Abstract
Background and Objective
Emicizumab is a monoclonal antibody that bridges activated coagulation factor IX and factor X to restore effective hemostasis in persons with hemophilia A. It is indicated for routine prophylaxis of bleeding episodes in persons with hemophilia A. The aim of the present study is to describe the exposure–response relationship between emicizumab concentrations and bleeding frequency, and to confirm adequate bleeding control of the investigated dosing regimens 1.5 mg/kg once weekly, 3 mg/kg every 2 weeks, and 6 mg/kg every 4 weeks.
Methods
Treated bleeding events were pooled from 445 persons with hemophilia A with and without inhibitors against factor VIII, participating in six clinical studies. Emicizumab concentrations were predicted using a previously developed population pharmacokinetic model. A count model was used to quantify the exposure–response relationship. These models were used to illustrate the relationship between emicizumab concentrations and cumulative count of bleeding over 1 year (annualized bleeding rate).
Results
The final exposure–response model, based on a generalized Poisson distribution and an inhibitory Emax relationship, adequately describes the relationship between daily emicizumab concentrations and daily bleed frequency. A significant effect of factor VIII prophylaxis among persons with hemophilia A without inhibitors was found. Annualized bleeding rate simulations show that the three emicizumab dosing regimens maintain the concentrations close to the plateau of the effect. At the average steady-state concentration across all regimens (53.5 µg/mL), the predicted mean annualized bleeding rate is 1.28, corresponding to a 94.0% reduction from baseline.
Conclusions
These results confirm that average emicizumab concentrations achieved with all three emicizumab dosing regimens provide adequate bleeding control.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-021-01006-0.
Key Points
| A count data model described the exposure-dependent, bleeding-prophylactic effect of emicizumab in persons with hemophilia A with or without factor VIII inhibitors. |
| Concentrations above 30 µg/mL are predicted to provide clinically meaningful control of bleeding. |
| The dosing regimens of 1.5 mg/kg once weekly, 3 mg/kg every 2 weeks, or 6 mg/kg every 4 weeks achieve average concentrations that provide a mean reduction of annualized bleeding rate of 94.0%. |
Introduction
Hemophilia A is a chromosome X-linked recessive deficiency of factor VIII (FVIII) activity resulting in lifelong bleeding. Severe disease was accompanied by high morbidity and mortality due to recurrent spontaneous bleeds [1].
The standard of care for hemophilia A includes either episodic infusion of a FVIII concentrate in response to symptoms of a bleed or scheduled prophylactic FVIII administration in an effort to prevent bleeds from occurring. Factor VIII prophylaxis has been proven to minimize bleeding events and complications; however, the prophylactic regimen requires intravenous infusions of standard half-life FVIII products at least twice a week because of their short elimination half-life (8–19 h) [2–5], which can impose a substantial burden of treatment on patients [6–8]. The emergence of extended half-life products has allowed for longer intervals between prophylactic infusions [9, 10]. Up to 30% of persons with hemophilia A (PwHA) develop neutralizing alloantibodies (inhibitors) against FVIII [11, 12], typically within the first 50 exposure days to FVIII therapy [13–15], which renders treatment with FVIII ineffective. These inhibitors can be eradicated with immune tolerance induction. Those who express high titer inhibitors are treated with bypassing agents, such as activated prothrombin complex concentrate or recombinant activated FVII (FVIIa), which have decreased efficacy relative to FVIII.
Emicizumab is a recombinant, humanized, bispecific monoclonal antibody that simultaneously binds to FIXa and FX, thereby mimicking the cofactor function of activated FVIII [16–18]. Preclinical investigations suggested that emicizumab could address an unmet need in hemophilia A treatment, with a high subcutaneous bioavailability [19], a longer elimination half-life compared with existing treatments, effectiveness regardless of the presence or absence of FVIII inhibitors, and a lack of FVIII inhibitor induction [16, 17, 20, 21]. Data collected in the early clinical program confirmed those characteristics [22, 23]. Based on an exposure–repeated time to event (RTTE) model [24] developed from limited bleeding count data collected in 18 Japanese male PwHA who received emicizumab [22], three dosing regimens were selected for phase III trials: 1.5 mg/kg once weekly (QW), 3 mg/kg every 2 weeks (Q2W), or 6 mg/kg every 4 weeks (Q4W).
Following confirmation of clinical efficacy in recent phase III trials [25–28], these three emicizumab dosing regimens have been approved in PwHA with or without inhibitors. The population pharmacokinetic (PK) model used in the multiple ascending dose study [24] was subsequently updated [29] using data from the phase III trials [25–28].
The pharmacometric analysis presented herein was performed to (1) further characterize the exposure–bleed count relationship in PwHA enrolled in phase I–III clinical studies; (2) identify factors that could contribute to between-patient variability in the treatment response, and (3) assess the impact of different dosing regimens (QW, Q2W, or Q4W) on the treatment response.
This was accomplished by the development of an exposure–response model that links emicizumab exposure to the daily bleeding count. The model was used to simulate the relationship between emicizumab concentration and the cumulative count of bleeding events over 1 year (equivalent to the annualized bleed rate [ABR]).
Methods
Clinical Studies, Subjects, and Data
The population exposure–efficacy analysis described here used data from one Japanese phase I/II study [22, 23] and four phase III studies: HAVEN 1 [25], HAVEN 2 [26], HAVEN 3 [27], and HAVEN 4 [28], as well as a non-intervention study [30–32]. The designs, baseline characteristics, and clinical findings of those studies are summarized in Table 1. The data set included data from a total of 445 PwHA; 60 of them only participated in the non-intervention studies [30–32], thus data from 385 emicizumab-treated PwHA were included. Study arms where emicizumab was not given were included in the analysis to provide more information on the distribution of bleeds in the absence of emicizumab. Among PwHA treated with emicizumab, 64 had previously participated in the non-intervention study [30–32] and later enrolled in the interventional trials. In addition, in studies HAVEN 1 and HAVEN 3 [25, 27], 6-month data from the study period pre-emicizumab introduction were collected. Thus, the non-intervention period varies considerably in duration between PwHA in the non-intervention studies, and for a number of PwHA, no non-intervention data were available (see Table 1). Only treated bleeds, as defined previously [25, 33], were considered in the present analysis.
