N Engl J Med 2000;342:1156–62. [DOI] [PubMed] [Google Scholar]
- 20.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium polyp prevention study group. N Engl J Med 1999;340:101–7. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg ER, Baron JA, Tosteson TD, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp prevention study group. N Engl J Med 1994;331:141–7. [DOI] [PubMed] [Google Scholar]
- 22.Alberts DS, Martínez ME, Hess LM, et al. Phase iii trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst 2005;97:846–53. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans affairs cooperative study group 380. N Engl J Med 2000;343:162–8. [DOI] [PubMed] [Google Scholar]
- 24.Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009;136:832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Løberg M, Kalager M, Holme O, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014;371:799–807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.