Abstract
Meniscal injuries represent one of the most common orthopedic injuries. The most frequent treatment is partial resection of the meniscus, or meniscectomy, which can affect joint mechanics and health. For this reason, the field has shifted gradually towards suture repair, with the intent of preservation of the tissue. “Save the Meniscus” is now a prolific theme in the field; however, meniscal repair can be challenging and ineffective in many scenarios. The objectives of this review are to present the current state of surgical management of meniscal injuries and to explore current approaches being developed to enhance meniscal repair. Through a systematic literature review, we identified meniscal tear classifications and prevalence, approaches being used to improve meniscal repair, and biological- and material-based systems being developed to promote meniscal healing. We found that biologic augmentation typically aims to improve cellular incorporation to the wound site, vascularization in the inner zones, matrix deposition, and inflammatory relief. Furthermore, materials can be used, both with and without contained biologics, to further support matrix deposition and tear integration, and novel tissue adhesives may provide the mechanical integrity that the meniscus requires. Altogether, evaluation of these approaches in relevant in vitro and in vivo models provides new insights into the mechanisms needed to salvage meniscal tissue, and along with regulatory considerations, may justify translation to the clinic. With the need to restore long-term function to injured menisci, biologists, engineers, and clinicians are developing novel approaches to enhance the future of robust and consistent meniscal reparative techniques.
Keywords: biologics, materials, meniscectomy, meniscus, repair
1 |. INTRODUCTION
The menisci are fibrocartilaginous, crescent-shaped wedges located between the femoral condyle and tibial plateau of the knee joint that enable load transmission, stability, and lubrication.1–4 The ability to withstand and distribute joint forces is attributed to its complex composition and organization. The menisci are comprised primarily of water, collagen, and proteoglycans (PGs),5 with the majority of the dry weight being circumferentially oriented type I collagen fibers.2,5 These fibers allow the meniscus to convert axial compressive stresses to circumferential hoop stresses,6,7 preventing meniscal extrusion and rupture. The menisci also contain radial fibers, which interdigitate amongst the circumferential fibers to prevent their longitudinal splitting.7–9 The inner third of the menisci, where compressive load predominates, is enriched with type II collagen and PG, exhibiting a more cartilage-like composition.10 The low amount of vascularization and resident cells (meniscal fibrochondrocytes [MFCs]) in these inner areas,11–13 especially with aging, decrease the endogenous healing capacity of the menisci following injury.14
Despite an elegantly complex structure and composition, meniscus injury is common, with an annual incidence of 66 tears per 100,000 persons.15,16 To provide symptomatic relief from the mechanical irritation (catching, locking) of a torn meniscus, damaged tissue is removed in a procedure called meniscectomy, approximately 850,000 procedures of which are carried out in the United States annually. However, both partial and total meniscectomy result in increased cartilage contact stresses and these mechanical changes are known to accelerate joint degeneration and lead to osteoarthritis. Despite clinical concerns, meniscectomy is indicated as the treatment of choice for many meniscal tear conditions (e.g., complex, degenerative, avascular), and performed at a rate 5–25 times higher than meniscus repair.17–19 While both meniscectomy and repair have similar short-term (<2 years) patient-reported outcomes, repairing the menisci better restores joint biomechanics and presumably confers a better long-term prognosis. While the ratio of meniscus repairs to meniscectomy remains low (~10%–15%), the prevalence of repairs has improved globally in the past decade.17,19–22 As a sign of increasing awareness of the importance of meniscal preservation, the phrase “Save the Meniscus” has become a popular moniker: with 10 manuscripts in the past 10 years23–32 featuring the phrase in their title, numerous conference proceedings, and a prolific social media handle (#savethemeniscus). Therefore, meniscus repair is clearly gaining in philosophical popularity, but given current limitations, it is not always physically possible or effective.
A multitude of factors influence this potential of meniscal repair. The geometry and location of the tear are highly influential in healing potential, yet the relative rates of types of tears are not readily available or consistent. Furthermore, augmentation of these repair environments has garnered much attention recently, both clinically and preclinically, to enhance and accelerate wound healing. A thorough review of model systems and outcome measures when evaluating these approaches is also lacking.
The goal of this review is to capture the current landscape of meniscus repair and augmentation strategies. We establish: (i) an examination of pertinent literature on meniscal tear classification and incidence rates, (ii) a systematic review on contemporary literature to highlight general trends, approaches, injury types, and models in meniscal repair, (iii) clinical techniques and advances to repair torn menisci, (iv) biologic- and biomaterial-based approaches being explored to augment the reparative process, and (v) model systems, considerations, and challenges for new augmentation procedures before clinical realization.
2 |. MENISCAL TEAR CLASSIFICATION AND OBSERVED INCIDENCE
2.1 |. Tear classification
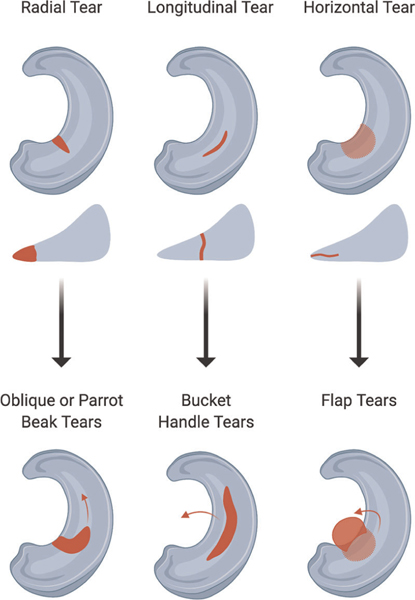
Treatment type (meniscectomy vs. repair), method of repair, and healing potential are dependent on a variety of tear characteristics including type, orientation, location, size, and severity.16,33 A number of classifications related to the shape and orientation of the tear exist: radial, longitudinal, horizontal, flap, bucket handle, complex/oblique, degenerative, and root tears (Figure 1). These tear types, and their prognoses, are discussed further in the Supporting Information. The location of the tear is often classified by circumferential axis position (anterior, body, posterior) and radial axis position (inner, middle, outer). The circumferential classification can have an impact on arthroscopic access, choice of suture repair technique, and stresses faced (greatest in posterior region). The radial classification is also crucial, as the microvasculature of the meniscus is concentrated around the periphery (red-red) and dissipates sharply toward the inner portion (white-white).11 When not treated, smaller and more localized tears may progress to more substantial injuries; for example, radial tears can extend along the circumferential axis, progressing to oblique or parrot beak tears.
FIGURE 1.
Schematics of prevalent meniscal tears. Schematics of radial, longitudinal, and horizontal tears, in both axial and cross-sectional views. Arrows indicate the progression of these smaller tears to larger and more complex versions
2.2 |. Observed incidence
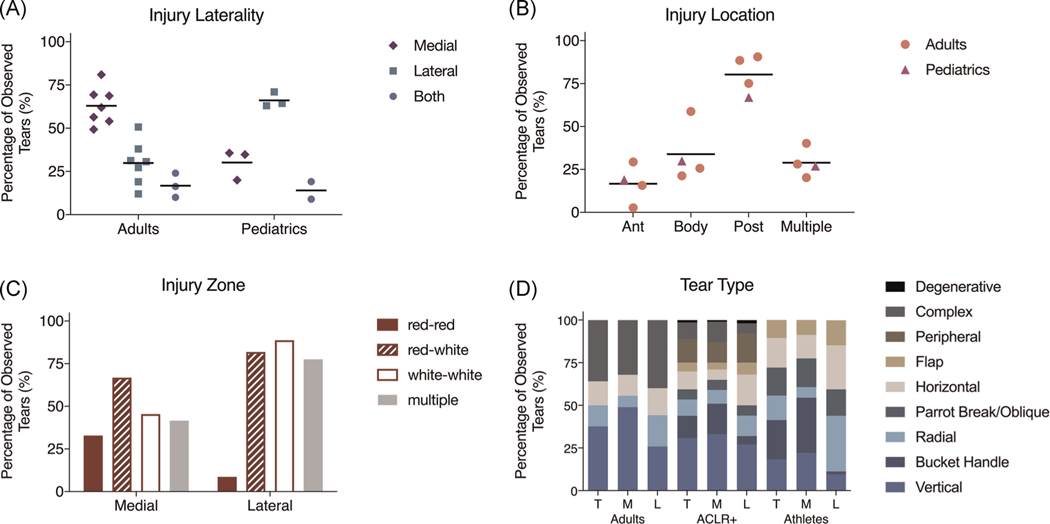
While studies reporting the incidence of meniscal tears exist, tears can be asymptomatic until they progress to a larger size. Identification of a meniscus tear using standard magnetic resonance imaging may not be conclusive, without the addition of advanced imaging34 and/or arthroscopic visualization. This situation may explain the variability in the reporting incidence of meniscus tears. Nonetheless, in adults, several studies have reported that the medial meniscus is injured more frequently than the lateral meniscus at a 2:1 ratio (Figure 2A) but in pediatric cases the inverse is true.35–37 It is also clear that these ratios often vary with acute versus traumatic injuries and concomitant injuries, as the biomechanics related to traumatic events will overstress one meniscus or region over others.44 In both adult and juvenile patients, tears are predominantly located in the posterior region (67.0%–90.6%) but can also span multiple regions (20.3%–40.2%) (Figure 2B). Specifically, shown in adults, the medial meniscus is more likely to exhibit posterior tears (93.1%–97.7%) than the lateral meniscus (34.5%–80.7%).38–40 Along the radial axis, more than half of tears span multiple zones (red-red, red-white, white-white). The medial meniscus experiences more tears in the red-red tears zone (32.8% vs. 8.6%) but less in the red-white (66.8% vs. 81.9%) and white-white (45.4% vs. 88.8%) zones than the lateral meniscus (Figure 2C; note: some tears involve multiple zones, explaining a total % >100%).40 Based on our review of the clinical data, longitudinal (18.2%–37.6%) and bucket handle (13.1%–24.0%) appear to be most common (Figure 2D). The medial meniscus has also been reported to be more susceptible to longitudinal tears, while the lateral meniscus is more susceptible to radial tears.38,40,43 Based on these relative observation rates (Figure 2), and the outcomes of healing (Supporting Information), certain tear scenarios are still problematic and may require augmentation to improve repair quality. Inner margin tears are clearly an issue due to the inherently deficient repair capacity of the avascular tissue. Radial tears are quite common in the avascular zone and disrupt the circumferential fiber network, an additional consideration which must be addressed to improve definitive treatment. Root tears are also common; they negate axial to hoop stress conversion and long-term outcomes are either inconsistent or not yet available. Regardless, repair of all types of tears is not always successful, and improved healing may improve outcomes. Thus, we wish to systematically review and subsequently highlight the recent scientific advances that may benefit the current state of meniscal repair.
