Abstract
Concerns for anaphylaxis may hamper severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunization efforts. We convened a multidisciplinary group of international experts in anaphylaxis composed of allergy, infectious disease, emergency medicine, and front-line clinicians to systematically develop recommendations regarding SARS-CoV-2 vaccine immediate allergic reactions. Medline, EMBASE, Web of Science, the World Health Organizstion (WHO) global coronavirus database, and the gray literature (inception, March 19, 2021) were systematically searched. Paired reviewers independently selected studies addressing anaphylaxis after SARS-CoV-2 vaccination, polyethylene glycol (PEG) and polysorbate allergy, and accuracy of allergy testing for SARS-CoV-2 vaccine allergy. Random effects models synthesized the data to inform recommendations based on the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) approach, agreed upon using a modified Delphi panel. The incidence of SARS-CoV-2 vaccine anaphylaxis is 7.91 cases per million (n = 41,000,000 vaccinations; 95% confidence interval [95% CI] 4.02-15.59; 26 studies, moderate certainty), the incidence of 0.15 cases per million patient-years (95% CI 0.11-0.2), and the sensitivity for PEG skin testing is poor, although specificity is high (15 studies, very low certainty). We recommend vaccination over either no vaccination or performing SARS-CoV-2 vaccine/excipient screening allergy testing for individuals without history of a severe allergic reaction to the SARS-CoV-2 vaccine/excipient, and a shared decision-making paradigm in consultation with an allergy specialist for individuals with a history of a severe allergic reaction to the SARS-CoV-2 vaccine/excipient. We recommend further research to clarify SARS-CoV-2 vaccine/vaccine excipient testing utility in individuals potentially allergic to SARS-CoV2 vaccines or their excipients.
Key words: SARS-CoV-2, COVID-19, Vaccination, Adenovirus-vector vaccine, mRNA vaccine, Anaphylaxis, Allergic reactions, Polyethylene glycol, Polysorbate 80, Skin testing, Shared decision making, GRADE, Allergy, Allergy specialist
Abbreviations used: BCC, Brighton Collaboration criteria; CDC, U.S. Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; EUA, Emergency use authorization; GRADE, Grading of Recommendation, Assessment, Development, and Evaluation; IgE, Immunoglobulin E; PEG, Polyethylene glycol; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; VAERS, Vaccine Adverse Event Reaction System
Introduction
Through the middle of April 2021, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) coronavirus and subsequent coronavirus disease 2019 (COVID-19) global pandemic has caused over 140 million infections and 3 million fatalities.1 , 2 The COVID-19 pandemic has caused unprecedented international social and economic disruption, resulting in a global economic cost in 2020 of $11 trillion, with estimates of an additional cost of $10 trillion in 2021.3
Vaccines are considered the most effective strategy to end the pandemic. By early 2021, 3 adenovirus-vector vaccine agents, 2 mRNA vaccine agents, and 2 inactivated viral vaccine agents completed phase 2/3 trials and were approved for emergency use in multiple countries.4 Through the middle of April 2021, greater than 878,900,000 doses of these vaccines have been administered globally.5 These vaccines have an efficacy from 62% to 95% in reducing symptomatic SARS-CoV-2 infection, and greater than 95% efficacy in reducing severe/critical infections.6 , 7 Data have also shown that vaccination can reduce hospitalization. Adverse events were reported in all of the vaccine trials, including hypersensitivity reactions, but no fatalities due to hypersensitivity reactions were reported. During the phase 3 studies for the currently approved vaccines, there was no significant difference in hypersensitivity rates between vaccine and placebo trial arms, with 0 to 1 cases of anaphylaxis per million doses reported.7, 8, 9, 10, 11
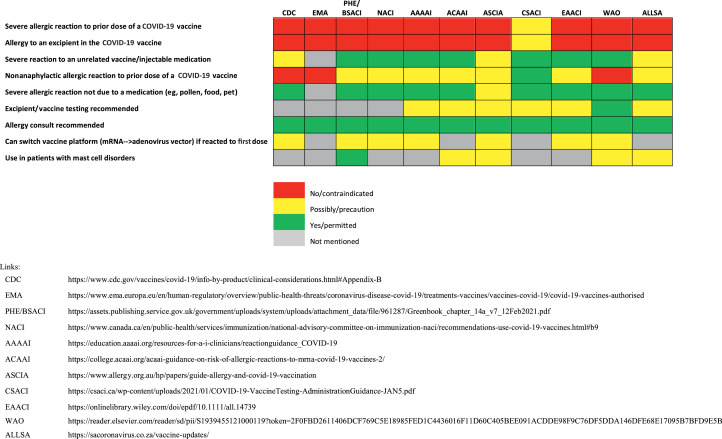
On December 9, 2020, there were 2 severe allergic reactions reported after administration of the first 500 Pfizer-BioNTech vaccinations in the United Kingdom; both patients responded to treatment with intramuscular epinephrine.12 In response, the U.K. Medicines and Healthcare Products Regulatory Agency (MHRA) temporarily recommended a contraindication for the Pfizer-BioNTech vaccine in individuals with a history of a severe allergic reaction to any vaccine, medication, or food.13 This recommendation was modified in early January 2021 to a contraindication only for those with a history of a severe allergic reaction to the SARS-CoV-2 mRNA vaccine or vaccine component/excipient (polyethylene glycol [PEG] in mRNA vaccines, and polysorbate 80, a related excipient contained in the adenovirus vaccines, which is considered potentially cross-reactive with PEG).8 , 9 , 14, 15, 16, 17, 18 Similar contraindications have been adopted in multiple other countries by public health regulatory agencies and allergy/immunology societies as detailed in Figure 1 .4 , 19, 20, 21 The evidence base for many of these recommendations remains uncertain, however.
Figure 1.
Recommendations regarding precautions and contraindications regarding SARS-CoV-2 vaccination. AAAAI, American Academy of Allergy, Asthma, and Immunology; ACAAI, American College of Allergy, Asthma, and Immunology; ALLSA, Allergy Society of South Africa; ASCIA, Australasian Society of Clinical Immunology and Allergy; CDC, U.S. Centers for Disease Control and Prevention; CSACI, Canadian Society of Allergy and Clinical Immunology; EAACI, European Academy of Allergy and Clinical Immunology; EMA, European Medicines Agency; NACI, National Advisory Committee on Immunizations; PHE/BSACI, Public Health England/British Society for Allergy and Clinical Immunology; WAO, World Allergy Organization.
A handful of publications have detailed potentially allergic reactions to SARS-CoV-2 mRNA vaccines, through the early stages of their emergency use authorization (EUA). These reports provide the specific reported symptoms, treatment, comorbid allergic history, and discussion of the confidence that the reaction constituted anaphylaxis as adjudicated by U.S. Centers for Disease Control and Prevention (CDC) physicians. This was based on adjudication of the data reported to the Vaccine Adverse Event Reaction System (VAERS) using the Brighton Collaboration criteria (BCC) for vaccine anaphylaxis.22, 23, 24 The CDC has continued to periodically update these estimates based on VAERS data.25 , 26 Through the first month after the U.S. EUA for both mRNA vaccines, the CDC described 66 anaphylaxis events in 17,524,676 vaccinations, or approximately 3.7 events per million.25 , 26 One large U.S. academic medical center reported 16 cases of anaphylaxis among 64,900 vaccinations (250 cases per million vaccinations) over a 2-month period, based on electronic health record review (by allergy investigators) of reported symptoms within 3 days of vaccination.27 These reports all indicate that anaphylactic reactions to the SARS-CoV-2 mRNA vaccines may occur but are rare.
Reports of severe allergic reactions, including anaphylaxis, have prompted concern that the new mRNA vaccine platform has the potential to cause allergic reactions (including anaphylaxis) at a greater rate than other vaccines. A lesser degree of concern is shared for adenovirus-vector vaccines because there is more experience with this platform. These concerns likely contribute to vaccine hesitancy and must be weighed against the life-saving and disease-mitigating benefits of SARS-CoV-2 vaccination. Given the lack of an established international evidence-based approach to reactions attributable to these vaccines, this group of international experts in the diagnosis and management of anaphylaxis has organized an evidence-based, consensus guidance for the diagnosis and management of severe allergic reactions, including anaphylaxis, to SARS-CoV-2 vaccines.
Methods
An ad hoc panel of clinical experts from the United States, Canada, United Kingdom, Russia, Europe (Ireland, Germany, Italy), South Africa, and Australia came together to synthesize the current evidence regarding severe allergic reactions to the SARS-CoV-2 vaccines and to provide consensus recommendations regarding the epidemiology, risk factors, assessment, and management of patients at possible risk for a severe allergic reaction to a SARS-CoV-2 vaccine from a societal perspective. The panel was chosen based on expertise in anaphylaxis diagnosis, management, and policy. The group included representation of infectious disease clinicians, clinicians serving as front-line vaccinators, and allergy specialists with prior expertise in policy related to adverse reactions to vaccines for infectious diseases. Direct financial and industry-related conflicts of interest (eg, direct or indirect involvement with the development or clinical trials related to SARS-CoV-2 vaccines) were not permitted and were considered disqualifying. Work unrelated to vaccine development with companies (eg, asthma, allergic rhinitis, atopic dermatitis) was not considered either a direct or an indirect conflict, but such involvement was disclosed. The development of this guidance did not include any industry input, funding, or financial or nonfinancial contribution. No member of the guidance panel received honoraria or remuneration for any role in the guidance development process.
A primary draft, inclusive of 4 focused questions, was iteratively developed by the senior authors (M.G., M.S., E.M.A., D.B.K.G., D.K.C., D.K.) using the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) format for evidence synthesis from an individual perspective with secondary consideration for the health care perspective. This draft was circulated and revised/edited iteratively over multiple draft versions by the workgroup. A modified Delphi panel among the members was used to rate agreement and consensus with the final recommendations.28 The GRADE methodology is explained in detail elsewhere,29, 30, 31 and the full details of the methods for this analysis are detailed in the Appendix E1 (available in this article’s Online Repository at www.jaci-inpractice.org). An electronic REDCap survey (Research Electronic Data Capture, Nashville, TN) was sent to panel members who were asked to rate their level of agreement with recommendations (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 = strongly agree).32 In addition, panel members were encouraged to submit free text comments for statements, and could choose “not applicable” if they did not feel they had the expertise to answer a question. A priori consensus was defined as agreement of greater than 75% for recommendations; “strongly disagree” and “disagree” were grouped together and “strongly agree” and “agree” were grouped together. For recommendations in which consensus was not achieved after the initial survey, an iterative discussion was undertaken with the intent to resolve the key points and adjust language. Subsequent surveys were then sent to panel members incorporating the deidentified responses from the prior survey with the goal of reaching consensus. This process was repeated until consensus was reached or, if no consensus was achieved by the third round, the statement was categorized as “no consensus reached.”
Outcomes prioritized in this systematic review and meta-analysis were anaphylaxis to the vaccine and vaccine excipients, as well as the diagnostic accuracy of allergy testing to the vaccine and vaccine excipients. The literature searched included (1) global coronavirus databases for country by country vaccine allergy or anaphylaxis reporting (2) national passive vaccine safety reporting systems, and (3) PubMed/Medline and clinical trials registries related to SARS-CoV-2 vaccine clinical trials and published case series related to SARS-CoV-2 vaccine adverse events. Systematic reviews and literature searches (Supplementary Methods, available in this article's Online Repository at www.jaci-inpractice.org) were conducted by 5 authors (M.G., M.S., D.K.C., A.W.-B., A.B.) using Covidence (Cochrane Group, Brighton, UK). Searches and manuscript selections are detailed in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagrams in Figure E1 (available in this article’s Online Repository at www.jaci-inpractice.org). Specific inclusion and exclusion criteria for each search are detailed in the Supplementary Methods. We considered but did not rely on indirect evidence from similar event reporting from other vaccines, given differences in the context and technology of the vaccines. However, we considered indirect evidence for management strategies of those with vaccine allergy because this comprises the extant literature regarding vaccine allergy, and the acute management of severe allergic reactions, including anaphylaxis, to vaccines is sufficiently similar to the experience with diagnosis and management of anaphylaxis to foods, drugs, and insect stings. We used the European Commission Guidance for Industry of Adverse Drug Reactions threshold for what was considered a rare event as between 1 case/1,000 to 10,000 individuals, and very rare as less than 1 case/10,000 individuals.33 Although not systematically reviewed, we examined vaccine efficacy as an outcome because this was identified as being of major importance to patients.
We use the wording “we recommend” for strong recommendations and “we suggest” for conditional recommendations. The implications of the recommendation strength are presented in Table E1 (available in this article’s Online Repository at www.jaci-inpractice.org), the certainty of evidence in Table E2 (available in this article’s Online Repository at www.jaci-inpractice.org), and the risk of bias assessment in Figures E2 to E4 (available in this article’s Online Repository at www.jaci-inpractice.org). The final list of recommendations was developed by panel discussion and consensus. We present the guideline statements and recommendations in Table I . The Evidence to Decision Framework supplement (Appendix E2; available in this article’s Online Repository at www.jaci-inpractice.org) provides a summary reflection of the evidence in the context of the clinical recommendation. The results of the modified Delphi panel for each recommendation are shown in the Table E3 (available in this article’s Online Repository at www.jaci-inpractice.org).
Table I.
