Abstract
Perineuronal nets (PNNs) are specialized extracellular matrix structures that primarily surround fast-spiking parvalbumin (PV)-containing interneurons within the PFC. They regulate PV neuron function and plasticity to maintain cortical excitatory/inhibitory balance. For example, reductions in PNN intensity are associated with reduced local inhibition and enhanced pyramidal neuron firing. We previously found that exposure to dietary high fat reduced PNN intensity within the PFC of male Sprague-Dawley (SD) rats. However, how high fat affects PNNs in the PFC of females or in obesity-vulnerable vs. -resistant models is unknown. Therefore, we gave male and female SD, selectively bred obesity-prone (OP), and obesity-resistant rats (OR) free access to standard lab chow or 60% high fat for 21 days. We then measured the number of PNN positive cells and PNN intensity (determined by Wisteria floribunda agglutinin [WFA] staining) as well as the number of PV positive neurons using immunohistochemistry. We found sex and region-specific effects of dietary high fat on PNN intensity, in the absence of robust changes in cell number. Effects were comparable in SD and OP but differed in OR rats. Specifically, high fat reduced PNN intensities in male SD and OP rats but increased PNN intensities in female SD and OP rats. In contrast, effects in ORs were opposite, with males showing increases in PNN intensity and females showing a reduction in intensity. Finally, these effects were also region specific, with diet-induced reductions in PNN intensity found in the prelimbic PFC (PL-PFC) and ventral medial orbital frontal cortex (vmOFC) of SD and OP males in the absence of changes in the infralimbic PFC (IL-PFC), and increases in PNN intensity in the IL-PFC of SD and OP females in the absence of changes in other regions. These results are discussed in light of roles PNNs may play in influencing PFC neuronal activity and the differential role of these sub-regions in food-seeking and motivation.
Keywords: Perineuronal nets, High fat, Prefrontal cortex, Obesity-prone
1. INTRODUCTION
Obesity continues to threaten the health of more than 2 billion people worldwide by increasing the risk for many chronic and potentially lethal diseases such as type II diabetes, heart disease, stroke, and cancer [1–3]. A contributing factor to the development of obesity is the overconsumption of processed, calorically dense foods that are high in fat [4, 5]. The prefrontal cortex (PFC) regulates many complex cognitive functions including monitoring inhibitory control, self-regulation/control, and plays a critical role in motivational behaviors [6–13]. Furthermore, both preclinical and human studies suggest that obesity and consumption of diets high in fats and sugars alters PFC function, which hampers voluntary food restriction and reduces cognitive control to maintain weight loss [14–19].S
The PFC has numerous connections with other cortical and sub-cortical areas [20] and sends excitatory projections to brain areas critical for the expression of motivated behaviors, including the nucleus accumbens [21–23] and amygdala [24]. The activity of pyramidal neurons within the PFC is tightly regulated by a minority (<10% of neurons) of parvalbumin (PV)-containing GABAergic interneurons [25, 26]. Despite their relatively small number, PV interneurons heavily modulate local pyramidal neuron excitability, thereby regulating PFC output [27]. Activity of PV interneurons themselves are partially regulated by perineuronal nets (PNNs) that are part of specialized extracellular matrix structures [28]. PNNs contribute to synaptic stabilization [29], protect against oxidative stress [30], regulate the ionic microenvironment [31], and suppress plasticity of excitatory synapses [32, 33]. In addition, pharmacological degradation of PNNs reduces PV-interneuron firing resulting in enhanced activity of pyramidal output neurons of the PFC [34, 35]. Thus, alterations in PNNs that ensheathe PV-interneurons have the potential to dynamically regulate PFC function, experience-induced plasticity, and ultimately, behavioral output [36, 37].
Data from preclinical obesity models show that consumption of calorically dense diets produce functional and structural alterations in PFC cells and the extracellular matrix. For example, exposure to a high fat “cafeteria” diet decreased inhibitory synaptic transmission within the PFC [38] and impaired cognitive function in male rats [39]. Studies in our lab have shown that exposure to 60% high fat reduced thin dendritic spines within the infralimbic-PFC (IL-PFC) and also reduced the staining intensity of the PNN marker, Wisteria floribunda agglutinin (WFA), in the prelimbic-PFC (PL-PFC) of outbred (Sprague-Dawley, SD) male rats [40, 41]. Within the PFC these cells are predominantly PV-containing fast spiking interneurons (FSIs), which impacts local circuit activity within the PFC [35]. Hence, changes in PNN intensity may alter the electrophysiological properties of the cells they surround and cells they synapse on [35, 42]. However, relative to other reinforcing sub-stances like drugs of abuse, what is known regarding the impact of high fat diets on cortical PNN plasticity is poorly understood. Furthermore, human and preclinical data also show that differences in individual susceptibility to obesity can result in qualitative and quantitative differences in diet-induced plasticity in target regions of the PFC like the NAc [43–46]. Finally, despite increased risk of obesity in women [47], the impact of diet manipulation on PNNs within the PFC of females is understudied.
This current study fills this gap in knowledge by characterizing how consumption of a 60% high fat diet affects PNN intensity, PNN number, and PV+ neuron number within the PL- and IL-PFC, and ventral medial orbitofrontal cortex (vmOFC) of adult male and female outbred SD and selectively bred obesity-prone (OP) and obesity-resistant (OR) rats. We find that high fat-induced changes in PNNs are consistent across SD and OP rats but differ in OR groups and that these effects are both sex and region specific. Specifically, SD and OP males show a decrease in PNN intensity in the PL-PFC and vmOFC following high fat diet. In contrast, OR males fail to demonstrate high fat-induced PNN plasticity in the PL-PFC but instead show an increase in PNN intensity in the vmOFC. In females, both SD and OP rats display an increase, while OR females show a decrease in PNN intensity in the IL-PFC. These results suggest that male and female rats exhibit distinct high fat-induced modifications to PV-containing interneurons in separate PFC subregions under conditions of nutritional excess.
2. MATERIALS AND METHODS
2.1. Subjects
All procedures were performed in accordance with the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals and with approval from the Institutional Animal Care and Use Committee (IACUC) at the University of Wyoming (UW). All rats were bred in house and were 60–80 days old at the start of dietary manipulation. Sprague-Dawley (SD) breeding pairs were originally obtained from Charles River and established breeding pairs of obesity-prone and obesity-resistant rats were obtained from Dr. Ferrario’s colonies at the University of Michigan. All rats were maintained in a temperature-controlled (25°C) vivarium with ad libitum access to food (prior to diet manipulation) and water under a standard 12-hour light/dark cycle (0700–1900).
2.2. Diet Manipulation
Male and female rats from each strain were randomly assigned to control chow (Rodent Diet 5001, LabDiet, St. Louis, MO; 3.36 kcal/g) or 60% high fat diet (Research Diets, New Brunswick, NJ; 5.24 kcal/g) conditions (n=4–5/group) and then singly housed for the remainder of the study. Single housing was used to enable measurement of daily food intake. All rats had free access to these diets in their home cage for 21–24 days and were weighed every other day. This duration of diet exposure was selected because it has been previously demonstrated to produce significant weight gain and adiposity in male SD rats relative to those maintained on standard chow [40, 41]. The chow diet comprises 29% protein, 58% carbohydrate, and 13% fat (in % kilocalories), whereas the lard-based high fat diet comprises 20% protein, 20% carbohydrate, and 60% fat. In addition, the estrous cycle was monitored in females by vaginal smearing as described previously [48] throughout dietary manipulation. Tissue collection from females was timed such that rats were euthanized during the diestrus phase of the cycle. This phase was chosen in part because food intake is greater during diestrus than during estrus [49], and because behavioral differences between OP and OR females have been observed during the diestrus phase of the cycle [43, 50]. In addition, motivational response can be influenced by ovarian hormones [51–53]. Thus, we decided to control for the cycle by examining effects in diestrus.
2.3. Tissue collection and Perineuronal net staining
Tissue collection, PNN staining, and analysis were conducted as previously described by our lab [41]. Briefly, rats were anesthetized with isoflurane prior to cardiac perfusion with ice cold phosphate buffered saline (PBS 200 mL) followed by 4% paraformaldehyde (PFA) in PBS (300 mL). Whole brains were then extracted and placed into 4% PFA for 24 hrs (4℃) before being transferred to 20% sucrose solution (in PBS, 4°C) for an additional 24 hrs. Brains were then removed from the sucrose solution, frozen on dry ice, and stored at −80°C until cryo-sectioning. 30 μm coronal sections of the PFC were obtained using a Leica 3050 cryostat (−20°C). Free floating slices were then rinsed in PBS at room temperature (RT) 3 times for 5 min each, 50% alcohol for 30 min, PBS 3x for 5 min each, and incubated in 3% blocking serum (PBS plus 3% normal goat serum [v/vol], S-1000, Vector Laboratories) for 1 hour (RT). Slices were then sequentially labeled using anti-PV antibody (SAB4200545, Sigma Aldrich; 1:1000) followed by staining of PNNs with fluorescein conjugated WFA (Vector Laboratories, Burlingame, CA; 1:500) as previously described [42]. Briefly, slices were incubated overnight in 2% blocking serum containing anti-PV antibody (4°C), rinsed in PBS (3×10min), and then incubated with goat anti-mouse IgG (H+L) secondary antibody (A-11005, ThermoFisher; 1:500) in 2% blocking serum (2 hrs at RT). Slices were then rinsed again (PBS, 3×10min) and incubated in PBS containing WFA for 2 hrs (RT). Finally, slices were rinsed as above, mounted on glass slides with ProLong Gold Antifade mounting media containing DAPI (Vector Laboratories), and stored in the dark at 4°C until the time of imaging. These yielded sections labeled for PV in red, PNNs in green, and cell bodies in blue.
2.4. Image acquisition and analysis
Image analysis was done as previously described [35, 41, 54]. Briefly, the experimenter was blind to group conditions during all image acquisition and analysis. Images of PV labeling, PNN, and DAPI staining were acquired using a Zeiss 710 scanning confocal microscope (40X oil immersion objective, NA 0.55, 10x zoom) and Zen imaging software using identical acquisition settings for each label/stain. Images of sections containing the PL-PFC, IL-PFC (approximately AP from bregma=+3.00 mm to 4.68 mm) and vmOFC (AP from bregma=+3.00 mm to 4.68 mm; 1–2 mm from midline; 4–6 mm from the skull [55]) were obtained. All images were acquired as Z-stacks comprising 25 optical sections, each 1 μm in thickness. For analysis, sequences of the raw images within the z-stack were projected into a sum slices image for each label or stain with no manipulation to brightness or contrast. Background subtraction from each summed image was then conducted. Each visible PNN in the image was assigned as a region of interest (ROI), including the cell body and proximal dendrites. The average intensity above background for each ROI (PNN) was calculated. Staining was then quantified only from those ROIs surrounding PV+ neurons. The total number of PV positive cells were counted manually for each image. Studies were conducted in two separate cohorts; therefore, to account for possible heterogeneity across cohorts, the data were normalized to chow fed groups (control) within each cohort as previously performed [41].