FIGURE 2.
Meniscus tear observed incidence rates (% of observed tears within each study) from the literature. (A) Rates for affected meniscus (medial, lateral, or both) in adult (including adults with ACL reconstruction (+ACLR) and Athletes) and pediatric populations. Data from Jackson et al. (2019), Robinson et al. (2011), El Mansori et al. (2018), Kim et al. (2019), Ridley et al. (2017), Christino et al. (2019), Baker et al. (1985), Terzidis et al. (2006).35–42 (B) Rates by region (anterior, body, posterior, multiple) in adult (including +ACLR and Athlete) and pediatric populations. Data from Jackson et al. (2019), El Mansori et al. (2018), Kim et al. (2019), Terzidis et al. (2006).35,38–40 (C) Rates by vascular zone (outer = red-red, intermediate = red-white, inner = white-white, multiple) in the medial and lateral menisci in adult populations. Data from Terzidis et al. (2006).40 (D) Rates by tear type and affected meniscus (T, M, and L) in general adults, ACLR+ adults, and adults who are athletes. Data from Jiang et al. (2017), El Mansori et al. (2018), Terzidis et al. (2006).38,40,43 ACL, anterior cruciate ligament; L, lateral; M, medial; T, total incidence
3 |. SYSTEMATIC REVIEW
3.1 |. Systematic review methods
To highlight experimental meniscal tear/repair papers and focus on full-length peer-reviewed publications in the preclinical space, a systematic review was performed. Literature was identified and screened by using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.45 PubMed was searched on May 1, 2020, for full-length peer-reviewed journal articles on meniscus repair that were published between December 31, 1979, and May 1, 2020 (Supporting Information—PRISMA Flow Diagram). The following search criteria were agreed on before executing the search: “meniscus repair” OR “meniscal repair” NOT “arthroscopy” NOT “arthroscopic” NOT “acl” NOT “temporomandibular.” Initial results yielded a total of 423 articles across Pubmed. Titles and abstracts were then screened to exclude repeats. Full-text articles were assessed and those studies whose focus was not a basic science perspective to test meniscus repair were excluded. Based on the application of these inclusion/exclusion criteria, a total of 107 full-length peer-reviewed manuscripts were identified. Each article was coded for advancement type (“Technique,” “Biological,” “Material,” or “Hybrid,” combined biological/material intervention), intervention details, experimental model (cells, explant, small animal, or large animal), and animal species (“bovine,” or cow, “canine,” or dog, “caprine” or goat, “lapine,” or rabbit, “murine,” or mouse, “ovine,” or sheep. “porcine,” or pig, “rattus,” or rat, or “human”). The type of induced injury was also noted to determine the prevalent models utilized. A complete list of these studies is provided in the Supporting Information.
3.2 |. Systematic review general findings
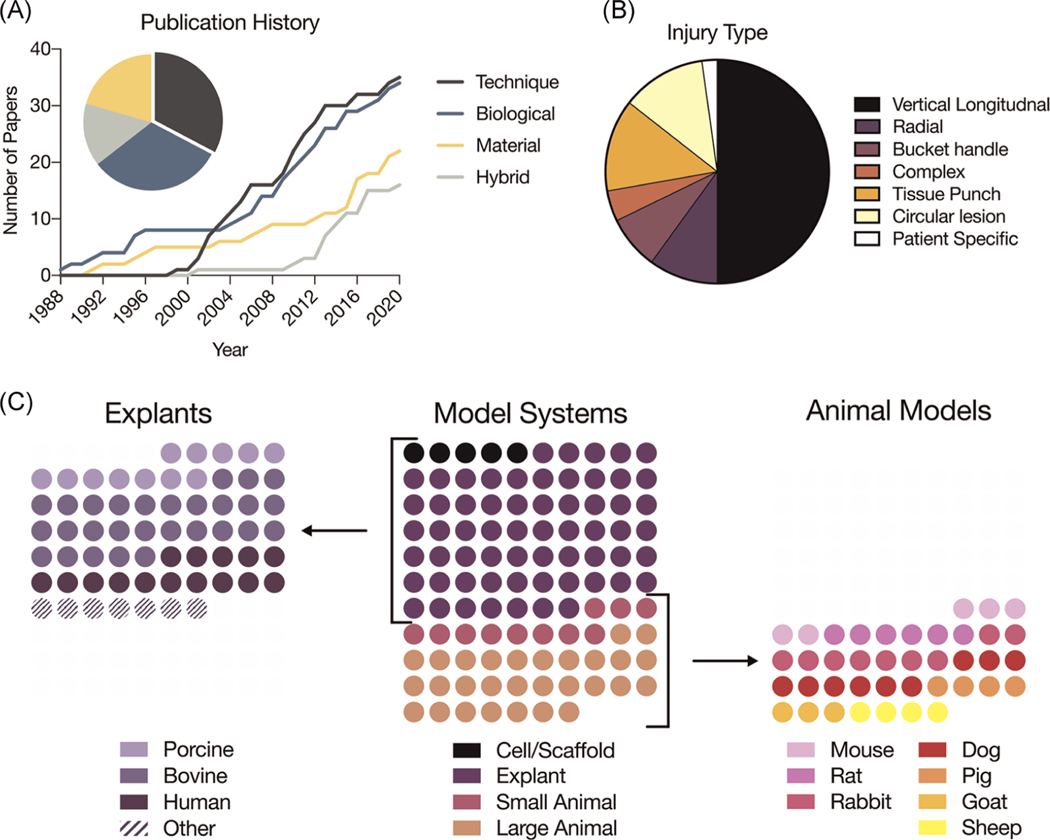
Thirty-five technique-focused studies (32.7%), 34 biologic interventions (31.8%), 22 material interventions (20.5%), and 16 hybrid (biological + material) interventions (15.0%) were identified (Figure 3A). Longitudinal tears were most prevalent tear type studied, appearing in 45 of 86 publications (52.3%) followed by radial (10.5%) and bucket handle (8.1%) tears (Figure 3B). Other experimental designs included circular lesions created within the body of the meniscus (12.8%) and an annulus model encompassing a cored-out disc of meniscus (9.3%).
FIGURE 3.
Summary of systematic review findings. (A) Publication history of papers included in the systematic review from 1988 to 2020. Papers categorized across technique, biological, material, and hybrid (combined biological/material) interventions. (B) Graph of prevalent injuries/models (annulus model and circular lesion) utilized in studies. (C) Graph of model systems used in studies in systematic review (middle) and expansions of explant species (left) and large animal model species (right). Each circle presents one study that has utilized that system or model (n = 107)
The remaining studies used migration assays, subcutaneous implantation, cell injection, or lap-jointed tissue, among other methods. The majority of studies were in explant models (57.9%), with tissue primarily from bovine (26.1%), human (14.0%), and porcine (11.2%) sources. Large animal models (27.1%) were more prevalent than small animal models (10.2%). Cell-based studies were least prevalent (4.7%) (Figure 3C). The remainder of this review focuses on these three categories of repair (technique, biological, material), and highlights methods for improvement in repair in these categories.
4 |. CLINICAL TEAR MANAGEMENT AND ADVANCES IN TECHNIQUE
4.1 |. Current management
Due to the spatially variant vasculature of the meniscus, treatment type often depends on the location of the tear along the radial axis.46 The outer third of the meniscus receives perfusion from the peripheral capsular plexus, while the inner two-thirds is diffusion dependent. Thus, red-red and red-white tears are most frequently repaired, whereas white-white tears are usually excised to alleviate mechanical discomfort.47 Mounting evidence challenges this practice. Rubman et al. reported white zone repairs failed in only 36% of cases, enough evidence to encourage white-zone repair for young, athletic patients.48 Similarly, Cinque et al. reported that while white-white repairs were inferior to red-red and red-white repairs, they still improved disability, pain, and functional scores relative to preoperative levels.49 These results indicate that repair should be attempted in all three zones when possible.49,50
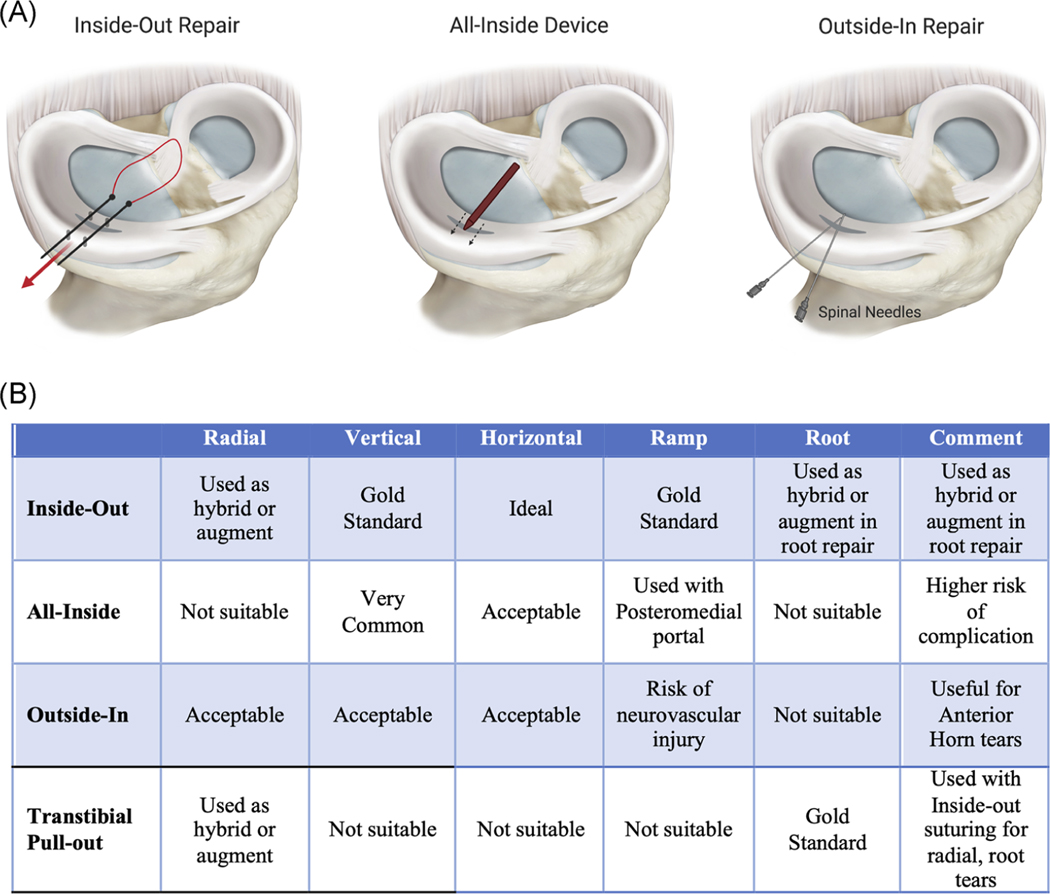
Suture repair techniques include “inside-out,” “all-inside,” and “outside-in” techniques (Figure 4). Inside-out suture repair is the current gold standard, avoiding the introduction of structures which could alter meniscal shape or damage articular cartilage.61 It also allows for compact suture spacing (~3 mm apart), smaller puncture holes, and secure longitudinal or horizontal mattress suturing perpendicular to the tear. However, the inside-out method requires an open surgical incision and a skilled assistant to protect the soft tissues when passing sutures.61 Alternatively, an all-inside device can simplify the procedure and decrease operative time, perhaps the reason that many providers prefer all-inside devices. Though reported to be relatively equivalent in outcomes to the inside-out technique,62,63 the all-inside approach has higher risks of new tear or tear propagation, chondral damage, and local irritation necessitating meniscal removal.64,65 Finally, the outside-in technique can be advantageous in specific scenarios (e.g., anterior horn tears55) or for most types of tears due to its cost effectiveness.55,66
FIGURE 4.
