Abstract
The pathogenesis of autoimmune complications triggered by SARS‐CoV2 has not been completely elucidated. Here, we performed an analysis of the cellular immune status, cell ratios, and monocyte populations of patients with COVID‐19 treated in the intensive care unit (ICU) (cohort 1, N = 23) and normal care unit (NCU) (cohort 2, n = 10) compared with control groups: patients treated in ICU for noninfectious reasons (cohort 3, n = 30) and patients treated in NCU for infections other than COVID‐19 (cohort 4, n = 21). Patients in cohort 1 presented significant differences in comparison with the other cohorts, including reduced frequencies of lymphocytes, reduced CD8+T‐cell count, reduced percentage of activated and intermediate monocytes and an increased B/T8 cell ratio. Over time, patients in cohort 1 who died presented with lower counts of B, T, CD4+T, CD8+T‐lymphocytes, NK cells, and activated monocytes. The B/T8 ratio was significantly lower in the group of survivors. In cohort 1, significantly higher levels of IgG1 and IgG3 were found, whereas cohort 3 presented higher levels of IgG3 compared to controls. Among many immune changes, an elevated B/T8‐cell ratio and a reduced rate of activated monocytes were mainly observed in patients with severe COVID‐19. Both parameters were associated with death in cohort 1.
Keywords: Autoimmunity ⋅ B/T‐cell ratio ⋅ COVID‐19 ⋅ Lymphocytes ⋅ Monocytes
Patients with severe COVID‐19 present a particular set of immune changes in comparison to patients with mild disease and controls. These include consumption of certain monocyte and lymphocyte populations and an elevated B/T8 Ratio. COVID‐19 patients in general share a proinflammatory immunoglobulin profile with elevated proportions of IgG1 and/or IgG3.
Introduction
Since its outbreak in December, 2019 the Corona Virus Disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has been responsible for hundreds of thousands of deaths worldwide. Even though the virus directly targets the respiratory tract, the immune response triggered by the infection, also called the host response, can provide further explanation for the development of severe disease with multiple complications such as thromboembolic events, liver failure, and autoimmune manifestations [1, 2].
Viral replication in infected cells causes cell death, leading to activation of alveolar epithelial cells and alveolar macrophages, resulting in a local inflammatory response. The release of proinflammatory cytokines from these cells, such as interleukin 6 (IL‐6), interferon (IFN)‐γ, monocyte chemoattractant protein‐1 (MCP1), and Interferon gamma‐induced protein 10 (IP‐10) [3, 4], further stimulates a type 1 helper T cell (Th1) response, with migration of T lymphocytes and monocytes to the site of infection. Whereas this immune reaction is responsible for viral clearance in the majority of individuals, many patients with severe disease present an abnormal immune cell activation with overexpression of proinflammatory cytokines leading to the so called cytokine release syndrome [1, 3–5]. This overshooting inflammation, similar to septic disease, can lead to organ damage and death.
Lymphopenia has been described as an important characteristic and is of prognostic value in patients with COVID‐19 [4, 6, 7] since it seems to be more pronounced in patients with a severe course of disease [8, 9, 10]. Of note, T cells appear to be particularly reduced in patients with severe disease [5]. CD8+ and CD4+ T cells are markedly reduced in patients with a complicated course and are also being identified as independent risk factors for poor prognosis [5, 11–13]. Furthermore, SARS‐CoV‐2 infection seems to induce a differentiation of CD4+ T lymphocytes into pathogenic Th1 cells with high expression of GM‐CSF and IFN‐γ. This cytokine milieu, is also involved in the differentiation of intermediate monocytes (CD14+, CD16+), whose presence could accelerate tissue damage [14, 15]. However, the role of monocytic activation in viral infections, especially in COVID‐19, is yet to be determined.
Most research efforts focusing on COVID‐19 are describing the immune reaction associated with SARS‐CoV‐2 extensively but data comparing COVID‐19 patients with healthy subjects and control patients suffering from infections and diseases other than COVID‐19 is sparse. Thus, it remains unknown whether the reported immune dysregulation is specific to COVID‐19 patients or if it can also be attributed to reactions of the immune system provoked by severe illness in general.
In addition, as compared to T cells, the role of B cells in the pathogenesis especially of severe disease has been less characterized so far. Studies have established that levels of specific circulating antibodies seem to be higher in patients with severe disease [16] which may be due to causal linkage to antibody‐dependent immune enhancement.
Due to the fundamental role of immune cells in COVID‐19 and their prognostic importance, our study aimed to further characterize the changes in lymphocyte and monocyte populations over time as well as other parameters such as immunoglobulin profile, C reactive protein (CRP), IL‐6, and ferritin in European patients with severe and mild COVID‐19. To further elucidate whether these changes are specific to COVID‐19, we compared these data to patients treated in the normal care unit (NCU) and intensive care unit (ICU) for other reasons than COVID‐19 in a university hospital in Germany.
Results
Mean age was similar in all groups with longest hospitalization times in the ICU COVID+ cohort
In total, 87 patients were included in the study and divided into four different cohorts: cohort 1 contained 24 patients treated in the ICU for COVID‐19 (ICU COVID+), cohort 2 contained 10 patients in the NCU with COVID‐19 (NCU COVID+), cohort 3 consisted of 30 patients treated in the ICU for other reasons than COVID 19 (ICU COVID‐), and cohort 4 included 21 patients treated in the NCU for an infection other than COVID‐19 (NCU COVID‐). Table 1 summarizes patient characteristics and treatment parameters of all cohorts. The ICU COVID+ cohort had the longest hospitalization time with an average time of 38 days, whereas cohort 4 (NCU COVID−) had the shortest time of hospitalization with only 5 days. The highest percentage of male patients with 87.5% was found in the ICU COVID+ cohort.
Table 1.
Patient characteristics and treatment parameters
| ICU COVID+ | NCU COVID+ | ICU COVID‐ | NCU COVID‐ | |
|---|---|---|---|---|
| Total, n | 24 | 10 | 30 | 21 |
| Age, mean (years) a | 65 | 61 | 68 | 66 |
| Male, n (%) | 21 (87.5) | 7 (66%) | 23 (76%) | 11 (53%) |
| Mechanical ventilation | 21 (87.5%) | 0 | 14 (43%) | 0 |
| Extracorporal circulation | 8 (33%) | 0 | 0 | 0 |
| Days of hospitalization | 38 | 11 | 13 | 5 |
|
In hospital mortality Reason for hospitalization Cardiovascular Event Bleeding |
8 (33%) |
0 |
10 (33%) 16 4 |
0 3 3 |
| Infections | 5 | 6 | ||
| Gastroenterological and hepatic disorders | 2 | 5 | ||
| Nephrological disorders | 1 | 1 | ||
| Pulmonary disorders | 1 | 0 | ||
| Other | 1 | 3 | ||
| COVID‐19 Spectrum | ||||
| Mild COVID‐19 | 1 | 4 | ||
| Pneumonia | 2 | 4 | ||
| Moderate ARDS | 7 | 0 | ||
| Severe ARDS | 14 | 0 | ||
| Asymptomatic | 0 | 2 | ||
| Use of antibiotics | 18 | 5 | ||
| Nonbacterial secondary Infections c | 6 | |||
| Immune suppression | ||||
| Previous | 2 | 3 | 0 | 2 |
| During the study b | 20 | 2 | 0 | 1 |
Differences are not statistically significant.