2.5. Statistical Analyses
All statistical analyses were conducted in Prism 6 (GraphPad Software). Comparisons were made between chow and high fat groups within each strain (SD, OP, OR). Because it was not possible to process all tissue at the same time, data have been normalized to chow groups within each strain to facilitate comparisons of relative changes compared to chow controls. Weight analysis was done using Two-way repeated measures ANOVA with a Sidak’s multiple comparisons posttest when appropriate. For average WFA intensity, WFA number, and PV number the data were analyzed with unpaired t test comparing chow vs high fat. Differences in the cumulative distributions of normalized PNN intensities were analyzed using the nonparametric Kolmogorov-Smirnov test (KST). All data are presented as the mean ± standard error of the mean (S.E.M.).
3. RESULTS
3.1. Dietary Manipulation
Consumption of high fat for 21–24 days promoted significant weight gain in SD males compared to chow fed males (Figure 1B: diet x time interaction F(1,6)=11.44, p<0.02, n=4 per diet condition). High fat also produced significant weight gain in female OP rats (Figure 1F: main effect of diet F(1,8)=6.52, p<0.04, n=5 per diet condition). There were strong trends in weight gain in both OP male (Figure 1C, n=5 per diet condition) and SD female (Figure 1E, n=5 per condition) groups exposed to high fat but neither of these groups reached significance. In contrast, OR male (Figure 1D, n=5 per condition) and female (Figure 1G, n=5 per condition) high fat fed groups did not show any significant weight gain or trends in weight gain compared to chow fed controls. For simplicity, only SD data are shown for caloric intake, but all strains and sexes showed the same profile. SD male rats fed high fat reduced their caloric intake between day 1 (81.24 kcal) and day 21 (67.83 kcal) relative to their chow fed counterparts (day 1=83.75 kcal and day 21=90.45 kcal; data not shown, diet x time interaction F(1,6)=108.0).
Figure 1.
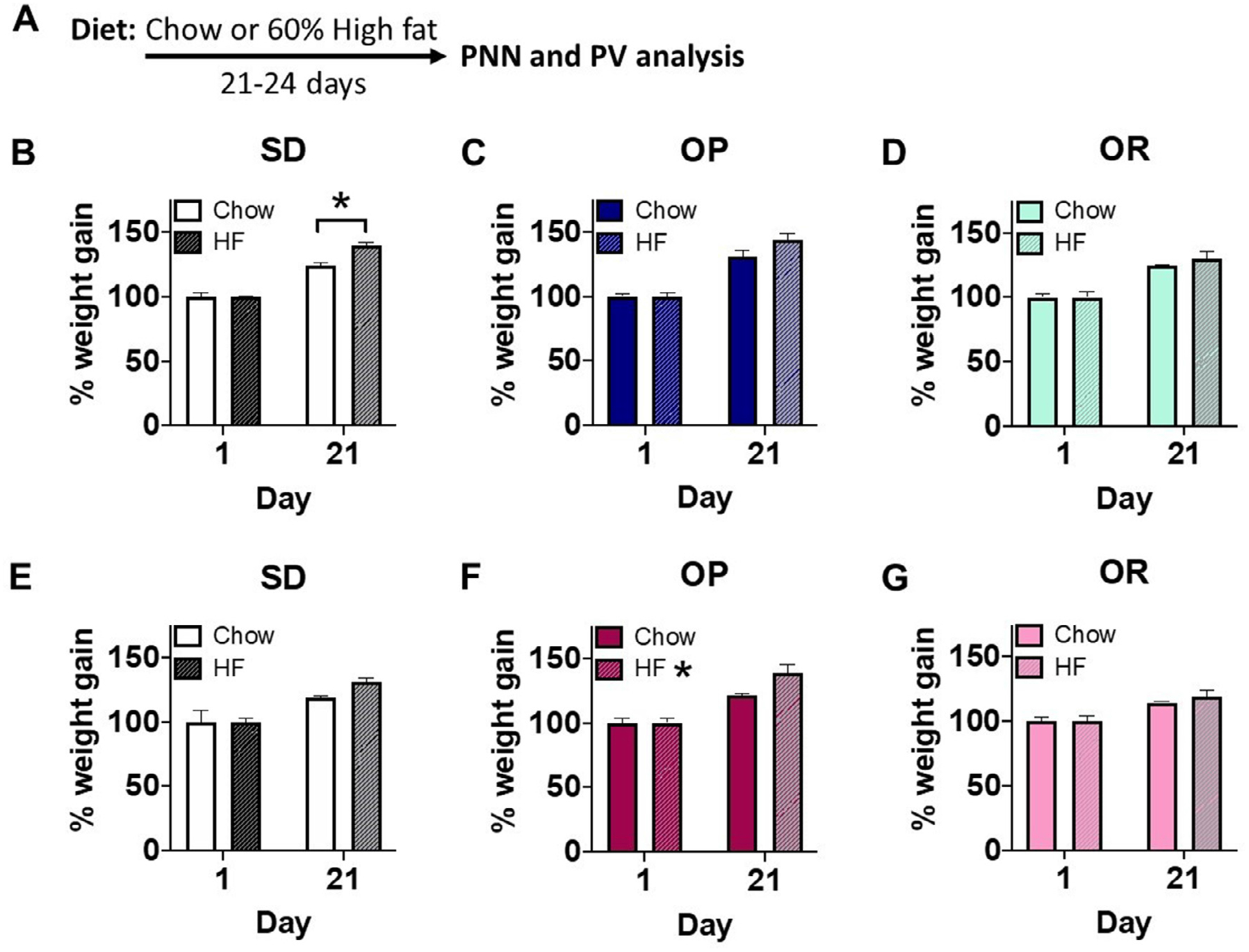
Dietary manipulation. A. Experimental timeline. Male and female rats of Sprague-Dawley (SD) and selectively bred obesity-prone (OP) and obesity-resistant (OR) strains were maintained on chow or high-fat (HF) for 21–24 days after which tissue was collected for analysis. B-D. Male weight gain, expressed as percent increase within animals from day 1 (D1) to D21 of dietary manipulation among SD, OP, and OR fed chow (left; white, dark blue, and light blue bars, respectively) and SD, OP, OR males fed HF (right; dashed white, dashed dark blue, and dashed light blue bars, respectively). E-G. Female weight gain among SD, OP, and OR fed chow (left; white, dark pink, and light pink bars, respectively) and SD, OP, OR females fed HF (right; dashed white, dashed dark pink, and dashed light pink bars, respectively). Values represent the mean ± S.E.M. (*p<0.05).
3.2. Effects of High-fat diet on DAPI ± Cells
The number of DAPI + cells was not affected by high fat in any sex, strain, or brain region (data not shown). Thus, high fat does not alter total cell number.
3.3. Effects of High-fat diet on PNNs in Males
All representative images shown (Figures 2–7) are Z-stacks re-constructed as sum sliced images using ImageJ software (NIH). In the PL-PFC of SD rats, there was a significant leftward shift in the cumulative distribution of WFA intensities in high fat vs chow groups (Figure 2B; KST, p<0.01). Consistent with this, overall WFA intensity was significantly reduced in male SD high fat vs chow groups (Figure 2C; p<0.05). High fat did not affect the number of WFA+/PV+ or PV+ neurons (Figure 2D, E). In the IL-PFC of SD males, there was a small but significant leftward shift in the cumulative distribution of WFA intensities in high fat vs chow groups (Figure 2H; KST, p<0.05). This was accompanied by a small, and variable overall reduction in WFA intensity (Figure 2I; p = 0.075). Again, high fat did not alter the number of WFA+/PV+ or PV+ neurons (Figure 2J, K). In the vmOFC of SD males, high fat again produced a significant leftward shift in the cumulative distribution of WFA intensities compared to chow fed controls (Figure 2N; KST, p<0.05) that was accompanied by a significant reduction in overall WFA intensity in high fat vs. chow groups (Figure 2O; p<0.05). In contrast to effects in the PL- and IL-PFC, high fat also produced a significant reduction in the number of WFA+/PV+ neurons (Figure 2P; p<0.05) and PV+ neurons (Figure 2Q; p<0.05). We do not believe the reduction in PV+ cells were due to a reduction in cell number as there was no reduction in DAPI staining (data not shown). Instead, we propose that there may be a reduction in the amount of PV protein within existing cells that results in fewer labeled PV+ cells. Overall, effects of high fat on PNNs of SD males replicate previous results from our laboratory [41].
Figure 2.