Table 1.
Baseline characteristics and clinical study findings for PwHA included in the dataset
| Study | Japanese phase I/II study [22, 23] | HAVEN 1 [25] | Non-intervention study [30–32] | HAVEN 2 [26] | HAVEN 3 [27] | HAVEN 4 [28] | Total |
|---|---|---|---|---|---|---|---|
| ClinicalTrials.gov or clinicaltrials-jp identifier | JapicCTI-121934 and JapicCTI-132195 | NCT02622321 | NCT02476942 | NCT02795767 | NCT02847637 | NCT03020160 | |
| Emicizumab dosing regimen |
Arm 1: 1 mg/kg SC followed by 0.3 mg/kg QW SCa Arm 2: 3 mg/kg SC followed by 1 mg/kg QW SC Arm 3: 3 mg/kg QW SC |
Arms A–D: 3 mg/kg SC for 4 weeks followed by 1.5 mg/kg QW SCb | No emicizumab given | 3 mg/kg SC for 4 weeks followed by 1.5 mg/kg QW SC |
Arm A/D: 3.0 mg/kg SC for 4 weeks followed by 1.5 mg/kg QW SC Arm B/C: 3.0 mg/kg SC followed by 3.0 mg/kg Q2W SC |
Run-in Arm: 6.0 mg/kg Q4W SC Expansion Arm: 3.0 mg/kg QW SC for 4 weeks followed by 6.0 mg/kg Q4W SC |
|
| Clinical cut-off date | 31 August 2017 | 8 September 2017 | 2 November 2016 | 5 October 2017 | 15 September 2017 | 15 December 2017 | |
| Number of evaluable PwHA in dataset | 16 | 109c | 60d | 61e | 151f | 48 | 445 |
| Age, years, median (range) | 34.5 (12–58) | 28 (12–75) | 26 (2–75) | 7.12 (1.22–15.7) | 38 (13–77) | 38 (14–68) | 29 (1.22–77) |
| BW, kg, median (range) | 63.4 (40.8–81.7) | 70 (40.1–156) | 65 (13.5–111) | 22.6 (9.5–53) | 76.5 (43–139) | 74.2 (43.3–102) | 68.5 (9.5–156) |
| FVIII inhibitors, present/absent, n | 9/7 | 109/0 | 40/20 | 61/0 | 0/151 | 8/40 | 227/218 |
| Number of bleeding events per individual during non-intervention, median (range) | 9.5 (0–38) | 5 (0–35) | 4 (1-33) | 5 (1–12) | 0.5 (0–36) | – | 4 (0–38) |
| ABR during non-intervention, median (range) | 20 (0–81.6) | 7.8 (0–75.6) | 21.6 (1.58–97) | 10.2 (2.47–32.9) | 0.656 (0–50.4) | – | 9.03 (0–97) |
| Number of bleeding events per individual during intervention, median (range) | 2 (0–74) | 0 (0–65) | – | 0 (0–2) | 0 (0–13) | 0 (0–18) | 0 (0–74) |
| Study non-intervention period duration, days, median (range) | 168 (168–186) | 145 (0–650) | 90 (0–294) | 0 (0-312) | 168 (0–382) | – | 85 (0–650) |
| Study intervention period duration, days, median (range) | 1348 (248–1570) | 434 (27–659) | – | 202 (56-440) | 206 (0–351) | 168 (164–308) | 210 (0–1570) |
ABR annualized bleeding rate, BW body weight, FVIII factor VIII, PK pharmacokinetic, PwHA persons with hemophilia A, QW once weekly, Q2W once every 2 weeks Q4W once every 4 weeks, SC subcutaneously
aTwo PwHA up-titrated their maintenance doses to 1 mg/kg QW SC (following a loading dose of 3 mg/kg SC) and thereafter 3 mg/kg QW SC because of suboptimal bleeding control
bFive PwHA up-titrated their maintenance doses to 3 mg/kg QW because of suboptimal bleeding control
c64 of these PwHA previously participated in the non-intervention study[30]
dIn addition, 64 PwHA participated that went on to later participate in HAVEN 1[25]
eTwo PwHA were excluded from model development because of very low albumin values at baseline[31]
fFour PwHA in this study were not included in the PK model development [31] as no emicizumab doses were administrated or no PK information was collected. However, data from the non-intervention period in these four PwHA were included
Persons with hemophilia A (with or without factor VIII inhibitors) were divided into three arms in the Japanese phase I/II study [22, 23]. Those in Arm 1 were given 1 mg/kg subcutaneously (SC) followed by 0.3 mg/kg QW SC. Participants in Arm 2 were given 3 mg/kg SC followed by 1 mg/kg QW SC. Those in Arm 3 were given 3 mg/kg QW SC.
In phase III studies, PwHA started emicizumab treatment with loading doses of 3 mg/kg QW for 4 weeks followed by a QW, Q2W, or Q4W dosing regimen. In HAVEN 1 [24], PwHA with factor VIII inhibitors were enrolled and randomized into two arms. Arm A was the main arm, where PwHA with FVIII inhibitors previously on episodic treatment with bypassing agents were given maintenance doses of 1.5 mg/kg QW SC. Arm B was the control arm, where a similar PwHA subset did not receive emicizumab until the primary 24-week efficacy period was completed. In addition, Arm C included non-randomized participants who were previously on a prophylactic bypassing agent, and Arm D included participants who were previously on an episodic or prophylactic bypassing agent, but unable to enroll in the other arms prior to their closure. All PwHA in Arms A, B, C, and D were given a maintenance dose of 1.5 mg/kg QW SC.