Most patients with severe disease received hydroxychloroquine. Steroids were not administered routinely, thus, only a small proportion of patients received it during the study. In the ICU COVID+ Cohort 1, patient received Tocilizumab.
Five patients had evidence of Aspergillus sp. in the bronchoscopy and one patient had evidence of Candida sp. in the blood culture.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
COVID‐19 patients present different immunoglobulin profiles
Initial laboratory parameters were collected and compared between the cohorts ICU COVID+ (cohort 1) versus ICU COVID− (cohort 3), ICU COVID+ (cohort 1) versus NCU COVID+ (cohort 2), and NCU COVID+ (cohort 2) versus NCU COVID− (cohort 4). Table 2 summarizes the comparisons between all groups. The ICU COVID+ cohort showed the most severe absolute lymphopenia among all groups, with numerically lower counts of CD4+ T lymphocytes, CD8+ T lymphocytes, B lymphocytes, and NK lymphocytes. However, the frequency of B lymphocytes was highest in the ICU COVID+ group.
Table 2.
Laboratory parameters at baseline
| ICU COVID+ | ICU COVID‐ | p | ICU COVID+ | NCU COVID+ | p | NCU COVID+ | NCU COVID‐ | p | |
|---|---|---|---|---|---|---|---|---|---|
| Leukocytes a | 11 641 | 10 929 | 0.48 | 11 641 | 7438 | <0.01 | 7438 | 8965 | 0.47 |
| Lymphocytes a | 700 | 1208 | 0.09 | 700 | 1467 | 0.09 | 1467 | 1217 | 0.84 |
| Lymphocytes (%) | 7.0 | 13 | 0.02 | 7.0 | 20 | <0.01 | 20 | 16 | 0.39 |
| B lymphocytes (%) | 22 | 16 | 0.16 | 22 | 7.6 | <0.01 | 7.6 | 10 | 0.44 |
| B lymphocytes a | 132 | 300 | 0.4 | 132 | 134 | 0.59 | 134 | 117 | 0.32 |
| T lymphocytes (%) | 67 | 74 | 0.04 | 67 | 74 | 0.12 | 74 | 72 | 0.71 |
| T lymphocytes a | 475 | 784 | 0.02 | 475 | 1130 | 0.09 | 1130 | 864 | 0.65 |
| Activated T lymphocytes (%) | 13 | 10 | 0.44 | 13 | 12 | 0.80 | 12 | 11 | 0.74 |
| CD4+ lymphocytes (%) | 50 | 54 | 0.39 | 50 | 40 | <0.01 | 40 | 54 | 0.01 |
| CD4+ lymphocytes a | 359 | 533 | 0.04 | 359 | 602 | 0.25 | 602 | 630 | 0.85 |
| CD8+ lymphocytes (%) | 16 | 19 | 0.19 | 16 | 31 | <0.01 | 31 | 19 | 0.01 |
| CD8+ lymphocytes a | 114 | 232 | 0.01 | 114 | 494 | <0.01 | 494 | 228 | 0.15 |
| NK cells (%) | 11 | 9.2 | 0.53 | 11 | 18 | 0.26 | 18 | 17 | 0.84 |
| NK‐cells a | 79 | 103 | 0.57 | 79 | 199 | 0.07 | 199 | 224 | 0.88 |
| T4/T8‐Ratio | 4.3 | 3.4 | 0.45 | 4.3 | 1.5 | <0.01 | 1.5 | 3.9 | 0.01 |
| B/T8‐Ratio | 1.8 | 0.98 | 0.01 | 1.8 | 0.34 | <0.01 | 0.34 | 0.67 | 0.03 |
| Monocytes a | 609 | 607 | 0.9 | 609 | 497 | 0.10 | 497 | 728 | 0.06 |
| Monocytes (%) | 4.8 | 4.8 | 0.8 | 4.8 | 5.0 | 0.97 | 5.0 | 10 | 0.04 |
| HLA‐DR+CD14+ monocytes (%) | 17 | 34 | <0.01 | 17 | 40 | <0.01 | 40 | 46 | 0.35 |
| Classical monocytes (%) | 82 | 80 | 0.42 | 82 | 75 | 0.13 | 75 | 77 | 0.73 |
| Intermediate monocytes (%) | 5.6 | 13 | <0.01 | 5.6 | 14 | 0.03 | 14 | 15 | 0.73 |
| Nonclassical monocytes (%) | 3.0 | 1.5 | 0.25 | 3.0 | 1.4 | 0.3 | 1.4 | 3.2 | 0.21 |
| C Reactive Protein (mg/L) | 180 | 119 | 0.03 | 180 | 37 | <0.01 | 37 | 51 | 0.33 |
| Platelets a | 239 | 182 | 0.01 | 239 | 252 | 0.94 | 252 | 220 | 0.53 |
| Hamoglobin (g/dL) | 10 | 10 | 0.81 | 10 | 13 | <0.01 | 13 | 11 | 0.09 |
| IgA | 325.3 | 320.3 | 0.74 | 325.3 | 281.6 | 0.12 | 281.7 | 272.9 | 0.8 |
| IgM | 119.1 | 60.5 | <0.01 | 119.1 | 155.5 | 0.86 | 155.5 | 75.1 | 0.17 |
| IgG | 1074.8 | 883.6 | 0.08 | 1074.8 | 1129.8 | 0.69 | 1129.8 | 1042.2 | 0.5 |
| IgG1 | 758.6 | 557.3 | <0.01 | 758.6 | 777.8 | 0.82 | 777.8 | 669.6 | 0.41 |
| IgG2 | 219.5 | 230.5 | 0.9 | 219.5 | 276.3 | 0.15 | 276.3 | 282.6 | 0.94 |
| IgG3 | 59.6 | 31.6 | <0.01 | 59.6 | 49.2 | 0.42 | 49.2 | 25.5 | 0.02 |
| IgG4 | 37.0 | 64.2 | 0.11 | 37 | 26.6 | 0.53 | 26.6 | 64.4 | 0.06 |
Values shown are mean cells/μL.