Summary recommendations
| Question | Recommendation | Recommendation trength | Evidence ertainty |
|---|---|---|---|
| What is the risk of SARS-CoV-2 vaccine anaphylaxis in a patient with no history of anaphylaxis to a SARS-CoV-2 vaccine or its excipients? | For patients with no history of a previous severe allergic reaction to a SARS-CoV-2 vaccine or its excipients, the risk of SARS-CoV-2 vaccine–induced anaphylaxis is very rare and we recommend vaccination over no vaccination based on this risk. | Strong | High |
| For patients with a history of a severe allergic reaction, including anaphylaxis, unrelated to a SARS-CoV-2 vaccine or excipient but no history of a previous severe allergic reaction to a SARS-CoV-2 vaccine or its excipients, the requirement for additional observation beyond standard wait time (eg, recommended by local health authorities for the general population) provides a minimal absolute risk reduction in severe allergic reaction outcomes and may also contribute to vaccine hesitancy. Therefore, we suggest against prolonged observation in those with a history of severe allergic reactions unrelated to a SARS-CoV-2 vaccine or excipient. | Conditional | Low | |
| In patients without a history of anaphylaxis to a SARS-CoV-2 vaccine or its excipients, should allergy skin testing to SARS-CoV-2 vaccines or its excipients be performed? | In patients with no history of a severe allergic reaction, including anaphylaxis, to SARS-CoV-2 vaccines or its excipients, we recommend against vaccine or vaccine excipient testing prior to vaccination in an attempt to predict the rare individual who will have a severe allergic reaction to vaccination. | Strong | Low |
| In patients with a history of anaphylaxis to a SARS-CoV-2 vaccine or its excipients, should allergy skin testing to SARS-CoV-2 vaccines or its excipients be performed to determine whether vaccine withholding is needed? | We suggest against the clinician routinely performing skin or in vitro testing using SARS-CoV-2 vaccines or excipients outside of the research setting for the purpose of vaccine withholding, given such testing has unknown sensitivity/specificity in predicting severe allergic reactions, including anaphylaxis, to SARS-CoV-2 vaccines. | Conditional | Low |
| Should SARS-CoV-2 mRNA or adenovirus-vector vaccines be administered to an individual who had an immediate allergic reaction to the first dose of the vaccine (defined as a generalized, systemic allergic reaction with acute onset occurring within 4 hours of vaccine administration) or given as a first dose to an individual who is suspected to have reacted previously to an excipient ingredient that is also present in the SARS-CoV-2 mRNA or adenovirus-vector vaccines? | We recommend a shared decision-making paradigm of care favoring vaccination through full or graded dosing (with or without additional observation time postvaccination) or changing vaccine platforms to another agent over no vaccination because there is no single best approach to assessment and management of the patient with a suspected SARS-CoV-2 mRNA or adenovirus-vector vaccine reaction or the patient with an allergy to an excipient in either of these vaccines who has not yet been vaccinated. | Strong | Moderate |
| In patients with a suspected immediate allergic reaction to SARS-CoV-2 vaccine whose standard schedule requires more than 1 dose, we recommend referral to an allergist for assessment of additional vaccination over no vaccination/vaccination being withheld. In resource-limited settings in which specialist referral is not readily available, alternatives may be presented in a shared decision-making context to provide assessment and opportunity for vaccination by remote consultation, use of alternative vaccine products, or delay in vaccination until a solution can be determined. | Strong | Moderate | |
| In patients with a suspected or confirmed but remote past medical history of reaction to a SARS-CoV-2 vaccine excipient, we recommend referral to an allergist for assessment of additional vaccination over no vaccination/vaccination being withheld. In resource-limited settings in which specialist referral is not readily available, alternatives may be presented in a shared decision-making context to provide assessment and opportunity for vaccination by remote consultation, use of alternative vaccine products, or delay in vaccination until a solution can be determined. | Strong | Moderate | |
| In patients with a definite/confirmed recent allergic reaction to SARS-CoV-2 vaccine and/or excipient, we recommend referral to an allergist for assessment of additional vaccination over no vaccination/vaccination being withheld. In resource-limited settings in which specialist referral is not readily available, alternatives may be presented in a shared decision-making context to provide assessment and opportunity for vaccination by remote consultation, use of alternative vaccine products, or delay in vaccination until a solution can be determined. | Strong | Low | |
| Whereas all vaccines should be administered in facilities capable of treating anaphylaxis, particularly for individuals with a prior immediate systemic allergic reaction to a SARS-CoV-2 vaccine or vaccine excipient, we recommend the clinician should administer SARS-CoV-2 mRNA or adenovirus-vector vaccine in a setting equipped to manage anaphylaxis (eg, hospital, mass immunization clinic, specialist office), under the supervision of personnel trained in the recognition and management of anaphylaxis. In resource-limited settings in which specialist referral is not readily available, alternatives may be presented in a shared decision-making context to provide assessment and opportunity for vaccination by remote consultation, use of alternative vaccine products, or delay in vaccination until a solution can be determined. | Strong | Moderate | |
| We suggest against routine H1-antihistamine or systemic corticosteroid premedication prior to vaccination because it has low-certainty evidence in preventing anaphylaxis, and theoretically, corticosteroid premedication could diminish the immune response. | Conditional | Low | |
| We recommend in favor of globally coordinated research studies being conducted to address (1) vaccine and vaccine excipient testing diagnostic accuracy for allergy to SARS-CoV-2 vaccines; (2) administration of the vaccine to individuals with prior anaphylaxis to the vaccine or vaccine excipient; (3) the necessity and efficacy of graded vaccine administration in the context of a patient with possible SARS-CoV-2 vaccine allergy; (4) the safety, efficacy, and necessity of mixing SARS-CoV-2 vaccine platforms; and (5) the incremental benefit of additional doses of an mRNA or certain adenovirus-vector vaccines following an initial dose. | Research recommendation |
Results
Part 1: Recommendations for individuals before their initial SARS-CoV-2 vaccination who do not have a history of a severe allergic reaction, including anaphylaxis, to SARS-CoV-2 vaccine or its excipients
Question 1
What is the risk of a severe allergic reaction, including anaphylaxis, to a SARS-CoV-2 vaccine in a patient with no history of a severe allergic reaction to a SARS-CoV-2 vaccine or its excipients?
Recommendation 1
For patients with no history of a previous severe allergic reaction to a SARS-CoV-2 vaccine or its excipients, the risk of SARS-CoV-2 vaccine–induced anaphylaxis is very rare and we recommend vaccination over no vaccination based on this risk.
Strong recommendation; high certainty of evidence.
Recommendation 2
For patients with a history of a severe allergic reaction, including anaphylaxis, unrelated to a SARS-CoV-2 vaccine or excipient, the requirement for additional observation beyond standard wait time (eg, recommended by local health authorities for the general population) provides a minimal absolute risk reduction in severe allergic reaction outcomes and may also contribute to vaccine hesitancy. Therefore, we suggest against prolonged observation in those with a history of severe allergic reactions unrelated to a SARS-CoV-2 vaccine or excipient.
Conditional recommendation; low certainty of evidence.
Rationale
Systematic review and meta-analysis
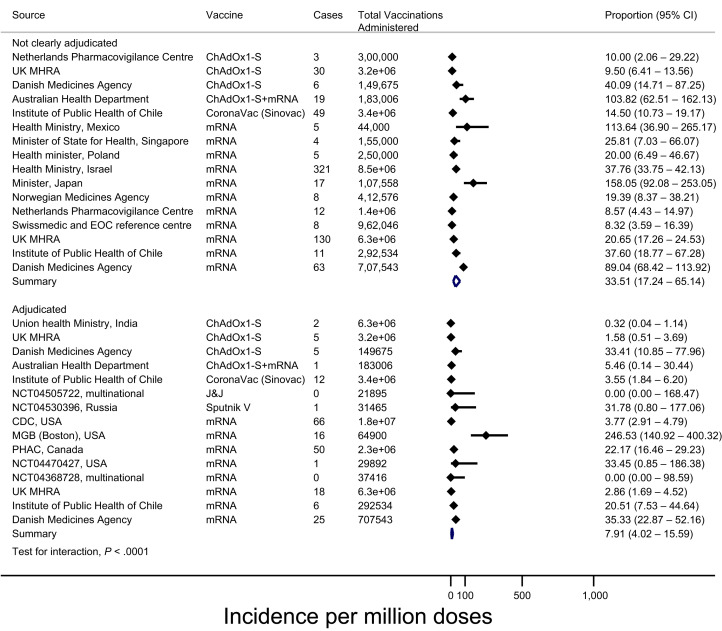
We searched the World Health Organization (WHO) Global Coronavirus database, the COVID vaccine randomized controlled trial living evidence map, government Websites, medical literature, and press releases for all estimates of anaphylaxis induced by SARS-CoV-2 vaccines up to March 19, 2021, identifying 46 total reports meeting inclusion criteria (Supplementary Methods).10 , 26 , 27 , 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72 We performed a random effects meta-analysis of proportions using hierarchical logistic regression and Clopper-Pearson confidence intervals (CIs). Among 26 reports involving reported cases adjudicated to meet BCC for anaphylaxis with a sample size of at least 20,000 doses, the meta-analyzed incidence of anaphylaxis was 7.91 per million (95% confidence interval [95% CI 4.02-15.59), and no anaphylaxis-related fatalities were reported. In multivariable meta-regression, we found that reports of anaphylaxis without adjudication were associated with a higher reported rate of anaphylaxis (odds ratio [OR] 5.53; 95% CI 4.01-7.61), and compared with mRNA-based vaccines, lower rates of anaphylaxis were associated with vaccines using adenoviral vectors (OR 0.47; 95% CI 0.33-0.68) and inactivated virus (OR 0.31; 95% CI 0.18-0.53). Findings were similar when analyzing all 46 reports (eg, regardless of sample size) (Figure 2 ). Table E2 details the certainty of evidence for this estimate, and Figure E2 the risk of bias assessment.
Figure 2.
Adjudicated and nonadjudicated internationally reported rates of anaphylaxis to SARS-CoV-2 vaccines. EOC, Ente Ospedaliero Cantonale; MGB, Mass General Brigham; PHAC, Public Health Agency of Canada; U.K. MHRA, U.K. Medicines and Healthcare Products Regulatory Agency.
Discussion
Vaccine-related fatalities from severe allergic reactions, including anaphylaxis, are very rare. Anaphylactic reactions to vaccines are historically cited at about 1.3 events per million doses.73 , 74 A 2019 study by Su et al75 using the VAERS noted a cumulative incidence rate of 17 cases of anaphylaxis per million vaccinations from 1990 to 2016. By comparison, the published rate for anaphylaxis to penicillin is 5 to 10 cases per million persons,76 and in the general population, drug allergy fatalities occur on the order of 0.1 to 1 event per million persons.77 Moreover, the estimated fatality rate from SARS-CoV-2 viral infection as of early March 2021 ranges from 36.4 to 1,852.7 per 1,000,000 persons in countries represented by authors on this consensus document.2 Data from a physician-reported national Canadian vaccine registry showed a rate of allergic reactions of any severity to vaccines of approximately 370 reactions per million doses, with most being attributable to influenza vaccines.78 Slightly older data have noted the estimated rate of allergic reactions of any severity (including anaphylaxis) ranges between 1 and 20 per million persons.77 Because SARS-CoV-2 mRNA vaccines are novel, historical estimates of severe reactions to these agents are not available. Although fatality attributed to SARS-CoV-2 vaccines has occurred, none has been the result of a severe allergic reaction.79
Comparing reported rates of anaphylaxis following vaccination is hampered by variation in anaphylaxis definitions across regions and between organizations, as shown in Table E4 (available in this article’s Online Repository at www.jaci-inpractice.org). This causes reactions considered to be anaphylaxis by some definitions to not be considered anaphylaxis by other definitions. For example, although the BCC are the accepted standard for anaphylaxis from vaccines and are used by VAERS,24 some of the cases in the Morbidity and Mortality Weekly Reports would not meet other international diagnostic criteria, such as either the National Institutes of Allergy and Infectious Diseases (NIAID) criteria or those from the World Allergy Organization (WAO).80, 81, 82, 83 Table II demonstrates how the cases published in the medical literature by the CDC could be rated differently based on what classification system for anaphylaxis was used.84 One study has compared the NIAID criteria and BCC, noting agreement of the criteria on 92 of 128 cases (72%), which based on a kappa statistic of 0.414, is at the lower limit of what is considered statistical “moderate” agreement.85
Table II.
Comparison of reported U.S. cases of mRNA vaccine anaphylaxis using different anaphylaxis rating criteria
| Anaphylaxis definition | Certainty | Pfizer-BioNTech December 14–23, 2020 |
Moderna December 21, 2020– January 10, 2021 |
Non-COVID-19 vaccines 2009-2011, United States |
|---|---|---|---|---|
| BCC (as reported) | Levels 1-3 | 21 cases in 1,893,360 doses 11.1/million (95% CI 6.9-17.0) |
10 cases in 4,041,396 doses 2.5/million (95% CI 1.2-4.6) |
33 cases in 25,173,965 doses 1.3/million (95% CI 0.9-1.8) |
| BCC (reassessed) | Levels 1-3 | 15 cases 7.9/million (95% CI 4.4-13.1) |
5 cases 1.2/million (95% CI 0.4-2.9) |
31 cases 1.2/million (95% CI 0.8-1.8) |
| NIAID | Probable | 4 cases 2.1/million (95% CI 0.6-5.4) |
3 cases 1.5/million (95% CI 0.2-2.2) |
18 cases 0.7/million (95% CI 0.4-1.1) |
| Possible | Up to 8 cases 4.2/million (95% CI 1.8-8.3) |
Up to 6 cases 1.5/million (95% CI 0.5-3.2) |
Up to 31 cases 1.2/million (95% CI 0.8-1.8) |
|
| WAO | Probable | 10 cases 5.3/million (95% CI 2.5-9.7) |
4 cases 1.0/million (95% CI 0.3-2.5) |
25 cases 1.0 /million (95% CI 0.6-1.5) |
| Possible | Up to 12 cases 6.3/million (95% CI 3.2-11.1) |
Up to 7 cases 1.7/million (95% CI 0.7-3.6) |
Up to 31 cases 1.2/million (95% CI 0.8-1.8) |
NIAID, National Institute of Allergy and Infectious Disease; WAO, World Allergy Organization.