The non-intervention study [30–32] and HAVEN 2 [26] were single-arm studies. Persons with hemophilia A with factor VIII inhibitors were enrolled in HAVEN 2. At the cut-off date used for the present analysis, all PwHA in the pediatric HAVEN 2 were given a maintenance dose of 1.5 mg/kg QW SC. In study HAVEN 3 [27], PwHA (FVIII < 1%) without FVIII inhibitors who received episodic treatment with FVIII prior to study entry were randomized in a 2:2:1 ratio to either an emicizumab maintenance dose of 1.5 mg/kg QW SC (Arm A) or 3 mg/kg Q2W SC (Arm B), or to a non-prophylaxis control arm (Arm C). Participants in Arm C were switched to receive the same emicizumab regimen as Arm B after the initial study period was completed. In addition, PwHA who received FVIII prophylaxis prior to study entry were enrolled in Arm D to receive emicizumab at a maintenance dose of 1.5 mg/kg QW.
In HAVEN 4 [28], those with either severe congenital hemophilia A without FVIII inhibitors or hemophilia A with FVIII inhibitors were initially assigned to a “run-in” Arm receiving emicizumab at 6 mg/kg Q4W SC without loading doses, and later on to an “expansion” Arm, where emicizumab was administered, after loading doses of 3 mg/kg QW during 4 weeks, using the same regimen as in the run-in Arm.
Population PK Predictions
A population PK model was previously developed [29] using emicizumab PK data from PwHA included in the Japanese phase I/II study [23, 23] and in the four HAVEN studies described in Sect. 2.1. It consisted of a linear one-compartment model with first-order absorption and elimination processes. The model included covariate effects of body weight and albumin on the apparent clearance, of body weight and ethnicity on the apparent distribution volume, as well as an effect of age on bioavailability. For the exposure–response analysis of the bleeding events, daily individual emicizumab concentrations were derived using recorded dosing, covariate, and individual post-hoc PK estimates. Daily concentrations were calculated every 24 h over the entire individual observation period, starting at time 0 after the first dose. Considering the long elimination half-life of emicizumab (26.8 days), low PK variations are expected within a day, justifying this use of an every 24 h-concentration in the exposure–response analysis.
Exposure–Response Model
A longitudinal count data approach [34–37] was applied. For each person, the bleeding events were summarized as the individual number of events per day and included in the data set.
Base Bleeding Events Distribution
Several distributions could be used to describe count data, such as Poisson, generalized Poisson, zero-inflated Poisson, negative binomial, or zero-inflated negative binomial. The assumption of equi-dispersion, which is a characteristic of the Poisson distribution, was first checked on the complete count data by comparing the individual mean and variance estimates, as well as by graphical analysis. See the ESM for more details.
A parametric approach was used to model the bleeding events on a given day in the absence of emicizumab treatment (i.e., potentially in the presence of FVIII treatment). The instantaneous rate of events (hazard) was modeled using a constant rate λ0 (the mean daily number of events when no emicizumab prophylaxis was given), and associated with a lognormal inter-individual variability, such that:
| 1 |
with being the estimated mean daily number of events in individual i in the absence of treatment and ηi being the approximate proportional deviation from for individual i. At the population level, the random effect was sampled from a normal distribution with mean value 0:
| 2 |
with ω2 being the inter-individual variance among the n PwHA.
Base Exposure–Response Model
In a second step, data from the intervention period under emicizumab administrations were added, and a model implementing the selected bleeding count distribution was fit to the full data set.
Several structural forms for the effect of emicizumab concentrations on bleed counts were tested, such as linear, power, and inhibitory Emax (Imax) functions.
Covariate Model
Following the establishment of a basic exposure–response model structure, the following covariates were tested: inhibitor status (yes/no), prior prophylaxis with FVIII (yes/no), prior prophylaxis with bypassing agents (yes/no), body weight, FIX and FX antigen levels at baseline, baseline ABR during the 6 months prior to study enrollment, ethnicity (Asian/white/black/other), and dosing regimen (QW, Q2W, or Q4W). Continuous covariates on parameter R were tested as follows:
| 3 |
in which is the population value for parameter R, Xi represents the value of covariate X for individual i, and is the observed median of the covariate in the study population. The covariate effect is described by θx.
Categorical covariates were tested as follows:
| 4 |
in which represents the fractional change in R for category m (m in 2...M) of covariate X, and is an indicator variable with value 1 if subject i falls in category m, and 0 otherwise. Note that this implies that RTV is the value of the parameter for the first level of X.
In the model, the predicted emicizumab concentration was allowed to exert an effect on a bleeding additive to the one of FVIII during the first week of treatment. No additional prophylaxis with FVIII was allowed when 1 week had passed after the commencement of emicizumab administration.
The covariate search was performed using a stepwise covariate model search procedure, as implemented in Perl-speaks NONMEM (“PsN”) [38]. More details on the implementation of the covariate search are provided in the ESM.
Model Selection and Qualification
Model selection was made based on overall judgment, taking into account Akaike information criterion values [39], the posterior distribution of the log likelihood function, physiological plausibility of parameter values, successful numerical convergence, and standard error of parameter estimates, and on outcomes of a visual predictive check [40] (see the ESM).