Bold values represent statistic significant comparisons.
p values are not adjusted for multiple comparisons.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
We also found significant differences in the immunoglobulin concentrations at baseline between COVID patients and non‐COVID patients. ICU COVID+ patients had higher concentrations of IgM (119.1 vs. 60.5 mg/dL, p = 0.002), IgG1 (758.6 vs. 557.3 mg/dL, p = 0.008), and IgG3 (59.6 vs. 26.43 mg/dL, p = 0.006) in comparison with ICU COVID‐ patients. Additionally, NCU COVID+ patients showed higher levels of IgG3 (49.2 vs. 25.5 mg/dL, p = 0.02) in comparison with NCU COVID– patients. There were no significant differences between both COVID+ cohorts or with regard to outcome (Table 2).
ICU COVID+ patients show more pronounced CD8+ T lymphopenia and higher B/T8 cell ratios
Comparisons between cohorts 1 (ICU COVID+) and 3 (ICU COVID‐) showed significant differences in CRP levels (180 vs. 119 mg/l, p = 0.03), percentage of lymphocytes (7 vs. 13 %, p = 0.02), T‐lymphocyte count (475 vs. 784 cells/μL, p = 0.02) and percentage (67 vs. 74%, p = 0.04), CD4+ T‐lymphocyte count (359 vs. 533 cells/μL, p = 0.04) and platelets (239 vs. 182 platelets/nL, p = 0.01), respectively. CD8+ T lymphopenia was observed in both groups, but was significantly more pronounced in the ICU COVID+ group (114 vs. 232 cell/μL, p = 0.01).
An exploratory analysis of the B cell to CD8+ T cell ratio showed a significant difference between ICU COVID+ and ICU COVID− patients (1.80 vs. 0.98, p = 0.01).
Intermediate and activated monocytes are consumed in the ICU COVID+ cohort
Comparing percentages of monocytes, the ICU COVID+ cohort showed significantly lower rates of intermediate monocytes compared to the ICU COVID− group (5.6 vs. 13%, p < 0.01), which was also true for activated HLA‐DR/CD14 positive monocytes (17 vs. 34 %, p < 0.01).
Complex immune changes are observed in ICU COVID+ but not in NCU COVID+ patients
Comparisons between ICU COVID+ and NCU COVID+ patients showed significant differences in leukocyte counts (11 641 vs. 7438 cells/μL, p < 0.01), proportion of lymphocytes (7 vs. 20%, p < 0.01), percentage of B lymphocytes (22 vs. 7.6%, p < 0.01), and CD4+ lymphocytes (50 vs. 40%, p < 0.01), absolute CD8+ T lymphocyte count (114 vs. 494 cells/μL, p < 0.01), proportion of CD8+ T lymphocytes (16 vs. 31%, p < 0.01), and T4/T8 ratio (4.3 vs. 1.5, p < 0.01). In addition, we found significant differences in CRP (180 vs. 37 mg/L, p < 0.01) and hemoglobin levels (10 vs. 13 g/dL, p < 0.01). Marked differences not reaching significance were observed for lymphocyte counts (700 vs. 1467 cell/μL, p = 0.09), T lymphocytes (475 vs. 1130 cell/μL, p = 0.09), CD4+ T lymphocytes (359 vs. 602 cells/μL, p = 0.25), and NK cells (79 vs. 199 cells/μL, p = 0.07).
Evaluating monocyte subsets, ICU COVID+ patients as compared to NCU COVID+ patients showed a significant smaller percentage of intermediate monocytes (5.6 vs. 14%, p = 0.03) as well as activated HLA‐DR/CD14 positive monocytes (17 vs. 40%, p < 0.01).
The exploratory analysis of the B cell to CD8+ T cell ratio showed a significant difference between COVID positive patients treated in the ICU and the NCU (1.8 vs. 0.34, p < 0.01).
NCU COVID+ (cohort 2) versus NCU COVID− (cohort 4)
Comparisons between baseline laboratory findings of cohorts 2 versus 4 revealed differences in CD4+ lymphocyte proportion (40 vs. 54%, p = 0.01), percentage of CD8+ lymphocytes (31 vs. 19%, p = 0.01), T4/T8 ratio (1.5 vs. 3.9, p = 0.01), B/T8 ratio (0.34 vs. 0.67, p = 0.03), and the frequency of monocytes (5.0 vs. 10%, p = 0.04).
Patients in the ICU COVID+ cohort with dismal outcome showed impaired immune recovery
Longitudinal measures were only performed in cohort 1 due to the longer time of hospitalization. In this group, a subgroup analysis compared patients with a favorable course of disease who were discharged from hospital due to clinical improvement (positive outcome) with patients who died due to COVID‐19 (negative outcome). Figures 1 and 2 summarize the linear regressions from this cohort (ICU COVID+). Total leucocyte count, B lymphocyte count, total monocytes, classical monocytes, and intermediate monocytes showed no significant changes over time.
Figure 1.
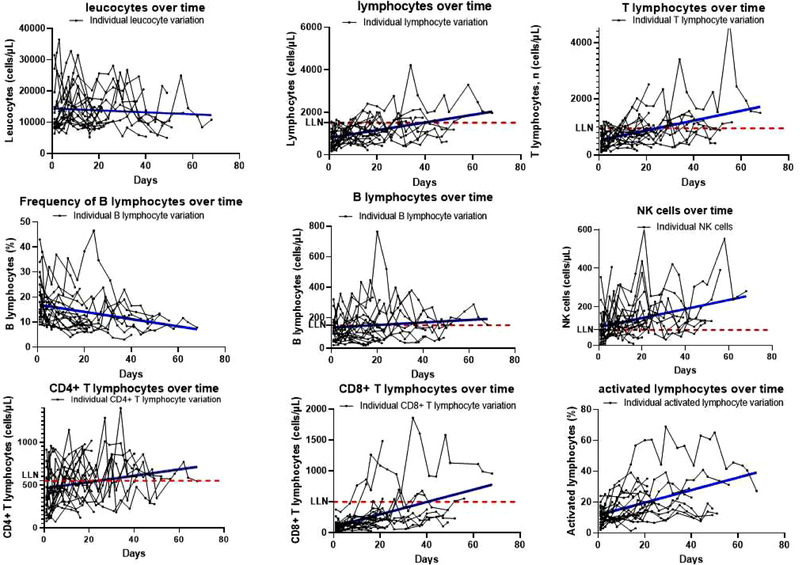
Lymphocyte variations over time in the ICU COVID+ Cohort. Spaghetti plots represent individual variations over time. Single points represent individual counts/frequencies of cells over time. All linear regressions show p < 0.05 for slopes different than zero, except for leucocytes and B lymphocytes for which the variation over time was not statistically significant. LLN, lower limit of normal. Samples were collected twice weekly until hospital discharge due to improvement or death. For every sample, one single experiment was performed since measurements were integrated into our routine diagnostic procedures. Each point in the plot corresponds to the measurements performed from one sample (N = 24 patients).