Schematic and recommendations for meniscus repair techniques. (A) (Left) inside-out, (middle), all-inside, and (right) outside-in techniques. (B) Repair techniques (inside-out, all-inside, outside-in) recommended for major tear types discussed (radial, longitudinal, horizontal, ramp [longitudinal tear in the peripheral capsular attachment of the posterior horn], root). Relative use (acceptable, very common, not suitable, etc.), gold standards, and risks are also detailed. Recommendations obtained from the following sources for inside-out (Muckenhirn et al., 2017; Nelson et al., 2013; Kang et al., 2019)51–53, all-inside (Kang et al., 2019; Negrin et al., 2018)53,54, outside-in (Menge et al., 2016; Steiner et al., 2018; Dave et al., 2012; Thompson et al., 2014)55–58, and transtibial techniques (Chahla et al., 2016; LaPrade et al., 2015)59,60
4.2 |. Systematic review of technique improvement studies
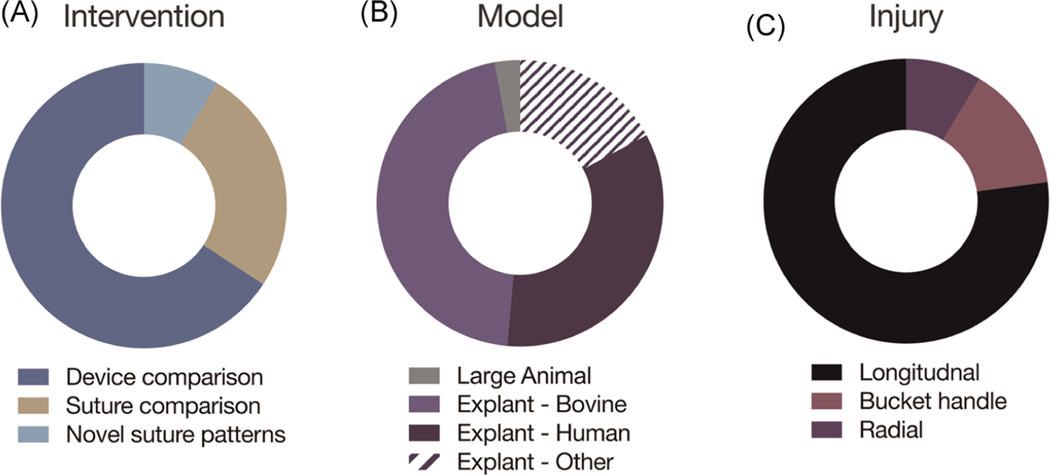
Recent improvements in these three traditional repair approaches, as well as new techniques in the clinical space, were identified through our systematic review. Within “Technique” studies, articles were coded further into sub-categories (“novel suture pattern,” “suture comparison,” “device comparison”). The majority (23 of 35, 65.7%) tested commercial medical devices, including arrows, anchors, and all-inside devices marketed by medical device companies. Suture patterns were biomechanically evaluated in 9 of 35 studies (25.7%) and novel patterns were detailed in only 3 (8.6%) (Figure 5A). These surgical advances include cross-stitch, rebar, and transtibial techniques, which have been used to address radial or root tears.67–69 All but one technique study used explant models, which were primarily bovine or human cadaveric in origin (Figure 5B), and no technique-oriented studies utilized small animal models. “Technique” studies primarily utilized longitudinal tears (27 of 35, 77.1%), over bucket handle (14.3%) and radial tears (8.6%) (Figure 5C). These novel repair devices and techniques may help to improve instantaneous structural integrity, thereby aiding in the restoration of the load-distributing capabilities of the meniscus. However, additional factors may also be needed to enhance the long-term bridging of the two ends of the tear, during and following suture resorption.
FIGURE 5.
Statistics regarding technique-based studies for meniscus repair. (A) Technique advancement by category, (B) model system, and (C) injury type
5 |. BIOLOGICS IN MENISCAL REPAIR
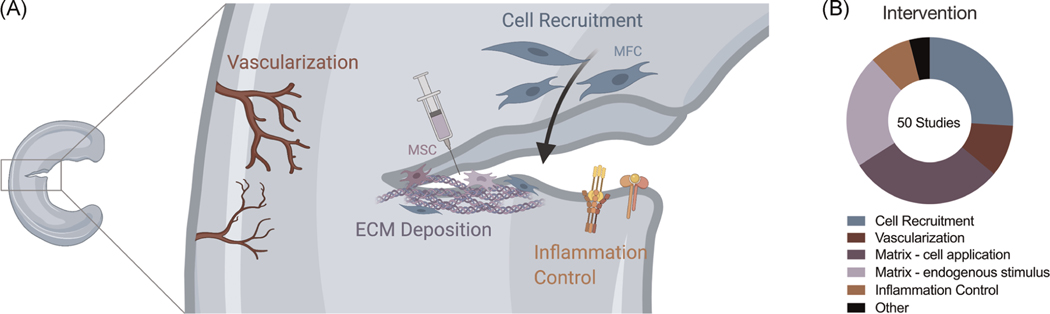
The use of biologics in orthopedics (“orthobiologics”), has garnered increased interest, especially in meniscal tear management. Biologics are intended to aid in defect closure by introducing additional cells and/or bioactive factors to the interface. These augmentations may be especially beneficial in avascular tears, which have limited endogenous healing capacity. Biologics used in recent clinical studies include fibrin clots, platelet-rich plasma (PRP), and mesenchymal stem cells (MSCs). A more thorough explanation of these factors can be found in the Supporting Information. As biologics for meniscal repair gain clinical traction, laboratory studies are focused on quantifying and characterizing the factors contained within biologic augmentations, the impact these factors have on the formation of meniscal tissue, and the mechanisms driving these processes. Furthermore, while not yet used clinically, growth factors and other bioactive cues can be implemented to improve various steps of meniscal repair. We identified four biologic-based functions (Figure 6) needed to enhance the various stages of meniscal repair: cell recruitment (26.0%), vascularization (10.0%), matrix deposition (52.0%), and inflammation control (8.0%).
FIGURE 6.
A look at the intended functions of biological intervention. (A) Schematic of four major themes in biological repair. (B) Statistics reflecting the prevalence of each theme
5.1 |. Cell recruitment
Due to the relatively avascular and acellular nature of the meniscus, the first step of repair augmentation involves the recruitment of cells with healing potential. In addition to the commonly used bone-marrow and adipose-derived MSCs, synovial stem cells,70,71 blood vessel-derived72 stem cells, and even chondrocytes,73 have been delivered locally in preclinical models of meniscal suture repair. Nakagawa et al.70 isolated and expanded synovial stem cells and injected them into longitudinal defects repaired with suture. Importantly, cells were localized to the defect site after only 10 min and led to enhanced cellular proliferation and wound closure. Cells may be applied directly to the site of injury during the meniscal repair procedure, whether open or arthroscopic, via injection. Growth factors and scaffold-based cell delivery (see Section 6) can also be utilized at the site of injury to improve exogenous cell localization.74 Alternatively, cells from the meniscus itself can be harnessed to promote healing.75 Meniscal progenitor cells and even MFCs have the capacity to produce robust matrix to heal meniscus tears76,77; however, their migration to the defect site through the dense extracellular matrix (ECM) presents a challenge. Techniques to improve the migration of meniscus progenitors and fibrochondrocytes include softening the ECM via localized collagenase release,78 improving cell motility via growth factor78,79 or serum80 delivery, and easing cellular migration through nuclear softening.81 Thus, whether exogenously added or endogenously recruited, supplementary cells at the injury site may be pivotal in the healing of meniscal lesions. Once recruited, these cells can then be guided towards a specified behavior, including vascularization, matrix deposition, or inflammatory relief. Certainly, the optimal type of cell for meniscal regeneration at the repair site is up for debate, and more head-to-head studies comparing cell types for meniscal repair may be required.
5.2 |. Vascularization
The variance in healing between the outer and inner meniscus is almost always attributed to differences in regional vascularity. Clinicians have improved blood flow to the inner meniscus via radial perforations,82,83 but only found a slight, insignificant improvement in patient outcomes. Bioabsorbable conduits have also been suggested to enhance vascular tissue ingrowth.84 Anatomically, the outer vascularized third of the meniscus contains blood-vessel derived stem cells (CD34+, CD146+), which when isolated and mobilized enhanced avascular tear repair in a rat model.72 King et al.85 increased neovascularization more than fivefold via treatment with angiogenin, a proangiogenic factor, in a rabbit defect model. Similarly, endothelial growth factors can promote vascularization of the inner meniscus86 and may promote cellular proliferation, granular tissue formation, and bridging of the defect.86,87 Thus, the formation of new blood vessels, or angiogenesis, and their impact on the healing of avascular injury sites need investigation. Collectively, both cell recruitment and vascularization may need to be directed to better encourage ECM deposition/organization.
