To the Editor,
The COVID‐19 pandemic has led to devastating health outcomes with the death toll exceeding two million cases as of February 2021. However, there is still limited data on long‐term immunity against SARS‐CoV‐2. Long‐term immunity can be analysed by SARS‐CoV‐2‐specific memory T and B cell formation. We and others have reported on SARS‐CoV‐2‐specific memory T‐cell responses in acute infection and long‐term follow up. 1 , 2 To our knowledge, however, only studies with Australian and US‐American cohorts assessed long‐term BMEMORY‐cells for more than 6 months. 2 , 3 Memory B (BMEMORY)‐cells can persist lifelong, and upon reinfection can be triggered to immediately start forming plasma cells secreting neutralizing antibodies. 4 Thus, quantifying BMEMORY‐cell levels may be used as an indicator of long‐term immunity in convalescent patients. Therefore, this study aimed to delineate SARS‐CoV‐2 spike (S)‐protein‐specific BMEMORY‐cells in a well‐characterized cohort of central‐European COVID‐19‐patients up to several months after infection.
Between April and October 2020, a cohort of 27 convalescent COVID‐19 patients and 14 healthy donors were included in the study in three German centers (Berlin, Bochum, Essen). Baseline characteristics are provided in Table S1. All patients gave written informed consent. The ethical committee of the Ruhr‐University Bochum approved the study (20–6886). A schematic presentation of the protocol is depicted in Figure S1, and materials are listed in Table S2–S3.
COVID‐19 patients were included at a median time of 53 days after diagnosis or onset of symptoms (range 15–214). SARS‐CoV‐2‐specific B cell response was analysed by characterizing levels of IgD−CD27+ BMEMORY, IgD+CD27+unswitched BMEMORY, IgD+CD27− BNAIVE, and CD27++CD38++plasmablasts (Figure 1). Overall B cell composition was not affected by SARS‐CoV‐2‐infection in convalescent patients (Figure 2A). We did not observe T‐cell lymphopenia in reconvalescent COVID‐19 patients (Figure 2B).
FIGURE 1.
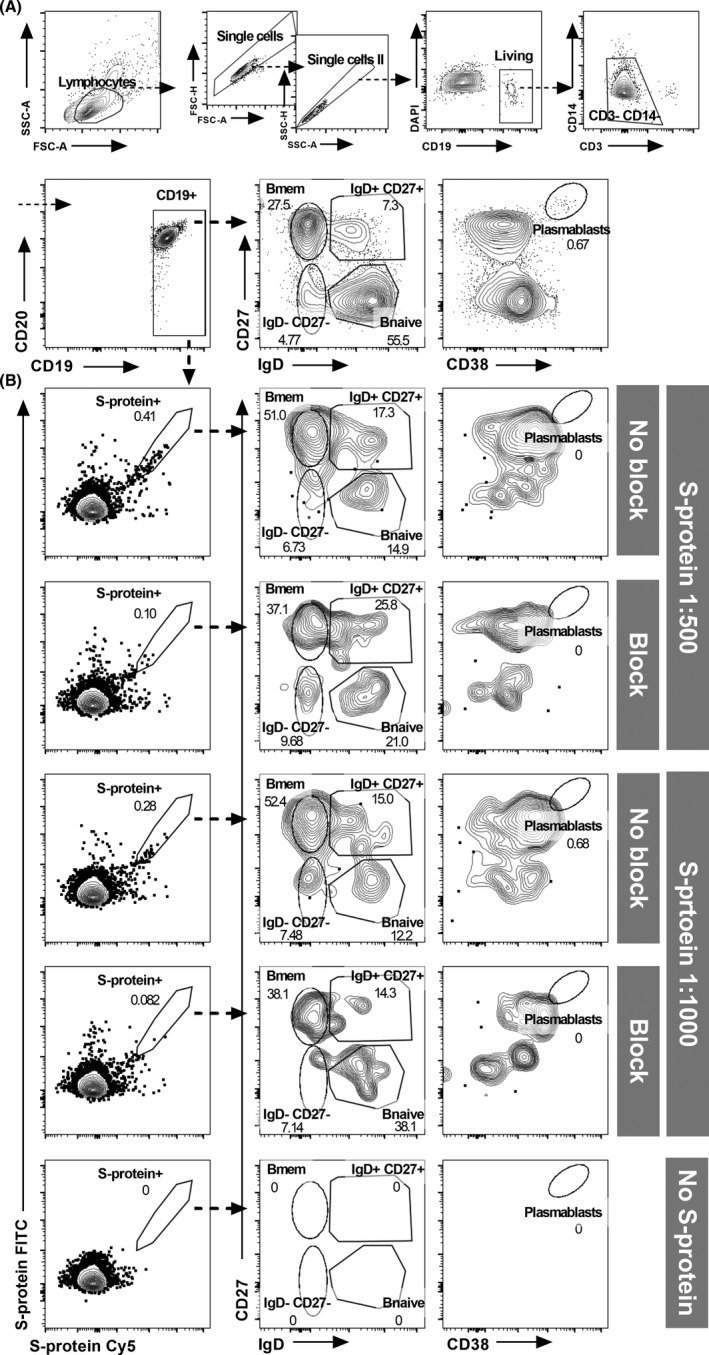
Detection of SARS‐CoV‐2‐specific <>B cell subsets. (A) Gating strategy for the detection of B cell subsets. (B) Representative example for the detection of dual‐labelled SARS‐CoV‐2 S‐protein‐binding B cells and quantification of antigen‐specific B cell subsets. Comparison of samples without fluorochrome‐coupled SARS‐CoV‐2 protein (“No S‐protein”) and SARS‐CoV‐2 S‐protein in concentrations of 1:500 (200 ng of each labelled protein in 100 µl PBS) and 1:1000 (100 ng of