Reprinted with permission from Hourihane JOB, Byrne AM, Blumchen K, Turner PJ, Greenhawt M. Ascertainment bias in anaphylaxis safety data of COVID-19 vaccines. J Allergy Clin Immunol Pract 2021;9:2562-6.84
Importantly, VAERS is a passive case reporting system that allows the public, manufacturers, and health care workers to report signs and symptoms considered to be adverse events occurring around vaccine administration. The VAERS and other similar reporting systems in other countries (eg, Yellow Card Reporting in the United Kingdom) are not designed to determine causality or validity of the event in question. Such reporting makes it difficult to definitively discern objective versus subjective symptoms, making it impossible to exclude other common causes that can mimic symptoms of an acute allergic reaction. Thus, based on evidence to date, whereas severe allergic reactions, including anaphylaxis, may occur with SARS-CoV-2 vaccination, this is a rare event and represents a very low (but not completely absent) risk according to pooled adjudicated data.25 In formulating recommendations 1 and 2, we weighed the potential benefits and harms of vaccination against each other, along with consideration of patient values, preferences, and cost. Whereas we acknowledge that well-informed individuals may have values and preferences that could lead to vaccine refusal in spite of very high certainty of evidence regarding the rarity of severe allergic reactions to these vaccines, it was felt that most informed individuals would be highly likely to choose vaccination over vaccine refusal based on the very rare risk of a severe allergic reaction and the benefit of vaccination in this situation.
Risk-stratification based on allergic history
In the phase 2 and 3 clinical trials for the currently approved vaccines, participants were excluded from participation if they had a known or suspected allergy or history of anaphylaxis, urticaria, or other significant adverse reaction to the vaccine or its excipients, including PEG and polysorbate 80, but not for any other baseline allergic or atopic condition.4 , 8, 9, 10, 11 , 48 , 69 , 71 , 86 Of note, a high percentage of individuals reporting severe allergic reactions to the SARS-CoV-2 mRNA vaccines in the CDC reports also had a self-reported history of an underlying allergic condition including past medication and unrelated vaccine allergy, and were female; however, the significance and validity of these allergy diagnoses or male/female differences are difficult to assess whether these signify a risk signal or not.22 , 23 , 87 Allergic conditions are very common in the general population and self-reported allergy is typically much higher than confirmed allergy. As well, the rate of individuals with similar allergic comorbidity who successfully tolerated their vaccine has not been calculated, and this is needed to properly quantify a risk ratio related to a general or specific type of allergic history (or sex). Mechanistically, it is unclear why allergies to food, venom, or other non–PEG/mRNA-containing vaccines/medications would pose any hazard requiring additional postvaccination observation or contraindication to vaccine administration. Therefore, pending emergence of additional data showing there is a clearly increased risk relative to a control population, allergic or atopic comorbidity should not be considered a risk greater than the general population for a vaccine-associated allergic reaction, and inclusion of these precautions risks harm in promoting vaccine hesitancy.14 , 18 , 88 An ongoing study is exploring risks of SARS-CoV-2 vaccination related to allergic comorbidity (www.clinicaltrials.gov, NCT04761822).
Shaker et al89 modeled the cost effectiveness of applying broad restrictions based on underlying allergic history in the United States, and the impact of extended wait time as a precaution. Using Markov modeling and microsimulation, universal vaccination was associated with a cost savings of $503,596,316 and saved 7,607 lives versus a risk-stratified approach until vaccine-associated anaphylaxis rates were less than 0.8%. Stratified postvaccination extended observation time (comparing a standard of 15 minutes recommended by the CDC for the general population vs 30 minutes for persons considered potentially at risk for a vaccine-associated allergic reaction) by anaphylaxis history was not cost effective without greater than 1% anaphylaxis case-fatality and less than 6% risk of vaccination-associated anaphylaxis. Furthermore, withholding of a second mRNA vaccine dose after a first reaction was not cost effective unless first-dose protection was very high (meaning there was limited value of additional doses) and risk for vaccine-associated anaphylaxis with an additional dose was high90 , 91 (Figure E5 and Table E5; available in this article’s Online Repository at www.jaci-inpractice.org). Emerging data from a real-world U.S. study of both mRNA vaccines in 3,950 recipients noted an 80% relative risk reduction in SARS-CoV-2 infection risk 14 days after a single dose of either vaccine and may help to enhance this model.92
As shown in Figure 1, multiple international public health agencies and allergy organizations around the world have issued guidance related to concerns for severe allergic reactions specifically to the mRNA vaccines that generally echo the CDC recommendations. Many also emphasize shared decision-making and all suggest consideration of allergy-immunology consultation. Most lack explicit language formally recommending testing to excipients (including PEG) or the vaccine itself as part of the evaluation (owing to the absence of evidence and predictive values for the testing).88 , 93 Only the Australasian Society of Clinical Immunology and Allergy (ASCIA) and WAO suggest consideration for skin testing to the vaccine or its components (eg, PEG, polysorbate) in some cases.8 , 24 A recommendation for such patients to undergo evaluation by an allergist may or may not imply consideration for testing as part of this evaluation, although the nature of what should be part of such evaluation is not specified. Threshold agreement was achieved for the voting on these recommendations on the first round of voting (Table E3).
Question 2
In patients without a history of a severe allergic reaction, including anaphylaxis, to a SARS-CoV-2 vaccine or its excipients, should allergy skin testing to SARS-CoV-2 vaccines or its excipients be performed?
Recommendation 3
In patients with no history of a severe allergic reaction, including anaphylaxis, to SARS-CoV-2 vaccines or its excipients, we recommend against vaccine or vaccine excipient testing prior to vaccination in an attempt to predict the rare individual who will have a severe allergic reaction to vaccination.
Strong recommendation; low certainty of evidence.
Rationale
Systematic review and meta-analysis
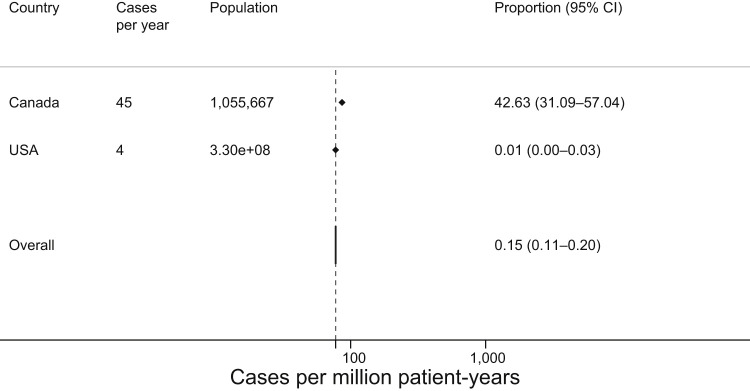
Systematic review regarding the incidence and/or prevalence of PEG or polysorbate allergy identified 1 study among 608 citations, with an additional study under review also identified.16 In a review of a Canadian national physician-reported drug allergy database entries from 2015 to 2018, Abrams et al78 found allergy to PEG or medications containing PEG/polysorbate in 135 cases out of 1,055,667 entries of allergic drug reactions, for an incidence of 42.6 cases per million person-years. This report described no cases in which PEG-allergic individuals had reported cross-reactivity to any polysorbate-containing medicine (and the individuals with polysorbate allergy only reacted to a single agent).78 In the other report, Stone et al16 compiled a review of the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database from 1989 through 2017, finding 53 probable cases of anaphylaxis to PEG among 25,905 reports, for an estimated incidence of 4 cases of PEG allergy per year (0.012 cases per million person-years). To allow for pooling, we presumed all cases in the Abrams et al78 report were anaphylaxis, and the combined incidence rate between these studies is 0.15 cases per million person-years (Figure 3 ). No studies describing a polysorbate allergy rate were identified. Table E2 details the certainty of evidence for this estimate and Figure E3 the risk of bias assessment.
Figure 3.
Pooled incidence of reported PEG allergy, in cases per million person-years.
Discussion
Historically, vaccines have contained inert excipients such as food proteins (eg, egg, gelatin, yeast) or other substances (eg, thimerosal, formaldehyde, latex, neomycin, adjuvants), as well as viral proteins and bacterial toxoids, to which reported adverse reactions, including allergic reactions of varying severity have been attributed.94 The mRNA vaccines utilize newer technology that differs from existing vaccines in that they do not contain historically commonly used excipients. Because these vaccines involve nanotechnology to deliver mRNA to recipient cells, they use other excipients, most notably PEG 2000 but also additional lipids and cholesterol.8 , 9 The AstraZeneca, Sputnik, and Johnson & Johnson vaccines each contain recombinant adenovirus and polysorbate 80.10 , 11 , 86 Adenovector virus vaccines are not novel technologies. Table III details the excipients in the SARS-CoV-2 vaccines.
Table III.
Comparison of SARS-CoV-2 vaccines and excipients
| Vaccine | Availability | Vaccine type | Excipients |
|---|---|---|---|
| CoronaVac (Sinovac, China) | Brasil, China (essential workers and high-risk groups), Indonesia, Turkey | Inactivated vaccine (formalin with alum adjuvant) | Aluminum hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride |
| Convidicea Ad5-nCoV (CanSino Biologics, Beijing Inst. Biotech., NPO Petrovax) | China (limited to military use only), Mexico, Pakistan | Recombinant adenovirus type 5 vector against spike RBD protein | NA |
| BBIBP-CorV (Sinopharm, Beijing Institute and Wuhan Institute of Biological Products) | China, Bahrain, Egypt, United Arab Emirates | Inactivated SARS-CoV-2 (vero cells) + aluminum hydroxide adjuvant | Aluminum hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, sodium hydroxide, sodium bicarbonate, M199 |
| Pfizer-BioNTech BNT162b2 | Argentina, Australia, Bahrain, Canada, Chile, Costa Rica, Ecuador, European Union, Israel, Japan, Jordan, Kuwait, Mexico, Oman, Panama, Saudi Arabia, Singapore, Switzerland, United Kingdom, United States, WHO. | mRNA-based vaccine (encoding the viral spike (S) glycoprotein) | (4-hydroxybutyl) azanediyl)bis (hexane-6,1-diyl)bis(2-hexyldecanoate)] (ALC-0315), 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159),1,2-Distearoyl-sn-glycero-3-phosphocholine cholesterol, potassium chloride, potassium dihydrogen phosphate, sodium chloride, disodium hydrogen phosphate dihydrate, sucrose, water for injection |
| Moderna mRNA-1273 | Canada, European Union, Israel, Switzerland, United Kingdom, United States | mRNA-based vaccine (encoding the prefusion stabilized spike (S) glycoprotein) | Lipids (SM-102, 1,2-dimyristoyl-rac-glycero3-methoxypolyethylene glycol-2000 [PEG2000-DMG], cholesterol, and 1,2-distearoyl-snglycero-3-phosphocholine [DSPC]), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate, and sucrose |
| ChAdOx1 (Oxford/AstraZeneca; Covishield in India) | Argentina, Australia, Canada, Dominican Republic, El Salvador, European Union, India, Mexico, Morocco, United Kingdom | Replication-deficient viral vector vaccine (adenovirus from chimpanzees) | L-Histidine, L-histidine hydrochloride monohydrate, magnesium chloride hexahydrate, polysorbate 80, ethanol, sucrose, sodium chloride, disodium edetate dihydrate, water for injection |
| Covaxin (BBV152) (Bharat Biotech, India) |
India | Inactivated vaccine | NA |
| Sputnik V (Gamaleya Research Institute) | Russia, Palestine | Nonreplicating, 2-component vector (adenovirus) against spike (S) glycoprotein | Tris (hydroxymethyl) aminomethane, sodium chloride, sucrose, magnesium chloride hexahydrate, sodium EDTA, polysorbate 80, ethanol, water for injection |
| EpiVacCorona (Federal Budgetary Research Institution State Research Ctr, Russia) | Russia | Peptide vaccine with alum adjuvant | Aluminum hydroxide, potassium dihydrogen phosphate, potassium chloride, sodium hydrogen phosphate dodecahydrate, sodium chloride, water for injection |
EDTA, Ethylenediaminetetraacetic acid; NA, Not applicable; RBD, receptor binding domain; WHO, World Health Organization,
Adapted with permission from Turner PJ, Ansotegui IJ, Campbell DE, Cardona V, Ebisawa M, El-Gamal Y, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J 2021;14:100517.4
From a societal standpoint, the population prevalence of PEG and polysorbate allergies are very rare. In addition, data regarding population-based skin test screening using SARS-CoV-2 vaccine are sparse—at the time of writing only 1 published study detailing SARS-CoV-2 prick testing prior to initial vaccination for persons (who had not yet received SARS-CoV-2 vaccine) with a general history of anaphylaxis, which noted no positive skin tests.95 The very rare rate of adjudicated cases of SARS-CoV-2 vaccine anaphylaxis and a lack of evidence that COVID-19 vaccine reactions are attributable to PEG or polysorbate strongly suggest against any population-based approach that would involve screening for preexisting specific immunoglobulin E (IgE) against PEG or polysorbate as a means to predict risk of a severe allergic reaction to a SARS-CoV 2 vaccine.14 , 18 Threshold agreement was achieved for the voting on this recommendation on the first round of voting (Table E3).
Part 2: Recommendations for individuals before their initial or additional SARS-CoV-2 vaccination who have a history of an allergic reaction to SARS-CoV-2 vaccine or its excipients
Question 3
In patients with a history of a severe allergic reaction, including anaphylaxis, to a SARS-CoV-2 vaccine or its excipients, should allergy skin testing to SARS-CoV-2 vaccines or its excipients be performed to determine whether vaccine withholding is needed?
Recommendation 4
We suggest against the clinician routinely performing skin or in vitro testing using SARS-CoV-2 vaccines or excipients outside of the research setting for the purpose of vaccine withholding, given such testing has unknown sensitivity/specificity in predicting severe allergic reactions, including anaphylaxis, to SARS-CoV-2 vaccines.
Conditional recommendation; low certainty of evidence.
