Abstract
Background
Infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) may be life‐threatening, and specific antiviral drugs are currently not available. However, first studies indicated that convalescent plasma treatment might improve the clinical outcome of coronavirus disease 2019 (COVID‐19) patients.
Study Design and Methods
In the current study, we investigated the efficacy of convalescent plasma treatment in eight COVID‐19 patients. All the patients were critically ill, and seven of them were SARS‐CoV‐2 RNA–positive when starting treatment. SARS‐CoV‐2–specific antibodies were determined by an enzyme‐linked immunosorbent assay detecting immunoglobulin G (IgG) antibodies against the S1 protein (Euroimmun), and the neutralizing titers were determined with a cell‐culture‐based neutralization assay. Plasma treatment started between 4 and 23 days after the onset of symptoms. The patients were usually treated by three plasma units, each containing 200–280 ml, which was applied at day 1, 3, and 5.
Results
Donor sera had on average lower IgG antibody ratios and neutralizing titers than the COVID‐19 patients before the onset of treatment (median ratio of 5.8 and neutralizing titer of 1:320 vs. 7.5 and 1:640, respectively). Nevertheless, we observed an increase of antibody ratios in seven and of neutralizing titers in five patients after treatment; which did, however, not correlate with patient survival. Plasma treatment was effective in three patients, but five deceased despite treatment. Patients who deceased had a later treatment onset than survivors and finally died from multiple organ failure.
Conclusion
Our data indicate that the efficacy of convalescent plasma treatment of critically ill COVID‐19 patients who already had developed strong antiviral immune responses and organ complications is limited.
Keywords: FFP transfusion, infectious disease testing, intravenous immunoglobulin
1. INTRODUCTION
A novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) responsible for the coronavirus disease 2019 (COVID‐19) was newly identified in the Hubei province, PR China, before becoming a pandemic causing enormous health and socioeconomic implications. SARS‐CoV‐2 is readily transmitted via aerosols in humans. 1 , 2 The symptoms of a SARS‐CoV‐2 infection range from cold‐like symptoms accompanied by cough and fever to severe pneumonia or disseminated infection, which may be fatal. 3 Individuals of any age may be infected with SARS‐CoV‐2, but the elderly and patients with underlying morbidities particularly suffer from COVID‐19. 4 , 5 , 6 No specific antiviral drug has been proven effective for treatment of patients with COVID‐19, apart from supportive care such as ventilation support. Of note, the effect of corticosteroids was studied in an open‐label randomized controlled trial (RCT) with hospitalized COVID‐19 patients receiving standard of care (N = 4321) compared with additional low‐dose dexamethasone (N = 2104). In the entire cohort, application of dexamethasone showed a significant reduction in 28‐day mortality. 7
However, quickly available treatment options are still urgently needed. The use of convalescent plasma for the treatment of potentially lethal infections represents an effective strategy that has been applied for other viral diseases such as Ebola or influenza A (H5N1). 8 , 9 In patients suffering from coronavirus infection with SARS‐CoV‐1, the use of convalescent plasma could lead to an improvement of the clinical course and reduced mortality. 10 Therefore, convalescent plasma has been proposed to treat COVID‐19 patients. 11 Only few studies described the use of convalescent plasmas in COVID‐19 patients by now. 12 , 13 , 14 The clinical condition and survival probability were improved in patients undergoing plasma treatment. 12 , 13 , 14 Because critically ill patients were additionally treated with experimental antiviral drugs, the contribution of plasma to improved clinical outcome of COVID‐19 needs further investigation. 12 Moreover, less is known about the antibody kinetics in convalescent plasma donors and recipients. Convalescent plasma with high neutralizing titers may constitute one possible treatment option for COVID‐19 patients. 15 Numerous enzyme‐linked immunosorbent assay (ELISA) kits to detect antibodies against SARS‐CoV‐2 are available on the market. Although recent studies demonstrated that there is a good correlation between the immunoglobulin G (IgG) titers, 16 , 17 a classical neutralization assay is still the method of choice to determine the antiviral activity of serum or plasma antibodies against SARS‐CoV‐2. 18 According to an EU guidance for COVID‐19 convalescent plasma collection and transfusion, it is strongly recommended that defined SARS‐CoV‐2 neutralizing antibody titers be measured in the donated plasma. The neutralizing titers should be optimally greater than 1:320, when available, as recommended by the European Commission for health and food safety. 19 The dynamics of IgG‐antibody titers and neutralizing titers in convalescent plasma donors and recipients are still poorly investigated. The primary aim of the current study was to assess humoral immune responses against SARS‐CoV‐2 in convalescent blood donors and critically ill COVID‐19 patients before and after the onset of plasma treatment. The secondary aim was to investigate the effectivity of convalescent plasma treatment in critically ill COVID‐19 patients. Therefore, humoral immune responses were tested by IgG antibody ELISA and a standard neutralization assay. The distribution of antibody responses and the correlation of results to ELISA and neutralization assay were analyzed. Furthermore, we investigated the effectivity of convalescent plasma treatment in eight critically ill COVID‐19 patients and monitored the IgG‐ELISA and neutralizing antibody titers.
