Abstract
Aims
The role of renin‐angiotensin‐aldosterone system (RAAS) blockers on the course of coronavirus disease 2019 (COVID‐19) is debated. We assessed the association between chronic use of RAAS blockers and mortality among inpatients with COVID‐19 and explored reasons for discrepancies in the literature.
Methods and results
We included adult hypertensive patients from a prospective nationwide cohort of 3512 inpatients with COVID‐19 up to June 30, 2020. Cox proportional hazard models with various adjustment or propensity weighting methods were used to estimate the hazard ratios (HR) of 30‐day mortality for chronic users versus non‐users of RAAS blockers. We analyzed data of 1160 hypertensive patients: 719 (62%) were male and 777 (67%) were older than 65 years. The main comorbidities were diabetes (n = 416, 36%), chronic cardiac disease (n = 401, 35%), and obesity (n = 340, 29%); 705 (61%) received oxygen therapy. We recorded 135 (11.6%) deaths within 30 days of diagnosis. We found no association between chronic use of RAAS blockers and mortality (unadjusted HR = 1.13, 95% CI [0.8–1.6]; propensity inverse probability treatment weighted HR = 1.09 [0.86‐1.39]; propensity standardized mortality ratio weighted HR = 1.08 [0.79–1.47]). Our comprehensive review of previous studies highlighted that significant associations were mostly found in unrestricted populations with inappropriate adjustment, or with biased in‐hospital exposure measurement.
Conclusion
Our results do not support previous concerns regarding these drugs, nor a potential protective effect as reported in previous poorly designed studies and meta‐analyses. RAAS blockers should not be discontinued during the pandemic, while in‐hospital management of these drugs will be clarified by randomized trials. NCT04262921.
Keywords: angiotensin antagonists, COVID‐19, hypertension, mortality, propensity score, RAAS blockers
Abbreviations
- ACE
angiotensin‐converting enzyme
- ACE2
angiotensin‐converting enzyme 2
- ARBs
angiotensin receptor blockers
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- MICE
multiple imputation by chain equations
- RAAS
renin‐angiotensin‐aldosterone system
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The potential influence of renin‐angiotensin‐aldosterone system (RAAS) blockers on the course of coronavirus disease 2019 (COVID‐19) has been a matter of controversy.
Early in the pandemic, it has been suggested that cardiovascular comorbidities such as hypertension, diabetes, and coronary heart disease were risk factors for severe COVID‐19 [1, 2, 3] with the potential explanation that these conditions are frequently treated with RAAS blockers. The underlying rationale came from animal studies showing an increased expression of angiotensin‐converting enzyme 2 (ACE2), the receptor for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), in the presence of angiotensin‐converting enzyme (ACE) inhibitors and/or angiotensin receptor blockers (ARBs) [4, 5]. However, other animal[6] and human [7] studies did not confirm these findings, and more importantly, there are no data demonstrating an increased expression of the transmembrane ACE2 protein in the lungs [8].
Conversely, robust animal data showed that ACE2 might be beneficial in the context of acute lung injury [9, 10]. Indeed, ACE2 converts angiotensin II to angiotensin 1–7, a peptide with vasodilating, antifibrotic, and anti‐inflammatory properties, opposite to those of angiotensin II. Therefore, modulating ACE‐angiotensin II and ACE2‐angiotensin (1–7) pathways using RAAS blockers might actually be beneficial in patients with COVID‐19 [11], especially as ACE2 might be downregulated during SARS‐CoV‐2 infection [8, 10].
While several clinical trials are ongoing to establish whether RAAS blockers should be maintained, discontinued, or even introduced de novo in patients with COVID‐19, a number of observational studies have attempted to establish the association between chronic use of RAAS blockers and the risk of contracting the infection and/or developing a severe or lethal form of COVID‐19. Several large studies consistently ruled out a significant association between the chronic use of RAAS blockers and the risk of a positive test for SARS‐CoV‐2 [12, 13, 14]. Conversely, studies on the association between exposure to RAAS blockers and severity of the disease have yielded discrepant results, largely explained by disparities in study design, selected populations, study size, exposure measurement, and adjustment methodologies—if any [14, 15, 16, 17, 18, 19, 20, 21, 22, 23].
The aim of this pharmacoepidemiologic study was to analyze the association between the chronic use of RAAS blockers and mortality of COVID‐19 in a large national multicenter prospective cohort of hospitalized patients in France.
2. METHODS
The French COVID Cohort (ClinicalTrials NCT04262921) of inpatients with RT‐PCR virologically confirmed COVID‐19 was set up at the end of January 2020 in 198 centers in France [24]. Patients were included at admission and followed daily during hospital stay, and at 1, 3 and 6 months after discharge. The study was sponsored by INSERM (the French national institute for health and medical research). As at end of June 2020, 3512 patients admitted to hospital with COVID‐19 were included in the cohort. We analyzed data from adult patients with a history of hypertension. The diagnosis of hypertension was gathered from the medical history based on the medical interview at admission, independently of blood pressure measurement at admission, and of the use of antihypertensive medication. In patients with hypertension, chronic treatment with RAAS blockers was collected. Patients with missing data regarding treatment prior to admission and pregnant women were excluded from the present analyses (Figure 1). Exposure was defined as the chronic use of RAAS blockers (i.e., either ACE inhibitors or ARBs reported as part the chronic treatment at inclusion in the cohort). Patients reporting neither ACE inhibitors nor ARBs were considered as non‐users. The outcome was death within 30 days of diagnosis.
FIGURE 1.
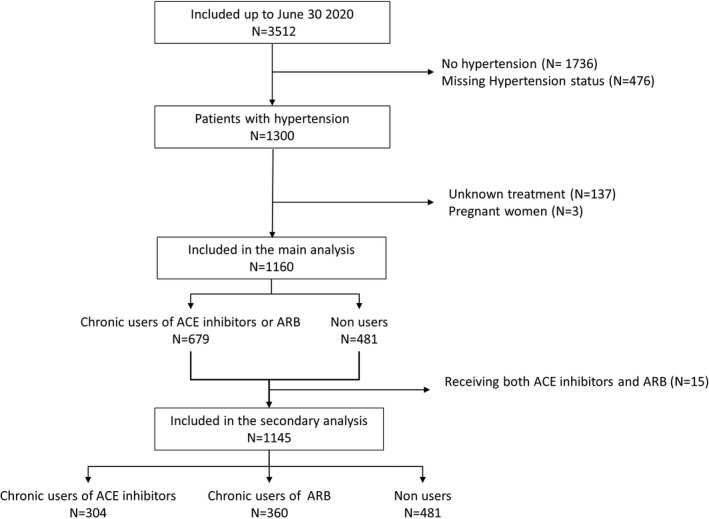
Flowchart of patients’ selection from the French COVID Cohort
2.1. Statistical analyses
Baseline characteristics were reported according to the chronic use of any RAAS blocker. Propensity score for the use of RAAS blockers was calculated by logistic regressions performed on five imputed datasets. Multiple imputations by chain equations (MICE) were performed for baseline characteristic with less than 20% missing values. Propensity score included the following variables collected at admission and assumed to be associated with the outcome: age, gender, healthcare worker, microbiology laboratory worker, geographical region of inclusion, chronic cardiac disease, chronic pulmonary disease, chronic kidney disease, immunosuppressive therapy, immunosuppressive disease, chronic neurological disorder including dementia, obesity, diabetes, malnutrition, and number of days with symptoms before positive RT‐PCR. Overlapping of propensity score distributions among users and non‐users of RAAS blockers was checked graphically. The propensity score distribution among treated and non‐treated patients showed good overlap (Figure S1). After weighting, no more imbalances are observed (Figure S2).
Hazard ratios of mortality for patients with chronic use of RAAS blockers versus non‐use and their 95% confidence interval (CI) were estimated using Cox proportional hazard models with adjustment and two types of weighting (inverse probability of treatment and standardized mortality ratio), in order to control for confounding.
In secondary analyses, patients receiving both ACE inhibitors and ARBs were excluded, and ACE inhibitors and ARBs were analyzed separately.
A sensitivity analysis included additional variables in the propensity score, which reflected severity at admission, and may be intermediaries in the causal diagram leading to overfitting: symptoms at admission (respiratory rate ≥30/min; systolic blood pressure <90 mm Hg or diastolic blood pressure ≤60 mm Hg), need for oxygen therapy within 2 days after admission, and level of C‐reactive protein at admission.
All P‐values are two‐sided. Statistically significant level was predefined at 5%. Detailed methods are described in the Methods S1.
2.2. Comprehensive review of the literature
We performed a review of observational studies and meta‐analyses—excluding preprints—on the association between RAAS blockers and outcome of COVID‐19 published until November 2020. We systematically searched PubMed with no language restriction and starting from January 2020, using the terms “Renin‐angiotensin‐(aldosterone)‐system,” “angiotensin converting enzyme 2,” “angiotensin converting enzyme inhibitors,” ”angiotensin receptor blockers,” “coronary artery disease” or their acronyms, and synonyms or various combinations of those words to identify systematic reviews, observational studies, trials, and meta‐analyses describing the relationship between RAAS blockers and outcome in COVID‐19. We also screened cited reports from the full texts of original articles for other relevant research, and all individual studies from reviews and meta‐analyses were analyzed for potential relevance. We screened reports by title and abstract, and title and full text in letters, to identify articles relevant for the study aim. Two authors independently extracted structured information on design, setting, population, exposure, outcomes, statistical methods, results, and conclusion of the authors.
3. RESULTS
Between February 28 and June 30, 2020, 3512 patients were included in the French COVID Cohort, of whom 1160 hypertensive patients were analyzed (Figure 1). Median age was 70 years (interquartile range 61;78), 62% (n = 719) were male. The most common comorbidities, besides hypertension, were diabetes (n = 416, 36%), chronic cardiac disease (n = 401, 35%), and obesity (n = 340, 29%); 61% (n = 705) received oxygen therapy within 2 days of admission (Table 1). Detailed laboratory results are reported in Table S1. Median follow‐up was 57 (95% CI [46‐77]) days, and 135 (11.6%) patients died within 30 days of diagnosis.
TABLE 1.