each labelled protein in 100 µl PBS) with and without excess unlabelled protein to block SARS‐CoV‐2‐reactive B cell receptors. Gates were set according to staining controls. To increase specificity and exclude unspecific fluorochrome‐binding cells, only B cells double‐positive for FITC and Cy5 labelled S‐protein were considered as S‐protein binding. In median, nearly 1 million lymphocytes were recorded per sample (minimum of three samples per patient, blocked and unblocked S‐protein samples and staining controls). Of these, B cells contributed about 5% (median 48,611 cells, IQR 31,141–74,189 cells). The numbers of recorded antigen‐specific cells varied greatly in the individuals. In the recovered COVID‐19 patients, we recorded in median 73 S‐protein binding cells in unblocked samples (IQR 25–163 cells)
FIGURE 2.
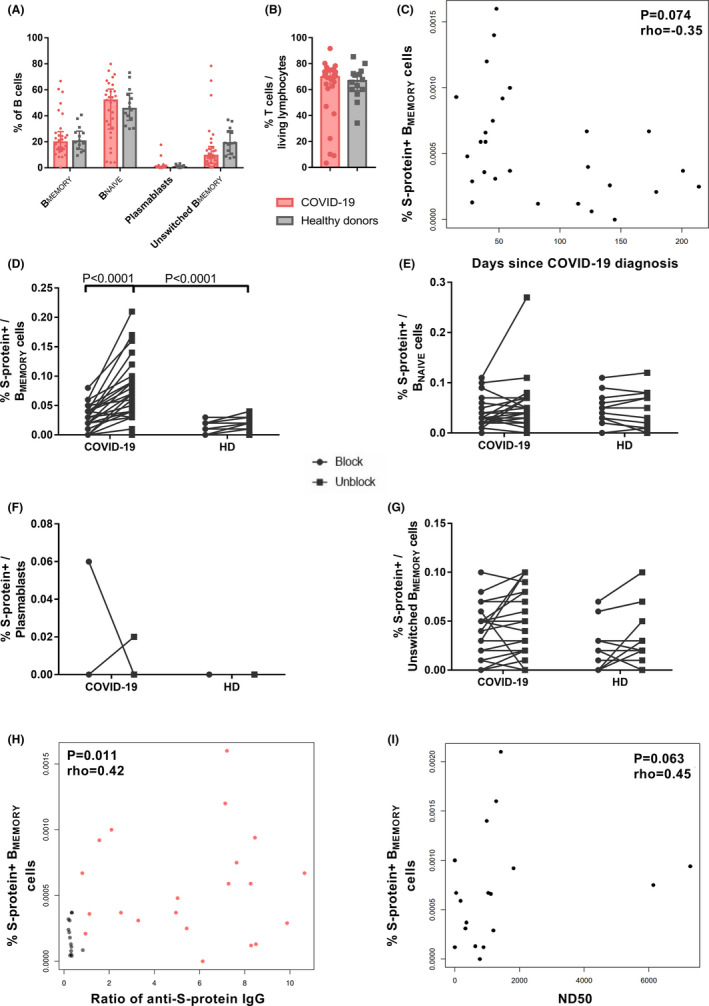
Detection of COVID‐19‐specific BMEMORY cells using spike (S)‐protein. Peripheral blood mononuclear cells of convalescent COVID‐19 patients (n = 26) and healthy donors (n = 14) were incubated with 200 ng dissolved in 100 µl PBS (1:500) Cy5 as well as FITC fluorochrome‐labelled SARS‐CoV‐2 S‐protein with or without excess unlabelled protein to block labelling. For three COVID‐19 patients, follow‐up samples from two different time points were included. All but six samples of COVID‐19 patients were directly processed without prior freezing and storage. (A) Percentage of IgD− CD27+ BMEMORY, IgD+ CD27+unswitched BMEMORY, IgD+ CD27− BNAIVE and CD27++ CD38++plasmablasts within the entire B cell population analysed for COVID‐19 disease and healthy cohorts. Bars show median with interquartile range. Repeated measurements two‐way ANOVA did not detect significant differences between COVID‐19 patients and healthy donors. (B) Percentage of CD3+ T cells within living lymphocytes of COVID‐19 and healthy donors. Bars show median with interquartile range. Parametric distribution was assessed with Shapiro–Wilk normality test and two‐tailed Mann–Whitney test used for statistical comparison. (C) Correlation of fluorochrome labelled SARS‐CoV‐2 S‐protein binding B cells and days after COVID‐19 diagnosis. N = 27 samples of 24 COVID‐19 patients (three patients with two samples collected at different time points). The analysis was performed with Spearman's rank coefficient, rho = −0.34, p = .095. (D)–(G) Frequencies of S‐protein binding BMEMORY (D), BNAIVE (E), plasmablasts (F), and unswitched BMEMORY cells (G) after staining with preincubation of unlabelled