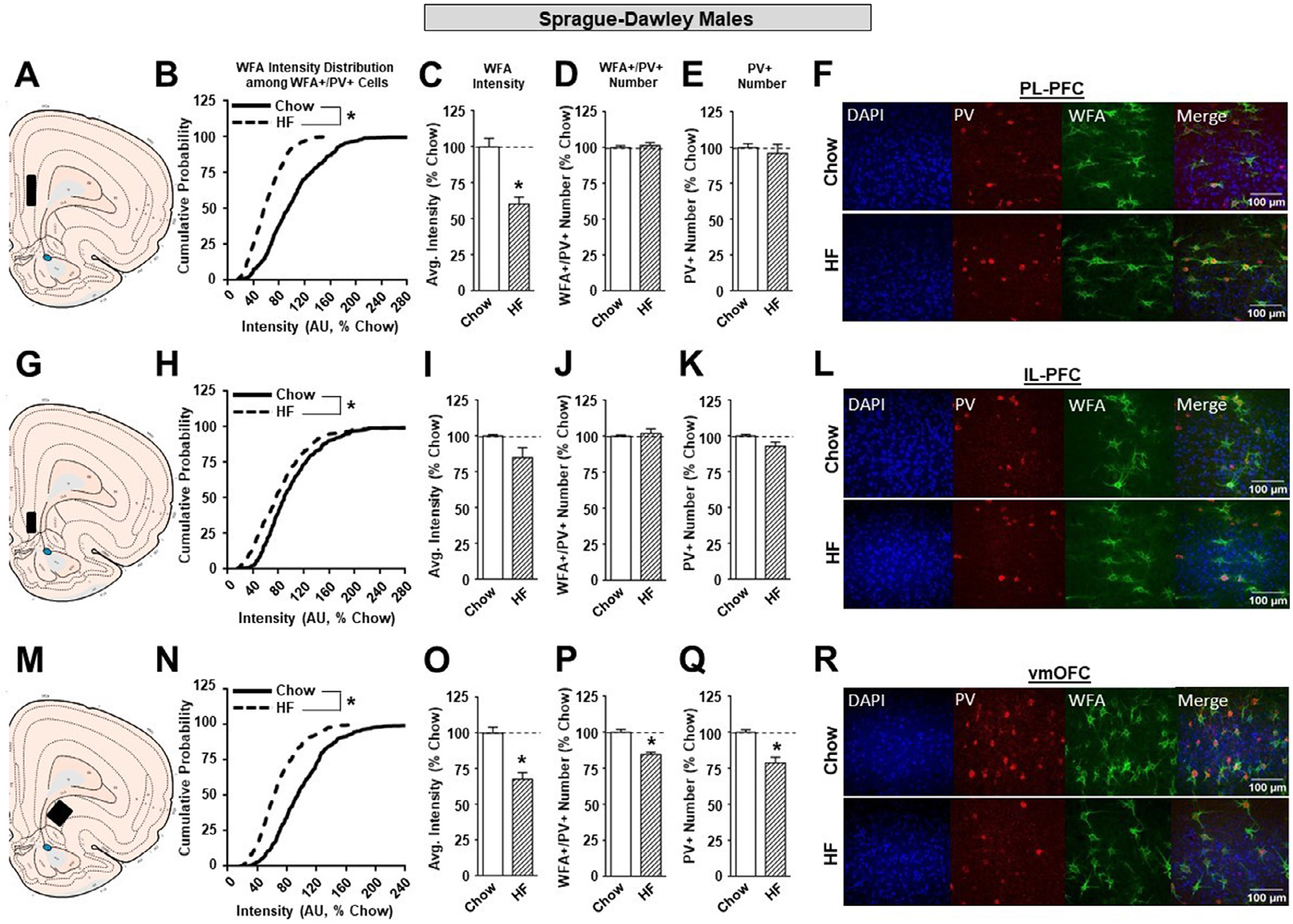
The effects of a HF diet on structural plasticity of PV-containing cells in the prefrontal cortex of male SD rats. A. Schematic of region analyzed. B. PNN intensity expressed as a cumulative probability for chow (black solid line) and high fat (black dotted line) fed rats in the PL-PFC. C. Average PNN intensity among chow (white bar) and high fat (white dashed bar) fed groups in the PL-PFC. D. PNN number among chow and high fat fed groups. E. Number of PV-containing cells in the PL-PFC. F. Representative images from the PL-PFC. G. Schematic of region analyzed. H. PNN intensity expressed as a cumulative probability for chow and high fed rats in the IL-PFC. I. Average PNN intensity among chow and HF fed groups in the IL-PFC. J. PNN number among chow and HF fed groups. K. Number of PV-containing cells in the IL-PFC. L. Representative images from the IL-PFC. M. Schematic of region analyzed. N. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the vmOFC. O. Average PNN intensity among chow and HF fed groups in the vmOFC. P. PNN number intensity among chow and HF fed groups. Q. Number of PV-containing cells in the vmOFC. R. Representative images from the vmOFC. Values represent the mean ± S.E.M. (*p<0.05).
Figure 7.
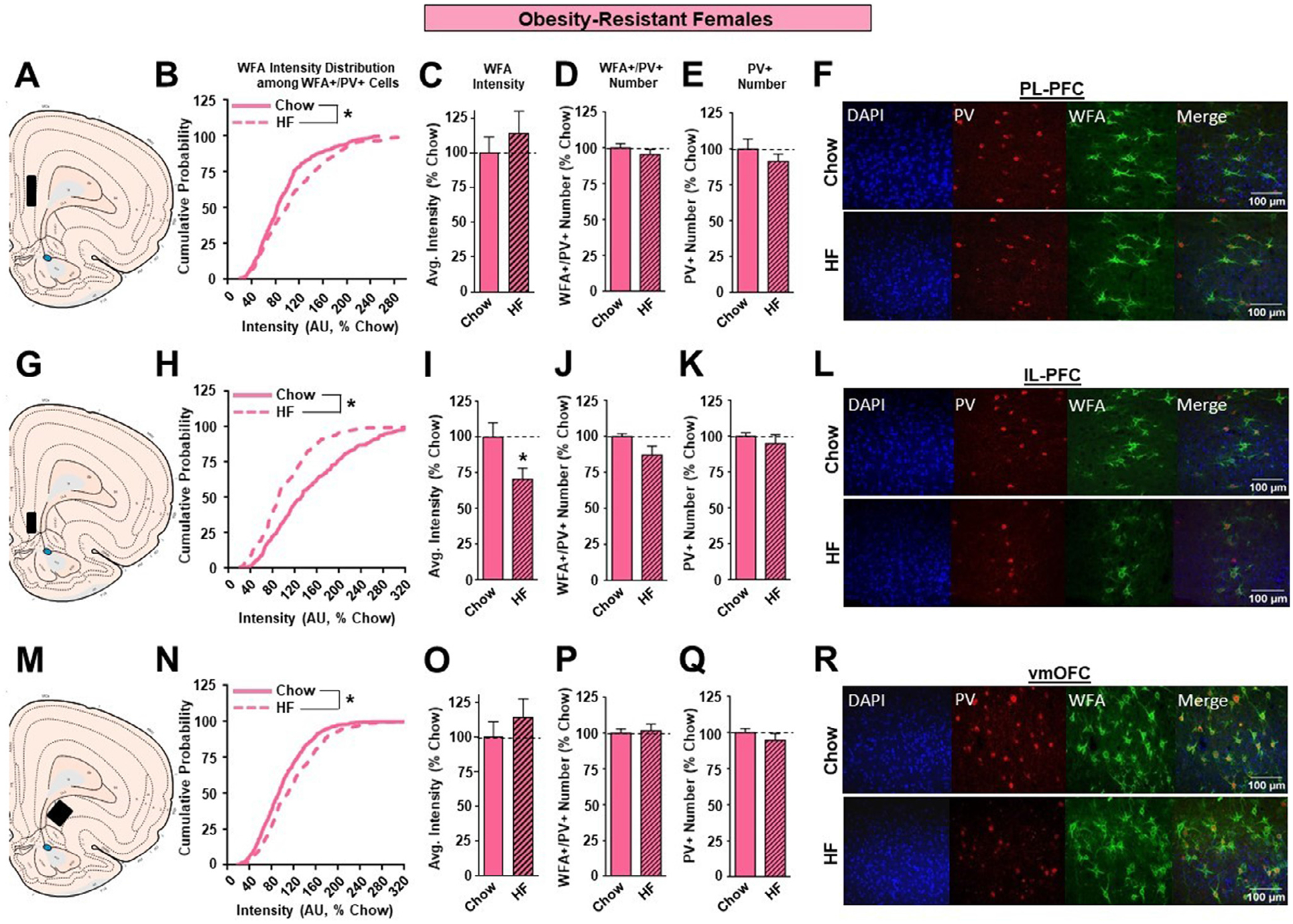
The effects of a HF diet on structural plasticity of PV-containing cells in the prefrontal cortex of female OR rats. A. Schematic of region analyzed. B. PNN intensity expressed as a cumulative probability for chow (light pink solid line) and HF (light pink dotted line) fed rats in the PL-PFC. C. Average PNN intensity among chow (light pink bar) and HF (light pink dashed bar) fed groups in the PL-PFC. D. PNN number among chow and HF fed groups. E. Number of PV-containing cells in the PL-PFC. F. Representative images from the PL-PFC. G. Schematic of region analyzed. H. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the IL-PFC. I. Average PNN intensity among chow and HF fed groups in the IL-PFC. J. PNN number among chow and HF fed groups. K. Number of PV-containing cells in the IL-PFC. L. Representative images from the IL-PFC. M. Schematic of region analyzed. N. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the vmOFC. O. Average PNN intensity among chow and HF fed groups in the vmOFC. P. PNN number intensity among chow and HF fed groups. Q. Number of PV-containing cells in the vmOFC. R. Representative images from the vmOFC. Values represent the mean ± S.E.M. (*p<0.05).
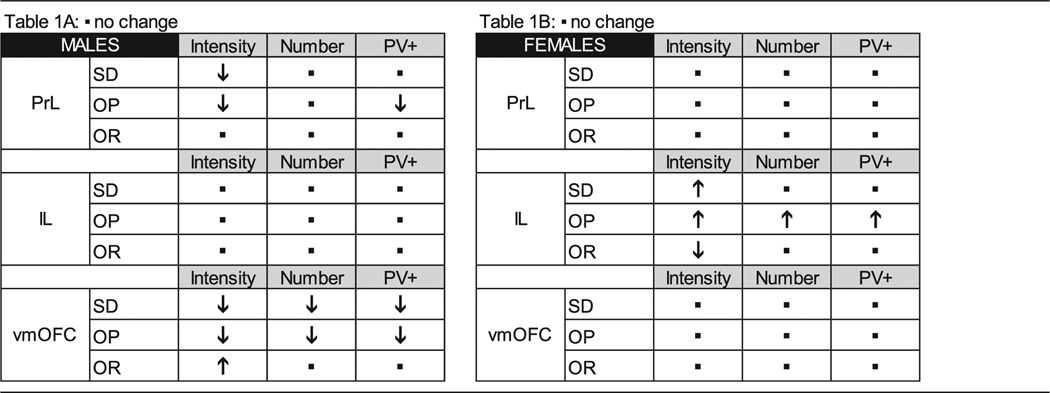
The effects of high fat diet on WFA intensity in OP males were generally like those described for SD males above (see also Table 1A). Specifically, there was a significant leftward shift in the cumulative distribution of WFA intensities of high fat vs. chow fed rats in the PL-PFC of OP males (Figure 3B; KST, p<0.01), and a concomitant significant reduction in WFA intensity (Figure 3C; p<0.05). High fat did not affect the number of WFA+/PV+ neurons in the PL-PFC (Figure 3D). However, there was a significant reduction in the number of PV+ neurons in high fat vs. chow fed OP groups (Figure 3E; p<0.05). Thus, while there was a reduction in total PV+ neurons, the number of these neurons that were surrounded by PNNs was not affected. In the IL-PFC of OP males, we found no effects of high fat diet on any measure (Figure 3H–K), consistent with results in SD males above. In the vmOFC, there was again a significant leftward shift in the cumulative distribution of WFA intensities in high fat vs. chow groups (Figure 3N; KST, p<0.01) that was accompanied by a reduction on overall WFA intensity (Figure 3 3O; p<0.06) that was similar in magnitude to that found in SDs above (Fig. 2O). As in SDs, high fat reduced WFA intensity within the vmOFC of OP males that was accompanied by a reduced number of WFA+/PV+ neurons (Figure 3P; p<0.05). High fat did not affect the number of PV+ neurons (Figure 3Q). Thus, high fat diet resulted in similar reductions in WFA intensity in the PL-PFC and vmOFC of OP and outbred male SD rats and similar reductions in the number of WFA+/PV+ and PV+ neurons within the vmOFC of these groups compared to chow-fed controls (see also Table 1A).