Patients’ characteristics at admission in the total population and in users versus non‐users of RAAS blockers
|
Total N = 1160 |
Non‐users N = 481 |
Chronic users N = 679 |
|
|---|---|---|---|
| Age (years) | 70 [61:78] | 70 [60:80] | 70 [61:77] |
| Older than 65 years | 777 (67) | 314 (65.3) | 463 (68.2) |
| Male | 719 (62) | 279 (58) | 440 (64.8) |
| Healthcare worker (39 imputed data for missing values) | 59 (5.1) | 30 (6.2) | 28 (4.2) |
| Microbiology laboratory worker (40 imputed data for missing values) | 6 (0.6) | 3 (0.7) | 3 (0.4) |
| French region of hospitalization | |||
| East | 98 (8.4) | 33 (6.9) | 65 (9.6) |
| North | 117 (10.1) | 47 (9.8) | 70 (10.3) |
| Other | 610 (52.5) | 262 (54.5) | 348 (51.3) |
| Parisian area | 335 (28.9) | 139 (28.9) | 196 (28.9) |
| Smoking status (150 imputed data for missing values) | |||
| Current smoker | 65 (5.6) | 32 (6.74) | 33 (4.8) |
| Former smoker | 383 (33.03) | 136 (28.36) | 247 (36.35) |
| Never smoked | 712 (61.36) | 312 (64.91) | 400 (58.85) |
| Performance status a (293 missing values) | |||
| 0 | 423 (48.79) | 157 (44.86) | 266 (51.45) |
| 1 | 226 (26.07) | 89 (25.43) | 137 (26.5) |
| 2 | 98 (11.3) | 47 (13.43) | 51 (9.86) |
| 3 | 73 (8.42) | 34 (9.71) | 39 (7.54) |
| 4 | 47 (5.42) | 23 (6.57) | 24 (4.64) |
| Obesity (28 imputed data for missing values) | 340 (29.28) | 126 (26.15) | 214 (31.55) |
| Diabetes (1 imputed data for missing value) | 416 (35.9) | 152 (31.68) | 264 (38.88) |
| Chronic cardiac disease (4 imputed data for missing values) | 401 (34.6) | 158 (32.8) | 243 (35.7) |
| Chronic pulmonary disease or asthma (3 imputed data for missing values) | 231 (19.9) | 96 (20) | 135 (19.9) |
| Chronic kidney disease (4 imputed data for missing values) | 199 (17.2) | 83 (17.3) | 115 (17) |
| Malnutrition (19 imputed data for missing values) | 36 (3.1) | 17 (3663) | 19 (2.8) |
| Chronic neurological disorder or dementia (2 imputed data for missing values) | 120 (10.3) | 62 (12.8) | 58 (8.5) |
| Immunosuppressive disease | 305 (26.29) | 144 (29.94) | 161 (23.71) |
| Immunosuppressive therapy (23 imputed data for missing values) | 78 (6.81) | 45 (9.56) | 33 (4.86) |
| Time from first symptoms to positive RT‐PCR, median [Q1:Q3] (46 imputed data for missing values) | 6 [3:9] | 6 [2:9] | 6 [3:10] |
| Anosmia (260 missing values) | 94 (10.44) | 33 (8.8) | 61 (11.62) |
| Agueusia (259 missing values) | 115 (12.76) | 40 (10.61) | 75 (14.31) |
| Oxygen therapy (116 imputed data for missing values) | 705 (60.76) | 276 (57.38) | 429 (63.15) |
| eGFR† (ml/min/1.73 m2), median [Q1:Q3] (646 missing values) | 78.8 [53.57:91.71] | 80.17 [57.18:91.67] | 77.72 [48.93:91.83] |
| Creatinine (µmol/L), median [Q1:Q3] (417 missing values) | 84 [67:112] | 82.5 [65.88:110.55] | 86 [69.2:114.2] |
| C‐reactive protein (mg/L), median [Q1:Q3] (352 missing values) | 77.5 [32.98:135.18] | 82.6 [31:138] | 74.3 [36.6:132] |
Abbreviations: Q1: first quartile; Q3: third quartile; SD, standard deviation.
Performance status was collected according to the Eastern Cooperative Oncology Group (ECOG) [53].
According to the Chronic Kidney Disease EPIdemiology collaboration [54]. Data are expressed with N (%) unless otherwise specified.
We found no significant association between chronic use of RAAS blockers and mortality, with any of the adjustment methods. Consistent results were found for the risk of mortality with the chronic use of ACE inhibitors or ARBs analyzed separately (Table 2), as well as in the sensitivity analyses (Table S3).
TABLE 2.
Hazard ratios of 30‐day mortality in users versus non‐users of RAAS blockers
| HR [95% CI] | p‐value | |
|---|---|---|
| Chronic users of ACE inhibitors or ARBs versus non‐users | ||
| Unadjusted | 1.13 [0.8–1.6] | 0.48 |
| Adjusted a | 1.07 [0.75–1.52] | 0.71 |
| IPT weighted | 1.09 [0.86–1.39] | 0.46 |
| SMR weighted | 1.08 [0.79–1.47] | 0.65 |
| Chronic users of ACE inhibitors versus non‐users | ||
| Unadjusted | 1.15 [0.76–1.76] | 0.50 |
| Adjusted a | 1.04 [0.68–1.6] | 0.85 |
| IPT weighted | 1.06 [0.79–1.42] | 0.72 |
| SMR weighted | 1.14 [0.71–1.82] | 0.58 |
| Chronic users of ARBs versus non‐users | ||
| Unadjusted | 1.14 [0.76–1.7] | 0.53 |
| Adjusted a | 1.13 [0.75–1.69] | 0.55 |
| IPT weighted | 1.12 [0.85–1.48] | 0.43 |
| SMR weighted | 1.07 [0.7–1.64] | 0.75 |
Adjusted for age, gender, diabetes, obesity, chronic heart disease, renal failure, region of inclusion.
Our comprehensive review of the literature included 65 published studies, of which 11 were meta‐analyses (Figures S3 and Table S4). Among the 56 individual studies (two of which combined an individual study and a meta‐analysis [25, 26]), 12 included both in‐ and outpatients and 44 included only inpatients. Among the 44 inpatients studies, 30 assessed the effect of chronic use and 12 the effect of in‐hospital use, 3 of which assessed both chronic and in‐hospital use [27, 28, 29]. Timing of exposure measurement was uncertain in five of 44. Only 34 of 56 individual studies reported results among hypertensive patients. A significant (deleterious) association between RAAS blockers and prognosis of COVID‐19 was found in most studies conducted in unselected populations before or after insufficient adjustment, but not among hypertensive patients or in properly adjusted studies. Conversely, a significant association in favor of a seemingly protective effect of treatment was found in most studies based on in‐hospital use of treatment.
4. DISCUSSION
In this multicenter hospital‐based cohort of 1160 hypertensive patients with COVID‐19, using various adjustment strategies to reduce bias due to potential confounders, chronic use of RAAS blockers was not associated with an increased risk of mortality.
Previous studies conducted among inpatients with hypertension, although smaller‐scaled, had mostly yielded similar conclusions [14, 15, 20, 30, 31, 32]. Most large studies conducted in both outpatients and inpatients with hypertension were also in line with our results [14, 31, 33], except for one study reporting a protective effect of RAAS blockers among outpatients [34].
Of note, in our study, careful adjustment for comorbidities and age had no major impact on the results since even unadjusted analyses did not show a significant association between exposure to RAAS blockers and mortality. This is in line with previous studies conducted in patients with hypertension (Table S4). Indeed, RAAS blockers are among first‐line antihypertensive therapies [35], so that patients receiving RAAS blockers do not drastically differ from other hypertensive patients with regard to baseline characteristics. Conversely, analyses conducted in unrestricted populations showed an increased unadjusted risk for a severe or fatal outcome of the disease in patients chronically treated with RAAS blockers [12, 15, 19, 22, 36]. However, this increased risk was systematically ironed out when proper adjustment was performed [12, 15, 19, 20]. In unrestricted populations, patients receiving RAAS blockers had more comorbidities such as hypertension, diabetes, and chronic kidney disease and were older than patients without these medications, so that baseline characteristics between users and non‐users markedly differed. This important methodological consideration is best illustrated by studies that reported analyses both in the total population (not restricted to hypertensive patients) and in the subgroup of patients with hypertension. For instance, in a nationwide population‐based cohort study using the Korean Health Insurance Review and Assessment including 5179 confirmed COVID‐19 cases among whom 1157 had hypertension, the unadjusted OR for mortality in users was 3.88 (95% CI 2.48‐6.05) in the total population and 0.74 (0.43‐1.28) in hypertensive patients, whereas these ORs were 0.88 (0.53–1.44) and 0.71 (0.40‐1.26) after adjustment [20].
The setting of the study—unrestricted population versus patients with an indication for RAAS blockers (hypertension in the vast majority of the studies)—is therefore crucial to take into account when interpreting the results. In our review, several meta‐analyses pooled studies including total populations and hypertensive patients [37, 38], which call their conclusions into question. In emulated trials, harmonization of selection criteria is one of the major recommendations when attempting to estimate the causal effect of treatments [39].
In our study, as in many previous studies, the selection of hospitalized patients may generate a collider bias, whereby patients with RAAS blockers may be admitted for less severe disease and hence have better outcomes [28, 40, 41, 42]. This bias is much more likely to occur in even more specific settings (such as intensive care unit admission) [43], or in studies conducted in unrestricted populations (i.e., with no indication for RAAS blockers), due to a larger baseline imbalance, as explained above. Still, to account for potential different severity at inclusion between groups of exposure, we performed a separate adjustment for baseline severity, which did not modify the results. This sensitivity analysis has been separated from the main adjustment model because baseline severity may actually lie on the causal pathway between exposure and outcome, in which case, taking it into account would lead to overfitting.
In contrast with the studies mentioned above, others have shown results in favor of a protective effect of RAAS blockers, giving the impression of overall discrepant results. However, careful analysis of study designs showed that the vast majority of these studies did not rely in chronic exposure to treatment, before diagnosis, but rather on “in‐hospital” use [23, 44] or, very similarly, on chronic use continued in‐hospital or after diagnosis [27, 45, 46]. Such study design generates a major bias because RAAS blockers are continued in patients with less severe disease and discontinued in patients with the most severe forms of the disease (for reasons such as hypotension, acute kidney injury, or ICU admission). A combination of reverse causality and immortal time bias, or the so‐called “healthy user‐sick stopper” bias, therefore explains this seemingly protective effect [28].
We have outlined how meta‐analyses have incorporated data from different populations, with unadjusted results in unselected patients weighting toward an increased risk associated with RAAS blockers. Conversely, and probably even worse with regard to methodological considerations, most meta‐analyses [37, 38, 47, 48, 49, 50] have pooled studies based on chronic exposure together with studies based on in‐hospital exposure. This erroneous study selection led a number of meta‐analysis to conclude in favor of a beneficial effect of RAAS blockers[38, 47, 48, 50]. Further meta‐analyses should take into account study populations and design as well as classification of exposure before pooling the results.
Altogether, our results, combined with a comprehensive analysis of previous studies, allow concluding that chronic use of RAAS blockers is not associated with outcome of COVID‐19.
As of November 2020, seven randomized clinical trials (e.g., REPLACE‐COVID in the United States, NCT04338009, BRACE CORONA in Argentina, NCT04364893, or ACORES‐2 in France, NCT04329195) have been designed to study whether these drugs should be continued or discontinued upon hospital admission in chronically treated patients. The recently published results of the REPLACE‐COVID and BRACE CORONA trials did not support discontinuing treatment with RAAS blockers in patients with COVID‐19 admitted to hospital [51, 52].
Conversely, based on solid experimental evidence mostly published after the SARS‐CoV‐1 outbreak [9, 10, 11], other authors have argued that antagonizing the RAAS may actually be beneficial in patients with COVID‐19 and that these drugs should not only be continued, but maybe even introduced do novo in previously untreated SARS‐CoV‐2 patients. As of November 2020, 21 trials have been designed to randomize patients with COVID‐19, to receive an ARB or a comparator (e.g., CLARITY in Australia, NCT04394117, COVID‐Aging, NCT04359953, and COVERAGE NCT04356495, in France, or STAR‐COVID in Mexico, NCT04510662). Results of these randomized trials will be crucial to help clinicians managing ARBs and ACE inhibitors after hospital admission.
Strengths of our study include its size and multicenter design. In addition, the French COVID Cohort was implemented very early in the pandemic, so that the entire “first wave” period is reflected in the study, whereas previous studies were very often restricted to a shorter period of observation. Overall, our results reflect real‐word data on the association between chronic use of RAAS blockers and outcome of the disease in patients with hypertension admitted for COVID‐19 and are not biased by inappropriate measurement of exposure and have therefore an important impact for patient care.
Our study has some limitations. First, hypertension was collected from the medical interview of the patient, so that misclassification may have occurred. However, we have no reason to believe a potential imprecision in the diagnosis of hypertension would introduce a major bias in our analysis. Importantly, blood pressure measurement at admission was not included in the definition of hypertension as we believe this stressful context may have induced elevated values in otherwise normotensive patients. Another limitation of the study is that the use of ACE inhibitors and ARBs was only collected in patients with a history of hypertension, so that their potential influence on outcome in patients with other indications for these treatments could not be assessed. However, as previously shown in several studies, most of the patients with an indication for RAAS blockers have hypertension. Moreover, blood pressure‐lowering treatments other that ACEIs and ARBs were not collected, so that the association between other antihypertensive treatments, such as mineralocorticoid antagonists, and outcome could not be analyzed. In addition, as in all previous studies based on chronic prescription of treatment, real adherence to treatment could not be ascertained. Furthermore, our sample is far from exhaustive in France, but we ensured a nationwide coverage of centers and provide a far more representative sample than a single hospital or regional database. Finally, as our cohort did not include outpatients, the association between treatment and outcome was only assessed in the most severe patients, requiring hospital admission. Such a selection of the population may alter the association between the exposure to RAAS blockers and outcome.