5.3 |. Matrix deposition
The afore-mentioned techniques routinely lead to increased granulation tissue.86 Any new tissue that bridges the opposing ends of the tear must withstand considerable stresses, and thus robust and appropriate matrix deposition is required. Perhaps the factor most utilized in meniscus regeneration is transforming growth factor β3 (TGF-β3). When added to cultures of stem cells or meniscus fibrochondrocytes, it increased both collagen and PG production and integration strength between two edges of a meniscus tear.74,88 Similarly, connective tissue growth factor74,87 and insulin-like growth factor89,90 led to mechanically superior repair integrity. The biggest challenge for growth factor use is localized and sustained delivery, which can be achieved with scaffold delivery (see Section 6). Finally, an exciting biologic direction is gene therapy with endogenous or exogenous cells.89,91 For example, a vector to overexpress TGF-β3 in MFCs resulted in increased cell proliferation and matrix synthesis without delivery of the growth factor itself.91 Thus, application of genetic vectors or genetically modified cells to meniscus tears may expedite ECM incorporation, though further preclinical and clinical exploration is required.92
5.4 |. Inflammation control
The synovial environment of the knee joint presents a harsher setting than is typically mimicked in vitro. Inflammation is a natural response to trauma and injury and if not harnessed to augment the repair, will likely inhibit the regenerative process. While inflammatory cytokines that are upregulated in osteoarthritic conditions93 can promote granular tissue formation, this tissue is mechanically inferior and vulnerable to retear. In vitro, interleukin-1 (IL-1) significantly decreased repair strength, cell migration, and tissue formation,94,95 but inhibition of IL-1 via a receptor antagonist enhanced integrative repair. Furthermore, other proinflammatory cytokines, such as tumor necrosis factor α and matrix metalloprotease, can be inhibited to improve meniscal healing.95–97 These findings suggest inflammation leads to a catabolic state in the meniscus, and inflammation control may be needed to maximize repair success, especially in joints with concomitant injury or a more degenerative and/or inflammatory state. Regardless, the spatiotemporal introduction and distribution of these biologic methods are often difficult to control and may need to be combined with material approaches.
6 |. MATERIALS IN MENISCAL REPAIR
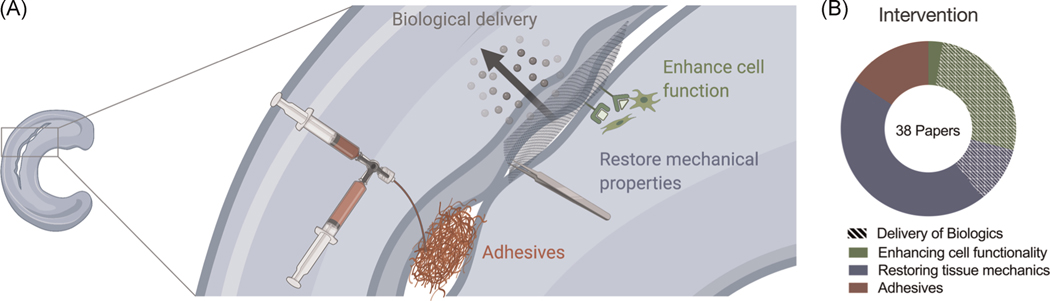
Historically, meniscus scaffolds have been directed at recreating the native anatomy and its mechanical properties following meniscectomy. As the focus has shifted toward repairing the injured tissue, bioactive materials that integrate with native tissue and include cell-instructive cues to promote physiological healing are paramount. Fibrin glue and collagen wraps are clinically available “materials” to augment meniscal repair, yet there are still no biomaterial-based FDA-approved products for this indication. Below, we highlight material strategies to target specific functions needed to augment repair: biologic delivery to promote cell migration and function, restoration of mechanical properties, and wound closure via tissue adhesives (Figure 7). Additional information is provided in Supporting Information.
FIGURE 7.
A schematic of the types of material intervention for repair. (A) Schematic of four major themes in materials-based repair. (B) Statistics reflecting the prevalence of each theme
6.1 |. Biologic delivery
As highlighted previously, localized delivery of proteins, nucleic acids, and cells is advantageous in augmenting meniscus regeneration. For success at the time scale needed for repair, sustained retention and release to maintain therapeutic concentrations may be required. Scaffold fabrication techniques alone can control the release of single or multiple factors. For example, biopolymer-based hydrogel scaffolds releasing TGF-β3 increased fibrochondrogenic differentiation and tissue integration.98,99 Additional introduction of stromal cell-derived factor α (SDF-1)78 enhanced cell migration, similarly noticed with platelet-derived growth factor79 and PRP.100 Further, various material carriers (e.g., microspheres, nanocapsules) can improve factor release.101 For example, TGF-β3 release from poly (lactic-co-glycolic acid) microspheres promoted extended differentiation and ECM production.74 These material strategies to incorporate and release biologics increased matrix production, collagen fiber alignment, and mechanical properties, producing a more physiological repair. Finally, these material and scaffolds can be incorporated with cells (e.g., MSCs), allowing for retention of cells at the defect site, increasing their likelihood to deposit meniscus-specific ECM and heal tears.102–104
6.2 |. Materials to promote cell adhesion and function
Material-augmented repair provides a biomimetic structure with requisite chemo-mechanical cues to promote cell migration, proliferation, and functional ECM production for wound closure of meniscus tears. Decellularized meniscus extracellular matrix (dECM) is an exciting material in the field, as the major ECM components of the native meniscus tissue (collagen I, collagen II, and glycosaminoglycans) are retained and provide a substrate for cell migration and proliferation.105–108 The maintenance of meniscus-specific biochemical cues promote cell growth and behavior, in particular the upregulation of fibrochondrogenic markers (ACAN, COL1A2, COL2A1, and COL10A1).108,109 Furthermore, its porosity allows nutrients and oxygen to diffuse into the scaffold and promote cell growth.106 Perhaps the only tradeoffs with dECM for meniscal repair are the relatively inadequate mechanical properties compared to native tissue and the potential variability in sourcing and composition.
Material fabrication properties and functionalization with bioactive agents can promote cell adhesion and function. Biocompatible materials can allow cell attachment and stimulate collagen deposition, increasing integration strength.110,111 Baek et al. fabricated core-shell electrospun fibers; a collagen shell to promote cell attachment and new matrix synthesis, and a core of poly(lactic acid) for mechanical strength. Histological and mechanical assessment illustrated increased integration with native meniscus tissue. Further, scaffold mechanics, and specifically the fibers used, can enhance cellular invasion and collagen deposition, illustrating the importance of scaffold microenvironment on cell sensation, deformation, and migration.112 Finally, routinely used polymers (e.g., polycaprolactone, poly(vinyl alcohol)) can be functionalized with unique biomolecules113,114 or peptides115,116 for cellular recruitment and adhesion, often the first step in augmenting meniscus repair.
6.3 |. Matching native mechanical properties
One of the most critical components of meniscal repair is recapitulating the circumferential and radial alignment of collagen fibers, permitting physiological stress distribution across the tissue and across the articular cartilage, which it protects.117 Electrospinning is a unique scaffold fabrication method that produces nanofibers that mimic the native collagen fibril diameter and arrangement, and can be tuned to create circumferential and radial fiber alignment to match native tissue.118–120 Moreover, new collagen production from seeded MSCs followed the electrospun fiber direction, enhancing mechanical properties in the circumferential direction. Polyester and collagen solutions can also be electrospun and organized to achieve mechanical properties similar to the native meniscus.111,118 Electrospun scaffolds have improved the degree of meniscal repair in both in vitro and in vivo models,78,121,122 yet the integration of the scaffold with tissue to produce a continue, mechanically function interface remains challenging.
6.4 |. Tissue adhesives
For many of the materials options discussed previously, arthroscopic implementation may be complicated with current surgical tools. In response, a relatively new arena in augmenting meniscal repair is the development and use of “bioadhesives.”123 Two potential materials for tissue adhesion and integration are fibrin glue and synthetic polymers. Fibrin glues are used clinically, utilize crosslinking pathways of fibrin clots for tissue adhesion,124,125 and can increase cell migration and integration of ECM components, but it is mechanically inferior in sealing defect edges. Alternatively, synthetic polymers can maintain cell viability (e.g., isocyanate-terminated polymers), while significantly enhancing shear adhesive strength relative to fibrin glue.126,127 While prefabricated scaffolds require an incision to be inserted into place,106,110 material adhesives can be introduced arthroscopically and crosslinked in vivo,74 and thus may be especially useful for tear locations that are not readily accessible for suture repair.
7 |. MODEL SYSTEMS FOR AUGMENTED MENISCUS REPAIR
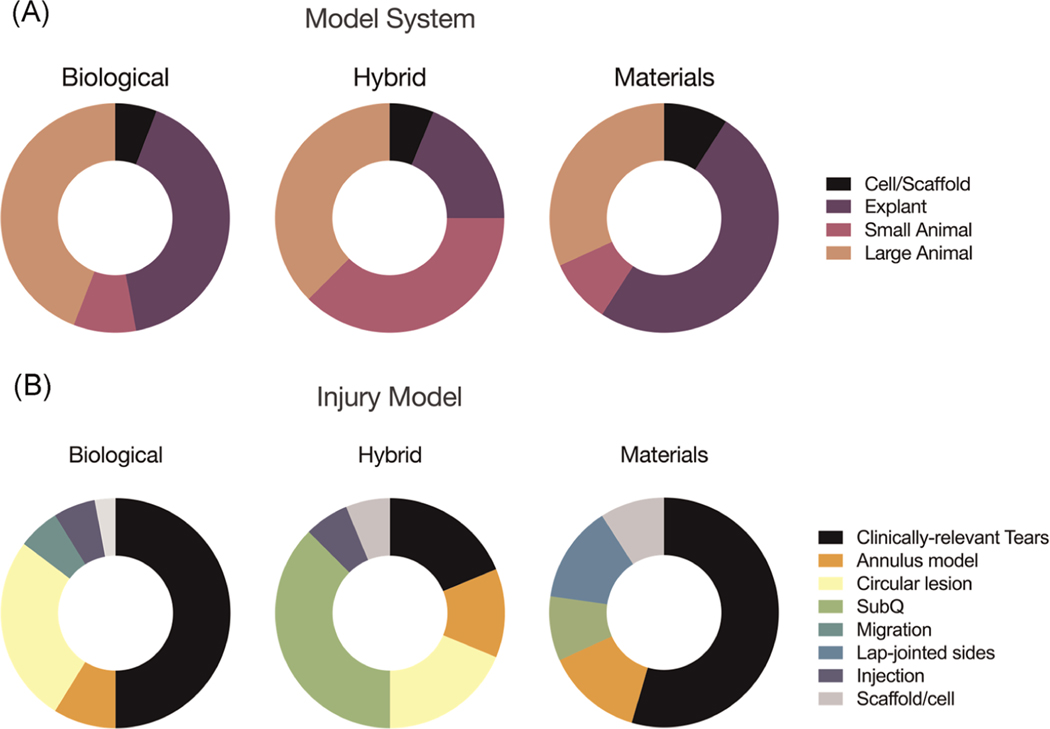
To evaluate biologic- and material-based augmentation approaches preclinically, appropriately integrated preclinical test platforms and evaluation criteria are required.128 We revisited our systematic review within the “Biological,” “Hybrid,” and “Materials” studies, and articles were further analyzed (Figure 8A). Regarding model systems, all three categories had a similar percentage of cell/scaffold (5.8%–9.1%) and large animal (31.8%–44.1%) studies. Interestingly, biologic and material interventions utilized explant models (41.2% and 50.0%, respectively) much more often than hybrid interventions (18.8%), which instead used small animal models (37.5%) more than biologic or material studies (8.8% and 9.1%, respectively). This may speak to the need for hybrid models to prove both biological efficacy and effective delivery, which are more clinically relevant in animal models compared to explants. Moreover, the individual components of these hybrid approaches may have already been investigated in in vitro and explant models, and thus the combination can undergo translation more quickly.