Figure 2.
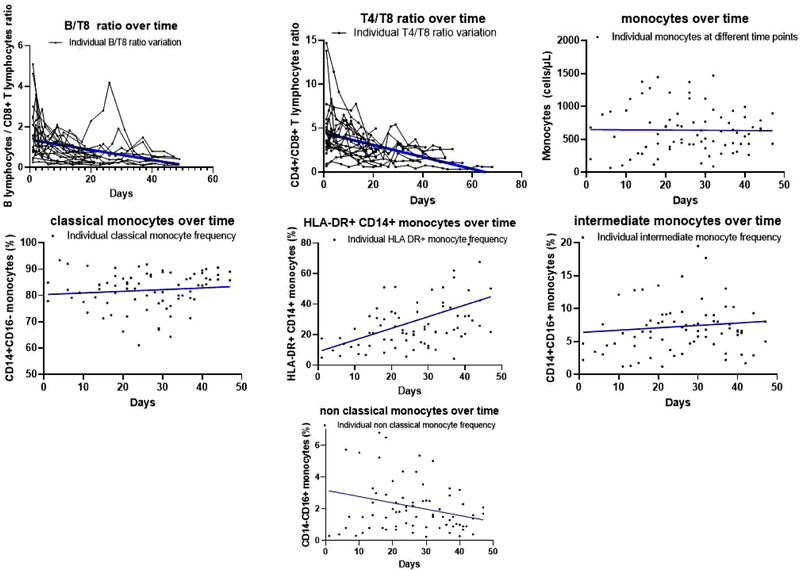
Monocyte and cell ratio variations over time in the ICU COVID+ Cohort. Plots represent cell ratio and cell variation over time. Single points represent individual counts/frequencies of cells over time. All linear regressions show p < 0.05 for slopes different than zero, except for monocyte count, monocyte frequency, intermediate monocytes, and classical monocytes for which the variation over time was not statistically significant. Samples were collected twice weekly until hospital discharge due to improvement or death. For every sample, one single experiment was performed as part of our routine diagnostic workup. Each point in the plot corresponds to the measurements performed from one sample. Data shown for cell ratios are from N = 24 patients and for monocytes are from N = 15 patients. The smaller sample size in monocytes is due to COVID‐19 related supply shortages of diagnostic material.
Linear regression analysis of absolute cell counts of lymphocytes, T lymphocytes, CD4+ T cells, CD8+ T cells, and NK cells as well as percentage of activated (HLA‐DR+) lymphocytes and monocytes showed a significant increase in respective values toward normalization. On the opposite, this was not observed for nonclassical monocyte and B‐lymphocyte percentages, which decreased over time. Also, cell ratios of T4/T8 and B/T8 declined significantly during the study period.
Patients with dismal outcome presented with more pronounced immune changes compared to survivors
A larger proportion of patients in the positive outcome group reached normalization of lymphocytes and its subpopulations than in the group of patients with negative outcome (Table 3). However, a small proportion of patients in the positive outcome group did not achieve normalization of cell counts before hospital discharge. In the group with negative outcome, only one patient reached a normal CD8+ T‐lymphocyte count. The positive outcome group showed faster normalization of all cell populations (Table 3, Fig. 3). In the ICU COVID+ group with favorable outcome, it took 12 days for lymphocytes, 2.9 days for B cells, 7.3 days for CD4 T cells, 19 days for CD8 T cells, and 3.4 days for NK cells to recover. Since only one patient in the group with negative outcome reached normal counts of CD8 lymphocytes, we performed a comparison of the time to a twofold increase, which was also shorter in the group of survivors.
Table 3.
Mean time to normalization according to outcome: Days (SD)
| Positive outcome a | N a (%) | Negative outcome a | N a (%) | |
|---|---|---|---|---|
| Total patients | 15 | 8 | ||
| Lymphocytes | 12 (7.7) | 9 (60%) | 22 (5.3) | 3 (37.5%) |
| B lymphocytes | 2.9 (4.9) | 10 (66%) | 5.0 (5.8) | 4 (50%) |
| T lymphocytes | 12 (9.4) | 10 (66%) | 23 (7) | 2 (25%) |
| CD4+ T lymphocytes | 7.3 (7) | 12 (80%) | 16 (20) | 5 (62.5%) |
| CD8+ T lymphocytes b | 19 (7) | 6 (40%) | 52 (0) | 1 (12.5%) |
| CD8+ T lymphocytes (twofold increase) | 12 (12) | 12 (80%) | 20 (18) | 6 (75%) |
| NK cells | 3.4 (10) | 10 (66%) | 8.8 (2.9) | 5 (62.5%) |
Only patients reaching normal values were included in this analysis. Single abrupt elevations followed by abnormal values were not counted as normalization.
Only one patient in the group with negative outcome reached normal CD8+ values.
In the group with positive outcome, two patients were lost to follow‐up after hospital discharge with only two measures at the point of censoring.
Normal values according to local laboratory definitions: Lymphocytes > 1500 cells/μL, B lymphocytes > 150/μL, T lymphocytes > 950/μL, CD4+ T lymphocytes > 550/μL, CD8+ T lymphocytes > 500/μL, NK cells > 80/μL.
SD, standard deviation.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 3.
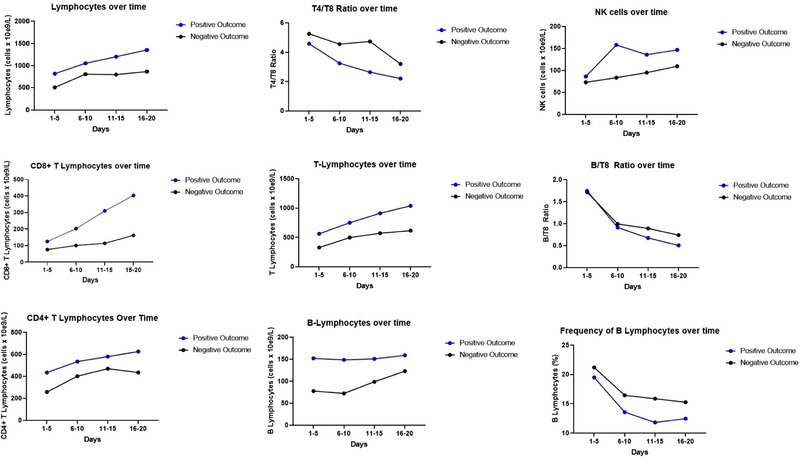
Comparisons of parameters per outcome over time until day 20. Graphs show cell counts or cell frequencies in four different time points for two subgroups of the ICU COVID+ cohort. Blue dots are means of the subgroup with positive outcome (survival) (N = 16) and black dots are means of the subgroup with negative outcome (death) (N = 8). Comparisons made using mixed effect model showed no significant differences between curves. A statistically significant interaction of cell count and outcome was verified for lymphocytes, T lymphocytes, and CD8+ lymphocytes.