2. MATERIALS AND METHODS
2.1. Volunteers and patients
Volunteers were selected as convalescent plasma donors if their SARS‐CoV‐2 infection was polymerase chain reaction (PCR)‐confirmed, their antibody ratio was >3, they fulfilled all criteria as blood donor, and they were blood group identical to the patient. Between April 24 and June 7, 2020, eight SARS‐CoV‐2–infected patients with respiratory insufficiency requiring intensive care treatment who themselves or their legal guardians provided informed consent were recruited for this study. The treatment was carried out as a series of individual healing attempts, whereby the indication in each case was determined by an experienced intensivist and transfusionist. The study was approved by the local ethics committee and was performed in accordance with the ethical standards noted in the 1964 Declaration of Helsinki and its later amendments or comparable ethics standards. All volunteers and patients/their legal guardians provided informed consent to participate in the study.
2.2. Antibody ELISA
IgG antibodies against SARS‐CoV‐2 were determined by a semiquantitative ELISA (Euroimmun, Lübeck, Germany), according to the manufacturer's instructions. The ELISA plates were coated with recombinant SARS‐CoV‐2 spike protein (S1 domain). Results are given as a ratio (patient sample/control sample). An antibody ratio of ≥1.1 was considered positive, ≥0.8 to <1.1 as borderline, and <0.8 as negative.
2.3. Cells and virus
Vero E6 cells were purchased from ATCC (Manassas, Virginia, USA; ATCC® CRL‐1586™) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% fetal calf serum (FCS), penicillin (100 IU/ml), and streptomycin (100 μg/ml). SARS‐CoV‐2 was isolated from a nasopharyngeal swab of a patient suffering from COVID‐19 as previously described. 20 The virus was propagated on Vero E6 cells cultured in DMEM containing 10% (v/v) FCS and supplemented with penicillin (100 IU/ml), streptomycin (100 μg/ml), ciprofloxacin (10 μg/ml), and amphotericin B (2.5 μg/ml). Viral titers were determined by endpoint dilution assay and expressed as 50% tissue culture infective dose (TCID50)/ml.
2.4. Neutralization
Neutralization capacity of sera from potential plasma donors or from COVID‐19 patients was determined by endpoint dilution assay. Therefore, serial dilutions (1:20 to 1:2560) of the respective sera were pre‐incubated with 100 TCID50 of SARS‐CoV‐2 for 1 h at 37°C and added afterward to confluent Vero E6 cells cultured in 96‐well microtiter plates. On day 3 after infection, the cells were stained with crystal violet (Roth, Karlsruhe, Germany) solved in 20% methanol (Merck, Darmstadt, Germany), and the appearance of cytopathic effects (CPEs) was analyzed by light microscopy. The neutralizing titer was defined as the reciprocal of the highest serum dilution at which no CPE breakthrough in any of the triplicate testing wells was observed.
2.5. Plasmapheresis Procedures
By August 10, 2020, 22 plasmapheresis procedures were performed in 11 volunteers, yielding 66 units of plasma (200–280 ml each). We used the cell separators Amicus™ (Fresenius Kabi, Bad Homburg, Germany), Trima Accel ™ (Terumo BCT, Eschborn, Germany), and Haemonetics MCS+ TM (Haemonetics GmbH, München, Germany). The production of the convalescent plasma was possible due to the permission by the county government (AZ24.05.05.02, Gestattung gemäß §79 Absatz 5 AMG). Additionally, serum samples were collected at the time of plasma donation to measure the ELISA and neutralizing antibody titers.
2.6. Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0.1 (San Diego, CA, United States). For the analysis of neutralization titers, the reciprocal value was chosen (1/x). Detectable neutralization below a titer of 1:20 was set as 1:10. We used Spearman correlation and linear regression analysis for numerical variables, that is, for ELISA results and neutralizing antibody titers. Two‐sided p values <.05 were considered significant.