FIGURE 8.
Statistics of biological, hybrid, and material interventions for meniscus repair. (A) Model systems and (B) injury models in each type of intervention
Regarding injury model, biological and material studies utilized clinically relevant tears (50.0% and 54.5%, respectively) at more than twice the rate of hybrid interventions (18.8%), likely due to the relative “youth” of hybrid techniques, which may require proof-of-concept experimentation. All three types of studies used the “annulus” model (8.8%–13.64%), yet circular punch defects were utilized only in biological and hybrid studies (26.5% and 18.8%, respectively), and not in materials-based solutions. The difficulties of creating circular scaffolds and the lack of clinical relevance may inform the lack of circular models in materials interventions. Meanwhile, subcutaneous evaluation is common in material and hybrid studies (9.1% and 37.5%, respectively) but not present in biological studies, likely because material safety and biocompatibility are essential to novel biomaterials. Lastly, two injury models were only seen in one type of study. Biological studies uniquely used migration assays (5.9%) given their emphasis on enhancing migration, and materials studies uniquely employed “lap-jointed” tissue models (13.6%) to assess integration strength (Figure 8B).
For preliminary testing of these approaches, an annulus model is frequently employed; evaluation of the repair interface should involve both mechanical (push-out testing96,129) and biological (histology and subsequent scoring,130 matrix characterization) outcomes. However, since each tear pattern compromises different meniscal fiber networks, these annular models may need to account for the circumferential versus radial axis. As translation moves to small and large animals, functional, mechanical, and biological outputs remain important.131,132 Since the majority of in vivo repair studies have involved vertical longitudinal tear models, which already have relatively successful clinical outcomes, preclinical models that introduce radial and root defects may better address more problematic tear types.
8 |. LOOKING TO THE FUTURE
Meniscal repair is a developing field. A PubMed search for meniscus/meniscal repair yielded over 3100 manuscripts, with nearly half in the past 5 years. Tear management has shifted away from meniscectomy towards procedures to salvage native tissue. Technique-based innovations like transtibial tunnel repairs133,134 have improved time-zero repair mechanics, yet some scenarios remain problematic (e.g., avascular and root tears) and bridging of the tear can be elusive. However, laboratory research has developed methods that may improve functional outcomes. Here, we performed a systematic review to highlight new techniques and trends (biologic- and material-based) to suggest the future landscape of meniscal repair. Certainly, while this systematic review may have missed much of the cutting-edge work in augmenting meniscal repair in the clinic, we were able to emphasize much of the preclinical work that can better characterize and evaluate specific elements of repair. In particular, cellular recruitment (exogenous or endogenous) and control upon arrival, and scaffolds and adhesives that help bridge tears, are exciting directions, both alone and in combination.
The discussed augmentation strategies are indeed promising, utilizing technical advances in sports medicine, innovative biological strategies such as gene editing and cell localization, and novel material chemistries and strategies to enhance the healing process. One of the biggest question marks that remains is reestablishing the natural geometry of collagen fibers that were severed during the initial tear. While studies have shown enhanced integration strength with augmentation, these properties are often a fraction of native tissue, leaving the repair site susceptible to retear. Another avenue of future exploration involves precise control of cells at the injury site; the introduction of a tear perturbs the mechanical and biochemical microenvironment, which may need to be accounted for when designing new therapies. Furthermore, many of these approaches do not account for some meniscal deterioration that can occur quite rapidly post-injury. The loss of matrix elements, mainly PGs around the free defect edge, may compromise the mechanical integrity of tissue around the repair site. Thus, techniques that fortify meniscal tissue around the site of injury may be advantageous.
While novel sutures and repair devices have been approved for clinical use, the biologics and materials discussed may require appropriate safety and efficacy testing, complicating the regulatory pathway. Currently, the only meniscus-related products in the market, other than sutures, are for replacement after partial or full meniscectomy (ActiFit, CMI, Trammpolin, FibroFix, NUSurface), and are comprised of widely characterized biological or synthetic materials. Utilizing predicate materials/factors, and minimal manipulation of biologics, would ease translation. Furthermore, therapies that harness current clinical augmentations (e.g., PRP) would be advantageous, and research to understand the basic science implications of these biologics would be fruitful.
Additional variables including sex, age, pre-existing conditions, and concomitant injuries play a role in meniscal injury and healing. Personalizing the approach represents a future direction in clinical management, even more so as we start to appreciate knee-to-knee variability in joint mechanics.135 Indeed, we should not lose sight of the fact that any potential solution should restore the ability of the meniscus to distribute load across the articular surfaces of the knee joint. Moreover, the rehabilitation process is also integral; strict timetables for early phases protect the surgical repair, especially as new tissue is deposited to bridge the tear.136 Earlier weight-bearing has been attempted, but higher re-tear rates are observed with weight-bearing under 6 weeks. Thus, the development of augmentation techniques should observe and potentially accelerate healing during this timeline.
One limitation of the systematic review and the preclinical literature is the emphasis on traumatic tears, and thus the disregard for degenerative tears. Degenerative tears are often observed with other articular pathologies or are within aging joints that complicate the healing process and reduce the possibility of successful repair.137,138 Typically treated conservatively first with physical therapy and, as a last resort, with partial meniscectomy,139–141 the long-term prognosis of these injuries is bleak. Additionally, degenerative menisci have likely experienced significant matrix loss and aberrant cellular behavior; thus, to save the meniscus in these case, fortification and stabilization techniques142,143 may better restore healthy meniscus function when combined with repair. Finally, in more severe cases, while note clinically recommended, degenerative tears may need to be treated with replacement allografts or scaffolds,144–146 since the remaining meniscal tissue may be too compromised. These approaches could achieve greater success if coupled with inflammatory relief to combat the likely upregulated cytokine concentration that inhibit meniscal regeneration. In general, combination approaches that slow the degeneration of both the meniscus and surrounding joint environment may be clinically beneficial in the management of these more degenerative injuries.147
In conclusion, we summarized the current state of meniscal tear management and highlighted basic science approaches that may improve repair. The meniscus remains a heavily researched tissue, as evidenced by increased journal publications and conference presentations. The Orthopaedic Research Society established a Meniscus Section in 2016, highlighting the variety of research in the field and leading to more collaborative efforts between biologists, engineers, and clinicians. We hope that these efforts yield new and exciting meniscal repair techniques to improve management following tears.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the Orthopaedic Research Society Meniscus Section for their support and guidance on this manuscript.