Rationale
Systematic review and meta-analysis
The utility of PEG testing to inform SARS-CoV-2 vaccine risk of allergic reaction is uncertain. However, data are available to understand how well such testing performs in evaluating persons with a known or suspected allergic reaction to PEG or polysorbate in other contexts. A systematic review identified 21 studies (all case reports/series) out of 548 citations (excluding duplicates) that described skin testing (skin prick or intradermal) to PEG of any molecular weight and/or polysorbate 80, in which control subjects were also tested.15 , 16 , 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114 A positive case was defined as any method of skin testing performed with PEG or polysorbate concentration in which the authors deemed the test positive for identification of a wheal and/or flare, without any specific or uniform threshold reported. For PEG, there were 15 reports detailing skin testing to varying agents and concentrations.15 , 16 , 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108 Following removal of 7 positive skin tests that were duplicated from 5 other studies in 1 large case series of 37 cases since 1977, we calculated a pooled sensitivity of 58.8% (30 true positive, 21 false negative) and specificity of 99.5% (247 true negative, 1 false positive). However, the specificity may be falsely high owing to the case series/report study designs. As well, among the reports, not all patients were challenged to confirm the history of PEG reaction, the diagnostic gold standard, which lowers the certainty of evidence owing to indirectness of the testing. There were 6 reports detailing 7 total patients with suspected allergy to polysorbate that had skin testing (no false positives, and 57 controls tested that were nonreactive).109, 110, 111, 112, 113, 114 Polysorbate testing sensitivity/specificity were not calculated given the low number of patients tested. Table E2 details the certainty of evidence for this estimate and Figure E4 the risk of bias assessment.
The same systematic review identified no studies that investigated vaccine or excipient testing compared with negative controls in individuals with a known or suspected allergic reaction of any severity to a SARS-CoV-2 vaccine. However, 4 case reports (without controls) were identified describing testing with SARS-CoV-2 mRNA vaccine or PEG/polysorbate in persons with reported severe allergic reactions to the vaccine, and vaccine testing in negative controls to establish a nonirritating testing concentration.115, 116, 117 The first report details negative vaccine skin testing among 4 persons with reported allergic reactions to their initial vaccination, 3 of whom elected to receive a second dose and did so without reaction.116 The second report details negative PEG 3350 testing in 8 persons with reported allergic reactions their initial vaccine, 7 of whom elected to receive a second dose and did so without reaction; as well as negative polysorbate 80 testing in 6 of 7 persons tested prior to their initial vaccination, with all 6 receiving their initial dose without reacting.115 The third report details no irritant response detected among 55 negative controls tested to SARS-CoV mRNA vaccine.117 The fourth detailed 2 adult patients with severe allergic reactions to their initial mRNA (Moderna) vaccine, who underwent skin testing to PEG, polysorbate, and the mRNA vaccine itself. In both patients, excipient skin testing was negative but intradermal skin tests to the Moderna vaccine were positive. However, both tolerated graded dosing administration of the vaccine without issue.118 Lastly, 1 additional case report of PEG testing was identified in a female patient with a past history of skin and oropharyngeal reactions to PEG-containing products, who sought evaluation after experiencing a reported systemic reaction to her initial dose of the Pfizer-BioNTech vaccine that was treated with epinephrine but was not associated with tryptase elevation. While undergoing testing, approximately 12 minutes after PEG 4000 prick skin test was applied, she developed a multisystem systemic reaction treated with epinephrine, which also was not associated with tryptase elevation. Skin testing to the Pfizer-BioNTech vaccine and to PEG molecular weight less than 4,000 were all negative, and neither vaccine rechallenge nor challenge to PEG 4000 or any other molecular weight PEG were attempted.119 One other case report was noted regarding an atopic female with a past episode of urticaria attributable to a topical product possibly containing PEG, who developed an erythematous rash, then slurred mouth and hoarseness over the course of 5 hours after her initial Pfizer-BioNTech vaccine (although she had no symptoms at 30 minutes). Her serum tryptase level was normal, and this reaction occurred despite steroid premedication starting 14 hours before vaccination and antihistamine an hour before vaccination. During evaluation by an allergy specialist 2 weeks after the event, whereas no PEG or vaccine skin testing was reported, she was noted to have an elevated basophil activation test (14.79 CD203chigh%) to PEG 4000 at 0.2mg/mL (and no activation seen in 5 negative control patients).120
Discussion
PEG-2000 is speculated to be a potential culprit in allergic reactions to SARS-CoV-2 mRNA vaccines.14 , 121 PEG and polysorbate are ubiquitous compounds, found in many health and beauty products and in foods and beverages, and are routinely used and tolerated in daily life.14 There are limited case reports in the medical literature of allergic reactions attributable to PEG contained in therapeutic agents and radiocontrast media.15 , 16 , 96 , 122, 123, 124 PEG is available in different molecular weights and structural formats. Allergic reactions may be more likely with higher molecular weight PEG agents, higher concentrations, or parenteral administration. It is uncertain whether the concentration and molecular weight of PEG in SARS-CoV-2 mRNA vaccines would be sufficient to cause reactions.15 , 16 There is theoretical concern, but limited evidence, that PEG may cross-react with certain polysorbates.16 Polysorbate 80 in adenovirus-vector vaccines was not implicated as causing allergic reactions in their trials, and there is limited reporting of allergic reactions of any severity with these vaccines.
Evaluation for suspected PEG allergy is largely based on history. Use of high molecular weight PEG solutions as skin testing agents has not consistently demonstrated the ability to elicit wheal and flare responses, and it remains unclear whether this approach would reliably indicate the presence (or absence) of specific IgE.123 In this meta-analysis, the pooled sensitivity of PEG skin testing is only 58.8%, but the pooled specificity is 99.5%, which implies that the test will miss identifying the presence of PEG-specific IgE in a significant number of PEG-allergic patients, but non–PEG-allergic individuals do not appear to have false-positive tests. There have been few cases of anaphylaxis reported to intradermal skin testing, mainly using PEG with molecular weight greater than 3,000. Other nonvalidated methods of detection for PEG-induced allergic response have been described, as has PEG oral provocation challenge.15 , 16 , 96 In individuals with a high pretest probability of PEG allergy, testing to PEG from these data in the meta-analysis show a very uncertain (very serious risk of bias) high positive likelihood ratio to confirm the diagnosis. However, this is distinct from the suggestion that PEG testing can inform the likelihood of a SARS-CoV-2 vaccine reaction (very serious indirectness). This was noted in case series to date in which 10 persons who reacted to their mRNA vaccine, in fact, had negative PEG skin testing, and in which 2 patients additionally were skin tested to PEG as well as to both polysorbate (negative) and the Moderna vaccine (positive intradermal test) and tolerated readministration.115 , 118 In the case of a person with a mRNA vaccine reaction in which PEG sensitization was detected, this was only to PEG 4000, and not the vaccine or the concentration of PEG in the vaccine. There is not yet any convincing evidence to confirm PEG as a causative agent in the reported SARS-CoV-2 mRNA vaccine reactions and the diagnostic utility of PEG and polysorbate allergy testing is highly uncertain.14 , 18 , 88 , 121 Of note, skin testing to the vaccines is not approved under any of the EUAs. Further research regarding excipient and vaccine skin testing in this context is needed.
Other mechanisms might potentially cause allergic symptoms following administration of SARS-CoV-2 mRNA vaccines. Complement Activation–Related Pseudoallergy (CARPA) has been theorized as a possible mechanism, in particular with lipids used in drug delivery.125 , 126 The mRNA particles themselves may also potentially be a causative agent, acting as a direct mast cell degranulation agent. There is 1 report that trometamol, a buffer specific to the Moderna vaccine, was associated with anaphylaxis to gadolinium-based contrast agents, also by a direct degranulation mechanism.127 Toll-like receptors 3, 7, and 8 can be directly activated by double-stranded and single-stranded RNA particles from viruses and can cause mast cell degranulation.128 , 129 Although the likelihood of such events from a SARS-CoV-2 mRNA vaccine is unclear, these pathways should be considered among the potential mechanisms for allergic reactions to these vaccines.130
Caution should be exercised in considering excipients as a provoking cause of vaccine reactions. Whereas excipients have occasionally caused allergic reactions with other vaccines, historically many excipients have also been wrongly implicated based on theoretical rather than actual risk, most prominently egg (ovalbumin) in modern influenza vaccines.73 , 94 , 131 This may be due to excipient concentrations being well below a threshold necessary to provoke a reaction.132 , 133 This may also prove to be the case for excipients that are suspected triggers for the SARS-CoV-2 vaccine reactions.
There is 1 published approach for SARS-CoV-2 mRNA vaccination with guidance based on skin testing to excipients.14 , 18 Notably, if methylprednisolone acetate is used for testing, the clinician should be aware this may contain both PEG and polysorbate 80, and should test only with brands of methylprednisolone that do not also contain polysorbate 80.14 The clinician should also be aware that the quantity of PEG administered by intradermal testing using this agent could exceed the quantity of PEG in the Pfizer-BioNTech mRNA vaccine, which only contains 0.05 mg PEG 2000 per dose (Table E6; available in this article’s Online Repository at www.jaci-inpractice.org). Therefore, even in persons with known PEG allergy who have positive PEG skin testing, it is likely that graded or full dose SARS-CoV-2 mRNA vaccine would be well tolerated if no systemic allergic reaction occurred during skin testing. The positive PEG test is less problematic to interpret, given this specificity, but no data exist to specify that positive PEG sensitization definitively infers a significant risk that such patients will not tolerate PEG-containing vaccines. As such, there is no clear role for the use of skin testing to PEG to inform a decision to withhold the vaccine or not, which is consistent with the last published vaccine allergy practice parameter, which suggests excipient- or vaccine-sensitized individuals should be offered a graded-dose challenge or multistep desensitization instead of withholding the vaccine.14 , 94 Data regarding skin testing to SARS-CoV-2 vaccine itself are scant. There are a small number of patients with suspected SARS-CoV-2 vaccine reactions who have undergone vaccine testing, most with negative results, but all who were willing to receive a second dose tolerated it without reaction irrespective of positive vaccine skin testing.115 , 116 , 118 The 3 individuals with a history of mRNA reaction with sensitization to either the vaccine or PEG either declined or were not offered the opportunity to receive a second dose. If vaccine testing is to be performed in persons hesitant to proceed with vaccination, the vaccine should be prepared per manufacturer instructions with regard to storage, thawing, and diluting for reconstitution. Consistent with the last published vaccine practice parameter, patients with positive skin testing to a SARS-CoV-2 vaccine may receive vaccination through graded or full dosing (see later).118 Limited safety data are available for testing to the vaccine or the vaccine excipients in this context. Further details regarding international variation about recommendations for vaccine/excipient testing are detailed in Figure 1.
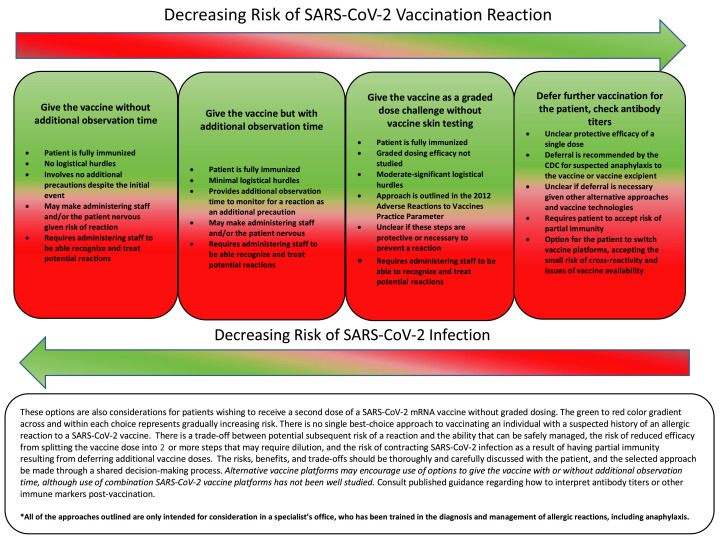
Whereas testing may be considered as a preference-sensitive option in shared decision making, options for cautious administration of vaccine (Figure 4 ) or the use of an alternative vaccine with a different platform and excipient may be additional considerations. Testing for PEG has not been shown to be necessary or of value in individuals with a history of severe allergic reactions, including anaphylaxis, to mRNA SARS-CoV-2 vaccines, nor has PEG yet been demonstrated as the causative agent of mRNA SARS-CoV-2 vaccine–allergic reactions. Moreover, it has poor sensitivity in evaluating those with known severe allergic reactions to PEG. However, PEG testing may be considered in the very narrow context of shared decision-making in individuals who would otherwise be unwilling to be vaccinated and would decline vaccination if not offered testing. Testing-specific guidance could change if and when convincing evidence emerges for a role for PEG as a culprit agent. The PEG 2000, the actual excipient, would likely be preferable to higher molecular weight PEG as a testing agent, if available. Given the low mortality of anaphylaxis (<0.5%) in general,134 and the higher mortality/morbidity of COVID-19, the risk of withholding SARS-CoV-2 vaccination is highly likely to exceed the risk of a severe allergic reaction, including anaphylaxis, after administration of the vaccine for many patients. However, this consideration regarding withholding additional vaccine doses could change if additional estimates emerge of potentially high efficacy of a single dose of an mRNA vaccine, which could allow more time for decision making regarding the risk/reward of additional dosing.92 Threshold agreement was achieved for the voting on this recommendation on the first round of voting (Table E3).
Figure 4.
Considerations for options in the approach to SARS-CoV-2 vaccination in patients with known or suspected reaction to a SARS-CoV-2 vaccine or vaccine excipient.
Question 4
Should SARS-CoV-2 mRNA or adenovirus-vector vaccines be administered to an individual who had an immediate allergic reaction to the first dose of the vaccine (defined as a generalized, systemic allergic reaction with acute onset occurring within 4 hours of vaccine administration) or given as a first dose to an individual who is suspected to have reacted previously to an excipient ingredient that is also present in the SARS-CoV-2 mRNA or adenovirus-vector vaccines?
Recommendation 5
We recommend a shared decision-making paradigm of care favoring vaccination through full or graded dosing (with or without additional observation time post-vaccination) or changing vaccine platforms to another agent over no vaccination because there is no single best approach to assessment and management of the patient with a suspected SARS-CoV-2 mRNA or adenovirus-vector vaccine reaction or the patient with an allergy to an excipient in either of these vaccines who has not yet been vaccinated.