3. RESULTS
3.1. Recruitment of CP donors and COVID‐19 patients
The study includes 61 potential plasma donors (43 males, 18 females) who recovered from COVID‐19 infection. SARS‐CoV‐2 infection was previously confirmed by PCR. The mean age of the donors was 45 years (19–65), and their mean body mass index, 26.4 kg m−2 (22.2–36.8 kg m−2). Thirty‐six stayed in a risk area, and the mean interval to onset of symptoms was 55 days (22–100). An experienced physician examined the volunteers for their suitability as blood donor. The cohort included 26 with blood group O, 24 with A, five with B, and four with AB. Two were not tested for their blood group because they have directly been excluded from blood donation due to medical reasons. Donor sera were tested for IgG antibodies against SARS‐CoV‐2 by ELISA and a standard neutralization assay. Twenty 3‐year‐old retention samples of blood donors served as negative controls. Eight COVID‐19 patients were treated under intensive care conditions at the University Hospital Essen and received convalescent plasma as an individual healing attempt (Table 1). All patients were critically ill and required mechanical ventilation. Six patients received extracorporeal membrane oxygenation (ECMO). ECMO therapy was chosen in case of a severe disturbance of oxygenation and carboxylation, if the ventilation pressure was too high and a prone position could not improve the disorder. Five patients received convalescent plasma from Essen and one from Aachen, one from Düsseldorf, and one from Bad Oeynhausen, respectively. We started testing for antibodies on April 9, 2020, the first plasma collection was performed on April 15, 2020, and the first administration of convalescent plasma was on April 24, 2020.
TABLE 1.
Clinical characteristics of convalescent plasma–treated COVID‐19 patients
| Internal ID | Sex/Age /blood group | SARS‐CoV‐2 PCR (ct) a | RKP (day) b | Pre‐existing comorbidity | Acute comorbidity c /Cause of death | Other COVID‐19 therapies | Horowitz Index (mmHg) | Outcome d |
|---|---|---|---|---|---|---|---|---|
| COVP003 | M/26/A | 22.63 | 23 | Adipositas (BMI = 44 kg/m2) and nicotine abuse | Multisystem organ failure | Anticoagulation, corticosteroids, hydroxychloroquine | 101 | D |
| COVP004 | M/29/A | 25.38 | 11 | Prior nicotine and cannabis abuse | Intermittent renal dysfunction, blurring of vision | Anticoagulation, corticosteroids | 255 | A |
| COVP006 | M/65/O | 16.59 | 7 | Coronary heart disease with myocardial infarctions 2007 and 2012, pulmonary adenocarcinoma treated with radiochemotherapy, nicotine abuse | Tachycardic atrial fibrillation | Anticoagulation, corticosteroids | 120 | A |
| COVP007 | M/51/B | 28.32 | 13 | Arterial hypertension, adipositas | Multisystem organ failure, bacterial sepsis | Anticoagulation, corticosteroids | 54 | D |
| COVP008 | M/51/B | n.d. | >20 | Adipositas | Multisystem organ failure, fungal sepsis | Anticoagulation | 47 | D |
| COVP009 | M/30/B | 19.13 | 4 | Chronic dilated cardiomyopathy, mitral and tricuspid valve incompetence, pulmonary hypertension | End stage cardiomyopathy treated with an artificial heart, acute pancreatitis, multisystem organ failure | Anticoagulation | 277 | D |
| COVP014 | F/70/O | 23.01 | 11 | Cerebral ischemia (right arteria cerebri media), arterial hypertension, hyperthyroidism, rheumatoid arthritis | Cerebellar ischemia with focal hemorrhage, pulmonary hypertension, multisystem organ failure, sepsis | Anticoagulation, corticosteroids | 204 | D |
| COVP025 | M/60/A | 28.00 | 10 | Unknown | Supraventricular tachycardia, spontaneous pneumothorax, bacterial sepsis | Anticoagulation, corticosteroids, remdesivir | 171 | A |
SARS‐CoV‐2 PCR ct‐value before the onset of plasma treatment.
Onset of treatment with convalescent plasma (RKP) given as day after onset of symptoms. Horowitz index: <300–>200 = mild lung injury; 200–100 = moderately severe lung injury; below 100 = severe lung injury.
Illness immediately prior to or during ICU admission.
D = death; A = alive.
3.2. SARS‐CoV‐2 IgG ELISA and neutralizing antibody titers in convalescent plasmas
Sera from 61 healthy volunteers who recovered from COVID‐19 infection were tested for SARS‐CoV‐2 IgG ELISA and neutralizing antibody titers. Forty‐nine volunteers (80%) displayed an antibody ratio classified as positive (ODRatio ≥ 1.1), five (8%) as borderline (ODRatio ≥ 0.8 to <1.1), and seven (11%) as negative (ODRatio < 0.8) (Figure 1). The median ratio was ODRatio = 2.88 (range 0.24–10.32). Additionally, twenty 3‐year‐old retention plasma samples of blood donors were tested. As expected, all samples acquired a long time before the onset of the SARS‐CoV‐2 pandemic were negative for SARS‐CoV‐2–specific IgG (median ratio: ODRatio = 0.18, range 0.10–0.36) (Figure 1). There was no time dependency of SARS‐CoV‐2 IgG antibodies (r = 0.14 and p = .3) observed within this time period, as indicated by Spearman's correlation analysis (Figure 2). The content of neutralizing antibodies is clearly a critical factor in convalescent plasma treatments. Therefore, we determined the neutralizing activity of the sera toward SARS‐CoV‐2 in cell culture. In total, 52 out of 61 convalescent plasmas completely neutralized a viral load of 100 TCID50 SARS‐CoV‐2 at dilutions ranging from 1:20 to 1:640, and the median neutralizing titer was 1:160 (Figure 3). Interestingly, neutralizing antibodies could not be detected in nine of 61 donors, despite a previous PCR‐confirmed SARS‐CoV‐2 infection (Figure 3). The detection limit was at a dilution of 1:20. Next, we analyzed the correlation between the SARS‐CoV‐2 spike S1 IgG antibody ratios and neutralizing titers. We found a significant correlation (r = 0.69 and p < .0001) between the IgG ELISA results and neutralizing antibody titers (Figure 4).