In conclusion, our analysis conducted in a multicenter prospective French cohort of patients hospitalized with COVID‐19 found no significant association between chronic use of RAAS blockers and mortality of COVID‐19 in hypertensive patients. These results, combined with a comprehensive review of all related studies published up to the end of November 2020 enabling us to provide epidemiological explanations for seemingly discrepant results, provide solid data to support that these treatments should not be discontinued during the pandemic.
ETHICS APPROVAL, PATIENT CONSENT
The research complies with the Declaration of Helsinki. The study protocol was approved by the French Ethics Committee (CPP‐Ile‐de‐France VI, ID RCB: 2020‐A00256‐33), and we obtained the consent of each participant or its surrogate.
CONFLICT OF INTERESTS
MR received travel funding from Pfizer, outside the submitted work. APHP, which employs JSH, has received research grants from Bioserenity, Sanofi, Servier, and Novo Nordisk. JSH received speaker, advisory board, or consultancy fees from Amgen, Astra Zeneca, Bayer, Bristol‐Myers Squibb, Novartis, Novo Nordisk, WeHealth, outside the submitted work. BL reports travel funding from ViiV (2019) and Gilead (2020), outside the submitted work. PR reports personal fees (consulting) from Idorsia and G3P and honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Fresenius, Grunenthal, Novartis, Novo Nordisk, Sequana medical, Servier, Stealth Peptides, Ablative Solutions, Corvidia, Relypsa, Vifor, and Vifor Fresenius Medical Care Renal Pharma; outside the submitted work. PR is the cofounder of CardioRenal (outside the submitted work). DC reports HIV grants from Janssen (2017‐2018, 2019‐2020) and MSD France (2015‐2017), personal fees from Janssen (2018), MSD France (2017) and Gilead (2018, 2020) for lectures on HIV, and personal fees from Merck Switzerland (2017) for consultancy on multiple sclerosis, outside the submitted work. EVP received fees and travel funding from Servier, outside the submitted work.
AUTHOR CONTRIBUTIONS
NG, MEF, MR, JI, AC, EP, CL, PR, DC, and EVP contributed to the conception or design of the work. All authors contributed to the acquisition, analysis, or interpretation of data for the work. NG, MEF, MR, JI, EP, JSH, CL, PR, DC, and EVP drafted the manuscript. All authors critically revised the manuscript and gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
The authors thank the scientific advisory board: Dominique Costagliola, Patrick Yeni, Astrid Vabret, Hervé Raoul, and Laurence Weiss.
THE FRENCH COVID COHORT STUDY GROUP (SCIENTIFIC, EXECUTIVE AND STEERING COMMITTEES)
| Name | Last name | Mail address @ | Affiliation |
|---|---|---|---|
| Marie | BARTOLI | marie.bartoli@anrs.fr | ANRS, Paris, France |
| Alpha | DIALLO | alpha.diallo@inserm.fr | ANRS, Paris, France |
| Soizic | LE MESTRE | soizic.le mestre@inserm.fr | ANRS, Paris, France |
| Noémie | MERCIER | noemie.mercier@inserm.fr | ANRS, Paris, France |
| Christelle | PAUL | christelle.paul@inserm.fr | ANRS, Paris, France |
| Ventzislava | PETROV‐SANCHEZ | ventzislava.petrov-sanchez@anrs.fr | ANRS, Paris, France |
| Catherine | CHIROUZE | catherine.chirouze@univ-fcomte.fr | CHRU Jean Minjoz, Besançon, France |
| Claire | ANDREJAK | andrejak.claire@chu-amiens.fr | CHU Amiens, France |
| Denis | MALVY | denis.malvy@chu-bordeaux.fr | CHU Bordeaux, France |
| François | DUBOS | Francois.DUBOS@CHRU-LILLE.FR | CHU Lille, France |
| Patrick | ROSSIGNOL | p.rossignol@chru-nancy.fr | CHU Nancy, France |
| Manuel | ETIENNE | Manuel.Etienne@chu-rouen.fr | CHU Rouen, France |
| François | BOMPART | fbompart@dndi.org | Drugs for Neglected Diseases initiative, Geneva, Switzerland |
| Tristan | GIGANTE | T.GIGANTE@chru-nancy.fr | F‐CRIN INI‐CRCT, Nancy, France |
| Morgane | GILG | M.GILG@chru-nancy.fr | F‐CRIN INI‐CRCT, Nancy, France |
| Bénédicte | ROSSIGNOL | B.ROSSIGNOL@chru-nancy.fr | F‐CRIN INI‐CRCT, Nancy, France |
| Claire | LEVY‐MARCHAL | claire.levy-marchal@inserm.fr | F‐CRIN INI‐CRCT, Paris, France |
| Marine | BELUZE | marine.beluze@aphp.fr | F‐CRIN Partners Platform, Paris, France |
| Delphine | BACHELET | delphine.bachelet@aphp.fr | Hôpital Bichat, Paris, France |
| Krishna | BHAVSAR | krishna.bhavsar@aphp.fr | Hôpital Bichat, Paris, France |
| Lila | BOUADMA | lila.bouadma@aphp.fr | Hôpital Bichat, Paris, France |
| Anissa | CHAIR | anissa.chair@aphp.fr | Hôpital Bichat, Paris, France |
| Camille | COUFFIGNAL | camille.couffignal@aphp.fr | Hôpital Bichat, Paris, France |
| Charlene | DA SILVEIRA | charlene.dasilveira@aphp.fr | Hôpital Bichat, Paris, France |
| Marie‐Pierre | DEBRAY | marie-pierre.debray@aphp.fr | Hôpital Bichat, Paris, France |
| Diane | DESCAMPS | diane.descamps@aphp.fr | Hôpital Bichat, Paris, France |
| Xavier | DUVAL | xavier.duval@aphp.fr | Hôpital Bichat, Paris, France |
| Philippine | ELOY | philippine.eloy@aphp.fr | Hôpital Bichat, Paris, France |
| Marina | ESPOSITO‐FARESE | marina.esposito-farese@aphp.fr | Hôpital Bichat, Paris, France |
| Nadia | ETTALHAOUI | nadia.ettalhaoui@aphp.fr | Hôpital Bichat, Paris, France |
| Nathalie | GAULT | nathalie.gault@aphp.fr | Hôpital Bichat, Paris, France |
| Jade | GHOSN | jade.ghosn@aphp.fr | Hôpital Bichat, Paris, France |
| Isabelle | GORENNE | isabelle.gorenne@aphp.fr | Hôpital Bichat, Paris, France |
| Isabelle | HOFFMANN | isabelle.hoffmann@aphp.fr | Hôpital Bichat, Paris, France |
| Ouifiya | KAFIF | ouifiya.kafif@aphp.fr | Hôpital Bichat, Paris, France |
| Sabrina | KALI | sabrina.kali@aphp.fr | Hôpital Bichat, Paris, France |
| Antoine | KHALIL | antoine.khalil@aphp.fr | Hôpital Bichat, Paris, France |
| Cédric | LAOUÉNAN | cedric.laouenan@aphp.fr | Hôpital Bichat, Paris, France |
| Samira | LARIBI | samira.laribi@aphp.fr | Hôpital Bichat, Paris, France |
| Minh | LE | minh.le@aphp.fr | Hôpital Bichat, Paris, France |
| Quentin | LE HINGRAT | quentin.lehingrat@aphp.fr | Hôpital Bichat, Paris, France |
| François‐Xavier | LESCURE | xavier.lescure@aphp.fr | Hôpital Bichat, Paris, France |
| Jean Christophe | LUCET | jean-christophe.lucet@aphp.fr | Hôpital Bichat, Paris, France |
| France | MENTRÉ | france.mentre@inserm.fr | Hôpital Bichat, Paris, France |
| Jimmy | Mullaert | jimmy.mullaert@inserm.fr | Hôpital Bichat, Paris, France |
| Nathan | PEIFFER‐SMADJA | nathan.peiffer-smadja@inserm.fr | Hôpital Bichat, Paris, France |
| Gilles | PEYTAVIN | gilles.peytavin@aphp.fr | Hôpital Bichat, Paris, France |
| Carine | ROY | carine.roy@aphp.fr | Hôpital Bichat, Paris, France |
| Marion | SCHNEIDER | marion.schneider2@aphp.fr | Hôpital Bichat, Paris, France |
| Nassima | SI MOHAMMED | nassima.simohammed@aphp.fr | Hôpital Bichat, Paris, France |
| Lysa | TAGHERSET | lysa.tagherset@aphp.fr | Hôpital Bichat, Paris, France |
| Coralie | TARDIVON | coralie.tardivon@aphp.fr | Hôpital Bichat, Paris, France |
| Marie‐Capucine | TELLIER | marie-capucine.tellier@aphp.fr | Hôpital Bichat, Paris, France |
| Jean‐François | TIMSIT | jean-francois.timsit@aphp.fr | Hôpital Bichat, Paris, France |
| Théo | TRIOUX | theo.trioux@aphp.fr | Hôpital Bichat, Paris, France |
| Sarah | TUBIANA | sarah.tubiana@aphp.fr | Hôpital Bichat, Paris, France |
| Benoit | VISSEAUX | benoit.visseaux@aphp.fr | Hôpital Bichat, Paris, France |
| Yazdan | YAZDANPANAH | yazdan.yazdanpanah@aphp.fr | Hôpital Bichat, Paris, France |
| Dominique | DEPLANQUE | Dominique.DEPLANQUE@chru-lille.fr | Hôpital Calmette, Lille, France |
| Jean‐Sébastien | HULOT | jean-sebastien.hulot@aphp.fr | Hôpital Européen Georges Pompidou, Paris, France |
| Noémie | VANEL | Noemie.VANEL@ap-hm.fr | Hôpital la Timone, Marseille, France |
| Romain | BASMACI | romain.basmaci@aphp.fr | Hôpital Louis Mourier, Colombes, France |
| Olivier | PICONE | olivier.picone@aphp.fr | Hôpital Louis Mourier, Colombes, France |
| François | ANGOULVANT | francois.angoulvant@aphp.fr | Hôpital Necker, Paris, France |
| Florentia | KAGUELIDOU | florentia.kaguelidou@aphp.fr | Hôpital Robert Debré, Paris, France |
| Justine | PAGES | justine.pages@aphp.fr | Hôpital Robert Debré, Paris, France |
| Christelle | TUAL | christelle.tual@chu-rennes.fr | Inserm CIC‐1414, Rennes, France |
| Aurélie | VEISLINGER | Aurelie.VEISLINGER@chu-rennes.fr | Inserm CIC‐1414, Rennes, France |