Table 1.
Summary of high fat-induced modifications to PNN intensity.
|
Figure 3.
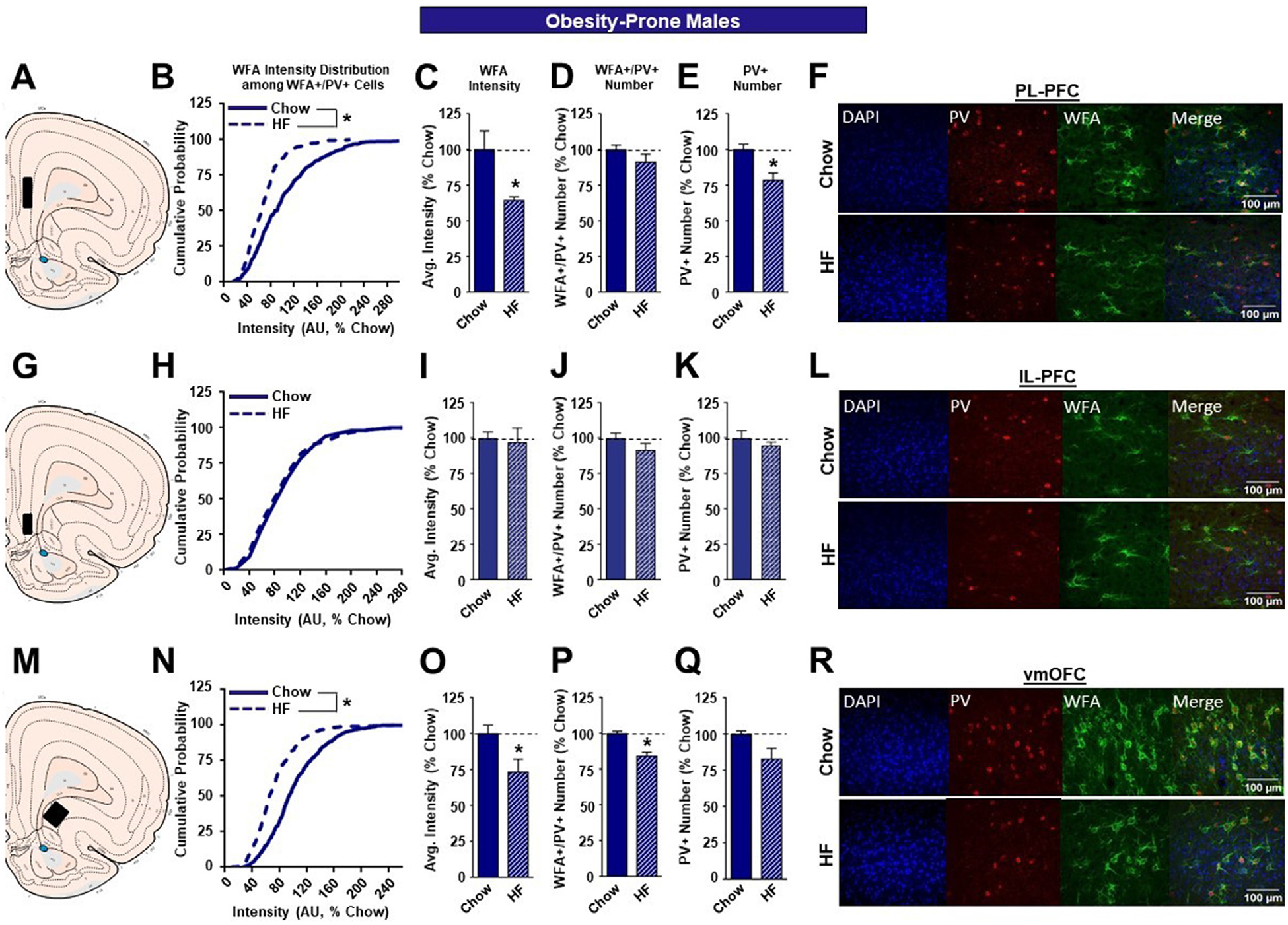
The effects of a high fat diet on structural plasticity of PV-containing cells in the prefrontal cortex of male OP rats. A. Schematic of region analyzed. B. PNN intensity expressed as a cumulative probability for chow (dark blue solid line) and high fat (dark blue dotted line) fed rats in the PL-PFC. C. Average PNN intensity among chow (dark blue bar) and HF (dark blue dashed bar) fed groups in the PL-PFC. D. PNN number among chow and HF fed groups. E. Number of PV-containing cells in the PL-PFC. F. Representative images from the PL-PFC. G. Schematic of region analyzed. H. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the IL-PFC. I. Average PNN intensity among chow and HF fed groups in the IL-PFC. J. PNN number among chow and HF fed groups. K. Number of PV-containing cells in the IL-PFC. L. Representative images from the IL-PFC. M. Schematic of region analyzed. N. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the vmOFC. O. Average PNN intensity among chow and HF fed groups in the vmOFC. P. PNN number intensity among chow and HF fed groups. Q. Number of PV-containing cells in the vmOFC. R. Representative images from the vmOFC. Values represent the mean ± S.E.M. (*p<0.05).
The effects of high fat diet in OR males were quite different from those found for outbred SD and OP males. Specifically, no effects of high fat diet were found in the PL-PFC or IL-PFC for any measure (Figure 4 B–K), while in the vmOFC increases in WFA intensity were found, as indicated by both a shift to the right in the cumulative distribution of WFA intensities in high fat vs. chow fed OR males (Figure 4N; KST, p<0.01) and a significant increase in total WFA intensity between these groups (Figure 4O; p<0.05). Furthermore, increases in vmOFC intensity were not accompanied by changes in the number of WFA+/PV+ (Figure 4P) or PV+ neurons (Figure 4Q).
Figure 4.
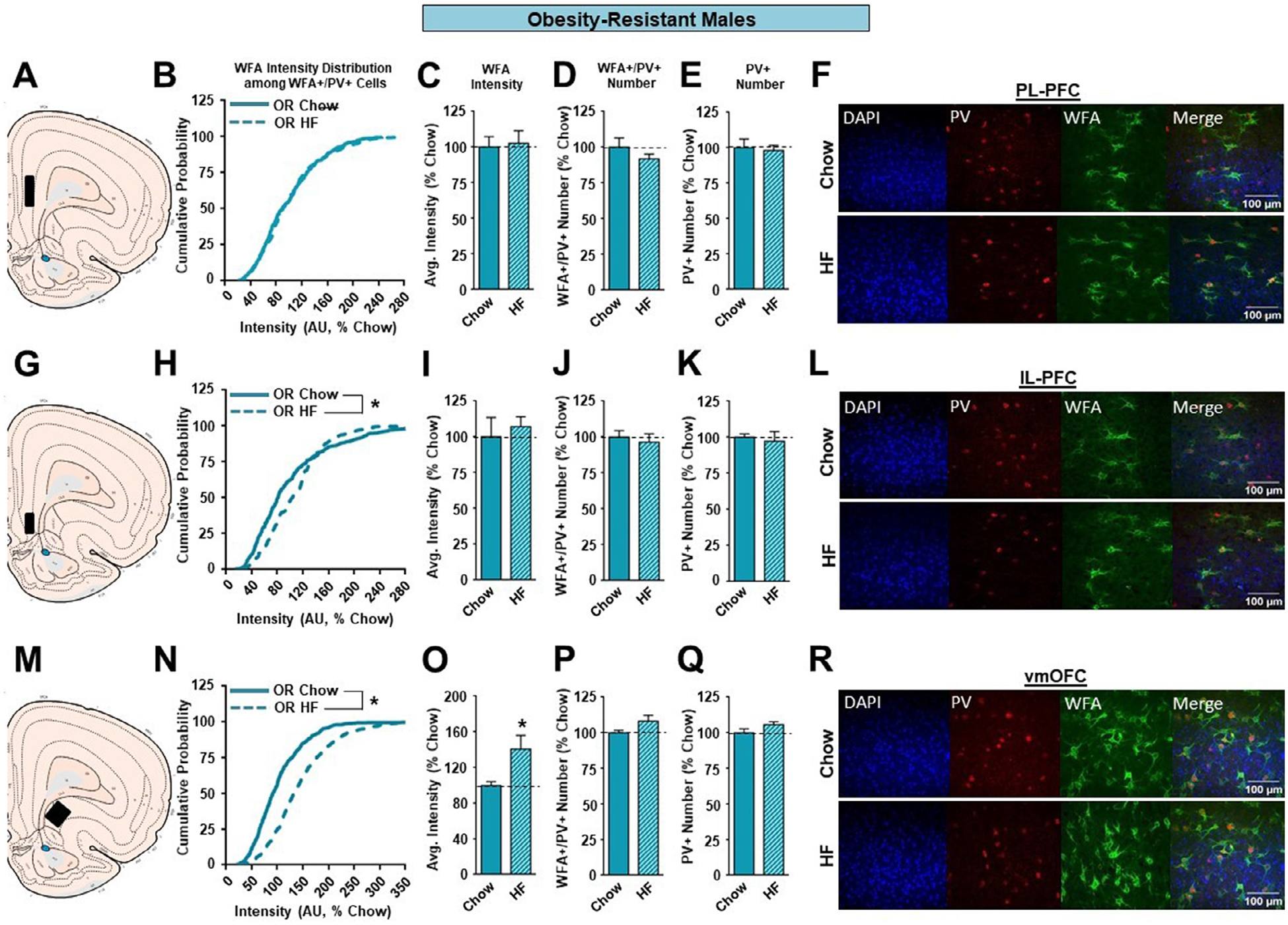
The effects of a HF diet on structural plasticity of PV-containing cells in the prefrontal cortex of male OR rats. A. Schematic of region analyzed. B. PNN intensity expressed as a cumulative probability for chow (light blue solid line) and HF (light blue dotted line) fed rats in the PL-PFC. C. Average PNN intensity among chow (light blue bar) and HF (light blue dashed bar) fed groups in the PL-PFC. D. PNN number among chow and HF fed groups. E. Number of PV-containing cells in the PL-PFC. F. Representative images from the PL-PFC. G. Schematic of region analyzed. H. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the IL-PFC. I. Average PNN intensity among chow and HF fed groups in the IL-PFC. J. PNN number among chow and HF fed groups. K. Number of PV-containing cells in the IL-PFC. L. Representative images from the IL-PFC. M. Schematic of region analyzed. N. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the vmOFC. O. Average PNN intensity among chow and HF fed groups in the vmOFC. P. PNN number intensity among chow and HF fed groups. Q. Number of PV-containing cells in the vmOFC. R. Representative images from the vmOFC. Values represent the mean ± S.E.M. (*p<0.05).