FIGURE 1.
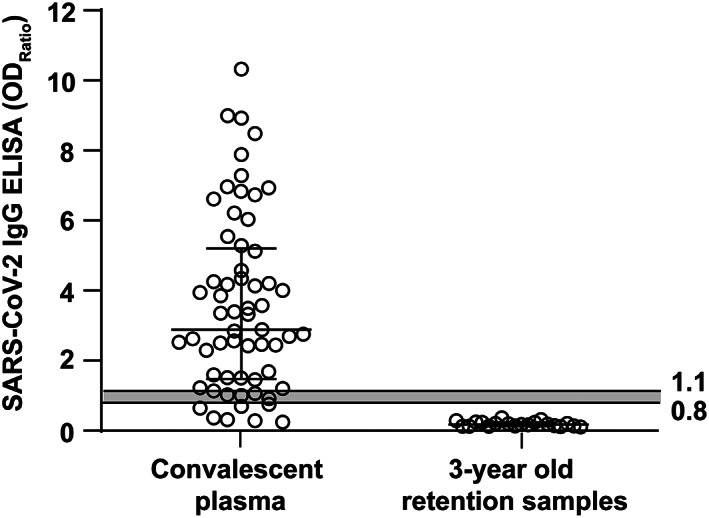
SARS‐CoV‐2 IgG ELISA (Euroimmun) ratios in sera of convalescent volunteers and in control samples. In total, 61 sera from convalescent, potential blood donors with a SARS‐CoV‐2 PCR‐confirmed infection (left panel) and in twenty 3‐year‐old retention samples from our blood bank (right panel) were tested for SARS‐CoV‐2 spike S1 domain–specific IgG. Samples were classified as positive (ODRatio ≥ 1.1), borderline (ODRatio ≥ 0.8 to <1.1), or negative (ODRatio < 0.8). Horizontal bars represent the median and the interquartile range. The gray shaded area indicates borderline results
FIGURE 2.
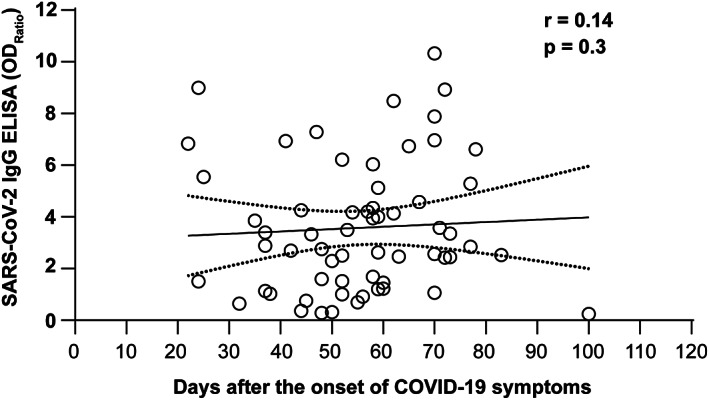
Distribution of antibody ratios in sera of convalescent volunteers acquired at different periods after the disease onset. SARS‐CoV‐2 spike S1 antibodies were measured by ELISA (Euroimmun) in sera from convalescent, potential blood donors with PCR‐confirmed SARS‐CoV‐2 infection. Correlation analysis was performed by Spearman's test. The continuous line shows the regression line, and the broken lines show the 95% confidence interval
FIGURE 3.