Strong recommendation; moderate certainty of evidence.
Recommendation 6
In patients with a suspected immediate allergic reaction to SARS-CoV-2 vaccine whose standard schedule requires more than 1 dose, we recommend referral to an allergist for assessment of additional vaccination over no vaccination/vaccination being withheld. In resource-limited settings in which specialist referral is not readily available, alternatives may be presented in a shared decision-making context to provide assessment and opportunity for vaccination by remote consultation, use of alternative vaccine products, or delay in vaccination until a solution can be determined.
Strong recommendation; moderate certainty of evidence.
Recommendation 7
In patients with a suspected or confirmed but remote past medical history of reaction to a SARS-CoV-2 vaccine excipient, we recommend referral to an allergist for assessment of additional vaccination over no vaccination/vaccination being withheld. In resource-limited settings in which specialist referral is not readily available, alternatives may be presented in a shared decision-making context to provide assessment and opportunity for vaccination by remote consultation, use of alternative vaccine products, or delay in vaccination until a solution can be determined.
Strong recommendation; moderate certainty of evidence.
Recommendation 8
In patients with a definite/confirmed recent allergic reaction to SARS-CoV-2 vaccine and/or excipient, we recommend referral to an allergist for assessment of additional vaccination over no vaccination/vaccination being withheld. In resource-limited settings in which specialist referral is not readily available, alternatives may be presented in a shared decision-making context to provide assessment and opportunity for vaccination by remote consultation, use of alternative vaccine products, or delay in vaccination until a solution can be determined.
Strong recommendation; low certainty of evidence.
Recommendation 9
Whereas all vaccines should be administered in facilities capable of treating anaphylaxis, particularly for individuals with a prior immediate systemic allergic reaction to a SARS-CoV-2 vaccine or vaccine excipient, we recommend the clinician should administer SARS-CoV-2 mRNA or adenovirus-vector vaccine in a setting equipped to manage anaphylaxis (eg, hospital, mass immunization clinic, specialist office), under the supervision of personnel trained in the recognition and management of anaphylaxis. In resource-limited settings in which specialist referral is not readily available, alternatives may be presented in a shared decision-making context to provide assessment and opportunity for vaccination by remote consultation, use of alternative vaccine products, or delay in vaccination until a solution can be determined.
Strong recommendation; moderate certainty of evidence.
Recommendation 10
We suggest against routine H1-antihistamine or systemic corticosteroid premedication prior to vaccination because it has low-certainty evidence in preventing anaphylaxis, and theoretically, corticosteroid premedication could diminish the immune response.
Conditional recommendation; low certainty of evidence.
Recommendation 11
We recommend in favor of globally coordinated research studies being conducted to address (1) vaccine and vaccine excipient testing diagnostic accuracy for allergy to SARS-CoV-2 vaccines; (2) administration of the vaccine to individuals with prior anaphylaxis to the vaccine or vaccine excipient; (3) the necessity and efficacy of graded vaccine administration in the context of a patient with possible SARS-CoV-2 vaccine allergy; (4) the safety, efficacy, and necessity of mixing SARS-CoV-2 vaccine platforms; and (5) the incremental benefit of additional doses of an mRNA or certain adenovirus-vector vaccines following an initial dose.
Research recommendation.
Rationale/discussion
Almost all allergy organizations recommend that the routine administration of SARS-CoV-2 vaccines are contraindicated in individuals with a suspected immediate allergic reaction to the SARS-CoV-2 vaccines or their components (ie, PEG or polysorbate 80), although all allow consideration of referral to an allergist-immunologist to determine the likelihood the initial reported reaction was consistent with an allergic reaction of any severity, and if so, whether a SARS-CoV-2 vaccine can be safely administered (Figure 1). There is similar consistency in the precaution for patients with nonanaphylactic reactions to the initial vaccine dose (and for the CDC, a history of immediate allergic reactions to other vaccines or injectable medications) to be considered for vaccination under observation.86 , 135 The Sputnik vaccine is contraindicated in people with a hypersensitivity to any of the vaccine components or any vaccine containing similar components, as well as contraindicated in people with a history of severe allergic reaction to the vaccine or its excipients. In addition, the second dose of the Sputnik vaccine is contraindicated where severe complications, such as anaphylactic shock, severe generalized allergic reaction, seizures, or fever over 40°C were noted after the first dose.136 The CDC has issued a precaution to receiving the Johnson & Johnson vaccine in persons in whom the SARS-CoV-2 mRNA vaccine is contraindicated and suggested an option for administration of the Johnson & Johnson vaccine at least 28 days after the initial SARS-CoV-2 mRNA vaccine dose, although the incremental safety and effectiveness of such a substitution is unknown.137 The Johnson & Johnson vaccine carries with it a risk of thrombotic events (as does the AstraZeneca vaccine), which should be a consideration in this decision.138 , 139
Preference-sensitive care is defined by scenarios in which there are multiple treatment options having significant trade-offs and varying potential outcomes, where treatment decisions reflect the patient’s personal values and preferences.140 Despite general international agreement that evaluation by an allergist-immunologist be considered for patients with suspected risk of allergic reaction to the vaccine, there is no elaboration on how the allergy specialist should evaluate these patients to determine whether vaccination is advisable and how to proceed. The allergist can be of central importance in this evaluation process by taking a detailed history that thoroughly investigates the nature and timing of the reported reaction and carefully considers the nature of the reported symptoms and whether they are the result of an immune-mediated process and not the result of any mimicking condition that could be the result of a nonallergic process. This step alone is of critical importance and may represent the greatest value that the allergist can offer as an expert in such evaluation. Also critical to involvement of the allergist in this process is for the persons providing vaccination to be trained to ask questions regarding risk of reaction and help facilitate referral to the allergist if there is a contraindication to receiving a dose or in the case of a suspected allergic reaction occurring at their site. If possible, patients with a history of a possible allergy to PEG, polysorbate, other vaccines, or even their initial SARS-CoV-2 vaccine should be encouraged to be proactive in discussing this with their primary care provider before seeking initial or additional SARS-CoV-2 vaccine doses.
The 2012 Adverse Reactions to Vaccine Practice Parameter (from the American Academy of Allergy Asthma and Immunology [AAAAI] and American College of Allergy Asthma and Immunology [ACAAI] Joint Task Force on Practice Parameters) outlines a general approach for a patient with a known or suspected history of a vaccine/vaccine excipient reaction.94 This includes possible vaccine and excipient testing and several contextualized ways for vaccines to be administered. This approach recognizes that, whereas administration of a vaccine in routine medical settings (eg, nonallergy specialist) is contraindicated, with allergy specialist evaluation and supervision, the vaccine can still potentially be given. The ACAAI COVID-19 task force outlines, but does not endorse, an approach of graded vaccine challenge that could be considered for certain individuals. At least 1 case report has demonstrated this is both safe and feasible, although it is unclear whether this is necessary.118 The ASCIA, Canadian Society of Allergy and Clinical Immunology (CSACI) and European Academy of Allergy and Clinical Immunology (EAACI) provide guidance for graded challenges in these patients and are consistent with the 2012 Practice Parameter.21 , 141 , 142 However, none of these recommendations have data denoting safety.
The COVID-19 pandemic and ramifications of SARS-CoV-2 infection represent a highly unique situation relative to other infectious diseases in terms of the global burden of this disease, and an imperative international public health need to attain herd immunity through vaccination. Any potential for vaccine refusal or the vaccine being withheld in a pandemic must be approached from an ethical perspective that considers whether SARS-CoV-2 infection and viral propagation are riskier outcomes than vaccination in a setting in which severe allergic reactions can be managed.143 Whereas no single best choice in these contexts exists, it can be addressed through an individualized, shared decision-making approach reviewing treatment options and the risk-to-benefit trade-offs related to vaccine reactions versus SARS-CoV-2 infection risk. Regardless of how the vaccine is administered, we recommend against routine premedication prior to vaccination (particularly with glucocorticosteroids) because it has low-certainty evidence in preventing anaphylaxis, and glucocorticosteroid premedication (or glucocorticosteroid treatment of any suspected allergic reactions to SARS-CoV-2 vaccines including anaphylaxis) could diminish the immune response.144 , 145
Figure 4 outlines preference-sensitive approaches to the individual who had an allergic reaction to the initial dose of the vaccine. The allergist’s role here is central as well in helping a patient clarify their values as to their choices and the outcomes of those choices in proceeding with or declining vaccination. These approaches are only recommended for trained experts in the diagnosis of medication allergy (including allergic reactions to vaccines and medication-provocation challenge), in facilities staffed with personnel skilled and trained to be able to assess and treat an allergic reaction resulting from testing or vaccine administration. Resources must be available to provide direct observation of patients for a minimum of 30 minutes after the vaccine is administered. The outlined approaches all involve some degree of trade-off, and the ultimate choice must be informed by the values, goals, and preferences of patients. These issues must be balanced against increased infection risk, with increased morbidity and mortality, which becomes a risk from vaccine refusal or withholding vaccination. Graded vaccine challenge does offer some practical reassurance to the patient and clinician reluctant to proceed with single-dose vaccination, although full-dose vaccination may be just as safe as a graded approach in many circumstances. In resource-limited settings or if consultation with an allergy specialist is not readily available, such as in rural settings or in low- and middle-income countries, such shared decision making does not need to be exclusively provided by a specialist. Although specialist referral would be preferred, any clinician may engage the patient in shared decision-making based on the options in Figure 4, allowing for local context in decision making, with input to the patient from the practitioners who are trusted by the local population. In particular, if first-dose protection of any SARS-CoV-2 vaccine is high, or there is availability of an alternative vaccine platform, particular preference may be given for vaccine refusal/withholding the vaccine or administering this alternative agent in certain local and regional settings.
Experience with graded challenge with SARS-CoV-2 mRNA vaccines is limited at present to 1 very small case series. The efficacy of graded vaccination is unknown, although 1 report has shown this can be successful.118 It is unknown whether splitting doses and/or diluting the vaccine outside of the initial manufacturer recommendation would disrupt the vaccine integrity or impair immune response. However, these variables may potentially contribute to unforeseen issues in trying to adapt previously recommended approaches for vaccine administration where there is concern of possible allergy, rendering this option for graded-dose administration less preferable than single-dose administration.94 Switching from one vaccine platform to another is an additional alternative to consider in this decisional matrix that may reduce reliance on graded dosing and may be a more feasible office-based choice, which is endorsed by a number of international regulatory health agencies on which multiple vaccine options are approved (Figure 1).137
The evaluation of individuals with a known or suspected PEG allergy (including anaphylaxis) but who have not yet received a vaccine is distinct. An algorithm for excipient testing has been published, but without evidence that the vaccine excipients are the cause of any vaccine reactions or that excipient allergic/sensitized individuals cannot tolerate the vaccine. Our meta-analysis suggests skin testing to excipients as a screening measure prior to vaccination has low sensitivity and is unlikely to reliably identify persons at risk. A choice to test and then recommend a sensitized person not receive an mRNA vaccine could unnecessarily withhold an intervention based on speculation of risk or force choice of an alternative vaccine platform (if available) that may have different nonimmunological risks. However, this could be a preference-sensitive option that concerned patients elect, and a negative skin test could be viewed as reassuring to proceed with use of an mRNA vaccination, despite the test having poor sensitivity. Similarly, it is reasonable to administer an mRNA vaccine following a shared decision-making process with an allergy specialist, as either a single or a graded dose, or to provide an adenovirus-vector vaccine (with precaution given polysorbate content) without testing. For individuals with a polysorbate allergy, it is reasonable to administer an adenovirus-vector vaccine following a shared decision-making process with an allergy specialist, as either a single or a graded dose, or to provide an mRNA vaccine (with precaution given PEG content) without testing. Either vaccine should be given in a setting in which the patient can be monitored for up to 30 minutes after vaccination and that is staffed and equipped to treat potential vaccine-associated anaphylaxis.
Withholding or refusing a second dose of an mRNA vaccine (either Moderna or Pfizer-BioNTech) has been considered by some, in the belief that the initial dose will provide adequate protection. Although we lack precise estimates of what constitutes durable protective adaptive immunity postvaccination, data from the EUA suggest there is 50% or greater protection at 2 or more weeks after the first dose (prior to administration of the second dose). Israeli reports suggest 33% to 60% protection more than 2 weeks after the first dose up to the time of the second dose.146 Additional studies have suggested first-dose protection of up to 93% but with wide ranges of protection reported.9 , 58 , 90 , 92 , 147, 148, 149 However, the duration of this partial protection is unknown, but likely shorter-lived than the immunity achieved after 2 doses or achieved naturally from SARS-CoV-2 infection. Optimal longer-term protection would be ensured by complete vaccination. Partial immunity at a community level is of concern because it might theoretically enable the spread of more virulent variants of the virus. Even within a shared decision-making paradigm, vaccine refusal or withholding a vaccine should remain an option of last resort, and all steps taken to avoid this outcome. Withholding/refusal, if chosen, should be clearly documented in the medical record, and the decision to do so made collaboratively by the patient after having been provided data regarding the nature of the risks.90
Threshold agreement was achieved for the voting on these recommendations on the first round of voting (Table E3).