3.4. Effects of High-fat diet on PNNs in Females
In outbred and OP females, high fat diet did not produce alterations in either the PL-PFC (Figure 5B–E, Fig. 6B–E) or vmOFC (Figure 5N–Q, Fig 6N–Q) as it had in males. Instead, high fat resulted in marked changes in the IL-PFC that varied with susceptibility to diet-induced obesity. Specifically, in outbred SD and OP females, the cumulative distribution of WFA intensities was significantly shifted to the right (SD: Figure 5H; KST, p<0.01; OP: Figure 6H; KST, p<0.01). This was accompanied by a significant increase in overall WFA intensity in both strains following high fat diet (SD: Figure 5I; p<0.05; OP: Figure 6I; p<0.05). In addition, there was an increase in the number of WFA+/PV+ neurons in OP females given high fat vs. chow (Figure 6J; p<0.05), but not SD females (Figure 5J). Finally, there was no effect of high fat diet on the number of PV+ neurons in SD or OP females (Figure 5K, 6K). See also Table 1B.
Figure 5.
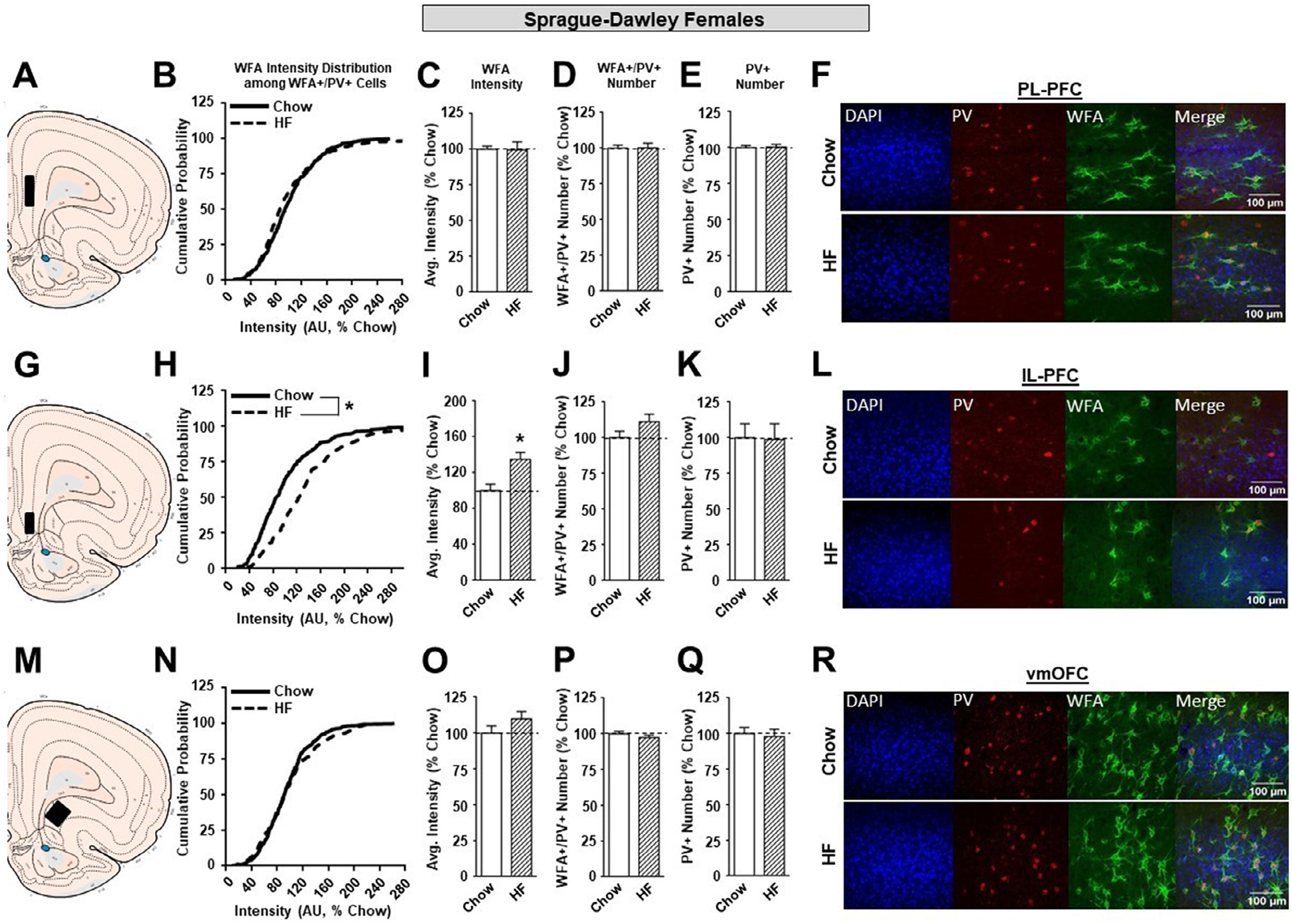
The effects of a HF diet on structural plasticity of PV-containing cells in the prefrontal cortex of female SD rats. A. Schematic of region analyzed. B. PNN intensity expressed as a cumulative probability for chow (black solid line) and HF (black dotted line) fed rats in the PL-PFC. C. Average PNN intensity among chow (white bar) and HF (white dashed bar) fed groups in the PL-PFC. D. PNN number among chow and HF fed groups. E. Number of PV-containing cells in the PL-PFC. F. Representative images from the PL-PFC. G. Schematic of region analyzed. H. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the IL-PFC. I. Average PNN intensity among chow and HF fed groups in the IL-PFC. J. PNN number among chow and HF fed groups. K. Number of PV-containing cells in the IL-PFC. L. Representative images from the IL-PFC. M. Schematic of region analyzed. N. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the vmOFC. O. Average PNN intensity among chow and HF fed groups in the vmOFC. P. PNN number intensity among chow and HF fed groups. Q. Number of PV-containing cells in the vmOFC. R. Representative images from the vmOFC. Values represent the mean ± S.E.M. (*p<0.05).
Figure 6.
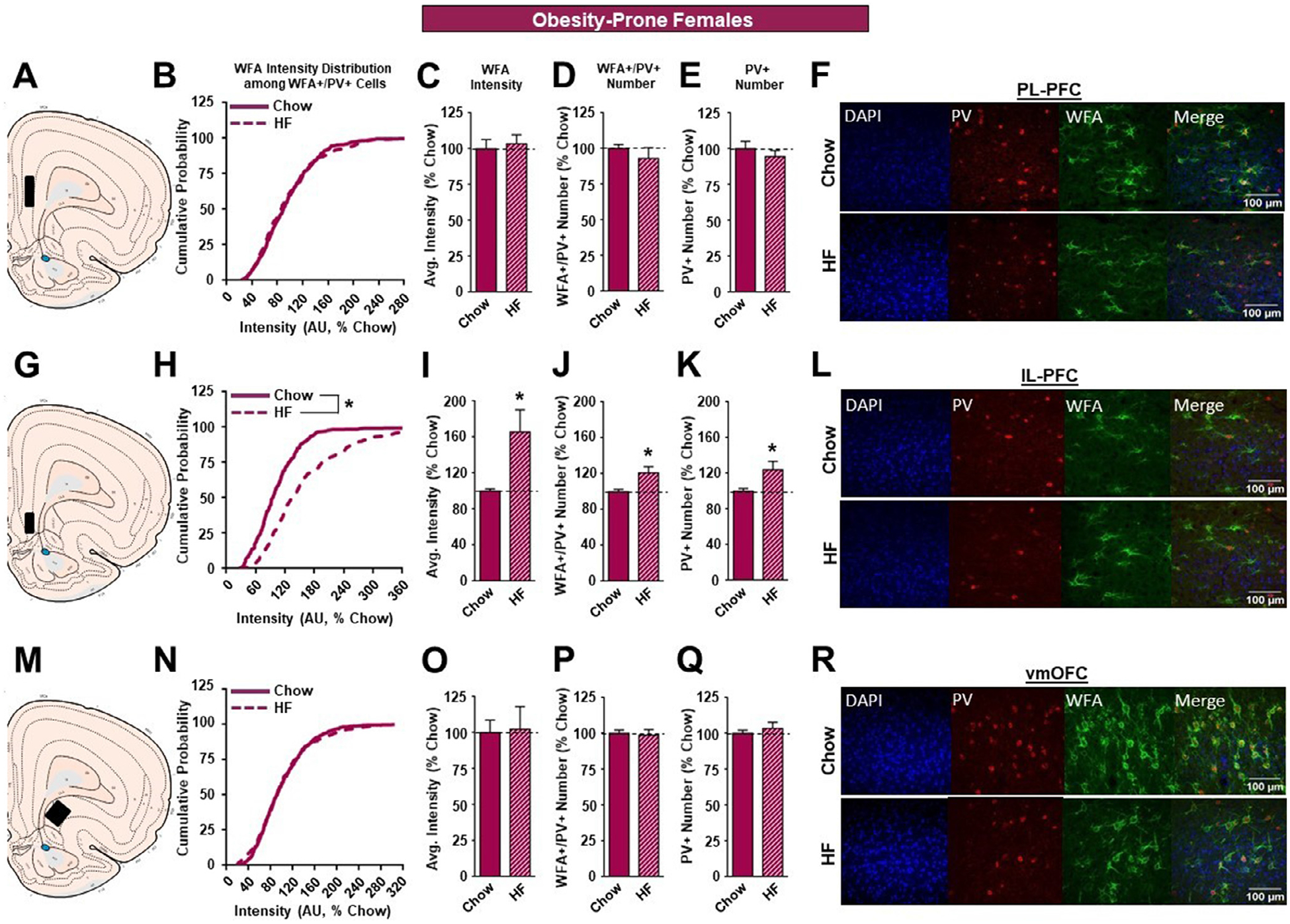
The effects of a HF diet on structural plasticity of PV-containing cells in the prefrontal cortex of female OP rats. A. Schematic of region analyzed. B. PNN intensity expressed as a cumulative probability for chow (dark pink solid line) and HF (dark pink dotted line) fed rats in the PL-PFC. C. Average PNN intensity among chow (dark pink bar) and HF (dark pink dashed bar) fed groups in the PL-PFC. D. PNN number among chow and HF fed groups. E. Number of PV-containing cells in the PL-PFC. F. Representative images from the PL-PFC. G. Schematic of region analyzed. H. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the IL-PFC. I. Average PNN intensity among chow and HF fed groups in the IL-PFC. J. PNN number among chow and HF fed groups. K. Number of PV-containing cells in the IL-PFC. L. Representative images from the IL-PFC. M. Schematic of region analyzed. N. PNN intensity expressed as a cumulative probability for chow and HF fed rats in the vmOFC. O. Average PNN intensity among chow and HF fed groups in the vmOFC. P. PNN number intensity among chow and HF fed groups. Q. Number of PV-containing cells in the vmOFC. R. Representative images from the vmOFC. Values represent the mean ± S.E.M. (*p<0.05).