Footnotes
CONFLICT OF INTERESTS
Sonia Bansal is a consultant for AGelity Biomechanics Corp. Jorge Chahla received consulting fees from Smith & Nephew, Arthrex, Ossur, Linvatec Corp, and DePuy Synthes Products, and hospitality payments from Medical Devices Business Services Inc, Stryker Corp, and Medwest Associates. Robert F. LaPrade is a consultant for Arthrex, Ossur, Linvatec Corp, and Smith & Nephew, received royalties from Arthrex, Ossur, and Smith & Nephew, received research grants from Ossur and Smith & Nephew, and is on the editorial board of the AJSM, JEO, KSSTA. Suzanne A. Maher is a founder of AGelity Biomechanics Corp. Jay M. Patel is a consultant for NovoPedics Inc.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Kambic HE, McDevitt CA. Spatial organization of types I and II collagen in the canine meniscus. J Orthop Res. 2005;23(1): 142–149. [DOI] [PubMed] [Google Scholar]
- 2.Chevrier A, Nelea M, Hurtig MB, Hoemann CD, Buschmann MD. Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. J Orthop Res. 2009;27(9):1197–1203. [DOI] [PubMed] [Google Scholar]
- 3.Setton LA, Mow VC, Müller FJ, Pita JC, Howell DS. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. J Bone Jt Surg. 1994;12(4):451–463. [DOI] [PubMed] [Google Scholar]
- 4.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009; 1(6):461–468. http://www.ncbi.nlm.nih.gov/pubmed/23015907%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/23015907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams ME, Hukins DWL. The Extracellular Matrix of the Meniscus. In: Mow VC, Arnoczky SP, Jackson DW, eds. Knee Meniscus: Basic and Clinical Foundations. New York: Raven Press; 1992:15–28. [Google Scholar]
- 6.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structurefunction, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skaggs DL, Warden WH, Mow VC. Radial tie fibers influence the tensile properties of the bovine medial meniscus. J Orthop Res. 1994;12(2):176–185. [DOI] [PubMed] [Google Scholar]
- 8.Andrews SHJ, Rattner JB, Abusara Z, Adesida A, Shrive NG, Ronsky JL. Tie-fibre structure and organization in the knee menisci. J Anat. 2014;224(5):531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullough PG, Munuera L, Murphy J, Weinstein AM. The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br. 1970;52(3):564–567. [PubMed] [Google Scholar]
- 10.Han WM, Heo S-J, Driscoll TP, et al. Microstructural heterogeneity directs micromechanics and mechanobiology in native and engineered fibrocartilage. Nat Mater. 2016;15(4):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnoczky SP. Gross and Vascular Anatomy of the Meniscus and its Role in Meniscal Healing, Regeneration and Remodeling. In: Mow VC, Arnoczky VP, Jackson DW, eds. Knee Meniscus: Basic and Clinical Foundations. New York: Raven Press; 1992:1–14. [Google Scholar]
- 12.Petersen W, Tillmann B. Age-related blood and lymph supply of the knee menisci: a cadaver study. Acta Orthop Scand. 1995;66(4):308–312. [DOI] [PubMed] [Google Scholar]
- 13.Ionescu LC, Lee GC, Garcia GH, et al. Maturation state-dependent alterations in meniscus integration: implications for scaffold design and tissue engineering. Tissue Eng Part A. 2011;17(1–2):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu F, Pintauro MP, Haughan JE, et al. Repair of dense connective tissues via biomaterial-mediated matrix reprogramming of the wound interface. Biomaterials. 2015;39:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merriam AR, Patel JM, Culp BM, Gatt CJ, Dunn MG. Successful total meniscus reconstruction using a novel fiber-reinforced scaffold: a 16- and 32-week study in an ovine model. Am J Sports Med. 2015;43(10):2528–2537. [DOI] [PubMed] [Google Scholar]
- 16.Mordecai SC. Treatment of meniscal tears: an evidence based approach. World J Orthop. 2014;5(3):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung KS, Ha JK, Kim YS, et al. National trends of meniscectomy and meniscus repair in Korea. J Korean Med Sci. 2019;34(32):e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery SR, Zhang A, Ngo SS, Wang JC, Hame SL. Cross-sectional analysis of trends in meniscectomy and meniscus repair. Orthopedics. 2013;36:e1007–e1013. [DOI] [PubMed] [Google Scholar]
- 19.Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333–2339. [DOI] [PubMed] [Google Scholar]
- 20.Parker BR, Hurwitz S, Spang J, Creighton R, Kamath G. Surgical trends in the treatment of meniscal tears. Am J Sports Med. 2016; 44:1717–1723. [DOI] [PubMed] [Google Scholar]
- 21.DeFroda SF, Yang DS, Donnelly JC, Bokshan SL, Owens BD, Daniels AH. Trends in the surgical treatment of meniscal tears in patients with and without concurrent anterior cruciate ligament tears. Phys Sportsmed. 2020;48:229–235. [DOI] [PubMed] [Google Scholar]
- 22.Katano H, Koga H, Ozeki N, et al. Trends in isolated meniscus repair and meniscectomy in Japan, 2011–2016. J Orthop Sci. 2018; 23:676–681. [DOI] [PubMed] [Google Scholar]
- 23.Lubowitz JH, Poehling GG. Save the meniscus. Arthroscopy. 2011; 27(3):301–302. 10.1016/j.arthro.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 24.Vidal AF. The save the meniscus society: commentary on an article by Jeffrey J. Nepple MD, et al. : “meniscal repair outcomes at greater than five years. a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(24):e186. http://europepmc.org/abstract/MED/23318625 [DOI] [PubMed] [Google Scholar]
- 25.Seil R, Becker R. Time for a paradigm change in meniscal repair: save the meniscus! Knee surgery. Sport Traumatol Arthrosc. 2016; 24(5):1421–1423. 10.1007/s00167-016-4127-9 [DOI] [PubMed] [Google Scholar]
- 26.Beaufils P, Pujol N. Management of traumatic meniscal tear and degenerative meniscal lesions. Save the meniscus. Orthop Traumatol Surg Res. 2017;103(8S):S237–S244. http://www.sciencedirect.com/science/article/pii/S1877056817302311 [DOI] [PubMed] [Google Scholar]
- 27.Pujol N, Beaufils P. Save the meniscus again! Knee surgery. Sport Traumatol Arthrosc. 2019;27(2):341–342. 10.1007/s00167-018-5325-4 [DOI] [PubMed] [Google Scholar]
- 28.Kurzweil PR. Editorial commentary: a stitch in time may save the meniscus…but not the articular cartilage? Arthroscopy. 2019;35(5): 1517–1519. 10.1016/j.arthro.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 29.Lee WQ, Gan JZ-W, Lie DTT. Save the meniscus—clinical outcomes of meniscectomy versus meniscal repair. J Orthop Surg. 2019;27(2): 2309499019849813. 10.1177/2309499019849813 [DOI] [PubMed] [Google Scholar]
- 30.Kim JG. Editorial commentary: save the meniscal root, why not? Arthroscopy. 2019;35(7):2207–2210. 10.1016/j.arthro.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 31.Momaya A. Editorial commentary: save the meniscus? show me the money!. Arthroscopy. 2019;35(12):3287–3288. 10.1016/j.arthro.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 32.Razi M, Mortazavi SMJ. Save the meniscus, a good strategy to preserve the knee. Arch Bone Jt Surg. 2020;8(1):1–4. https://pubmed.ncbi.nlm.nih.gov/32090138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48(8):2178–2187. [DOI] [PubMed] [Google Scholar]
- 34.Koff MF, Shah P, Pownder S, et al. Correlation of meniscal T2* with multiphoton microscopy, and change of articular cartilage T2 in an ovine model of meniscal repair. Osteoarthr Cartil. 2013;21:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson T, Fabricant PD, Beck N, Storey E, Patel NM, Ganley TJ. Epidemiology, injury patterns, and treatment of meniscal tears in pediatric patients: a 16-year experience of a single center. Orthop J Sport Med. 2019;7:232596711989032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christino M, Willimon SC, Perkins C, et al. The rate of meniscus tears in association with anterior cruciate ligament injuries increases with age. Orthop J Sport Med. 2019;7(3 Suppl): 2325967119S00171. [Google Scholar]
- 37.Ridley TJ, McCarthy MA, Bollier MJ, et al. Age differences in the prevalence of isolated medial and lateral meniscal tears in surgically treated patients. Iowa Orthop J. 2017;37:91–94. [PMC free article] [PubMed] [Google Scholar]
- 38.Mansori AE, Lording T, Schneider A, Dumas R, Servien E, Lustig S. Incidence and patterns of meniscal tears accompanying the anterior cruciate ligament injury: possible local and generalized risk factors. Int Orthop. 2018;42:2113–2121. [DOI] [PubMed] [Google Scholar]
- 39.Kim S-G, Kim S-H, Baek J-H, et al. High incidence of subsequent reoperation following treatments for medial meniscus tears combined with anterior cruciate ligament reconstruction: second-look arthroscopic study. Knee Surg Relat Res. 2019;31:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terzidis IP, Christodoulou A, Ploumis A, Givissis P, Natsis K, Koimtzis M. Meniscal tear characteristics in young athletes with a stable knee: arthroscopic evaluation. Am J Sports Med. 2006;34: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 41.Robinson S, Nixon M, Hakkalamani S, Parkinson R. Meniscal tears: epidemiology and correlation between clinical and arthroscopic findings. Orthop Proc. 2011;93-B(SUPP_II):161. 10.1302/0301-620X.93BSUPP_II.0930161a [DOI] [Google Scholar]
- 42.Baker BE, Peckham AC, Pupparo F, Sanborn JC. Review of meniscal injury and associated sports. Am J Sports Med. 1985;13:1–4. [DOI] [PubMed] [Google Scholar]
- 43.Jiang D, Luo X, Ao Y, et al. Risk of total/subtotal meniscectomy for respective medial and lateral meniscus injury: correlation with tear type, duration of complaint, age, gender and ACL rupture in 6034 Asian patients. BMC Surg. 2017;17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arner JW, Irvine JN, Zheng L, et al. The effects of anterior cruciate ligament deficiency on the meniscus and articular cartilage: a novel dynamic in vitro pilot study. J Sport Med. 2016;4(4):2325967116639895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6:e1000097. [PMC free article] [PubMed] [Google Scholar]
- 46.Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–95. [DOI] [PubMed] [Google Scholar]
- 47.Hutchinson ID, Moran CJ, Potter HG, Warren RF, Rodeo SA. Restoration of the meniscus: form and function. Am J Sports Med. 2013; 42(4):987–998. 10.1177/0363546513498503 [DOI] [PubMed] [Google Scholar]
- 48.Rubman MH, Noyes FR, Barber-Westin SD. Arthroscopic repair of meniscal tears that extend into the avascular zone. A review of 198 single and complex tears. Am J Sports Med. 1998;26:87–95. [DOI] [PubMed] [Google Scholar]