Simulation-Based Exploration of Target Efficacious Exposure and Doses
Using the final exposure–response model, simulations were performed to explore the predicted relationship between ABR and the emicizumab concentrations, assuming that no other prophylaxis was given. For each model parameter, population values were sampled from the vector of standard errors to account for uncertainty in the parameter estimates as if new trials were replicated. The number of trial replicates M was set at 1500. Then, within each replicate, individual parameters for 1000 people were obtained by sampling the random effects (η:s) from the variance–covariance matrix. For each replicated trial and each simulated PwHA, the treatment period was set to 365.25 days to obtain annual bleeding rates. The values of n and M were not formally determined through simulation but taken as common replicate and sample sizes.
Software
All modeling and simulation analyses were performed using NONMEM [41] Version 7.3.0 (ICON Development Solutions, Ellicott City, MD, USA) assisted by Perl-speaks-NONMEM Version 3.5.3 (http://psn.sourceforge.net/) [38] on workstations with Intel® Core. i7 processors, Windows 7 Professional and the GNU gfortran compiler (Version 4.5.0, ftp://ftp.globomaxnm.com/Public/nonmem7/compilers). Post-processing of NONMEM analysis results was carried out in R Version 3.2.2 (R Development Core Team, 2008).
Estimation Methods
Stochastic approximation of the Expectation–Maximization (EM) algorithm followed by a Monte Carlo-Markov chain Bayesian estimation [36, 37] was used. See the ESM for more details.
Results
Exposure–Response Data
The data set is summarized in Table 1. Median age was 29 years (range 1–77 years), and 227 (51%) persons had FVIII inhibitors.
Population PK Predictions
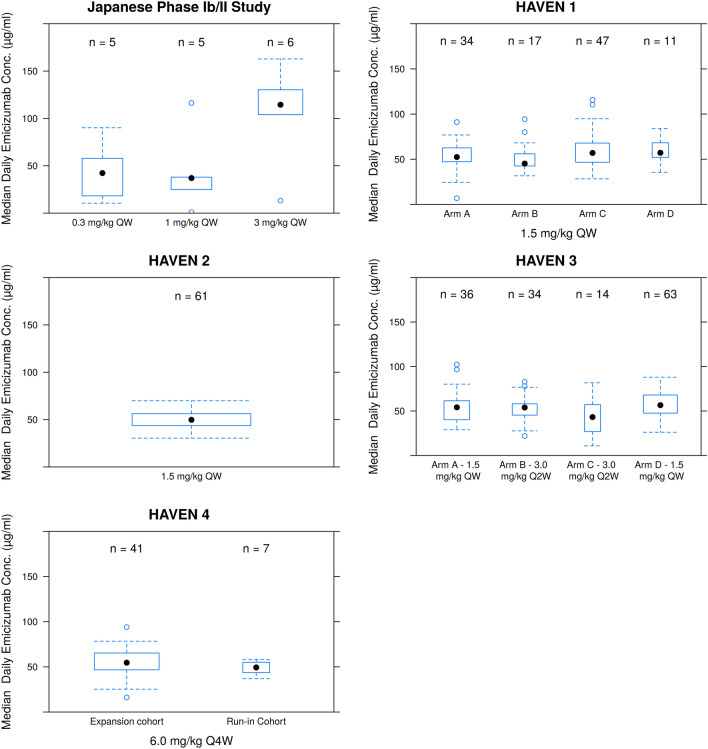
The medians of the daily emicizumab concentrations during active treatment in each arm are summarized in Fig. 1. The daily concentrations in the overall population ranged between 0 (during the pre-intervention period) and 162 µg/mL. Only those who were exposed to emicizumab were included in the plot. For the purpose of this calculation, people remained assigned to their initial dosing cohort. Dosing interruptions and up-titration occurred, and monitoring time varied between individuals in the current data set. As dose up-titrations occurred in the 0.3- and 1.0-mg/kg QW cohorts in the Japanese phase I/II study, the concentrations in these cohorts appear to be disproportionately high.
Fig. 1.
Distribution of predicted median daily emicizumab concentrations during intervention period by arm. The solid dot is the median of predicted median concentrations, the boxes confine the interquartile range and the whiskers extend to 1.5 times the interquartile range, and the open dots are predicted median concentrations outside the whiskers. For details on the study design, see [23–28]. For the purpose of this plot, individuals remained assigned to their initial dosing cohort, ignoring dose up-titration. QW once weekly, Q2W once every 2 weeks, Q4W once every 4 weeks
Exposure–Response Model
A generalized Poisson distribution was selected. See the ESM for more details on the distribution and structural model selection. The final model included an Imax relationship for the effect of daily emicizumab concentrations on the daily bleed frequency (), with Imax fixed to 1. Previous prophylaxis with FVIII was included in the model a priori, adapted from the previous exposure–RTTE model [21]. None of the covariates tested was found to be significant. The parameter estimates of the final model are presented in Table 2.
Table 2.
Parameter estimates of the developed exposure–response model
| Parameter | Unit | Estimate | 95% CIa |
|---|---|---|---|
| Model structure | |||
| λ0 (expected mean daily bleed count) | Event/day | 0.0238 | 0.0206–0.0270 |
| δ (dispersion factor for generalized Poisson distribution) | – | 0.0244 | 0.0164–0.0324 |
| IC50 (concentration to achieve 50% of drug effect) | μg/mL | 3.58 | 3.03–4.14 |
| Covariate effect | |||
| ΘPLXI (effect of prophylaxis with FVIII treatment among PwHA without FVIII inhibitorsb) | – | 0.811 | 0.757–0.865 |
| Inter-individual variability | |||
| ω2, variance of λ | – | 1.73 | 1.37–2.09 |
λ0 baseline bleeding hazard, δ dispersion parameter, CI confidence interval, FVIII Factor VIII, IC50 the plasma emicizumab concentration to reduce λ by half, PwHA persons with hemophilia A
aDerived from 2000 samples from the Bayesian posterior distribution
bModeled according to Eq. (5)
The final model can be written as follows:
| 5 |
| 6 |
where is the expected bleeding count on day t for individual I, is the baseline λ for individual I, is the predicted plasma concentration of emicizumab on day t for individual I, is the concentration to achieve 50% of the maximum drug effect, is the status of ongoing/no use of FVIII treatment at time t for individual i, taking the value 1 on days where FVIII treatment was given, 0 otherwise, and is the effect of FVIII prophylaxis on bleeding hazard. is the probability of observing count on day t for individual i. is the dispersion factor for the generalized Poisson distribution described in Eq. (6). Inter-individual variability was successfully estimated for λ0, while inclusion of inter-individual variability for the other parameters ( and ) provided numerically unstable results, indicating that these effects were not supported by the data. The estimate of θPLX 0.811, which denotes the effect of prophylaxis with FVIII, corresponds to a 44.7% reduction in bleeding hazard, as calculated from Eq. (5).