| Sandrine | COUFFIN‐CADIERGUES | sandrine.couffin-cadiergues@inserm.fr | Inserm sponsor, Paris, France |
| Hélène | ESPEROU | helene.esperou@inserm.fr | Inserm sponsor, Paris, France |
| Ikram | HOUAS | ikram.houas@inserm.fr | Inserm sponsor, Paris, France |
| Salma | JAAFOURA | salma.jaafoura@inserm.fr | Inserm sponsor, Paris, France |
| Aurélie | PAPADOPOULOS | aurelie.papadopoulos@inserm.fr | Inserm sponsor, Paris, France |
| Alexandra | COELHO | alexandra.coelho@inserm.fr | Inserm UMR 1018, Paris, France |
| Alphonsine | DIOUF | alphonsine.diouf@inserm.fr | Inserm UMR 1018, Paris, France |
| Alexandre | HOCTIN | alexandre.hoctin@inserm.fr | Inserm UMR 1018, Paris, France |
| Marina | MAMBERT | marina.mambert@inserm.fr | Inserm UMR 1018, Paris, France |
| Maude | BOUSCAMBERT | maude.bouscambert-duchamp@chu-lyon.fr | Inserm UMR 1111, Lyon, France |
| Alexandre | GAYMARD | alexandre.gaymard@chu-lyon.fr | Inserm UMR 1111, Lyon, France |
| Bruno | LINA | bruno.lina@chu-lyon.fr | Inserm UMR 1111, Lyon, France |
| Manuel | ROSA‐CALATRAVA | manuel.rosa-calatrava@univ-lyon1.fr | Inserm UMR 1111, Lyon, France |
| Olivier | TERRIER | olivier.terrier@univ-lyon1.fr | Inserm UMR 1111, Lyon, France |
| Dehbia | BENKERROU | dehbia.benkerrou@iplesp.upmc.fr | Inserm UMR 1136, Paris, France |
| Céline | DORIVAL | celine.dorival@iplesp.upmc.fr | Inserm UMR 1136, Paris, France |
| Amina | MEZIANE | amina.meziane@iplesp.upmc.fr | Inserm UMR 1136, Paris, France |
| François | TÉOULÉ | francois.teoule@iplesp.upmc.fr | Inserm UMR 1136, Paris, France |
| Jérémie | GUEDJ | jeremie.guedj@inserm.fr | Inserm UMR 1137, Paris, France |
| Hervé | LE NAGARD | herve.lenagard@inserm.fr | Inserm UMR 1137, Paris, France |
| Guillaume | LINGAS | guillaume.lingas@inserm.fr | Inserm UMR 1137, Paris, France |
| Nadège | NEANT | nadege.neant@inserm.fr | Inserm UMR 1137, Paris, France |
| Laurent | ABEL | laurent.abel@inserm.fr | Inserm UMR 1163, Paris, France |
| Mathilde | DESVALLÉE | mathilde.desvallees@u-bordeaux.fr | Inserm UMR 1219, Bordeaux, France |
| Coralie | KHAN | coralie.khan@u-bordeaux.fr | Inserm UMR 1219, Bordeaux, France |
| Sylvie | BEHILILL | sylvie.behillil@pasteur.fr | Pasteur Institute, Paris, France |
| Vincent | ENOUF | vincent.enouf@pasteur.fr | Pasteur Institute, Paris, France |
| Hugo | MOUQUET | hugo.mouquet@pasteur.fr | Pasteur Institute, Paris, France |
| Sylvie | VAN DER WERF | sylvie.van-der-werf@pasteur.fr | Pasteur Institute, Paris, France |
| Minerva | CERVANTES‐GONZALEZ | minerva.cervantes@inserm.fr | REACTing, Paris, France |
| Eric | d’ORTENZIO | eric.dortenzio@inserm.fr | REACTing, Paris, France |
| Oriane | PUÉCHAL | oriane.puechal@inserm.fr | REACTing, Paris, France |
| Caroline | SEMAILLE | caroline.semaille@anses.fr | REACTing, Paris, France |
| Marion | NORET | mnoret@ch-annecygenevois.fr | RENARCI, Annecy, France |
| Yves | LEVY | yves.levy@inserm.fr | Vaccine Research Institute (VRI), Inserm UMR 955, Créteil, France |
| Aurélie | WIEDEMANN | aurelie.wiedemann@inserm.fr | Vaccine Research Institute (VRI), Inserm UMR 955, Créteil, France |
THE FRENCH COVID COHORT INVESTIGATORS GROUP
| Name | Last name | Mail address @ | Affiliation |
|---|---|---|---|
| Mélanie | RORIZ | Rorizm@ch-agen-nerac.fr | Agen ‐ Médecine Interne |
| Patrick | RISPAL | rispalp@ch-agen-nerac.fr | Agen ‐ Médecine Interne |
| Sarah | REDL | redls@ch-agen-nerac.fr | Agen ‐ Médecine Interne |
| Laurent | LEFEBVRE | llefebvre@ch-aix.fr | Aix en Provence ‐ SMIT |
| Pascal | GRANIER | pgranier@ch-aix.fr | Aix en Provence ‐ SMIT |
| Laurence | MAULIN | lmaulin@ch-aix.fr | Aix en Provence ‐ SMIT |
| Cédric | JOSEPH | joseph.cedric@chu-amiens.fr | Amiens ‐ SMIT/Réanimation |
| Julien | MOYET | moyet.julien@chu-amiens.fr | Amiens ‐ SMIT/Réanimation |
| Cinthia | RAMES | rames.cinthia@chu-amiens.fr | Amiens ‐ SMIT/Réanimation |
| Rafael | MAHIEU | Rafael.Mahieu@chu-angers.fr | Angers ‐ SMIT |
| Alexandra | DUCANCELLE | alexandra.ducancelle@univ-angers.fr | Angers ‐ SMIT |
| Vincent | DUBEE | vincent.dubee@chu-angers.fr | Angers ‐ SMIT |
| Stéphane | SALLABERRY | ssallaberry@ch-annecygenevois.fr | Annecy ‐ Réanimation |
| Aldric | MANUEL | amanuel@ch-annecygenevois.fr | Annecy ‐ SMIT |
| Gabriel | MACHEDA | gmacheda@ch-annecygenevois.fr | Annecy ‐ SMIT |
| Mylène | MAILLET | mmaillet@ch-annecygenevois.fr | Annecy ‐ SMIT |
| Patrick | IMBERT | pimbert@ch-annecygenevois.fr | Annecy ‐ SMIT |
| Amélie | VALRAN | avalran@ch-annecygenevois.fr | Annecy ‐ SMIT |
| Jean‐Charles | GAGNARD | jeancharles.gagnard@gmail.com | Antony ‐ Médecine interne |
| Guillermo | GIORDANO | GIORDANO.Guillermo@ch-avignon.fr | Avignon ‐ SMIT |
| Clara | MOUTON PERROT | mouttonperrot.clara@gmail.com | Avignon ‐ SMIT |
| Vincent | PESTRE | PESTRE.Vincent@ch-avignon.fr | Avignon ‐ SMIT |
| Cécile | FICKO | cecile.ficko@gmail.com | Bégin ‐SMIT |
| Marie | GOMINET | marie.gominet@intradef.gouv.fr | Bégin ‐SMIT |
| Aurore | BOUSQUET | aurorebousquet@yahoo.fr | Bégin ‐SMIT |
| Charline | VAUCHY | cvauchy@chu-besancon.fr | Besancon ‐ SMIT |
| Kévin | BOUILLER | kbouiller@chu-besancon.fr | Besancon ‐ SMIT |
| Maïder | PAGADOY | mpagadoy@chu-besancon.fr | Besancon ‐ SMIT |
| Quentin | LEPILLER | q1lepiller@chu-besancon.fr | Besancon ‐ SMIT |
| Noémie | TISSOT | noemie.tissot@univ-fcomte.fr | Besancon ‐ SMIT |
| Cyril | LE BRIS | cyril.le-bris@ch-beziers.fr | Beziers ‐ SMIT/Réanimation |
| Benoit | THILL | benoit.thill@ch-beziers.fr | Beziers ‐ SMIT/Réanimation |
| Marie‐Laure | CASANOVA | marie-laure.casanova@ch-beziers.fr | Beziers ‐ SMIT/Réanimation |
| Georges | LE FALHER | georges.le-falher@ch-beziers.fr | Beziers ‐ SMIT/Réanimation |
| Eric | OZIOL | eric.oziol@ch-beziers.fr | Beziers ‐ SMIT/Réanimation |
| Hugues | CORDEL | hugues.cordel@aphp.fr | Bobigny ‐ Avicenne ‐ SMIT |
| Nathalie | DOURNON | nathaliedournon@gmail.com | Bobigny ‐ Avicenne ‐ SMIT |
| Olivier | BOUCHAUD | olivier.bouchaud@aphp.fr | Bobigny ‐ Avicenne ‐ SMIT |
| Duc | NGUYEN | duc.nguyen@chu-bordeaux.fr | Bordeaux ‐ SMIT |
| Segolene | GREFFE | segolene.greffe@aphp.fr | Boulogne Billancourt ‐ A. Paré ‐Médecine interne |
| Camille | BOUISSE | cbouisse@ch-bourg01.fr | Bourg en Bresse ‐ Infectiologie/Réanimation |
| Nicholas | SEDILLOT | nsedillot@ch-bourg01.fr | Bourg en Bresse ‐ Infectiologie/Réanimation |
| Damien | BOUHOUR | dbouhour@ch-bourg01.fr | Bourg en Bresse ‐ Infectiologie/Réanimation |
| Camille | CHASSIN | cchassin@ghnd.fr | Bourgoin‐Jallieu ‐ Médecine interne |
| Erwan | L'HER | erwan.lher@chu-brest.fr | Brest ‐ Réanimation |
| Laetitia | BODENES | Laetitia.bodenes@chu-brest.fr | Brest ‐ Réanimation |
| Nicolas | FERRIERE | nicoferriere@yahoo.fr | Brest ‐ Réanimation |
| Séverine | ANSART | severine.ansart@chu-brest.fr | Brest ‐ SMIT |
| Cécile | TROMEUR | cecile.tromeur@chu-brest.fr | Brest ‐ SMIT |
| Dewi | GUELLEC | dewi.guellec@chu-brest.fr | Brest ‐ SMIT |
| Antoine | MERCKX | antoine.merckx@ch-cahors.fr | Cahors ‐ SMIT |
| Felix | DJOSSOU | felix.djossou@ch-cayenne.fr | Cayenne ‐ SMIT/Réanimation |
| Mayka | MERGEAYFABRE | mayka.mergeayfabre@ch-cayenne.fr | Cayenne ‐ SMIT/Réanimation |
| Arsène | KPANGON | amadohoue.kpangon@ch-cayenne.fr | Cayenne ‐ SMIT/Réanimation |
| Vincent | PEIGNE | vincent.peigne@ch-metropole-savoie.fr | Chambery ‐ SMIT |
| Carola | PIEROBON | carola.pierobon@ch-metropole-savoie.fr | Chambery ‐ SMIT |
| Marie‐Christine | CARRET | mariechristine.carret@ch-metropole-savoie.fr | Chambery ‐ SMIT |
| Florence | JEGO | florence.jego@ch-metropole-savoie.fr | Chambery ‐ SMIT |
| Margaux | ISNARD | margaux.isnard@ch-metropole-savoie.fr | Chambery ‐ SMIT |
| Johann | AUCHABIE | johann.auchabie@ch-cholet.fr | Chollet ‐ Réanimation |
| Anthony | Lemeur | anthony.lemeur@ch-cholet.fr | Chollet ‐ Réanimation |
| Thierry | MAZZONI | thierry.mazzoni@ch-cholet.fr | Chollet ‐ Réanimation |
| Roxane | COURTOIS | roxane.courtois@ch-cholet.fr | Chollet ‐ SMIT |
| Olivier | LESENS | olesens@chu-clermontferrand.fr | Clermont‐Ferrand ‐ SMIT |
| Martin | MARTINOT | Martin.martinot@ch-colmar.fr | Colmar ‐ SMIT |
| Jeanne | SIBIUDE | Jeanne.sibiude@aphp.fr | Colombes ‐ Louis Mourier ‐ Gynécologie |
| Laurent | MANDELBROT | laurent.mandelbrot@aphp.fr | Colombes ‐ Louis Mourier ‐ Gynécologie |
| Marie | LACOSTE | mlacoste@ch-alpes-leman.fr | Contamine sur Arve ‐ Infectiologie/Réanimation |