In OR females, although there was a significant, but modest, shift to the right in the cumulative distribution of WFA intensities in the PL-PFC (Figure 7B; KST, p<0.01), this was not accompanied by alterations in overall WFA intensity (Figure 7C), or number of WFA+/PV+ or PV+ neurons (Figure 7D, E). A similar pattern was found in the vmOFC, with a significant but slight shift to the right in the cumulative distribution of WFA intensities (Figure 7N; KST, p<0.05) and no change in overall WFA intensity (Figure 7O), or number of WFA+/PV+ or PV+ neurons (Figure 7P,Q). Thus, high fat diet did not produce robust alterations in the PL-PFC or vmOFC in OR females, like results found for outbred and OP females above. However, in the IL-PFC, high fat diet produced marked reductions in PNN intensity of OR females, with both a significant shift to the left in the cumulative distribution of WFA intensities in high fat vs. chow groups (Figure 7H; KST, p<0.01) and an overall reduction in WFA intensity (Figure 7I; p<0.05). This is opposite to effects seen in outbred SD and OP females and is consistent with the generally opposite effects of high fat diet found in in both female and male OR rats compared to outbred SD and OP groups. Finally, no effects of high fat on the number of WFA+/PV+ or PV+ neurons were found in the IL-PFC (Figure 7J, K) of OR females.
In sum, high fat diet consumption produced similar patterns of changes in PNN intensity, and the number of WFA+/PV+, and PV+ neurons in OP and outbred SD rats of the same sex, but had opposite effects in OR rats of the same sex. Furthermore, high fat diet produced opposite and region-specific effects in males and females, with reductions occurring in the PL-PFC and vmOFC of male OPs and SDs but increases in the IL-PFC of females of these strains. Of course, this does not preclude potential differential effects across the cycle, and/or differences in the magnitude of potential sex differences had measurements been made in proestrus/estrus. [56]. Finally, for the most part, effects of high fat diet also varied by susceptibility to obesity, with opposite effects within the same brain regions found in OR vs. OP rats of the same sex.
4. DISCUSSION
In general, high fat diet reduced PNN intensity in the PL-PFC and vmOFC of males similarly to what we have reported previously [41], but not females. Instead, in females high fat diet increased PNN intensity in the IL-PFC. Furthermore, effects in OP and SD rats were similar overall. Thus, it appears the OR rats are the “odd ones out” showing an increase in vmOFC intensity in males, and reductions in IL-PFC intensity in females. In addition, there were small and variable effects of high fat diet in the IL-PFC of SD males that are similar to previously observed trends [41]. This work replicates previous findings [41] and expands them by demonstrating opposing effects of high fat diet in females vs males and in OP vs OR rats.
Although we did not assess function directly, we can speculate about how adaptions in PNNs may influence local circuit activity and pyramidal neuron function. Changes in WFA staining intensity are traditionally used as an indirect measure of PNN maturity/integrity [54], which can affect the electrophysiological properties of the PV interneurons they surround [35, 42]. That is, a reduction in PNN (WFA) intensity is associated with a reduction in the firing of PV interneurons (principally FSIs in the PFC), which they predominantly surround in the PFC. Previous studies have shown that complete degradation of PNNs with chondroitinase ABC (Ch-ABC), an enzyme that degrades chondroitin sulfate proteoglycans, reduces the excitability of FSIs in slices from the medial nucleus of the trapezoid body [34]. In addition, our lab has shown that degradation of PNNs with Ch-ABC in the PL-PFC increases excitability of pyramidal neurons [35]. We previously speculated that the increase in pyramidal excitability was due to an attenuation in FSI function resulting in a decrease in inhibitory tone onto the pyramidal neurons. Hence, we have seen a correlation between PNN intensity, FSI firing properties, and pyramidal neuron output [35, 42]. Therefore, although our data does not experimentally show changes in electrophysiological function, reductions in PNN intensity found here would be expected to induce plasticity of PV-FSIs in the PFC that may disrupt the excitatory:inhibitory balance within prefrontal regions. This idea is supported by recent studies showing that high fat consumption reduces GABA concentrations in the frontal cortex [57] and release probability of GABAergic inputs to the OFC of male Long-Evans rats [38].
There is also evidence that diet-induced reductions in PNN number can affect cognition. For example, the antibody, Cat316 attenuates PNN formation when injected into the perirhinal cortex and enhances long-term object recognition memory in mice [58]. Additionally, Reichelt et al., 2019 found that a high-fat high-sugar diet given intermittently during adolescence (PND 28–57) impaired social recognition memory, and the degree of impairment corresponded to degree of reduction in the number of PNNs within the IL-PFC [59]. While effects here were predominantly on PNN intensity, we did find some evidence for loss of PNNs in some regions. The nature of PNN changes (intensity vs loss of PNN+ neurons) could be due to differences in age and/or how the diet was administered (intermittent access vs ad libitum), these data nonetheless support the idea that diet manipulation can alter PNNs in a way that may likely affects PFC function and cognitive processes.
In this study OP males displayed similar patterns of changes to SD males in all three regions analyzed (PL, IL, and vmOFC). Specifically, there was a reduction in WFA intensity within the PL-PFC and vmOFC for both SD and OP rat (Figures 2B–E and 3B–E) strains with OP rats also showing a significant reduction in PV+ neurons (Figure 3E). This reduction in PV+ neurons may suggest that OP male rats are also having a loss of PV+ cells that are not surrounded by PNNs. Given that PNNs can act as a buffer, the absence of PNNs may render these neurons more susceptible to damage caused by inflammation and reactive oxygen species induced by high fat [30, 60]. This may ultimately result in cell death, thereby disrupting the regulation of local circuits within the PL-PFC and vmOFC. Furthermore, the loss of PV+ neurons in OP but not SD males is also consistent with the enhanced sensitivity of OPs to metabolic dysregulation [61], which is associated with neuroinflammation. This would also suggest that given enough high fat diet exposure, similar alterations could occur in SDs. This should be examined in the future.
Significant, though small, reductions in the cumulative distribution of WFA intensities within the IL-PFC were found in SD males (Figure 2H), although only trends were observed when these data were summarized to examine average intensity (Figure 2I). Recently, Reichelt et al., (2019) reported that male rats exposed to a high fat and high sugar diet for 2 hours/day showed a significant reduction in WFA+ neurons in the IL-PFC in adolescent rats [59]. It’s possible that effects are more robust when fat/sugar combinations are used, rather than the high fat only diet used here. Additionally, the small shifts in WFA intensity observed here could have functional consequences, as one PV+ neuron innervates many nearby pyramidal output neurons. This possibility, as well as potential differences in the effects of fat vs sugar diets on PNNs should be examined in future.
Several adaptations could result in a decrease in PNN intensity within the PFC. WFA stains PNNs by selectively binding to carbohydrate moieties on proteoglycans that comprise PNNs [59]. Thus, reductions in PNN intensity could be due to a total loss of PNNs (which are comprised of several different proteins including aggrecan, brevican, and Z), a reduction in proteoglycan composition within the PNNs, or potentially alterations to protein-protein interactions within the PNNs that prevent WFA binding to proteoglycans. [62]. Unfortunately, there are very limited tools available to examine these possibilities, as WFA is the gold standard for visualizing PNNs. Although the reduction in WFA staining intensity is a reliable but indirect measure of PNN maturity; mature PNNs show a high intensity of WFA staining, while less intense WFA staining is reflective of immature PNNs [54]. However, future studies could use selective antibodies for specific proteoglycans such as aggrecan or brevican to determine the nature of reductions in WFA intensity. Second, the reduction in PNN intensity may reflect PNN degradation and subsequent circuit remodeling and indicate critical windows of plasticity. We favor the latter alternative as PNNs have been shown to play critical roles in development and experience-dependent plasticity [31, 63]. Hence, we hypothesize that a reduction in PNN intensity would have a similar functional effect on PV-FSI excitability as Ch-ABC degradation. Consequently, pyramidal neurons within the PL-PFC and vmOFC would be in an excitable state due to a reduction of inhibitory tone from the PV-FSIs having immature or compromised PNNs. This hypothesis aligns with what has been seen in the OFC [38, 43] and preliminarily from our lab (unpublished observations TEB).
The role of the PL-PFC in the context of food reward remains poorly understood. It has been shown however, that lesions of the PL-PFC reduces licking responsiveness (number of licks) to a sucrose solution, suggesting that PL-PFC activation is required for food reward seeking [64]. Activation of muscarinic and D1-like receptors in the PL-PFC are also required for social transmission of food preference [65] and glucose preference [66], respectively. Thus, any disruption to inhibitory control within PL-PFC may alter food preference and/or seeking and contribute to obesogenic behaviors. Therefore, high fat-induced reduction of PNN intensity within the PL-PFC and vmOFC may reduce GABAergic PV-FSI neuronal firing among SD and OP males and would be predicted to increase prefrontal output to target regions like the nucleus accumbens. This may further exacerbate maladaptive seeking behaviors.