This document has several limitations. First, SARS-CoV-2 vaccination is a new and evolving topic, and data to date regarding allergic reactions, potential provoking causes of these reactions, and best management practices are limited. As such, there is intent to continue to reevaluate and update these recommendations as more data are published regarding reactions and their management. Second, GRADE is one evidence-rating approach and has well-described limitations. Third, in this context, several recommendations are conditional, and have low certainty of evidence, which underscores the first limitation. This in particular may impact recommendations regarding excipient and/or direct vaccine testing, as well as considerations about withholding/refusing vaccination (initial or subsequent doses) or the best initial choice of a vaccine given a patient’s history. Fourth, to perform the meta-analysis and data pooling for questions 2 and 3, we made 2 major assumptions: (1) we considered all reactions coded in a Canadian drug allergy database as allergy to PEG or PEG-containing medications as anaphylaxis; and (2) we considered any positive PEG skin test, by any method (prick or intradermal), to any listed concentration, as the same for the purposes of denoting a positive result to assess testing sensitivity and specificity. Both these assumptions allow for a liberal consideration of what defines a case, and increases the potential sensitivity of the analysis, at the risk of inflating both estimates. However, given limited data, we feel these are justified. Fifth, whereas our approach and recommendations were developed through systematic evidence synthesis, in some instances, these recommendations contradict more empirical approaches from allergy professional societies and governmental health authorities. It is of note that no approach or recommendation—ones made in this document or made previously by any society or governmental health authority—have been proven safe by high certainty of evidence in the context of evaluating suspected allergic reactions to these vaccines. Lastly, our recommendations may be limited to the populations that have been studied. Our approaches and considerations may be more or less relevant in particular countries or regions, depending on government policy, access to vaccination, access to allergy specialists for evaluation of potential reactions, and availability of resources for some of the options in our shared decision-making paradigm. In this analysis, there were no studies identified that addressed this issue in the context of low- to middle-income areas or resource-limited areas, and most of the studies on vaccine anaphylaxis are from tertiary-care centers, which by definition are areas with access to specialty care, including allergists. In areas without access to an allergist, remote health care options or involvement of a health care provider who can assess and manage vaccine reactions could be considered, although this does not address individuals without access to care or locations lacking in some of the resources described. It is possible that recommendations made by an allergy specialist who is not actually administering the vaccine to another care provider who is doing so may not necessarily be accepted by others who may have less experience in treating anaphylaxis, or with administering a vaccine to someone who has been evaluated for a vaccine-associated allergic reaction. These factors may modify the recommendations stated. The Evidence to Decision Framework supplement provides a summary reflection of the evidence in the context of the clinical recommendation and helps balance the recommendations in light of these limitations.
Conclusion
The SARS-CoV-2 is a devastating global pandemic with limited treatment options, which has caused more than 3,000,000 global fatalities and more than $10 trillion in global economic costs.3 To achieve an optimal population herd immunity and mitigate the impact of this infection, vaccination represents the optimal global strategy. Multiple vaccines have proven to be safe and effective in clinical trials and are now in use. Potential allergic reactions, including anaphylaxis, have been reported, but these have occurred very rarely. No uniform recommendations exist on an approach to the assessment and management of individuals with a known or suspected allergy to the excipients or the vaccine itself. This document provides evidence-based (where available) expert consensus that incorporates guidance from clinical specialists in Australia, Canada, Europe, Russia, South Africa, the United States, and the United Kingdom stressing a patient-centered approach involving consideration of the risks and benefits of receiving SARS-CoV-2 vaccination, the value and cost effectiveness of testing and precautionary measures, and shared decision making in recognition of multiple preference-sensitive approaches to this issue from an international perspective. There is no clear evidence that any preexisting factor, or testing with the vaccine or excipients, can predict risk of reaction to the vaccine. Because this is the first experience with these vaccines, this is intended to be a living document that will require periodic updating owing to still-emerging needs assessment, including further research data on the nature of vaccine-associated reactions and the necessity of potential risk-assessment measures. There are continued knowledge gaps and unmet needs outlined in Table IV that must be addressed as the vaccine roll-out continues. Given an imperative global need to vaccinate as many people in as many countries as possible, the clinician should be familiar with the risk of potential reactions to these vaccines and the approach to their evaluation and management.
Table IV.
Knowledge gaps and unmet needs regarding SARS-CoV-2 vaccination and risk of allergic reactions
| Knowledge gaps |
| Definitive identification of an immunological mechanism for reactions |
| Determination of a known excipient(s) as an allergen |
| Determination of risk for receiving SARS-CoV-2 vaccines containing an excipient to which a recipient is allergic |
| Determination of risk in receiving a second dose of a SARS-CoV-2 vaccine after an allergic reaction to the first dose |
| Establish test sensitivity, specificity, and reliability for use of the vaccine and/or vaccine excipients as a testing reagent |
| Accurate determination of the incidence of allergic reactions, including anaphylaxis |
| Identification of potential risk factors associated with allergic reactions |
| Necessity of testing or how test results may influence patient hesitancy to receive vaccination |
| Necessity of single vs graded/split dosing for risk assessment |
| Necessity of additional postvaccination observation time for risk assessment |
| Efficacy of mixed vaccine platform schedule |
| Stability of graded /split dosing for mRNA vaccines |
| Determination of durable immunity conferred by first dose of a vaccine to assist in determining risk/reward of additional doses |
| Unmet needs |
| Consensus on reporting standards for anaphylaxis related to vaccines (BCC vs NIAID or WAO criteria) |
| Development of an active surveillance system for vaccine reactions |
| Preparedness and training of personnel at vaccination clinics to properly identify and treat potential anaphylaxis. |
| Consideration for use of placebo dosing, under a shared decision-making paradigm, for determining validity of a reaction in patients with underlying anxiety |
| Evidence-based algorithmic approach to individuals with reactions to the first dose of a SARS-CoV-2 vaccine or who have a history of SARS-CoV-2–vaccine excipient allergy |
| Handling of assessment of patients with a vaccine reaction or an excipient allergy in settings in which there is reduced access to specialist care or persons with experience in managing anaphylaxis (eg, rural, low-/middle-income countries) |
NA, Not applicable; NIAID, National Institutes of Allergy and Infectious Diseases; WHO, World Health Organization.
Acknowledgments
P. A. Frischmeyer-Guerrerio’s work on this manuscript was supported, in part, by the Division of Intramural Research, National Institutes of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
No funding has been received for this study.
Conflicts of interest: M. Greenhawt is a consultant for Aquestive; is a member of physician/medical advisory boards for DBV Technologies, Sanofi/Regeneron, Genentech, Nutricia, Novartis, Acquestive, Allergy Therapeutics, Pfizer, US World Meds, Allergenis, Aravax, and Prota, all unrelated to vaccines/vaccine development; is a member of the scientific advisory council for the National Peanut Board; is an associate editor for the Annals of Allergy, Asthma, and Immunology; is member of the Joint Taskforce on Allergy Practice Parameters; and has received honorarium for lectures from ImSci and MedLearningGroup. E. M. Abrams is a collaborator with the Institute for Health Metrics and Evaluation. M. Shaker is a member of the Joint Taskforce on Allergy Practice Parameters; has a family member who is CEO of Altrix Medical; and serves on the Editorial Board of the Journal of Food Allergy and the Annals of Allergy, Asthma, and Immunology. D. Khan is the president elect of the American Academy of Allergy, Asthma, and Immunology. J. Bernstein has received financial support from Sanofi Regeneron, AstraZeneca (unrelated to vaccines/vaccine development), Merck, Optinose, Takeda, CSL Behring, BioCryst, Pharming, the National Institutes of Health (NIH), Taylor Francis, INEOS; is Editor in Chief of the Journal of Asthma; is INEOS Medical Immunosurveillance Director, Vice Chair and Lectureship Chair of the American Academy of Allergy, Asthma, and Immunology (AAAAI) Foundation, Chairman of AFI, American College of Asthma, Allergy, and Immunology (ACAAI) Asthma Chair, Scientific Chair, and Young Investigator Award Chair; and serves of the Board of Directors and Scientific Committee of Interasma. D. Campbell is a part-time employee of DBV Technologies; reports grants from National Health and Medical Research Council of Australia and personal fees from Allergenis, Westmead Fertility Centre, and Financial Markets Foundation for Children. A. K. Ellis has participated in advisory boards for ALK Abello, AstraZeneca (unrelated to vaccines/vaccine development), Aralez, Bausch Health, LEO Pharma, Merck, Novartis, and Pfizer (unrelated to vaccines/vaccine development); has been a speaker for ALK Abello, The ACADEMY, Aralez, AstraZeneca, Medexus, and Mylan; her institution has received research grants from ALK Abello, Aralez, AstraZeneca, Bayer LLC, Green Cross Pharmaceuticals, Medexus, Novartis, Sanofi, and Regeneron; has also served as an independent consultant to Bayer LLC and Regeneron; and is also the Vice President of the Canadian Society of Allergy and Clinical Immunology. A. Fox is a member of the MHRA Expert Working Group of COVID Vaccine Reactions. M. H. Grayson has served on advisory boards for Aimmune, DBV, GSK, and Sanofi (not related to vaccines); has stock options in Invirsa, Inc; serves on the American Academy of Allergy, Asthma, and Immunology COVID-19 Task Force, and the Board of Directors for the American Board of Allergy and Immunology; is chair of the Medical Scientific Council and on the Board of Directors of the Asthma and Allergy Foundation of America; is a member of the Scientific Advisory Committee of the American Lung Association; and is the editor-in-chief elect for the Annals of Allergy, Asthma, and Immunology. C. H. Katelaris received an honorarium for Pfizer atopic dermatitis advisory board participation (not related to vaccines). D. Lang is on the Editorial Board for Allergy and Asthma Proceedings; Topic Editor for DynaMed; Associate Editor for Journal of Asthma; and Delegate to National Quality Forum representing the American Academy of Allergy, Asthma, and Immunology. J. Lieberman has received financial support from the American College of Asthma, Allergy, and Immunology, Aquestive, Aimmune, DBV, Biotest Pharma, and Regeneron; is associate editor of the Annals of Allergy, Asthma, and Immunology; Chair for the American College of Asthma, Allergy, and Immunology Food Allergy Committee; and Medical Director for Food Allergy Alliance of the MidSouth D. Mack is a member of the Board of Directors for the Canadian Society of Allergy and Clinical Immunology; serves on the Editorial Board of the Journal of Food Allergy; has provided consultation and speaker services for Pfizer, Aimmune, Merck, Covis, and Pediapharm; and has been part of an advisory board for Pfizer and Bausch Health; none of these works are related to vaccines. B. Mazer is the Associate Director of Canada’s COVID-19 Immunity Task Force but otherwise has no conflicts to declare. G. Mosnaim received research grant support from AstraZeneca (unrelated to vaccines/vaccine development), GlaxoSmithKline, and Propeller Health; owned stock in Electrocore; served as a consultant and/or member of a scientific advisory board for GlaxoSmithKline, Sanofi-Regeneron, Teva, Novartis, Astra Zeneca (unrelated to vaccines/vaccine development), Boehringer Ingelheim, and Propeller Health; and is the current president of the American Academy of Allergy, Asthma, and Immunology. D. Munblit reports giving paid lectures for Bayer and received funding from the 5-100 Russian Academic Excellence Project. S. Shahzad Mustafa is on the speakers bureaus for Genentech, Regeneron, GSK, AstraZeneca (unrelated to vaccines/vaccine development), and CSL Behring. J. Oppenheimer performs Research/Adjudication for AstraZeneca (unrelated to vaccines/vaccine development), GSK, Sanofi, Novartis; is a consultant for GSK, AstraZeneca (unrelated to vaccines/vaccine development), Sanofi; is associate editor of the Annals of Allergy Asthma Immunology and AllergyWatch; is section editor for Current Opinion of Allergy; receives royalties from UptoDate; and is a Board Liaison for the American Board of Internal Medicine for the American Board of Allergy and Immunology. A. Ramsey is on the speaker’s bureau for Sanofi/Regeneron and GSK. M. Rank has received financial support from the American College of Allergy, Asthma, and Immunology, the National Institutes of Health, and Levin Family Foundation; has served as chair of the American Academy of Allergy, Asthma, and Immunology Health Education Delivery Quality Interest Section; and is Research Director of the Phoenix Children’s Hospital Breathmobile. D. Stukus has received financial support from Aimmune, Before Brands, Abbott Nutrition, the American Academy of Pediatrics, American College of Allergy, Asthma, and Immunology; has served as committee chair for the American Academy of Allergy Asthma and Immunology and American College of Allergy, Asthma, and Immunology. M. L. K. Tang: has received speaker fees from Nestle Health Science, Abbot Nutrition; is a consultant to Bayer Pharmaceuticals and Abbot Nutrition; has received research Grants from National Health and Medical Research Council, Bayer Pharmaceuticals, and Abbott Nutrition; is an inventor on patents related to food allergy treatments; and is an employee at Prota Therapeutics. J. M. Tracy is a shareholder for Johnson & Johnson (purchased more than 10 years prior to this manuscript). P. J. Turner reports grants from UK Medical Research Council, National Institute for Health Research/Imperial Biomedical Research Center, UK Food Standards Agency, End Allergies Together, and Jon Moulton Charity Trust; personal fees and nonfinancial support from Aimmune Therapeutics, DBV Technologies, and Allergenis; personal fees and other from ILSI Europe and UK Food Standards Agency, outside the submitted work; is the current chairperson of the World Allergy Organization Anaphylaxis Committee, and joint chair of the Anaphylaxis Working group of the UK Resuscitation Council. D. Wallace is on the Kaleo-Advisory board; and aids Bryan-Consulting. J. Wang received institutional research funding from Regeneron, DBV, and Aimmune; consultancy fees from DBV, ALK Abello, and Genentech; and is an UpToDate author. M. Worm declares the receipt of honoraria or consultation fees by the following companies: ALK-Abelló Arzneimittel GmbH, Mylan Germany GmbH, Leo Pharma GmbH, Sanofi-Aventis Deutschland GmbH, Regeneron Pharmaceuticals, DBV Technologies S.A, Stallergenes GmbH, HAL Allergie GmbH, Allergopharma GmbH & Co.KG, Bencard Allergie GmbH, Aimmune Therapeutics UK Limited, Actelion Pharmaceuticals Deutschland GmbH, Novartis AG, Biotest AG., AbbVie Deutschland GmbH & Co. KG and Lilly Deutschland GmbH. T. K. Vander Leek has served on advisory boards and received honoraria from Aralez, Bausch Health, and Pfizer (unrelated to vaccines). D. B. K. Golden received speakers bureau honoraria from Genentech, Kaleo; clinical trial support from Genentech, Novartis, Pfizer (unrelated to vaccine/vaccine development), GSK, Regeneron; consulting fees from Aquestive; and royalties from UpToDate (section editor). The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary Data