- 49.Cinque ME, DePhillipo NN, Moatshe G, et al. Clinical outcomes of inside-out meniscal repair according to anatomic zone of the meniscal tear. Orthop J Sport Med. 2019;7(7):2325967119860806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nepple JJ, Dunn WR, Wright RW. Meniscal repair outcomes at greater than five years: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94:2222–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muckenhirn KJ, Kruckeberg BM, Cinque ME, et al. Arthroscopic inside-out repair of a meniscus bucket-handle tear augmented with bone marrow aspirate concentrate. Arthrosc Tech. 2017;6: e1221–e1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson CG, Bonner KF. Inside-out meniscus repair. Arthrosc Tech. 2013;2:e453–e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang D-G, Park Y-J, Yu J-H, Oh JB, Lee DY. A systematic review and meta-analysis of arthroscopic meniscus repair in young patients: comparison of all-inside and inside-out suture techniques. Knee Surg Relat Res. 2019;31:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Negrín R, Reyes NO, Iñiguez M, Pellegrini JJ, Wainer M, Duboy J. Meniscal ramp lesion repair using an all-inside technique. Arthrosc Tech. 2018;7:e265–e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5:e1111–e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steiner SRH, Feeley SM, Ruland JR, Diduch DR. Outside-in repair technique for a complete radial tear of the lateral meniscus. Arthrosc Tech. 2018;7:e285–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson SM, Spalding T, Church S. A novel and cheap method of outside-in meniscal repair for anterior horn tears. Arthrosc Tech. 2014;3:e233–e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dave LYH, Caborn DNM. Outside-in meniscus repair: the last 25 years. Sports Med Arthrosc. 2012;20:77–85. [DOI] [PubMed] [Google Scholar]
- 59.Chahla J, Moulton SG, LaPrade CM, Dean CS, LaPrade RF. Posterior meniscal root repair: the transtibial double tunnel pullout technique. Arthrosc Tech. 2016;5:e291–e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laprade CM, Laprade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43:899–904. [DOI] [PubMed] [Google Scholar]
- 61.Dean CS, Chahla J, Matheny LM, Mitchell JJ, LaPrade RF. Outcomes after biologically augmented isolated meniscal repair with marrow venting are comparable with those after meniscal repair with concomitant anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45:1341–1348. [DOI] [PubMed] [Google Scholar]
- 62.Fillingham YA, Riboh JC, Erickson BJ, Bach BR, Yanke AB. Inside-out versus all-inside repair of isolated meniscal tears: an updated systematic review. Am J Sports Med. 2017;45:234–242. [DOI] [PubMed] [Google Scholar]
- 63.Turman KA, Diduch DR, Miller MD. All-inside meniscal repair. Sports Health. 2009;1:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant JA, Wilde J, Miller BS, Bedi A. Comparison of inside-out and all-inside techniques for the repair of isolated meniscal tears: a systematic review. Am J Sports Med. 2012;40:459–468. [DOI] [PubMed] [Google Scholar]
- 65.Tachibana Y, Sakaguchi K, Goto T, Oda H, Yamazaki K, Iida S. Repair integrity evaluated by second-look arthroscopy after arthroscopic meniscal repair with the FasT-Fix during anterior cruciate ligament reconstruction. Am J Sports Med. 2010;38: 965–971. [DOI] [PubMed] [Google Scholar]
- 66.Rodeo SA. Arthroscopic meniscal repair with use of the outside-in technique. Instr Course Lect. 2000;82:127–141. [PubMed] [Google Scholar]
- 67.Matsubara H, Okazaki K, Izawa T, et al. New suture method for radial tears of the meniscus: biomechanical analysis of cross-suture and double horizontal suture techniques using cyclic load testing. Am J Sports Med. 2012;40:414–418. [DOI] [PubMed] [Google Scholar]
- 68.Massey P, McClary K, Parker D, Barton RS, Solitro G. The rebar repair for radial meniscus tears: a biomechanical comparison of a reinforced suture repair versus parallel and cross-stitch techniques. J Exp Orthop. 2019;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhatia S, Civitarese DM, Turnbull TL, et al. A novel repair method for radial tears of the medial meniscus: biomechanical comparison of transtibial 2-tunnel and double horizontal mattress suture techniques under cyclic loading. Am J Sports Med. 2016;44:639–645. [DOI] [PubMed] [Google Scholar]
- 70.Nakagawa Y, Muneta T, Kondo S, et al. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthr. Cartil 2015;23:1007–1017. [DOI] [PubMed] [Google Scholar]
- 71.Horie M, Driscoll MD, Sampson HW, et al. Implantation of allogenic synovial stem cells promotes meniscal regeneration in a rabbit meniscal defect model. J Bone Joint Surg Am. 2012;94:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osawa A, Harner CD, Gharaibeh B, et al. The use of blood vessel-derived stem cells for meniscal regeneration and repair. Med Sci Sports Exerc. 2013;45:813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinand C, Peretti GM, Adams SB, Bonassar LJ, Randolph MA, Gill TJ. An allogenic cell-based implant for meniscal lesions. Am J Sports Med. 2006;34:1779–1789. [DOI] [PubMed] [Google Scholar]
- 74.Tarafder S, Gulko J, Sim KH, Yang J, Cook JL, Lee CH. Engineered healing of avascular meniscus tears by stem cell recruitment. Sci Rep. 2018;8:8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chahla J, Papalamprou A, Chan V, et al. Assessing the resident progenitor cell population and the vascularity of the adult human meniscus. Arthroscopy. 2020;37(1):252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seol D, Zhou C, Brouillette MJ, et al. Characteristics of meniscus progenitor cells migrated from injured meniscus. J Orthop Res. 2017; 35(9):1966–1972. https://pubmed.ncbi.nlm.nih.gov/27813166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muhammad H, Schminke B, Bode C, et al. Human migratory meniscus progenitor cells are controlled via the TGF-β pathway. Stem Cell Reports. 2014;3(5):789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qu F, Holloway JL, Esterhai JL, Burdick JA, Mauck RL. Programmed biomolecule delivery to enable and direct cell migration for connective tissue repair. Nat Commun. 2017;8(1):1780. 10.1038/s41467-017-01955-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee KI, Olmer M, Baek J, D’Lima DD, Lotz MK. Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears. Acta Biomater. 2018;76: 126–134. https://pubmed.ncbi.nlm.nih.gov/29908335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Freymann U, Endres M, Goldmann U, Sittinger M, Kaps C. Toward scaffold-based meniscus repair: effect of human serum, hyaluronic acid and TGF-ß3 on cell recruitment and re-differentiation. Osteoarthr Cartil. 2013;21:773–781. [DOI] [PubMed] [Google Scholar]
- 81.Heo S-J, Song KH, Thakur S, et al. Nuclear softening expedites interstitial cell migration in fibrous networks and dense connective tissues. Sci Adv. 2020;6(25):eaax5083. http://advances.sciencemag.org/content/6/25/eaax5083.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henning CE, Lynch MA, Clark JR. Vascularity for healing of meniscus repairs. Arthrosc J Arthrosc Relat Surg. 1987;3(1):13–18. http://www.sciencedirect.com/science/article/pii/S074980638780004X [DOI] [PubMed] [Google Scholar]
- 83.Scott GA, Jolly BL, Henning CE. Combined posterior incision and arthroscopic intra-articular repair of the meniscus. An examination of factors affecting healing. JBJS. 1986;68(6):847–861. https://journals.lww.com/jbjsjournal/Fulltext/1986/68060/Combined_posterior_incision_and_arthroscopic.6.aspx [PubMed] [Google Scholar]
- 84.Cook JL, Fox DB. A novel bioabsorbable conduit augments healing of avascular meniscal tears in a dog model. Am J Sports Med. 2007; 35:1877–1887. [DOI] [PubMed] [Google Scholar]
- 85.King TV, Vallee BL. Neovascularisation of the meniscus with angiogenin: an experimental study in rabbits. J Bone Joint Surg Br. 1991;73(4):587–590. [DOI] [PubMed] [Google Scholar]
- 86.Hashimoto J, Kurosaka M, Yoshiya S, Hirohata K. Meniscal repair using fibrin sealant and endothelial cell growth factor: an experimental study in dogs. Am J Sports Med. 1992;20:537–541. [DOI] [PubMed] [Google Scholar]
- 87.He W, Liu YJ, Wang ZG, et al. Enhancement of meniscal repair in the avascular zone using connective tissue growth factor in a rabbit model. Chin Med J (Engl). 2011;124(23):3968–3975. [PubMed] [Google Scholar]
- 88.Ionescu LC, Lee GC, Huang KL, Mauck RL. Growth factor supplementation improves native and engineered meniscus repair in vitro. Acta Biomater. 2012;8(10):3687–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang H, Leng P, Zhang J. Enhanced meniscal repair by overexpression of hIGF-1 in a full-thickness model. Clin Orthop Relat Res. 2009;467(12):3165–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Izal I, Ripalda P, Acosta CA, Forriol F. In vitro healing of avascular meniscal injuries with fresh and frozen plugs treated with TGF-beta1 and IGF-1 in sheep. Int J Clin Exp Pathol. 2008;1(5): 426–434. [PMC free article] [PubMed] [Google Scholar]
- 91.Cucchiarini M, Schmidt K, Frisch J, Kohn D, Madry H. Overexpression of TGF-β via rAAV-mediated gene transfer promotes the healing of human meniscal lesions ex vivo on explanted menisci. Am J Sports Med. 2015;43:1197–1205. [DOI] [PubMed] [Google Scholar]
- 92.Cucchiarini M, McNulty AL, Mauck RL, Setton LA, Guilak F, Madry H. Advances in combining gene therapy with cell and tissue engineering-based approaches to enhance healing of the meniscus. Osteoarthr Cartil. 2016;24(8):1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stone AV, Loeser RF, Vanderman KS, Long DL, Clark SC, Ferguson CM. Pro-inflammatory stimulation of meniscus cells increases production of matrix metalloproteinases and additional catabolic factors involved in osteoarthritis pathogenesis. Osteoarthr Cartil. 2014;22:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. J Orthop Res. 2008;26(4):504–512. http://europepmc.org/abstract/MED/18050309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McNulty AL, Moutos FT, Weinberg JB, Guilak F. Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor α. Arthritis Rheum. 2007;56: 3033–3043. [DOI] [PubMed] [Google Scholar]
- 96.McNulty AL, Weinberg JB, Guilak F. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res. 2009;467:1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riera KM, Rothfusz NE, Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Interleukin-1, tumor necrosis factor-alpha, and transforming growth factor-beta 1 and integrative meniscal repair: influences on meniscal cell proliferation and migration. Arthritis Res Ther. 2011;13:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romanazzo S, Vedicherla S, Moran C, Kelly DJ. Meniscus ECM-functionalised hydrogels containing infrapatellar fat pad-derived stem cells for bioprinting of regionally defined meniscal tissue. J Tissue Eng Regen Med. 2018;12(3):e1826–e1835. [DOI] [PubMed] [Google Scholar]
- 99.Sasaki H, Rothrauff BB, Alexander PG, et al. In vitro repair of meniscal radial tear with hydrogels seeded with adipose stem cells and TGF-β3. Am J Sports Med. 2018;46:2402–2413. [DOI] [PubMed] [Google Scholar]