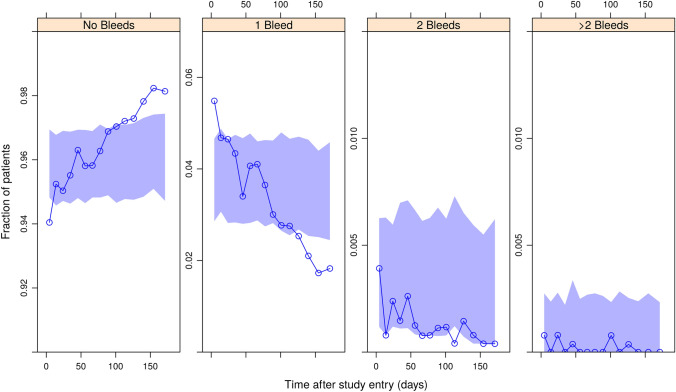
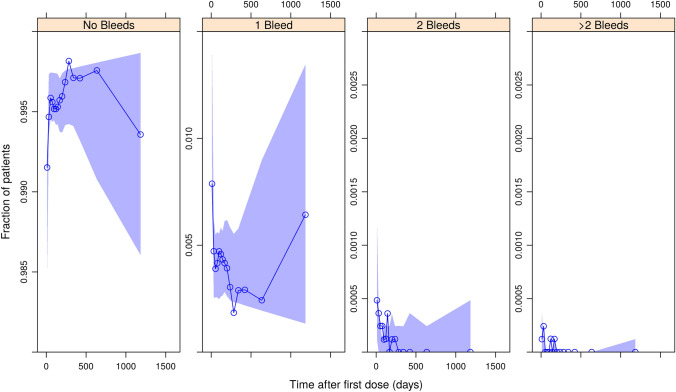
The estimated half maximal inhibitory concentration (IC50), 3.58 µg/mL, was low compared to the expected concentration ranges at steady state under the intended dosing regimens, distributed around 50 µg/mL (Fig. 1). The model adequately predicted the bleeding onset over time both before and after the start of emicizumab prophylaxis, as shown in the visual predictive check (Figs. 2, 3, S2–S9 of the ESM). The central tendency of the data was captured well, and the effect of emicizumab is visualized clearly by the observed bleed counts. In Fig. 2, the proportion of persons not receiving an intervention and experiencing a single daily bleed fluctuates between 2 and 5%. In contrast, in Fig. 3, where data from PwHA treated with emicizumab are presented, bleed frequencies are considerably lower. Likewise, in the top panel of Fig. S4 of the ESM, the proportion of PwHA experiencing a single daily bleed after being dosed with 0.3 mg/kg QW varies between 1 and 4%. In contrast, the proportion of PwHA in the bottom panel, treated with 1 mg/kg QW, experiencing a single daily bleed never exceeds 1%. This illustrates the exposure–response relationship in the data, which is well captured by the model.
Fig. 2.
Visual predictive check plot for the count data model: non-intervention study period, all data pooled. Circles and solid blue lines are the observed fractions of persons with hemophilia A experiencing the respective number of daily bleeds in each period, shaded areas are the simulated 95% prediction intervals, derived using the final model
Fig. 3.
Visual predictive check plot for the count data model: emicizumab period, all regimens pooled. Circles and solid blue lines are the observed fractions of persons with hemophilia A experiencing the respective number of daily bleeds in each period, shaded areas are the simulated 95% prediction intervals, derived using the final model
Simulation of Clinical Outcome
The simulated bleed counts as a function of emicizumab concentration are presented in Fig. 4, in which the predicted ranges of daily mean steady-state concentrations for PwHA treated with maintenance doses of 1.5 mg/kg QW, 3 mg/kg Q2W, and 6 mg/kg Q4W are overlaid.
Fig. 4.
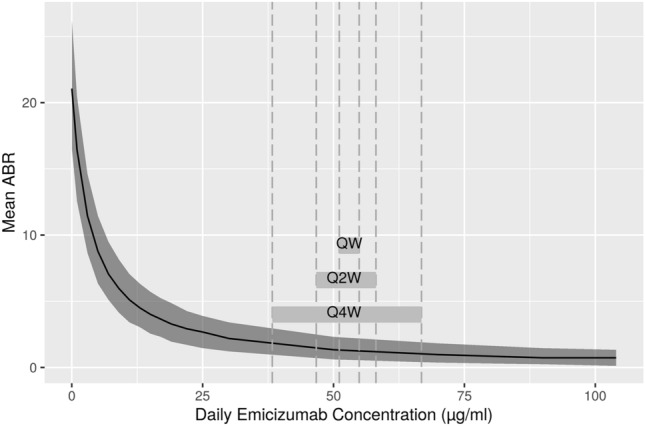
Simulated exposure–response analysis of emicizumab. Gray shading around the simulated mean plot lines indicate the 2.5th and 97.5th percent boundaries of the prediction intervals. Gray horizontal bars indicate the simulated range of steady-state mean trough concentrations and mean maximum concentrations over a dosing interval at maintenance doses of 1.5 mg/kg once weekly (QW), 3 mg/kg once every 2 weeks (Q2W), and 6 mg/kg once every 4 weeks (Q4W). ABR annualized bleeding rate
The simulated mean ABR was 21.1 in the absence of emicizumab or any other prophylactic treatment. Annualized bleed rate decreased with an increasing emicizumab concentration, with a relatively flat relationship for concentrations above approximately 30 µg/mL. At steady state, the fifth percentiles of trough concentrations at the three regimens are 28.4, 24.9, and 17.7 µg/mL, respectively [29]. As the treatment effect is linear, the protective effect at concentrations below 30 µg/mL would be considerable, keeping in mind that the estimated IC50 was 3.58 µg/mL.