In addition to adaptations in neuronal function that may be associated with PNN modulation we cannot rule out the neuroprotective role PNNs may be playing as a barrier against oxidative stress and inflammatory cytokine activity generated by high fat consumption [67–69]. Although beyond the scope of this study, future studies will determine whether supplementation with antioxidants and/or reducing neuroinflammation within the PFC “normalizes” high fat diet-induced changes in PNN intensity to determine whether the changes in PNNs are directly related to adaptations in neuronal function or are compensatory responses to diet/obesity-induced elevations in oxidative stress and/or inflammation.
Unlike OP males, OR males did not exhibit any diet-induced alterations within the PL-PFC (Figures 4B–E). However, OR males showed a rightward shift and an increase in PNN intensity following high fat consumption in the vmOFC (Figures 4N, O). These results cannot be attributed to differences in food consumption as OR males consumed the same kilocalories/day as SD and OP strains maintained on the same diet (data not shown). These data indicate that male OR rats exhibit a lack of high fat-induced PNNs changes in the PL-PFC and opposite change in the vmOFC compared to OP and SD rats. Given the proposed functions of the PFC these differences in response to high fat diet may contribute to their obesity-resistant phenotype. However, it should be noted that studies of acquisition or performance of PFC-mediated tasks are lacking in the OP/OR model and should be addressed in the future.
Unlike male rats, females exhibited no significant changes in WFA intensity and the number of WFA+/PV+ and PV+ neurons in the PL-PFC or vmOFC after high fat exposure. However, consumption of a high fat diet elicited a rightward shift in the cumulative distribution of PNN intensity (Figures 5H and 6H) as well as an increase in PNN average intensity (Figures 5I and 6I) among SD and OP females in the IL-PFC. In addition to this, high fat fed OP females exhibited an increase in the number of WFA+/PV+ neurons compared to their chow-fed counterparts (Figure 6J), which was accompanied by an increase in the total number of PV+ neurons (Figure 6K) in the IL-PFC. Much like the PL-PFC, the functional roles of the IL-PFC are diverse and range from reward valuation [70] to decision-making [71]. However, the PL and IL-PFC also have distinct roles in mediating behavior. For instance, pharmacological inhibition and lesion studies suggest that the PL-PFC plays role in behavioral flexibility [72, 73], while the IL-PFC may be involved in habit formation and impulsivity [74–76]. In addition, IL-PFC inactivation delays the collection of earned sucrose pellets [77], impairs cue-induced food consumption [78], and is recruited for the learning of cocaine extinction [79]. Together this suggests that the IL-PFC plays a significant yet independent role from the PL-PFC in the expression of motivated reward seeking behaviors. The high fat-induced increase in PNN intensity observed in the SD and OP females and increase in the number of WFA+/PV+ neurons in OP females may facilitate an increase in PV-FSI function and ultimately decrease IL-PFC activity and promote reward-driven behaviors. Alternatively, increases in PNN intensity in the IL-PFC have been observed previously in animals trained to self-administer sucrose when exposed to environmental enrichment during the abstinence period, which may render food cues less salient and reduce food seeking behaviors [80]. Further, research is needed to understand the casual relationships between PNNs and the impact on feeding behaviors.
In contrast, high fat fed OR females showed a decrease in PNN intensity in the IL-PFC (Figure 7H–I) compared to chow fed controls and may increase the excitatory drive from the IL-PFC to promote control of food seeking behaviors. While the mechanisms underlying this sex difference is not known, there is substantive evidence for sex differences in diet-induced plasticity across many brain regions and circuits, with some evidence for roles of ovarian hormones [81]. Furthermore, behavioral data suggest that mechanisms of reversal learning (which relies in part on PFC) differ in males and females [82]. Although speculative, this nonetheless suggests that future studies should assess potential organizational vs activational roles of sex differences in PNN plasticity and brain function [83].
In sum, results here expand upon our previous work, which showed that exposure to a high fat diet resulted in an attenuation in PNN intensity within the PL and vmOFC of male SD rats [41]. Specifically, this study characterizes diet-induced changes in PNNs in females as well as animals from selectively bred OP and OR strains. Our observations in general indicate that OP rats, both male and female, display similar high fat-induced alterations to PNNs within the PFC as their SD counterparts. This suggests that OP phenotypes are present in SD, not surprising, as OPs were derived from selective breeding SD rats [84] in order to amplify naturally occurring variance in the population. Our results also indicate that the high fat-induced modifications to PNNs and PV+ cells observed in OR animals are different and often opposite to those observed in SD and OP strains, which begs the question of whether OR rats possess genetic modifications that enable the observed weight gain profiles and PNN phenotypes in the face of nutritional excess. Our laboratories are currently investigating this possibility. Finally, our results reveal interesting sex differences; namely, females appear to exhibit fat-induced changes to PNNs in the IL-PFC while males do so in the PL-PFC and vmOFC. However, as the PL and IL-PFC project to distinct layers within the nucleus accumbens [22] the overall effect of local circuit modulation between males and females may ultimately result in similar behavioral outputs. For instance, high fat exposure in male SD and OP rats may increase PL output and drive maladaptive food seeking behaviors while SD and OP females exposed to high fat my reduce IL output and inhibitory control of maladaptive behaviors. Hence, the net effect of diet manipulation on food seeking behaviors could be similar between males and females but mediated by different neural alterations and cognitive strategies. This hypothesis needs to be examined in the future. Finally, it is worthwhile to note that dietary manipulation during critical periods of development (when PNNs are not yet mature) may yield different results in both male and females as there are age-dependent effects on PNN expression in rats [17, 56, 85]. Taken together, these results further elucidate GABAergic-associated neuroplasticity within the PFC after high fat consumption and may guide future therapeutic targets for the treatment of obesity.
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Emily Jorgensen and Ms. Macy Watson for their assistance with animal care. We would also like to thank Ms. Georgia Kirkpatrick for her assistance on the manuscript. This worked was supported by the National Institute on Drug Abuse R01DA040965 [T.E.B., B.A.S.], R21DA045277 [T.E.B., C.R.F.], and R01DK106188 [C.R.F.] Research reported in this publication was supported in part by the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM121310 [Z.Z., T.E.B.]. The authors declare no conflicts of interest regarding the publication of this article.
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- [1].Hales CM, et al. , Prevalence of Obesity Among Adults and Youth: United States, 2015–2016, NCHS Data Brief (288) (2017) 1–8. [PubMed] [Google Scholar]
- [2].Kopelman P, Health risks associated with overweight and obesity, Obes Rev 8 (Suppl 1) (2007) 13–7. [DOI] [PubMed] [Google Scholar]
- [3].Ng M, et al. , Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013, Lancet 384 (9945) (2014) 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gibson SA, Are high-fat, high-sugar foods and diets conducive to obesity? Int J Food Sci Nutr 47 (5) (1996) 405–415. [DOI] [PubMed] [Google Scholar]
- [5].Poti JM, Braga B, Qin B, Ultra-processed Food Intake, Obesity, What Really Matters for Health-Processing or Nutrient Content? Curr Obes Rep 6 (4) (2017) 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Badre D, Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes, Trends Cogn Sci 12 (5) (2008) 193–200. [DOI] [PubMed] [Google Scholar]
- [7].Coutlee CG, Huettel SA, The functional neuroanatomy of decision making: prefrontal control of thought and action, Brain Res 1428 (2012) 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Godefroy O, et al. , Control functions of the frontal lobes, Modularity of the central-supervisory system? Cortex 35 (1) (1999) 1–20. [DOI] [PubMed] [Google Scholar]
- [9].Kim S, Lee D, Prefrontal cortex and impulsive decision making, Biol Psychiatry 69 (12) (2011) 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Knoch D, Fehr E, Resisting the power of temptations: the right prefrontal cortex and self-control, Ann N Y Acad Sci 1104 (2007) 123–134. [DOI] [PubMed] [Google Scholar]
- [11].Ochsner KN, Gross JJ, The cognitive control of emotion, Trends Cogn Sci 9 (5) (2005) 242–249. [DOI] [PubMed] [Google Scholar]
- [12].Pinto L, Dan Y, Cell-Type-Specific Activity in Prefrontal Cortex during Goal-Directed Behavior, Neuron 87 (2) (2015) 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rorie AE, Newsome WT, A general mechanism for decision-making in the human brain? Trends Cogn Sci 9 (2) (2005) 41–3. [DOI] [PubMed] [Google Scholar]
- [14].Brooks SJ, Cedernaes J, Schioth HB, Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies, PLoS One 8 (4) (2013) e60393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Davids S, et al. , Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli, Int J Obes (Lond) 34 (1) (2010) 94–104. [DOI] [PubMed] [Google Scholar]
- [16].Le DS, et al. , Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity, Am J Clin Nutr 84 (4) (2006) 725–731. [DOI] [PubMed] [Google Scholar]
- [17].Lowe CJ, Morton JB, Reichelt AC, Adolescent obesity and dietary decision making-a brain-health perspective, Lancet Child Adolesc Health (2020). [DOI] [PubMed] [Google Scholar]
- [18].Lowe CJ, Reichelt AC, Hall PA, The Prefrontal Cortex and Obesity: A Health Neuroscience Perspective, Trends Cogn Sci 23 (4) (2019) 349–361. [DOI] [PubMed] [Google Scholar]
- [19].Klesges RC, et al. , A longitudinal analysis of the impact of dietary intake and physical activity on weight change in adults, Am J Clin Nutr 55 (4) (1992) 818–822. [DOI] [PubMed] [Google Scholar]
- [20].Groenewegen HJ, Wright CI, Uylings HB, The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia, J Psychopharmacol 11 (2) (1997) 99–106. [DOI] [PubMed] [Google Scholar]
- [21].Britt JP, et al. , Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens, Neuron 76 (4) (2012) 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ma YY, et al. , Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections, Neuron 83 (6) (2014) 1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Suska A, et al. , Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine, Proc Natl Acad Sci U S A 110 (2) (2013) 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Little JP, Carter AG, Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala, J Neurosci 33 (39) (2013) 15333–15342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Meyer HS, et al. , Inhibitory interneurons in a cortical column form hot zones of inhibition in layers 2 and 5A, Proc Natl Acad Sci U S A 108 (40) (2011) 16807–16812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tremblay R, Lee S, Rudy B, GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits, Neuron 91 (2) (2016) 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Markram H, et al. , Interneurons of the neocortical inhibitory system, Nat Rev Neurosci 5 (10) (2004) 793–807. [DOI] [PubMed] [Google Scholar]