Covid‐19 antigen (blocked, left) and without preincubation (not blocked staining, right) samples. N = 26 COVID‐19 patients and n = 14 healthy donors. Only the first sample of patients with multiple samples was included. Statistical comparison was done with two‐way repeated measurements ANOVA and Sidak's multiple comparisons test. (H) Correlation of fluorochrome labelled SARS‐CoV‐2 S‐protein binding BMEMORY‐cells and anti‐S1/S2‐IgG. Red dots: 21 samples of 19 COVID‐19 patients (two patients with two samples collected at different time points). Grey dots: 14 healthy donors. Analysis was performed with Spearman's rank coefficient, rho = .42, p = .011. (I) Correlation of fluorochrome labelled SARS‐CoV‐2 S‐protein binding BMEMORY‐cells and serum 50% neutralization dose titre (ND50). Virus neutralization was assessed using a propagation incompetent vesicular stomatitis pseudovirus system bearing SARS‐CoV‐2 S‐protein. N = 18 samples of 17 patients (one patient with two samples collected at different time points). Analysis was performed with Spearman's rank coefficient, rho = .45, p = .063
For flow‐cytometric analysis of specific B cells, SARS‐CoV‐2‐S‐protein was labelled with two different fluorochromes, and double‐positive B cells were determined. Blocking of specific staining by excess unlabelled S protein demonstrates specificity of labeling. 5 , 6 Unlabelled S‐protein in class‐switched BMEMORY‐cells could significantly block binding in COVID‐19 patients, but not in healthy donor samples, indicating only minimal cross‐reactivity in healthy donors (Figures 1B, 2D). Accordingly, the frequency of S‐protein‐specific BMEMORY‐cells was significantly higher in the COVID‐19‐cohort compared to healthy individuals (Figure 2D). SARS‐CoV‐2‐specific BMEMORY‐cells were also detectable 200 days after COVID‐19 diagnosis with a tendency of lower frequencies in samples collected at later time points (Figure 2C). Specific labelling with SARS‐CoV‐2 S‐protein and difference between COVID‐19 patients and healthy individuals was restricted to BMEMORY‐cells and not observed for other B cell subsets (Figure 2D–G). S‐protein‐specific BMEMORY‐cells have been described to be pivotal for effective antibody responses. 4 SARS‐CoV‐2‐specific BMEMORY‐cells correlated moderately with anti‐S‐protein IgG‐antibodies, but correlation with neutralizing antibodies did not achieve statistical significance (Figure 2H–I). Thus, we demonstrate specific detection of SARS‐CoV‐2‐S‐protein‐binding BMEMORY‐cells over 6 months post‐infection.