Discussion
The daily bleed count following emicizumab administration was well described using a generalized Poisson model and an Imax relationship between daily emicizumab concentrations and daily bleed frequency. A similar relationship was used in the previous RTTE analysis [24]. The effects of a number of covariates were tested, and the use of FVIII prophylaxis among PwHA without inhibitors was found to significantly reduce the bleed count at baseline. The model showed good predictive performance and was used to simulate the relationship between emicizumab concentration and ABR.
The simulation results indicate that the recommended emicizumab dosing regimens produce average steady-state exposures that correspond to a clinically meaningful reduction of ABR (Fig. 4). Although the bleeding prophylactic effect is driven by the concentration of emicizumab and the three dosing regimens lead to different concentration–time profiles, the generated exposures are on average at the plateau of the exposure–response relationship. A lower dose, hence concentration, may lead to a lower efficacy. Annualized bleed rate decreased with an increasing emicizumab concentration, with a relatively flat relationship for concentrations above approximately 30 µg/mL. Such effective concentrations were generally achieved on average in the population with all three dosing regimens. For instance, at a concentration of 53.5 µg/mL (the concentration achieved on average over all three regimens [29]), the predicted mean ABR is 1.28, which corresponds to a 94% reduction in ABR from baseline. In addition, it should be noted that the fifth percentile of the individual steady-state trough concentrations following QW, Q2W, or Q4W dosing regimens (see Table 5 in [29]) includes values slightly lower than 30 µg/mL. This indicates that a lower dose concentration may lead to a loss of therapeutic benefit for some PwHA.
The simulated mean ABR in the absence of prophylactic treatment with FVIII or emicizumab was 21.1. This is close to the observed ABR among emicizumab-naïve PwHA, for which an average of 24 was calculated based on the observed mean baseline ABR in the study population.
The phase III dosing regimens had not been tested in the preceding phase I–I/II studies, but was selected based on the RTTE model [24]. In the present study, using a larger patient database, the similar efficacy of these dosing regimens was confirmed. In conjunction with the earlier findings, the same dose of emicizumab to either adult or adolescent populations with or without FVIII inhibitors was also supported.
Prophylaxis with FVIII had a profound effect on the bleeding rate, with a 44.7% reduction in bleeding hazard. This effect is substantially larger than the previous RTTE estimate [24], which was 23.9% (θPLX = 0.314). However, the previous estimate of θPLX was associated with a very wide 95% confidence interval, 0.00314–1.96, corresponding to a 0.31–66% reduction. Lack of randomization in the selection of PwHA receiving FVIII prophylaxis, as the study was not designed to assess this effect, could also explain this finding. Likewise, the present estimate of IC50, 3.58 µg/mL, is larger than the IC50 derived from the RTTE model, 1.19 µg/mL, but within the previous confidence limits of 0.308–3.77 µg/mL.
In the Japanese phase I/II study, the clock time of each bleed was precisely collected, contrary to the HAVEN studies [25–28], where only the day of each bleed was collected, leading potentially to multiple simultaneous events per day. Thus, as an alternative to the RTTE approach, a longitudinal count data modeling approach [34–37] was used here to characterize the exposure–response relationship of emicizumab in PwHA. This could be considered an extension of the RTTE approach, where a similar model has been applied to data with fewer restrictions in terms of recording the exact time points of each bleed.
There were limitations in the range of exposure concentrations in the population available for this exposure–response analysis. The majority of PwHA in the current database were receiving maintenance doses of 1.5 mg/kg QW, 3 mg/kg Q2W, or 6 mg/kg Q4W, with the exception of PwHA from the Japanese phase I/II study that encompassed only a few percent of the total population (see Table 1). This results in exposure concentrations mostly between 25 and 75 µg/mL, which is close to the exposure concentration associated with the maximum effect (Fig. 4). The lack of data at lower exposure concentration could produce problems for the estimation of the exposure–response relationship in the bleed count model. However, data from the initial phase of emicizumab prophylaxis, where the concentrations were lower, were also included, thus providing access to data also outside the 25- and 75-µg/mL concentration range.
The dosing frequency was taken into account in the model by using individual dosing records and PK parameter estimates. Any additional effects of the regimen were also investigated in the covariate analysis, and no statistically significant effect of treatment under Q2W and Q4W regimens was found on IC50. This aligns with the mechanism of action of emicizumab, for which the plasma concentrations at a given time are expected to drive the effect. This was further confirmed by the ability of the exposure–response model, assuming a direct effect between the daily concentrations and risk of bleeding, to properly describe the daily bleed count. The detection of any significant regimen effects on IC50 would have been an indication of structural weakness in the model.