- [28].Hartig W, Brauer K, Bruckner G, Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons, Neuroreport 3 (10) (1992) 869–872. [DOI] [PubMed] [Google Scholar]
- [29].Wang D, Fawcett J, The perineuronal net and the control of CNS plasticity, Cell Tissue Res 349 (1) (2012) 147–160. [DOI] [PubMed] [Google Scholar]
- [30].Cabungcal JH, et al. , Perineuronal nets protect fast-spiking interneurons against oxidative stress, Proc Natl Acad Sci U S A 110 (22) (2013) 9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bruckner G, et al. , Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain, Glia 8 (3) (1993) 183–200. [DOI] [PubMed] [Google Scholar]
- [32].Carstens KE, et al. , Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons, J Neurosci 36 (23) (2016) 6312–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Frischknecht R, et al. , Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity, Nat Neurosci 12 (7) (2009) 897–904. [DOI] [PubMed] [Google Scholar]
- [34].Balmer TS, Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons, eNeuro 3 (4) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Slaker M, et al. , Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory, J Neurosci 35 (10) (2015) 4190–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Slaker M, Blacktop JM, Sorg BA, Caught in the Net: Perineuronal Nets and Addiction, Neural Plast 2016 (2016) 7538208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sorg BA, et al. , Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity, J Neurosci 36 (45) (2016) 11459–11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thompson JL, et al. , Obesity-Induced Structural and Neuronal Plasticity in the Lateral Orbitofrontal Cortex, Neuropsychopharmacology 42 (7) (2017) 1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Winocur G, et al. , Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity, Behav Neurosci 119 (5) (2005) 1389–1395. [DOI] [PubMed] [Google Scholar]
- [40].Dingess PM, et al. , Structural and Functional Plasticity within the Nucleus Accumbens and Prefrontal Cortex Associated with Time-Dependent Increases in Food Cue-Seeking Behavior, Neuropsychopharmacology 42 (12) (2017) 2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dingess PM, et al. , Consumption of a High-Fat Diet Alters Perineuronal Nets in the Prefrontal Cortex, Neural Plast 2018 (2018) 2108373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Slaker ML, et al. , Cocaine Exposure Modulates Perineuronal Nets and Synaptic Excitability of Fast-Spiking Interneurons in the Medial Prefrontal Cortex, eNeuro 5 (5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Alonso-Caraballo Y, et al. , Functional and structural plasticity contributing to obesity: roles for sex, diet, and individual susceptibility, Curr Opin Behav Sci 23 (2018) 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Oginsky MF, Ferrario CR, Eating “junk food” has opposite effects on intrinsic excitability of nucleus accumbens core neurons in obesity-susceptible versus -resistant rats, J Neurophysiol 122 (3) (2019) 1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vollbrecht PJ, et al. , Pre-existing differences and diet-induced alterations in striatal dopamine systems of obesity-prone rats, Obesity (Silver Spring) 24 (3) (2016) 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vollbrecht PJ, et al. , Pre-existing differences in motivation for food and sensitivity to cocaine-induced locomotion in obesity-prone rats, Physiol Behav 152 (Pt A) (2015) 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hruby A, Hu FB, The Epidemiology of Obesity: A Big Picture, Pharmacoeconomics 33 (7) (2015) 673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Westwood FR, The female rat reproductive cycle: a practical histological guide to staging, Toxicol Pathol 36 (3) (2008) 375–384. [DOI] [PubMed] [Google Scholar]
- [49].Becker JB, Hu M, Sex differences in drug abuse, Front Neuroendocrinol 29 (1) (2008) 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Alonso-Caraballo Y, et al. , Enhanced anxiety-like behavior emerges with weight gain in male and female obesity-susceptible rats, Behav Brain Res 360 (2019) 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Arnoni-Bauer Y, et al. , Is It Me or My Hormones? Neuroendocrine Activation Profiles to Visual Food Stimuli Across the Menstrual Cycle, J Clin Endocrinol Metab 102 (9) (2017) 3406–3414. [DOI] [PubMed] [Google Scholar]
- [52].Dreher JC, et al. , Menstrual cycle phase modulates reward-related neural function in women, Proc Natl Acad Sci U S A 104 (7) (2007) 2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Frank TC, et al. , Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues, Brain Res 1363 (2010) 81–92. [DOI] [PubMed] [Google Scholar]
- [54].Slaker ML, Harkness JH, Sorg BA, A standardized and automated method of perineuronal net analysis using Wisteria floribunda agglutinin staining intensity, IBRO Rep 1 (2016) 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Paxinos G, Watson C, The rat brain in stereotaxic coordinates, 6th ed, Academic Press/Elsevier, Amsterdam; Boston, 2007. [Google Scholar]
- [56].Griffths BB, et al. , Age-dependent sexual dimorphism in hippocampal cornu ammonis-1 perineuronal net expression in rats, Brain Behav 9 (5) (2019) e01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sandoval-Salazar C, et al. , A high-fat diet decreases GABA concentration in the frontal cortex and hippocampus of rats, Biol Res 49 (2016) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yang S, et al. , Antibody recognizing 4-sulfated chondroitin sulfate proteoglycans restores memory in tauopathy-induced neurodegeneration, Neurobiol Aging 59 (2017) 197–209. [DOI] [PubMed] [Google Scholar]
- [59].Reichelt AC, et al. , A high-fat high-sugar diet in adolescent rats impairs social memory and alters chemical markers characteristic of atypical neuroplasticity and parvalbumin interneuron depletion in the medial prefrontal cortex, Food Funct 10 (4) (2019) 1985–1998. [DOI] [PubMed] [Google Scholar]
- [60].Kim SY, et al. , TGFbeta signaling is associated with changes in inflammatory gene expression and perineuronal net degradation around inhibitory neurons following various neurological insults, Sci Rep 7 (1) (2017) 7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Levin BE, et al. , Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats, Am J Physiol 273 (2 Pt 2) (1997) R725–R730. [DOI] [PubMed] [Google Scholar]
- [62].Enwright JF, et al. , Reduced Labeling of Parvalbumin Neurons and Perineuronal Nets in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia, Neuropsychopharmacology 41 (9) (2016) 2206–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pizzorusso T, et al. , Structural and functional recovery from early monocular deprivation in adult rats, Proc Natl Acad Sci U S A 103 (22) (2006) 8517–8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Parent MA, et al. , The medial prefrontal cortex is crucial for the maintenance of persistent licking and the expression of incentive contrast, Front Integr Neurosci 9 (2015) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Boix-Trelis N, et al. , Muscarinic cholinergic receptor blockade in the rat prelimbic cortex impairs the social transmission of food preference, Neurobiol Learn Mem 87 (4) (2007) 659–668. [DOI] [PubMed] [Google Scholar]
- [66].Touzani K, Bodnar RJ, Sclafani A, Acquisition of glucose-conditioned flavor preference requires the activation of dopamine D1-like receptors within the medial prefrontal cortex in rats, Neurobiol Learn Mem 94 (2) (2010) 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Reichelt AC, et al. , Perineuronal Nets: Plasticity, Protection, and Therapeutic Potential, Trends Neurosci 42 (7) (2019) 458–470. [DOI] [PubMed] [Google Scholar]
- [68].Dutheil S, et al. , High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation, Neuropsychopharmacology 41 (7) (2016) 1874–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jeon BT, et al. , Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet, Diabetes 61 (6) (2012) 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Grabenhorst F, Rolls ET, Value, pleasure and choice in the ventral prefrontal cortex, Trends Cogn Sci 15 (2) (2011) 56–67. [DOI] [PubMed] [Google Scholar]
- [71].Euston DR, Gruber AJ, McNaughton BL, The role of medial prefrontal cortex in memory and decision making, Neuron 76 (6) (2012) 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Marquis JP, Killcross S, Haddon JE, Inactivation of the prelimbic, but not infralimbic, prefrontal cortex impairs the contextual control of response conflict in rats, Eur J Neurosci 25 (2) (2007) 559–566. [DOI] [PubMed] [Google Scholar]
- [73].Ragozzino ME, The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility, Ann N Y Acad Sci 1121 (2007) 355–375. [DOI] [PubMed] [Google Scholar]
- [74].Chudasama Y, et al. , Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity, Behav Brain Res 146 (1–2) (2003) 105–119. [DOI] [PubMed] [Google Scholar]
- [75].Murphy ER, Dalley JW, Robbins TW, Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task, Psychopharmacology (Berl) 179 (1) (2005) 99–107. [DOI] [PubMed] [Google Scholar]
- [76].Van den Oever MC, et al. , Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking, Nat Neurosci 11 (9) (2008) 1053–1058. [DOI] [PubMed] [Google Scholar]
- [77].Burgos-Robles A, Bravo-Rivera H, Quirk GJ, Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior, PLoS One 8 (2) (2013) e57575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Petrovich GD, et al. , Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats, J Neurosci 27 (24) (2007) 6436–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Peters J, LaLumiere RT, Kalivas PW, Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats, J Neurosci 28 (23) (2008) 6046–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Slaker M, et al. , Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex following Early and Late Abstinence from Sucrose Self-Administration in Rats, PLoS One 11 (12) (2016) e0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Marrocco J, McEwen BS, Sex in the brain: hormones and sex differences, Dialogues Clin Neurosci 18 (4) (2016) 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schuch CP, et al. , An RFID-based activity tracking system to monitor individual rodent behavior in environmental enrichment: Implications for post-stroke cognitive recovery, J Neurosci Methods 324 (2019) 108306. [DOI] [PubMed] [Google Scholar]
- [83].Arnold AP, Breedlove SM, Organizational and activational effects of sex steroids on brain and behavior: a reanalysis, Horm Behav 19 (4) (1985) 469–498. [DOI] [PubMed] [Google Scholar]
- [84].Levin BE, Govek E, Gestational obesity accentuates obesity in obesity-prone progeny, Am J Physiol 275 (4) (1998) R1374–R1379. [DOI] [PubMed] [Google Scholar]
- [85].Bruckner G, et al. , Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R, J Comp Neurol 428 (4) (2000) 616–629. [DOI] [PubMed] [Google Scholar]