The comparisons were made using the mixed‐effects model for repeated measures and showed no significant interactions of outcome and cell count or cell ratio over time. Therefore, no significant differences between graphs were observed. Significant interactions between outcome and cell count were observed for lymphocytes, T lymphocytes, and CD8+ T lymphocytes (p = 0.02, 0.02, and 0.04, respectively). For CD4+ T lymphocytes (p = 0.08) and B lymphocytes (p = 0.06), interactions between outcome and cell count failed to show significance. Figure 3 shows cell counts, cell ratios, and cell frequencies according to outcome over time in specific time intervals until day 20.
A comparative analysis of the mean values of all parameters collected over time showed significant differences between patients with positive and negative outcomes with respect to leucocytes (14 466 vs. 12 294 cells/μL), lymphocytes (1183 vs. 993 cells/μL), B lymphocytes (887 vs. 631 cells/μL), CD4+ lymphocytes (572 vs. 442 cells/μL), CD8 lymphocytes (294 vs. 172 cells/μL), NK cells (141 vs. 105 cells/μL), T4/T8 ratio (3 vs. 3.9) HLA‐DR+CD14+ monocytes (31.1 vs. 21.6%), and B/T8 ratio (0.93 vs. 1.16). We also found lower concentrations of IL 6 (624 vs. 1560 pg/mL), ferritin (1253 vs. 2295 μg/L), and CRP (125.6 vs. 197.6 g/dL) in the positive outcome group as well as higher platelet counts (262.9 vs. 178.4/nL). Table 4 provides a summary of pooled analyses of all parameters.
Table 4.
Pooled analysis of all measures over time from COVID+ ICU patients according to outcome
| ICU positive outcome | ICU negative outcome | p | |
|---|---|---|---|
| Patients (n) | 15 | 8 | |
| Leukocytes a | 14 466 | 12 293 | <0.01 |
| Lymphocytes a | 1183 | 993 | <0.01 |
| Lymphocytes (%) | 26.7 | 7.7 | 0.15 |
| B lymphocytes (%) | 14.4 | 16 | 0.74 |
| B lymphocytes a | 164 | 135 | <0.01 |
| T lymphocytes (%) | 72.8 | 70.5 | 0.625 |
| T lymphocytes a | 887 | 631 | <0.01 |
| Activated T lymphocytes (%) | 18.7 | 17.7 | 0.589 |
| CD4+ lymphocytes (%) | 49.6 | 51.3 | 0.340 |
| CD4+ lymphocytes a | 572 | 442 | <0.01 |
| CD8+ lymphocytes (%) | 21.4 | 17.5 | 0.01 |
| CD8+ lymphocytes a | 294 | 172 | <0.01 |
| NK cells (%) | 11.9 | 12.1 | 0.427 |
| NK‐cells a | 141 | 105 | <0.01 |
| T4/T8‐Ratio | 3.0 | 3.9 | 0.046 |
| Monocytes a | 668 | 637 | 0.694 |
| Monocytes (%) | 5.1 | 5.5 | 0.962 |
| HLA‐DR+CD14+ Monocytes (%) | 31.1 | 21.6 | 0.036 |
| Classical monocytes (%) | 83 | 80.8 | 0.310 |
| Intermediate monocytes (%) | 7.4 | 7.2 | 0.793 |
| Nonclassical monocytes (%) | 2.4 | 1.7 | 0.242 |
| C Reactive Protein (g/dL) | 125.6 | 197.6 | <0.01 |
| Thrombocytes a | 2 62 900 | 1 78 400 | <0.01 |
| Hamoglobin (g/dL) | 8.6 | 8.5 | 0.89 |
| Interleukin 6 (pg/mL) | 624 | 1560 | <0.01 |
| Ferritin (μg/L) | 1253 | 2295 | <0.01 |
| B/T8 | 0.93 | 1.16 | 0.045 |
Values shown are mean cells/μL.
Bold values represent statistic significant comparisons.
p values are not adjusted for multiple comparisons.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Discussion
The immune changes during COVID‐19 have been shown to play an important role, especially in severe disease. However, most studies conducted, thus, far have compared COVID‐19 patients with healthy subjects. To address this issue, we designed a prospective cohort study including four patient cohorts. We collected comprehensive immune cell data and serological parameters from all patient cohorts at the time of hospital admission. Data collection over time was only feasible for patients treated for severe COVID‐19 in the ICU since cohorts 2, 3, and 4 had considerably shorter times of hospitalization. Interestingly, two of the parameters that were only identified in patients with severe COVID‐19 during baseline measurements (elevated B/T8 ratio and low percentage of activated monocytes) were also found to correlate with poor prognosis in this patient cohort.
In our comparisons of baseline immunological parameters both ICU cohorts (ICU COVID+ and COVID‐) showed general lymphopenia as well as T cell and CD8+ T‐cell lymphopenia, with more severe changes observed in the ICU COVID+ cohort. CD4+ lymphopenia was only observed in the ICU COVID+ group. Even though some of these differences were not significant, these results are in line with recently published data [13, 14, 17, 18]. It is known that patients undergoing severe illness may develop nonviral lymphopenia, and studies have shown that for ICU patients persistent nonviral lymphopenia is an independent risk factor for 28‐day mortality [19, 20]. However, viral pathways and host response triggered by SARS‐CoV2 seem to induce a more specific and complex pattern of changes, which is supported by our data. The mechanisms involving immune dysfunction, especially of T lymphocytes, seem to be multifactorial and associated with a complex context of overstimulation, excessive migration, cell exhaustion, and simultaneous inhibition [4, 9, 14, 21–23]. Moreover, patients with severe disease seem to have higher levels of SARS‐CoV2‐specific CD4+ T lymphocytes [24, 25], as well as lymphocytes with reduced cytotoxic activity [26].