- 100.Kemmochi M, Sasaki S, Takahashi M, Nishimura T, Aizawa C, Kikuchi J. The use of platelet-rich fibrin with platelet-rich plasma support meniscal repair surgery. J Orthop. 2018;15:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel JM, Saleh KS, Burdick JA, Mauck RL. Bioactive factors for cartilage repair and regeneration: improving delivery, retention, and activity. Acta Biomater. 2019;93:222–238. http://www.sciencedirect.com/science/article/pii/S1742706119300832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McCorry MC, Puetzer JL, Bonassar LJ. Characterization of mesenchymal stem cells and fibrochondrocytes in three-dimensional co-culture: analysis of cell shape, matrix production, and mechanical performance. Stem Cell Res Ther. 2016;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCorry MC, Bonassar LJ. Fiber development and matrix production in tissue-engineered menisci using bovine mesenchymal stem cells and fibrochondrocytes. Connect Tissue Res. 2017;58: 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koch M, Achatz FP, Lang S, et al. Tissue engineering of large fullsize meniscus defects by a polyurethane scaffold: accelerated regeneration by mesenchymal stromal cells. Stem Cells Int. 2018; 2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ruprecht JC, Waanders TD, Rowland CR, et al. Meniscus-derived matrix scaffolds promote the integrative repair of meniscal defects. Sci Rep. 2019;9:8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen YC, Chen RN, Jhan HJ, et al. Development and characterization of acellular extracellular matrix scaffolds from porcine menisci for use in cartilage tissue engineering. Tissue Eng Part C Methods. 2015;21(9):971–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McNulty AL, Lyons LP, Perea SH, et al. Meniscus-derived matrix bioscaffolds: effects of concentration and cross-linking on meniscus cellular responses and tissue repair. Int J Mol Sci. 2020; 21(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhong G, Yao J, Huang X, et al. Injectable ECM hydrogel for delivery of BMSCs enabled full-thickness meniscus repair in an orthotopic rat model. Bioact Mater. 2020;5(4):871–879. http://www.sciencedirect.com/science/article/pii/S2452199X20300992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan X, Wei Y, Villasante A, et al. Stem cell delivery in tissue-specific hydrogel enabled meniscal repair in an orthotopic rat model. Biomaterials. 2017;132:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murakami T, Otsuki S, Nakagawa K, et al. Establishment of novel meniscal scaffold structures using polyglycolic and poly-l-lactic acids. J Biomater Appl. 2017;32:150–161. [DOI] [PubMed] [Google Scholar]
- 111.Baek J, Lotz MK, D’lima DD. Core-shell nanofibrous scaffolds for repair of meniscus tears. Tissue Eng Part A. 2019;25:1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song KH, Heo SJ, Peredo AP, Davidson MD, Mauck RL, Burdick JA. Influence of fiber stiffness on meniscal cell migration into dense fibrous networks. Adv Healthc Mater. 2020;9:1901228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pillai MM, Gopinathan J, Senthil Kumar R, et al. Tissue engineering of human knee meniscus using functionalized and reinforced silkpolyvinyl alcohol composite three-dimensional scaffolds: understanding the in vitro and in vivo behavior. J Biomed Mater Res A. 2018;106:1722–1731. [DOI] [PubMed] [Google Scholar]
- 114.Janarthanan G, Pillai MM, Kulasekaran SS, Rajendran S, Bhattacharyya A. Engineered knee meniscus construct: understanding the structure and impact of functionalization in 3D environment. Polym Bull. 2020;77:2611–2629. [Google Scholar]
- 115.Szojka A, Lalh K, Andrews SHJ, Jomha NM, Osswald M, Adesida AB. Biomimetic 3D printed scaffolds for meniscus tissue engineering. Bioprinting. 2017;8:1–7. [Google Scholar]
- 116.Li Z, Wu N, Cheng J, et al. Biomechanically, structurally and functionally meticulously tailored polycaprolactone/silk fibroin scaffold for meniscus regeneration. Theranostics. 2020;10(11): 5090–5106. http://www.thno.org/v10p5090.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maher SA, Wang H, Koff MF, Belkin N, Potter HG, Rodeo SA. Clinical platform for understanding the relationship between joint contact mechanics and articular cartilage changes after meniscal surgery. J Orthop Res. 2017;35(3):600–611. [DOI] [PubMed] [Google Scholar]
- 118.López-Calzada G, Hernandez-Martínez AR, Cruz-Soto M, et al. Development of meniscus substitutes using a mixture of biocompatible polymers and extra cellular matrix components by electrospinning. Mater Sci Eng C. 2016;61:893–905. [DOI] [PubMed] [Google Scholar]
- 119.Fisher MB, Henning EA, Söegaard N, Esterhai JL, Mauck RL. Organized nanofibrous scaffolds that mimic the macroscopic and microscopic architecture of the knee meniscus. Acta Biomater. 2013;9(1):4496–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y, Chen M, Zhou W, et al. Cell-free 3D wet-electrospun PCL/silk fibroin/Sr2+ scaffold promotes successful total meniscus regeneration in a rabbit model. Acta Biomater. 2020;113:196–209. [DOI] [PubMed] [Google Scholar]
- 121.Baek J, Sovani S, Glembotski NE, et al. Repair of avascular meniscus tears with electrospun collagen scaffolds seeded with human cells. Tissue Eng Part A. 2016;22:436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shimomura K, Bean AC, Lin H, Nakamura N, Tuan RS. In vitro repair of meniscal radial tear using aligned electrospun nanofibrous scaffold. Tissue Eng Part A. 2015;21:2066–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tarafder S, Park G, Lee CH. Explant models for meniscus metabolism, injury, repair, and healing. Connect Tissue Res. 2020;61: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ishimura M, Tamai S, Fujisawa Y. Arthroscopic meniscal repair with fibrin glue. Arthrosc J Arthrosc Relat Surg. 1991;7:177–181. [DOI] [PubMed] [Google Scholar]
- 125.Scotti C, Pozzi A, Mangiavini L, et al. Healing of meniscal tissue by cellular fibrin glue: an in vivo study. Knee Surg Sports Traumatol Arthrosc. 2009;17:645–651. [DOI] [PubMed] [Google Scholar]
- 126.Bochyńska AI, Hannink G, Verhoeven R, Grijpma DW, Buma P. Evaluation of novel biodegradable three-armed- and hyper-branched tissue adhesives in a meniscus explant model. J Biomed Mater Res A. 2017;105:1405–1411. [DOI] [PubMed] [Google Scholar]
- 127.Bochyńska AI, Van Tienen TG, Hannink G, Buma P, Grijpma DW. Development of biodegradable hyper-branched tissue adhesives for the repair of meniscus tears. Acta Biomater. 2016;32:1–9. [DOI] [PubMed] [Google Scholar]
- 128.Maher SA, Rodeo SA, Potter HG, Bonassar LJ, Wright TM, Warren RF. A pre-clinical test platform for the functional evaluation of scaffolds for musculoskeletal defects: the meniscus. HSS J. 2011;7:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qu F, Lin JMG, Esterhai JL, Fisher MB, Mauck RL. Biomaterial-mediated delivery of degradative enzymes to improve meniscus integration and repair. Acta Biomater. 2013;9(5):6393–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Longo UG, Loppini M, Romeo G, Maffulli N, Denaro V. Histological scoring systems for tissue-engineered, ex vivo and degenerative meniscus. Knee Surgery, Sport Traumatol Arthrosc. 2013;21(7): 1569–1576. 10.1007/s00167-012-2142-z [DOI] [PubMed] [Google Scholar]
- 131.Brzezinski A, Ghodbane SA, Patel JM, Perry BA, Gatt CJ, Dunn MG. The ovine model for meniscus tissue engineering: considerations of anatomy, function, implantation, and evaluation. Tissue Eng Part C Methods. 2017;23:829–841. 10.1089/ten.TEC.2017.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bansal S, Keah NM, Neuwirth AL, et al. Large animal models of meniscus repair and regeneration: a systematic review of the state of the field. Tissue Eng Part C Methods. 2017;23:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cinque ME, Geeslin AG, Chahla J, Dornan GJ, LaPrade RF. Two-tunnel transtibial repair of radial meniscus tears produces comparable results to inside-out repair of vertical meniscus tears. Am J Sports Med. 2017;45:2253–2259. [DOI] [PubMed] [Google Scholar]
- 134.Feucht MJ, Kühle J, Bode G, et al. Arthroscopic transtibial pullout repair for posterior medial meniscus root tears: a systematic review of clinical, radiographic, and second-look arthroscopic results. Arthroscopy. 2015;31:1808–1816. [DOI] [PubMed] [Google Scholar]
- 135.Guo H, Santner TJ, Lerner AL, Maher SA. Reducing uncertainty when using knee-specific finite element models by assessing the effect of input parameters. J Orthop Res. 2017;35:2233–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mueller BT, Moulton SG, O’Brien L, LaPrade RF. Rehabilitation following meniscal root repair: a clinical commentary. J Orthop Sports Phys Ther. 2016;46:104–113. [DOI] [PubMed] [Google Scholar]
- 137.Beaufils P, Becker R, Kopf S, et al. Surgical management of degenerative meniscus lesions: the 2016 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc. 2017;25:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brophy RH, Sandell LJ, Rai MF. Traumatic and degenerative meniscus tears have different gene expression signatures. Am J Sports Med. 2017;45(1):114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aprato A, Sordo L, Costantino A, et al. Outcomes at twenty years after meniscectomy in patients aged between 50-70 years. Arthroscopy. 2021;S0749-8063(20):31054–31059. [DOI] [PubMed] [Google Scholar]
- 140.Kopf S, Sava M-P, Stärke C, Becker R. The menisci and articular cartilage: a life-long fascination. EFORT Open Rev. 2020;5(10): 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sihvonen R, Paavola M, Malmivaara A, et al. Arthroscopic partial meniscectomy for a degenerative meniscus tear: a 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. Br J Sports Med. 2020;54(22): 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mäkelä JTA, Cooper BG, Korhonen RK, Grinstaff MW, Snyder BD. Functional effects of an interpenetrating polymer network on articular cartilage mechanical properties. Osteoarthr Cartil. 2018; 26(3):414–421. http://www.sciencedirect.com/science/article/pii/S1063458418300025 [DOI] [PubMed] [Google Scholar]
- 143.McGann ME, Bonitsky CM, Jackson ML, Ovaert TC, Trippel SB, Wagner DR. Genipin crosslinking of cartilage enhances resistance to biochemical degradation and mechanical wear. J Orthop Res. 2015;33:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pereira H, Fatih Cengiz I, Gomes S, et al. Meniscal allograft transplants and new scaffolding techniques. EFORT Open Rev. 2019;4:279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patel JM, Merriam AR, Culp BM, Gatt CJ, Dunn MG. One-year outcomes of total meniscus reconstruction using a novel fiber-reinforced scaffold in an ovine model. Am J Sports Med. 2015;44(4): 898–907. [DOI] [PubMed] [Google Scholar]
- 146.Lee CH, Rodeo SA, Fortier LA, Lu C, Erisken C, Mao JJ. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med. 2014;6(266):266ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kamimura T, Kimura M. Meniscal repair of degenerative horizontal cleavage tears using fibrin clots: clinical and arthroscopic outcomes in 10 cases. Orthop J Sport Med. 2014;2(11):2325967114555678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.