References
- 1.Del Rio C., Malani P.N. COVID-19—new insights on a rapidly changing epidemic. JAMA. 2020;323:1339–1340. doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- 2.Johns Hopkins University Coronavirus Resource Center. https://coronavirusjhuedu/maphtml Available from:
- 3.Global Preparedness Monitoring Board . World Health Organization; Geneva: 2020. A World in Disorder. Global Preparedness Monitoring Board Annual Report 2020.https://apps.who.int/gpmb/assets/annual_report/GPMB_AR_2020_EN.pdf Licence: CC BY-NC-SA 3.0 IGO. Available from: [Google Scholar]
- 4.Turner P.J., Ansotegui I.J., Campbell D.E., Cardona V., Ebisawa M., El-Gamal Y., et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14:100517. doi: 10.1016/j.waojou.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html Available from: [PubMed]
- 6.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., Shcheblyakov D.V., Dzharullaeva A.S., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraborty S., Mallajosyula V., Tato C.M., Tan G.S., Wang T.T. SARS-CoV-2 vaccines in advanced clinical trials: where do we stand? Adv Drug Deliv Rev. 2021;172:314–338. doi: 10.1016/j.addr.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfizer-BioNTech Vaccines and Related Biological Products Advisory Committee Meeting December 10, 2020. https://www.fda.gov/media/144245/download Available from:
- 9.ModernaTX Vaccines and Related Biological Products Advisory Committee Meeting December 17, 2020. https://www.fda.gov/media/144673/download Available from:
- 10.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaccines and Related Biological Products Advisory Committee Meeting, February 26, 2021. FDA Briefing Document: Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. https://www.fda.gov/media/146217/download Available from:
- 12.Kirka D. UK probes whether COVID-19 vaccine caused allergic reactions. https://www.yahoo.com/news/uk-investigates-possible-allergic-reactions-105421617.html Available from:
- 13.Mahase E. Covid-19: people with history of significant allergic reactions should not receive Pfizer vaccine, says regulator. BMJ. 2020;371:m4780. doi: 10.1136/bmj.m4780. [DOI] [PubMed] [Google Scholar]
- 14.Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenande E., Garvey L.H. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 16.Stone C.A., Jr., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medicines and Healthcare Products Regulatory Agency. COVID-19 vaccines (Pfizer/BioNTech and COVID-19 Vaccine AstraZeneca): current advice. https://www.gov.uk/drug-safety-update/covid-19-vaccines-pfizer-slash-biontech-and-covid-19-vaccine-astrazeneca-current-advice Available from:
- 18.Banerji A., Wolfson A.R., Wickner P.G., Cogan A.S., McMahon A.E., Saff R., et al. COVID-19 vaccination in patients with reported allergic reactions: updated evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:2135–2138. doi: 10.1016/j.jaip.2021.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Government of Canada Recommendations on the use of COVID-19 vaccines. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html#b9 Available from:
- 20.Shimabukuro T. COVID-19 vaccine safety updateAdvisory Committee on Immunization Practices (ACIP) January 27, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-01/06-COVID-Shimabukuro.pdf Available from:
- 21.Guide: Allergy and COVID-19 Vaccination. https://www.allergy.org.au/hp/papers/guide-allergy-and-covid-19-vaccination Available from:
- 22.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325:780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC COVID-19 Response Team; Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 Vaccine–United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggeberg J.U., Gold M.S., Bayas J.M., Blum M.D., Bonhoeffer J., Friedlander S., et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 25.Shimabukuro T. COVID-19 vaccine safety update. Advisory Committee on Immunization Practices (ACIP) January 27, 2021. CDC COVID-19 Vaccine Task Force Vaccine Safety Team. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-01/06-COVID-Shimabukuro.pdf Available from:
- 26.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020–January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B., et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brouwers M.C., Kho M.E., Browman G.P., Burgers J.S., Cluzeau F., Feder G., et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brozek J.L., Akl E.A., Alonso-Coello P., Lang D., Jaeschke R., Williams J.W., et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64:669–677. doi: 10.1111/j.1398-9995.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 30.Brozek J.L., Akl E.A., Jaeschke R., Lang D.M., Bossuyt P., Glasziou P., et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy. 2009;64:1109–1116. doi: 10.1111/j.1398-9995.2009.02083.x. [DOI] [PubMed] [Google Scholar]
- 31.Brozek J.L., Akl E.A., Compalati E., Kreis J., Terracciano L., Fiocchi A., et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: part 3 of 3. The GRADE approach to developing recommendations. Allergy. 2011;66:588–595. doi: 10.1111/j.1398-9995.2010.02530.x. [DOI] [PubMed] [Google Scholar]
- 32.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornelius V.R., Liu K., Peacock J., Sauzet O. Variation in adverse drug reactions listed in product information for antidepressants and anticonvulsants, between the USA and Europe: a comparison review of paired regulatory documents. BMJ Open. 2016;6:e010599. doi: 10.1136/bmjopen-2015-010599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan J. 4 out of 155,000 vaccinated people experienced severe allergic reactions, all have recovered: Janil Puthucheary. The benefits of vaccination outweighs the potential side effects. https://mothership.sg/2021/02/anaphylaxis-incidence-rate-covid-19-vaccine Available from:
- 35.Poland sets up compensation fund to cover adverse effects of vaccines. https://www.thefirstnews.com/article/poland-sets-up-compensation-fund-to-cover-adverse-effects-of-vaccines-18997 Available from:
- 36.Jaffe-Hoffman M. COVID-19 vaccination: 73 cases of facial paralysis, 7 anaphylactic shock. Serious symptoms of the vaccine include blurred vision, inflammation of the pulmonary pleura, inflammation of the heart, heart attack, or even liver damage. https://www.jpost.com/health-science/covid-19-vaccination-73-cases-facial-paralysis-7-anaphylactic-shock-661073 Available from:
- 37.Reported suspected adverse reactions of covid-19 vaccines. https://legemiddelverket.no/english/covid-19-and-medicines/vaccines-against-covid-19/reported-suspected-adverse-reactions-of-covid-19-vaccines#click-here-to-see-previous-weekly-reports Available from:
- 38.Ofiteru A. De ce sunt mai multe reacții adverse la vaccinul AstraZeneca. Răspunsul lui Valeriu Gheorghiță. https://romania.europalibera.org/a/de-ce-sunt-mai-multe-reac%c8%9bii-adverse-la-vaccinul-astrazeneca-r%c4%83spunsul-lui-valeriu-gheorghi%c8%9b%c4%83/31117691.html Available from:
- 39.Update van bijwerkingen. https://www.lareb.nl/pages/update-van-bijwerkingen Available from:
- 40.Swissmedic Media Unit Side effects of COVID-19 vaccines in Switzerland – update. https://www.admin.ch/gov/en/start/documentation/media-releases.msg-id-82668.html Available from:
- 41.COVID-19 Switzerland | Coronavirus | Dashboard. Available from: https://www.covid19.admin.ch/en/epidemiologic/vacc-doses?vaccrel=abs#showdetail. Accessed March 27, 2021.
- 42.Bijwerkingen Centrum Lareb. Reported side effects for COVID-19. https://laegemiddelstyrelsen.dk/en/news/themes/reported-side-effects-for-covid-19 Available from:
- 43.Covid vaccinations have produced 110 allergic reactions. Mexico News Daily. January 5, 2021. https://mexiconewsdaily.com/news/coronavirus/covid-vaccinations-have-produced-110-allergic-reactions Available from: Mexico News Daily.
- 44.Janssen Pharmaceuticals Vaccines and Related Biological Products Advisory Committee Meeting February 26, 2021. FDA Briefing Document: Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. https://www.fda.gov/media/146217/download Available from: 62.
- 45.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Government of Canada. Reported side effects following COVID-19 vaccination in Canada Summary Weekly report. https://health-infobase.canada.ca/covid-19/vaccine-safety/index.html Available from:
- 47.Chappell K.J., Mordant F.L., Li Z., Wijesundara D.K., Ellenberg P., Lackenby J., et al. Social Science Research Network; Rochester, NY: 2021. First report of a phase 1 randomised trial of molecular clamp-stabilised spike protein-based and MF59-adjuvanted vaccine for SARS-CoV-2. SSRN Scholarly Paper. Report No.: ID 3769210. [Google Scholar]
- 48.Che Y., Liu X., Pu Y., Zhou M., Zhao Z., Jiang R., et al. Randomized, double-blinded, placebo-controlled phase 2 trial of an inactivated severe acute respiratory syndrome coronavirus 2 vaccine in healthy adults. Clin Infect Dis. Published online November 9, 2020. [DOI] [PMC free article] [PubMed]
- 49.Chu L., McPhee R., Huang W., Bennett H., Pajon R., Nestorova B., et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39:2791–2799. doi: 10.1016/j.vaccine.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ella R., Reddy S., Jogdand H., Sarangi V., Ganneru B., Prasad S., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21:950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ella R., Vadrevu K.M., Jogdand H., Prasad S., Reddy S., Sarangi V., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Formica N, Mallory R, Albert G, Robinson M, Plested JS, Cho I, et al. Evaluation of a SARS-CoV-2 vaccine NVX-CoV2373 in younger and older adults [preprint published online March 1, 2021]. medRxiv. 10.1101/2021.02.26.21252482. [DOI] [PMC free article] [PubMed]
- 53.Goepfert P.A., Fu B., Chabanon A.L., Bonaparte M.I., Davis M.G., Essink B.J., et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1-2, dose-ranging study. Lancet Infect Dis. 2021;21:1257–1270. doi: 10.1016/S1473-3099(21)00147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore C. How 19 Aussies have now suffered allergic reactions to the Covid vaccines after 200,000 jabs - but health boss says: ‘That's normal’. Daily Mail Australia. March 16, 2021. https://www.dailymail.co.uk/news/article-9370477/Nineteen-Australians-suffer-allergic-reactions-Covid-19-vaccinations.html Available from:
- 57.Kydo News. Japan sees high rate of anaphylaxis after taking Pfizer vaccine. ABS CBN News. March 10, 2021. https://news.abs-cbn.com/overseas/03/10/21/japan-sees-high-rate-of-anaphylaxis-after-taking-pfizer-vaccine Available from:
- 58.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pu J., Yu Q., Yin Z., Zhang Y., Li X., Li D., et al. An in-depth investigation of the safety and immunogenicity of an inactivated SARS-CoV-2 vaccine. [preprint published online October 6, 2020]. medRxiv. [DOI]
- 60.Richmond P., Hatchuel L., Dong M., Ma B., Hu B., Smolenov I., et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shinde V., Bhikha S., Hoosain Z., Archary M., Bhorat Q., Fairlie L., et al. Preliminary efficacy of the NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. [preprint published online March 3, 2021]. medRxiv. [DOI] [PMC free article] [PubMed]
- 63.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward B.J., Gobeil P., Séguin A., Atkins J., Boulay I., Charbonneau P.-Y., et al. Phase 1 trial of a candidate recombinant virus-like particle vaccine for Covid-19 disease produced in plants. [preprint published online November 6, 2020]. medRxiv. [DOI]
- 66.Kamath P. ‘No deaths due to COVID-19 vaccination’: health ministry cites AEFI committee findings. Republic World. February 9, 2021. https://www.republicworld.com/india-news/general-news/no-deaths-due-to-covid-19-vaccination-health-ministry-cites-aefi-committee-findings.html Available from:
- 67.Wu Z., Hu Y., Xu M., Chen Z., Yang W., Jiang Z., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang S., Li Y., Dai L., Wang J., He P., Li C., et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21:1107–1119. doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., et al. Immunogenicity and safety of a recombinant adenovirus type-5–vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dreskin S.C., Halsey N.A., Kelso J.M., Wood R.A., Hummell D.S., Edwards K.M., et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9:32. doi: 10.1186/s40413-016-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stratton K.R., Howe C.I., Johnston R.B. National Academy Press; Washington: 1994. Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality. [PubMed] [Google Scholar]
- 75.Su J.R., Moro P.L., Ng C.S., Lewis P.W., Said M.A., Cano M.V. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990-2016. J Allergy Clin Immunol. 2019;143:1465–1473. doi: 10.1016/j.jaci.2018.12.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blumenthal K.G., Peter J.G., Trubiano J.A., Phillips E.J. Antibiotic allergy. Lancet. 2019;393:183–198. doi: 10.1016/S0140-6736(18)32218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner P.J., Jerschow E., Umasunthar T., Lin R., Campbell D.E., Boyle R.J. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5:1169–1178. doi: 10.1016/j.jaip.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abrams E.M., Greenhawt M., Shaker M., Kosowan K., Singer A. Primary care reported prevalence of vaccine and polyethylene-glycol allergy in Canada. Ann Allergy Asthma Immunol. Published online May 15, 2021. [DOI] [PMC free article] [PubMed]