| Jean‐Daniel | LELIEVRE | jean-daniel.lelievre@aphp.fr | Créteil ‐ Mondor ‐ SMIT |
| Brigitte | ELHARRAR | Brigitte.elharrar@chicreteil.fr | Créteil CHIC ‐ Médecine interne |
| Valerie | GARRAIT | valerie.garrait@chicreteil.fr | Créteil CHIC ‐ Médecine interne |
| Isabelle | DELACROIX | isabelle.delacroix@chicreteil.fr | Créteil CHIC ‐ Médecine interne |
| Thomas | MAITRE | thomas.maitre@chicreteil.fr | Créteil CHIC ‐ Médecine interne |
| Jean Baptiste | ASSIE | jean-baptiste.assie@inserm.fr | Créteil CHIC ‐ Médecine interne |
| Elsa | NYAMANKOLLY | NYAMANKOLLYe@ch-dax.fr | Dax ‐ SMIT/Réanimation |
| Adrien | AUVET | auveta@ch-dax.fr | Dax ‐ SMIT/Réanimation |
| Anne‐Hélène | BOIVIN | helene.boivin@ght40.fr | Dax ‐ SMIT/Réanimation |
| Younes | KERROUMI | ykerroumi@hopital-dcss.org | Diaconesses CSS ‐ Médecine interne |
| Vanina | MEYSSONNIER | vmeyssonnier@hopital-dcss.org | Diaconesses CSS ‐ Médecine interne |
| Oryane | MABIALA | omabiala@for.paris | Diaconesses CSS ‐ Médecine interne |
| François Xavier | CATHERINE | francois-xavier.catherine@chu-dijon.fr | Dijon ‐ SMIT |
| Mathieu | BLOT | mathieu.blot@chu-dijon.fr | Dijon ‐ SMIT |
| Sophie | MAHY | sophie.mahy@chu-dijon.fr | Dijon ‐ SMIT |
| Marielle | BUISSON | marielle.buisson@chu-dijon.fr | Dijon ‐ SMIT |
| Lionel | PIROTH | lionel.piroth@chu-dijon.fr | Dijon ‐ SMIT |
| Valentine | CAMPANA | Valentine.CAMPANA@chu-martinique.fr | Fort de France ‐ SMIT |
| Jérémie | PASQUIER | jeremie.pasquier@chu-martinique.fr | Fort de France ‐ SMIT |
| André | CABIE | andre.cabie@chu-martinique.fr | Fort de France ‐ SMIT |
| Pierre‐François | SANDRINE | sandrine.pierre-francois@chu-martinique.fr | Fort de France ‐ SMIT |
| Jean‐Marie | TURMEL | jean-marie.turmel@chu-martinique.fr | Fort de France ‐ SMIT |
| Simon | BESSIS | simon.bessis@aphp.fr | Garches ‐ SMIT |
| Olivier | EPAULARD | OEpaulard@chu-grenoble.fr | Grenoble ‐ SMIT |
| Nicolas | TERZI | nterzi@chu-grenoble.fr | Grenoble ‐ SMIT |
| Jean‐François | PAYEN | JFPayen@chu-grenoble.fr | Grenoble ‐ SMIT |
| Laurence | BOUILLET | lbouillet@chu-grenoble.fr | Grenoble ‐ SMIT |
| Rebecca | HAMIDFAR | rhamidfar@chu-grenoble.fr | Grenoble ‐ SMIT |
| Marion | LE MARECHAL | mlemarechal@chu-grenoble.fr | Grenoble ‐ SMIT |
| Elodie | CURLIER | elodie.curlier@chu-guadeloupe.fr | Guyane ‐ Guadeloupe ‐Réanimation ‐ SMIT |
| Rachida | OUISSA | rachida.ouissa@chu-guadeloupe.fr | Guyane ‐ Guadeloupe ‐Réanimation ‐ SMIT |
| Isabelle | FABRE | mfabre@ghnd.fr | Guyane ‐ Guadeloupe ‐Réanimation ‐ SMIT |
| Pierre‐Marie | ROGER | pierre-marie.roger@chu-guadeloupe.fr | Guyane ‐ Guadeloupe ‐Réanimation ‐ SMIT |
| Samuel | Markowicz | samuel.markowicz@chu-guadeloupe.fr | Guyane ‐ Guadeloupe ‐Réanimation ‐ SMIT |
| Olivier | PICONE | olivier.picone@aphp.fr | Gynécologie,Hôpital Louis Mourrier, Colombe |
| Cécile | GOUJARD | cecile.goujard@aphp.fr | Kremlin‐Bicêtre ‐SMIT/Médecine interne |
| Stéphane | JAUREGUIBERRY | stephane.jaureguiberry@aphp.fr | Kremlin‐Bicêtre ‐SMIT/Médecine interne |
| Antoine | CHERET | antoine.cheret@aphp.fr | Kremlin‐Bicêtre ‐SMIT/Médecine interne |
| Gwenhaël | COLIN | gwenhael.colin@chd-vendee.fr | La Roche Sur Yon ‐ Infectiologie |
| Romain | DECOURS | romain.decours@chd-vendee.fr | La Roche Sur Yon ‐ Infectiologie |
| Thomas | GUIMARD | thomas.guimard@chd-vendee.fr | La Roche Sur Yon ‐ Infectiologie |
| Vincent | Langlois | vincent.langlois@ch-havre.fr, | Le Havre ‐ MI / Pneumologie |
| Laure | GOUBERT | laure.goubert@ch-havre.fr | Le Havre ‐ MI / Pneumologie |
| Stéphanie | COUSSE | stephanie.cousse@ch-havre.fr | Le Havre ‐ MI / Pneumologie |
| Hikombo | HITOTO | hhitoto@ch-lemans.fr | Le Mans CH ‐ SMIT |
| Julien | POISSY | julien.poissy@chru-lille.fr | Lille ‐ Réanimation |
| Saad | NSEIR | saadalla.nseir@chru-lille.fr | Lille ‐ Réanimation |
| Sébastien | PREAU | sebastien.preau@chru-lille.fr | Lille ‐ Réanimation |
| Mercé | JOURDAIN | merce.jourdain@chru-lille.fr | Lille ‐ Réanimation |
| Raphaël | FAVORY | raphael.favory@chru-lille.fr | Lille ‐ Réanimation |
| Karine | FAURE | karine.faure@chru-lille.fr | Lille ‐ SMIT |
| Fanny | VUOTTO | Fanny.VUOTTO@CHRU-LILLE.FR | Lille ‐ SMIT |
| Marie‐Charlotte | CHOPIN | Mariecharlotte.CHOPIN@CHRU-LILLE.FR | Lille ‐ SMIT |
| Sarah | STABLER | stabler.sarah@gmail.com | Lille ‐ SMIT |
| Jules | BAUER | bauerjules@gmail.com | Lille ‐ SMIT |
| Marc | LAMBERT | Marc.LAMBERT@chru-lille.fr | Lille Calmette ‐ SMIT |
| Arnaud | SCHERPEREEL | Arnaud.SCHERPEREEL@chru-lille.fr | Lille Calmette ‐ SMIT |
| Ryadh | POKEERBUX | ryadh.pokeerbux@chru-lille.fr | Lille Calmette ‐ SMIT |
| Stéphanie | FRY | Stephanie.FRY@chru-lille.fr | Lille Calmette ‐ SMIT |
| Cécile | YELNIK | CECILE.YELNIK@chru-lille.fr | Lille Calmette ‐ SMIT |
| Laurent | BITKER | laurent.bitker@chu-lyon.fr | Lyon ‐ Réanimation |
| Mehdi | MEZIDI | mehdi.mezidi@chu-lyon.fr | Lyon ‐ Réanimation |
| Hodane | YONIS | hodane.yonis@chu-lyon.fr | Lyon ‐ Réanimation |
| Nicolas | BENECH | nicolas.benech@chu-lyon.fr | Lyon ‐ SMIT |
| Thomas | PERPOINT | thomas.perpoint@chu-lyon.fr | Lyon ‐ SMIT |
| Anne | CONRAD | anne.conrad@chu-lyon.fr | Lyon ‐ SMIT |
| Muriel | DORET‐DION | muriel.doret-dion@chu-lyon.fr | Lyon ‐ Hôpital Mère Enfant ‐ Gynécologie |
| Pierre‐Adrien | BOLZE | pierre-adrien.bolze@chu-lyon.fr | Lyon Sud ‐ Obstétrique |
| Simon‐Djamel | THIBERVILLE | thiberville.sd@ch-manosque.fr | Manosque ‐ SMIT |
| Moïse | MACHADO | mmachado@ghef.fr | Marne la Vallee‐ SMIT |
| Audrey | BARRELET | abarrelet@ghef.fr | Marne la Vallee‐ SMIT |
| Alexandra | BEDOSSA | abedossa@ghef.fr | Marne la Vallee‐ SMIT |
| Stanislas | REBAUDET | s.rebaudet@hopital-europeen.fr | Marseille ‐ SMIT |
| Frédérique | RETORNAZ | f.retornaz@hopital-europeen.fr | Marseille ‐ SMIT |
| Myriam | BENNANI | M.BENNANI@hopital-europeen.fr | Marseille ‐ SMIT |
| Hortense | DROUET | h.drouet@hopital-europeen.fr | Marseille ‐ SMIT |
| Bertrand | DUSSOL | bertrand.dussol@ap-hm.fr | Marseille conception ‐ Néphrologie |
| Marc | LEONE | marc.leone@ap-hm.fr | Marseille Nord ‐ La Timone ‐ Réanimation |
| Bruno | PASTENE | bruno.pastene@ap-hm.hm | Marseille Nord ‐ La Timone ‐ Réanimation |
| Karine | BEZULIER | karine.bezulier@ap-hm.fr | Marseille Nord ‐ La Timone ‐ Réanimation |
| Axelle | BRACONNIER | a.braconnier@hotmail.fr | Mayotte ‐ Gynécologie |
| Sylvain | DIAMANTIS | Sylvain.diamantis@ghsif.fr | Melun ‐ SMIT |
| Catherine | CHAKVEATZE | eka.chakvetadze@aphp.fr | Melun ‐ SMIT |
| Clara | FLATEAU | clara.flateau@ghsif.fr | Melun ‐ SMIT |
| Vincent | DINOT | v.dinot@chr-metz-thionville.fr | Metz ‐ Réanimation |
| Rostane | GACI | r.gaci@chr-metz-thionville.fr | Metz ‐ Réanimation |
| Nadia | OUAMARA | n.ouamara@chr-metz-thionville.fr | Metz ‐ Réanimation |
| Guillaume | LOUIS | g.louis@chr-metz-thionville.fr | Metz ‐ Réanimation |
| Cyril | CADOZ | c.cadoz@chr-metz-thionville.fr | Metz ‐ Réanimation |
| Hajnal‐Gabriela | ILLES | gabriela.illes@ch-mdm.fr | Mont de Marsan ‐ SMIT |
| Bouchra | LOUTFI | bouchra.loutfi@ch-mdm.fr | Mont de Marsan ‐ SMIT |
| Jérôme | DIMET | jerome.dimet@ght40.fr | Mont de Marsan ‐ SMIT |
| Vincent | LE MOING | v-le_moing@chu-montpellier.fr | Montpellier ‐ SMIT |
| Nathalie | PANSU | n-pansu@chu-montpellier.fr | Montpellier ‐ SMIT |
| Clément | LE BIHAN | c-lebihan@chu-montpellier.fr | Montpellier ‐ SMIT |
| Antoine | KIMMOUN | a.kimmoun@chru-nancy.fr | Nancy ‐ Réanimation |
| Bruno | LEVY | b.levy@chru-nancy.fr | Nancy ‐ Réanimation |
| Maximilen | SAINT GILLES | M.SAINTGILLES@chru-nancy.fr | Nancy ‐ Réanimation |
| François | GOEHRINGER | f.goehringer@chru-nancy.fr | Nancy ‐ SMIT |
| Christian | RABAUD | c.rabaud@chru-nancy.fr | Nancy ‐ SMIT |
| Sibylle | BEVILACQUA | s.bevilacqua@chru-nancy.fr | Nancy ‐ SMIT |
| Benjamin | LEFEVRE | B.LEFEVRE@chru-nancy.fr | Nancy ‐ SMIT |
| Anne | GUILLAUMOT | a.guillaumot@chru-nancy.fr | Nancy ‐ SMIT |
| Anne Sophie | BOUREAU | annesophie.boureau@chu-nantes.fr | Nantes ‐ Gériatrie |
| Clotilde | ALLAVENA | Clotilde.ALLAVENA@chu-nantes.fr | Nantes ‐ SMIT |
| Sabelline | BOUCHEZ | Sabelline.BOUCHEZ@chu-nantes.fr | Nantes ‐ SMIT |
| Romain | GUERY | dr.guery@groupeconfluent.fr | Nantes ‐ SMIT |
| Paul | LE TURNIER | Paul.LETURNIER@chu-nantes.fr | Nantes ‐ SMIT |
| Cécile | MEAR‐PASSARD | cecile.passard@chu-nantes.fr | Nantes ‐ SMIT |
| Christophe | RAPP | christophe.rapp@ahparis.org | Neuilly sur Seine ‐ Médecine Interne |