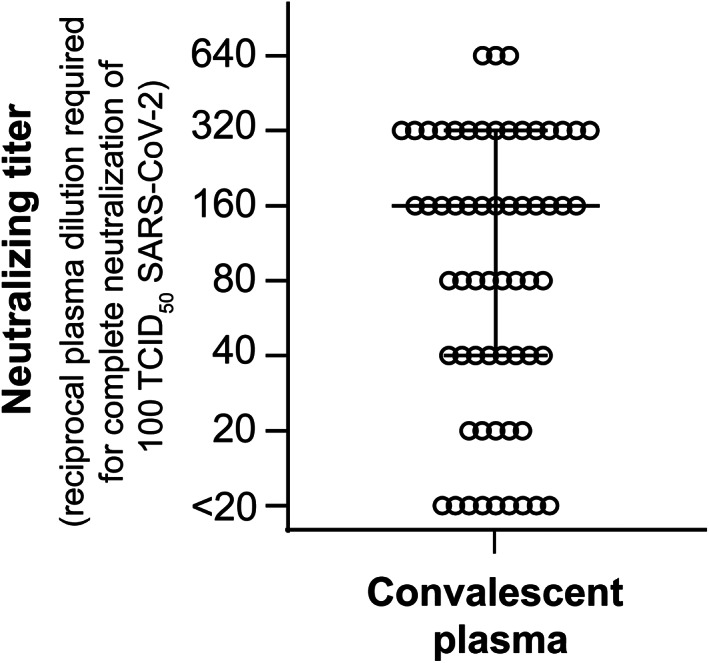
SARS‐CoV‐2 neutralizing antibody titers in sera of convalescent plasma donors. The neutralizing antibody titers in sera from 61 potential blood donors were determined by endpoint dilution assay. Serial dilutions (1:20 to 1:2560) of the respective plasma samples were pre‐incubated with 100 TCID50 of SARS‐CoV‐2 for 1 h at 37°C and subsequently incubated on Vero E6 cells for 3 days. Cytopathic effects (CPEs) were analyzed by light microscopy. The neutralizing titer is given as the reciprocal of the highest plasma dilution required for complete virus neutralization. The horizontal bars represent median and the interquartile range
FIGURE 4.
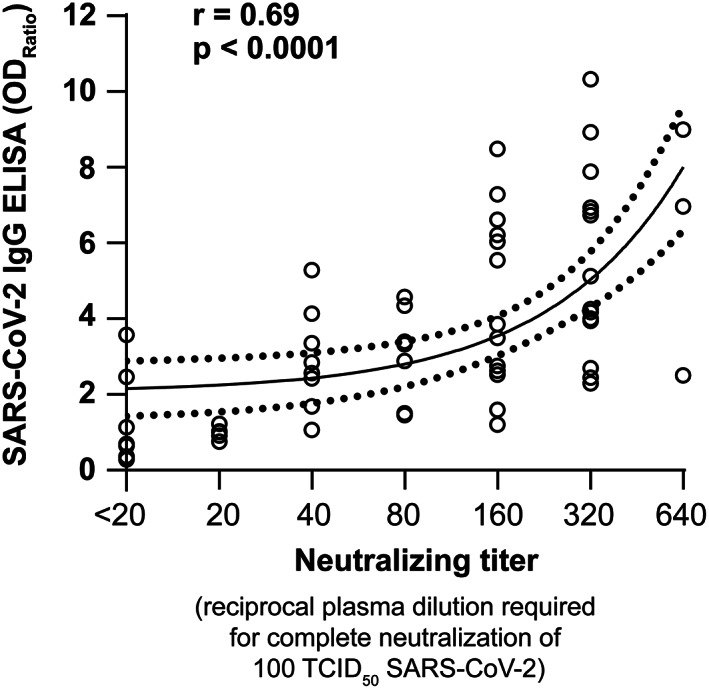
Correlation between SARS‐CoV‐2 IgG ELISA and neutralizing antibody titers. The correlation between the SARS‐CoV‐2 IgG ELISA ratios (Euroimmun) and neutralizing antibody titers in sera of 61 convalescent, potential blood donors with PCR‐confirmed infection was calculated using Spearman's rank correlation test
3.3. Clinical efficacy of convalescent plasma treatment of critically ill COVID‐19 patients
Starting in April 2020, eight critically ill COVID‐19 patients were treated with convalescent plasmas at intensive care units at the University Hospital Essen. The majority of patients received three plasma units, each containing 200–280 ml, which was infused at day 1, 3, and 5. However, one of the patients (COVP008) received only two units of convalescent plasma (CP) because after starting CP treatment, the PCR yielded a negative result for SARS‐CoV‐2 at baseline and because no further unit with blood group B was available. Convalescent plasma was well tolerated in all patients. The course of antibodies is shown in Figure 5(A) and (B). Six of eight patients were known to suffer from COVID‐19 since at least 1 week before the onset of plasma treatment (Table 1). Six patients showed critical comorbidities at the onset of COVID‐19 symptoms (Table 1). One patient showed no comorbidities at hospitalization (Table 1). Seven of the patients were SARS‐CoV‐2 RNA–positive before the onset of plasma treatment (Figure 5(C)). During the period of treatment, we monitored the SARS‐CoV‐2 IgG ELISA and neutralizing antibody titers of those patients. At the onset of treatment, five of the patients showed higher SARS‐CoV‐2 IgG ELISA ratios than those of the respective donors (Figure 5(A)). The median antibody ratio of the eight patients was 7.5, and that of their donors was 5.8. Accordingly, neutralizing antibody titers in sera from COVID‐19 patients were higher (median 1:640) than those in sera from donors whose convalescent plasmas were used for treatment (median 1:320) (Figure 5(B)). Nevertheless, we observed an increase of antibody ratios in seven patients after treatment with convalescent plasma. Neutralizing titers increased in four patients (COVP003, COVP004, COVP008, and COVP009) after receiving the first convalescent plasma treatment, reaching a neutralizing titer of up to 1:10,240 (Figure 5(B)). During the whole course after convalescent plasma treatment, five patients showed an increase of neutralizing antibody titers. However, the increase in antibody ratios or neutralizing titers did not correlate with patient survival.