- 79.Centers for Disease Control and Prevention. Selected adverse events reported after COVID-19 vaccination. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html Available from:
- 80.Sampson H.A., Munoz-Furlong A., Campbell R.L., Adkinson N.F., Jr., Bock S.A., Branum A., et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 81.de Silva D., Singh C., Muraro A., Worm M., Alviani C., Cardona V., et al. Diagnosing, managing and preventing anaphylaxis: systematic review. Allergy. 2021;76:1493–1506. doi: 10.1111/all.14580. [DOI] [PubMed] [Google Scholar]
- 82.Cardona V., Ansotegui I.J., Ebisawa M., El-Gamal Y., Fernandez Rivas M., Fineman S., et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13:100472. doi: 10.1016/j.waojou.2020.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner P.J., Worm M., Ansotegui I.J., El-Gamal Y., Rivas M.F., Fineman S., et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J. 2019;12:100066. doi: 10.1016/j.waojou.2019.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hourihane J.O.B., Byrne A.M., Blümchen K., Turner P.J., Greenhawt M. Ascertainment bias in anaphylaxis safety data of COVID-19 vaccines. J Allergy Clin Immunol Pract. 2021;9:2562–2566. doi: 10.1016/j.jaip.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erlewyn-Lajeunesse M., Dymond S., Slade I., Mansfield H.L., Fish R., Jones O., et al. Diagnostic utility of two case definitions for anaphylaxis: a comparison using a retrospective case notes analysis in the UK. Drug Saf. 2010;33:57–64. doi: 10.2165/11318970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 86.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shimabukuro T.T., Nguyen M., Martin D., DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2015;33:4398–4405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy K.R., Patel N.C., Ein D., Hudelson M., Kodoth S., Marshall G.D., Jr., et al. Insights from American College of Allergy, Asthma, and Immunology COVID-19 Vaccine Task Force: allergic reactions to mRNA SARS-CoV-2 vaccines. Ann Allergy Asthma Immunol. 2021;126:319–320. doi: 10.1016/j.anai.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shaker M., Abrams E.M., Greenhawt M. A cost-effectiveness evaluation of hospitalizations, fatalities, and economic outcomes associated with universal versus anaphylaxis risk-stratified COVID-19 vaccination strategies. J Allergy Clin Immunol Pract. 2021;9:2658–2668. doi: 10.1016/j.jaip.2021.02.054. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kadire S.R., Wachter R.M., Lurie N. Delayed second dose versus standard regimen for Covid-19 vaccination. N Engl J Med. 2021;384:e28. doi: 10.1056/NEJMclde2101987. [DOI] [PubMed] [Google Scholar]
- 91.Shaker M., Phillips E., Blumenthal K.G., Abrams E.M., Banerji A., Oppenheimer J., et al. The importance of a timely second dose of the 2021 COVID-19 mRNA vaccine depends on the protection afforded by a first dose and subsequent risk of anaphylaxis. J Allergy Clin Immunol Pract. 2021;9:2556–2561. doi: 10.1016/j.jaip.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight U.S. Locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.American College of Allergy, Asthma & Immunology. COVID-19 vaccine: ACAAI provider frequently asked questions. https://college.acaai.org/covid-19-vaccine-frequently-asked-questions/ Available from:
- 94.Kelso J.M., Greenhawt M.J., Li J.T., Nicklas R.A., Bernstein D.I., Blessing-Moore J., et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 95.Rojas-Perez-Ezquerra P., Crespo Quiros J., Tornero Molina P., Baeza Ochoa de Ocariz M.L., Zubeldia Ortuno J.M. Safety of new mRNA vaccines against COVID-19 in severely allergic patients. J Investig Allergol Clin Immunol. 2021;31:180–181. doi: 10.18176/jiaci.0683. [DOI] [PubMed] [Google Scholar]
- 96.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol–induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9:670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 97.Sanchez Moreno G.V., Vazquez Cortes S., Elices Apellaniz A., Davila G Chamorro Gomez M. Allergy to macrogols: a case report. Allergy. 2015;70:332–333. [Google Scholar]
- 98.Extremera Ortega A.M., Garcia Rodriguez R., Moreno Lozano L., Gomez Torrijos E., Alfaya Arias T., Urra Ardanaz J.M., et al. Immediate allergy to macrogol. Allergy. 2018;73:370–702. [Google Scholar]
- 99.Shah S., Prematta T., Adkinson N.F., Ishmael F.T. Hypersensitivity to polyethylene glycols. J Clin Pharmacol. 2013;53:352–355. doi: 10.1177/0091270012447122. [DOI] [PubMed] [Google Scholar]
- 100.Pizzimenti S., Heffler E., Gentilcore E., Raie A., Bussolino C., Nebiolo F., et al. Macrogol hypersensitivity reactions during cleansing preparation for colon endoscopy. J Allergy Clin Immunol Pract. 2014;2:353–354. doi: 10.1016/j.jaip.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 101.Kim T.S., Choi D.C., Lee B.J. Anaphylaxis due to polyethylene glycol: a case report. Allergy Asthma Respir Dis. 2018;6:274–276. [Google Scholar]
- 102.Sohy C., Vandenplas O., Sibille Y. Usefulness of oral macrogol challenge in anaphylaxis after intra-articular injection of corticosteroid preparation. Allergy. 2008;63:478–479. doi: 10.1111/j.1398-9995.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 103.Giangrande N., Garcia-Menaya J.M., Marcos-Fernandez M., Camara-Hijon C., Bobadilla-Gonzalez P. Anaphylaxis due to macrogol in a laxative solution with a positive basophil activation test. Ann Allergy Asthma Immunol. 2019;123:302–304. doi: 10.1016/j.anai.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 104.Bommarito L., Mietta S., Nebiolo F., Geuna M., Rolla G. Macrogol hypersensitivity in multiple drug allergy. Ann Allergy Asthma Immunol. 2011;107:542–543. doi: 10.1016/j.anai.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 105.Hyry H., Vuorio A., Varjonen E., Skytta J., Makinen-Kiljunen S. Two cases of anaphylaxis to macrogol 6000 after ingestion of drug tablets. Allergy. 2006;61:1021. doi: 10.1111/j.1398-9995.2006.01083.x. [DOI] [PubMed] [Google Scholar]
- 106.Jover Cerda V., Rodriguez Pacheco R., Domenech Witek J., Marco de la Calle F.M., de la Sen Fernandez M.L. Immediate hypersensitivity to polyethylene glycols in unrelated products: when standardization in the nomenclature of the components of drugs, cosmetics, and food becomes necessary. Allergy Asthma Clin Immunol. 2019;15:9. doi: 10.1186/s13223-019-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anton Girones M., Roan Roan J., de la Hoz B., Sanchez Cano M. Immediate allergic reactions by polyethylene glycol 4000: two cases. Allergol Immunopathol (Madr) 2008;36:110–112. doi: 10.1157/13120396. [DOI] [PubMed] [Google Scholar]
- 108.Badiu I., Guida G., Heffler E., Rolla G. Multiple drug allergy due to hypersensitivity to polyethylene glycols of various molecular weights. J Investig Allergol Clin Immunol. 2015;25:368–369. [PubMed] [Google Scholar]
- 109.Perez-Perez L., Garcia-Gavin J., Pineiro B., Zulaica A. Biologic-induced urticaria due to polysorbate 80: usefulness of prick test. Br J Dermatol. 2011;164:1119–1120. doi: 10.1111/j.1365-2133.2011.10220.x. [DOI] [PubMed] [Google Scholar]
- 110.Wagner N., Podda M. High volume of polysorbate-containing (Tween(R) 80) solutions induces false-positive results in intradermal test. J Eur Acad Dermatol Venereol. 2018;32:1972–1976. doi: 10.1111/jdv.14999. [DOI] [PubMed] [Google Scholar]
- 111.Badiu I., Geuna M., Heffler E., Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5797. bcr0220125797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shelley W.B., Talanin N., Shelley E.D. Polysorbate 80 hypersensitivity. Lancet. 1995;345:1312–1313. doi: 10.1016/s0140-6736(95)90963-x. [DOI] [PubMed] [Google Scholar]
- 113.Coors E.A., Seybold H., Merk H.F., Mahler V. Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann Allergy Asthma Immunol. 2005;95:593–599. doi: 10.1016/S1081-1206(10)61024-1. [DOI] [PubMed] [Google Scholar]
- 114.Limaye S., Steele R.H., Quin J., Cleland B. An allergic reaction to erythropoietin secondary to polysorbate hypersensitivity. J Allergy Clin Immunol. 2002;110:530. doi: 10.1067/mai.2002.126460. [DOI] [PubMed] [Google Scholar]
- 115.Pitlick M.M., Sitek A.N., Kinate S.A., Joshi A.Y., Park M.A. Polyethylene glycol and polysorbate skin testing in the evaluation of COVID-19 vaccine reactions: early report. Ann Allergy Asthma Immunol. 2021;126:735–738. doi: 10.1016/j.anai.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kelso J.M. Misdiagnosis of systemic allergic reactions to mRNA COVID-19 vaccines. Ann Allergy Asthma Immunol. 2021;127:133–134. doi: 10.1016/j.anai.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marcelino J., Farinha S., Silva R., Didenko I., Proença M., Tomáz E. Non-irritant concentrations for skin testing with SARS-CoV-2 mRNA vaccine. J Allergy Clin Immunol Pract. 2021;9:2476–2477. doi: 10.1016/j.jaip.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mustafa S.S., Ramsey A., Staicu M.L. Administration of a second dose of the Moderna COVID-19 vaccine after an immediate hypersensitivity reaction with the first dose: two case reports. Ann Intern Med. 2021;174:1177–1178. doi: 10.7326/L21-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sellaturay P., Nasser S., Islam S., Gurugama P., Ewan P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51:861–863. doi: 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Restivo V., Candore G., Barrale M., Caravello E., Graziano G., Onida R., et al. Allergy to polyethilenglicole of anti-SARS CoV2 vaccine recipient: a case report of young adult recipient and the management of future exposure to SARS-CoV2. Vaccines (Basel) 2021;9:412. doi: 10.3390/vaccines9050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang Q., Jacobs T.M., McCallen J.D., Moore D.T., Huckaby J.T., Edelstein J.N., et al. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal Chem. 2016;88:11804–11812. doi: 10.1021/acs.analchem.6b03437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Z.H., Stone C.A., Jr., Jakubovic B., Phillips E.J., Sussman G., Park J., et al. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021;9:1731–1733.e3. doi: 10.1016/j.jaip.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Y., Smith C.A., Panetta J.C., Yang W., Thompson L.E., Counts J.P., et al. Antibodies predict pegaspargase allergic reactions and failure of rechallenge. J Clin Oncol. 2019;37:2051–2061. doi: 10.1200/JCO.18.02439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Szebeni J., Alving C.R., Rosivall L., Bunger R., Baranyi L., Bedocs P., et al. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J Liposome Res. 2007;17:107–117. doi: 10.1080/08982100701375118. [DOI] [PubMed] [Google Scholar]
- 126.Klimek L., Novak N., Cabanillas B., Jutel M., Bousquet J., Akdis C.A. Allergenic components of the mRNA-1273 vaccine for COVID-19: possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy. Published online March 3, 2021. [DOI] [PMC free article] [PubMed]
- 127.Lukawska J., Mandaliya D., Chan A.W.E., Foggitt A., Bidder T., Harvey J., et al. Anaphylaxis to trometamol excipient in gadolinium-based contrast agents for clinical imaging. J Allergy Clin Immunol Pract. 2019;7:1086–1087. doi: 10.1016/j.jaip.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 128.Lund J.M., Alexopoulou L., Sato A., Karow M., Adams N.C., Gale N.W., et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kulka M., Alexopoulou L., Flavell R.A., Metcalfe D.D. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 130.Risma K.A., Edwards K.M., Hummell D.S., Little F.F., Norton A.E., Stallings A., et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147:2075–2082.e2. doi: 10.1016/j.jaci.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rouleau I., De Serres G., Drolet J.P., Banerjee D., Lemire C., Moore A., et al. Allergic symptoms after pandemic influenza vaccination rarely mediated by vaccine-specific IgE. J Allergy Clin Immunol. 2012;130:1423–1426. doi: 10.1016/j.jaci.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 132.Greenhawt M., Turner P.J., Kelso J.M. Administration of influenza vaccines to egg allergic recipients: a practice parameter update 2017. Ann Allergy Asthma Immunol. 2018;120:49–52. doi: 10.1016/j.anai.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 133.Gagnon R., Primeau M.N., Des Roches A., Lemire C., Kagan R., Carr S., et al. Safe vaccination of patients with egg allergy with an adjuvanted pandemic H1N1 vaccine. J Allergy Clin Immunol. 2010;126:317–323. doi: 10.1016/j.jaci.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 134.Ma L., Danoff T.M., Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:1075–1083. doi: 10.1016/j.jaci.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html Available from:
- 136.Contraindications to Sputnik V vaccine set. https://www.kazpravda.kz/en/news/society/contraindications-to-sputnik-v-vaccine-set Available from:
- 137.MacNeil J. Clinical considerations for use of COVID-19 vaccines. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/28-03-01/03-COVID-MacNeil.pdf Available from:
- 138.Centers for Disease Control and Prevention. CDC recommends use of Johnson & Johnson’s Janssen COVID-19 vaccine resume. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html Available from:
- 139.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keirns C.C., Goold S.D. Patient-centered care and preference-sensitive decision making. JAMA. 2009;302:1805–1806. doi: 10.1001/jama.2009.1550. [DOI] [PubMed] [Google Scholar]
- 141.Canadian Society of Allergy and Clinical Immunology. COVID-19 vaccine testing & administration guidance for allergists/immunologists from the CSACI. https://csaci.ca/wp-content/uploads/2021/01/COVID-19-VaccineTesting-AdministrationGuidance-JAN5.pdf Available from: [DOI] [PMC free article] [PubMed]
- 142.Klimek L., Jutel M., Akdis C.A., Bousquet J., Akdis M., Torres M.J., et al. ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines—an EAACI-ARIA position paper. Allergy. 2021;6:1624–1628. doi: 10.1111/all.14726. [DOI] [PubMed] [Google Scholar]
- 143.COVID-19 Treatment Guidelines Panel, National Institutes of Health Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/ Available from: [PubMed]
- 144.Shaker M.S., Wallace D.V., Golden D.B.K., Oppenheimer J., Bernstein J.A., Campbell R.L., et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145:1082–1123. doi: 10.1016/j.jaci.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 145.Deepak P., Kim W., Paley M.A., Yang M., Carvidi A.B., El-Qunni A.A., et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. [preprint published online April 7, 2021]. medRxiv. [DOI]
- 146.Petter E., Mor O., Zuckerman N., Oz-Levi D., Younger A., Aran D., et al. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. [preprint published online February 8, 2021]. medRxiv. [DOI]
- 147.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Skowronski D.M., De Serres G. Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1576–1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 149.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.