In addition to the clinical efficacy observed in phase III HAVEN studies [25–28], this analysis allowed a comprehensive characterization of the relationship between emicizumab plasma concentrations and the probability of bleeding events in PwHA with or without FVIII inhibitors. It further supported the adequacy of the three emicizumab dosing regimens to provide clinically meaningful control of bleeding events. Following confirmation of clinical efficacy in phase III trials [25–28] and supported by the present exposure–response analysis, all three emicizumab dosing regimens have been approved in PwHA with or without FVIII inhibitors.
Conclusions
A pharmacokinetic–bleeding count data model was developed using pooled data from six studies to characterize the exposure-dependent, bleeding-prophylactic effect of emicizumab among PwHA with or without FVIII inhibitors. It shows that ABR decreased rapidly with an increasing emicizumab plasma concentration (IC50 = 3.58 µg/mL), with a relatively flat relationship for concentrations above approximately 30 µg/mL. Such concentrations were achieved on average with the three approved therapeutic dosing regimens of 1.5 mg/kg QW, 3 mg/kg Q2W, and 6 mg/kg Q4W.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the patients and their families and the volunteers who participated in the clinical studies; investigators and staff at the study institutions; and colleagues at qPharmetra LLC and F. Hoffmann-La Roche Ltd.
Declarations
Funding
This research was sponsored by F. Hoffmann-La Roche Ltd. Open access publication of this article was sponsored by qPharmetra and F. Hoffmann-La Roche Ltd.
Conflict of interest
Fredrik Jonsson is an employee of qPharmetra and reports consultancy fees from F. Hoffmann-La Roche Ltd. Christophe Schmitt, Claire Petry, Francois Mercier, Nicolas Frey, and Sylvie Retout are employees and hold stock ownership in F. Hoffmann-La Roche Ltd.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Code availability
Not applicable.
Authors’ contributions
FJ, SR, FM, CP, NF, and CS designed the research. FJ analyzed the data and wrote the manuscript. All authors interpreted the data and contributed to the manuscript.
References
- 1.Plug I, Van Der Bom JG, Peters M, Mauser-Bunschoten EP, De Goede-Bolder A, Heijnen L, et al. Mortality and causes of death in patients with hemophilia, 1992–2001: a prospective cohort study. J Thromb Haemost. 2006;4(3):510–516. doi: 10.1111/j.1538-7836.2006.01808.x. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 3.Collins PW, Fischer K, Morfini M, Blanchette VS, Björkman S. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17:2–10. doi: 10.1111/j.1365-2516.2010.02370.x. [DOI] [PubMed] [Google Scholar]
- 4.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 5.Collins P, Chalmers E, Chowdary P, Keeling D, Mathias M, O’Donnell J, et al. The use of enhanced half-life coagulation factor concentrates in routine clinical practice: guidance from UKHCDO. Haemophilia. 2016;22:487–498. doi: 10.1111/hae.13013. [DOI] [PubMed] [Google Scholar]
- 6.Geraghty S, Dunkley T, Harrington C, Lindvall K, Maahs J, Sek J. Practice patterns in haemophilia A therapy: global progress towards optimal care. Haemophilia. 2006;12:75–81. doi: 10.1111/j.1365-2516.2006.01189.x. [DOI] [PubMed] [Google Scholar]
- 7.De Moerloose P, Urbancik W, Van Den Berg HM, Richards M. A survey of adherence to haemophilia therapy in six European countries: results and recommendations. Haemophilia. 2008;14:931–938. doi: 10.1111/j.1365-2516.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 8.du Treil S, Rice J, Leissinger CA. Quantifying adherence to treatment and its relationship to quality of life in a well-characterized haemophilia population. Haemophilia. 2007;13:493–501. doi: 10.1111/j.1365-2516.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 9.Tiede A. Half-life extended factor VIII for the treatment of hemophilia A. J Thromb Haemost. 2015;13(Suppl. 1):S176–S179. doi: 10.1111/jth.12929. [DOI] [PubMed] [Google Scholar]
- 10.Mahlangu J, Powell JS, Ragni MV, Chowdary P, Josephson NC, Pabinger I, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325. doi: 10.1182/blood-2013-10-529974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer K, Lewandowski D, van den Berg HM, Janssen MP. Validity of assessing inhibitor development in haemophilia PUPs using registry data: the EUHASS project. Haemophilia. 2012;18:e241–e246. doi: 10.1111/j.1365-2516.2011.02687.x. [DOI] [PubMed] [Google Scholar]
- 12.Peyvandi F, Mannucci PM, Garagiola I, El-Beshlawy A, Elalfy M, Ramanan V, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374:2054–2064. doi: 10.1056/NEJMoa1516437. [DOI] [PubMed] [Google Scholar]
- 13.Calvez T, Chambost H, Claeyssens-Donadel S, FranceCoag Network et al. Recombinant factor VIII products and inhibitor development in previously untreated boys with severe hemophilia A. Blood. 2014;124(23):3398–3408. doi: 10.1182/blood-2014-07-586347. [DOI] [PubMed] [Google Scholar]
- 14.Gouw SC, van der Bom JG, Ljung R, PedNet and RODIN Study Group et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 2013;368(3):231–239. doi: 10.1056/NEJMoa1208024. [DOI] [PubMed] [Google Scholar]
- 15.Gouw SC, van der Bom JG, van den Berg HM. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007;109(11):4648–4654. doi: 10.1182/blood-2006-11-056291. [DOI] [PubMed] [Google Scholar]
- 16.Kitazawa T, Igawa T, Sampei Z, Muto A, Kojima T, Soeda T, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18:1570–1574. doi: 10.1038/nm.2942. [DOI] [PubMed] [Google Scholar]