FIGURE 5.
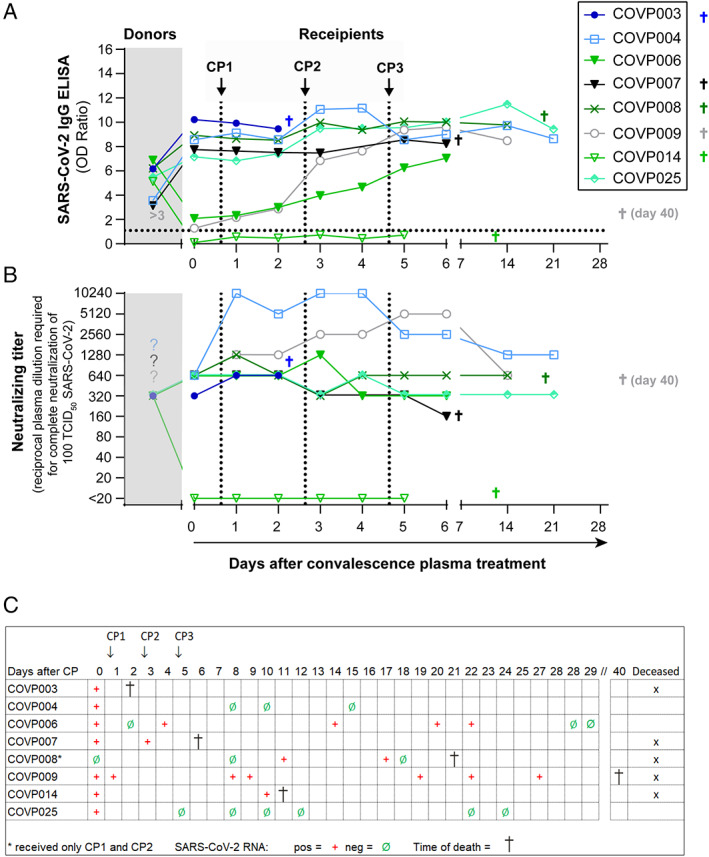
Impact of convalescent plasma treatment of COVID‐19 patients on survival and antibody titers. SARS‐CoV‐2 IgG ELISA (A) and neutralizing antibody titers (B) of eight severely ill COVID‐19 patients undergoing convalescent plasma treatment were analyzed and compared with the titers of the corresponding convalescence plasmas used for treatment of the indicated patients. Dotted lines indicate the time points of plasma applications (CP1, CP2, and CP3). (C) Clinical outcomes of convalescent plasma–treated COVID‐19 patients and SARS‐CoV‐2 RNA results at distinct time points before and upon plasma treatment [Color figure can be viewed at wileyonlinelibrary.com]
The clinical course improved in three patients (COVP004, COVP006, and COVP025) shortly after receiving convalescent plasma, and all of them finally recovered from infection (Figure 5(C)). Notably, patient COVP006 suffered from a coronary heart disease and pulmonary adenocarcinoma (Table 1). Two of the patients who finally recovered from COVID‐19 (COVP004 and COVP006) became SARS‐CoV‐2 RNA–negative rapidly after plasma transfusion, and one on day 28 after the onset of treatment (Figure 5(C)). Four patients (COVP003, COVP007, COVP009, and COVP014) remained SARS‐CoV‐2 RNA–positive and died despite convalescent plasma treatment and high levels of neutralizing antibody titers up to 1:5120 (Figure 5(B) and (C)). All these patients showed severe, life threatening comorbidity at the onset of the convalescent plasma treatment such as multiple organ failure or end‐stage cardiomyopathy (COVP003, COVP007, COVP009, and COVP014) (Table 1). One patient (COVP008) died on day 21 despite receiving two plasma infusions and becoming RNA negative by day 18 after the onset of treatment. The patients whose survival seemed to be associated with plasma therapy received convalescent plasma on day 10 and those who deceased on day 13 (median after onset of symptoms). Furthermore, the survivors suffered from fewer comorbidities.
Taken together, convalescent plasma treatment was associated with survival in three of eight (38%) critically ill COVID‐19 patients.
4. DISCUSSION
In the present study, we analyzed the SARS‐CoV‐2 IgG ELISA and neutralizing antibody titers in plasmas from 61 donors who recovered from SARS‐CoV‐2 infection and from eight critically ill intensive care COVID‐19 patients undergoing convalescence plasma therapy as individual healing attempt.