| Stéphane | LASRY | stephane.lasry@ahparis.org | Neuilly sur Seine ‐ Médecine Interne |
| Thierry | CARMOI | thierry.carmoi@ahparis.org | Neuilly sur Seine ‐ Médecine Interne |
| Elisa | DEMONCHY | demonchy.e@chu-nice.fr | Nice ‐ SMIT |
| Céline | MICHELANGELLI | michelangeli.c@chu-nice.fr | Nice ‐ SMIT |
| Karine | RISSO | risso.k@chu-nice.fr | Nice ‐ SMIT |
| Paul | LOUBET | Paul.LOUBET@chu-nimes.fr | Nimes ‐ SMIT |
| Alberto | SOTTO | albert.sotto@chu-nimes.fr | Nimes ‐ SMIT |
| Didier | Laureillard | didier.laureillard@chu-nimes.fr | Nimes ‐ SMIT |
| Etienne | DE MONTMOLLIN | etienne.demontmollin@aphp.fr | Paris ‐ Bichat ‐ Réanimation |
| Juliette | PATRIER | juliette.patrier@aphp.fr | Paris ‐ Bichat ‐ Réanimation |
| Paul Henri | WICKY | paul-henri.wicky@aphp.fr | Paris ‐ Bichat ‐ Réanimation |
| Lucie | LE FEVRE | lucie.lefevre@aphp.fr | Paris ‐ Bichat ‐ Réanimation |
| Pierre | JACQUET | Pierre.jaquet@aphp.fr | Paris ‐ Bichat ‐ Réanimation |
| Raphael | BORIE | raphael.borie@aphp.fr | Paris ‐ Bichat ‐ SMIT |
| Tiphaine | GOULENOK | tiphaine.goulenok@aphp.fr | Paris ‐ Bichat ‐ SMIT |
| Dominique | LUTON | dominique.luton@aphp.fr | Paris ‐ Bichat ‐ SMIT |
| Lauren | DECONINCK | bastien.deconninck@aphp.fr | Paris ‐ Bichat ‐ SMIT |
| Sylvie | LE GAC | sylvie.legac@aphp.fr | Paris ‐ Bichat ‐ SMIT |
| Cecile | AZOULAY | cecile.azoulay@aphp.fr | Paris ‐ Cochin ‐ CIC Vaccinologie |
| Nicolas | CARLIER | nicolas.carlier@aphp.fr | Paris ‐ Cochin ‐ CIC Vaccinologie |
| Liem | LUONG | liem.luong@aphp.fr | Paris ‐ Cochin ‐ CIC Vaccinologie |
| Marie | LACHATRE | marie.lachatre@aphp.fr | Paris ‐ Cochin ‐ CIC Vaccinologie |
| Odile | LAUNAY | odile.launay@aphp.fr | Paris ‐ Cochin ‐ CIC Vaccinologie |
| Jean‐Luc | DIEHL | jean-luc.diehl@aphp.fr | Paris ‐ HEGP ‐ Réanimation |
| Marine | LIVROZET | marine.livrozet@aphp.fr | Paris ‐ HEGP ‐ Réanimation |
| Bernard | CHOLLEY | bernard.cholley@aphp.fr | Paris ‐ HEGP ‐ Réanimation |
| Jean‐Benoit | ARLET | jean-benoit.arlet@aphp.mssante.fr | Paris ‐ HEGP ‐ Réanimation |
| Olivier | SANCHEZ | manuel.sanchez@aphp.fr | Paris ‐ HEGP ‐ Réanimation |
| Victoria | MANDA | victoria.manda@aphp.fr | Paris ‐ Lariboisière ‐ SMIT |
| Laurène | AZEMAR | laurene.azemar@aphp.fr | Paris ‐ Lariboisière ‐ SMIT |
| Guylaine | CASTOR‐ALEXANDRE | guylaine.alexandre@aphp.fr | Paris ‐ Lariboisière ‐ SMIT |
| Jeanne | TRUONG | jeanne.truong@aphp.fr | Paris ‐ Robert Debré ‐ Pédiatrie |
| Karine | LACOMBE | karine.lacombe2@aphp.fr | Paris ‐ Saint Antoine ‐ SMIT |
| Thibault | CHIARABINI | Thibault.chiarabini@aphp.fr | Paris ‐ Saint Antoine ‐ SMIT |
| Bénédicte | LEFEBVRE | benedicte.lefebvre2@aphp.fr | Paris ‐ Saint Antoine ‐ SMIT |
| Nathalie | DE CASTRO | nathalie.de-castro@aphp.fr | Paris ‐ Saint Louis ‐ Réanimation |
| Geoffrey | LIEGEON | geoffroy.liegeon@aphp.fr | Paris ‐ Saint Louis ‐ Réanimation |
| Diane | PONSCARME | diane.ponscarme@aphp.fr | Paris ‐ Saint Louis ‐ Réanimation |
| Julie | CHAS | julie.chas@aphp.fr | Paris ‐ Tenon ‐SMIT |
| Valérie | GABORIEAU | valerie.gaborieau@ch-pau.fr | Pau ‐ SMIT/Réanimation |
| Eve | LE COUSTUMIER | eve.lecoustumier@ch-pau.fr | Pau ‐ SMIT/Réanimation |
| Walter | PICARD | walter.picard@ch-pau.fr | Pau ‐ SMIT/Réanimation |
| Jean‐Benoit | ZABBE | marion.zabbe@ch-perigueux.fr | Perigueux ‐ SMIT |
| Florent | PEELMAN | florent.peelman@ch-perigueux.fr | Perigueux ‐ SMIT |
| Edouard | SOUM | edouard.soum@ch-perigueux.fr | Perigueux ‐ SMIT |
| Hugues | AUMAÎTRE | hugues.aumaitre@ch-perpignan.fr | Perpignan ‐ SMIT |
| Blandine | RAMMAERT | blandine.rammaert@chu-poitiers.fr | Poitiers ‐ SMIT |
| Gwenaël | Le Moal | Gwenaël.LEMOAL@chu-poitiers.fr | Poitiers ‐ SMIT |
| Isabelle | PIRONNEAU | Isabelle.PIRONNEAU@chu-poitiers.fr | Poitiers ‐ SMIT |
| Anne Sophie | RESSEGUIER | annesophie.resseguier@ch-lepuy.fr | Puy en Velay ‐ Médecine interne |
| Nadia | SAIDANI | n.saidani@ch-cornouaille.fr | Quimper ‐ MIIS |
| Firouzé | BANI‐SADR | fbanisadr@chu-reims.fr | Reims ‐ SMIT |
| Maxime | HENTZIEN | mhentzien@chu-reims.fr | Reims ‐ SMIT |
| Yohan | N'GUYEN | ynguyen@chu-reims.fr | Reims ‐ SMIT |
| Juliette | ROMARU | jromaru@chu-reims.fr | Reims ‐ SMIT |
| Kévin | DIDIER | kdidier@chu-reims.fr | Reims ‐ SMIT |
| Isabelle | ENDERLE | Isabelle.ENDERLE@chu-rennes.fr | Rennes ‐ Gynécologie |
| Fabrice | LAINE | Fabrice.Laine@chu-rennes.fr | Rennes ‐ SMIT |
| Matthieu | LESOUHAITIER | mathieu.LESOUHAITIER@chu-rennes.fr | Rennes ‐ SMIT |
| Matthieu | REVEST | matthieu.revest@chu-rennes.fr | Rennes ‐ SMIT |
| Pierre | TATTEVIN | pierre.tattevin@chu-rennes.fr | Rennes ‐ SMIT |
| Jean‐Marc | CHAPPLAIN | jean-marc.chapplain@chu-rennes.fr | Rennes ‐ SMIT |
| Manuel | ETIENNE | Manuel.Etienne@chu-rouen.fr | Rouen ‐ SMIT |
| Véronique | LEMEE | Veronique.Lemee@chu-rouen.fr | Rouen ‐ SMIT |
| Eglantine | FERRAND DEVOUGE | E.Ferrand-Devouge@chu-rouen.fr | Rouen ‐ SMIT |
| Kévin | ALEXANDRE | kevin.alexandre@chu-rouen.fr | Rouen ‐ SMIT |
| Elise | ARTAUD‐MACCARI | Elise.Artaud-Macari@chu-rouen.fr | Rouen ‐ SMIT |
| Nathalie | ALLOU | nathalie.allou@chu-reunion.fr | Saint Denis ‐ Saint Pierre ‐ SMIT |
| Marie | LAGRANGE | marie.lagrange-xelot@chu-reunion.fr | Saint Denis ‐ Saint Pierre ‐ SMIT |
| Julien | JABOT | jabot974@gmail.com | Saint Denis ‐ Saint Pierre ‐ SMIT |
| Elisabeth | BOTELHO‐NEVERS | elisabeth.botelho-nevers@chu-st-etienne.fr | Saint Etienne ‐ SMIT |
| Amandine | GAGNEUX‐BRUNON | amandine.gagneux-brunon@chu-st-etienne.fr | Saint Etienne ‐ SMIT |
| Tiffany | TROUILLON | tiffany.trouillon@chu-st-etienne.fr | Saint Etienne ‐ SMIT |
| Corinne | DANIEL | c.daniel@chsaintmartin.fr | Saint Martin ‐ Médecine UDSMT |
| Benoît | ROZE | b.roze@ch-saintonge.fr | Saintes ‐ Réanimation |
| Delphine | BREGEAUD | d.bregeaud@ch-saintonge.fr | Saintes ‐ Réanimation |
| Younes | AIT TAMLIHAT | y.ait-tamlihat@ch-saintonge.fr | Saintes ‐ Réanimation |
| Ali | HACHEMI | ali.hachemi@ch-soissons.fr | Soissons ‐ Infectiologie |
| Hélène | SALVATOR | h.salvator@hopital-foch.org | Suresnes ‐ Hopital Foch ‐ DRCI |
| Erwan | FOURN | e.fourn@hopital-foch.org | Suresnes ‐ Hopital Foch ‐ DRCI |
| David | ZUCMAN | d.zucman@hopital-foch.org | Suresnes ‐ Hopital Foch ‐ DRCI |
| Marie‐Laure | CHABI‐CHAVILLAT | ml.chabi-charvillat@hopital-foch.com | Suresnes ‐ Hopital Foch ‐ DRCI |
| Aurélie | MARTIN | a.martin@hopital-foch.com | Suresnes ‐ Hopital Foch ‐ DRCI |
| Eric | DELAVEUVE | e.delaveuve@chr-metz-thionville.fr | Thionville ‐ Bel Air ‐ SMIT/Réanimation |
| Coline | JAUD‐FISCHER | C.JAUDFISCHER@chru-nancy.fr | Thionville ‐ Bel Air ‐ SMIT/Réanimation |
| Paul | DUNAND | paul.m.dunand@gmail.com | Thionville ‐ Bel Air ‐ SMIT/Réanimation |
| François | BISSUEL | f-bissuel@ch-hopitauxduleman.fr | Thonon les Bains ‐ Pneumologie |
| Karen | DELAVIGNE | delavigne.karen@iuct-oncopole.fr | Toulouse ‐ Hématologie/Médecine interne |
| Alexa | DEBARD | debard.a@chu-toulouse.fr | Toulouse ‐ SMIT |
| Pierre | DELOBEL | delobel.p@chu-toulouse.fr | Toulouse ‐ SMIT |
| Benjamine | SARTON | sarton.b@chu-toulouse.fr | Toulouse ‐ SMIT |
| Stella | Rousset | rousset.st@chu-toulouse.fr | Toulouse ‐ SMIT |
| Guillaume | MARTIN‐BLONDEL | martin-blondel.g@chu-toulouse.fr | Toulouse ‐ SMIT |
| Laurent | GUILLEMINAULT | guilleminault.l@chu-toulouse.fr | Toulouse Larrey ‐ Pneumologie |
| Marlène | MURRIS | murris.m@chu-toulouse.fr | Toulouse Larrey ‐ Pneumologie |
| Agnès | SOMMET | agnes.sommet@univ-tlse3.fr | Toulouse Larrey ‐ Pneumologie |
| Olivier | LAIREZ | lairez.o@chu-toulouse.fr | Toulouse‐cardiologie |
| Eric | SENNEVILLE | esenneville@ch-tourcoing.fr | Tourcoing ‐ SMIT |
| Olivier | ROBINEAU | olivier.robineau82@gmail.com | Tourcoing ‐ SMIT |
| Agnès | MEYBECK | ameybeck@ch-tourcoing.fr | Tourcoing ‐ SMIT |
| Denis | GAROT | d.garot@chu-tours.fr | Tours ‐ Réanimation |
| Laurent | PLANTIER | laurent.plantier@univ-tours.fr | Tours ‐ Réanimation |
| Valérie | GISSOT | valerie.gissot@univ-tours.fr | Tours ‐ Réanimation |
| Emmanuelle | MERCIER | emercier@med.univ-tours.fr | Tours ‐ Réanimation |
| Charlotte | SALMON GANDONNIERE | charlotte.salmon.gandonniere@gmail.com | Tours ‐ Réanimation |
| Adrien | LEMAIGNEN | adrien.lemaignen@chu-tours.fr | Tours ‐ SMIT |
| Julie | MANKIKIAN | J.MANKIKIAN@chu-tours.fr | Tours ‐ SMIT |
| Thomas | FLAMENT | T.FLAMENT@chu-tours.fr | Tours ‐ SMIT |