Even though the ICU COVID+ cohort presented with a marginally reduced absolute B‐lymphocyte count, relative counts showed higher B‐cell frequencies in comparison to all other groups, leading to the assumption of increased B‐cell activity. Similar findings have been reported by others, also demonstrating higher frequencies of B cells in patients with severe disease as compared to patients with milder disease [10]. Since in cohort 1, the CD8+ T lymphocytes were reduced to a greater extent compared with other cell populations, we performed an explorative analysis of the B/T8 ratio, which showed differences between the ICU COVID+ (cohort 1) and ICU COVID‐ (cohort 3) groups as well as between the groups ICU COVID+ (cohort 1) and NCU COVID+ (cohort 2), suggesting that higher ratios could not only predict a more severe course of disease, but also illustrate more precisely the immune changes caused by SARS‐CoV2. Interestingly, the pooled analysis of all measures over time in ICU COVID+ subgroups also showed a higher B/T8 ratio in the group with dismal outcome, which could help to understand the involvement of B cells in severe COVID‐19. Even though neutralizing antibodies play an important role in viral clearance and immunity, the overstimulation of B cells and the production of non‐neutralizing antibodies may lead to antibody‐dependent enhancement (ADE) worsening the infection [27, 28]. Moreover, non‐neutralizing antibodies can lead to formation of immune complexes, favoring complement activation and tissue damage [28]. Recent reports of patients undergoing B‐cell depleting therapies provide interesting insights into the roles of B cells in the disease. Even though a prolonged course has been reported, many patients developed only mild disease, despite their underlying hematological diseases [23, 29]. All our patients presented an inversion of the ratio (from >1 to <1) over time with a trend toward immune reconstitution, suggesting that immune dysfunction is more pronounced at early stages. In addition, the immunoglobulin profile in COVID patients could also be linked to overshooting immune response. In our study, we found statistically significant higher levels of IgG3 and IgG1 in patients with COVID compared to controls. Both IgG subclasses are known to be linked with intensification of inflammation with enhanced complement activation [26]. Even though we could not find differences between both COVID+ cohorts, these findings support the development of an intrinsic proinflammatory, complement‐activating immunoglobulin milieu and agree with recent published data [26]. Even though almost all patients in the ICU COVID+ cohort received immunomodulatory agents, mainly hydroxychloroquine, our results agree with most of the literature published, thus far, following the conclusion that these drugs probably did not influence the observed results. Hydroxychloroquine is known to have an immunomodulatory effect, rather than immunosuppressive and it is mainly prescribed in systemic lupus erythematosus, which is a complex autoimmune disease with predominance of autoantibodies [30]. Our findings support an important role of B lymphocytes in severe COVID‐19, which was found despite the use of hydroxychloroquine. Steroids were used in only a small percentage of patients and for short periods, rendering them unlikely to have influenced our results.
Previous work suggested that the cytokine environment in patients with severe disease stimulates intermediate monocytes (CD14+/CD16+) enhancing inflammation and tissue damage [15]. In our data, we could not demonstrate increased cell counts of intermediate monocytes. In contrast, intermediate monocytes and activated monocytes (HLA‐DR+/CD14+) were reduced in the ICU COVID+ cohort in comparison to the other groups when measured at baseline. Since many patients were referred to our hospital in an already critically‐ill condition, monocyte measurements were probably performed at a time point during the disease course when tissue damage was already established through migrated monocytes. Interestingly, activated monocytes increased significantly over time, which may favor the notion of “consumption” of these cells in the earlier stages of disease, which is in line with other recent published data [31]. In contrast to this, nonclassical monocytes (CD14‐, CD16+) were increased in the ICU COVID+ cohort with decreasing trend over time. Notably, the subgroup of ICU COVID+ patients with negative outcome had lower counts of monocytes at baseline compared to survivors, which might indicate tissue migration. When comparing activated monocytes over time, ICU COVID+ patients with favorable outcome showed higher counts than patients with negative outcome.
The limitations of our study include the small sample size, especially of the NCU COVID+ collective, which was due to the structure of care implemented in Germany, with our center receiving mainly complex and severe cases. For the same reason, our baseline measures at the day of hospital admission often do not correspond to the onset of symptoms, which could have provided important information about the immune changes at the early stages of the disease.
Notwithstanding these limitations, we were able to demonstrate that the immune changes induced by COVID‐19 follow a specific dynamic profile and even though some characteristics may be similar to severe disease in general, SARS‐CoV2 infection has distinct features. The T‐cell compartment is particularly affected and dramatic changes in CD8+ and CD4+ T lymphocytes might play a prognostic role. Moreover, monocytic subsets and the dynamics of these cells appear to correlate with outcome. Additionally, we present the B/T8 ratio as novel marker of immune changes in severe COVID‐19 and report a complement‐activating immunoglobulin profile in COVID patients. Considering this immune profile of COVID‐19, we suggest that monitoring the immune changes in T‐cell compartments, monocytes, the B/T8 ratio, as well as immunoglobulin subclasses could be a useful tool in monitoring the disease course. Our findings might potentially be helpful in the future decision‐making process of immune modulatory therapies, based on the specific level of immune dysfunction.
Materials and Methods
Study design and patients
We performed a prospective cohort study assessing the immune response in four patient cohorts admitted to the Saarland University Medical Center from March 2020 to July 2020. Patients were followed up until death or hospital discharge, or until the end of July 2020. One patient was still hospitalized by the time of final censoring and was counted as survivor. Cohorts 1 and 2 consisted of hospitalized patients diagnosed with SARS‐CoV‐2 infection, confirmed by reverse transcriptase polymerase chain reaction (RT‐PCR) from nasopharyngeal swabs, admitted to the ICU (cohort 1 = ICU COVID+) or NCU (cohort 2 = NCU COVID+), respectively.
Cohort 3 (ICU COVID‐) comprised hospitalized patients admitted to ICU from April 2020 until July 2020 without COVID‐19 infection, confirmed by negative RT‐PCR results in nasopharyngeal swabs. Diagnosis at admission was variable between patients and no specific inclusion criteria were used in this cohort. Cohort 4 (NCU COVID‐) included patients admitted to the NCU between April 2020 and July 2020 for reasons other than SARS‐CoV‐2. Cohorts 3 and 4 served as control groups.
Patients undergoing chemotherapy or with active hematological malignancies were excluded from the cohorts.
Patient characteristics included age, sex, presence of comorbidities, time of hospitalization, and number of patients in need of mechanical ventilation or extracorporeal membrane oxygenation (ECMO) at any point during the observation period. Acute respiratory distress syndrome (ARDS) was defined and classified according to the Berlin definition [32] with categorization into mild (PaO2/FiO2 200 to 300 mmHg), moderate (PaO2/FiO2 100 to 200 mmHg), and severe (PaO2/FiO2 less than 100 mmHg).
The study was part of the CORSAAR register study and was approved by the Ethics Committee of Saarland University, Homburg, Germany.