Overall, there was a good correlation between the IgG‐S1‐ELISA ratios and neutralizing titers (r = 0.69). The SARS‐CoV‐2 IgG ELISA (Euroimmun) test measures antibody binding to the spike‐S1 domain of the SARS‐CoV‐2 spike protein. This region harbors the ACE‐2 receptor‐binding site, which is crucial for the infection of the cell. 21 , 22 Antibodies that bind the receptor‐binding site may neutralize the virus, which would explain the correlation between S1‐specific antibody titers and neutralizing titers. 23 , 24 The median neutralizing antibody titer we found in convalescent donors was 1:160 (range 1:20–1:640). Similar average neutralizing titers in people who recovered from COVID‐19 were recently described by others. 25 Interestingly, we found that neutralizing antibody titers in sera from recovered donors were markedly lower (mean = 1:160) than those in sera from critically ill COVID‐19 patients taken at least 7 days after the onset of COVID‐19 symptoms (median = 1:640). In addition, neutralizing titers in convalescent plasma donors were lower or equal when compared with those of the COVID‐19 patients included in this study. Because the plasma donors reported only mild symptoms or were asymptomatic, there seems to be a clear correlation between neutralizing antibody titers and the severity of disease. This finding is consistent with recent studies reporting that patients with more severe disease contain relatively higher neutralizing antibody titers than people recovered from a mild disease or those with an asymptomatic course of disease. 26 , 27 , 28 , 29 Furthermore, our observation is in line with recent studies investigating the antibody titers in symptomatic and asymptomatic people with a PCR‐confirmed SARS‐CoV‐2 infection. The authors reported that IgG‐ELISA and neutralizing antibody titers were significantly lower in the asymptomatic cohort. 27 Moreover, a recent study showed that the average neutralizing antibody titers in outpatients or convalescent plasma donors are up to 3000 times lower than those in hospitalized patients. 30 The reason for the different antibody kinetics is not fully understood. The seroconversion rates and neutralizing antibody titers in severe cases may be associated with prolonged viral shedding, and low antibody responses in mild cases may be due to short‐lived infections. 31 However, the clinical meaning of the neutralizing antibody titers against SARS‐CoV‐2 warrants further studies.
Despite the presence of neutralizing antibodies, seven of eight patients were SARS‐CoV‐2 RNA–positive when receiving the first plasma application. All the patients were in a critical condition and required mechanical ventilation or ECMO. The patients received the first convalescent plasma application between at least 7 and >22 days after the onset of disease, and most of the patients already had high neutralizing antibody titers, which were higher than those of the convalescent plasma. The mortality rate in this cohort was 62.5% (5/8 patients deceased). In comparison, a comprehensive study published in April 2020 reported a mortality rate of 76.4% in the 18–65 age group and that of 97.2% in the >65 age group of patients who received mechanical ventilation but no convalescent plasma transfusion. 32 However, due to the limited number of patients included in our study, it was impossible to determine whether CP treatment might have a beneficial effect on the overall survival. Notably, recent studies from China, United States, and India (PLACID trial) published between August and November 2020 reported no significant differences in clinical status or overall mortality between patients treated with convalescent plasma and those without CP treatment. 33 , 34 , 35 In our study, only three of eight patients recovered from COVID‐19, confirming that there is no significant benefit of convalescence plasma therapy started in advanced stage of infection, when the patients already have high titers of neutralizing antibodies in the blood stream. Our data are consistent with other recent and past literature, demonstrating that passive antibody‐based immunotherapies are most efficacious when administrated early in disease. 14 , 36 , 37 , 38
In summary, we report that the efficacy of convalescent plasma treatment of critically ill COVID‐19 patients at late stage of disease, when the patients already have high levels of neutralizing antibodies, is less effective. Starting a convalescent plasma treatment in high‐risk patients at earlier stage of disease could improve the effectiveness of the therapy.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
ACKNOWLEDGMENTS
The authors thank Stiftung Universitätsmedizin Essen (grant awarded to AK), and the Rudolf Ackermann Foundation (grant awarded to OW) for financial support. The authors would like to thank Babette Große‐Rhode and Martina Filipovic for their excellent technical assistance and Angelina Giorgio for the management of data from the potential blood donors. This study was funded by the Stiftung Universitätsmedizin Essen (Awarded to AK), and the Rudolf Ackermann Foundation (Awarded to OW).
Open access funding enabled and organized by Projekt DEAL.