| Grégory | CORVAISIER | gregory.corvaisier@ch-bretagne-atlantique.fr | Vannes ‐ SMIT |
| Delphine | LARIVIERE | delphine.lariviere@ch-bretagne-atlantique.fr | Vannes ‐ SMIT |
| Marie | LANGELOT‐RICHARD | marie.langelot-richard@ch-bretagne-atlantique.fr | Vannes ‐ SMIT |
| Pauline | CARAUX PAZ | pauline.caraux-paz@chiv.fr | Villeneuve Saint Georges ‐ SMIT |
| Laurent | RICHIER | laurent.richier@aphp.fr | Villeneuve Saint Georges ‐ SMIT |
| Danielle | JAAFAR | danielle.jaafar@chiv.fr | Villeneuve Saint Georges ‐ SMIT |
| Claudine | BADR | claudine.Badr@chiv.fr | Villeneuve Saint Georges ‐ SMIT |
| Fara | DIOP | Fara.Diop@chiv.fr | Villeneuve Saint Georges ‐ SMIT |
Gault N, Esposito‐Farèse M, Revest M, et al. Chronic use of renin‐angiotensin‐aldosterone system blockers and mortality in COVID‐19: A multicenter prospective cohort and literature review. Fundam Clin Pharmacol. 2021;35:1141-1158. 10.1111/fcp.12683
Funding information
This work was supported by the REACTing (REsearch & ACtion emergING infectious diseases) consortium and by the French Ministry of Health [grant number PHRC20‐0424]. The sources of funding had no role in the design, steering, interpretation of results, and decision for publication of the study. The sponsor, INSERM, had the responsibility for the research, getting ethical and regulatory approvals, data quality monitoring, and patient safety.
Contributor Information
Nathalie Gault, Email: nathalie.gault@aphp.fr.
Emmanuelle Vidal‐Petiot, Email: emmanuelle.vidal-petiot@aphp.fr.
the French‐Covid cohort investigators, study group:
Marie Bartoli, Alpha Diallo, Soizic Le Mestre, Noémie Mercier, Christelle Paul, Ventzislava Petrov‐Sanchez, Claire Andrejak, Denis Malvy, François Dubos, François Bompart, Tristan Gigante, Morgane Gilg, Bénédicte Rossignol, Claire Léy‐Marchal, Marine Beluze, Delphine Bachelet, Krishna Bhavsar, Lila Bouadma, Anissa Chair, Camille Couffignal, Charlene Da Silveira, Marie‐Pierre Debray, Diane Descamps, Xavier Duval, Philippine Eloy, Marina Esposito‐Farèse, Nadia Ettalhaoui, Isabelle Gorenne, Isabelle Hoffmann, Ouifiya Kafif, Sabrina Kali, Antoine Khalil, Cédric Laouénan, Samira Laribi, Minh Lê, Quentin Le Hingrat, François‐Xavier Lescure, Jean‐Christophe Lucet, France Mentré, Jimmy Mullaert, Nathan Peiffer‐Smadja, Gilles Peytavin, Carine Roy, Marion Schneider, Nassima Si Mohammed, Lysa Tagherset, Coralie Tardivon, Marie‐Capucine Tellier, Jean‐François Timsit, Théo Trioux, Sarah Tubiana, Benoît Visseaux, Yazdan Yazdanpanah, Dominique Deplanque, Noémie Vanel, Romain Basmaci, Olivier Picone, François Angoulvant, Florentia Kaguelidou, Justine Pages, Christelle Tual, Aurélie Veislinger, Sandrine Couffin‐Cadiergues, Hélène Esperou, Ikram Houas, Salma Jaafoura, Aurélie Papadopoulos, Alexandra Coelho, Alphonsine Diouf, Alexandre Hoctin, Marina Mambert, Maude Bouscambert, Alexandre Gaymard, Bruno Lina, Manuel Rosa‐calatrava, Olivier Terrier, Dehbia Benkerrou, Céline Dorival, Amina Meziane, François Téoulé, Jérémie Guedj, Hervé Le Nagard, Guillaume Lingas, Nadège Neant, Laurent Abel, Mathilde Desvallée, Coralie Khan, Sylvie Behilill, Vincent Enouf, Hugo Mouquet, Sylvie Van der werf, Minerva Cervantes‐gonzalez, Eric D’Ortenzio, Oriane Puéchal, Caroline Semaille, Marion Noret, Yves Levy, Aurélie Wiedemann, Mélanie Roriz, Patrick Rispal, Sarah Redl, Laurent Lefebvre, Pascal Granier, Laurence Maulin, Cédric Joseph, Julien Moyet, Cinthia Rames, Rafael Mahieu, Alexandra Ducancelle, Vincent Dubee, Stéphane Sallaberry, Aldric Manuel, Gabriel Macheda, Mylène Maillet, Patrick Imbert, Amélie Valran, Jean‐Charles Gagnard, Guillermo Giordano, Clara Mouton Perrot, Vincent Pestre, Cécile Ficko, Marie Gominet, Aurore Bousquet, Charline Vauchy, Kévin Bouiller, Maïder Pagadoy, Quentin Lepiller, Noémie Tissot, Cyril Le Bris, Benoit Thill, Marie‐Laure Casanova, Georges Le Falher, Hugues Cordel, Nathalie Dournon, Olivier Bouchaud, Duc Nguyen, Segolène Greffe, Camille Bouisse, Nicholas Sedillot, Damien Bouhour, Camille Chassin, Erwan L'her, Laetitia Bodenes, Nicolas Ferriere, Séverine Ansart, Cécile Tromeur, Dewi Guellec, Antoine Merckx, Felix Djossou, Mayka Mergeayfabre, Arsène Kpangon, Vincent Peigne, Carola Pierobon, Marie‐Christine Carret, Florence Jego, Margaux Isnard, Johann Auchabie, Anthony Lemeur, Thierry Mazzoni, Roxane Courtois, Olivier Lesens, Martin Martinot, Jeanne Sibiude, Laurent Mandelbrot, Marie Lacoste, Jean‐Daniel Lelievre, Brigitte Elharrar, Valerie Garrait, Isabelle Delacroix, Thomas Maitre, Jean‐Baptiste Assie, Elsa Nyamankolly, Adrien Auvet, Anne‐Hélène Boivin, Younes Kerroumi, Vanina Meyssonnier, Oryane Mabiala, François Xavier Catherine, Mathieu Blot, Sophie Mahy, Marielle Buisson, Valentine Campana, Jérémie Pasquier, Pierre‐François Sandrine, Jean‐Marie Turmel, Simon Bessis, Nicolas Terzi, Jean‐François Payen, Laurence Bouillet, Rebecca Hamidfar, Marion Le Marechal, Elodie Curlier, Rachida Ouissa, Isabelle Fabre, Pierre‐Marie Roger, Samuel Markowicz, Olivier Picone, Cécile Goujard, Stéphane Jaureguiberry, Antoine Cheret, Gwenhaël Colin, Romain Decours, Thomas Guimard, Vincent Langlois, Laure Goubert, Stéphanie Cousse, Hikombo Hitoto, Saad Nseir, Sébastien Preau, Mercé Jourdain, Raphaël Favory, Karine Faure, Fanny Vuotto, Marie‐Charlotte Chopin, Sarah Stabler, Jules Bauer, Marc Lambert, Arnaud Scherpereel, Ryadh Pokeerbux, Stéphanie Fry, Cécile Yelnik, Laurent Bitker, Mehdi Mezidi, Hodane Yonis, Nicolas Benech, Thomas Perpoint, Anne Conrad, Muriel Doret‐Dion, Pierre‐Adrien Bolze, Simon‐Djamel Thiberville, Moïse Machado, Audrey Barrelet, Alexandra Bedossa, Stanislas Rebaudet, Frédérique Retornaz, Myriam Bennani, Hortense Drouet, Bertrand Dussol, Marc Leone, Bruno Pastene, Karine Bezulier, Axelle Braconnier, Sylvain Diamantis, Catherine Chakveatze, Clara Flateau, Vincent Dinot, Rostane Gaci, Nadia Ouamara, Guillaume Louis, Cyril Cadoz, Hajnal‐Gabriela Illes, Bouchra Loutfi, Jérôme Dimet, Vincent Le Moing, Nathalie Pansu, Clément Le Bihan, Antoine Kimmoun, Bruno Levy, Maximilen Saint Gilles, François Goehringer, Christian Rabaud, Sibylle Bevilacqua, Benjamin Lefèvre, Anne Guillaumot, Anne‐Sophie Boureau, Clotilde Allavena, Sabelline Bouchez, Romain Guery, Paul Le Turnier, Cécile Mear‐Passard, Christophe Rapp, Stéphane Lasry, Thierry Carmoi, Elisa Demonchy, Céline Michelangelli, Karine Risso, Paul Loubet, Alberto Sotto, Didier Laureillard, Etienne De Montmollin, Juliette Patrier, Paul Henri Wicky, Lucie Lefevre, Pierre Jacquet, Raphael Borie, Tiphaine Goulenok, Dominique Luton, Laurène Deconinck, Sylvie Le Gac, Cecile Azoulay, Nicolas Carlier, Liem Luong, Marie Lachatre, Odile Launay, Marine Livrozet, Bernard Cholley, Jean‐Benoit Arlet, Olivier Sanchez, Victoria Manda, Laurène Azemar, Guylaine Castor‐Alexandre, Jeanne Truong, Karine Lacombe, Thibault Chiarabini, Bénédicte Lefebvre, Nathalie De Castro, Geoffrey Liegeon, Diane Ponscarme, Julie Chas, Valérie Gaborieau, Eve Le Coustumier, Walter Picard, Jean‐Benoit Zabbe, Florent Peelman, Edouard Soum, Hugues Aumaître, Blandine Rammaert, Gwenaël Le Moal, Isabelle Pironneau, Anne Sophie Resseguier, Nadia Saidani, Firouzé Bani‐Sadr, Maxime Hentzien, Yohan N'guyen, Juliette Romaru, Kévin Didier, Isabelle Enderle, Fabrice Laine, Matthieu Lesouhaitier, Pierre Tattevin, Jean‐Marc Chapplain, Véronique Lemee, Eglantine Ferrand Devouge, Kévin Alexandre, Elise Artaud‐Maccari, Nathalie Allou, Marie Lagrange, Julien Jabot, Elisabeth Botelho‐Nevers, Amandine Gagneux‐Brunon, Tiffany Trouillon, Corinne Daniel, Benoît Roze, Delphine Bregeaud, Younes Ait Tamlihat, Ali Hachemi, Hélène Salvator, Erwan Fourn, David Zucman, Marie‐Laure Chabi‐Chavillat, Aurélie Martin, Eric Delaveuve, Coline Jaud‐Fischer, Paul Dunand, François Bissuel, Karen Delavigne, Alexa Debard, Pierre Delobel, Benjamine Sarton, Stella Rousset, Guillaume Martin‐Blondel, Laurent Guilleminault, Marlène Murris, Agnès Sommet, Olivier Lairez, Eric Senneville, Olivier Robineau, Agnès Meybeck, Denis Garot, Laurent Plantier, Valérie Gissot, Emmanuelle Mercier, Charlotte Salmon Gandonniere, Adrien Lemaignen, Julie Mankikian, Thomas Flament, Grégory Corvaisier, Delphine Lariviere, Marie Langelot‐Richard, Pauline Caraux Paz, Laurent Richier, Danielle Jaafar, Claudine Badr, and Fara Diop
DATA AVAILABILITY STATEMENT
Data can be made available upon reasonable request.