We acknowledge limitations of our study. Subsequent studies should enrol larger patient cohorts with longer follow‐up periods. Using bifluorescent tetramer‐based staining may increase sensitivity to detect SARS‐Cov2‐specific B cells. 6 Control patients were significantly younger than the COVID‐19‐cohort. Nevertheless, we did not observe specific staining in the control cohort as evidenced by the lack of significant blocking of the staining.
In conclusion, evaluating the long‐term immunity in a cohort of convalescent COVID‐19 patients, we demonstrated SARS‐COV‐2‐specific BMEMORY‐cells in individuals both early as well as over 6 months after infection. Thus, our study performed on a central‐European cohort is in line with the data on the recently published US‐American and Australian cohorts and accordingly, confirms and extends the knowledge on the B cell response against SARS‐CoV‐2. 2 , 3 Demonstrating the persistence of SARS‐CoV‐2‐specific B cell response, our results point towards an additional hallmark of immunisation beyond specific serum antibodies.
CONFLICT OF INTERESTS
All authors declare no competing interests.
Supporting information
App S1
ACKNOWLEDGEMENTS
We are grateful to the patients who donated their blood samples and clinical data for this project. We would like to acknowledge the excellent technical assistance as well as the expertise of the immune diagnostic laboratory of the Center for Translational Medicine at Marien Hospital Herne. Mr. Thieme has nothing to disclose. Dr. Abou‐el‐Enein has nothing to disclose and received funding from the Arab‐German Young Academy of Sciences (AGYA) which is supported by the German Federal Ministry of Education and Research (BMBF) grant 01DL20003. Mr. Fritsche has nothing to disclose. Dr. Anft has nothing to disclose. Ms. Paniskaki has nothing to disclose. Ms. Skrzypczyk has nothing to disclose. Mr. Doevelaar has nothing to disclose. Mr. Elsallab has nothing to disclose. Dr. Brindle has nothing to disclose. Dr. Blazquez‐Navarro has nothing to disclose. Dr. Seibert has nothing to disclose. Ms. Meister has nothing to disclose. Dr. Pfänder has nothing to disclose. Dr. Steinmann has nothing to disclose. Dr. Witzke has received research grants for clinical studies, speaker’s fees, honoraria and travel expenses from Amgen, Alexion, Astellas, Basilea, Biotest, Bristol‐Myers Squibb, Correvio, Chiesi, Gilead, Hexal, Janssen, Dr. F. KöhlerChemie, MSD, Novartis, Roche, Pfizer, Sanofi, TEVA, and UCB. Dr. Westhoff has nothing to disclose. Dr. Stervbo has nothing to disclose. Dr. Heine reports personal fees from Allergopharma, Biotest, Eli Lilly, Sanofi, and Abbvie, outside the submitted work. Dr. Roch has nothing to disclose. Dr. Babel has nothing to disclose and received funding from the Mercator Foundation, the German Federal Ministry of Education and Research (BMBF) e:KID (01ZX1612A), and BMBF NoChro (FKZ 13GW0338B).
Thieme, Abou‐el‐Enein, Heine, Roch and Babel equal contribution
REFERENCES
- 1. Thieme CJ, Anft M, Paniskaki K, et al. Robust T Cell Response Toward Spike, Membrane, and Nucleocapsid SARS‐CoV‐2 Proteins Is Not Associated with Recovery in Critical COVID‐19 Patients. Cell Reports Med. 2020;1(6):100092. 10.1016/j.xcrm.2020.100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartley GE, Edwards ESJ, Aui PM, et al. Rapid generation of durable B cell memory to SARS‐CoV‐2 spike and nucleocapsid proteins in COVID‐19 and convalescence. Sci Immunol. 2020;5(54):eabf8891. 10.1126/sciimmunol.abf8891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson P, Stamper C, Dugan H, et al. Distinct B cell subsets give rise to antigen‐specific antibody responses against SARS‐CoV‐2. Res Sq. 2020. Preprint 10.21203/rs.3.rs-80476/v1 [DOI] [Google Scholar]
- 5. Roch T, Giesecke‐Thiel C, Blazquez‐Navarro A, et al. Generation of HBsAg‐reactive T‐ and B‐cells following HBV vaccination in serological non‐responders under hemodialysis treatment. Eur J Immunol. 2021;51(5):1278‐1281. [DOI] [PubMed] [Google Scholar]
- 6. Cossarizza A, Chang HD, Radbruch A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol. 2019;49 : 1457‐1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