- 17.Sampei Z, Igawa T, Soeda T, Okuyama-Nishida Y, Moriyama C, Wakabayashi T, et al. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PLoS ONE. 2013;8:e57479. doi: 10.1371/journal.pone.0057479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitazawa T, Esaki K, Tachibana T, Ishii S, Soeda T, Muto A, et al. Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb Haemost. 2017;117:1348–1357. doi: 10.1160/TH17-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotani N, Yoneyama K, Kawakami N, Shimuta T, Fukase H, Kawanishi T. Relative and absolute bioavailability study of emicizumab to bridge drug products and subcutaneous injection sites in healthy volunteers. Clin Pharmacol Drug Dev. 2019;8(6):702–712. doi: 10.1002/cpdd.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muto A, Yoshihashi K, Takeda M, Kitazawa T, Soeda T, Igawa T, et al. Anti-factor IXa/X bispecific antibody (ACE910): hemostatic potency against ongoing bleeds in a hemophilia A model and the possibility of routine supplementation. J Thromb Haemost. 2014;12:206–213. doi: 10.1111/jth.12474. [DOI] [PubMed] [Google Scholar]
- 21.Muto A, Yoshihashi K, Takeda M, Kitazawa T, Soeda T, Igawa T, et al. Anti-factor IXa/X bispecific antibody ACE910 prevents joint bleeds in a long-term primate model of acquired hemophilia A. Blood. 2014;124:3165–3171. doi: 10.1182/blood-2014-07-585737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, et al. Long-term safety and efficacy of emicizumab in a phase 1/2 study in hemophilia A patients with or without inhibitors. Blood Adv. 2017;1(22):1891–1899. doi: 10.1182/bloodadvances.2017006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, et al. Factor VIII-mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374:2044–2053. doi: 10.1056/NEJMoa1511769. [DOI] [PubMed] [Google Scholar]
- 24.Yoneyama K, Schmitt C, Kotani N, Levy GG, Kasai R, Iida S, et al. A pharmacometric approach to substitute for a conventional dose-finding study in rare diseases: example of phase III dose selection for emicizumab in hemophilia A. Clin Pharmacokinet. 2018;57:1123–1134. doi: 10.1007/s40262-017-0616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–818. doi: 10.1056/NEJMoa1703068. [DOI] [PubMed] [Google Scholar]
- 26.Young G, Liesner R, Sidonio R, Jr, Oldenburg J, Jiménez Yuste V, Mahlangu J, et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134(24):2127–2138. doi: 10.1182/blood.2019001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahlangu J, Oldenburg J, Paz-Priel I, Negrier C, Niggli M, Mancuso ME, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811–822. doi: 10.1056/NEJMoa1803550. [DOI] [PubMed] [Google Scholar]
- 28.Pipe SW, Shima M, Lehle M, Shapiro A, Chebon S, Fukutake K, et al. A multicentre, open-label, phase 3 study to evaluate the efficacy, safety, and pharmacokinetics of emicizumab prophylaxis administered every 4 weeks in persons with haemophilia A. Lancet Haematol. 2019;6(6):e295–305. doi: 10.1016/S2352-3026(19)30054-7. [DOI] [PubMed] [Google Scholar]
- 29.Retout S, Schmitt C, Petry C, Mercier F, Frey N. Population pharmacokinetic analysis and exposure-bleeding events relationship of emicizumab in adult, adolescent and pediatric patients with hemophilia A. Clin Pharmacokinet. 2020;59(12):1611–1625. doi: 10.1007/s40262-020-00904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruse-Jarres R, Oldenburg J, Santagostino E, Shima M, Kempton CL, Kessler CM, et al. Bleeding and safety outcomes in persons with haemophilia A without inhibitors: results from a prospective non-interventional study in a real-world setting. Haemophilia. 2019;25(2):213–220. doi: 10.1111/hae.13655. [DOI] [PubMed] [Google Scholar]
- 31.Oldenburg J, Shima M, Kruse-Jarres R, Santagostino E, Mahlangu J, Lehle M, et al. Outcomes in children with hemophilia A with inhibitors: results from a noninterventional study. Pediatr Blood Cancer. 2020;9:e28474. doi: 10.1002/pbc.28474. [DOI] [PubMed] [Google Scholar]
- 32.Mahlangu J, Oldenburg J, Callaghan MU, Shima M, Mancuso ME, Trask P, et al. Health-related quality of life and health status in persons with haemophilia A with inhibitors: a prospective, multicentre, non-interventional study (NIS) Haemophilia. 2019;25(3):382–391. doi: 10.1111/hae.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
- 34.Thall PF. Mixed Poisson likelihood regression models for longitudinal interval count data. Biometrics. 1988;44(1):197–209. doi: 10.2307/2531907. [DOI] [PubMed] [Google Scholar]
- 35.Gupta SK, Sathyan G, Lindemulder EA, Ho PL, Sheiner LB, Aarons L. Quantitative characterization of therapeutic index: application of mixed-effects modeling to evaluate oxybutynin dose-efficacy and dose-side effect relationships. Clin Pharmacol Ther. 1999;65(6):672–684. doi: 10.1016/S0009-9236(99)90089-9. [DOI] [PubMed] [Google Scholar]
- 36.Plan EL. Modeling and simulation of count data. CPT Pharmacomet Syst Pharmacol. 2014;3:e129. doi: 10.1038/psp.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prins, NH, Dykstra, KD, Darekar, A, van der Graaf, PH. Use of a generalized Poisson model to describe micturition frequency in patients with overactive bladder disease [abstract 2201]. PAGE Meeting; Athens; 2011: p. 20. https://www.page-meeting.org/?abstract=2201. Accessed Sep 2020.
- 38.Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit: a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Akaike H. A new look at the statistical model identification. IEEE Transact Autom Control. 1974;19(6):716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 40.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boeckmann AJ, Sheiner LB, Beal SL. NONMEM users guide: part VIII: help guide. ICON Development Solutions; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.