Immune characterization and laboratory parameters
Measurements of leucocytes, hemoglobin, and platelets were performed using a Sysmex XN‐L™ automated hematology analyzer.
Immune characterization of lymphocytes was performed by flowcytometry using a BD FACSCanto™ II cell analyzer. Flow cytometry was performed according to the “Guidelines for the use of flow cytometry and cell sorting in immunological studies” [33]. Monocyte subpopulations were characterized using a Beckman–Coulter Navios™ Analyzer. Total counts of lymphocytes and monocyte subpopulations were therefore calculated using a dual‐platform system. Tests were performed twice weekly until hospital discharge due to clinical improvement or death. Cellular parameters included lymphocytic differentiation into NK cells (CD56+, CD16+), B lymphocytes (CD19+), T lymphocytes (CD3+), CD4+ T lymphocytes (CD3+, CD4+), and CD8+ T lymphocytes (CD3+, CD8+), as well as differentiation of monocytes into the subsets classical, intermediate, and nonclassical (CD14+ CD16‐, CD14+ CD16+, CD14‐ CD16+). Of note, monocyte measurements were repeatedly interrupted throughout the study due to COVID‐19 related supply shortages of diagnostic antibodies resulting in gaps of monocyte data.
Cell ratios of B cells to CD8+ cells and of CD4+ to CD8+ T lymphocytes were calculated from total cell counts of each patient and are given as mean of all individual ratios.
Laboratory parameters included complete blood cell count, CRP, Ferritin, and IL‐6 and were also obtained twice weekly until hospital discharge due to clinical improvement or death. The level of immunoglobulins IgA, IgM, IgG, and IgG subclasses (1, 2, 3, and 4) were measured once at baseline.
Statistical analysis
Data were collected using Microsoft® Excel® 2016. Statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). Trends over time were analyzed using linear regression. Comparisons per outcome over time were analyzed using repeated measures mixed‐effect models. Nonparametric tests (Mann–Whitney U) were used to compare median ranks between groups, because most variables were not distributed normally. Comparisons between cohorts were performed using baseline values. Comparisons per outcome in the ICU COVID+ group (cohort 1) were performed at baseline and as pooled analysis of all individual values collected over time. For this, cohort 1 was divided into two groups of patients with positive outcome (discharge from hospital due to clinical improvement) and patients with negative outcome (death due to COVID‐19). Baseline was determined as the date of admission to NCU or ICU, which does not necessarily correspond to the onset of symptoms.
Conflict of Interest
Igor Age Kos, Benedikt Balensiefer, Vadim Lesan, Dominic Kaddu‐Mulindwa, Lorenz Thurner, Konstantinos Christofyllakis, Joerg Thomas Bittenbring, Manfred Ahlgrimm, Martina Seiffert, Stefan Wagenpfeil, Yvonne Bewarder, Frank Neumann, Torben Rixecker, Sigrun Smola, Andreas Link, Marcin Krawczyk, Frank Lammert, Phillipp M. Lepper, Robert Bals, Stephan Stilgenbauer, and Moritz Bewarder declare to have no conflict of interest.
Ethics
The study does not contain experiments using animals, however, it contains human studies. The project was approved by the local Ethical Committee (University of Saarland).
Patients consent
Patient information was obtained according to the authorization of the local Ethical Committee.
Author's contributions
Igor Age Kos, Benedikt Balensiefer, Vadim Lesan, Dominic Kaddu‐Mulindwa, Lorenz Thurner, Konstantinos Christofyllakis, Joerg Thomas Bittenbring, Manfred Ahlgrimm, Frank Neumann, Torben Rixecker, Stephan Stilgenbauer, and Moritz Bewarder performed the research and designed the study.
Martina Seiffert, Yvonne Bewarder, Frank Neumann, Torben Rixecker, Sigrun Smola, Andreas Link, Marcin Krawczyk, Frank Lammert, Phillipp M. Lepper, Robert Bals contributed essential tools or patient information.
Igor Age Kos, Vadim Lesan, Dominic Kaddu‐Mulindwa, Lorenz Thurner, Konstantinos Christofyllakis, Joerg Thomas Bittenbring, Manfred Ahlgrimm, Marcin Krawczyk, Frank Lammert, Phillipp M. Lepper, Robert Bals, Stephan Stilgenbauer, and Moritz Bewarder analysed the data.
Igor Age Kos, Benedikt Balensiefer, Vadim Lesan, Dominic Kaddu‐Mulindwa, Lorenz Thurner, Konstantinos Christofyllakis, Joerg Thomas Bittenbring, Manfred Ahlgrimm, Martina Seiffert, Stefan Wagenpfeil, Yvonne Bewarder, Frank Neumann, Torben Rixecker, Sigrun Smola, Andreas Link, Marcin Krawczyk, Frank Lammert, Phillipp M. Lepper, Robert Bals, Stephan Stilgenbauer, and Moritz Bewarder wrote, revised, and approved the article.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202049163
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CRP
C reactive protein
- COVID‐19
Corona Virus Disease 2019
- ECMO
extracorporeal membrane oxygenation
- ICU
intensive care unit
- NCU
normal care unit
- RT‐PCR
reverse transcriptase polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus type 2
- Th1
type 1 helper T cell
Supporting information
Supporting Information
Acknowledgments
This study was supported by grants of the Rolf M. Schwiete Stiftung, the Saarland University, and the State of Saarland (CORSAAR) to Robert Bals.