Lindemann M, Lenz V, Knop D, et al. Convalescent plasma treatment of critically ill intensive care COVID‐19 patients. Transfusion. 2021;61:1394–1403. 10.1111/trf.16392
Funding information Rudolf Ackermann Stiftung; Stiftung Universitätsmedizin Essen
REFERENCES
- 1. Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS‐CoV‐2 transmission. Proc Natl Acad Sci U S A. 2020;117:11875–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morawska L, Cao J. Airborne transmission of SARS‐CoV‐2: The world should face the reality. Environ Int. 2020;139:105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonanad C, Garcia‐Blas S, Tarazona‐Santabalbina F, Sanchis J, Bertomeu‐Gonzalez V, Facila L, et al. The effect of age on mortality in patients with COVID‐19: A meta‐analysis with 611,583 subjects. J Am Med Dir Assoc. 2020;21:915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jordan RE, Adab P, Cheng KK. Covid‐19: Risk factors for severe disease and death. BMJ. 2020;368:m1198. [DOI] [PubMed] [Google Scholar]
- 7. Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Dexamethasone in Hospitalized Patients with Covid‐19. N Engl J Med. 2021;384(8):693–704. 10.1101/2020.06.22.20137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1. [DOI] [PubMed] [Google Scholar]
- 9. van Griensven J, Edwards T, de Lamballerie X, Semple MG, Gallian P, Baize S, et al. Evaluation of convalescent plasma for ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis. 2020;20:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. Treatment of COVID‐19 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190(11):2290–2303. 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X, Guo X, Xin Q, Pan Y, Hu Y, Li J, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 in patients and convalescent patients. Clin Infect Dis. 2020;71(10):2688–94. 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Müller L, Ostermann PN, Walker A, Wienemann T, Mertens A, Adams O, et al. Sensitivity of anti‐SARS‐CoV‐2 serological assays in a high‐prevalence setting. Eur J Clin Microbiol Infect Dis. 2021. 10.1007/s10096-021-04169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stroemer A, Grobe O, Rose R, Fickenscher H, Lorentz T, Krumbholz A. Diagnostic accuracy of six commercial SARS‐CoV‐2 IgG/total antibody assays and identification of SARS‐CoV‐2 neutralizing antibodies in convalescent sera. medRxiv. 2020. 10.1101/2020.06.15.20131672. [DOI] [Google Scholar]
- 18. Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Clinical performance of different SARS‐CoV‐2 IgG antibody tests. J Med Virol. 2020;92(10):2243–47. 10.1002/jmv.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Commission D‐GfHaFS . An EU programme of COVID‐19convalescent plasma collection and transfusion. Available from: https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf. 2020.
- 20. Heilingloh CS, Aufderhorst UW, Schipper L, Dittmer U, Witzke O, Yang D, et al. Susceptibility of SARS‐CoV‐2 to UV irradiation. Am J Infect Control. 2020;48:1273–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181:281–92.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffmann M, Kleine‐Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kreer C, Zehner M, Weber T, Ercanoglu MS, Gieselmann L, Rohde C, et al. Longitudinal isolation of potent near‐germline SARS‐CoV‐2‐neutralizing antibodies from COVID‐19 patients. Cell. 2020;182:1663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, et al. Detection of SARS‐CoV‐2‐specific humoral and cellular immunity in COVID‐19 convalescent individuals. Immunity. 2020;52:971–7.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gharbharan A, Jordans CCE, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FPN, et al. Convalescent plasma for COVID‐19. A randomized clinical trial. medRxiv. 2020. 10.1101/2020.07.01.20139857. [DOI] [Google Scholar]
- 26. Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS‐CoV‐2 in a COVID‐19 recovered patient cohort and their implications. medRxiv. 2020. 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 27. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26:845–8. [DOI] [PubMed] [Google Scholar]
- 28. Wang P, Liu L, Nair MS, Yin MT, Luo Y, Wang Q, et al. SARS‐CoV‐2 neutralizing antibody responses are more robust in patients with severe disease. Emerg Microbes Infect. 2020;9:2091–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu STH, Lin HM, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID‐19: A propensity score‐matched control study. Nat Med. 2020;26:1708–13. [DOI] [PubMed] [Google Scholar]
- 30. Dogan M, Kozhaya L, Placek L, Gunter C, Yigit M, Hardy R, et al. Novel SARS‐CoV‐2 specific antibody and neutralization assays reveal wide range of humoral immune response during COVID‐19. medRxiv. 2020. 10.1101/2020.07.07.20148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang AT, Garcia‐Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richardson S, Hirsch JS, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323:2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A Randomized Trial of Convalescent Plasma in Covid‐19 Severe Pneumonia. N Engl J Med. 2021;384(7):619–29. 10.1056/nejmoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P, et al. Convalescent plasma in the management of moderate covid‐19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID trial). BMJ. 2020;371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: A randomized clinical trial. JAMA. 2020;324:460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hung IFN, KKW T, Lee CK, Lee KL, Yan WW, Chan K, et al. Hyperimmune IV immunoglobulin treatment: A multicenter double‐blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144:464–73. [DOI] [PubMed] [Google Scholar]
- 38. Liu STH, Lin H, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID‐19: a propensity score–matched control study. Nat Med. 2020;26(11):1708–13. 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]