REFERENCES
- 1. Richardson S, Hirsch JS, Narasimhan M et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrario CM, Jessup J, Chappell MC et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 5. Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398–F405. [DOI] [PubMed] [Google Scholar]
- 6. Wu C, Ye D, Mullick AE et al. Effects of renin‐angiotensin inhibition on ACE2 and TMPRSS2 expression: insights into COVID‐19. Hypertension. 2020;76:e29–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sama IE, Ravera A, Santema BT et al. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J. 2020;41:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kreutz R, Algharably EAE, Azizi M et al. Hypertension, the renin‐angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID‐19. Cardiovasc Res. 2020;116:1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imai Y, Kuba K, Rao S et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuba K, Imai Y, Rao S et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with covid‐19. N Engl J Med. 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fosbøl EL, Butt JH, Østergaard L et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reynolds HR, Adhikari S, Pulgarin C et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrea C, Francesco M, Antonio N et al. Renin‐angiotensin‐aldosterone system inhibitors and outcome in patients with SARS‐CoV‐2 pneumonia: a case series study. Hypertension. 2020;76:e10–e12. [DOI] [PubMed] [Google Scholar]
- 16. Bean DM, Kraljevic Z, Searle T et al. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust. Eur J Heart Fail. 2020;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feng Y, Ling Y, Bai T et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Z, Cao J, Yao Y et al. The effect of RAS blockers on the clinical characteristics of COVID‐19 patients with hypertension. Ann Transl Med. 2020;8:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M. Age and multimorbidity predict death among COVID‐19 patients: results of the SARS‐RAS study of the Italian Society of Hypertension. Hypertension. 2020;76:366–372. [DOI] [PubMed] [Google Scholar]
- 20. Jung Sun‐Young, Choi Jae Chol, You Seung‐Hun, Kim Won‐Young. Association of Renin‐angiotensin‐aldosterone System Inhibitors With Coronavirus Disease 2019 (COVID‐19)‐ Related Outcomes in Korea: A Nationwide Population‐based Cohort Study. Clinical Infectious Diseases. 2020;71 (16):2121–2128. 10.1093/cid/ciaa624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for Coronavirus Disease 2019 (COVID‐19) Infection in Wuhan. China. JAMA Cardiol. 2020;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehta N, Kalra A, Nowacki AS et al. Association of use of angiotensin‐converting Enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang P, Zhu L, Cai J et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yazdanpanah Y. Impact on disease mortality of clinical, biological and virological characteristics at hospital admission and over time in COVID‐19 patients. J Med Virol. 2021;93:2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen C, Wang F, Chen P et al. Mortality and pre‐hospitalization use of renin‐angiotensin system inhibitors in hypertensive covid‐19 patients. J Am Heart Assoc. 2020;9:e017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao C, Cai Y, Zhang K et al. Association of hypertension and antihypertensive treatment with COVID‐19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaudhri I, Koraishy FM, Bolotova O et al. Outcomes associated with the use of RAAS Blockade in hospitalized patients with SARS‐CoV‐2 infection. Kidney360. 2020;1:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lahens A, Mullaert J, Gressens S et al. Association between RAAS blockers and outcome in COVID‐19: analysing in‐hospital exposure generates a biased seemingly protective effect of treatment. J Hypertens. 2021;39:367–375. [DOI] [PubMed] [Google Scholar]
- 29. Martínez‐Del Río J, Piqueras‐Flores J, Martín N‐S et al. Comparative analysis between the use of renin‐angiotensin system antagonists and clinical outcomes of hospitalized patients with COVID‐19 respiratory infection. Med Clin (Engl Ed). 2020;155:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tedeschi S, Giannella M, Bartoletti M et al. Clinical impact of renin‐angiotensin system inhibitors on in‐hospital mortality of patients with hypertension hospitalized for COVID‐19. Clin Infect Dis. 2020;71:899–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Son M, Seo J, Yang S. Association between renin‐angiotensin‐aldosterone system inhibitors and COVID‐19 infection in South Korea. Hypertension. 2020;76:742–749. [DOI] [PubMed] [Google Scholar]
- 32. Lafaurie M, Martin‐Blondel G, Delobel P, Charpentier S, Sommet A, Moulis G. Outcome of patients hospitalized for COVID‐19 and exposure to angiotensin‐converting enzyme inhibitors and angiotensin‐receptor blockers in France: results of the ACE‐CoV study. Fundam Clin Pharmacol. 2021;35:194–203. [DOI] [PubMed] [Google Scholar]
- 33. de Abajo FJ, Rodríguez‐Martín S, Lerma V et al. Use of renin‐angiotensin‐aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet. 2020;395:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Savarese Gianluigi, Benson Lina, Sundström Johan, Lund Lars H.. Association between renin–angiotensin–aldosterone system inhibitor use and COVID‐19 hospitalization and death: a 1.4 million patient nationwide registry analysis. European Journal of Heart Failure. 2020. 10.1002/ejhf.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams B, Mancia G, Spiering W et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 36. Liabeuf Sophie, Moragny Julien, Bennis Youssef, Batteux Benjamin, Brochot Etienne, Schmit Jean Luc, Lanoix Jean‐Philippe, Andrejak Claire, Ganry Olivier, Slama Michel, Maizel Julien, Mahjoub Yazine, Masmoudi Kamel, Gras‐Champel Valérie. Association between renin–angiotensin system inhibitors and COVID‐19 complications. European Heart Journal ‐ Cardiovascular Pharmacotherapy. 2020. 10.1093/ehjcvp/pvaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grover Abhinav, Oberoi Mansi. A systematic review and meta‐analysis to evaluate the clinical outcomes in COVID‐19 patients on angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers. European Heart Journal ‐ Cardiovascular Pharmacotherapy. 2021;7 (2):148–157. 10.1093/ehjcvp/pvaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo X, Zhu Y, Hong Y. Decreased mortality of COVID‐19 with renin‐angiotensin‐aldosterone system inhibitors therapy in patients with hypertension: a meta‐analysis. Hypertension. 2020;76:e13–e14. [DOI] [PubMed] [Google Scholar]
- 39. Lodi S, Phillips A, Lundgren J et al. Effect estimates in randomized trials and observational studies: comparing apples with apples. Am J Epidemiol. 2019;188:1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cole SR, Platt RW, Schisterman EF et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen JB, D'Agostino‐McGowan L, Jensen ET, Rigdon J, South AM. Evaluating sources of bias in observational studies of angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker use during coronavirus disease 2019: beyond confounding. J Hypertens. 2021;39:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Griffith GJ, Morris TT, Tudball MJ et al. Collider bias undermines our understanding of COVID‐19 disease risk and severity. Nat Commun. 2020;11:5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jung C, Bruno RR, Wernly B et al. Inhibitors of the renin‐angiotensin‐aldosterone system and Covid‐19 in critically ill elderly patients. Eur Heart J Cardiovasc Pharmacother. 2021;16:76–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou F, Liu YM, Xie J et al. Comparative impacts of ACE (angiotensin‐converting enzyme) inhibitors versus angiotensin II receptor blockers on the risk of COVID‐19 mortality. Hypertension. 2020;76:e15–e17. [DOI] [PubMed] [Google Scholar]
- 45. Cannata F, Chiarito M, Reimers B et al. Continuation versus discontinuation of ACE inhibitors or angiotensin II receptor blockers in COVID‐19: effects on blood pressure control and mortality. Eur Heart J Cardiovasc Pharmacother. 2020;6:412–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lam KW, Chow KW, Vo J et al. Continued in‐hospital angiotensin‐converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID‐19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baral R, White M, Vassiliou VS. Effect of renin‐angiotensin‐aldosterone system Inhibitors in patients with COVID‐19: a systematic review and meta‐analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID‐19: A systematic review and meta‐analysis. Pharmacol Res. 2020;158:104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bavishi C, Whelton PK, Mancia G, Corrao G, Messerli FH. Renin‐angiotensin‐system inhibitors and all‐cause mortality in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. J Hypertens. 2021;39:784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chu Chang, Zeng Shufei, Hasan Ahmed A., Hocher Carl‐Friedrich, Krämer Bernhard K., Hocher Berthold. Comparison of infection risks and clinical outcomes in patients with and without SARS‐CoV‐2 lung infection under renin–angiotensin–aldosterone system blockade: Systematic review and meta‐analysis. British Journal of Clinical Pharmacology. 2020. 10.1111/bcp.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cohen JB, Hanff TC, William P et al. Continuation versus discontinuation of renin‐angiotensin system inhibitors in patients admitted to hospital with COVID‐19: a prospective, randomised, open‐label trial. Lancet Respir Med. 2021;9:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lopes RD, Macedo AVS, de Barros ESPGM et al. Effect of discontinuing vs continuing angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID‐19: a randomized clinical trial. JAMA. 2021;325:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 54. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;5:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data can be made available upon reasonable request.