Open access funding enabled and organized by Projekt DEAL.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Vabret, N. , Britton, G. J. , Gruber, C. , Hegde, S. , Kim, J. , Kuksin, M. , Levantovsky, R. et al., Immunology of COVID‐19: current state of the science. Immunity. 2020. 52: 910–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tay, M. Z. , Poh, C. M. , Rénia, L. , MacAry, P. A. and Ng, L. F. P. , The trinity of COVID‐19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020. 20: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wan, S. , Yi, Q. , Fan, S. , Lv, J. , Zhang, X. , Guo, L. , Lang, C. et al., Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 2020. 10.1101/2020.02.10.20021832 [DOI] [Google Scholar]
- 4. Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Yi , Zhang, Li et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020.. 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diao, B. , Wang, C. , Tan, Y. , Chen, X. , Liu, Y. , Ning, L. , Chen, L. et al., Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Front. Immunol. 2020. 11: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang, D. , Hu, B. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , Wang, B. et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020. 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan, W.‐J. , Ni, Z.‐Y. , Hu, Y. , Liang, W.‐H. , Ou, C.‐Q. , He, J.‐X. , Liu, L. et al., Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020. 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He, R. , Lu, Z. , Zhang, L. , Fan, T. , Xiong, R. , Shen, X. , Feng, H. et al., The clinical course and its correlated immune status in COVID‐19 pneumonia. J. Clin. Virol. 2020. 127: 104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deng, Z. , Zhang, M. , Zhu, T. , Zhili, N. , Liu, Z. , Xiang, R. , Zhang, W. et al., Dynamic changes of peripheral blood lymphocytes subsets in adult patients with COVID‐19. Int. J. Infect. Dis. 2020. 98 : 353‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song, J.‐W. , Zhang, C. , Fan, X. , Meng, F.‐P. , Xu, Z. , Xia, P. , Cao, W.‐J. et al., Immunological and inflammatory profiles in mild and severe cases of COVID‐19. Nat. Commun. 2020. 11: 3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nie, S. , Zhao, X. , Zhao, K. , Zhang, Z. , Zhang, Z. and Zhang, Z. , Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID‐19): a retrospective study. medRxiv. 2020. 10.1101/2020.03.24.20042283 [DOI] [Google Scholar]
- 12. Zeng, Q. , Li, Y. , Huang, G. , Wu, W. , Dong, S. and Xu, Y. , Mortality of COVID‐19 is associated with cellular immune function compared to immune function in Chinese Han population. medRxiv. 2020. 10.1101/2020.03.08.20031229 [DOI] [Google Scholar]
- 13. Zheng, M. , Gao, Y. , Wang, G. , Song, G. , Liu, S. , Sun, D. , Xu, Y. et al., Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell. Mol. Immunol. 2020. 17: 533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu, B. , Fan, C.‐Y. , Wang, A.‐L. , Zou, Y.‐L. , Yu, Y.‐H. , He, C. , Xia, W.‐G. et al., Suppressed T cell‐mediated immunity in patients with COVID‐19: a clinical retrospective study in Wuhan, China. J. Infect. 2020. 81: e51–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou, Y. , Fu, B. , Zheng, X. , Wang, D. , Zhao, C. , Qi, Y. , Sun, R. et al. Pathogenic T‐cells and inflammatory monocytes incite inflammatory storms in severe COVID‐19 patients. Natnl. Sci. Rev. 2020. 7: 998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao, J. , Yuan, Q. , Wang, H. , Liu, W. , Liao, X. , Su, Y. , Wang, X. et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020. 71:2027‐2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ganji, A. , Farahani, I. , Khansarinejad, B. , Ghazavi, A. and Mosayebi, G. , Increased expression of CD8 marker on T‐cells in COVID‐19 patients. Blood Cells Mol. Dis. 2020.. 83: 102437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi, H. , Wang, W. , Yin, J. , Ouyang, Y. , Pang, L. , Feng, Y. , Qiao, L. et al., The inhibition of IL‐2/IL‐2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1‐STAT5 in critical patients with COVID‐19 pneumonia. Cell. Death Dis. 2020.11: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang, J. , Du, H. , Su, Y. , Li, X. , Zhang, J. , Chen, M. , Ren, G. et al., Nonviral infection‐related lymphocytopenia for the prediction of adult sepsis and its persistence indicates a higher mortality. Medicine (Baltimore). 2019. 98: e16535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adrie, C. , Lugosi, M. , Sonneville, R. , Souweine, B. , Ruckly, S. , Cartier, J.‐C. , Garrouste‐Orgeas, M. et al., Persistent lymphopenia is a risk factor for ICU‐acquired infections and for death in ICU patients with sustained hypotension at admission. Ann. Intensive Care. 2017. 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao, M. , Liu, Y. , Yuan, J. , Wen, Y. , Xu, G. , Zhao, J. , Chen, L. et al., The landscape of lung bronchoalveolar immune cells in COVID‐19 revealed by single‐cell RNA sequencing. medRxiv. 2020. 10.1101/2020.02.23.20026690. [DOI] [Google Scholar]
- 22. Wang, F. , Nie, J. , Wang, H. , Zhao, Q. , Xiong, Y. , Deng, L. , Song, S. et al., Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J. Infect. Dis. 2020. 221: 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kos, I. , Balensiefer, B. , Roth, S. , Ahlgrimm, M. , Sester, M. , Schmidt, T. , Thurner, L. et al., Prolonged course of COVID‐19‐associated pneumonia in a B‐cell depleted patient after rituximab. Front. Oncol. 2020. 10: 1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schub, D. , Klemis, V. , Schneitler, S. , Mihm, J. , Lepper, P. , Wilkens, H. , Bals, R. et al. High levels of SARS‐CoV‐2 specific T‐cells with restricted functionality in patients with severe course of COVID‐19. JCI Insight. 2020.. 5: e142167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mazzoni, A. , Maggi, L. , Capone, M. , Spinicci, M. , Salvati, L. , Colao, M. G. , Vanni, A. et al., Cell‐mediated and humoral adaptive immune responses to SARS‐CoV‐2 are lower in asymptomatic than symptomatic COVID‐19 patients. Eur. J. Immunol. 2020. 50: 2013–2024. [DOI] [PubMed] [Google Scholar]
- 26. Mazzoni, A. , Salvati, L. , Maggi, L. , Capone, M. , Vanni, A. , Spinicci, M. , Mencarini, J. et al. Impaired immune cell cytotoxicity in severe COVID‐19 is IL‐6 dependent. J. Clin. Invest. 2020. 130: 4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tetro, J. A. , Is COVID‐19 receiving ADE from other coronaviruses? Microbes Infect. 2020. 22: 72–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee, W. S. , Wheatley, A. K. , Kent, S. J. and Dekosky, B. J. , Antibody‐dependent enhancement and SARS‐CoV‐2 vaccines and therapies. Nat. Microbiol. 2020. 5: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 29. Treon, S. P. , Castillo, J. J. , Skarbnik, A. P. , Soumerai, J. D. , Ghobrial, I. M. , Guerrera, M. L. , Meid, K. et al., The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID‐19 infected patients. Blood. 2020. 135: 1912–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peruzzi, B. , Bencini, S. , Capone, M. , Mazzoni, A. , Maggi, L. , Salvati, L. , Vanni, A. et al., Quantitative and qualitative alterations of circulating myeloid cells and plasmacytoid DC in SARS‐CoV‐2 infection. Immunology. 2020. 161: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ranieri, V. M. , Rubenfeld, G. D. , Thompson, B. T. , Ferguson, N. D. , Caldwell, E. , Fan, E. , Camporota, L. et al., Acute respiratory distress syndrome: the Berlin definition. J. Am. Med. Assoc. 2012. 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 32. Cossarizza, A. , Chang, H.‐D. , Radbruch, A. , Acs, A. , Adam, D. , Adam‐Klages, S. , Agace, W. W. et al., Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019. 49: 1457–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


